- 1Division of Allergology and Clinical Immunology, Department of Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa
- 2Allergy and Immunology Unit, University of Cape Town Lung Institute, Cape Town, South Africa
- 3The Division of Epidemiology and Biostatistics, Department of Global Health, Stellenbosch University, Stellenbosch, South Africa
- 4The Modelling and Simulation Hub, Africa, Department of Statistical Sciences, University of Cape Town, Cape Town, South Africa
- 5District 6 Day Hospital, Western Cape, Cape Town, South Africa
- 6The Division of Nephrology and Hypertension, Department of Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa
Introduction: Angiotensin converting enzyme inhibitors (ACEI) have proven mortality and morbidity benefit in hypertension, ischemic heart disease, heart failure, and renal disease and are among the most prescribed medications globally. ACEI angioedema (AE-ACEI) is a potentially life-threatening adverse drug reaction that is reported to occur more frequently in African American populations. However, the clinical profile of AE-ACEI is poorly characterized in diverse African populations.
Methods: A case-controlled cohort study with enrolment of AE-ACEI cases and drug-tolerant controls in Cape Town, South Africa. Univariable and multivariable analysis was performed. Controls were defined as patients tolerating ACEI for a minimum of two years. Cases were defined as patients who had angioedema while using an ACEI, patients with a history of angioedema while not on an ACEI were excluded. Cases and controls were recruited from the same demographic areas, including both hospitals and clinics. Information regarding demographics and clinical history was captured via both in person interviews and folder review.
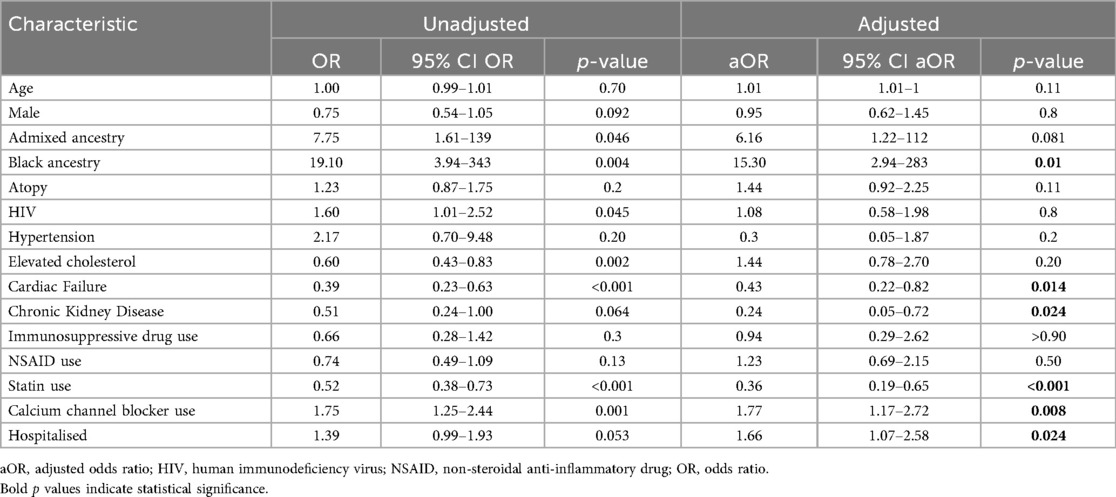
Results: A total of 237 AE-ACEI cases, and 466 ACEI tolerant controls were enrolled from seven sites in Cape Town. Features of IgE-mediated immediate drug hypersensitivity were present in 24 cases, which excluded them from analysis. The median age was 58 years (IQR: 47; 67) and 57% were female. AE-ACEI cases more frequently had Black genetic ancestry compared to controls [53% (81/154), vs. 29% (146/407), p < 0.001]. AE-ACEI occurred within 30 days of initiating ACEI therapy in only 31.1% (70/225), with median treatment time to AE-ACEI of 6.9 years (IQR: 2.9; 13). The ACEI tolerant controls were using ACEI for median 9.5 years (IQR: 5; 15.5). All AE-ACEI cases developed swelling above the shoulders, involving the lips and tongue in 72% (165/213) and 50% (107/213) cases respectively. Hospitalisation for AE-ACEI was required in 82% (175/213), however only two patients were intubated, and there were no mortalities. In multivariable analysis traditional risk factors of age, gender, immunosuppression and atopy did not differ between cases and controls. Black genetic ancestry [aOR 15.4 (95% CI 2.94–283), p value = 0.01] and calcium channel blocker use [aOR 1.77 (95% CI 1.17–2.72), p value = 0.008] were significant risk factors for developing AE-ACEI. Cardiac failure, chronic kidney disease, and statin use reduced the risk of AE-ACEI in this model.
Conclusion: In this South African population, Black genetic ancestry and calcium channel blocker use were the major risk factors for AE-ACEI. The majority of AE-ACEI occurred after several years of treatment, with most cases involving the lip and/or tongue. Long-term follow-up, genetic, and further mechanistic studies are warranted in additional diverse African populations.
Introduction
Angiotensin converting enzyme inhibitors (ACEI) are a class of antihypertension medications that inhibit the Angiotensin Converting Enzyme (ACE; also known as kinase II). ACE is responsible for the conversion of angiotensin I to angiotensin II, as well as degradation of the vasoactive peptide bradykinin. ACEI are one of the most widely used medications globally as a result of their affordability, and many large studies have demonstrated their efficacy in the treatment of hypertension (1), cardiac failure (2, 3), ischemic heart disease (4), and chronic renal disease (5). ACEI also show improved efficacy, when compared to angiotensin receptor blockers (ARBs), in preventing hypertension related cardiovascular events, and all-cause mortality (6). Baptiste et al. found that when comparing ACEI to ARBs in hypertensive patients that in the Black African cohort ARB use was associated with a higher risk of cardiovascular related death when compared to ACEI [HR 1.2 (95% CI: 1.02–1.4)]. The two major adverse drug reactions with ACEI are cough and angioedema.
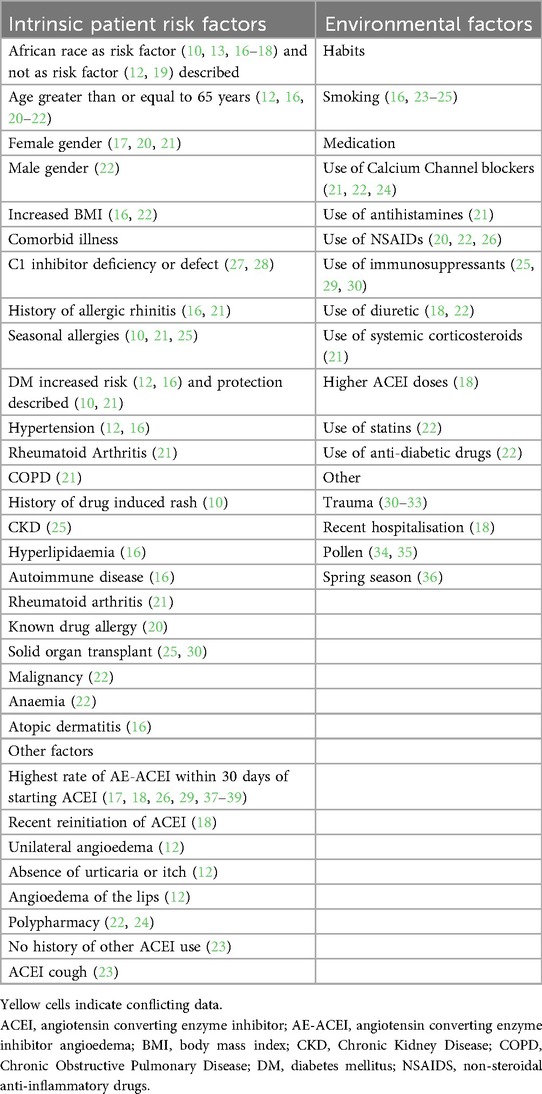
Angioedema is defined as localised swelling in subcutaneous and submucosal tissues. The frequency of AE-ACEI varies from 0.2% to 1% (7), but ubiquitous use of these medications means that AE-ACEI is the most common single cause of angioedema globally, including in South Africa (8–11). AE-ACEI most commonly affects the head and neck (12–14), with mortality rates reported as high as 11%, and intubation rates at 22% internationally (9, 12, 15). Several studies have investigated epidemiological and clinical risk factors for AE-ACEI (see Table 1) with consistent identified risk factors including: African American ancestry (with a 3–5 times increased AE-ACEI angioedema frequency) (10, 13, 16–18, 20, 23, 26, 40), female gender (17, 20, 21), older age (12, 16, 20–22), use of immunosuppression (25, 29, 30), and seasonal allergies (16, 21, 36). Additional identified risk factors (with some conflicting findings) include a variety of concomitant medications (18, 20–22, 25, 30, 41, 42), current smoking status (16, 23–25), and obesity (16, 22). The recent initiation of ACEI and first 30 days of treatment with ACEI have previously been identified as the highest risk period for AE-ACEI (17, 18, 26, 29, 37–39). Most of these epidemiological studies of AE-ACEI are from High Income Countries (HICs) and predominantly European populations, despite the reported increased risk in African Americans. Thus, the aim of this study was to examine the clinical profile and assess risk factors for AE-ACEI in an African setting.
Methods
This is a case control study comparing patients who tolerate ACEI vs. patients who have developed AE-ACEI. We aim to describe this cohort of patients and identify significant risk factors for AE-ACEI in this community. AE-ACEI cases were defined as participants who developed angioedema while on an ACEI, with no history of angioedema while not using an ACEI. ACEI tolerant controls were defined as participants who had safely tolerated an ACEI for at least two years, with no history of angioedema. In this study, AE-ACEI cases were identified both prospectively (through referral) and retrospectively (through folder review). Cases and matched controls were recruited from seven sites: District 6 Clinic, Victoria Wynberg Hospital, Mitchell's Plain District Hospital, Heideveld Emergency Centre, Green Point Clinic, The University of Cape Town Lung Institute Allergy and Immunology Clinic, and Groote Schuur Hospital (GSH) (allergy division, emergency unit, hypertension clinic, and general medical wards). All the above facilities are primary or secondary level centers that refer to GSH and the demographics of this cohort match the demographics of the Western Cape Province (see Supplement for more information about each facility, a detailed description of recruitment at each site as well as consort diagrams, Supplementary Figures 1, 2). The Western Cape Provincial Health Data Centre (PHDC) team assisted with accessing HECTIS admission data (43). All cases and controls were interviewed in person, and all data was assessed by an Allergist/Allergy Medical Officer. Information regarding angioedema history, medical history, and demographics was collected. This study is part of the Angioedema Biomarkers in Africa project, which has been approved by the University of Cape Town Human Research Ethics Committee (HREC 057/2020).
We acknowledge that neither self-reported race nor Fitpatrick skin tone accurately captures genetic ancestry. Therefore, we have decided to group patients based on our available genetic ancestry data into White ancestry, Admixed ancestry, and Black ancestry based on location and grouping in the Principle Component Analysis plot (Supplementary Figure 3) from our previous genome wide association study in this cohort. We did not have genotype data for 118/679 (17.4%) of patients.
Proportions and frequencies were used to describe categorical variables. Normally distributed variables, tested using the Shapiro Wilk test of normality, were described using mean and standard deviation or else median and interquartile range was used. The mean difference test in continuous variables that were normally distributed between groups was done using the analysis of variance (ANOVA) or else the Kruskal Wallis test was used. Proportion difference test for all categorical variables that had at least 5 observations across all groups was done using the Chi square test of independence, or else the Fisher's exact test was applied. All statistical tests were done at 5% level of significance, and Stata 15.1 software was used for the analyses (44). Multivariable analysis was performed for this cohort with AE-ACEI as the outcome. Patients were stratified as “hospitalised” (including patients in general medical wards, intensive care or high care units, and the emergency center) or “not hospitalised” (patients from outpatient departments.). The following covariates were included: age, gender, skin tone, atopy, Human immunodeficiency virus (HIV), hypertension, elevated cholesterol, cardiac failure, and chronic kidney disease. Key selected medications included: immunosuppression, NSAIDs, Statins, and Calcium channel blockers. Cardiac disease was removed from the model as there was evidence of collinearity with cardiac failure. Multicollinearity was assessed using The Variance Inflation Factor (VIF) (Supplementary Table 2), and all results were less than 5. Adjusted odds ratios (aOR) with respective 95% confidence intervals (CI) were reported. There was minimal missing data in chosen co-variates (see Supplementary Table 6). We performed Multivariable analysis for this cohort with AE-ACEI within 30 days of initiating treatment as the outcome, but no covariates reached significance (Supplementary Table 3).
Results
A total of 237 AE-ACEI cases and 466 ACEI tolerant controls were enrolled. Between June 2021 and December 2024, 49 cases of acute AE-ACEI were referred to the GSH Allergy team, while 188 participants with a history of prior AE-ACEI were retrospectively enrolled via folder review and interview. In the AE-ACEI angioedema cases, 24 participants were excluded, as they had evidence of immediate drug hypersensitivity (urticaria n = 10; pruritis n = 13; anaphylaxis n = 1) as determined by two allergists (Supplementary Figures 1, 2). Most of our cohort were treated with the ACEI enalapril [99.7%, (699/701)]. In the ACEI tolerant controls the duration on an ACEI at the time of enrollment was median 9.5 years (IQR: 5; 15.5 years).
In the overall cohort, the median age was 58 years (IQR: 47; 67), 57.5% were female, and 94.8% (532/561) were classified as Admixed or Black ancestry (see Table 2). The cases and controls were similar in terms of age and gender, but significantly more AE-ACEI cases were classified as Black ancestry when compared to controls [53% (81/154), vs. 29%% (119/407), p < 0.001]. With regards to comorbid illness, 97.5% of patients had hypertension. HIV was more prevalent in the AE-ACEI cases [16.9% (36/213) vs. 11.4% (53/466), p = 0.043] but immunosuppressive treatment did not differ between cases and controls. Compared to cases the controls had significantly higher rates of hypercholesterolaemia [51.9% (242/466) vs. 39.0% (83/213), p = 0.001], cardiac disease [21.9% (102/466) vs. 12.2% (26/213), p = 0.006], or had previously tested positive for COVID-19 [16.3% (76/466) vs. 12.2% (26/213), p = 0.001]. Atopy was reported in 32.3% (69/213) of cases and 27.6% (126/466) of controls but the results did not reach significance (p = 0.201). The cases had significantly higher rates of asthma [42.0% (29/69) vs. 20.9% (27/129), p = 0.001], while atopic dermatitis was more common in the controls [31% (40/129) vs. 8.6% (8/69), p = 0.023]. Calcium channel blockers use was significantly greater in the cases [135/213 (63%) vs. 232/466 (50%), p < 0.001] while simvastatin use was significantly more common in the controls [267/466 (57%) vs. 88/213 (41%), p < 0.001]. In multivariable analysis Black ancestry (aOR 15.3; 95% CI 2.94–283, p = 0.01), calcium channel blocker usage (aOR 1.77; 95% CI: 1.17–2.72, p = 0.008) were significant risk factors for developing AE-ACEI (Table 3). Chronic kidney disease, cardiac failure, and statin use were protective in this model.
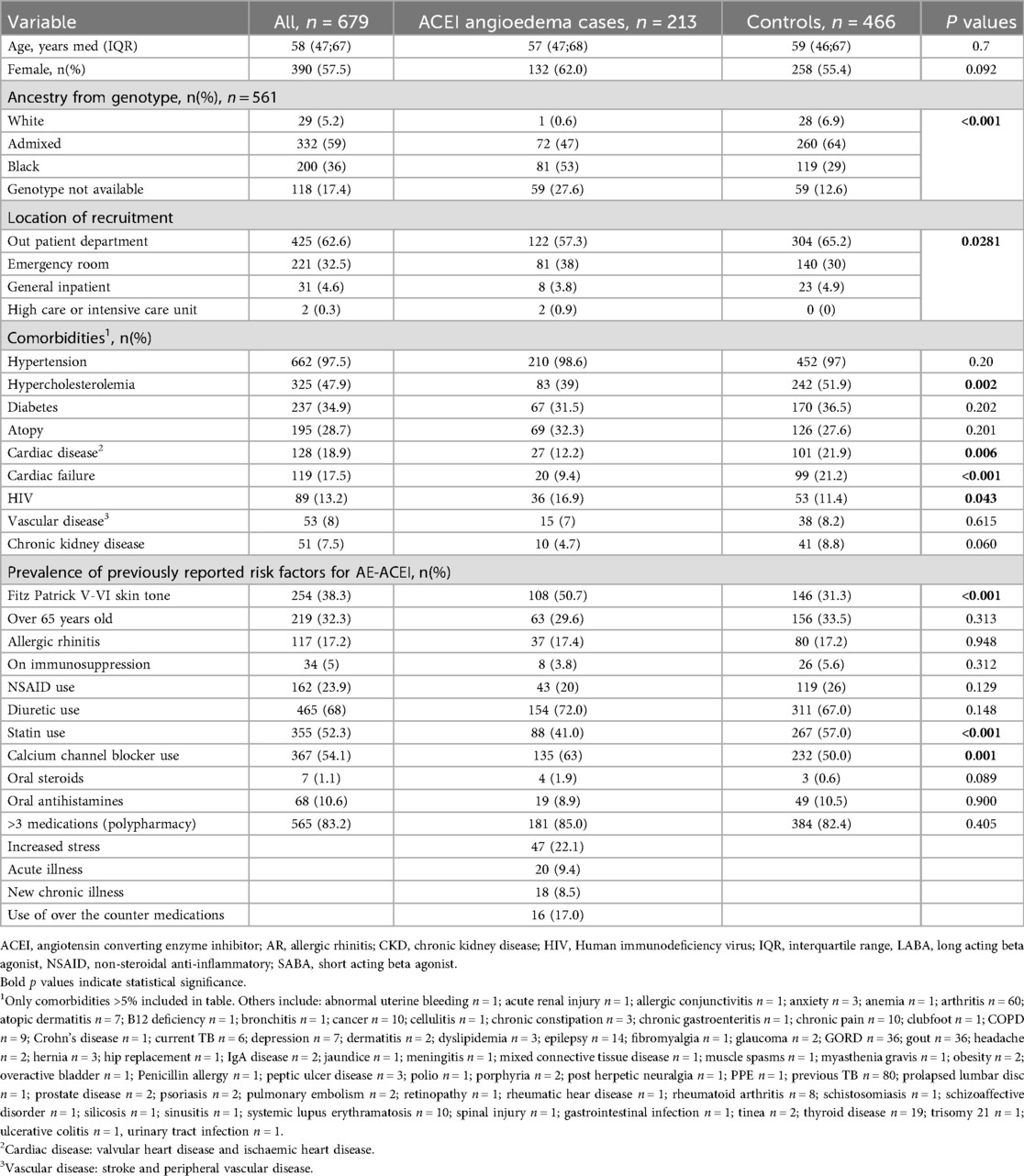
Table 2. Univariate comparison of self-reported clinical variables between ACEI angioedema cases and ACEI tolerant controls and prevalence of previously reported risk factors.
In our cohort the duration of ACEI treatment before developing angioedema median of 6.9 years (IQR: 2.9; 13.0 years) with 31.5% (67/213) of patients developing angioedema within 30 days of ACEI initiation. The median duration of AE-ACEI was 48 h (IQR: 24; 72 h). Multivariable analysis for developing AE-ACEI within 30 days of initiating treatment found no significant covariates (Supplementary Table 3).
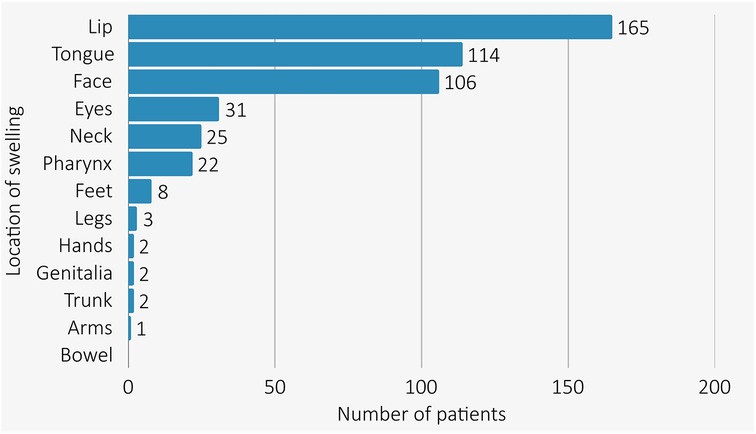
All AE-ACEI cases had swelling above the shoulders (see Figure 1) with the lip [72.3% (154/213)] and tongue [50.2% (107/213)] being the most common sites of swelling. Overall, 22 (10.3%) patients developed pharyngeal angioedema (10/22 with concomitant tongue and lip swelling), with two patients requiring intubation for airway protection (one emergency orotracheal intubation, and one emergency cricothyroidotomy both after reinitiation of an ACEI in patients with known AE-ACEI). There were no angioedema related deaths. Hospitalisation was required in 82.2% (175/213) of AE-ACEI cases. Information about treatment of AE-ACEI was available for 41.8% of cases (89/213). Therapy with fresh frozen plasma was given in 11 cases (11/89, 12.4%) while 73% (65/89) were treated with antihistamines, 64% (57/89) with corticosteroids, and 12.4% (12/89) required adrenaline for airway protection. One patient received Icatibant peri-intubation after discussion with our Angioedema Hotline.
Discussion
AE-ACEI is a common and potentially life-threatening complication of ACEI use, and the global rate of acute ACEI requiring care in emergency centers is rising (45, 46). To date there are no available tests or biomarkers to predict which patients are at risk of developing AE-ACEI. Currently, clinical risk factors remain the best clues to identify at-risk groups—however, these risk factors may be population-specific with limited data from diverse African countries. This large South African cohort of AE-ACEI and drug-tolerant controls were examined for traditional and novel risk factors. AE-ACEI cases more commonly had Black genetic ancestry, a higher prevalence of HIV, and lower rates of concomitant metabolic and non-hypertensive cardiovascular diseases. Notably, the majority of AE-ACEI occurred following prolonged periods of treatment, and there was increased occurrence during the spring/summer seasons. Interestingly, we also found that all patients had angioedema of the face and neck. Despite high rates of hospitalisation there were low rates of intubation (0.9%) for airway protection, and no deaths. This is contradictory to international data where 22% (37) to 32% (13) of patients with AE-ACEI required intubation, and 11% demised (37). Unlike international groups, we did not find that older age, female gender, allergic rhinitis, or immunosuppressive therapy were associated with AE-ACEI. However, similar to international data, we found that calcium channel blockers were a significant risk factor (21, 22, 24).
In 1996, Brown et al. (18) were the first to describe an increased risk for AE-ACEI in African Americans with a relative risk of 4.5 compared to white Americans. They also found that African patients were more likely to have severe hypertensive disease as they required higher doses of ACEI, as well as having more severe AE-ACEI with higher rates of hospitalisation and intubation for airway protection. Later studies in the USA (10, 13, 16, 17, 20, 23, 41) and a systematic review (40) found that black and Hispanic patients had a significantly higher risk of AE-ACEI compared with patients with paler skin tone (41). However, other authors from the USA have found that African race were not associated with AE-ACEI (12, 19). Studies completed in Sweden, the United Kingdom, and Thailand either did not describe racial demographics (21), or had no patients with darker skin tones (24, 39) (see Supplementary Table 5 for a summary of these studies). Our data from an African Middle Income Country (MIC) setting does find a significantly higher prevalence of Black genetic ancestry in AE-ACEI cases with a high aOR of 15.3 (p = 0.01 (Table 3). This result aligns with African American data and suggests that examining African populations for genetic risk factors for AE-ACEI is warranted. We have recently published a GWAS from this cohort and we replicated findings for the single nucleotide polymorphisms (SNP) rs500766 on chromosome 10 (previously linked to AE-ACEI). We also found SNPs located close to the genes PRKCQ (protein kinase C theta), RAD51B (RAD51 Paralog B), and RIMS1 (regulating synaptic membrane exocytosis 1), which have previously been linked with drug-induced angioedema. Additionally, SNPs near the CSMD1 (CUB and sushi multiple domains 1) gene, which has been linked to ACEI cough (47), were identified. We have highlighted that further work across diverse African populations is justified, as given African genomic diversity extrapolation to all darker skinned populations would be flawed (48).
AE-ACEI can occur immediately upon drug exposure, or years into therapy, and its occurrence cannot be predicted. Some authors have found that AE-ACEI is most common within the first 30 days of starting treatment, reporting that 48.6–53.0% of AE-ACEI cases occur within that window (17, 18, 39), while others have found much lower AE-ACEI rates of 10.2% within the first month of treatment (15, 39, 41). Our cohort had a long drug latency, with only 31.1% developing AE-ACEI within the first 30 days of drug initiation, which is within range of global rates. In AE-ACEI this prolonged drug latency raises the question of whether there is an additional factor/second hit event in the late presenters which i) either effects other enzymes that metabolise bradykinin, such as neprilysin, or aminopeptidase P, ii) may represent another factor that leads to increased flux through the bradykinin pathway, potentially even at the tissue-level, or iii) represents the onset of a chronic urticaria/angioedema variant independent of AE-ACEI. A recent publication by Bocquet et al. found that, on review of reported AE-ACEI angioedema cases that almost 50% of patients were excluded, and that these patients likely had mast cell mediated angioedema rather than AE-ACEI (49).
All of our AE-ACEI cases presented with swelling above the shoulders, most commonly affecting the lips and tongue, aligning with global findings (14, 36). Previous studies have identified tongue swelling in AE-ACEI as a marker associated with poor clinical outcomes and increased likelihood of intensive care admissions (13, 15). Other forms of bradykinin mediated angioedema—such as hereditary angioedema and acquired angioedema—can affect multiple body sites, including the oropharynx, abdominal viscera, limbs, and genitalia (50). If AE-ACEI is also bradykinin mediated, one would expect it to involve a broader range of anatomical sites. The density of mast cells in tissue is higher in the face than other anatomical areas (51); so a possible explanation is that ACEI treatment is unmasking mast cell mediated angioedema (i.e., ACEI are acting as a cofactor). This is supported by recent findings of high rates of mast cell mediated angioedema in patients who developed recurrent angioedema after exposure to ACEI. Douillard et al. found that 41% of patients with a suspected ACEI/ARB angioedema still had recurrent angioedema without urticaria more than 6 months after stopping the drug, indicating a diagnosis of mast cell mediated angioedema rather than bradykinin mediated angioedema in these patients (52). These findings are important and underline the need for future investigations into the pathomechanisms of AE-ACEI, and potentially cross-talk between mast-cells and kallikrein-kinin pathways.
This study is the largest study to date describing AE-ACEI in an African MIC. Our limitations include prospective case finding, which was reliant on referrals from local sites; as well as retrospective case finding which is dependent on existing ICD10 coding and local databases. We encountered difficulties in contacting retrospective cases to schedule in-person interviews because of a highly migrant population, and incorrect or outdated contact details. Our cohort had higher rates of hypertension and dyslipidaemia than reported in South Africa (53), which likely reflects our recruitment from hospitals and local clinics, rather than the general population. However, ACEI are most commonly prescribed for hypertension, so high hypertension rates (and associated metabolic conditions) in this cohort are not an unexpected finding. The case-controlled nature of the study cannot exclude potential for unintentional selection bias; however, cases and controls were recruited from the same hospitals and drainage populations. It is also important to note that as this study is nested in the government funded state sector, almost all our cohort were using Enalapril, which is the ACEI on government tender. We did have some variables with high rates of missing data—especially with regards to treatment of AE-ACEI—as participants either did not know or could not recall the treatment that was received at the time of the event.
In summary, we have shown that Black genetic ancestry, calcium channel blocker use, and HIV co-infection are important risk factors for AE-ACEI—and that previously identified risk factors including older age, female sex, and allergic rhinitis were not risk factors in this cohort. We have also identified a long drug latency compared to international cohorts. These findings highlight the need for further epidemiological and clinical studies on AE-ACEI in diverse ethnic backgrounds and LMIC settings to provide a true global clinical understanding of AE-ACEI.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Cape Town Human Research Ethics Committee (HREC 057/2020). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LM: Formal analysis, Software, Writing – review & editing. MR: Data curation, Methodology, Writing – review & editing. MD: Data curation, Writing – review & editing. CM: Data curation, Writing – review & editing. AE: Project administration, Writing – review & editing. EJ: Data curation, Project administration, Writing – review & editing. SP: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. JP: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. CD has received funding for her PhD from the Discovery Foundation, and the South African Medical Council Division of Research Capacity Development Programme. The Degree from which this study emanated was funded by the South African Medical Research Council through its Division of Research Capacity Development under the Clinician Researcher Development Programme with funding received from the South African National Department of Health. The content hereof is the sole responsibility of the authors and does not necessarily represent the official views of the SAMRC or the funders. Research reported in this manuscript was supported by the South African Medical Research Council with funds received from the South African Department of Science and Innovation. JP is supported by the NIHR global research professorship program. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Acknowledgments
Thank you to our patients, without whom this work would not be possible. Thank you to Mrs. Wisahl Wallace, Mrs. Thandokazi Bezana, and Mrs. Nomafu Jayiya for all administrative assistance. Appreciation is extended by the authors to the Provincial Health Data Centre (PHDC) team for their valuable contributions. Situated within the Western Cape Government: Department of Health and Wellness, the Provincial Health Data Centre functions as a Health Information Exchange, established to positively impact patient care and health outcomes by leveraging data and technology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2025.1664354/full#supplementary-material
References
1. Williams GH. Converting-enzyme inhibitors in the treatment of hypertension. N Engl J Med. (1988) 319(23):1517–25. doi: 10.1056/NEJM198812083192305
2. Swedberg K, Kjekshus J, CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. (1987) 316(23):1429–35. doi: 10.1056/NEJM198706043162301
3. Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. (1991) 325(5):293–302. doi: 10.1056/NEJM199108013250501
4. TAIREA Study. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The acute infarction ramipril efficacy (AIRE) study investigators. Lancet. (1993) 342(8875):821–8. doi: 10.1016/0140-6736(93)92693-N
5. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The collaborative study group. N Engl J Med. (1993) 329(20):1456–62. doi: 10.1056/NEJM199311113292004
6. Peresuodei TS, Gill A, Orji C, Reghefaoui M, Saavedra Palacios MS, Nath TS. A comparative study of the safety and efficacy between angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the management of hypertension: a systematic review. Cureus. (2024) 16(2):e54311. doi: 10.7759/cureus.54311
7. Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the omapatrilat cardiovascular treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. (2004) 17(2):103–11. doi: 10.1016/j.amjhyper.2003.09.014
8. Maurer M, Magerl M. Differences and similarities in the mechanisms and clinical expression of bradykinin-mediated vs. Mast cell-mediated angioedema. Clin Rev Allergy Immunol. (2021) 61(1):40–9. doi: 10.1007/s12016-021-08841-w
9. Day C, van der Walt J, Crombie K, Hendrikse C, Peter J. Acute angioedema in Cape Town emergency centres and a suggested algorithm to simplify and improve management. S Afr Med J. (2023) 113(8):51–7. doi: 10.7196/SAMJ.2023.v113i8.717
10. Kostis JB, Kim HJ, Rusnak J, Casale T, Kaplan A, Corren J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. (2005) 165(14):1637–42. doi: 10.1001/archinte.165.14.1637
11. Hoover T, Lippmann M, Grouzmann E, Marceau F, Herscu P. Angiotensin converting enzyme inhibitor induced angio-oedema: a review of the pathophysiology and risk factors. Clin Exp Allergy. (2010) 40(1):50–61. doi: 10.1111/j.1365-2222.2009.03323.x
12. Bluestein HM, Hoover TA, Banerji AS, Camargo CA Jr, Reshef A, Herscu P. Angiotensin-converting enzyme inhibitor-induced angioedema in a community hospital emergency department. Ann Allergy Asthma Immunol. (2009) 103(6):502–7. doi: 10.1016/S1081-1206(10)60267-0
13. Chan NJ, Soliman AM. Angiotensin converting enzyme inhibitor-related angioedema: onset, presentation, and management. Ann Otol Rhinol Laryngol. (2015) 124(2):89–96. doi: 10.1177/0003489414543069
14. Zingale LC, Beltrami L, Zanichelli A, Maggioni L, Pappalardo E, Cicardi B, et al. Angioedema without urticaria: a large clinical survey. Can Med Assoc J. (2006) 175(9):1065–70. doi: 10.1503/cmaj.060535
15. Agah R, Bandi V, Guntupalli KK. Angioedema: the role of ACE inhibitors and factors associated with poor clinical outcome. Intensive Care Med. (1997) 23(7):793–6. doi: 10.1007/s001340050413
16. Kamil RJ, Jerschow E, Loftus PA, Tan M, Fried MP, Smith RV, et al. Case-control study evaluating competing risk factors for angioedema in a high-risk population. Laryngoscope. (2016) 126(8):1823–30. doi: 10.1002/lary.25821
17. Reichman ME, Wernecke M, Graham DJ, Liao J, Yap J, Chillarige Y, et al. Antihypertensive drug associated angioedema: effect modification by race/ethnicity. Pharmacoepidemiol Drug Saf. (2017) 26(10):1190–6. doi: 10.1002/pds.4260
18. Brown NJ, Ray WA, Snowden M, Griffin MR. Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther. (1996) 60(1):8–13. doi: 10.1016/S0009-9236(96)90161-7
19. Chiu AG, Newkirk KA, Davidson BJ, Burningham AR, Krowiak EJ, Deeb ZE. Angiotensin-converting enzyme inhibitor-induced angioedema: a multicenter review and an algorithm for airway management. Ann Otol Rhinol Laryngol. (2001) 110(9):834–40. doi: 10.1177/000348940111000906
20. Garcia-Saucedo JC, Trejo-Gutierrez JF, Volcheck GW, Park MA, Gonzalez-Estrada A. Incidence and risk factors of angiotensin-converting enzyme inhibitor-induced angioedema: a large case-control study. Ann Allergy Asthma Immunol. (2021) 127(5):591–2. doi: 10.1016/j.anai.2021.07.028
21. Mahmoudpour SH, Baranova EV, Souverein PC, Asselbergs FW, de Boer A, Maitland-van der Zee AH. Determinants of angiotensin-converting enzyme inhibitor (ACEI) intolerance and angioedema in the UK clinical practice research datalink. Br J Clin Pharmacol. (2016) 82(6):1647–59. doi: 10.1111/bcp.13090
22. Stauber T, Confino-Cohen R, Goldberg A. Life-threatening angioedema induced by angiotensin-converting enzyme inhibitors: characteristics and risk factors. Am J Rhinol Allergy. (2014) 28(1):54–8. doi: 10.2500/ajra.2014.28.3989
23. Morimoto T, Gandhi TK, Fiskio JM, Seger AC, So JW, Cook EF, et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract. (2004) 10(4):499–509. doi: 10.1111/j.1365-2753.2003.00484.x
24. Hallberg P, Nagy J, Karawajczyk M, Nordang L, Islander G, Norling P, et al. Comparison of clinical factors between patients with angiotensin-converting enzyme inhibitor-induced angioedema and cough. Ann Pharmacother. (2017) 51(4):293–300. doi: 10.1177/1060028016682251
25. Byrd JB, Woodard-Grice A, Stone E, Lucisano A, Schaefer H, Yu C, et al. Association of angiotensin-converting enzyme inhibitor-associated angioedema with transplant and immunosuppressant use. Allergy. (2010) 65(11):1381–7. doi: 10.1111/j.1398-9995.2010.02398.x
26. Banerji A, Clark S, Blanda M, LoVecchio F, Snyder B, Camargo CA Jr. Multicenter study of patients with angiotensin-converting enzyme inhibitor-induced angioedema who present to the emergency department. Ann Allergy Asthma Immunol. (2008) 100(4):327–32. doi: 10.1016/S1081-1206(10)60594-7
27. Cichon S, Martin L, Hennies HC, Müller F, Van Driessche K, Karpushova A, et al. Increased activity of coagulation factor XII (hageman factor) causes hereditary angioedema type III. Am J Hum Genet. (2006) 79(6):1098–104. doi: 10.1086/509899
28. Bowen T, Cicardi M, Bork K, Zuraw B, Frank M, Ritchie B, et al. Hereditary angiodema: a current state-of-the-art review, VII: canadian Hungarian 2007 international consensus algorithm for the diagnosis, therapy, and management of hereditary angioedema. Ann Allergy Asthma Immunol. (2008) 100(1 Suppl 2):S30–40. doi: 10.1016/S1081-1206(10)60584-4
29. Byrd JB, Touzin K, Sile S, Gainer JV, Yu C, Nadeau J, et al. Dipeptidyl peptidase IV in angiotensin-converting enzyme inhibitor associated angioedema. Hypertension. (2008) 51(1):141–7. doi: 10.1161/HYPERTENSIONAHA.107.096552
30. Abbosh J, Anderson JA, Levine AB, Kupin WL. Angiotensin converting enzyme inhibitor-induced angioedema more prevalent in transplant patients. Ann Allergy Asthma Immunol. (1999) 82(5):473–6. doi: 10.1016/S1081-1206(10)62723-8
31. Megerian CA, Arnold JE, Berger M. Angioedema: 5 years’ experience, with a review of the disorder’s presentation and treatment. Laryngoscope. (1992) 102(3):256–60. doi: 10.1288/00005537-199203000-00005
32. Vleeming W, van Amsterdam JG, Stricker BH, de Wildt DJ. ACE inhibitor-induced angioedema. Incidence, prevention and management. Drug Saf. (1998) 18(3):171–88. doi: 10.2165/00002018-199818030-00003
33. Schiller PI, Messmer SL, Haefeli WE, Schlienger RG, Bircher AJ. Angiotensin-converting enzyme inhibitor-induced angioedema: late onset, irregular course, and potential role of triggers. Allergy. (1997) 52(4):432–5. doi: 10.1111/j.1398-9995.1997.tb01024.x
34. Wilson M, Frohna W, Trent G, Sauter D. Evaluating for seasonal variation in angiotensin-converting enzyme inhibitor- and angiotensin receptor blocker-induced angioedema. Ann Allergy Asthma Immunol. (2014) 112(2):178–9. doi: 10.1016/j.anai.2013.11.016
35. Straka B, Nian H, Sloan C, Byrd JB, Woodard-Grice A, Yu C, et al. Pollen count and presentation of angiotensin-converting enzyme inhibitor-associated angioedema. J Allergy Clin Immunol Pract. (2013) 1(5):468–73.e1–4. doi: 10.1016/j.jaip.2013.05.003
36. Pfaue A, Schuler PJ, Mayer B, Hoffmann TK, Greve J, Hahn J. Clinical features of angioedema induced by renin-angiotensin-aldosterone system inhibition: a retrospective analysis of 84 patients. J Community Hosp Intern Med Perspect. (2019) 9(6):453–9. doi: 10.1080/20009666.2019.1698259
37. Slater EE, Merrill DD, Guess HA, Roylance PJ, Cooper WD, Inman WH, et al. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA. (1988) 260(7):967–70. doi: 10.1001/jama.1988.03410070095035
38. Hedner T, Samuelsson O, Lunde H, Lindholm L, Andrén L, Wiholm BE. Angio-oedema in relation to treatment with angiotensin converting enzyme inhibitors. Br Med J. (1992) 304(6832):941–6. doi: 10.1136/bmj.304.6832.941
39. Win TS, Chaiyakunapruk N, Suwankesawong W, Dilokthornsakul P, Nathisuwan S. Renin angiotensin system blockers-associated angioedema in the Thai population: analysis from Thai national pharmacovigilance database. Asian Pac J Allergy Immunol. (2015) 33(3):227–35. doi: 10.12932/AP0556.33.3.2015
40. McDowell SE, Coleman JJ, Ferner RE. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. Br Med J. (2006) 332(7551):1177–81. doi: 10.1136/bmj.38803.528113.55
41. Banerji A, Blumenthal KG, Lai KH, Zhou L. Epidemiology of ACE inhibitor angioedema utilizing a large electronic health record. J Allergy Clin Immunol Pract. (2017) 5(3):744–9. doi: 10.1016/j.jaip.2017.02.018
42. Hallberg P, Persson M, Axelsson T, Cavalli M, Norling P, Johansson HE, et al. Genetic variants associated with angiotensin-converting enzyme inhibitor-induced cough: a genome-wide association study in a Swedish population. Pharmacogenomics. (2017) 18(3):201–13. doi: 10.2217/pgs-2016-0184
43. Boulle A, Heekes A, Tiffin N, Smith M, Mutemaringa T, Zinyakatira N, et al. Data centre profile: the provincial health data centre of the Western Cape Province, South Africa. Int J Popul Data Sci. (2019) 4(2):1143. doi: 10.23889/ijpds.v4i2.1143
45. Cicardi M, Suffritti C, Perego F, Caccia S. Novelties in the diagnosis and treatment of angioedema. J Investig Allergol Clin Immunol. (2016) 26(4):212–21; quiz two pages after page 21. doi: 10.18176/jiaci.0087
46. Smith A, Ray M, Jain N, Zhang H, Sebelik M. The burden of angioedema on United States emergency departments: 2006–2010. Laryngoscope. (2017) 127(4):828–34. doi: 10.1002/lary.26336
47. Mugo JW, Day C, Choudhury A, Deetlefs M, Freercks R, Geraty S, et al. A GWAS of angiotensin-converting enzyme inhibitor-induced angioedema in a South African population. J Allergy Clin Immunol Glob. (2025) 4(3):100464. doi: 10.1016/j.jacig.2025.100464
48. Peter JG, Ntusi NAB, Ntsekhe M. Are recommendations that favor other agents over angiotensin-converting enzyme inhibitors in Africans with hypertension justified? Circulation. (2024) 149(11):804–6. doi: 10.1161/CIRCULATIONAHA.123.065887
49. Bocquet A, Marmion N, Boccon-Gibod I, Bouillet L. Angiotensin-converting enzyme inhibitor-induced angioedema: proposal for a diagnostic score. World Allergy Organ J. (2025) 18(3):101037. doi: 10.1016/j.waojou.2025.101037
50. Day C, Peter J. The South African ACARE centre approach to recurrent angioedema without urticaria. Curr Allergy Clin Immunol. (2023) 36(3):148–53.
51. Weber A, Knop J, Maurer M. Pattern analysis of human cutaneous mast cell populations by total body surface mapping. Br J Dermatol. (2003) 148(2):224–8. doi: 10.1046/j.1365-2133.2003.05090.x
52. Douillard M, Deheb Z, Bozon A, Raison-Peyron N, Dereure O, Moulis L, et al. Over diagnosis of bradykinin angioedema in patients treated with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. World Allergy Organ J. (2023) 16(8):100809. doi: 10.1016/j.waojou.2023.100809
Keywords: angioedema, angiotensin converting enzyme inhibitor, ACEI angioedema, Africa, drug latency
Citation: Day C, Mapahla L, Ribeiro M, Deetlefs M, McDougall C, Engelbrecht A, Jones E, Pedretti S and Peter J (2025) Risk factors for angiotensin converting enzyme inhibitor angioedema in a South African population. Front. Allergy 6:1664354. doi: 10.3389/falgy.2025.1664354
Received: 11 July 2025; Accepted: 22 September 2025;
Published: 20 October 2025.
Edited by:
Blanca Del Rio-Navarro, Hospital Infantil de México Federico Gómez, MexicoReviewed by:
José J. Leija-Martínez, Autonomous University of San Luis Potosí, MexicoNatasa Teovska Mitrevska, Remedika General Hospital Dermatology Department, North Macedonia
Copyright: © 2025 Day, Mapahla, Ribeiro, Deetlefs, McDougall, Engelbrecht, Jones, Pedretti and Peter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cascia Day, Y2FzY2lhLmRheUB1Y3QuYWMuemE=; Jonny Peter, am9ubnkucGV0ZXJAdWN0LmFjLnph
 Cascia Day
Cascia Day Lovemore Mapahla
Lovemore Mapahla Melissa Ribeiro2
Melissa Ribeiro2 Sarah Pedretti
Sarah Pedretti Jonny Peter
Jonny Peter