- Department of Agricultural Biology, Colorado State University, Fort Collins, CO, United States
Alfalfa mosaic virus (Bromoviridae: Alfamovirus) (AMV) is an emerging pathogen of peppers (Capsicum spp. L.) in Colorado that has caused significant yield losses. The virus is transmitted non-persistently by aphids, and few management options are available to suppress the virus. Thus, the goal of this work was to evaluate the effectiveness of host plant resistance against AMV. We evaluated 20 commercially available pepper cultivars for resistance to AMV in the greenhouse and validated resistance in a subset of these under field conditions on commercial farms in Pueblo, CO. Virus infection was assessed by quantifying symptom severity and measuring incidence of AMV symptoms and disease incidence through ELISAs. We found in the greenhouse that 20% of cultivars were asymptomatic to AMV despite being infected. Additionally, 10% of cultivars displayed symptoms but never tested positive for the virus. Field trials on commercial farms revealed that half of the tested cultivars (‘Bayonet’, ‘Masivo’, ‘Mosco’, and ‘Revolution’) maintained low symptom severity and infection incidence throughout the season. Cultivar responses were consistent between greenhouse and field settings, and temporal changes in susceptibility were also observed in two cultivars (‘Masivo’ and ‘Lumbre’). Our findings identify several pepper cultivars with resistance to AMV that could be integrated into pest management programs. These results also provide a foundation for future research aimed at breeding AMV-resistant peppers and developing comprehensive management strategies for this emerging viral disease.
1 Introduction
In 2019, Alfalfa mosaic virus (Bromoviridae: Alfamovirus) (AMV) was documented for the first time in peppers (Capsicum spp. L.) in Colorado (Amiri-Kazaz et al., 2025). Since then, it has contributed to significant yield losses, threatening yield and profitability (Amiri-Kazaz et al., 2025). Symptoms of AMV in peppers present as vein deformities, leaf curling, and mosaic chlorosis and necrosis of leaves, leading to reduced photosynthetic capacity (Burgmans et al., 1986; Trucco et al., 2022). It can also cause blotchiness and deformities of fruit, making them unmarketable (Burgmans et al., 1986; Kenyon et al., 2014). Alfalfa mosaic virus is transmitted by aphids (Hemiptera: Aphididae), and there are over 25 species confirmed as vectors (Kenyon et al., 2014; Trucco et al., 2022). It can also be transmitted through seed in many crops, including pepper (Šutič, 1959), alfalfa (Medicago sativa L.) (Frosheiser, 1973; Hemmati and McLean, 1977), chickpea (Cicer arietinum L.), and lentil (Lens culinaris Medik. subsp. Culinaris) (Jones and Coutts, 1996; Latham et al., 2004). With a broad host range of over 600 species, AMV is one of the most important crop viruses worldwide (Trucco et al., 2022).
The virus’s widespread impact is largely due to its transmission by aphids in a non-persistent manner, enabling quick and efficient spread among plants (Kenyon et al., 2014). Non-persistently transmitted viruses are retained only on the insect mouthpart and parts of the foregut (Ng and Perry, 2004). Vectors of non-persistently transmitted viruses typically take seconds to minutes to transmit the virus from an infected host to an uninfected host (Watson and Roberts, 1939). Thus, viruses transmitted non-persistently do not require habitual feeding for transmission to occur (Perring et al., 1999). Most often, non-persistently transmitted viruses are spread by non-colonizing aphids that probe hosts with their mouthparts as they transiently move through fields (Perring et al., 1999). Peppers in Colorado are an example of this, as aphids are rarely found on peppers in fields with severe incidence and symptoms of AMV. Management of AMV, especially when the vectors are rarely found in the field, poses a unique challenge for Colorado pepper farmers.
Mitigation of infection and subsequent yield losses through cultural controls act as a first line of defense against many pathogens (Stout, 2014). Host plant resistance is a promising cultural management tactic for farmers as it offers a cost-effective and sustainable approach to minimizing the impact of AMV on pepper production (Stout, 2014). Resistance of a plant to a pathogen depends on the response (or lack thereof) within the plant (Ausubel et al., 1995). Plants are considered resistant to pathogens when they limit pathogen multiplication and thus reduce the effect of infection on their fitness (Pagán and García-Arenal, 2018). They can also be tolerant of pathogens, whereby the host reduces the effects of infection regardless of the level of pathogen multiplication (Pagán and García-Arenal, 2018). Plants can also be susceptible to a pathogen, whereby the pathogen and plant are compatible, and infection occurs (Walling, 2009). Investigating resistance amongst pepper cultivars can provide producers with a valuable disease management tool (Stout, 2014). Moreover, host plant resistance is a tactic that is compatible with other methods of management, reinforcing its importance in an integrated pest management program (Stout, 2014).
Host plant resistance has been employed as a tool against viral plant pathogens in several cropping systems. Host plant resistance has been successfully used for the suppression of Bean yellow mosaic virus (Potyviridae: Patatavirales) in peas (Pisum sativum L.) (Van Leur et al., 2013) and Chile leaf curl virus (Geplafuvirales: Geminiviridae) in several chile pepper cultivars (Thakur et al., 2019). Host plant resistance is also a powerful tactic against vectors of viruses. Lower infection rates of Tomato chlorosis virus (Martellivirales: Closteroviridae) were observed in tomato (Solanum lycopersicum L.) cultivars that are resistant to the virus vector, Bemisia tabaci (Hemiptera: Aleyrodidae) (Fortes et al., 2020). Genomic studies have also revealed specific genes that provide resistance against certain pathogens. Boyd et al. (2013) found XA21, a resistant gene to the rice blight pathogen, Xanthomonas oryzae pv. oryzae (Xanthomonadales: Xanthomonadaceae), by recognizing the corresponding avirulence gene AX21. AX21 has been observed in multiple Xanthomonas species including those of economically important crops such as soybeans (Glycine max L.) and citrus (Citrus spp. L.). As research on host plant resistance advances, new insights into plant immunity and pathogen biology will be available for the protection of crops.
Since the discovery of AMV in 2019, Colorado pepper farmers have been left with negligible management tools for improving yields. Discovering resistant and/or tolerant pepper cultivars to AMV is a crucial first step in disease management. By investigating host plant resistance, we can provide farmers with an inexpensive and environmentally friendly tool to improve yields. Moreover, testing cultivars in the field will evaluate host plant resistance in an applied setting and confirm its robustness as a management tactic. Thus, our objectives were to phenotype peppers cultivars for resistance to AMV in the greenhouse and to validate greenhouse results in the field. The research presented here could provide farmers with a long-term solution for increased yields while contributing to more sustainable farming practices.
2 Materials and methods
2.1 Plant cultivation and virus colony maintenance
All plants used in the greenhouse experiments were grown from seed at the Colorado State University Insectary in Fort Collins, Colorado. Peppers were sown into 128-cell plug trays and each cell received one pearl of Osmocote® Plus 15:9:12 N-P-K slow-release fertilizer. Plug trays were kept in metal trays in a growth chamber for germination and bottom-watered ad libitum. Once seeds began to germinate, trays were fertilized with liquid fertilizer once a week (JR Peters Inc., 15-16–17 Peat Lite, Allentown, PA, USA). Some cultivars had low germination in both experimental blocks and thus, those cultivars had lower replications than others. Due to the number of cultivars tested and space available, the highest replication of cultivars was 10 plants. When plants developed four true leaves, two to ten plants for each cultivar were transplanted into four-inch pots and kept in mesh cages (75 x 75 x 115 cm with an aperture of 680 µm mesh; MegaView Science Co. Ltd. Taichung, Taiwan) on greenhouse benches.
To maintain a virus colony for inoculations, pepper plants displaying AMV symptoms were collected from the Colorado State University Arkansas Valley Research Station in Rocky Ford, Colorado on 8 September 2023. Leaves of symptomatic plants were used to inoculate a commercially available cultivar of chile peppers, ‘Joe Parker’, that is highly susceptible to AMV (Janecek, 2023). Once symptoms appeared, PCR was used to confirm AMV presence in each plant. Total RNA was extracted from symptomatic leaf tissues using the RNeasy Plus Mini kit (Qiagen Inc., Valencia, CA, USA) following the manufacturer’s instructions. RNA quantity and quality were assessed using a Nanodrop One spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA) and a Qubit 3.0 fluorometer (ThermoFisher Scientific). DNA contamination was removed from the RNA using the TURBO DNA-Free kit (Invitrogen, Waltham, MA, USA) in 15 μl reactions following the manufacturer’s instructions. Following DNAse treatment and quantification, one μg of total RNA was used to synthesize cDNA using Verso cDNA synthesis kit (ThermoFisher Scientific) according to manufacturer’s instructions. The RT-PCR was performed using DreamTaq Green PCR Master Mix (2X) (ThermoFisher Scientific) with primers specific for a region of AMV coat protein (CP) -CP (5’- ATCATGAGTTCTTCACAAAAGAA-3’ and 5’- TCAATGACGATCAAGATCGTC-3’) (Xu and Nie 2006). The amplification cycle consisted of 2 min at 95 °C, 35 cycles of 30 sec at 95 °C, 30 sec at 58 °C and 1 min at 72 °C followed by 5 min at 72 °C. The PCR products (669 bp) were visualized on a 1% agarose gel. The sequences of each PCR product showed 99% identity to Aq isolate of AMV-CP (GenBank accession JX112758). These plants were maintained in the greenhouse and used for all further inoculations of both experimental and additional colony plants.
2.2 Greenhouse experiment
The experiment was conducted in two sequential time blocks from April to July 2024. Seeds of twenty different pepper cultivars (Table 1) were sown and maintained as described above. Mechanical inoculations consisted of harvesting highly symptomatic leaves from an infected pepper plant from the AMV virus colony and grinding them into a paste using a sodium phosphate buffer (0.1M Na3PO4, pH 7.2-7.4). For each gram of leaf tissue, 1 mL of buffer was used. For the plants that were to be inoculated, carborundum was dusted onto two leaves using a paintbrush. The paste was then rubbed onto both leaves for 12–15 s per leaf. Plants were inoculated once between the four and six true leaf stages.
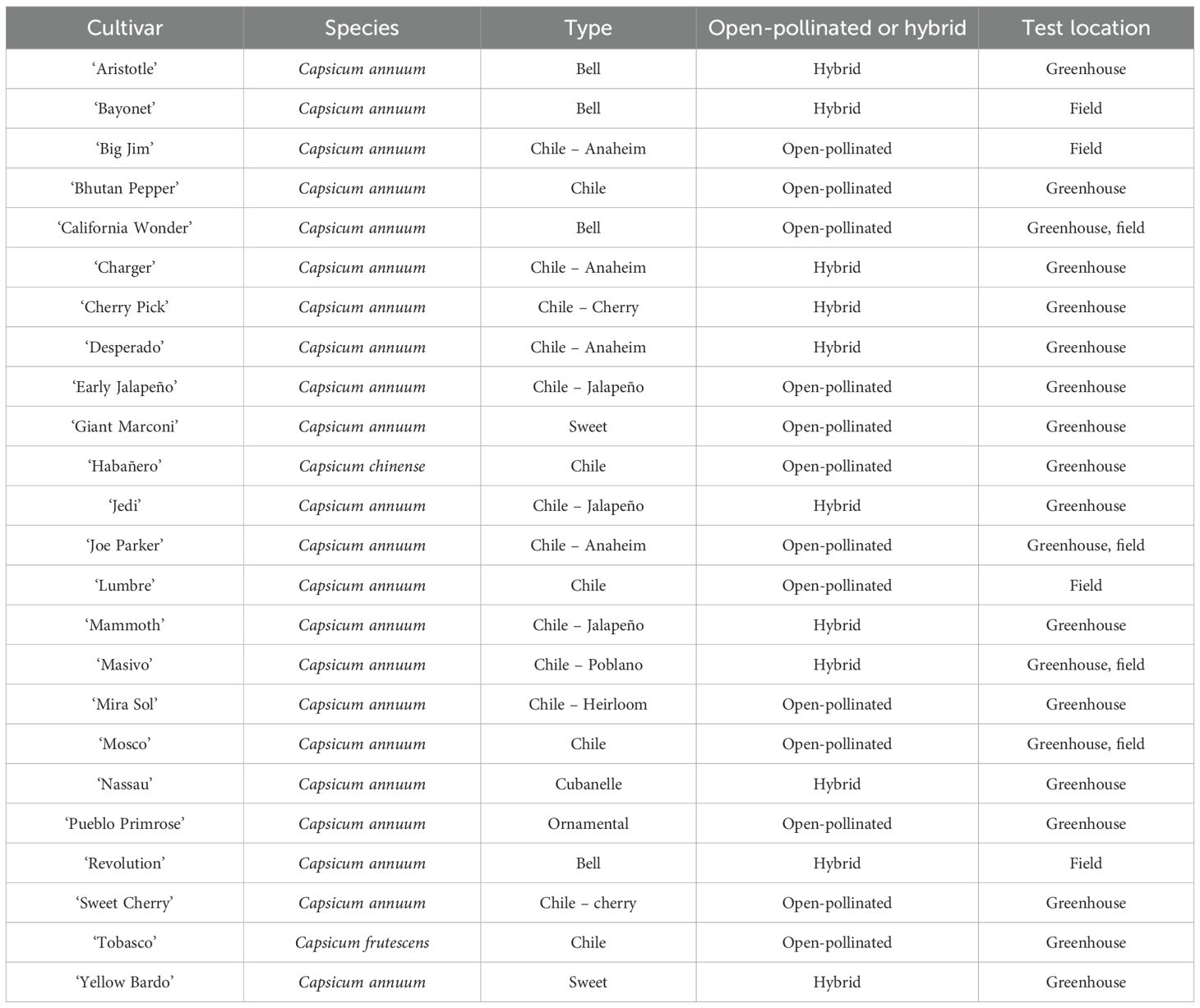
Table 1. Pepper cultivars, their species, pepper type, and the location tested for both greenhouse and field experiments.
After inoculations, plants were maintained in mesh cages on the greenhouse bench in a complete randomized design. Pots were only labeled with a number that corresponded to the cultivar to maintain randomization of cultivars within the cages. Each cage can hold 16 pots, and 14–16 pots were randomly placed within cages depending on the total number of pots for each experimental block. Plants were watered ad libitum and fertilized once a week with liquid fertilizer (JR Peters Inc., 15-16–17 Peat Lite, Allentown, PA, USA). Three to four weeks after inoculation, symptom severity of AMV was recorded as the number of leaves with symptoms divided by total number of leaves per plant. Additionally, one leaf per plant was collected on the same day. To keep sampling consistent, the youngest fully developed leaf was chosen for collection. Leaf tissue was collected in 2.0 mL centrifuge tubes and kept in a -80 °C freezer until processed with enzyme-linked immunosorbent assays (ELISA). Agdia® AMV ELISA protocol and Agdia® Compound-ELISA reagent and buffer sets were used to process and test for the presence of AMV. A 96-well plate was used for each assay and consisted of two samples per collected leaf. Using an ELx800 Universal Microplate Reader (Agilent technologies Inc, Santa Clara, CA, USA) at 450 nm, plates were assessed for optical density (OD) values. Plants were considered positive for AMV if the average OD value of their two samples was over double the average of the negative control. Negative and positive ELISA outcomes were recorded as 0 and 1 for disease incidence, respectively.
2.3 Field experiment
The field experiment was conducted from June to August 2024 on two commercial pepper farms owned and operated by DiSanti Farms, in Pueblo, Colorado: Farm 1 (38°13’12.3”N 104°27’35.8”W) and Farm 2 (38°14’10.3”N 104°27’34.0”W). Farm 1 (11 ha) cultivated peppers in addition to other vegetables, including tomatoes and squash, whereas Farm 2 (7 ha) only grew peppers. Both farms were irrigated using furrow irrigation. The farms were located less than one mile apart, and both farms were adjacent to alfalfa fields. Each farm was treated as a block.
Pepper cultivars ‘Joe Parker’, ‘Masivo’, ‘Bayonet’, ‘Revolution’, and ‘California Wonder’ were produced on Farm 1 and cultivars ‘Joe Parker’, ‘Big Jim’, ‘Lumbre’, and ‘Mosco’ were produced on Farm 2. As the field experiment was conducted on commercial farms, we were unable to choose the cultivars planted. However, four cultivars, namely ‘Joe Parker’, ‘California Wonder’, ‘Masivo’ and ‘Mosco’, were the same cultivars tested in the greenhouse experiments described above (Table 1).
All peppers except for ‘Bayonet’ and ‘Revolution’ were direct seeded on 8 April 2024. Seed costs of ‘Bayonet’ and ‘Revolution’ were significantly greater than all the other cultivars and thus were seeded in the greenhouse and transplanted into the field on 13 and 14 May 2024. Each cultivar was sampled within an area of 60 meters across rows. The number of rows was different for each cultivar, ranging from 18 to 108; however, the first and last three rows for each cultivar were excluded from sampling. Each of these areas was divided into three plots (replicates) measuring 20 meters from edge to edge with a 15-meter buffer in between. Five plants from each section were randomly sampled, resulting in fifteen plants from each cultivar. Data were collected twice throughout the growing season to explore if symptom severity and disease incidence change over time. Data collection took place on 25 June 2024 when plants had reached just about 30 cm in height, and 24 July 2024 when plants were 45–60 cm in height. At the time of the first collection date, plants were still in their vegetative stage and by the second sampling date, most were in their bolting stage (transition) with only a few plants flowering. Symptom severity (estimated percentage of leaves showing AMV symptoms) and incidence of AMV symptoms (0 for no symptoms and 1 for symptoms) were recorded for each plant. Additionally, a leaf from each plant was harvested and collected in a 2.0 mL centrifuge tube. Tubes were kept on ice during transportation from Pueblo to Fort Collins where they were placed in a -80 °C freezer. Leaf tissue collected at both time points was used to evaluate the presence or absence of AMV through ELISA tests using the same Agdia® AMV ELISA protocol and Agdia® Compound-ELISA reagent and buffer kits mentioned earlier.
2.4 Statistical analyses
Statistical analyses were conducted using RStudio Version 2024.12.0 + 467. For symptom severity assessed in greenhouse experiments and field trials, data did not meet the assumptions of ANOVA and thus, were non-parametric. We used the Scheirer-Ray-Hare test, a non-parametric alternative to a two-way ANOVA via the “rcompanion” package (Sokal and Rohlf, 1995) to assess if the interaction between block and treatment was significant. For incidence of AMV symptoms and disease incidence in both the greenhouse and field, data were binomial. Thus, we fit and compared two generalized linear models (GLMs).
For symptom severity in the greenhouse, we found that the interaction was not significant and combined both blocks to run a single Kruskal-Wallis test for symptom severity. For incidence of AMV symptoms and disease incidence, we fit and compared two generalized linear models (GLMs): an interaction model (cultivar*block) and an additive model (cultivar+block) for each parameter. We found no significant interaction and combined both blocks to run a single GLM for incidence of AMV symptoms and disease incidence, respectively.
Statistical analysis comparing symptom severity in the field included testing the interaction between cultivar and farm (i.e., block) using the Scheirer-Ray-Hare test and found that it was not significant. We then used the Scheirer-Ray-Hare test to test the interaction between cultivar and sampling date. Where the interactions were not significant, we combined the data for both blocks (i.e., farms) and used Kruskal-Wallis tests to individually test the effect of cultivar and sampling date on symptom severity. For incidence of AMV symptoms, we compared a GLM with an interaction effect between cultivar and sampling date (cultivar*sampling date) to a GLM with an additive effect between cultivar and sampling date (cultivar+sampling date). We found no significant interaction between the models and thus used the additive model to analyze the effect of cultivar on incidence of AMV symptoms across farms (i.e., blocks) and sampling date. We ran a similar analysis for disease incidence and found that there was a significant difference between the interaction and the additive model. Thus, we analyzed positive outcomes by sampling date, running separate models for each.
2.5 Criteria for tolerance and resistance classification
To create a criterion for which cultivars we deem tolerant versus resistant in our experiments, we used Cooper and Jones’ (1983) and Jeger’s (2023) definitions to guide our classifications. Cultivars that showed moderate to high levels of infection but maintained low symptom severity were classified as tolerant, while cultivars that experienced low or no disease incidence were classified as resistant.
3 Results
3.1 Greenhouse experiment
We found no significant interactive effect of cultivar and time block on the severity of AMV symptoms (Sheirer Ray Hare, df = 19, H = 9.23, p = 0.969), thus we combined both blocks. We found that cultivar had a significant effect on AMV symptom severity (Kruskal-Wallis χ² = 38.83, df = 19, P = 0.005; Figure 1A). ‘Joe Parker’ was the most symptomatic cultivar, with an average of 37% of leaves on each plant showing symptoms of the disease. All other cultivars that had AMV symptoms did not surpass an average of 20% of leaves affected by the virus. Cultivars ‘Desperado’, ‘Masivo’, ‘Miral Sol’, and ‘Habañero’ did not show any AMV symptoms (Figure 1A).
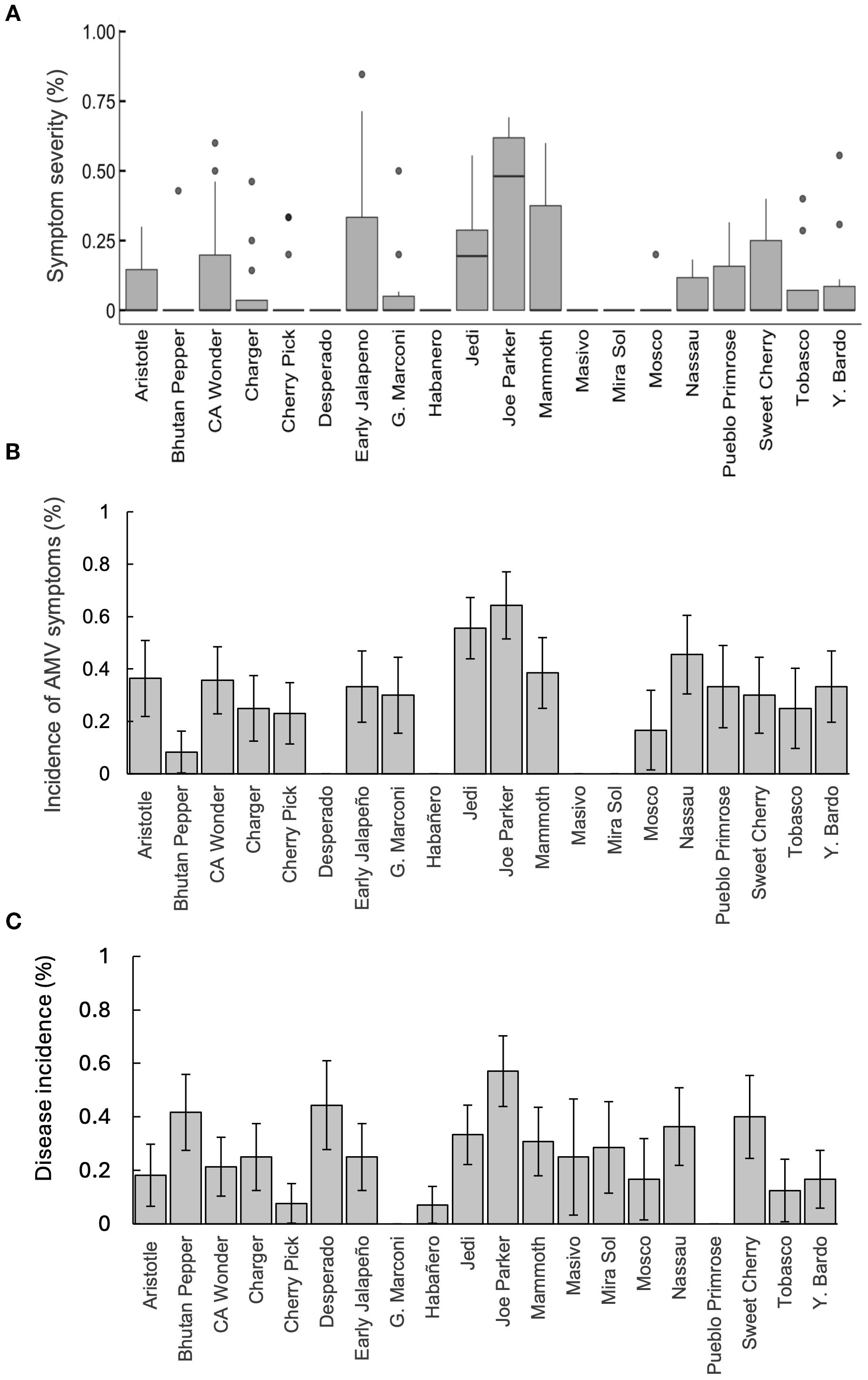
Figure 1. (A). Severity of AMV symptoms across cultivars in the greenhouse. The x-axis represents the 20 cultivars tested and the y-axis represents the severity of symptoms (number of leaves with symptoms divided by the total number of leaves). Boxes represent the interquartile range for each cultivar. Median is indicated by the horizontal line within the box, and whiskers show the range excluding outliers. Outliers are shown as individual points. The most symptomatic cultivar was ‘Joe Parker,’ while four cultivars (‘Desperado’, ‘Habañero’, ‘Masivo’ and ‘Mira Sol’) did not show any symptoms. Some cultivars showed low overall symptom severity but had individual plants with unusually high symptoms (e.g., ‘Bhutan Pepper’, ‘Cherry Pick’, among others). (B). Average incidence of AMV symptoms across cultivars in the greenhouse. Bars represent means ± 1 SEM. Cultivars tested are on the x-axis and the proportion of symptomatic plants for each cultivar is on the y-axis. These results correspond to the severity of AMV symptoms (Figure 1A), as ‘Joe Parker’ has the highest incidence of AMV symptoms while cultivars ‘Desperado’, ‘Habañero’, ‘Masivo’, and ‘Mira Sol’ did not show any symptoms throughout the experiment. Several cultivars had high incidence while symptoms on plants remained mild (e.g. ‘Nassau’, ‘Yellow Bardo’, among others). (C). Disease incidence for plants tested in the greenhouse. Bars represent means ± 1 SEM. Although four cultivars did not show any AMV symptoms, they all still tested positive for AMV through ELISAs (‘Desperado’, ‘Habañero’, ‘Masivo’, and ‘Mira Sol’). There were two cultivars, ‘Giant Marconi’ and ‘Pueblo Primrose’ that showed symptoms but did not test positive for AMV through ELISAs. ‘Habañero’ had the lowest disease incidence, closely followed by ‘Cherry Pick’ with only 7% and 8% of plants testing positive for AMV, respectively.
The incidence of AMV symptoms in the greenhouse was evaluated using a generalized linear model (GLM) to examine the effect of cultivar and block on the presence of symptoms. We found no significant effect of the interaction between cultivar and block (likelihood ratio χ² = 15.28, df = 19, p = 0.704) on incidence of AMV symptoms and thus combined both blocks for analysis. We found that cultivar had significant effects on the incidence of AMV symptoms (likelihood ratio χ² = 42.96, df = 19, p = 0.001) (Figure 1B). Cultivars ‘Joe Parker’ and ‘Jedi’ had the highest proportion of plants showing AMV symptoms with 64.3% and 55.6%, respectively (Figure 1B). Similarly, the interaction between cultivar and block did not have a significant effect on disease incidence (likelihood ratio χ² = 18.91, df = 19, p = 0.463) and both blocks were combined for analysis. We found that the cultivar again had a significant effect on disease incidence (likelihood ratio χ² = 30.87, df = 19, p = 0.042) (Figure 1C). The cultivar with the highest proportion of plants testing positive for AMV was ‘Joe Parker’, consistent with the severity and incidence of AMV symptoms (Figure 1). Based on ELISA results, ‘Joe Parker’ had an infection rate of 57% while no other cultivars exceeded 40%. Only two cultivars, ‘Giant Marconi’ and ‘Pueblo Primrose’ did not test positive for AMV, while exhibiting AMV symptoms (Figure 1).
3.2 Field experiment
We found significant differences in AMV symptom severity and incidence among cultivars grown on commercial pepper farms. A Scheirer-Ray-Hare test was performed to assess the effects of cultivar, farm, and their interaction on AMV symptom severity of peppers. The interaction between cultivar and farm (i.e., block) was not significant (Scheirer Ray Hare, df = 261, H = 172.45, p = 0.999), thus we combined the data from both farms (i.e., blocks). We then performed an additional Scheirer-Ray-Hare test to assess the effects of cultivar, sampling date, and their interaction on AMV symptom severity. We again found no significant interaction (Scheirer-Ray-Hare, df = 7, H = 11.35, p = 0.124), indicating that the overall changes in symptom severity for each cultivar were consistent over time. We used Kruskal-Wallis tests to individually analyze the effects of cultivar and sampling date on AMV symptom severity. We found that cultivar had a significant effect on AMV symptom severity (Kruskal-Wallis χ² = 93.34, df = 7, p-value < 0.001) as did sampling date (Kruskal-Wallis χ² = 15.28, df = 1, p-value < 0.001). Specifically, plants sampled later in the season had higher symptom severity compared to plants sampled earlier (Wilcoxon rank-sum, W = 6668, p < 0.001; Figure 2). After performing Bonferroni correction, we found that two cultivars, ‘Joe Parker’ (adjusted p-value < 0.001) and ‘Big Jim’ (adjusted p-value = 0.002) had significant increases in symptom severity between sampling dates.
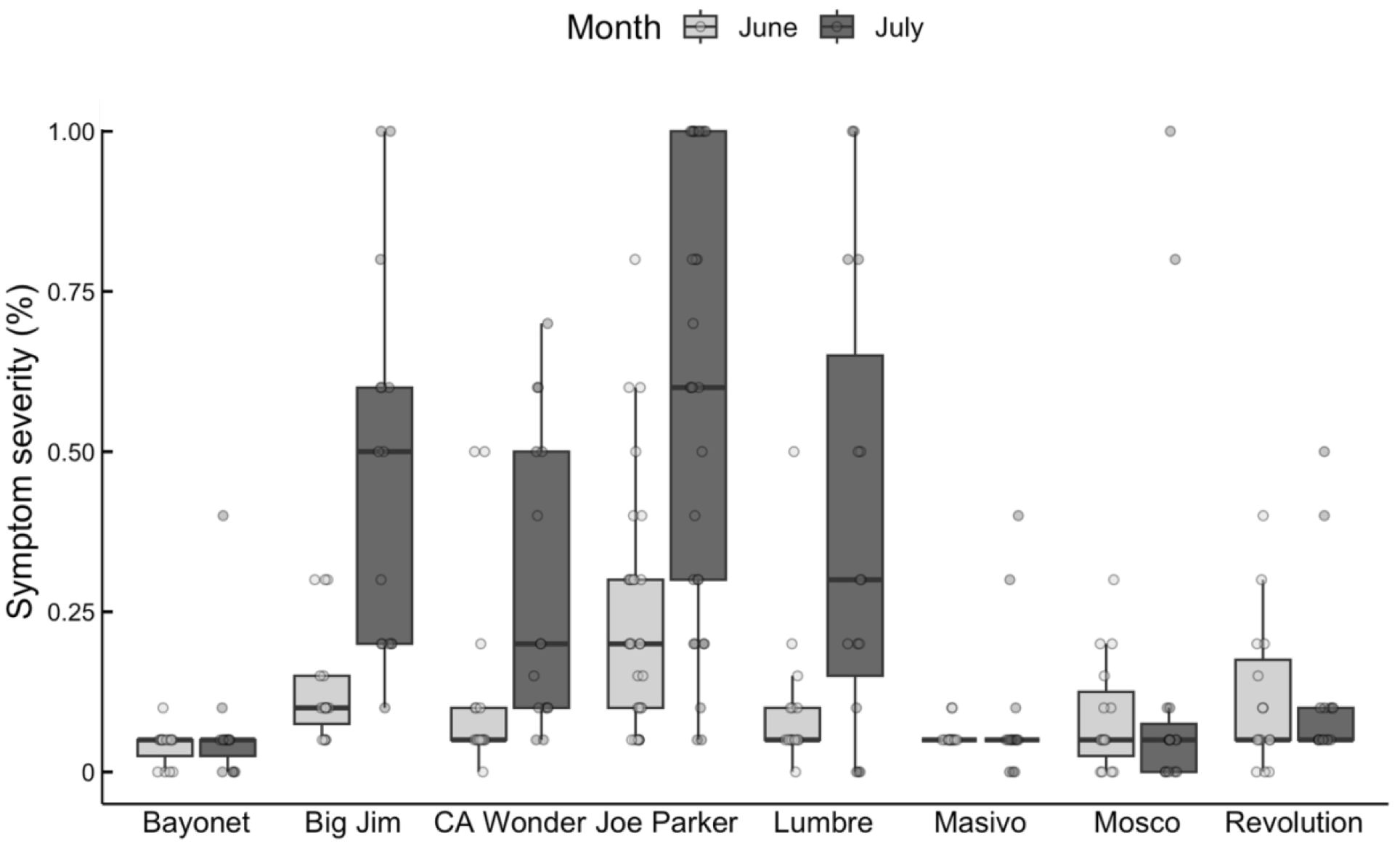
Figure 2. Severity of AMV symptoms across pepper cultivars on commercial farms in June (light grey) and July (dark grey). Cultivars are on the x-axis, and symptom severity shown as an estimated percentage of symptomatic leaves is on the y-axis. Boxes represent the interquartile range for each cultivar. Median is indicated by the horizontal line within the box, and whiskers show the range excluding outliers. Outliers are shown as individual points. ‘Joe Parker’ and ‘Big Jim’ are the only cultivars that had a significant increase in AMV symptoms between June and July (p<0.05), as indicated by an asterisk.
A generalized linear model (GLM) was used to evaluate the incidence of AMV symptoms and disease incidence across cultivars on commercial pepper farms throughout the growing season. For both measurements, we first fitted and compared an interaction model and additive model between cultivar and month, with farm (i.e., block) as an additive effect. For the incidence of AMV symptoms, we found no significant interaction between the models (ΔDeviance = 11.88, Δdf = 7, p = 0.105). Thus, we used the additive model to assess the individual contribution of each factor to AMV symptom incidence. Cultivar had a significant effect on the incidence of AMV symptoms (likelihood ratio χ² = 34.66, df = 7, p < 0.001; Figure 3A), whereas sampling date did not (likelihood ratio χ² = 0.42, df = 1, p = 0.52), indicating that infection of AMV across cultivars did not change throughout the season. Block (i.e., farm) also did not significantly affect disease incidence (likelihood ratio χ² = 0.0, p = 1).
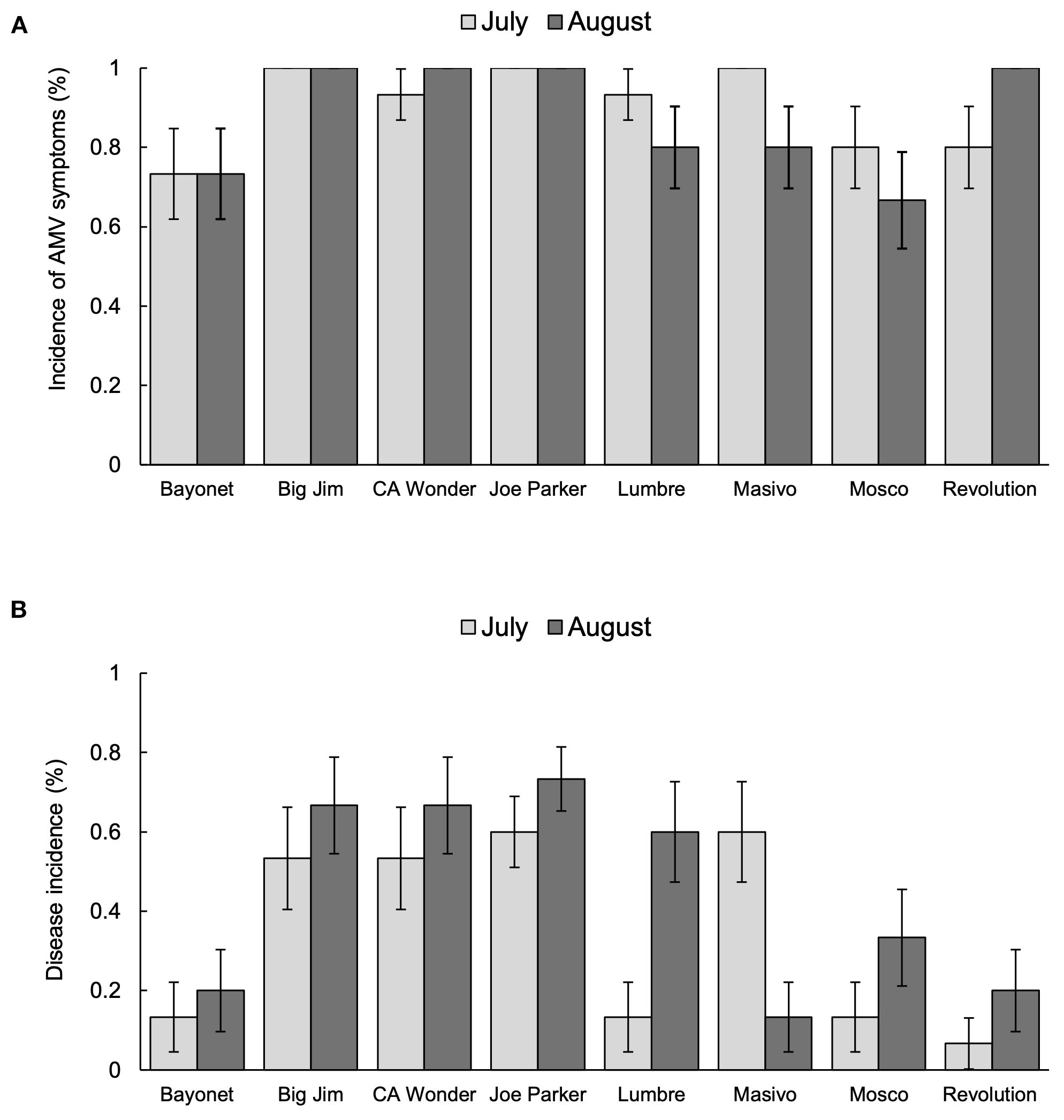
Figure 3. Incidence of AMV symptoms (A) and disease incidence (B) for cultivars tested on commercial pepper farms. Cultivars are listed on the x-axis and incidence rate is on the y-axis. Light grey bars represent the mean incidence of AMV symptoms in June and dark grey bars represent mean incidence of AMV symptoms in July. Errors bars represent standard error (± 1 SEM). ‘Big Jim’ and ‘Joe Parker’ showed 100% incidence throughout the growing season. Cultivars ‘California Wonder’ and ‘Revolution’ showed increases throughout the season while ‘Lumbre’, ‘Masivo’, and ‘Mosco’ showed decreased positive outcomes. However, these differences were not significant. ‘Bayonet’ was the only cultivar to have a constant incidence rate throughout the season (A). On the other hand, the incidence of AMV reflected in the positive ELISA results tended to increase throughout the season for most cultivars; however, this increase was only significant for ‘Lumbre’. ‘Masivo’ was the only cultivar to show a significant decrease in positive outcomes for AMV (B).
On the other hand, the interaction model significantly improved the fit of the logistic regression (ΔDeviance = 16.8, Δdf = 7, p = 0.019) for disease incidence. This indicated that the effect of cultivar on this measure of incidence depended on sampling time, with more plants testing positive for the virus later in the season (Figure 3B). We ran separate GLMs for both sampling date and found that cultivar had a significant effect on disease incidence in both June (likelihood ratio χ² = 33.18, df = 7, p < 0.001) and July (likelihood ratio χ² = 26.64, df = 7, p < 0.001). However, the only cultivar to have a significant increase in disease incidence was ‘Lumbre’ (estimate = 2.28, SE = 0.92, z = -2.46, p = 0.014). There was one cultivar, ‘Masivo’, that had a significant decrease in disease incidence throughout the season (estimate = -2.28, SE = 0.924, z = 2.46, p = 0.014).
4 Discussion
Host plant resistance to AMV emerged as a strong theme in greenhouse and field screenings of pepper cultivars. We found that 10% of the 20 cultivars screened in the greenhouse had strong resistance to AMV, displaying minimal symptoms and never testing positive for AMV, while an additional 20% of the cultivars demonstrated tolerance to the virus, maintaining low infection rates and symptoms. This result highlights that commercially available cultivars possess strong genetic resistance to AMV. While not all 20 cultivars used in the greenhouse were tested in the field experiments owing to limitations imposed by on-farm research, we found that the four cultivars that overlapped between field and greenhouse studies showed similar responses to AMV across systems. Further, half of all the cultivars used in the field also showed strong tolerance to AMV with minimal symptom severity throughout the season and infection rates of less than 40% by the end of the season. We did not find any cultivars that exhibited resistance in the field, which may be attributed to the variability of field conditions and additional stressors that may compromise plant defenses. It is noteworthy that for the cultivars we used in both the greenhouse and in the field, i.e., ‘California Wonder’, ‘Joe Parker’, ‘Masivo’, and ‘Mosco’, the disease incidence was generally greater when plants were exposed to the virus via aphid vectors in the field. The incidence of disease in the bell pepper cultivar ‘California Wonder’ was more than twice the levels we noted in the greenhouse. This could be attributed to continuous exposure to infected aphids in the field as well as the abiotic factors such as heat stress and suboptimal irrigation that may further hamper plants’ responses to infection. Interestingly, the cultivar ‘Joe Parker’ had similarly high infection rates of AMV in the greenhouse and field, suggesting this cultivar is highly susceptible to AMV, which has been demonstrated previously as well (Janecek, 2023).
The disease incidence and severity of symptoms of AMV we observed in the greenhouse and field highlight the wide variation in resistance and tolerance to the virus. How tolerance is defined has evolved since its first use to describe rust-enduring wheat cultivars by Cobb (1894). Generally, a host is considered tolerant to a pathogen if it can reduce the negative effects of infection as opposed to resistance, which is the ability to limit pathogen replication (Pagán and García-Arenal, 2020). Metrics used to evaluate tolerance to pathogens typically focus on yield and quality of product for crops, host mortality, and fecundity (Read, 1994; Alizon et al., 2009; Alizon and Michalakis, 2015; Pagán and García-Arenal, 2020). However, metrics such as symptom development and their severity regularly correlate with mortality or fecundity metrics and are often easier to quantify (Doumayrou et al., 2013; Pagán and García-Arenal, 2020). There is evidence for all classes of plant pathogens that disease severity is correlated with pathogen replication within a host (Read, 1994; Anderson et al., 1996; Fargette et al., 2002; Alizon and Michalakis, 2015; Pagán and García-Arenal, 2020). However, this is less common for viruses as many studies have shown that virus pathogenesis is largely dependent on the deregulation of host gene expression (Pallás and García, 2011). Moreover, “tolerant” and “sensitive” have been utilized as opposite ends of a scale representing the reaction of a host plant to a viral infection as compared to “resistant” and “susceptible” that more accurately describe a scale of the host plants’ effect on virus infection, replication, and invasion (Cooper and Jones, 1983; Jeger, 2023). Based on these definitions and metrics of resistance and tolerance, we conclude that tolerance is a much more widespread mechanism of resistance in our system, and the majority of the cultivars we tested displayed responses congruent with tolerance to AMV.
Our study is the first to screen pepper cultivars specifically for AMV. Interestingly, other research indicated that some of the same cultivars we used in our experiments were also resistant to other diseases. For example, Suzuki et al. (2003) found that ‘Tobasco’ showed resistance to Cucumber mosaic virus Y in greenhouse experiments, which is in the same virus family as AMV. Maruthi et al. (2003) found that only 3% of the bell pepper cultivar ‘California Wonder’ had any incidence of Tomato leaf curl Bangalore virus–Bangalore strain 4 (Geplafuvirales: Geminiviridae) in greenhouse experiments. In another study, other bell pepper cultivars ‘Revolution’ and ‘Aristotle’ were screened for phytophthora blight, Phytophthora capsici (Peronosporales: Peronosporaceae), in field trials in New York state. ‘Aristotle’ showed lower disease incidence with just over 20% of plants infected compared to almost 100% for ‘Revolution’ (Dunn et al., 2013). Strong resistance to Tomato spotted wilt virus (Elliovirales: Tospoviridae) was also evident in five Capsicum species (C. annum, C. baccatum, C. chinense, C. frutescens, and C. pubescens) (Cebolla-Cornejo et al., 2003). Our research adds to the body of literature showing strong innate resistance to a wide range of pathogens across cultivars and species of peppers, and future work focused on elucidating mechanism of potential cross resistance will advance our ability to select for robust resistance to multiple pathogens.
Our results also highlighted distinct differences in how commercially available individual pepper cultivars responded to AMV. For example, ‘Desperado’, ‘Masivo’, and ‘Mira Sol’ did not exhibit any AMV symptoms, but their infection rates were much higher than ‘Habañero’ which also remained asymptomatic throughout the experiment. Moreover, cultivars ‘Giant Marconi’ and ‘Pueblo Primrose’ had low levels of severity but did not test positive for AMV. This suggests that ‘Desperado’, ‘Masivo’, and ‘Mira Sol’ may be tolerant to AMV, whereas ‘Giant Marconi’ and ‘Pueblo Primrose’ have a higher level of resistance to AMV, potentially affecting the virus’s ability to replicate and/or translocate through the plant. Additionally, ‘Nassua’ had high incidence of AMV symptoms and high infection rates compared to other cultivars but maintained low symptom severity. This response suggests that this cultivar may also possess tolerance rather than resistance to AMV. Moreover, we found interesting temporal patterns in the susceptibility of certain cultivars to the virus. For example, the cultivar ‘Masivo’ exhibited increasing resistance to AMV over time, which was evident in the field experiments. Specifically, the early-season infection rates were high in ‘Masivo’ peppers, suggesting that the host was unable to prevent infection. However, the subsequent reduction later in the season coupled with low symptom severity both early and late in the season imply that the plants were able to employ effective mechanisms for resistance against the virus over time. On the other hand, ‘Lumbre’ was the only cultivar to have a significant increase in infection rate with only 20% of plants infected in June and 60% infected by the end of July, demonstrating a decrease in tolerance to AMV at a different stage than most others.
Generally, host plants are most susceptible to a pathogen in an earlier growth stage and could acquire resistance over time as the host increases their ability to mitigate the infection (Whalen, 2005; Develey-Rivière and Galiana, 2007). This could explain why ‘Masivo’, even with a high infection rate early on had minimal symptoms and a lower infection rate later in the season. However, ‘Lumbre’ was unique in that it was more susceptible to AMV infection at an older growth stage. This would be contrary to what is typically seen, as resistance generally persists throughout the rest of the hosts’ lifecycle (Develey-Rivière and Galiana, 2007). At the time of second sampling, pepper plants were transitioning from their vegetative stages to flowering (bolting), as flower buds were developed but had not yet bloomed for most. This change in allocation of resources to reproductive tissues could have altered any acquired resistance ‘Lumbre’ may have possessed earlier in the season and/or increased its susceptibility to AMV. Melero et al. (2023) explored this idea and found that infection of Turnip mosaic virus (Stelpaviricetes: Potyviridae) in Arabidopsis proceeded faster in mature, post-bolting plants as compared to juvenile and bolting plants. These results, along with our own, add to a growing body of literature highlighting the complexity of virus-plant interactions, and reveal important differences between viral pathogenesis from other plant disease systems.
While AMV has been recognized as a pathogen of peppers for some time, it has only recently become a concern for growers in Colorado (Amiri-Kazaz et al., 2025). As a result, currently available commercial cultivars were not developed with resistance as a targeted trait. However, our greenhouse and field screenings demonstrate the complexity of innate resistance and tolerance mechanisms, providing a foundation for the future development of AMV-resistant peppers. The discovery of resistance genes and their use in crop breeding has often begun with field and greenhouse screenings to reveal cultivars with potentially useful levels of resistance. For example, García-Neria and Rivera-Bustamante’s (2011) work in discovering the two genes relaying resistance to Pepper golden mosaic virus (PepGMV) (Geplafuvirales: Geminiviridae) in a C. chinense accession was based on previous work by Godínez-Hernández et al. (2001) and Anaya-López et al. (2003) that screened for PepGMV in field and glasshouse experiments, respectively. Moreover, an open-field screening program of over 307 Capsicum genotypes to leaf curling most likely caused by Pepper leaf curl Varanasi virus (PepLCVaV) (Geplafuvirales: Geminiviridae) (Kumar et al., 2006) later led to the identification of a hybrid species that showed resistance through a single recessive gene (Rai et al., 2014). Through our experiments, we identified cultivars that remained asymptomatic despite being infected with AMV (‘Habañero’, ‘Desperado’, ‘Masivo’, ‘Mira Sol’) and cultivars that although showed symptoms did not test positive for AMV (‘Giant Marconi’ and ‘Pueblo Primrose’) in the greenhouse. We also identified one cultivar in the field, ‘Masivo’, that seemingly was able to suppress AMV infection throughout the growing season, resulting in significantly lower infection rates late in the season. These results may contribute to the identification of genetic markers that confer different types and levels of resistance, which could ultimately be used to develop accessions for managing AMV.
Results from our study show that several commercially available pepper cultivars should be considered for integrated pest management programs to combat high infection rates of AMV. Additionally, our screening experiments in both the greenhouse and field can provide a valuable starting point for the development of AMV-resistant pepper cultivars. Validating tolerance on commercial pepper farms provided evidence that incorporating promising cultivars can be seamlessly implemented in commercial production. Several limitations of our study was the low number of replications for certain cultivars in the greenhouse, as well as the lack of quantitative molecular tests across the greenhouse and field. Moreover, we were not able to record yield data as our research was conducted on commercial farms. Additional research is needed to quantify resistance in these cultivars and evaluate them using metrics such as yield and fruit quality to determine whether their tolerance to AMV translates into significant economic value for producers. As we were unable to choose which cultivars were tested on commercial farms, additional field experiments exploring promising cultivars from the greenhouse experiment in a more applied setting would provide valuable insights to their performance against AMV infection. Furthermore, other cultural tactics such as barrier crops or increased distance between peppers and AMV reservoirs such as alfalfa could contribute to the development of robust IPM programs for AMV management. This study highlights the potential of several pepper cultivars with innate resistance to AMV that should be considered for current production as well as future research directions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
LA-K: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. PN: Funding acquisition, Writing – review & editing. AS: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was funded by the Colorado Department of Agriculture Specialty Crop Block Grant Program to A.S. and P.N. (23SCBPCO1177); the United States Department of Agriculture, National Institute of Food and Agriculture Grant to A.S. (Number: 2024-70006-43565), and Hatch project COL00411 (accession number 7001931). The Colorado Department of Agriculture Specialty Crop Block Grant Program funds were applied towards salary, materials, and travel. The United States Department of Agriculture, National Institute of Food and Agriculture Grant and Hatch project COL00411 funds were applied toward salary.
Acknowledgments
We would like to thank M. Bartolo for information on Alfalfa mosaic virus in peppers in Colorado and D. DiSanti and J. DiSanti for their continued collaboration and access to pepper farms in Pueblo, CO. We would also like to thank N. Panwar, B. Martin, R. Baldwin, and A. Walker for their assistance in gathering data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alizon S., Hurford A., Mideo N., and Van Baalen M. (2009). Virulence evolution and the trade-off hypothesis: History, current state of affairs and the future. J. Evol. Biol. 22, 245–59 2. doi: 10.1111/j.1420-9101.2008.01658.x
Alizon S. and Michalakis Y. (2015). Adaptive virulence evolution: The good old fitness-based approach. Trends Ecol. Evol. 30, 248–254. doi: 10.1016/j.tree.2015.02.009
Amiri-Kazaz L. M., Pastrana A. M., Daugovish O., and Szczepaniec A. (2025). Alfalfa mosaic virus in Chile peppers: status, management prospects, and research needs. J. Integr. Pest Manage. 16, 24–30. doi: 10.1093/jipm/pmaf022
Anaya-López J. L., Torres-Pacheco I., González-Chavira M., GarzónTiznado J. A., Pons-Hernández J. L., Guevara-González R. G., et al. (2003). Resistance to geminivirus mixed infections in Mexican wild peppers. HortScience 38, 251–255. doi: 10.21273/HORTSCI.38.2.251
Anderson E. J., Kline A. S., Morelock T. E., and McNew R. W. (1996). Tolerance to Blackeye cowpea mosaic potyvirus not correlated with decreased virus accumulation or protection from cowpea stunt disease. Plant Dis. 80, 847–852. doi: 10.1094/PD-80-0847
Ausubel F. M., Katagiri F., Mindrinos M., and Glazebrook J. (1995). Use of Arabidopsis thaliana defense-related mutants to dissect the plant response to pathogens. Proc. Natl. Acad. Sci. 92, 4189–4196. doi: 10.1073/pnas.92.10.4189
Boyd L. A., Ridout C., O’Sullivan D. M., Leach J. E., and Leung H. (2013). Plant–pathogen interactions: Disease resistance in modern agriculture. Trends Genet. 29, 233–240. doi: 10.1016/j.tig.2012.10.011
Burgmans J. L., Fry P. R., and Sunde R. G. (1986). Peppers: Survey of virus diseases of Capsicum annuum in Hawke’s Bay and Poverty Bay. N.Z J. Exp. Agric. 14, 459–463. doi: 10.1080/03015521.1986.10423066
Cebolla-Cornejo J., Soler S., Gomar B., Soria M. D., and Nuez F. (2003). Screening Capsicum germplasm for resistance to tomato spotted wilt virus (TSWV). Ann. Appl. Biol. 143, 143–152. doi: 10.1111/j.1744-7348.2003.tb00280.x
Cobb N. (1894). Contributions to an economic knowledge of Australian rusts (Uredineae). Agric. Gaz. N. S. W. 5, 239–250. doi: 10.3389/fimmu.2019.02974
Cooper J. I. and Jones A. T. (1983). Responses of plants to viruses: Proposals for the use of terms. Phytopathology 73, 127–128. doi: 10.1094/Phyto-73-127
Develey-Rivière M. and Galiana E. (2007). Resistance to pathogens and host developmental stage: a multifaceted relationship within the plant kingdom. New Phytol. 175, 405–416. doi: 10.1111/j.1469-8137.2007.02130.x
Doumayrou J., Leblaye S., Froissart R., and Michalakis Y. (2013). Reduction of leaf area and symptom severity as proxies of disease-induced plant mortality: The example of the Cauliflower mosaic virus infecting two Brassicaceae hosts. Virus Res. 176, 91–100. doi: 10.1016/j.virusres.2013.05.008
Dunn A. R., Wyatt L. E., Mazourek M., Reiners S., and Smart C. D. (2013). Performance and tolerance to Phytophthora blight of bell pepper varieties. HortTechnology 23, 382–390. doi: 10.21273/HORTTECH.23.3.382
Fargette D., Pinel A., Traoré O., Ghesquiére A., and Konaté G. (2002). Emergence of resistance-breaking isolates of Rice yellow mottle virus during serial inoculations. Eur. J. Plant Pathol. 108, 585–591. doi: 10.1023/A:1019952907105
Fortes I. M., Fernández-Muñoz R., and Moriones E. (2020). Host plant resistance to Bemisia tabaci to control damage caused in tomato tlants by the emerging Crinivirus Tomato Chlorosis Virus. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.585510
Frosheiser F. I. (1973). Alfalfa mosaic virus transmission to seed through alfalfa gametes and longevity in alfalfa seed. Phytophathology 64, 102–105. doi: 10.1094/phyto-64-102
García-Neria M. A. and Rivera-Bustamante R. F. (2011). Characterization of Geminivirus resistance in an accession of Capsicum chinense Jacq. Mol. Plant-Microbe Interac. 24, 172–182. doi: 10.1094/MPMI-06-10-0126
Godínez-Hernández Y., Anaya-López J. L., Díaz-Plaza R., González-Chavira M., Torres-Pacheco I., Rivera-Bustamante R. F., et al. (2001). Characterization of resistance to Pepper Huasteco Geminivirus in chili peppers from Yucatán, México. HortScience 36, 139–142. doi: 10.21273/HORTSCI.36.1.139
Hemmati K. and McLean D. L. (1977). Gamete-seed transmission of Alfalfa mosaic virus and its effect on seed germination and yield in alfalfa plants. Phytopathology 77, 576. doi: 10.1094/Phyto-67-576
Janecek T. (2023). Developing integreated pest management tactics for Alfalfa mosaic virus and its aphid vectors in Chile peppers [Master’s dissertation]. Social Science Research Network. (Colorado State University). Available online at: https://api.mountainscholar.org/server/api/core/bitstreams/eca7ff64-1dca-42b3-849a-e75f9f14e016/content (Accessed July 18, 2025).
Jeger M. J. (2023). Tolerance of plant virus disease: Its genetic, physiological, and epidemiological significance. Food Energy Security. 12, e440. doi: 10.1002/fes3.440
Jones R. A. C. and Coutts B. A. (1996). Alfalfa mosaic and cucumber mosaic virus infection in chickpea and lentil: incidence and seed transmission. Ann. Appl. Biol. 129, 491–506. doi: 10.1111/j.1744-7348.1996.tb05771.x
Kenyon L., Kumar S., Tsai W.-S., and Hughes J. (2014). “Virus diseases of peppers (Capsicum spp.) and their control,” in Advances in Virus Research, eds. Loebenstein G. and Katis N. (Elsevier), vol. 90, p. 297–354.
Kumar S., Kumar S., Singh M., Singh A. K., and Rai M. (2006). Identification of host plant resistance to pepper leaf curl virus in chilli (Capsicum species). Sci. Horticult. 110, 359–361. doi: 10.1016/j.scienta.2006.07.030
Latham L. J., Jones R. A. C., and Coutts B. A. (2004). Yield losses caused by virus infection in four combinations of non-persistently aphid-transmitted virus and coolseason crop legume. Aus. J. Exp. Agric. 44, 57. doi: 10.1071/EA03060
Maruthi M., Muniyappa V., Green S., Colvin J., and Hanson P. (2003). Resistance of tomato and sweet-pepper genotypes to Tomato leaf curl Bangalore virus and its vector Bemisia tabaci. Intern. J. Pest Mngmt. 49, 297–303. doi: 10.1080/09670870310001593353
Melero I., González R., and Elena S. F. (2023). Host developmental stages shape the evolution of a plant RNA virus. Philos. Trans. R. Soc B. Biol. Sci. 378, 20220005. doi: 10.1098/rstb.2022.0005
Ng J. C. K. and Perry K. L. (2004). Transmission of plant viruses by aphid vectors. Mol. Plant Pathol. 5, 505–511. doi: 10.1111/j.1364-3703.2004.00240.x
Pagán I. and García-Arenal F. (2018). Tolerance to plant pathogens: Theory and experimental evidence. Intern. J. Mol. Sci. 19, 810. doi: 10.3390/ijms19030810
Pagán I. and García-Arenal F. (2020). Tolerance of plants to pathogens: A unifying view. Annu. Rev. Phytopatho. 58, 77–96. doi: 10.1146/annurev-phyto-010820-012749
Pallás V. and García J. A. (2011). How do plant viruses induce disease? Interactions and interference with host components. J. Gen. Virol. 92, 2691–2705. doi: 10.1099/vir.0.034603-0
Perring T. M., Gruenhagen N. M., and Farrar C. A. (1999). Management of plant virus disease through chemical control of insect vectors. Annu. Rev. Entomol. 44, 457–481. doi: 10.1146/annurev.ento.44.1.457
Rai V. P., Kumar R., Singh S. P., Kumar S., Kumar S., Singh M., et al. (2014). Monogenic recessive resistance to Pepper leaf curl virus in an interspecific cross of Capsicum. Sci. Horticult. 172, 34–38. doi: 10.1016/j.scienta.2014.03.039
Read A. F. (1994). The evolution of virulence. Trends Microbiol. 2, 73–76. doi: 10.1016/0966-842X(94)90537-1
Sokal R. R. and Rohlf F. J. (1995). Biometry: The Principles and Practice of Statistics in Biological Research. 3rd Edition (New York: W.H. Freeman and Co.).
Stout M. J. (2014). “Host-plant resistance in pest management,” in Integrated pest management. ed. Abrol, D. P (Elsevier), 1–21.
Šutič D. (1959). Die Rolle des Paprikasamens bei der Virusübertragung. J. Phytopathol. 36, 84–93. doi: 10.1111/j.1439-0434.1959.tb01845.x
Suzuki K., Kurado T., Miura Y., and Murai J. (2003). Screening and field trials of virus resistant sources in Capsicum spp. Plant Dis. 87, 779–783. doi: 10.1094/PDIS.2003.87.7.779
Thakur H., Jindal S. K., Sharma A., and Dhaliwal M. S. (2019). A monogenic dominant resistance for leaf curl virus disease in chilli pepper (Capsicum annuum L.). Crop Protect. 116, 115–120. doi: 10.1016/j.cropro.2018.10.007
Trucco V., Castellanos Collazo O., Vaghi Medina C. G., Cabrera Mederos D., Lenardon S., and Giolitti F. (2022). Alfalfa mosaic virus (AMV): genetic diversity and a new natural host. J. Plant Pathol. 104, 349–356. doi: 10.1007/s42161-021-00961-8
Van Leur J., Kumari S., Aftab M., Leonforte A., and Moore S. (2013). Virus resistance of Australian pea (Pisum sativum) varieties. N.Z. J. Crop Hortic. Sci. 41, 86–101. doi: 10.1080/01140671.2013.781039
Walling L. L. (2009). “Adaptive defense responses to pathogens and insects.” in Advances in Botanical Research, eds. In: Kader J. C. and Delseny M., (Elsevier), p. 551–612.
Watson M. A. and Roberts F. M. (1939). A comparative study of the transmission of Hyoscyamus virus 3, potato virus Y and cucumber virus 1 by the vectors Myzus persicae (Sulz), M. circumflexus (Buckton), and Macrosiphum gei (Koch). Proc. R. Soc Lond. Ser. B. Biol. Sci. 127, 543–576. doi: 10.1098/rspb.1939.0039
Whalen M. C. (2005). Host defense in a developmental context. Mol. Plant Pathol 6, 347–360. doi: 10.1111/j.1364-3703.2005.00286.x
Keywords: Bromoviridae, AMV, tolerance, disease management, host plant resistance, integrated pest management
Citation: Amiri-Kazaz LM, Nachappa P and Szczepaniec A (2025) Evaluation of host plant resistance to Alfalfa mosaic virus in peppers in greenhouse and field. Front. Agron. 7:1679604. doi: 10.3389/fagro.2025.1679604
Received: 04 August 2025; Accepted: 19 September 2025;
Published: 02 October 2025.
Edited by:
Kris De Jonghe, Institute for Agricultural, Fisheries and Food Research (ILVO), BelgiumReviewed by:
Eduardo Rodríguez-Román, Instituto Venezolano de Investigaciones Científicas, VenezuelaHerbaud Zohoungbogbo, World Vegetable Center, Benin
Copyright © 2025 Amiri-Kazaz, Nachappa and Szczepaniec. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lara M. Amiri-Kazaz, TGFyYS5BbWlyaS1LYXphekBjb2xvc3RhdGUuZWR1
 Lara M. Amiri-Kazaz
Lara M. Amiri-Kazaz Punya Nachappa
Punya Nachappa Adrianna Szczepaniec
Adrianna Szczepaniec