- 1Department of Ecological, Plant and Animal Sciences, La Trobe Institute for Sustainable Agriculture and Food, La Trobe University, Melbourne, VIC, Australia
- 2Faculty of Science, Plant Breeding Institute, The University of Sydney, Cobbitty, NSW, Australia
- 3Federal Research Centre for Cultivated Plants, Institute for Resistance Research and Stress Tolerance, Julius Kühn-Institut (JKI), Brandenburg, Kleinmachnow, Germany
- 4Centre for Cardiovascular Biology and Disease Research, La Trobe Institute for Molecular Science, La Trobe University, Melbourne, VIC, Australia
- 5Department of Biochemistry and Chemistry, School of Agriculture, Biomedicine and Environment, La Trobe University, Melbourne, VIC, Australia
- 6Organogenesis and Cancer Program, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia
- 7Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, Australia
Introduction: Barley leaf rust (BLR), caused by Puccinia hordei, is a devastating fungal disease that significantly reduces barley (Hordeum vulgare subsp. vulgare) yields worldwide. Genetic resistance offers an effective and sustainable control strategy; however, reliance on single resistance genes often results in the rapid breakdown of resistance due to the emergence of virulent P. hordei races. Durable resistance in elite, high-yielding cultivars requires pyramiding multiple genes with diverse mechanistic actions. To achieve this, predictive molecular markers are essential for tracking individual resistance genes throughout the breeding cycle. Unlike most classical major resistance genes in the Triticeae, the recently cloned Rph7 gene encodes a NAC transcription factor containing a zinc-finger BED domain that mediates a novel, pathogen-induced resistance mechanism by enhancing basal defence. Rph7 represents a valuable target for gene-stacking strategies, particularly when deployed in combination with other effective BLR resistance genes.
Methods: To support predictive marker-assisted selection, three single-nucleotide polymorphisms located within the conserved flanking gene HvPG1 were converted into Kompetitive Allele Specific PCR (KASP) co-dominant markers.
Results and discussion: These markers were validated across a core panel of barley accessions known to either lack or contain Rph7 resistance, with the best performing marker subsequently evaluated in 173 accessions, including lines from the latest pangenome, elite Australian and international cultivars, and experimental lines carrying or lacking Rph7. One marker was deemed a reliable, breeder-friendly, high-throughput KASP marker for tracking Rph7 resistance, enabling the efficient development of BLR-resistant cultivars and supporting improved disease management in barley breeding programs.
Introduction
Cultivated barley (Hordeum vulgare L.) is the world’s fourth most important cereal crop behind maize, rice, and wheat, primarily grown for malt production, animal feed, and, to a lesser extent, as a food staple in mountainous regions of Central and Southwest Asia, the Andes, and Northern Africa (Dracatos et al., 2023). Despite its versatility and economic value, barley productivity is threatened by various biotic stresses. Pests and diseases account for an estimated 19% loss in Australian barley production (Murray and Brennan, 2010), a challenge further exacerbated by climate change and monoculture practices that promote the emergence of new, more aggressive pathogen variants. Among the diseases affecting barley, rusts caused by Puccinia spp. are particularly destructive. P. hordei Otth., the causal agent of barley leaf rust (BLR), is the most widespread and damaging rust disease of barley (Park et al., 2015). In highly susceptible cultivars, yield losses can reach up to 62% (Cotterill et al., 1992). In Australia, average annual losses due to BLR are estimated at $12 million AUD, but under severe epidemic conditions have the potential to escalate to $197 million AUD (Murray and Brennan, 2010; Park et al., 2015), highlighting its substantial economic impact.
Current disease control strategies for BLR include agronomic management, chemical fungicides, and genetic resistance (Walters et al., 2012). Agronomic practices such as crop rotation and removal of alternate hosts can reduce inoculum pressure but provide only partial protection. Chemical treatments, while effective, are costly, environmentally damaging, and contribute to the development of fungicide-resistant strains (Edlinger et al., 2022; Jørgensen et al., 2018). Genetic resistance is therefore considered the most sustainable, cost-effective, and environmentally friendly approach (Park et al., 2015). Host resistance, particularly through the deployment of Rph genes (Reaction to Puccinia hordei), offers durable protection, especially when used in combination (Park et al., 2015; Rothwell et al., 2020; Singh et al., 2015; Chen et al., 2023). Two major forms of resistance are recognised: all-stage resistance (ASR), governed by Rph genes that provide strong, race-specific protection throughout the plant’s lifecycle, and adult plant resistance (APR), typically conferred by quantitative trait loci offering partial, generally non-race-specific resistance expressed later in development (Niks et al., 2015). To date, 28 Rph genes have been mapped: 25 confer ASR (Rph1–Rph19, Rph21, Rph22, Rph25–28), and three (Rph20, Rph23, Rph24) confer APR (Hickey et al., 2012; Kavanagh et al., 2017; Rothwell et al., 2020; Singh et al., 2021; Ziems et al., 2017, 2014), with additional QTL identified for partial resistance at the seedling stage. While these discoveries have broadened the resistance gene pool, the durability of ASR is often compromised by pathogen evolution (McDonald and Linde, 2002), and deployment using marker-assisted selection (MAS) has been constrained by the paucity of predictive, co-segregating markers.
Prior to the cloning of the Rph7 gene, several molecular markers were employed to approximate its chromosomal location, including restriction fragment length polymorphism (RFLP), cleaved amplified polymorphic site (CAPS), gel-based sequence-tagged site (STS) and simple sequence repeat (SSR) markers (Brunner and Feuillet, 2003; Graner et al., 2000, 2000). These tools aided genetic mapping but were constrained by low throughput/reproducibility and labour-intensive workflows and have limited utility in modern breeding programs. Kompetitive Allele Specific PCR (KASP) markers interrogating target SNP loci are codominant, high-throughput, fluorescence-based genotyping platforms that are preferred in crop breeding programs due to their reliability and accuracy (Semagn et al., 2014).
The BLR resistance gene Rph7, which confers ASR to P. hordei, was first identified in the Argentinian barley cultivar Cebada Capa (Roane and Starling, 1970). Rph7 was mapped near the telomere on the short arm of chromosome 3H within a region of high haplotypic divergence that revealed a 100 kb insertion unique to Cebada Capa relative to the BLR susceptible Morex (Graner et al., 2000; Scherrer et al., 2005). Recent fine mapping, supported by RNA-Seq, mutational analysis, and transgenic complementation, confirmed that Rph7 is a non-canonical resistance gene encoding a NAC transcription factor with a zinc-finger BED domain, structurally distinct from typical disease resistance genes in the Triticeae (Chen et al., 2023; Jensen et al., 2010). Its broad-spectrum effectiveness across diverse P. hordei races is likely attributed to historically limited deployment in commercial cultivars, thereby reducing the selection pressure for virulent races (Ziems et al., 2014). Nevertheless, virulence to Rph7 has been detected in some races from Australia, North America, Spain, and the Near East (Park et al., 2015; Shtaya et al., 2006; Steffenson, 1993), indicating a risk of resistance breakdown if deployed in isolation (Park et al., 2015). Despite these reports, the overall rarity of virulence and novel mechanistic action make Rph7 an attractive component for pyramiding strategies aimed at durable BLR control.
Comparative analysis of 20 pangenome lines (v1;), revealed that most lacked the Rph7 gene due to a 100 kb insertion-deletion event, and four accessions carried a non-functional susceptibility homologue of Rph7 (Chen et al., 2023; Scherrer et al., 2005). This structural variation limits the utility of gene-based markers, as absence of amplification could reflect either gene absence or susceptibility. In contrast, the neighbouring gene HvPG1, located 50 kb from Rph7, showed complete co-segregation with the Rph7 resistance phenotype in prior mapping studies, and is conserved among pan-genome accessions (Chen et al., 2023; Scherrer et al., 2005). Given the low recombination frequency expected in breeding populations, HvPG1 provides a stable target for predictive marker design.
In the present study, we have developed a breeder-friendly KASP marker targeting SNPs within HvPG1, closely linked to Rph7, validated their accuracy and robustness across diverse germplasm panels, including elite cultivars and pangenome lines, and identified the most reliable assay for application in MAS to support the deployment of Rph7-mediated resistance in breeding programs.
Materials and methods
Plant materials
Marker validation was conducted using various panels of Hordeum accessions, including: BLR differential Bowman near isogenic lines (NILs); (Martin et al., 2020), the Australian BLR differential set (Park et al., 2015), elite and historical Australian cultivars with mostly known resistance status, and v2 barley pangenome accessions, comprising landraces, wild barley and globally important cultivars and experimental lines. All accessions genotyped in this study, along with their postulated genetic complements, are listed in Supplementary File 1.
For definitive controls, we included: four biological replicates of the Bowman NIL carrying Rph7 (BW758, Bowman+Rph7.g), four technical replicates of Bowman (susceptible), three technical replicates of an artificial heterozygote (equal DNA mix of Bowman and BW758), two technical replicates of Rph7-carrying cultivars (Ellinor, La Estanzuela, Galaxy), and two technical replicates of Morex as negative controls.
DNA extraction
Genomic DNA was extracted from seedling leaves using a modified crude extraction method. Briefly, 2–3 segments (~2 cm each) of leaf tissue were homogenised in 200 µL of extraction buffer (1 M Tris-HCl, pH 8.0; 2.55 M KCl; 100 mM EDTA) using two tungsten carbide beads in a TissueLyser II (Qiagen) for 2 minutes. The homogenate was centrifuged at 16,000 × g for 10 min, and the resulting supernatant was transferred to fresh tubes. DNA was precipitated by adding 170 µL of isopropanol, incubating for 5 min, and centrifuging again (16,000 × g, 10 min). Pellets were washed with 500 µL of 70% (v/v)_ethanol, re-centrifuged (16,000 × g, 5 min), air-dried, and resuspended in sterile TE buffer (10 mM Tris-HCl, 1 mM EDTA). DNA concentration was quantified using a Qubit fluorometer 4 (Invitrogen) and adjusted to a working concentration of 20 ng/µL.
Development of KASP primers
Genomic sequences flanking Rph7, delineated by Chen et al. (2023) between HvGAD1 and HvPG4, were aligned from nine susceptible accessions (Barke, HOR9043, Golden Promise, RGT Planet, Akashinriki, Igri, Morex and Bowman) against the 184 kb BAC sequence from Cebada Capa (AY642926) using the map to reference function in Geneious Prime 2024.1 (Supplementary Table S1).
As HvPG1 was previously shown to co-segregate with the Rph7 phenotype (Scherrer et al., 2005; Chen et al., 2023), it was selected as the most reliable flanking locus for codominant marker development. Comparative SNP discovery within HvPG1 identified polymorphisms unique to Cebada Capa and absent across all nine non-Rph7 accessions (Supplementary Table S2). Three SNPs demonstrating consistent allelic variation were selected, and 50 bp of flanking sequence were extracted and submitted to Geneworks for KASP primer design and synthesis (Supplementary Table S3). Details of the SNPs and corresponding primers are provided in Table 1 and illustrated in Figure 1.
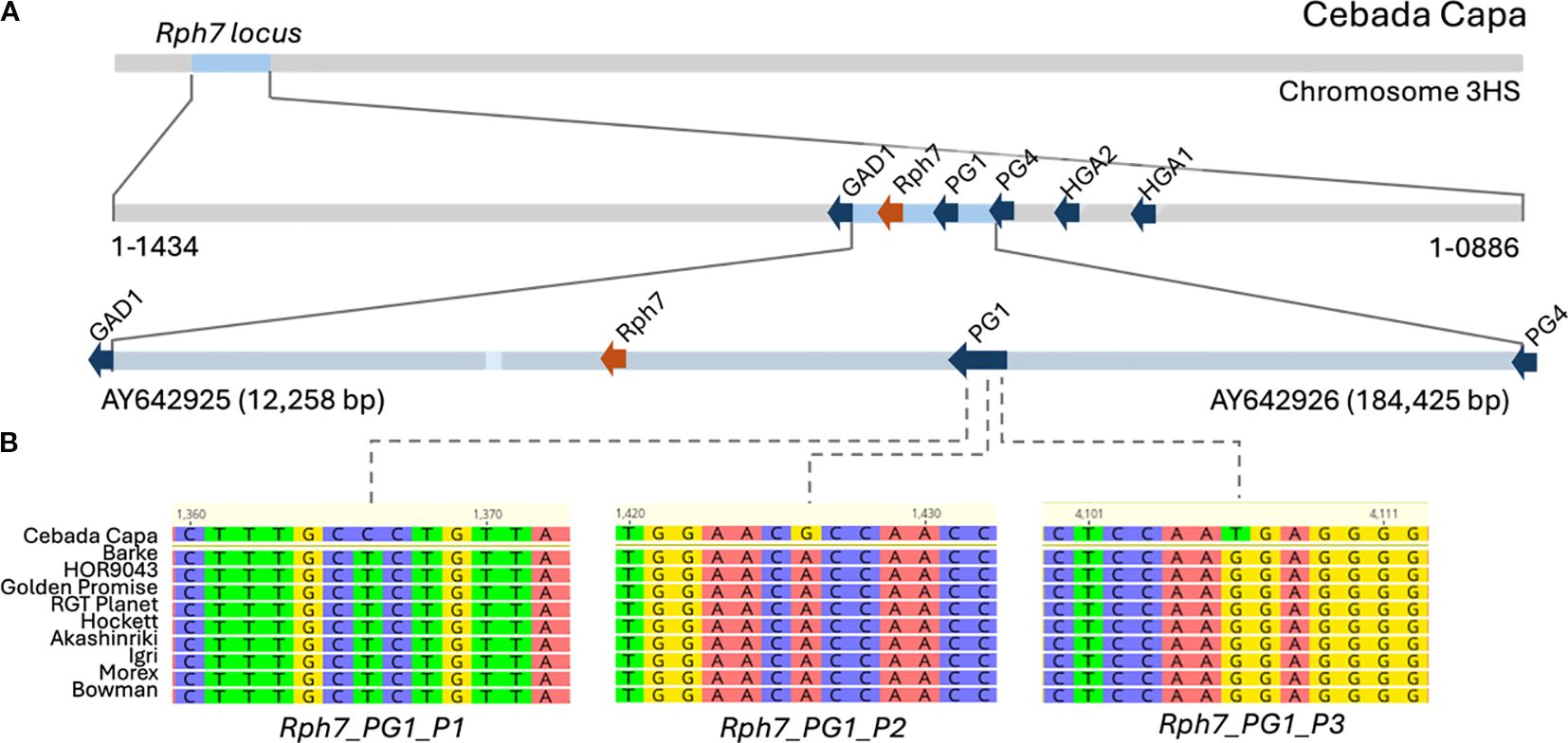
Figure 1. KASP marker design at the Rph7 locus. (A) Schematic representation of the previously defined Rph7 locus, showing sequenced BAC contigs (Scherrer et al., 2005) alongside the position of the identified Rph7 gene (orange), located between the flanking genes HvGAD1, HvPG1, and HvPG4 (blue) (Chen et al., 2023). (B) Genomic alignment of SNPs selected for KASP marker development within the conserved HvPG1 gene.
Bioinformatic analysis of Rph7 conservation across the barley pangenome
To assess the conservation of the Rph7 genomic region and evaluate the applicability of the developed KASP markers, the Rph7 and HvPG1 sequences from Cebada Capa were used as BLASTn queries against the PanBARLEX v2 resource, which comprises chromosome-scale assemblies of 76 diverse Hordeum accessions, including H. spontaneum landraces, and cultivars. This analysis enabled the evaluation of haplotypic variation at the selected SNP loci, assessment of presence-absence variation across 76 accessions relative to the 20 accessions surveyed in Chen et al. (2023), and detection of potential paralogous sequences in other chromosomal regions.
KASP genotyping
KASP assays were performed in 96-well plates with 5 µL reaction volumes containing 50 ng of genomic DNA and 2.5 µL genotyping mix (2× KASP Master Mix and 0.07 µL primer mix). PCR amplification was performed on an ABI ViiA 7 Real-Time PCR System (Applied Biosystems) using the following thermal profile: 94°C for 15 min; 10 cycles of 94°C for 20 sec and 61°C to 55°C touchdown (1.6°C per cycle) for 60 sec; followed by 26 cycles of 94°C for 20 sec and 55°C for 60 sec. Fluorescence detection was performed at 30°C, and genotype calls were was conducted using the QuantStudio™ software.
PACE genotyping
PCR Allele Competitive Extension (PACE) assays were performed in 96-well plates with a total reaction volume of 8 µL. Each reaction contained 25–50 ng of genomic DNA and 4 µL of the PACE Genotyping Master Mix (LGC), containing 0.12 pmol of the two allele-specific primers and 0.3 pmol common reverse primer. PCR amplification was carried out on an ABI 7500 Real-Time PCR System (Applied Biosystems) using the following cycling conditions: an initial denaturation at 94°C for 15 minutes, followed by 10 touchdown cycles of 94°C for 20 seconds and annealing/extension from 61°C to 55°C for 60 seconds with a decrement of 1.6°C per cycle. This was followed by 26 cycles of 94°C for 20 seconds and 55°C for 60 seconds. A post-read stage was conducted at 30°C. Fluorescence detection was performed on the same instrument, and genotype calls were assigned using QuantStudio™ software (Applied Biosystems).
Results
Development of KASP markers for Rph7
The Rph7 locus, conferring resistance to BLR, is located between the HvGAD1 and HvPG1 genes on chromosome 3HS (Figure 1). The Rph7 CDS was used as a BLASTn query against all 76 barley pangenome (v2) accessions using the PanBARLEX online resource, revealing that 11 (14%) carried non-functional susceptibility alleles and the remaining 65 accessions lacked the gene entirely due to a presence/absence variation (Supplementary File S1). Because Rph7-mediated resistance is conferred by a presence-absence variation, marker design within the gene itself would yield a dominant marker, as many genotypes lacking the gene would appear as null alleles. In contrast, alignment of HvPG1 gene across the 76 barley accessions revealed a high degree of conservation on chromosome 3H across both wild and cultivated barley, confirming its suitability for co-dominant KASP marker development. A divergent paralogous copy of HvPG1 was also identified on chromosome 7H; however, the sequence alignment showed low conservation at the primer binding sites, suggesting that the developed KASP marker is specific to the 3H HvPG1 gene.
Comparative sequence analysis between the Rph7 donor Cebada Capa BAC sequence (AY642926) and nine non-Rph7 accessions revealed three high-confidence SNPs within HvPG1 at positions 1,366 (T/C), 1,426 (A/G), and 4,106 (G/T). These polymorphisms were monomorphic across all nine non-Rph7 carrying accessions but uniquely present in Cebada Capa. This consistent predictive haplotype strongly associates these polymorphisms with the presence of Rph7, making them suitable SNPs for interrogating KASP marker development. Each SNP was supported by conserved flanking regions, allowing robust primer design. The three resulting KASP markers (Rph7_PG1_1, Rph7_PG1_2, Rph7_PG1_3) were hypothesised to reliably discriminate homozygous resistant, homozygous susceptible, and heterozygous genotypes across cultivated barley germplasm and breeding material germplasm. Details of the identified SNPs and corresponding primer sequences are provided in Table 1, with their genomic locations illustrated in Figure 1.
Validation of the KASP markers
Initial validation of the Rph7-linked KASP markers (Rph7_PG1_P1, Rph7_PG1_P2, and Rph7_PG1_P3) was performed on a panel of 22 barley genotypes comprising eight Rph7-carrying lines, 13 non-Rph7 genotypes, three artificial heterozygotes, and two no-template controls (Supplementary File S1). All three KASP markers exhibited clear allelic clustering patterns indicative of co-dominant allele amplification, enabling unambiguous discrimination between Rph7 carrying homozygotes and non-carrier alleles (Figure 2; Supplementary File S1). Notably, only Rph7_PG1_P3 reliably detected artificial heterozygote samples, highlighting its superior sensitivity for distinguishing heterozygous states.
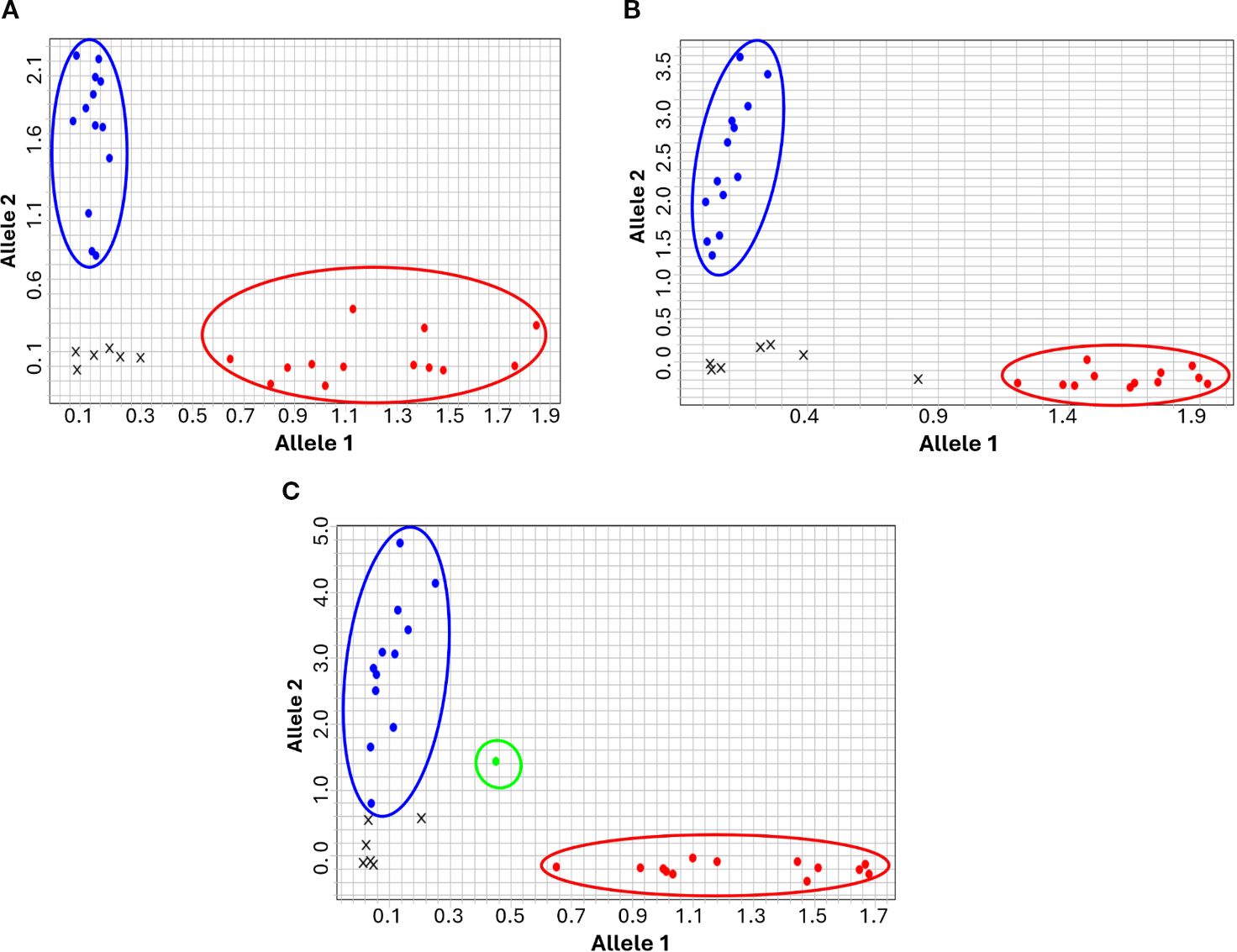
Figure 2. Genotype clustering plots for the Rph7_PG1_P1 (A), Rph7_PG1_P2 (B), and Rph7_PG1_P3 (C) KASP markers initially validated against a panel of barley lines known to either carry or lack Rph7-mediated resistance. Blue dots (upper left) represent homozygous wild-type non-Rph7 genotypes, red dots (lower right) represent homozygous Rph7 carriers, and green dots (central cluster) indicate heterozygous genotypes. Black crosses denote no-template controls (NTCs) or samples with failed amplification.
Expanded validation across Hordeum germplasm
To further evaluate the reliability and diagnostic accuracy of the Rph7_PG1_P3 KASP marker for detecting Rph7, genotyping was tested on an expanded panel of diverse barley accessions comprising landraces, international and Australian cultivars and BLR differential stocks with known BLR resistance genes (Supplementary File S1). Allelic discrimination was visualised using fluorescence-based clustering. All known Rph7-carrying controls were correctly genotyped, with no false negatives observed (Figure 3). The majority of non-Rph7 lines formed a distinct susceptible cluster, reinforcing the specificity of the marker. Additionally, a subset of samples displayed intermediate fluorescence signals indicative of heterozygosity, confirming the markers’ ability to distinguish segregating alleles. Only five samples yielded undetermined calls; three showed no amplification signal, (Supplementary Table S1). These results confirm that Rph7_PG1_P3 is robust, sensitive and suitable for tracking the presence of Rph7-mediated resistance.
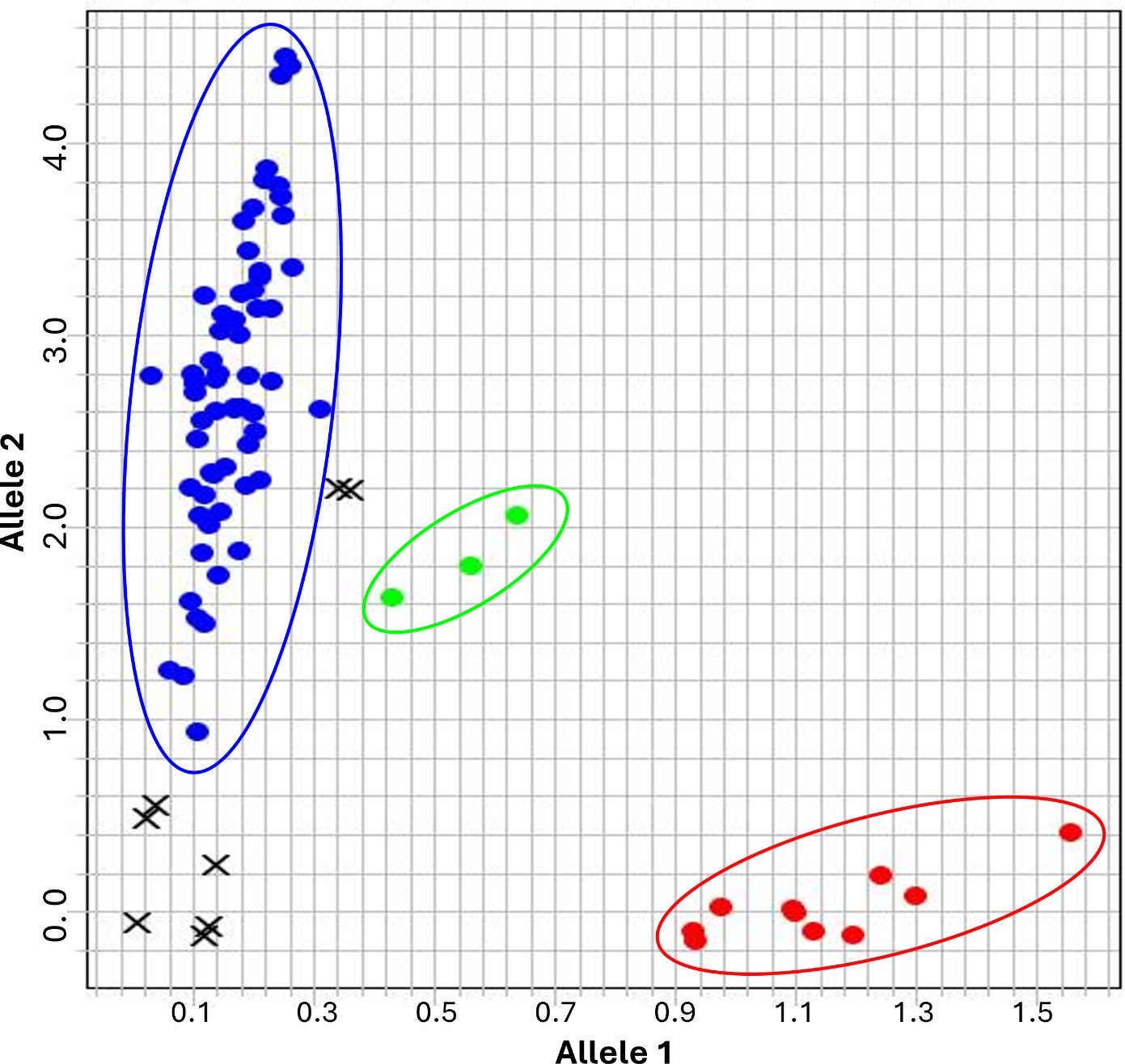
Figure 3. Genotype clustering plots for the Rph7_PG1_P3 KASP marker against a diverse panel of barley lines. The plot illustrates the segregation of the Rph7 allele among lines known to carry or lack the Rph7. Blue dots (upper left) represent the homozygous non-Rph7 wild type allele, red dots (lower right) represent homozygous Rph7 carriers, and green dots (central cluster) indicate heterozygous genotypes. Black crosses denote no-template controls (NTCs) or samples with failed amplification.
Independent validation using PACE chemistry
To further demonstrate the utility and transferability, the Rph7_PG1_P3 marker was tested at the Institute for Resistance Research and Stress Tolerance, Julius Kühn-Institute (JKI - Germany) using PACE chemistry across 144 diverse barley germplasm, including 73 barley pangenome (v2) accessions as detailed in Supplementary File S1. The results were fully consistent with our KASP assays, with the Rph7_PG1_P3 marker successfully distinguishing between lines known to carry Rph7 and those lacking the gene. Notably, 15 accessions (9%) were identified as homozygous Rph7 carriers. Of the 15 positive accessions nine were wild barleys and four diverse landrace accessions whereas the remaining were positive control lines expected to carry Rph7 mediated resistance. One H. spontaneum line (HID357) produced a heterozygous call (Figure 4; Supplementary File S1).
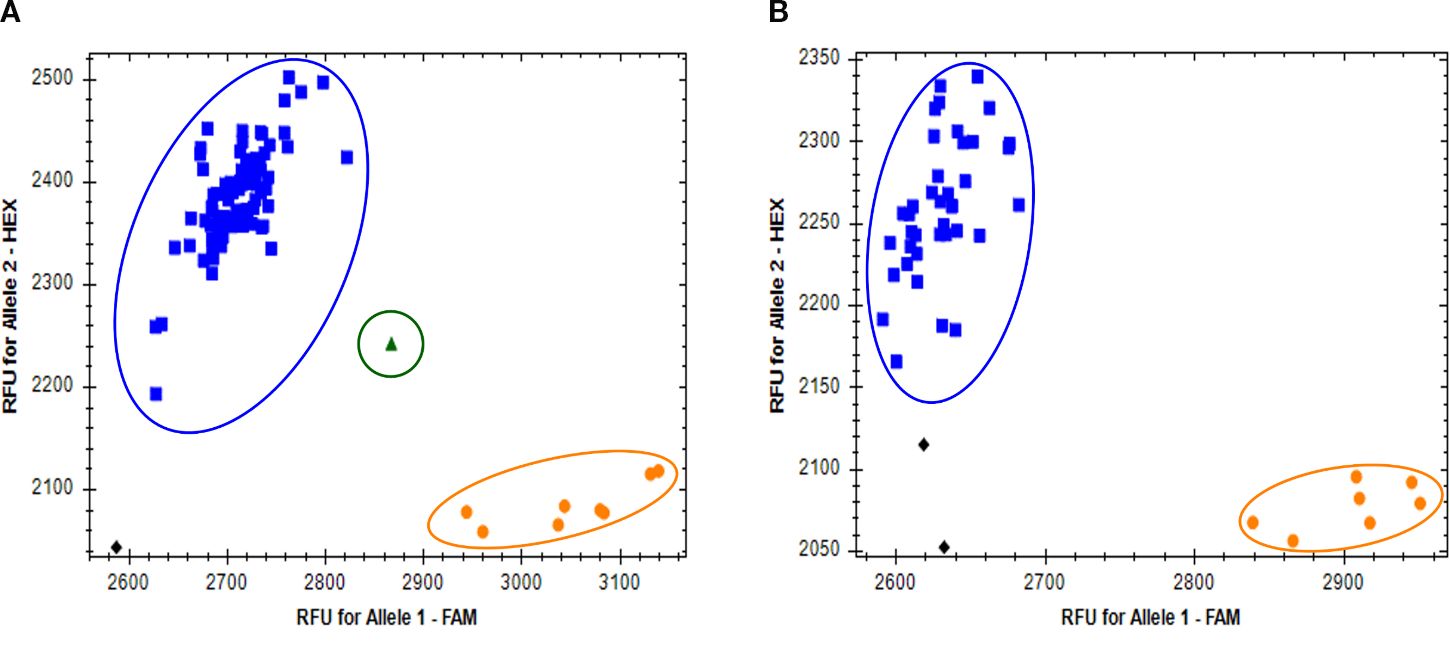
Figure 4. Genotype clustering plots for the Rph7_PG1_P3 PACE marker against a diverse panel of 144 barley lines. The plots (A, B) show the clustering of the Rph7 allele among lines known to carry or lack Rph7. Blue squares (upper left) represent homozygous non-Rph7 genotypes, orange circles (lower right) represent homozygous Rph7 carriers, and green triangles (central cluster) indicate heterozygous genotypes. Black diamonds denote no-template controls (NTCs) or samples with failed amplification.
Discussion
Plants deploy multilayered defence mechanisms against rust pathogens, including both passive barriers and inducible responses (Kaur et al., 2022). In barley, the recently cloned resistance gene Rph7 functions as a positive regulator of basal defence, conferring resistance to a wide range of P. hordei races (Chen et al., 2023; Niks et al., 2015). For durable control of BLR, pyramiding multiple functionally diverse resistance genes with broad-spectrum efficacy remains a key strategy (Mahesha et al., 2022; Dracatos et al., 2023). The inclusion of Rph7 in BLR resistance gene stacks is therefore an attractive target for integration into breeding programs. To achieve this effectively, robust, high-throughput, and breeder-friendly co-dominant molecular markers are essential to accelerate MAS (Collard and Mackill, 2008; Kumar et al., 2024).
KASP technology has emerged as a benchmark for SNP genotyping due to its accuracy, scalability, and cost-effectiveness. KASP assays have been widely applied across cereals including wheat, rice, and barley (Dipta et al., 2024; Makhoul and Obermeier, 2022; Semagn et al., 2014; Yang et al., 2019). Their utility in tracking resistance loci is well established, with applications including tracking Wsm2 for wheat streak mosaic virus resistance (Tan et al., 2017), YrAS2388 for wheat stripe rust resistance (Hu et al., 2021), and BLR resistance genes Rph13 and Rph15 (Chen et al., 2021; Jost et al., 2020; Mehnaz et al., 2022; Chen et al., 2021). More recently, the capacity to multiplex KASP assays has enabled simultaneous identification of multiple alleles, as demonstrated for Fhb7 and Pm21, conferring resistance to Fusarium head blight and powdery mildew, respectively (Wang et al., 2025). Collectively, these examples highlight the versatility of KASP for accelerating gene discovery, deployment, and pyramiding in breeding programs. Our study builds on this foundation by developing and validating novel KASP markers tightly linked to Rph7, leveraging pangenome-wide sequence conservation. We demonstrate that pangenome-guided SNP discovery in conserved neighbouring genes can provide a powerful strategy for designing robust, KASP assays to track effective resistance genes in breeding programs.
Comparative sequence analysis of the Rph7 genomic region identified three high-confidence SNPs within the conserved HvPG1 gene. These polymorphisms were absent in nine Rph7-susceptible reference genomes but divergent in the resistant donor line Cebada Capa, providing a strong basis for marker development. Leveraging this conservation resulted in KASP assays that produced clear, co-dominant clustering, enabling reliable discrimination between homozygous resistant, susceptible and heterozygous genotypes. Among the three developed assays, Rph7_PG1_3 emerged as the most diagnostically robust, exhibiting superior sensitivity for detecting heterozygotes and consistent amplification across diverse germplasm. The genotypic evaluation of Australian and international barley cultivars using the Rph7_PG_3 marker confirmed the rarity of the Rph7-associated KASP marker TT allele, corresponding to the presence of the Rph7 resistance gene the barley germplasm assessed in this study. The homozygous Rph7-associated KASP allele was predominantly restricted to known Rph7 resistance donors and a single Australian cultivar ‘Galaxy’. This scarcity highlights the importance of MAS for efficiently incorporating Rph7-mediated resistance into elite breeding pools, particularly where existing Rph genes may mask its detection through phenotypic screening (Figueroa et al., 2023; Mapari and Mehandi, 2024).
Independent cross chemistry and lab validation (PACE vs KASP chemistries) further reinforced the reliability and utility of Rph7_PG1_3 for tracking or incorporating Rph7 into elite barley breeding material. The high conservation of the HvPG1 flanking region, together with the absence of interference from paralogous HvPG1-like sequences on chromosome 7H in cultivated germplasm, further supports the robustness and specificity of the Rph7_PG1_3 marker. Despite this, genotyping the 76 barley pangenome (v2) accessions with Rph7_PG1_3 identified several false positives in lines known to lack the Rph7 resistance allele including mainly wild barleys but also four exotic landraces. These results highlight that the frequency of false positives is likely to be higher in wild barley and diverse landraces when genotyping large diverse germplasm collections found in global gene banks. This suggests that Rph7_PG1_3, while robust and specific in cultivated germplasm, may not reliably predict the presence of Rph7 in wild barley or exotic landraces, due to the higher levels of polymorphism and historical recombination within the 50 kb region between Rph7 and HvPG1.
The validated Rph7_PG1_3 KASP marker developed here provides breeders with a reliable, high-throughput diagnostic tool enables more efficient MAS, facilitating Rph7 introgression and pyramiding alongside other BLR resistance genes (Dipta et al., 2024; Dracatos et al., 2023; Kumar et al., 2024, 2022). For example, tracking Rph7 from the adapted background of BW758 into the commercial high-yielding barley cultivar RGT Planet would be enhanced by using Rph7_PG1_3. RGT Planet already carries Rph3, and APR genes Rph20 and Rph24 (Singh et al., 2020). Incorporating Rph7 into this background is expected to significantly enhance the durability of BLR resistance, despite the widespread virulence against Rph3 in Australian pathogen populations (Crété et al., 2020; Dracatos et al., 2023; Jost et al., 2023).
Conclusion
This study reports on the development and validation of a breeder-friendly, reliable co-dominant KASP marker tightly linked to the cloned Rph7 resistance gene on chromosome 3H. By enabling efficient pyramiding with other BLR resistance genes, Rph7_PG1_3 provides breeders with a powerful tool for enhancing the durability of BLR resistance. The integration of high-throughput, predictive markers will be critical for acceleratingresistance gene deployment and safeguarding global barley production. Further research should focus on identifying the origin, prevalence and specific variants associated with the Rph7 resistance haplotype utilizing global gene bank genomic sequence data.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
DW: Data curation, Investigation, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing, Formal analysis, Methodology, Validation. KG: Formal analysis, Methodology, Software, Visualization, Writing – review & editing. ET: Formal analysis, Visualization, Writing – review & editing. RP: Investigation, Writing – review & editing. YC: Formal analysis, Writing – review & editing. BV: Formal analysis, Writing – review & editing. KO: Funding acquisition, Resources, Writing – review & editing. DP: Formal analysis, Funding acquisition, Validation, Writing – review & editing. PD: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The authors would like to thank the Alexander von Humboldt foundation, The Grains Research and Development Corporation project grant number ULA2307-001RTX and the IdeMoDeResBar project grant number FKZ: 031B0199B.
Acknowledgments
The authors thank Dr. Lesley Cheng Sim at La Trobe University for providing access to the ABI ViiA 7 Real-Time PCR System, Ms Katy Niedung for technical assistance during the project, Prof Nils Stein and Prof Brian Steffenson for the availability of seed of barley pan genome accessions and Bowman near isogenic lines for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The reviewer MA declared a past co-authorship with the author(s) RP, DP, PMD to the handling editor.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2025.1670733/full#supplementary-material
References
Brunner S. and Feuillet C. (2003). A large rearrangement involving genes and low-copy DNA interrupts the microcollinearity between rice and barley at the Rph7 locus. Genetics 164, 673–683. doi: 10.1093/genetics/164.2.673
Chen C., Jost M., Clark B., Martin M., Matny O., Steffenson B. J., et al. (2021). BED domain-containing NLR from wild barley confers resistance to leaf rust. Plant Biotechnol. J. 19, 1206–1215. doi: 10.1111/pbi.13542
Chen C., Jost M., Outram M. A., Friendship D., Chen J., Wang A., et al. (2023). A pathogen-induced putative NAC transcription factor mediates leaf rust resistance in barley. Nat. Commun. 14 (1), 5468. doi: 10.1038/s41467-023-41021-2
Collard B. C. Y. and Mackill D. J. (2008). Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. B: Biol. Sci. 363, 557-572. doi: 10.1098/rstb.2007.2170
Cotterill P. J., Rees R. G., Platz G. J., and Dill-Mucky R. (1992). Effects of leaf rust on selected Australian barleys. Aust. J. Exp. Agric. 32 (6), 747-751. doi: 10.1071/EA9920747
Crété R., Pires R. N., Barbetti M. J., and Renton M. (2020). Rotating and stacking genes can improve crop resistance durability while potentially selecting highly virulent pathogen strains. Sci. Rep. 10, 19752. doi: 10.1038/s41598-020-76788-7
Dipta B., Sood S., Mangal V., Bhardwaj V., Thakur A. K., Kumar V., et al. (2024). KASP: a high-throughput genotyping system and its applications in major crop plants for biotic and abiotic stress tolerance. Mol. Biol. Rep., 19752. doi: 10.1007/s11033-024-09455-z
Dracatos P. M., Lu J., Sánchez-Martín J., and Wulff B. B. H. (2023). Resistance that stacks up: engineering rust and mildew disease control in the cereal crops wheat and barley. Plant Biotechnol. J. 21, 1938-1951. doi: 10.1111/pbi.14106
Edlinger A., Garland G., Hartman K., Banerjee S., Degrune F., García-Palacios P., et al. (2022). Agricultural management and pesticide use reduce the functioning of beneficial plant symbionts. Nat. Ecol. Evol. 6, 1145–1154. doi: 10.1038/S41559-022-01799-8
Figueroa M., Dodds P. N., Henningsen E. C., Sperschneider J., Figueroa M., Dodds P. N., et al. (2023). Global landscape of rust epidemics by Puccinia species: current and future perspectives 391–423. doi: 10.1007/978-3-031-16503-0_17
Graner A., Streng S., Drescher A., Jin Y., Borovkova I., and Steffenson B. J. (2000). Molecular mapping of the leaf rust resistance gene Rph7 in barley. Plant Breed. 119, 389–392. doi: 10.1046/j.1439-0523.2000.00528.x
Hickey L. T., Lawson W., Platz G. J., Dieters M., and Franckowiak J. (2012). Origin of leaf rust adult plant resistance gene Rph20 in barley. Genome 55, 396–399. doi: 10.1139/G2012-022
Hu Y., Huang X., Wang F., He Y., Feng L., Jiang B., et al. (2021). Development and validation of gene-specific KASP markers for YrAS2388R conferring stripe rust resistance in wheat. Euphytica 217, 206. doi: 10.1007/s10681-021-02937-2
Jensen M. K., Kjaersgaard T., Nielsen M. M., Galberg P., Petersen K., O’shea C., et al. (2010). The Arabidopsis thaliana NAC transcription factor family: structure-function relationships and determinants of ANAC019 stress signalling. Biochem. J. 426, 183–196. doi: 10.1042/BJ20091234
Jørgensen L., Oliver R., and Heick T. (2018). Occurrence and avoidance of fungicide resistance in cereal diseases (Cambridge, United Kingdom: Burleigh Dodds Science Publishing). doi: 10.1201/9780429201219-15
Jost M., Outram M. A., Dibley K., Zhang J., Luo M., and Ayliffe M. (2023). Plant and pathogen genomics: essential approaches for stem rust resistance gene stacks in wheat. Front. Plant Sci. 14, 1223504. doi: 10.3389/fpls.2023.1223504
Jost M., Singh D., Lagudah E., Park R. F., and Dracatos P. (2020). Fine mapping of leaf rust resistance gene Rph13 from wild barley. Theor. Appl. Genet. 133, 1887–1895. doi: 10.1007/s00122-020-03564-6
Kaur S., Samota M. K., Choudhary M., Choudhary M., Pandey A. K., Sharma A., et al. (2022). How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 28, 485–504. doi: 10.1007/S12298-022-01146-Y/FIGURES/4
Kavanagh P. J., Singh D., Bansal U. K., and Park R. F. (2017). Inheritance and characterization of the new and rare gene Rph25 conferring seedling resistance in Hordeum vulgare against Puccinia hordei. Wiley Online Library 136, 908–912. doi: 10.1111/PBR.12535
Kumar R., Das S. P., Choudhury B. U., Kumar A., Prakash N. R., Verma R., et al. (2024). Advances in genomic tools for plant breeding: harnessing DNA molecular markers, genomic selection, and genome editing. Biol. Res. 57, 1 57, 1–23. doi: 10.1186/S40659-024-00562-6
Kumar S., Pradhan A. K., Kumar U., Dhillon G. S., Kaur S., Budhlakoti N., et al. (2022). Validation of Novel spot blotch disease resistance alleles identified in unexplored wheat (Triticum aestivum L.) germplasm lines through KASP markers. BMC Plant Biol. 22, 618. doi: 10.1186/s12870-022-04013-w
Mahesha H. S., Saini R. P., Singh T., Singh A. K., and Srinivasan R. (2022). “Potential breeding strategies for developing disease-resistant barley: progress, challenges, and applications,” in Cereal Diseases: Nanobiotechnological Approaches for Diagnosis and Management Springer Nature, Singapore, 163–181. doi: 10.1007/978-981-19-3120-8_9/FIGURES/3
Makhoul M. and Obermeier C. (2022). “Development of breeder-friendly KASP markers from genome-wide association studies results,” in Methods in Molecular Biology (Humana Press Inc.), 287–310. doi: 10.1007/978-1-0716-2237-7_17
Mapari A. R. and Mehandi S. (2024). Enhancing crop resilience: advances and challenges in marker-assisted selection for disease resistance. J. Adv. Biol. Biotechnol. 27, 569–580. doi: 10.9734/jabb/2024/v27i71018
Martin M. J., Chicaiza O., Caffarel J. C., Sallam A. H., Druka A., Waugh R., et al. (2020). Development of barley introgression lines carrying the leaf rust resistance genes Rph1 to Rph15. Crop Sci. 60, 282–302. doi: 10.1002/csc2.20057
McDonald B. A. and Linde C. (2002). The population genetics of plant pathogens and breeding strategies for durable resistance. Euphytica 124, 163–180. doi: 10.1023/A:1015678432355/METRICS
Mehnaz M., Dracatos P. M., Dinh H. X., Forrest K., Rouse M. N., Park R. F., et al. (2022). A novel locus conferring resistance to Puccinia hordei maps to the genomic region corresponding to Rph14 on barley chromosome 2HS. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.980870
Murray G. M. and Brennan J. P. (2010). Estimating disease losses to the Australian barley industry. Australas. Plant Pathol. 39, 85–96. doi: 10.1071/AP09064
Niks R. E., Qi X., and Marcel T. C. (2015). Quantitative resistance to biotrophic filamentous plant pathogens: concepts, misconceptions, and mechanisms. Annu. Rev. Phytopathol. 53, 445–470. doi: 10.1146/ANNUREV-PHYTO-080614-115928/CITE/REFWORKS
Park R. F., Golegaonkar P. G., Derevnina L., Sandhu K. S., Karaoglu H., Elmansour H. M., et al. (2015). Leaf rust of cultivated barley: pathology and control. Annu. Rev. Phytopathol. 53, 565–589. doi: 10.1146/annurev-phyto-080614-120324
Roane C. and Starling T. (1970). Inheritance of reaction to Puccinia hordei in barley. III. Genes in the cultivars Cebada Capa and Franger. Phytopathology 60, 788. doi: 10.1094/Phyto-60-788
Rothwell C. T., Singh D., Dracatos P. M., and Park R. F. (2020). Inheritance and characterization of Rph27: A third race-specific resistance gene in the barley cultivar Quinn. Am. Phytopath Soc. 110, 1067–1073. doi: 10.1094/PHYTO-12-19-0470-R
Scherrer B., Isidore E., Klein P., Kim J. S., Bellec A., Chalhoub B., et al. (2005). Large intraspecific haplotype variability at the Rph7 locus results from rapid and recent divergence in the barley genome. Plant Cell 17, 361–374. doi: 10.1105/tpc.104.028225
Semagn K., Babu R., Hearne S., and Olsen M. (2014). Single nucleotide polymorphism genotyping using Kompetitive Allele Specific PCR (KASP): Overview of the technology and its application in crop improvement. Mol. Breed. 33, 1–14. doi: 10.1007/S11032-013-9917
Shtaya M. J. Y., Sillero J. C., and Rubiales D. (2006). Identification of a new pathotype of Puccinia hordei with virulence for the resistance gene Rph7. Eur. J. Plant Pathol. 116, 103–106. doi: 10.1007/S10658-006-9043-2/METRICS
Singh D., Dracatos P., Derevnina L., Zhou M., and Park R. F. (2015). Rph23: A new designated additive adult plant resistance gene to leaf rust in barley on chromosome 7H. Wiley Online Library 134, 62–69. doi: 10.1111/PBR.12229
Singh D., Mehnaz M., Dracatos P., and Park R. (2020). Australian barley cultivar pedigree and leaf rust seedling and adult plant resistance genotype information (Sydney Institute of Agriculture Cereal Rust Report Plant Breeding Institute) 17 (1), 1-9.
Singh L., Park R. F., Dracatos P., Ziems L., and Singh D. (2021). Understanding the expression and interaction of Rph genes conferring seedling and adult plant resistance to Puccinia hordei in barley. Can. J. Plant Pathol. 43, S218–S226. doi: 10.1080/07060661.2021.1936649
Steffenson B. J. (1993). Pathotypes of Puccinia hordei with virulence for the barley leaf rust resistance gene Rph 7 in the United States. Plant Dis. 77, 867. doi: 10.1094/PD-77-0867
Tan C. T., Assanga S., Zhang G., Rudd J. C., Haley S. D., Xue Q., et al. (2017). Development and validation of kasp markers for wheat streak mosaic virus resistance gene wsm2. Crop Sci. 57, 340–349. doi: 10.2135/cropsci2016.04.0234
Walters D. R., Avrova A., Bingham I. J., Burnett F. J., Fountaine J., Havis N. D., et al. (2012). Control of foliar diseases in barley: towards an integrated approach. Eur. J. Plant Pathol. 133, 1 133, 33–73. doi: 10.1007/S10658-012-9948-X
Wang Y., Yang J., Gao Y., Ma H., Dai Y., and Ma H. (2025). Development and application of a cost-effective multiplex Kompetitive Allele-Specific polymerase chain reaction assay for pyramiding resistant genes of fusarium head blight and powdery mildew in wheat. BMC Plant Biol. 25, 963. doi: 10.1186/s12870-025-07005-8
Yang G., Chen S., Chen L., Sun K., Huang C., Zhou D., et al. (2019). Development of a core SNP arrays based on the KASP method for molecular breeding of rice. Rice 12, 21. doi: 10.1186/s12284-019-0272-3
Ziems L. A., Hickey L. T., Hunt C. H., Mace E. S., Platz G. J., Franckowiak J. D., et al. (2014). Association mapping of resistance to Puccinia hordei in Australian barley breeding germplasm. Theor. Appl. Genet. 127, 1199–1212. doi: 10.1007/S00122-014-2291-1/FIGURES/5
Keywords: barley leaf rust, resistance, Kompetitive Allele Specific Primers, Rph7, marker assisted selection
Citation: Wiles D, Gurung K, Tongson E, Park RF, Cai Y, Viradia B, Okuda KS, Perovic D and Dracatos PM (2025) Pangenome-assisted development and validation of a predictive KASP marker for the barley leaf rust resistance gene Rph7. Front. Agron. 7:1670733. doi: 10.3389/fagro.2025.1670733
Received: 22 July 2025; Accepted: 22 September 2025;
Published: 23 October 2025.
Edited by:
Congcong Jiang, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Md Arifuzzaman, Hajee Mohammad Danesh Science and Technology University, BangladeshNaveed Rehman, Huazhong Agricultural University, China
Mir Muhammad Nizamani, Shantou University, China
Copyright © 2025 Wiles, Gurung, Tongson, Park, Cai, Viradia, Okuda, Perovic and Dracatos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter M. Dracatos, cC5kcmFjYXRvc0BsYXRyb2JlLmVkdS5hdQ==
 Danielle Wiles1
Danielle Wiles1 Robert F. Park
Robert F. Park Kazuhide Shaun Okuda
Kazuhide Shaun Okuda Dragan Perovic
Dragan Perovic Peter M. Dracatos
Peter M. Dracatos