- Department of Allergy, Tangshan Workers Hospital, Tangshan, Hebei, China
Objective: This study analyzes a case with a JAK1 (Janus Kinase 1) inhibitor was successfully employed to treat a patient with glucocorticoid-resistant acute severe urticaria (ASU), with the aim of improving clinical understanding of this condition.
Methods: A retrospective analysis was conducted on the clinical data, diagnosis, treatment, and prognosis of a patient with acute severe urticaria, who was admitted to the Allergy Department of Tangshan Workers’ Hospital on March 10, 2025.
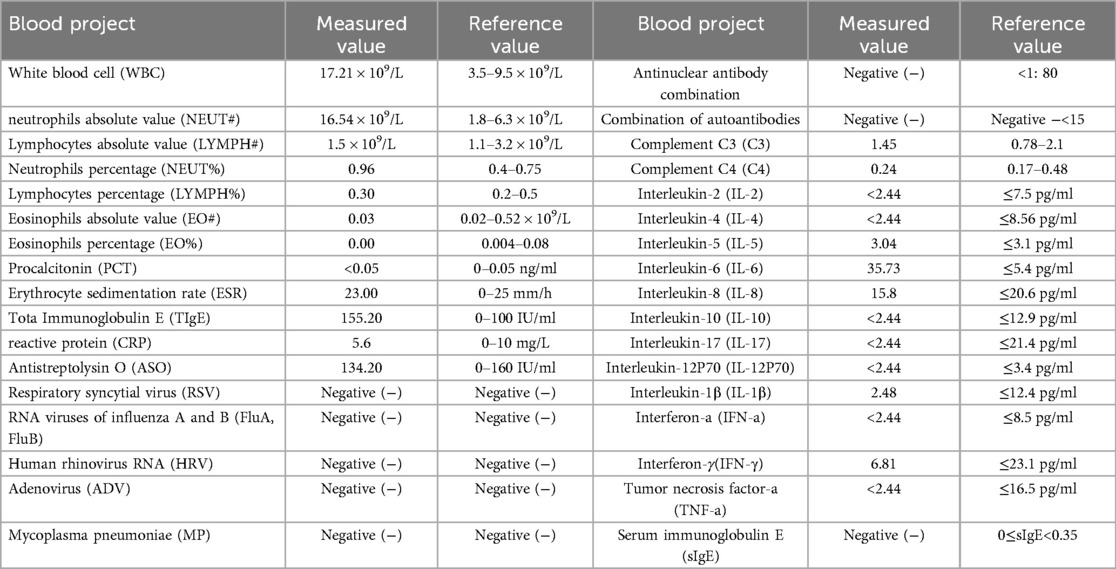
Results: The patient was a 50-year-old female who presented with widespread skin wheals and itching, along with a sensation of throat obstruction for two days. Upon admission, the patient had a body temperature of 38.5°C. Large, irregularly shaped wheals, up to 10 cm in diameter, were observed on the skin. These wheals were bright red with surrounding erythema and increased upon scratching. Laboratory tests indicated elevated levels of white blood cells (WBC), neutrophils percentage, neutrophils absolute value, total IgE, and interleukin-6 (IL-6). A diagnosis of acute severe urticaria was made. Prior to admission, the patient had been administered with betamethasone sodium phosphate, dexamethasone sodium phosphate, methylprednisolone succinate, diphenhydramine, and calcium gluconate at the emergency department without relief in wheals and itching. Upon admission, the patient was treated with glucocorticoids and JAK1 inhibitors, resulting in the complete regression of the rash and normalization of laboratory indicators.
Conclusion: This case suggests that JAK1 inhibitors can achieve satisfactory results in treating glucocorticoid-resistant acute severe urticaria.
1 Introduction
Acute spontaneous urticaria is defined as the occurrence of spontaneous wheals, angioedema or both for less than 6 weeks (1). Acute urticaria often presents with multi-system symptoms, including respiratory and digestive manifestations such as nausea, vomiting, abdominal pain, diarrhea, chest tightness, and throat obstruction (2). These patients exhibit a Th2 cell-dominated T-helper cell imbalance (3). Th2 cells play roles in allergic diseases, not limited to promoting B-cell IgE production, but also in generating cytokines like IL-4, 5, and 13 (4). These cytokines signal via the JAK-STAT pathway, mediating inflammation and itching. Over 50 cytokines have been identified as signaling through the JAK-STAT pathway, with JAK receptors possibly linked to multiple cytokine receptors (5). By inhibiting JAK1 kinase activation, JAK1 inhibitors block cytokine signaling, providing rapid anti-inflammatory and anti-itch action, notably in treating refractory or immune-mediated inflammatory diseases. To date, no cases have been reported on the use of JAK1 inhibitors for glucocorticoid-resistant acute severe urticaria. This article presents such a case to enhance understanding of this treatment.
2 Clinical data
2.1 General information
The patient, a 50-year-old female, was admitted for widespread skin wheals with severe itching and throat obstruction sensation on March 10, 2025. The onset was March 8, marked by large skin wheals, intolerable itching, and persistent symptoms not relieved by self-care. There was no throat pain, cough, sputum, abdominal pain, diarrhea, nausea, vomiting, urinary issues, or joint pain. The wheals were unrelated to cold or heat stimuli. Previously treated at the emergency room with intravenous medications such as betamethasone sodium phosphate, dexamethasone sodium phosphate, methylprednisolone succinate, diphenhydramine, and calcium gluconate with minimal relief from throat obstruction and persistent wheals and itching. No past medical history of hepatitis, tuberculosis, or other infectious diseases was reported. The patient denied any drug or food allergies, with no family history of similar illnesses.
2.2 Physical examination and auxiliary tests
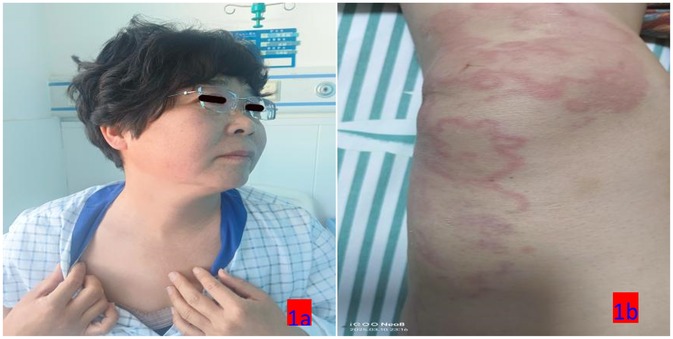
Upon admission: mental status was poor, consciousness clear, temperature 37.9°C, pulse 96 beats/min, respiratory rate 20 breaths/min, blood pressure 110/75 mmHg. The patient displayed large skin wheals, up to 10 cm, irregularly shaped, bright red with surrounding erythema, as shown in Figures 1a,b. Elevated skin temperature, absence of sweating or joint deformation, and no swollen lymph nodes were noted. The Urticaria Activity Score (UAS7) (6) was 36, and the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL) (7) scored 35. Auxiliary tests on the admission day are shown in Table 1.
2.3 Diagnosis and treatment process
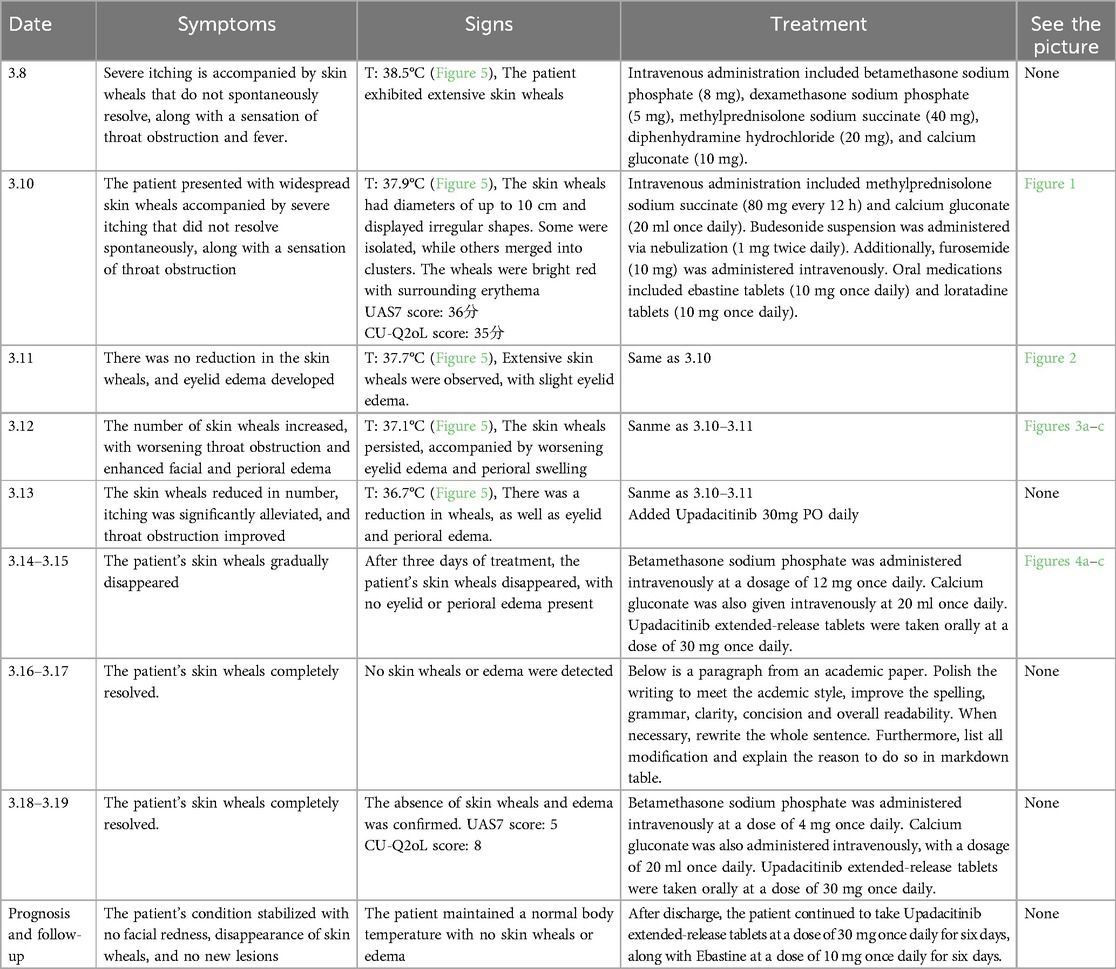
Following comprehensive evaluation and exclusion of contraindications, the diagnosis of acute severe urticaria was confirmed. Details of diagnosis and treatment are listed in Table 2 (Figures 2–5).

Figure 2. On the second day of hospitalization (March 11, 2025), the patient exhibited swelling in the eyelids and face, along with a rash on the neck.
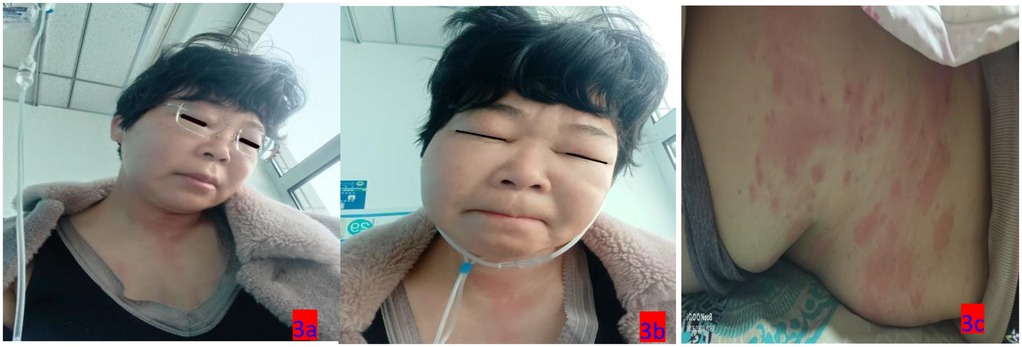
Figure 3. (a–c) On the third day of hospitalization (March 12, 2025), the patient exhibited swelling in the eyelids and face, along with truncal rashes.

Figure 4. (a–c) On the sixth day of hospitalization (March 16, 2025), the patient exhibited a reduction in eyelid and facial rashes, as well as improvement in skin lesions throughout the body.
3 Discussion
The primary etiologies of acute urticaria are infections, medications, and foods (8). Infections are closely related to its onset, with bacterial, fungal, viral, and parasitic factors (9). Acute infectious urticaria presents with fever, throat pain, joint pain, chest tightness, and abdominal pain; lab tests frequently show elevated inflammatory markers. A Japanese study set the following diagnostic criteria for acute infectious urticaria: widespread wheals with fever (≥37°C), resistance to antihistamine treatment requiring glucocorticoid and antibiotic combinations, and meeting two of three lab abnormalities (WBC >10,000/mm3, neutrophils percentage >70%, CRP >0.5 mg/dl) (10). In this case, the patient's widespread, intractable wheals were bright red and merged into larger patches, causing intense itching. Glucocorticoid treatment saw no symptom relief but induced fever, worsening throat obstruction, eyelid, and facial edema. No infection was observed clinically or in lab tests except elevated WBC, neutrophils, and IL-6. The presentation aligns with infectious urticaria diagnostic criteria, though wheals preceded fever with a low fever magnitude suggesting possible severe allergy or nosocomial infection. Literature shows increased white blood cells and neutrophils in patients treated with glucocorticoids (11), making symptom and lab tests insufficient for classifying infectious urticaria. Without comprehensive differentiation between infectious/non-infectious urticaria, symptoms resolved rapidly with Upadacitinib, highlighting its importance in early infectious/non-infectious urticaria treatment.
Upadacitinib is a selective JAK1 inhibitor affecting CD4+ T cells, neutrophils, dendritic cells, and reducing inflammatory cytokines like IL-6, IL-17, IL-2, IL-23, IL-36, IFN-α, IFN-β, and IFN-γ (12), influencing Th2 cell differentiation and inflammation cell infiltration, thereby reducing mast cell activation, inflammatory mediator release, and alleviating urticaria symptoms. Upadacitinib not only inhibits inflammatory pathways but also modulates immune cell functions, aiding immune balance recovery. This dual regulatory role aids in controlling urticaria's inflammatory state.
In summary, this case reports the successful use of the JAK1 inhibitor Upadacitinib for glucocorticoid-resistant acute severe urticaria, achieving satisfactory results. JAK1 inhibitors could become highly promising treatment options for acute severe urticaria patients. No thorough differentiation was made for infectious urticaria in this case; thus, no anti-infective treatment was administered. Further studies on the long-term efficacy and safety of JAK1 inhibitors in acute and chronic severe urticaria are warranted.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YW: Visualization, Funding acquisition, Validation, Writing – review & editing, Data curation. L-fW: Conceptualization, Validation, Writing – original draft, Software. H-nY: Project administration, Methodology, Writing – original draft. C-xK: Project administration, Writing – original draft, Resources. XG: Formal analysis, Funding acquisition, Writing – original draft. G-dH: Validation, Visualization, Supervision, Investigation, Writing – review & editing, Resources, Software.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Hebei Province Administration of Traditional Chinese Medicine research project (2023413).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zuberbier T, Aberer W, Asero R. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. (2018) 12(73):1393–414. doi: 10.1111/all.13397
2. Urticaria Research Center, Dermatology and Venereology Branch, Chinese Medical Association. Chinese guidelines for the diagnosis and treatment of urticaria (2022 edition). Chin J Dermatol. (2022) 55(12):1041–9.
3. Yicheng Y, Haizhen X, Haibin W, Hui S, Min F, Hua C, Jianping S. Detection of Serum IL-4, IL-17 and IFN-γ levels in patients with urticaria. Chin J Dermatol Venereol. (2014) 28(11):1124–6.
4. Romagmani S. The role of lymphacytes in allergic disease. Allergy Clin Immunol. (2000) 105(3):399–408. doi: 10.1067/mai.2000.104575
5. Iznardo H, Roé E, Serra-Baldrich E, Puig L. Efficacy and safety of JAK11inhibitor abrocitinibin atopic dermatitis. Pharmaceutics. (2023) 15(2):385. doi: 10.3390/pharmaceutics15020385
6. Młynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T, Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. (2008) 63(6):777–80. doi: 10.1111/j.1398-9995.2008.01726.x
7. Sánchez J, Zakzuk J, Cardona R. Evaluation of a guidelines-based approach to the treatment of chronic spontaneous urticaria. J Allergy Clin Immunol Pract. (2018) 6(1):177–82.28709817
8. Zuberbier T. Acute urticaria: clinical aspects and therapeutic responsiveness. Acta Dermatovenereol. (1996) 76(12):135–9.
9. Minciullo PL, Cascio A, Barberi G, Gangemi S. Urticaria and bacterial infections. Allergy Asthma Proc. (2014) 35(4):295–302. doi: 10.2500/aap.2014.35.3764
10. Takahashi T, Minami S, Teramura K, Tanaka T, Fujimoto N. Four cases of acute infectious urticaria showing significant elevation of plasma D-dimer level. J Dermatol. (2018) 45(8):1013–6. doi: 10.1111/1346-8138.14481
11. Ronchetti S, Ricci E, Migliorati G, Gentili M, Riccardi C. How glucocorticoids affect the neutrophil life. Int J Mol Sci. (2018) 19(12):129–31. doi: 10.3390/ijms19124090
Keywords: acute severe urticaria, JAK1 inhibitors, upadacitinib, glucocorticoid-resistant, successful treatment
Citation: Wu Y, Wang L-f, Yang H-n, Kan C-x, Guo X and Hao G-d (2025) Successful treatment of glucocorticoid-resistant acute severe urticaria with JAK1 inhibitor: case report. Front. Allergy 6:1657164. doi: 10.3389/falgy.2025.1657164
Received: 1 July 2025; Accepted: 13 August 2025;
Published: 19 September 2025.
Edited by:
Luisa Ricciardi, University of Messina, ItalyReviewed by:
Michael Rudenko, London Allergy and Immunology Centre, United KingdomYashdeep Pathania, All India Institute of Medical Sciences, India
Copyright: © 2025 Wu, Wang, Yang, Kan, Guo and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-dong Hao, aGdkZ3htQHNpbmEuY29t
 Ying Wu
Ying Wu Long-fei Wang
Long-fei Wang