- 1Department of Dermatovenereology, Tianjin Medical University General Hospital/Tianjin Institute of Sexually Transmitted Disease, Tianjin, China
- 2Department of Dermatology, Ulanqab Central Hospital, Inner Mongolia, Ulanqab, China
- 3Department of Dermatology, Gem Flower Xi'an Chang Qing Staff Hospital, Xi’an, China
Background: Traditional Chinese Medicine (TCM) has demonstrated certain efficacy in the treatment of psoriasis, a chronic papulosquamous skin disease. However, in rare instances, the use of TCM may exacerbate cutaneous lesions. This study aims to explore whether the methotrexate (MTX) component in TCM may increase the risk of cutaneous ulceration in patients with psoriasis. MTX is also an immunosuppressive agent widely used in the treatment of various dermatologic conditions.
Methods: We retrospectively analyzed the medical records of five patients who developed painful skin ulcers at primary psoriatic sites after taking TCM. Evaluated indicators included hemocytopenia, liver function, serum MTX concentration, and ABCB1 gene polymorphisms. Histopathological examination was also performed on ulcerative tissue samples.
Results: Among the five patients, two developed hemocytopenia and two had abnormal liver function. Serum MTX concentrations in two patients ranged from 0.03 to 0.11 μmol/L (<0.1 μmol/L at 72 h after MTX administration). The ABCB1 genotypes AA and AG were detected in two different patients. Histopathological findings revealed dyskeratosis of keratinocytes, dermal vasodilation, and inflammatory cell infiltration. Rescue treatment with oral folic acid was administered to three patients, leading to complete healing of all lesions within two weeks. The remaining two patients showed gradual improvement in skin ulcers after discontinuing TCM.
Conclusions: TCM containing MTX may induce skin ulceration in rare cases among patients with psoriasis.
Introduction
Methotrexate (MTX) is an anti-folate drug possessing immunosuppressive, anti-proliferative, and anti-inflammatoryactivities (1). This drug has been used to treat many diseases including cancers, ectopic pregnancy, and rheumatoid arthritis (1–3). Psoriasis is a chronic, immune-mediated papulosquamous skin disease (4). MTX has a significant therapeutic effect on various types of psoriasis and is considered the first-line drug for systemic treatment of psoriasis (5, 6). The recommended maximum dose of MTX varies in different diseases. High-dose of MTX is used in patients with aggressive B-cell lymphoma (2), whereas low-dose of MTX is used to treat rheumatoid arthritis and psoriasis (7).
MTX can cause a variety of adverse effects, such as bone marrow suppression, hepatotoxicity, nephrotoxicity, and skin damage (8–10). Although relatively rare, several cases of cutaneous ulceration have been reported in psoriasis patients treated with low-dose MTX (11–13). Berna, R. et al. reviewed 114 cases of methotrexate-associated skin ulcers through the PubMed and Embase databases, of which 19 cases (16.7%) presented as localized ulcers (14). ABCB1 gene polymorphisms can influence MTX transport under different pathological conditions (15). In patients with psoriasis, ABCB1 gene polymorphisms play a significant role in the efficacy of MTX.
Traditional Chinese medicine (TCM) is an alternative and effective treatment option for patients with psoriasis (16, 17). However, there have also been individual cases in which cutaneous lesions worsened after taking TCM (18). The mechanisms underlying TCM-induced adverse skin reactions remain unclear. This study aims to investigate whether the MTX component contained in TCM may increase the risk of cutaneous ulceration in patients with psoriasis.
Materials and methods
This study retrospectively analyzed the medical records of five psoriasis patients who presented with painful skin ulcers as the main manifestation after oral administration of TCM from April 2019 to February 2025. The inclusion criteria were as follows: a confirmed diagnosis of psoriasis; clinical presentation primarily characterized by painful skin ulcers; a clear history of compound TCM use, with either detectable MTX content in the compound TCM taken by the patient, or detectable serum MTX after TCM administration, or histopathological findings consistent with MTX-induced skin toxicity, or complete healing of skin ulcers within two weeks after folic acid supplementation; all enrolled patients signed written informed consent permitting the disclosure of their case-related information.
Laboratory evaluations included assessments of hemocytopenia, liver function, and serum MTX concentration. Two patients underwent ABCB1 gene polymorphism testing. The procedure was as follows: Genomic DNA was extracted from the peripheral blood of the subjects using the MagPure Buffy Coat DNA Midi KF Kit. The DNA was then randomly fragmented into segments primarily ranging from 100 to 500 base pairs using BGI's enzyme kit (Segmentase, BGI). Magnetic bead enrichment was performed to select DNA fragments between 280 and 320 base pairs. After end repair and A-tailing, T4 DNA ligase was used to ligate adapters containing barcode sequences. The ligated products were purified using Axygen beads, followed by LM-PCR amplification to enrich DNA fragments flanked by the adapters, thereby constructing individual DNA libraries for each patient. The libraries were hybridized and enriched using a capture chip. The enriched products were quantified with an Agilent 2,100 Bioanalyzer and BMG, then pooled in equimolar amounts. A total of 160 ng of the pooled DNA was used for single-strand separation and circularization, yielding single-stranded circular DNA libraries with one adapter. Finally, gene sequencing was performed using the MGISEQ-2000 platform developed by BGI. In addition, ulcer biopsies from two patients were subjected to histopathological analysis.
We quantified the methotrexate content in the compound capsules of traditional Chinese medicine (TCM) taken by the patients. The analysis was conducted using an ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) system (Waters ACQUITY UPLC H-Class system coupled with a Waters Xevo TQ-S micro mass spectrometer), employing the MRM (multiple reaction monitoring) method for quantification. Detailed methodology: The TCM capsules were ground into a powder, and 0.5 g of the powder was weighed and mixed with 10 ml of methanol. The mixture underwent ultrasonic extraction for one hour, followed by centrifugation at 12,000 rpm for 10 min at 4°C. For liquid capsules, the supernatant was directly subjected to mass spectrometric analysis.
Results
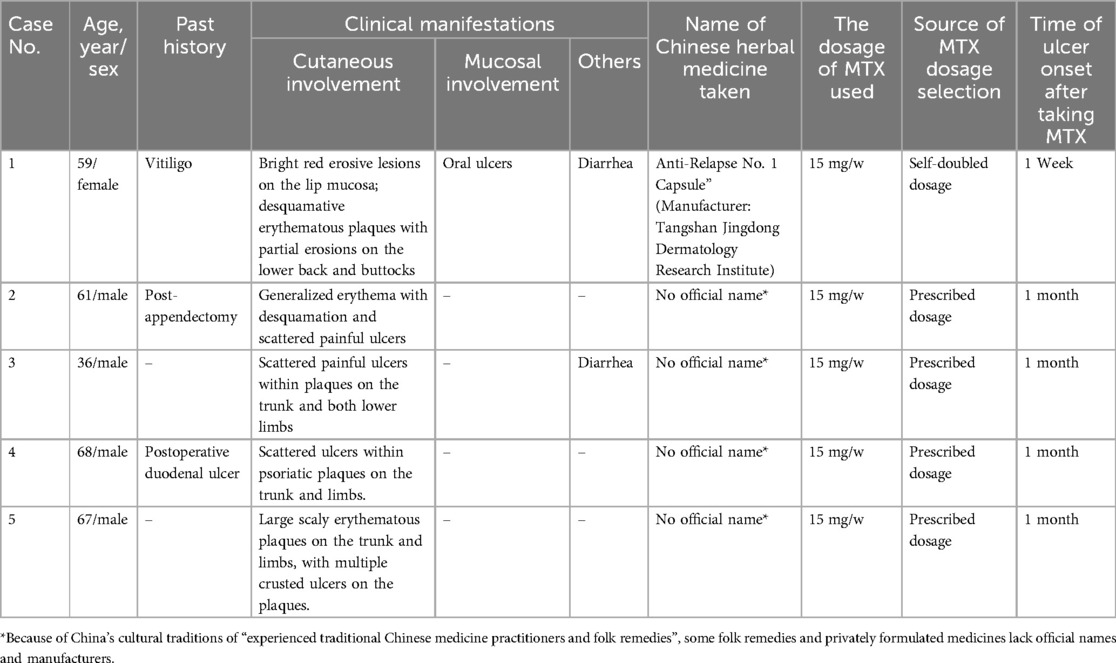
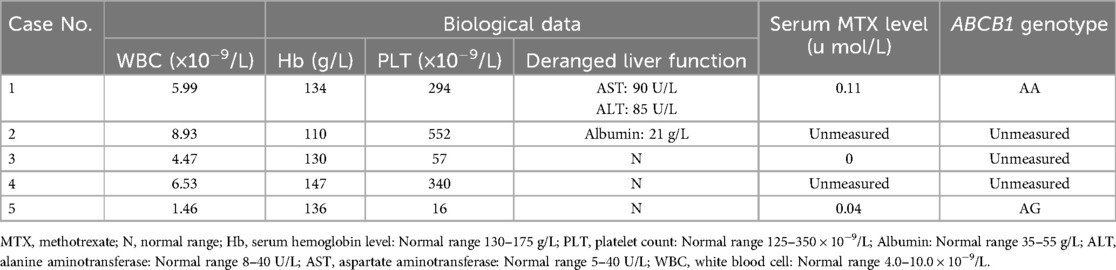
Tables 1, 2 list the main clinical findings observed in the five patients. In addition to the ulceration in primary psoriatic lesions, one case showed oral ulceration and two cases gastrointestinal discomfort (i.e., diarrhea and nausea). Laboratory examinations revealed two cases of hemocytopenia and two abnormal liver function. Two cases showed serum MTX concentration of 0.03–0.11 μmol/L (<0.1 mol/L 72 h after MTX intaking), while the remaining patients had undetectable levels, likely due to the prolonged interval since MTX discontinuation. Among the two patients tested for ABCB1polymorphisms, one patient had the ABCB1genotype AA and the other AG, the remaining three patients refused examination due to financial reasons. Analysis of ulcer biopsies showed dyskeratosis of keratinocytes, focal dermis-epidermal separation, dermal vasodilation, and infiltration of multiple inflammatory cells including neutrophils, eosinophils, and lymphocytes.
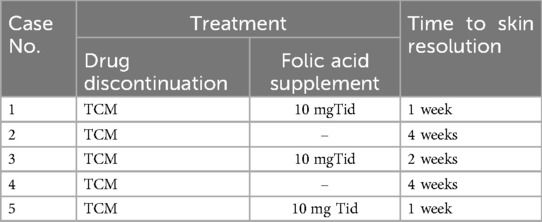
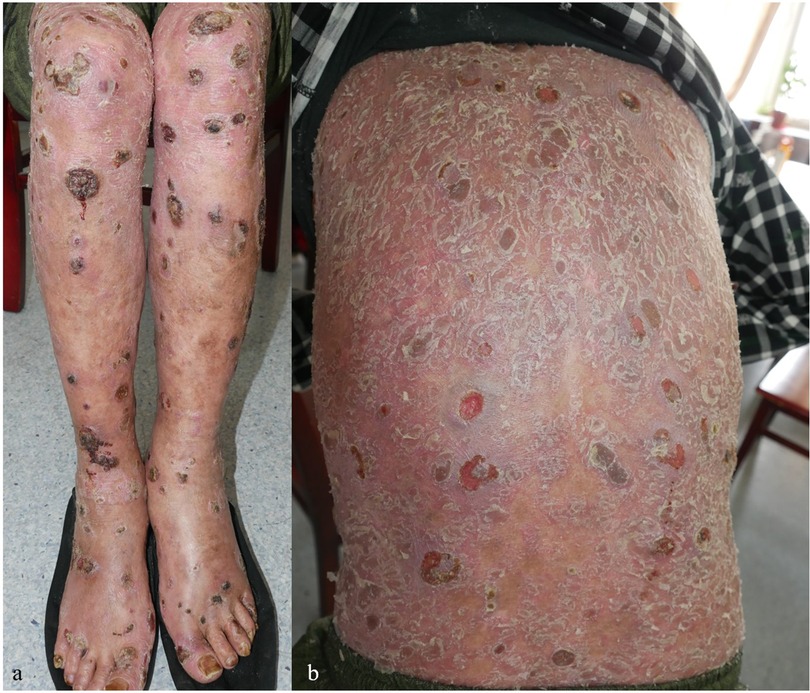
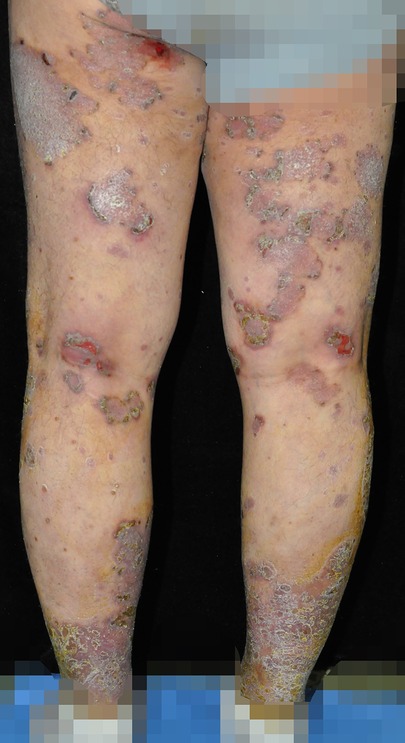
Oral folic acid was given in three patients, resulting in complete healing of all lesions within two weeks (Table 3). The remaining two patients showed gradual improvement after discontinuing the TCM. Figures 1–5 shows the lesion changes in these five cases before and after treatment with folic acid. Figure 6 demonstrates the histopathological images for Case 4.
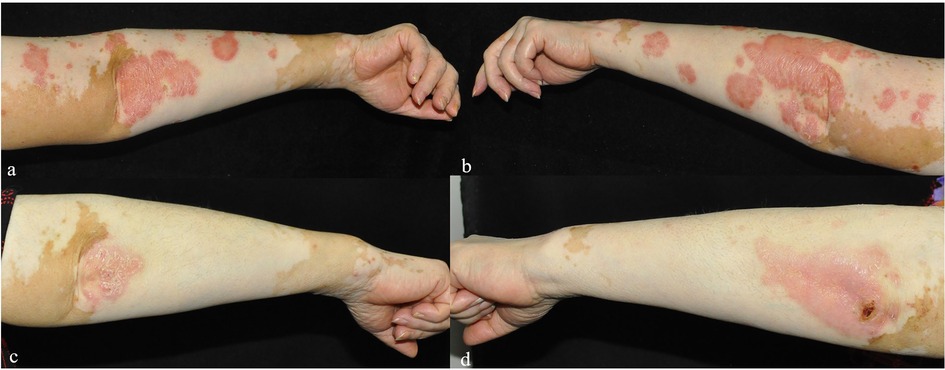
Figure 1. (a–d) The changes in the bilateral upper limb lesions in Case 1 before (a,b) and one week after folic acid supplementation (c,d).
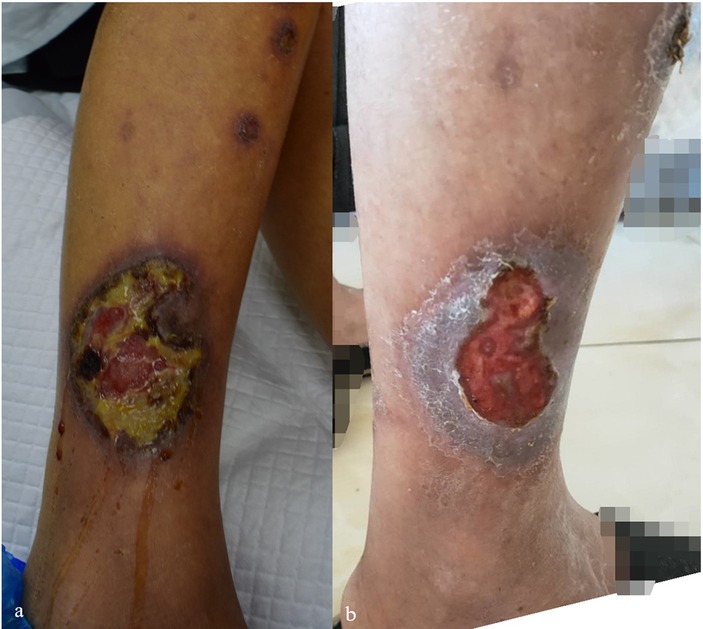
Figure 4. (a,b) Skin lesion characteristics of Case 4 before treatment and 3 weeks after discontinuing TCM compound capsules containing MTX.
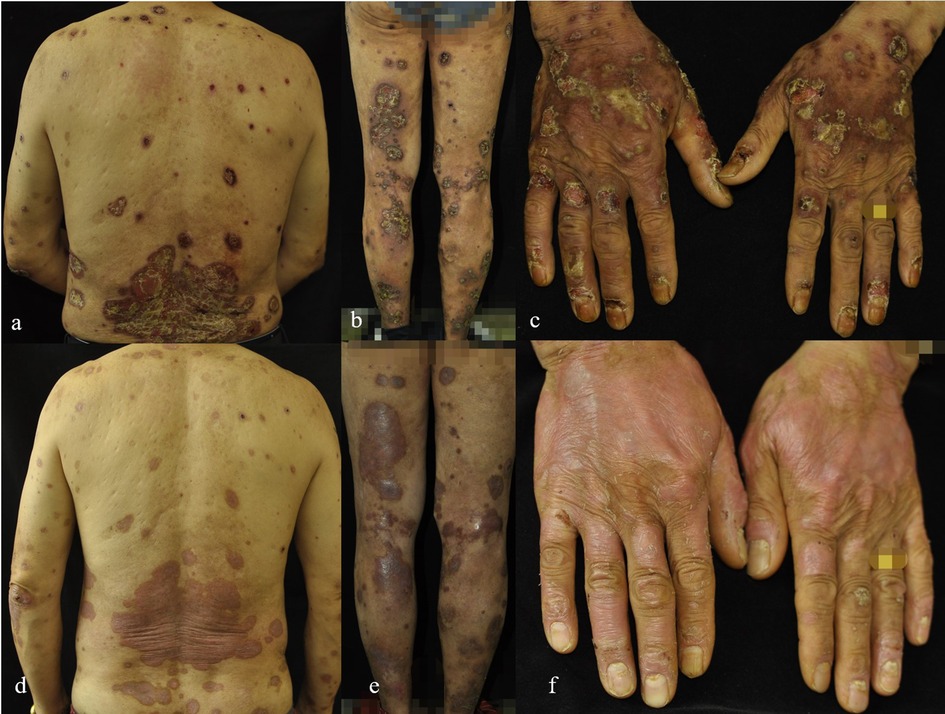
Figure 5. (a–f) The changes in the lesions on the back, flexor sides of both lower limbs, and dorsum of both hands in Case 5 before (a–c) and one week after folic acid supplementation treatment (d–f).
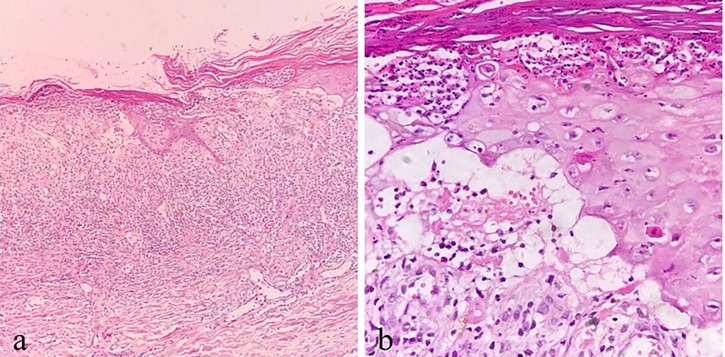
Figure 6. A(40×), b(100×): illustrative histopathological images of Case 4. (a) Thick parakeratosis, epidermal necrosis, ulcer formation, dermal vasodilation, and inflammatory cells in the dermis. (b) High-power view of parakeratosis with neutrophilic abscess, dyskeratosis of keratinocytes, focal dermis-epidermal separation, and scattered lymphocytes in the dermis.
Discussion
This study reports five cases of psoriasis patients who developed skin ulcers after taking MTX-containing TCM. MTX is a cytotoxic drug structurally similar to folic acid. After entering cells via the reduced folate carrier (RFC-1), MTX irreversibly binds to the enzyme dihydrofolate reductase (DHFR), preventing the formation of tetrahydrofolate (19). As a result, it competitively inhibits the S phase of cell division and suppresses cell proliferation, thus preferentially affecting actively proliferating cells (20). In psoriasis, proliferating lymphocytes and epithelial cells may become targets of MTX (19). Folate deficiency within psoriatic lesions may lead to the selective accumulation of MTX, thereby enhancing its cytotoxic effects (21). MTX-induced skin ulcers in psoriasis can be classified into two types (22): type I ulcers occur on psoriatic plaques, while type II ulcers appear outside of psoriatic lesions. Type I ulcers are generally shallower than type II ulcers. In this study, all five patients exhibited type I ulcers after oral administration of TCM.
MTX toxicity is influenced by multiple factors including drug dose, renal insufficiency, advanced age, folate level, serum albumin reduction, and gene polymorphism (10, 11, 23). As the kidney is responsible for the elimination of MTX, decreased renal function with age contributes to MTX toxicity (23). In this study, four of the five patients were over 55 years old. Therefore, MTX toxicity tends to occur in older patients. Two cases showed serum MTX concentration of 0.03–0.11 μmol/L (<0.1 mol/L 72 h after MTX intaking), suggesting that MTX overdose may also account for the painful skin ulcers observed. However, MTX dermal toxicity was still observed in 1 case who had an undetectable level of serum MTX. Therefore, serum MTX concentration may not be a reliable predictor for the MTX toxicity.
TCM-related skin ulceration is rarely encountered in psoriasis patients. Genetic background of patients may be an important determinant of TCM-related adverse reactions. Compelling evidence indicates that the transporter ABCB1polymorphisms affect the transport of MTX in different pathological contexts (24–26). The P-glycoprotein (P-gp) encoded by the ABCB1 gene is an important transmembrane transporter located on the cell membrane, which can influence the transmembrane transport, tissue distribution, and clearance rate of various drugs, including methotrexate (MTX) (24). In patients with psoriasis, ABCB1 gene polymorphisms have a significant impact on the therapeutic efficacy of MTX. Patients with certain genotypes (such as AA and AG) tend to have lower expression levels of P-gp, leading to increased accumulation of MTX in tissues, which in turn exacerbates drug toxicity, including severe adverse effects such as skin ulceration. In contrast, patients with the GG genotype generally exhibit higher P-gp expression, resulting in enhanced drug efflux, lower MTX levels in the body, and consequently a reduced risk of adverse reactions (27). Our results suggest that the deleterious effects incurred by MTX-containing TCM are also affected by the ABCB1 gene polymorphisms. Among the two patients tested for ABCB1 polymorphisms, one was the AA genotype, and the other AG genotype. The ABCB1 AA or AG genotype seems to be more susceptible than the GG genotype to MTX toxicity. Therefore, when using MTX-containing traditional Chinese medicine in patients with psoriasis, the polymorphisms of the ABCB1 gene may need to be taken into consideration. Detection of ABCB1 gene polymorphisms holds promise for guiding individualized MTX therapy in clinical practice. This is especially important for patients using MTX-containing traditional Chinese medicine, as dosage and monitoring strategies can be tailored according to the patient's genotype, thereby reducing the risk of adverse reactions and improving treatment safety.
TCM is composed of a large number of bioactive compounds. Although our data indicate the increased risk of dermal toxicity by MTX, we cannot exclude the possibility that other chemical agents from TCM may also contribute to skin ulceration observed in psoriasis patients. Nevertheless, discontinuation of TCM or supplementation of folic acid is useful in resolving the TCM-related skin ulcers. The limitations of this study include a small sample size and the lack of comprehensive genetic data. In the future, it will be necessary to expand the sample size and incorporate a broader range of genetic backgrounds and multicenter data to further investigate the predictive and interventional significance of ABCB1 gene polymorphisms in MTX-induced cutaneous toxicity.
Conclusion
In summary, our work demonstrates that MTX-containing TCM play a role in skin ulceration in rare cases of psoriasis patients. Understanding the mechanism for TCM-induced dermal toxicity is important to improve the safety of TCM-based therapy against psoriasis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics statement
The research/study was approved by the Institutional Review Board at Tianjin Medical University General Hospital, numberIRB2024-YX-006-01, dated 2023-01. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZ: Software, Conceptualization, Resources, Writing – original draft, Visualization, Funding acquisition, Investigation, Writing – review & editing, Formal analysis, Validation, Methodology, Project administration, Data curation, Supervision. YD: Validation, Data curation, Supervision, Project administration, Conceptualization, Investigation, Resources, Funding acquisition, Writing – original draft, Visualization, Formal analysis. RW: Supervision, Investigation, Data curation, Conceptualization, Methodology, Software, Funding acquisition, Resources, Validation, Writing – original draft, Project administration. SL: Formal analysis, Funding acquisition, Visualization, Resources, Project administration, Supervision, Methodology, Writing – review & editing, Validation, Conceptualization, Data curation, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (No. 81773319) and Basic Research Projects in Ulanqab City (2021JC302).
Acknowledgments
The authors are grateful to all the participants who cooperated in the skin lesion photography for this study. The authors would also like to thank the relevant dermatology teams for their support and collaboration.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Torres RP, Santos FP, Branco JC. Methotrexate: implications of pharmacogenetics in the treatment of patients with rheumatoid arthritis. ARP Rheumatol. (2022) 1(3):225–9.35724450
2. Lewis KL, Jakobsen LH, Villa D, Smedby KE, Savage KJ, Eyre TA, et al. High-dose methotrexate as CNS prophylaxis in high-risk aggressive B-cell lymphoma. J Clin Oncol. (2023) 41(35):5376–87. doi: 10.1200/JCO.23.00365
3. Grosen A, Kelsen J, Hvas CL, Bellaguarda E, Hanauer SB. The influence of methotrexate treatment on male fertility and pregnancy outcome after paternal exposure. Inflamm Bowel Dis. (2017) 23(4):561–9. doi: 10.1097/MIB.0000000000001064
4. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. (2021) 397(10281):1301–15. doi: 10.1016/S0140-6736(20)32549-6
5. Fougerousse AC, Mery-Bossard L, Parier J, Taieb C, Bertolotti A, Maccari F, et al. Use of methotrexate in the treatment of moderate to severe plaque psoriasis in France: a practice survey. Clin Cosmet Investig Dermatol. (2021) 14:389–93. doi: 10.2147/CCID.S311269
6. van Huizen AM, Sikkel R, Caron AGM, Menting SP, Spuls PI. Methotrexate dosing regimen for plaque-type psoriasis: an update of a systematic review. J Dermatolog Treat. (2022) 33(8):3104–18. doi: 10.1080/09546634.2022.2117539
7. Muanda FT, Blake PG, Weir MA, Ahmadi F, McArthur E, Sontrop JM, et al. Low-dose methotrexate and serious adverse events among older adults with chronic kidney disease. JAMA Netw Open. (2023) 6(11):e2345132. doi: 10.1001/jamanetworkopen.2023.45132
8. Hu Q, Wang H, Xu T. Predicting hepatotoxicity associated with low-dose methotrexate using machine learning. J Clin Med. (2023) 12(4):1599. doi: 10.3390/jcm12041599
9. Khuwaja S, Lyons M, Zulfiqar B. A rare case of acute methotrexate toxicity leading to bone marrow suppression. Case Rep Rheumatol. (2024) 2024:7693602. doi: 10.1155/2024/7693602
10. Zuber M, Harikrishna , Vidhyashree , Chhabra M, Venkataraman R, Kumar S, et al. Methotrexate related cutaneous adverse drug reactions: a systematic literature review. J Basic Clin Physiol Pharmacol. (2021) 33(5):549–65. doi: 10.1515/jbcpp-2021-0165
11. Weidmann A, Foulkes AC, Kirkham N, Reynolds NJ. Methotrexate toxicity during treatment of chronic plaque psoriasis: a case report and review of the literature. Dermatol Ther. (2014) 4(2):145–56. doi: 10.1007/s13555-014-0056-z
12. Pradhan S, Sirka CS, Rout AN, Dash G, Sahu K. Acute methotrexate toxicity due to overdosing in psoriasis: a series of seven cases. Indian Dermatol Online J. (2019) 10(1):64–8. doi: 10.4103/idoj.IDOJ_157_18
13. Fridlington JL, Tripple JW, Reichenberg JS, Hall CS, Diven DG. Acute methotrexate toxicity seen as plaque psoriasis ulceration and necrosis: a diagnostic clue. Dermatol Online J. (2011) 17(11):2.22136858
14. Berna R, Rosenbach M, Margolis DJ, Mitra N, Baumrin E. Methotrexate cutaneous ulceration: a systematic review of cases. Am J Clin Dermatol. (2022) 23(4):449–57. doi: 10.1007/s40257-022-00692-1
15. Huang J, Fan H, Qiu Q, Liu K, Lv S, Li J, et al. Are gene polymorphisms related to adverse events of methotrexate in patients with rheumatoid arthritis? A retrospective cohort study based on an updated meta-analysis. Ther Adv Chronic Dis. (2020) 11:2040622320916026. doi: 10.1177/2040622320916026
16. Wang G, Liu Y. Traditional Chinese medicine is effective and safe in the treatment of psoriasis. Int J Dermatol. (2004) 43(7):552. doi: 10.1111/j.1365-4632.2004.02181.x
17. Li T, Gao S, Han W, Gao Z, Wei Y, Wu G, et al. Potential effects and mechanisms of Chinese herbal medicine in the treatment of psoriasis. J Ethnopharmacol. (2022) 294:115275. doi: 10.1016/j.jep.2022.115275
18. Pietrzak A, Bartosińska J, Dreiher J, Szepietowski JC, Gawlik-Dziki U, Maciejewski R, et al. Deleterious effects of traditional Chinese medicine preparations on the course of psoriasis–a case report. Ann Agric Environ Med. (2013) 20(4):816–8.24364460
19. Jeffes EW 3rd, McCullough JL, Pittelkow MR, McCormick A, Almanzor J, Liu G, et al. Methotrexate therapy of psoriasis: differential sensitivity of proliferating lymphoid and epithelial cells to the cytotoxic and growth-inhibitory effects of methotrexate. J Invest Dermatol. (1995) 104(2):183–8. doi: 10.1111/1523-1747.ep12612745
20. Yang M, Kim JS, Kim J, Kim SH, Kim JC, Kim J, et al. Neurotoxicity of methotrexate to hippocampal cells in vivo and in vitro. Biochem Pharmacol. (2011) 82(1):72–80. doi: 10.1016/j.bcp.2011.03.020
21. Brazzelli V, Grasso V, Fornara L, Moggio E, Gamba G, Villani S, et al. Homocysteine, vitamin B12 and folic acid levels in psoriatic patients and correlation with disease severity. Int J Immunopathol Pharmacol. (2010) 23(3):911–6. doi: 10.1177/039463201002300327
22. Lawrence CM, Dahl MG. Two patterns of skin ulceration induced by methotrexate in patients with psoriasis. J Am Acad Dermatol. (1984) 11(6):1059–65. doi: 10.1016/s0190-9622(84)70259-3
23. Tett SE, Triggs EJ. Use of methotrexate in older patients. A risk-benefit assessment. Drugs Aging. (1996) 9(6):458–71. doi: 10.2165/00002512-199609060-00008
24. Jiang B, Yan LJ, Wu Q. ABCB1 (C1236T) polymorphism affects P-glycoprotein-mediated transport of methotrexate, doxorubicin, actinomycin D, and etoposide. DNA Cell Biol. (2019) 38(5):485–90. doi: 10.1089/dna.2018.4583
25. Zaruma-Torres F, Lares-Asseff I, Reyes-Espinoza A, Loera-Castañeda V, Chairez-Hernández I, Sosa-Macías M, et al. Association of ABCB1, ABCC5 and xanthine oxidase genetic polymorphisms with methotrexate adverse reactions in Mexican pediatric patients with ALL. Drug Metab Pers Ther. (2015) 30(3):195–201. doi: 10.1515/dmpt-2015-0011
26. Srisuttayasathien M, Areepium N, Lertkhachonsuk R. ABCB1 And SLCO1B1 gene polymorphisms predict methotrexate-resistant for low-risk gestational trophoblastic neoplasia. Per Med. (2021) 18(2):107–14. doi: 10.2217/pme-2020-0089
Keywords: psoriasis, methotrexate toxicity, skin ulceration, traditional Chinese, immunosuppressive
Citation: Zheng Y, Dubai Y, Wang R and Luo S (2025) Painful skin ulcers in psoriasis patients after taking methotrexate-containing traditional Chinese medicine: a retrospective case series. Front. Allergy 6:1634055. doi: 10.3389/falgy.2025.1634055
Received: 23 May 2025; Accepted: 9 June 2025;
Published: 23 June 2025.
Edited by:
Lili Zhi, Shandong Provincial Qianfoshan Hospital, ChinaReviewed by:
Qiri Mu, Inner Mongolia International Hospital, ChinaYan Zheng, The First Affiliated Hospital of Xi'an Jiaotong University, China
Copyright: © 2025 Zheng, Dubai, Wang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suju Luo, bHVvc3VqdUB0bXUuZWR1LmNu
†These authors have contributed equally to this work
 Yunhong Zheng1,2,†
Yunhong Zheng1,2,† Suju Luo
Suju Luo