- 1Cranfield Environment Centre, Cranfield University, Bedford, United Kingdom
- 2School of Biosciences, University of Nottingham, Loughborough, United Kingdom
- 3Centre for Soil, Agrifood and Biosciences, Cranfield University, Bedford, United Kingdom
Targeted crop selection offers a promising potential pathway to reduce greenhouse gas (GHG) emissions from global croplands. Yet, the influence of crop genotypes on GHG emissions remains poorly studied, limiting our ability to understand its global potential. To address this challenge, we conducted a global synthesis of GHG and crop yield data from 42 field experiments across 180 genotypes of major cereal (predominantly rice) and oilseed crops (soybeans and canola) and nitrogen (N) fertilisation rates (40kg ha-1 to 390kg ha-1) (n =390). To test the influence of genotype, we removed measurements from genotypes with fewer than three independent replicates (n = 97) and apply linear mixed-effects models to control for study and latitude effects. Across a range of environmental and experimental conditions, we analysed the influence of N application rate on crop nitrous oxide (N2O) and methane (CH4) emissions, alongside yield. We found significant differences in N2O-N cumulative fluxes between crop types and mean annual precipitation ranges. When expressed per unit of crop yield, N2O-N and CH4-C cumulative fluxes revealed a significant difference between N application rate groups (a = < 50, b = 50-100, c = 100-150, d = 150-200, e = 200-250, f = 250-300, g = > 300), with a positive yield response to N fertilisation. While yield-scaled N2O-N cumulative fluxes declined with N application rate, yield-scaled CH4-C cumulative fluxes increased; however, all CH4 measurements were derived from rice systems. Regression relationships between cumulative N2O, CH4, crop yield and N application rate were consistent with previous global syntheses, showing that N2O and CH4 emissions increased exponentially with N application, while crop yield exhibited a quadratic response. Our results indicate that N application rate was the primary driver of N2O emissions and crop yield, while genotypic differences significantly influenced CH4 emissions. These findings underscore the importance of integrating genotype selection with nitrogen management to improve GHG mitigation while optimising crop productivity.
Introduction
Agricultural intensification to meet global food demand has made farming one of the largest contributors to anthropogenic greenhouse gas (GHG) emissions (Lynch et al., 2021; Tilman et al., 2011), contributing approximately 58% of global non-carbon dioxide (CO2) GHG emissions. GHGs such as nitrous oxide (N2O) and methane (CH4) further have global warming potentials 273 and 81 times greater than CO2, respectively over a 20-year time period (Beach et al., 2008; IPCC, 2023). Agricultural GHG emissions exacerbate climate-related environmental problems, including biodiversity loss, eutrophication and reductions in soil carbon stocks (Johnson et al., 2014; Vitousek et al., 1997). Several cost-effective and sustainable agricultural mitigation opportunities and practices are accessible for agriculture compared to other sectors (Lai et al., 2022; Smith, 2012; Vergé et al., 2007; Zutshi and Creed, 2015).
Nitrogen (N) fertilisation remains a cornerstone of yield maximisation in modern cropping systems but is also a major source of N2O emissions (Guo et al., 2022; Sun and Huang, 2012). Projections suggest that N fertiliser use could triple by 2050 (Khampuang et al., 2021) and so there is an urgent need to improve Nitrogen Use Efficiency (NUE) in crops to mitigate the negative effects of N application (Zhang et al., 2015). Previous meta-analyses have identified optimal N application rates for key global crops such as wheat, rice, and maize in the range of 130 to 200 kg N ha-¹, emphasising not only rate optimisation but also soil, climate, and genotype selections as complementary mitigation strategies (Guo et al., 2022). A promising strategy for reducing agricultural GHG emissions is the selective breeding for crop genotypes that exhibit higher NUE, enabling lower fertiliser inputs while maintaining high yield (Shcherbak et al., 2014; Swarbreck et al., 2019). Empirical studies have shown that crop genotypes can significantly vary in their N2O and CH4 emissions due to physiological and morphological characteristics such as transpiration rate, xylem vessel diameter, and root architecture (Borah and Baruah, 2016; Chen et al., 2021). Modern wheat genotypes have demonstrated lower N2O emissions, potentially due to physiologically more efficient N uptake rather than observable shifts in plant morphology (Chen et al., 2021). Other traits such as flag-leaf senescence and nitrogen accumulation at anthesis have also been linked to NUE (Gaju et al., 2011).
Optimal N fertilisation management can play an important role in NUE for oilseed rape genotypes (Berry et al., 2010). Whilst a large portion of these studies show a significant difference in N2O and CH4 emissions between genotypes, the resulting data and conclusions are frequently limited due to a limited number of genotypes used or low levels of replication (Chen et al., 2021; Gogoi and Baruah, 2012). Many studies which observe GHG emissions between different crop varieties investigate other factors which may be affected by the crop variety such as water management, further complicating direct comparisons (Ma et al., 2012; Phungern et al., 2023; Vo et al., 2024).
Genotypic variation can influence soil nitrification and denitrification by actively changing soil properties, such as N availability (through N uptake) and pH change via root exudation, respiration and NUE capacity (Philippot et al., 2013). Different genotypes can also be more or less effective at transporting N2O from the soil via root uptake and releasing into the atmosphere (Baruah et al., 2010; Verma et al., 2006). The varieties with a n optimal root architecture will have greater NUE and have a reduced quantity of N2O byproduct. Plants can also produce N2O directly via N assimilation (Smart and Bloom, 2001), and different genotypes can have variable N2O production during nitrogen assimilation (Oszvald et al., 2022). Genotypic variation can also influence plant-mediated transfer of CH4 from the soil to the atmosphere due to differences in aerenchyma, root exudates and root oxygen (Aulakh et al., 2000; Girkin et al., 2018, 2020). Optimisation of agricultural management practices through selective breeding for crop genotypes with optimised NUE, sustained crop yields and low GHG emissions would assist in achieving a more sustainable agricultural produce (Ceccarelli and Grando, 2020).
Various studies have reported genotypic variation in crop GHG emissions, but their findings are limited by narrow genotypic comparisons and inconsistent experimental replications, often focusing on a small number of genotypes within a single environment (Gogoi and Baruah, 2012; Chen et al., 2021; Phungern et al., 2023). To uncover the role of genotypic variation in GHG emissions from crops, especially under variable N fertilisation, a broader data synthesis is needed. While the concept that genotype impacts on GHG emissions are context-dependent and most pronounced at intermediate or suboptimal N rates is established in the literature. Here we provide the first global synthesis quantifying the magnitude and consistency of the genotypic effect on GHG emissions across studies.
Methods
Literature search and data collection
We conducted a systematic literature review using the Scopus database following PRISMA guidelines (Haddaway et al., 2022). We searched for peer-reviewed journal articles published up to April 2024 using the following keywords: (wheat OR rice OR barley OR oat OR linseed OR “oil seed rape” OR maize OR corn) AND (genotype OR genotype OR variety OR varieties) AND (“nitrous oxide” OR N2O) AND NOT (livestock OR cattle). These crops were selected due to their high importance for global food systems and crop rotation systems. From 221 initially identified records, 42 studies met the inclusion criteria (Supplementary Figure 1), reporting GHG emissions from at least two genotypes of the same crop species under field conditions and provided sufficient data to quantify cumulative N2O or CH4 fluxes. See Supplementary Table 2 for a summary of the 42 studies included.
Many studies primary focus was not genotypic variation in greenhouse gas emissions and instead their primary focus was on specific management interventions. These interventions were not considered due to insufficient information across studies. A significant proportion of our compiled dataset came from studies focused on rice systems (35 of 42 studies) and there was a limited number of studies that included more than one crop (Supplementary Table 2). Most sources also studied a limited number of genotypes (< 3). This data sparsity limited our genotype analysis as many genotypes across several studies would be optimal for robust analyses. Many studies were conducted over multiple years however, providing replication of genotypes within studies.
Data extraction and standardisation
From each of the 42 studies identified, we extracted relevant metadata and experimental data including: crop species, genotype names, N fertiliser application rates, mean annual precipitation (MAP, mm), mean annual temperature (MAT,°C), measurement techniques, cumulative and daily GHG fluxes (N2O-N, CH4-C, and CO2 where available), study duration, and crop yield (where reported). Data were extracted directly from tables, text, figures (via WebPlotDigitizer), or Supplementary Materials. Where necessary, units were standardised to kg ha-¹ for cumulative emissions. Cumulative N2O-N fluxes were derived by converting N2O mass to nitrogen mass using a factor of 14/44.01, and CH4-C fluxes were converted using 12/16 from CH4 mass to carbon mass (IPCC, 2023). For cases where only cumulative N2O-N or CH4-C were reported, average daily fluxes were back-calculated based on the reported measurement period. The compiled dataset included 391 data entries across 180 unique genotypes. Most data entries were for rice (n=319), followed by wheat (n=27), maize (n=20), canola (n=18) and soybean (n=3). Geographically, most data entries were from studies conducted in China (n=171), followed by Vietnam (n=80), India (n=75), Canada (n=18), Indonesia (n=16), USA (n=12), Japan (n=11) and Brazil (n=4) (Figure 1). Four data entries were removed as obvious outliers as they were associated with atypical treatments (e.g. chemical nitrate transport inhibitors) that distorted emission outcomes (Iqbal et al., 2023).
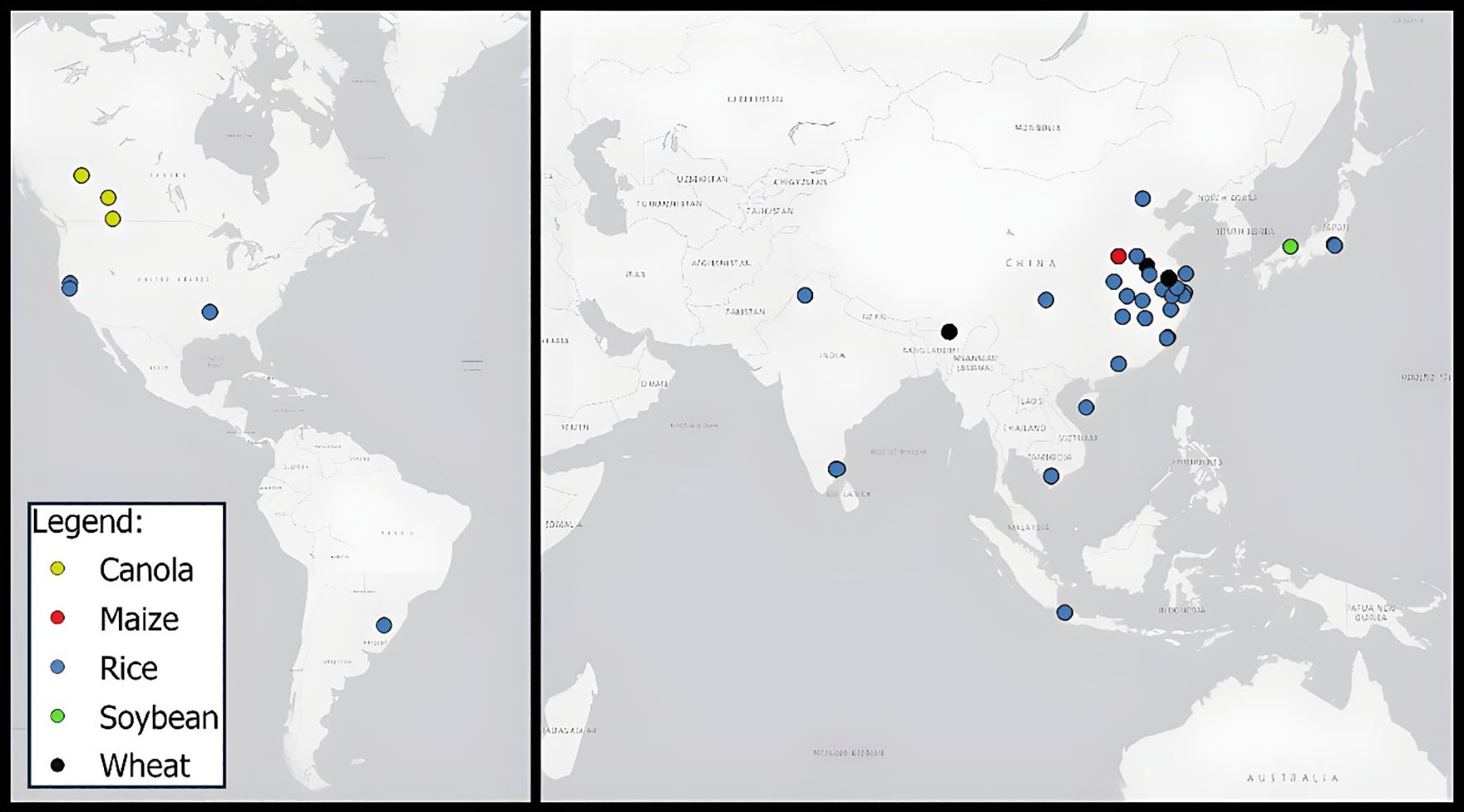
Figure 1. Distribution of studies reporting crop genotypic variation effects on GHG emissions, compiled in this study, showing a bias towards Asia and rice (crop type indicated by symbol colour).
Study locations were restricted, with 23 from China, 9 from India, 3 from the USA, 3 from Japan and 1 from Vietnam, Brazil, Canada and Indonesia (Figure 1). This sampling bias reflects the high proportion of studies focusing on rice systems, as 90% of global rice production is conducted within Asian countries, which make up the majority of the results in our study (Fukagawa and Ziska, 2019). The majority of studies in our dataset used static chambers for GHG measurements (n=41) and few studies employed automated systems (n=1). Static chambers may underestimate episodic fluxes due to limited temporal coverage.
Data analysis
All analyses were performed in R 4.2.2 (R Core Team, 2024). Descriptive statistics and one-way ANOVAs were used to compare N2O-N cumulative and CH4-C cumulative fluxes across crop type, measurement method, chamber type, and N fertilisation, MAT and MAP groups categorised using terciles to assess potential climatic and management effects. One-way ANOVA’s and Tukey’s T-tests for pairwise comparisons were used to assess the significance of study factors on GHG emissions (p < 0.05). General linear models were applied to test the form and significance of N application rate on crop yield, N2O and CH4 emissions across all available measurements in the compiled dataset. To test the influence of genotype, measurements from genotypes with fewer than three independent replicates were excluded to ensure model robustness, resulting in 20 genotypes across 97 observations. To evaluate whether the linear mixed-effects models (LMMs) presented in Table 1 were constrained by dataset size due to the exclusion of genotypes with fewer than three replicates, we tested additional models. These included LMMs with genotypes having ≥2 replicates, multilevel LMMs incorporating genotype as a random effect, and meta-regressions using all available genotypes with constant variance assumptions due to the absence of standard error data. Model performance was assessed using marginal (R²m) and conditional (R²c) R-squared values, with results reported in Supplementary Table 1. The threshold of three replicates per genotype was selected to reduce the risk of overfitting in mixed models. We fitted LMMs using the “lme4” package, with crop genotype and N application rate as fixed effects, and Latitude and Study as random intercepts to account for geographical and experimental heterogeneity. Model selection was based on an increased goodness of fit of the LMMs to the data according to the Akaike Information Criterion (AIC) where ΔAIC was < -2 for each additional degree of freedom.
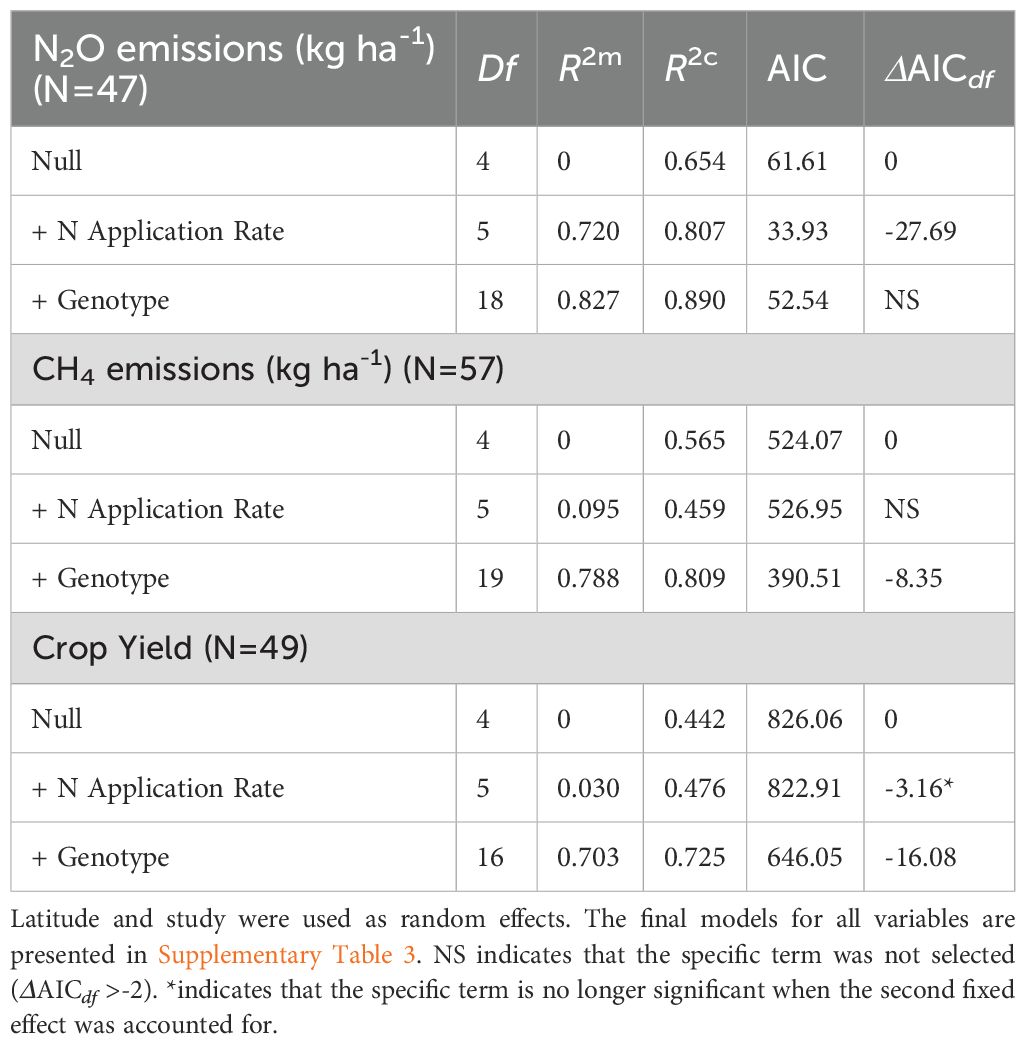
Table 1. Summary of linear mixed effect model results, testing the influence of N application rate and genotype on N2O emissions, CH4 emissions and crop yield as fixed effects.
Results
Environmental and experimental drivers of N2O emissions
Note that flux data were log-transformed prior to analysis to normalize residuals and stabilize variance. Back-transformed values approximate relative changes (fold differences) rather than absolute changes, which should be considered during interpretation. N2O-N cumulative fluxes varied significantly among the crops (Figure 2A). Rice exhibited more variable fluxes, while soybean showed the lowest, and maize and wheat exhibited the highest fluxes, respectively. N2O-N cumulative fluxes measured in field conditions were significantly higher than those from pot experiments (Figure 2B), indicating that pot studies may underestimate field-scale N2O emissions, possibly due to constrained root growth in pots or altered microclimate effects. There was no statistically significant difference in N2O-N cumulative fluxes between open-system and closed-system chambers, suggesting that chamber design does not bias overall emission estimates in our dataset (Figure 2C). No significant difference was observed across MAT categories (<10°C, 10–20°C, >20°C), indicating that temperature alone may not be a strong forecaster of N2O-N cumulative fluxes (Figure 2D). However, N2O-N cumulative fluxes were significantly influenced by MAP groups, particularly between moderate precipitation conditions (500–1000 mm) compared to dry regions (<500 mm) and wetter regions (>1000 mm) (Figure 2E), suggesting that moderate moisture levels may support microbial activity leading to greater N2O emissions. Surprisingly, no significant difference was observed among N application rate groups (<100, 100–200, >200 kg ha-¹), suggesting that the relationship between N input and emissions may be nonlinear or masked by crop-specific or environmental interactions (Figure 2F).
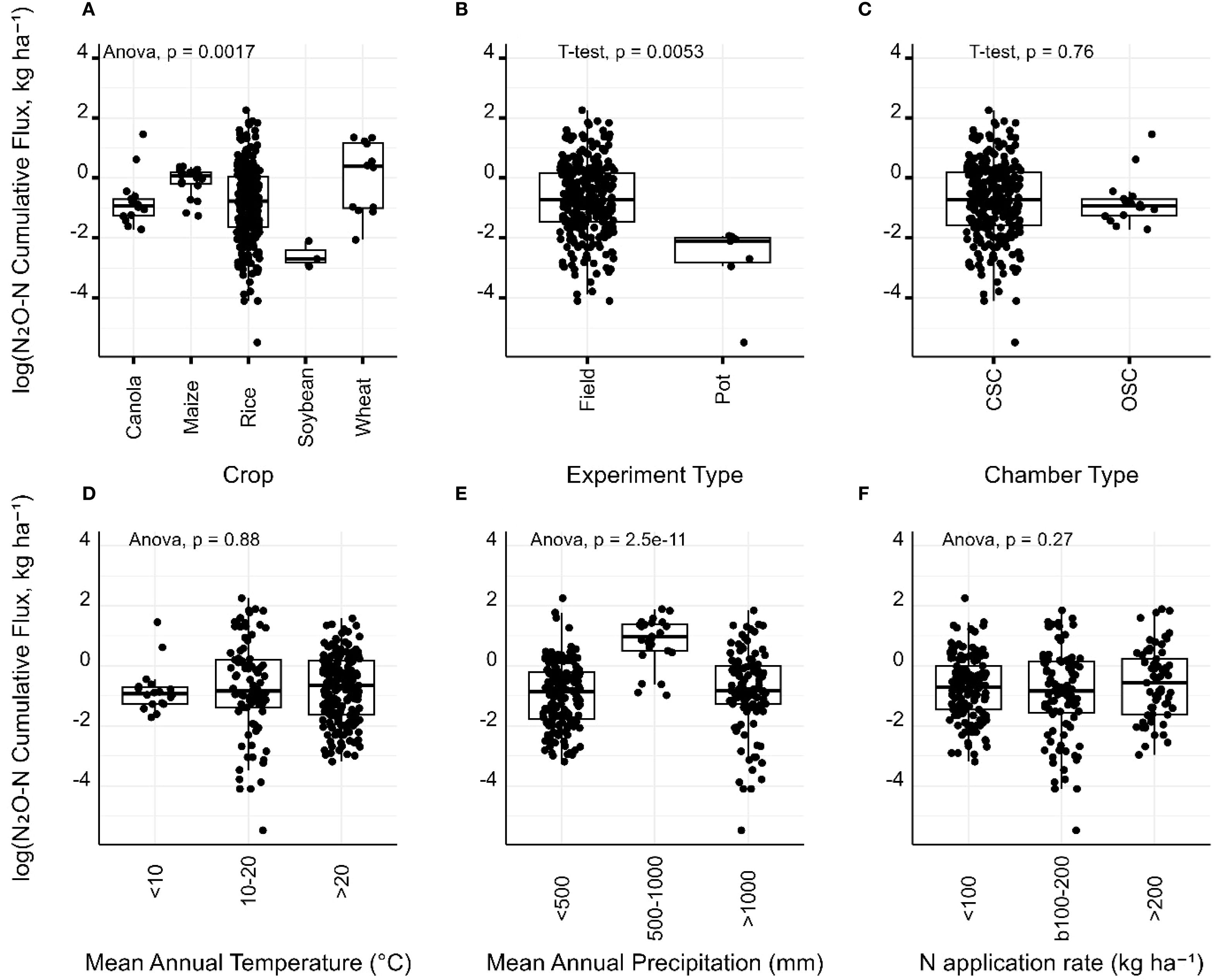
Figure 2. Boxplots showing variation in log-transformed cumulative N2O-N fluxes across (A) crop types, (B) experimental setting (field vs. pot), (C) chamber type (closed system chamber (CSC) vs. open system chamber (OSC)), (D) mean annual temperature (MAT), (E) mean annual precipitation (MAP), and (F) nitrogen fertiliser application rate. Anova and t-test p values indicate significance levels between groups, and boxes represent interquartile ranges, horizontal lines indicate medians and symbols denote individual data points. Flux data was log-transformed to normalise residuals and stabilise variance.
Yield and emissions efficiency relative to nitrogen application rates
Crop yield and yield-adjusted GHG emissions were explored across different N application rate groups (Figure 3). Crop yield enhanced significantly with increasing nitrogen fertilisation, although no increase was observed between 100–200 and >200 kg N ha-¹ (Figure 3A). The <100 kg N ha-¹ group produced the lowest yields, suggesting a yield-limiting nitrogen deficit in many low-input trials. No significant difference was observed for CH4-C cumulative fluxes across N application rates (Figure 3B), similar to N2O-N cumulative flux responses (Figure 2F). Yield-scaled N2O fluxes (Figure 3C) and CH4 emissions (Figure 3D), however, did show a significant response to N application rates. Yield-scaled N2O emissions decreased significantly with increasing N fertiliser input (Figure 3B). The <100 kg N ha-¹ group exhibited the highest emissions per unit of yield compared to sharply lower values in the 100–200 and >200 kg N ha-¹ groups. Conversely, yield-scaled CH4 emissions increased significantly with N application rate, although no measurements were available for the low (<100 kg ha-1) N application group (Figure 3D).
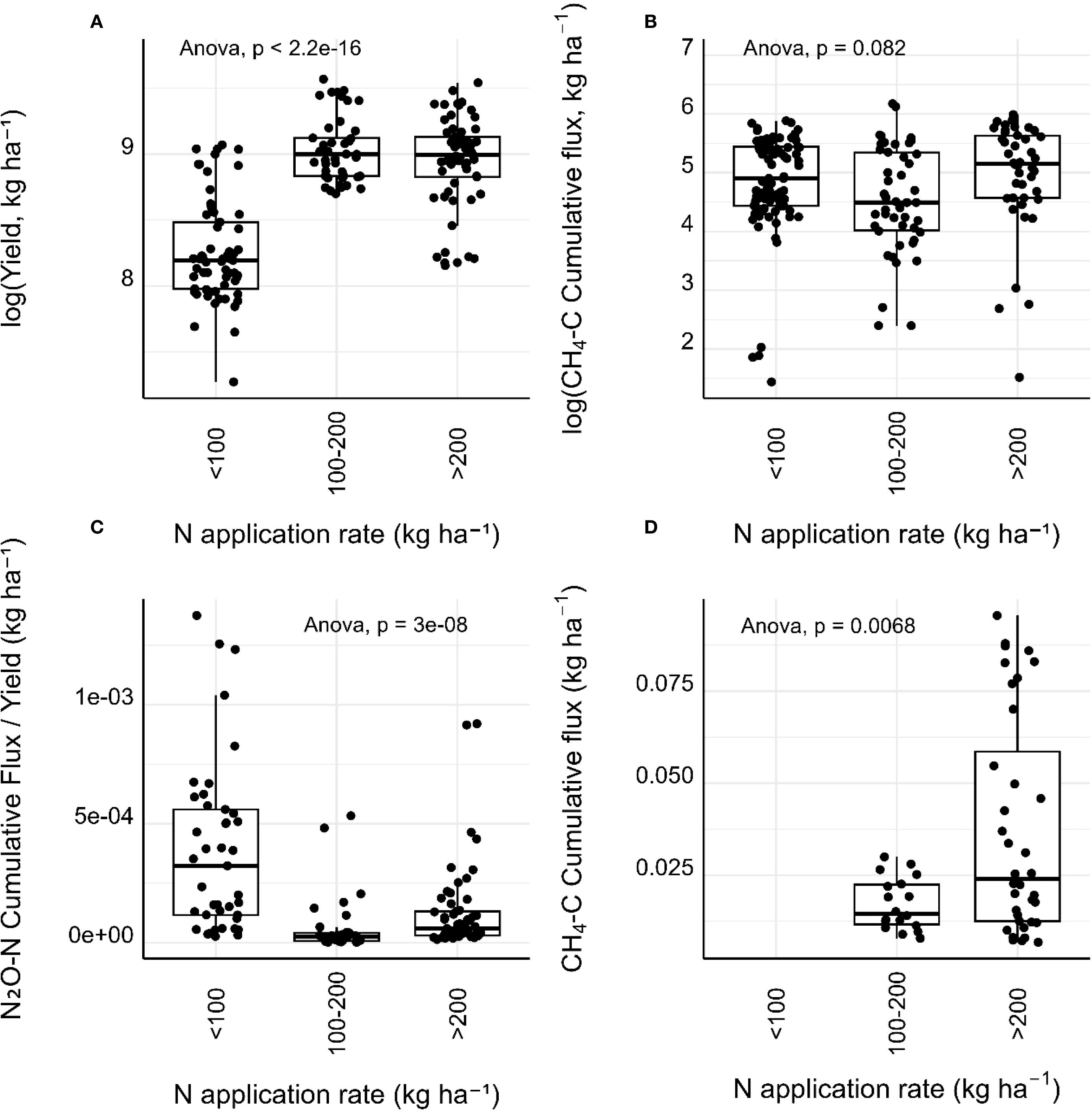
Figure 3. Boxplots showing (A) log-transformed crop yield (kg ha-¹), (B) cumulative CH4-C emissions, (C) cumulative N2O-N emissions per unit yield, and (D) cumulative CH4-C emissions per unit yield, across nitrogen application rate groups. Anova p values indicate significance levels between groups, and boxes represent interquartile ranges, horizontal lines indicate medians and symbols denote individual data points. Flux data was log-transformed to normalise residuals and stabilise variance.
Relationships between crop yield, GHG emissions and nitrogen application
Regression analyses examining the relationships between N fertilisation rate with N2O emissions, crop yield, and CH4 emissions revealed different N responses (Figure 4). N2O emissions increased exponentially with nitrogen application rate, showing a strong nonlinear relationship (Figure 4A, adjusted R²=0.284, p < 0.0001). Crop yield exhibited a curvilinear response to nitrogen input (Figure 4B, R²=0.158, p < 0.0001), peaking around 180–200 kg N ha-¹. Below this threshold, yields increased steeply with added N, while beyond this threshold yield gains diminished or plateaued. CH4 emissions also increased with nitrogen application, though the relationship was weaker than for N2O and yield (Figure 4C, adjusted R²=0.159, p < 0.0001).
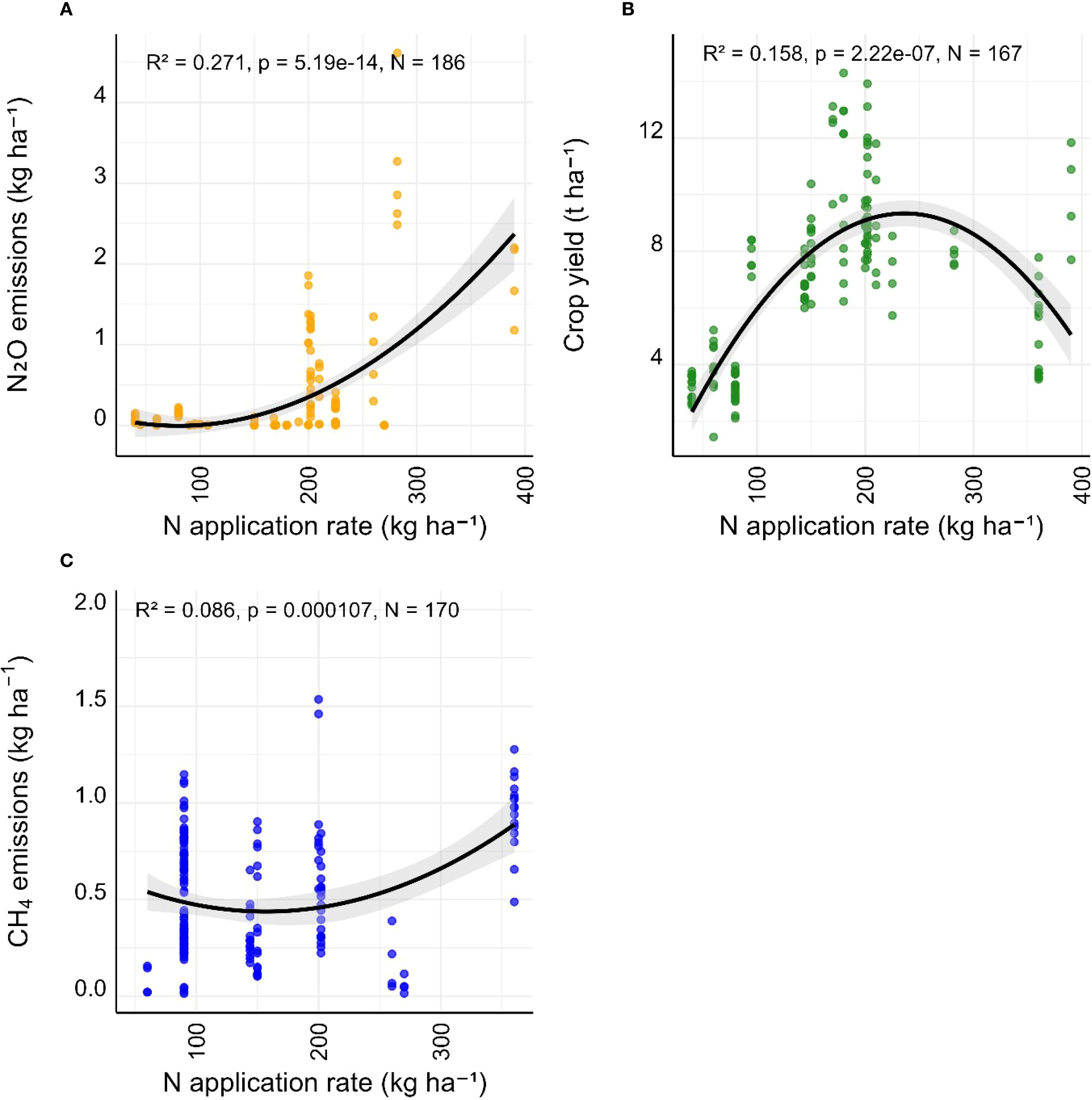
Figure 4. Regression relationships between nitrogen application rate (kg ha-¹) and: (A) N2O emissions, (B) crop yield (t ha-¹), and (C) CH4 emissions. Each panel shows fitted regressions with 95% confidence intervals (grey shading). Statistical parameters from the model fits are shown in each panel.
Influence of N application and genotypic variation on GHG emissions and crop yield
Twenty genotypes, with at least three replicates each, were recorded in our dataset (N=97). We applied LMMs to the filtered genotype dataset, with latitude and study as random effects, and tested null models against models with N application rate and genotype as fixed effects (Table 1). When this dataset was further filtered to include available measurements for N2O emissions and crop yield with N application rate measurements, n=47, n=57 and n=49 measurements were available to fit the N2O, CH4 and crop yield models, respectively. Selected models, based on goodness of fit and model parsimony, included N application rate for N2O and crop yield, and genotype effect for CH4 and crop yield (Table 1). All models showed good explanatory power of the fixed effects (R²m=0.720, R²m=0.788, R²m=0.703). However, while N application rate explained much of the variation in N2O emissions (ΔAICdf=–27.69), genotype variation had a much greater explanatory power for CH4 (ΔAICdf=–8.35) and crop yields (ΔAICdf=–16.08, Table 1).
Observed and predicted values from the best-fitting LMMs for N2O emissions and crop yield are compared in Figure 5. Comparison of the LMMs in Table 1 with alternative models, including those with genotypes having ≥2 replicates, multilevel LMMs with genotype as a random effect, and meta-regressions, did not indicate a consistent improvement in explanatory power for N2O emissions (Supplementary Table 1). For N2O, the original model with ≥3 replicates yielded the highest conditional R-squared, suggesting robust capture of variance by fixed and random effects. In contrast, the explanatory power for CH4 flux improved with dataset size, with the LMM including genotypes with ≥2 replicates achieving higher marginal R-squared, indicating a stronger influence of genotype when more data were included. For crop yield, the original models maintained high explanatory power, with minimal gains from alternative approaches. Meta-regressions, limited by constant variance assumptions, provided limited additional insight due to convergence issues for N2O and yield.
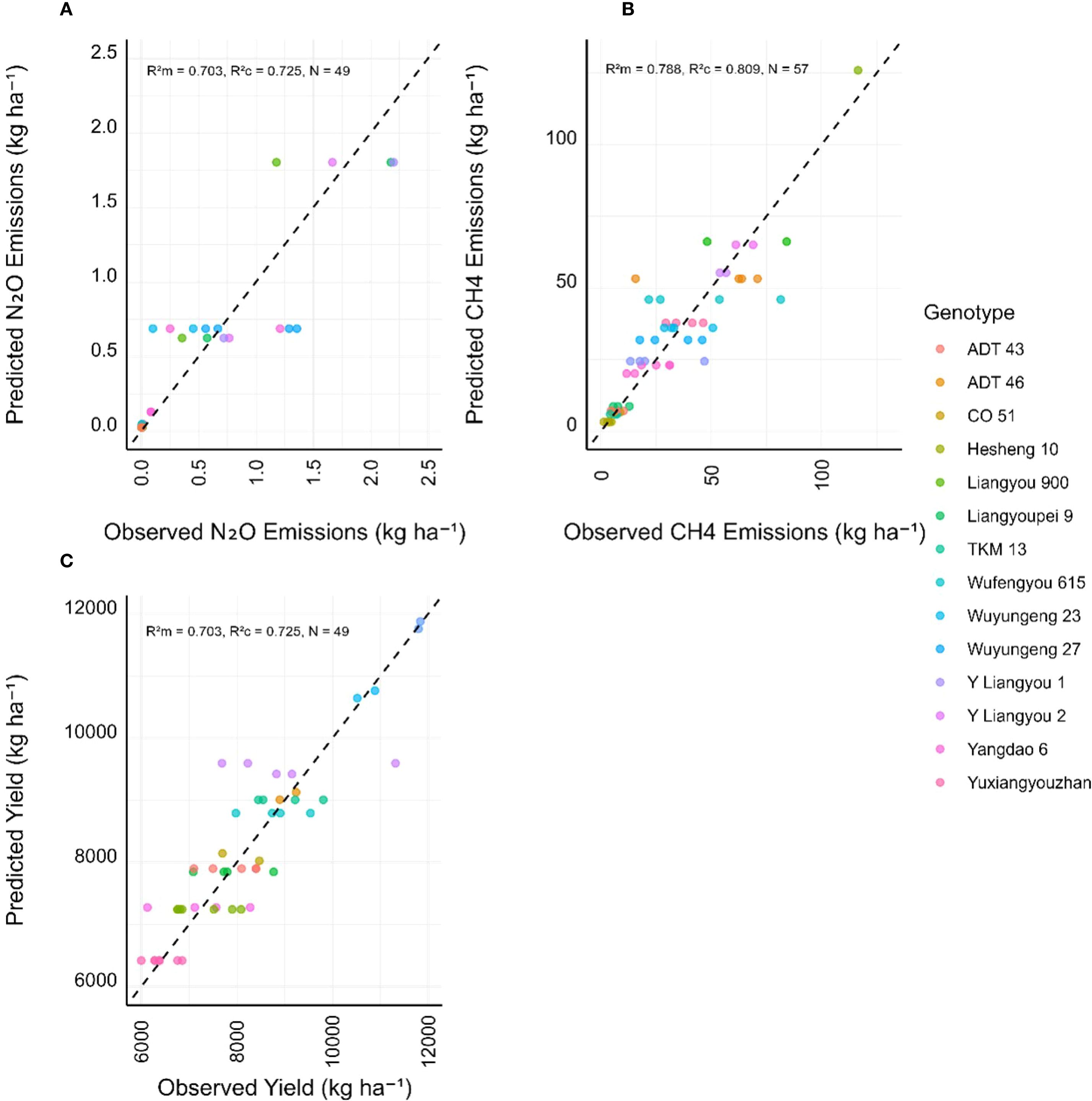
Figure 5. Predicted vs observed (A) N2O emissions, (B) CH4 emissions and (C) crop yield for the best fitting LMMs identified in Table 1. Dashed lines represent a perfect 1:1 fit, and marginal and conditional R2 values are shown. Random effects in the models were latitude and study for all variables, with (A) N application rate, (B) N application rate and genotype and (C) genotype selected as fixed effects. Symbol colour represents different genotypes.
The observed clustering of many genotypes near the x-axis and below the 1:1 line for N2O predictions (Figure 5A) suggests the model tended to underpredict higher N2O values, particularly in genotypes or contexts with elevated emissions. In comparison, predictions for CH4 (Figure 5B) and crop yield (Figure 5C) were more tightly aligned along the 1:1 line and together with Table 1 suggests that genotypic differences meaningfully capture variability in both response variables.
Discussion
Our global synthesis highlights the complexity of factors influencing N2O and CH4 emissions from widely grown cereal and oilseed cropping systems, particularly under variable N fertilisation regimes. While environmental variables such as MAP and experimental setting explained a substantial portion of the variability in cumulative N2O fluxes (Figure 2), N application rate effects were more apparent when fluxes were scaled by crop yield (Figure 3). Across crops, N2O emissions increased exponentially with N fertiliser rate and this trend is consistent with previous meta-analysis studies examining the response of N2O emissions to N fertiliser rate (Guo et al., 2022; Shcherbak et al., 2014). Crop yield followed a saturation curve, and CH4 emissions showed a weaker but significant positive trend with increasing nitrogen inputs (Figure 4). Given the prevalence of rice systems in our dataset, however, these patterns likely reflect conditions typical of flooded paddy cultivation. Across genotypes with sufficient replication, we found that while genotype has a pronounced effect on CH4 emissions and crop yield, the role of genotypes in explaining N2O emissions is comparatively limited, with emissions primarily driven by fertiliser rate and contextual factors (Figure 5) (Hansen et al., 2019). Genotype had a strong effect on CH4 but not N2O, likely because CH4 flux is strongly plant-mediated via root aerenchyma development, radial oxygen loss, and exudates, which vary markedly between genotypes. By contrast, N2O emissions are more tightly coupled to soil microbial processes (nitrification/denitrification) and strongly masked by environmental drivers (moisture, N input), thereby obscuring genotype effects. Research shows stronger genotype effects on N2O only under controlled or single-site studies, suggesting environment × genotype interactions are key (Chen et al., 2021; Peyrard et al., 2016). These biases in our dataset (overrepresentation of rice and temperate regions) likely amplify genotype effects on CH4 in flooded systems while limiting detection of N2O effects in uplands. Our findings reinforce the need for mitigation strategies in global croplands that integrate genetic, environmental, and management dimensions, and the need to address several research gaps for more comprehensive analyses of genotypic effects on crop GHG emissions, including an improved understanding of the underlying physiological and microbial mechanisms.
Several plausible mechanisms may underlie the influence of crop genotype on N2O emissions (Baggs et al., 2023). Genotypic differences in root morphology and architecture can influence N uptake, soil aeration, and microbial habitat conditions, thereby regulating the balance between nitrification and denitrification. Root exudates and rhizosphere pH modification may also alter microbial community composition and activity, further contributing to variation in fluxes (de Klein and Di, 2018). Differences in nitrogen assimilation efficiency and senescence traits may additionally affect the timing and magnitude of N2O release (Wingler and Soualiou, 2025). Future research should investigate these mechanisms in interdisciplinary studies combining plant physiology and soil microbial ecology (Philippot et al., 2009).
Environmental and experimental context effects
Cumulative N2O emissions varied widely across crop types and experimental settings (Figure 2), with rice exhibiting lower emissions than maize or wheat, consistent with differences in aeration, soil moisture, and denitrification potential between flooded and upland systems. In contrast to rice systems, oilseed studies in our dataset reported either neutral or slightly positive CH4 fluxes. This is consistent with evidence that nitrogen fertilisation can suppress methane oxidation, thereby reducing the soil sink capacity (Sun et al., 2016). Such dynamics highlight the need for broader research beyond rice, particularly in upland systems where methane oxidation plays a larger role in net fluxes. The absence of a clear temperature effect but significant differences across precipitation categories suggests that water availability may be a more direct driver of microbial N2O production than temperature in these systems, which could be further influenced by approximately 80% of our dataset consisting of genotypes grown in flooded rice paddies. Prior studies have suggested that soil moisture is a key driver of N2O production predominantly through control of nitrification and denitrification (Butterbach-Bahl et al., 2013; C. Wang et al., 2021), however these studies have also emphasised how temperature influences N2O production. Additionally, the higher emissions observed under field conditions compared to pot studies should be considered when pot trial datasets are used to estimate field-scale GHG dynamics.
Interestingly, differences in N2O-N emissions across N application groups were not statistically significant (Figure 2F), but more significant relationships were detected by weighting fluxes by crop yield (Figure 3). Regression models demonstrate a non-linear, exponential increase in N2O with increasing N input (Figure 4A), a finding that aligns with prior meta-analyses (Guo et al., 2022; Shcherbak et al., 2014). This non-linearity is often amplified under wet soil conditions where denitrification dominates, leading to higher N2O fluxes (Girkin and Cooper, 2022; Stehfest and Bouwman, 2006). Such environmental modulation may also obscure genotype-level effects, suggesting that genotyped-based mitigation of GHG emissions will be most effective when combined with optimised N management strategies.
Trade-offs between yield and GHG emissions
Crop yield increased with nitrogen input as expected (Figure 3A), but diminishing returns were evident beyond ~200 kg N ha-¹ (Figure 4B), consistent with known saturation effects (Singh, 2024; Biswas and Ma, 2016; Sun et al., 2020). Importantly, emissions per unit yield, an indicator of NUE, declined significantly with moderate N inputs (Figures 3C, D), suggesting an emissions-efficiency threshold in the 100–200 kg N ha-¹ range. This result supports repeated calls to optimise, rather than maximise, N application rates to balance productivity with environmental sustainability (McLellan et al., 2018).
A disproportionate acceleration of N2O emissions as N fertiliser rate exceeded crop uptake capacity was observed (Figure 4A) and this was likely due to increased nitrification and denitrification activity as the excess N fertiliser acts as a substrate for these processes (Long et al., 2021; Ma et al., 2023). A similar response of CH4 emissions suggests nitrogen-induced shifts in soil redox conditions or crop-mediated CH4 dynamics, particularly in anaerobic cropping systems like rice (Figure 4C). The curvilinear response of crop yield to N application rate in Figure 4B reflects diminishing returns at high N inputs and indicate over-fertilisation in some systems, a finding which is consistent with what has already been described previously (Hu et al., 2022; Song et al., 2022).
Genotypic effects on GHG emissions and yield
Our LMMs revealed contrasting roles of species genotypes in explaining N2O emissions, CH4 emissions and crop yield. For N2O emissions, inclusion of genotype effects in the model did not significantly improve fit (ΔAIC=-18.6), and explained variance was largely captured by nitrogen input and study-level random effects (Figure 5A). This suggests that genotype might influence N2O emissions, however, those effects are obscured by environmental variability and methodological heterogeneity across the investigated studies. This can be seen when comparing separate datasets from individual studies which suggested that N2O emissions were affected directly by genotypic variation (Chen et al., 2021; Ma et al., 2012), primarily due to different genotype’s effects on soil organic carbon, the soil microbial community and a crop’s NUE (Chen et al., 2021; Gogoi and Baruah, 2012; Manco et al., 2024). On the contrary, others suggested that N2O emissions were not influenced by the genotypic variation (Phungern et al., 2023; Z.-H. Wang et al., 2021) but instead were primarily affected by other factors such as nitrogen application rate, water management or tillage type (Feng et al., 2021; Oo et al., 2018; Zhao et al., 2024). By contrast, we found genotype to have a large effect on CH4 emissions (Figure 5B) and crop yield (Figure 5C). Genotypic effect on CH4 can occur for several reasons, including varying root biomass and root radial oxygen loss (Qi et al., 2024) as well as special genotypes which develop larger aerenchyma tissues which facilitate CH4 transport from the soil to the atmosphere (Kludze et al., 1993). Many of the analysed datasets used for this study concluded that genotypic variation indeed does have an effect on CH4 emissions (Ding et al., 2022; Kou et al., 2018; Satpathy et al., 1998). Selecting genotypes for improved crop yield has been discussed extensively within the literature (Bailey-Serres et al., 2019; Stella et al., 2023), with greater resistance to disease and pests as well as greater NUE being cited as key factors for greater crop yield (Gaju et al., 2011; Tooker and Frank, 2012).
Implications for climate-smart breeding and future research needs
Taken together, our study demonstrates that N2O emissions are primarily driven by N input rate, while CH4 emissions are more genotype-driven. This highlights the importance of yield-weighted emissions as a breeding metric to balance mitigation with productivity. Breeding for low-emission genotypes may benefit from prioritising yield-weighted emissions as selection criteria, particularly under moderate N inputs where genotypic differentiation is more pronounced (Chen et al., 2021; Das and Kim, 2024). At present, we also largely lack an integrated and mechanistic understanding of all the processes by which plants regulate the soil environment, and thus the production and emissions of CH4 and N2O (Cooper et al., 2024; Snyder et al., 2009).
Future work should prioritise standardised, multi-genotype field trials of various crops that control for environmental confounders, and thus support development of new tools and models to better elucidate pathways and processed. Such experimental designs would improve detection of subtle genotypic effects on GHG emissions and facilitate integration of GHG emissions related traits into conventional or precision breeding pipelines. Such studies will also enhance global synthesis studies, by resolving critical data gaps and sampling biases that are present within this study, such as crop variety, location bias and crop treatment.
Limitations
A key limitation is the reliance on static chamber measurements in most studies, which may miss episodic N2O events and underestimate total fluxes. Future work should prioritize automated chambers for higher-resolution data, standardised trial protocols, harmonised GHG reporting and increased multi-crop coverage. Furthermore, our dataset reflects the current evidence base, with a heavy bias toward rice systems (35 of 42 studies) and temperate climates, limiting generalizability to other crops and regions. This overrepresentation likely amplifies detected genotype effects on CH4 (prevalent in flooded rice systems) while constraining insights into N2O in upland systems. Findings are thus most robust for rice and temperate zones, underscoring the urgent need for broader crop coverage and multi-location trials.
Conclusion
This global synthesis provides evidence that N application rates are primary drivers for N2O, with an exponential increase in emissions and a critical inflection point where emissions rise sharply with greater N inputs. A limited direct effect of genotype was found on absolute N2O emissions, whereas genotype was a significant explanatory variable for CH4 emissions and crop yield. Focus on yield-weighted emissions rather than absolute emissions is key to developing genotypes that mitigate GHGs while optimizing productivity. For CH4, water management and soil carbon (not studied here) are major drivers alongside genotype. The biases in the current dataset, particularly the overrepresentation of rice and temperate climate studies, underscore a critical need for globally coordinated research efforts, such as multi-location trials with a standardized set of diverse genotypes, to provide robust, balanced data for more definitive conclusions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
CW: Investigation, Visualization, Software, Data curation, Validation, Writing – review & editing, Formal Analysis, Conceptualization, Methodology, Writing – original draft, Project administration. NG: Funding acquisition, Supervision, Writing – review & editing, Resources, Methodology, Conceptualization, Project administration. ZK: Methodology, Writing – review & editing, Supervision. AJ: Conceptualization, Resources, Writing – review & editing, Project administration, Data curation, Methodology, Software, Supervision, Formal Analysis.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was funded by the Cranfield Industrial Partnership PhD Programme and Premium Crops awarded to NG. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2025.1669002/full#supplementary-material
References
Aulakh M. S., Wassmann R., Rennenberg H., and Fink S. (2000). Pattern and amount of aerenchyma relate to variable methane transport capacity of different rice cultivars. Plant Biol. 2, 182–194. doi: 10.1055/s-2000-9161
Baggs E. M., Cairns J. E., Mhlanga B., Petroli C. D., Chamberlin J., Karwat H., et al. (2023). Exploiting crop genotype-specific root-soil interactions to enhance agronomic efficiency. Front. Soil Sci. 3. doi: 10.3389/fsoil.2023.1125604
Bailey-Serres J., Parker J. E., Ainsworth E. A., Oldroyd G. E. D., and Schroeder J. I. (2019). Genetic strategies for improving crop yields. Nature 575, 109–118. doi: 10.1038/s41586-019-1679-0
Baruah K. K., Gogoi B., Gogoi P., and Gupta P. K. (2010). N2O emission in relation to plant and soil properties and yield of rice varieties. Agron. Sustain. Dev. 30, 733–742. doi: 10.1051/agro/2010021
Beach R. H., DeAngelo B. J., Rose S., Li C., Salas W., and DelGrosso S. J. (2008). Mitigation potential and costs for global agricultural greenhouse gas emissions1. Agric. Econ 38, 109–115. doi: 10.1111/j.1574-0862.2008.00286.x
Berry P. M., Spink J., Foulkes M. J., and White P. J. (2010). The physiological basis of genotypic differences in nitrogen use efficiency in oilseed rape (Brassica napus L.). Field Crops Res. 119, 365–373. doi: 10.1016/j.fcr.2010.08.004
Biswas D. K. and Ma B.-L. (2016). Effect of nitrogen rate and fertilizer nitrogen source on physiology, yield, grain quality, and nitrogen use efficiency in corn. Can. J. Plant Sci. 96, 392–403. doi: 10.1139/cjps-2015-0186
Borah L. and Baruah K. K. (2016). Nitrous oxide emission and mitigation from wheat agriculture: Association of physiological and anatomical characteristics of wheat genotypes. Environ. Sci. pollut. Res. 23, 709–721. doi: 10.1007/s11356-015-5299-4
Butterbach-Bahl K., Baggs E. M., Dannenmann M., Kiese R., and Zechmeister-Boltenstern S. (2013). Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B: Biol. Sci. 368, 20130122. doi: 10.1098/rstb.2013.0122
Ceccarelli S. and Grando S. (2020). Evolutionary plant breeding as a response to the complexity of climate change. iScience 23, 101815. doi: 10.1016/j.isci.2020.101815
Chen H., Zheng C., Chen F., Qiao Y., Du S., Cao C., et al. (2021). Less N2O emission from newly high-yielding cultivars of winter wheat. Agriculture Ecosyst. Environ. 320, 107557. doi: 10.1016/j.agee.2021.107557
Cooper H., Davidson S., Gauci V., and Girkin N. (2024). Plant controls over tropical wetland nitrous oxide dynamics: A review. Available online at: https://eartharxiv.org/repository/view/6820/ (Accessed July 9, 2025).
Das S. and Kim P. J. (2024). Rice breeding for low methane and high yields. Plant Commun. 5, 100924. doi: 10.1016/j.xplc.2024.100924
de Klein C. and Di H. (2018). Assessment of current understanding of the effects of plant species on nitrous oxide emissions. Wellington, New Zealand: Ministry for Primary Industries.
Ding H., Hu Q., Cai M., Cao C., and Jiang Y. (2022). Effect of dissolved organic matter (DOM) on greenhouse gas emissions in rice varieties. Agriculture Ecosyst. Environ. 330. doi: 10.1016/j.agee.2022.107870
Feng Z. Y., Qin T., Du X. Z., Sheng F., and Li C. F. (2021). Effects of irrigation regime and rice variety on greenhouse gas emissions and grain yields from paddy fields in central China. Agric. Water Manage. 250, 106830. doi: 10.1016/j.agwat.2021.106830
Fukagawa N. K. and Ziska L. H. (2019). Rice: importance for global nutrition. J. Nutr. Sci. Vitaminol. 65, S2–S3. doi: 10.3177/jnsv.65.S2
Gaju O., Allard V., Martre P., Snape J. W., Heumez E., LeGouis J., et al. (2011). Identification of traits to improve the nitrogen-use efficiency of wheat genotypes. Field Crops Res. 123, 139–152. doi: 10.1016/j.fcr.2011.05.010
Girkin N. and Cooper H. (2022). “Nitrogen and ammonia in soils,” in Reference Module in Earth Systems and Environmental Sciences. California, United States: EarthAirXiv. doi: 10.1016/B978-0-12-822974-3.00010-0
Girkin N. T., Turner B. L., Ostle N., Craigon J., and Sjögersten S. (2018). Root exudate analogues accelerate CO2 and CH4 production in tropical peat. Soil Biol. Biochem. 117, 48–55. doi: 10.1016/j.soilbio.2017.11.008
Girkin N. T., Vane C. H., Turner B. L., Ostle N. J., and Sjögersten S. (2020). Root oxygen mitigates methane fluxes in tropical peatlands. Environ. Res. Lett. 15, 064013. doi: 10.1088/1748-9326/ab8495
Gogoi B. and Baruah K. K. (2012). Nitrous oxide emissions from fields with different wheat and rice varieties. Pedosphere 22, 112–121. doi: 10.1016/S1002-0160(11)60197-5
Guo C., Liu X., and He X. (2022). A global meta-analysis of crop yield and agricultural greenhouse gas emissions under nitrogen fertilizer application. Sci. Total Environ. 831, 154982. doi: 10.1016/j.scitotenv.2022.154982
Hansen S., Berland Frøseth R., Stenberg M., Stalenga J., Olesen J. E., Krauss M., et al. (2019). Reviews and syntheses: Review of causes and sources of N2O emissions and NO3 leaching from organic arable crop rotations. Biogeosciences 16, 2795–2819. doi: 10.5194/bg-16-2795-2019
Haddaway N. R., Page, M. J., Pritchard C. C., and McGuinness L. A. (2022). PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev 18, e1230. doi: 10.1002/cl2.1230
Hu J., Chen G., Hassan W. M., Lan J., Si W., Wang W., et al. (2022). The impact of fertilization intensity on soil nematode communities in a Tibetan Plateau grassland ecosystem. Appl. Soil Ecol. 170, 104258. doi: 10.1016/j.apsoil.2021.104258
IPCC (2023). Climate Change 2021 – The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. 1st ed (Cambridge, United Kingdom: Cambridge University Press). doi: 10.1017/9781009157896
Iqbal M. F., Zhang Y., Kong P., Wang Y., Cao K., Zhao L., et al. (2023). High-yielding nitrate transporter cultivars also mitigate methane and nitrous oxide emissions in paddy. Front. Plant Sci 14, 1133643. doi: 10.3389/fpls.2023.1133643
Johnson J. A., Runge C. F., Senauer B., Foley J., and Polasky S. (2014). Global agriculture and carbon trade-offs. Proc. Natl. Acad. Sci. 111, 12342–12347. doi: 10.1073/pnas.1412835111
Khampuang K., Rerkasem B., Lordkaew S., and Prom-u-thai C. (2021). Nitrogen fertilizer increases grain zinc along with yield in high yield rice varieties initially low in grain zinc concentration. Plant Soil 467, 239–252. doi: 10.1007/s11104-021-05090-w
Kludze H. K., DeLaune R. D., and Patrick W. H. Jr. (1993). Aerenchyma formation and methane and oxygen exchange in rice. Soil Sci. Soc. America J. 57, 386–391. doi: 10.2136/sssaj1993.03615995005700020017x
Kou T., Hang X., Lam S. K., Chen D., and He J. (2018). Ozone pollution increases CO2 and N2O emissions in ozone-sensitive wheat system. Agron. J. 110, 496–502. doi: 10.2134/agronj2017.09.0514
Lai Y. Y., Christley E., Kulanovic A., Teng C. C., Björklund A., Nordensvärd J., et al. (2022). Analysing the opportunities and challenges for mitigating the climate impact of aviation: A narrative review. Renewable Sustain. Energy Rev. 156, 111972. doi: 10.1016/j.rser.2021.111972
Long G., Li L., Wang D., Zhao P., Tang L., Zhou Y., et al. (2021). Nitrogen levels regulate intercropping-related mitigation of potential nitrate leaching. Agriculture Ecosyst. Environ. 319, 107540. doi: 10.1016/j.agee.2021.107540
Lynch J., Cain M., Frame D., and Pierrehumbert R. (2021). Agriculture’s contribution to climate change and role in mitigation is distinct from predominantly fossil CO2-emitting sectors. Front. Sustain. Food Syst. 4. doi: 10.3389/fsufs.2020.518039
Ma Q., Qian Y., Yu Q., Cao Y., Tao R., Zhu M., et al. (2023). Controlled-release nitrogen fertilizer application mitigated N losses and modified microbial community while improving wheat yield and N use efficiency. Agriculture Ecosyst. Environ. 349, 108445. doi: 10.1016/j.agee.2023.108445
Ma Y., Wang J., Zhou W., Yan X., and Xiong Z. (2012). Greenhouse gas emissions during the seedling stage of rice agriculture as affected by cultivar type and crop density. Biol. Fertility Soils 48, 589–595. doi: 10.1007/s00374-011-0656-z
Manco A., Giaccone M., Zenone T., Onofri A., Tei F., Farneselli M., et al. (2024). An overview of N2O emissions from cropping systems and current strategies to improve nitrogen use efficiency. Horticulturae 10, Article 7. doi: 10.3390/horticulturae10070754
McLellan E. L., Cassman K. G., Eagle A. J., Woodbury P. B., Sela S., Tonitto C., et al. (2018). The nitrogen balancing act: tracking the environmental performance of food production. BioScience 68, 194–203. doi: 10.1093/biosci/bix164
Oo A. Z., Sudo S., Inubushi K., Chellappan U., Yamamoto A., Ono K., et al. (2018). Mitigation potential and yield-scaled global warming potential of early-season drainage from a rice paddy in tamil nadu, India. Agronomy 8, 202. doi: 10.3390/agronomy8100202
Oszvald M., Hassall K. L., Hughes D., Torres-Ballesteros A., Clark I., Riche A. B., et al. (2022). Genetic diversity in nitrogen fertiliser responses and N gas emission in modern wheat. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.816475
Peyrard C., Mary B., Perrin P., Véricel G., Gréhan E., Justes E., et al. (2016). N2O emissions of low input cropping systems as affected by legume and cover crops use. Agriculture Ecosyst. Environ. 224, 145–156. doi: 10.1016/j.agee.2016.03.028
Philippot L., Hallin S., Börjesson G., and Baggs E. M. (2009). Biochemical cycling in the rhizosphere having an impact on global change. Plant Soil 321, 61–81. doi: 10.1007/s11104-008-9796-9
Philippot L., Raaijmakers J. M., Lemanceau P., and van der Putten W. H. (2013). Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 11, Article 11. doi: 10.1038/nrmicro3109
Phungern S., Azizan S. N. F., Yusof N. B., and Noborio K. (2023). Effects of water management and rice varieties on greenhouse gas emissions in central Japan. Soil Syst. 7, Article 4. doi: 10.3390/soilsystems7040089
Qi Z., Guan S., Zhang Z., Du S., Li S., and Xu D. (2024). Effect and mechanism of root characteristics of different rice varieties on methane emissions. Agronomy 14, Article 3. doi: 10.3390/agronomy14030595
R Core Team (2024). R: the R project for statistical computing. Available online at: https://www.r-project.org/ (Accessed November 5, 2024).
Satpathy S. N., Mishra S., Adhya T. K., Ramakrishnan B., Rao V. R., and Sethunathan N. (1998). Cultivar variation in methane efflux from tropical rice. Plant Soil 202, 223–229. doi: 10.1023/A:1004385513956
Shcherbak I., Millar N., and Robertson G. P. (2014). Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. 111, 9199–9204. doi: 10.1073/pnas.1322434111
Singh B. (2024). Long-term fertilizer nitrogen management—Soil health conundrum. Pedosphere 34, 23–25. doi: 10.1016/j.pedsph.2023.09.013
Smart D. R. and Bloom A. J. (2001). Wheat leaves emit nitrous oxide during nitrate assimilation. Proc. Natl. Acad. Sci. United States America 98, 7875–7878. doi: 10.1073/pnas.131572798
Smith P. (2012). Agricultural greenhouse gas mitigation potential globally, in Europe and in the UK: What have we learnt in the last 20 years? Global Change Biol. 18, 35–43. doi: 10.1111/j.1365-2486.2011.02517.x
Snyder C. S., Bruulsema T. W., Jensen T. L., and Fixen P. E. (2009). Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agriculture Ecosyst. Environ. 133, 247–266. doi: 10.1016/j.agee.2009.04.021
Song Q., Fu H., Shi Q., Shan X., Wang Z., Sun Z., et al. (2022). Overfertilization reduces tomato yield under long-term continuous cropping system via regulation of soil microbial community composition. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.952021
Stehfest E. and Bouwman L. (2006). N2O and NO emission from agricultural fields and soils under natural vegetation: Summarizing available measurement data and modeling of global annual emissions. Nutrient Cycling Agroecosystems 74, 207–228. doi: 10.1007/s10705-006-9000-7
Stella T., Webber H., Eyshi Rezaei E., Asseng S., Martre P., Dueri S., et al. (2023). Wheat crop traits conferring high yield potential may also improve yield stability under climate change. In Silico Plants 5, diad013. doi: 10.1093/insilicoplants/diad013
Sun W. and Huang Y. (2012). Synthetic fertilizer management for China’s cereal crops has reduced N2O emissions since the early 2000s. Environ. pollut. 160, 24–27. doi: 10.1016/j.envpol.2011.09.006
Sun J., Li W., Li C., Chang W., Zhang S., Zeng Y., et al. (2020). Effect of different rates of nitrogen fertilization on crop yield, soil properties and leaf physiological attributes in banana under subtropical regions of China. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.613760
Sun B., Zhao H., Lü Y., Lu F., and Wang X. (2016). The effects of nitrogen fertilizer application on methane and nitrous oxide emission/uptake in Chinese croplands. J. Integr. Agric. 15, 440–450. doi: 10.1016/S2095-3119(15)61063-2
Swarbreck S. M., Wang M., Wang Y., Kindred D., Sylvester-Bradley R., Shi W., et al. (2019). A roadmap for lowering crop nitrogen requirement. Trends Plant Sci. 24, 892–904. doi: 10.1016/j.tplants.2019.06.006
Tilman D., Balzer C., Hill J., and Befort B. L. (2011). Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. 108, 20260–20264. doi: 10.1073/pnas.1116437108
Tooker J. F. and Frank S. D. (2012). Genotypically diverse cultivar mixtures for insect pest management and increased crop yields. J. Appl. Ecol. 49, 974–985. doi: 10.1111/j.1365-2664.2012.02173.x
Vergé X. P. C., De Kimpe C., and Desjardins R. L. (2007). Agricultural production, greenhouse gas emissions and mitigation potential. Agric. For. Meteorol. 142, 255–269. doi: 10.1016/j.agrformet.2006.06.011
Verma A., Tyagi L., Yadav S., and Singh S. N. (2006). Temporal changes in N2O efflux from cropped and fallow agricultural fields. Agriculture Ecosyst. Environ. 116, 209–215. doi: 10.1016/j.agee.2006.02.005
Vitousek P. M., Aber J. D., Howarth R. W., Likens G. E., Matson P. A., Schindler D. W., et al. (1997). Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 7, 737–750. doi: 10.1890/1051-0761(1997)007[0737:HAOTGN]2.0.CO;2
Vo T. B. T., Johnson K., Wassmann R., Sander B. O., and Asch F. (2024). Varietal effects on Greenhouse Gas emissions from rice production systems under different water management in the Vietnamese Mekong Delta. J. Agron. Crop Sci. 210, e12669. doi: 10.1111/jac.12669
Wang C., Amon B., Schulz K., and Mehdi B. (2021). Factors that influence nitrous oxide emissions from agricultural soils as well as their representation in simulation models: A review. Agronomy 11, Article 4. doi: 10.3390/agronomy11040770
Wang Z.-H., Wang L.-H., Liang H., Peng T., Xia G.-P., Zhang J., et al. (2021). Methane and nitrous oxide emission characteristics of high-yielding rice field. Environ. Sci. pollut. Res. 28, 15021–15031. doi: 10.1007/s11356-020-11641-y
Wingler A. and Soualiou S. (2025). Overcoming physiological trade-offs between flowering time and crop yield: Strategies for a changing climate. J. Exp. Bot. 76, 2646–2658. doi: 10.1093/jxb/eraf110
Zhang X., Davidson E. A., Mauzerall D. L., Searchinger T. D., Dumas P., and Shen Y. (2015). Managing nitrogen for sustainable development. Nature 528, 51–59. doi: 10.1038/nature15743
Zhao J., Hu Y., Gao W., Chen H., Yang M., Quan Z., et al. (2024). Effects of long-term conservation tillage on N2 and N2O emission rates and N2O emission microbial pathways in Mollisols. Sci. Total Environ. 908, 168440. doi: 10.1016/j.scitotenv.2023.168440
Keywords: nitrous oxide, methane, climate change mitigation, nitrogen fertilisation, greenhouse gas emissions, genotypic variation
Citation: Walthall C, Girkin NT, Kevei Z and Johnston ASA (2025) A global synthesis of genotypic variation in crop greenhouse gas emissions under variable nitrogen fertilisation. Front. Agron. 7:1669002. doi: 10.3389/fagro.2025.1669002
Received: 18 July 2025; Accepted: 15 September 2025;
Published: 24 September 2025.
Edited by:
Sangeeta Lenka, Indian Institute of Soil Science (ICAR), IndiaReviewed by:
Ashim Datta, Central Soil Salinity Research Institute (ICAR), IndiaArti Bhatia, Indian Council of Agricultural Research (ICAR), India
Copyright © 2025 Walthall, Girkin, Kevei and Johnston. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Conor Walthall, Y29ub3Iud2FsdGhhbGxAY3JhbmZpZWxkLmFjLnVr; Alice S. A. Johnston, YS5zLmpvaG5zdG9uQGNyYW5maWVsZC5hYy51aw==
 Conor Walthall
Conor Walthall Nicholas T. Girkin
Nicholas T. Girkin Zoltan Kevei
Zoltan Kevei Alice S. A. Johnston
Alice S. A. Johnston