- 1.School of Organic Farming, Punjab Agricultural University, Ludhiana, Punjab, India
- 2ICAR-Indian Institute of Farming System Research (IIFSR), Meerut, Uttar Pradesh, India
Prolonged cultivation of the rice–wheat system in the Indo-Gangetic Plain (IGP) has led to soil degradation, groundwater depletion, and reduced input use efficiency, necessitating resilient and diversified cropping systems. Therefore, a 6-year field experiment (from 2017–2018 to 2022–2023) was conducted at Punjab Agricultural University (PAU), Ludhiana, Punjab, India, using a randomized complete block design with four replications to evaluate 10 cropping systems (CS). The results revealed that the legume-integrated cropping system of maize–peas–spring groundnut (CS6), being statistically at par with other legume-based systems (i.e., CS3, CS4, CS5, and CS8), showed a significantly lower bulk density (1.32 g/cm3) and higher availability of macronutrients [nitrogen (250.88 kg/ha), phosphorus (27.87 kg/ha), and potassium (194.12 kg/ha)] and micronutrients [zinc (2.85 mg/kg), iron (25.02 mg/kg), copper (2.67 mg/kg), and manganese (12.35 mg/kg)], as well as a substantially improved soil biological health, as indicated by the increased microbial populations [bacteria (132.56 × 106 CFU/g), fungi (25.34 × 103 CFU/g), actinomycetes (35.01 × 104 CFU/g), and diazotrophs (97.32 × 104 CFU/g)], enzymatic activity [dehydrogenase (62.22 µg TPF/g soil/h), alkaline phosphatase (10.78 µg PNP/g soil/h), and urease (16.96 µ/g soil/h)], and microbial biomass carbon (255.21 mg/kg) and nitrogen (20.01 mg/kg). The correlation analysis showed significant interrelationships (p ≤ 0.01 and 0.05), while the principal component analysis (PCA) identified the legume-based systems as key contributors to improved soil health. The cropping system CS6 produced 68.97% higher rice equivalent yield (199.88 q/ha) than the rice–wheat system (118.29 q/ha), consequently resulting in higher gross returns (₹391,770/ha), net returns (₹233,193/ha), benefit/cost (BC) ratio (1.47), and economic efficiency (₹639/ha/day), making it the most economically and ecologically sustainable system recommended for adoption.
1 Introduction
Conventional intensified agricultural practices threaten ecosystem services and the sustainability of agroecosystems due to soil erosion, agrochemical contamination, greenhouse gas emissions, and biodiversity loss (Venter et al., 2016), leading to a shift toward more environment-friendly and sustainable measures, such as organic agriculture (Nautiyal et al., 2010), agroecosystems (Bhagat et al., 2024), functional agrobiodiversity (Chenu et al., 2019), and crop diversification (Nunes et al., 2018; Almagro et al., 2023). Crop diversification with sustainable cropping systems enhances the agroecosystem stability by improving soil health and increasing resilience to climatic and biotic stresses, thereby promoting sustainability (Makate et al., 2016; Kumari et al., 2024). Thus, diversification through suitable cropping systems is directly sustainably linked to increased food production (Wang et al., 2021; Douyon et al., 2022), with improved soil fertility, particularly with leguminous crops (Akshit et al., 2023), maintenance of the soil structure, suppression of weeds, and disruption of pest cycles (Smith et al., 2008). This improvement is attributed to rhizosphere stimulation, increased root biomass, and legume-mediated nitrogen fixation, which enhances microbial diversity (Saha et al., 2017). The cropping system has a significant impact on the soil properties and the crop yield potential. Intensive cropping without replenishing nutrients leads to excessive soil nutrient depletion (Akshit et al., 2023). Crop diversification also determines the soil organic carbon (OC) storage by altering the rate of the decomposition of organic matter in the soil, as extending the crop cover duration can increase the net annual carbon input (Hazra et al., 2019). Therefore, diversifying cropping systems through sustainable crop rotation restores the soil nutrients, improves its structure, and enhances long-term sustainable production (Shah et al., 2021; Zou et al., 2024). Soil microorganisms primarily drive these processes through their complex biochemical processes (Gougoulias et al., 2014; Jacoby et al., 2017). The stability of soil microbial communities (a key factor in the functionality and sustainability of soil ecosystems) is primarily governed by the abundance and the diversity of microbial populations, and these parameters influence critical ecosystem processes such as nutrient cycling, organic matter decomposition, and disease suppression (Pan et al., 2017; Song et al., 2023). Soil biological health is also determined by the soil microbial biomass carbon (MBC), microbial biomass nitrogen (MBN), and enzymatic activity (Borase et al., 2020). Moreover, the microbial population structure is further affected by the physicochemical properties of the soil (Cai et al., 2019; Song et al., 2023). Sustainable cropping systems improve soil health by positively influencing the fertility status and the extent of crop residues incorporated into the soil, and increasing the residues enhances the soil nutrients, and legumes, by atmospheric nitrogen fixation (Sainju and Alasinrin, 2020). Therefore, understanding how different crop rotations affect the physicochemical and biological properties is essential for achieving long-term sustainability. Kurdyś-Kujawska et al. (2021) reported that sustainable crop diversification plays a vital role in managing the risks and uncertainties associated with climate change, thereby enhancing farmers’ resilience and improving income stability.
The dominant rice (Oryza sativa L.)–wheat (Triticum aestivum L.) cropping system in the Indo-Gangetic Plain (IGP) has resulted in groundwater depletion, hard plough pan formation, soil health decline, yield stagnation, waterlogging, greenhouse gas emissions, and increased pest and disease pressure (Sharma et al., 2023). Moreover, the productivity of the cereal–cereal systems in the IGP is on a plateau due to soil degradation, which is aggravated by the lack of organic matter, the absence of diversification, and imbalanced fertilization (Singh et al., 2020; Islam et al., 2023). Diversifying the prevalent cropping system with a sustainable alternative is a pressing need in the region in order to address these challenges. The inclusion of legume crops in the cropping systems can significantly enhance the soil properties and make crop diversification possible against cereal rotations to improve the quality of the soil, the productivity, and the long-term sustainability (Hazra et al., 2019; Borase et al., 2020; Akshit et al., 2023). Such diversification can enhance soil health and productivity by varying the nutrient requirements, root structures, and carbon allocation to the soil, thereby reducing the environmental impacts and increasing the overall system resilience (Yang et al., 2023; Liu et al., 2024). Despite the potential benefits, diversified systems may face challenges, including increased management complexity, labor demand, and market limitations for certain crops, necessitating location-specific validation of their feasibility. Several studies have investigated the impact of crop diversification across different agroecological regions worldwide (Hufnagel et al., 2020; Kurdyś-Kujawska et al., 2021; Guorui et al., 2024). However, the majority have focused either on productivity or on a limited set of soil parameters, hence lacking a holistic assessment that integrates the soil physical, chemical, and biological indicators with system-level productivity and economic performance. Moreover, long-term evaluations of legume-integrated, multi-seasonal cropping sequences in the IGP remain limited, particularly those that address the combined goals of soil health restoration and economic sustainability. To address these gaps, this study aimed to: i) assess the effects of diversified cropping systems on the soil physical, chemical, and biological properties; ii) evaluate system productivity; and iii) determine the economic viability of these systems relative to the conventional system (i.e., rice–wheat). The cropping systems selected for evaluation represent a diverse set of regionally adapted, legume-integrated, and seasonally optimized combinations of cereals, pulses, vegetables, and fodder crops, designed to enhance soil productivity and the profitability of the farmers in the region. We hypothesized that diversification through legume-based cropping systems would significantly improve the soil health indicators, the system productivity, and the economic returns compared with the conventional rice–wheat system in the IGP of northwestern India.
2 Materials and methods
2.1 Description of the experimental location, climate, and site characteristics
The present research was conducted at the research farm of the School of Organic Farming, PAU, Ludhiana, Punjab, India, located at 30°56′ N, 75°52′ E, 247 m.a.s.l. The map of the experimental area is depicted in Figure 1. The research study was undertaken for six consecutive years (from 2017–2018 to 2022–2023) under the “All India Coordinated Research Project on Integrated Farming Systems.” The experimental site is located in the central plain zone of Punjab, within India’s trans-Gangetic region. The region’s climate is semi-arid and subtropical with distinct seasons. It is dry and hot from April to June and changes to a humid and warm monsoon weather from July to September. October and November bring mild early winters, followed by colder winter months from December to February. Moreover, the annual rainfall averages 755 mm, with approximately 70% of the total occurring from July to September due to the southwest monsoon. The soil in the experimental site was characterized as sandy loam, falling under the Typic Ustochrept order of the Samana series (Piper, 1966). Initially, the soil had a normal pH (7.15) and electrical conductivity (0.270 dS/m), as measured using a pH meter in a 1:2 soil-to-water suspension. Electrical conductivity was recorded from the supernatant of the same suspension using a Systronics direct reading conductivity meter (Jackson, 1973). The OC (0.39%) was determined using Walkley and Black’s rapid titration method (Walkley and Black, 1934). The available nitrogen, measured using the modified alkaline permanganate method (Subbiah and Asija, 1956), was categorized as low (204.89 kg/ha). The available phosphorus was quantified using Olsen’s method (Olsen et al., 1954), with color development in the extract using ascorbic acid. The absorbance measured at 760 nm on a spectrophotometer (Olsen et al., 1954) fell under the medium level (20.16 kg/ha). The available potassium (145.28 kg/ha) was extracted with a neutral normal ammonium acetate solution (Piper, 1966) and measured via flame photometry. The diethylenetriaminepentaacetic acid (DTPA)-extractable micronutrients were analyzed from a 1:2 soil-to-extractant ratio using a DTPA-TEA (triethanolamine) buffer (0.005 M DTPA + 0.001 M CaCl2 + 0.1 M TEA, pH 7.3) as described by Lindsay and Norvell (1978). The micronutrient concentrations were determined using atomic absorption spectrophotometry (AAS), and the concentrations of zinc, iron, copper, and manganese were 2.40, 18.97, 2.05, and 8.88 mg/kg, respectively. Bulk density (1.46 g/cm3) was assessed with undisturbed soil cores placed in a 100-cm3 steel cylinder (Blake and Hartge, 1986), while the water holding capacity (WHC) (32.67%) was measured using the method proposed by Richard (1954) (Keen’s box method).
2.2 Methodology and agronomic management
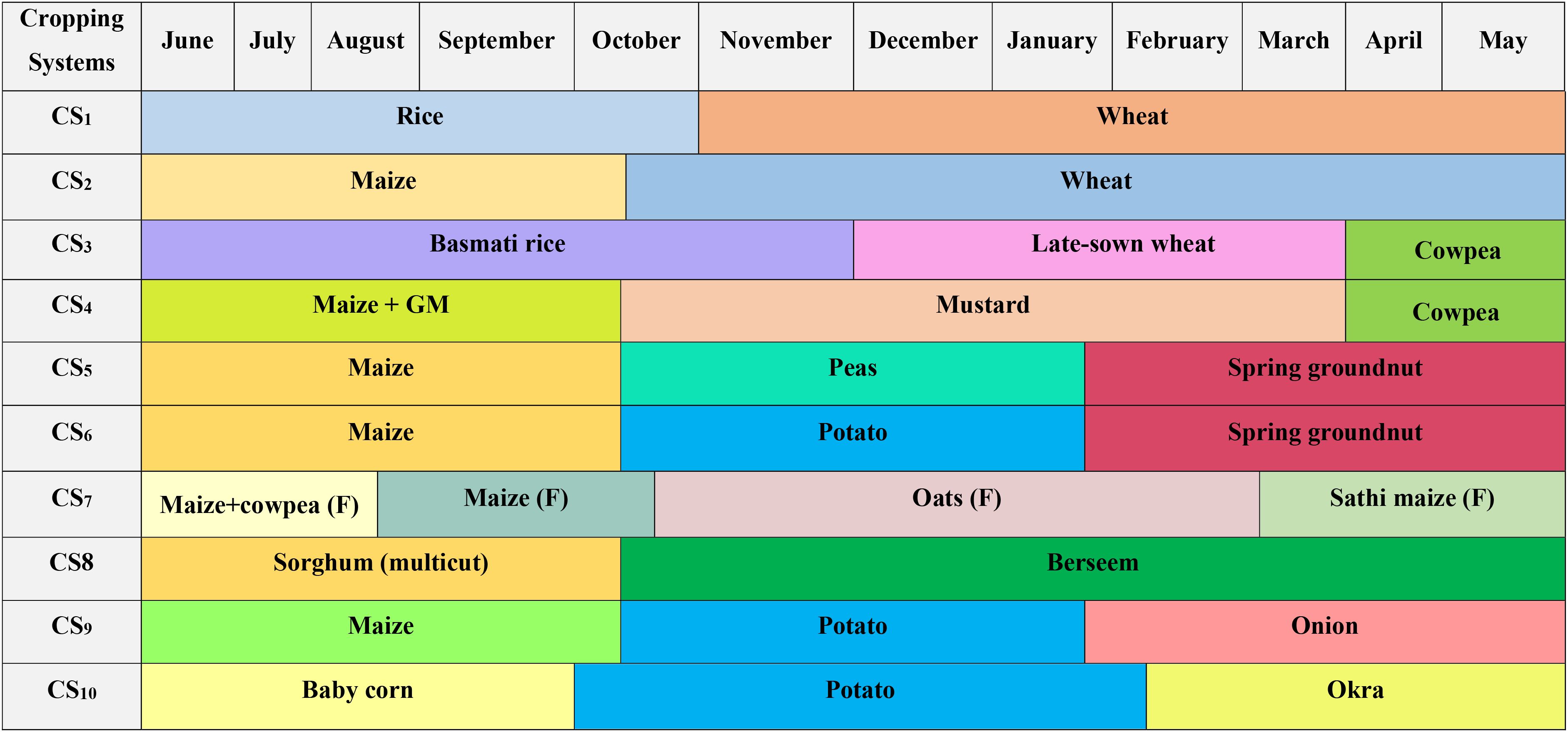
The field study was conducted from 2017–2018 to 2022–2023 with 10 different cropping systems as treatments: rice–wheat (CS1), maize (Zea mays)–wheat (CS2), basmati rice–late-sown wheat–cowpea (Vigna unguiculata) (CS3), maize + (green manure)–mustard (Brassica juncea)–cowpea (CS4), maize–potato (Solanum tuberosum)–spring groundnut (Arachis hypogaea) (CS5), maize–peas (Pisum sativum)–spring groundnut (CS6), maize + cowpea (fodder)–maize (fodder)–oats (Avena sativa) (fodder)–Sathi maize (fodder) (CS7), Sorghum multicut (Sorghum bicolor)–berseem (Trifolium alexandrium) (CS8), maize–potato–onion (Allium cepa) (CS9), and baby corn–potato–okra (CS10). The experiment was conducted using a randomized complete block design with four replications in a plot size of 10 m × 9 m. The crop calendar for the 10 cropping systems is depicted in Figure 2.
All cropping systems were implemented following standardized agronomic recommendations detailed in the Package of Practices of PAU, Ludhiana. Rice (cv. PR 126) was transplanted at 20 cm × 15 cm spacing using a seed rate of 20 kg/ha and fertilized with 105 kg nitrogen (N), 30 kg phosphorus (P), and 30 kg potassium (K) per hectare, with nitrogen split at 7, 21, and 35 days after transplanting (DAT). Basmati rice (cv. Pusa Basmati 1509) received 42 kg N/ha in two equal splits at 21 and 42 DAT. Wheat (cv. Unnat PBW 373) and late-sown wheat (cv. PBW 752) were sown at a seed rate of 100 kg/ha with 15–20 cm and 15 cm × 5 cm spacing, respectively, and both received 125 kg N and 62.5 kg P per hectare at sowing. Maize (cv. PMH-1) was planted at 60 cm × 20 cm spacing using a 25-kg/ha seed rate and fertilized with 50 kg N, 60 kg P, and 30 kg K per hectare, with nitrogen applied in three equal splits. In the maize + cowpea intercropping system, 87.5 kg N and 30 kg P per hectare were applied at sowing. Potato (cv. Kufri Pukhraj) was planted at 60 cm × 10 cm spacing and received 188 kg N, 63 kg P, and 63 kg K per hectare, with half the nitrogen applied at earthing-up (30 DAS). Cowpea (cv. CL 367) and mustard (B. juncea cv. GSC 7) were sown at 45 cm × 15 cm spacing and were fertilized with 50 kg N, 40 kg P, and 25 kg K per hectare and with 100 kg N and 30 kg P per hectare, respectively. The nitrogen in mustard was split between sowing and first irrigation (30 DAS). Groundnut (cv. TG 37A) and pea (cv. Punjab 89) were sown at 80 and 75 kg/ha, respectively, and fertilized with the recommended doses of nitrogen and phosphorus at sowing. Oats (cv. OL 11) and berseem (cv. BL 43) were sown at 20 cm and 20 cm × 10 cm spacing, respectively. A 62.5-kg/ha seed rate was used for oats, and berseem received 25 kg N and 75 kg P per hectare. Sorghum multicut (cv. Punjab Sudax Chari 4) received split nitrogen applications across successive cuttings. Onion (cv. Punjab Naroya) and baby corn (Baby Corn 1) were fertilized at transplanting, with supplemental nitrogen applied post-establishment. Okra (cv. Punjab Suhawani) was sown at 45 cm × 15 cm spacing and was fertilized with 92 kg N per hectare, applied half at sowing and half following the first fruit set.
2.3 Soil parameters, productivity, and economic parameters
After 6 years of experimentation, the soil properties were analyzed in 2023 for all cropping systems. Samples from treatment plots at depths of 0–15 cm were mixed, shade-dried, sieved (2 mm), and assessed for the physical and chemical properties of the soil using the standard procedures mentioned above. Stored at 4° C, samples were used to quantify the microbial populations as colony-forming units (CFU) through triplicate plate counts on specific media (nutrient agar for bacteria, glucose yeast extract for fungi, Kenknight and Munaires for actinomycetes, and Jensen’s medium for diazotrophs). The media were sterilized at 15 psi and 121° C for 20 min. For homogeneity, 10 g of fresh soil was agitated in sterile distilled water, with serial dilutions to a factor of 10 (Subba Rao, 1986). The enzyme activity, i.e., dehydrogenase, alkaline phosphatase, and urease, was assessed following the methods of Tabatabai (1982); Tabatabai and Bremner (1969), and Douglas and Bremner (1970), respectively. The MBC and MBN were calculated following the methods proposed by Jenkinson and Ladd (1981) and Jenkinson (1988), respectively. The harvested produce from each cropping system was weighted for the calculation of economic yield (in kilograms per hectare) and rice equivalent yield (REY; in quintals per hectare). REY was derived by converting the economic yield with crop-specific market prices, relative to rice. The cultivation cost (in rupees per hectare) was calculated as the sum of the input costs (i.e., seeds, fertilizers, plant protection, and irrigation) and the operational costs (e.g., hired and family labor, land preparation, sowing, weeding, and harvesting). Gross returns (in rupees per hectare) were calculated by multiplying the economic yield by market price with the net returns (in rupees per hectare) derived by subtracting the cultivation costs. The benefit/cost (BC) ratio was obtained by taking the ratio of the net returns to the cultivation costs. Economic efficiency (in rupees per hectare per day) was calculated by dividing the net returns by 365.
2.4 Statistical analysis
The data were subjected to statistical analysis using the general linear model to estimate the standard error of the mean (±SEM) for economic yield. The mean values of the key parameters, i.e., REY and the physical, chemical, and biological properties under different cropping systems, were analyzed for significance at the 5% probability level (p = 0.05) using Duncan’s multiple range test (DMRT) following the methodology of Cochran and Cox (1967) with SPSS software (version 20.0; IBM Corporation, Armonk, NY, USA). Correlation analysis and principal component analysis (PCA) were performed using OriginPro 2024 (version 10.1; OriginLab Corporation, Northampton, MA, USA) to evaluate associations among the soil properties and to identify the major sources of variation.
3 Results
3.1 Soil properties
3.1.1 Effect of the different cropping systems on the physical properties of the soil
A 6-year assessment of the soil physical properties under diversified cropping systems revealed statistically significant variations (Table 1), highlighting the significance of sustainable system diversification and legume integration in modulating the soil quality parameters. The data revealed that the maize–peas–spring groundnut cropping system recorded a significantly lower bulk density (1.32 g/cm3), followed by the cropping systems of maize–potato–spring groundnut (1.33 g/cm3), maize + green manure (cowpea)–mustard–cowpea (1.35 g/cm3), basmati rice–late-sown wheat–cowpea (1.36 g/cm3), and Sorghum (multicut)–berseem (1.38 g/cm3). However, a significantly higher bulk density was observed under the rice–wheat (1.44 g/cm3) system. In contrast, the maize–peas–spring groundnut cropping system significantly improved the WHC (42.87%), indicating a 26.05% increase over the rice–wheat system (34.01%). Furthermore, the other legume-based cropping systems, i.e., basmati rice–late-sown wheat–cowpea (41.70%), maize–potato–spring groundnut (41.52%), maize + green manure (cowpea)–mustard–cowpea (41.23%), and Sorghum (multicut)–berseem (41.18%), showed significant WHC enhancement of 22.61%, 22.08%, 21.23%, and 21.08%, respectively.
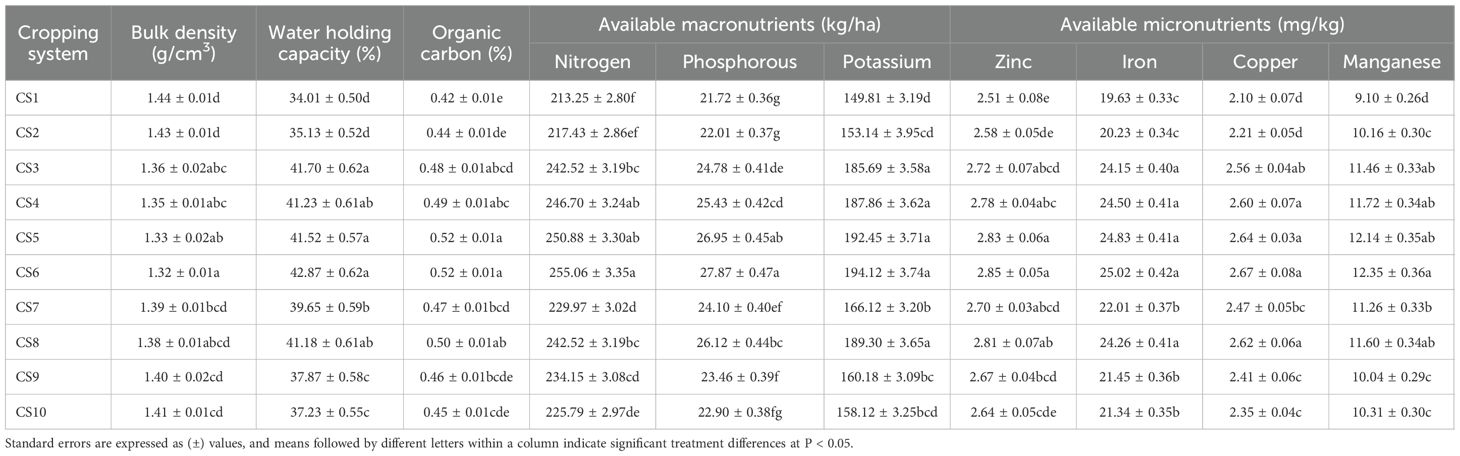
Table 1. Effect of the different cropping systems on the physical and chemical properties of the soil (after the 6-year experiment).
3.1.2 Effect of the different cropping systems on the chemical properties of the soil
Substantial improvements in the soil chemical properties were observed under the legume-integrated cropping systems (Table 1). A significantly higher OC content was recorded in the maize–peas–spring groundnut (0.52%) cropping system compared with the other cropping systems, but which was found statistically at par with the cropping systems of maize–potato–spring groundnut (0.52%), maize + green manure (cowpea)–mustard–cowpea (0.49%), Sorghum (multicut)–berseem (0.50%), and basmati rice–late-sown wheat–cowpea (0.48%). Similarly, a significant enhancement in available nitrogen was also observed in the legume-based cropping systems of maize–potato–spring groundnut (255.06 kg/ha), maize–peas–spring groundnut (250.88 kg/ha), maize + green manure (cowpea)–mustard–cowpea (246.70 kg/ha), Sorghum (multicut)–berseem (242.52 kg/ha), and basmati rice–late-sown wheat–cowpea (242.52 kg/ha) over the other treatments. Furthermore, the available phosphorus and potassium contents were substantially higher under the maize–peas–spring groundnut cropping system (27.87 and 194.12 kg/ha, respectively), followed by maize–potato–spring groundnut (26.95 and 192.45 kg/ha, respectively), Sorghum (multicut)–berseem (26.12 and 189.30 kg/ha, respectively), maize + green manure (cowpea)–mustard–cowpea (25.43 and 187.86 kg/ha, respectively), and basmati rice–late-sown wheat–cowpea (24.78 and 185.69 kg/ha, respectively). In contrast, the rice–wheat cropping system recorded the lowest OC (0.42%) and available nitrogen (213.25 kg/ha), phosphorus (21.72 kg/ha), and potassium (149.81 kg/ha).
3.1.3 Effect of the different cropping systems on the biological properties of the soil
The effects of the different cropping systems on the soil biological properties were evaluated (Table 2). The data showed a significant increase in the total microbial population under the legume-based cropping systems, with significantly higher plate counts of bacteria, fungi, actinomycetes, and diazotrophs observed: maize–peas–spring groundnut (132.56 × 106, 25.34 × 103, 35.01 × 104, and 97.32 × 104 CFU/g soil, respectively), maize–potato–spring groundnut (130.81 × 106, 24.72 × 103, 34.18 × 104, and 96.56 × 104 CFU/g soil, respectively), maize + green manure (cowpea)–mustard–cowpea (129.25 × 106, 24.13 × 103, 33.79 × 104, and 94.88 × 104 CFU/g soil, respectively), Sorghum (multicut)–berseem (126.74 × 106, 24.16 × 103, 33.68 × 104, and 93.11 × 104 CFU/g soil, respectively), and basmati rice–wheat–cowpea (126.32 × 106, 24.00 × 103, 33.09 × 104, and 92.82 × 104 CFU/g soil, respectively). The enzyme activity also differed significantly among cropping systems, with the maize–peas–spring groundnut cropping system recording significantly higher values for dehydrogenase [62.22 µg triphenylformazan (TPF) /g soil/h], alkaline phosphatase [10.78 µg p-nitrophenol (PNP) /g soil/h], and urease activity (16.96 µg/g soil/h) compared with the other cropping systems, but was found statistically similar with the crop rotations of basmati rice–wheat–cowpea (58.75 µg TPF/g soil/h, 9.95 µg PNP/g soil/h, and 16.08 µ/g soil/h, respectively), maize + green manure (cowpea)–mustard–cowpea (59.47 µg TPF/g soil/h, 10.58 µg PNP/g soil/h, and 15.97 µg/g soil/h, respectively), maize–potato–spring groundnut (61.97 µg TPF/g soil/h, 10.43 µg PNP/g soil/h, and 16.53 µg/g soil/h, respectively), and Sorghum (multicut)–berseem (58.91 µg TPF/g soil/h, 10.21 µg PNP/g soil/h, and 16.12 µg/g soil/h, respectively). Moreover, the MBC and MBN were also substantially higher in the maize–peas–spring groundnut system (255.21 and 20.01 mg/kg, respectively), followed by maize–potato–spring groundnut (253.96 and 19.11 mg/kg, respectively), maize + green manure (cowpea)–mustard–cowpea (249.67 and 19.87 mg/kg, respectively), basmati rice–wheat–cowpea (246.46 and 18.92 mg/kg, respectively), and Sorghum (multicut)–berseem (247.56 and 18.96 mg/kg, respectively).
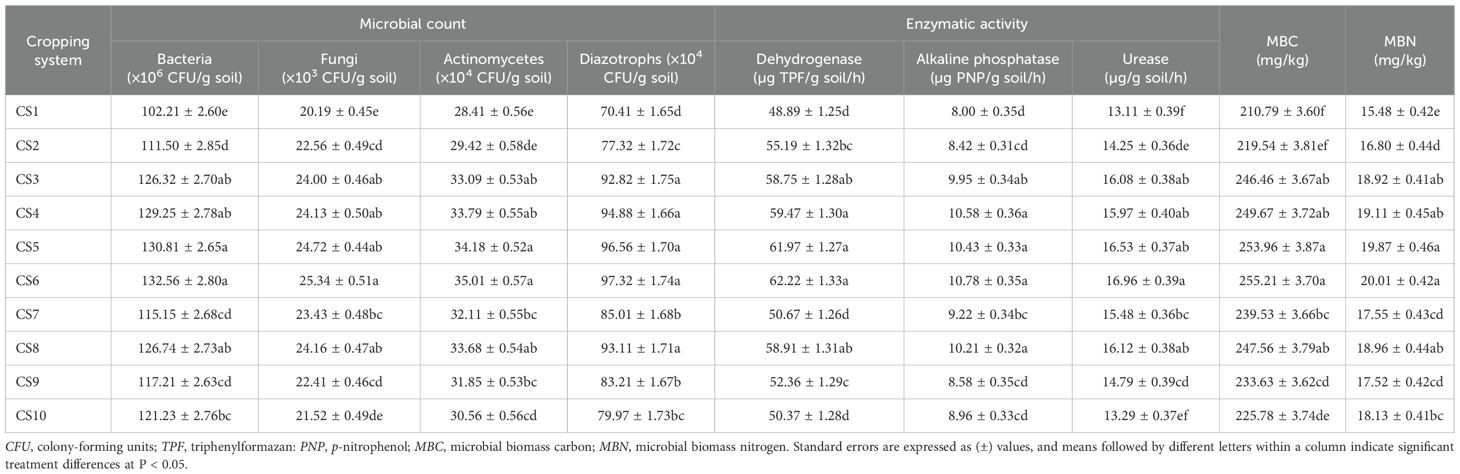
Table 2. Effect of the different cropping systems on the biological properties of the soil (after the 6-year experiment).
3.2 Interrelationships between soil properties
The correlation matrix (Figure 3) revealed significant and strong positive relationships among the different soil properties across the evaluated cropping systems. The soil OC showed a highly significant and positive correlation with WHC; available nitrogen, phosphorus, and potassium; and micronutrients (zinc, iron, copper, and manganese). The biological indicators, i.e., MBC and MBN, also exhibited significant positive correlations with OC. Furthermore, the MBC and MBN were highly correlated with the microbial groups. All three enzyme activities, i.e., dehydrogenase, alkaline phosphatase, and urease, exhibited strong positive correlations with the soil OC and microbial populations (fungi, bacteria, actinomycetes, and diazotrophs). PCA was employed to identify the key soil quality indicators explaining the variability among the different cropping systems (Figure 4). The first two principal components explained 96.07% of the total variance, with PC1 alone accounting for 94.43% and PC2 for only 1.64% (Figure 4A). The scree plot (Figure 4B) confirmed a sharp decline in the eigenvalues after the first principal component, indicating that PC1 captures the majority of the variation in the dataset. The PCA biplot revealed strong associations of the soil biological and chemical parameters, e.g., MBC, MBN, OC, and WHC, and the available macronutrients (nitrogen, phosphorus, and potassium) and micronutrients (zinc, iron, copper, and manganese) with PC1, suggesting their significant contribution to system-level differentiation. The enzyme activity and the microbial populations also showed positive loadings along PC1, indicating their collective influence on soil quality. Furthermore, the cropping systems CS6, CS5, CS3, and CS4 clustered closer to the direction of the vectors representing the soil health indicators, highlighting their superior performance in enhancing the biological and chemical properties. In contrast, CS1, CS2, CS9, and CS10 were positioned far from these vectors, indicating their relatively lower contributions to the key soil properties.
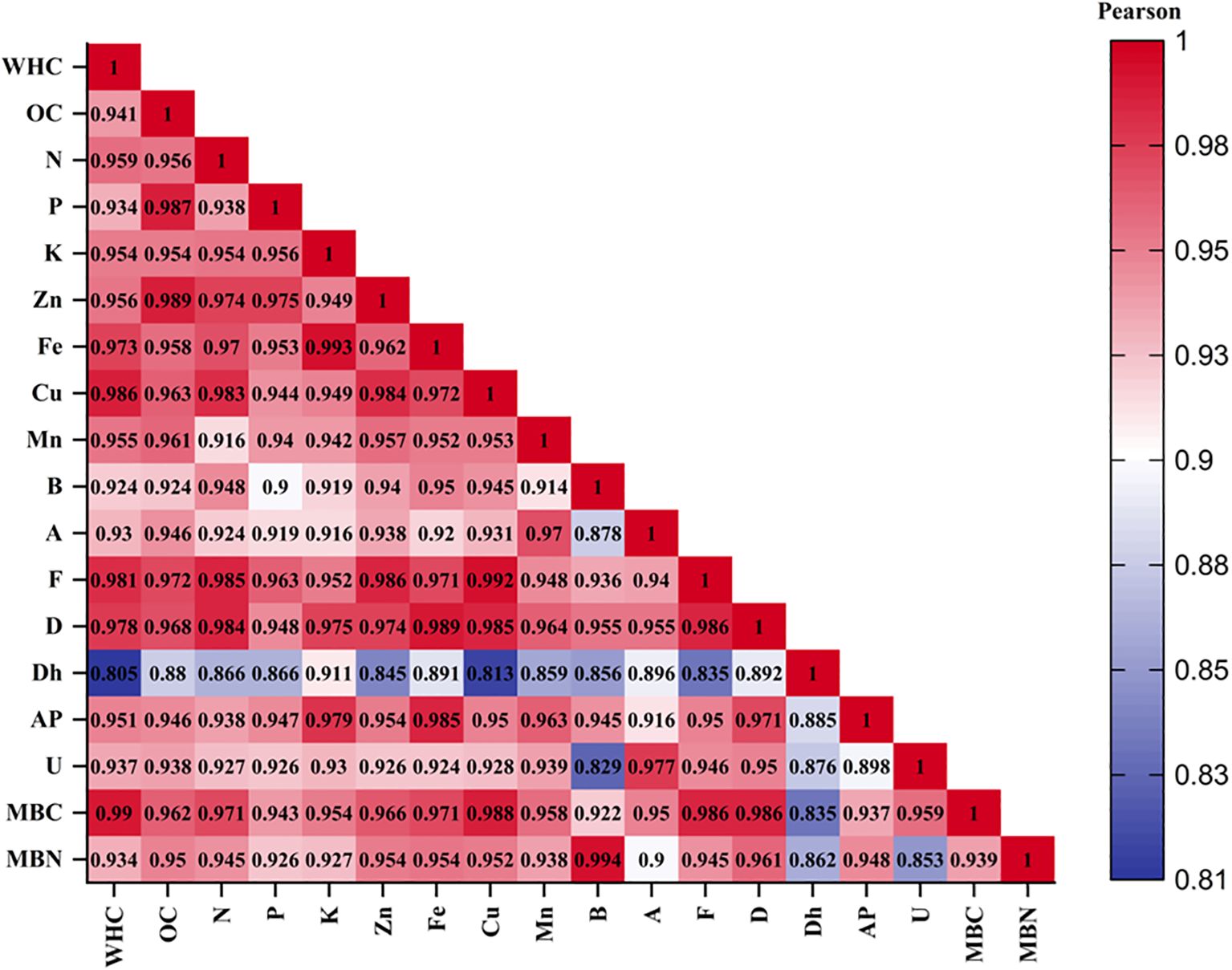
Figure 3. Correlation matrix of the soil properties (Pearson’s). WHC, water holding capacity; OC, organic carbon; N, available nitrogen; P, available phosphorus; K, available potassium; Zn, zinc; Fe, iron; Cu, copper; Mn, manganese; B, bacteria; F, fungi; A, actinomycetes; D, diazotrophs; AP, alkaline phosphatase; U, urease; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen.
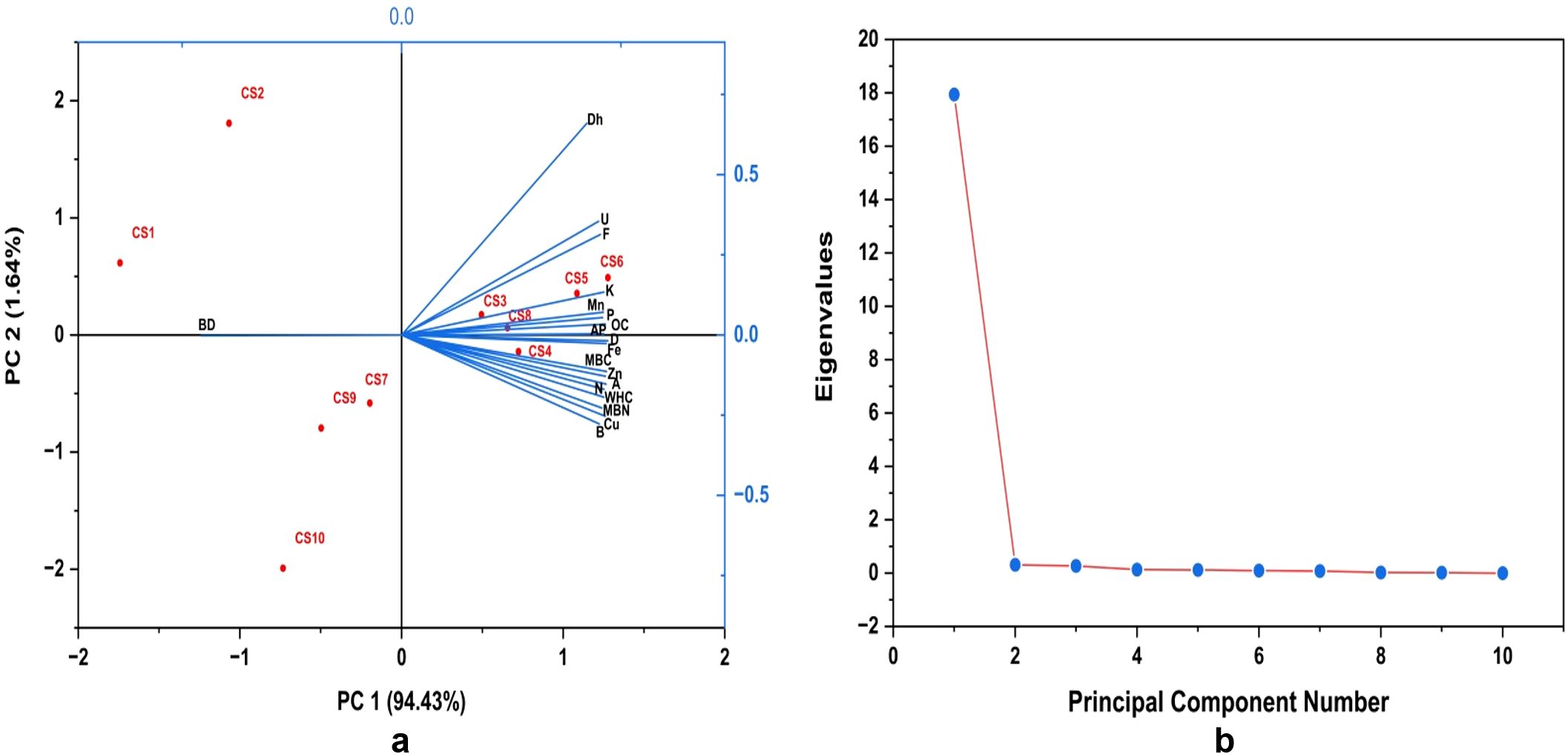
Figure 4. Principal component analysis (PCA) biplot (A) and scree plot (B) of soil health under diversified cropping systems. WHC, water holding capacity; OC, organic carbon; N, available nitrogen; P, available phosphorus; K, available potassium; Zn, zinc; Fe, iron; Cu, copper; Mn, manganese; B, bacteria; F, fungi; A, actinomycetes; D, diazotrophs; AP, alkaline phosphatase; U, urease; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen. .
3.3 Productivity of the diversified cropping systems
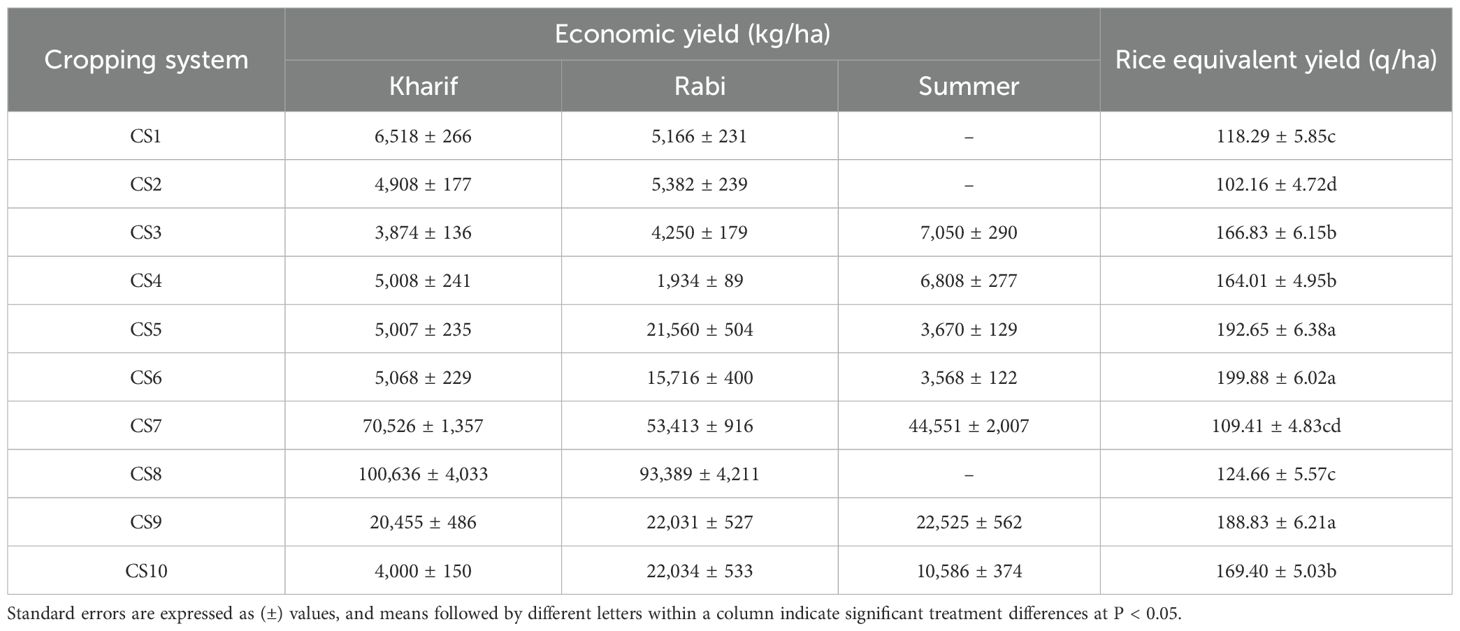
The equivalent yields differed significantly across the different cropping systems, as presented in Table 3. The pooled data of 6 years showed that the maize–peas–spring groundnut cropping system recorded a substantially higher REY of 199.88 q/ha, with economic yields of 5,068 ± 229 kg/ha (maize), 1,5716 ± 400 kg/ha (peas), and 3,568 ± 122 kg/ha (spring groundnut), over the other cropping systems, but was found statistically at par with the maize–potato–spring groundnut crop rotation, which recorded a REY of 192.65 q/ha and had economic yields of 5,007 ± 235 kg/ha (maize), 21,560 ± 504 kg/ha (potato), and 3,670 ± 129 kg/ha (spring groundnut), and the maize–potato–onion rotation, which recorded a REY of 188.83 q/ha with economic yields of 20,455 ± 486 kg/ha (maize), 22,031 ± 527 kg/ha (potato), and 22,525 ± 562 kg/ha (onion).
3.4 Profitability of the diversified cropping systems
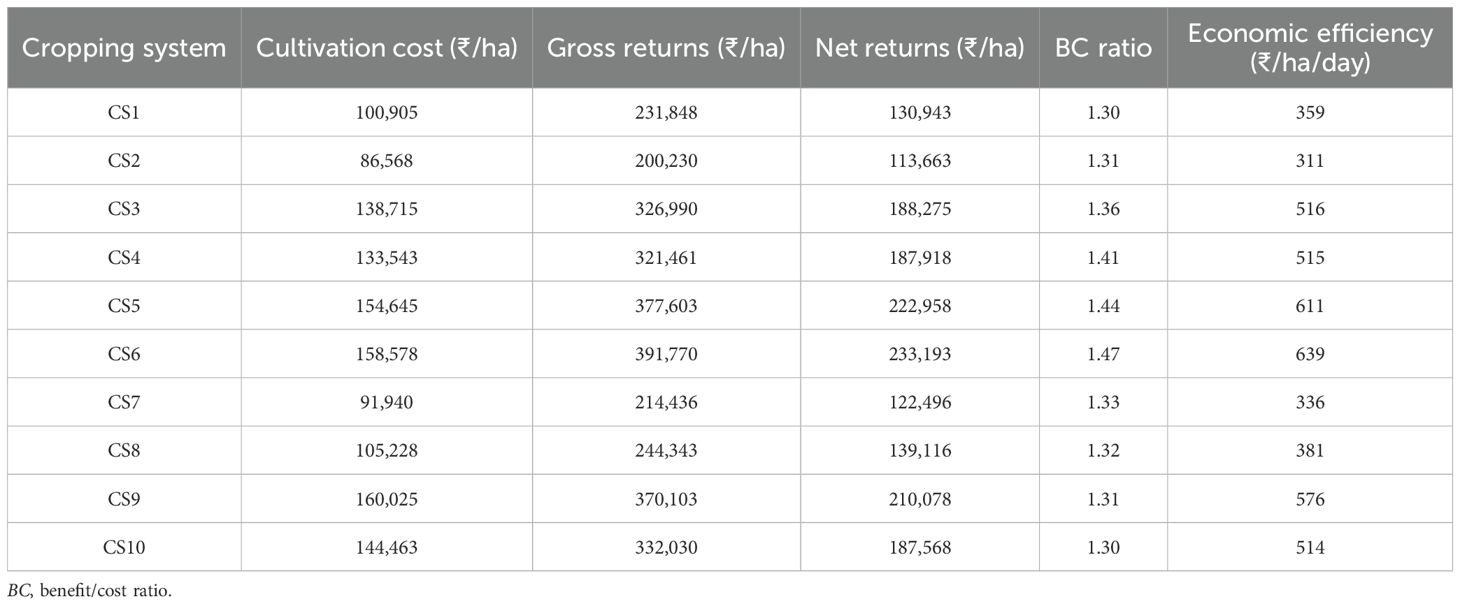
The economic analysis of the various cropping systems is presented in Table 4. The data showed higher gross and net returns recorded under the maize–peas–spring groundnut system (₹391,770 and ₹233,193/ha, respectively), followed by maize–potato–spring groundnut (₹377,603 and ₹222,958/ha, respectively) and maize–potato–onion (₹370,103 and ₹210,078/ha, respectively). Furthermore, the maize–peas–spring groundnut cropping system recorded the highest BC ratio (1.47) and economic efficiency (₹639/ha/day) compared with the other cropping systems.
4 Discussion
4.1 Improvement in soil health due to the various cropping systems
The present 6-year study (from 2017–2018 to 2022–2023), which aimed to identify a sustainable cropping system to diversify the dominant rice–wheat cropping system in IGP, revealed that the different legume-based cropping systems significantly and positively influenced the overall health (physicochemical and biological properties) of the soil. Moreover, the findings of the study on the different cropping systems showed that the different legume-based cropping systems had significantly less bulk density and higher soil WHC, which is due to the improvement in the soil organic matter content (Ananda et al., 2022; Ma et al., 2025; Bhagat et al., 2024). The deep root systems of leguminous crops, the root activities, and leaf fall improve the soil structure by increasing the macropores and macroaggregates through decomposition of leaf litter, root biomass, and rhizodeposition (Nath et al., 2023). The results of the study suggest that the high decomposable organic litter falls by the legumes in the cropping system enhanced the organic matter on the upper surface (0–15 cm) of the soil and maintained the structural stability of the soil, as reported by Bhagat et al. (2024). Moreover, the polysaccharide complexes and the ligno-protein from fresh leaves and the lower C/N of leguminous crop residues enhance the soil aggregate cohesion and stability, thus reducing the bulk density (Kavdir et al., 2008; Udom and Omovbude, 2019; Nath et al., 2023). Similarly, Hazra et al. (2019) also highlighted that the introduction of legumes in cropping sequences results in a lower bulk density, consequently leading to a higher WHC. Bhagat et al. (2024) also reported that the legume-based cropping system showed a lower bulk density and a higher WHC due to the addition of more biolitters by legume crops into the soil. Similarly, the legume-based cropping systems enhanced the soil chemical properties by fixing atmospheric nitrogen through symbiosis with nitrogen-fixing bacteria and the addition of organic matter, which improved the nitrogen available in the soil (Ma et al., 2025). Lu et al. (2011) also observed that increasing the crop rotations with legumes enhanced nutrient release, resulting in higher nitrogen content. Similar results were observed by Yan et al. (2024), who indicated the beneficial effects of legumes on the cropping system for overall improved soil health. The presence of leguminous crops in cropping systems also increased the phosphorus availability by releasing organic acids and root exudates that solubilize the bound phosphorus in the soil, making it more accessible for plant uptake, while the decomposition of legume residues further enhanced the phosphorus availability through mineralization (Dong et al., 2024). Legumes increase available potassium by accessing the subsoil reserves through their deep root systems and enhancing nutrient release during the decomposition of their residues while also promoting microbial activity, resulting in potassium mineralization. Similarly, Bhagat et al. (2024) reported that introducing legume crops into cropping systems enhanced the soil OC and increased the amount of available nitrogen, phosphorus, and potassium in the soil, and these improvements were attributed to the biological nitrogen fixation capability of legumes. The cultivation of legumes is primarily valued for improving soil fertility rather than for their yield, as these crops are largely self-sufficient in supplying nitrogen (Porpavai et al., 2011). Therefore, legume-based cropping systems also show a positive effect on soil biodiversity. The predominance of microbial communities in legume-based systems is largely linked to the traits of the legume crops, which include symbiotic relationships with nitrogen-fixing bacteria and higher rates of exudation by the roots that supply carbon to soil microbes. These factors likely stimulate the microbial growth and diversity in the legume rhizosphere, enhancing nutrient cycling and soil aggregation and encouraging better soil health, thereby contributing to the sustainability of the system. Song et al. (2023) also reported that rotation with legumes improved the amount of organic matter in the soil, consequently enriching the soil OC and ultimately increasing the microbial population. The microbial communities showed significant correlations with organic matter and available nitrogen, with the external carbon and nitrogen inputs into the system markedly influencing the microbial community structure (Fierer et al., 2012). Legume-based rotations improved the soil microbial biomass and activity (Congreves et al., 2015; Yan et al., 2024), encouraging the secretion of essential extracellular enzymes through increased plant diversity, thereby improving soil quality (Zhang et al., 2024). Soil enzymes are primarily derived from exudates of plant roots and microbial metabolites, with the levels of enzymes largely determined by the abundance and the composition of soil microorganisms. Positive correlations are thus established between microbial abundance, soil moisture, enzyme activity, and nutrient content in the soil rhizosphere (Richardson et al., 2009). Yan et al. (2024) further noted that the lower C/N of the legume residues in the soil can stimulate microbial activity, thereby enhancing enzymatic activity. The results of this study will provide a foundation for the regulation of the soil nutrient profiles and the microbial community structures, informing cropping system choices and supporting soil ecosystem protection.
4.2 Productivity of the different cropping systems
Diversifying crops with legume-based systems is recommended to improve the yield and the functions and services of agroecosystems (Yan et al., 2024). The residues left by legumes in the soil contribute to its overall health, creating a favorable environment for subsequent crops in the rotation, in turn enhancing the potential yield of the entire cropping system. The cropping systems of maize–peas–spring groundnut and maize potato–spring groundnut resulted in higher REY, which was attributed to the complementary nature of diverse crops, with legumes (peas and groundnut) fixing nitrogen and improving the fertility of the soil (Jiang et al., 2024), in turn maximizing the potential yields of the subsequent crops (Zhang et al., 2025). The research by Rose et al. (2024) further supports that the inclusion of legumes in various cropping systems tends to increase the yield of the subsequent crops more effectively than non-legume cover crops. Similarly, Abdalla et al. (2019) and Allam et al. (2023) observed higher productivity primarily in legume-based cropping systems rather than in cereal-based cropping systems.
4.3 Profitability of the different cropping systems
Food security remains the primary goal of cropping systems; however, unsustainable practices are increasingly threatening productivity, particularly in the current climate change context (Bhagat et al., 2024). The inclusion of legumes into intensified cropping systems has been demonstrated to increase the net returns and the BC ratios sustainably. This is achieved by the optimal utilization of resources, enhancement of the biodiversity, and ensuring additional returns for farmers. Diversification through the integration of legumes and value-added crops enhanced market prospects, leading to the overall profitability and sustainability of the cropping system. Legume crops enhance soil fertility by providing additional nitrogen to subsequent crops, leading to yield benefits and increased farm profitability in legume–cereal rotations. The studies of Xing et al. (2017) and Bitew et al. (2019) also showed that legume-based cropping systems generate greater economic returns than monoculture cereal production. Similarly, Yigezu et al. (2019) and Bhagat et al. (2024) found that legume-based systems are more remunerative and economically viable than other systems. Therefore, adopting legume-based cropping systems offers an economically remunerative approach toward a sustainable agriculture.
5 Conclusion
This 6-year field investigation conducted in the IGP of Punjab, India, showed that legume-integrated cropping systems are sustainable alternatives to the conventional rice–wheat system. Diversification through maize–peas–spring groundnut and maize–potato–spring groundnut systems is recommended as these significantly improved the soil properties (physical, chemical, and biological) and enhanced the productivity in terms of REY and increased profitability, with higher gross and net returns, BC ratio, and economic efficiency. These findings highlight the potential of legume-based diversification not only to enhance environmental sustainability but also to provide valuable guidance for policy formulation and encourage the wider adoption of resilient cropping systems in similar agroecological regions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NeR: Data curation, Formal Analysis, Methodology, Software, Validation, Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Visualization. NaR: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RB: Data curation, Formal Analysis, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. TK: Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. KK: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
The authors acknowledge the support and resources provided by Punjab Agricultural University, Ludhiana, Punjab, India and the AICRP on Integrated Farming Systems, ICAR-IIFSR, Modipuram, Meerut, UP, India.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdalla M., Hastings A., Cheng K., Yue Q., Chadwick D., Espenberg M., et al (2019). A critical review of the impacts of cover crops on nitrogen leaching, net greenhouse gas balance and crop productivity. Glob. Chang. Boil. 25, 2530–2543. doi: 10.1111/gcb.14644
Akshit, Kumar S., Sheoran N., Devi P., Sharma K., Kamboj E., et al. (2023). Legumes in cropping systems: A way toward agricultural sustainability and diversification. Commun. Soil Sci. Plant Anal. 55, 596–608. doi: 10.1080/00103624.2023.2274035
Allam M., Radicetti E., Ben Hassine M., Jamal A., Abideen Z., and Mancinelli R. (2023). A meta-analysis approach to estimate the effect of cover crops on the grain yield of succeeding cereal crops within European cropping systems. Agriculture 13, 1714. doi: 10.3390/agriculture13091714
Almagro M., Re P., Díaz-Pereira E., Boix-Fayos C., Sánchez-Navarro V., Zornoza R., et al. (2023). Crop diversification effects on soil organic carbon and nitrogen storage and stabilization is mediated by soil management practices in semiarid woody crops. Soil Tillage Res. 233, 105815. doi: 10.1016/j.still.2023.105815
Ananda M. R., Vaiahnav S., Naide P. R., and Aruna N. V. (2022). Long-term benefits of legume-based cropping systems on soil health and productivity: An overview. Int. J. Environ. Climate Change 12, 299–315. doi: 10.9734/ijecc/2022/v12i930767
Bhagat R., Walia S. S., Dheri G. S., Singh G., and Sharma K. (2024). Pear (Pyrus communis)-based agroecosystem improves soil nutrient dynamics, microbial biomass, enzymatic activity, farm productivity, and profitability. Sci. Hortic. 336, 113398. doi: 10.1016/j.scienta.2024.113398
Bitew Y., Alemayehu G., Adego E., and Assefa A. (2019). Boosting land use efficiency, profitability and productivity of finger millet by intercropping with grain legumes. Cogent Food Agric. 5, 1702826. doi: 10.1080/23311932.2019.1702826
Blake G. R. and Hartge K. H. (1986). “Bulk density,” in Methods of Soil Analysis, Part 1 – Physical and Mineralogical Methods, 2nd. Ed. Klute A.Agronomy Monograph 9 (ASA–SSSA, Madison), 363–382.
Borase D. N., Nath C. P., Hazra K. K., Senthilkumar M., Singh S. S., Praharaj C. S., et al. (2020). Long-term impact of diversified crop rotations and nutrient management practices on soil microbial functions and soil enzyme activity. Ecol. Indic. 114, 106322. doi: 10.1016/j.ecolind.2020.106322
Cai A., Xu M., Wang B., Zhang W., Liang G., Hou E., et al. (2019). Manure acts as a better fertilizer for increasing crop yields than synthetic fertilizer does by improving soil fertility. Soil Tillage Res. 189, 168–175. doi: 10.1016/j.still.2018.12.022
Chenu C., Angers D. A., Barré P., Derrien D., Arrouays D., and Balesdent J. (2019). Increasing organic stocks in agricultural soils: Knowledge gaps and potential innovations. Soil Tillage Res. 188, 41–52. doi: 10.1016/j.still.2018.04.011
Congreves K. A., Hayes A., Verhallen E. A., and Van Eerd L. L. (2015). Long-term impact of tillage and crop rotation on soil health at four temperate agroecosystems. Soil Tillage Res. 152, 17–28. doi: 10.1016/j.still.2015.03.012
Dong R., Hu W., Bu L., Cheng H., and Liu G. (2024). Legume cover crops alter soil phosphorus availability and microbial community composition in mango orchards in karst areas. Agric. Ecosyst. Environ. 364, 108906. doi: 10.1016/j.agee.2024.108906
Douglas C. and Bremner N. (1970). Soil organic carbon management for sustainable land use in the Sudano-Sahelian West Africa. Nutr. Cycl. Agroecosyst. 61, 131–142.
Douyon A., Worou O. N., Diama A., Badolo F., Denou R. K., Touré S., et al. (2022). Impact of crop diversification on household food and nutrition security in southern and central Mali. Front. Sustain. Food Syst. 5. doi: 10.3389/fsufs.2021.751349
Fierer N., Leff J. W., Adams B. J., Nielsen U. N., Bates S. T., and Lauber C. L. (2012). Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. U. S. A. 109, 21390–21395. doi: 10.1073/pnas.1215210110
Gougoulias C., Clark J. M., and Shaw L. J. (2014). The role of soil microbes in the global carbon cycle: Tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J. Sci. Food Agric. 94, 2362–2371. doi: 10.1002/jsfa.6577
Guorui L., Chaoyang Y., Shen P., Hou Y., Ren Z., Li N., et al. (2024). Crop diversification promotes soil aggregation and carbon accumulation in global agroecosystems: A meta-analysis. J. Environ. Manage. 350, 119661. doi: 10.1016/j.jenvman.2023.119661
Hazra K. K., Nath C. P., Singh U., Praharaj C. S., Kumar N., Singh S. S., et al. (2019). Diversification of maize–wheat cropping system with legumes and integrated nutrient management increases soil aggregation and carbon sequestration. Geoderma 353, 308–319. doi: 10.1016/j.geoderma.2019.06.039
Hufnagel J., Reckling M., and Ewert F. (2020). Diverse approaches to crop diversification in agricultural research: A review. Agron. Sustain. Dev. 40, 14. doi: 10.1007/s13593-020-00617-4
Islam M. A., Sarkar D., Alam M. R., Jahangir M. M. R., Ali M. O., Sarker D., et al. (2023). Legumes in conservation agriculture: A sustainable approach in rice-based ecology of the Eastern Indo-Gangetic Plain of South Asia – an overview. Technol. Agron. 3, 1–17. doi: 10.48130/TIA-2023-0003
Jackson M. L. (1973). Soil Chemical Analysis. 1st (New Delhi: Prentice Hall of India Pvt. Ltd.), 498.
Jacoby R., Peukert M., Succurro A., Koprivova A., and Kopriva S. (2017). The role of soil microorganisms in plant mineral nutrition—Current knowledge and future directions. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01617
Jenkinson D. S. (1988). “The determination of microbial biomass carbon and nitrogen in soil,” in Advances in Nitrogen Cycling in Agricultural Ecosystems. Ed. Wilson J. R. (CAB Int., Wallingford), 368–386.
Jenkinson D. S. and Ladd J. N. (1981). Microbial biomass in soil: measurement and turnover. Soil Biochem. 5, 415–417.
Jiang S., Xue D., Feng W., Wang K., Wang S., Wang T., et al. (2024). Long-term organic fertilization alters soil microbial community structure and its influence on faba bean production in a six-crop rotation system. Plant Soil. 513, 1–17. doi: 10.1007/s11104-024-07169-6
Kavdir Y., Hellebrand H. J., and Kern J. (2008). Seasonal variations of nitrous oxide emission in relation to nitrogen fertilization and energy crop types in sandy soil. Soil Tillage Res. 98, 175–186. doi: 10.1016/j.still.2007.11.002
Kumari V. V., Balloli S. S., Kumar M., Ramana D. B. V., Prabhakar M., Osman M., et al. (2024). Diversified cropping systems for reducing soil erosion and nutrient loss and for increasing crop productivity and profitability in rainfed environments. Agric. Syst. 217, 103919. doi: 10.1016/j.agsy.2024.103919
Kurdyś-Kujawska A., Strzelecka A., and Zawadzka D. (2021). The impact of crop diversification on the economic efficiency of small farms in Poland. Agriculture 11, 250. doi: 10.3390/agriculture11030250
Liu M., Zhou X., Huang G., and Li Y. (2024). The increasing water stress projected for China could shift the agriculture and manufacturing industry geographically. Commun. Earth Environ. 5, 396. doi: 10.1038/s43247-024-01560-y
Lu J., Kang L., He X., and Xu D. (2011). Multilocus sequence analysis of the Rhizobia from five woody legumes in southern China. Afr. J. Microbiol. Res. 5, 5343–5353. doi: 10.5897/AJMR11.826
Ma J., Yin B., Gao T., He K., Huang X., Jiang T., et al. (2025). Legume–non-legume cover crop mixtures enhance soil nutrient availability and physical properties: A meta-analysis across Chinese agroecosystems. Agronomy 15, 1756. doi: 10.3390/agronomy15081756
Makate C., Wang R., Makate M., and Mango N. (2016). Crop diversification and livelihoods of smallholder farmers in Zimbabwe: Adaptive management for environmental change. SpringerPlus 5, 1–18. doi: 10.1186/s40064-016-2802-4
Nath C. P., Kumar N., Dutta A., Hazra K. K., Praharaj C. S., Singh S. S., et al. (2023). Pulse crop and organic amendments in cropping system improve soil quality in rice ecology: Evidence from a long–term experiment of 16 years. Geoderma 430, 116334. doi: 10.1016/j.geoderma.2023.116334
Nautiyal C. S., Chauhan P. S., and Bhatia C. R. (2010). Changes in soil physico-chemical properties and microbial functional diversity due to 14 years of conversion of grassland to organic agriculture in semi-arid agroecosystem. Soil Tillage Res. 109, 55–60. doi: 10.1016/j.still.2010.04.008
Nunes M. R., van Es H. M., Schindelbeck R., Ristow A. J., and Ryan M. (2018). No-till and cropping system diversification improve soil health and crop yield. Geoderma 328, 30–43. doi: 10.1016/j.geoderma.2018.04.003
Olsen S. R., Cole C. V., Watanabe F. S., and Dean L. A. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circ. 939, 19.
Pan W. L., Schillinger W. F., Young F. L., Kirby E. M., Yorgey G. G., Borrelli K. A., et al. (2017). Integrating historic agronomic and policy lessons with new technologies to drive farmer decisions for farm and climate: the case of inland Pacific Northwestern US. Front. Environ. Sci. 5. doi: 10.3389/fenvs.2017.00076
Porpavai S., Devasenapathy P., Siddeswaran K., and Jayaraj T. (2011). Impact of various rice-based cropping systems on soil fertility. J. Cereal Oilseeds 2, 43–46.
Richard L. A. (1954). Diagnosis and improvement of saline and alkali soils. Vol. 60 (Washington, D.C.: USDA Agric. Handb), 107–108.
Richardson A. E., Barea J. M., McNeill A. M., and Prigent-Combaret C. (2009). Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321, 305–339. doi: 10.1007/s11104-009-9895-2
Rose T. J., Parvin S., McInnes J., van Zwieten L., Gibson A. J., Kearney L. J., et al. (2024). Summer cover crop and temporary legume–cereal intercrop effects on soil microbial indicators, soil water and cash crop yields in a semi-arid environment. Field Crops Res. 312, 109384. doi: 10.1016/j.fcr.2024.109384
Saha B., Saha S., Das A., Bhattacharyya P. K., Basak N., Sinha A. K., et al. (2017). “Biological nitrogen fixation for sustainable agriculture,” in Agriculturally Important Microbes for Sustainable Agriculture, vol. 2. Applications in Crop Production and Protection (Springer, Singapore), 81–128. doi: 10.1007/978-981-10-5343-6_4
Sainju U. M. and Alasinrin S. Y. (2020). Changes in soil chemical properties and crop yields with long-term cropping system and nitrogen fertilization. Agrosyst. Geosci. Environ. 3, e20019. doi: 10.1002/agg2.20019
Shah K. K., Modi B., Pandey H. P., Subedi A., Aryal G., Pandey M., et al. (2021). Diversified crop rotation: An approach for sustainable agriculture production. Adv. Agric. 2021, 8924087. doi: 10.1155/2021/8924087
Sharma K., Walia S. S., Dhaliwal S. S., Saini K. S., and Bhagat R. (2023). Residual effect of nitrogen management on succeeding summer moong (Vigna radiata) under maize-wheat-moong rotation. Indian J. Agric. Sci. 93, 762–767. doi: 10.56093/ijas.v93i7.134678
Singh S. R., Yadav P., Singh D., Tripathi M. K., Bahadur L., Singh S. P., et al. (2020). Cropping systems influence microbial diversity, soil quality and crop yields in Indo-Gangetic Plains of India. Eur. J. Agron. 121, 126152. doi: 10.1016/j.eja.2020.126152
Smith P., Martino D., Cai Z., Gwary D., Janzen H., Kumar P. M., et al. (2008). Greenhouse gas mitigation in agriculture. Philos. Trans. R. Soc B Biol. Sci. 363, 789–813. doi: 10.1098/rstb.2007.2184
Song M., Li J., Gao L., and Tian Y. (2023). Comprehensive evaluation of effects of various carbon-rich amendments on overall soil quality and crop productivity in degraded soils. Geoderma 436, 116529. doi: 10.1016/j.geoderma.2023.116529
Subba Rao N. S. (1986). Soil Micro-organisms and Plant Growth (New Delhi: Oxford & IBH Publ. Co. Pvt. Ltd.).
Subbiah B. V. and Asija G. L. (1956). A rapid procedure for the estimation of available nitrogen in soils. Curr. Sci. 25, 259–260.
Tabatabai M. A. and Bremner J. M. (1969). Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1, 301–307. doi: 10.1016/0038-0717(69)90012-1
Udom B. E. and Omovbude S. (2019). Soil physical properties and carbon/nitrogen relationships in stable aggregates under legume and grass fallow. Acta Ecol. Sin. 39, 56–62. doi: 10.1016/j.chnaes.2018.05.008
Venter Z. S., Jacobs K., and Hawkins H. J. (2016). The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia 59, 215–223. doi: 10.1016/j.pedobi.2016.04.001
Walkley A. J. and Black I. A. (1934). An examination of the Degtjareff method for determination soil organic matter and a proposed modification of the chromic acid Titration method. Soil Sci. 37, 29–38.
Wang T., Jin H., Fan Y., Obembe O., and Li D. (2021). Farmers’ adoption and perceived benefits of diversified crop rotations in the margins of U.S. Corn Belt. J. Environ. Manage. 293, 112903. doi: 10.1016/j.jenvman.2021.112903
Xing H., Li Liu D., Li G., Wang B., Anwar M. R., Crean J., et al (2017). Incorporating grain legumes in cereal-based cropping systems to improve profitability in southern New South Wales, Australia. Agric. Syst. 154, 112–123. doi: 10.1016/j.agsy.2017.03.010
Yan Z., Chu J., Nie J., Qu X., Sánchez-Rodríguez A. R., Yang Y., et al. (2024). Legume-based crop diversification with optimal nitrogen fertilization benefits subsequent wheat yield and soil quality. Agric. Ecosyst. Environ. 374, 109171. doi: 10.1016/j.agee.2024.109171
Yang M., Wang G., Sun Y., You L., and Anyah R. (2023). Water stress dominates the projected maize yield changes in Ethiopia. Glob. Planet Change 228, 104216. doi: 10.1016/j.gloplacha.2023.104216
Yigezu Y. A., El-Shater T., Boughlala M., Bishaw Z., Niane A. A., Maalouf F., et al. (2019). Legume-based rotations have clear economic advantages over cereal monocropping in dry areas. Agron. Sustain. Dev. 39, 58. doi: 10.1007/s13593-019-0602-2
Zhang J., Dyck M., Quideau S. A., and Norris C. E. (2024). Assessment of soil health and identification of key soil health indicators for five long-term crop rotations with varying fertility management. Geoderma 443, 116836. doi: 10.1016/j.geoderma.2024.116836
Zhang L., Liu C., Yao W., Shao J., Peixoto L., Yang Y., et al. (2025). Legume-based rotation benefits crop productivity and agricultural sustainability in the North China Plain. Soil Tillage Res. 250, 106502. doi: 10.1016/j.still.2025.106502
Keywords: cropping rotation, enzymatic activity, microbial population, productivity, soil health, sustainability
Citation: Walia SS, Rani N, Ravisankar N, Bhagat R, Kaur T and Kaur K (2025) Legume-based crop rotation sustain the soil biodiversity, fertility levels, productivity, and profitability: evidence from a long-term study under Indian subtropical conditions. Front. Agron. 7:1681733. doi: 10.3389/fagro.2025.1681733
Received: 07 August 2025; Accepted: 01 October 2025;
Published: 20 October 2025.
Edited by:
Venkatesh Paramesha, Central Coastal Agricultural Research Institute (ICAR), IndiaReviewed by:
Jian-Wei Guo, Kunming University, ChinaPrakash Chand Ghasal, Indian Council of Agricultural Research (ICAR), India
Copyright © 2025 Walia, Rani, Ravisankar, Bhagat, Kaur and Kaur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rakshit Bhagat, cmFrc2hpdC5iaGFnYXQyMzJAZ21haWwuY29t
†ORCID: Rakshit Bhagat, orcid.org/0000-0003-2127-1101
 Sohan Singh Walia1
Sohan Singh Walia1 Rakshit Bhagat
Rakshit Bhagat