- 1Epidemiology, GSK, Mississauga, ON, Canada
- 2Analysis Group, Inc., Boston, MA, United States
- 3Division of Allergy and Immunology, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati School of Medicine, Cincinnati, OH, United States
- 4Global Real-World Evidence & Health Outcomes Research, GSK, Collegeville, PA, United States
Background: Limited data exist on the burden of myeloproliferative, lymphocytic and idiopathic subtypes of hypereosinophilic syndrome (M-HES, L-HES and I-HES) and the characteristics of patients with HES receiving biologic therapies. This analysis aimed to further characterize these subtypes and explore the impact of biologics in a real-world European setting.
Methods: This was a post hoc subgroup analysis of a retrospective, non-interventional, chart review (GSK ID: 214657) across five European countries. Index date was first clinical visit during January 2015–December 2019 (after or at time of HES diagnosis). Patients with HES aged ≥6 years with ≥1-year follow-up from index were included. Demographics, disease characteristics, diagnostic assessments, comorbidities, types of treatment, clinical manifestations, clinical outcomes and HES-related healthcare resource utilization were summarized for HES overall and subtypes. Oral corticosteroid (OCS) use and clinical manifestations/outcomes were assessed 12-months pre- and post-biologics.
Results: The analysis included 280 patients with I-HES (n = 155), M-HES (n = 66), L-HES (n = 42) and chronic eosinophilic leukemia (n = 2). The most common clinical manifestations were fatigue (54.2% I-HES, 52.4% L-HES, 42.4% M-HES), skin itch (36.4% M-HES, 35.7% L-HES, 33.5% I-HES) and pain (31.0% L-HES, 30.3% M-HES, 27.1% I-HES). Biologic use was highest with L-HES (64.3%), followed by I-HES (43.9%) and M-HES (34.8%). Clinical response rates were highest for the I-HES subtype (75.5%; 66.7% L-HES, 63.6% M-HES). Hospitalizations were highest for L-HES (45.2%; 30.3% M-HES, 25.8% I-HES). The annualized rate of OCS prescriptions reduced by 56.8% (0.44–0.19 per person-year) and the proportion of patients with ≥1 clinical response increased 3.6-fold (6.5%–23.4%) between the pre- and post-biologics periods.
Conclusions: All HES subtypes had a substantial disease burden and were commonly associated with fatigue, skin itch and pain. I-HES appeared to be more responsive to treatment than L-HES and M-HES. Biologic use for HES led to more patients experiencing clinical responses and was OCS-sparing.
1 Introduction
Hypereosinophilic syndromes (HES) are rare and heterogenous blood disorders characterized by persistent hypereosinophilia (>1,500 cells/µl) and eosinophilic tissue infiltration leading to organ damage (1–3) HES prevalence has been estimated to be 1.5 cases per 100,000 people in Europe (4), but regional estimates vary (North America: 0.32–6.3 cases per 100,000; UK: 0.15–0.89 cases per 100,000) (5, 6). HES is diagnosed when known causes of secondary hypereosinophilia are excluded (1, 3, 7, 8) and can be classified into several subtypes, including myeloproliferative, lymphocytic and idiopathic variants (M-HES, L-HES and I-HES, respectively), based on distinct molecular characteristics (1, 3, 7, 9).
M-HES is diagnosed in patients whose hypereosinophilia is caused by a defined myeloid malignancy or who have clinical characteristics consistent with one (7). L-HES is a form of reactive HES, diagnosed when patients present with an aberrant immunophenotype in ≥1 T-cell subset, increased type 2 cytokine production and signs of HES-related organ damage; clonal T-cell receptor gene rearrangement may or may not be evident (3). I-HES is a diagnosis of exclusion, made when the criteria for HES are fulfilled and all known primary and secondary causes of hypereosinophilia have been excluded (1).
HES-related disease flares, defined as a period of worsening of HES-related symptoms, have a significant adverse impact on health and quality of life and may be life-threatening. The goals of HES treatment are to control disease symptoms and minimize tissue damage (10). Therapy typically relies on high-dose maintenance oral corticosteroids (OCS), while immunosuppressant and/or cytotoxic therapies may be added for OCS-resistant HES (11). OCS can be associated with adverse effects and may be less effective in patients with M-HES and L-HES (11–13). In the first-line setting, M-HES may also be treated with tyrosine kinase inhibitors such as imatinib (14). Several biologics have demonstrated reductions in circulating eosinophils resulting in clinical benefits (14, 15). Mepolizumab is a biologic targeting interleukin (IL)-5, which is approved in Europe and the US for inadequately-controlled HES without an identifiable non-hematologic cause (16, 17). In a Phase III trial, mepolizumab was associated with a statistically significant reduction in the proportion of patients experiencing flares, or having to withdraw from the trial, vs. placebo (18). Targeted therapies, such as mepolizumab, can contribute to OCS-sparing strategies and could improve clinical outcomes for patients (19–21).
A retrospective, non-interventional study of patients with HES from France, Germany, Italy, Spain and the UK, identified a substantial disease burden including comorbidities such as asthma, anxiety or depression, hypertension and chronic sinusitis with nasal polyps (22). Additionally, high rates of healthcare resource utilization (HCRU) were reported, with approximately 30% of patients requiring hospitalization, 26% requiring emergency department visits and 87% requiring outpatient visits for HES-related reasons over a median follow-up of 2.4 years (22). These findings highlight the need for optimized HES management strategies.
Given that HES is a rare disorder there are limited data available on clinical outcomes and treatment patterns for patients and there are very few published studies reporting such data in patients with M-HES, L-HES or I-HES subtypes (9, 13, 23, 24). There is some evidence that symptoms differ according to subtype. For example, I-HES appears to be particularly associated with left-ventricular abnormalities, respiratory issues and gastrointestinal symptoms; M-HES with splenomegaly, anemia and fatigue; and L-HES with skin lesions/rashes and gastrointestinal symptoms (9). Additionally, there are limited data globally from clinical trials or real-world usage of emerging biologic therapies for HES and their clinical benefits, including OCS-sparing (15, 18, 19, 25, 26). Therefore, this post hoc subgroup analysis aimed to characterize the burden of HES by disease subtype, to gain better understanding of the characteristics of patients with HES receiving biologic therapies and to explore the clinical impact of biologic therapy in a real-world European setting.
2 Materials and methods
2.1 Study design and population
These were post hoc subgroup analyses of a retrospective, non-interventional, longitudinal, physician panel-based chart review study (GSK ID: 214657) of patients diagnosed with HES across five European countries (Germany, Italy, France, Spain and the UK), the methodology of which has been reported previously (22). Briefly, diagnosis date was defined as the date of HES diagnosis and index date was defined as the first clinical visit for any reason occurring between January 2015 and December 2019 (patients could have an existing diagnosis of HES or be newly diagnosed at the index date). For patients diagnosed before the index date, a pre-index period was utilized from which patient demographics and baseline clinical characteristics were identified. Follow-up included the period from index date to the earliest occurrence of death, loss to follow-up or end date of chart abstraction. The last date of follow-up was 14 July, 2021. The study included patients with a physician-confirmed HES diagnosis aged ≥6 years at time of diagnosis with ≥1-year follow-up from index, except where follow-up ended due to death.
2.2 Data source and data collection
Longitudinal patient-level data were obtained from demographically and geographically diverse, nationally representative populations. Physicians (N = 121) were the primary healthcare provider for ≥1 eligible patient with HES, with access to their medical records. Physicians abstracted medical charts of randomly selected patients. Physicians were from different practice settings (academic or community-based) and included targeted specialties (allergy, hematology, immunology, internal medicine, pulmonology and rheumatology) from each participating country. Only anonymized data were collected. Physicians were blind to the identity of the study sponsor and vice versa.
Demographic and baseline characteristic information were obtained from patient charts from the pre-index period (between HES diagnosis and the index date for patients diagnosed before index; these characteristics were also collected for patients diagnosed at index). Clinical manifestations and outcomes of HES and HCRU were assessed from the index date until the end of follow-up. Comorbid conditions and treatment patterns were assessed from HES diagnosis until end of follow-up, unless otherwise stated.
2.3 Subgroup analysis of patient characteristics and outcomes by HES subtype
Data were summarized for the overall HES population and M-HES, L-HES and I-HES subtypes (defined post hoc) as follows: patient demographics and disease characteristics (including age at diagnosis, age at index and disease duration), diagnostic assessments, comorbidities, types of treatment (e.g., OCS, immunosuppressant/cytotoxic agents, biologics or other therapies), clinical manifestations, clinical outcomes and HES-related HCRU. Clinical outcomes included the occurrence of flares, defined as a worsening of HES-related symptoms or blood eosinophil counts requiring therapy escalation (dose increase or additional/new therapy) and responses to treatment. A complete response was defined as physician-reported improved or resolved symptoms and a normal eosinophil count (≤500 cells/ml) (27). Partial response reflected improved symptoms and eosinophil count that was improved but was not in the reference range and required more therapy.
2.4 Subgroup analysis of patients receiving biologic therapy
Demographics, disease characteristics, comorbidities and HCRU were summarized for the subgroup of patients who had received biologic treatment between HES diagnosis and end of follow-up. For patients with a non-missing date for ≥1 biologics prescription pre- and post-biologic initiation periods were defined. The pre-biologics period was defined as 12 months before and including the initiation of biologics. Only events and person-years after HES diagnosis were included. The post-biologics period was defined as 12 months after the initiation of biologics, or until death or end of follow-up. The following outcomes were summarized for ≤12 months pre- and post- biologics initiation: OCS use (annualized rate of prescriptions and proportion of patients receiving ≥1 prescription), clinical manifestations (proportion of patients with manifestation) and clinical outcomes (flares and clinical response to treatment).
2.5 Sample size and statistical analysis
The subgroup analyses were defined post hoc. All study outcomes were summarized using descriptive statistics and no comparative testing was performed. Real-world flare-free survival (FFS) was assessed over the 6 years following the initial diagnosis date (6-year restricted mean survival time) using Kaplan–Meier analysis. Data analysis was performed using SAS Enterprise Guide Version 7.15 (SAS Institute Inc., Cary, NC, USA).
3 Results
3.1 Patient characteristics and outcomes by HES subtype
Of the 280 patients for whom data were collected, 265 had a physician-defined subtype and 15 had unknown disease subtype. Of those with a physician-defined subtype, 155 had I-HES, 66 had M-HES, 42 had L-HES and 2 had chronic eosinophilic leukemia. Overall patient demographics and physician characteristics have been published previously (22). HES subtype distribution varied between countries, although in all countries most patients had I-HES [51.9% in Spain (lowest), 61.5% in Italy (highest); 55.4% overall]. The highest proportion of patients with M-HES was in Spain (34.6%; 23.6% overall) and with L-HES was in the UK (22.6%; 15% overall).
A higher proportion of patients with M-HES were male (75.8%) and were older at diagnosis (mean age 47.0 years) than in the overall population (65.0% and 42.4 years, respectively) and other subtypes (Table 1). The median number of diagnostic assessments was similar across HES subtypes; however, types of testing performed varied according to disease subtype (Table 1). For example, allergy testing (83.3%) and kidney function testing (95.2%) were performed most frequently among patients with L-HES, whereas bone marrow aspiration and biopsy (80.3%) and molecular genetic testing (71.2%) were more frequent in patients with M-HES. Median duration of HES (2.5–2.9 years) and length of follow-up (2.3–2.5 years) were similar across subtypes. The highest median [interquartile range (IQR)] blood eosinophil count was observed in the M-HES subtype [2,300.0 (850.0, 4,500.0) cells/µl]; counts in L-HES and I-HES were 1,570.0 and 1,890.0 cells/µl, respectively (Table 1).
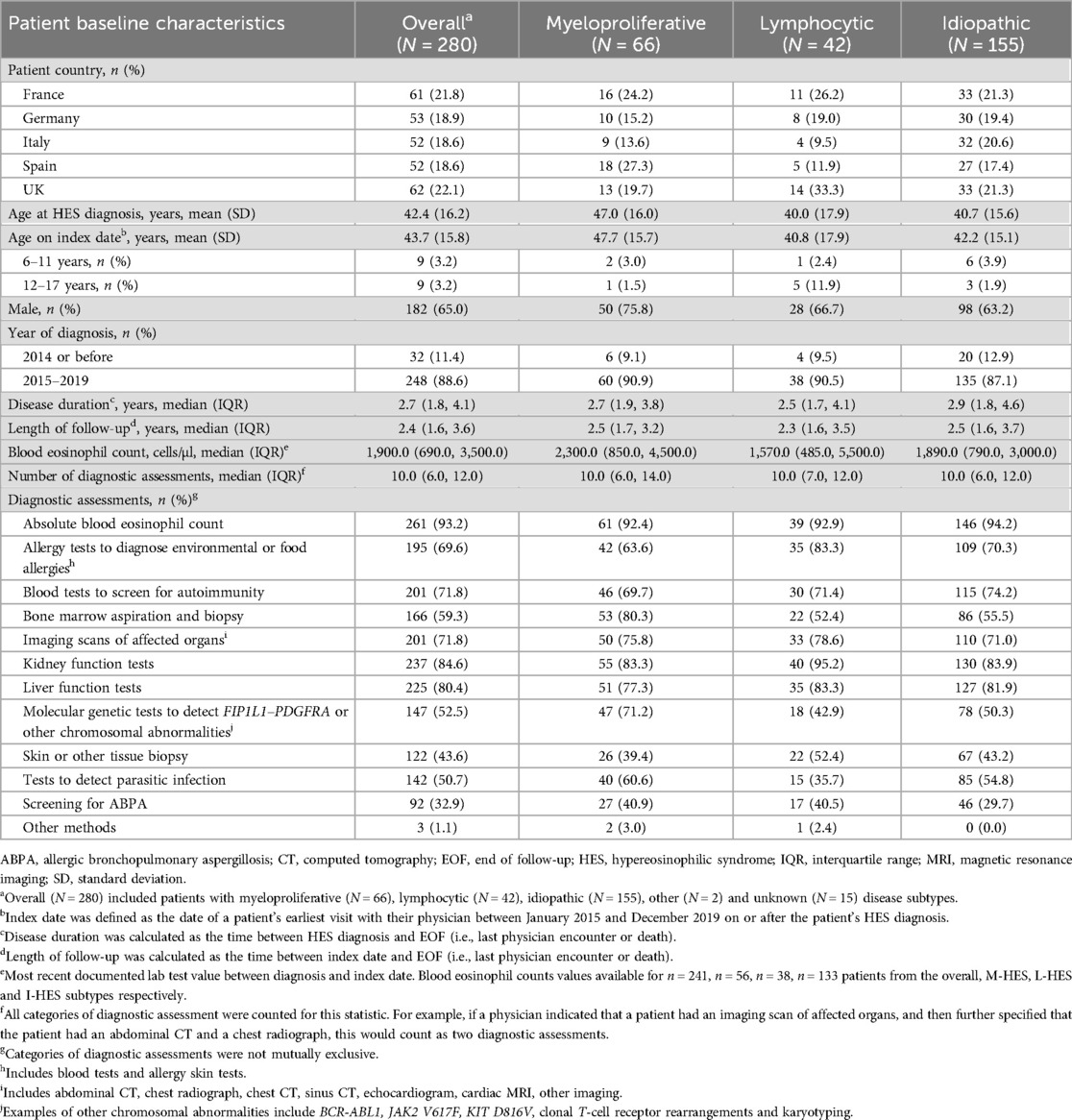
Table 1. Baseline patient demographics and disease characteristics across HES subtypes (22).
Asthma, anxiety or depression, nasal polyps and hypertension were the most common comorbidities across subtypes (Table 2). Asthma was most common in I-HES (52.3%) and less common in M-HES and L-HES subtypes (25.8%–47.6%, respectively). Anxiety or depression affected more patients with M-HES (48.5%) than L-HES (33.3%) or I-HES (34.2%). Nasal polyps were most common with I-HES (38.7%) and less common with the other subtypes (23.8%–27.3%); while rates of hypertension were reported in around one-third of patients (31.6%–36.4%) across subtypes (Table 2).
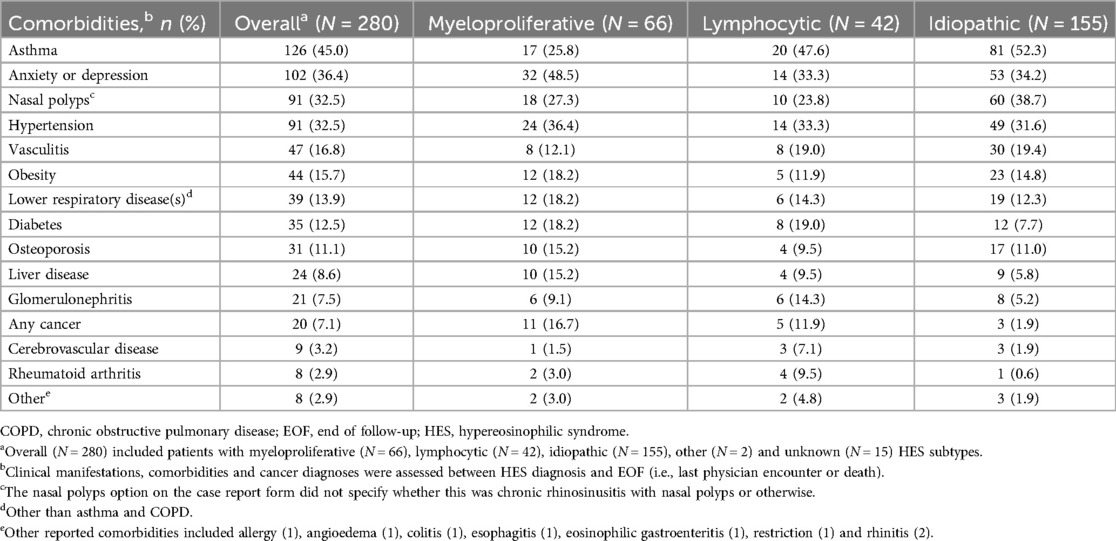
Table 2. Patient comorbidities across HES subtypes (assessed between HES diagnosis and end of follow-up).
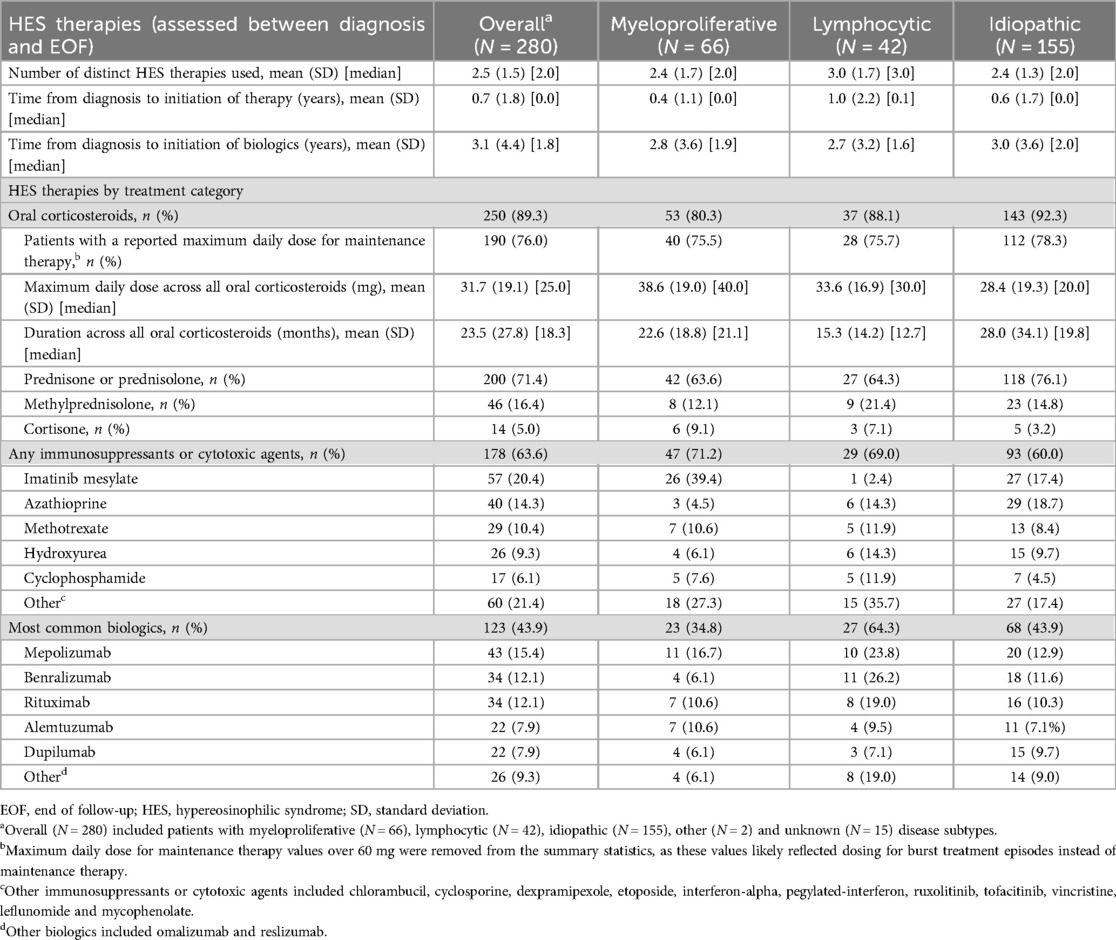
Mean [standard deviation (SD)] time from diagnosis to initiation of HES therapy was shortest for M-HES [0.4 (1.1) years] and longest for L-HES [1.0 (2.2) years; Table 3]. OCS were received by 80.3%–92.3% of patients across all subtypes and were most commonly used among patients with I-HES; however, the mean (SD) maximum daily OCS dose was lowest in the I-HES subtype [28.4 (19.3) mg] and highest with the M-HES subtype [38.6 (19.0) mg; Figure 1; Table 3]. The use of immunosuppressive and cytotoxic therapies was most common among the M-HES (71.2%) and L-HES (69.0%) subtypes. This included the targeted therapy imatinib, which was used by 39.4% of patients with M-HES, 2.4% with L-HES and 17.4% with I-HES (Table 3). Biologic use was highest in patients with L-HES (64.3%), followed by I-HES (43.9%) and M-HES (34.8%). The most common biologic therapies were mepolizumab (15.4%), benralizumab (12.1%) and rituximab (12.1%). Mean (SD) time from HES diagnosis to biologic initiation ranged from 2.7 (3.2) years for L-HES to 3.0 (3.6) years for I-HES (Table 3). The most common therapies reported as ongoing at end of follow-up were OCS in I-HES, immunosuppressants/cytotoxic therapies in M-HES and biologic therapies in L-HES (Figure 1; Supplementary Table 1).
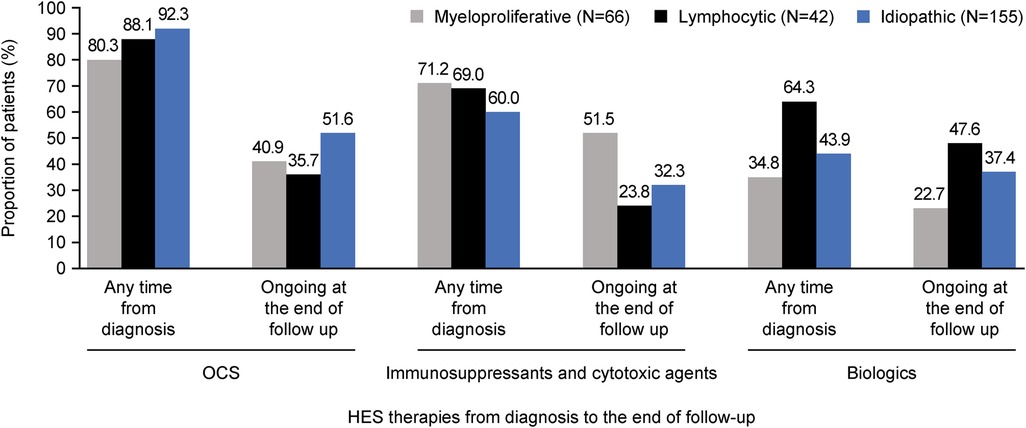
Figure 1. Summary of proportion of patients with OCS, immunosuppressive/cytotoxic and biologic use at any time from diagnosis and ongoing at end of follow-up, across HES subtypes. HES, hypereosinophilic syndrome; OCS, oral corticosteroids.
A median (IQR) of 3.0 (1.0, 5.0) distinct clinical manifestations was observed for each HES subtype. The patterns of organ involvement varied across subtypes but constitutional, lung and skin manifestations were common across all (Figure 2). Gastrointestinal involvement was highest in M-HES (34.8%), cardiovascular involvement was highest in L-HES (26.2%) and neuropsychiatric involvement was highest in I-HES (16.8%). The most common distinct clinical manifestations were fatigue (54.2% I-HES, 52.4% L-HES and 42.4% M-HES), skin itch (36.4% M-HES, 35.7% L-HES and 33.5% I-HES) and pain (31.0% L-HES, 30.3% M-HES and 27.1% I-HES; Supplementary Table 2). These clinical manifestations were most commonly of moderate severity in each of the HES subtypes (moderate fatigue: 71.4% M-HES, 63.6% L-HES and 63.1% I-HES; moderate skin itch: 53.3% L-HES, 50.0% M-HES, 42.3% I-HES; and moderate pain: 80.0% M-HES, 73.8% I-HES, 69.2% L-HES; Supplementary Table 3).
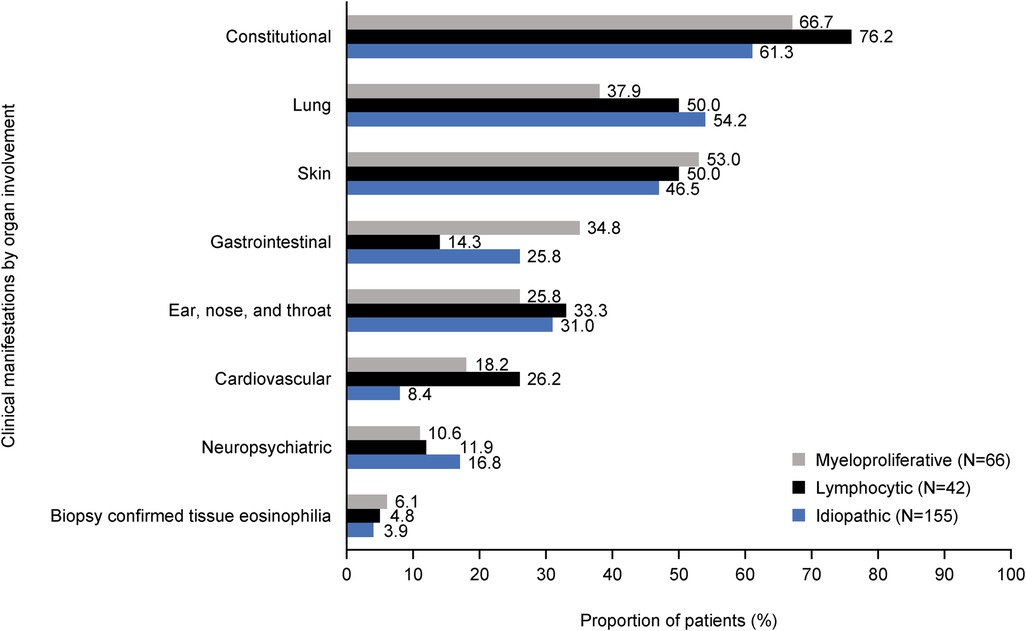
Figure 2. Clinical manifestations* by organ involvement across HES subtypes. *Clinical manifestations were assessed between index date and EOF (i.e., last physician encounter or death). Index date was defined as the date of a patient's earliest visit with their physician between January 2015 and December 2019 on or after the patient's HES diagnosis. Categories are ordered by the highest to lowest frequency of clinical manifestations in any HES subtype. EOF, end of follow-up; HES, hypereosinophilic syndrome.
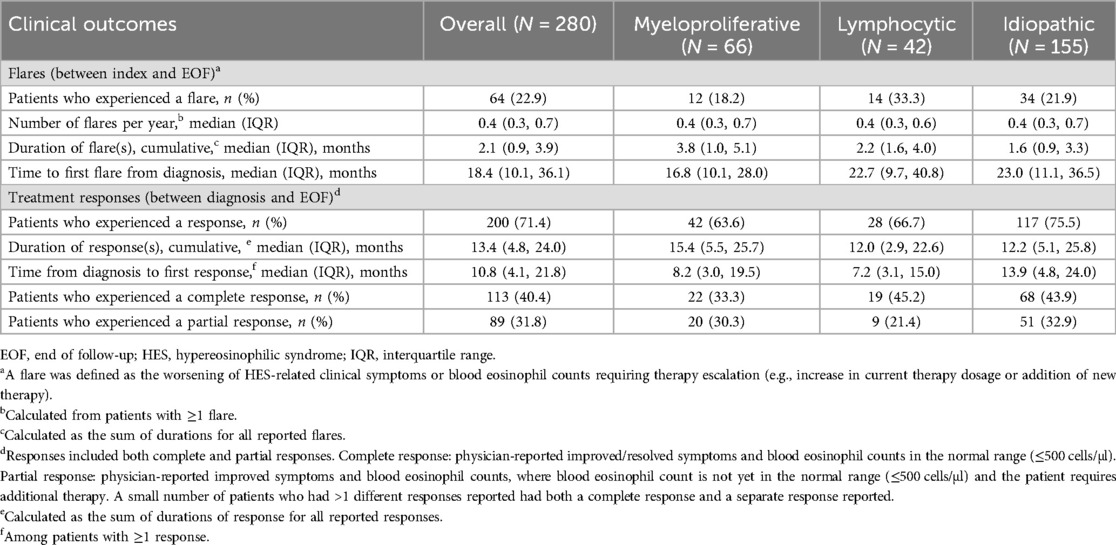
The pattern of flare frequency and length, as well as clinical response, differed between subtypes (Table 4). The proportion of patients who experienced ≥1 flare/s was highest in the L-HES subtype (33.3%) and lowest in the M-HES subtype (18.2%). However, median flare duration was longest for M-HES (3.8 months) and shortest for I-HES (1.6 months). Clinical response rates were highest for I-HES (75.5%) than other subtypes (63.6%–66.7%) but with longer median time to first response with I-HES (13.9 months) than L-HES (7.2 months) or M-HES (8.2 months). Over 6 years of follow-up, mean FFS was 4.91 years overall, and 5.05, 4.24 and 5.04 years with the M-HES, L-HES and I-HES subtypes, respectively (Supplementary Figure 1). Over the same follow-up period, there were 6 deaths across all subtypes (Supplementary Figure 2).
HES-related hospitalizations were highest for L-HES (45.2%) than other subtypes (25.8%–30.3%) and the mean length of stay was longest with L-HES at 13.6 days, compared with 10.2 and 11.2 days for I-HES and M-HES, respectively (Supplementary Table 4). Emergency department visits were also most common with L-HES (47.6%) than other subtypes (22.6%–24.2%). The proportions reporting outpatient visits were similar (88%) across subtypes.
3.2 Characteristics and outcomes of patients with HES who received biologics
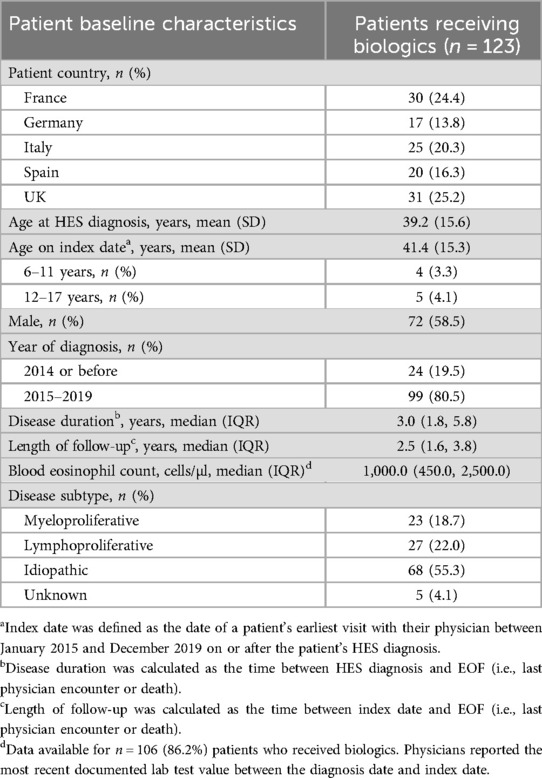
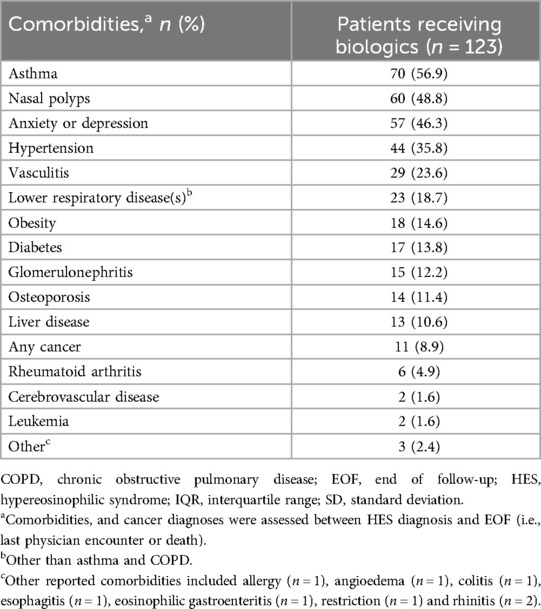
A total of 123 (43.9%) patients received biologics between HES diagnosis and end of follow-up. These patients had a mean age of 39.2 years at diagnosis, 58.5% were male and median blood eosinophil count was 1,000 cells/µl (Table 5). Over half of patients receiving biologics had I-HES (55.3%), while 22.0% had L-HES and 18.7% had M-HES. Asthma (56.9%), nasal polyps (48.8%), anxiety/depression (46.3%), hypertension (35.8%) and vasculitis (23.6%) were the most common comorbidities reported in patients receiving biologics (Table 6).
In total, 41.5% of patients with HES receiving biologics were hospitalized between the index date and end of follow-up, with an average stay of >11 days (Supplementary Table 5). Thirty-nine percent of patients required an emergency department visit and 89.4% attended outpatient visits between the index date and end of follow-up.
Clinical manifestations, OCS use and clinical outcomes were analyzed in the pre-biologic and post-biologic periods among patients who received biologics and who had non-missing dates for ≥1 biologics record (n = 107). The incidence of fatigue was lower in the 12-month post-biologic period (8.4%) than the pre-biologic period (15.9%), whereas the incidence of gastrointestinal manifestations, itching and pain were slightly higher in the post-biologic period (11.2%, 10.3% and 8.4%, respectively) than in the pre-biologic period (7.5%, 6.5% and 2.8%, respectively; Table 7). It should be noted, however, that patient numbers for each of these clinical manifestations were low.
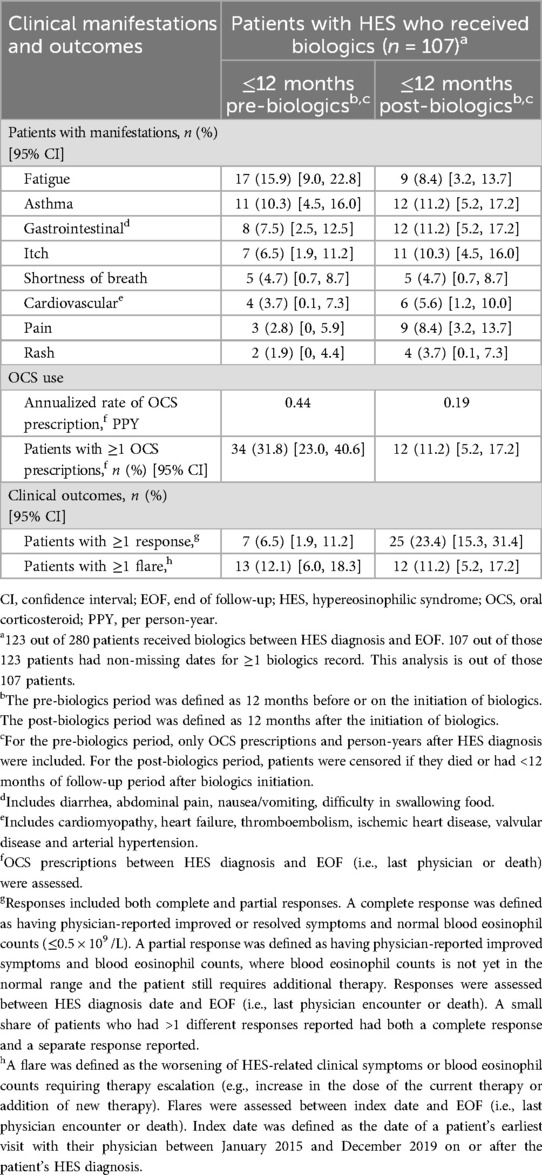
Table 7. Clinical manifestations, OCS reduction and clinical outcomes 12-months pre-/post-biologics.
The annualized rate of OCS prescriptions was reduced by 56.8% from 0.44 to 0.19 per person-year and the proportion of patients with ≥1 OCS prescription decreased by 64.7% (31.8% pre-biologics and 11.2% post biologics; Table 7). In terms of clinical outcomes, the proportion of patients with ≥1 response (complete or partial) was 3.6 times higher in the post-biologics period (23.4%) than in the pre-biologics period (6.5%), while the proportion of patients experiencing ≥1 flare was similar in the pre- and post-biologics periods (12.1% and 11.2%, respectively; Table 7).
4 Discussion
This subgroup analysis of real-world European data provides new insights into patient demographics, HES disease characteristics, treatment patterns and outcomes across different HES subtypes, raising awareness and adding to existing limited data. The analysis also provided real-world data regarding biologic use and treatment outcomes, for which there are limited data. This analysis indicated that patients with I-HES were more treatment responsive compared with patients with L-HES and M-HES, with a greater proportion of patients achieving a clinical response. This is consistent with a previous retrospective chart review study, which indicated that M-HES and L-HES had significantly worse odds of achieving a clinical response with OCS compared with I-HES (odds ratio [95% confidence interval] 0.34 [0.15–0.81], p = 0.015 for L-HES and 0.013 [0.0013–0.118, p = 0.0001 for M-HES) (13). There are nuances in the data from the current analysis, as the cumulative duration of responses was highest for M-HES, the proportion with complete responses was similar for I-HES and L-HES but lower for M-HES, and time to first response was longer for I-HES than the other subtypes. However, despite these factors, results indicate I-HES may be more treatment responsive than the other subtypes. Additionally, a lower proportion of patients in the current study with I-HES needed hospital or emergency department visits than patients with L-HES and M-HES, suggesting I-HES may be less severe than the other subtypes.
All HES subtypes have a substantial burden of disease, with constitutional; lung; skin; gastrointestinal; ear, nose and throat (ENT); cardiovascular; and neuropsychiatric issues affecting 8.4%–76.2% of patients in this study. This is consistent with a previous retrospective study of 188 patients with HES, which identified skin, pulmonary and gastrointestinal adverse impacts as being the most common clinical manifestations (28). The current analysis identified cardiovascular manifestations in 8% of patients with I-HES and up to 26% in those with L-HES; this is consistent with a case study review, which reported cardiac, rather than cardiovascular, manifestations in 12.9% of patients with L-HES, 14.9% with M-HES and 22.4% with I-HES (9).
There were also differences between HES subtypes in the prevalence of clinical manifestations within the organ domains of constitutional, cardiovascular, gastrointestinal and lung in this study. A higher proportion of patients with L-HES experienced constitutional manifestations such as pain, ENT manifestations such as sinus headache/facial pain/pressure and cardiovascular manifestations such as heart failure and thromboembolism, than with the other subtypes. Patients with M-HES experienced skin manifestations such as rash and gastrointestinal manifestations such as diarrhea, abdominal pain and nausea/vomiting slightly more frequently than patients with other subtypes. While overall, M-HES was the subtype with the highest prevalence of skin manifestations, the prevalence of hives/urticaria was highest in patients with L-HES. This corresponds with a case review study based on data from the French Reference Center for Hypereosinophilic Syndromes (CEREO), which reported eczema-like lesions, angioedema and urticaria in over 26% of patients with L-HES (24). However, while our data suggested that around half of patients with L-HES had skin manifestations, a retrospective study of 21 patients with L-HES initiated by the French Eosinophil Network, reported that 81% had skin manifestations (23). Finally, patients with I-HES had the highest prevalence of lung manifestations such as asthma and dyspnea and neuropsychiatric manifestations, such as sensory neuropathy, than the other subtypes.
Along with clinical manifestations, the high dosage of OCS received by patients in this study also underscores the substantial disease burden (mean maximum daily dose across all patients was 31.7 mg). The use of OCS, immunosuppressant/cytotoxic agents and biologics differed between HES subtypes. At baseline, the proportion receiving OCS was highest in patients with I-HES, the proportion receiving immunosuppressants/cytotoxic agents was highest in patients with M-HES and biologics were used by more patients with L-HES than the other subtypes. This is in accordance with findings from a previous review article that included individual case and aggregated data (9). This treatment pattern was seen with ongoing treatments at end of follow-up, with OCS being most used in I-HES, immunosuppressants/cytotoxic agents in M-HES and biologics in L-HES, with each prevalent drug class being used by approximately half of patients in their respective subtype.
Compared with the pre-biologic period, following post-biologic initiation there was a 56.8% reduction in the annualized rate of OCS prescriptions and a 64.7% decrease in the proportion of patients with ≥1 OCS prescription. Clinical response to treatment was also improved; 3.6 times as many patients had ≥1 clinical response in the ≤12-month post-biologic period, compared with pre-biologic. These findings are consistent with randomized controlled trials and open-label extensions that support the OCS-sparing effect, efficacy and safety of mepolizumab and benralizumab in the treatment of HES (18–20, 29, 30). The proportion of patients experiencing ≥1 flare was similar in the pre- and post-biologics periods, which may have reflected the fact that the study did not differentiate between different types of biologics used, including anti-IL-5, anti-IL-5 receptor, anti-cluster of differentiation (CD)20, anti-CD52, anti-IL-4 and anti-IL-13 therapies. A range of studies, however, have demonstrated that biologics targeting IL-5 or the IL-5 receptor can significantly reduce flares in HES (18, 25, 26, 31, 32). Additionally, there are clear knowledge gaps in terms of the efficacy of biologic therapies targeting eosinophilic inflammation in different HES subtypes (15). Therefore, examining the impact of different biologics on clinical outcomes in each HES subtypes, including those targeting IL-5/IL-5 receptor, would be an interesting topic to explore in future studies.
A strength of this study is the real-world nature of the data, which were collected across five European countries, meaning the findings may be generalizable across these healthcare systems and potentially more widely. Limitations of the study include that these were descriptive post hoc analyses with no statistical comparisons made, and there was no control population for the characterization of the biologics group. Therefore, the results should be seen as providing preliminary insights with the goal of providing hypotheses for further investigations.
5 Conclusion
Different HES subtypes are associated with differing disease burdens, treatment patterns and clinical outcomes. Biologic use appeared to be associated with numerically reduced OCS use and improved clinical responses, although these findings were not stratified by HES subtype. To improve patient care, further research is needed to optimize awareness regarding the differing clinical needs associated with each HES subtype and further characterize the impact of different biologic treatment across different subtypes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The study was submitted to the Western Copernicus Group Institutional Review Board and was granted exemption status with a waiver of authorization issued (December 23, 2020; #1-1383121-1), in accordance with the local legislation and institutional requirements. Only anonymized data were collected and the reported data reflect aggregate analyses without patient identification. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
JH: Conceptualization, Writing – review & editing, Visualization, Writing – original draft. LH: Conceptualization, Data curation, Investigation, Formal analysis, Writing – original draft, Visualization, Writing – review & editing. WdCJ: Writing – review & editing, Writing – original draft, Formal analysis, Visualization. MER: Visualization, Writing – original draft, Writing – review & editing. MSD: Writing – review & editing, Writing – original draft, Formal analysis, Conceptualization, Visualization, Data curation, Investigation. RA-C: Writing – review & editing, Conceptualization, Writing – original draft, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank Lee Baylis and Anamika Khanal for their contributions to this study. Medical writing support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Alice Rees, PhD, at Fishawack Indicia Ltd, UK, part of Avalere Health, and was funded by GSK.
Conflict of interest
JH and RA-C are employed by GSK and hold financial equities in GSK. LH, WdCJ and MSD are employees of the Analysis Group, Inc.; Analysis Group, Inc. received payment from GSK to conduct the study. MER is a consultant for Pulm One, Spoon Guru, ClostraBio, Serpin Pharm, Allakos, Celldex, Uniquitybio, Santa Ana Bio, EnZen Therapeutics, Bristol Myers Squibb, AstraZeneca, Pfizer, GSK, Regeneron/Sanofi, Revolo Biotherapeutics and Guidepoint, and has an equity interest in the first nine listed and royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust) and UpToDate. MER is an inventor of patents owned by Cincinnati Children's Hospital.
The authors declare that these post hoc analyses and the parent study were funded by GSK [GSK ID: 214657]. The sponsor was involved in study design and implementation, as well as data collection, analysis, interpretation, writing the study report and reviewing this manuscript. All authors had full access to the data upon request and had final responsibility for the decision to submit for publication.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2025.1605397/full#supplementary-material
References
1. Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. (2022) 97:129–48. doi: 10.1002/ajh.26352
2. Leru PM. Eosinophilic disorders: evaluation of current classification and diagnostic criteria, proposal of a practical diagnostic algorithm. Clin Transl Allergy. (2019) 9:36. doi: 10.1186/s13601-019-0277-4
3. Valent P, Klion AD, Roufosse F, Simon D, Metzgeroth G, Leiferman KM, et al. Proposed refined diagnostic criteria and classification of eosinophil disorders and related syndromes. Allergy. (2023) 78:47–59. doi: 10.1111/all.15544
4. Orphanet Report Series. Prevalence of rare diseases: Bibliographic data. (2022). Available at: https://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf (Accessed January 18, 2022).
5. Crane MM, Chang CM, Kobayashi MG, Weller PF. Incidence of myeloproliferative hypereosinophilic syndrome in the United States and an estimate of all hypereosinophilic syndrome incidence. J Allergy Clin Immunol. (2010) 126:179–81. doi: 10.1016/j.jaci.2010.03.035
6. Requena G, Logie J, Gibbons DC, Steinfeld J, Van Dyke MK. The increasing incidence and prevalence of hypereosinophilic syndrome in the United Kingdom. Immun Inflamm Dis. (2021) 9:1447–51. doi: 10.1002/iid3.495
7. Curtis C, Ogbogu P. Hypereosinophilic syndrome. Clin Rev Allergy Immunol. (2016) 50:240–51. doi: 10.1007/s12016-015-8506-7
8. Kahn JE, Groh M, Lefevre G. (A critical appraisal of) classification of hypereosinophilic disorders. Front Med (Lausanne). (2017) 4:216. doi: 10.3389/fmed.2017.00216
9. Requena G, van den Bosch J, Akuthota P, Kovalszki A, Steinfeld J, Kwon N, et al. Clinical profile and treatment in hypereosinophilic syndrome variants: a pragmatic review. J Allergy Clin Immunol Pract. (2022) 10:2125–34. doi: 10.1016/j.jaip.2022.03.034
10. Shomali W, Gotlib J. World health organization and international consensus classification of eosinophilic disorders: 2024 update on diagnosis, risk stratification, and management. Am J Hematol. (2024) 99:946–68. doi: 10.1002/ajh.27287
11. Roufosse F. Management of hypereosinophilic syndromes. Immunol Allergy Clin North Am. (2015) 35:561–75. doi: 10.1016/j.iac.2015.05.006
12. Klion AD. How I treat hypereosinophilic syndromes. Blood. (2015) 126:1069–77. doi: 10.1182/blood-2014-11-551614
13. Khoury P, Abiodun AO, Holland-Thomas N, Fay MP, Klion AD. Hypereosinophilic syndrome subtype predicts responsiveness to glucocorticoids. J Allergy Clin Immunol Pract. (2018) 6:190–5. doi: 10.1016/j.jaip.2017.06.006
14. Dispenza MC, Bochner BS. Diagnosis and novel approaches to the treatment of hypereosinophilic syndromes. Curr Hematol Malig Rep. (2018) 13:191–201. doi: 10.1007/s11899-018-0448-8
15. Kuang FL, Khoury P, Weller PF, Wechsler ME, Klion AD. Biologics and hypereosinophilic syndromes: knowledge gaps and controversies. J Allergy Clin Immunol Pract. (2023) 11:2666–71. doi: 10.1016/j.jaip.2023.07.026
16. EMA. Mepolizumab (Nucala) summary of product characteristics. European Medicines Agency (2022). Available at: https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_en.pdf (Accessed January 18, 2024).
17. Food and Drug Administration (FDA). Mepolizumab (Nucala) prescribing information. Food and Drug Administration (2023). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125526Orig1s021,761122Orig1s011Corrected_lbl.pdf (Accessed June 12, 2024).
18. Roufosse F, Kahn JE, Rothenberg ME, Wardlaw AJ, Klion AD, Kirby SY, et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: a phase iii, randomized, placebo-controlled trial. J Allergy Clin Immunol. (2020) 146:1397–405. doi: 10.1016/j.jaci.2020.08.037
19. Gleich GJ, Roufosse F, Chupp G, Faguer S, Walz B, Reiter A, et al. Safety and efficacy of mepolizumab in hypereosinophilic syndrome: an open-label extension study. J Allergy Clin Immunol Pract. (2021) 9:4431–40.e1. doi: 10.1016/j.jaip.2021.07.050
20. Kuang FL, Legrand F, Makiya M, Ware J, Wetzler L, Brown T, et al. Benralizumab for pdgfra-negative hypereosinophilic syndrome. N Engl J Med. (2019) 380:1336–46. doi: 10.1056/NEJMoa1812185
21. Kuang FL, Klion AD. Biologic agents for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol Pract. (2017) 5:1502–9. doi: 10.1016/j.jaip.2017.08.001
22. Hwee J, Huynh L, Du S, Kwon N, Jakes RW, Alfonso-Cristancho R, et al. Hypereosinophilic syndrome in Europe: retrospective study of treatment patterns, clinical manifestations, and healthcare resource utilization. Ann Allergy Asthma Immunol. (2023) 130:768–75. doi: 10.1016/j.anai.2023.02.022
23. Lefevre G, Copin MC, Staumont-Salle D, Avenel-Audran M, Aubert H, Taieb A, et al. The lymphoid variant of hypereosinophilic syndrome: study of 21 patients with Cd3-Cd4+ aberrant T-cell phenotype. Medicine (Baltimore). (2014) 93:255–66. doi: 10.1097/MD.0000000000000088
24. Laurent C, Lefevre G, Kahn JE, Staumont-Salle D, Felten R, Puget M, et al. Cutaneous manifestations of lymphoid-variant hypereosinophilic syndrome. Br J Dermatol. (2022) 187:1011–3. doi: 10.1111/bjd.21782
25. Pane F, Lefevre G, Kwon N, Bentley JH, Yancey SW, Steinfeld J. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. (2022) 13:935996. doi: 10.3389/fimmu.2022.935996
26. Rothenberg ME, Roufosse F, Faguer S, Gleich GJ, Steinfeld J, Yancey SW, et al. Mepolizumab reduces hypereosinophilic syndrome flares irrespective of blood eosinophil count and interleukin-5. J Allergy Clin Immunol Pract. (2022) 10:2367–74.e3. doi: 10.1016/j.jaip.2022.04.037
27. Kuang FL. Approach to patients with eosinophilia. Med Clin North Am. (2020) 104:1–14. doi: 10.1016/j.mcna.2019.08.005
28. Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. (2009) 124:1319–25.e3. doi: 10.1016/j.jaci.2009.09.022
29. Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. (2008) 358:1215–28. doi: 10.1056/NEJMoa070812
30. Roufosse FE, Kahn JE, Gleich GJ, Schwartz LB, Singh AD, Rosenwasser LJ, et al. Long-term safety of mepolizumab for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol. (2013) 131:461–7.e1-5. doi: 10.1016/j.jaci.2012.07.055
31. Reiter A, Lefevre G, Cid MC, Kwon N, Mavropolou E, Yancey SW, et al. Association between baseline therapy and flare reduction in mepolizumab-treated patients with hypereosinophilic syndrome. Front Immunol. (2022) 13:840974. doi: 10.3389/fimmu.2022.840974
Keywords: hypereosinophilic syndrome, burden of disease, biologics, mepolizumab, retrospective study, corticosteroid
Citation: Hwee J, Huynh L, da Costa W Jr, Rothenberg ME, Duh MS and Alfonso-Cristancho R (2025) Steroid-sparing benefits of biologic use in hypereosinophilic syndrome and substantial disease burden across subtypes. Front. Allergy 6:1605397. doi: 10.3389/falgy.2025.1605397
Received: 3 April 2025; Accepted: 21 April 2025;
Published: 23 May 2025.
Edited by:
Igor Kaidashev, Poltava State Medical University, UkraineReviewed by:
Sergii Zaikov, Shupyk National Medical Academy of Postgraduate Education, UkraineSvitlana Zubchenko, Danylo Halytsky Lviv National Medical University, Ukraine
Copyright: © 2025 Hwee, Huynh, da Costa, Rothenberg, Duh and Alfonso-Cristancho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeremiah Hwee, amVyZW1pYWgueC5od2VlQGdzay5jb20=
 Jeremiah Hwee
Jeremiah Hwee Lynn Huynh
Lynn Huynh Wilson da Costa Jr
Wilson da Costa Jr Marc E. Rothenberg
Marc E. Rothenberg Mei Sheng Duh
Mei Sheng Duh Rafael Alfonso-Cristancho4
Rafael Alfonso-Cristancho4