- 1Department of Pediatrics, The Affiliated Wuxi Second People’s Hospital of Jiangnan University, Wuxi Medical School, Jiangnan University, Wuxi, Jiangsu, China
- 2Department of Pediatrics, Wuxi Second People’s Hospital, Nanjing Medical University, Wuxi, Jiangsu, China
- 3Department of Pediatrics, The Affiliated Wuxi Second People’s Hospital of Jiangnan University, Wuxi, Jiangsu, China
Dust mites are ubiquitous in human living environments and represent the primary source of indoor air allergens worldwide. They are capable of triggering allergic rhinitis, conjunctivitis, asthma, atopic dermatitis, and other allergic conditions. Long-term avoidance of dust mite allergens should decrease sensitization, significantly improves skin lesions, and reduces both the development and severity of respiratory diseases. Therefore, early diagnosis of dust mite allergy is critical for effective treatment and intervention. This review summarizes the existing methods for detecting dust mite allergy, which include both in vivo and in vitro approaches—such as skin prick testing(SPT), atopy patch testing(APT), provocation tests, basophil activation test (BAT), and molecular component-resolved diagnostics(CRD)—and analyzes the underlying principles, advantages, and limitations of each method to serve as a reference for the development of future detection methods.
1 Introduction
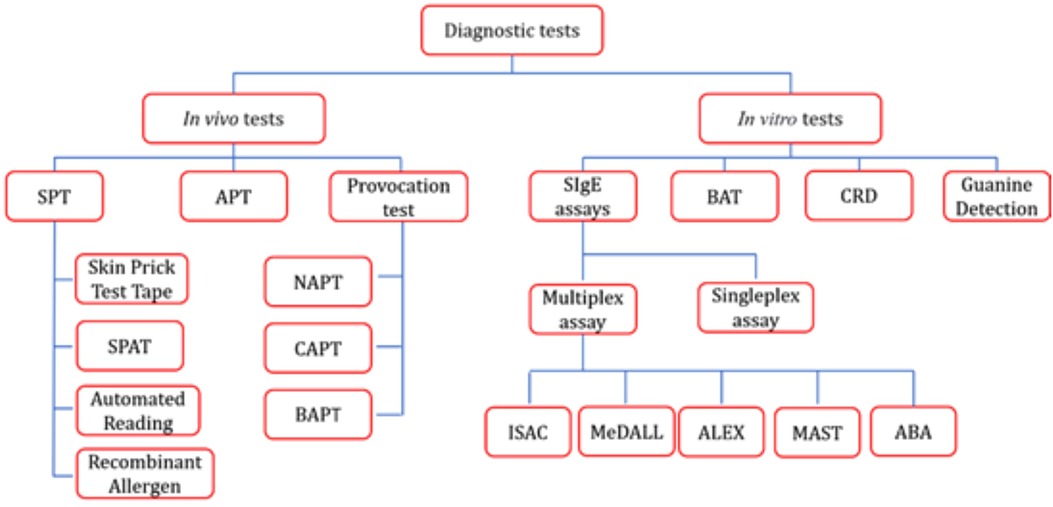
In 1964 and 1967, physicians and biologists first elucidated the classification of house dust mites (HDM) and demonstrated that they are the primary source of house dust allergens, thereby revealing the critical role of dust mites in allergic diseases (1). Approximately 1%–2% of the global population—ranging from 65 to 130 million people—is affected (2). HDM unique habits enable them to colonize a wide range of human habitats, and their products predispose them to trigger both innate and adaptive immune responses (3). When dust mite allergens contact the conjunctiva, skin, respiratory tract, or intestinal tract, they can trigger atopic sensitization and related symptoms, including allergic rhinitis, conjunctivitis, asthma, and atopic dermatitis. Dust mite sensitization can be diagnosed based on patient history, SPT, provocation tests, and/or allergen-specific IgE (sIgE) assays, thereby providing a crucial basis for timely treatment and intervention, such as allergen avoidance, pharmacotherapy, and allergen-specific immunotherapy (AIT). This review describes both in vivo and in vitro detection methods for mite allergy (e.g., Figure 1), analyzes the advantages and disadvantages of each approach, and offers a reference for clinical diagnosis and decision-making as well as for the future development of detection techniques.
2 Immunological mechanisms of dust mite allergy
Dust mite allergy can be classified into IgE-mediated and non-IgE-mediated immune responses. In IgE-mediated immune responses, upon exposure to dust mite allergens, the immune system produces sufficient amounts of sIgE antibodies. Non-IgE-mediated immune responses primarily arise from other properties of mite allergens, such as dust mite protein hydrolases (4–10), activators of natural immune response pattern recognition receptors (11), and polysensitization promoters (12, 13).
When environmental dust mite allergens reach a certain concentration, they directly enhance allergen permeability by disrupting tight junctions between epithelial cells and activate epithelial cells to secrete secretion of IL-25, IL-33, and TSLP (thymic stromal lymphopoietin), which activate localized dendritic cells (DCs) and intrinsic lymphocytes (ILC2). DCs recognize dust mite allergens through pattern recognition receptors (e.g., TLR, CLR) recognize dust mite allergens, uptake and presentation of antigens to the lymph nodes. Meanwhile, the protease activity of dust mite allergens inhibits the production of IL-12 by DCs, prompting them to secrete IL-4 and IL-5, which induces the differentiation of Th0 cells towards Th2. IL-33 signaling further strengthens the Th2 polarization ability of DCs. Th2 cells, which are the core of the regulation of allergic responses, secrete key cytokines such as IL-4, IL-5, and IL-13, and their secretion of IL-4 will further reinforce the Th0 cells’ ability to polarize to Th2. IL-4 will further enhance the differentiation of Th0 cells toward Th2 and inhibit the expression of Th1-related genes (e.g., IFN-γ) (14, 15). IL-4 drives B-cell IgE class switching by binding to the IL-4 receptor on the surface of the B cell, thereby promoting IgE production (16, 17), and by increasing the expression of CD23 (a low-affinity IgE receptor) on the surface of the B cell, it promotes IgE binding to the B cell and enhances the antigen-presenting capacity, further amplifying the allergic response.
SIgE produced by B cells binds to the surface of mast cells and basophils via the FcεRI receptor. Upon re-exposure to the allergen, sIgE cross-linking triggers degranulation, releasing mediators such as histamine, leukotrienes (LTs), and prostaglandins (PGD2) and causing acute symptoms (e.g., vasodilatation, smooth muscle contraction). IL-5 promotes the differentiation, survival, and recruitment of eosinophils to the site of inflammation, and eosinophils release major basic protein (MBP), eosinophil cationic protein (ECP), which directly damages the epithelium and secretes IgE. IL-13 and TGF-β to promote chronic inflammation and airway remodeling (14). IL-13 induces cuprocyte chemotaxis, increased mucus secretion, and airway hyperresponsiveness. IL-4, IL-5, and IL-13 maintain the activation of Th2 cells and ILC2 through autocrine/paracrine secretion, memory Th2 cells expand rapidly upon re-exposure, and pro-fibrotic factors exacerbate tissue damage and further maintain chronic inflammation.
SIgE production in dust mite allergy requires the breaching of multiple thresholds, including concentration of allergen exposure, Th2 cytokine concentration, and individual immune status. In clinical practice, a sIgE level of 0.35 kUA/L is the threshold for diagnosis of sensitization. SIgE levels below this threshold (e.g., 0.10–0.35 kUA/L) may not trigger clinical symptoms but may indicate a potential sensitization risk. Individual responses are modulated by genetics, environmental microorganisms, and history of previous exposure.
3 Detection methods
Dust mite allergen specificity detection methods commonly used in clinical and research settings include two types of tests: in vivo and in vitro. In vivo tests involve the direct application of allergens to the human body, with the reaction observed to make a diagnosis. In contrast, in vitro tests involve exposing blood or other bodily fluids to the allergens in isolation and evaluating the reaction based on the results.
3.1 In vivo tests
3.1.1 SPT
SPT is the simplest in vivo tests for assessing IgE sensitization in humans (18). In dust mite sensitization testing, SPT is considered positive if the wheal diameter is at least 3 mm larger than that of the negative control, indicating the presence of mite sIgE in the body (19). Currently, there is no fully standardized criterion for recording and assessment. Most clinical studies measure the wheal size by calculating its mean diameter [(D + d)/2, where D is the largest diameter and d is the diameter perpendicular to D] (20). The criteria for interpreting SPT results are shown in Table 1. The interpretation methods used in Tables 1A, B streamline the evaluation process, enabling rapid screening of allergens and thus are more widely applicable in clinical practice. In contrast, the approaches described in Tables 1C, D provide a quantitative assessment of allergen reactivity, minimize subjective bias, and are therefore more appropriate for scientific research.
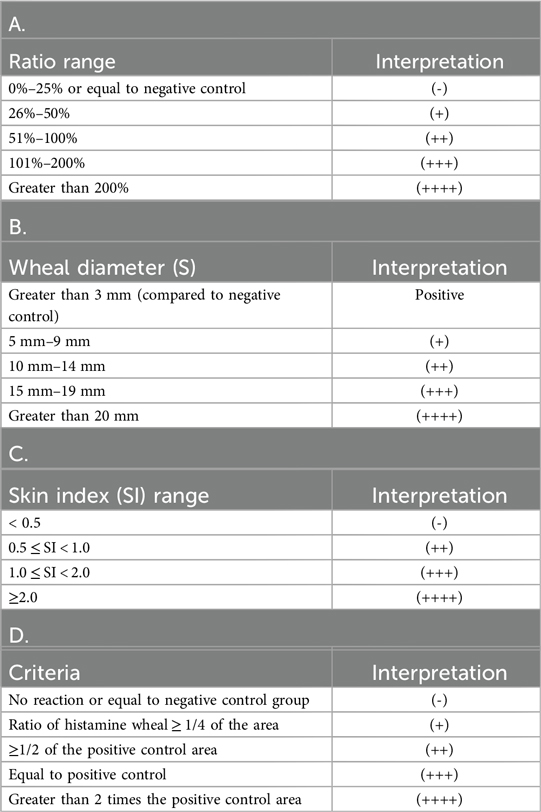
Table 1. Interpretation criteria for SPT results: A. Ratio Judgment Method Based on Different Criteria; B. Wheal and Erythema Diameter Judgment Method; C. Skin Index (SI, SI = Average diameter of allergen wheals/Average diameter of wheals in the positive control group) Judgment Method; D. Other Judgment Criteria.
When positive SPT results are combined with patient history, IgE-mediated allergic diseases can be definitively diagnosed with a positive predictive value of 95%–100% (21–23). This method offers advantages such as ease of operation, rapid visualization of results, time efficiency, reproducibility, cost-effectiveness, and high sensitivity. SPT is generally safe, with few adverse reactions—typically localized to the skin and very rarely systemic (24, 25).
The disadvantages of SPT primarily stem from a high rate of false-positive and false-negative results, which are attributed to factors such as the operator's skill, the type and shape of the puncture device, and the applied force (26). SPT results are also influenced by medications such as antihistamines, tricyclic antidepressants, tranquilizers, anti-IgE monoclonal antibodies, and H2 receptor antagonists. The measurement of wheal size involves a certain degree of subjectivity, and there are time differences in measurement and assessment among subjects of different ages and body mass indices (27). The lack of standardization in selecting antigen reagents and determining puncture reagent concentrations can also affect results. SPT can cause anxiety and pain in some subjects, particularly younger children (28).
Recent developments in SPT for clinical applications and research include innovations such as skin prick tape, which is less painful and more acceptable while reducing cross-contamination during multi-allergen testing and offering similar biological effects; however, it has not yet been fully empirically validated for clinical use (29). The Skin Prick Automated Test (SPAT) device (30) demonstrates higher reproducibility and tolerability, reduces human error, and decreases experimental variability compared to conventional SPT. It also saves testing time and reduces the consumption of allergen solutions (31). Technologies such as 2D scanners, blood flow measurements, skin impedance, thermography, photography, and 3D scanners enable automated reading of test results; however, issues related to time, cost, and accuracy currently limit their use in clinical practice (32). The use of pure allergens overcomes batch-to-batch variability in natural allergen extracts by offering a clear composition, high purity, and the removal of non-allergenic components, thereby improving test specificity and reducing cross-reactivity (33).
3.1.2 APT
APT uses protein allergens known to elicit an IgE-mediated immediate-type allergic reaction and evaluates the test site for an eczematous delayed-type reaction after 48–72 h (34, 35). It can be used for allergen detection in hay fever, asthma, urticaria, atopic dermatitis, etc. APT has a high degree of specificity and is an important tool for identifying allergens that cause atopic eczema and dermatitis syndrome (AEDS) (36), and it is also suitable for identifying atopic dermatitis caused by mite allergy (37). A study found that patients with both endogenous and exogenous atopic dermatitis (AD) showed a positive response to APT to house dust mites (38). APT has been used in the detection of mite allergens associated with respiratory diseases mainly to assess its value in the diagnosis of allergic rhinitis and asthma. APT is able to recapitulate the pathophysiology of the T-cell-mediated allergic response, and in children with allergic rhinitis or asthma patients showing high positivity rates ranging from 25% to 56% (39). In dust mite allergy testing, approximately 10% of patients are positive only by APT, avoiding misclassification as non-allergic if negative in conventional SPT or in vitro IgE testing and reducing the risk of untimely intervention or inappropriate management. APT has a high safety profile, with fewer side effects, most of which are mild reactions such as localized skin rashes, contact urticaria, and localized pruritus (40).
APT also has limitations in mite allergy testing. Standardizing the substances, concentrations, vehicles, interpretation times, and procedural techniques used in the APT is difficulty (41); skin conditions at the test site and age differences also affect APT results, adult and adolescent patients reacting positively to APT for mite allergens significantly is more often than children (38). Pharmacologic factors such as steroids, cyclosporine A, tacrolimus, and antihistamines can affect the test results; the test itself is time-consuming; and the stimulus reaction of the APT itself may also lead to false-positive results. And heterogeneity between different studies, although APT shows higher sensitivity and specificity in some cases, test results should be interpreted with caution (37).
3.1.3 Allergen provocation test
Allergen provocation test is one of the most important methods for diagnosing allergic diseases, which can visually demonstrate the clinical correlation between allergens and the symptoms and severity of allergic diseases. When the history suggests allergy and serum sIgE is not detected or SPT is negative, provocation tests are feasible (42–44). The provocation tests used for mite allergy detection include nasal allergen provocation test (NAPT), conjunctival allergen provocation test (CAPT), and bronchial allergen provocation test (BAPT).
The NAPT is currently the only available test to confirm nasal reactivity to allergens. It is safe and highly reproducible (45). NAPT is a valuable test for confirming the diagnosis of dust mite allergy when the SPT test result is negative, and the symptoms following NAPT for dust mites are also of high value in predicting perennial allergic rhinitis (46). Compared to SPT, dust mite NAPT has a lower sensitivity and higher specificity in the diagnosis of allergic asthma (47).
The CAPT is the only test capable of determining the relationship between ocular manifestations and sIgE, with a diagnostic sensitivity and specificity of 90% and 100%, respectively, in a study to diagnose HDM-induced allergic conjunctivitis, attesting to its high antigenic quality (48). The CAPT can provide valuable clinical information, and the lack of more thorough evaluation of safety aspects has not been fully utilized in practice (49). CAPT can provide valuable clinical information, lacking a more thorough evaluation of safety, and is not fully utilized in practice (46).
Dust mite allergen is one of the common allergens in many patients suffering from asthma and co-morbid AR (50), and BAPT is one of the most important tools for the diagnosis of allergic asthma (51–53). The absence of standardized protocols and equipment in bronchial provocation testing diminishes its reproducibility. Moreover, bronchial provocation testing exhibits lower safety compared to other in vivo tests, as it may trigger adverse effects—including acute bronchospasm, asthma attacks, laryngeal edema, and, in rare cases, anaphylactic shock—which further restrict its clinical use (54). A study proposed the use of NAPT instead of BAPT as a diagnostic tool by comparing bronchial and nasal allergen provocation tests in patients with bronchial asthma and mite sensitization, and showed that NAPT could be used to confirm the relevance of HDM sensitization in the majority of asthma cases prior to BAPT; in NAPT-negative patients, the use of BAPT was still recommended to rule out an HDM-induced asthma reactions (55).
The provocation tests have high sensitivity, specificity and validity, and accuracy is also high relative to skin tests. Because of its time-consuming operation, high cost and equipment requirements, technical difficulty, and the need for skilled personnel for operation and measurement, provocation tests are generally not used as an initial screening tool for allergy, instead has been more widely used in the study of pathogenesis and pathophysiology (56, 57), and is also used to assess the effectiveness of treatments such as the efficacy assessment of immunotherapy for house dust mite allergens (58).
3.2 In vitro test
3.2.1 BAT
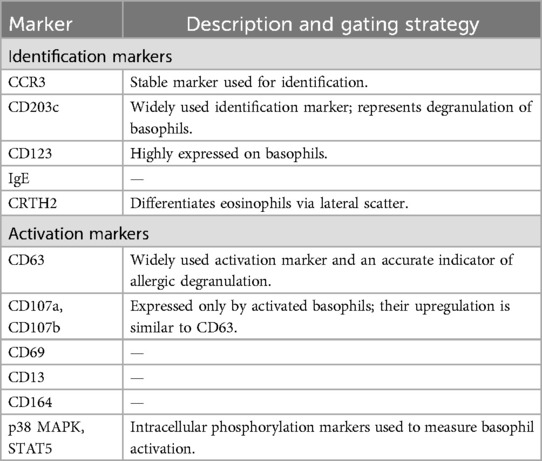
Basophils and mast cells are the key effector cells of immediate allergic reaction. The process of basophil degranulation is known as basophil activation. With the development and popularization of flow cytometry, and the discovery of unique markers such as CD63, CD203, and unique markers for identifying basophils, the BAT has gradually become a universally accepted auxiliary allergic reaction detection method (59). The BAT measures the expression of activation markers on the surface of basophils by means of flow cytometry, for example, CD63, a membrane protein localized to the same secreted lysosomal granules containing histamine, it is a precise marker for allergenic desmoplasia by regulating cytokinesis after allergen-mediated activation of mast cells and basophils (60), and the release of histamine in the activation of basophils correlates well with the upregulation of CD63, which was measured by flow cytometry on the CD63 expressed on basophils is detected and evaluated by flow cytometry to determine whether basophils are activated and the level of activation to make a diagnosis of allergy. Common basophil recognition markers and activation markers are shown in Table 2 (61).
The utility of basophil activation test in dust mite allergy has been studied and analyzed through natural extracts, purified extracts, and recombinant allergen fractions of dust mites, and its overall performance is good (62). This test reduces the risk of severe allergic reactions by detecting 150–2,000 basophils in less than 0.1 ml of fresh blood in response to allergen crosslinked IgE, which is more reproducible and less stressful for the patient compared to other tests.
The limitations of BAT are: ideally, whole blood BAT should be performed within 4 h after blood collection to maximize basophil viability and function, because basophil reactivity decreases significantly with time, when it appears that it takes a long time from blood collection to BAT, blood needs to be processed and preserved, and there is no standardized time and conditions for preservation, and the optimal preservation conditions need to be further explored and researched; whole blood BAT can be interfered by serum components such as blocking antibodies; basophil enrichment and purification can cause cell loss and in vitro activation, which can affect the results of the assay; the source of allergens is another key factor in the application of BAT in clinical and research applications, and there is also the problem of the lack of standardization of allergens (61); systemic application of steroids and cyclosporine A can affect the results of the BAT assay as well (63); how to choose the gating strategy for identifying basophils according to different conditions also needs to be further investigated (64); additionally, the high cost, specialized equipment, and requirement for trained personnel limit the clinical application of the basophil activation test (BAT). The clinical application of BAT still needs to be further optimized and standardized, especially in the control of allergen selection and pharmacological interventions; and cost barriers can be mitigated by sharing equipment, optimizing processes, and implementing standardization.
3.2.2 Sige test
The serum sIgE test detects IgE antibodies against specific allergens (e.g., dust mites) in patients' serum using in vitro immunological techniques. Studies have demonstrated that the sIgE test for dust mite allergy has a sensitivity of 85%–98.8% and a specificity of 89.6%–97.9%, with a significant positive correlation with the skin prick test (SPT) (r = 0.506–0.737) (65, 66). Additionally, the sIgE test can be quantitatively graded: an sIgE level of ≥0.35 kUA/L is considered positive, while a level of ≥3.5 kUA/L (grade 3) indicates moderate-to-severe sensitization, which partially correlates with clinical symptom severity (67). Compared with other detection methods, the six-class classification of sIgE provides an objective standard for allergy diagnosis by quantifying the degree of sensitization, and has become a key tool for AIT. Additionally, sIgE testing eliminates the confounding effects of skin condition, age, and medication use on test results, and it is associated with a very high safety profile (68).
Currently, more than 4,000 scientific articles have demonstrated the clinical value of ImmunoCAP, which is considered the “reference standard” for in vitro IgE detection (69). With the emergence and development of allergenic molecules, the application of this test has introduced allergen research into the field of precision medicine (70). The ImmunoCAP test for individual allergens is based on the coupling of sIgE from serum or other body fluids to solid-phase allergens, followed by detection of bound sIgE using enzyme-labeled anti-human IgE, with the level of sIgE indicated by fluorescence intensity.
The main advantages of the ImmunoCAP assay for individual allergens are the quantitative detection of allergen-specific antibodies based on the total IgE standard calibration system of the WHO human reference preparation; by immobilizing a larger number of allergens on the surface of the ImmunoCAP to ensure complete binding of the antibodies, the high sensitivity of the assay and a wide linear detection range are achieved, with good precision and reproducibility. The limit of detection is as low as 0.1 kUA/L (range 0.1–90 kUA/L); there is no interference from allergen-specific IgG antibodies, which improves the accuracy of the IgE assay and somewhat reduces the use of provocation tests, etc., in the diagnosis of allergies; a retrospective study has shown that the ImmunoCAP testing is the most suitable standalone method for confirming allergies to nuts, wheat, and other specific foods. Additionally, it is applicable for detecting allergic reactions to a broad range of allergens (71); its limitations are mainly the small number of allergen molecules available, the incomplete spectrum of IgE responses obtained from a single or a few tests, and the high cost of multiple tests and the large amount of serum samples required.
The proof-of-concept that proteomics microarray methods can be applied to the diagnosis of allergic sensitization was validated in 2002 (72), and subsequent literature has successively validated the same arrays (73–75), commonly referred to as the Immuno Solid-phase Allergen Chip (ISAC) system, which is based on the same principles as the individual ImmunoCAP assay test. The ISAC assay is a highly reproducible and accurate method (76), as a more complete assay platform, ISAC can simultaneously measure sIgE against more than 100 allergens with a micro-volume of serum, while its assay performance is stable and has been evaluated at 23 sites worldwide by different operators, essentially obtaining the same results irrespective of the analytical site, laboratory conditions, operator and microarray batch, and to a certain extent, can distinguish cross-reactivity. The disadvantages of the ISAC assay include lower analytical sensitivity and higher cost per assay, which limits its use in allergy research. Due to the high cost of the test, ISAC testing is currently only performed in a subset of the population in most clinical services in the UK, i.e., patients whose diagnosis remains unclear after SPT and ImmunoCAP testing. However, some researchers still believe that the test is expected to become routine (77).
Microarray technologies have been progressively refined, incorporating recombinant allergens (78), and leading to the development of platforms such as the MeDALL chip, Allergy Explorer (ALEX), Multiple Allergen Simultaneous Testing (MAST), Allergen Micro-Bead Array (ABA), and a novel immunofluorescence chromatography strategy (D-FILA). The MeDALL chip has demonstrated higher sensitivity in detecting sensitizations compared to ImmunoCAP sIgE or SPT (79, 80). ALEX employs nanoparticle technology to immobilize a comprehensive panel of allergen extracts and molecular components on a solid phase, enabling both second-level diagnostics (represented by extract allergens) and third-level diagnostics (represented by single molecules) (81), and is associated with the Allergenius system developed for the interpretation of ISAC results, making it a good diagnostic tool for “bottom-up” allergy diagnosis (82). MAST based on immunoblotting techniques, such as EUROLINE, also represents a valid diagnostic option since MAST and ImmunoCAP were found to be in general agreement with respect to inhalant, food and venom allergens when compared to ImmunoCAP (83). ABA are a good diagnostic tool in the diagnosis of allergies (84), which quantifies IgE binding levels by flow cytometry detection of fluorescent signals from microbeads, ABA can be used to detect IgE responses to inhalant allergens such as dust mites (e.g., Der p 1, Der s 1) and pollen (e.g., Bet v 1, Phl p 5), which can help in the diagnosis of allergic rhinitis and asthma (84, 85). D-FILA based on quantum dot immunochromatography has also been used for sIgE detection, especially for detection of dust mite allergy has a higher sensitivity and accuracy compared to the conventional ImmunoCAP detection system and does not require stringent patient conditions and specialized equipment, which reduces the economic burden for both laboratories and patients (86).
3.2.3 CRD
CRD is a specific diagnostic method based on recombinant or purified allergen components, which is centered on the precise identification of sensitizing proteins through the detection of sIgE against a single allergen component (rather than crude extracts) in the patient's serum (87). Compared to other diagnostic methods, specific sensitizing components can be precisely identified. There are significant differences in the sensitizing proteins of dust mites in different regions and populations, and CRD of dust mite allergens makes up for the lack of other diagnostic strategies in accurately identifying the sensitizing fractions, which is an important guide to accurately formulate dust mite immunotherapy protocols. The recombinant or purified dust mite allergen fractions that are currently widely used in clinical or research applications are shown in Table 3. The current HMD CRD mainly cover the core fractions Der p 1, Der p 2, and Der p 23, which have been validated for their high specificity and phenotypic correlation, and the minor fractions Der p 5, Der p 7, and Der p 21, which have complementary value in specific populations (e.g., patients with negative conventional tests). In the future, the clinical significance of emerging components (e.g., Der p 11, 18) needs to be further validated and the standardization of multi-component combination assays needs to be promoted.

Table 3. Recombinant or purified dust mite allergen components in clinical or research applications.
CRD employs molecular-level identification of key house dust mite allergen components to resolve the cross-reactivity and component ambiguity inherent in traditional extract-based assays. Its core values include: precise differentiation of cross-reactive IgE responses to minimize misdiagnosis; prediction of disease severity and risk of complications; and guidance for personalized AIT to achieve significantly enhanced treatment efficacy. Patients selected for AIT based on CRD profiles demonstrate significantly higher response rates compared to those chosen via conventional diagnostic methods (88).
3.2.4 Environmental allergen testing—guanine testing
Guanine is the end product of nitrogen metabolism in dust mite feces, and its content is significantly and positively correlated with dust mite population density and the concentration of key allergens (e.g., Der p 1). The guanine test is suitable for the rapid assessment of dust mite contamination in households and public places. It is easy to perform, requires no specialized equipment and is suitable for use by non-technical personnel. The results are presented in a semi-quantitative form to facilitate risk classification, detection of dust mite allergens (e.g., Der p 1) or guanine in household dust, and assessment of exposure risk. The guanine test is low-cost and low-cost and time-efficient (results in 15–20 min). However, guanine is also a metabolic byproduct of various arthropods, and biological residues containing guanine can also be found in the environment; these factors may all affect the accuracy of results, resulting in false positives, and it has low sensitivity (limit of detection about 500 ng/g dust) (89).
4 Conclusion and prospects
Technological advances in testing have rendered detection methods more convenient, accurate, and efficient. At the same time, there are many bottlenecks and challenges. The extraction of allergens used for testing, molecular components, operation, detection thresholds and units of different testing methods have not yet been fully standardized, which affects the comparability of results; dust mite allergens contain more than 40 protein fractions, which cannot be fully covered by traditional testing methods, and at the same time, there is cross-reactivity, which makes accurate identification difficult; the development of new technologies is rapid, and there is a lack of clinical validation; the existing tests cannot reflect the change of allergic status or treatment effect in real time; in the future, it is necessary to break through the bottlenecks of cross-reactivity, technology standardization and resource accessibility, high cost, and to optimize the whole chain from diagnosis to management by relying on multi-omics, nano-materials and artificial intelligence.
Although the diagnostic methods for dust mite allergy discussed in this review are supported by existing literature, some studies may exhibit methodological biases due to limitations in sample size, geographic variability, and testing standards. Furthermore, discrepancies in result interpretation and evaluation criteria across different studies underscore the need for future research to establish standardized diagnostic protocols and assessment systems.
Overall, in vivo and in vitro tests have their own advantages and disadvantages. With technological advances and deeper interdisciplinary cooperation, future testing platforms are expected to achieve multimodal data fusion, which is truly accurate, intelligent, and individualized, providing more comprehensive and reliable support for early diagnosis, treatment monitoring, and prognosis assessment of dust mite allergy.
Author contributions
MH: Writing – review & editing, Methodology, Writing – original draft, Investigation. JL: Writing – original draft, Software. WZ: Software, Writing – original draft. SW: Writing – original draft, Investigation. YZ: Writing – review & editing, Investigation. YY: Writing – review & editing, Methodology. XG: Supervision, Funding acquisition, Writing – review & editing, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by General Project under the Scientific Research Program of the Wuxi Municipal Health Commission, grant number M202223.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Spieksma FTM, Dieges PH. The history of the finding of the house dust mite. J Allergy Clin Immunol. (2004) 113(3):573–6. doi: 10.1016/j.jaci.2003.10.064
2. Calderón MA, Linneberg A, Kleine-Tebbe J, De Blay F, de Rojas DHF, Virchow JC, et al. Respiratory allergy caused by house dust mites: what do we really know? J Allergy Clin Immunol. (2015) 136(1):38–48. doi: 10.1016/j.jaci.2014.10.012
3. Miller JD. The role of dust mites in allergy. Clin Rev Allergy Immunol. (2019) 57(3):312–29. doi: 10.1007/s12016-018-8693-0
4. Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. (1999) 104(1):123–33. doi: 10.1172/JCI5844
5. Nakamura T. Reduction of skin barrier function by proteolytic activity of a recombinant house dust mite major allergen der f 1. J Invest Dermatol. (2006) 126:2719–23. doi: 10.1038/sj.jid.5700584
6. Grunstein MM, Veler H, Shan X, Larson J, Grunstein JS, Chuang S. Proasthmatic effects and mechanisms of action of the dust mite allergen, Der p 1, in airway smooth muscle. J Allergy Clin Immunol. (2005) 116(1):94–101. doi: 10.1016/j.jaci.2005.03.046
7. Trian T, Allard B, Dupin I, Carvalho G, Ousova O, Maurat E, et al. House dust mites induce proliferation of severe asthmatic smooth muscle cells via an epithelium-dependent pathway. Am J Respir Crit Care Med. (2015) 191(5):538–46. doi: 10.1164/rccm.201409-1582OC
8. Kato T, Takai T, Fujimura T, Matsuoka H, Ogawa T, Murayama K, et al. Mite serine protease activates protease-activated receptor-2 and induces cytokine release in human keratinocytes. Allergy. (2009) 64(9):1366–74. doi: 10.1111/j.1398-9995.2009.02023.x
9. Shakib F, Ghaemmaghami AM, Sewell HF. The molecular basis of allergenicity. Trends Immunol. (2008) 29(12):633–42. doi: 10.1016/j.it.2008.08.007
10. Pfeffer PE, Corrigan CJ. An imbalance between proteases and endogenous protease inhibitors in eosinophilic airway disease. Am J Respir Crit Care Med. (2017) 195(6):707–8. doi: 10.1164/rccm.201610-2020ED
11. Wills-Karp M, Nathan A, Page K, Karp C. New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunol. (2010) 3(2):104–10. doi: 10.1038/mi.2009.138
12. Hammad H, Smits HH, Ratajczak C, Nithiananthan A, Wierenga EA, Stewart GA, et al. Monocyte-derived dendritic cells exposed to Der p 1 allergen enhance the recruitment of Th2 cells: major involvement of the chemokines TARC\CCL17 and MDC\CCL22. Eur Cytokine Network. (2003) 14(4):219–28. doi: 10.1164/rccm.201610-2020ED
13. Ghaemmaghami A, Gough L, Sewell H, Shakib F. The proteolytic activity of the major dust mite allergen der p 1 conditions dendritic cells to produce less interleukin-12: allergen-induced Th2 bias determined at the dendritic cell level. Clinical & Experimental Allergy. (2002) 32(10):1468–75. doi: 10.1046/j.1365-2745.2002.01504.x
15. Park BL, Cheong HS, Kim LH, Choi YH, Namgoong S, Park H-S, et al. Association analysis of signal transducer and activator of transcription 4 (STAT4) polymorphisms with asthma. J Hum Genet. (2005) 50(3):133–8. doi: 10.1007/s10038-005-0232-1
16. Sivangala R, Sumanlatha G. Cytokines that mediate and regulate immune responses. Innovative Immunology. (2015):1–26.
17. Ryff J-C, Pestka S. Interferons and Interleukins. Pharmaceutical Biotechnology: Fundamentals and Applications. London: Springer (2013). p. 413–37.
18. Ansotegui IJ, Melioli G, Canonica GW, Caraballo L, Villa E, Ebisawa M, et al. Ige allergy diagnostics and other relevant tests in allergy, a world allergy organization position paper. World Aller Organ J. (2020) 13(2):100080. doi: 10.1016/j.waojou.2019.100080
19. Topal SG, Karaman BF, Aksungur VL. Variables affecting interpretation of skin prick test results. Indian J Dermatol Venereol Leprol. (2017) 83:200. doi: 10.4103/0378-6323.192956
20. Wöhrl S, Vigl K, Binder M, Stingl G, Prinz M. Automated measurement of skin prick tests: an advance towards exact calculation of wheal size. Exp Dermatol. (2006) 15(2):119–24. doi: 10.1111/j.1600-0625.2006.00388.x
21. Smith JM. Skin tests and atopic allergy in children. Clin Exper Allergy. (1973) 3(3):269–75. doi: 10.1111/j.1365-2222.1973.tb01333.x
22. Kumar R, Gupta N, Kanuga J, Kanuga M. A comparative study of skin prick test versus serum-specific IgE measurement in Indian patients with bronchial asthma and allergic rhinitis. Indian J Chest Dis Allied Sci. (2015) 57(2):81–5.26591967
23. Portnoy J. Diagnostic testing for allergies. Ann Allergy Asthma Immunol. (2006) 96(1):3–4. doi: 10.1016/s1081-1206(10)61031-9
24. Heinzerling L, Mari A, Bergmann K-C, Bresciani M, Burbach G, Darsow U, et al. The skin prick test–European standards. Clin Transl Allergy. (2013) 3:1–10. doi: 10.1186/2045-7022-3-3
25. Reid MJ, Lockey RF, Turkeltaub PC, Platts-Mills TA. Survey of fatalities from skin testing and immunotherapy 1985–1989. J Allergy Clin Immunol. (1993) 92(1):6–15. doi: 10.1016/0091-6749(93)90030-J
26. Chiaranairungroj M, Chatchatee P, Srituravanich W. The effect of applied force and device design on skin prick test performance. Ann Allergy Asthma Immunol. (2023) 130(3):312–6. doi: 10.1016/j.anai.2022.11.014
27. Beken B, Celik V, Gokmirza Ozdemir P, Yazicioglu M. Think twice before interpreting the skin prick test as age, body mass index, and atopy affect reaction time and size. Int Arch Allergy Appl Immunol. (2021) 182(9):835–43. doi: 10.1159/000515414
28. Karaatmaca B, Sahiner Ü, Soyer Ö, Sekerel B. The impact of skin prick testing on pain perception and anxiety in children and parents. Allergol Immunopathol (Madr). (2021) 49(2):72–9. doi: 10.15586/aei.v49i2.68
29. Gong Z, Yang Z, Wu R, Ye H, Jia M, Zhang N, et al. Comparison of a new skin prick test tape with the conventional skin prick test. J Allergy Clin Immunol. (2019) 143(1):424–7. doi: 10.1016/j.jaci.2018.08.036
30. Kahveci M, Karabulut E, Soyer O, Sahiner UM, Buyuktiryaki B, Sekerel BE. Fine-tuning the use of a skin prick test device. World Allergy Organ J. (2020) 13(5):100122. doi: 10.1016/j.waojou.2020.100122
31. Gorris S, Uyttebroek S, Backaert W, Jorissen M, Schrijvers R, Thompson MJ, et al. Reduced intra-subject variability of an automated skin prick test device compared to a manual test. Allergy. (2023) 78(5):1366–8. doi: 10.1111/all.15619
32. Justo X, Díaz I, Gil J, Gastaminza G. Prick test: evolution towards automated Reading. Allergy. (2016) 71(8):1095–102. doi: 10.1111/all.12921
33. Niederberger V, Eckl-Dorna J, Pauli G. Recombinant allergen-based provocation testing. Methods. (2014) 66(1):96–105. doi: 10.1016/j.ymeth.2013.07.037
34. Mitchell EB, Chapman M, Pope FM, Crow J, Jouhal S, Platts-Mills TE. Basophils in allergen-induced patch test sites in atopic dermatitis. Lancet. (1982) 319(8264):127–30. doi: 10.1016/S0140-6736(82)90379-8
35. Ring J, Kunz B, Bieber T, Vieluf D, Przyilla B. The atopy patch test with aeroallergens in atopic eczema (AE). J Allergy Clin Immunol. (1989) 83(1):195.
36. Fuiano N, Incorvaia C. The atopy patch test: is it time to redefine its significance? Annals of allergy. Asthma & Immunology. (2011) 106(4):278–82. doi: 10.1016/j.anai.2011.01.004
37. Liu Y, Peng J, Zhou Y, Cui Y. Comparison of atopy patch testing to skin prick testing for diagnosing mite-induced atopic dermatitis: a systematic review and meta-analysis. Clin Transl Allergy. (2017) 7:1–12. doi: 10.1186/s13601-017-0178-3
38. Dou X, Kim J, Ni C, Shao Y, Zhang J. Atopy patch test with house dust mite in Chinese patients with atopic dermatitis. J Eur Acad Dermatol Venereol. (2016) 30(9):1522–6. doi: 10.1111/jdv.13655
39. Incorvaia C. The role of the atopy patch test in the diagnosis of allergy. J Allergy Ther. (2015) 06(04). doi: 10.4172/2155-6121.1000e109
40. Darsow U, Laifaoui J, Kerschenlohr K, Wollenberg A, Przybilla B, Wüthrich B, et al. The prevalence of positive reactions in the atopy patch test with aeroallergens and food allergens in subjects with atopic eczema: a European multicenter study. Allergy. (2004) 59(12):1318–25. doi: 10.1111/j.1398-9995.2004.00556.x
41. Turjanmaa K, Darsow U, Niggemann B, Rancé F, Vanto T, Werfel T. EAACI/GA2LEN position paper: present status of the atopy patch test. Allergy. (2006) 61(12):1377–84. doi: 10.1111/j.1398-9995.2006.01136.x
42. Agache I, Bilò M, Braunstahl GJ, Delgado L, Demoly P, Eigenmann P, et al. In vivo diagnosis of allergic diseases—allergen provocation tests. Allergy. (2015) 70(4):355–65. doi: 10.1111/all.12586
43. Kirerleri E, Guler N, Tamay Z, Ones U. Evaluation of the nasal provocation test for its necessity in the diagnosis of nasal allergy to house dust mite. Asian Pac J Allergy Immunol. (2006) 24(2-3):117.17136876
44. Xiao H, Jia Q, Zhang H, Zhang L, Liu G, Meng J. The importance of nasal provocation testing in the diagnosis of dermatophagoides pteronyssinus-induced allergic rhinitis. Am J Rhinol Allergy. (2022) 36(2):191–7. doi: 10.1177/19458924211037913
45. Eguiluz-Gracia I, Testera-Montes A, González M, Pérez-Sánchez N, Ariza A, Salas M, et al. Safety and reproducibility of nasal allergen challenge. Allergy. (2019) 74(6):1125–34. doi: 10.1111/all.13728
46. Chusakul S, Phannaso C, Sangsarsri S, Aeumjaturapat S, Snidvongs K. House-dust mite nasal provocation: a diagnostic tool in perennial rhinitis. Am J Rhinol Allergy. (2010) 24(2):133–6. doi: 10.2500/ajra.2010.24.3441a
47. Choi IS, Kim S-J, Won J-M, Park M-S. Usefulness of house dust mite nasal provocation test in asthma. Allergy Asthma Immunol Res. (2017) 9(2):152–7. doi: 10.4168/aair.2017.9.2.152
48. Bertel F, Mortemousque B, Sicard H, Andre C. Conjunctival provocation test with dermatophagoides pteronyssinus in the diagnosis of allergic conjunctivitis from house mites. J Fr Ophtalmol. (2001) 24(6):581–9.11460053
49. Fauquert JL, Jedrzejczak-Czechowicz M, Rondon C, Calder V, Silva D, Kvenshagen B, et al. Conjunctival allergen provocation test: guidelines for daily practice. Allergy. (2017) 72(1):43–54. doi: 10.1111/all.12986
50. Resch Y, Michel S, Kabesch M, Lupinek C, Valenta R, Vrtala S. Different IgE recognition of mite allergen components in asthmatic and nonasthmatic children. J Allergy Clin Immunol. (2015) 136(4):1083–91. doi: 10.1016/j.jaci.2015.03.024
51. Dente F, Bacci E, Di Franco A, Giannini D, Conti I, Macchioni P, et al. Reproducibility of early and late asthmatic responses to allergen challenge in a large group of asthmatics. Respir Med. (2000) 94(5):441–7. doi: 10.1053/rmed.1999.0760
52. Boulet LP, Gauvreau G, Boulay ME, O’Byrne P, Cockcroft D. The allergen bronchoprovocation model: an important tool for the investigation of new asthma anti-inflammatory therapies. Allergy. (2007) 62(10):1101–10. doi: 10.1111/j.1398-9995.2007.01499.x
53. Gauvreau GM, El-Gammal AI, O'Byrne PM. Allergen-induced airway responses. Eur Respir J. (2015) 46(3):819–31. doi: 10.1183/13993003.00536-2015
54. Schulze J, Reinmüller W, Herrmann E, Rosewich M, Rose MA, Zielen S. Bronchial allergen challenges in children–safety and predictors. Pediatr Allergy Immunol. (2013) 24(1):19–27. doi: 10.1111/pai.12031
55. Fischl A, Eckrich J, Passlack V, Klenke S-K, Hartmann D, Herrmann E, et al. Comparison of bronchial and nasal allergen provocation in children and adolescents with bronchial asthma and house dust mite sensitization. Pediatr Allergy Immunol. (2020) 31(2):143–9. doi: 10.1111/pai.13147
56. Boulet L-P, Côté A, Abd-Elaziz K, Gauvreau G, Diamant Z. Allergen bronchoprovocation test: an important research tool supporting precision medicine. Curr Opin Pulm Med. (2021) 27(1):15–22. doi: 10.1097/MCP.0000000000000742
57. Gauvreau GM, Davis BE, Scadding G, Boulet L-P, Bjermer L, Chaker A, et al. Allergen provocation tests in respiratory research: building on 50 years of experience. Eur Respir J. (2022) 60(2):2102782. doi: 10.1183/13993003.02782-2021
58. Han J, Lu M, Cheng L. Research progress and clinical application of allergen nasal provocation test. J Clin Otorhinolaryngol Head Neck Surg. (2023) 37(6):415. doi: 10.13201/j.issn.2096-7993.2023.06.003
59. Hoffmann H, Santos A, Mayorga C, Nopp A, Eberlein B, Ferrer M, et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy. (2015) 70(11):1393–405. doi: 10.1111/all.12698
60. MacGlashan D Jr. Expression of CD203c and CD63 in human basophils: relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clinical & Experimental Allergy. (2010) 40(9):1365–77. doi: 10.1111/j.1365-2222.2010.03572.x
61. Hemmings O, Kwok M, McKendry R, Santos AF. Basophil activation test: old and new applications in allergy. Curr Allergy Asthma Rep. (2018) 18:1–12. doi: 10.1007/s11882-018-0831-5
62. Ebo DG, Bridts CH, Mertens CH, Sabato V. Principles, potential, and limitations of ex vivo basophil activation by flow cytometry in allergology: a narrative review. J Allergy Clin Immunol. (2021) 147(4):1143–53. doi: 10.1016/j.jaci.2020.10.027
63. Iqbal K, Bhargava K, Skov PS, Falkencrone S, Grattan CE. A positive serum basophil histamine release assay is a marker for ciclosporin-responsiveness in patients with chronic spontaneous urticaria. Clin Transl Allergy. (2012) 2(1):19. doi: 10.1186/2045-7022-2-19
64. Sonder SU, Plassmeyer M, Loizou D, Alpan O. Towards standardizing basophil identification by flow cytometry. Front Allergy. (2023) 4:1133378. doi: 10.3389/falgy.2023.1133378
65. Soleha W, Iswanti FC. Innate immune response to house dust mite allergens in allergic asthma. Mol Cell Biomed Sci. (2021) 5(3):104–14. doi: 10.21705/mcbs.v5i3.217
66. Rockwood J, Morgan MS, Arlian LG. Proteins and endotoxin in house dust mite extracts modulate cytokine secretion and gene expression by dermal fibroblasts. Exper Appl Acarol. (2013) 61:311–25. doi: 10.1007/s10493-013-9703-9
67. Frati F, Incorvaia C, David M, Scurati S, Seta S, Padua G, et al. Requirements for acquiring a high-quality house dust mite extract for allergen immunotherapy. Drug Des Devel Ther. (2012) 6:117–23. doi: 10.2147/dddt.s30908
68. Jacquet A. The role of the house dust mite-induced innate immunity in development of allergic response. Int Arch Allergy Appl Immunol. (2011) 155(2):95–105. doi: 10.1159/000320375
69. Panel N-SE. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. (2010) 126(6):S1–S58. doi: 10.1016/j.jaci.2010.10.007
70. van Hage M, Hamsten C, Valenta R. ImmunoCAP assays: pros and cons in allergology. J Allergy Clin Immunol. (2017) 140(4):974–7. doi: 10.1016/j.jaci.2017.05.008
71. Griffiths RL, El-Shanawany T, Jolles SR, Selwood C, Heaps AG, Carne EM, et al. Comparison of the performance of skin prick, ImmunoCAP, and ISAC tests in the diagnosis of patients with allergy. Int Arch Allergy Appl Immunol. (2017) 172(4):215–23. doi: 10.1159/000464326
72. Hiller R, Laffer S, Harwanegg C, Huber M, Schmidt WM, Twardosz A, et al. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. (2002) 16(3):414–6. doi: 10.1096/fj.01-0711fje
73. Untersmayr E, Lukschal A, Hemmer W, Harwanegg C, Breiteneder H, Jarisch R, et al. Exercise with latex sport bands represents a risk for latex allergic patients. Immunol Lett. (2008) 115(2):98–104. doi: 10.1016/j.imlet.2007.10.008
74. Wöhrl S, Vigl K, Zehetmayer S, Hiller R, Jarisch R, Prinz M, et al. The performance of a component-based allergen-microarray in clinical practice. Allergy. (2006) 61(5):633–9. doi: 10.1111/j.1398-9995.2006.01078.x
75. Ott H, Schroeder C, Stanzel S, Merk H, Baron J. Microarray-based IgE detection in capillary blood samples of patients with atopy. Allergy. (2006) 61(9):1146–7. doi: 10.1111/j.1398-9995.2006.01074.x
76. Lambert C, Sarrat A, Bienvenu F, Brabant S, Nicaise-Roland P, Alyanakian MA, et al. The importance of EN ISO 15189 accreditation of allergen-specific IgE determination for reliable in vitro allergy diagnosis. Allergy. (2015) 70(2):180–6. doi: 10.1111/all.12546
77. Antonicelli L, Massaccesi C, Braschi M, Cinti B, Bilo M, Bonifazi F. Component resolved diagnosis in real life: the risk assessment of food allergy using microarray-based immunoassay. Eur Ann Allergy Clin Immunol. (2014) 46(1):30–4.24702871
78. Williams P, Önell A, Baldracchini F, Hui V, Jolles S, El-Shanawany T. Evaluation of a novel automated allergy microarray platform compared with three other allergy test methods. Clinical & Experimental Immunology. (2016) 184(1):1–10. doi: 10.1111/cei.12721
79. Javaloyes G, Goikoetxea MJ, Nuñez IG, Aranda A, Sanz ML, Blanca M, et al. Pru p 3 acts as a strong sensitizer for peanut allergy in Spain. J Allergy Clin Immunol. (2012) 130(6):1432–4.e3. doi: 10.1016/j.jaci.2012.08.038
80. Skrindo I, Lupinek C, Valenta R, Hovland V, Pahr S, Baar A, et al. The use of the me DALL-chip to assess IgE sensitization: a new diagnostic tool for allergic disease? Pediatr Allergy Immunol. (2015) 26(3):239–46. doi: 10.1111/pai.12366
81. Heffler E, Puggioni F, Peveri S, Montagni M, Canonica GW, Melioli G. Extended IgE profile based on an allergen macroarray: a novel tool for precision medicine in allergy diagnosis. World Allergy Organ J. (2018) 11:7. doi: 10.1186/s40413-018-0186-3
82. Melioli G, Spenser C, Reggiardo G, Passalacqua G, Compalati E, Rogkakou A, et al. Allergenius, an expert system for the interpretation of allergen microarray results. World Allergy Organ J. (2014) 7:15. doi: 10.1186/1939-4551-7-15
83. Konopka E, Ceregra A, Maciorkowska E, Surowska B, Trojanowska I, Roszko-Kirpsza I, et al. Specific IgE antibodies in young children with atopic dermatitis–correlation of multiple allergen simultaneous immunoblot test and ImmunoCap system. Clin Lab. (2016) 62(5):815–21. doi: 10.7754/Clin.Lab.2015.150816
84. Pomponi D, Bernardi ML, Liso M, Palazzo P, Tuppo L, Rafaiani C, et al. Allergen micro-bead array for IgE detection: a feasibility study using allergenic molecules tested on a flexible multiplex flow cytometric immunoassay. PLoS One. (2012) 7(4):e35697. doi: 10.1371/journal.pone.0035697
85. Lupinek C, Wollmann E, Baar A, Banerjee S, Breiteneder H, Broecker BM, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. (2014) 66(1):106–19. doi: 10.1016/j.ymeth.2013.10.008
86. Liang Z-Y, Deng Y-Q, Tao Z-Z. A quantum dot-based lateral flow immunoassay for the rapid, quantitative, and sensitive detection of specific IgE for mite allergens in sera from patients with allergic rhinitis. Anal Bioanal Chem. (2020) 412:1785–94. doi: 10.1007/s00216-020-02422-0
87. Valenta R, Lidholm J, Niederberger V, Hayek B, Kraft D, Grönlund H. The recombinant allergen-based concept of component-resolved diagnostics and immunotherapy (CRD and CRIT). Clin Exper Allergy. (1999) 29(7):896–904. doi: 10.1046/j.1365-2222.1999.00653.x
88. Rodríguez-Domínguez A, Berings M, Rohrbach A, Huang H-J, Curin M, Gevaert P, et al. Molecular profiling of allergen-specific antibody responses may enhance success of specific immunotherapy. J Allergy Clin Immunol. (2020) 146(5):1097–108. doi: 10.1016/j.jaci.2020.03.029
89. Platts-Mills TA, de Weck AL, Aalberse R, Bessot J, Bjorksten B, Bischoff E, et al. Dust mite allergens and asthma—a worldwide problem. J Allergy Clin Immunol. (1989) 83(2):416–27. doi: 10.1016/0091-6749(89)90128-0
90. Henriquez OA, Beste KD, Hoddeson EK, Parkos CA, Nusrat A, Wise SK. House dust mite allergen der p 1 effects on sinonasal epithelial tight junctions. Int Forum Allergy Rhinol. (2013) 3(8):630–5. doi: 10.1002/alr.21168
91. Zhang J, Chen J, Newton GK, Perrior TR, Robinson C. Allergen delivery inhibitors: a rationale for targeting sentinel innate immune signaling of group 1 house dust mite allergens through structure-based protease inhibitor design. Mol Pharmacol. (2018) 94(3):1007–30. doi: 10.1124/mol.118.112730
92. Thongdee D, Rabablert J, Muninnobpamas T, Wasuwat P, Pipatchaipaisan R, Tiewchareon S, et al. T cell responses to der f2 mite allergens in Thai allergic patients. Health. (2011) 3(07):423. doi: 10.4236/health.2011.37070
93. Pawankar R, Holgate ST, Rosenwasser LJ. Allergy Frontiers: Epigenetics, Allergens and Risk Factors. Japan: Springer (2009).
94. Chruszcz M, Chapman MD, Vailes LD, Stura EA, Saint-Remy J-M, Minor W, et al. Crystal structures of mite allergens der f 1 and der p 1 reveal differences in surface-exposed residues that may influence antibody binding. J Mol Biol. (2009) 386(2):520–30. doi: 10.1016/j.jmb.2008.12.049
95. Sundaru H. House dust mite allergen level and allergen sensitization as risk factors for asthma among student in central Jakarta. Med J Indonesia. (2006) 15(1):55–9. doi: 10.13181/mji.v15i1.213
96. Dabbaghzadeh A, Ghaffari J, Feridoni M, Alipour A. House dust mite allergen levels of der p1 and der f1 in houses of asthmatic children. J Pediatr Rev. (2020) 8(4):267–74. doi: 10.32598/jpr.8.4.28.13
97. Cui Y, Yu L, Teng F, Wang N, Zhou Y, Zhang C, et al. Expression of recombinant allergen, der f 1, der f 2 and der f 4 using baculovirus-insect cell systems. Arch Med Sci. (2018) 14(6):1348–54. doi: 10.5114/aoms.2018.79005
98. Jiménez-Feijoo R, Pascal M, Moya R, Riggioni C, Domínguez O, Lózano J, et al. Molecular diagnosis in house dust mite allergic patients suggests clinical relevance of der p 23 in asthmatic children. J Investig Allergol Clin Immunol. (2019) 30(4):10–18176. doi: 10.18176/jiaci.0431
99. Paul WE, Zhu J. How are TH2-type immune responses initiated and amplified? Nat Rev Immunol. (2010) 10(4):225–35. doi: 10.1038/nri2735
100. Zhang Z, Cai Z, Hou Y, Hu J, He Y, Chen J, et al. Enhanced sensitivity of capture IgE-ELISA based on a recombinant Der f 1/2 fusion protein for the detection of IgE antibodies targeting house dust mite allergens. Mol Med Rep. (2019) 19(5):3497–504. doi: 10.3892/mmr.2019.10050
101. Tscheppe A, Breiteneder H. Recombinant allergens in structural biology, diagnosis, and immunotherapy. Int Arch Allergy Appl Immunol. (2017) 172(4):187–202. doi: 10.1159/000464104
102. Hamilton RG. Microarray technology applied to human allergic disease. Microarrays. (2017) 6(1):3. doi: 10.3390/microarrays6010003
103. Wills-Karp M. Allergen-specific pattern recognition receptor pathways. Curr Opin Immunol. (2010) 22(6):777–82. doi: 10.1016/j.coi.2010.10.011
104. Karp CL. Guilt by intimate association: what makes an allergen an allergen? J Allergy Clin Immunol. (2010) 125(5):955–60. doi: 10.1016/j.jaci.2010.03.002
105. Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a toll-like receptor complex protein. Nature. (2009) 457(7229):585–8. doi: 10.1038/nature07548
106. Kim IS, Lee NR, Lee J-S. Suppressive effect of Der p 2 on constitutive neutrophil apoptosis by cytokine secretion of normal and allergic lymphocytes. Korean J Clin Lab Sci. (2016) 48(2):102–8. doi: 10.15324/kjcls.2016.48.2.102
107. Chen KW, FockeTejkl M, Blatt K, Kneidinger M, Gieras A, Dall'Antonia F, et al. Carrier-bound nonallergenic D er p 2 peptides induce I g G antibodies blocking allergen-induced basophil activation in allergic patients. Allergy. (2012) 67(5):609–21. doi: 10.1111/j.1398-9995.2012.02794.x
109. Hsin L, Varese N, Aui PM, Wines BD, von Borstel A, Mascarell L, et al. Accurate determination of house dust mite sensitization in asthma and allergic rhinitis through cytometric detection of Der p 1 and Der p 2 binding on basophils (CytoBas). J Allergy Clin Immunol. (2024) 153(5):1282–91.e10. doi: 10.1016/j.jaci.2024.02.002
110. Vrtala S, Huber H, Thomas WR. Recombinant house dust mite allergens. Methods. (2014) 66(1):67–74. doi: 10.1016/j.ymeth.2013.07.034
111. Villalta D, Scala E, Asero R, Da Re M, Conte M, Buzzulini F. Evaluation and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens using a new multiplex assay: a real-life experience on an Italian population. Eur Ann Allergy Clin Immunol. (2022) 54(3):117–22. doi: 10.23822/EurAnnACI.1764-1489.195
112. Mueller GA, Randall TA, Glesner J, Pedersen LC, Perera L, Edwards LL, et al. Serological, genomic and structural analyses of the major mite allergen Der p 23. Clin Exper Allergy. (2016) 46(2):365–76. doi: 10.1111/cea.12680
113. Semedo FM, Dorofeeva Y, Pires A, Tomaz E, Taborda BL, Inácio F, et al. Der p 23: clinical relevance of molecular monosensitization in house dust mite allergy. J Investig Allergol Clin Immunol. (2019) 29(4):314–6. doi: 10.18176/jiaci.0392
114. Romero-Sánchez L, Otero A, González-Rivas M, Lojo S, González-Quintela A, Vidal C. Der p 23 sensitisation in patients with house dust mite respiratory allergy. Eur Ann Allergy Clin Immunol. (2022) 10:1764–489.264. doi: 10.1111/pai.13829
115. Becker S, Schlederer T, Kramer MF, Haack M, Vrtala S, Resch Y, et al. Real-life study for the diagnosis of house dust mite allergy-the value of recombinant allergen-based IgE serology. Int Arch Allergy Appl Immunol. (2016) 170(2):132–7. doi: 10.1159/000447694
116. An S, Shen C, Liu X, Chen L, Xu X, Rong M, et al. Alpha-actinin is a new type of house dust mite allergen. PLoS One. (2013) 8(12):e81377. doi: 10.1371/journal.pone.0081377
117. Wang X, Pu X, Chen L, Guo M, Zheng C, Wang H. Profiles of IgE sensitization to dust mite allergen components in patients with allergic rhinitis and asthma. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi-J Clin Otolaryngol Head Neck Surg. (2022) 36(8):576–81. Chinese. doi: 10.13201/j.issn.2096-7993.2022.08.002
118. Matricardi PM, KleineTebbe J, Hoffmann H, Valenta R, Hilger C, Hofmaier S, et al. EAACI Molecular allergology user’s guide. Pediatr Allergy Immunol. (2016) 27:1–250. doi: 10.1111/pai.12563
119. Resch Y, Weghofer M, Seiberler S, Horak F, Scheiblhofer S, Linhart B, et al. Molecular characterization of der p 10: a diagnostic marker for broad sensitization in house dust mite allergy. Clin Exper Allergy. (2011) 41(10):1468–77. doi: 10.1111/j.1365-2222.2011.03798.x
120. Khemili S, Kwasigroch JM, Hamadouche T, Gilis D. Modelling and bioinformatics analysis of the dimeric structure of house dust mite allergens from families 5 and 21: Der f 5 could dimerize as Der p 5. J Biomol Struct Dyn. (2012) 29(4):663–75. doi: 10.1080/073911012010525018
121. Vieira CJ, Silva RC, Silveira EF, Fernandes AM, Jaramillo-Hernández DA, Garcés LF, et al. Removal of N-terminal peptide impacts structural aspects of an IgE-reactive recombinant Der p 5. Allergies. (2023) 3(3):184–201. doi: 10.3390/allergies3030012
122. Lahiani S, Dumez M-E, Khemili S, Bitam I, Gilis D, Galleni M. Cross-reactivity between major IgE epitopes of family 5 allergens from dermatophagoides pteronyssinus and Blomia tropicalis. Int Arch Allergy Appl Immunol. (2019) 178(1):10–8. doi: 10.1159/000492871
123. Mueller GA, Edwards LL, Aloor JJ, Fessler MB, Glesner J, Pomés A, et al. The structure of the dust mite allergen der p 7 reveals similarities to innate immune proteins. J Allergy Clin Immunol. (2010) 125(4):909–17.e4. doi: 10.1016/j.jaci.2009.12.016
124. Choina M, Kowal K, Markut-Miotła E, Majsiak E. Dermatophagoides pteronyssinus proteins and their role in the diagnostics and management of house dust mite allergy: exploring allergenic components. Adv Dermatol Allergology/Postępy Dermatologii I Alergologii. (2024) 41(1):339–49. doi: 10.5114/ada.2024.142390
125. Aggarwal P, Senthilkumaran S. Dust mite allergy. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing (2023).
Keywords: dust mite allergy, skin testing, molecular diagnostics, in vivo experiment, in vitro experiments, detection methods
Citation: Han M, Luo J, Zhou W, Wen S, Zhou Y, Ye Y and Ge X (2025) From skin testing to molecular diagnostics: the precision leap in dust mite allergy diagnosis and clinical translation challenges. Front. Allergy 6:1598575. doi: 10.3389/falgy.2025.1598575
Received: 23 March 2025; Accepted: 13 May 2025;
Published: 5 June 2025.
Edited by:
Andrijana Nesic, University of Applied Sciences, GermanyReviewed by:
Domingo Barber, Universidad CEU San Pablo, SpainCristina Rivas- Juesas, Hospital de Sagunto, Spain
Fifa Argentina, Sriwijaya University, Indonesia
Isidora Protic-Rosic, Medical University of Vienna, Austria
Copyright: © 2025 Han, Luo, Zhou, Wen, Zhou, Ye and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoli Ge, YW15YmFjQDEyNi5jb20=
 Ming Han
Ming Han Jindan Luo2
Jindan Luo2