- 1Faculty of Animal Science and Technology, Maejo University, Chiang Mai, Thailand
- 2Tropical Feed Resources Research and Development Center (TROFREC), Faculty of Agriculture, Khon Kaen University, Khon Kaen, Thailand
- 3Faculty of Food and Agricultural Technology, Animal Science, Pibulsongkram Rajabhat University, Phitsanulok, Thailand
This study aimed to investigate the effect of tannin extract from mangosteen peel (MANGTAN) in soybean meal on the in vitro gas production kinetics, degradability, and intestinal digestion of proteins using a three-step in vitro procedure. A completely randomized design (CRD) was used as the experimental design with nine treatments. The dietary treatments involved spraying different amounts of mangosteen peel extract (MANGTAN; mL) onto soybean meal (SBM; gDM) in a mixture, with the ratios being 0:100, 5:100, 10:100, 15:100, 20:100, 25:100, 30:100, 35:100, and 40:100. It was found that MANGTAN did not influence the gas production kinetics (P < 0.05). However, MANGTAN did influence the gas production from the immediately soluble fraction (a), which was found to have the highest gas volume at the level of 15 mL of MANGTAN/100 gDM of soybean meal. In vitro dry matter at 12 h decreased when using 5–40 mL of MANGTAN/100 gDM of soybean meal. Organic matter degradability at 12 and 24 h was reduced when using MANGTAN on soybean meal (P < 0.05). Analysis of crude protein degradability using a three-step in vitro procedure found that increasing the levels of 5 to 40 mL of MANGTAN/100 gDM of soybean meal resulted in reduced crude protein degradation in the rumen (24 h), but the intestinal digestion of proteins was improved. In addition, the levels of 15 mL of MANGTAN/100 gDM of soybean meal and above influenced the decrease in total protein degradation (P < 0.05). Based on the results, using 15 mL of MANGTAN/100 gDM of soybean meal could modify gas production kinetics, increase in vitro degradability, protect ruminal crude protein degradability, and increase intestinal crude protein degradability.
1 Introduction
Feeds are a necessary factor for determining a product’s quality. The protein content in feed consists of rumen undegradable protein (RUP) and rumen degradable protein (RDP). When RDP enters the rumen, it is broken down by microorganisms into peptides, ammonia, and amino acids. RDPs are found in raw materials, such as fish meal and soybeans, while RUP or bypass protein is very important in animal production as it can serve as an amino acid in ruminants. Currently, the use of bypass protein in the ruminant industry is popular in animals that require high energy and protein, such as dairy cows that produce high milk yields. Tannins can increase the RUP amount in the diet. When it is combined with high-quality proteins, it can improve nitrogen utilization and milk yield by increasing the passage of amino acids to the small intestine. The production of bypass proteins is a process in which the physical or chemical properties of proteins change, such as extruded soybeans, soybean meal (SBM) treated at 140°C, soybean meal treated with formaldehyde, and the mechanism of the tannin–protein complex. Tannins are secondary compounds found mainly in tropical plants such as cassava leaves and acacia; in particular, plants with an astringent flavor are high in tannins, such as mangosteen peels. Mangosteen is a source of phenolic compounds. The important phenolic compounds that are found in mangosteen are xanthones, tannins, and proanthocyanins. Tannins are polyphenols that have been used as feed additives to manipulate rumen fermentation and microbiota (Charoenphun et al., 2020). Foiklang et al. (2016b) revealed that using mangosteen peel powder as a feed additive for dairy steers did not affect feed intake but can increase nutrient degradability and increase the microbial population and rumen fermentation in dairy steers. Bouchard et al. (2015) reported that dietary tannin concentrations between 20 and 40 gCT/kgDM resulted in rumen methane production, and Bueno et al. (2015) reported that tannin deficiency affected methane release and phenolic compounds can inhibit methanogenic processes both in vitro and in vivo, with the efficacy largely dependent on the type, concentration, and source of tannin condensate. Patra and Saxena (2010) reported that rumen methane concentrations were decreased with tannin supplementation. Tannins bind with proteins/enzymes in the rumen and reduce protein degradation, and if included in small concentrations in supplements, they can increase the flexibility of urea inclusions (Martello et al., 2020). The inclusion of hydrolyzed tannins reduced the total short-chain fatty acid concentration. Within a dose range of 10 to 40 g/kg of a condensed tannin extract, the total short-chain fatty acid concentration was reduced by 0.804 mM in the rumen fluid (Brutti et al., 2023). Moreover, Phesatcha et al. (2021) reported that when dairy cows received mangosteen peel liquid-protected soybean meal (MPLP-SBM), milk composition was increased in terms of 3.5% FCM (fat-corrected milk) and milk protein, which could be due to the condensed tannins and soybean meal creating a tannin–protein complex bond to protect the digestion of protein in the rumen, resulting in moving the bypass protein to the duodenum, where cows receive amino acids from the digestion of bypass protein and use it to produce milk.
The digestion of proteins in ruminants is different from that of other animals, particularly in protein degradation in the rumen. Protein and non-protein nitrogen compounds in feed, when entering the body through the mouth, are mixed with saliva in the stomach. They are swallowed via the esophagus and transported to the rumen for digestion. Approximately 70% of proteins are digested by proteolytic microorganisms. It has been found that more than 40% of the total microbial population in the rumen can digest proteins, especially bacteria, with numbers up to 1010–11 cells/mL. The proteolytic bacteria, namely Bacteroides spp., Butyrivibrio spp., and Selenomonas spp., produce protease enzymes to digest proteins and nitrogen compounds through the hydrolysis process, while some protozoa also digest proteins, such as Entodinium and Eudiplodinium. The products of protein digestion by microbiota are peptides and amino acids. The remaining 30% of proteins avoid digestion and bypass the small intestine, and are thus named RUPs (Wanapat, 2015). How much of the protein in the feed is absorbed in the small intestine? This question can be answered using a three-step in vitro method, simulating the degradability of proteins in the rumen, abomasum, and small intestine according to the method of Gargallo et al. (2006). Therefore, the current study aimed to study the process of extracting tannins from mangosteen peels and examining the effect of this mangosteen peel extract supplementation on in vitro gas production kinetics, degradability, and intestinal protein digestion using a three-step in vitro procedure.
2 Materials and methods
All the procedures involving animals providing rumen fluid collection were approved by the ethics committee of Maejo University, following the guidelines of the Institutional Animal Care and Use Committee of Maejo University, Chiang Mai, Thailand (Approval No. MACUC 006A/2567).
2.1 Experimental design and dietary treatments
The experimental design was a completely randomized design. The dietary treatments were mangosteen peel extract (MANGTAN; mL) sprayed on soybean meal (gDM) in a concentrate mixture at ratios of 0:100, 5:100, 10:100, 15:100, 20:100, 25:100, 30:100, 35:100, and 40:100, respectively. Mangosteen peel was bought from the local market in Chiang Mai Province, Thailand. Samples of the dietary treatment were dried at 60°C for 72 h, ground to pass a 1-mm sieve, and used for further analysis. The tannin extraction method involves soaking the solution mixed with water and ethanol, modified from the method of Im et al. (2017). The steps were as follows: 1) Tannin extract preparation used a solution of 50% ethanol as a solvent. 2) The mangosteen peel powder was soaked in the extract solution using the ratio of mangosteen peel powder to extract solution of 1:50, soaking for 24 hours at room temperature. The resulting extract was called MANGTAN. 3) The amount of MANGTAN was then analyzed by adapting the methods of Hou et al. (2003) and Ye et al. (1999). Thus, 1 mL of the MANGTAN sample was put into a test tube, and 1 mL of 1N Folin-Ciocalteu reagent and 1 mL of 7.5% sodium carbonate (Na2CO3) were added, mixed well, and left at room temperature for 1 hour. The amount of tannin compounds in the extract was calculated by comparing it with the standard curve of gallic acid at 0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, and 4 mL. The absorbance was measured at a wavelength of 745 nm using a UV-vis spectrophotometer (Benassi et al., 2008). A Biochrom Libra 5516v2.02 spectrophotometer was used. The average total tannin content was detected at 31.69 ppm/mL, which is 0.03169 mg/mL or 0.003169%w/v. The dry matter (DM), ash, and crude protein (CP) of all the dietary samples were analyzed using the procedures of the AOAC Official Method 950.02 Animal Feed, 2023, and neutral detergent fiber (NDF) and acid detergent fiber were analyzed according to Van Soest et al. (1991). The ingredients and chemical compositions of the concentrate and rice straw used in the in vitro experiment are shown in Table 1.
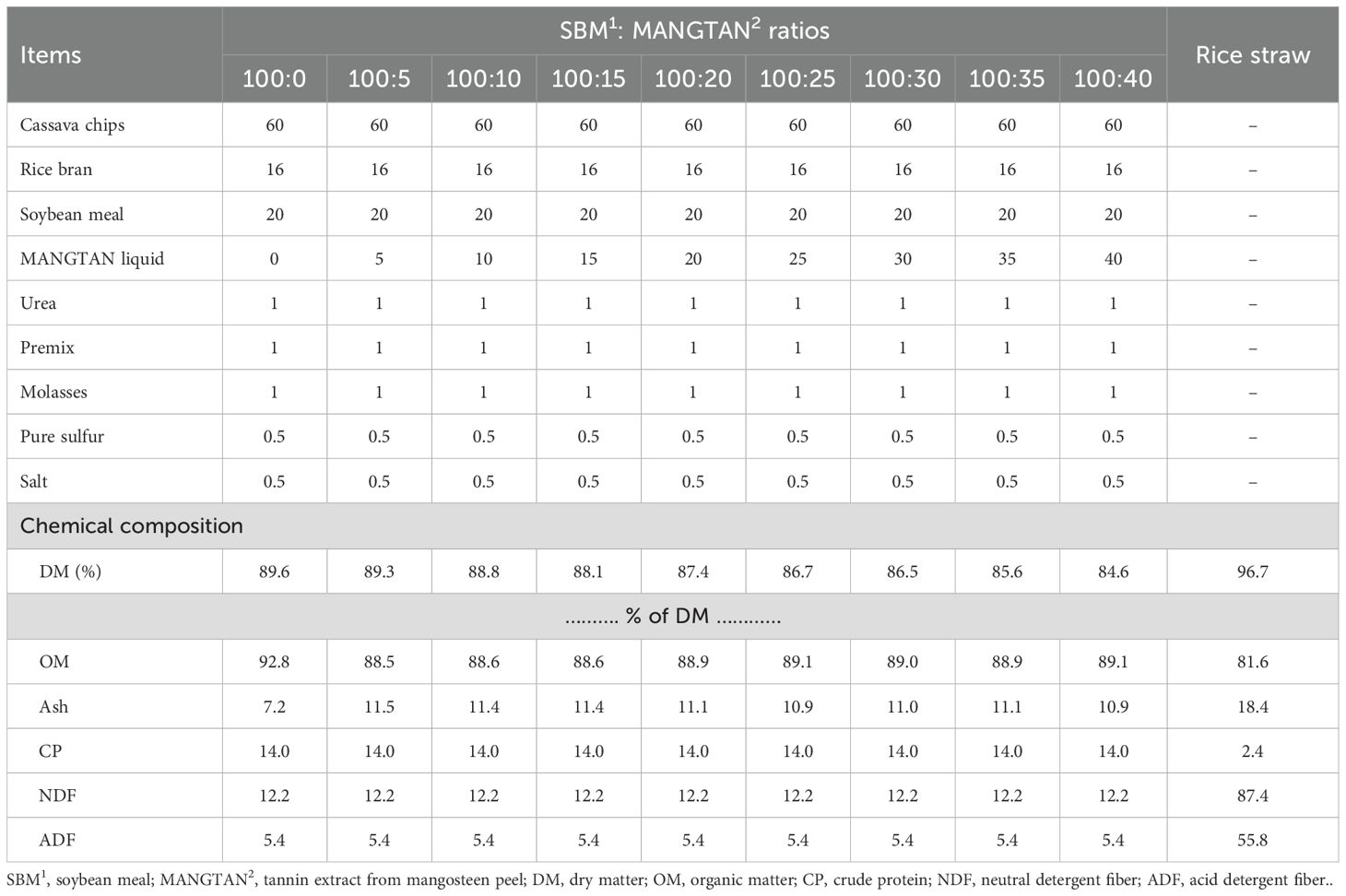
Table 1. Ingredients and chemical composition of the concentrates and rice straw used in the experiment (kg dry matter, DM).
2.2 Rumen inoculum preparation
Rumen fluid was collected from two rumen-fistulated beef cattle with an average body weight of 230 ± 30 kg and an age of 4 years that were fed with concentrate feed at 0.5% BW and ad libitum rice straw. The animals were kept in individual pens, and clean, fresh water and mineral blocks were offered with free access. The animals received the diets for the adjusting period of 14 days before the rumen fluid was collected. On day 14, 1,000 mL of rumen fluid was obtained from each animal before the morning feeding. The rumen fluid was filtered through four layers of cheesecloth into pre-warmed thermoflasks and then transported to the laboratory.
The medium preparation per run (2,911 mL) was described by Makkar et al. (1995). The reduced medium (2,251 mL) consists of 1,095 mL of distilled water, 730 mL of rumen buffer solution, 365 mL of macro-mineral solution, 0.23 mL of micromineral solution, 1 mL of resazurine, and 60 mL of freshly prepared reduction solution. Then, 660 mL of rumen fluid from beef cattle was mixed with the reduced medium as described by Makkar et al. (1995). The mixture was continuously stirred under CO2 at 39°C using a magnetic stirrer fitted with a hot plate under continuous flushing with CO2. A portion (40 mL) of the rumen fluid medium was transferred into each bottle and incubated in an incubator at 39°C.
2.3 In vitro rumen incubation, gas production kinetics, and degradability
Samples of the experimental 0.5 g of rice straw and concentrate mixture ratios (60:40) were weighed into 50-mL serum bottles. Each incubation was performed in triplicate with three blanks. Ruminal fluid from each animal was mixed with the artificial saliva solution of Menke (1988) at 39°C under continuous flushing with CO2. Bottles were sealed with rubber stoppers and aluminum caps and incubated at 39°C (120 h) for an in vitro gas test. The bottles were gently mixed 30 min after starting the incubation and then mixed three times every 3 h. For each sampling time, three bottles containing only the rumen inoculum were included within each run, and the mean gas production values of these bottles were used as blanks. The blank values were subtracted from each measured value to give the net gas production. Gas production kinetics during the incubation and the gas production were measured immediately after incubation at 0, 0.5, 1, 2, 4, 6, 8, 12, 18, 24, 48, 72, and 96 h according to the method of Foiklang et al. (2016a). A glass syringe fitted with an 18-gauge needle was used to punch a hole in the rubber stopper on the fermentation bottles, which were then placed in the incubator. We then noted that the gas volume was growing and used the fit curve program to explain the kinetics of the gas production. Cumulative gas production data were fitted to the model of Ørskov and McDonald (1979) as follows: y = a + b (1–e(-ct)); where a = the gas production from the immediately soluble fraction, b = the gas production from the insoluble fraction, c = the gas production rate constant for the insoluble fraction (b), t = incubation time, (a + b) = the potential extent of gas production, and y = gas produced at time ‘t’.
After 0 h and 4 h of incubation, serum bottles were removed from the incubator and the pH immediately measured using a HANNA pH meter (HANNA Instruments, Nusfalau, Romania). Then, the fermentation liquid was filtered through four layers of cheesecloth and was used for NH3-N and volatile fatty acid (VFA) concentration analysis. The samples were centrifuged for 15 min at 16,000× g, and then the supernatant was collected and kept at -20°C until analysis. The concentration of NH3-N was analyzed using the micro-Kjeldahl method (AOAC Official Method 950.02 Animal Feed, 2023), and VFA concentration, including acetic acid (C2), propionic acid (C3), and butyric acid (C4), was measured using gas chromatography (GC; Shimadzu, Model: GC-2014, Kyoto, Japan) according to So et al. (2022).
In vitro degradability was determined after the termination of incubation at 12 and 24 h. Each treatment was immediately refrigerated at -20°C to stop microbial activity and wait for subsequent digestion analysis. We then removed the samples from the freezer and allowed them to thaw at room temperature before analyzing. The samples were filtered through Whatman filter paper No. 4 and rinsed with distilled water. We placed the residual samples in the crucible and baked them at 100°C for 2 h. The residual DM was estimated. We then calculated the in vitro dry matter degradability (IVDMD) using the following equation: IVDMD (%) = {[(RS100 - C) - (RB100 - C)]/WS} · 100, where RS100 is the weight of the crucible and the residue after drying at 100°C, RB100 is the weight of the crucible and the chemical reagent residue after drying at 100°C (blank), C is the weight of the dried crucible, and WS is the DM weight of the sample (before incubation). The dried feed sample and residue left above were ashed at 600°C for 4 h and determined for in vitro organic matter degradability (IVOMD) (Tilley and Terry, 1963).
2.4 The three-step in vitro procedure
A three-step in vitro procedure was used to determine the intestinal digestion of proteins using the protocol of the Daisy II procedure (Gargallo et al., 2006) as follows: First step: Weigh approximately 5 g of feed (ground through a 2-mm screen) into 5 × 10 cm nylon bags (AnkomR510, pore size 50 µm; Ankom, Fairport, NY) and suspend them in the rumen for 12 h. After ruminal incubation, rinse the bags for 5 min three times in an automatic washing machine. Allow them to drain and dry in an oven at 55°C for 48 h. Pool the residues from the bags, combine them with feedstuffs, and determine the N content using the Kjeldahl method (AOAC Official Method 950.02 Animal Feed, 2023). Second step: Weigh 0.5 to 5 g of rumen-exposed residue into nylon bags and heat-seal. Place up to 30 bags in each incubation bottle in a Daisy II incubator (Ankom). Add 2 L of a prewarmed 0.1 N HCl solution (pH 1.9) containing 1 g/L of pepsin (P-7000, Sigma, St. Louis, MO) and incubate with constant rotation at 39°C for 1 h. After incubation, drain all the liquid and rinse the bags with tap water until the runoff is clear. Third step: Introduce the bags into the incubation bottles (a maximum of 30 bags per bottle) and add 2 L of a prewarmed pancreatin solution (0.5 M KH2PO4 buffer, pH 7.75, containing 50 ppm of thymol and 3 g/L of pancreatin; Sigma P-7545). Incubate the bags for 24 h with constant rotation at 39°C. After incubation, drain all the liquid and rinse the bags with tap water until the runoff is clear. Allow the bags to drain and dry in an oven at 55°C for 48 h. Record the dry weight. Analyze the N content of the residue in all the bags using the Kjeldahl method (AOAC Official Method 950.02 Animal Feed, 2023). Calculate the pepsin–pancreatin digestion of protein as the amount of the sample N (rumen-exposed residue) minus the N remaining after pepsin–pancreatin incubation, divided by the amount of sample N.
2.5 Statistical analysis
Triplicate in vitro data were pooled per substrate. The triplicate results for each substrate per run were averaged before the statistical analysis. The experimental unit for the in vitro measurements was the value of the average data (fermentation bottles). All data were statistically analyzed by analysis of variance (ANOVA) using PROC GLM, and the mean difference of the experimental group was compared using Duncan’s new multiple range test (Steel and Torrie, 1960) in the SAS Institute (2016). Data for the in vitro experiment were analyzed for each MANGTAN level in a complete randomized design (CRD), with the concentration of MANGTAN as the fixed effect and the run as the random effect. Differences among means with P < 0.05 were regarded as statistically significant differences.
3 Results and discussion
3.1 In vitro gas production kinetics and degradability
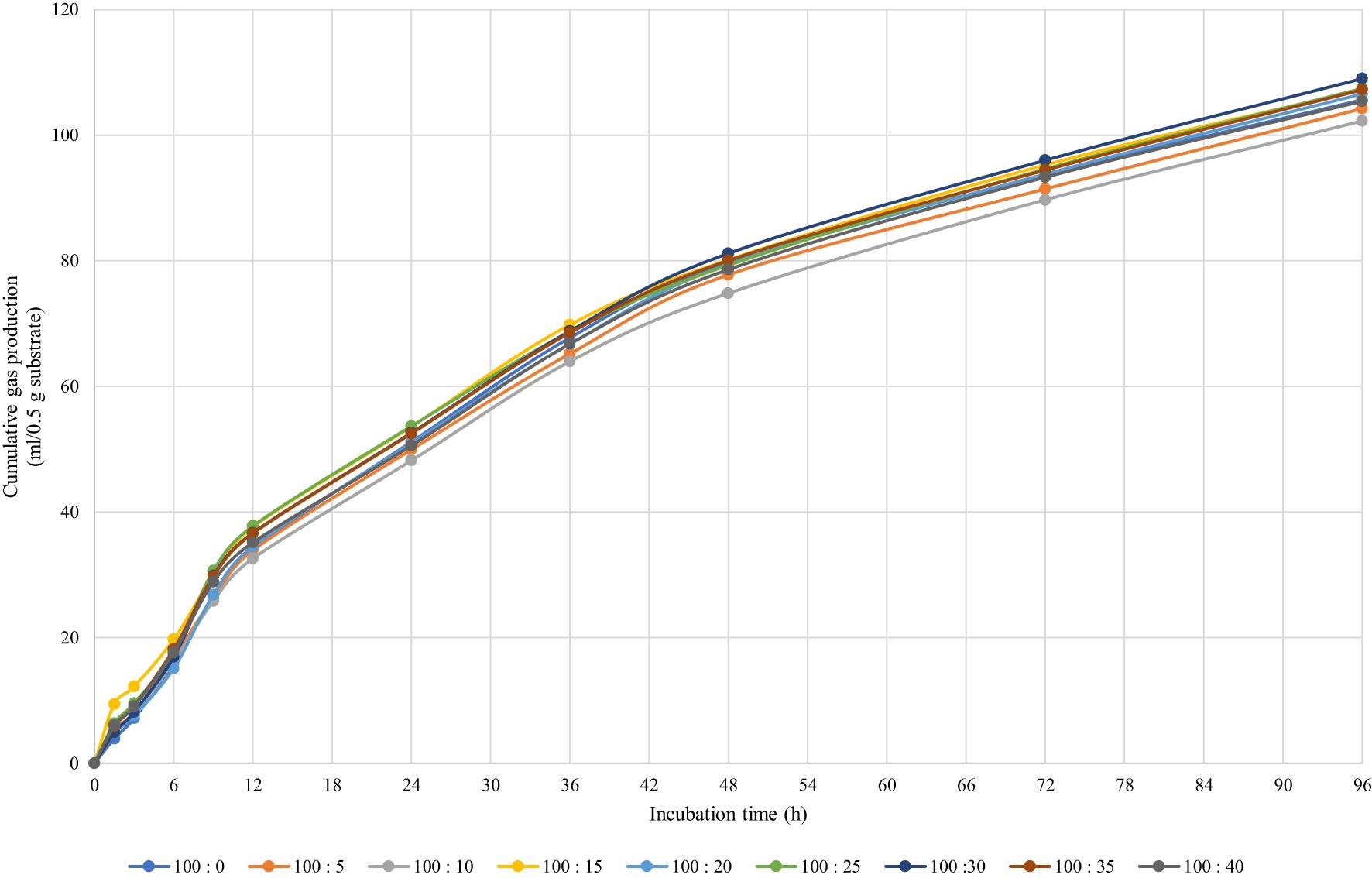
Table 2 shows the values obtained from the kinetics of the gas production models for the substrates studied. It was found that gas production from soluble fractions (a) ranged from 1.35 to 6.73 mL/0.5 g, and supplementation with 20 mL of MANGTAN/100 gDM of soybean meal did not affect gas production compared to the control group (P < 0.05). The gas production from the insoluble fraction (b) and the potential extent of gas production (a+b) were also not altered when increasing the level of MANGTAN in soybean meal (P > 0.05). The gas production rate constants for the insoluble fraction (c) ranged from 0.021 to 0.023 mL/0.5 g, which was similar among treatments (P > 0.05). Cumulative gas production (at 96 h of incubation) ranged from 109.2 to 117.3 mL/0.5g, which was similar when comparing MANGTAN in soybean meal and the control group (Figure 1). The in vitro gas production kinetics is an indication of the total amount of gas produced. The majority of the gas is VFAs, which are gases mainly produced from the degradation of carbohydrates. To know how much protein in feed is degradable, it must be analyzed by the three-step in vitro method. Chamadia et al. (2020) reported the effect of in vitro gas production and digestibility levels of tannic acid on the protein protection of soybean meal. Soybean meal was treated with different levels of tannic acid, i.e., 1%, 2%, 3%, and 4%, and it was found that gas production kinetics did not vary significantly. Phesatcha et al. (2021) investigated the use of liquid-containing tannin in dairy cows as a dietary additive to reduce rumen protein degradation. They reported that when supplemented with MPLP-SBM, methane content was reduced because the condensation tannins inhibited the methanogens attached to the protozoa. Norrapoke et al. (2012) reported that mangosteen peel powder supplementation in dairy cows’ diets reduced methanogens and methane production. Shokryzadan et al. (2016) found that when mangosteen peel powder was mixed with concentrated alfalfa grass, it decreased methane yields.
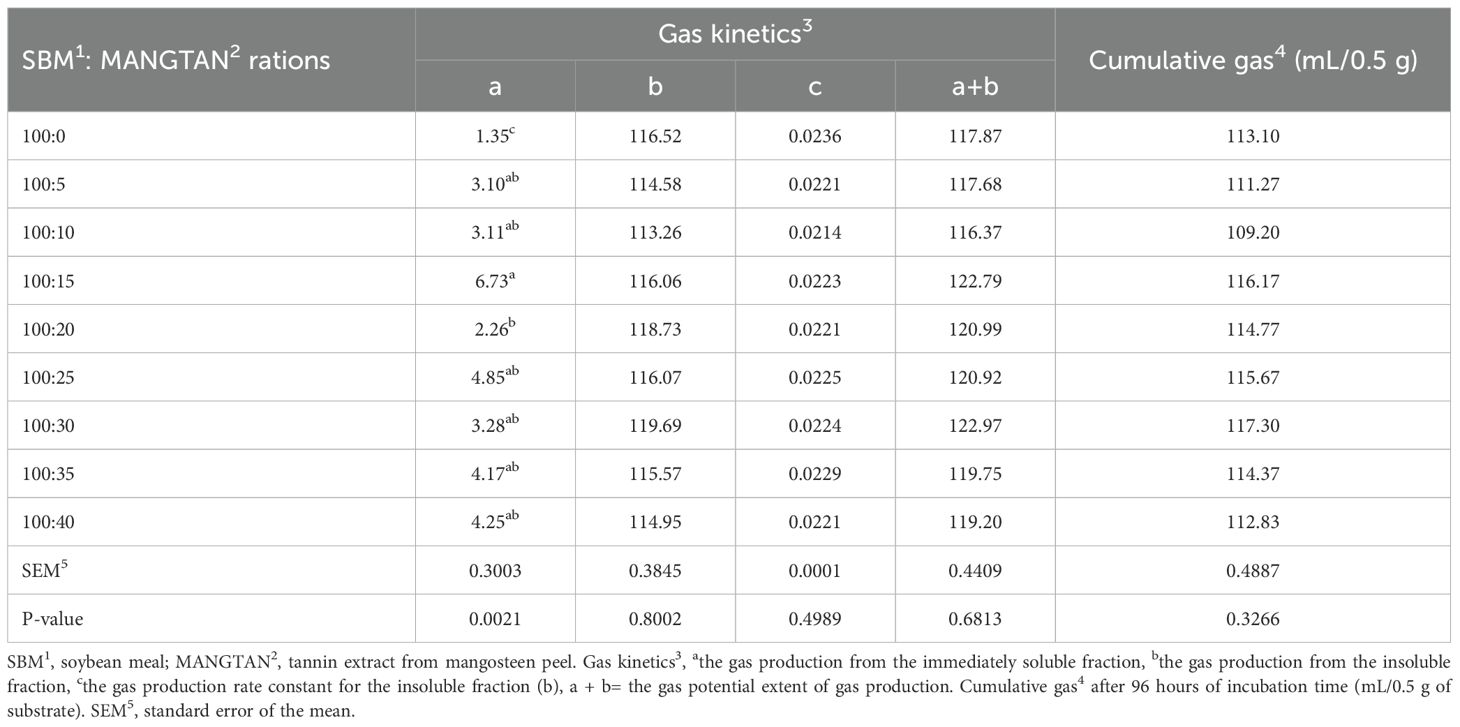
Table 2. Effect of mangosteen peel extract supplementation on in vitro gas production kinetics and cumulative gas production at 96 h after incubation.
The in vitro dry matter digestibility (IVDMD) and in vitro true dry matter digestibility (IVTDMD) values at 12 and 24 h in treatments supplemented with MANGTAN 25 mL/100 gDM of soybean meal were 75.59% and 80.48% higher than all the supplement levels and tended to decrease with increasing MANGTAN supplementation levels. IVOMD at 12 and 24 hours in the control group (without MANGTAN) showed digestibility values that were 76.23% and 80.24% higher than all the supplement levels, as shown in Table 3, and tended to decrease with increasing MANGTAN supplementation levels. From the experimental results, when the level of MANGTAN in soybean meal was increased, the degradability in the rumen decreased, which was as expected, as the application of tannins on soybean meal can protect protein degradation in the rumen. This result was similar to the experiment of Phesatcha et al. (2021), where tannins were extracted by soaking the mangosteen peel and spraying the liquid on the soybean meal, which was reported to have reduced digestibility when supplemented with larger amounts of condensed tannins. It also resulted in reduced protein digestibility when the MPLP level was increased. When supplementing with further increased MANGTAN levels, tannin toxicity may result. When animals are fed excessive amounts of tannins, they inhibit the production of digestive enzymes in the abomasum. Besharati et al. (2021) investigated the beneficial effect of monensin, tannic acid, and cinnamon essential oil supplementation on sesame meal degradability using the three-step in vitro method. The treatments with monensin, tannic acid, and cinnamon essential oil showed the highest rate of organic matter degradability in the total tract.
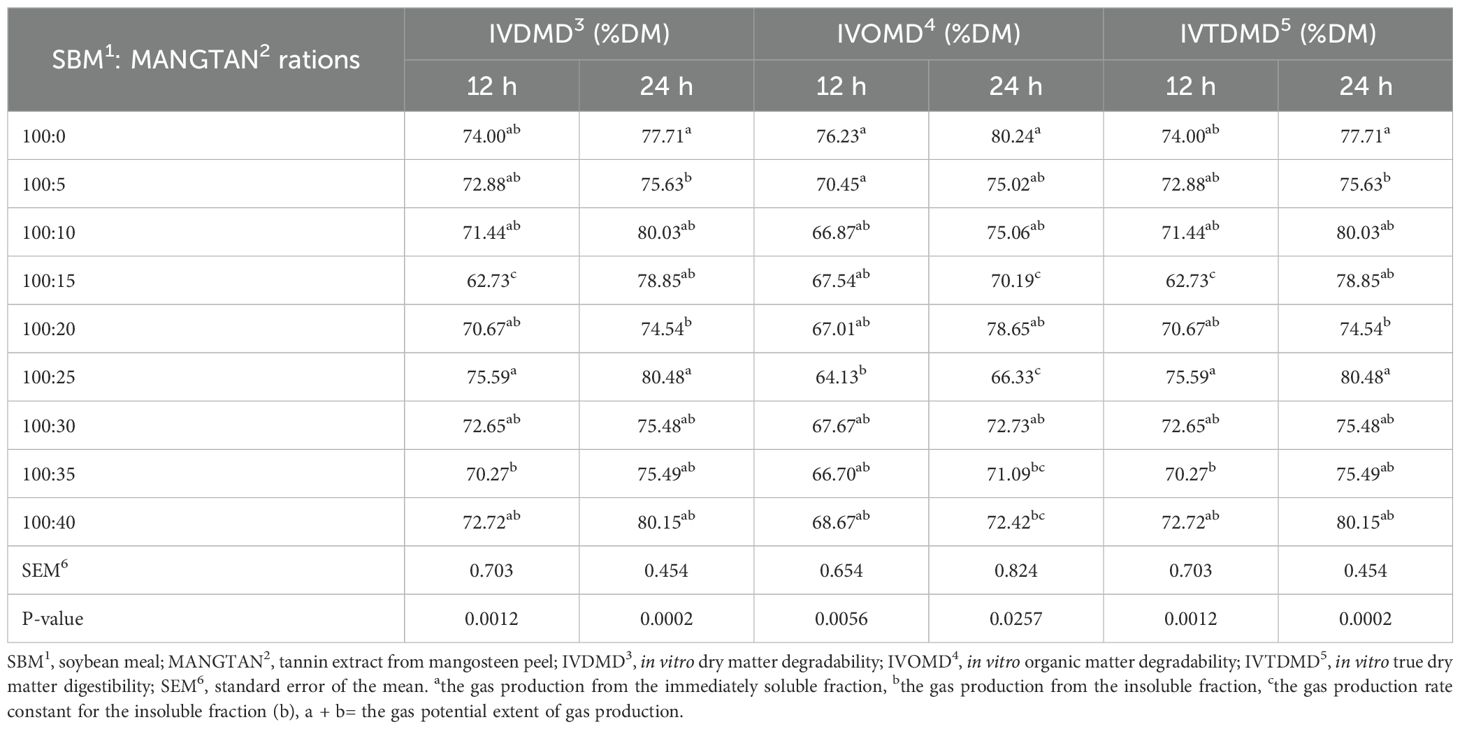
Table 3. Effect of mangosteen peel extract supplementation on the in vitro degradability of dry matter and organic matter, and in vitro true dry matter digestibility.
3.2 Three-step in vitro procedure to determine the intestinal digestion of proteins
In this method, the substrates are sprayed with MANGTAN on the SMB only, with no other feed ingredients. Table 4 shows the in vitro dry matter degradability values obtained from the rumen models for the substrates studied. It was found that the in vitro dry matter degradability and degradability of crude protein at 24 h incubation (rumen) decreased when supplemented with 5 to 40 mL of MANGTAN/100 gDM of soybean meal.
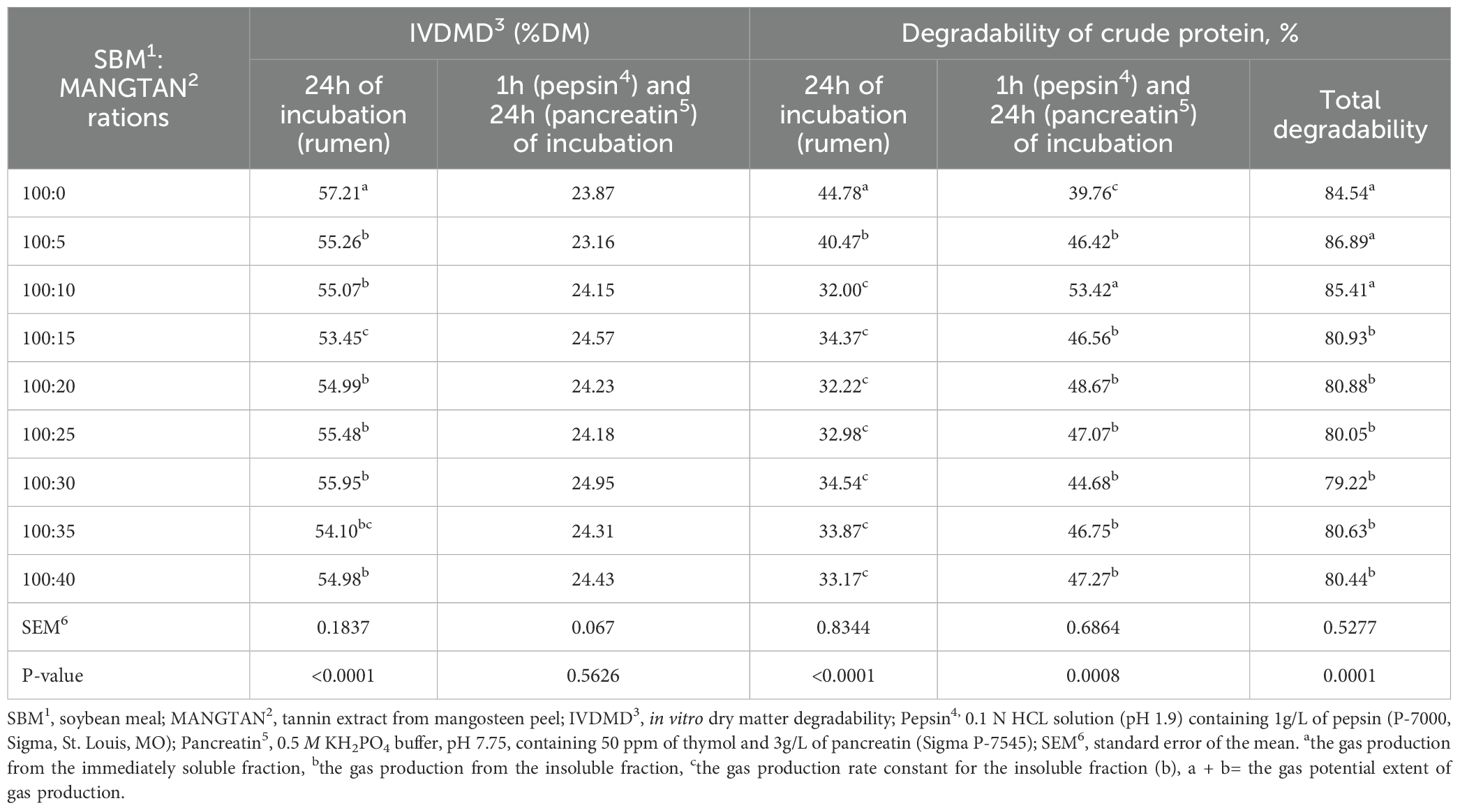
Table 4. Effect of mangosteen peel extract supplementation on intestinal digestion of proteins using a three-step in vitro procedure.
(P < 0.0001). Furthermore, after incubation at 1 h in pepsin and 24 h in pancreatin, the degradability of crude protein increased when supplemented with 5 to 40 mL of MANGTAN/100 gDM of soybean meal (P < 0.001). From the experimental results, MANGTAN was found to protect against protein degradation by microorganisms in the rumen. Because the degradability of crude protein in the rumen decreased, the amount of rumen undegradable protein increased, and this increased the crude protein degradation (RUP) by pepsin from the abomasum and pancreatin from the small intestine. Loregian et al. (2023) reported the effects of incorporating varying levels of tannins into three protein sources (cottonseed, peanut, and soybean meals) on ruminal kinetic parameters, ruminal fermentation, and intestinal digestibility by three in situ experiment methods. They found that the effects of tannin treatment in soybean meal at 20 g/kg increased the proportion of RUP and decreased the dry matter degradability and intestinal degradability. Tannin treatment in peanut meal at 20 to 60 g/kg did not affect dry matter degradability and intestinal degradability, but tannin treatment at 60 g/kg increased the proportion of RUP. The increase in RUP implies a decrease in the quantity of substrates accessible for microbial fermentation within the ruminal environment (Chamadia et al., 2020). Thus, we hypothesized that tannin treatment could increase the RUP content of the tested feeds without impacting the intestinal degradability of protein. In fact, the MANNGTAN treatment in soybean meal indicates that the protein–tannin complexes were sufficiently robust to shield the protein from rumen fermentation but not strong enough to prevent their breakdown after passing through the rumen. The complexes formed for feed protection between proteins and tannins should be reversible when exposed to pH values lower than the ruminal pH, such as those found in the abomasum and small intestine (Verma et al., 2021). Thus, the application of MANGTAN on soybean meal can be used as a bypass protein. Besharati et al.’s (2021) study investigated the beneficial effect of monensin, tannic acid, and cinnamon essential oil supplementation on sesame meal degradability using the three-step in vitro method. The effect of experimental additives on the degradability of sesame meal in the rumen, after the rumen, and in the whole gastrointestinal tract was significant. The in vitro ruminal and intestinal digestibility of sesame meal crude protein with experimental additives was in the range of 76% to 84% and 49% to 60%, respectively. The intestinal degradability of crude protein increased with the addition of tannic acid (approximately 10%). The addition of monensin, tannic acid, and cinnamon essential oil significantly increased the degradability of NDF and ADF in the rumen, intestines, and the whole gastrointestinal tract. The results showed that the addition of tannic acid (100 mg/L) decreased the disappearance of crude protein in the rumen, while it increased crude protein degradation after the rumen. Caroprese et al. (2020) reported that tannic acid has successfully been used to reduce methane production, ruminal protein decomposition, and ammonia production and increase ruminal protein for further digestion in the gut, improving ruminal fermentation efficiency.
3.3 Ruminal fermentation end-product parameters
Table 5 shows the values obtained for the in vitro VFAs, methane production, and ammonia-nitrogen (NH3-N) concentration. The ammonia nitrogen concentration values in bottled serum decreased when supplemented with 15 to 40 mL of MANGTAN per 100 g of soybean meal, ranging from 4.06 to 6.51 mg/100 mL, compared to the control group with an ammonia nitrogen concentration of 7.28 mg/100 mL (P<0.0001). Similarly, Mohammadabadi and Chaji (2012) reported the mean values of NH3-N concentrations were 31.6, 19.2, 15.3, 19.5, and 18.2 mg/dL for untreated SBM, SBM treated with 30 g/kg DM oak fruit tannin, oak leaves tannin, pistachio hull tannin, and pistachio leaves tannin, respectively. This shows that the concentration of ammonia nitrogen decreased when tannins were supplemented in soybean meal. Phesatcha et al. (2021) reported that the rumen NH3-N concentration of the dairy cows fed dietary treatments with MPLP-SBM decreased. This could be due to increased microbial protein synthesis that can consequently reduce the NH3-N concentration in the rumen. However, CTs have a high capacity for CP binding in the rumen and would reduce dietary protein loss by ammonia production, thus improving protein utilization (Adejoro et al., 2018). El-Waziry et al. (2013) found that the concentration of ruminal NH3-N decreased with protected SBM. Reducing NH3-N in the rumen means that treated SBM reduced peptide degradation, proteolysis, and amino acid deamination in the rumen. Ampapon et al. (2019) stated that plant phytonutrients such as CT exhibited selective suppression of cellulolytic bacteria in the rumen. Due to the complexation of protein–tannin, MANGTAN supplementation in the diet has beneficial effects, reducing the availability of feed protein for possible ruminal degradation to produce ammonia–nitrogen since tannins are polyphenolic compounds that have a high affinity for protein. Under normal rumen conditions, these active components can form stable rumen complexes and protect dietary proteins from degradation (Zhang et al., 2019). The result of condensed tannin supplementation is less gastric protein degradation, which may reduce rumen ammonia nitrogen (NH3-N) concentrations (Focant et al., 2019). However, Shokryzadan et al. (2016) found that the ammonia production in the rumen fluid samples using 25% and 50% mangosteen peel replacing alfalfa did not affect ammonia production after 24 hours of incubation. Similarly, Bahrami-Yekdangi et al. (2016) observed that the concentration of NH3-N in the diet was unchanged. Thus, it may depend on the amount of tannin concentration used and the source of the tannin.
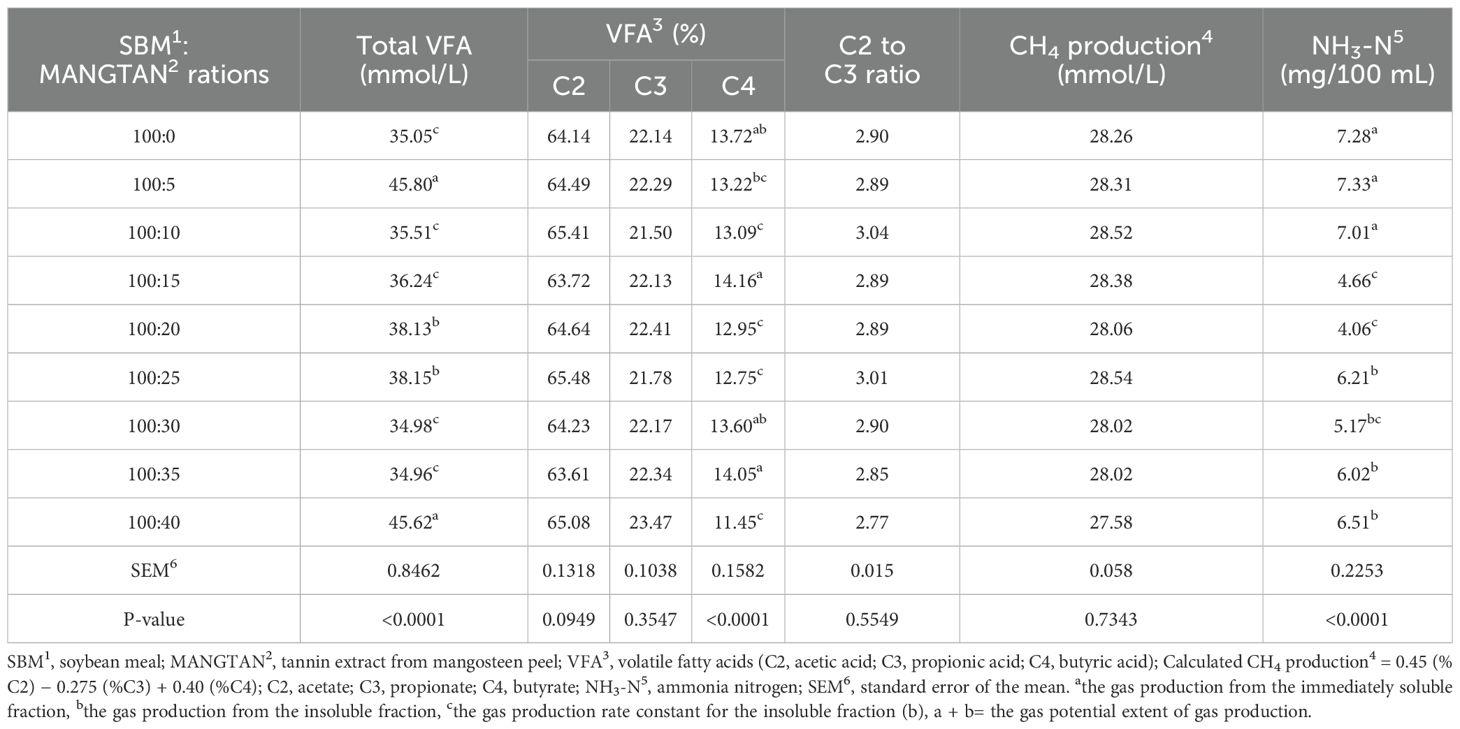
Table 5. Effect of mangosteen peel extract on in vitro volatile fatty acids (VFAs), methane production, and ammonia–nitrogen (NH3-N) concentration.
The proportion of VFAs in the rumen had concentrations of acetic acid (C2) of 63.61%–65.48%, propionic acid (C3) of 21.50%–23.47%, and butyric acid (C4) of 11.45%–14.16%. The molar ratio of C2:C3, C2, and C3 was insignificant (P>0.05) when supplemented with 5 to 40 mL of MANGTAN/100 gDM of soybean meal. These results were similar to those reported by Phesatcha et al. (2021), whose study investigated the use of liquid-containing tannin in dairy cows as a dietary additive to reduce rumen protein degradation. They reported that when supplemented with MPLP-SBM, the proportion of volatile fatty acids in the rumen had a concentration of acetate of 55%–65%, propionate of 20%–25%, and butyrate of 10%–15%. The concentrations of total VFA and C3 were increased by protein level or by MPLP inclusion. Wanapat et al. (2014) reported that the mangosteen peel supplementation in buffaloes impacted total rumen VFA production and increased the C3 concentration. Total volatile fatty acids, acetic acid (C2), and butyric acid were similar between treatments while reducing C2:C3 and CH4 production. Additionally, Polyorach et al. (2016) reported that the use of condensed tannin from mangosteen peel powder (MSP) in dairy cows can increase total VFA concentration, especially C3. Increasing the level of MSP supplement decreases rumen methane production from 27.5 to 23.7 mmol/100 ml3. Moreover, in the present study, CH4 production was not statistically significantly different (p > 0.05) after MANGTAN supplementation, with methane production ranging from 27.58 to 28.54 mmol/L. Poungchompu et al. (2009) revealed that plants containing condensed tannins and saponin greatly improved ruminal feed degradation by mitigating the C2 concentration and CH4 production, enhancing the C3 concentration. The value of methane production was not significantly different (P>0.05) when supplementing with 5 to 40 mL of MANGTAN/100 gDM of soybean meal. Brutti et al. (2023) analyzed the effects of the use of tannins on the ruminal fermentation of cattle from the 27 studies and concluded that tannins affect the concentration of short-chain fatty acids, ammonia nitrogen, dry matter digestibility, and methane production. Tannins associated with balanced protein–energy diets can reduce ruminal protein degradability and methane production, which can benefit animal metabolism and the environment. Many previous studies have stated that condensed tannins and saponin or their extracts are effective in reducing CH4 production in both in vitro and in vivo studies (Abdalla et al., 2012). MANGTAN supplementation resulted in a reduction in NH3-N and CH4 production.
4 Conclusions and recommendations
Based on this experiment, it can be concluded that 15 mL of MANGTAN per 100 gDM of soybean meal can be supplemented in concentrate diets with no negative effect on gas kinetics and rumen fermentation. It reduces in vitro degradability and protein degradability in the rumen and increases protein degradability in the abomasum and small intestine. Therefore, its use in animal feeding contributes to an increase in bypass protein. However, these findings should be applied in further in vivo experiments to estimate animal performance.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
All procedures involving animals providing rumen fluid collection were approved by the ethics committee of Maejo University, with authorization from the Maejo University’s guidelines of the Institutional Animals Care and Use Committee of Maejo University, Chiang Mai, Thailand (Approval No. MACUC 006A/2567). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
CS: Data curation, Methodology, Writing – original draft. SF: Supervision, Writing – review & editing, Conceptualization, Investigation. AC: Supervision, Writing – review & editing. AP: Supervision, Writing – review & editing. NL: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was also supported by the National Research Council of Thailand (NRCT) contract grant NRCT5-RRI63013-P18 (Research and Researcher for Industry, RRI).
Acknowledgments
The authors would like to express our sincere thanks to the laboratory staff, the Faculty of Animal Science and Technology, Maejo University, for the use of their research facilities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdalla A. L., Louvandini H., Sallam S. M. A. H., Bueno I. C. D. S., Tsai S. M., and Figueira A. V. D. O. (2012). In vitro evaluation, in vivo quantification, and microbial diversity studies of nutritional strategies for reducing enteric methane production. Trop. Anim. Health Prod. 44, 953–964. doi: 10.1007/s11250-011-9992-0
Adejoro F. A., Hassen A., and Thantsha M. S. (2018). Preparation of acacia tannin loaded lipid microparticles by solid-in-oil-in-water and melt dispersion methods, their characterization and evaluation of their effect on ruminal gas production in vitro. PloS One 13, e0206241. doi: 10.1371/journal.pone.0206241
Ampapon T., Phesatcha K., and Wanapat M. (2019). Effects of phytonutrients on ruminal fermentation, digestibility, and microorganisms in swamp buffaloes. Animals 9, 671. doi: 10.3390/ani9090671
AOAC Official Method 950.02 Animal Feed (2023). Official Methods of Analysis of AOAC INTERNATIONAL. 22nd Edition. (New York: AOAC Publications). doi: 10.1093/9780197610145.001.0001
Bahrami-Yekdangi M., Ghorbani G. R., Khorvash M., Khan M. A., and Ghaffari M. H. (2016). Reducing crude protein and rumen degradable protein with a constant concentration of rumen undegradable protein in the diet of dairy cows: Production performance, nutrient digestibility, nitrogen efficiency, and blood metabolites. J. Anim. Sci. 94, 718–725. doi: 10.2527/jas.2015-9947
Benassi R., Ferrari E., Lazzari S., Spagnolo F., and Saladini M. (2008). Theoretical study on curcumin: a comparison of calculated spectroscopic properties with NMR, UV–vis and IR experimental data. J. Mol. Struct. 892 (1-3), 168–176. doi: 10.1016/j.molstruc.2008.05.024
Besharati M., Palangi V., Taghizadeh A., Kaya A., and Abachi S. (2021). Determining the effect of natural inhibitors on sesame meal degradability using in vitro three step method. Vet. Arh. 91, 513–521. doi: 10.24099/vet.arhiv.1138
Bouchard K., Wittenberg K. M., Legesse G., Krause D. O., Khafipour E., Buckley K. E., et al. (2015). Comparison of feed intake, body weight gain, enteric methane emission and relative abundance of rumen microbes in steers fed sainfoin and lucerne silages under western C anadian conditions. Grass Forage Sci. 70, 116–129. doi: 10.1111/gfs.12105
Brutti D. D., Canozzi M. E. A., Sartori E. D., Colombatto D., and Barcellos J. O. J. (2023). Effects of the use of tannins on the ruminal fermentation of cattle: A meta-analysis and meta-regression. Anim. Feed. Sci. Technol. 306, 115806. doi: 10.1016/j.anifeedsci.2023.115806
Bueno I. C., Brandi R. A., Franzolin R., Benetel G., Fagundes G. M., Abdalla A. L., et al. (2015). In vitro methane production and tolerance to condensed tannins in five ruminant species. Anim. Feed. Sci. Technol. 205, 1–9. doi: 10.1016/j.anifeedsci.2015.03.008
Caroprese M., Ciliberti M. G., and Albenzio M. (2020). “Application of aromatic plants and their extracts in dairy animals,” in Feed Additives (Academic Press), 261–277. doi: 10.1016/B978-0-12-814700-9.00015-7
Chamadia B., Grewal R. S., Lamba J. S., Kaur J., and Kashyap N. (2020). Effect of varying levels of tannins treatment on in vitro degradability of soybean meal. Int. J. Curr. Microbiol. Appl. Sci. 9, 3991–4000. doi: 10.20546/ijcmas.2020.907.469
Charoenphun N., Setarnawat S., and Sai-Ut S. (2020). Chemical composition and trends in utilization of by-products and wastes from 4 types of tropical fruit processing. TJST 28, 113–128. doi: 10.14456/tstj.2020.10
El-Waziry A. M., AlKoaik F., Khalil A. I., Metwally H., and Al-Mahasneh M. A. (2013). Estimation of degradability kinetics, energy and organic matter digestibility of date palm (Phoenix dactylifera L.) leaves silage by in vitro gas production technique. AJAVA 8, 814–820 ref. 28. doi: 10.3923/ajava.2013.814.820
Focant M., Froidmont E., Archambeau Q., Van Q. D., and Larondelle Y. (2019). The effect of oak tannin (Quercus robur) and hops (Humulus lupulus) on dietary nitrogen efficiency, methane emission, and milk fatty acid composition of dairy cows fed a low-protein diet including linseed. J. Dairy Sci. 102, 1144–1159. doi: 10.3168/jds.2018-15479
Foiklang S., Wanapat M., and Norrapoke T. (2016a). In vitro rumen fermentation and degradability of buffaloes as influenced by grape pomace powder and urea-treated rice straw supplementation. Anim. Sci. J. 87, 370–377. doi: 10.1111/asj.12428
Foiklang S., Wanapat M., and Norrapoke T. (2016b). Effect of grape pomace powder, mangosteen peel powder and monensin on nutrient degradability, rumen fermentation, nitrogen balance and microbial protein synthesis in dairy steers. Asian-Australas. J. Anim. Sci. 29, 1416. doi: 10.5713/ajas.15.0689
Gargallo S., Calsamiglia S., and Ferret A. (2006). A modified three-step in vitro procedure to determine intestinal digestion of proteins. J. Anim. Sci. 84, 2163–2167. doi: 10.2527/jas.2004-704
Hou W. C., Lin R. D., Cheng K. T., Hung Y. T., Cho C. H., Chen C. H., et al. (2003). Free radical-scavenging activity of Taiwanese native plants. Phytomedicine 10, 170–175. doi: 10.1078/094471103321659898
Im A., Kim Y. M., Chin Y. W., and Chae S. (2017). Protective effects of compounds from Garcinia mangostana L.(mangosteen) against UVB damage in HaCaT cells and hairless mice. Int. J. Mol. Med. 40, 1941–1949. doi: 10.3892/ijmm.2017.3188
Loregian K. E., Pereira D. A., Rigon F., Magnani E., Marcondes M. I., Baumel E. A., et al. (2023). Effect of tannin Inclusion on the enhancement of rumen undegradable protein of different protein sources. Ruminants 3, 413–424. doi: 10.3390/ruminants3040034
Makkar H. P. S., Blümmel M., and Becker K. (1995). Formation of complexes between polyvinyl pyrrolidones or polyethylene glycols and tannins, and their implication in gas production and true digestibility in in vitro techniques. Br. J. Nutr. 73, 897–913. doi: 10.1079/BJN19950095
Martello H. F., De Paula N. F., Teobaldo R. W., Zervoudakis J. T., Fonseca M. A., Cabral L. S., et al. (2020). Interaction between tannin and urea on nitrogen utilization by beef cattle grazing during the dry season. Livest. Sci. 234, 103988. doi: 10.1016/j.livsci.2020.103988
Menke K. (1988). Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 28, 7–55. Available at: https://ci.nii.ac.jp/naid/10025840911/ (Accessed March 7, 2025).
Mohammadabadi T. and Chaji M. (2012). The Influence of the plant tannins on in vitro ruminal degradation and improving nutritive value of sunflower meal in ruminants. Pak. Vet. J. 32, 225–228.
Norrapoke T., Wanapat M., and Wanapat S. (2012). Effects of protein level and mangosteen peel pellets (Mago-pel) in concentrate diets on rumen fermentation and milk production in lactating dairy crossbreds. Asian-Australas. J. Anim. Sci. 25, 971. doi: 10.5713/ajas.2012.12053
Ørskov E. R. and McDonald I. (1979). The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 92, 499–503. doi: 10.1017/s0021859600063048
Patra A. K. and Saxena J. (2010). Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 91, 24–37. doi: 10.1002/jsfa.4152
Phesatcha K., Phesatcha B., and Wanapat M. (2021). Mangosteen peel liquid-protected soybean meal can shift rumen microbiome and rumen fermentation end-products in lactating crossbred holstein friesian cows. Front. Vet. Sci. 8. doi: 10.3389/fvets.2021.772043
Polyorach S., Wanapat M., Cherdthong A., and Kang S. (2016). Rumen microorganisms, methane production, and microbial protein synthesis affected by mangosteen peel powder supplement in lactating dairy cows. Trop. Anim. Health Prod. 48, 593–601. doi: 10.1007/s11250-016-1004-y
Poungchompu O., Wanapat M., Wachirapakorn C., Wanapat S., and Cherdthong A. (2009). Manipulation of ruminal fermentation and methane production by dietary saponins and tannins from mangosteen peel and soapberry fruit. Arch. Anim. Nutr. 63, 389–400. doi: 10.1080/17450390903020406
SAS Institute (2016). Statistical Analysis Software (SAS) user’s guide version 9.4 (Cary, NC SAS Institute, Inc. - References - Scientific Research Publishing). Available online at: https://www.scirp.org/reference/referencespapers?referenceid=2877863.
Shokryzadan P., Rajion M. A., Goh Y. M., Ishak I., Ramlee M. F., Jahromi M. F., et al. (2016). Mangosteen peel can reduce methane production and rumen biohydrogenation in vitro. S Afr. J. Anim. Sci. 46, 419–431. doi: 10.4314/sajas.v46i4.10
So S., Cherdthong A., and Wanapat M. (2022). Growth performances, nutrient digestibility, ruminal fermentation and energy partition of Thai native steers fed exclusive rice straw and fermented sugarcane bagasse with Lactobacillus, cellulase and molasses. J. Anim. Physiol. Anim. Nutr. 106, 45–54. doi: 10.1111/jpn.13563
Steel R. G. D. and Torrie J. H. (1960). Principles and procedures of statistics. (with special reference to the Biological Sciences.) (New York, Toronto, London: McGraw-Hill Book Company). doi: 10.1002/bimj.19620040313
Tilley J. M. and Terry R. A. (1963). A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 18, 104–111. doi: 10.1111/j.1365-2494.1963.tb00335.x
Van Soest P. J., Robertson J. B., and Lewis B. A. (1991). Methods for dietary fiber neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Verma S., Taube F., and Malisch C. S. (2021). Examining the variables leading to apparent incongruity between antimethanogenic potential of tannins and their observed effects in ruminants—a review. Sustainability 13, 2743. doi: 10.3390/su13052743
Wanapat M. (2015). Dietary sources and their effects on animal production and Environmental Sustainability’. Anim. Nutr. 1, 96–103. doi: 10.1016/j.aninu.2015.07.004
Wanapat M., Chanthakhoun V., Phesatcha K., and Kang S. (2014). Influence of mangosteen peel powder as a source of plant secondary compounds on rumen microorganisms, volatile fatty acids, methane and microbial protein synthesis in swamp buffaloes. Livest. Sci. 162, 126–133. doi: 10.1016/j.livsci.2014.01.025
Ye S., Lu J., He S., Chen L., and Hu J. (1999). Studies on tannin and hydrolysate in three species of Chinese Caesalpinia plants. Zhongguo Zhong yao za zhi= Zhongguo Zhongyao Zazhi= China J. Chin. Materia Med. 24, 525–527. Available at: https://europepmc.org/article/med/12205895 (Accessed March 7, 2025).
Keywords: tannin extract, mangosteen peel, in vitro degradability, gas production kinetics, three-step in vitro
Citation: Sonthongdaeng C, Foiklang S, Cherdthong A, Paserakung A and Laorodphan N (2025) Effect of mangosteen peel extract (Garcinia mangostana L.) supplementation on the in vitro gas kinetics, degradability, and intestinal digestion of proteins using a three-step in vitro procedure. Front. Anim. Sci. 6:1545531. doi: 10.3389/fanim.2025.1545531
Received: 15 December 2024; Accepted: 02 June 2025;
Published: 08 July 2025.
Edited by:
Yafeng Huang, Anhui Agricultural University, ChinaReviewed by:
Tiurma Pasaribu, National Research and Innovation Agency (BRIN), IndonesiaHeru Sasongko, Sebelas Maret University, Indonesia
Copyright © 2025 Sonthongdaeng, Foiklang, Cherdthong, Paserakung and Laorodphan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suban Foiklang, YnVuZ3VuZ0Bob3RtYWlsLmNvbQ==
 Chutikan Sonthongdaeng
Chutikan Sonthongdaeng Suban Foiklang
Suban Foiklang Anusorn Cherdthong
Anusorn Cherdthong Anon Paserakung1
Anon Paserakung1