- Department of Animal Science, Shabestar Branch, Islamic Azad University, Shabestar, Iran
The present study aimed to determine the dietary lysine (Lys) requirements of Gilan native ducks (mallards) up to seven weeks of age. A total of 300 male ducklings were randomly assigned to five dietary treatments with six replicates of ten birds each, in a completely randomized design. Birds received 0.7, 0.8, 0.9, 1.0, and 1.1% lysine during the starter phase (1–14 days) and 0.45, 0.55, 0.65, 0.75, and 0.85% lysine during the grower phase (15–49 days). The results showed that during the starter phase, feed intake (FI) and body weight gain (BWG) significantly increased, and feed conversion ratio (FCR) decreased in ducks fed 1.0% and 1.1% lysine compared to the National Research Council recommended level (0.9%) (P<0.05). In the grower phase, ducks receiving 0.75% lysine showed enhanced FI and BWG, while those fed 0.85% lysine demonstrated improved FCR (P<0.05). Higher lysine levels had no significant effect on spleen, bursa of fbricius, or breast weight, crypt diameter, villus height, heterophile percentage, H/L ratio, monocyte count, MCHC, glutathione peroxidase activity, or Newcastle antibody titer (P>0.05). However, lysine levels ≥0.65% significantly increased thigh and wing weights, glucose, cholesterol, HDL, LDL, total protein, albumin, uric acid, hematological indices (hematocrit, RBC, WBC, hemoglobin, lymphocytes, MCH, MCV, eosinophils), crypt depth, villus thickness, and superoxide dismutase activity (P<0.05). In contrast, ducks receiving 0.45% lysine exhibited higher liver and intestinal weights, triglyceride, urea, and VLDL levels (P<0.05). In conclusion, lysine levels above National Research Council recommendations improved growth performance, carcass characteristics, serum biochemical indices, and intestinal morphology in Gilan native ducks. Based on these results, dietary lysine levels of 1.0–1.1% during the starter phase and 0.75–0.85% during the grower phase are recommended for optimal performance.
Introduction
The green-headed duck is classified as a dabbling duck, which feeds on the water’s surface. It belongs to the subfamily Anatinae, family Anatidae, and order Anseriformes, which includes many wild waterfowl species. Green-headed ducks are widely distributed across the globe as migratory birds (Johnson and Sorenson, 1999). Their dietary requirements can be influenced by various factors, including species, age, anatomical structure, and physiological conditions (Purba et al., 2017).
l-Lysine is recognized as the second limiting amino acid in poultry diets (Erickson et al., 2007). It serves as an essential precursor for l-carnitine biosynthesis and plays a vital role in lipid metabolism. Dietary lysine supplementation increases the carnitine levels, modulates the activity of lipid-related coenzymes, and promotes the β-oxidation of fatty acids. Several studies have reported that growing chickens fed low-protein, lysine-deficient diets exhibit increased hepatic fat infiltration, elevated serum cholesterol, and greater abdominal fat deposition (Schmeisser et al., 1983). Inadequate protein synthesis under lysine deficiency may impair the enzyme systems involved in lipid metabolism and simultaneously lead to hepatic lipid accumulation. In this context, Ruan et al. (2019) reported that lysine supplementation in laying ducks increased the relative expression of the peroxisome proliferator-activated receptors (e.g., PPAR-α), carnitine palmitoyltransferase 1A (CPT1A), and apolipoprotein-II (ApoVLDL-II) both linearly and quadratically. On the other hand, the expression of the very low-density lipoprotein receptor (VLDLR), as well as the triglyceride and hepatic cholesterol levels, decreased quadratically. A diet containing 8.6 g/kg lysine enhanced the protein turnover and lipid metabolism in laying duck breeders, leading to improved productivity and reproductive performance.
Xing et al. (2015) reported that feeding Chinese Linwu ducks a lysine-producing probiotic for 63 days increased the villus height in the jejunum and ileum, decreased the crypt depth in the jejunum, and reduced the Lactobacillus counts in the cecum. Wu et al. (2024) demonstrated that the dietary crude protein levels could be reduced in growing Pekin ducks (21–42 days old) housed in cascading cages with supplementation of crystalline amino acids. However, the cage design affected the growth performance of the ducks. Using broken-line regression models, the optimal dietary crude protein level for maximum weight gain and feed efficiency was estimated at approximately 15%.
Mirzaei et al. (2022) investigated the effects of dietary supplementation with different levels of l-carnitine and/or lysine–methionine (Lys-Met) on the reproductive performance of breeder ducks. Their results showed that Lys-Met above 100% of the National Research Council (NRC, 1994) recommendations, with or without l-carnitine, improved feed utilization. The serum glucose increased and the total cholesterol decreased in ducks fed either 100% Lys-Met without l-carnitine or 110% Lys-Met with 150 mg l-carnitine. Conversely, 75 mg l-carnitine combined with 100% Lys-Met reduced glucose but increased total cholesterol. Increasing Lys-Met without l-carnitine decreased the serum protein. Albumin and alanine aminotransferase (ALT) increased with 75 mg l-carnitine–100% Lys-Met and decreased with 150 mg l-carnitine–120% Lys-Met. No interaction effects were found on globulin, uric acid, or aspartate aminotransferase (AST).
Purba et al. (2022) found that supplementation with essential amino acids (e.g., lysine, methionine, threonine, arginine, and tryptophan) at 10% above the standard level, along with pelleted feed, was the most effective in improving performance and reducing the feed intake and feed conversion ratio (FCR) in Alabio ducks during the starter period.
Helmbrecht and Hou (2013) conducted an experiment to determine the lysine requirement of Pekin ducks aged 35–49 days using a quadratic regression model. Growth performance showed a significant response to commercial lysine supplementation. The breast meat yield was significantly improved with increasing dietary lysine levels, indicating a high demand for lysine in muscle protein synthesis. Other carcass traits were not significantly affected. The negative effects of lysine deficiency were clearly observed in the study. The optimal dietary lysine level was determined to be 0.68% for body weight gain (BWG) and 0.70% for FCR. In addition, the optimal lysine level maximizing the breast meat yield was estimated to exceed 0.83%. Analysis of these data indicated that the NRC (1994) recommendation of 0.63% lysine during the growth stage is below the actual requirement for optimal performance in Pekin ducks.
Souza et al. (2014) suggested that the nutrient levels in dose–response trials should be distributed across both the response and plateau phases, enabling proper evaluation of the impact of nutrient increases on animal performance.
Based on this background, the present study aimed to determine the optimal dietary l-lysine requirement for Gilan native ducks up to 7 weeks of age.
Materials and methods
This study was conducted to determine the optimal dietary level of l-lysine for Gilan native ducks (mallards) up to 7 weeks of age.
A total of 300 male Gilan native ducklings with an average initial weight of 40 g were used. Birds were fed formulated diets based on the nutritional requirements for ducks (NRC, 1994). Feed and water were provided ad libitum throughout the experimental period. The l-lysine (purity, 98.5%) used in the diets was sourced from ThreAMINO® (Evonik, Essen, Germany).
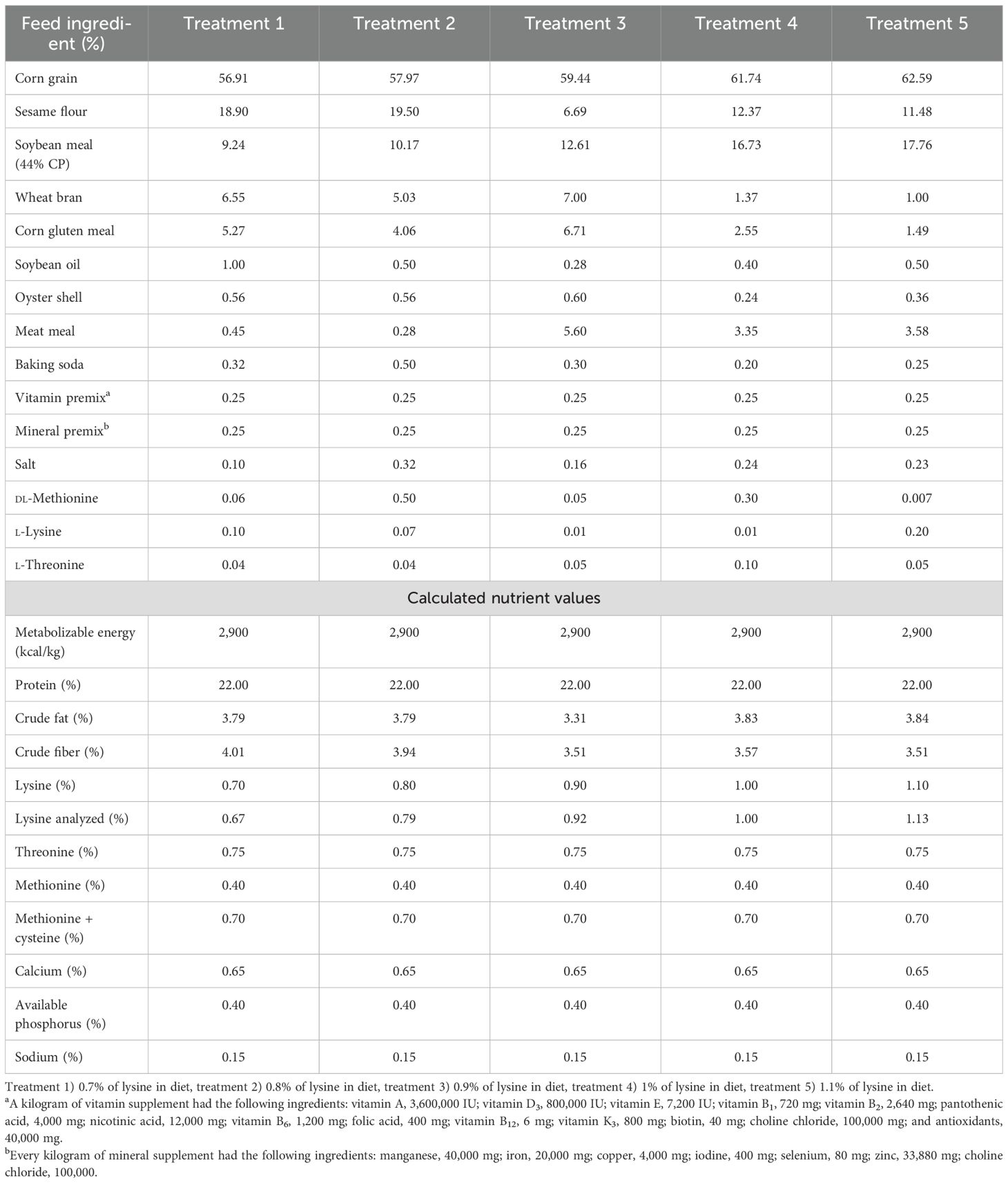
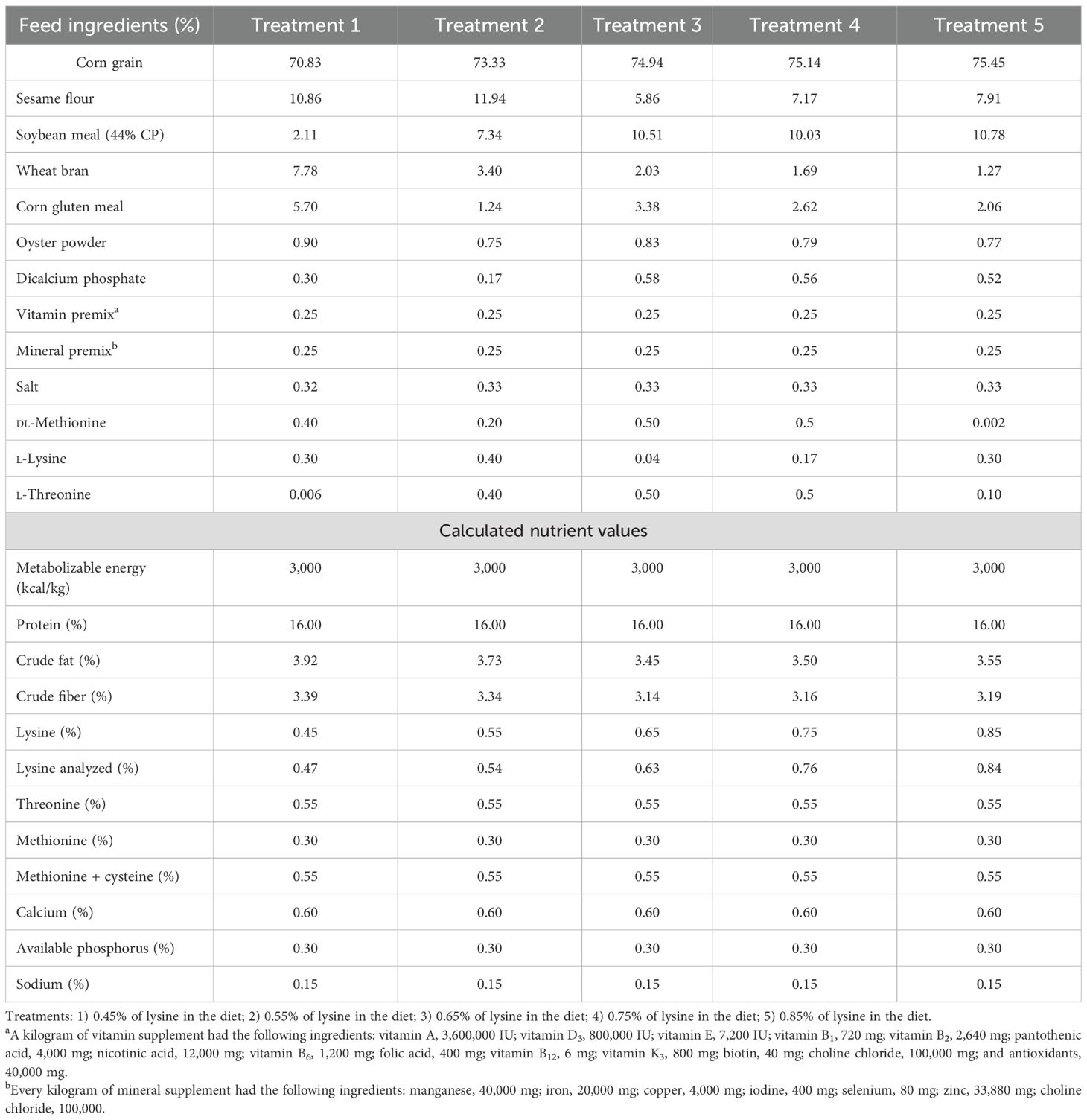
The birds were allocated into five treatments using a completely randomized design, with six replications per treatment and 10 ducklings per replicate. The trial lasted for 7 weeks. The composition and nutrient content of the experimental diets are provided in Tables 1 and 2.
The experimental treatments included five dietary lysine levels during the starter phase (days 1–14), as follows: 0.70%, 0.80%, 0.90% (NRC, 1994 recommendation), 1.00%, and 1.10% l-lysine. During the grower phase (days 15–49), five l-lysine levels were also examined: 0.45%, 0.55%, 0.65% (NRC, 1994 recommendation), 0.75%, and 0.85% l-lysine.
The estimation and the measurement of lysine in the experimental diets were based on total lysine. The composition of each experimental diet was calculated, and the l-lysine content of each treatment diet was analyzed and presented in the corresponding tables.
Limitations section
In the current study, the primary goal was to determine the lysine requirements of Gilan native ducks, a local genotype for which no commercial nutrient recommendations currently exist. Therefore, the dietary lysine levels were systematically varied around the NRC (1994) values, while the level of methionine was intentionally kept constant across all treatments (0.40% in the starter phase and 0.30% in the grower phase). This allowed isolating the effect of lysine levels on the performance and physiological responses.
However, it should be noted that not adjusting the methionine and arginine levels alongside lysine may have led to imbalances, particularly in the higher lysine treatments. Methionine is the first limiting amino acid in poultry, and such an imbalance could result in suboptimal protein utilization, altered nitrogen metabolism, or confounding physiological responses. Similarly, the lysine-to-arginine ratio was not optimized, which may have influenced the immune function or nutrient absorption.
The relationship between lysine and other amino acids in native ducks warrants careful consideration, particularly due to the critical role of lysine as a limiting amino acid influencing the overall protein synthesis and metabolic processes. Lysine acts synergistically with essential amino acids, including methionine, threonine, and tryptophan, to optimize the growth performance, feed efficiency, and physiological health of native ducks. Previous studies have demonstrated that an imbalance in the lysine levels relative to other amino acids may negatively affect nitrogen retention, muscle development, and immune function. Therefore, ensuring appropriate dietary lysine concentrations alongside balanced levels of complementary essential amino acids is crucial. Future research should focus on determining precise amino acid-to-lysine ratios that maximize the genetic potential of native ducks, enhancing productivity and health outcomes. Investigating these interrelationships further could provide practical dietary recommendations that support optimal growth performance and economic efficiency in native duck farming.
Performance
During the experimental period, the performance parameters including BWG, feed intake, mortality rate, and FCR were recorded weekly and calculated on a daily basis. To ensure accuracy, the birds were fasted for 4 h prior to weighing in order to clear the digestive tract of feed residues. Body weight was measured at the beginning of the trial and at the end of each experimental period. Weighing was performed on a group basis, and the average body weight was calculated accordingly. To determine the daily feed intake, the amount of feed offered to each replicate was recorded at the beginning of each period (1–14 and 15–49 days). The leftover feed was weighed at the end of the same period, and the difference was considered as the feed intake. FCR was calculated by dividing the amount of feed consumed by the total BWG during each experimental period.
Carcass traits
To investigate the characteristics of the carcass at the end of the course, one duck from each repetition was selected based on the average weight of each unit, weighed, and then euthanized using the CO2 gas method (Nicolau et al., 2015).
Thereafter, the carcass was dissected and the relative weights of the carcass, liver, spleen, kidney, bursa, thigh, breast, wing, and the different parts of the small intestine, the duodenum (the beginning of the small intestine to the end of the duodenum), the jejunum (the end of the duodenum to Michael’s appendix), and the ileum (Michel’s excess to the junction of the cecum with the small intestine) were measured using a digital scale with an accuracy of 0.001 g. The weight of each of these organs was calculated as a percentage of live weight (Li et al., 2024).
Blood biochemical parameters
At the end of the experiment, one duck per replicate was randomly selected, and 3 ml of blood was collected from the wing vein. The serum levels of total protein, cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides, uric acid, urea, albumin, and glucose were measured using an automatic analyzer (Rayto Chemray 800 Auto Analyzer, Rayto, China). Commercial colorimetric detection kits (Pars Azmoun Co., Tehran, Iran) were used for all biochemical analyses (Jerabek et al., 2018).
To evaluate the heterophil and lymphocyte counts, 0.5 µl of blood was collected from the wing vein of a bird with body weight closest to the group average. After staining with Giemsa dye (1:10 ratio; 1 ml dye + 9 ml water), blood smears were examined under a light microscope using a ×100 objective lens. A total of 100 leukocytes were counted and the percentage of heterophils and lymphocytes calculated (Thiam et al., 2022).
The activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) were determined using commercial assay kits (Randox Laboratories Ltd., Crumlin, UK) according to the manufacturer’s instructions (Liu et al., 2019).
Examination of intestinal morphology
At the end of the experiment, a 0.5-cm segment of the jejunum was collected and fixed in 10% neutral buffered formalin. The tissue was embedded in paraffin, sectioned using a microtome at a thickness of 5 µm, and stained on glass slides. Histological evaluation was performed using a light microscope equipped with suitable lenses and analysis software. The following parameters were measured:
● Villus height (VH): from the tip of the villus to the lamina propria.
● Crypt depth (CD): from the base between the adjacent villi to the bottom of the crypt.
● Villus width (VW): measured at the base of the villus.
The villus cross-sectional area (VCSA) was calculated using the formula:
In addition, the thickness of the intestinal wall was evaluated across 10 villi using ×40 and ×100 magnifications (Liu et al., 2019).
Statistical model
Data were analyzed using SAS software version 9.1 (SAS Institute Inc., 2004). NRM 1.4.xls software was used to fit the appropriate models for each trait (Vedenov and Pesti, 2008). The following models were used for fitting the best model selected using R2.
● Linear: y = a + bx (two parameters)
● Quadratic: y = a + bx + cx2 (three parameters)
● Broken line: y = a + bx if x ≤ c; else, y = a + bc (three parameters)
● Quadratic broken line: y = a + bx + cx2 if x ≤ d; else, y = a + bd + cd2 (four parameters)
● Saturation kinetics: y = a * x/(b + x) (two parameters)
● Logistic three-parameter: y = a/(1 + b * exp (−c * x)) (three parameters)
● Logistic four-parameter: y = d + (a − d)/(1 + (x/c)^b) (four parameters)
● RNB model 1: Richards model, y = a/(1 + b * exp (−c * (x − d)))^(1/e) (five parameters)
● RNB model 2: Gompertz model, y = a * exp (−b * exp (−c * x)) (three parameters)
The best model was selected using R2 statistics.
Results and discussion
Effect of dietary l-lysine levels on the performance of Gilan native ducks
In this study, during the starter phase (days 1–14), the ducks fed diets with 1.0% or 1.1% lysine, which were slightly higher than the 0.9% recommended by the National Research Council (NRC, 1994), ate more and gained significantly more weight (p< 0.01) compared with those on a 0.9% lysine diet. On the other hand, cutting lysine to 0.7% (0.2% below the NRC recommendation) led to a noticeable drop in both the feed intake and weight gain during the same period (p< 0.01). Ducks on the 1.1% lysine diet also used feed more efficiently, showing a better FCR than those on 0.9% lysine (p< 0.05) (see Table 3). In the grower period (days 15–49), boosting the lysine levels from 0.45% to 0.85% had a clear impact on growth. Ducks fed 0.75% lysine (0.1% above the NRC’s 0.65% recommendation) ate more and gained more weight, while those on 0.85% lysine (0.2% above that of the NRC) showed better feed efficiency (p< 0.05) (see Table 4).
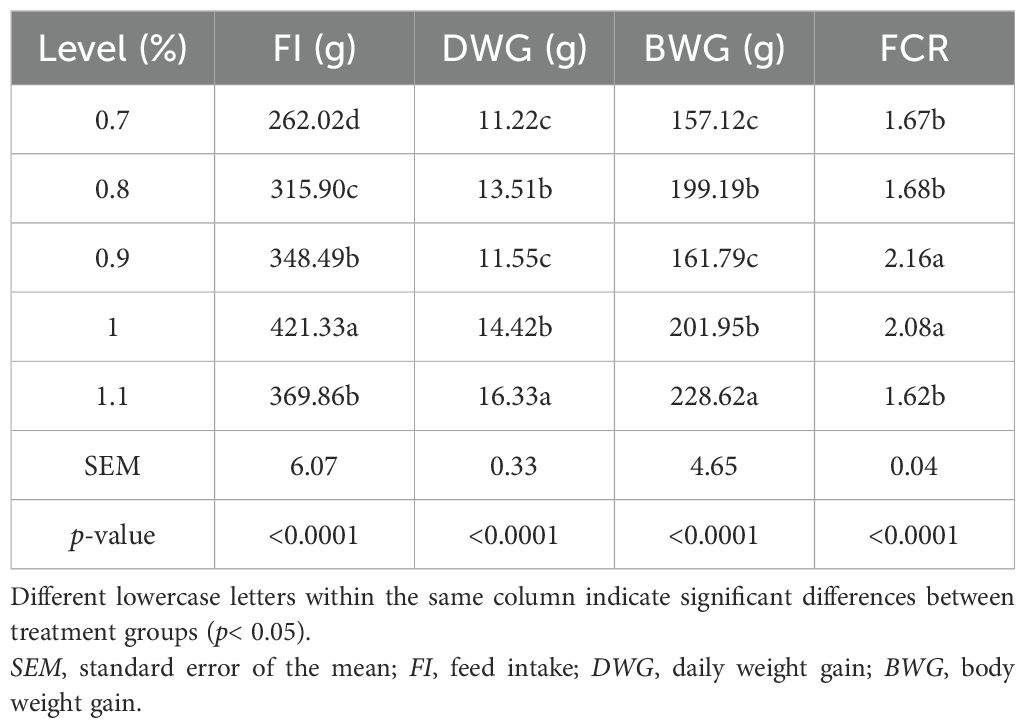
Table 3. Effect of the addition of different levels of l-lysine amino acid on the performance of Gilan native ducks at 1–14 days.

Table 4. Effect of the addition of different levels of l-lysine amino acid on the performance of Gilan native ducks aged 15–49 days.
The NRC (1994) suggests that White Pekin ducks need 0.9% lysine in the starter phase and 0.65% in the grower period. However, research on broilers, native chickens, quails, turkeys, White Pekin ducks, and Korean native ducks indicates that higher lysine levels can boost the daily weight gain, feed intake, and feed efficiency (Fouad et al., 2018). Consistent with our findings, An et al. (2022) found that increasing the lysine from 7.5 to 12.5 g/kg improved growth in broiler chickens aged 21–28 days. Ishii et al. (2019) showed that giving broilers more lysine than recommended by the NRC (1994) during the finishing phase (21–38 days) boosted their performance. This suggests that diets with higher lysine could be a smart move for poultry farming. Yu et al. (2024) found that White Pekin ducklings in the first 3 weeks after hatching do best with standardized ileal digestible (SID) lysine levels of around 1.05%, 1.05%, and 1.04% for maximum body weight, daily weight gain, and feed efficiency, respectively. Similarly, Wickramasuriya et al. (2016) reported that increasing lysine from 0.52% to 1.22% during the starter phase improved the weight gain and feed efficiency of Korean native ducklings. Mehri et al. (2015) also saw benefits in growing quails, with lysine levels increased from 0.84% to 1.29% leading to heavier birds and better feed efficiency. On the other hand, Watanabe et al. (2017) noted that cutting lysine by 10% below the NRC (1994) levels led to worse feed efficiency in 28-day-old broilers. However, Mousa et al. (2023) warned that going too high—20% above the recommended lysine levels—impaired the broiler growth during the growing and finishing stages. The authors suggested that excess lysine might negatively affect how other amino acids are used. Surprisingly, however, the high lysine level still improved the FCR. There is a need for more research to understand the puzzling combination of increased feed intake but reduced weight gain with too much lysine.
In another study, Wu et al. (2024) determined that a 15% crude protein diet was ideal for maximizing the weight gain and feed efficiency of growing Pekin ducks. Purba et al. (2024) found that boosting the essential amino acids (e.g., lysine, methionine, threonine, arginine, and tryptophan) by 10% above the standard levels, combined with pelleted feed, improved the performance and cut down the feed intake and FCR in Alabio ducks during the starter phase. However, Fouad et al. (2018) examined five lysine levels (i.e., 0.75%, 0.80%, 0.85%, 0.90%, and 0.95%) and found no significant impact on the FCR. These mixed results across studies might stem from the differences in bird genetics, study design, what the research measured, or the mathematical models used (Zhou et al., 2017). The response to lysine also appears to be dependent on the levels of other amino acids in the diet. Overall, previous research showed that how well lysine supplementation works depends on the starting lysine level, the age, the breed of the birds, the duration of the study, and other factors (Fouad et al., 2018).
Effect of dietary l-lysine levels on the carcass characteristics of native ducks
In the present study, the dietary lysine levels had no significant effect on the relative weights of the spleen, bursa of Fabricius, and breast muscle in Gilan native ducks (p > 0.05) (Table 5).
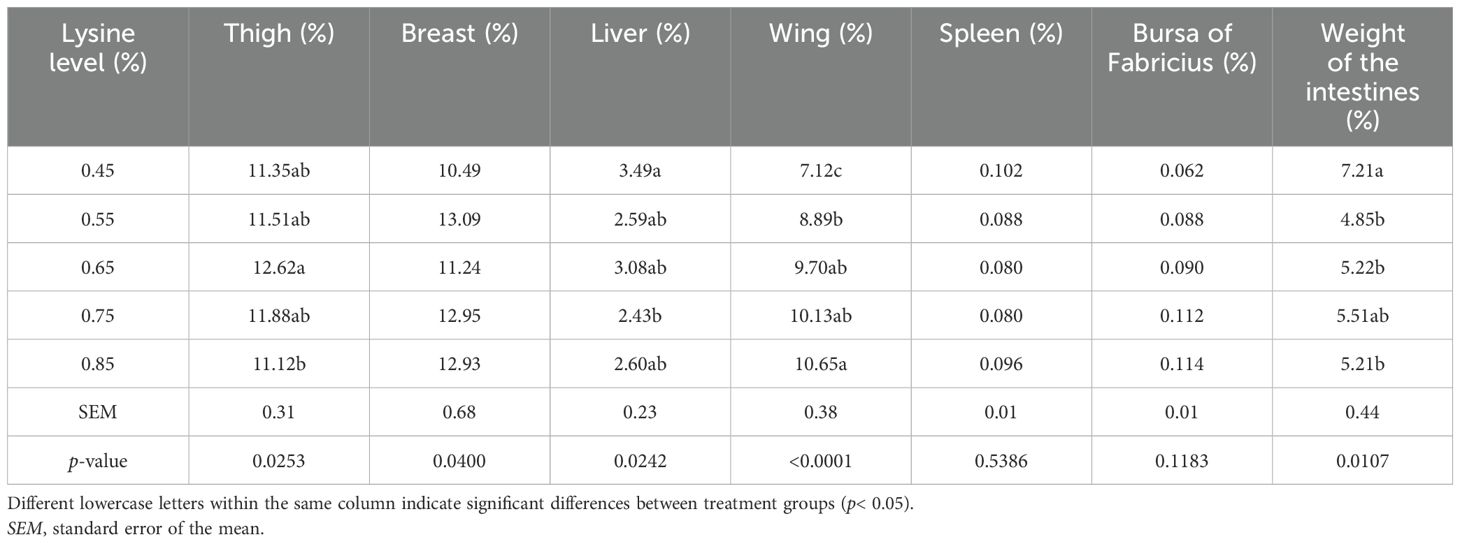
Table 5. Effect of the addition of different levels of l-lysine amino acid on the carcass traits of Gilan native ducks at the age of 49 days (percent of live body weight).
It is well established that feed additives such as amino acids can stimulate the immune system and improve overall health. Several studies have demonstrated that the lymphoid structures associated with the digestive system are significantly influenced by the dietary amino acid levels (Ruth and Field, 2013). Amino acids play a critical role in both humoral and cellular immunity by supporting the development of the germinal center in the bursa of Fabricius and by promoting the synthesis of the effector molecules such as immunoglobulins, nitric oxide, lysozyme, and complement, as well as the regulatory molecules including cytokines and eicosanoids (Swain and Johri, 2000).
In the study by Wu et al. (2024), reducing the dietary crude protein increased the abdominal fat yield, while the breast and thigh meat yields remained unaffected. In contrast, some earlier studies reported that lysine deficiency resulted in a significant reduction in carcass development, particularly in lymphoid organs such as the spleen and bursa of Fabricius.
In addition, reductions in antibody synthesis, alterations in thyroid and growth hormone secretion, and impaired growth performance can significantly affect bone development and structure (Fouad et al., 2018). Mulyantini (2014) reported that high levels of digestible lysine during the starter phase increased the weights of the spleen and bursa of Fabricius and enhanced the cellular immune function. Similarly, Chen (2002) observed that dietary lysine deficiency negatively affected the body weight and the weights of the lymphoid organs such as the thymus, spleen, and bursa of Fabricius. Body weight reduction has been consistently reported as a key symptom of lysine deficiency in broilers. According to Marquardt et al. (1985), the smaller size of immune organs is likely linked to the reduced body weight under lysine-deficient conditions.
Sigolo et al. (2019) found that supplementing diets with 10% excess lysine, with or without additional methionine, tended to result in the highest relative weight of the bursa of Fabricius.
The findings of the present study also align with those of Hesabi et al. (2006), who reported that dietary lysine levels of 110% and 115% did not significantly affect the breast muscle weight during the starter period in male broilers. Rosa et al. (2014) similarly noted that increased levels of digestible lysine in the diet had no effect on the breast meat yield in male broilers.
However, many studies have emphasized the essential role of lysine in improving the body weight and breast muscle yield in broilers, attributing this to its high concentration in pectoral muscle fibers (Nasr and Kheiri, 2012). In support, Helmbrecht and Hou (2013) reported that increasing the dietary lysine in Pekin ducks enhanced the breast meat yield, highlighting the crucial role of lysine in muscle protein synthesis. The authors concluded that the optimal lysine level for maximizing breast meat yield should exceed 0.83%.
These findings may be attributed to the increased availability of lysine, an essential amino acid that plays a key role in muscle growth, particularly in the pectoral muscles, due to its involvement in muscle protein synthesis.
In the present study, the dietary lysine levels significantly affected the relative weights of the wings, thighs, liver, and intestines in native ducks (p< 0.05). Specifically, the thigh weight was significantly higher in the 0.65% lysine treatment (NRC-recommended level), the wing weight in the 0.85% lysine group (0.2% above NRC), and both the liver and intestine weights in the 0.45% lysine group (0.2% below NRC) compared with other treatments (p< 0.05) (Table 5).
Evidence indicates that the liver, gizzard, and intestines develop rapidly, as these organs play crucial roles in early growth (Purba and Sinurat, 2018). Bouyeh (2012) reported that feeding Ross 308 male broilers with diets containing 40% more lysine and methionine than recommended increased the thigh weight and carcass yield. Bouyeh and Gevorgyan (2011) also found that the heart and liver weights increased when the dietary lysine exceeded the recommendations by 30%.
In the study by Sigolo et al. (2019), the highest relative weight of the cloaca was observed in birds fed a diet containing 100% lysine. In addition, broilers fed standard diets exhibited the highest liver weight.
The present findings align with those of Nasr and Kheiri (2012), who reported that increasing the dietary lysine by 10% above the NRC recommendations significantly improved the carcass and thigh weights in broilers.
These improvements in carcass and organ yields are likely due to the primary role of lysine in protein deposition, being one of the most abundant amino acids in muscle protein (Brasil et al., 2018). Moreover, lysine deficiency has been associated with the reduced production of organic bone matrix, potentially leading to leg abnormalities in broilers with ascites (Franco et al., 2006). Lysine may also contribute to bone formation by enhancing the calcium absorption in the intestines and reducing fecal calcium excretion (D’Mello, 2003).
In contrast, the findings of the present study do not align with the results reported by Purba and Sinurat (2018). In their study on EPMp broiler ducks, the different dietary lysine levels had no significant effect on the relative percentages of the liver, gizzard, thigh, wing, or intestines. They evaluated four dietary lysine levels (i.e., 0.60%, 0.70%, 0.80%, and 0.90%) in ducks raised up to 10 weeks of age and found no significant changes in the average carcass yield or the relative weights of the thigh, wing, liver, gizzard, and intestine.
Effect of adding different levels of l-lysine amino acid on the biochemical parameters of native duck blood
Blood plasma proteins and amino acids play a vital role in maintaining colloidal osmotic pressure and supporting immune function. They contribute to the amino acid balance through rapid replenishment of essential amino acids, help stabilize the blood glucose levels via gluconeogenesis, and are involved in the synthesis of various functional enzymes (Ma et al., 2018). Therefore, plasma proteins and amino acids are essential for the maintenance of physiological homeostasis.
In addition, blood albumin serves as a primary reservoir of amino acids for tissue protein synthesis, particularly during periods of rapid physical growth in birds and under restricted feeding conditions (Filipovic et al., 2007).
In the present study, increasing the dietary lysine levels significantly influenced the blood biochemical parameters, including glucose, cholesterol, triglycerides, HDL, LDL, very low-density lipoprotein (VLDL), total protein, albumin, uric acid, and urea (p< 0.05). Ducks that received lysine levels above the NRC recommendations exhibited significant increases in serum glucose (0.65% treatment), cholesterol, HDL, and LDL (0.75% treatment), total protein and albumin (0.75% and 0.85% treatments), and uric acid (0.85% treatment) compared with the other groups (p< 0.05). In contrast, the serum triglycerides, urea, and VLDL levels were significantly increased in ducks that received 0.45% lysine supplementation (lower than the NRC level) (p< 0.05; Table 6).
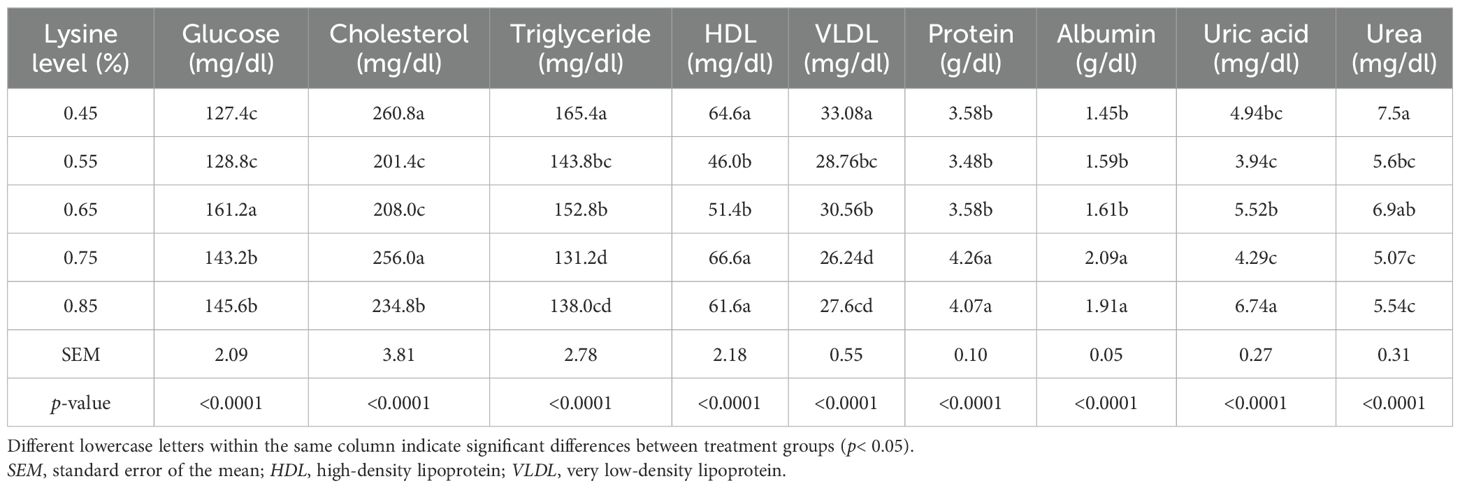
Table 6. Effect of the addition of different levels of l-lysine amino acid on the blood biochemical parameters of 49-day-old Gilan native ducks.
In accordance with the present findings, Sigolo et al. (2019) reported that supplementing broiler diets with 10% excess lysine resulted in the highest concentration of uric acid. In birds, uric acid is the main end product of nitrogen metabolism. Excess or unbalanced dietary amino acids are metabolized into carbon skeletons and ammonia, and the highly toxic ammonia is subsequently converted into uric acid (Sigolo et al., 2019).
The results of the current study also align with those of Fakhraei et al. (2011), who demonstrated that increasing the dietary lysine levels elevated the serum albumin and total protein concentrations in broiler chickens. This increase in albumin may be due to the reduced catabolism of albumin to compensate for lysine deficiency. According to Tian et al. (2019), lysine deficiency suppresses hepatic albumin synthesis, resulting in lower serum albumin concentrations.
Accordingly, the increase in serum total protein observed with higher lysine levels appears reasonable. Cemin et al. (2017) emphasized that dietary lysine promotes hepatic protein synthesis, as the liver is the primary site of protein production and lysine is one of the key amino acids involved in this process. Similarly, Sahir et al. (2006) reported that the serum total protein concentration increased with higher dietary lysine intake. Several studies have also shown that growing chickens fed low-protein, lysine-deficient diets exhibit increased hepatic lipid accumulation, elevated serum cholesterol, and higher abdominal fat deposition (Schmeisser et al., 1983). Inadequate protein synthesis under lysine-deficient conditions may impair the lipid metabolizing enzyme systems, leading to hepatic lipid accumulation. Lysine also serves as a metabolic precursor to l-carnitine, which plays a key role in lipid and energy metabolism. l-Carnitine has been shown to reduce the serum concentrations of cholesterol, triglycerides, free fatty acids, phospholipids, and VLDL while increasing the levels of HDL, intermediate-density lipoprotein (IDL), and LDL.
Sigolo et al. (2019) reported that excess dietary lysine led to significant increases in plasma cholesterol, LDL, and HDL in broilers. In line with these findings, the present study showed that increasing the dietary lysine levels was associated with higher plasma concentrations of cholesterol, LDL, and HDL and reduced the triglyceride and VLDL levels.
Furthermore, reductions in the serum triglyceride levels have also been reported in birds supplemented with l-carnitine (Xu et al., 2003). Chang et al. (2018), who studied five dietary lysine levels (i.e., 0.68%, 0.72%, 0.76%, 0.80%, and 0.84%) in laying pigeons found that lysine significantly influenced the serum total cholesterol and total protein concentrations. Based on the serum biochemical parameters, they concluded that the optimal lysine level for laying pigeons was 0.76%.
Bouyeh and Gevorgyan (2011) reported increased blood glucose levels in birds supplemented with dietary lysine. In contrast, Akbari Moghaddam et al. (2016) found that dietary lysine had no significant effect on the plasma total cholesterol or HDL-C levels in laying hens.
In the study by Baghban-Kanani et al. (2020), sesame seed flour containing 2.7% lysine was found to reduce the serum low-density lipoprotein cholesterol (LDL-C), total cholesterol, and the atherogenic index while increasing high-density lipoprotein cholesterol (HDL-C). These effects were attributed to the enhanced cholesterol binding to bile acids, the inhibition of micelle formation, and the promotion of short-chain fatty acid production via fermentation. Such mechanisms have been proposed to explain the cholesterol-lowering potential of lysine-containing feed components. The liver is the primary site of fatty acid and cholesterol synthesis in birds. Previous studies have shown that low dietary lysine stimulates de novo lipogenesis and increases fat deposition, whereas lysine supplementation can modulate lipogenesis and reduce fat accumulation (Carew et al., 2005).
However, in the present study, increasing the dietary lysine levels was associated with elevated serum cholesterol, HDL, and LDL levels. These findings are consistent with those of Ruan et al. (2019), who reported that increasing the dietary lysine (6.4–10.4 g/kg) reduced the hepatic triglyceride content and the VLDLR expression. The authors believed that dietary lysine concentrations influence the expression of the genes involved in protein and lipid metabolism, potentially enhancing the protein and lipid mobilization in poultry.
Conversely, the current findings differ from those of Khakpour-Irani et al. (2015) and Akbari Moghaddam et al. (2016). Khakpour-Irani et al. (2015) found that supplementing broiler diets with 90%, 100%, and 110% of the Ross 308 lysine requirement had no significant effects on the triglyceride levels or the total blood protein. Similarly, Akbari Moghaddam et al. (2016) reported that five levels of digestible lysine (i.e., 0.657%, 0.707%, 0.757%, 0.807%, and 0.857%) had no significant effect on the serum triglyceride, cholesterol, HDL, LDL, uric acid, and total protein levels.
Contrary to the present findings, some researchers suggest that increased dietary lysine may upregulate the expression of the genes involved in electron transport and mitochondrial energy production. This enhanced energy availability may contribute to elevated hepatic triglyceride synthesis and, consequently, increased serum triglyceride levels (Cemin et al., 2017).
Conversely, other studies have reported that a higher lysine intake downregulates the genes associated with fat synthesis, which may reduce the lipid storage in adipose tissue while increasing circulating free triglycerides (Tian et al., 2019).
The serum biochemical data obtained in this study indicate that lysine supplementation did not adversely affect the physiological status of the birds, as the serum protein values remained within the normal physiological range.
Effect of adding different levels of l-lysine amino acid on the number of native duck blood cells
The results of the present study showed that varying dietary lysine levels did not significantly affect the percentages of heterophils, the heterophil-to-lymphocyte (H/L) ratio, monocytes, and the mean corpuscular hemoglobin concentration (MCHC) (p > 0.05).
However, increasing the dietary lysine levels above the NRC recommendations significantly increased the hematocrit percentage, the red blood cell (RBC) and white blood cell (WBC) counts, the hemoglobin concentration, the lymphocyte percentage, the mean corpuscular hemoglobin (MCH), and the mean corpuscular volume (MCV) (p< 0.05). The highest eosinophil percentage was observed in the group that received 0.65% lysine, which was significantly different from the 0.55%, 0.75%, and 0.85% lysine treatments (p< 0.05) (Table 7).
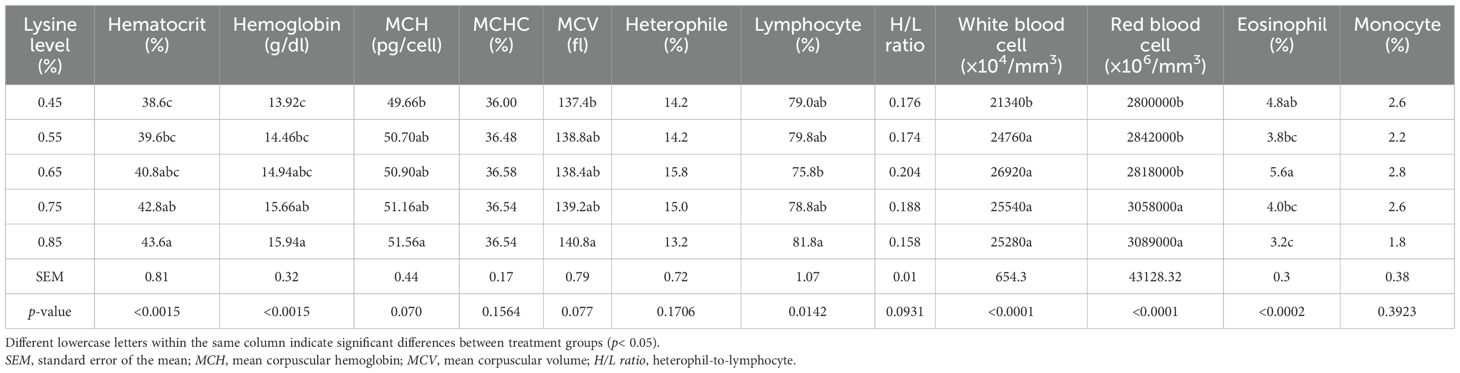
Table 7. Effect of the addition of different levels of l-lysine on the blood cells of Gilan native ducks at the age of 49 days.
The serum biochemical results from this study indicate that lysine supplementation did not negatively impact the birds’ physiological status, as the serum protein concentrations remained within the normal physiological range.
Numerous studies have demonstrated that a deficiency of amino acids, including lysine, impairs protein synthesis and compromises immune function, thereby affecting the growth and productivity of birds. In addition to genetic selection, non-genetic factors such as the dietary amino acid concentrations can influence the gene expression related to immune responses by altering immune system development and the antibody production against pathogens.
Studies in broiler chickens by Mirzaaghatabar et al. (2011) and Hosseini et al. (2012) reported that increasing the dietary amino acid levels enhances immune function. Amino acids play a central role in both humoral and cellular immunity by promoting the proliferation of lymphocytes, the recruitment of monocytes and heterophils from the bone marrow, and the synthesis of effector molecules such as immunoglobulins, nitric oxide, lysozyme, and complement (Swain and Johri, 2000).
Amino acid deficiency weakens the cellular immune responses by reducing the thymic T-cell populations, lowering the serum interleukin-2 levels, and increasing the apoptotic cell counts (Wu et al., 2012). It also leads to a reduction in the bursa weight and the lymphocyte numbers in follicles (Wu et al., 2013). Konashi et al. (2000) reported that lysine is involved in cytokine synthesis, lymphocyte proliferation, and optimal immune function. Similarly, Faluyi et al. (2015) found that hematological indices, including the erythrocyte sedimentation rate (ESR), MCH, and MCV, were significantly affected by the dietary lysine levels (1.12%, 1.13%, 1.14%). Birds fed diets containing 1.13% lysine showed the highest antibody titer. Glick et al. (1981) conducted similar research on amino acid deficiency and immune response in chickens. They observed that the serum protein levels were significantly reduced in birds fed amino acid-deficient diets, which was linked to impaired humoral immunity. Similarly, Liao et al. (2015) demonstrated that inadequate lysine intake leads to suppressed cellular immune responses in chickens. Chen et al. (2003) also reported reductions in both the antibody production and the cellular immune function due to lysine deficiency.
In agreement with these findings, Bouyeh (2012) showed that increasing dietary lysine above the NRC (1994) recommendations enhanced the immune system function in broilers. Bouyeh and Gevorgyan (2011) found that supplementing Ross 308 broiler diets with lysine at levels 30% above the catalog recommendations during the starter and grower phases significantly increased the serum lymphocyte counts, the spleen size, and the size of the heart and liver.
Furthermore, Bouyeh (2012) reported that supplementing Ross 308 broiler diets with lysine and methionine at 30% and 40% above NRC recommendations, respectively, significantly increased the blood lymphocyte counts and decreased the heterophil counts and the H/L ratio, a known stress indicator. A linear increase in the Newcastle disease antibody titers was also observed by day 42 in response to elevated dietary lysine and methionine levels.
In the study conducted by Mahdavi et al. (2012), six levels of digestible lysine (i.e., 0.77%, 0.84%, 0.91%, 0.98%, 1.05%, and 1.12%) were evaluated. The results showed that dietary lysine significantly increased the plasma free lysine, albumin, total protein, immunoglobulins, and the H/L ratio. Based on their findings, the lysine requirement for optimal immune response appeared to be higher than that required for general performance in broilers.
Similarly, Chen (2002) reported that birds that received sufficient lysine produced significantly more antibodies than those in the lysine-deficient groups. Their results also indicated that lysine deficiency impaired the T-cell function in response to mitogenic stimulation. However, in contrast to the present findings, Chen (2002) did not observe significant differences between the control and lysine-deficient groups in the lymphocyte proliferation assays, regardless of the Newcastle disease vaccination status. The researchers suggested that lysine deficiency did not have a statistically significant effect on cellular immune function. They also noted that the lymphocyte proliferation data had high standard deviations across all groups, possibly due to individual variability or experimental inconsistencies.
Effect of adding different levels of l-lysine amino acid on the intestinal morphology of native ducks
In this study, the intestinal morphometric parameters of Gilan native ducks were evaluated under the influence of different dietary lysine levels. As shown in Table 8, lysine supplementation significantly affected the CD and villus thickness, with the highest villus thickness observed in the 0.75% lysine group (0.1% above the NRC recommendation). However, there was no significant difference in the villus thickness between the 0.45% and 0.85% treatments (p > 0.05). The CD increased significantly in the 0.55% treatment (0.1% below the NRC level) compared with the 0.45%, 0.65%, and 0.85% treatments (p< 0.05). No significant differences were observed in the crypt diameter or VH among the groups (p > 0.05).
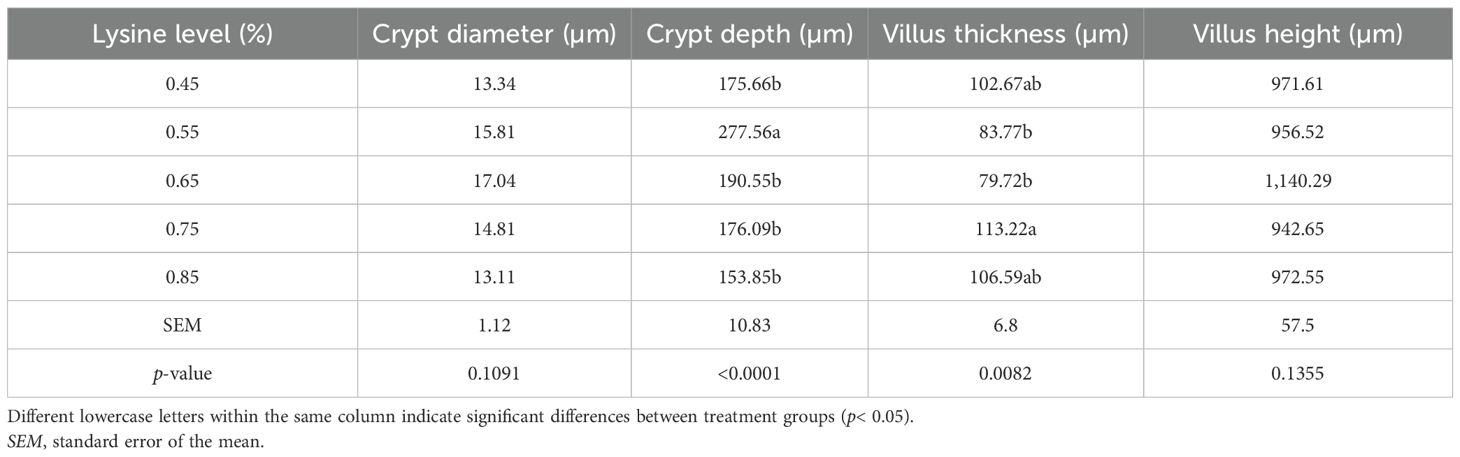
Table 8. Effect of the addition of different levels l-lysine on the intestinal morphometry of Gilan native ducks at the age of 49 days.
These findings are consistent with Franco et al. (2006), who reported increased duodenal and jejunal CD in 7-day-old broilers fed diets containing 11.4–12.7 g/kg of lysine. Similarly, Vaezi et al. (2011) noted that higher lysine levels increased the CD and the VH-to-CD ratio. Ojediran et al. (2017) also concluded that lysine supplementation enhances the villus length, improving amino acid digestibility.
Ebrahimi et al. (2017) demonstrated that in ovo injection of 20 mg l-lysine improved chick weight at hatch, the VH, and the VH-to-CD ratio in the duodenum, jejunum, and ileum, as well as the diameter of the duodenal and jejunal crypts. The current results are in agreement with these findings. The observed improvements can be attributed to the role of lysine as an essential amino acid with protective effects on the digestive system and muscular structure. Lysine also plays a significant role in enhancing intestinal calcium absorption. Deficiency in lysine may impair nutrient absorption by reducing the villus length, as the intestine utilizes dietary lysine for protein synthesis and other metabolic activities.
Konieczka et al. (2021) found that high-lysine diets (10% above turkey requirements) lowered the intestinal pH, increased the concentration of short-chain fatty acids (SCFAs) in the ceca, and enhanced the microbial activity. These changes suggest that lysine may influence the extracellular enzymatic activity via microbiota modulation.
On the other hand, the findings of the present experiment contradict the results of the study by Ebrahimi et al. (2017). The authors reported a significant reduction in CD in the duodenum, jejunum, and ileum, as well as a decrease in VW in the duodenum and ileum. Similarly, the findings of Mateos et al. (2014); Hung et al. (2020), and Xing et al. (2015) do not support the results of the current study. Mateos et al. (2014) found that increasing the dietary lysine levels did not influence the villus morphometry in birds between 1 and 21 days of age. Hung et al. (2020) reported that supplementation with 0.1%–0.3% extra lysine improved the VH. Xing et al. (2015) observed that feeding Chinese Linwu ducks with lysine-producing probiotics for 63 days increased the VH in the jejunum and ileum, reduced the CD in the jejunum, and decreased the Lactobacillus population in the cecum.
In another study, Vaezi et al. (2011) reported that higher dietary lysine levels increased the VH. These findings are also in contrast to those of Ebrahimi et al. (2017), highlighting that the results of the present study are not consistent with several previous reports.
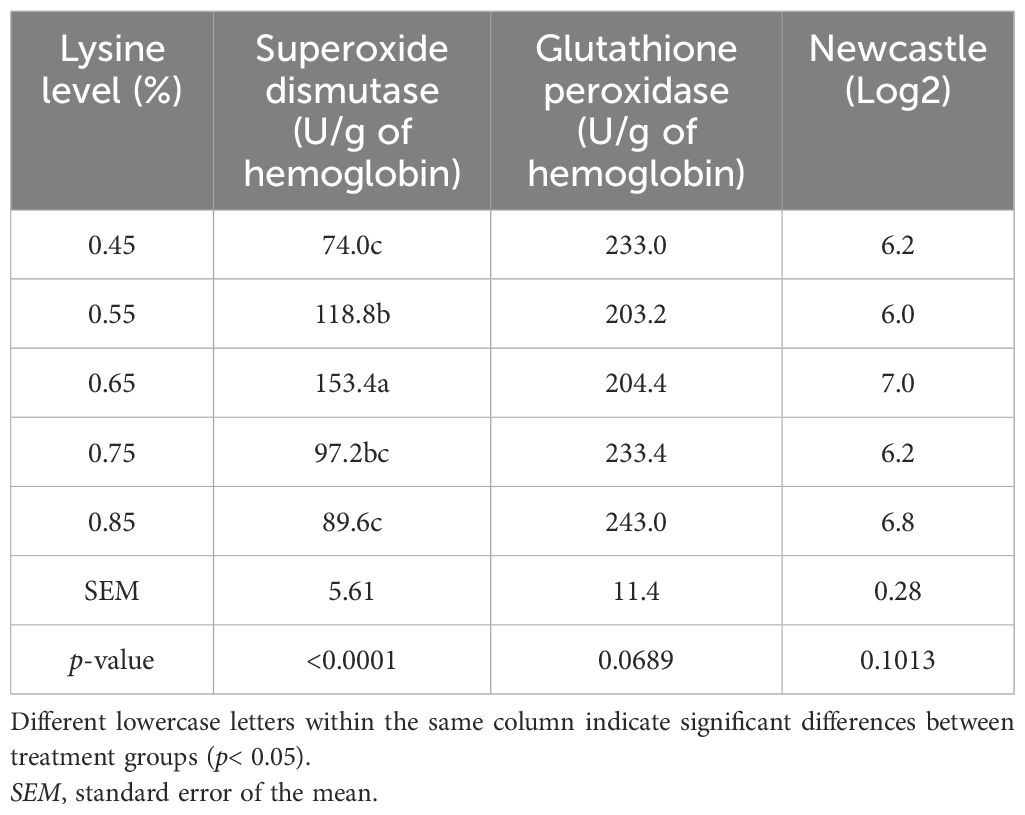
Effect of adding different levels of l-lysine amino acid on the antioxidant parameters of native ducks
The additive effect of dietary lysine on the blood antioxidant parameters is presented in Table 9. The experimental treatments did not significantly affect the GSH-Px activity or the antibody titers against Newcastle disease. However, a significant increase in SOD activity was observed in birds that received 0.65% dietary lysine compared with other treatments.
It has been suggested that supplementation with compounds possessing antioxidant properties can alleviate oxidative stress caused through reactive oxygen species (ROS), particularly under heat stress conditions, thereby enhancing performance (Zhang et al., 2013).
Similarly, Baghban-Kanani et al. (2020) reported that the total antioxidant capacity and serum SOD activity in laying hens increased with the dietary inclusion of sesame seed meal containing 2.7% lysine. These effects may be attributed to the antioxidant potential of sesame seed flour.
Lysine, as a precursor of l-carnitine, may play a key role in enhancing the glutathione levels, increasing the thiol groups (−SH), and reducing the acetyl-CoA accumulation—a precursor of lipid peroxidation—by chelating iron ions and inhibiting free radical production. Consequently, lysine supports the activity of antioxidant enzymes such as catalase, SOD, and GSH-Px, helping to preserve these enzymes and reduce oxidative damage (Adabi et al., 2011).
El-Bahr et al. (2021) evaluated the effects of diets containing optimal lysine + flaxseed oil, optimal lysine + linseed oil, high lysine + flaxseed oil, and high lysine + linseed oil over a 35-day period on the oxidative stress biomarkers. Their findings showed that the liver SOD activity and glutathione concentration were significantly higher in birds fed the high lysine + linseed oil diet compared with those in the other groups, indicating a strong antioxidant potential. Similarly, the antioxidant potential in the breast muscle was elevated in birds that received the high lysine + linseed oil combination.
However, the results of the current study are inconsistent with those of Khakpour Irani et al. (2015), who reported that supplementation of broiler diets with 90%, 100%, and 110% of the Ross 308 lysine requirement did not significantly affect the serum SOD activity in birds fed the 100% lysine level.
In contrast, the GSH-Px activity was reduced in birds fed certain lysine levels compared with the other treatment groups. It has been suggested that providing the recommended dietary lysine level for Ross 308 broilers during heat stress helps maintain the antioxidant capacity. However, excessive lysine may increase arginine excretion, resulting in elevated lipid peroxidation and delayed weight gain.
Newcastle disease is one of the most prevalent viral infections in poultry, with an estimated prevalence rate of 28.9%. Effective vaccination, balanced nutrition, and immune modulation are essential strategies for enhancing immunity and preventing disease outbreaks (Faluyi et al., 2015).
In contrast to some studies, Bouyeh (2012) reported that supplementing broiler diets with lysine and methionine at 30% and 40% above the NRC (1994) recommendations, respectively, led to a linear increase in the Newcastle antibody titers by day 42. Conversely, Chen et al. (2003) found that broilers fed a diet containing 1.24% lysine had a reduced antibody response to Newcastle vaccination, as measured by ELISA.
Faluyi et al. (2015) further showed that birds fed 1.13% lysine had the highest mean antibody titers against Newcastle disease, while those that did not receive lysine supplementation exhibited the lowest. Under stress or disease conditions, the immune response levels become critical in determining the effectiveness of vaccination and the resistance to infections.
The absence of significant effects of the experimental treatments on the Newcastle disease antibody titers in the present study may be attributed to the lack of environmental stress and the overall normal conditions under which the experiment was conducted.
Mahdavi et al. (2012) reported that, across six levels of digestible lysine, the requirement for achieving the maximum antibody titer against Newcastle disease virus in male broilers exceeded the highest lysine levels tested. Dietary lysine significantly increased the immunoglobulin levels and antibody titers against both sheep RBCs and Newcastle virus.
These findings suggest that the lysine requirement for optimal immune response may be higher than that required for growth performance. Moreover, the broken-line regression analysis in their study showed that the plasma free lysine concentration is a reliable physiological indicator for determining the digestible lysine requirements in both male and female broilers. In general, requirement estimates derived from exponential models tend to be higher than those obtained from broken-line models.
Model fitting for optimization of the lysine level for different traits
Table 10 summarizes the model fitting for optimization of the lysine level for different traits in Iranian native ducks.

Table 10. Model fitting for optimization of the lysine level for different trait in Iranian natives’ ducks.
For performance traits in early growth (1–15 days), the feed intake, body weight, and daily weight gain exhibited logistic four-parameter model fits (R2 = 0.858, 0.828, and 0.704, respectively) with a consistent optimal lysine level of 1.10, indicating a high requirement for rapid growth and a sigmoidal response. The FCR (1–15 days) was best modeled by quadratic broken line (R2 = 0.354, optimal lysine = 0.748), suggesting moderate lysine optimizes the feed efficiency despite variability. In later growth (15–49 days), the feed intake followed RNB model 1 (R2 = 0.861, optimal lysine = 0.787), while the body weight and daily weight gain used quadratic broken-line models (R2 = 0.693 and 0.814, respectively; optimal lysine = 0.706), indicating peak responses at moderate levels. The FCR (15–49 days) showed an excellent quadratic broken-line fit (R2 = 0.994, optimal lysine = 0.641), highlighting high lysine sensitivity for feed efficiency. Organ weights showed quadratic models for the thigh (R2 = 0.7086, optimal lysine = 0.65) and the wing (R2 = 0.9855, optimal lysine = 0.85) across datasets, with strong muscle development at higher lysine. The breast and liver followed the broken-line models (R2 = 0.5955 and 0.6916, respectively; optimal lysine = 0.46), suggesting minimal lysine needs, while the spleen (quadratic; R2 = 0.9724, optimal lysine = 0.67) and the bursa of Fabricius (broken line; R2 = 0.9439, optimal lysine = 0.77) indicated parabolic and threshold responses, respectively.
The blood biochemical parameters, including glucose, cholesterol, protein, albumin, uric acid, and urea, were best fitted by logistic four-parameter models (R2 = 0.562–0.952, optimal lysine = 0.787–0.850), showing sigmoidal responses with metabolic plateaus at higher lysine levels. Triglyceride and VLDL followed saturation kinetics (R2 = 0.711, optimal lysine = 0.850, 0.544), indicating lipid metabolism saturation, while HDL and LDL had poor logistic three-parameter fits (R2 = 0.072 and 0.156, respectively, optimal lysine = 0.850), suggesting weak lysine dependence. The hematological parameters such as hematocrit (quadratic broken line; R2 = 0.999, optimal lysine = 0.787) and hemoglobin (broken line; R2 = 0.995, optimal lysine = 0.810) showed precise responses, while the WBC count, MCH, MCV, and immune parameters (i.e., heterophil, lymphocyte, H/L ratio, and eosinophil; R2 = 0.927–0.989, optimal lysine = 0.544–0.850) predominantly followed logistic four-parameter models. The weight of the intestines likely followed a quadratic model (optimal lysine = 0.65), aligning with muscle traits. These findings highlight trait-specific lysine requirements, guiding targeted nutritional strategies for poultry production.
The lysine requirement levels and their effects on the growth performance, the carcass traits, and the physiological parameters observed in the current study align well with findings from other researchers; however, there are some differences in the optimal lysine concentrations and response patterns due to variations in the experimental conditions, broiler genetics, and dietary formulations. In white Pekin ducks, Bons et al. (2002) reported that 1.06% lysine was optimal for maximizing the BWG during the first 3 weeks of age. From 3 to 7 weeks, 1.02% lysine was required to achieve peak BWG, carcass yield, and breast yield. Xie et al. (2009) found that male white Pekin ducklings (7–21 days) needed 0.84%, 0.90%, and 0.98% lysine for optimal BWG, feed efficiency, and breast meat proportion, respectively. In male Korean native ducklings (1–21 days), the optimal BWG and feed efficiency were attained with 0.71% and 1.01% lysine, respectively (Wickramasuriya et al., 2016) For Longyan laying ducks (22–38 weeks), diets with 0.80% lysine optimized the egg production, egg weight, egg mass, FCR, and eggshell quality (Fouad et al., 2017).
Lysine supplementation significantly influences the carcass traits in broilers and ducks, with the optimal levels varying by trait and species. In broilers, our datasets showed the thigh and wing optimized at 0.65%–0.85% lysine (quadratic models), while the breast and liver required 0.46% (broken line). The literature suggests higher lysine (0.95%–1.15%) for broiler breast yield, reflecting genetic advancements, but aligns with our thigh and wing levels. In ducks, the breast and carcass yield require 0.98%–1.02% lysine, which is higher than broiler breast, but comparable to that of the wing, indicating species-specific muscle demands. The quadratic and broken-line models in our data mirrored nonlinear responses in the literature, with broilers showing stronger fits (e.g., R2 = 0.9855 for wing). These findings guide lysine supplementation strategies, emphasizing moderate levels for broiler muscle traits and higher levels for duck breast and carcass yield, with adjustments for breed, age, and lysine source (Leclercq, 1998; Wickramasuriya et al., 2016; Ruan et al., 2019). Bahadur et al. (2010) noted that lysine supplementation increased the breast meat yield and reduced abdominal fat, consistent with our breast findings (optimal lysine = 0.46), although our lower requirement may reflect a plateau in growth. A study on l-lysine sulfate supplementation confirmed improved breast meat yield (p< 0.05) and protein accretion, supporting our muscle development findings, but did not specify optimal levels. Our spleen (quadratic; R2 = 0.9724, optimal lysine = 0.67) and bursa of Fabricius (broken line; R2 = 0.9439, optimal lysine = 0.77) results suggest immune tissue sensitivity to lysine, a topic less explored in the literature, although one study noted the immunological benefits of lysine without quantifying levels.
Conclusion
This study demonstrated that dietary l-lysine supplementation significantly influenced the growth performance, carcass traits, hematological parameters, intestinal morphology, and blood biochemistry of Gilan native ducks. The optimal lysine levels (1.0%–1.1% during the starter phase and ~0.75% during the grower phase) improved the feed intake, BWG, and feed efficiency. In addition, lysine supplementation enhanced the wing, thigh, liver, and intestinal weights, along with the CD and villus thickness in the intestine. Increasing the level of lysine in the diet compared with the NRC (1994) recommendation increased the total protein, albumin, cholesterol, LDL, HDL, and uric acid, suggesting improved protein and lipid metabolism without impairing physiological homeostasis. Furthermore, lysine improved the RBC and WBC counts, hemoglobin concentration, and antioxidant enzyme activity (SOD), indicating enhanced hematological and oxidative status. Although no significant changes in the Newcastle antibody titer were observed, the absence of stress may explain this result. Overall, lysine levels above the NRC (1994) recommendations appear beneficial for improving the health and productivity of native ducks. This integrated analysis of 42 poultry traits demonstrates the critical role of lysine in optimizing the growth, organ development, and physiological parameters. Logistic four-parameter and quadratic broken-line models were the most effective, capturing sigmoidal and threshold responses. The optimal lysine levels ranged from 0.46 (breast and liver) to 1.10 (early growth), guiding precise nutritional interventions. Future studies should validate these findings across breeds and explore amino acid interactions to enhance poultry production efficiency.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal studies were approved by Department of Animal Science, Shabestar Branch, Islamic Azad University, Shabestar, Iran. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
HS: Conceptualization, Methodology, Writing – review & editing. MG: Data curation, Investigation, Writing – original draft. YE: Investigation, Methodology, Validation, Writing – original draft. TF: Data curation, Supervision, Writing – original draft. TV: Project administration, Writing – original draft. AG: Formal Analysis, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adabi S. G., Cooper R., Ceylan N., and Corduk M. (2011). L-carnitine and its functional effects in poultry nutrition. Worlds Poult. Sci. J. 67, 277–296. doi: 10.1017/S0043933911000304
Akbari Moghaddam Kakhki R., Golian A., and Zarghi H. (2016). Effect of dietary digestible lysine concentration on performance, egg quality, and blood metabolites in laying hens. J. Appl. Poult. Res. 25, 506–517. doi: 10.3382/japr/pfw032
An S. H., Kang H.-K., and Kong C. (2022). Standardized ileal digestible lysine requirements of 21–28 days old male broilers. Anim. Feed. Sci. Technol. 292, 115409. doi: 10.5187/jast.2023.e139
Baghban-Kanani P., Hosseintabar-Ghasemabad B., Azimi-Youvalari S., Seidavi A., Laudadio V., Mazzei D., et al. (2020). Effect of dietary sesame (Sesame indicum L) seed meal level supplemented with lysine and phytase on performance traits and antioxidant status of late-phase laying hens. Asian-Australas J. Anim. Sci. 33, 277. doi: 10.5713/ajas.19.0107
Bahadur V., Haldar S., and Ghosh T. K. (2010). Assessment of the efficacy of L-lysine sulfate vis-à-vis Llysine hydrochloride as sources of supplemental lysine in broiler chickens. Vet. Med. Int. 2010, 964076. doi: 10.4061/2010/964076
Bons A., Timmler R., and Jeroch H. (2002). Lysine requirement of growing male Pekin ducks. Br. Poult. Sci. 43, 677–686. doi: 10.1080/0007166021000025073
Bouyeh M. (2012). Effect of excess lysine and methionine on immune system and performance of broilers. Annals Biol. Res. 3, 3218–3224.
Bouyeh M. and Gevorgyan O. K. (2011). Influence of excess lysine and methionine on cholesterol, fat and performance of broiler chicks. J. Anim. Vet. Adv. 10, 1546–1550. doi: 10.3923/javaa.2011.1546.1550
Brasil R., Lima C., MaChado N., Curvello F., Quaresma D., Vieites F., et al. (2018). Digestible lysine requirements the performance, carcass traits and breast meat quality of slowgrowing broilers. Braz. J. Poult. Sci. 20, 555–564. doi: 10.1590/1806-9061-2017-0676
Carew L., McMurtry J., and Alster F. (2005). Effects of lysine deficiencies on plasma levels of thyroid hormones, insulin-like growth factors I and II, liver and body weights, and feed intake in growing chickens. Poul. Sci. 84, 1045–1050. doi: 10.1093/ps/84.7.1045
Cemin H. S., Vieira S. L., Stefanello C., Kipper M., Kindlein L., and Helmbrecht A. (2017). Digestible lysine requirements of male broilers from 1 to 42 days of age reassessed. PloS One 12, e0179665. doi: 10.1371/journal.pone.0179665
Chang L.-L., Xie P., Bu Z., Wang Q., Fu S.-y., and Mu C.-y. (2018). Effect of dietary lysine level on performance, egg quality and serum biochemical indices of laying pigeons. J. Appl. Poult. Res. 27, 152–158. doi: 10.3382/japr/pfx047
Chen C. F. (2002). The affect of dietary lysine and phosphorus deficiency on immune function in broiler chicken: University of Georgia. (Georgia: Master of Science Athens).
Chen C., Sander J., and Dale N. (2003). The effect of dietary lysine deficiency on the immune response to Newcastle disease vaccination in chickens. Avian. Dis. 47, 1346–1351. doi: 10.1637/7008
D’Mello J. (2003). “Responses of growing poultry to amino acids,” in Amino Acids in Animal Nutrition, 2nd Edition (CAB International, Wallingford), 237–263. doi: 10.1079/9780851996547.0237
Ebrahimi M., Janmohammadi H., Kia H. D., Moghaddam G., Rajabi Z., Rafat S. A., et al. (2017). The effect of L-lysine in ovo feeding on body weight characteristics and small intestine morphology in a day-old Ross broiler chicks. Revue Med. Vet. 168, 116–124.
El-Bahr S. M., Shousha S., Alfattah M. A., Al-Sultan S., Khattab W., Sabeq I. I., et al. (2021). Enrichment of broiler chickens’ meat with dietary linseed oil and lysine mixtures: Influence on nutritional value, carcass characteristics and oxidative stress biomarkers. Foods 10, 618. doi: 10.3390/foods10030618
Erickson A., Li X., Zabala-Díaz I., and Ricke S. (2007). Potential for measurement of lysine bio availability in poultry feeds by rapid microbiological assays - A review. J. Rapid Meth. Aut. Mic. 10, 1–8. doi: 10.1111/j.1745-4581.2002.tb00004.x
Fakhraei J., Lotfollahian H., Shivazad M., and Chamani M. (2011). Effects of different levels of lysine amino acid in Arian broiler breeders diets on immunity and some blood biochemical traits. Vet. Res. Biol. Product. 24, 48–57.
Faluyi O., Agbede J., and Adebayo I. (2015). Growth performance and immunological response to Newcastle disease vaccinations of broiler chickens fed lysine supplemented diets. J. Vet. Med. Anim. Health 7, 77–84. doi: 10.5897/JVMAH2014.0328
Filipovic N., Stojevic Z., Milinkovic-Tur. S., Ljubic B. B., and Zdelar-Tuk M. (2007). Changes in concentration and fractions of blood serum proteins of chickens during fattening. Vet. Arh. 77, 319.
Fouad A. M., Chen W., Ruan D., Shuan W., Weiguan X., and Zheng C. T. (2017). Effects of dietary lysine supplementation on performance, egg quality and development of reproductive system in egglaying ducks. J. Appl. Anim. Res. 1, 386–391. doi: 10.1080/09712119.2017.1308868
Fouad A. M., Chen W., Ruan D., Wang S., Xia W., and Zheng C. (2018). Effects of dietary lysine supplementation on performance, egg quality, and development of reproductive system in egg-laying ducks. J. Appl. Anim. Res. 46 (1), 386–391. doi: 10.1080/09712119.2017.1308868
Franco J. R. G., Murakami A. E., Natali M. R. M., Garcia E., and Furlan A. C. (2006). Influence of delayed placement and dietary lysine levels on small intestine morphometrics and performance of broilers. Braz. J. Poult. Sci. 8, 233–241. doi: 10.1590/S1516635X2006000400006
Glick B., Day E., and Thompson D. (1981). Calorie-protein deficiencies and the immune response of the chicken I. Humoral immunity. Poul. Sci. 60, 2494–2500. doi: 10.3382/ps.0602494
Helmbrecht A. and Hou S. S. (2013). “Lysine requirement of Pekin ducks from 35 to 49 days of age,” in Proceedings 5th World Waterfowl Conference of the Asian Pacific Federation of the World Poul. Sci Assoc, November. (Paper presented at the conference).
Hesabi A., Nasiri H., and Birjandi M. (2006). Effect of supplemental methionine and lysine on performance and carcass yield characteristics in broiler chicks. World Poul. Sci. Assoc. 16, 112–117.
Hosseini S. A., Zaghari M., Lotfollahian H., Shivazad M., and Moravaj H. (2012). Reevaluation of methionine requirement based on performance and immune responses in broiler breeder hens. J. Poult. Sci. 49, 26–33. doi: 10.2141/jpsa.011021
Hung L., Lan L., Thu N., Phong N., Nhan N., and Ngu N. (2020). Effects of dietary lysine on apparent amino acid digestibility and carcass characteristics of Noi broilers. Livest Res. Rural Dev. 32, 8.
Ishii T., Shibata K., Kai S., Noguchi K., Hendawy A. O., Fujimura S., et al. (2019). Dietary supplementation with lysine and threonine modulates the performance and plasma metabolites of broiler chicken. J. Poult. Sci. 56, 204–211. doi: 10.2141/jpsa.0180104
Jerabek M., Suchy P., Strakova E., Kudelkova L., Simek V., Jakesova P., et al. (2018). Selected blood biochemical indicators of Cherry Valley ducks undergoing fattening in relation to their diet and sex. Vet. Med. (Praha) 63 (9), 420–432. doi: 10.17221/81/2018-VETMED
Johnson K. P. and Sorenson M. D. (1999). Phylogeny and biogeography of dabbling ducks (genus: Anas): a comparison of molecular and morphological evidence. Auk. 116, 792–805. doi: 10.2307/4089339
Khakpour Irani F., Daneshyar M., and Najafi R. (2015). Growth and antioxidant status of broilers fed supplemental lysine and pyridoxine under high ambient temperature. Vet. Res. Forum. 6, 189–195.
Konashi S., Takahashi K., and Akiba Y. (2000). Effects of dietary essential amino acid deficiencies on immunological variables in broiler chickens. Br. J. Nutr. 83, 449–456. doi: 10.1017/S0007114500000556
Konieczka P., Mikulski D., Ognik K., Juśkiewicz J., Zduńczyk Z., and Jankowski J. (2021). Increased dietary inclusion levels of lysine are more effective than arginine in supporting the functional status of the gut in growing Turkeys. Animals 11, 2351. doi: 10.3390/ani11082351
Leclercq B. (1998). Lysine: Specific effects of lysine on broiler production: comparison with threonine and valine. Poult. Sci. 77, 118–123. doi: 10.1093/ps/77.1.118
Li F., Lu Y., He Z., Yu D., Zhou J., Cao H., et al. (2024). Analysis of carcass traits, meat quality, amino acid and fatty acid profiles between different duck lines. Poult. Sci. 103, 103791. doi: 10.1016/j.psj.2024.103791
Liao S. F., Wang T., and Regmi N. (2015). Lysine nutrition in swine and the related monogastric animals: muscle protein biosynthesis and beyond. SpringerPlus 4, 1–12. doi: 10.1186/s40064-015-0927-5
Liu Y., Zhu X., Huang L., Jia Y., and Xia Z. (2019). Effects of force-feeding on immunology, digestive function and oxidative stress in the duodenal and jejunal mucosa of Pekin ducks. Animal 13, 21992206. doi: 10.1017/S1751731119000612
Ma N., Guo P., Zhang J., He T., Kim S. W., Zhang G., et al. (2018). Nutrients mediate intestinal bacteria–mucosal immune crosstalk. Front. Immunol. 9. doi: 10.3389/fimmu.2018.00005
Mahdavi A., Shivazad M., Alemi F., Zaghari M., Moravej H., and Darabighane B. (2012). Digestible lysine requirement of broilers based on practical diet. Ital. J. Anim. Sci. 11, e13. doi: 10.4081/ijas.2012.e13
Marquardt W., Snyder D., Savage P., Kadavil S., and Yancey F. (1985). Antibody response to Newcastle disease virus given by two different routes as measured by ELISA and hemagglutination-inhibition test and associated tracheal immunity. Avian. Dis. 29, 71–79. doi: 10.2307/1590695
Mateos G., Mohiti-Asli M., Borda E., Mirzaie S., and Frikha M. (2014). Effect of inclusion of porcine mucosa hydrolysate in diets varying in lysine content on growth performance and ileal histomorphology of broilers. Anim. Feed. Sci. Technol. 187, 53–60. doi: 10.1016/J.AnifeedSci.2013.09.013
Mehri M., Kasmani F. B., and Asghari-Moghadam M. (2015). Estimation of lysine requirements of growing Japanese quail during the fourth and fifth weeks of age. Poul. Sci. 94, 1923–1927. doi: 10.3382/ps/pev153
Mirzaaghatabar F., Saki A., Zamani P., Aliarabi H., and Hemati Matin H. (2011). Effect of different levels of diet methionine and metabolisable energy on broiler performance and immune system. Food. Agr. Immunol. 22, 93–103. doi: 10.1080/09540105.2010.530249
Mirzaei M., Bouyeh M., Zahedi A., Seidavi A., Khan R. U., Tufarelli V., et al. (2022). Influence of dietary L-carnitine and lysine–methionine levels on reproductive performance and blood metabolic constituents of breeder ducks. Reprod. Domest. Anim. 57, 253–261. doi: 10.1111/rda.14047
Mousa M. A., Asman A. S., Ali R. M., Sayed R. K., Majrashi K. A., Fakiha K. G., et al. (2023). Impacts of dietary lysine and crude protein on performance, hepatic and renal functions, biochemical parameters, and histomorphology of small intestine, liver, and kidney in broiler chickens. Vet. Sci. 10, 98. doi: 10.3390/vetsci10020098
Mulyantini N. G. (2014). The antibody and organ immune responses of broiler starter Fed diets with graded levels of digestible lysine. Media Peternakan 37, 57–60. doi: 10.5398/medpet.2014.37.1.57
Nasr J. and Kheiri F. (2012). Effects of lysine levels of diets formulated based on total or digestible amino acids on broiler carcass composition. Braz. J. Poult. Sci. 14, 249–258. doi: 10.1590/S1516635X2012000400004
Nicolau J. P., Pinto M. F., Ponsano E. H. G., Perri S. H. V., and Garcia Neto M. (2015). Exposure to carbonic gas enriched atmosphere or electrical water bath to stun or kill chickens. Braz. J. Poult. Sci. 17, 341–346. doi: 10.1590/1516-635x1703341-346
Ojediran T., Oloruntade T., Durojaye B., Saka R., and Emiola I. (2017). Blood parameters, carcass yield, organ weight and villi morphometrics of broilers fed low protein diet in excess of dietary lysine. Trakia J. Sci. 2, 121–127. doi: 10.15547/tjs.2017.02.004
Purba M. and Sinurat A. P. (2018). Performance of EPMp broiler ducks feed with various levels of dietary lysine up to 10 weeks of age. J. Ilmu. Ternak. Dan. Vet. 22, 1–8. doi: 10.14334/jitv.v22i1.1606
Purba M., Sinurat A. P., and Pasaribu T. (2022). Effect of essential amino acid supplementation and feed form on performance of Alabio ducks during starter phase. J.Poult. Sci. Technol. 40, 115–123.
Purba M., Sinurat A. P., Pasaribu T., and Kostaman T. (2024). “Supplementation of essential amino acids with the provision different types of feed to the performance of Alabio ducks (Anas plathyrynchos Borneo) for the starter periods,” in AIP Conference Proceedings, Vol. 2957 (1).
Purba M., Sinurat A. P., and Susanti T. (2017). Effect of different lysine and energy levels in diets on carcass percentage of three strains of broiler duck (International Seminar on Livestock Production and Veterinary Technology). doi: 10.14334/Proc.Intsem.LPVT-2016-p.395-407
Rosa E. P. M., Kiefer C., Souza K. M. R. D., Silva J. B. D., Ozelame A. M., Gomes E. N. O., et al. (2014). Níveis de lisina digestível para frangos de corte tipo caipira de 28 a 56 dias de idade. Rev. Bras. Saude Prod. Anim. 15, 872–880. doi: 10.1590/S1519-99402014000400012
Ruan D., Fouad A., Zhang Y., Wang S., Chen W., Xia W., et al. (2019). Effects of dietary lysine on productivity, reproductive performance, protein and lipid metabolism-related gene expression in laying duck breeders. Poul. Sci. 98, 5734–5745. doi: 10.3382/ps/pez361
Ruth M. R. and Field C. J. (2013). The immune modifying effects of amino acids on gut-associated lymphoid tissue. J. Anim. Sci. Biotechnol. 4, 1–10. doi: 10.1186/2049-1891-4-27
Sahir M., Shariatmadari F., Mirhadi S., and Chwalibog A. (2006). The effect of lysine supplements on haematology and serum biochemical indices of broiler breeders. Arch. Geflugelk. 70, S74–S79.
Schmeisser D. D., Kummerow F. A., and Baker D. H. (1983). Effect of excess dietary lysine on plasma lipids of the chick. J. Nutr. 113, 1777–1783. doi: 10.1093/jn/113.9.1777
Sigolo S., Deldar E., Seidavi A., Bouyeh M., Gallo A., and Prandini A. (2019). Effects of dietary surpluses of methionine and lysine on growth performance, blood serum parameters, immune responses, and carcass traits of broilers. J. Appl. Anim. Res. 47, 146–153. doi: 10.1080/09712119.2019.1583571
Souza F., Malheiros E. B., and Carneiro P. R. O. (2014). Positioning and number of nutritional levels in dose-response trials to estimate the optimal-level and the adjustment of the models. Cienc. Rural. 44, 1204–1209. doi: 10.1590/0103-8478cr20130694
Swain B. and Johri T. (2000). Effect of supplemental methionine, choline and their combinations on the performance and immune response of broilers. Bri. Poult. Sci. 41, 83–88. doi: 10.1080/00071660086457
Thiam M., Wang Q., Barreto Sánchez A. L., Zhang J., Ding J., Wang H., et al. (2022). Heterophil/Lymphocyte ratio level modulates salmonella resistance, cecal microbiota composition and functional capacity in infected chicken. Front. Immunol. 13, 816689. doi: 10.3389/fimmu.2022.816689
Tian D., Guo R., Li Y., Chen P., Zi B., Wang J., et al. (2019). Effects of lysine deficiency or excess on growth and the expression of lipid metabolism genes in slow-growing broilers. Poul. Sci. 98, 2927–2932. doi: 10.3382/ps/pez041
Vaezi G., Teshfam M., Bahadoran S., Farazyan H., and Hosseini S. (2011). Effects of different levels of lysine on small intestinal villous morphology in starter diet of broiler chickens. Glob. Vet. 7, 523–526.
Vedenov D. and Pesti G. M. (2008). A comparison of methods of fitting several models to nutritional response data. J. Anim. Sci. 86, 500–507. doi: 10.2527/jas.2007-0536
Watanabe G., Kobayashi H., Shibata M., Kubota M., Kadowaki M., and Fujimura S. (2017). Reduction of dietary lysine increases free glutamate content in chicken meat and improves its taste. Anim. Sci. J. 88, 300–305. doi: 10.1111/asj.12577
Wickramasuriya S., Yi Y., Yoo J., Kim J., Heo K., and Heo J. (2016). Lysine requirements of Korean native ducklings for three weeks after hatch. J. Appl. Poult. Res. 25, 464–473. doi: 10.3382/japr/pfw019
Wu B.-y., Cui H.-m., Peng X., Fang J., Cui W., and Liu X. (2013). Pathology of bursae of Fabricius in methionine-deficient broiler chickens. Nutrients 5, 877–886. doi: 10.3390/nu5030877
Wu B.-y., Cui H.-m., Xi P., Jing F., Wei C., and Liu X.-d. (2012). Effect of methionine deficiency on the thymus and the subsets and proliferation of peripheral blood T-cell, and serum IL-2 contents in broilers. J. Integr. Agric. 11, 1009–1019. doi: 10.1016/S1671-2927(00)8625
Wu Y., Feng Y., Cao J., Jiang Y., Wang Q., Hou S., et al. (2024). Dietary crude protein reduction with addition of crystalline amino acids in growing pekin ducks housed in cascading cages: influence on growth performance, carcass traits, and apparent nutrient digestibility. Agriculture 14, 1102. doi: 10.3390/agriculture14071102
Xie M., Guo Y., Zhang T., Hou S., and Huang W. (2009). Lysine requirement of male white pekin ducklings from seven to twenty-one days of age. Asian-Australas. J. Anim. Sci. 22, 1386–1390. doi: 10.5713/ajas.2009.90142
Xing Y., Wang S., Fan J., Oso A., Kim S., Xiao D., et al. (2015). Effects of dietary supplementation with lysine-yielding Bacillus subtilis on gut morphology, cecal microflora, and intestinal immune response of Linwu ducks. J. Anim. Sci. 93, 3449–3457. doi: 10.2527/jas.2014-8090
Xu Z., Wang M., Mao H., Zhan X., and Hu C. (2003). Effects of L-carnitine on growth performance, carcass composition, and metabolism of lipids in male broilers. Poult. Sci. 82, 408–413. doi: 10.1093/ps/82.3.408
Yu M., Kim Y. B., Cho H. M., Hong J. S., Nawarathne S. R., Oketch E. O., et al. (2024). Standardized ileal digestible lysine requirements based on growth performance of White Pekin ducks for 21 days after hatch. J. Anim. Sci. Technol. 67 (2), 383–392. doi: 10.5187/jast.2024.e25
Zhang H., Piao X., Zhang Q., Li P., Yi J., Liu J., et al. (2013). The effects of Forsythia suspensa extract and berberine on growth performance, immunity, antioxidant activities, and intestinal microbiota in broilers under high stocking density. Poult. Sci. 92, 1981–1988. doi: 10.3382/ps.2013-03081
Keywords: L-Lysine, native duck of Gilan (mallard), performance, Biochemical parameters, carcass traits, intestinal morphology
Citation: Ghorab MJ, Shahryar HA, Ebrahimnezhad Y, Farahvash T, Vahdatpour T and Ghorbani A (2025) Evaluation of the required level of l-lysine amino acid in the diet of Gilan native ducks up to the age of 7 weeks. Front. Anim. Sci. 6:1500706. doi: 10.3389/fanim.2025.1500706
Received: 23 September 2024; Accepted: 07 July 2025;
Published: 18 September 2025.
Edited by:
Massimo Bionaz, Oregon State University, United StatesReviewed by:
Mürsel Özdoğan, AydınAdnan Menderes University, TürkiyeYongbao Wu, Chinese Academy of Agricultural Sciences, China
Hector Leyva, UAH, United States
Copyright © 2025 Ghorab, Shahryar, Ebrahimnezhad, Farahvash, Vahdatpour and Ghorbani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: H. Aghdam Shahryar, aGFfc2hhaHJ5YXJAeWFob28uY29t
 M. Jamili Ghorab
M. Jamili Ghorab H. Aghdam Shahryar
H. Aghdam Shahryar Y. Ebrahimnezhad
Y. Ebrahimnezhad T. Farahvash
T. Farahvash T. Vahdatpour
T. Vahdatpour A. Ghorbani
A. Ghorbani