- 1Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 3Shanghai Literature Institute of Traditional Chinese Medicine, Shanghai, China
Background: Allergic rhinitis (AR) is a prevalent allergic disorder. Acupuncture has been widely utilized to alleviate allergic symptoms, and numerous studies have investigated its therapeutic effects on AR. However, due to the challenges associated with establishing appropriate placebo controls, few studies have directly compared acupuncture with sham acupuncture for AR treatment. This trial investigates the comparative effectiveness and tolerability of acupuncture vs. placebo needling for allergic rhinitis patients.
Methods: This clinical trial features a stratified randomization scheme with 1:1 allocation, single-blind assessment, and a total sample size of 84 participants. After screening for inclusion, qualified subjects with perennial allergic rhinitis will be randomly allocated to treatment group(accepting acupuncture, n = 42) or control group (accepting sham acupuncture, n = 42). The intervention will last over a 4-week period. The main efficacy outcome is the change in main symptom severity assessed by the Visual Analogue Scale (VAS) after each week of treatment. Secondary outcomes include the Total Nasal Symptom Score (TNSS), Efficacy Index (%) after each treatment session, time to onset of effect, Rhinitis Quality of Life Questionnaire (RQLQ) scores after each week of treatment, and the additional use rate of anti-allergic medications.
Conclusion: The findings of this study aims to evaluate the effectiveness and safety of acupuncture in treating perennial allergic rhinitis through comprehensive assessment of symptom relief, time-effect relationships, quality of life improvements, and reduction in anti-allergic medication use.
Trial Registration: Chinese Clinical Trial Registry (ChiCTR2400086227).
1 Introduction
Allergic rhinitis (AR) is a non-infectious inflammatory disease characterized by symptoms such as sneezing, rhinorrhea, nasal congestion, and nasal pruritus. The prevalence of self-reported AR ranges from approximately 2%–25% in children and 1%–40% in adults (1, 2). Based on the timing and type of allergen exposure, AR is classified into seasonal allergic rhinitis (SAR), typically triggered by outdoor allergens such as pollens or molds, and perennial allergic rhinitis (PAR), commonly caused by indoor allergens like house dust mites, molds, cockroaches, and animal dander (3, 4). Although non-life-threatening, AR significantly impairs quality of life, work/school performance, and in children, facial and vocal development (5, 6). In addition, AR patients frequently experience mental health issues, including insomnia, anxiety, depression,stress and suicidal, impair cognitive function, and contribute to fatigue, irritability, and reduced quality of life (7). AR also association with comorbidities like asthma underscores the need for effective treatments (8). Current pharmacotherapy (antihistamines, corticosteroids, leukotriene antagonists, etc.) provides rapid but transient symptom relief, often with side effects (9–11). Symptoms typically recur after discontinuation. While immunotherapy can modify disease progression, its months-to-years duration challenges patient compliance. Most patients use medications only intermittently for severe symptoms, highlighting the urgent need for safer, more sustainable therapies to improve long-term outcomes.Acupuncture,as an important therapy in traditional Chinese medicine, has been used for centuries to treat nasal disorders. In 2015, the American Academy of Otolaryngology-Head and Neck Surgery recommended acupuncture as an alternative treatment for AR (12). Our previous study (13) demonstrated that a 10-day course of acupuncture significantly reduced the severity of major nasal and ocular symptoms (P < 0.05). Building on these findings, we designed a single-blinded, randomized, controlled trial with an extended treatment duration to assess the effectiveness and safety of acupuncture in managing AR.
2 Methods and analysis
2.1 Study design and setting
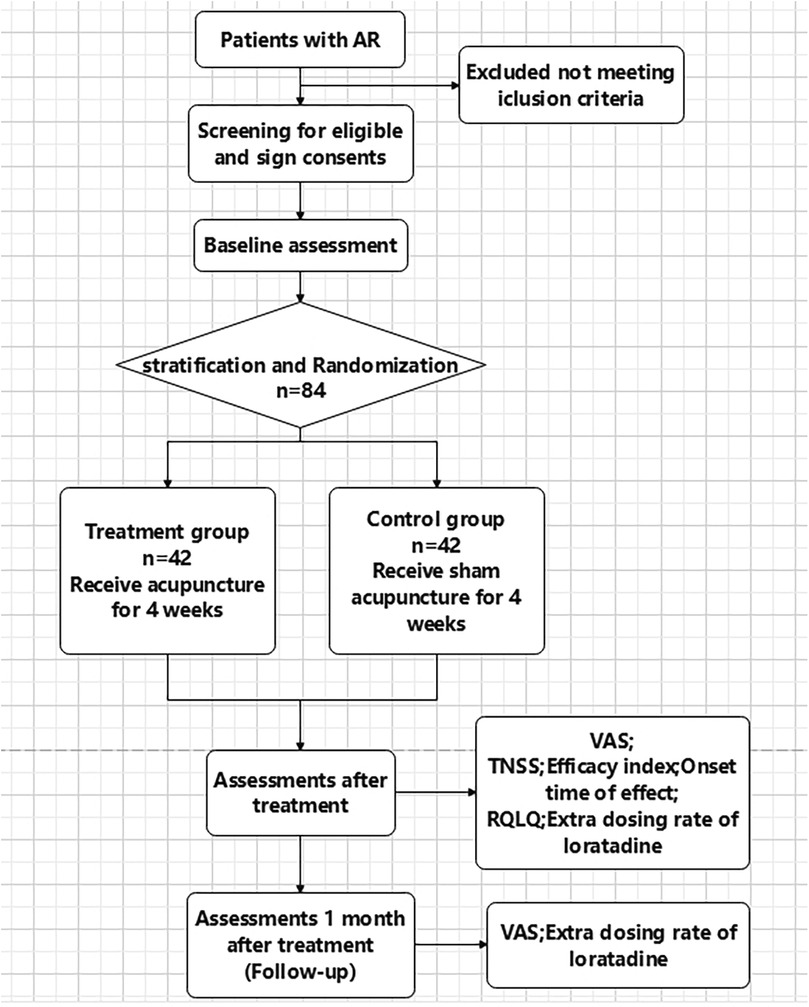
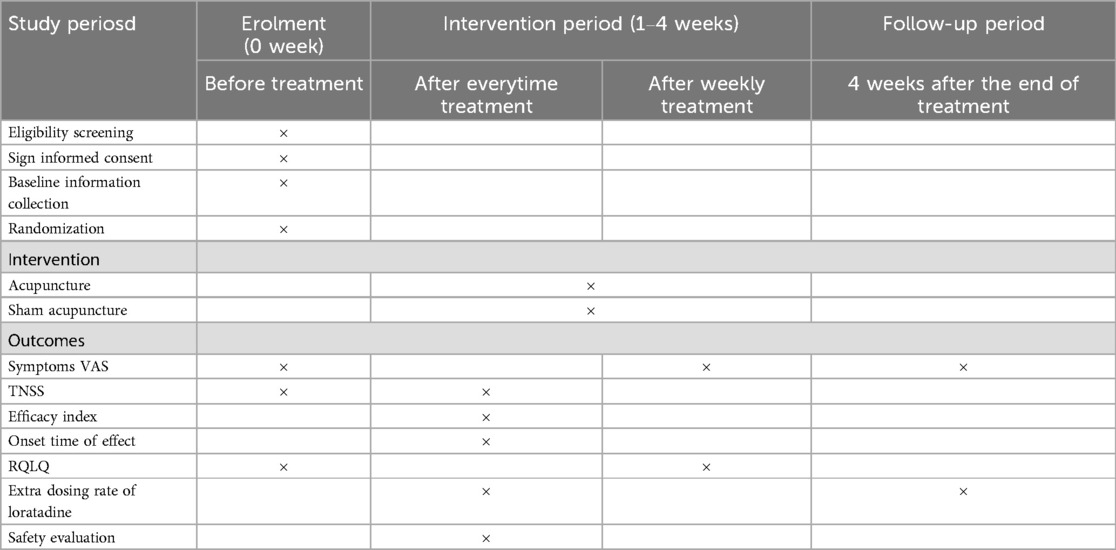
This is a stratified, randomized, single-blinded, controlled trial. The study flowchart is presented in Figure 1, and the schedule of enrolment, interventions, and assessments is presented in Table 1. The study sets in acupuncture department of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, China.
2.2 Participant and consumer involvement
Inclusion criteria includes a diagnosis of perennial allergic rhinitis (14), between the age of 18 and 60. All participants will sign consent documents before participation. Exclusion criteria includes: Severe comorbidities (e.g.,uncontrolled hypertension, diabetes, malignancies, organ dysfunction).Nasal abnormalities, sinusitis, asthma, or prior nasal surgery. Recent use of corticosteroids/Antihistamines (1 month) or immunotherapy (1 year). Prior acupuncture experience or pregnancy/lactation. Withdrawal Criteria includes using rescue medicine 3 times/week,rescue medicine-related Adverse Event ≥Grade 2 (CTCAE v5.0); experiencing severe complications or other serious illnesses and is unable to continue cooperation. Data were analyzed by intention-to-treat (ITT).
All participants provided informed consent and could withdraw anytime. Data were used solely for research purposes.
2.3 Acupoint selection
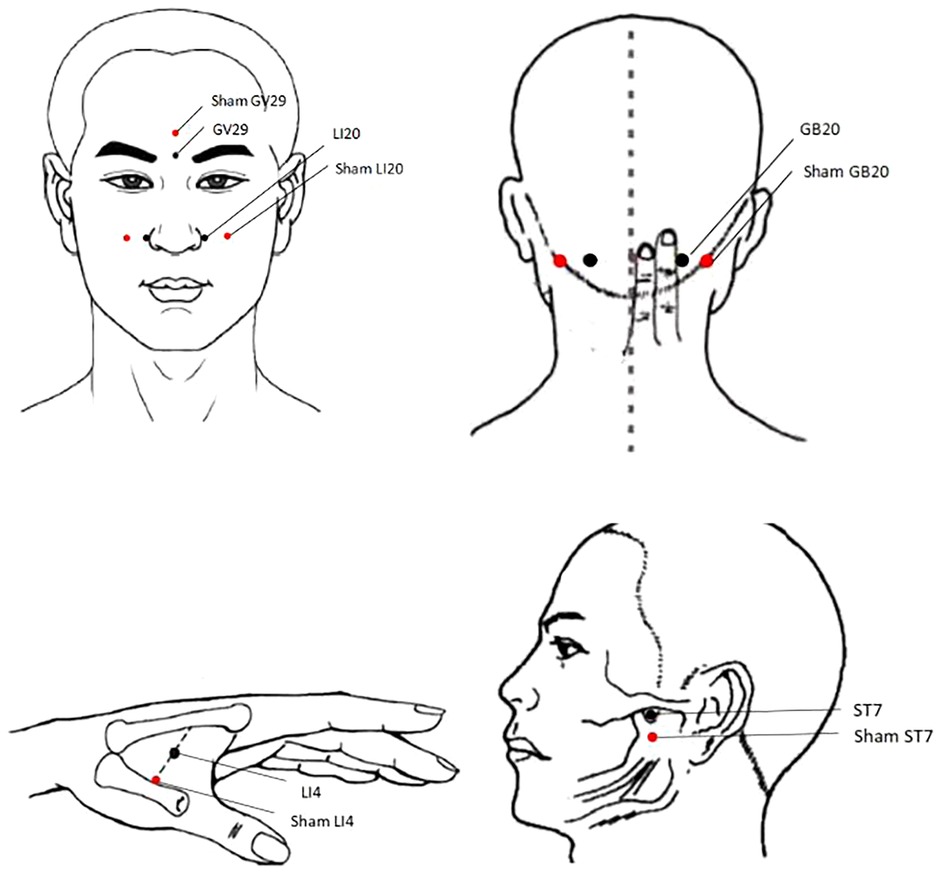
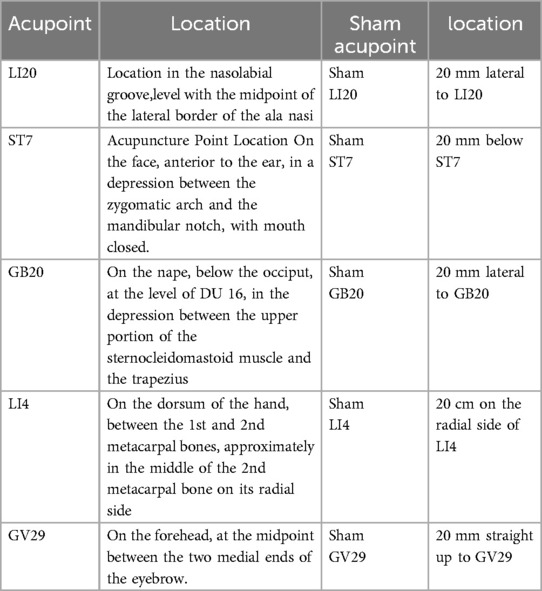
Acupoints: LI20, ST7, GB20, LI4, and GV29 on both side (Figure 2 and Table 2) were selected based on the following rationale: (1) Acupoints for allergic rhinitis recommended in textbooks “Acupuncture” (China Traditional Chinese Medicine Press, 11th Edition) (2) Effective acupoint combinations validated in our previous clinical studies. To ensure standardization and reproducibility of the protocol, we have adopted a fixed acupoint prescription without individualized modifications. While this approach may not fully align with the traditional Chinese medicine principle of individualized treatment based on syndrome differentiation, it enhances the internal validity of our research by allowing assessment of a specific acupoint combination.
2.4 Operator qualifications and standardization
All acupuncture procedures will be performed by three licensed acupuncturists with at least 5 years of clinical experience, each holding nationally recognized certification in acupuncture practice. Prior to study commencement, all operators will undergo a one-week standardization training program covering acupoint location, needle insertion depth, manipulation techniques, and safety protocols. Following training, operator consistency will be evaluated through simulated treatment sessions, requiring at least 85% concordance in key operational parameters (e.g., acupoint location accuracy, needle insertion depth, manipulation frequency) among operators.
2.5 Interventions
Both groups will undergo 12 sessions with eye masks. Participants in the treatment group will undergo acupuncture by 0.25 × 40 mm disposable needles (Guizhou Andy Pharmaceutical Equipment Co., Ltd., Guizhou, China). Following skin sterilization, aseptic adhesive pads will be positioned GB20 on both sides, and acupuncture needles are inserted through the pads to a depth of roughly 10–15 mm, following the direction of the nasal tip. Upon needle placement, gentle and uniform twirling at a frequency of approximately 60 rotations per minute, with a rotation angle of approximately 180°-360° (STRICTA 2010 guidelines) are performed to achieve de qi (a characteristic sensations encompassing soreness, numbness,fullness, and a heavy feeling), which is regarded as a crucial factor for therapeutic effectiveness in acupuncture. The needles are then retained at GB20. Subsequently, aseptic adhesive pads are placed on bilateral LI20, ST7, LI4, and GV29. Acupuncture needles are inserted through the pads to depths of 10–20 mm (10 mm for LI20 and GV29; 20 mm for ST7 and LI4), following the direction of the nasal tip. After insertion, twirling manipulations are performed on all needles to achieve de qi. All needles are retained for 20 minutes.
Participants in the control group will receive sham acupuncture using pragmatic placebo needles (0.25 mm in diameter × 30 mm; “Andy” brand disposable sterile flat-head needles; Guizhou Andy Pharmaceutical Equipment Co., Ltd.) at non-acupoint locations (Figure 2 and Table 2). The procedures and treatment settings in the control group are identical to those in the treatment group,with the exception that neither skin penetration nor needle manipulation to elicit de qi is administered.
Both procedures follow Liu's research (15) and SHARE guidelines (16).
2.6 Rescue medication
In case participants experience any of the following conditions:VAS score ≥7 for any of the primary nasal symptoms for >2 hours;≥10 sneezing episodes within a 24-hour period;Complete nasal obstruction affecting sleep;Symptoms subjectively evaluated by the patient as severely affecting daily activities or work and becoming intolerable.Supplemental loratadine (Xuesu Shanghai Haini Pharmaceutical Co., LTD, Shanghai, China) will be administered 10 mg every day. All investigators and participants must systematically document: (1) All instances of rescue medication including timing, dosage, and reason for use (2) Adverse reactions to rescue medication including symptom description, severity, duration, assessment of relatedness to the rescue medication, and management measures.
These measures ensure patient safety while maintaining treatment efficacy assessment integrity. Collected data will inform comprehensive therapeutic outcome analyses. Participants will be instructed to refrain from using rescue medication for 24 hours prior to Primary outcomes assessment; If a participant has used rescue medication within 24 hours before a scheduled assessment, the assessment will be postponed until at least 24 hours after the last dose. And rescue medication use will be incorporated as a covariate for both primary and secondary outcomes. This approach adjusts its potential confounding effects on symptom scores. To ensure robustness, we will conduct sensitivity analyses. Besides, we will also do exploratory subgroup analysis stratified by whever they use rescue medication or not. All results will be interpreted with this limitation explicitly stated in the Discussion.
2.7 Outcome measures
2.7.1 Primary outcome
Participants will self-report symptom intensity scores before the first treatment and after weekly treatments during the trial. The participants are required to self assess the severity of their four nasal symptoms (sneezing, watery rhinorrhea, nasal congestion, and nasal itching) and 4 non-nasal symptoms (ocular itching, watering, redness, itching of the ears, and/or palate) by using a 10-cm Visual Analogue Scale (VAS) (17) that ranges from “no symptoms” to “worst symptoms ever”. Scores will be assessed at baseline (before treatment initiation) and after each week of treatment.
2.7.2 Secondary outcome
1. The participants self assess the four nasal symptoms (nasal obstruction, rhinorrhea, sneezing, and itching) involved in assigning the total nasal symptom score (TNSS) (18). The symptoms were graded on a five-point scale (where 0 indicates no symptoms, a score of 1 for mild symptoms that are easily tolerated, 2 for awareness of symptoms which are bothersome but tolerable and 3 is reserved for severe symptoms that are hard to tolerate and interfere with daily activity). Score will be calculated before the treatment and after everytime treatment.
2. The efficacy was evaluated according to the improvement of TNSS score
Efficacy index (%) = (TNSS total score before treatment-total score after treatment)/ total score before treatment ×100% (19).
Obvious effect: the nasal symptoms were significantly improved after treatment, and the curative effect was >76%;
Effective: Nasal symptoms improved after treatment, 26%≤curative effect ≤76%;
Ineffective: Nasal symptoms did not significantly reduce after treatment, and the curative effect was <26%.
3. Onset time of effect
After the treatment begin, the number of treatments will be recorded when the Efficacy index reaches 26%.
4. Quality of life score of AR patients:
The participants self assess symtoms by using the Rhinitis Quality of Life Questionnaire (RQLQ) (20), which has 28 questions in 7 items ranked from 0 to 6.
Score will be calculated before the treatment and after weekly treatment.
5. Safety evaluation
Adverse events (AEs) will be recorded on the Case Report Form (CRF), including the initial symptom presentation date, recovery timeline, intensity grading, treatment-associated occurrences, and final outcome status (resolution or persistence). AEs associated with acupuncture include needle stagnation, needle fainting, hematoma, or other discomfort. In the event of AEs, the research team will administer necessary medical care and provide corresponding reimbursement, while simultaneously notifying the Data and Safety Monitoring Board for documentation and review.
2.8 Data analysis
2.8.1 Sample size calculation
The sample size calculation was determined through a priori power analysis conducted with PASS (v15.0.5, NCSS, LLC). Using a two-tailed alpha level of 0.05% and 90% statistical power, with equal group allocation (1:1), the computation was derived from the change in the AR symptoms VAS scores after treatment. Preliminary data indicated mean VAS scores of 33.6 (SD = 5.2) and 29.4 (SD = 4.8) for the treatment and control group, respectively. Accounting for a 20% dropouot rate, each group required 33 participants, yielding a total target enrollment of 84 patients.
2.8.2 Randomization
The randomization sequence was generated by an independent statistician using SAS version 9.4 (SAS Institute, Cary, NC, USA) with a block randomization algorithm (block sizes of 4 and 6) to ensure balanced allocation between groups. Participants were stratified by disease severity into two strata (mild vs. moderate-to-severe) according to the ARIA guidelines (2016 revision) (14), Within each stratum, participants were randomized 1:1 to either the treatment or control group. The randomization sequence was concealed in opaque, sequentially numbered envelopes, which were opened only after baseline data collection to ensure allocation concealment (21). To ensure baseline comparability between groups, we monitored the distribution of key variables, including age (18–40 vs. 41–60 years), sex, disease duration (≤5 vs. >5 years), and baseline VAS scores. Any significant imbalances detected during the study were adjusted for in the final analysis using appropriate statistical methods.
2.8.3 Blinding
To maintain blinding integrity, all investigators will undergo standardized training and adhere to the institutional segregation protocol. All study personnel—including participants, outcome evaluators, and statisticians—remain unaware of group assignments,except the acupuncturist. After the whole treatment period, blinding efficacy will be assessed by asking participants: “What kind of treatment do you think you received? (a) Real acupuncture, (b) Sham acupuncture, (c) Unsure, (d) Not caring.” The success of blinding will then be evaluated using Bang's blinding index (22).
2.8.4 Analytical methods
Statistical analyses will be conducted utilizing the SAS software package, with statistical significance defined as P < 0.05. For continuous data following normal distribution, results will be reported as mean ± SD; non-normally distributed data will be presented as median (IQR). Continuous variables will be assessed by the t-test or Mann–Whitney U-test. Categorical data will be summarized as frequencies (percentages) and evaluated through χ2 tests or Fisher's exact tests. Additionally, time-by-treatment interactions will be analyzed employing generalized estimating equations or mixed-effects models. All statistical tests will use a 95% confidence level, considering results significant when P values are below 0.05.Rescue medication use will be incorporated as a covariate. To ensure robustness, we will conduct sensitivity analyses. Besides, we wil also do exploratory subgroup analysis stratified by whever they use rescue medication or not.
3 Discussion
Allergic rhinitis (AR), the most prevalent allergic disorder globally, remains challenging to manage effectively despite its non-life-threatening nature compared to other medical conditions. This chronic condition significantly impairs patients’ overall health and quality of life, with symptoms extending beyond classical nasal manifestations to include palatal pruritus, postnasal discharge, and persistent coughing. Based on the unified airway theory, AR is strongly associated with asthma, with 15%–38% of AR patients experiencing concurrent asthma (23, 24). Additionally, the relationship between AR and various forms of chronic rhinosinusitis has been increasingly investigated (25).While direct treatment costs for AR are substantial (8, 26), indirect costs—particularly those related to lost work productivity—far exceed direct expenditures (27, 28).
Although pharmacotherapy remains a standard treatment for AR symptom relief, its limitations, such as the need for prolonged use and potential adverse effects, diminish its long-term efficacy. Consequently, there is a growing demand for safer and more effective therapeutic alternatives. It was reported that about 27%–46% of AR patients prefered to complementary and alternative treatments for their allergic symptoms (29–31). Recent studies have demonstrated that alternative therapies can effectively relieve allergic rhinitis symptoms (32, 33),with efficacy comparable to Western medicine (34). In 2023, ICAR emphasized evidence-based therapies as primary strategies, with alternative therapies playing a potential supportive role in select cases (35).
Traditional Chinese medicine first documented AR (“Biqiu”) over 2000 years ago. The Systematic Classic of Acupuncture and Moxibustion (265–316 AD) records LI20 (Yingxiang) acupuncture for Biqiu treatment. Recent studies found that acupuncture may relieve both nasal and non-nasal symptoms of AR patients,improve their life quality (36–38) and in our previous parallel control studies also found that after 10 days of treatment, the scores of major nasal and eye symptoms were cumulative lower than before (13, 39). And the immediate effective rate of the first treatment of acupuncture was 52.1% (40).
The mechanism of acupuncture treatment for allergic rhinitis is still unclear, but studies suggest it may involve the following aspects: (1) Neuromodulation: Stimulation of specific acupoints may modulate both sympathetic and parasympathetic nervous system activity, potentially reducing nasal inflammation, congestion, and hypersecretion (41, 42). (2) Immunomodulation: Acupuncture may suppress inflammatory mediators (43, 44) and help restore Th1/Th2 immune balance, potentially reducing IgE-mediated allergic responses (45, 46). (3) Neuropeptide regulation: Modulation of substance P may reduce nasal hyperreactivity and symptom severity (43).
Now existing studies lacked control groups or combined acupuncture with other treatments, potentially confounding its efficacy in allergic rhinitis (AR). Additionally, few studies have compared acupuncture with sham acupuncture, and the lack of standardized sham methods may obscure its true therapeutic effects. To address these issues, we designed a randomized, single-blind, sham-controlled trial following <The SHARE:SHam Acupuncture REporting guidelines and a checklist in clinical trials > (16), using pragmatic placebo needles on sham acupoints to assess acupuncture's specific effects. This study will also evaluates changes in AR patients’ quality of life and may provide the time-effect relationship to optimize treatment frequency and duration. To ensure ethical and realistic efficacy assessment, participants may use anti-allergic drugs for intolerable symptoms, with detailed medication records.
This study has several limitations that to acknowledged. First, the single-center design may restrict the generalizability of our findings to other clinical settings and more diverse populations,as cultural factors, patient expectations, and practitioner expertise unique to our institution could potentially influence treatment outcomes. Additionally, while the homogeneous patient population and standardized treatment protocols at our center help ensure internal validity, they may not fully capture the variability encountered in real-world clinical practice. To address these limitations and strengthen the evidence base for acupuncture in treating perennial allergic rhinitis, future research should employ multi-center trial designs incorporating diverse healthcare settings, different cultural contexts, and practitioners with varying levels of expertise. Such studies would significantly enhance the external validity of the findings.
Ethics statement
This trial is officially registered with the Chinese Clinical Trial Registry (registration ID: ChiCTR2400086227). The study protocol was developed in accordance with the Standard Protocol Items: Recommendations for Intervention Trials (SPIRIT) 2013 guidelines. This studies involving humans were approved by Medical Ethics Committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine. This studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
J-xY: Writing – original draft, Writing – review & editing. S-rM: Data curation, Writing – review & editing. HC: Formal analysis, Writing – review & editing. Y-lC: Project administration, Supervision, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research is supported by the Shanghai Famous Old Chinese Medicine Experts Academic Experience Research Studio Construction Project (SHGZS-202232) and the Construction of Traditional Chinese Medicine Inheritance and Innovation Development Demonstration Pilot Projects in Pudong New Area - High-Level Research-Oriented Traditional Chinese Medicine Hospital Construction (YC2023-0901).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the world health organization, GA(2)LEN and AllerGen). Allergy. (2008) 63(86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x
2. Katelaris CH, Lee BW, Potter PC, Maspero JF, Cingi C, Lopatin A. Prevalence and diversity of allergic rhinitis in regions of the world beyond Europe and North America. Clin Exp Allergy. (2012) 42:186–207. doi: 10.1111/j.1365-2222.2011.03891.x
3. Dykewicz MS. 7. Rhinitis and sinusitis. J Allergy Clin Immunol. (2003) 111(1):520–9. doi: 10.1067/mai.2003.82
4. Van Cauwenberge P, Bachert C, Passalacqua G, Bousquet J, Canonica GW, Durham SR, et al. Consensus statement on the treatment of allergic rhinitis. European academy of allergology and clinical immunology. Allergy. (2000) 55(2):116–34. doi: 10.1034/j.1398-9995.2000.00526.x
5. Zhu WJ, Liu Y, Wang G, Zheng X, Li X, Wang Y, et al. Interaction effects of asthma and rhinitis control on work productivity and activity impairment: a cross-sectional study. Allergy Asthma Proc. (2022) 42:409–16. doi: 10.2500/aap.2021.42.210052
6. Vandenplas O, Vinnikov D, Blanc PD, Bousquet J, Canonica GW, Carlsen KH, et al. Impact of rhinitis on work productivity: a systematic review. J Allergy Clin Immunol Pract. (2018) 6:1274–86. doi: 10.1016/j.jaip.2017.09.002
7. Safia A, Elhadi UA, Karam M, Merchavy S, Khater A. A meta-analysis of the prevalence and risk of mental health problems in allergic rhinitis patients. J Psychosom Res. (2024) 184:111813. doi: 10.1016/j.jpsychores.2024.111813
8. Akar-Ghibril N, Casale T, Custovic A, Phippatanakul W, Denburg JA, Fokkens WJ, et al. Allergic endotypes and phenotypes of asthma. J Allergy Clin Immunol Pract. (2020) 8:429–40. doi: 10.1016/j.jaip.2019.11.008
9. Brozek JL, Bousquet J, Baena - Cagnani CE, Georgitis SV, Lang DA, Pedersen SE, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. (2010) 126:466–76. doi: 10.1016/j.jaci.2010.06.047
10. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis and management. Lancet. (2000) 356:1255–59. doi: 10.1016/S0140-6736(00)02799-9
11. Lingjaerde O, Ahlfors UG, Bech P, Elmståhl I, von Knorring L, Borg LO, et al. The UKU side effect rating scale: a new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. (1987) 334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x
12. Seidman MD, Gurgel RK, Lin SY, Schwartz SR, Baroody FM, Bonner JR, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. (2015) 152(1, Suppl.):S1–S43. doi: 10.1177/0194599815577560.13
13. Yang JX, Chen YL, Guo X, Wang X. Short-term curative effect of acupuncture on allergic rhinitis. China J Tradit Chin Med Pharm. (2018) 33(3):134–6. doi: 10.1016/S0254-6272(13)60115-6
14. Brozek JL, Bousquet J, Agache I, Passalacqua G, Sterk PJ, Levy O, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. (2017) 140(4):950–8. doi: 10.1016/j.jaci.2017.03.050
15. Liu Z, Liu Y, Xu H, Cao X, Li Y, Li X, et al. Effect of electroacupuncture on urinary leakage among women with stress urinary incontinence:a randomized clinical trial. JAMA. (2017) 317(24):2493–501. doi: 10.1001/jama.2017.7220
16. Ma P, Liu X, Liu , Guo Y, Zhou K, Bian Z, et al., SHARE Workgroup, et al. The SHARE: sham acupuncture REporting guidelines and a checklist in clinical trials. J Evid Based Med. (2023) 16(4):428–31. doi: 10.1111/jebm.12560
17. Del Cuvillo A, Santos V, Montoro J, Sánchez-Bayuela MJ, Marín MÁ, Sánchez-Bayuela FJ. Allergic rhinitis severity an be assessed using a visual analogue scale in mild, moderate and severe. Rhinology. (2017) 55(1):34–8. doi: 10.4193/Rhin16.025
18. Yonekura S, Okamoto Y, Sakurai D, Iinuma T, Sakurai T, Yoneda R, et al. Efficacy of desloratadine and levocetirizine in patients with cedar pollen-induced allergic rhinitis: a randomized, double-blind study. Int Arch Allergy Immunol. (2019) 180:274–83. doi: 10.1159/000503065
19. Er Z, Yan B, Tou H, Wai J, Za K, Bian Z, et al. Diagnosis and treatment of allergic rhinitis and a recommended scheme (2004 Lanzhou). Zhong Hua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2005) 40:166–7.
20. Watelet JB, Gevaert P, Holtappels G, Bachert C, Leynen C, Schröckh S, et al. Collection of nasal secretions for immunological analysis. European Archives Otorhinolaryngol. (2004) 261(5):242–6. doi: 10.1007/s00405-003-0691-y
21. Schulz KF, Altman DG, Higgins JPT, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel-group randomised trials. Br Med J. (2010) 340:c332. doi: 10.1136/bmj.c332
22. Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials. Control Clin Trials. (2004) 25(2):143–56. doi: 10.1016/j.cct.2003.10.016
23. Leynaert B, Bousquet J, Neukirch C, Enander D, Pirinen E, Simpson A, et al. Perennial rhinitis: an independ-ent risk factor for asthma in nonatopic subjects: results from the European community respiratory health survey. J Allergy Clin Immunol. (1999) 104:301–4. doi: 10.1016/S0091-6749(99)70370-2
24. Chitsuthipakorn W, Hoang MP, Kanjanawasee D, Wanitpongpan P, Kietpeerakool K. Combined medical therapy in the treatment of allergic rhinitis: systematic review and meta-analyses. Int Forum Allergy Rhinol. (2022) 12:1480–502. doi: 10.1002/alr.23015
25. Zuberbier T, Lotvall J, Simoens S, Agacheva S, Cecchi M, GA(2)LEN Executive Committee. Economic burden of inadequate management of allergic diseases in the European union: a GA(2) LEN review. Allergy. (2014) 69:1275–9. doi: 10.1111/all.12470
26. Haahtela T, Valovirta E, Hannuksela M, Uusitalo K, Kulmala M, Laippala R. Finnish allergy programme 2008 - 2018 - time to act and change the course. Allergy. (2008) 63:634–45. doi: 10.1111/j.1398-9995.2008.01712.x
27. Thanaviratananich S, Cho SH, Ghoshal A G, Sritamras S, Sritamras P, APBORD Study Group. Burden of respiratory disease in Thailand: results from the APBORD observational study. Medicine (Baltimore). (2016) 95:4090. doi: 10.1097/MD.0000000000004090
28. Yoo KH, Ahn HR, Park JK, Shin SH, Kim HJ, Lee HJ, et al. Burden of respiratory disease in Korea: an observational study on allergic rhinitis, asthma, COPD, and rhinosinusitis. Allergy Asthma Immunol Res. (2016) 8:527–34. doi: 10.4168/aair.2016.8.6.527
29. Schafer T, Riehle A, Wichmann HE, Ring J. Alternative medicine in allergies- prevalence, patterns of use, and costs. Allergy. (2002) 57:694–700. doi: 10.1034/j.1398-9995.2002.23469.x
30. Passalacqua G, Bousquet PJ, Carlsen K-H, Kemp J, Lockey RF, Niggemann B, et al. ARIA Update: i-systematic review of complementary and alternative medicine for rhinitis and asthma. J Allergy Clin Immunol. (2006) 117:1054–62. doi: 10.1016/j.jaci.2005.12.1308
31. Krouse JH, Krouse HJ. Patient use of traditional and complementary therapies in treating rhinosinusitis before consulting an otolaryngologist. Laryngoscope. (1999) 109:1223–7. doi: 10.1097/00005537-199908000-00007
32. Briskey D, Ebelt P, Rao A. The effect of levagen+ (palmitoylethanolamide) supplementation on symptoms of allergic rhinitis-a double-blind placebo-controlled trial. Nutrients. (2023) 15(23):5202. doi: 10.3390/nu15234940
33. Jo HR, Sung WS, Jung CY, Kim SH, Lee HJ, Park JW. Effectiveness and safety of electric heating moxibustion for perennial allergic rhinitis: a pilot, randomized, assessor-blind trial. Complement Ther Med. (2022) 68:102835. doi: 10.1016/j.ctim.2022.102835
34. Yamprasert R, Chanvimalueng W, Mukkasombut N, Thongprayoon D, Kullavanijaya P. Ginger extract versus loratadine in the treatment of allergic rhinitis: a randomized controlled trial. BMC Complement Med Ther. (2020) 20(1):119. doi: 10.1186/s12906-020-2875-z
35. Wise SK, Damask C, Roland LT, Arshad SH, Bachert C, Bousquet J, et al. International consensus statement on allergy and rhinology: allergic rhinitis – 2023.international. Forum Allergy Rhinol. (2023) 13(4):293–859. doi: 10.1002/alr.23090
36. Kang J, Son M, Kim Y, Shin JH, Park HJ, Lee HJ, et al. Intranasal low-level laser therapy versus acupuncture treatment for allergic rhinitis: a randomized, noninferiority trial. Explore (NY). (2022) 18(6):676–82. doi: 10.1016/j.explore.2022.02.006
37. Li YJ, Zong M, Ding LF, Cui YQ, Sun XM. Efficacy of Chinese medicine acupoint application combined with montelukast on children with perennial allergic rhinitis: a randomized controlled trial. Chin J Ntegr Med. (2020) 26(11):845–52. doi: 10.1007/s11655-020-3099-2
38. Liu LL, Gong Z, Tang L, Wang Y, Zhang X, Li Y, et al. A novel and alternative therapy for persistent allergic rhinitis via intranasal acupuncture: a randomized controlled trial. Eur Arch Otorhinolaryngol. (2023) 280(6):2773–83. doi: 10.1007/s00405-022-07793-x
39. Yang JX, Liu LM, Hu Junwei , Chen YL. Observation on the cumulative effect of acupuncture in the treatment of allergic rhinitis. Beijing J Tradit Chin Med Sci. (2022) 41(6):602–5.
40. Jin Q, Chen YL, Yang JX, Zheng NZ, Feng SY. Immediate effect of sensory transfer technique in the treatment of allergic rhinitis with superficial acupuncture. Shanghai J Acupuncture Moxibustion. (2020) 39(5):570–5.
41. McDonald JL, Cripps AW, Smith PK, Smith CA, Xue CC, Golianu B. The anti-inflammatory effects of acupuncture and their relevance to allergic rhinitis: a narrative review and proposed model. Evid Based Complement Alternat Med. (2013) 2013:591796. doi: 10.1155/2013/591796
42. Li YW, Li W, Wang ST, Liu CZ. The autonomic nervous system: a potential link to the efficacy of acupuncture. Front Neurosci. (2022) 16:1038945. doi: 10.3389/fnins.2022.1038945
43. McDonald ER, Smith PK, Smith CA, Xue CC, Cripps AW. Effect of acupuncture on house dust mite specific IgE, substance P, and symptoms in persistent allergic rhinitis. Ann Allergy Asthma Immunol. (2016) 117(6):497–505. doi: 10.1016/j.anai.2016.04.002
44. Sun D, Jiang M, Lu M, Ma X, Shang G, Wang Y. The effect of warm acupuncture on EOS, IgE, inflammatory factors, and T lymphocyte subsets in patients with allergic rhinitis of lung qi deficiency and cold-type. Altern Ther Health Med. (2023) 29(8):271–7.37573600
45. Rao YQ, Han NY. Therapeutic effect of acupuncture on allergic rhinitis and its effects on immunologic function. Chin J Otorhinolaryngol Integr Trad Western Med. (2006) 14(4):287–9.
Keywords: acupuncture, allergic rhinitis, sham acupuncture, efficacy, life quality
Citation: Yang J-x, Ming S-r, Chen H and Chen Y-l (2025) Clinical efficacy on acupuncture for perennial allergic rhinitis: a study protocol for a randomized clinical trial. Front. Allergy 6:1600032. doi: 10.3389/falgy.2025.1600032
Received: 25 March 2025; Accepted: 26 May 2025;
Published: 16 June 2025.
Edited by:
Pongsakorn Tantilipikorn, Mahidol University, ThailandReviewed by:
Qingwu Wu, Third Affiliated Hospital of Sun Yat-sen University, ChinaQinwei Fu, McMaster University, Canada
Copyright: © 2025 Yang, Ming, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue-lai Chen, Y2hlbnl1ZWxhaUAxNjMuY29t
†These authors have contributed equally to this work
 Jia-xin Yang
Jia-xin Yang Shu-ren Ming
Shu-ren Ming Hui Chen3
Hui Chen3 Yue-lai Chen
Yue-lai Chen