- Faculty of Agricultural, Environmental and Food Sciences, Free University of Bolzano, Bolzano, Italy
Background and aims: Climate change is intensifying abiotic stresses in viticulture, particularly through increased drought due to erratic rainfall. Meanwhile, copper (Cu2+) toxicity, a legacy of phytosanitary treatments, may be aggravated by these environmental shifts. This study evaluated the physiological and ionomic responses of young Vitis vinifera cv. Pinot gris plants, grafted onto three rootstocks (M4, 1103 Paulsen, SO4), under controlled drought, Cu2+ toxicity, and their combined effects.
Methods: Plants were grown under greenhouse conditions and subjected to individual and combined stress treatments. Morpho-physiological traits, biomass distribution, and nutrient profiles were assessed to determine genotype-specific responses.
Results: Drought markedly reduced gas exchange and photosystem II efficiency (Fv/Fm), especially in SO4, while M4 maintained better physiological performance. Cu2+ toxicity alone had limited physiological impacts but significantly altered root ionomic profiles. Combined stress exacerbated water-state impairment, chlorophyll reduction, and nutrient imbalances, especially in SO4. The PCA analysis of ionomic data revealed clear separation of stress treatments among rootstocks, with M4 exhibiting the most distinct and balanced nutrient profile. In contrast, plants grafted on 1103 Paulsen and SO4 showed less coordinated nutrient responses and reduced recovery capacity.
Conclusions: Rootstock genotype strongly affected grapevine resilience under multifactorial stress. M4 emerged as the most tolerant, suggesting its suitability for future viticultural conditions marked by drought and soil contamination. These results emphasize the critical importance of belowground traits in selecting more resilient grapevine plants, integrating physiological and ionomic assessments, to enhance resilience against multifactorial stresses under climate change.
1 Introduction
Among all food and agricultural commodities, whether consumed fresh or processed into derived products and beverages, wine stands out as one of the most significant value-added products globally (OIV, 2022), with a cultivated area of 792 kha in the Italian peninsula as of 2023 (OIV, 2024). However, at present several threats correlated with climate change (i.e., increase in temperatures, altered precipitation patterns, and enhanced frequency of extreme weather events, (Cesco et al., 2024) might seriously affect its production. In particular, the rising incidence of emerging diseases (Anderson et al., 2004; Fisher et al., 2012; Ristaino and Records, 2020) coupled with prolonged drought conditions (Masson-Delmotte et al., 2022) is causing significant pressure on grapevine cultivation. This impact can be especially pronounced during the early stages of the vineyard establishment, when grapevines are still young and particularly vulnerable (Palliotti et al., 2014; Poni et al., 2018).
From a general point of view, grapevines, unlike other crops, are known to be relatively resistant to moderate degrees of water deficit due to various physiological adaptive responses mainly based on antioxidant enzyme activities and the synthesis of secondary metabolites (Keller, 2020). These physiological responses may have negative effects on fruit yields, but, on the other hand, can positively affect berry quality, particularly when referring to the obtained wine (Medrano et al., 2003; Deluc et al., 2009; Leeuwen et al., 2009; Carbonell-Bejerano et al., 2014; Liu et al., 2015). However, under prolonged drought conditions, the ability of grapevine plants to sustain their physiological functions declines, resulting in marked effects on their physical and biological characteristics, such as slowed development, wilting, reduced water potential and turgor pressure, and impaired cell expansion (Lovisolo et al., 2010; Khan, 2011). Therefore, the grapevine ability to recover from drought conditions is a key agronomic trait, as it influences canopy regrowth, fruit set, yield stability, and overall vineyard performance across growing seasons (Gambetta et al., 2020). From the perspective of adaptive response mechanisms, the regulation of intrinsic water-use efficiency (iWUE), defined as the ratio between carbon assimilation and stomatal water loss (Briggs and Shantz, 1913; Hatfield and Dold, 2019), represents a particularly crucial strategy for grapevines under water deficit conditions. Values of iWUE increase under mild drought conditions, and subsequently decline with more severe or prolonged drought, primarily due to damage or inhibition of photosynthetic activity (Bota et al., 2016). This decline is further exacerbated by a decrease in the mesophyll conductance, which impairs CO2 diffusion and limits photosynthetic capacity (Ouyang et al., 2017). Thus, while iWUE may initially increase under mild drought, severe or prolonged water deficits impair photosynthetic processes, ultimately reducing iWUE. Under such conditions, plants activate additional drought-response mechanisms, including osmotic adjustment, which is regulated by endogenous hormones such as abscisic acid (ABA) and methyl jasmonate (Su et al., 2020), and involves the accumulation of organic osmolytes like sugars and quaternary ammonium compounds (Jogaiah et al., 2014).
While originally developed to confer resistance to biotic stressors, rootstock selection has progressively evolved to include adaptative traits against abiotic constraints, including edaphic imbalances (such as high pH and calcareous soils), waterlogging, salinity, and drought (Rahemi et al., 2022). Noteworthy, in the context of climate change, where enhancing grapevine resilience is a growing priority, increasing attention is being directed toward the role of rootstocks in supporting grafted-plant adaptation. Among current strategies, the use of drought-resistant rootstocks has emerged as a key agronomic approach to improving vineyard performance under increasing water scarcity. Their drought tolerance is largely attributed to specific traits, including depth and branching pattern, and has been associated with the maintenance of yield, reduced irrigation requirements, and the preservation of key berry quality traits (Zhang et al., 2016). A clear example is observed in arid regions or in areas subject to irrigation restrictions, where rootstocks like 1103 Paulsen (V. rupestris × V. berlandieri) are widely used for their deep taproots and resilience (Fregoni et al., 1978; Ollat et al., 2016; Zhang et al., 2016). In contrast, the newer M4 one (41B × V. berlandieri Resseguier No. 4) shows promise due to its quicker recovery after re-watering, slower physiological decline under water stress, and higher hydraulic conductance (Meggio et al., 2014) when compared to the more drought-sensitive SO4 (Galbignani et al., 2016). Additionally, rootstocks can influence the regulation of various physiological mechanisms, such as ABA synthesis and signaling pathways. It is interesting to note that these physiological effects differ significantly between self-rooted Vitis vinifera grapevines (e.g., Cabernet Sauvignon) and grapevines grafted onto selected rootstocks (Prinsi et al., 2021) in the whole plant. Grapevine rootstocks are therefore not only a structural support but also a functional component of the whole plant system, with a critical influence on vineyard efficiency. In fact, they not only supply water and mineral nutrients to the scion, but they are also crucial in determining the final tree vigor (Cookson and Ollat, 2013; Nimbolkar et al., 2016; Rossdeutsch et al., 2021).
Although significant efforts have been made to select rootstocks and scion varieties capable of withstanding individual abiotic or biotic stresses, grapevines grown under field conditions are rarely exposed to isolated stressing factors. On the contrary, in vineyard they typically face complex and simultaneous environmental challenges. A clear example is observed in temperate regions, where grapevines are often affected not only by soil-fertility related constraints including drought, but also by biotic pressures such as downy mildew (Plasmopara viticola) (Gessler et al., 2011), or powedry mildew (Erysiphe necator) (Rienth et al., 2021). To prevent these fungal infections, copper (Cu)-based fungicides are routinely applied as part of grapevine plant protection programs. However, the repeated use of Cu-based fungicides over successive growing seasons has led to the long-term accumulation of Cu2+ in vineyard soils, particularly in the upper layers (Kandeler et al., 1996; Merrington et al., 2002; Brunetto et al., 2016; Keiblinger et al., 2018) often exceeding the trace levels sufficient to support an equilibrate plant growth (Pietrzak and McPhail, 2004; Brunetto et al., 2016) and, in some cases, exceeding the maximum limits established for agricultural soils (Komárek et al., 2010). In this respect it should be mentioned that the Cu2+ excess in soils poses serious threats to environmental sustainability as well as vineyard productivity. High Cu2+ levels interfere with root development, callus formation, and overall plant establishment, which leads to lower success rates during propagation, making it more difficult to establish healthy young vines, complicating replanting efforts (Cesco et al., 2021). This element primarily accumulates in roots, with uptake and translocation varying by concentration (Nan and Cheng, 2001; Chaignon et al., 2002; Benimeli et al., 2010; Guan et al., 2011). When present at high availability levels, it disrupts root nutrient acquisition capacity, inhibit plant development, and affect key physiological and biochemical processes (Marastoni et al., 2019b; Feil et al., 2020, 2023). In response to this nutritional disorder, grapevines activate a range of tolerance mechanisms that include morphological alterations (e.g., stunted growth, leaf chlorosis), physiological adjustments (e.g., activation of antioxidant systems, accumulation of osmo-protectants, and metal chelators/ligands), and biochemical responses (e.g., enhanced detoxifying enzyme activity), which intensify proportionally with soil Cu2+ levels (Cesco et al., 2021, 2022; Kosakivska et al., 2021). Overall, these findings highlight the complexity of grapevine responses to soil Cu²+ excess. They also illustrate how multiple stress factors often occur simultaneously, challenging grapevine resilience and long-term vineyard productivity. It is important to note that, during natural selection, plants have adapted to both abiotic and biotic challenges, developing a wide range of coordinated tolerance mechanisms. Whilst considerable information on cross-tolerance of plants to either multiple abiotic or multiple biotic stresses is available in literature (Cao et al., 1998; Warren et al., 1998; Wen et al., 2008; Krattinger et al., 2009; Priyanka et al., 2010; Ramegowda et al., 2012, 2014, 2017; Qin et al., 2015; Carvalho et al., 2016; Ju et al., 2021; Straffelini et al., 2024), the grapevine responses to combined abiotic and biotic stresses is quite complex to understand. Several authors have indeed highlighted that the interaction between the two types of stresses (i.e., biotic and abiotic) can have either antagonistic or synergistic effects (Atkinson and Urwin, 2012; Atkinson et al., 2014; Kissoudis et al., 2014; Ramegowda and Senthil-Kumar, 2015), thus making the prediction of grapevine plants response challenging on the bases of previously acquired data (Rizhsky et al., 2002, 2004). A notable example of such interactions is represented by vine age, an often overlooked but agronomically relevant factor. Older grapevines, thanks to their deeper root systems, are better able to avoid the upper topsoil layers (0–20 cm), where heavy metals like Cu2+ tend to be accumulated (Pham, 2024), and can access water from deeper horizons, improving their resilience to both metal toxicity and drought (Galet, 2000; Pourtchev, 2003; Lehnart et al., 2008). In contrast, newly planted vines, due to standard root pruning practices (~10 cm) aimed at stimulating new root formation (Fregoni, 2013), can remain confined to the most contaminated and driest soil layers during the critical establishment phase. This condition is very likely to increase the susceptibility of young vineyards to the co-occurrence of multiple stressors, further complicating replanting efforts in historically cultivated areas and, at least partially, explaining the difficulties often observed in these contexts, challenges that are expected to intensify under ongoing climate change.
Based on these premises, this work was aimed at deepening our understanding of the complex interaction between abiotic stresses (i.e., water stress and Cu2+ toxicity) that grapevine plants may simultaneously face in the current context of climate change. To this scope, three different rootstocks (i.e., 1103 Paulsen, M4 and SO4) grafted with Pinot gris have been exposed either to drought stress, Cu2+ toxicity or the combination of the two stresses within a pot experiment in greenhouse conditions. The three rootstocks were selected based on their different levels of tolerance to drought stress (Supplementary Table S1). With regard to Cu2+ tolerance, several publications are available for 1103 Paulsen and SO4 (Trentin et al., 2022, 2024); however, no studies have yet been conducted for M4. Physiological and biochemical parameters have been monitored throughout the experimental period to assess the differential impact of abiotic stresses on the three rootstocks under investigation. The outcomes of this study are intended to support rootstock selection strategies aimed at improving vineyard resilience under multifactorial stress conditions, particularly in the context of replanting programs in areas affected by water scarcity and long-term soil accumulation of Cu2+.
2 Materials and methods
2.1 Plant material and experimental design
The experiment was conducted in the greenhouse of the Free University of Bozen-Bolzano in Laimburg (Alto Adige, Italy 46.3827° N, 11.2877° E) during the vegetation period (from May to November 2023) using grapevine plants obtained from Vivai Cooperativi Rauscedo (Rauscedo, Italy).
The vine plants tested were a combination of the same cultivar (Pinot gris) grafted on three different rootstocks, i.e., M4, 1103 Paulsen and SO4.
The grafted plants were stored in a refrigerated room at 6°C and prepared for vegetative awakening. The plants were soaked in water for 24 h, and afterwards the roots have been shortened to a length of about 10 cm and then transplanted in 6 L pots, filled with approximately 5 kg of substrate, composed of River Sand (60%), Perlite (20%), and Peat mixture (20%). The lower part of the plastic pots hosted several holes to drain excessive water from the pot, preventing any anoxia condition at the root level; moreover, the upper surface of the pots was covered with felt pads to prevent water loss by direct soil evaporation.
The pot experiment was then conducted in greenhouse with a mean air temperature of 28 ± 2°C during the day and 20° ± 2°C during the night, controlled lighting (500 µmol m2/s) and with controlled irrigation. Protection against diseases was carried out only with Cu-free preparations.
Before the end of the growing period, each vine was pruned to retain two shoots, which were then supported using stakes.
The experiment followed a completely randomized block design (CRBD) with a minimum of nine replicates per treatment. Plants were organized in rows of 16 and randomly assigned to treatments within each block. To control spatial and positional effects, plants were rotated weekly both within a single row and between rows. This regular repositioning helped ensure uniform exposure to environmental conditions and minimized location-based bias. Plants were divided in four treatments: 1- Control (no treatments applied); 2- Cu2+ and drought stresses (Cu+DS); 3- drought stress (DS); 4- Cu stress (Cu). The Cu was applied as CuSO4 and the first Cu2+ dose of 120 mg/kg was applied at ~55 days after the transplanting. Based on preliminary analyses, a maintenance Cu2+ dose of 60 mg/kg was applied a week after the first Cu2+ application. The copper dosage was selected by comparing data from previous studies with concentrations currently reported in contaminated vineyards (Toselli et al., 2009; Komárek et al., 2010; Baldi et al., 2018; Marastoni et al., 2019a, 2019b), with the objective of inducing Cu2+ toxicity without being lethal to grapevine. In the same moment, drought stress was induced in Cu+DS and DS plants by withholding the irrigation, until severe drought stress was reached (corresponding to approximately -1.2 MPa midday stem water potential). The other plants were manually irrigated until soil saturation. After reaching severe drought stress, the plants were watered to induce the recovery. During the different stages of the experiment — (start of the experiment (T0), induction of drought stress (T1), peak of drought stress (T2), recovery (T3)) — morpho-physiological data have been recorded, and root, leaves and soil samples were collected and stored for further chemical, biochemical and molecular analysis.
2.2 Measurements of morpho-physiological parameters
Every other day during the experimental period the midday stem water potential (Ψstem - MPa) was measured. To mitigate the effect of repeated leaf destructive samplings on the total vine leaf area, measurements were taken on each date from only one fully expanded leaf per vine. The leaves were enclosed in transparent plastic bags, covered with aluminum foil at noon, detached after approximately one hour, and immediately inserted into a pressure chamber (Pump-up Pressure Chamber, PMS Instrument Comp. USA) for the reading.
Using a portable infrared gas analyzer (LC-pro ADC, Hoddesdon Bioscientific, Ltd., Herts, United Kingdom), measurements were conducted for the photosynthetic rate (A, μmol CO2 m−2 s−1), transpiration rate (E, mmol H2O m−2 s−1), and stomatal conductance (gs, mol H2O m−2 s−1). The measurements were performed on the same day as the Ψstem assessments to ensure consistency in plants’ water status. Morning readings (9:00–11:00 am) were taken under saturating light conditions (PPFD of 1800 μmol photons m−2 s−1 provided by a LED array unit) and ambient CO2 levels (382–438 ppm), always selecting a fully expanded leaf per vine located in the intermediate section of the shoots. A and gs values were used to calculate the intrinsic water use efficiency (iWUE= A/gs). A total of ten measurements were collected over the course of the experiment, from the first copper application to the recovery of the plants.
Chlorophyll fluorescence parameters were measured every other day, on the same days as other physiological parameters (leaf gas exchange and midday Ψstem). Readings were obtained in the morning (9:00-11:00) utilizing a portable chlorophyll fluorometer Handy PEA (Hansatech Instruments, UK) with an excitation light at 650 nm. For each measurement, one mature and healthy leaf per vine was selected and prepared for assessment. The measurements were taken after the leaves were fully dark-adapted for at least 20 minutes, achieved by covering them with a leaf clip that occupied a total diameter of 4mm, ensuring proper illumination.
Leaf chlorophyll content was indirectly evaluated with a SPAD-502 Chlorophyll Meter (Konica Minolta, Tokyo, Japan). Measurements were performed once per week on each plant. SPAD values are reported as an average of three measurements on the third to fifth leaf of each shoot. The instrument calculated a numerical SPAD value, which correlates with the chlorophyll content in leaf tissues.
Soil humidity (m3/m3) from a depth of 0 to 6 cm was measured every other day during the trial using a Theta Probe ML3 (Delta-T Devices Ltd, Cambridge, England).
The total leaf area of each plant was estimated using a Leaf Area Meter (LI-3000 Leaf Area Meter coupled with the Li-3050 leaf charger, Li-Cor Inc., Nebraska, USA) by collecting all the available leaves that were present at the end of the experiment. The harvested grapevine roots (only the new growth), branches, and leaves were oven dried at 60°C for a week, and the dry weight of each sample was recorded.
2.3 ICP-MS analysis
For each treatment, all leaves and all roots from a plant were collected, dried at 65°C, and ground to a fine powder using a TissueLyser II. Three biological replicates were prepared for both leaf and root tissues. Approximately 0.2–0.3 g of each sample was weighed and acid digested with 69% ultrapure HNO3 (Carlo Erba, Milano, Italy) in a single reaction chamber microwave digestion system (UltraWAVE, Milestone, Shelton, CT, USA). The digested samples were diluted to 2% HNO3 with ultrapure grade water (18.2 MW·cm at 25°C), and the concentrations were then determined using an inductively coupled plasma–mass spectrometer (ICP-MS, iCAP™ RQ, Thermo Scientific). Element quantification was carried out using certified multi-element standards (CPI International, https://cpiinternational.com). NIST standard reference materials 1573a (tomato leaves) and 1570a (spinach leaves) were used as external certified references, which were digested and analyzed the same way as the samples.
2.4 Cu accumulation assessment
The methodology proposed by Lai et al. (2010) and employed by Vršič et al. (2023) was used to characterize the rate of Cu2+uptake from the soil into the grapevine grafts in our experimental setup.
In accordance with the definition proposed by (Juang et al., 2012), the bioaccumulation factor (BAF) is established as the ratio of Cu2+concentrations in the grapevine leaf (Cgl) to the Cu2+concentration in the substrate (Csu), expressed by the equation BAFl = Cgl/Csu, where BAFl represents the bioaccumulation factor for the grapevine leaf, Cgl is the Cu2+ concentration in the leaf (mg kg−1), and Csu is the Cu2+concentration in the substrate (mg kg−1).
The translocation factors (TFs) represent the ratios of Cu2+ concentrations in the roots to those in other organs of grapevine grafts. Therefore, the TFs for Cu2+ translocation from roots to trunk and canes were estimated according to the equation TFl = Cleaf/Croot (Chopin et al., 2008; Busuioc et al., 2011), where Cleaf is the Cu2+concentration in the leaf (mg kg-1), Croot is the Cu concentration in the roots, and TFl signifies the translocation factor through the root to the leaf.
2.5 Data treatment, statistical analysis and data visualization
Statistical analyses were performed using R software (Team, 2025). Differences among the days of leaf gas exchanges (A, E, gs), chlorophyll parameters (SPAD and florescence) and Ψstem were tested by one-way ANOVA. Mean separation was performed with the Tukey HSD test (p < 0.05). Biometric values were also tested by one-way ANOVA and the mean separation was performed with the Tukey HSD test (p < 0.001). Both ANOVA and Tukey HSD tests were conducted using the stats package (v4.5.0), and post hoc analyses were performed using the agricolae package (de Mendiburu, 2023).
Principal component analysis (PCA) was performed to evaluate patterns in the dataset using the prcomp function in R. PCA visualization was carried out using the ggfortify package (Horikoshi and Tang, 2018) and plotted with ggplot2 (Wickham, 2016). Data wrangling and preparation were managed with dplyr (Wickham et al., 2023) and readxl (Wickham and Bryan, 2025).
For visualization of mean comparisons (e.g., bar plots), customized plotting was performed using ggplot2 (Wickham, 2016) and enhanced with the ggpattern package (Mike et al., 2025) for pattern fills. Thematic styling was applied using ggthemes (Arnold, 2024), and multi-panel plot arrangements were constructed using the patchwork package (Pedersen, 2024). Additional data manipulation and formatting tasks were handled with the plyr (Wickham, 2011), stringr (Wickham, 2023) and tidyr (Wickham et al., 2024) packages.
All base functions and core graphical capabilities were supported by R’s native base, graphics, grDevices, methods, utils, and datasets packages (v4.5.0; R Core Team, 2025).
3 Results
3.1 Soil humidity
Soil moisture measurements began at the onset of the drought stress (T1), as the soil was fully irrigated prior to this point. In the fully irrigated treatments (Control, Cu), soil humidity remained consistently high and constant throughout the entire growing period (Table 1), ranging from approximately 0.27 to 0.39 m³/m³ between the initial and final measurements (T1–T3), indicating that the soil could guarantee a sufficient availability of water for the request of the plants. In contrast, in non-irrigated treatments (DS, Cu+DS),soil humidity reaching minimum values between ~0.02 and ~0.1 m³/m³ at the peak of the stress (T2), indicated a severe water deficit. Following rewatering (T3), soil moisture in these treatments recovered to levels between 0.31 and 0.42 m³/m³. Statistical analysis confirmed significant differences in soil moisture across treatments during stress and recovery phases, although no substantial rootstock-specific differences were observed for this parameter.
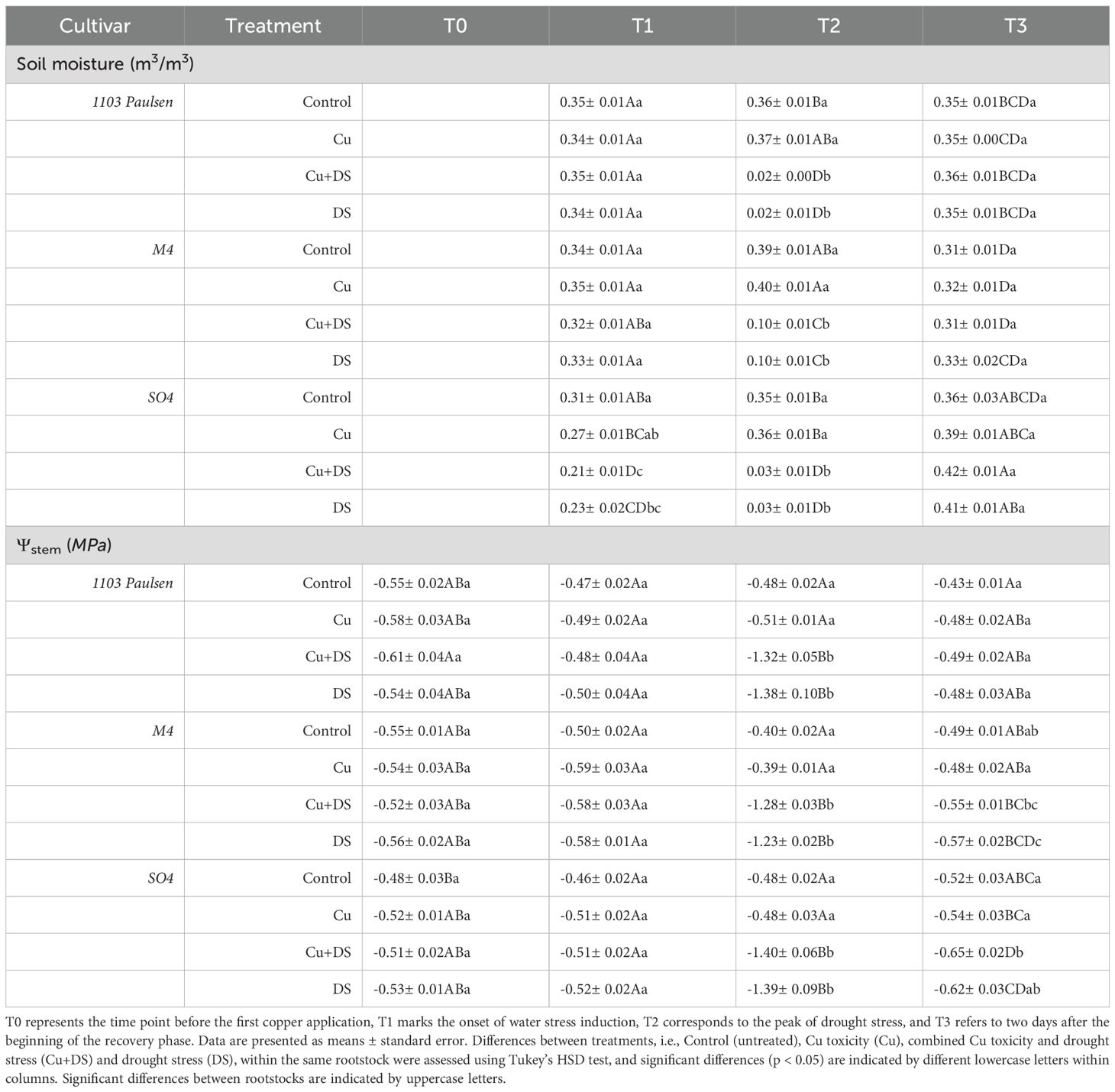
Table 1. Soil moisture and Midday stem water potential (Ψstem) measured from the start till the end of the experiment.
3.2 Assessment of grapevine plants’ physiological status
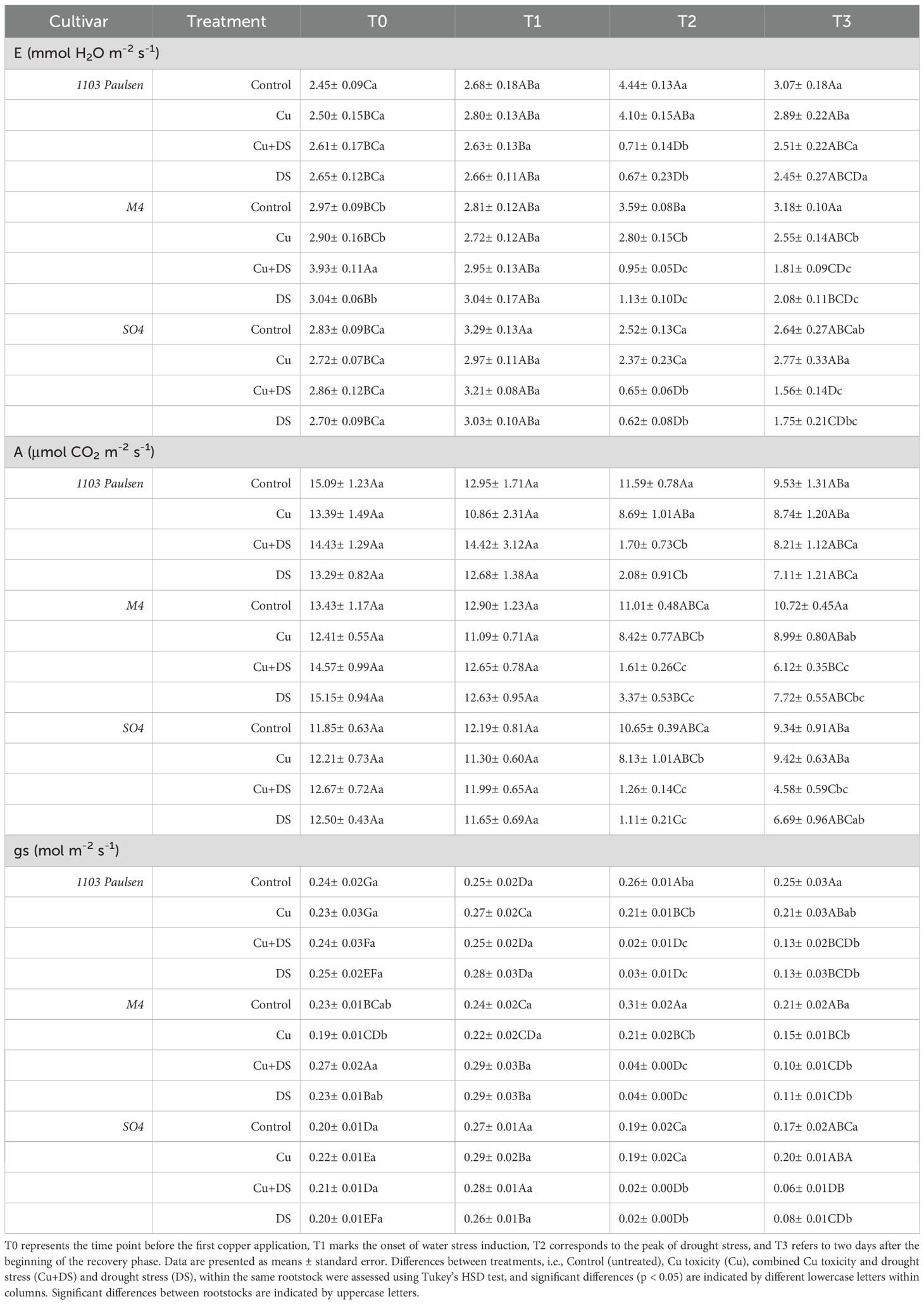
As far as Ψstem is concerned (Table 1), in the fully irrigated treatments (Control, Cu), the Ψstem ranged over the whole growing period from -0.3 to -0.6 MPa, confirming adequate water availability throughout the cycle. Under drought stress (DS, Cu+DS), Ψstem values dropped to -0.8 - -1.7 MPa, indicating severe stress (Table 1). Upon rewatering, values partially recovered to -0.4 - -0.7 MPa. At the peak of drought stress, M4 maintained much less negative Ψstem to soil moisture ratios (MPa/m³/m³), reaching about –12.6 under DS and –13.1 under Cu+DS, while SO4 dropped to –62.0 and –67.7 and 1103P to –105.0 and –77.2, indicating that M4 sustained higher Ψstem at the same soil moisture and therefore displayed higher water uptake efficiency.
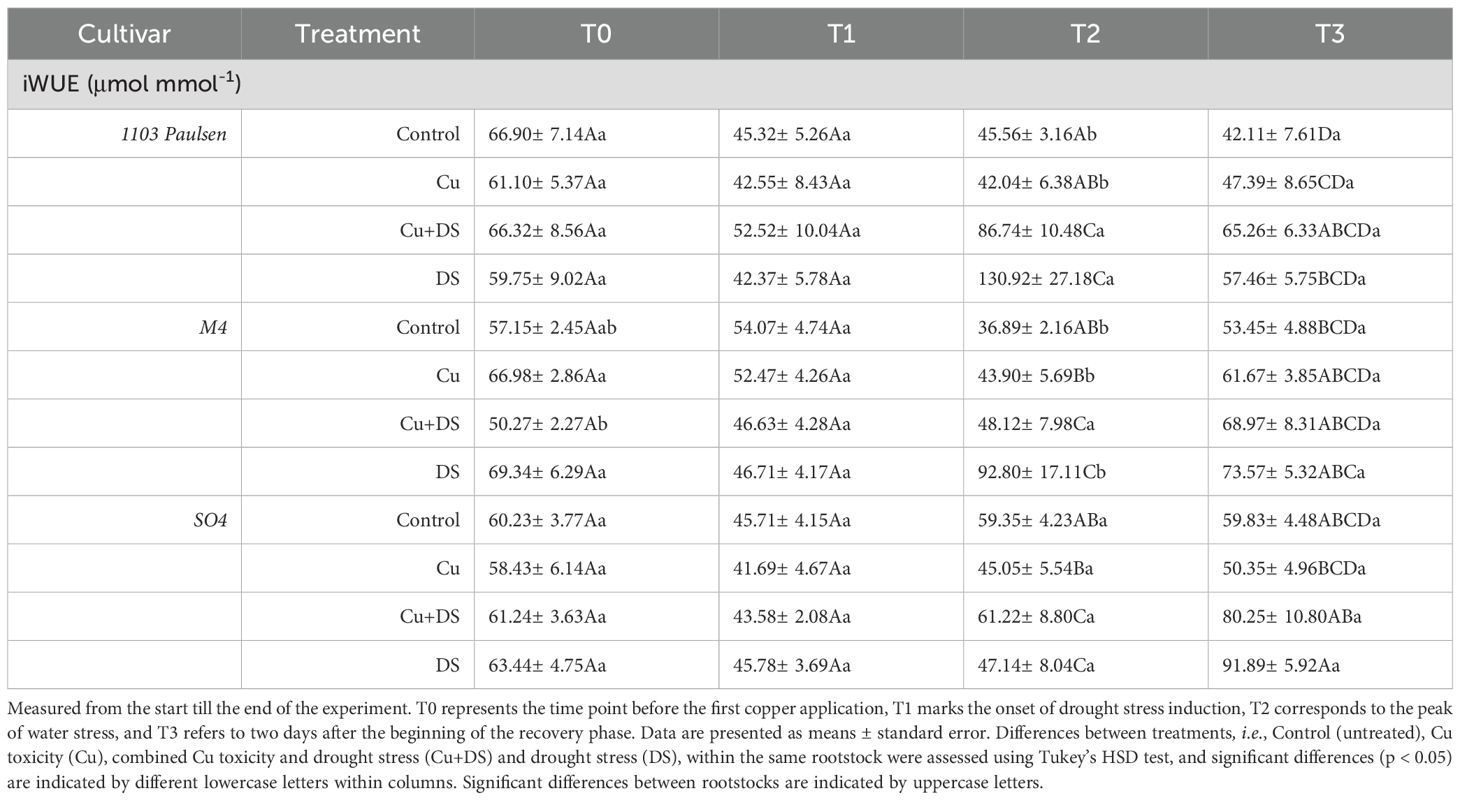
Stomatal conductance, transpiration rate and photosynthetic rate (Table 2) exhibited consistently high intensity throughout the entire cycle under full irrigation conditions (Control, Cu), while in drought-stressed treatments (DS, Cu+DS), all three parameters progressively declined until rewatering. At the peak of drought stress, no statistically significant differences were observed between rootstocks under drought-stressed conditions (DS and Cu+DS), whereas among fully irrigated plants (Control and Cu), 1103 Paulsen exhibited the highest transpiration rate compared to M4 and SO4. Additionally, M4 plants treated with Cu2+ showed noticeably lower values of both transpiration and stomatal conductance compared to the control. After recovery, a general trend toward restoration was observed, though the extent varied by rootstock (Table 2). Intrinsic water use efficiency (iWUE) (Table 3) remained unaffected until the peak of drought stress in 1103 Paulsen and M4, consistent with their reported drought tolerance. Interestingly, even when subjected to combined stresses (Cu+DS), vines grafted on M4 showed values of iWUE that were not different from those of vines exposed to drought or Cu2+stress only. In SO4, on the other hand, iWUE showed no significant change across treatments. After rehydration, differences among treatments largely disappeared (Table 3).
The Chlorophyll Fluorescence (Fv/Fm) values (Table 4) generally ranged from 0.75 to 0.9 and remained stable in irrigated treatments (Control, Cu) in all the rootstocks considered. In contrast, drought-stressed treatments (DS and Cu+DS) caused a decline in Fv/Fm, particularly at the peak of drought stress (T2). The most severe reduction was observed in the 1103 Paulsen rootstock under DS, where Fv/Fm dropped to 0.63 at T2. A similar trend, albeit with a lower extent, was noted in SO4 and M4 under DS and Cu+DS conditions. After rewatering (T3), Fv/Fm values showed partial recovery in most treatments, although values remained slightly lower than the pre-stress phase, especially in SO4.
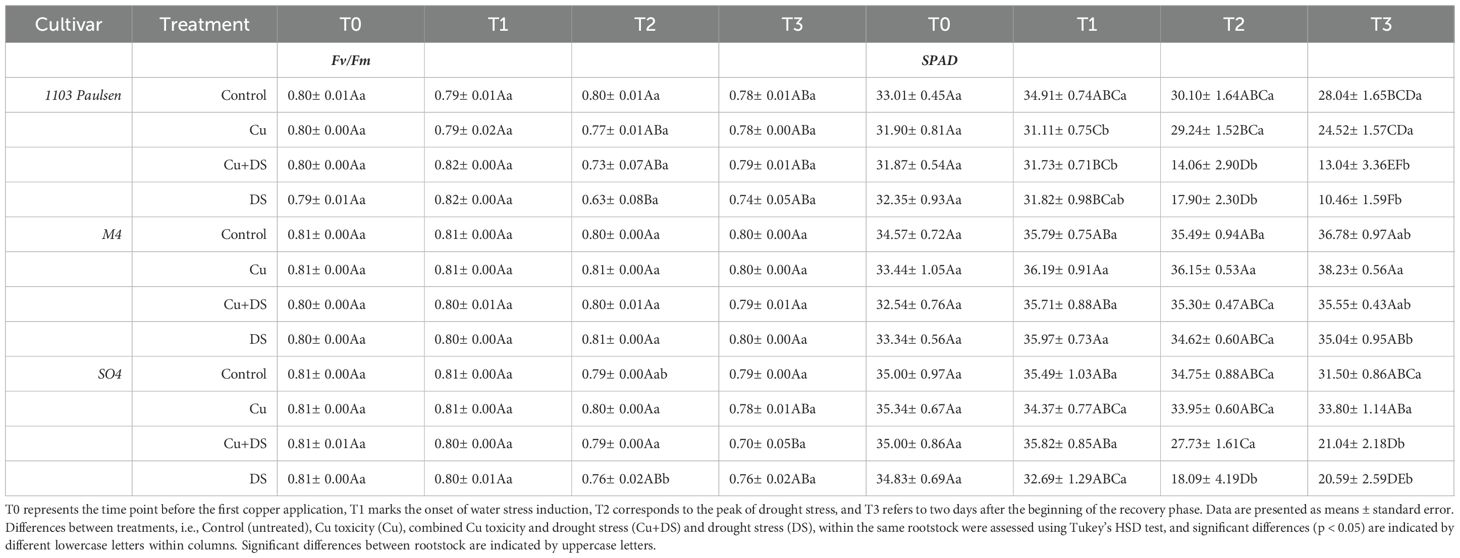
Table 4. Chlorophyll fluorescence (Fv/Fm) and SPAD measured from the start till the end of the experiment.
Leaf relative chlorophyll content, expressed as SPAD values (Table 4), varied in response to drought and Cu stress, showing rootstock-specific patterns. In 1103 Paulsen and SO4, a significant decrease in SPAD values was observed under drought (DS) and combined Cu+DS treatments, especially at the peak of drought stress (T2) and after partial recovery (T3). In contrast, M4 maintained relatively stable SPAD values across all treatments, with no significant reduction under drought stress or Cu2+ application. Under fully irrigated conditions (Control and Cu), all rootstocks showed minimal variation in SPAD values throughout the experimental period.
3.3 Leaf area and dry biomass
The biometric data, reported in Table 5, demonstrated that water deprivation (DS and Cu+DS) had a significant negative impact on both leaf area (LA) and dry shoot biomass across all three rootstocks. In contrast, Cu2+application alone did not significantly affect biomass accumulation at either the root or shoot level.
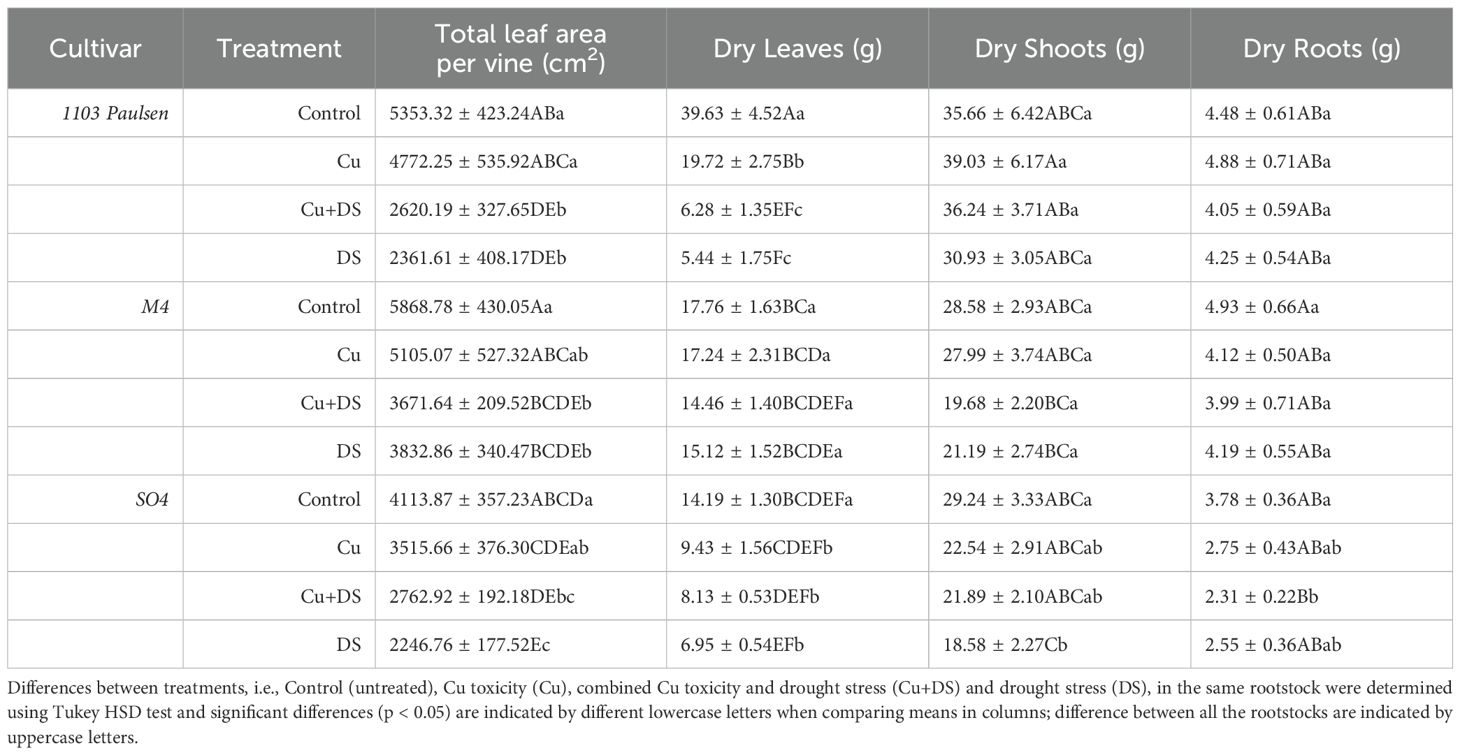
Table 5. Leaf area, leaves dry weight, shoot dry weight, root dry weight measured at the end of the experiment.
In terms of LA, 1103 Paulsen and SO4 showed the most pronounced reduction under drought conditions. For example, 1103 Paulsen decreased from 5353.3 cm² (Control) to 2361.6 cm² (DS) and 2620.2 cm² (Cu+DS), while SO4 declined from 5002.7 cm² (Control) to 1954.4 cm² (DS). Although M4 was also affected, it retained higher LA values under stress (e.g., 4216.5 cm² under DS), indicating a greater capacity to maintain leaf development under water limitation (Table 5).
Regarding dry leaf biomass, M4 retained higher values under drought compared to SO4 and 1103 Paulsen. Notably, leaf dry weight of SO4 under DS fell to 6.95 g, and 1103 Paulsen dropped to 5.44 g, M4 instead showed 15.12 g, suggesting a greater tolerance. For shoot dry weight, only SO4 under drought stress showed a significant reduction of dry biomass (Table 5). At the root level, M4 and 1103 Paulsen did not exhibit significant differences across treatments, maintaining root dry biomass between 4.0 and 5.1 g, including under drought. In contrast, SO4 showed a marked reduction in root biomass, from 3.78 g (Control) to 2.31 g (DS), reflecting lower drought resilience in below-ground organs.
3.4 Copper bioaccumulation and translocation
The ionomic data has been investigated to calculate Cu2+bioaccumulation and translocation factors, to shed light on possible different behaviors depending on the stressing conditions and the rootstocks genotype. The analysis of copper content (Figure 1A), bioaccumulation factor (Figure 1B), and translocation factor (Figure 1C) revealed distinct rootstock-specific responses to copper supplementation and drought stress. Across all treatments, Cu2+accumulation in root tissues was significantly higher in Cu and Cu+DS conditions compared to the control and DS treatments (p < 0.05), whilst it remained largely unaffected in leaves, except for the specific case of drought and heavy metal stressed 1103 Paulsen (Figure 1A). Among rootstocks, SO4 accumulated the highest Cu2+ levels in roots, particularly under Cu+DS, with root concentrations exceeding ~1400 mg/kg, reflecting its higher sensitivity to combined stress. In contrast, M4 exhibited the lowest root Cu2+accumulation under both Cu and Cu+DS treatments (e.g., ~290 mg/kg), suggesting a superior exclusion or sequestration capacity at the root level (Figure 1A).
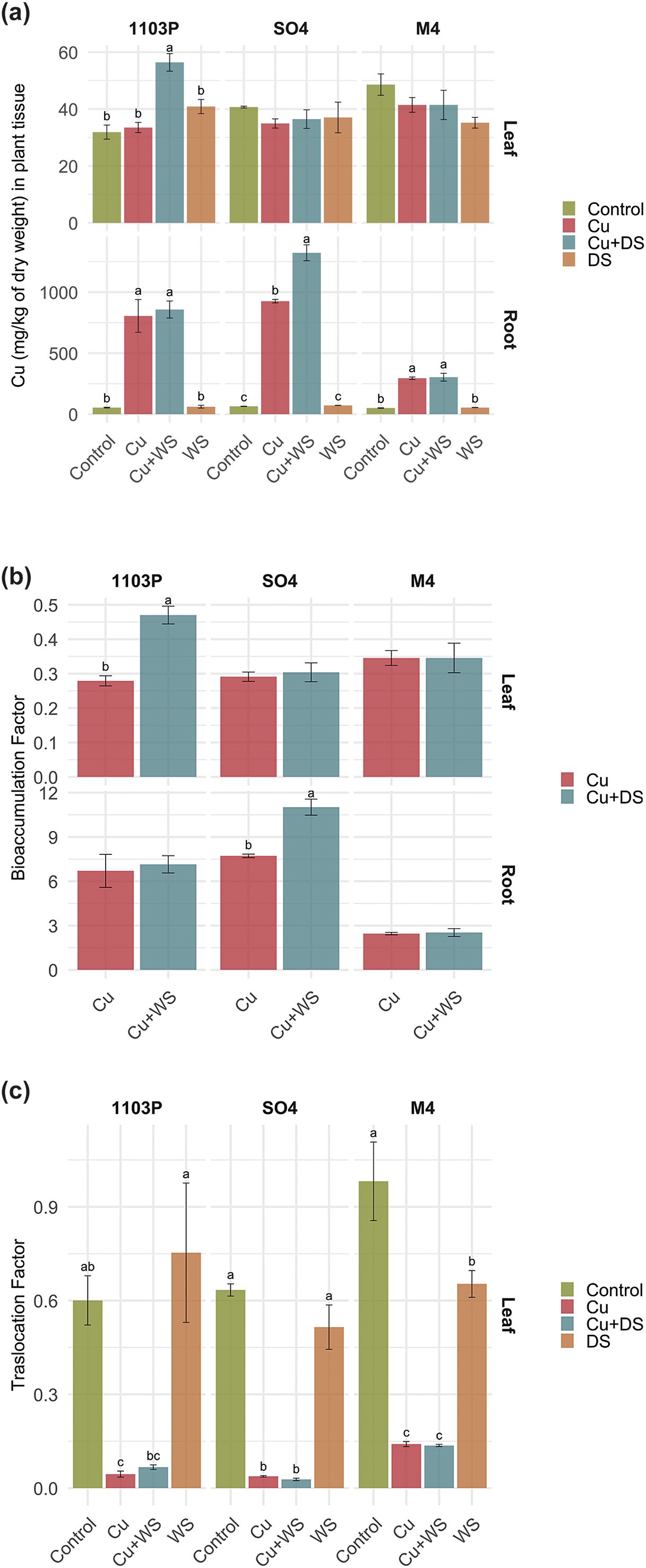
Figure 1. Copper concentration (mg kg−1 dry weight) (a), Bioaccumulation Factor (b) and Translocation Factor of Copper from roots to leaves (c) in grapevine plants subjected to different treatments, i.e., Control (untreated), Cu toxicity (Cu), combined Cu toxicity and drought stress (Cu+DS) and drought stress (DS). SO4, 1103 Paulsen and M4 are the grapevine rootstock used. Data are reported as means ± SE, n = 3. Differences between treatments in the same rootstock were determined using Tukey HSD test and significant differences (p < 0.05) are indicated by different lowercase letters comparing means in columns.
These trends were mirrored by the Bioaccumulation factor (BAF), where M4 consistently showed lower BAF values, indicating limited translocation from soil to roots. For instance, M4 under Cu treatment had a BAF in the root of approximately ~2.45, significantly lower than the ~7.70 observed in SO4 under the same condition, and values of ~ 11 under Cu+DS (Figure 1B). The translocation factor (TF) from roots to leaves also differed between rootstocks (Figure 1C). Whilst all Cu-treated plants displayed lower TFs compared to controls, M4 maintained relatively higher TF values under Cu+DS and Cu, with values of ~0.14, possibly due to reduced root accumulation. Conversely, SO4 and 1103 Paulsen showed strong Cu2+retention in roots with limited translocation to aerial parts.
3.5 Ionomics
To gain a better understanding about the effects of treatments on plant nutrients balance, ionomic data (Supplementary Table S2) obtained from the leaves and roots were subjected to multivariate statistical analyses, specifically principal component analysis (PCA). The PCA model obtained for root samples explained approximately 66% of the total variance in the dataset and revealed a clear separation of samples along PC1 (Figure 2A), determined by the different rootstock’s genotype. Notably, the M4 treatment exhibited distinct separation, primarily driven by phosphorus (P), manganese (Mn), and potassium (K). Similarly, samples obtained from 1103 Paulsen rootstock clustered together, independently from the treatments, while those of SO4 were separated primarily along PC2 (Figure 2A). When the ionome of shoot tissue is considered, the PCA model generated explained 62% of the total variance and showed a distinct behavior depending on the rootstock considered (Figure 2B). In particular, SO4 samples created a single cluster that was partially overlapping with 1103 Paulsen samples. Nevertheless, 1103 Paulsen samples showed a separation in two distinct groups within the cluster (Figure 2B). On the other hand, M4 samples showed a clear separation with respect to SO4 and 1103 Paulsen along the PC1, albeit a significant segregation of M4 samples was also observed along PC2 (Figure 2B).
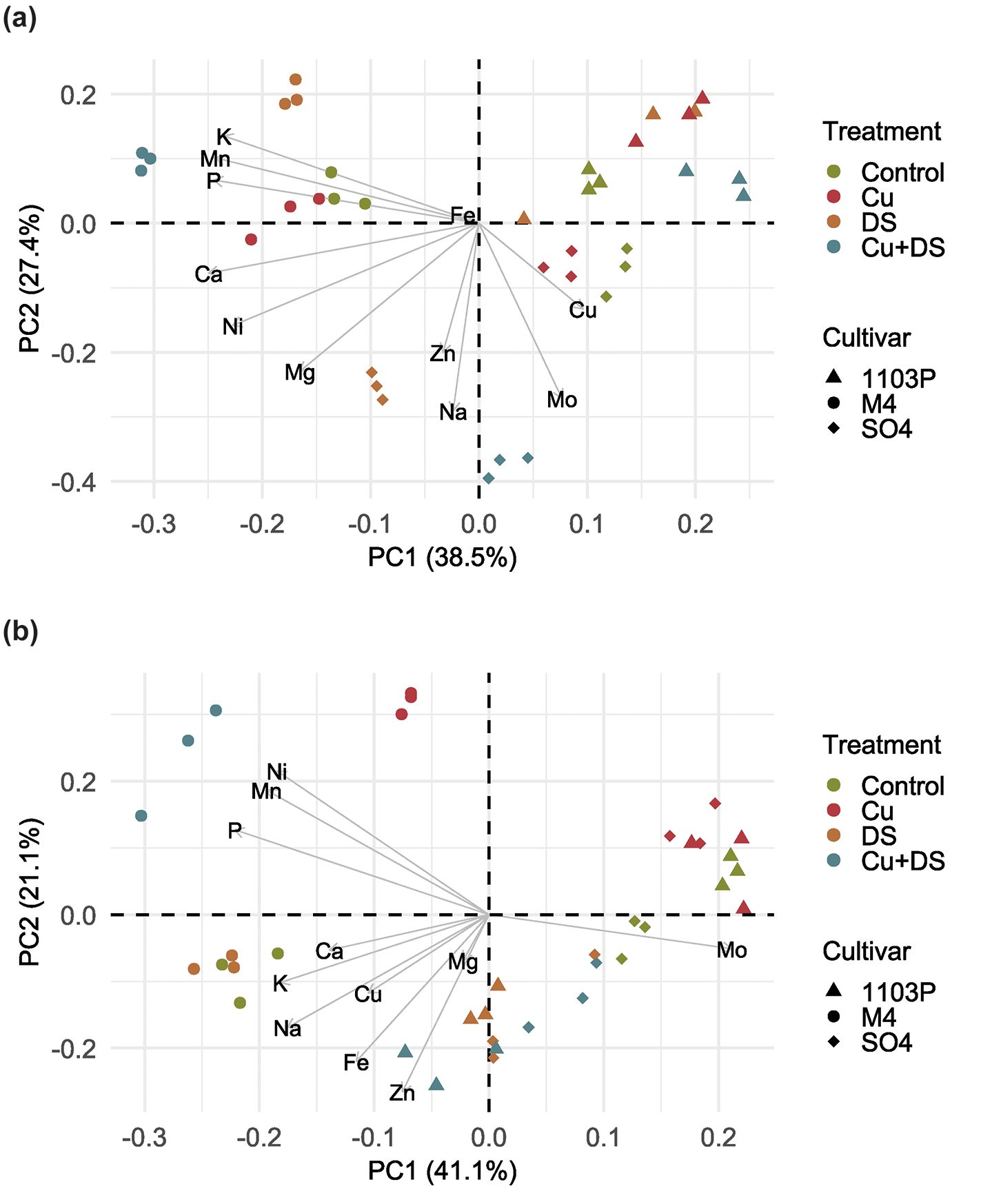
Figure 2. Principal component analysis of ionomic data of root (a) and leaf (b) samples of SO4, 1103 Paulsen and M4 grapevine rootstock. Each point on the plot represents an individual tissue sample, with shapes representing different grapevine cultivars and color representing different treatments, i.e., Control (untreated), Cu toxicity (Cu), combined Cu toxicity and drought stress (Cu+DS) and drought stress (DS).
To further investigate the treatment effects with a focus on the different rootstocks, additional PCA were performed by subsetting the dataset according to rootstock type (Figure 3). The model generated with SO4 roots dataset explained approximately 73% of the total variance (Figure 3A). Along PC1, drought-stressed samples (i.e., DS and Cu+DS) resulted separated from the others, suggesting a higher hierarchy of the stress respect to Cu. Nevertheless, the overall samples distribution showed the separation of independent clusters (Figure 3A), possibly suggesting that each single treatment could represent a peculiar condition, as also confirmed by the alteration in the correlations between nutrients (Supplementary Figure S1). Interestingly, a similar behavior was also observed at leaf level (Figure 3B and Supplementary Figure S2).
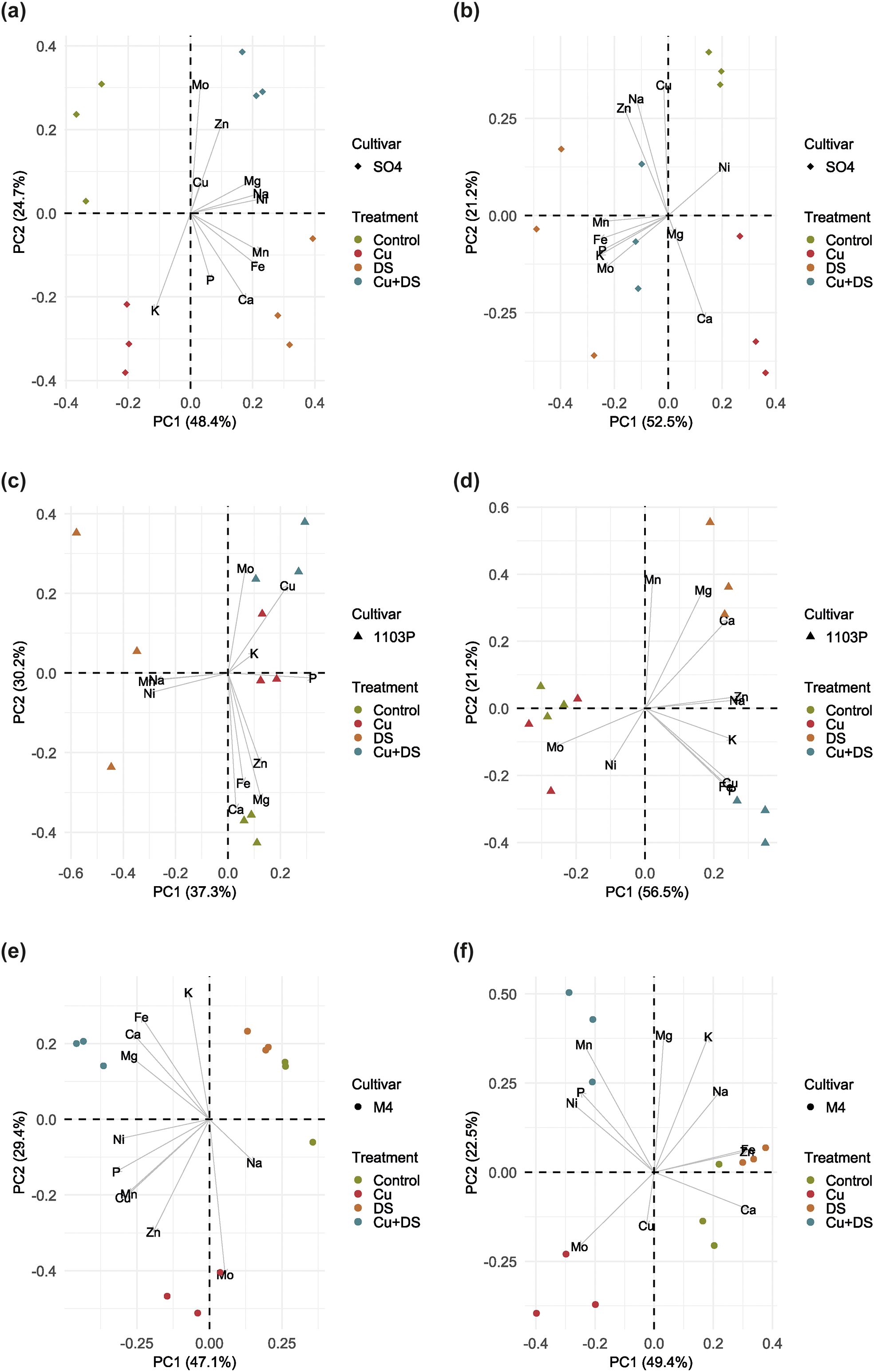
Figure 3. Principal component analysis of ionomic data of root and leaf samples of SO4 (a, b), 1103 Paulsen (c, d) and M4 (e, f) grapevine rootstock. Each point on the plot represents an individual tissue sample, with colors indicating different treatment groups, i.e., Control (untreated), Cu toxicity (Cu), combined Cu toxicity and drought stress (Cu+DS) and drought stress (DS), and shapes representing different rootstocks. The proximity of points on the plot suggests similarities or differences in elemental composition among the samples.
The PCA model obtained for the roots of 1103 Paulsen explained approximately 67% of the dataset variance and showed a main sample separation along the PC1 (Figure 3C). In particular, roots subjected to drought stress (DS) showed a significantly different ionome as compared to the other samples. Interestingly, when correlations among elements were examined, they showed only synergisms (Supplementary Figure S1). On the other hand, Control, Cu and Cu+DS samples separated along the PC2, with Cu and Cu+DS samples being richer in Cu2+as expected (Figure 3C). Nevertheless, the reciprocal dynamics established by mineral nutrients within Cu and Cu+DS samples are different, highlighting different physiological outcomes of single and combined stresses (Supplementary Figure S1). This distribution could suggest that in 1103 Paulsen rootstock, at root levels, the Cu2+stress might have prevalence on drought stress, at least when the uptake of mineral nutrients is concerned. Contrarily, at leaf level, a clear separation of samples driven by the DS (i.e., along PC1) was observed (Figure 3D and Supplementary Figure S2).
The PCA model generated with the M4 roots dataset explained approximately 77% of the total variance and showed the separation of samples in three independent clusters (Figure 3E). The first group encompassed Control and DS samples, whereas Cu and Cu+DS samples were separated and independent. Such observation further confirms that M4 rootstock is tolerant to drought stress and highlights that single Cu or combined Cu+DS stress can have significantly different effects in the accumulation of minerals, as also confirmed by the correlations analyses (Supplementary Figure S1). Interestingly, a coherent behavior has been also observed at leaf level (Figure 3F and Supplementary Figure S2).
4 Discussion
The present study investigated the physiological, biochemical, and ionomic responses of grapevines (Pinot gris variety) grafted onto three rootstocks (i.e., M4, SO4, and 1103 Paulsen), following exposure to drought, Cu2+toxicity, or their combination under controlled greenhouse conditions. The results are then discussed in terms of genotype-specific stress-response strategies, with particular attention to how stress interactions affect plant water relations, photosynthetic recovery, and nutrient homeostasis, providing insights into rootstock selection for viticultural systems increasingly exposed to multifactorial stress.
Results reported in Table 1 and referred to the midday Ψstem measurements in leaves of Pinot gris vines grafted onto the three different rootstocks show that, as expected, fully irrigated vines maintained stable and adequate water status throughout the experiment, whereas non-irrigated plants experienced a clear and pronounced drought stress. Following rewatering, only a partial recovery of Ψstem values was observed, suggesting that the effects of severe water limitation were only partially reversible within the short time frame of this study. Interestingly, the decline in Ψstem values under drought stress did not consistently mirror changes in soil moisture content, particularly during the early phases of drought (data not shown). This decoupling indicates a degree of physiological buffering or delayed stress perception, supporting previous findings that grapevines may exhibit a lag between decreasing soil water availability and measurable physiological responses at the leaf or whole-plant level (Chaves et al., 2010). Such temporal uncoupling may reflect the ability of rootstocks to temporarily compensate the deficit through hydraulic redistribution, osmotic adjustment, or internal water reserves. Notably, in treatments subjected to both drought and Cu stress, the reduction in Ψstem was, on average, 2% more pronounced, and its recovery appeared slightly limited compared to drought alone. This suggests that Cu2+toxicity may exacerbate hydraulic dysfunction, possibly by damaging root tissues, inhibiting water uptake, or interfering with aquaporin activity (Fatemi et al., 2020). Copper-induced root stress may therefore amplify drought effects by limiting the plant’s capacity to maintain water transport and recover after rehydration, particularly in more sensitive genotypes such as SO4. Therefore, these findings highlight how crucial strong hydraulic resilience in rootstocks is to withstand the compounded effects of drought and Cu2+ toxicity, which can severely impair water transport and recovery capacity.
Results in Table 2 showed that drought stress mainly impacted leaf-level biochemical and physiological processes across all rootstocks used, leading to significant decrease in transpiration, photosynthetic rate and stomatal conductance. These declines were most evident at the peak of drought stress and are consistent with classical drought response mechanisms aimed at limiting water loss (Benyahia et al., 2023; Shtai et al., 2024). Following rehydration, recovery of gas exchange was only partial after three days in plants grafted onto M4 and SO4 rootstocks, suggesting persistent inhibition or delayed reactivation of both stomatal and mesophyll conductance (Flexas et al., 2006). In contrast, those grafted onto1103 Paulsen exhibited a faster recovery, which may be ascribed to its diminished leaf area post-stress, potentially lowering transpiration demand, and a more conservative water-use strategy. This observation aligns with previous findings (Pou et al., 2008), which indicates that the full stomatal recovery in water-stressed vines may require up to two weeks, particularly under midday Ψstem values comparable to those imposed in this work (−1.4 to −1.5 MPa). Additionally, in the presence of combined Cu2+ toxicity and drought stress, recovery was even more limited compared to drought stress alone, particularly in plant grafted onto SO4, with drops of approximately 11% in E, 31% in A, and 23% in gs, suggesting that Cu2+ toxicity may exacerbate impairment of stomatal function or delay hydraulic reactivation during the recovery phase (Tashakorizadeh et al., 2023). More in general, the incomplete recovery of gas exchange parameters after rehydration may reflect persistent impairments in mesophyll conductance (Flexas et al., 2008), delayed reactivation of aquaporins (Pou et al., 2008), or continued ABA-mediated stomatal regulation (Zhang et al., 2006). The physiological bottlenecks here highlighted can limit the re-establishment of full photosynthetic capacity even when water availability is restored. These observations emphasize the need to consider rootstock-specific recovery dynamics, as the persistence of stress effects, especially under combined drought and Cu2+ toxicity, may affect short-term physiological functionality and, consequently, vineyard management decisions.
The intrinsic water use efficiency (iWUE)was introduced to compare photosynthetic properties regardless of the evaporative demand (Osmond et al., 1980). The increase of iWUE values at the peak of drought stress is coherent with the drought-related traits of 1103 Paulsen and M4 rootstocks (Bianchi et al., 2020), which appear to reduce stomatal conductance as a conservative strategy to limit water loss, while maintaining relatively stable rates of photosynthesis. This adaptive response suggests a preferential regulation of gas exchange in these genotypes, enabling sustained C assimilation under conditions of reduced water availability (Bertolino et al., 2019; Barl et al., 2025). In contrast, the lack of significant changes of iWUE values in plants grafted onto SO4 under similar stress conditions may indicate a less effective stomatal regulation or a decline in photosynthetic capacity, reinforcing its classification as a drought-sensitive genotype (Bertolino et al., 2019; Barl et al., 2025). Consistently, the normalization of iWUE values after rehydration might indicate partial recovery of stomatal function and photosynthetic activity, although with varying speeds among rootstocks (Table 3). From an agronomic perspective, these results underscore the importance of selecting rootstocks that support efficient water use regulation under drought, as demonstrated by grafted plants onto M4 and 1103 Paulsen.
The Fv/Fm ratio, a widely used indicator of the maximum quantum efficiency of PSII, decreased under increasing drought stress, as previously described in other studies focused on grapevine (Murchie and Lawson, 2013; Mashilo et al., 2018; Giorgi et al., 2019; Díaz-Barradas et al., 2020; Benyahia et al., 2023; Shtai et al., 2024). Under our experimental conditions, statistically significant differences in Fv/Fm were evident only for plants grafted onto 1103 Paulsen and SO4 at the peak of drought stress and for the latter also after the recovery (Table 4). These findings are consistent with existing literature, where Fv/Fm ratios typically range from 0.6 to 0.8 under drought and heat stress (Palliotti et al., 2015; Ju et al., 2018; Bernardo et al., 2022). Indeed, our results indicate that photosystem II efficiency is impaired under severe drought conditions, especially in plants grafted onto the more sensitive rootstock SO4 and align with previously reported thresholds for photoinhibition under abiotic stress in grapevines (Tzortzakis et al., 2020; Benyahia et al., 2023). The relatively stable Fv/Fm values in M4-grafted plants, even under the stresses, further support its already mentioned drought resilience. Overall, chlorophyll fluorescence proved to be a sensitive early indicator of drought stress across genotypes. On the other hand, stable SPAD values in fully irrigated plants could suggest that Cu2+ application alone did not significantly impair chlorophyll biosynthesis or N status, at least at the concentration used for this work. However, under drought (DS) and combined stress (Cu+DS), SPAD values dropped significantly in both plants grafted onto 1103 Paulsen and SO4, particularly at the peak of water deficit (T2) and following the recovery phase (T3). The observed decline likely reflects stress-induced chlorophyll degradation or impaired N metabolism, consistent with previous reports of drought-associated decrease in leaf greenness (Monteoliva et al., 2021; Hu et al., 2023; Zhao et al., 2023). In contrast, vines grafted onto M4 rootstock maintained stable SPAD values across all treatments, with no significant variation even under severe stress, suggesting a greater capacity to preserve chlorophyll integrity and N assimilation under adverse conditions. From an agronomic perspective, these results clearly confirm the M4 potential as a resilient rootstock for sustaining vine performance in increasingly harsh growing conditions, like under drought and combined (Cu2+ toxicity and drought) stress.
Biometric data (Table 5) indicate that plants grafted onto M4 and 1103 Paulsen maintained the root biomass accumulation under drought and combined drought-Cu2+ stresses, suggesting robust below-ground resilience. This is consistent with previous studies highlighting the importance of deep, well-developed root systems in drought-tolerant rootstocks such as 1103 Paulsen (Zhang et al., 2016). However, 1103 Paulsen exhibited a marked decrease in leaf area and shoot biomass under drought stress, suggesting a resource allocation strategy that prioritizes root maintenance over canopy development. Such a trade-off is characteristic of isohydric behavior, where water-conserving responses, like stomatal closure and growth inhibition, limit shoot expansion to preserve hydraulic integrity (Schultz, 2003; Lovisolo et al., 2010). This strategy may help ensure survival under prolonged drought but could impair C assimilation and delay post-stress recovery (Pou et al., 2008). Therefore, evaluating rootstock performance under stress should consider not only the ability to sustain root function but also the capacity to support rapid shoot regrowth once favorable conditions return, that is particularly important for young vines establishing their canopy in challenging environments (Cuneo et al., 2021). Copper stress alone, on the other hand, reduced shoot biomass in vines grafted onto SO4 and 1103 Paulsen but had minimal effects on the M4-grafted ones. This suggests that SO4 rootstock may lack effective Cu2+ detoxification or metal exclusion mechanisms, leading to impaired shoot function (Marastoni et al., 2019c; Cesco et al., 2021). Interestingly, the ability of M4 to preserve both root and shoot biomass under stress, including combined Cu2+ toxicity and drought, might underscores its integrated stress tolerance. Previous research has associated the rootstock M4 with high hydraulic conductance, enhanced rewatering recovery, and better physiological stability under water deficit (Meggio et al., 2014; Bianchi et al., 2020). Altogether these elements highlight the agronomic relevance of selecting rootstocks capable of sustaining both root and shoot growth under abiotic stress, with the M4 one demonstrating integrated stress resilience and representing a promising candidate for vineyard replanting in drought- and Cu-affected soils, while 1103 Paulsen may limit canopy recovery due to its conservative growth strategy.
Although drought drove the largest shifts, Cu²+ imposed a root-centered constraint that became clear under co-stress with an extra drop in Ψstem and slower post-rewatering recovery of E, A and gs, strongest in SO4. This pattern fits oxidative damage and aquaporin inhibition lowering root hydraulic conductivity; at our dose, Cu²+ alone didn’t reduce SPAD. Ionomically, Cu²+ shifted nutrient balance: PCA highlighted K, P, Mn. M4 maintained higher root K–P–Mn with lower Cu accumulation but higher translocation (chelation/compartmentation), sustaining Fv/Fm, biomass, and a faster rebound; 1103P was intermediate; SO4 showed dispersed ionomics and weak K/P/Mn control, matching its largest photochemical and gas-exchange losses.
Ionomic analyses and PCA (Figure 2) revealed rootstock-specific responses to Cu2+ toxicity and water scarcity. In vines grafted onto 1103 Paulsen, Cu2+ stress had a more pronounced impact on root ionome, while drought stress dominated leaf responses. M4-grafted plants displayed clear separation between Cu, Cu+DS, and DS treatments in both roots and leaves, indicating distinct effects of combined stresses and a strong tolerance to water scarcity. Plants grafted onto SO4 rootstock showed specific and distinct responses to each stressor, with minimal interaction between Cu2+ and drought stress, although drought stress was more impactful at the leaf level. The PCA loadings indicated that PC1 in the root datasets was primarily driven by elements such as potassium (K), phosphorus (P), and manganese (Mn), which are crucial for osmoregulation, membrane integrity, and antioxidant activity under stress (Hasanuzzaman et al., 2018; Alejandro et al., 2020; Sardans and Peñuelas, 2021; Khan et al., 2023). In vines grafted onto M4, the clustering of Cu and Cu+DS samples away from Control and DS along PC1 suggests that Cu2+ availability strongly modulated the uptake of these key elements, likely due through adaptive regulation of root transporters (Marastoni et al., 2019b). Differently, the close grouping of 1103 Paulsen root samples under Cu and Cu+DS along PC2 may reflect Cu2+ accumulation rather than active redistribution. For rootstock SO4, the less distinct clustering and more dispersed samples might suggest a weaker nutrient homeostasis and a limited capacity to prioritize specific elemental adjustments under stress (Marastoni et al., 2019a). Therefore, rootstock choice proves crucial in shaping nutrient uptake and maintaining homeostasis under combined drought and Cu2+ stress, with M4 showing the most favorable response.
The physiological effects of Cu2+ stress were less pronounced than those of drought, as also demonstrated by the iWUE, although interesting trends were observed in mineral translocation patterns (Figure 1C). In vines exposed to Cu2+ stress, translocation of Cu2+ appeared limited, possibly due to protective mechanisms that limit its systemic diffusion or impair translocation pathways. Notably, M4 exhibited a higher Cu2+ translocation factor compared to other rootstocks, which could be attributed to its lower Cu2+ accumulation factor. This may reduce the toxic response in M4, enabling greater tolerance to Cu2+ exposure. Several physiological mechanisms might be responsible of these observations. Indeed, one key strategy could be represented by sequestration of complexed Cu2+ into vacuoles through tonoplast-localized transporters, thus reducing its cytoplasmic toxicity (Ejaz et al., 2023). Moreover, the exuded organic acids (e.g., citrate or malate) or phenolic compounds can chelate Cu2+ in the rhizosphere, decreasing its bioavailability (Marastoni et al., 2019c, 2019a). Beside these mechanisms, also a modulation of high-affinity Cu2+ transporters as well as other divalent cations’ transporters could contribute to a reduced Cu2+ uptake at root level (Marastoni et al., 2019b). These mechanisms, alone or in combination, could explain the lower Cu2+ accumulation factor observed in M4 (Kopittke and Menzies, 2006; Cesco et al., 2020). Additionally, the capacity of M4 rootstock to translocate Cu2+ without excessive accumulation in leaves might suggests an efficient chelation by ligands such as phytochelatins or glutathione, reducing free Cu2+ ions in the cytoplasm (Seregin and Kozhevnikova, 2023). In addition, Cu2+ toxicity has been reported to reduce the shoot content of macronutrients such as calcium (Ca), magnesium (Mg), K, and P, likely due to interference with ion uptake and translocation (Kopittke and Menzies, 2006). Similarly, drought stress can also impair nutrient dynamics by limiting mass flow and diffusion of ions in the soil, reducing root uptake efficiency, and altering translocation within the plant (Hsiao and Xu, 2000; Farooq et al., 2009). The combination of both stressors, as observed in our study, may thus create a complex physiological context where antagonistic or synergistic effects impact the ionomic balance in a unique manner, also depending on plants’ genotype (Mishra et al., 2024) highlighting the agronomic relevance of selecting rootstocks like M4 that ensure efficient Cu2+ detoxification and nutrient homeostasis under multifactorial stress conditions.
Among the vines grafted onto the three rootstocks considered, the M4-grafted ones demonstrated the most stable ionomic profile under stress conditions. Notably, M4 roots accumulated higher levels of K, P, and Mn under both Cu and Cu+DS treatments. These elements are critical in abiotic stress resilience: K regulates stomatal function and xylem hydraulic conductance (Brodersen et al., 2010), P supports ATP-driven transport processes and stress-related signaling (Khan et al., 2023), while Mn is a cofactor in antioxidant enzymes and lignin biosynthesis (Alejandro et al., 2020; Cesco et al., 2020). The higher root accumulation of these elements suggests that M4-grafted plants may actively prioritize root nutrient uptake and retention mechanisms to mitigate stress impact. In contrast, plants grafted onto SO4 exhibited pronounced ionomic imbalance under stress, particularly in roots, where the lack of coherent elemental clustering and greater dispersion in PCA plots suggest weaker ionomic regulation and a limited capacity to reprogram nutrient uptake under dual stress. Interestingly, vines grafted onto 1103 Paulsen presented an intermediate profile, thereby showing divergent responses across tissues. Most importantly, the analysis of the ionomic signature in root and leaves possibly highlighted a different hierarchy of drought and Cu2+ stress, underscoring the central role of rootstock genotype in determining nutrient plasticity and homeostatic regulation under combined abiotic stress.
Taken together, the physiological, biochemical, and ionomic responses observed in this study reveal distinct stress-response strategies among the vines grafted onto the three tested rootstocks. Those grafted onto M4 consistently exhibited the most balanced and integrated tolerance to both drought and Cu2+ toxicity, maintaining photosynthetic efficiency, biomass accumulation, and nutrient homeostasis across all treatments. This rootstock demonstrated strong physiological plasticity, likely supported by active regulation of mineral uptake and Cu2+ detoxification mechanisms (Figure 4). Although vines grafted onto 1103 Paulsen showed robust root maintenance and some drought resilience, the recovery of shoot functionality and mineral balance under stress was more limited, possibly reflecting a more conservative drought strategy prioritizing below-ground function (Figure 4). In contrast, the SO4-grafted vines were the most sensitive, with significant declines in both shoot and root biomass, reduced Fv/Fm ratios, and nutrient imbalances, particularly under combined Cu2+ toxicity and drought stress (Figure 4). Moreover, the rootstock-specific responses observed in this study have clear implications for vineyard management in areas increasingly affected by both drought stress and heavy metal accumulation.
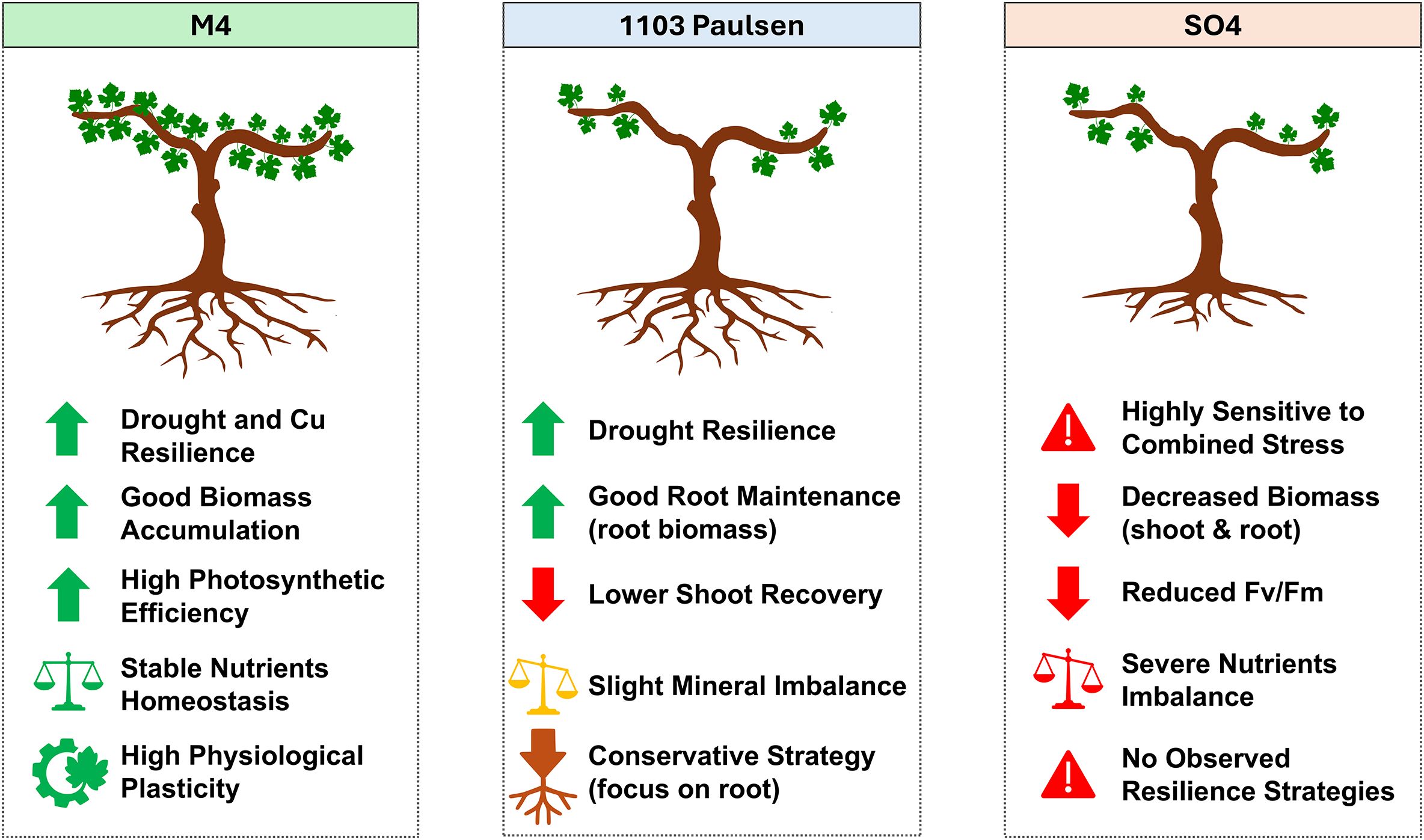
Figure 4. Schematic representation of the physiological response activated by the different rootstock towards the combined Cu and drought stress.
Specifically, M4 coupled stable Ψstem and gas exchange after rewatering with sustained SPAD and Fv/Fm under WSDS and Cu+DS and preserved both root and shoot biomass. Its ionomic signature, e.g.higher root K, P and Mn under Cu and Cu+DS with a lower Cu2+ accumulation but higher translocation factor, points to efficient compartmentation and nutrient prioritization that support osmotic balance, ATP supply and antioxidant capacity, explaining faster photosynthetic recovery. 1103 Paulsen behaved as drought-hardy but more Cu-sensitive, thereby showing elevated iWUE and maintained roots, yet reduced leaf area/shoot biomass and weaker K/P retention under Cu conditions. SO4 showed the largest drops in Fv/Fm, SPAD, photosynthesis and stomatal conductance and the poorest recovery under Cu+DS; its dispersed PCA pattern and lack of coherent K/P/Mn adjustments indicate limited ionomic homeostasis and detoxification capacity, amplifying Cu2+-related hydraulic and photochemical impairment. These trait-linked differences might be used to inform site-specific rootstock choice: M4 for Cu2+ contaminated, drought-prone vineyards where rapid recovery is needed; 1103P for severe-drought, lower-Cu sites with canopy-supporting management; and SO4 avoided where water deficits and legacy Cu co-occur.
The sensitivity manifested by SO4 mirrors the behavior of its parental V. riparia which confers limited drought tolerance and a more conservative, isohydric stomatal regulation that depresses gas exchange as soil water potential declines, whereas V. rupestris confers drought hardiness, complemented by V. berlandieri adaptation to dry, calcareous soils (Serra et al., 2014; Keller, 2020; Lucini et al., 2020; Jiao et al., 2023).
The superior performance of M4 under combined stress conditions suggests that it could be a suitable candidate for replanting programs in Cu2+ contaminated soils and/or limited water resource environments, especially in Mediterranean basin where these stressors often co-occur. Additionally, understanding Cu2+ uptake and translocation patterns under field conditions is critical for developing strategies to reduce metal accumulation in edible plant parts and limit environmental toxicity. These findings emphasize the importance of selecting rootstocks not only for their resistance to individual stressors but also for their integrated performance under multiple abiotic challenges, an approach that is becoming increasingly relevant under future climate change scenarios. However, it should be noted that this study was conducted under short-term pot conditions in a greenhouse environment, which may not fully reproduce vineyard field dynamics. Future research under field conditions will be essential to confirm the stress-response patterns reported here.
5 Conclusion
Overall, this study highlights contrasting stress responses among grapevine rootstocks under drought and Cu2+ toxicity, identifying M4 as the most resilient genotype. Its ability to maintain physiological functions, photosynthetic efficiency, and ionomic stability under both single and combined stress conditions underscores its strong potential for use in viticultural systems increasingly affected by abiotic pressures and multifactorial environmental stress. By contrast, SO4 displayed the most pronounced sensitivity. From an agronomic perspective, these findings reinforce the importance of selecting rootstocks capable of supporting water uptake, nutrient homeostasis, and shoot growth recovery, particularly in young vineyards or replanting context where altered soil fertility and limited water availability are critical issues. These findings underline the importance of rootstock selection as a strategic tool to enhance vineyard resilience by optimizing the soil-plant functional system under multiple scenarios. The observed genotype-specific responses, especially at root-soil interface, demonstrate how root traits and transporter regulation can influence whole-plant resilience under complex environment. Future research should focus on elucidating the molecular mechanisms underpinning M4’s stress tolerance and validating these traits under long-term field conditions. The deployment of tolerant rootstocks such as M4 may represent a sustainable strategy for managing grapevine performance and soil resource use in marginal or contaminated areas, particularly within Mediterranean viticulture systems.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
RF: Visualization, Writing – review & editing, Methodology, Formal analysis, Writing – original draft, Data curation. TC: Investigation, Writing – review & editing, Methodology. FB: Investigation, Formal analysis, Writing – review & editing, Data curation, Methodology. MZ: Writing – review & editing, Investigation, Methodology. SM: Writing – review & editing, Methodology, Investigation. AA: Methodology, Writing – review & editing, Investigation. CA: Writing – review & editing, Conceptualization, Supervision, Funding acquisition. SC: Writing – review & editing, Project administration, Funding acquisition, Supervision, Conceptualization. YP: Conceptualization, Funding acquisition, Writing – original draft, Project administration, Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was carried out within the Agritech National Research Center and received funding from the European Union Next-Generation EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) -MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4 -D.D. 1032 17/06/2022, CN00000022 CUP:I53C22000730007). In particular, our study represents an original paper related to: Spoke 4 Multifunctional and resilient agriculture and forestry systems for the mitigation of climate change risks (SC, YP, CA). The present work was also supported by the Italian Ministry of University and Research, PRIN Research Projects of National Interest 2022, “From farm to glass: use of a winemaking waste-derived compost as fertilizer for grapevine plants: elucidation of the interaction between vine molecular response and microbial community for wine production - EMERGE” Codice progetto: 2022SCAN2Z - CUP: I53D23004560008.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The AI was used to revise the English language throughout the whole manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2025.1682753/full#supplementary-material
References
Alejandro S., Höller S., Meier B., and Peiter E. (2020). Manganese in plants: from acquisition to subcellular allocation. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00300
Anderson P. K., Cunningham A. A., Patel N. G., Morales F. J., Epstein P. R., and Daszak P. (2004). Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 19, 535–544. doi: 10.1016/j.tree.2004.07.021
Arnold J. B. (2024). Ggthemes: Extra Themes, Scales and Geoms for “ggplot2”. doi: 10.32614/cran.package.ggthemes
Atkinson N. J., Jain R., and Urwin P. E. (2014). Combined Stresses in Plants, Physiological, Molecular, and Biochemical Aspects. 181–201. Switzerland: Springer International Publishing, doi: 10.1007/978-3-319-07899-1_9
Atkinson N. J. and Urwin P. E. (2012). The interaction of plant biotic and abiotic stresses: from genes to the field. J. Exp. Bot. 63, 3523–3543. doi: 10.1093/jxb/ers100
Baldi E., Miotto A., Ceretta C. A., Quartieri M., Sorrenti G., Brunetto G., et al. (2018). Soil-applied phosphorous is an effective tool to mitigate the toxicity of copper excess on grapevine grown in rhizobox. Sci. Hortic. Amsterdam 227, 102–111. doi: 10.1016/j.scienta.2017.09.010
Barl L., Benato B. D., Genze N., Grimm D. G., Gigl M., Dawid C., et al. (2025). The combined effect of decreased stomatal density and aperture increases water use efficiency in maize. Sci. Rep. 15, 13804. doi: 10.1038/s41598-025-94833-1
Benimeli C. S., Medina A., Navarro C. M., Medina R. B., Amoroso M. J., and Gómez M. I. (2010). Bioaccumulation of copper by zea mays: impact on root, shoot and leaf growth. Water Air Soil pollut. 210, 365–370. doi: 10.1007/s11270-009-0259-6
Benyahia F., Campos F. B., Abdelkader A. B., Basile B., Tagliavini M., Andreotti C., et al. (2023). Assessing grapevine water status by integrating vine transpiration, leaf gas exchanges, chlorophyll fluorescence and sap flow measurements. Agronomy 13, 464. doi: 10.3390/agronomy13020464
Bernardo S., Rodrigo M. J., Vives-Peris V., Gómez-Cadenas A., Zacarías L., MaChado N., et al. (2022). Fine-tuning of grapevine xanthophyll-cycle and energy dissipation under Mediterranean conditions by kaolin particle-film. Sci. Hortic. 291, 110584. doi: 10.1016/j.scienta.2021.110584
Bertolino L. T., Caine R. S., and Gray J. E. (2019). Impact of stomatal density and morphology on water-use efficiency in a changing world. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00225
Bianchi D., Caramanico L., Grossi D., Brancadoro L., and Lorenzis G. D. (2020). How do novel M-rootstock (Vitis spp.) genotypes cope with drought? Plants 9, 1385. doi: 10.3390/plants9101385
Bota J., Tomás M., Flexas J., Medrano H., and Escalona J. M. (2016). Differences among grapevine cultivars in their stomatal behavior and water use efficiency under progressive water stress. Agric. Water Manag. 164, 91–99. doi: 10.1016/j.agwat.2015.07.016
Briggs L. J. and Shantz H. L. (1913). The Water Requirements of Plants. I. Investigation in the Great Plains in 1910 and 1911. US. Dep., Agr. Bur. Plant Indr. Bull. 284. 49 p.
Brodersen C. R., McElrone A. J., Choat B., Matthews M. A., and Shackel K. A. (2010). The dynamics of embolism repair in xylem: in vivo visualizations using high-resolution computed tomography. Plant Physiol. 154, 1088–1095. doi: 10.1104/pp.110.162396
Brunetto G., Melo G.W.B. De, Terzano R., Buono D. D., Astolfi S., Tomasi N., et al. (2016). Copper accumulation in vineyard soils: Rhizosphere processes and agronomic practices to limit its toxicity. Chemosphere 162, 293–307. doi: 10.1016/j.chemosphere.2016.07.104
Busuioc G., Elekes C. C., Stihi C., Iordache S., and Ciulei S. C. (2011). The bioaccumulation and translocation of Fe, Zn, and Cu in species of mushrooms from Russula genus. Environ. Sci. pollut. Res. 18, 890–896. doi: 10.1007/s11356-011-0446-z
Cao H., Li X., and Dong X. (1998). Generation of broad-spectrum disease resistance by overexpression of an essential regulatory gene in systemic acquired resistance. Proc. Natl. Acad. Sci. 95, 6531–6536. doi: 10.1073/pnas.95.11.6531
Carbonell-Bejerano P., Diago M.-P., Martínez-Abaigar J., Martínez-Zapater J. M., Tardáguila J., and Núñez-Olivera E. (2014). Solar ultraviolet radiation is necessary to enhance grapevine fruit ripening transcriptional and phenolic responses. BMC Plant Biol. 14, 183. doi: 10.1186/1471-2229-14-183
Carvalho L. C., Coito J. L., Gonçalves E. F., Chaves M. M., and Amâncio S. (2016). Differential physiological response of the grapevine varieties Touriga Nacional and Trincadeira to combined heat, drought and light stresses. Plant Biol. 18, 101–111. doi: 10.1111/plb.12410
Cesco S., Ascoli D., Bailoni L., Bischetti G. B., Buzzini P., Cairoli M., et al. (2024). Smart management of emergencies in the agricultural, forestry, and animal production domain: Tackling evolving risks in the climate change era. Int. J. Disaster Risk Reduct. 114, 105015. doi: 10.1016/j.ijdrr.2024.105015
Cesco S., Pii Y., Borruso L., Orzes G., Lugli P., Mazzetto F., et al. (2021). A smart and sustainable future for viticulture is rooted in soil: how to face cu toxicity. Appl. Sci. 11, 907. doi: 10.3390/app11030907
Cesco S., Terzano R., Astolfi S., Brunetto G., Vigani G., and Pii Y. (2022). “Nutrient and elemental toxicieties,” in Soil Constraints on Crop Production (Newcastle, UK: Cambridge Scholar Publishing), 218–243.
Cesco S., Tolotti A., Nadalini S., Rizzi S., Valentinuzzi F., Mimmo T., et al. (2020). Plasmopara viticola infection affects mineral elements allocation and distribution in Vitis vinifera leaves. Sci. Rep. UK 10, 18759. doi: 10.1038/s41598-020-75990-x
Chaignon V., Bedin F., and Hinsinger P. (2002). Copper bioavailability and rhizosphere pH changes as affected by nitrogen supply for tomato and oilseed rape cropped on an acidic and a calcareous soil. Plant Soil 243, 219–228. doi: 10.1023/a:1019942924985
Chaves M. M., Zarrouk O., Francisco R., Costa J. M., Santos T., Regalado A. P., et al. (2010). Grapevine under deficit irrigation: hints from physiological and molecular data. Ann. Bot. 105, 661–676. doi: 10.1093/aob/mcq030
Chopin E. I. B., Marin B., Mkoungafoko R., Rigaux A., Hopgood M. J., Delannoy E., et al. (2008). Factors affecting distribution and mobility of trace elements (Cu, Pb, Zn) in a perennial grapevine (Vitis vinifera L.) in the Champagne region of France. Environ. pollut. 156, 1092–1098. doi: 10.1016/j.envpol.2008.04.015
Cookson S. J. and Ollat N. (2013). Grafting with rootstocks induces extensive transcriptional re-programming in the shoot apical meristem of grapevine. BMC Plant Biol. 13, 147. doi: 10.1186/1471-2229-13-147
Cuneo I. F., Barrios‐Masias F., Knipfer T., Uretsky J., Reyes C., Lenain P., et al. (2021). Differences in grapevine rootstock sensitivity and recovery from drought are linked to fine root cortical lacunae and root tip function. New Phytol. 229, 272–283. doi: 10.1111/nph.16542
Deluc L. G., Quilici D. R., Decendit A., Grimplet J., Wheatley M. D., Schlauch K. A., et al. (2009). Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genomics 10, 212. doi: 10.1186/1471-2164-10-212
de Mendiburu F. (2023). Agricolae: Statistical Procedures for Agricultural Research (R package version 1.3-7). Comprehensive R Archive Network (CRAN). doi: 10.32614/CRAN.package.agricolae
Díaz-Barradas M. C., Gallego-Fernández J. B., and Zunzunegui M. (2020). Plant response to water stress of native and non-native Oenothera drummondii populations. Plant Physiol. Biochem. 154, 219–228. doi: 10.1016/j.plaphy.2020.06.001
Ejaz U., Khan S. M., Khalid N., Ahmad Z., Jehangir S., Rizvi Z. F., et al. (2023). Detoxifying the heavy metals: a multipronged study of tolerance strategies against heavy metals toxicity in plants. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1154571
Farooq M., Wahid A., Kobayashi N., Fujita D., and Basra S. M. A. (2009). Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev. 29, 185–212. doi: 10.1051/agro:2008021
Fatemi H., Zaghdoud C., Nortes P. A., Carvajal M., and Martínez-Ballesta M.D. C. (2020). Differential Aquaporin Response to Distinct Effects of Two Zn Concentrations after Foliar Application in Pak Choi (Brassica rapa L.) Plants. Agronomy 10, 450. doi: 10.3390/agronomy10030450
Feil S. B., Pii Y., Valentinuzzi F., Tiziani R., Mimmo T., and Cesco S. (2020). Copper toxicity affects phosphorus uptake mechanisms at molecular and physiological levels in Cucumis sativus plants. Plant Physiol. Bioch. 157, 138–147. doi: 10.1016/j.plaphy.2020.10.023
Feil S. B., Zuluaga M. Y. A., Cesco S., and Pii Y. (2023). Copper toxicity compromises root acquisition of nitrate in the high affinity range. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1034425
Fisher M. C., Henk D. A., Briggs C. J., Brownstein J. S., Madoff L. C., McCraw S. L., et al. (2012). Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194. doi: 10.1038/nature10947
Flexas J., Bota J., Galmés J., Medrano H., and Ribas‐Carbó M. (2006). Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiol. Plant 127, 343–352. doi: 10.1111/j.1399-3054.2006.00621.x
Flexas J., Ribas-Carbò M., Diaz-Espejo A., Galmés J., and Medrano H. (2008). Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ. 31, 602–621. doi: 10.1111/j.1365-3040.2007.01757.x
Fregoni (2013). Viticoltura di Qualita: Trattato dell’eccellenza da terroir (Milano: Tecniche nuove).
Fregoni M., Scienza A., and Miravalle R. (1978). Evaluation précoce de la résistance des porte-greffes à la secheresse. 2. In Génétique et amélioration de la vigne: Comptes rendus du IIᵉ Symposium International sur l’Amélioration de la Vigne (Bordeaux, 12–16 septembre 1977). Paris: INRA, pp. 287–296.
Galbignani M., Merli M. C., Magnanini E., Bernizzoni F., Talaverano I., Gatti M., et al. (2016). Gas exchange and water-use efficiency of cv. Sangiovese grafted to rootstocks of varying water-deficit tolerance. Irrig. Sci. 34, 105–116. doi: 10.1007/s00271-016-0490-z
Galet P. (2000). General Viticulture. 622–656. Berkeley: University of California Press, doi: 10.1525/9780520353183-026
Gambetta G. A., Herrera J. C., Dayer S., Feng Q., Hochberg U., and Castellarin S. D. (2020). The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance Physiological Regulation of Evapotranspiration View project Vineyard ecosystem vulnerability to pathogens and drought in the context of climate change View project. Article J. Exp. Bot. 71(16), 4658–4676. doi: 10.1093/jxb/eraa245
Gessler C., Pertot I., and Perazzolli M. (2011). Plasmopara viticola: a review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 50, 3–44.
Giorgi E. G., Sadras V. O., Keller M., and Peña J. P. (2019). Interactive effects of high temperature and water deficit on Malbec grapevines. Aust. J. Grape Wine Res. 25, 345–356. doi: 10.1111/ajgw.12398
Guan T. X., He H. B., Zhang X. D., and Bai Z. (2011). Cu fractions, mobility and bioavailability in soil-wheat system after Cu-enriched livestock manure applications. Chemosphere 82, 215–222. doi: 10.1016/j.chemosphere.2010.10.018
Hasanuzzaman M., Bhuyan M. H. M. B., Nahar K., Hossain M., Mahmud J. A., Hossen M., et al. (2018). Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 8, 31. doi: 10.3390/agronomy8030031
Hatfield J. L. and Dold C. (2019). Water-use efficiency: advances and challenges in a changing climate. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00103
Horikoshi M. and Tang Y. (2018). ggfortify: Data Visualization Tools for Statistical Analysis Results. Available online at: https://CRAN.R-project.org/package=ggfortify (Accessed July 10, 2025).
Hsiao T. C. and Xu L. (2000). Sensitivity of growth of roots versus leaves to water stress: biophysical analysis and relation to water transport. J. Exp. Bot. 51, 1595–1616. doi: 10.1093/jexbot/51.350.1595
Hu F., Zhang Y., and Guo J. (2023). Effects of drought stress on photosynthetic physiological characteristics, leaf microstructure, and related gene expression of yellow horn. Plant Signal. Behav. 18, 2215025. doi: 10.1080/15592324.2023.2215025
Jiao S., Zeng F., Huang Y., Zhang L., Mao J., and Chen B. (2023). Physiological, biochemical and molecular responses associated with drought tolerance in grafted grapevine. BMC Plant Biol. 23, 110. doi: 10.1186/s12870-023-04109-x
Jogaiah S., Ramteke S. D., Sharma J., and Upadhyay A. K. (2014). Moisture and salinity stress induced changes in biochemical constituents and water relations of different grape rootstock cultivars. Int. J. Agron. 2014, 1–8. doi: 10.1155/2014/789087
Ju Y., Min Z., Zhang Y., Zhang K., Liu M., and Fang Y. (2021). Transcriptome profiling provide new insights into the molecular mechanism of grapevine response to heat, drought, and combined stress. Sci. Hortic. 286, 110076. doi: 10.1016/j.scienta.2021.110076
Ju Y., Yue X., Zhao X., Zhao H., and Fang Y. (2018). Physiological, micro-morphological and metabolomic analysis of grapevine (Vitis vinifera L.) leaf of plants under water stress. Plant Physiol. Biochem. 130, 501–510. doi: 10.1016/j.plaphy.2018.07.036
Juang K.-W., Lee Y.-I., Lai H.-Y., Wang C.-H., and Chen B.-C. (2012). Copper accumulation, translocation, and toxic effects in grapevine cuttings. Environ. Sci. pollut. Res. 19, 1315–1322. doi: 10.1007/s11356-011-0657-3
Kandeler F., Kampichler C., and Horak O. (1996). Influence of heavy metals on the functional diversity of soil microbial communities. Biol. Fert. Soils 23, 299–306. doi: 10.1007/bf00335958
Keiblinger K. M., Schneider M., Gorfer M., Paumann M., Deltedesco E., Berger H., et al. (2018). Assessment of Cu applications in two contrasting soils—effects on soil microbial activity and the fungal community structure. Ecotoxicology 27, 217–233. doi: 10.1007/s10646-017-1888-y
Keller M. (2020). The science of grapevines. xi–xii. Academic Press, doi: 10.1016/b978-0-12-419987-3.00014-5
Khan M. S. (2011). The role of dreb transcription factors in abiotic stress tolerance of plants. Biotechnol. Biotec. Eq. 25, 2433–2442. doi: 10.5504/bbeq.2011.0072
Khan F., Siddique A. B., Shabala S., Zhou M., and Zhao C. (2023). Phosphorus plays key roles in regulating plants’ Physiological responses to abiotic stresses. Plants 12, 2861. doi: 10.3390/plants12152861
Kissoudis C., Wiel C.V. De, Visser R. G. F., and Linden G.V. D. (2014). Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00207
Komárek M., Čadková E., Chrastný V., Bordas F., and Bollinger J.-C. (2010). Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 36, 138–151. doi: 10.1016/j.envint.2009.10.005
Kopittke P. M. and Menzies N. W. (2006). Effect of cu toxicity on growth of cowpea (Vigna unguiculata). Plant Soil 279, 287–296. doi: 10.1007/s11104-005-1578-z
Kosakivska I. V., Babenko L. M., Romanenko K. O., Korotka I. Y., and Potters G. (2021). Molecular mechanisms of plant adaptive responses to heavy metals stress. Cell Biol. Int. 45, 258–272. doi: 10.1002/cbin.11503
Krattinger S. G., Lagudah E. S., Spielmeyer W., Singh R. P., Huerta-Espino J., McFadden H., et al. (2009). A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323, 1360–1363. doi: 10.1126/science.1166453
Lai H.-Y., Juang K.-W., and Chen B.-C. (2010). Copper concentrations in grapevines and vineyard soils in central Taiwan. Soil Sci. Plant Nutr. 56, 601–606. doi: 10.1111/j.1747-0765.2010.00494.x
Leeuwen C.V., Trégoat O., Choné X., Bois B., Pernet D., and Gaudillère J.-P. (2009). Vine water status is a key factor in grape ripening and vintage quality for red Bordeaux wine. How can it be assessed for vineyard management purposes? Oeno One 43, 121–134. doi: 10.20870/oeno-one.2009.43.3.798
Lehnart R., Michel H., Löhnertz O., and Linsenmeier A. (2008). Root dynamics and pattern of ‘Riesling’on 5C rootstock using minirhizotrons. Vitis 47, 197–200. doi: 10.5073/vitis.2008.47.197-200
Liu L., Gregan S., Winefield C., and Jordan B. (2015). UV‐B‐induced gene expression. Plant Cell Environ. 38, 905–919. doi: 10.1111/pce.12349
Lovisolo C., Perrone I., Carra A., Ferrandino A., Flexas J., Medrano H., et al. (2010). Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: a physiological and molecular update. Funct. Plant Biol. 37, 98–116. doi: 10.1071/fp09191
Lucini L., Miras-Moreno B., Busconi M., Marocco A., Gatti M., and Poni S. (2020). Molecular basis of rootstock-related tolerance to water deficit in Vitis vinifera L. cv. Sangiovese: A physiological and metabolomic combined approach. Plant Sci. 299, 110600. doi: 10.1016/j.plantsci.2020.110600
Marastoni L., Sandri M., Pii Y., Valentinuzzi F., Brunetto G., Cesco S., et al. (2019a). Synergism and antagonisms between nutrients induced by copper toxicity in grapevine rootstocks: Monocropping vs. intercropping. Chemosphere 214, 563–578. doi: 10.1016/j.chemosphere.2018.09.127
Marastoni L., Sandri M., Pii Y., Valentinuzzi F., Cesco S., and Mimmo T. (2019b). Morphological root responses and molecular regulation of cation transporters are differently affected by copper toxicity and cropping system depending on the grapevine rootstock genotype. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00946
Marastoni L., Tauber P., Pii Y., Valentinuzzi F., Astolfi S., Simoni A., et al. (2019c). The potential of two different Avena sativa L. cultivars to alleviate Cu toxicity. Ecotoxicol. Environ. Saf. 182, 109430. doi: 10.1016/j.ecoenv.2019.109430
Mashilo J., Odindo A. O., Shimelis H. A., Musenge P., Tesfay S. Z., and Magwaza L. S. (2018). Photosynthetic response of bottle gourd [Lagenaria siceraria (Molina) Standl.] to drought stress: Relationship between cucurbitacins accumulation and drought tolerance. Sci. Hortic. 231, 133–143. doi: 10.1016/j.scienta.2017.12.027
Masson-Delmotte V., Zhai P., Pörtner H.-O., Roberts D., Skea J., and Shukla P. R. (2022). Global Warming of 1.5°C: IPCC special report on impacts of global warming of 1.5°C above pre-industrial levels in context of strengthening response to climate change, sustainable development, and efforts to eradicate poverty. An IPCC Special Report on the impacts of global warming of, Cambridge, UK: Cambridge University Press 43–50.
Medrano H., Escalona J. M., Cifre J., Bota J., and Flexas J. (2003). A ten-year study on the physiology of two Spanish grapevine cultivars under field conditions: effects of water availability from leaf photosynthesis to grape yield and quality. Funct. Plant Biol. 30, 607–619. doi: 10.1071/fp02110
Meggio F., Prinsi B., Negri A. S., Lorenzo G. S. D., Lucchini G., Pitacco A., et al. (2014). Biochemical and physiological responses of two grapevine rootstock genotypes to drought and salt treatments. Aust. J. Grape Wine R 20, 310–323. doi: 10.1111/ajgw.12071
Merrington G., Rogers S. L., and Zwieten L. V. (2002). The potential impact of long-term copper fungicide usage on soil microbial biomass and microbial activity in an avocado orchard. Soil Res. 40, 749–759. doi: 10.1071/sr01084
Mike F., Davis T. L., and authors, ggplot2 (2025). ggpattern: “ggplot2” Pattern Geoms. doi: 10.32614/cran.package.ggpattern
Mishra G., Mohapatra S. K., and Rout G. R. (2024). Plant membrane transporters function under abiotic stresses: a review. Planta 260, 125. doi: 10.1007/s00425-024-04548-2
Monteoliva M. I., Guzzo M. C., and Posada G. A. (2021). Breeding for drought tolerance by monitoring chlorophyll content. Gene Technol. 10, 10.35248.
Murchie E. H. and Lawson T. (2013). Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J. Exp. Bot. 64, 3983–3998. doi: 10.1093/jxb/ert208
Nan Z. and Cheng G. (2001). Copper and Zinc Uptake by Spring Wheat (Triticum aestivum L.) and Corn (Zea mays L.) Grown in Baiyin Region. Bull. Environ. Contam. Toxicol. 67, 83–90. doi: 10.1007/s001280094
Nimbolkar P. K., Chander S., Awachare C., Reddy Y. T. N., and Hussain F. (2016). Role of Rootstocks in Fruit Production-A Review Crop regulation and source sink relation studies in Annona View project Organic farming practices for sustainable horticulture Vol 3 View project Role of Rootstocks in Fruit Production-A Review. J. Agric. Eng. Food Technol. 3, 183–188. Available online at: http://www.krishisanskriti.org/Publication.html (Accessed July 10, 2025).
OIV (2022). STATE OF THE WORLD VINE AND WINE SECTOR 2021- April 2022. Available online at: https://www.oiv.int/sites/default/files/documents/eng-state-of-the-world-vine-and-wine-sector-april-2022-v6_0.pdf (Accessed April 11, 2023).
OIV (2024). Available online at: https://www.oiv.int/sites/default/files/2024-04/OIV_STATE_OF_THE_WORLD_VINE_AND_WINE_SECTOR_IN_2023.pdf (Accessed July 10, 2025).
Ollat N., Bordenave L., Tandonnet J. P., Boursiquot J. M., and Marguerit E. (2016). Grapevine rootstocks: origins and perspectives. Acta Horticulturae, 1136, 11–22. doi: 10.17660/ActaHortic.2016.1136.2
Osmond C. B., Björkman O., and Anderson D. J. (1980). Physiological processes in plant ecology, toward a synthesis with atriplex. Ecol. Stud., 1–11. doi: 10.1007/978-3-642-67637-6_1
Ouyang W., Struik P. C., Yin X., and Yang J. (2017). Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 68, 5191–5205. doi: 10.1093/jxb/erx314
Palliotti A., Tombesi S., Frioni T., Silvestroni O., Lanari V., D’Onofrio C., et al. (2015). Physiological parameters and protective energy dissipation mechanisms expressed in the leaves of two Vitis vinifera L. genotypes under multiple summer stresses. J. Plant Physiol. 185, 84–92. doi: 10.1016/j.jplph.2015.07.007
Palliotti A., Tombesi S., Silvestroni O., Lanari V., Gatti M., and Poni S. (2014). Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 178, 43–54. doi: 10.1016/j.scienta.2014.07.039
Pedersen T. L. (2024). patchwork: the composer of plots (R package version 1.3.0). CRAN. doi: 10.32614/cran.package.patchwork
Pham N. T. H. (2024). Distribution and bioavailability of copper in the soil of a young steep vineyard applying different extraction procedures and pseudo-total digestion. Water Air Soil pollut. 235, 326. doi: 10.1007/s11270-024-07166-6
Pietrzak U. and McPhail D. C. (2004). Copper accumulation, distribution and fractionation in vineyard soils of Victoria, Australia. Geoderma 122, 151–166. doi: 10.1016/j.geoderma.2004.01.005
Poni S., Gatti M., Palliotti A., Dai Z., Duchêne E., Truong T.-T., et al. (2018). Grapevine quality: A multiple choice issue. Sci. Hortic. 234, 445–462. doi: 10.1016/j.scienta.2017.12.035
Pou A., Flexas J., Alsina M., Del M., Bota J., Carambula C., et al. (2008). Adjustments of water use efficiency by stomatal regulation during drought and recovery in the drought‐adapted Vitis hybrid Richter‐110 (V. berlandieri × V. rupestris). Physiol. Plant 134, 313–323. doi: 10.1111/j.1399-3054.2008.01138.x
Pourtchev P. (2003). To the problem of depth of root system penetration of grapevine. Soil Science, Agrochemistry and Ecology (Sofia), 2, 47–52.
Prinsi B., Simeoni F., Galbiati M., Meggio F., Tonelli C., Scienza A., et al. (2021). Grapevine rootstocks differently affect physiological and molecular responses of the scion under water deficit condition. Agronomy 11, 289. doi: 10.3390/agronomy11020289
Priyanka B., Sekhar K., Reddy V. D., and Rao K. V. (2010). Expression of pigeonpea hybrid‐proline‐rich protein encoding gene (CcHyPRP) in yeast and Arabidopsis affords multiple abiotic stress tolerance. Plant Biotechnol. J. 8, 76–87. doi: 10.1111/j.1467-7652.2009.00467.x
Qin Y., Tian Y., and Liu X. (2015). A wheat salinity-induced WRKY transcription factor TaWRKY93 confers multiple abiotic stress tolerance in Arabidopsis thaliana. Biochem. Bioph. Res. Co 464, 428–433. doi: 10.1016/j.bbrc.2015.06.128
Rahemi A., Peterson J. C. D., and Lund K. T. (2022). Grape Rootstocks and Related Species. 117–180. Springer Cham, doi: 10.1007/978-3-030-99407-5
Ramegowda V., Basu S., Krishnan A., and Pereira A. (2014). Rice Growt under Drought Kinase is Required for Drought Tolerance and Grain Yield under Normal and Drought Stress Conditions. Plant Physiol. 166, 1634–1645. doi: 10.1104/pp.114.248203
Ramegowda V., Gill U. S., Sivalingam P. N., Gupta A., Gupta C., Govind G., et al. (2017). GBF3 transcription factor imparts drought tolerance in Arabidopsis thaliana. Sci. Rep. UK 7, 9148. doi: 10.1038/s41598-017-09542-1
Ramegowda V. and Senthil-Kumar M. (2015). The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 176, 47–54. doi: 10.1016/j.jplph.2014.11.008
Ramegowda V., Senthil-Kumar M., Nataraja K. N., Reddy M. K., Mysore K. S., and Udayakumar M. (2012). Expression of a finger millet transcription factor, ecNAC1, in tobacco confers abiotic stress-tolerance. PloS One 7, e40397. doi: 10.1371/journal.pone.0040397
Rienth M., Vigneron N., Walker R. P., Castellarin S. D., Sweetman C., Burbidge C. A., et al. (2021). Modifications of grapevine berry composition induced by main viral and fungal pathogens in a climate change scenario. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.717223
Ristaino J. B. and Records A. (2020). Emerging Plant Diseases and Global Food Security. i–iii. Minnesota, U.S.A.: The American Phytopathological Society, doi: 10.1094/9780890546383.fm
Rizhsky L., Liang H., and Mittler R. (2002). The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 130, 1143–1151. doi: 10.1104/pp.006858
Rizhsky L., Liang H., Shuman J., Shulaev V., Davletova S., and Mittler R. (2004). When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 134, 1683–1696. doi: 10.1104/pp.103.033431
Rossdeutsch L., Schreiner R. P., Skinkis P. A., and Deluc L. (2021). Nitrate uptake and transport properties of two grapevine rootstocks with varying vigor. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.608813
Sardans J. and Peñuelas J. (2021). Potassium control of plant functions: ecological and agricultural implications. Plants 10, 419. doi: 10.3390/plants10020419
Schultz H. R. (2003). Differences in hydraulic architecture account for near‐isohydric and anisohydric behaviour of two field‐grown Vitis vinifera L. cultivars during drought. Plant Cell Environ. 26, 1393–1405. doi: 10.1046/j.1365-3040.2003.01064.x
Seregin I. V. and Kozhevnikova A. D. (2023). Phytochelatins: sulfur-containing metal(loid)-chelating ligands in plants. Int. J. Mol. Sci. 24, 2430. doi: 10.3390/ijms24032430
Serra I., Strever A., Myburgh P. A., and Deloire A. (2014). Review: the interaction between rootstocks and cultivars (Vitis vinifera L.) to enhance drought tolerance in grapevine. Aust. J. Grape Wine Res. 20, 1–14. doi: 10.1111/ajgw.12054
Shtai W., Asensio D., Kadison A. E., Schwarz M., Raifer B., Andreotti C., et al. (2024). Soil water availability modulates the response of grapevine leaf gas exchange and PSII traits to a simulated heat wave. Plant Soil 501, 537–554. doi: 10.1007/s11104-024-06536-7
Straffelini E., Wang W., and Tarolli P. (2024). European vineyards and their cultural landscapes exposed to record drought and heat. Agric. Syst. 219, 104034. doi: 10.1016/j.agsy.2024.104034
Su L., Fang L., Zhu Z., Zhang L., Sun X., Wang Y., et al. (2020). The transcription factor VaNAC17 from grapevine (Vitis amurensis) enhances drought tolerance by modulating jasmonic acid biosynthesis in transgenic Arabidopsis. Plant Cell Rep. 39, 621–634. doi: 10.1007/s00299-020-02519-x
Tashakorizadeh M., Golkar P., Vahabi M. R., and Ghorbanpour M. (2023). Physiological and biochemical mechanisms of grain yield loss in fumitory (Fumaria parviflora Lam.) exposed to copper and drought stress. Sci. Rep. 13, 17934. doi: 10.1038/s41598-023-45103-5
Team, R. C. (2025). R: A Language and Environment for Statistical Computing. Available online at: https://www.R-project.org/ (Accessed July 10, 2025).
Toselli M., Baldi E., Marcolini G., Malaguti D., Quartieri M., Sorrenti G., et al. (2009). Response of potted grapevines to increasing soil copper concentration. Aust. J. Grape Wine R 15, 85–92. doi: 10.1111/j.1755-0238.2008.00040.x
Trentin E., Ferreira P. A. A., Ricachenevsky F. K., Morsch L., Belles S. W., Hindersmann J., et al. (2024). Identifying grapevine rootstocks tolerant to copper excess. Horticulturae 10, 883. doi: 10.3390/horticulturae10080883
Trentin E., Ferreira P. A. A., Ricachenevsky F. K., Morsch L., Hindersmann J., Tarouco C. P., et al. (2022). The tolerance of grapevine rootstocks to copper excess and to the use of calcium and phosphorus to mitigate its phytotoxicity. Environ. Sci. pollut. Res. 29, 82844–82854. doi: 10.1007/s11356-022-21515-0
Tzortzakis N., Chrysargyris A., and Aziz A. (2020). Adaptive response of a native Mediterranean grapevine cultivar upon short-term exposure to drought and heat stress in the context of climate change. Agronomy 10, 249. doi: 10.3390/agronomy10020249
Vršič S., Gumzej M., Lešnik M., Perko A., and Pulko B. (2023). Patterns of copper bioaccumulation and translocation in grapevine grafts depending on rootstocks. Agriculture 13, 1768. doi: 10.3390/agriculture13091768
Warren R. F., Henk A., Mowery P., Holub E., and Innes R. W. (1998). A mutation within the leucine-rich repeat domain of the Arabidopsis disease resistance gene RPS5 partially suppresses multiple bacterial and downy mildew resistance genes. Plant Cell 10, 1439–1452. doi: 10.1105/tpc.10.9.1439
Wen X.-P., Pang X.-M., Matsuda N., Kita M., Inoue H., Hao Y.-J., et al. (2008). Over-expression of the apple spermidine synthase gene in pear confers multiple abiotic stress tolerance by altering polyamine titers. Transgenic Res. 17, 251–263. doi: 10.1007/s11248-007-9098-7
Wickham H. (2011). The split-apply-combine strategy for data analysis. J. Stat. Softw. 40, 1–29. Available online at: https://www.jstatsoft.org/v40/i01/ (Accessed July 10, 2025).
Wickham H. (2016). ggplot2: Elegant Graphics for Data Analysis. Available online at: https://ggplot2.tidyverse.org (Accessed July 10, 2025).
Wickham H. (2023). stringr: Simple, Consistent Wrappers for Common String Operations. doi: 10.32614/cran.package.stringr
Wickham H., François R., Henry L., Müller K., and Vaughan D. (2023). dplyr: A Grammar of Data Manipulation. doi: 10.32614/cran.package.dplyr
Wickham H., Vaughan D., and Girlich M. (2024). tidyr: Tidy Messy Data. doi: 10.32614/cran.package.tidyr
Zhang J., Jia W., Yang J., and Ismail A. M. (2006). Role of ABA in integrating plant responses to drought and salt stresses. Field Crop Res. 97, 111–119. doi: 10.1016/j.fcr.2005.08.018
Zhang L., Marguerit E., Rossdeutsch L., Ollat N., and Gambetta G. A. (2016). The influence of grapevine rootstocks on scion growth and drought resistance. Theor. Exp. Plant Phys. 28, 143–157. doi: 10.1007/s40626-016-0070-x
Keywords: grapevine rootstocks, copper toxicity, drought stress, ionomics, abiotic stress tolerance
Citation: Fattorini R, Caretta TO, Benyahia F, Zuluaga MYA, Monterisi S, Agostini A, Andreotti C, Cesco S and Pii Y (2025) Double trouble belowground: grapevine rootstocks face drought and copper toxicity. Front. Agron. 7:1682753. doi: 10.3389/fagro.2025.1682753
Received: 09 August 2025; Accepted: 09 October 2025;
Published: 28 October 2025.
Edited by:
Anket Sharma, Texas Tech University, United StatesReviewed by:
Chao Song, Ben-Gurion University of the Negev, IsraelShabnam Heidarpour, Urmia University, Iran
Copyright © 2025 Fattorini, Caretta, Benyahia, Zuluaga, Monterisi, Agostini, Andreotti, Cesco and Pii. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. Fattorini, Um9iZXJ0by5mYXR0b3JpbmlAc3R1ZGVudC51bmliei5pdA==; Y. Pii, eW91cnkucGlpQHVuaWJ6Lml0
 R. Fattorini
R. Fattorini T. O. Caretta
T. O. Caretta F. Benyahia
F. Benyahia M. Y. A. Zuluaga
M. Y. A. Zuluaga S. Monterisi
S. Monterisi A. Agostini
A. Agostini C. Andreotti
C. Andreotti S. Cesco
S. Cesco Y. Pii
Y. Pii