- Department of Pediatrics, Peking University Third Hospital, Beijing, China
Introduction: Food-induced anaphylactic shock is the most severe and life-threatening manifestation of food allergy in children. However, its underlying risk factors remain poorly defined, especially in pediatric populations. This study aimed to identify independent predictors of food-induced anaphylactic shock by analyzing clinical and immunological data from a tertiary hospital in Northern China.
Methods: This retrospective study included 68 children (aged 0–16 years) hospitalized for food-induced anaphylaxis at Peking University Third Hospital from May 2018 to May 2025. Demographic data, clinical manifestations, serum-specific immunoglobulin E (sIgE) levels, and comorbidities were collected. Univariate and multivariate logistic regression analyses were performed to identify independent predictors of anaphylactic shock.
Results: Of 68 patients with food-induced anaphylaxis, 14 (20.6%) experienced anaphylactic shock, with 78.6% of these cases occurring in children older than six years. Wheat and fruits were the most common triggers in the shock group (each 28.6%). Subgroup analysis revealed that 88.9% (8/9) of plant-food-triggered shock cases specifically clustered from April to October, aligning with mugwort pollen season. sIgE levels to inhalant allergens, especially mugwort, were significantly higher in the shock group (P < 0.05). In multivariate analysis, mugwort sensitization (sIgE levels exceeding 2.83 kU/L, OR = 9.91, 95% CI: 1.27–77.31, P = 0.029) and comorbid asthma (OR = 8.11, 95% CI: 1.29–50.98, P = 0.026) were identified as independent predictors of anaphylactic shock.
Conclusions: Mugwort sensitization and asthma are independent risk factors for food-induced anaphylactic shock in children. Early identification of these risk markers may support early risk stratification and seasonal dietary interventions.
1 Introduction
Food allergy (FA) is an adverse immune response to specific food antigens, and its global prevalence has risen substantially in recent decades, posing a significant public health challenge (1–4). When the respiratory or circulatory systems are involved, FA reactions can rapidly escalate to distributive shock, leading to multiorgan failure or death. Despite its severity, food-induced anaphylactic shock remains unpredictable in pediatric populations.
Previous studies have suggested that various factors may influence the severity of allergic reactions, including the type and number of food allergens (1, 5, 6), age (7), underlying atopic diseases [such as asthma (8, 9)], and cofactors such as exercise (10) or the use of nonsteroidal anti-inflammatory drugs (NSAIDs) (11). Moreover, up to 60% of FA reactions may be associated with cross-reactivity to inhalant allergens (12). In Northern China, plant-derived foods—especially wheat and fruits—are common food allergens (13) and are significantly linked to pollinosis (14). The prevalence of wheat allergy among children in Northern China is 3.6% (15), while the self-reported prevalence of fruit allergies in children aged 0–5 years is 1.55% (16). Aeroallergens like mugwort pollen are highly prevalent and may contribute to enhanced allergic responses due to molecular cross-reactivity with plant-derived foods (17). However, most current data are derived from adult populations, and there is a lack of pediatric-specific evidence, particularly regarding risk factors for the development of anaphylactic shock in children. This knowledge gap limits our ability to identify high-risk children and implement early intervention strategies.
In this retrospective study, we analyzed clinical and laboratory data from pediatric patients with food-induced anaphylaxis at a tertiary hospital in Northern China. Using univariate and multivariate logistic regression, we aimed to identify independent risk factors for food-induced anaphylactic shock in children, when compared to non-shock anaphylaxis cases. Our goal is to inform clinical risk stratification, guide preventive strategies, and identify high-risk children who may benefit from targeted education or desensitization therapy.
2 Materials and methods
2.1 Study design and participants
This retrospective study enrolled pediatric patients (aged 0–16 years) diagnosed with food-induced anaphylaxis and hospitalized at the Pediatric Department of Peking University Third Hospital from May 2018 to May 2025. The study protocol was approved by the hospital's Ethics Committee (Approval No. M20250454) and conducted in accordance with the Declaration of Helsinki.
2.2 Data collection
Patient data were retrieved from the hospital's electronic medical record system (Jiahe, Beijing, China). Variables collected included demographic characteristics (age, sex), month of symptom onset, latency between food exposure and symptom onset, clinical manifestations, history of allergic diseases, family history of allergies (first-degree relatives), and laboratory test results (total serum IgE, allergen-specific IgE, and skin prick test results).
2.3 Diagnostic criteria
Anaphylaxis was diagnosed based on the 2020 World Allergy Organization (WAO) criteria (18). Food-induced anaphylactic shock was defined as anaphylaxis accompanied by hypotension, corresponding to Grade 5 in the WAO classification. Hypotension was defined using the 2021 European Academy of Allergy and Clinical Immunology guidelines (19): systolic blood pressure below the age-specific threshold or a >30% decrease from baseline (e.g., <70 mmHg in infants aged 1 month–1 year, <70 + 2× age in years mmHg for children aged 1–10 years, <90 mmHg for adolescents aged 11–17 years). Food triggers were identified based on patient history and confirmed via skin prick testing and/or serum sIgE detection. In cases where NSAIDs were suspected as cofactors, medication history was reviewed, and oral provocation tests under fasting conditions were conducted to exclude NSAID hypersensitivity. All diagnoses were confirmed by board-certified allergy and immunology specialists.
2.4 Statistical analysis
Statistical analyses were performed using SPSS version 27.0. Continuous variables with non-normal distribution were expressed as median (interquartile range) [M (P25, P75)] and compared using the Mann–Whitney U test. Categorical variables were presented as counts (percentages) and compared using the chi-square test or Fisher's exact test as appropriate. A P-value <0.05 was considered statistically significant. To identify potential risk factors for food-induced anaphylactic shock in children, univariate analysis was first performed using the chi-square test. Receiver operating characteristic curves were generated for continuous variables to determine optimal cut-off values based on the Youden index, and variables were then dichotomized by these thresholds for further chi-square testing. Variables with P < 0.05 in univariate analysis were entered into a multivariate logistic regression model to identify independent risk factors, with significance set at P < 0.05.
3 Results
3.1 Patient characteristics
Among 10,837 pediatric admissions during the study period, 97 were diagnosed with anaphylaxis. Of these, 68 cases (70.1%) were attributed to food-induced anaphylaxis and included in the final analysis. Within this group, 14 patients (20.6%) developed anaphylactic shock, while 54 (79.4%) did not. A majority of patients were male (63.2%). Figure 1 summarizes the study enrollment process.
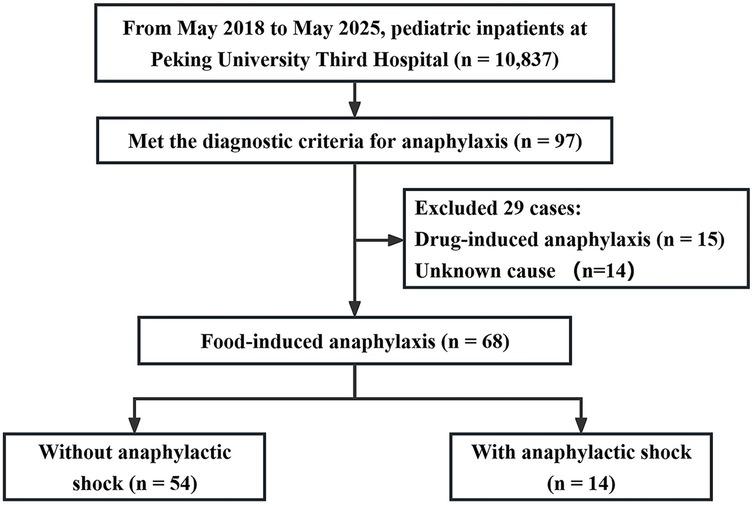
Figure 1. Flowchart of patient inclusion. Study enrollment diagram showing the screening and inclusion of 68 pediatric patients with food-induced anaphylaxis, including 14 cases with anaphylactic shock and 54 without shock.
3.2 Age distribution
The age distribution of children with food-induced anaphylaxis is shown in Figure 2. The non-shock group ranged from 1 month and 17 days to 15 years, while the shock group ranged from 4 months and 18 days to 15 years. Notably, most cases of shock occurred in children older than six years (11/14, 78.57%).
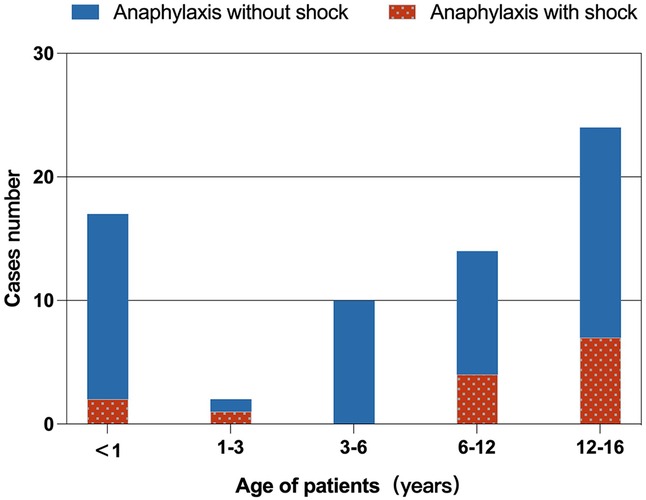
Figure 2. Age distribution of children with food-induced anaphylaxis. Anaphylactic shock occurred more frequently in children over 6 years of age (11/14, 78.6%).
3.3 Food triggers
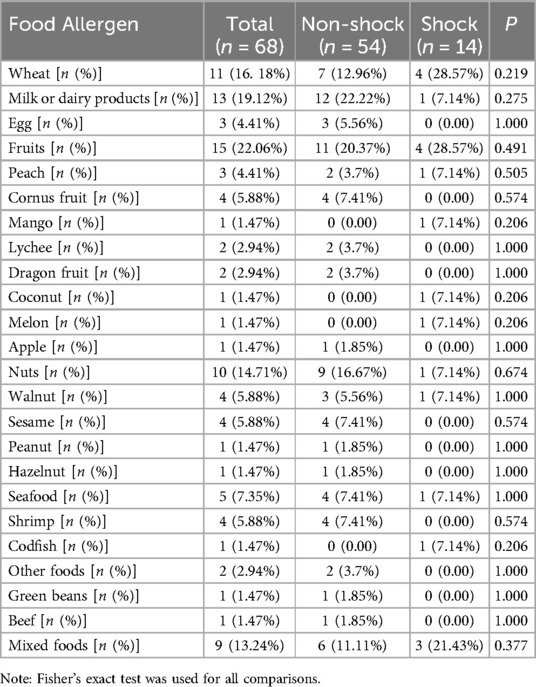
Table 1 presents the distribution of food triggers. Fruits (22.1%) and dairy products (19.1%) were the most common allergens overall. In the shock group, wheat and fruits were the predominant triggers (each 28.6%), whereas dairy products (12/54, 22.22%) and fruits (11/54, 20.37%) were most frequent in the non-shock group. No statistically significant differences in food allergen distribution were observed between the two groups (P > 0.05).
3.4 Seasonal patterns and aeroallergen sensitization
Figure 3A illustrates the monthly distribution of food-induced anaphylaxis cases, with a marked increase from April to October and a peak in September. While shock reactions were more equally distributed throughout the year, subgroup analysis revealed that 88.9% (8/9) of plant-food-triggered shock cases (including wheat, peach, walnut, melon, mango and coconut) specifically clustered from April to October. Figure 3B compares the median levels of sIgE to common inhalant allergens between the two groups. Mugwort sensitization was significantly more common in the shock group (P < 0.05), while no significant differences were found for other aeroallergens, including ragweed, birch, Japanese hop, animal dander, dust mites, and molds.
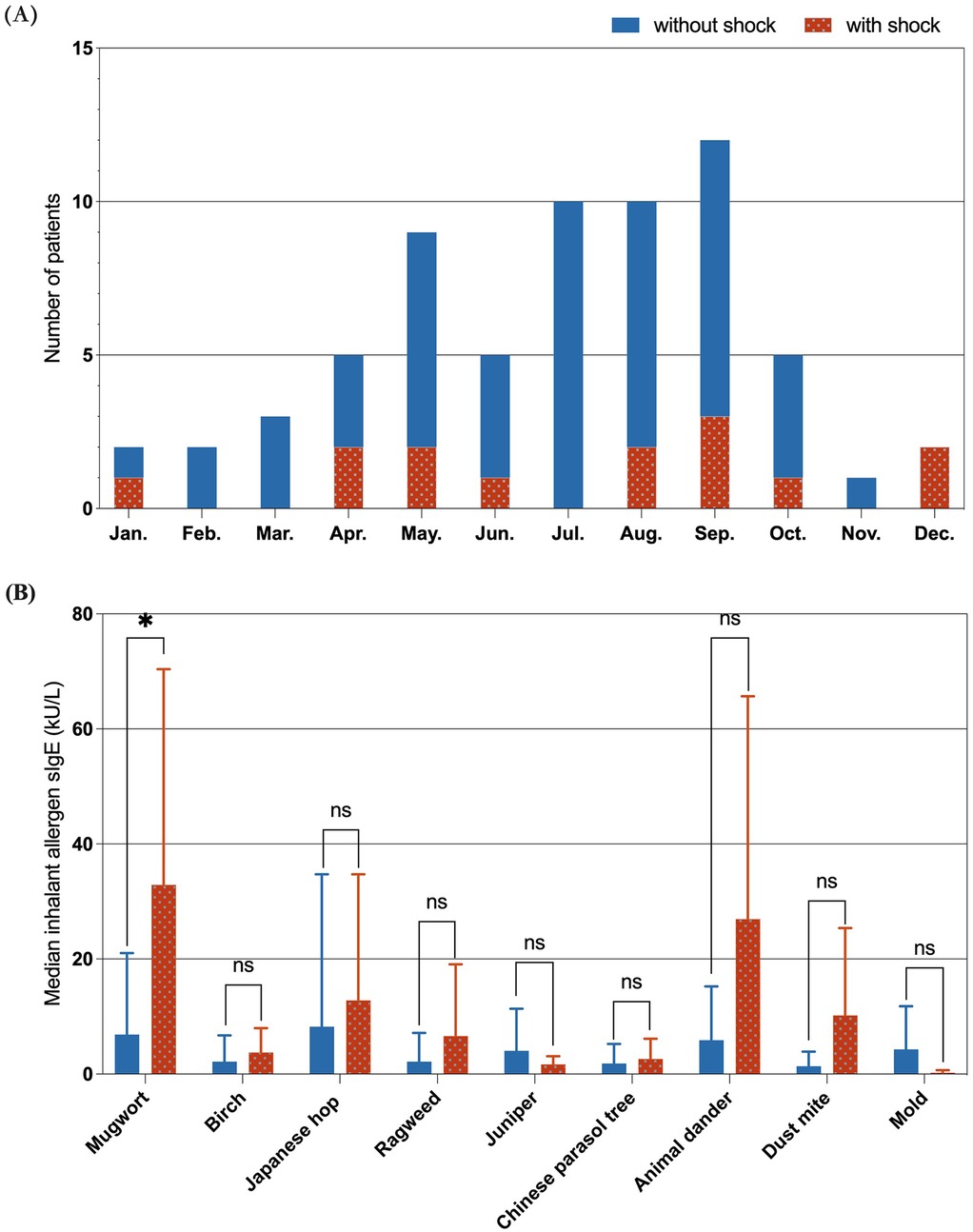
Figure 3. Seasonal distribution and inhalant allergen sensitization. (A) Cases were concentrated between April and October, with a peak in September. (B) Mugwort sensitization was significantly more common in the shock group (P < 0.05), other inhalant allergens showed no significant difference. (In the figure, “ns” represents P > 0.05; “*” represents P < 0.05).
3.5 Predictive value of mugwort sIgE: receiver operating characteristic (ROC) analysis
To assess the predictive performance of mugwort sIgE, ROC curve was generated (Figure 4). The area under the curve (AUC) was 0.749, indicating good discriminative ability. The optimal cut-off value for mugwort sIgE was determined to be 2.83 kU/L, with a sensitivity of 66.7% and specificity of 73.7% for predicting anaphylactic shock.
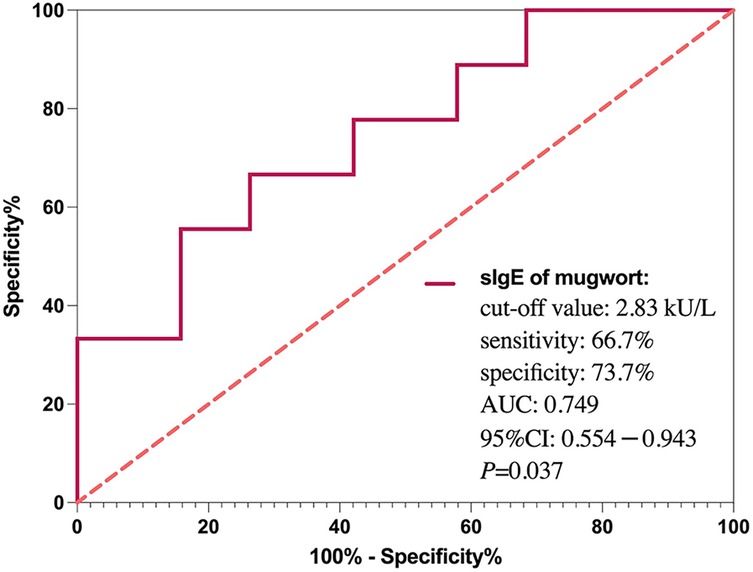
Figure 4. ROC curve for mugwort-specific IgE predicting anaphylactic shock. The area under the curve (AUC) was 0.749. A cut-off value of 2.83 kU/L yielded 66.7% sensitivity and 73.7% specificity.
3.6 Univariate analysis of risk factors for food-induced anaphylactic shock
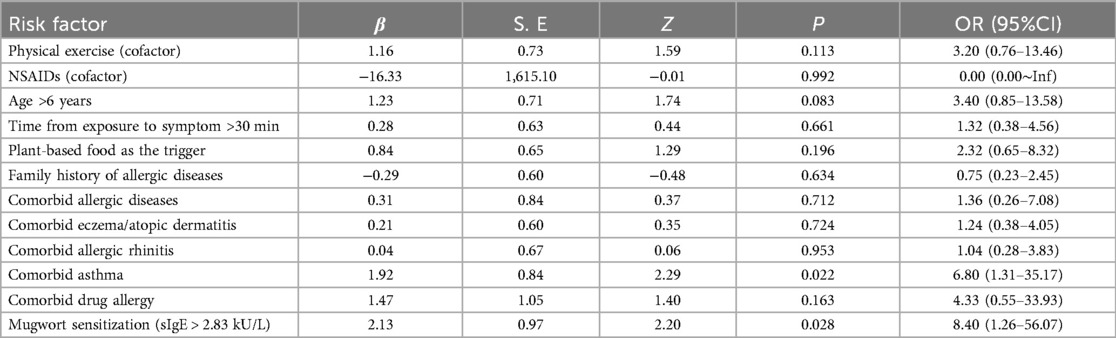
As shown in Table 2, univariate logistic regression identified comorbid asthma [Odds ratio [OR] = 6.80, 95% Confidence interval [CI]: 1.31–35.17, P = 0.022] and mugwort sensitization (sIgE levels exceeding 2.83 kU/L, OR = 8.40, 95% CI: 1.26–56.07, P = 0.028) as significant risk factors for anaphylactic shock. Other variables, including coexisting other allergic diseases (except asthma), cofactor exposure, age, food type and family history of allergic diseases, were not significantly associated with anaphylactic shock.
3.7 Multivariate analysis of risk factors for food-induced anaphylactic shock
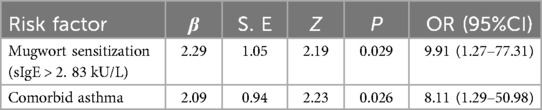
In multivariate logistic regression (Table 3), both mugwort sensitization (OR = 9.91, 95% CI: 1.27–77.31, P = 0.029) and asthma (OR = 8.11, 95% CI: 1.29–50.98, P = 0.026) remained independent predictors of anaphylactic shock.
4 Discussion
In this retrospective analysis of 68 pediatric cases of food-induced anaphylaxis, we identified two independent risk factors for anaphylactic shock in children from Northern China: elevated sensitization to mugwort pollen (sIgE levels exceeding 2.83 kU/L) and a history of asthma, when compared to non-shock anaphylaxis cases. In contrast, food allergen type, cofactor exposure (e.g., physical exertion or NSAIDs use), and other comorbid allergic diseases (except asthma) did not show significant associations with shock.
Regional variations in environmental exposures, dietary habits, and climate across China contribute to distinct allergic sensitization patterns (12). In Northern China, mugwort pollen is the predominant aeroallergen during late summer and early autumn (20). With over 180 Artemisia species documented (21), cross-reactive molecules such as non-specific lipid transfer proteins (nsLTPs) and profilins—shared between mugwort pollen and many plant-derived foods—can trigger allergic responses. These proteins are highly stable, resisting both digestion and heat, and are recognized as potent allergens (17, 22). In our study, plant-food-triggered shock cases specifically clustered from April to October, aligning with mugwort pollen season, suggesting a potential “priming–amplification” effect of seasonal inhalant sensitization on FA severity. Among the aeroallergens evaluated, only mugwort sensitization was independently associated with anaphylactic shock (P < 0.05). A mugwort sIgE level exceeding 2.83 kU/L was associated with nearly tenfold increased risk of shock (OR = 9.91), highlighting the clinical importance of mugwort screening. These findings underscore the need for targeted counseling and avoidance strategies in mugwort-sensitized children, especially regarding consumption of cross-reactive foods during high-pollen periods.
Asthma history also emerged as an independent predictor of anaphylactic shock. Many asthmatic patients in our cohort had poorly controlled disease and suboptimal adherence to inhaled corticosteroid therapy. FA and asthma may share common sensitization pathways and inflammatory mechanisms, with potential synergistic effects. Shared type 2 immune pathways and epithelial barrier dysfunction may contribute to enhanced allergen absorption and anaphylaxis (23). Additionally, elevated Th2 cytokines such as IL-4 and IL-13 in poorly controlled asthma may upregulate IgE synthesis and mast cell activation (24), further compounding the risk of anaphylactic shock. These insights support a dual-management approach in pediatric patients with asthma: on one hand, achieving optimal asthma control, such as improving adherence through single maintenance and reliever therapy to enhance asthma management; on the other hand, assessing and mitigating the risk of food-induced allergic reactions.
Although cofactors such as physical activity and NSAID use are recognized exacerbators of anaphylaxis in adults (25–27), our data did not show a significant association between these factors and anaphylactic shock in children. This aligns with findings from Casas-Saucedo et al. (28) and may reflect the lower intensity of physical exertion and limited NSAID use in pediatric populations. Furthermore, intrinsic host factors—such as genetic susceptibility (29) and reduced compensatory mechanisms (30, 31) —may play a more prominent role in pediatric anaphylaxis severity than external triggers.
Our results emphasize the importance of incorporating regional inhalant allergen profiles into pediatric allergy risk assessments. In areas like Northern China, where mugwort pollen exposure is common, measuring sIgE levels and evaluating cross-reactivity with food allergens should be part of routine care. Children with coexisting asthma and mugwort sensitization represent a particularly high-risk group and should be prioritized for early intervention, including seasonal dietary guidance and personalized management plans. Unlike Western cohorts, where peanut, tree nuts, or milk are primary triggers (32), our findings point to plant-based foods—particularly fruits and wheat—as the leading causes of food-induced anaphylactic shock in Northern Chinese children. This regional difference underscores the importance of context-specific prevention strategies.
Several limitations warrant consideration. First, the retrospective, single-center design and relatively small number of shock cases (n = 14) may limit statistical power and generalizability. Second, due to data limitations, we could not assess the influence of food type, quantity, or component-resolved allergen profiles. Future multicenter studies with larger sample sizes are needed to further explore the interaction between food allergens and environmental factors. Moreover, the integration of component-resolved diagnostics may enhance risk stratification by identifying specific sensitizing molecules and may facilitate the development of targeted immunotherapy or biologic interventions for high-risk populations.
5 Conclusion
This study identifies mugwort pollen sensitization and a history of asthma as independent risk factors for food-induced anaphylactic shock in children from Northern China. These findings underscore the need to incorporate regional aeroallergen profiles and comorbid respiratory conditions into pediatric allergy risk assessments. Routine screening for mugwort sensitization—particularly during peak pollen seasons—and strict asthma control may reduce the risk of life-threatening anaphylactic events. Region-specific strategies, including seasonal dietary counseling and personalized allergy management, are essential for high-risk populations. Future multicenter and prospective studies integrating molecular diagnostics are warranted to further refine risk prediction models and improve early intervention in pediatric food allergy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by This study was approved by the Ethics Committee of Peking University Third Hospital (Approval No. M20250454). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because As a retrospective review of clinical records without identifiable personal data or additional risk to patients, the requirement for informed consent was waived.
Author contributions
ZZ: Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft. JZ: Writing – review & editing, Supervision, Validation. ND: Methodology, Validation, Writing – review & editing. ZL: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Clinical Cohort Construction Program of Peking University Third Hospital, No. BYSYDL2022019.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
FA, food allergy; NSAIDs, non-steroidal anti-inflammatory drugs; sIgE, serum-specific immunoglobulin E; ROC, receiver operating characteristic; OR, odds ratio; CI, confidence interval.
References
1. Baseggio Conrado A, Ierodiakonou D, Gowland MH, Boyle RJ, Turner PJ. Food anaphylaxis in the United Kingdom: analysis of national data, 1998–2018. Br Med J. (2021) 372:n251. doi: 10.1136/bmj.n251
2. Goh SH, Yap GC, Cheng HY, Chiang WC, Soh JY, Chong KW, et al. Trends in childhood anaphylaxis in Singapore: 2015–2022. Clin Exp Allergy. (2024) 54(8):585–95. doi: 10.1111/cea.14528
3. Rueter K, Moseley N, Ta B, Bear N, Borland ML, Prescott SL. Increasing emergency department visits for anaphylaxis in very early childhood: a canary in the coal mine. Acta Paediatr. (2023) 112(10):2182–8. doi: 10.1111/apa.16917
4. Weber HC, Bassett GL, Sohal SS, Prior SJ. Self-reported food allergies in early childhood in rural Australia. Front Allergy. (2025) 6:1544496. doi: 10.3389/falgy.2025.1544496
5. Lieberman JA, Gupta RS, Knibb RC, Haselkorn T, Tilles S, Mack DP, et al. The global burden of illness of peanut allergy: a comprehensive literature review. Allergy. (2021) 76(5):1367–84. doi: 10.1111/all.14666
6. Gabriela ZI, Elena RMM, Carlos LT, Marta BL, Ochoa MLB. Clinical and sensitization profile in peach allergy due to ltp sensitization. Front Allergy. (2024) 5:1477364. doi: 10.3389/falgy.2024.1477364
7. Turner PJ, Arasi S, Ballmer-Weber B, Baseggio Conrado A, Deschildre A, Gerdts J, et al. Risk factors for severe reactions in food allergy: rapid evidence review with meta-analysis. Allergy. (2022) 77(9):2634–52. doi: 10.1111/all.15318
8. Carter MC, Park J, Vadas P, Worm M. Extrinsic and intrinsic modulators of anaphylaxis. J Allergy Clin Immunol Pract. (2023) 11(7):1998–2006. doi: 10.1016/j.jaip.2023.05.015
9. Kawahara T, Tezuka J, Ninomiya T, Honjo S, Masumoto N, Nanishi M, et al. Risk prediction of severe reaction to oral challenge test of cow’s milk. Eur J Pediatr. (2019) 178(2):181–8. doi: 10.1007/s00431-018-3274-z
10. Pouessel G, Alonzo S, Divaret-Chauveau A, Dumond P, Bradatan E, Liabeuf V, et al. Fatal and near-fatal anaphylaxis: the allergy-vigilance® network data (2002–2020). Allergy. (2023) 78(6):1628–38. doi: 10.1111/all.15645
11. Scala E, Abeni D, Villella V, Villalta D, Cecchi L, Pravettoni V, et al. Clinical severity of LTP syndrome is associated with an expanded IgE repertoire, FDEIA, FDHIH, and LTP mono reactivity. Eur Ann Allergy Clin Immunol. (2025) 57(3):128–39. doi: 10.23822/EurAnnACI.1764-1489.314
12. Werfel T, Asero R, Ballmer-Weber BK, Beyer K, Enrique E, Knulst AC, et al. Position paper of the EAACI: food allergy due to immunological cross-reactions with common inhalant allergens. Allergy. (2015) 70(9):1079–90. doi: 10.1111/all.12666
13. Leung AS, Jie S, Gu Y, Wong GW. Food allergy in children in China. Clin Exp Allergy. (2024) 55:634–647. doi: 10.1111/cea.14596
14. Zhao L, Ma T, Wang X, Wang H, Liu Y, Wu S, et al. Food-pollen cross-reactivity and its molecular diagnosis in China. Curr Allergy Asthma Rep. (2024) 24(9):497–508. doi: 10.1007/s11882-024-01162-w
15. Wang Y, Weng J, Zhu C, Ai R, Zhou J, Wang C, et al. Allergenicity assessment and allergen profile analysis of different Chinese wheat cultivars. World Allergy Organ J. (2021) 14(7):100559. doi: 10.1016/j.waojou.2021.100559
16. Ju LH, Zhao LY, Wei XQ, Fang HY, Li JX, Wu XX, et al. Prevalence and influencing factors on food allergy among children aged 0–5 years in China. Zhonghua Liu Xing Bing Xue Za Zhi. (2024) 45(6):817–23. doi: 10.3760/cma.j.cn112338-20231201-00329
17. Deng S, Yin J. Mugwort pollen-related food allergy: lipid transfer protein sensitization and correlation with the severity of allergic reactions in a Chinese population. Allergy Asthma Immunol Res. (2019) 11(1):116–28. doi: 10.4168/aair.2019.11.1.116.
18. Cardona V, Ansotegui IJ, Ebisawa M, El-Gamal Y, Fernandez Rivas M, Fineman S, et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. (2020) 13(10):100472. doi: 10.1016/j.waojou.2020.100472
19. Muraro A, Worm M, Alviani C, Cardona V, DunnGalvin A, Garvey LH, et al. EAACI guidelines: anaphylaxis (2021 update). Allergy. (2022) 77(2):357–77. doi: 10.1111/all.15032
20. Sun A, Sun X, Li X, Wu S, Ye C, Zhang H. Sensitization characteristics in allergic rhinitis and transport pathway for Artemisia pollen in northern Beijing, China. Sci Total Environ. (2023) 884:163795. doi: 10.1016/j.scitotenv.2023.163795
21. Zhao L, Fu W, Gao B, Liu Y, Wu S, Chen Z, et al. Variation in IgE binding potencies of seven Artemisia species depending on content of major allergens. Clin Transl Allergy. (2020) 10(1):50. doi: 10.1186/s13601-020-00354-7
22. Li JD, Du ZR, Liu J, Xu YY, Wang RQ, Yin J. Characteristics of pollen-related food allergy based on individual pollen allergy profiles in the Chinese population. World Allergy Organ J. (2020) 13(5):100120. doi: 10.1016/j.waojou.2020.100120
23. Berni Canani R, Caminati M, Carucci L, Eguiluz-Gracia I. Skin, gut, and lung barrier: physiological interface and target of intervention for preventing and treating allergic diseases. Allergy. (2024) 79(6):1485–500. doi: 10.1111/all.16092
24. Jutel M, Agache I, Zemelka-Wiacek M, Akdis M, Chivato T, Del Giacco S, et al. Nomenclature of allergic diseases and hypersensitivity reactions: adapted to modern needs: an EAACI position paper. Allergy. (2023) 78(11):2851–74. doi: 10.1111/all.15889
25. Bartra J, Turner PJ, Muñoz-Cano RM. Cofactors in food anaphylaxis in adults. Ann Allergy Asthma Immunol. (2023) 130(6):733–40. doi: 10.1016/j.anai.2023.03.017
26. Versluis A, van Os-Medendorp H, Kruizinga AG, Blom WM, Houben GF, Knulst AC. Cofactors in allergic reactions to food: physical exercise and alcohol are the most important. Immun Inflamm Dis. (2016) 4(4):392–400. doi: 10.1002/iid3.120
27. Oztop N, Vitus MV, Faihs V, Kugler C, Biedermann T, Brockow K. Test panel of hidden allergens for “idiopathic anaphylaxis” reveals wheat allergy dependent on augmentation factors as common final diagnosis. J Allergy Clin Immunol Pract. (2024) 12(9):2337–46. doi: 10.1016/j.jaip.2024.05.037
28. Casas-Saucedo R, de la Cruz C, Araujo-Sánchez G, Gelis S, Jimenez T, Riggioni S, et al. Risk factors in severe anaphylaxis: which matters the most, food or cofactors? J Investig Allergol Clin Immunol. (2022) 32(4):282–90. doi: 10.18176/jiaci.0698
29. Ribó P, Guo Y, Aranda J, Ainsua-Enrich E, Navinés-Ferrer A, Guerrero M, et al. Mutation in KARS: a novel mechanism for severe anaphylaxis. J Allergy Clin Immunol. (2021) 147(5):1855–64.e9. doi: 10.1016/j.jaci.2020.12.637
30. Muñoz-Cano RM, Casas R, Araujo G, de la Cruz C, Martin M, Roca-Ferrer J, et al. Prostaglandin E2 decreases basophil activation in patients with food-induced anaphylaxis. Allergy. (2021) 76(5):1556–9. doi: 10.1111/all.14615
31. Rastogi S, Willmes DM, Nassiri M, Babina M, Worm M. PGE2 Deficiency predisposes to anaphylaxis by causing mast cell hyperresponsiveness. J Allergy Clin Immunol. (2020) 146(6):1387–96.e13. doi: 10.1016/j.jaci.2020.03.046
Keywords: food allergy, anaphylactic shock, children, mugwort, asthma, risk prediction
Citation: Zhou Z, Zhang J, Dai N and Li Z (2025) Mugwort sensitization and asthma as predictors of food-induced anaphylactic shock in children: a retrospective study in Northern China. Front. Allergy 6:1658895. doi: 10.3389/falgy.2025.1658895
Received: 3 July 2025; Accepted: 6 August 2025;
Published: 21 August 2025.
Edited by:
Richard L. Wasserman, Medical City Children's Hospital, United StatesReviewed by:
Hugh Windom, University of South Florida, United StatesJulianne Doucette, Rush University, United States
Copyright: © 2025 Zhou, Zhang, Dai and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zailing Li, dG9wYmoxNjNAc2luYS4gY29t
 Zibei Zhou
Zibei Zhou Juan Zhang
Juan Zhang Nini Dai
Nini Dai Zailing Li
Zailing Li