- 1Department of Allergy and Clinical Immunology, Unidade Local de Saúde de São João, Porto, Portugal
- 2Department of Immunoallergology, Unidade Local de Saúde do Algarve, Faro, Portugal
- 3Department of Public Health and Forensic Sciences and Medical Education, Faculty of Medicine, University of Porto, Porto, Portugal
Hereditary angioedema (HAE) is a rare genetic disorder characterized by recurrent episodes of subcutaneous and/or submucosal angioedema. Pregnancy and breastfeeding may be associated with an increased frequency of attacks. Plasma-derived C1 inhibitor (pdC1INH) is the recommended first-line treatment for long-term prophylaxis (LTP) in these special populations. The pdC1INH currently available in Portugal is one intravenous (IV) formulation not approved for LTP, as are the other IV and subcutaneous (SC) formulations. This report documents the first cases of SC pdC1INH use during pregnancy and breastfeeding in Portugal. It describes two cases of 37-year-old women with HAE type 1 treated with SC pdC1INH as LTP during pregnancy and lactation. Both patients had been previously treated with tranexamic acid. In the first case, the patient was started on IV pdC1INH at 8 weeks' gestation due to clinical deterioration. Due to difficult IV access and inability to space out administrations, SC pdC1INH at a dose of 4,000 U (∼43.5 U/kg) every 72 h was started at 21 weeks' gestation. Administration intervals were progressively increased to 96 and later 120 h. LTP was continued throughout lactation. In the second case, LTP was not administered during pregnancy. However, after delivery, the patient experienced a worsening of angioedema episodes during breastfeeding, which persisted despite tranexamic acid treatment. SC pdC1INH was started six months postpartum at a dose of 2,000 U (∼45 U/kg) twice weekly. The administration interval was later increased to 120 h. Both patients remained free of angioedema episodes and reported no systemic adverse events. The safety of SC pdC1INH was consistent with reports in the literature. Overall, these positive results support the future use of SC pdC1INH in a broader population of pregnant and lactating women in clinical practice.
Introduction
Hereditary angioedema (HAE) is a rare genetic disorder characterized by recurrent episodes of localized cutaneous and submucosal angioedema (1). The most commonly affected areas include the skin (especially the extremities, face, and genitals) and the gastrointestinal tract. Other sites may be affected, and laryngeal attacks are of particular concern due to their potential to cause fatal airway obstruction (1, 2). The recurrent, unpredictable and potentially life-threatening nature of HAE attacks has a significant impact on patients' quality of life (3), resulting in physical, emotional, and psychological distress.
HAE can be classified into HAE due to C1 inhibitor (C1INH) deficiency (HAE-C1INH) and HAE with normal C1 inhibitor (HAE-nC1INH). Patients with HAE-C1INH may have low plasma levels of C1INH protein (HAE type 1) or normal to elevated plasma levels of dysfunctional C1INH protein (HAE type 2) (4).
Regarding treatment, the primary goals in HAE are to achieve complete disease control and enable patients to lead normal, unrestricted lives (1, 5). Often, this can only be achieved with the use of long-term prophylaxis (LTP). In Portugal, the following drugs are commercially available for LTP: lanadelumab and berotralstat as first-line therapies; danazol and antifibrinolytics (e.g., tranexamic acid), which are classified as second-line therapies according to international guidelines; and an intravenous (IV) plasma-derived C1INH (pdC1INH), which is only approved for on-demand treatment and short-term prophylaxis (STP) (1, 6).
Pregnancy may increase, decrease, or have no effect on the frequency and severity of HAE attacks (1). However, an increase in disease activity during pregnancy appears to be the most common scenario (7, 8). Notably, the course of HAE in a previous pregnancy does not reliably predict how the disease will evolve in subsequent pregnancies (7). The course of the disease during gestation is also unpredictable, with some studies reporting a worsening of angioedema attacks during the first trimester, and others reporting a higher frequency of attacks in the last trimesters (9–11).
During eutocic delivery, the risk of experiencing an angioedema attack is low, and STP is not routinely recommended (12). However, STP is recommended in cases of dystocic delivery, such as cesarean sections or instrumented vaginal deliveries (7).
Breastfeeding may be associated with an increased frequency of angioedema attacks in women with HAE, possibly related to elevated serum prolactin levels (10).
During pregnancy and lactation, therapeutic options for the management of HAE are limited. Attenuated androgens are absolutely contraindicated during pregnancy as they may lead to virilization of the female fetus (13). In addition, danazol should also be avoided during lactation, as it can potentially decrease milk production (14). The safety of lanadelumab and berotralstat during pregnancy and lactation has not been established (1, 15). Antifibrinolytics may be used during pregnancy despite lack of proven efficacy and potential increase in thrombotic risk (1, 7). Tranexamic acid appears to be safe during lactation (16). PdC1INH has been used during pregnancy and lactation with proven safety and efficacy and is the recommended LTP in these special populations (1).
In Portugal, only IV pdC1INH is currently available for on-demand prophylaxis and STP. Subcutaneous (SC) pdC1INH has not been approved for commercialization by INFARMED and can only be used by special authorization.
The aim of this report was to describe the first cases of women with HAE treated with SC pdC1INH for LTP during pregnancy and lactation in Portugal.
Case presentation
This report describes the cases of two women with HAE followed at the Department of Allergy and Clinical Immunology of two Portuguese centers, who were treated with SC pdC1INH during pregnancy and lactation. The first patient started treatment in March 2024 during pregnancy, and the second patient started treatment in June 2024 during lactation.
Case 1
Patient information, clinical findings, and diagnostic assessment
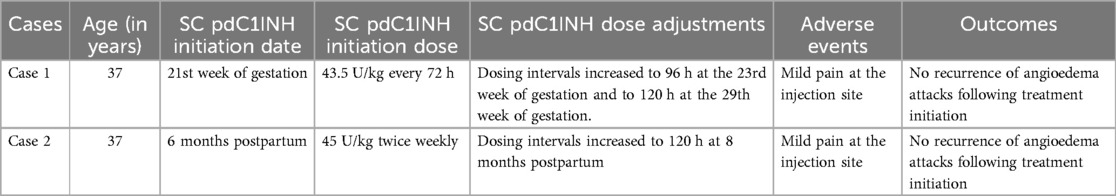
The first case is a 37-year-old woman with HAE type 1 (Table 1). Her diagnosis was established at the age of 20 by C4 and C1INH measurement and later confirmed by genetic testing, which identified the p.Ala275Thr variant in exon 5 of the SERPING1 gene.
At the age of 19, shortly after starting an estrogen-containing contraceptive pill, the woman experienced her first angioedema attack, which affected the abdomen and feet. During her first pregnancy at the age of 32, she experienced a moderate worsening of the disease, especially during the first trimester, with weekly abdominal HAE attacks. She was treated with on-demand IV pdC1INH, but declined the proposed option of LTP with the same drug.
At the age of 36, she was on LTP with tranexamic acid (1,000–1,500 mg/day) with only partial HAE control. She experienced angioedema attacks approximately every 2 months, primarily peripheral and typically induced by stress. This treatment was discontinued when she decided to become pregnant. After the first few weeks of pregnancy, there was a marked increase in HAE attacks, which significantly affected the patient's quality of life. In the 8th week of pregnancy, a joint decision was made to start IV pdC1INH at a dose of 1,500 U (∼16 U/kg) twice a week.
At 15 weeks' gestation, as the patient remained attack-free but had difficult IV access, the frequency of IV pdC1INH administration was reduced to once a week. However, due to the recurrence of HAE attacks, a special authorization for the use of SC pdC1INH was requested from INFARMED.
Therapeutic intervention and outcome
After INFARMED's approval, the patient started LTP with SC pdC1INH at a dose of 4,000 U (∼43.5 U/kg) every 72 h at 21 weeks' gestation. After three hospital administrations and patient and family education, the treatment was successfully transitioned to home administration.
At 24 weeks' gestation, the administration interval was increased to 96 h, and at 29 weeks' gestation, it was further increased to every 120 h (i.e., every five days). The patient remained attack free throughout this period and reported no side effects other than mild pain at the injection site.
A healthy baby boy was delivered at 38 weeks gestation. STP with IV pdC1INH 1,500 U was administered prior to cesarean section and the delivery was uneventful. After delivery and throughout lactation, LTP was continued with SC pdC1INH 4,000 U (∼43.5 U/kg) every five days. The patient remained free of angioedema attacks and with good disease control, as evidenced by an Angioedema Control Test (AECT) score of 15, an Angioedema Quality of Life Questionnaire (AE-QoL) score of 20, and an Angioedema Activity Score (AAS) of zero at five weeks postpartum, all collected in paper forms during medical appointments, as part of the routine clinical assessment.
Case 2
Patient information, clinical findings, and diagnostic assessment
The second case is another 37-year-old woman with HAE type 1 (Table 1). She experienced her first episode of angioedema at the age of 15 following a dental procedure, with swelling predominantly in the limbs and abdomen. The diagnosis was established at the age of 32 through the detection of C1-INH protein deficiency. Genetic testing further identified the SERPING1 c.1480C > T mutation. The patient was started on LTP with tranexamic acid, which resulted in a reduction in the frequency and severity of angioedema attacks.
During her first pregnancy at the age of 32, the disease remained under control without the need for LTP and delivery was uneventful. However, during breastfeeding, the frequency of angioedema attacks increased and LTP with tranexamic acid was initiated with a good clinical response.
During her second pregnancy, at the age of 36, the disease was managed exclusively with on-demand treatment. In the second trimester, the patient experienced an increase in the frequency of episodes, with abdominal angioedema occurring once or twice a week. These episodes resolved with medical leave and rest. Delivery occurred at 38 weeks' gestation under STP with IV pdC1INH and was uneventful.
During breastfeeding, the patient experienced an increase in the frequency of abdominal and peripheral angioedema attacks, prompting the initiation of LTP with tranexamic acid at a dose of 2,000 mg daily. Despite prophylaxis, she continued to experience weekly angioedema episodes lasting an average of two days each, with a significant impact on her quality of life. Special authorization for the use of SC pdC1-INH was requested and granted.
Therapeutic intervention and outcome
At six months postpartum, treatment with SC pdC1INH was initiated at a dose of 2,000 U (∼45 U/kg) administered twice weekly. After five in-hospital administrations and training, the patient transitioned to home administration. The only adverse event reported was tolerable pain in the injection site.
Following the introduction of SC pdC1INH LTP, the patient remained free of angioedema attacks. Two months later, based on the good clinical response as evidenced by an AECT score of 16, an AE-QoL score of zero, and an AAS of zero, combined with a reduction in breastfeeding frequency, the SC pdC1INH administration interval was increased to every 120 h (i.e., every five days).
Currently, both women continue to breastfeed, with treatment duration adjusted according to clinical progression.
Discussion
The efficacy and safety of SC pdC1INH as LTP at the doses of 40 and 60 U/kg administered twice weekly was previously demonstrated in the COMPACT (Clinical Study for Optimal Management of Preventing Angioedema with Low-Volume Subcutaneous C1-Inhibitor Replacement Therapy) open-label extension study (17). In this study, four women were exposed to SC pdC1INH during the first pregnancy trimester without complications, and all delivered healthy babies (17). Additionally, a few published case reports have further confirmed the effectiveness and safety of SC pdC1INH as HAE prophylaxis during pregnancy and breastfeeding (18, 19).
In this report, the first patient experienced an increase in angioedema attacks during the first pregnancy trimester. Prophylaxis with IV pdC1INH successfully achieved complete disease control. However, the need for twice-weekly hospital visits and the difficulty in IV access hampered treatment adherence. It should be noted that several IV pdC1INH preparations are available, but Berinert© is not approved for LTP (6) and Cinryze©, although approved for LTP, is not available in Portugal (20).
The second patient experienced an increase in the frequency of angioedema attacks during the second pregnancy trimester, but the symptoms were controlled with rest. However, during breastfeeding, she experienced weekly angioedema attacks before the introduction of SC pdC1INH.
The administered doses, 43.5 UI/kg in the first case and 45 UI/KG in the second, did not exactly match the recommended dosage of 60 UI/kg due to limitations related to the fixed-dose format of the syringes used for subcutaneous administration (1).
In both cases, stopping breastfeeding itself may have reduced the number of angioedema episodes in the postpartum period (7). Nevertheless, breastfeeding is widely recommended due to its significant benefits for the newborn (1).
Since the initiation of SC pdC1INH, both patients have remained completely asymptomatic, consistent with findings from a similar case report (18). The complete clinical response allowed the administration interval to be extended to every five days while maintaining effective symptom control. Apart from mild injection site pain, no relevant side effects were reported.
Although SC pdC1INH has been shown to be effective and safe during pregnancy and lactation, its use in Portugal is limited by the requirement to obtain it from abroad through a special authorization, resulting in delays in access to this life-changing treatment.
To the authors' knowledge, these are the first two cases of SC pdC1INH use in Portugal. The authors emphasize the importance of individualized treatment and highlight the potential of SC pdC1INH as a therapeutic option for other pregnant and lactating women in the country.
Patient perspective
Case 1: “When I started using subcutaneous Berinert®, the changes in my life were significant: I gained more independence (no need to go to the hospital for intravenous medication) and more security (my disease is under control with effective medication and no side effects). I feel that my life has returned to normal, which has had a very positive impact on both my physical and mental well-being.”
Case 2: “Berinert® has brought me a great improvement in my quality of life. Today, I feel more confident, more active, and freer.”
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ALP: Investigation, Writing – original draft, Writing – review & editing. NS: Conceptualization, Writing – review & editing. EDdC: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors declare that financial support was received from CSL Behring Portugal for the language editing and publication of this article.
Acknowledgments
The authors acknowledge the editorial support provided by Joana Cavaco-Silva, Ph.D. (jo.cvsilva@gmail.com).
Conflict of interest
NS has received advisory board and/or speaker fees from Abbvie, Novartis, CSL Behring, Leo Pharma and Sanofi and congress support from Medinfar, Biocryst, Bial, Roxall and Mylan. EDdC has received advisory board and/or lecture fees from Takeda, Kalvista, CSL Behring and Biocryst and congress support from Takeda and CSL Behring. ALP has received congress support from Biocryst, Takeda and CSL Behring.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maurer M, Magerl M, Betschel S, Aberer W, Ansotegui IJ, Aygoren-Pursun E, et al. The international WAO/EAACI guideline for the management of hereditary angioedema-the 2021 revision and update. Allergy. (2022) 77(7):1961–90. doi: 10.1111/all.15214
2. Busse PJ, Christiansen SC. Hereditary angioedema. N Engl J Med. (2020) 382(12):1136–48. doi: 10.1056/NEJMra1808012
3. Banerji A, Davis KH, Brown TM, Hollis K, Hunter SM, Long J, et al. Patient-reported burden of hereditary angioedema: findings from a patient survey in the United States. Ann Allergy Asthma Immunol. (2020) 124(6):600–7. doi: 10.1016/j.anai.2020.02.018
4. Reshef A, Buttgereit T, Betschel SD, Caballero T, Farkas H, Grumach AS, et al. Definition, acronyms, nomenclature, and classification of angioedema (DANCE): AAAAI, ACAAI, ACARE, and APAAACI DANCE consensus. J Allergy Clin Immunol. (2024) 154(2):398–411 e1. doi: 10.1016/j.jaci.2024.03.024
5. Maurer M, Aygoren-Pursun E, Banerji A, Bernstein JA, Balle Boysen H, Busse PJ, et al. Consensus on treatment goals in hereditary angioedema: a global Delphi initiative. J Allergy Clin Immunol. (2021) 148(6):1526–32. doi: 10.1016/j.jaci.2021.05.016
6. Direção-Geral da Saúde do Serviço Nacional de Saúde. NORMA CLÍNICA: 009/2019—Abordagem Diagnóstica e Terapêutica do Angioedema Hereditário (2019). Available at: https://normas.dgs.min-saude.pt/wp-content/uploads/2019/12/abordagem-diagnostica-e-terapeutica-do-angioedema-hereditario.pdf (Accessed December 01, 2024).
7. Caballero T, Canabal J, Rivero-Paparoni D, Cabanas R. Management of hereditary angioedema in pregnant women: a review. Int J Womens Health. (2014) 6:839–48. doi: 10.2147/IJWH.S46460
8. Caires A, Mesquita AM, Dias De Castro E. The impact of pregnancy in patients with hereditary angioedema—a Portuguese sample. Allergy. (2019) 74(Suppl. 106):188. doi: 10.1111/all.13959
9. Martinez-Saguer I, Rusicke E, Aygoren-Pursun E, Heller C, Klingebiel T, Kreuz W. Characterization of acute hereditary angioedema attacks during pregnancy and breast-feeding and their treatment with C1 inhibitor concentrate. Am J Obstet Gynecol. (2010) 203(2):131 e1–7. doi: 10.1016/j.ajog.2010.03.003
10. Czaller I, Visy B, Csuka D, Fust G, Toth F, Farkas H. The natural history of hereditary angioedema and the impact of treatment with human C1-inhibitor concentrate during pregnancy: a long-term survey. Eur J Obstet Gynecol Reprod Biol. (2010) 152(1):44–9. doi: 10.1016/j.ejogrb.2010.05.008
11. Chinniah N, Katelaris CH. Hereditary angioedema and pregnancy. Aust N Z J Obstet Gynaecol. (2009) 49(1):2–5. doi: 10.1111/j.1479-828X.2008.00945.x
12. Gonzalez-Quevedo T, Larco JI, Marcos C, Guilarte M, Baeza ML, Cimbollek S, et al. Management of pregnancy and delivery in patients with hereditary angioedema due to C1 inhibitor deficiency. J Investig Allergol Clin Immunol. (2016) 26(3):161–7. doi: 10.18176/jiaci.0037
13. Maurer M, Magerl M. Long-term prophylaxis of hereditary angioedema with androgen derivates: a critical appraisal and potential alternatives. J Dtsch Dermatol Ges. (2011) 9(2):99–107. doi: 10.1111/j.1610-0387.2010.07546.x
15. Hsu FI, Lumry W, Riedl M, Tachdjian R. Considerations in the management of hereditary angioedema due to C1-INH deficiency in women of childbearing age. Allergy Asthma Clin Immunol. (2022) 18:64. doi: 10.1186/s13223-022-00689-9
16. Gilad O, Merlob P, Stahl B, Klinger G. Outcome following tranexamic acid exposure during breastfeeding. Breastfeed Med. (2014) 9(8):407–10. doi: 10.1089/bfm.2014.0027
17. Craig T, Zuraw B, Longhurst H, Cicardi M, Bork K, Grattan C, et al. Long-term outcomes with subcutaneous C1-inhibitor replacement therapy for prevention of hereditary angioedema attacks. J Allergy Clin Immunol Pract. (2019) 7(6):1793–802 e2. doi: 10.1016/j.jaip.2019.01.054
18. Andarawewa S, Aygoren-Pursun E. Subcutaneous C1-inhibitor concentrate for prophylaxis during pregnancy and lactation in a patient with C1-INH-HAE. Clin Case Rep. (2021) 9(3):1273–5. doi: 10.1002/ccr3.3743
19. Mumneh N, Li HH, Dang J, Chiao J. Clinical experience with subcutaneous C1-esterase inhibitor prophylactic therapy in pregnant patients with hereditary angioedema. Allergy Asthma Proc. (2019) 40(5):358. doi: 10.2500/108854119827259875
20. European Medicines Agency. Cinryze. Available at: https://www.ema.europa.eu/en/documents/product-information/cinryze-epar-product-information_en.pdf
Keywords: hereditary angioedema, lactation, long-term prophylaxis, plasma-derived C1 inhibitor, pregnancy
Citation: Pinhal AL, Santos N and Dias de Castro E (2025) Case reports of subcutaneous pdC1INH in pregnancy and lactation: expanding treatment options for hereditary angioedema in Portugal. Front. Allergy 6:1604440. doi: 10.3389/falgy.2025.1604440
Received: 1 April 2025; Accepted: 10 June 2025;
Published: 2 July 2025.
Edited by:
Michael Rudenko, London Allergy and Immunology Centre, United KingdomReviewed by:
Eli Mansour, State University of Campinas, BrazilNatasa Teovska Mitrevska, Remedika General Hospital, North Macedonia
Copyright: © 2025 Pinhal, Santos and Dias de Castro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana Luísa Pinhal, YW5hbHVpc2FwaW5oYWxAZ21haWwuY29t
 Ana Luísa Pinhal
Ana Luísa Pinhal Natacha Santos
Natacha Santos Eunice Dias de Castro1,3
Eunice Dias de Castro1,3