- 1Institute for Immunological Research, University of Cartagena, Cartagena, Colombia
- 2Inmunotek, S.L., Madrid, Spain
There are more than 3,000 mosquito species. Aedes aegypti, Ae. communis, and C. quinquefasciatus are, among others, three of the most important mosquito allergen sources in the tropics, western, and industrialized countries. Several individuals are sensitized to mosquito allergens, but the epidemiological data indicates that the frequency of sensitization markedly differs depending on the geographical region. Additionally, the geographical localization of mosquito species has been affected by global warming and some mosquito species have invaded areas where they were not previously found, at the same time as other species have been displaced. This phenomenon has repercussions in the pathogenesis and the accuracy of the diagnosis of mosquito allergy. Allergic individuals are sensitized to mosquito allergens from two origins: saliva and body allergens. Exposure to saliva allergens occurs during mosquito bite and induces cutaneous allergic reactions. Experimental and clinical data suggest that body allergens mediate different manifestations of allergic reactions such as asthma and rhinitis. The most studied mosquito species is Ae. aegypti, from which four and five allergens of the saliva and body, respectively, have been reported. Many characterized allergens are homologs to arthropod-derived allergens, which cause strong cross-reactivity at the humoral and cellular level. The generalized use of whole body Ae. communis or C. quinquefasciatus extracts complicates the diagnosis of mosquito allergy because they have low concentration of saliva allergens and may result in poor diagnosis of the affected population when other species are the primary sensitizer. This review article discusses the current knowledge about mosquito allergy, allergens, cross-reactivity, and proposals of component resolved approaches based on mixtures of purified recombinant allergens to replace saliva-based or whole-body extracts, in order to perform an accurate diagnosis of allergy induced by mosquito allergen exposure.
Introduction
Mosquitoes are insects that belong to the family Culicidae, which includes more than 3,000 species distributed worldwide. Some species have the ability to adapt to different climatic conditions. Four species, Culex pipiens, Culex quinquefasciatus, Aedes aegypti, and the genera Anopheles have virtually populated all the planet and induce allergic reactions in atopic individuals (1).
Mosquito allergy occurs worldwide and is common in tropical and subtropical regions where mosquitoes are abundant, since the climatic conditions at these latitudes favor their life cycle and proliferation (2, 3), and increase the chances of interaction with humans. Early efforts to identify mosquito allergens focused mainly on the saliva because it was believed that biting was the unique mechanism of exposure and sensitization. However, some evidence suggests that proteins from the insect's body may remain in the environment as aerosols or in the dust after they die and induce and allergic responses when they are inhaled by atopic individuals, similarly as house dust mites (HDMs) do.
Mosquito allergy seems to be highly prevalent and variable, although there is not enough data to support such affirmation. Diagnosis criteria is different, dependent of the study design or clinicians team. In some studies, the diagnosis of mosquito allergy was defined by bite reactions or in severe cases, anaphylaxis and systemic symptoms after a witnessed mosquito bite. Diagnosis was also made in some cases by SPT to mosquito allergen extract or positive serum to mosquito saliva IgE (4). In Monterrey City, Mexico, a cross-sectional study reported that 82% of patients admitted to the allergy service had specific IgE to mosquitoes, although only 2.5% of them showed positive skin reactions (5). In a study performed in India, 47% of the population with asthma and/or allergic rhinitis were sensitized to mosquito allergens, as determined by skin prick tests (SPT), serum specific IgE antibodies and bronchial provocation tests with whole mosquito body extracts (6). In Guangzhou, China, a study showed that in a cohort of 7,047 allergic patients, 4% of them had detectable specific IgE levels to mosquito allergens, ranging from ≥0.35 to <3.5 IU/ml in most of the patients, with peaks of sensitization at age between 15 and 18 years (7).
About 20 IgE binding proteins are contained in whole body extracts or the saliva from Ae. aegypti, but only 10 have been recognized as allergens in the databases (8, 9). Allergens from the saliva induce cutaneous reactions or a systemic response, that rarely occur (10–13). Body allergens could be contained in emanations and mosquito detritus and, when inhaled, induce variable immune responses (14, 15). A small number of mosquito allergens have been obtained and characterized. More research remains to be performed to establish the complete allergenic spectrum of Ae. aegypti and other species.
Studies on the cross-reactivity among different mosquito species, and with other sources of allergens, are scarce. However, an important degree of cross-reactivity between mosquitoes and other arthropods is reported (9, 16). We have found that sera obtained from a cohort of patients residing in the Caribbean island of Martinique suffering from allergic respiratory symptoms after the inhalation of HDM allergens, recognized allergens from Ae. aegypti (16). These findings suggest that Ae. aegypti contains allergens that induce a Th2 response and subsequent allergic symptoms, or could modulate the response originally established against arthropods.
High occurrence of mosquitoes at patient's homes seems to reflect a higher prevalence of sensitization and may explain a more severe cutaneous reaction during SPTs. In a study performed on a south American population sensitized to cockroaches and mosquitoes, Sanchez et al. (17) found that the size of the wheal generated during SPTs with mosquito extracts is positively correlated with the density of these insects at their homes and directly related with allergy to HDMs. This finding is similar in other tropical countries where high occurrence of mosquitoes and HDMs results in high prevalence of allergic sensitization (18). The observations open questions about the magnitude of the clinical impact produced by sensitization to mosquitoes and postulate the need for developing diagnostic tests to properly identify individuals with mosquito allergy (19). In this context, the comparison of mosquito prevalence and the frequency of sensitization to their allergens in tropical and other regions around the world should be further addressed.
Mosquito Species: Geographical Distribution and Their Relationship With Allergies
Mosquitoes are arthropods that belong to the class Insecta, order Diptera and members of a family of the nematocerid flies Culicidae. Two subfamilies are widely accepted within the family Culicidae: Anophelinae and Culicinae. Some authors have proposed a third subfamily, Toxorhynchitinae, which includes only one genus (1). Nearly 400 and 2,600 species are included in Anophelinae and Culicinae, respectively. The females of many species of mosquitoes require blood-feeding to reproduce, for which they bite the skin, inject saliva, and then suck blood from vessels (20). Lysozymes, antibacterial glucosidases, anticoagulants, antiplatelet aggregating factors, and vasodilators are molecules contained in mosquito saliva (21–23). Some of these substances induce allergic skin reactions (10–13). We have hypothesized that non-salivary allergens might be contained in emanations and detritus of mosquitoes, and when inhaled, induce respiratory allergic responses (9).
The mosquito species distributed worldwide easily adapt to different environmental conditions helping them to distribute in nearly any latitude (1). Distribution of mosquitoes is generalized to three main geographical locations: Cosmopolitan, Old and New world. In all of these categories, there are species associated with allergic responses. Cosmopolitan: Anopheles (An.) stephensi, An. minimus, An. sinensis, Ochlerotatus (Oc.) triseriatus, Oc. hendersoni, Culex (Cx.) quinquefasciatus, Cx. tritaeniorhynchus, Cx. pipiens, Cx. pipiens pallens, and Cx. tarsalis. Old world (Africa, Asia, and Europe): Aedes (Ae.) aegypti, Ae. vexans, Ae. communis, Ae. togoi, Ae. albopictus, and Ae. triseriatus. New world (America): Culiseta inornate (Table 1).
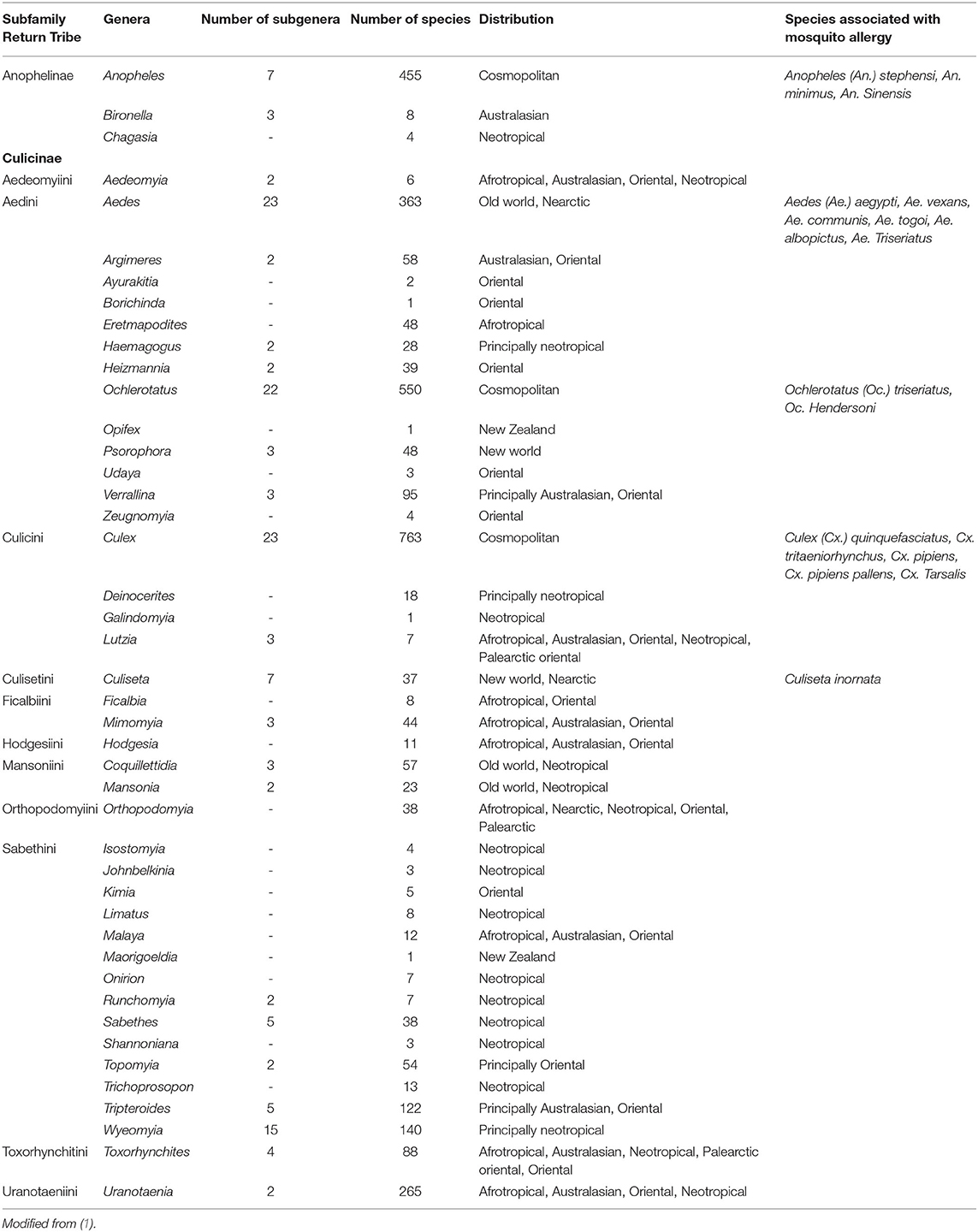
Table 1. Taxonomical classification and distribution of the main mosquito species associated with mosquito allergy.
Although several environmental factors affect the geographical distribution of mosquitoes, the main ones are temperature, humidity, rains, and solar radiation. As a result of global warming, the distribution of some mosquito species has already changed, and they found ways to move toward other geographical areas. This behavior apply for mosquitoes and other insects as more tropical species have invaded temperate habitats, and temperate species have disappeared when their natural habitats have become warmer (24, 25). Anthropic intervention such as urbanization and transportation also plays an important role (26). For instance, Ae. aegypti originated in the forest areas of sub-Saharan Africa as a “wild,” black-pigmented insect biting species Ae. aegypti formosus. Facilitated by human transportation and environmental conditions a new sub-species, Aedes aegypti (Ae. aegypti), evolved (27, 28) and is present in North, Central and South America, Africa, Asia and Oceania (29). It is very abundant throughout tropical and subtropical regions of America, Africa, and Asia, as well as in the Indian Ocean islands, and northern Australia (30).
Aedes spp.
Ae. aegypti and Ae. albopictus are the most important species within this genus. Other Aedes species such as Ae. vexans (31), are tightly associated to allergic sensitization to mosquito bites. Ae. aegypti and Ae. vexans usually share their geographical distribution and are present almost worldwide. Ae. aegypti is arguably the most studied mosquito species as an allergenic source. Four salivary and six non-salivary allergens from this species have been deposited in the WHO/IUIS Allergen Nomenclature Sub-Committee (http://www.allergen.org). Ae. aegypti is rapidly expanding its geographical distribution and is highly concentrated in the tropics and subtropics (29) and have developed a preference for biting humans (32, 33), probably by an evolutionary over-expression of odorant receptors (34). Frequency of sensitization to Ae. aegypti varies depending on the region and the nature of the preparation used for diagnosis. Saliva-based preparations are probably more reliable to identify patients allergic to mosquito bites but might not be useful when sensitization occurs to non-salivary allergens. In a cohort of 34 allergic patients residing in the tropical island of Martinique, a prevalence of 65% of IgE reactivity to whole body Ae. aegypti extract was found (21). In Monterrey, Mexico, the frequency of IgE sensitization to Ae. aegypti was reported in 17.6% (5), similar to mosquito sensitization in a ~18 years old allergic population from Guangzhou, China (7). Ae. albopictus has become a new threat to human health as it is getting spread to new tropical, sub-tropical and temperate areas (18, 35) where it is an epidemic driver of certain diseases (36). Only two allergens from Ae. albopictus, Aed al 2, and Aed al 3, are in the allergen database and reports of frequency of sensitization is scarce or non-existing.
Culex quinquefasciatus
Together with Aedes, species from Culex genera are above all other species as allergen sources. C. quinquefasciatus is a peridomestic insect that lives relatively farer from humans than Ae. aegypti. Native from west Africa, it feeds from birds, mammalians, and humans (37) and has spread out worldwide by commercial sailing, to warmer and temperate tropical and sub-tropical regions (38). At least 8 IgE reactive proteins have been detected in the saliva and 15 in whole body extracts from C. quinquefasciatus (15, 31) but only two allergens from this species, Cul q 2 and Cul q 3, have been reported in the databases (19). Epidemiologic data about allergy to C. quinquefasciatus is scarce. Seven out of 14 (50%) individuals from United States, Canada, Germany, Japan, and Switzerland who experienced systemic allergic reactions to mosquito bites were sensitized to this species (10). The high number of potential allergens found in whole body extracts of C. quinquefasciatus indicates that the role that this species may have in mosquito bite allergy or other clinical manifestations of allergy deserves to be studied.
An increase in the frequency of allergic sensitization to mosquitoes is expected to occur as a result of the environmental changes that have led to a global spreading of these insects. Temperature, relative humidity, and precipitations are the main factors that affect mosquito development, reproduction, and mortality. Temperature and relative humidity positively affect some mosquito species (39). High precipitations increase their population by maintaining their breeding (40). Allergies induced by mosquitoes and vector-borne diseases will become bigger threats for public health. The study of the pathophysiology and worsening of mosquito allergy will help to properly counteract the potential complications that will arise as a result of the increasing exposure to them.
Characterized Mosquito Allergens
Mosquito allergens are divided in two main groups: (a) salivary allergens (10) and (b) body-derived allergens (8). Exposure to allergens from either group results in different clinical manifestations of mosquito allergy. Salivary allergens are mainly related to cutaneous symptoms caused by mosquito bites. We hypothesized that body allergens induce respiratory allergic symptoms after inhalation of mosquito detritus (9, 16).
Saliva Allergens
Identification of salivary allergens is a difficult task and usually requires the extraction of saliva from the live mosquito or postmortem excision of the salivary gland which is used as the raw material to prepare allergenic extracts (41). Both methods are experimentally difficult (13, 41, 42) and result in low protein content. As an alternative, whole-body mosquito extracts could be used but salivary allergens are poorly represented in such preparations.
About 16 IgE-reactive bands (16-95 kDa) were detected by immunoblotting when saliva and salivary gland extracts from 10 different worldwide distributed mosquito species were analyzed (31). Sera from mosquito allergic individuals have specific IgE against 35.5, 32.5, and 22.5 kDa proteins present in the saliva of C. quinquefasciatus (42), and 14 proteins in salivary glands of Aedes togoi, Culex tritaeniorhynchus, and C. pipiens pallens with molecular weights ranging from 23 to 93 kDa (13). Some of these proteins induced an IgG1 response when used as recombinant molecules to immunize mice.
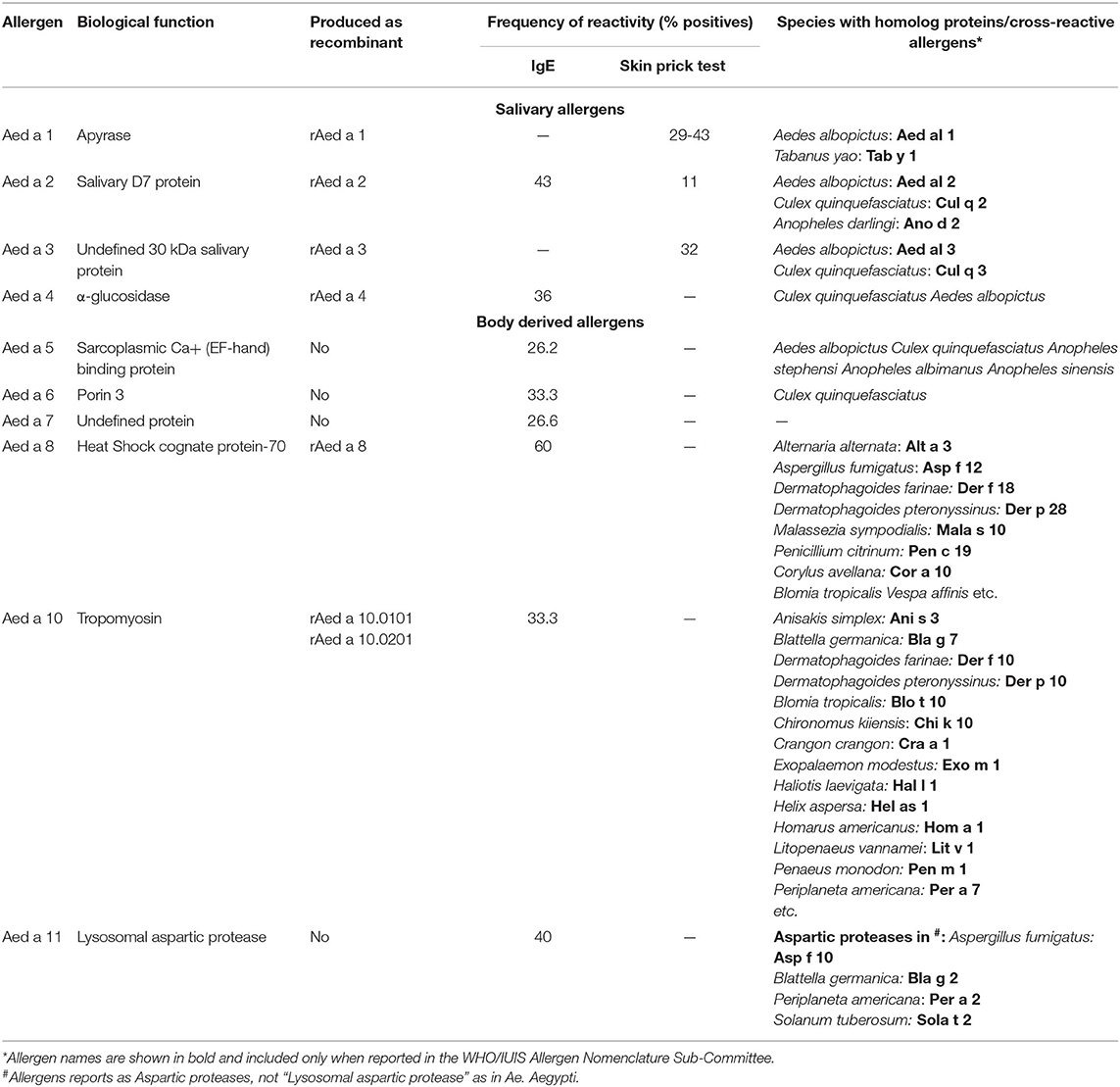
Some salivary allergens have been further characterized comprising groups 1-4 (Table 2). Usually, they needed to be produced as recombinant proteins because obtaining the natural version is a difficult task.
Group 1 Mosquito Allergens
The saliva apyrase (ATP di-phosphohydrolase) Aed a 1, from Ae. aegypti, is the only allergen from group 1 that has been accepted by the WHO/IUIS Allergen Nomenclature Sub-Committee. It corresponds to a 68 kDa enzyme with homology with the 5′-nucleotidase enzyme family (43) and interferes with platelet aggregation in human blood by hydrolyzing ADP and ATP released by the platelets and other cells (44). About 29% of Canadian individuals sensitized to mosquito bites had positive SPT to rAed a 1 (11). However, when tested in an allergic population from the tropics, living in urban and sub-urban areas, the IgE frequency of reactivity increased to 60% (19). B cell epitopes seem to be contained in the 150-562 amino acid region and react with the IgE and IgG from allergic individuals (45). Homolog molecules or apyrase enzymatic activity have been detected in the saliva from Ochlerotatus triseriatus, Ochlerotatus hendersoni (46), and Ae. albopictus (31, 47).
Group 2 Mosquito Allergens
It corresponds to allergens that belong to the family of proteins called D7, which are required by mosquitoes for feeding and reproduction, and are released together with the saliva during biting. They have structural homology with the protein THP12 from Tenebrio molitor, which is part of the family of pheromone-binding proteins and odorants and help transporting hydrophobic molecules (48). Allergens within this group have been reported in the WHO/IUIS allergen database from Ae. aegypti (49) and in Ae. albopictus, An. dirus, and C. quinquefasciatus (19). This group could also be present in other Aedes species and O. triseriatus (31).
Aed a 2, from Ae. aegypti, is a multi-domain protein with a N-terminal and a C-terminal domain that binds leukotrienes and biogenic amines released as a mechanism of protection in individuals that are getting bitten (50). In a group of 15 mosquito bite allergic individuals residing in the tropics the frequency of reactivity was 100%, studied by immunoblotting using salivary gland extracts (19). However, in a North American population seems to be 11% (31). Recombinant Aed a 2 expressed in insect cells infected with baculovirus retains the IgE-binding capacity and allergenicity, and immunogenicity as seen in immunized mice (51), suggesting that it can be used as a replacement of the natural protein.
Group 3 and 4 Mosquito Allergens
The WHO/IUIS allergen database reports allergens in groups 3 and 4 from the mosquito species Ae. aegypti (52, 53), Ae. albopictus and C. quinquefasciatus (19). Aed a 3 and Aed al 3 in Ae. aegypti and Ae. albopictus, respectively, are 30 kDa molecules. In C. quinquefasciatus, Cul q 3 is a 35 kDa molecule. Aed a 3 from Ae. aegypti shows collagen binding capacity and prevents its interaction with platelet glycoprotein IV, integrin α2β1 and von Willebrand factor (52). When used together with Aed a 1 and Aed a 2, about 60% of an allergic population could be accurately diagnosed (53). 40% of individuals from a tropical region react against Aed a 3. Aed a 4 is a 67 kDa α-glucosidase. About 36-46% of mosquito allergic individuals react against this allergen (19, 54).
Body-Derived Allergens
Allergic individuals have IgE against non-salivary body-derived mosquito proteins. For instance, in the subtropical city of Yazd, Iran, 33% of individuals with allergic rhinitis had positive skin test to whole body mosquito extracts (55). Similar observations were reported in India where 47% of the population with asthma and/or rhinitis were sensitized to mosquito allergens (6) and in Martinique with 65% of sensitization (16). Such observations strongly suggest that exposure to mosquito allergens occurs through the skin when the mosquito is biting, but also through the airways, leading to different manifestations of the allergic response such as asthma and rhinitis.
An important question to address is whether body-derived mosquito allergens are found in the dust or mattresses from the allergic individuals' residing places and in quantities enough to induce allergic symptoms. Although we don't know the answer yet, several studies have made important advances in this matter. To begin, extracts prepared from airborne particles collected in the homes of mosquito allergic individuals block the specific IgE reactivity of sera from such individuals to whole-body C. quinquefasciatus extract (14), which allows to hypothesize that mosquito allergens are present in house dust and retains antibody binding capacity. A weakness of this hypothesis is that it is based on immunoassays, and it cannot exclude that arthropod-derived allergens might be the molecules responsible of inhibiting the IgE binding capacity. It is already demonstrated that they are present in the dust from places where allergic individuals reside (56, 57). The DNA-based study of arthropod diversity in homes via high-throughput marker gene sequencing of 700 home's dust revealed that mosquito (Aedes spp) together with carpet beetle, dust mite and Aphid (Aphis spp) are common in home's dust (58). Quantitative analyses are necessary to establish whether the amounts of mosquito allergens in such samples are high enough to represent a potential primary sensitizer and inducer of allergic symptoms.
Different allergen composition has been observed depending on the sample and techniques used to detect IgE binding molecules. There are at least 11 IgE-binding proteins in whole-body Ae. aegypti extract, as detected by immunoblotting (16). Five of those proteins cross-react with allergens from HDM, cockroach and shrimp. Whole-body extracts are prepared by extraction with PBS and non-PBS soluble allergens could be missing. The analysis of the Ae. aegypti allergenome using proteomic tools revealed a set of 25 IgE-binding molecules corresponding to 10 different proteins and some of their variants or isoforms (8). Four of them were deposited in the WHO/IUIS Allergen Nomenclature Sub-Committee as Aed a 5.0101 (sarcoplasmic Ca+ (EF-hand) binding protein), Aed a 6.0101 (Porin 3), Aed a 7.0101 (undefined protein), Aed a 8.0101 (HSC-70), and Aed a 11.0101 (lysosomal aspartic protease). Notice that tropomyosin Aed a 10 was also identified. Only the HSC-70, Aed a 8 and tropomyosin Aed a 10 have been further studied (Table 2).
Group 8 Mosquito Allergens
Aed a 8 is the representative allergen of this group. Heat shock cognate protein-70 belongs to the highly conserved Heat shock protein-70 family (59), chaperones that help in protein folding maintaining their correct biological function under stress conditions (60). Homolog allergens are present in Dermatophagoides farinae (61) and cockroach (62). Aed a 8 reacted with the IgE in 9 out of 15 allergic individuals (60%) (8). Similar frequency of reactivity is reported for Der f 8 from D. farinae (61).
We obtained recombinant Aed a 8 as a 74 kDa by expression in Escherichia coli. Recombinant Aed a 8 inhibited 43% of the IgE reactivity of a mixture of human serum samples to the whole body extract of Ae. aegypti, indicating that the wild type Aed a 8 is present in such extract, and retains immunogenicity and the capacity to activate basophils. Six out of 14 sera from allergic individuals reacted to the recombinant and, when used to immunize mice, it induced specific antibody that also reacted against the natural counterpart, indicating that it retained biological activity (63).
Obtaining mosquito allergens is a difficult task, especially for proteins that are expressed in low levels, such as HSC-70 molecules. Using purified and biologically active recombinant allergens will help to overcome this problem and we strongly suggest using rAed a 8 for further analysis of mosquito allergy and study the clinical relevance of group 8 allergens in the physiopathology of mosquito allergy.
Group 10 Mosquito Allergens
Tropomyosin is a well-described allergen from diverse sources. Some of the allergenic sources are shrimps, lobsters, prawns, crabs, fish, mollusks, and snails. This allergen is also common in HDMs, helminths, cockroaches, and insects, and partially explains the existence of the cross-reactivity between them (64, 65). Ae. aegypti has 11 genes that encode different variants, or isoforms of tropomyosin. Four of them were detected, characterized and purified (66). Two tropomyosin isoforms, Aed a 10.0101 and Aed a 10.0201 are the most abundant and 33% of a population sensitized to Ae. aegypti had IgE against a mixture of them (66), suggesting that they are relevant molecules involved in IgE sensitization against Ae. aegypti tropomyosins.
The IgE frequency of sensitization to tropomyosin is variable, but usually low. Tropomyosin from shrimp species Penaus aztecus, Pen a 1, binds up to 75% of shrimp-specific IgE antibodies (67, 68). In Africa and South America, the prevalence of sensitization to mite tropomyosin is ~50% (69, 70), higher than that in developed countries (71, 72). The relatively high frequency of sensitization to tropomyosin in African and South American areas indicates that cross-reactivity with mosquito tropomyosin must be considered.
IgE Cross-Reactivity Mediated by Mosquito Allergens
The apparent geospatial differences of immune and allergic response to mosquito allergens have implications in the cross-reactivity phenomena. In regions where cutaneous allergic reactions to mosquito bites is frequent, saliva-derived allergens are the main cross-reactive molecules (15, 31, 73, 74). In contrast, in tropical areas, body allergens seem to be the main proteins associated to cross-reactivity with arthropods (8, 16) (Figure 1). These differences have clinical implications since preparations for diagnostic and immunotherapy based on salivary allergens would make sense to consider in western and industrialized countries. The case is different for tropical and subtropical countries where species specific and cross-reactive body-derived allergens might be the best targets to focus on. It is also possible that in these regions, body-based preparations could be a more effective tool to cope with allergies caused by mosquitoes and other arthropods.
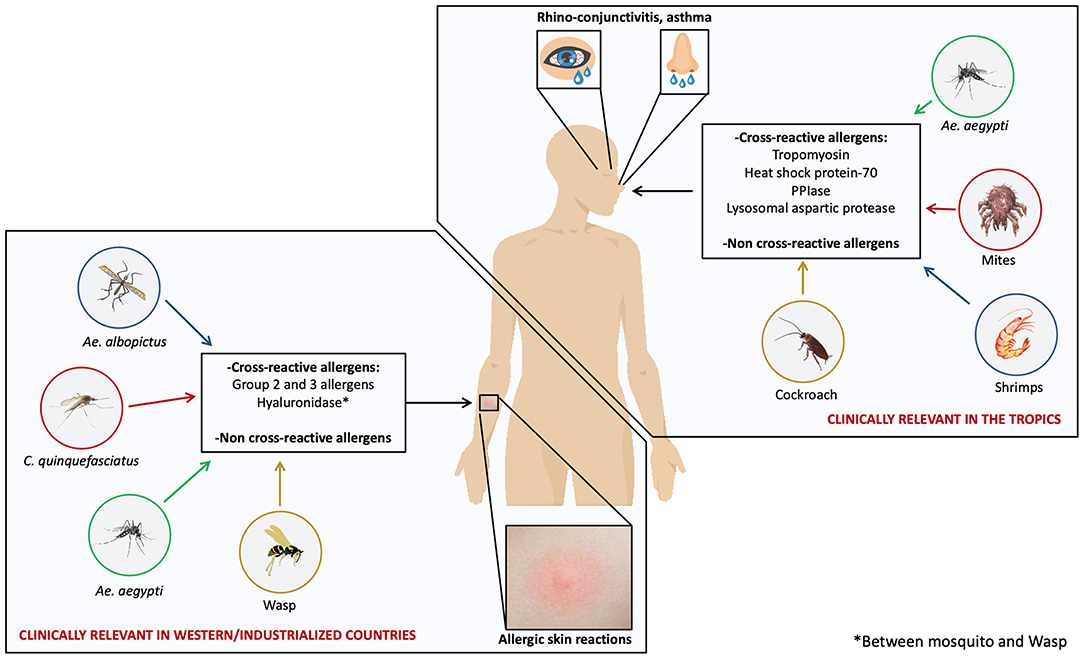
Figure 1. Unique and cross-reactive mosquito allergens induce different manifestations of mosquito allergy. Mosquito species such as Ae. aegypti, Ae. albopictus, and C. quinquefasciatus are distributed worldwide; however, some studies suggest that two kinds of allergic reactions induced by mosquito allergens are clinically relevant depending on the geospatial location: allergic skin reactions induced by salivary allergens and respiratory reactions induced by body-derived allergens. Skin reactions are common in western and industrialized countries and respiratory reactions are relevant in tropical areas. Cross-reactivity between mosquito species and with several species within Arthropods may play an important clinical role.
Cross-Reactivity Mediated by Saliva Allergens
Studies on animals indicate that sensitization to a mosquito salivary allergen induce antibodies that react against allergens from different mosquito species. Sera from rabbits immunized with rAed a 1 cross-react with extracts from Ae. vexans and Ae. albopictus (31). The finding of homologs of the apyrase Aed a 1 allergen in Ae. aegypti, O. triseriatus, and O. hendersoni indicates that this protein is conserved among several mosquito species and explains the above-mentioned observations. Similarly, immunization with rAed a 2 induces anti-sera that react with extracts of C. quinquefasciatus, O. triseriatus (46) and several species of Aedes (12, 31). It is plausible to assume that saliva proteins other than group 1 and 2 allergens are involved in the cross-reactivity among mosquito species.
Several studies show a similar phenomenon in humans. Individuals from Shanghai, China, have IgE-reactivity to Ae. vexans allergens, although this species is not indigenous in such area (31, 73). Contrarily, Ae. vexans is a major pest in Winnipeg, Manitoba (Canada) where individuals allergic to mosquitoes co-react with allergens from other mosquito species not found in Manitoba (73). The sera from individuals allergic to mosquito bites in Thailand react with several broad range molecular weight proteins present in the extracts from the C. quinquefasciatus, Ae. aegypti, Ae. albopictus, and An. minimus, common mosquitoes (15).
Saliva derived allergens from mosquitoes can also cross-react with proteins from wasps. The so-called “wasp/ mosquito syndrome,” involves an IgE cross-reactive 44-kDa hyaluronidase which is present in both insects (74). Cross-reactivity between salivary allergens occurs in western/industrialized countries as well as in tropical regions. However, it is necessary to evaluate the clinical implications that this may have. In countries like Canada where cross-reactivity among Ae. vexans and several other mosquito species is common (31, 73) and mosquito bite allergies are frequent, it is important to determine whether such cross-reactivity has implications in the physiopathology of allergic responses. However, in other regions like Brazil, cross-reactivity between endemic mosquito species also occur (48), but it involves antibodies from allergic and non-allergic individuals. This suggests that in such regions, broad sensitization to mosquito occurs but does not mean that it leads to a clinical manifestation of allergy and cross-reactivity might not be important.
Cross-Reactivity Mediated by Body-Derived Allergens
There are homolog proteins distributed in several species from the filum Arthropoda, including mosquitoes, that induce allergic reactions. It is widely accepted that in the tropics HDMs, cockroaches and shrimp are some of the most common sources of allergens (75).
in vitro studies and SPTs showing that individuals sensitized to one or several arthropod species had concomitant immunoreactivity against mosquito proteins or extracts led to the hypothesis that cross-reactivity involving allergens from mosquitoes and other sources occurs (76, 77) (Figure 1).
In our mentioned study with allergic individuals from Martinique (16), we identified four novel cross-reactive allergens in Ae. aegypti allergen extract and concluded that, these molecules could influence the manifestation of allergy to environmental allergens in the tropics. ELISA experiments showed that in this population D. pteronyssinus, Litopenaeus vannamei, Blomia tropicalis, and Periplaneta americana extracts inhibited the IgE reactivity to Ae. aegypti extract in 75.4-96.6%, and that the main allergen involved was tropomyosin (16), a well-known cross-reactive molecule within arthropods. Besides tropomyosin, other components are involved, especially a 17.9 kDa PPIase that has 81.1% identity in the amino acid sequence with Der f 29 allergen from D. farinae.
Tropomyosin is the main cross-reactivity allergen in Ae. aegypti, which is expressed as several variants and isoforms. Two of the more abundant are Aed a 10.0101 and Aed a 10.0201, which cross-react with rDer p 10 from D. pteronyssinus (78). In the Caribbean, 33% of a group of sera from allergic individuals had specific IgE to these two tropomyosins (9); a number that is evidently higher than the frequency of sensitization to tropomyosins from other sources typically observed in developed countries.
We demonstrated that cross-reactivity of Ae. aegypti tropomyosins leads to effector cell activation. We used basophils in the PBMCs from non-allergic donors where the membrane bound IgE was stripped away and re-sensitization with sera from allergic patients sensitized to the tropomyosin Der p 10. Challenging such cells with rDer p 10 or recombinant Ae. aegypti tropomyosins, induced dose dependent activation. In addition, splenocytes from mosquito tropomyosin immunized mice proliferate upon stimulus with rDer p 10 (78).
Diagnosis
Whole body extracts prepared from Ae. communis, C. pipiens or C. quinquefasciatus are currently the main preparations used for the purpose of mosquito allergy diagnose, although their use has some disadvantages. To begin, the accuracy of the diagnosis is compromised when the primary sensitizer is a species different to the one used to prepare the allergenic extract. Different geographical regions have different local mosquito species and having the appropriate mosquito extract that works for a specific population, is mandatory to achieve an appropriate diagnosis (19), but sometimes is not possible. For instance, Ae. communis is endemic in northern temperate zones but poorly present in tropical countries where Ae. aegypti and C. quinquefasciatus are abundant (18). The use of Ae. communis extract results in poor diagnosis of mosquito allergic individuals from the tropics (19, 79). In contrast to the case in Cuba, where mosquito allergy is frequently related to C. quinquefasciatus bites and using a high dose of standardize extract of this mosquito species in SPTs resulted in positive results that correlated in 100% of the patients (80). Second, whole body extracts may have poor representation of saliva allergens (15, 81), which could jeopardize the accuracy of such preparations to detect allergic individuals who are sensitized to the saliva (79). Wang et al. found that the diagnosis by the detection of specific IgE using salivary extracts provide higher specificity and sensitivity than using whole body extracts (82). Alternatively, using saliva-based preparations or salivary gland extracts, may provide 80% positivity result (4). However, this is not cost effective and requires complicated procedures that result in low recovery of allergens. Using whole-body extracts appear more attractive when the affected population is sensitized to non-salivary allergens.
Using recombinant allergens is especially convenient to circumvent the above mentioned problems as they are obtained in high amounts and purity. Additionally, they have the intrinsic advantages when used as a replacement of natural extracts, as they can be easily standardized, subjected to proper quality control analysis and allows component-resolved immunotherapy since it help to identify the set of allergens to which each individual is sensitized (83–85). Only a few recombinant mosquito allergens have been obtained and analyzed. Aed a 1, Aed a 2, and Aed a 3 have been well-characterized, obtained as recombinants and are an interesting tool to replace Ae. aegypti saliva since a mixture of the three allergens allows identifying 60% of the Ae. aegypti population allergic to mosquito bites (53). Evidently, clinically relevant mosquito allergens must be chosen to allow a better identification of allergic individuals (86). Obtaining recombinant saliva allergens from other species is also necessary to allow future development of more accurate diagnostic tests.
The situation is similar for individuals sensitized to non-salivary allergens. Very few body allergens have been detected and only two recombinant allergens from Ae. aegypti, rAed a 8, and rAed a 10 (9), have been produced and tested. We made some advances and proposed an alternative to replace whole body Ae. aegypti extracts for a mixture of three allergens, Aed a 6, Aed a 8, and Aed a 10, which may be enough to identify more than 80% of the allergic individuals (8). More efforts must be done to broadly identify and characterize saliva and body mosquito allergens from different species, obtain relevant allergens as recombinant proteins and confirm their potential as diagnostic tools in clinical studies with well-characterized populations.
Concluding Remarks
The concept of mosquito allergy should be re-evaluated as more allergens have been identified, revealing that they belong to the saliva and the insect's body. Mosquito body allergens seem to induce different types of allergic responses, such as asthma, allergic rhinitis, and probably conjunctivitis. The mechanisms of exposure to these allergens are not established yet but, may occur by inhalation of mosquito detritus suspended in the air. These observations have several implications and open many questionings: (1) Is there a relationship between the exposure to mosquito allergens and the onset of respiratory allergic reactions? (2) Do mosquito allergens induce manifestation of allergic responses different to the cutaneous or airway related symptoms? (3) Could mosquito allergens contained in the environment induce immunological responses?
The current knowledge has many unresolved issues. Only a few allergens have been identified and characterized, and they belong to a few species, mainly Ae. aegypti and C. quinquefasciatus. The diversity of mosquito species is quiet variable depending on the geographical region and it has continuously changed with global warming. Additionally, an important degree of cross-reactivity occurs among mosquitoes and several arthropod species. The effects that this phenomenon has on the pathophysiology of allergy diseases is still unknown.
The quest for answers to these questions will help to propose a more accurate definition of mosquito allergy and may pave the way to find solutions to the scientific and clinical challenges that will subsequently arise. More efforts must be done to identify and characterize saliva and mosquito body allergens from different species, obtain relevant allergens as recombinant proteins and confirm their potential as diagnostic tools in clinical studies with well-characterized populations.
Author Contributions
LP conceived the idea. LP and JC contributed equally to the preparation of the draft and final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the University of Cartagena (Grant 005-2019).
Conflict of Interest
JC was employed by the company Inmunotek, S.L.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Harbach R. The Culicidae (Diptera): a review of taxonomy, classification and phylogeny. Zootaxa. (2007) 47:591–688. doi: 10.11646/zootaxa.1668.1.28
2. Montoya-Lerma J, Solarte YA, Giraldo-Calderon GI, Quinones ML, Ruiz-Lopez F, Wilkerson RC, et al. Malaria vector species in Colombia: a review. Mem Ins Oswaldo Cruz. (2011) 106(Suppl 1):223–38. doi: 10.1590/S0074-02762011000900028
3. Morrone JJ. Biogeographic areas and transition zones of Latin America and the Caribbean islands based on panbiogeographic and cladistic analyses of the entomofauna. Ann Rev Entomol. (2006) 51:467–94. doi: 10.1146/annurev.ento.50.071803.130447
4. Kulthanan K, Wongkamchai S, Triwongwaranat D. Mosquito allergy: clinical features and natural course. J Dermatol. (2010) 37:1025–31. doi: 10.1111/j.1346-8138.2010.00958.x
5. Gonzalez Diaz SN, Cruz AA, Sedo Mejia GA, Rojas Lozano AA, Valenzuela EA, Vidaurri Ojeda AC. [Prevalence of reactions secundary to mosquito bites Aedes aegypti at en el Regional Center of Allergy and Clinical Immunology, University Hospital, de Monterrey, Nuevo Leon]. Rev Alerg Mex. (2010) 57:37–43.
6. Agarwal MK, Chaudhry S, Jhamb S, Gaur SN, Chauhan UP, Agarwal HC. Etiologic significance of mosquito (Anopheles stephensi) in respiratory allergy in India. Ann Allergy. (1991) 67:598–602.
7. Sun B-Q, Zheng Pei-yan, Zhang X-W, Huang H-M, Chen D-H, Zeng G-Q. Prevalence of allergen sensitization among patients with allergic diseases in Guangzhou, Southern China: a four-year observational study. Multidiscip Respir Med. (2014) 9:2. doi: 10.1186/2049-6958-9-2
8. Cantillo JF, Puerta L, Puchalska P, Lafosse-Marin S, Subiza JL, Fernandez-Caldas E. Allergenome characterization of the mosquito Aedes aegypti. Allergy. (2017) 72:1499–509. doi: 10.1111/all.13150
9. Cantillo JF, Puerta L, Lafosse-Marin S, Subiza JL, Caraballo L, Fernandez-Caldas E. Identification and characterization of IgE-binding tropomyosins in Aedes aegypti. Int Arch Allergy Immunol. (2016) 170:46–56. doi: 10.1159/000447298
10. Peng Z, Beckett AN, Engler RJ, Hoffman DR, Ott NL, Simons FE. Immune responses to mosquito saliva in 14 individuals with acute systemic allergic reactions to mosquito bites. J Allergy Clin Immunol. (2004) 114:1189–94. doi: 10.1016/j.jaci.2004.08.014
11. Peng Z, Xu W, James AA, Lam H, Sun D, Cheng L, et al. Expression, purification, characterization and clinical relevance of rAed a 1–a 68-kDa recombinant mosquito Aedes aegypti salivary allergen. Int Immunol. (2001) 13:1445–52. doi: 10.1093/intimm/13.12.1445
12. Peng Z, Xu W, Lam H, Cheng L, James AA, Simons FE. A new recombinant mosquito salivary allergen, rAed a 2: allergenicity, clinical relevance, and cross-reactivity. Allergy. (2006) 61:485–90. doi: 10.1111/j.1398-9995.2006.00985.x
13. Jeon SH, Park JW, Lee BH. Characterization of human IgE and mouse IgG1 responses to allergens in three mosquito species by immunoblotting and ELISA. Int Arch Allergy Immunol. (2001) 126:206–12. doi: 10.1159/000049515
14. Kausar MA, Vijayan VK, Bansal SK, Menon BK, Vermani M, Agarwal MK. Mosquitoes as sources of inhalant allergens: clinicoimmunologic and biochemical studies. J Allergy Clin Immunol. (2007) 120:1219–21. doi: 10.1016/j.jaci.2007.07.017
15. Wongkamchai S, Khongtak P, Leemingsawat S, Komalamisra N, Junsong N, Kulthanan K, et al. Comparative identification of protein profiles and major allergens of saliva, salivary gland and whole body extracts of mosquito species in Thailand. Asian Pac J Allergy Immunol. (2010) 28(2-3):162–9.
16. Cantillo JF, Puerta L, Lafosse-Marin S, Subiza JL, Caraballo L, Fernandez-Caldas E. Allergens involved in the cross-reactivity of Aedes aegypti with other arthropods. Ann Allergy Asthma Immunol. (2017) 118:710–8. doi: 10.1016/j.anai.2017.03.011
17. Sanchez J, Sanchez A, Cardona R. Exposure and sensitization to insects in allergic patients in the tropics. Biomedica. (2018) 3880–6. doi: 10.7705/biomedica.v38i3.3801
18. Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. ELife. (2015) 4:e08347. doi: 10.7554/eLife.08347
19. Opasawatchai A, Yolwong W, Thuncharoen W, Inrueangsri N, Itsaradisaikul S, Sasisakulporn C, et al. Novel salivary gland allergens from tropical mosquito species and IgE reactivity in allergic patients. World Allergy Organ J. (2020) 13:100099. doi: 10.1016/j.waojou.2020.100099
20. Hudson A, Bowman L, Orr CW. Effects of absence of saliva on blood feeding by mosquitoes. Science. (1960) 131:1730–1. doi: 10.1126/science.131.3415.1730
21. Sun D, McNicol A, James AA, Peng Z. Expression of functional recombinant mosquito salivary apyrase: a potential therapeutic platelet aggregation inhibitor. Platelets. (2006) 17:178–84. doi: 10.1080/09537100500460234
22. Valenzuela JG, Pham VM, Garfield MK, Francischetti IM, Ribeiro JM. Toward a description of the sialome of the adult female mosquito Aedes aegypti. Insect Biochem Mol Biol. (2002) 32:1101–22. doi: 10.1016/S0965-1748(02)00047-4
23. Arca B, Lombardo F, Francischetti IM, Pham VM, Mestres-Simon M, Andersen JF, et al. An insight into the sialome of the adult female mosquito Aedes albopictus. Insect Biochem Mol Biol. (2007) 37:107–27. doi: 10.1016/j.ibmb.2006.10.007
24. Bebber DP, Ramotowski MAT, Gurr SJ. Crop pests and pathogens move polewards in a warming world. Nat Climate Change. (2013) 3:985. doi: 10.1038/nclimate1990
25. Robinet C, Roques A. Direct impacts of recent climate warming on insect populations. Integr Zool. (2010) 5:132–42. doi: 10.1111/j.1749-4877.2010.00196.x
26. Rochlin I, Faraji A, Ninivaggi DV, Barker CM, Kilpatrick AM. Anthropogenic impacts on mosquito populations in North America over the past century. Nat Commun. (2016) 7:13604. doi: 10.1038/ncomms13604
27. Christophers SR. Aedes aegypti (L.) the Yellow Fever Mosquito: Its Life History, Bionomics and Structure. London: Cambridge University Press (1960).
28. Mattingly PF. Genetical aspects of the Aedes aegypti problem. I. Taxonom: and bionomics. Ann Trop Med Parasitol. (1957) 51:392–408. doi: 10.1080/00034983.1957.11685829
29. Kraemer MU, Sinka ME, Duda KA, Mylne A, Shearer FM, Brady OJ, et al. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci Data. (2015) 2:150035. doi: 10.1038/sdata.2015.35
30. Bousquet PJ, Chinn S, Janson C, Kogevinas M, Burney P, Jarvis D, et al. Geographical variation in the prevalence of positive skin tests to environmental aeroallergens in the European Community Respiratory Health Survey I. Allergy. (2007) 62:301–9. doi: 10.1111/j.1398-9995.2006.01293.x
31. Peng Z, Li H, Simons FE. Immunoblot analysis of salivary allergens in 10 mosquito species with worldwide distribution and the human IgE responses to these allergens. J Allergy Clin Immunol. (1998) 101:498–505. doi: 10.1016/S0091-6749(98)70357-4
32. Trpis M, Hausermann W. Demonstration of differential domesticity of Aedes aegypti (L) (Diptera, Culicidae) in Africa by mark-release-recapture. Bull Entomol Res. (1975) 65:199–208. doi: 10.1017/S0007485300005903
33. Gouck HK. Host preferences of various strains of Aedes aegypti and Aedes simpsoni as determined by an olfactometer. Bull World Health Organ. (1972) 47:680–3.
34. McBride CS, Baier F, Omondi AB, Spitzer SA, Lutomiah J, Sang R, et al. Evolution of mosquito preference for humans linked to an odorant receptor. Nature. (2014) 515:222–7. doi: 10.1038/nature13964
35. Kraemer MUG, Reiner RC Jr., Brady OJ, Messina JP, Gilbert M, Pigott DM, et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. (2019) 4:854–63. doi: 10.1038/s41564-019-0376-y
36. Vincent M, Larrieu S, Vilain P, Etienne A, Solet JL, François C, et al. From the threat to the large outbreak: dengue on Reunion Island, 2015 to 2018. Euro Surveill. (2019) 24:1900346. doi: 10.2807/1560-7917.ES.2019.24.47.1900346
37. Lounibos LP. Invasions by insect vectors of human disease. Annu Rev Entomol. (2002) 47:233–66. doi: 10.1146/annurev.ento.47.091201.145206
38. Farajollahi A, Fonseca DM, Kramer LD, Marm Kilpatrick A. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect Genet Evol. (2011) 11:1577–85. doi: 10.1016/j.meegid.2011.08.013
39. Al-Ghamdi KAkM, Mahyoub J. Role of climatic factors in the seasonal abundance of Aedes aegypti L. and dengue fever cases in Jeddah province of Saudi Arabia. Curr World Environ. (2017) 4:307–12. doi: 10.12944/CWE.4.2.07
40. Jemal Y, Al-Thukair AA. Combining GIS application and climatic factors for mosquito control in Eastern Province, Saudi Arabia. Saudi J Biol Sci. (2018) 25:1593–602. doi: 10.1016/j.sjbs.2016.04.001
41. Peng Z, Simons FE. Mosquito allergy: immune mechanisms and recombinant salivary allergens. Int Arch Allergy Immunol. (2004) 133:198–209. doi: 10.1159/000076787
42. Wongkamchai S, Techasintana P, Wisuthsarewong W, Kulthanan K, Suthipinittharm P, Eakpo P. Analysis of IgE-binding allergens in Culex quinquefasciatus saliva protein in mosquito bite allergic patients. Ann Allergy Asthma Immunol. (2007) 98:200–1. doi: 10.1016/S1081-1206(10)60698-9
43. Champagne DE, Smartt CT, Ribeiro JM, James AA. The salivary gland-specific apyrase of the mosquito Aedes aegypti is a member of the 5'-nucleotidase family. Proc Natl Acad Sci USA. (1995) 92:694–8. doi: 10.1073/pnas.92.3.694
44. Ribeiro JM, Sarkis JJ, Rossignol PA, Spielman A. Salivary apyrase of Aedes aegypti: characterization and secretory fate. Comp Biochem Physiol B. (1984) 79:81–6. doi: 10.1016/0305-0491(84)90081-6
45. Xu W, Simons FE, Peng Z. Expression and rapid purification of an Aedes aegypti salivary allergen by a baculovirus system. Int Arch Allergy Immunol. (1998) 115:245–51. doi: 10.1159/000023907
46. Reno HE, Novak RJ. Characterization of apyrase-like activity in Ochlerotatus triseriatus, Ochlerotatus hendersoni, and Aedes aegypti. Am J Trop Med Hyg. (2005) 73:541–5. doi: 10.4269/ajtmh.2005.73.541
47. Dong F, Fu Y, Li X, Jiang J, Sun J, Cheng X. Cloning, expression, and characterization of salivary apyrase from Aedes albopictus. Parasitol Res. (2012) 110:931–7. doi: 10.1007/s00436-011-2579-x
48. Malafronte Rdos S, Calvo E, James AA, Marinotti O. The major salivary gland antigens of Culex quinquefasciatus are D7-related proteins. Insect Biochem Mol Biol. (2003) 33:63–71. doi: 10.1016/S0965-1748(02)00168-6
49. James AA, Blackmer K, Marinotti O, Ghosn CR, Racioppi JV. Isolation and characterization of the gene expressing the major salivary gland protein of the female mosquito, Aedes aegypti. Mol Biochem Parasitol. (1991) 44:245–53. doi: 10.1016/0166-6851(91)90010-4
50. Calvo E, Mans BJ, Ribeiro JM, Andersen JF. Multifunctionality and mechanism of ligand binding in a mosquito antiinflammatory protein. Proc Natl Acad Sci USA. (2009) 106:3728–33. doi: 10.1073/pnas.0813190106
51. Peng Z, Lam H, Xu W, Cheng L, Chen YL, FER S. Characterization and clinical relevance of two recombinant mosquito Aedes aegypti salivary allergens, rAed a 1 and rAed a 2 [abstract]. J Allergy Clin Immunol. (1998) 101.
52. Xu W, Peng Z, Simons FER. Isolation of a cDNA encoding a 30 kDa IgE-binding protein of mosquito Aedes aegypti saliva. J Allergy Clin Immunol. (1998) 101:S203.
53. Becket AN, Sun W, Simons FER, Ma Y, Peng Z. Role of recombinant mosquito salivary allergens in the diagnosis of individuals with allergic reactions to mosquito bites. J Allergy Clin Immunol. (2004) 13:S7. doi: 10.1016/j.jaci.2003.12.239
54. Li Beckett AN, Simons FER, Li C, Zhang T, Peng Z. A new 67 kDa recombinant Aedes aegypti salivary allergen rAed a 4 in the diagnosis of mosquito allergy. J Allergy Clin Immunol. (2005) 115:S100. doi: 10.1016/j.jaci.2004.12.412
55. Bemanian MH, Alizadeh Korkinejad N, Shirkhoda S, Nabavi M, Pourpak Z. Assessment of sensitization to insect aeroallergens among patients with allergic rhinitis in Yazd City, Iran. Iran J Allergy Asthma Immunol. (2012) 11:253–8.
56. Wynn SR, Swanson MC, Reed CE, Penny ND, Showers WB, Smith JM. Immunochemical quantitation, size distribution, and cross-reactivity of lepidoptera (moth) aeroallergens in southeastern Minnesota. J Allergy Clin Immunol. (1988) 82:47–54. doi: 10.1016/0091-6749(88)90050-4
57. Swanson MC, Agarwal MK, Reed CE. An immunochemical approach to indoor aeroallergen quantitation with a new volumetric air sampler: studies with mite, roach, cat, mouse, and guinea pig antigens. J Allergy Clin Immunol. (1985) 76:724–9. doi: 10.1016/0091-6749(85)90678-5
58. Madden AA, Barberan A, Bertone MA, Menninger HL, Dunn RR, Fierer N. The diversity of arthropods in homes across the United States as determined by environmental DNA analyses. Mol Ecol. (2016) 25:6214–24. doi: 10.1111/mec.13900
59. Gupta RS, Singh B. Phylogenetic analysis of 70 kD heat shock protein sequences suggests a chimeric origin for the eukaryotic cell nucleus. Curr Biol. (1994) 4:1104–14. doi: 10.1016/S0960-9822(00)00249-9
60. Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. (2007) 581:3702–10. doi: 10.1016/j.febslet.2007.05.039
61. An S, Chen L, Long C, Liu X, Xu X, Lu X, et al. Dermatophagoides farinae allergens diversity identification by proteomics. Mol Cell Proteomics. (2013) 12:1818–28. doi: 10.1074/mcp.M112.027136
62. Chuang JG, Su SN, Chiang BL, Lee HJ, Chow LP. Proteome mining for novel IgE-binding proteins from the German cockroach (Blattella germanica) and allergen profiling of patients. Proteomics. (2010) 10:3854–67. doi: 10.1002/pmic.201000348
63. Cantillo JF, Puerta L, Fernandez-Caldas E, Subiza JL, Soria I, Lafosse-Marin S, et al. Expression and immunological characterization of a heat shock cognate-70 protein allergen, rAed a 8, from the mosquito species Aedes aegypti. Rev Alerg Mex. (2018) 65(Suplemento 1: XI Congreso ACAAI. Resumenes de trabajos libres/Immunology) 63:117–8.
64. Acevedo N, Sanchez J, Erler A, Mercado D, Briza P, Kennedy M, et al. IgE cross-reactivity between Ascaris and domestic mite allergens: the role of tropomyosin and the nematode polyprotein ABA-1. Allergy. (2009) 64:1635–43. doi: 10.1111/j.1398-9995.2009.02084.x
65. Diez S, Puerta L, Martínez D, Muñoz M, Hernández K, Sánchez J. Clinical Relevance of Shrimp Sensitization in Patients with Allergic Rhinitis: Anti-Der p 10 IgE as Predictor. Int Arch Allergy Immunol. (2021) 1–9. doi: 10.1159/000516005. [Epub ahead of print].
66. Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. (2007) 316:1718–23. doi: 10.1126/science.1138878
67. Reese G, Schicktanz S, Lauer I, Randow S, Luttkopf D, Vogel L, et al. Structural, immunological and functional properties of natural recombinant Pen a 1, the major allergen of Brown Shrimp, Penaeus aztecus. Clin Exp Allergy. (2006) 36:517–24. doi: 10.1111/j.1365-2222.2006.02454.x
68. Daul CB, Slattery M, Reese G, Lehrer SB. Identification of the major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosin. Int Arch Allergy Immunol. (1994) 105:49–55. doi: 10.1159/000236802
69. Westritschnig K, Sibanda E, Thomas W, Auer H, Aspock H, Pittner G, et al. Analysis of the sensitization profile towards allergens in central Africa. Clin Exp Allergy. (2003) 33:22–7. doi: 10.1046/j.1365-2222.2003.01540.x
70. Santos AB, Rocha GM, Oliver C, Ferriani VP, Lima RC, Palma MS, et al. Cross-reactive IgE antibody responses to tropomyosins from Ascaris lumbricoides and cockroach. J Allergy Clin Immunol. (2008) 121:1040–6 e1. doi: 10.1016/j.jaci.2007.12.1147
71. Satinover SM, Reefer AJ, Pomes A, Chapman MD, Platts-Mills TA, Woodfolk JA. Specific IgE and IgG antibody-binding patterns to recombinant cockroach allergens. J Allergy Clin Immunol. (2005) 115:803–9. doi: 10.1016/j.jaci.2005.01.018
72. Weghofer M, Thomas WR, Kronqvist M, Mari A, Purohit A, Pauli G, et al. Variability of IgE reactivity profiles among European mite allergic patients. Eur J Clin Invest. (2008) 38:959–65. doi: 10.1111/j.1365-2362.2008.02048.x
73. Peng Z, Simons FE. Cross-reactivity of skin and serum specific IgE responses and allergen analysis for three mosquito species with worldwide distribution. J Allergy Clin Immunol. (1997) 100:192–8. doi: 10.1016/S0091-6749(97)70224-0
74. Sabbah A, Hassoun S, Drouet M, Lauret MG, Doucet M. The wasp/mosquito syndrome. Allerg Immunol. (1999) 31:175–84.
75. Caraballo L, Zakzuk J, Lee BW, Acevedo N, Soh JY, Sánchez-Borges M, et al. Particularities of allergy in the Tropics. World Allergy Organ J. (2016) 9:20. doi: 10.1186/s40413-016-0110-7
76. Cabrerizo Ballesteros S, de Barrio M, Baeza ML, Rubio Sotes M. Allergy to chironomid larvae (red migde larvae) in non professional handlers of fish food. J Investig Allergol Clin Immunol. (2006) 16:63–8.
77. Adalsteinsdottir B, Sigurdardottir S, Gislason T, Kristensen T, Gislason D. What characterizes house dust mite sensitive individuals in a house dust mite free community in Reykjavik, Iceland? Allergol Int. (2007) 56:6. doi: 10.2332/allergolint.O-06-447
78. Cantillo JF, Puerta L, Fernandez-Caldas E, Subiza JL, Soria I, Wöhrl S, et al. Tropomyosins in mosquito and house dust mite cross-react at the humoral and cellular level. Clin Exp Allergy. (2018) 48:1354–63. doi: 10.1111/cea.13229
79. Manuyakorn W, Itsaradisaikul S, Benjaponpitak S, Kamchaisatian W, Sasisakulporn C, Jotikasthira W, et al. Mosquito allergy in children: clinical features and limitation of commercially-available diagnostic tests. Asian Pac J Allergy Immunol. (2017) 35:186–90. doi: 10.12932/AP0842
80. Castro-Almarales RL, Álvarez-Castelló M, Ronquillo-Díaz M, Rodríguez-Canosa JS, González-León M, Navarro-Viltre BI, et al. [Sensitivity and specificity of prick skin test with two concentrations of standardized extract of Culex quinquefasciatus in allergic children]. Rev Alerg Mex. (2016) 63:11–9. doi: 10.29262/ram.v63i1.114
81. Peng Z, Simons FE. Comparison of proteins, IgE, and IgG binding antigens, and skin reactivity in commercial and laboratory-made mosquito extracts. Ann Allergy Asthma Immunol. (1996) 77:371–6. doi: 10.1016/S1081-1206(10)63335-2
82. Wang Q, Beckett A, Simons FE, Peng Z. Comparision of the mosquito saliva-capture enzyme-linked immunosorbent assay and the unicap test in the diagnosis of mosquito allergy. Ann Allergy Asthma Immunol. (2007) 99:199–200. doi: 10.1016/S1081-1206(10)60650-3
83. Chapman MD, Smith AM, Vailes LD, Arruda LK, Dhanaraj V, Pomés A. Recombinant allergens for diagnosis and therapy of allergic disease. J Allergy Clin Immunol. (2000) 106:409–18. doi: 10.1067/mai.2000.109832
84. Eiringhaus K, Renz H, Matricardi P, Skevaki C. Component-resolved diagnosis in allergic rhinitis and asthma. J Appl Lab Med. (2019) 3:883–98. doi: 10.1373/jalm.2018.026526
85. Valenta R, Lidholm J, Niederberger V, Hayek B, Kraft D, Gronlund H. The recombinant allergen-based concept of component-resolved diagnostics and immunotherapy (CRD and CRIT). Clin Exp Allergy. (1999) 29:896–904. doi: 10.1046/j.1365-2222.1999.00653.x
Keywords: mosquito allergy, allergens, tropics, IgE, Aedes aegypti, cross reactivity
Citation: Cantillo JF and Puerta L (2021) Mosquitoes: Important Sources of Allergens in the Tropics. Front. Allergy 2:690406. doi: 10.3389/falgy.2021.690406
Received: 02 April 2021; Accepted: 03 June 2021;
Published: 08 July 2021.
Edited by:
Andreas L. Lopata, James Cook University, AustraliaReviewed by:
Sandip D. Kamath, James Cook University, AustraliaMayte Villalba, Complutense University of Madrid, Spain
Copyright © 2021 Cantillo and Puerta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leonardo Puerta, bHB1ZXJ0YWwxQHVuaWNhcnRhZ2VuYS5lZHUuY28=
 Jose Fernando Cantillo
Jose Fernando Cantillo Leonardo Puerta
Leonardo Puerta