- 1Animal and Poultry Science, School of Agricultural, Earth and Environmental Sciences, University of KwaZulu-Natal, Pietermaritzburg, South Africa
- 2Equitable Education and Economies Division, Human Sciences Research Council, Durban, South Africa
- 3Department of Animal Science, University of the Free State, Bloemfontein, South Africa
- 4Department of Agriculture, Faculty of Health and Applied Sciences, Mangosuthu University of Technology, Jacobs, South Africa
Introduction: This study aimed to compare the effects of sex, breed, and age on the body weight and internal organ weight of chickens reared in resource-poor communities. Understanding these factors is important for optimizing poultry production and enhancing food security in such environments.
Methods: A total of 120 chickens were used, comprising three breeds: broilers (n=40), Potchefstroom Koekoek (n=40), and a non-descriptive breed (n=40). Each breed group consisted of 20 males and 20 females, which were further categorized by age into 10 growers and 10 mature chickens per breed. Body weight and internal organ weights (liver, gizzard, heart, intestinal weight, and length) were measured and analyzed to assess the influence of breed, sex, and age.
Results: The results indicated that both grower and mature broilers had the highest body weights compared to Potchefstroom Koekoek and the non-descriptive breed. Breed significantly influenced body weight (p<0.001), and males were generally heavier than females, although this difference was not statistically significant (p>0.05). Liver weight was higher in females, but sex did not significantly affect liver weight. A linear relationship (p<0.05) was observed between body weight and the variables breed, sex, and age. Breed had a negative coefficient with body weight, while sex and age had positive coefficients. Age demonstrated the strongest relationship with both body weight and liver weight. The independent variables (breed, sex, and age) accounted for 64% of the variance in body weight.
Discussion: The findings demonstrate that breed and age are significant determinants of body weight and internal organ weight in chickens reared under resource-poor conditions, whereas sex has a lesser effect. These results highlight the importance of considering breed and age in poultry management strategies to maximize meat and organ yield, thereby contributing to food security. Further research is recommended to explore these relationships in greater depth, with a focus on the potential of chickens in resource-poor communities, regardless of breed, sex, or age.
1 Introduction
The global poultry population is approximately 19.46 billion, of which Africa is responsible for 8.08% (Faostat, 2012). There is a growing global poultry production and rising demand for high-quality protein, particularly in developing countries (Mengesha et al., 2011). From 2000 to 2007, chicken meat production was reported to have increased by 86% from 58.7 to 109.0 million tons (Faostat, 2019). Poultry production, including village chickens in South Africa, has experienced growth due to high demand, consumption, and acceptability. Their products are the most commonly consumed livestock products due to their affordability and accessibility. However, due to increasing population growth, there is a need to utilize underutilized poultry products, such as the internal organs of chickens (Biel et al., 2019) and village chicken products to meet animal protein demands (Sonaiya, 2007; Krawczyk, 2009).
Broiler farming has expanded rapidly compared to village chickens globally to meet the demand for animal protein (Alam et al., 2020). Although South Africa is the largest commercial poultry production country on the African continent, the poultry industry has challenges in maintaining competitiveness (Nkukwana, 2018). When comparing the production costs of village chickens with broilers, it is evident that village chickens are considerably less expensive, as farmers rear them as free-range using organic feed. In addition, there are diverse local chickens grouped according to breeds or ecotypes, but most are unclassified and result in extinction without potential contributions to food and nutrition security.
Village chickens, also known as indigenous or free-range chickens, are typically raised in rural areas under traditional scavenging systems. They are a vital resource for smallholder farmers, providing meat, eggs, and income with minimal input requirements. In contrast, broiler chickens are specifically bred for rapid growth and high meat yield. They dominate commercial poultry farming globally due to their efficiency in converting feed into body weight. Village chicken breeds are commonly used as layers and broilers in developing countries because they have not undergone artificial selection (Besbes, 2009). However, their internal organs are regarded as by-products that are higher in trace elements compared to the muscular tissues (Kandyliari et al., 2021) and they are regarded as slow-growing chickens compared to broilers.
In many developing countries, chickens serve as the primary source of sustenance and income generation. Thus, they are a key entry point for addressing malnutrition, food security, and poverty in resource-poor communities (Mulugeta et al., 2020). Both village chickens and broilers significantly contribute to food and nutrition, providing high-quality and safe agricultural products (Besbes, 2009) such as eggs and meat, for public consumption in these communities. However, according to Chowdhury (2013), consumers prefer the meat and eggs of village chickens because of their good taste and lean meat, but they are more expensive compared to broiler chickens. Moreover, the internal organs of chickens, as a source of animal food, are underutilized, and there is limited research available, particularly in comparison with other poultry.
There is a knowledge gap and limited information on comparing village chickens and broilers reared under a backyard production system in relation to the quantity or yield of chicken products, such as meat and internal organs, with the intention of these products competing in the mainstream market. Hence, Duah et al. (Duah et al., 2020) suggested that village chickens are more widely used in developing countries than commercial chickens, but little research and development is available. Comparing broilers and village chicken breeds may be the ideal model to understand the differences in the quantity of products and to contribute to challenges in Africa, such as poverty, hunger, and food security, and influence various global policies. In these areas, the preference in terms of consumption is a mature female due to cultural mores. The objective of this study was to compare the effect of sex, breed, and age on the body weight and internal organ weights of chickens reared in resource-poor communities. It was hypothesized that sex, breed, and age would have no significant effect on a chicken’s weight and internal organ weight in resource-poor communities.
2 Materials and methods
2.1 Study site, bird management, and data collection
The study complied with the standards required by the Animal Ethics Committee of the University of KwaZulu-Natal (protocol number: AREC/00004967/2022). Chickens (n=120) were purchased from the uMgungundlovu district municipality, KwaZulu-Natal province, South Africa (-29.617°S 30.383°E). Over 50% of the chickens were owned by women, and their age ranged from 20 to 70 years, with an average of 16 years of participation in rural farming. Their major source of income is selling these chickens in the Central Business District, and village chickens were more preferred than broiler chickens. The reasons why the village chickens were preferred were ranked as healthy > organic >long shelf life > tasty > income > low labour > quality of meat > availability > high meat yield. Farmers depended on indigenous knowledge with no formal education. They did not receive any agricultural production training to rear chickens, but were interested in enrolling in poultry training. These chickens were reared under a backyard production system in resource-poor communities where they scavenge for feed resources and broilers. Over 75% of the owners provided housing for the chickens and supplementary feed such as maize, kitchen waste, and commercial feed was provided depending on the availability of funds. Water was provided ad libitum.
The chickens were sexed and aged by visual observation of the comb and size of the wattle development and sickle feathers, which is one of the indigenous knowledge methods. Males possess larger red combs and wattles, and large spurs on the back of the shank, while females often have a small pale pink to yellowish coloration. The males have long, curving sickle feathers, and females exhibit shorter, rounder feathers. They were categorized into groups of 40 per breed (Potchefstroom Koekoek, boilers, and non-descriptive breed), sex (20 females and 20 males), and age (10 growers and 10 mature).
The chickens were weighed with a digital scale for live weight (EZ hang scale,099912 CF). The cervical region was cut using a sharp knife, dislocated, and then bled within 60 seconds by the chicken owners (Ncobela and Chimonyo, 2016). They were categorized by sex, breed, and age, and the harvested internal organs were placed in labeled bags inside a cooler box with ice and then transported to the Animal Science Department Laboratory for analysis (University of KwaZulu-Natal, Pietermaritzburg, South Africa). The liver, gizzard, heart, and intestinal weights were measured with a sensitive digital weighing balance scale (Mettler Toledo, PL203 CE) with an accuracy of 0.001 g. Furthermore, small and large intestinal lengths were measured using a flexible tape measure with an accuracy of 1 mm.
Data were analyzed using SAS 9.4 (2023). A general linear model was used to analyze the effect of breed, sex, and age on body weight and internal organ weight. All data were reported as least square means (LSM) ± standard error means (SEM). The data were expressed as significant differences in mean values, as determined by the Student–Newman–Keuls test (P<0.05) procedure of the statistical analysis system (Sirri et al., 2010). The degree of influence of breed, sex, and age on internal organ weight variables was determined using linear regression and a regression equation. The following linear model was used:
where Y is the response variable (body weight and internal organ weight); β0, β1, and β2 are the regression coefficients; D is the sex, breed, and age; and E is the residual error.
3 Results
4.1 Effect of breed on body weight and internal organs of chickens
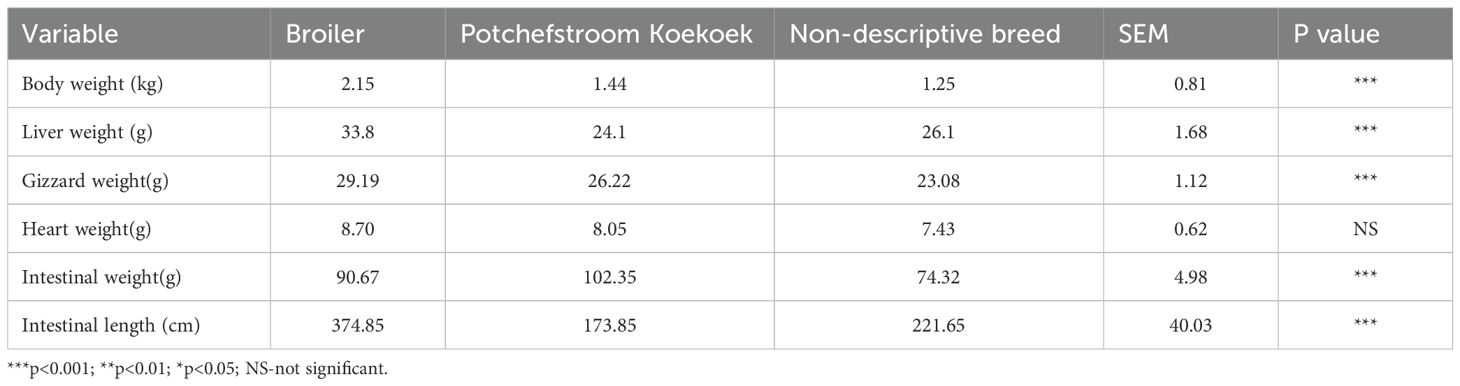
The effect of breed on body weight and internal organ weight is shown in Table 1. The results showed that broilers had a heavier (2.15 ± 0.81 kg) body weight compared to Potchefstroom Koekoek (1.44 ± 081 kg) and the non-descriptive breed (1.25 ± 0.81 kg). The significance level indicates that breed had an (p<0.001) effect on the body weight of chickens. There was an effect (p<0.001) of breed on the liver weight of chickens, and Potchefstroom Koekoek recorded a lower liver weight than broilers and the non-descriptive breed. Gizzard weight was the lowest for the non-descriptive breed compared to Potchefstroom Koekoek and broilers, and breed had an effect (p<0.001) on the gizzard weight.
There was no significant effect of breed recorded on the heart weight of broilers, non-descriptive breed, and Potchefstroom Koekoek. The intestinal weight for Potchefstroom Koekoek was the heaviest and the breed had an effect (p<0.001) on the heart weight. Intestinal weight and length of the total intestinal were significantly influenced by breed.
4.2 Effect of sex and age on body weight and internal organs of chickens
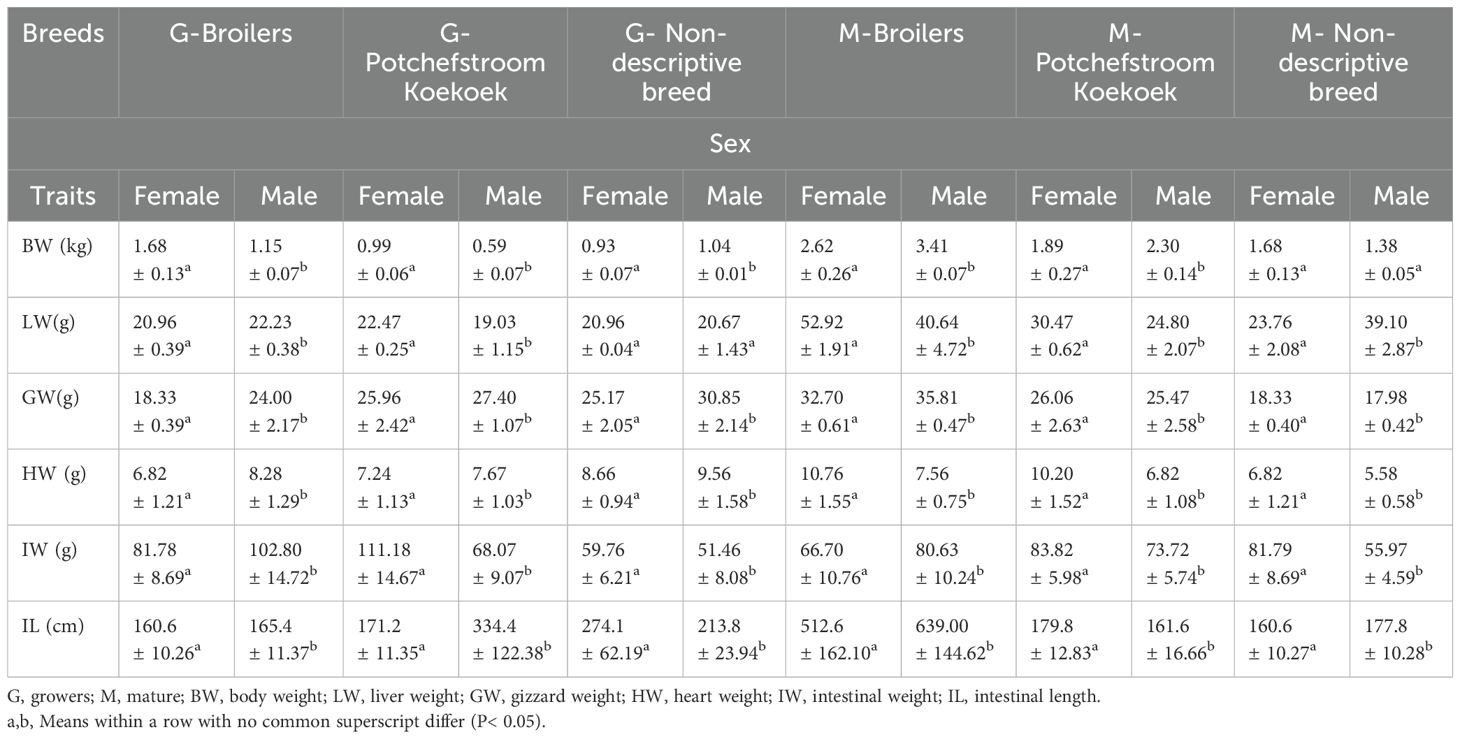
Table 2 shows the effect of sex and age on the chickens’ body weight and internal organ weight. Broiler grower females had the highest body weight, and non-descriptive breed males had the lowest. In grower broilers, there was a significant (P<0.05) average body weight of females (1.68 ± 0.13 kg) compared to males (1.15 ± 0.07 kg). The same was observed in Potchefstroom Koekoek females, but the non-descriptive breed had a significantly (P<0.05) higher average male body weight (1.04 ± 0.01 kg) compared to females (0.93 ± 0.07 kg).
Mature chicken broiler males had the highest body weight and the non-descriptive breed had the lowest. The male broilers recorded a significantly (P<0.05) average male body weight (3.41 ± 0.07 kg) compared to the females (2.62 ± 0.26 kg). Potchefstroom Koekoek males (2.30 ± 0.14 kg) had significantly (P<0.05) higher average body weight compared females (1.89 ± 0.27 kg). However, an insignificant (P<0.05) difference was recorded in the non-descriptive breed females (1.68 ± 0.13 kg) compared to the males (1.38 ± 0.05Kg).
Female growers had the largest liver weight, except for those of broiler chickens. Potchefstroom Koekoek females had the largest, followed by broiler males. Potchefstroom Koekoek chickens recorded the lowest liver weight, followed by non-descriptive breed males. Broiler males (22.23 ± 0.38 g) showed a significantly (P<0.05) higher liver weight than females (20.96 ± 0.39 g). Potchefstroom Koekoek males (19.03 ± 1.15g) recorded a significantly (P<0.05) lower liver weight compared to females (22.47 ± 0.25 g). Finally, the non-descriptive breed females (20.96 ± 0.04 g) had a non-significantly (P>0.05) higher liver weight compared to the males (20.67 ± 1.43 g).
In mature chicken broilers, the females had the highest liver weight, followed by the males. Potchefstroom Koekoek males and females of the non-descriptive breed had the lowest liver weight. Broiler females (52.92 ± 1.91 g) had a significantly (P<0.05) higher liver weight compared to the males (40.64 ± 4.72 g). In Potchefstroom Koekoek, the same trend was observed as the females (30.47 ± 0.62g) had a significantly (P<0.05) higher liver weight than the males (24.80 ± 2.07g). Non-descriptive breed followed a different trend, as the females recorded a significantly (P<0.05) lower liver weight (23.76 ± 2.08 g) than in the males (39.10 ± 2.87 g).
Gizzard weight was relatively the same between growers and mature chickens. Grower broiler males had significantly higher (<0.05) gizzard weight (24.00 ± 2.17 g) compared to the females (18.33 ± 0.39 g). Between the males and females of the non-descriptive breed, the males had a significantly (P<0.05) higher gizzard weight (30.85 ± 2.14g), followed by Potchefstroom Koekoek males (27.40 ± 1.07g).
Mature broiler males had the highest gizzard weight and the non-descriptive breed had the lowest gizzard weight. In mature chickens, females had a larger gizzard weight compared to males, but the opposite was found in broilers. Potchefstroom Koekoek females had a significantly higher (P<0.05) gizzard weight, which was followed by the non-descriptive breed (P<0.05) females (18.33 ± 0.40g) and broiler males had a higher gizzard weight (35.81 ± 0.47g) compared to the females.
Heart weight in the growing chickens was higher in the males compared to the females. The non-descriptive breed males had a significantly (P<0.05) higher heart weight (8.28 ± 1.29g), followed by broilers (P<0.05) males (8.28 ± 1.29 g) and finally, Potchefstroom Koekoek (P<0.05) males (7.67 ± 1.03 g) compared to the females (7.24 ± 1.13 g).
Surprisingly, in mature chickens, a higher heart weight was observed in the females than the males. Broiler females (10.76 ± 1.55 g) had the highest heart weight compared to all chickens and they were followed by Potchefstroom Koekoek females (10.20 ± 1.52 g). The non-descriptive breed males had the lowest heart weight (5.58 ± 0.58 g) compared to all chickens.
The intestinal weight and length of the growing chickens were affected by the sex of the chicken. The Potchefstroom Koekoek females had heavier (P<0.05) intestinal weight (111.18 ± 14.67g) and longer intestinal length (334.4 ± 122.38 cm) compared to the males. The non-descriptive breed followed the same trend, as a significantly heavier intestinal weight was observed in the females than in the males, with significantly (P<0.05) longer intestinal length. However, the male broilers had scientifically heavier intestinal weight and longer intestinal length than the females.
In the mature chickens, the females had heavier intestinal weights than broilers, whereas males had higher intestinal weights. The inverse was observed in the intestinal length, as broiler males (639.00 ± 144.62) had significantly (P<0.05) longer intestinal length, which was followed by Potchefstroom Koekoek females (179.8 ± 12.83) and lastly the males (177.8 ± 10.28) of the non-descriptive breed.
4.3 Interaction of breed, sex, and age on body weight and internal organs of chickens
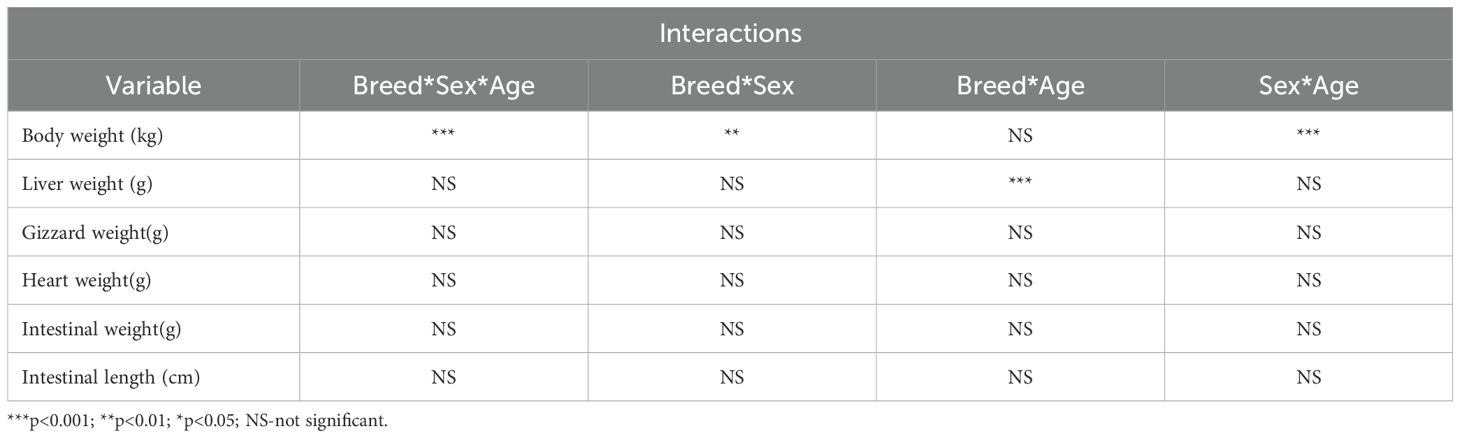
Table 3 indicates the interaction of the independent variables: breed, sex, and age. A (p<0.001) interaction between body weight and breed × sex × age, breed ×sex, and sex × age was recorded. An (p<0.01) interaction between liver weight and breed × sex was found.
4.4 Interaction of breed, sex, and age on body weight and internal organs of chickens
The relationship of breed, sex, and age to chicken body weight and internal organ weight is given in Table 4. A linear relationship (p<0.05) existed between body weight and breed, sex, and age. A negative coefficient was recorded between body weight and breed, but sex and age showed a positive coefficient for the body weight of chickens. Age had the strongest relationship with body and liver weight compared to breed and sex. A proportion of the variance in the dependent variables (body weight, liver weight, gizzard weight, heart weight, intestinal weight, and intestinal length) could be predicted from the independent variables of breed, sex, and age. A 64% variance in body weight could be explained by breed, sex, and age, therefore, there was a 64% association between body weight and breed, sex, and age.
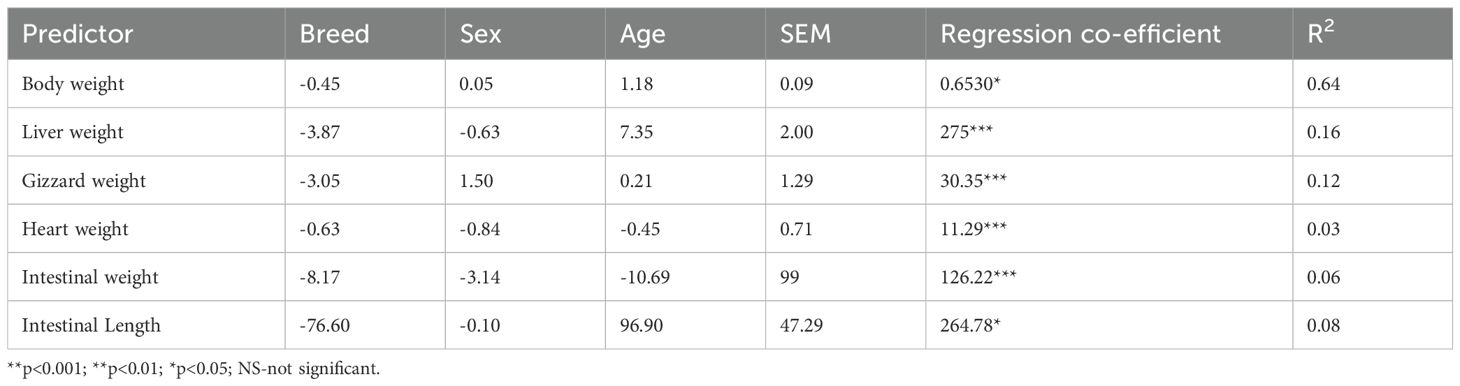
Table 4. The relationship between breed, sex, and age on the weight and internal organs of chickens.
A linear relationship (p<0.001) was observed between the relative liver weight and breed, sex, and age. Breed and sex showed a negative coefficient relative to liver weight, but a positive coefficient was observed between age and liver weight. In total, 16% of the variance in the liver weight could be predicted from breed, sex, and age, and there was a 16% association between liver weight and breed, sex, and age.
Sex had the strongest relationship with gizzard weight compared with the breed and sex of the chickens. Gizzard weight showed a negative coefficient with breed, but sex and age had a positive coefficient, and there was a significant linear relationship between these parameters. In total, 12% of the variance in the gizzard weight could be predicted from breed, sex, and age and there was a 16% association between gizzard weight and breed, sex, and age.
4 Discussion
In order to promote the production of village poultry on a large scale, it is important to have information on the carcass characteristics, including the weight of the organs (e.g., liver, heart, and intestines) that are frequently consumed within households and the level of acceptability of the meat (Dyubele et al., 2010). Limited information is available on how the weight of the vital organs of chickens varies with age, weight, strain, sex, and environment (Iwujia et al., 2022).The information could potentially be used to identify and develop informal markets, providing internal organs as a primary protein source from village chickens in resource-poor communities. Understanding the potential of utilizing internal organs as a protein source may influence informal market dynamics, generating new options for local farmers, vendors, and commercial producers. Furthermore, identifying breeds that are more suitable for local conditions may promote sustainable agricultural practices that improve food security and nutrition in resource-limited communities. The significant effect of breed on body weight suggests that genetic differences exist between breeds. Investigating the genetic differences between breeds can help identify those with superior traits for meat yield and organ development.
Village chickens generally have a slower growth rate compared to broiler chickens (Mwalusanya et al., 2002; Wattanachant et al., 2004). Notable differences between village and broiler chickens regarding their body weight and internal organs were observed. Broilers had a heavier body weight than village chickens, as Wattanachant et al. (Wattanachant et al., 2004) indicated that village chickens aged 16 weeks have a slow growth rate compared to broilers aged 28 days, as they both had a similar live weight of 1.5 ± 0.2 kg. Even though Potchefstroom Koekoek is regarded as a heavy breed with an average body weight ranging from 3 to 4 kg for mature males and 2.5 to 3.5 kg for females (Joubert, 1996). In broiler chickens, body growth consists of various components such as protein, water, and fat. Their deposition rate depends on the age, maturity stage, genotype, and environment (Vincek et al., 2012).
The higher body weight of broiler chickens compared with village chickens may have been due to broilers’ relatively superior genetic growth rate (Chaikuad et al., 2022). The digestive organs of broilers tend to increase drastically in weight (Ravindran et al., 2006). Moreover, Birteeb et al. (Birteeb et al., 2016) indicated that village chicken breeds tend to have comparatively lower growth rates and body weight. It is also argued that this may be due to genetic make-up or poor management (Mupeta et al., 2000). Halima et al. (Halima et al., 2006) showed that the growth rate of village chickens under good management is comparable with Rhode Island Red chickens. Village chickens exhibit a slow growth rate during the initial phase, followed by an exponential growth phase, and finally, a slow growth phase as they approach maturity (Ngeno, 2011; Gakige, 2015).
This pattern is reflected in their growth curve, which shows fluctuations (low and high points) in growth rates over time. The slow growth rate might be due to low and unbalanced nutrient intake, due to scavenging for feed resources in resource-poor environments (Raphulu and Jansen van Rensburg, 2018). Poor-quality feed limits their ability to achieve optimal body weight compared to chickens raised on formulated diets. Consequently, numerous subsistence farmers crossbreed their chickens with different breeds to enhance their overall body weight (Kanlisi et al., 2024).While crossbreeding improves productivity, it may also dilute some of the desirable traits of village chickens, such as their adaptability to harsh environments and resistance to diseases. However, a contributing factor to the development and anatomy of the internal organs and digestive tract in poultry feed (Auza et al., 2021).
It was evident in the current study that the breed of chicken had an effect on internal organ weights, which are mainly used for food security. In addition, Huang et al. (Huang et al., 2022) showed that the selection of traits such as body weight and feed efficiency affects the size of internal organs at all ages. Internal organ size in chickens is closely associated with body weight. In broilers, which are bred for rapid growth and high protein deposition, internal organ weights may be reduced compared to other chicken types, despite their higher overall body weight (Ramadan et al., 2014). This is attributed to selective breeding that prioritizes muscle growth over organ development. In contrast with the present study, broilers had higher internal organ weight in most organs except for intestinal weight. This could be because the feed is low in the plane of nutrition in resource-poor communities.
Resource-poor communities often rely on low-nutrition feed, which can influence the development of internal organs. The reduced nutritional plane may result in smaller organ sizes compared to chickens raised on nutrient-rich diets. This nutritional limitation could explain why broilers in these settings show reduced intestinal weights despite their higher body weight. In broiler chickens, internal organs tend to develop slower and remain in size compared to other chickens which results in various metabolic disorders (Julian, 2005). Broilers exhibit an accelerated growth rate compared to village chickens, which can lead to metabolic disorders due to the disproportionate development of organs relative to their body size. This rapid growth may compromise the health and functionality of internal organs. However, broilers have an increased growth rate and body weight that is usually also increased, while organ weights may be reduced (Dou et al., 2017). Even though broilers had a higher body weight than village chickens, it is suggested that both could participate in the mainstream value chain as an alternative during feed shortages and high demand.
Village chickens, often used for food security in resource-poor areas, have slower growth rates but more balanced organ development relative to body size (Tshovhote, 2015). Although they weigh less than broilers, their adaptability to low-nutrition environments makes them a viable option for sustainable food systems. Both broilers and village chickens could play complementary roles in the mainstream poultry value chain. Broilers can meet high-demand periods due to their rapid growth, while village chickens offer resilience during feed shortages or in low-resource settings. The interplay between body weight, organ development, and nutrition highlights the challenges and opportunities in poultry production for resource-poor communities. While broilers are efficient for meat production under optimal conditions, village chickens provide a sustainable alternative during feed shortages or economic constraints. Both types of chickens can contribute to food security if integrated thoughtfully into local and national value chains.
Liver weight was higher in broilers than in village chickens. Schmidt et al. (Schmidt et al., 2009) showed that the liver was heavy because it matures early with a longer length. A relationship exists between a chicken’s body weight and liver weight (Nurrahmandani et al., 2022). The weight of the gizzard, a preferred organ for many consumers, can be determined without the need to slaughter the chicken (Butzen et al., 2013). The weight of the gizzard was higher in broilers compared to village chickens, and breed had an effect. Broilers reared in intensive systems with optimized feed regimens develop larger gizzards. For instance, broilers fed 100% kitchen waste (with varied particle sizes) had heavier gizzards than those on a restricted diet (Bughio et al., 2021). Village chickens generally have smaller gizzards due to genetic differences and less controlled diets. Kadaknath and Aseel breeds (village chickens) showed 15%–20% lower gizzard weights compared to commercial broilers (Chickens, 2019).
The present study revealed that growers were lighter than mature chickens, and age was a factor. The results of the study of Osei-Amponsah et al. (Osei-Amponsah et al., 2012) indicated that body weight increases with age, especially during early ages, as does the growth rate, while age differed with breed type, and the environment may also be a factor. The same has been reported about broilers, and their growth rate begins with a slow growth rate that increases with age and goes up to a maximum rate, and then gradually decreases (Iwujia et al., 2022). It was notable in the current study that growers had higher intestinal weight than mature village and broiler chickens. This is in line with Kushch et al. (Kushch et al., 2019), who argued that the intestinal weight was higher in light breeds than heavy breeds.
The present study found that males were heavier than females. This aligns with the existing body of evidence which suggests that males typically have more significant body proportions than females in terms of sexual dimorphism (Ganbold et al., 2019). The variations in traits between males and females cannot be ascribed to a singular source. Multiple factors, including heightened rivalry for food, aggressive behavior exhibited by males, social dominance, disparities in nutritional needs, and the influence of hormones on development and fat accumulation, contribute to these variances (Wallner and Machatschke, 2009). This also agrees with Hassaan at al (Hassaan et al., 2009)., who found that when breeding village chickens, males were heavier compared to females.
The importance of understanding the effect of sex on body weight and internal organ weights of different chicken breeds is to determine their sexual differences in uniform environmental management parameters. These parameters can contribute significantly to providing solutions for resource-poor communities to achieve no poverty and zero hunger. The current study found that sex did not affect the liver weight of chickens. This is in contrast with the results of Tůmová and Chodová (2019), who suggested that there were differences in the liver weight of males and females of broilers. On the other hand, Moura et al.’s (Moura et al., 2016) study showed that the gizzard weight of broilers and layers was higher than that of females. The current study revealed no effect on the weight and internal organs of village and broiler chickens. This agrees with Omojola et al. (Omojola et al., 2004), who found that there were no sex differences in the weight and internal organs of broiler chickens.
The significant effect of the breed × sex × age, breed × sex, and sex × age interactions on body weight could be due to the growth rate of broilers and village chickens being different, males being heavier than females, and finally, growers being lighter compared to mature chickens. It is also hypothesized that the low nutrition plane of village chickens plays a significant factor in the lower body weight because the energy consumed is mainly used for an activity that includes scavenging for both feed and water, thus, body weight may be compromised. The insignificant interaction of breed × sex × age, breed × sex, breed × age, and sex ×age and the internal organ weight parameters except for liver weight in the current study indicated that the effect was the same regardless of breed, sex, and age and the weights tended to be similar for broilers and village chickens who are male and female in their growing or mature stage. The insignificant interaction effects on gizzard, heart, and intestinal weight and length showed no joint effect of breed and sex on these traits, and the two factors acted independently.
This agrees with Fernandes et al. (Fernandes et al., 2013), who found that breed had insignificant effects on carcass weight and giblet weight, except for liver weight. However, the study of Musa et al. (Musa et al., 2006) revealed significant effects on carcass weight and giblets in Anka and Rugao chicken breeds. The significant interaction effect of breed × sex on the body weight of different broiler breeds was also reported (Benyi et al., 2015). The highest coefficient for age in the present study indicated that age had the strongest relationship with the body weight of chickens compared to breed and sex. This could be due to the fact that the body weight of chickens is much different between growers and mature chickens in terms of body size and structure, as growers are still in the process of growing, while mature chickens are more likely to reach their growth process but are going through the fat deposition stage.
5 Conclusion
This study concludes that breed, sex, and age significantly influence the body weight and internal organ weights of chickens reared in resource-poor communities. Among the three breeds evaluated, i.e., broilers, Potchefstroom Koekoek, and non-descriptive indigenous chickens, broilers consistently exhibited higher body weight and internal organ mass compared to village chickens. Potchefstroom Koekoek outperformed non-descriptive breeds in most metrics, indicating its potential as a dual-purpose breed. Village and broiler chickens are superior in different parameters. The study emphasizes the need for further research into the productivity of village chickens and their internal organs, focusing on food security, irrespective of breed, sex, or age. This could enhance the understanding of how to optimize poultry production in these settings. The recommendations of this study are as follows: encourage breeding and management programs that improve both meat and organ yield in village chickens; promote training for rural poultry farmers, especially women (who are primary custodians of village chicken production), on basic poultry nutrition and health; integrate internal organ yield into poultry selection criteria for food security planning in resource-poor communities; support further research into nutrient supplementation and crossbreeding strategies to enhance growth performance without compromising disease resistance and adaptability; focus on improving the productivity of village chickens irrespective of breed, sex, or age due to their adaptability to harsh conditions and their role in food security; finally, study the genetic traits that enhance meat yield and organ quality in village chicken breeds.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by Animal Ethics Committee of the University of KwaZulu-Natal Protocol number (AREC/00004967/2022). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
TT: Writing – original draft, Writing – review & editing. LM: Formal Analysis, Methodology, Software, Visualization, Writing – review & editing. CN: Methodology, Supervision, Writing – review & editing. ZR: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Sustainable and Healthy Food Systems (SHEFS) program of the Welcome Trust’s Our Planet, Our Health program [Grant Number: 205200/Z/16/Z]. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alam M., Ullah M. O., Malik S. U. F., and Islam M. S. (2020). Broiler and indigenous chickens: a comparison through biochemical parameters. Int. J. Sustain. Agric. Res. 7, 228–233. doi: 10.18488/journal.70.2020.74.228.233
Auza F., Purwanti S., Syamsu J., and Natsir A. (Eds.) (2021). The relative weight of internal organs and digestive tract in native chickens age 12 weeks that are given various levels of BSF larvae meal (Hermetia illucens L) in the ration. IOP Conference Series: Earth and Environmental Science (Bristol, United Kingdom:IOP Publishing).
Benyi K., Tshilate T. S., Netshipale A. J., and Mahlako K. T. (2015). Effects of genotype and sex on the growth performance and carcass characteristics of broiler chickens. Trop. Anim. Health Prod. 47, 1225–1231. doi: 10.1007/s11250-015-0850-3
Besbes B. (2009). Genotype evaluation and breeding of poultry for performance under sub-optimal village conditions. World’s Poultry Sci. J. 65, 260–271. doi: 10.1017/S0043933909000221
Biel W., Czerniawska-Piątkowska E., and Kowalczyk A. (2019). Offal chemical composition from veal, beef, and lamb maintained in organic production systems. Animals 9, 489. doi: 10.3390/ani9080489
Birteeb P. T., Essuman A. K., and Adzitey F. (2016). Variations in morphometric traits of local chicken in Gomoa West district, southern Ghana. J. World’s Poultry Res. 6, 153–160.
Bughio E., Hussain J., Mahmud A., and Khalique A. (2021). Effects of production system and feeding regimen on carcass and meat quality traits of Naked Neck chicken. South Afr. J. Anim. Sci. 51, 250–261. doi: 10.4314/sajas.v51i2.13
Butzen F., Ribeiro A., Vieira M., Kessler A., Dadalt J., and Della M. (2013). Early feed restriction in broilers. I–Performance, body fraction weights, and meat quality. J. Appl. Poultry Res. 22, 251–259. doi: 10.3382/japr.2012-00639
Chaikuad N., Loengbudnark W., Chankitisakul V., and Boonkum W. (2022). Genetic comparisons of body weight, average daily gain, and breast circumference between slow-growing Thai native chickens (Pradu hang dum) raised on-site farm and on-station. Veterinary Sci. 10, 11. doi: 10.3390/vetsci10010011
Chickens A. (2019). Comparative study on carcass traits, meat quality and taste in broiler, broiler breeder and Revista Brasileira de Ciência Avícola. Braz. J. Poult. Sci. 21, 010. doi: 10.1590/1806-9061-2018-0770
Chowdhury S. (2013). Family poultry production in Bangladesh: is it meaningful or anaimless journey? World’s Poultry Sci. J. 69, 649–665.
Dou T., Zhao S., Rong H., Gu D., Li Q., Huang Y., et al. (2017). Biological mechanisms discriminating growth rate and adult body weight phenotypes in two Chinese indigenous chicken breeds. BMC Genomics 18, 1–12. doi: 10.1186/s12864-017-3845-9
Duah K. K., Essuman E. K., Boadu V. G., Olympio O. S., and Akwetey W. (2020). Comparative study of indigenous chickens on the basis of their health and performance. Poultry Sci. 99, 2286–2292. doi: 10.1016/j.psj.2019.11.049
Dyubele N., Muchenje V., Nkukwana T., and Chimonyo M. (2010). Consumer sensory characteristics of broiler and indigenous chicken meat: A South African example. Food Qual. Pref. 21, 815–819. doi: 10.1016/j.foodqual.2010.04.005
Faostat F. (2012). Disponível em. Available online at: http://faostat.fao.org (Accessed May 9, 2025).
Faostat F. (2019). Food and Agriculture Organization of the United Nations-Statistic Division. Available online at: https://www.fao.org/faostat/en/data.QC (Accessed May 9, 2025).
Fernandes J. I. M., Bortoluzzi C., Triques G. E., Garcez Neto A. F., and Peiter D. C. (2013). Effect of strain, sex and age on carcass parameters of broilers. Acta Scientiarum Anim. Sci. 35, 99–105. doi: 10.4025/actascianimsci.v35i1.13354
Gakige J. K. (2015). Effects of targeted phase supplementary feeding on performance of scavenging ecotypes of indigenous chickens in Kenya (Njoro, Kenya:Egerton University).
Ganbold O., Reading R. P., Wingard G. J., Paek W. K., Tsolmonjav P., Jargalsaikhan A., et al. (2019). Reversed sexual size dimorphism: body size patterns in sexes of lesser kestrels (Falco naumanni) in the Ikh Nart Nature Reserve, Mongolia. J. Asia-Pacific Biodiversity. 12, 363–368. doi: 10.1016/j.japb.2019.04.003
Halima H., NTD W. C., de Kock E., and van Marle-Koster (2006). Studies on the growth performance of native chicken ecotypes and RIR chicken under improved management system in north-west Ethiopia. Livestock Res. Rural Dev. 18, 112–118.
Hassaan S., Abdel-Fattah S., Elsalmoney A., and Hassan M. (2009). Relationship between some serum enzyme activities, liver functions and body weight in growing local chickens. Int. J. Poultry Sci. 8, 700–705. doi: 10.3923/ijps.2009.700.705
Huang Q., Wen C., Yan W., Sun C., Gu S., Zheng J., et al. (2022). Comparative analysis of the characteristics of digestive organs in broiler chickens with different feed efficiencies. Poultry Sci. 101, 102184. doi: 10.1016/j.psj.2022.102184
Iwujia T., Iheanachoa G., Ogambaa M., and Odunfab O. (2022). Relationship between live weight, internal organs, and body part weights of broiler chickens. Malay Anim. Husband J. 2, 64–66. doi: 10.26480/mahj.02.2022.64.66
Joubert J. (1996). The story of the indigenous domestic animals in South Africa. Agric. Res. Centre Private Bag. X. 2.
Julian R. J. (2005). Production and growth related disorders and other metabolic diseases of poultry–a review. Veterinary J. 169, 350–369. doi: 10.1016/j.tvjl.2004.04.015
Kandyliari A., Karavoltsos S., Sakellari A., Anastasiadis P., Asderis M., Papandroulakis N., et al. (2021). Trace metals in six fish by-products of two farmed fishes, the gilthead sea bream (Sparus aurata) and the meager (Argyrosomus regius): Interactions with the environment and feed. Hum. Ecol. Risk Assessment: Int. J. 27, 1126–1146. doi: 10.1080/10807039.2020.1799188
Kanlisi R. A., Amuzu-Aweh E. N., Naazie A., Otsyina H. R., Kelly T. R., Gallardo R. A., et al. (2024). Genetic architecture of body weight, carcass, and internal organs traits of Ghanaian local chickens. Front. Genet. 15, 1297034. doi: 10.3389/fgene.2024.1297034
Krawczyk J. (2009). Quality of eggs from Polish native Greenleg Partridge chicken-hens maintained in organic vs. backyard production systems. Anim. Sci. Pap Rep. 27, 227–235.
Kushch M., Kushch L., Byrka E., Byrka V., and Yaremchuk O. (2019). Morphological features of the jejunum and ileum of the middle and heavy goose breeds. Ukrainian J. Ecol. 9, 690–694. doi: 10.15421/2019_811
Mengesha M., Tamir B., and Dessie T. (2011). Village chicken constraints and traditional management practices in Jamma District, South Wollo, Ethiopia. Livestock Res. Rural Dev. 23, 30–37.
Moura A., Ledur M., Boschiero C., Nones K., Pinto L., Jaenisch F., et al. (2016). Quantitative trait loci with sex-specific effects for internal organs weights and hematocrit value in a broiler-layer cross. J. Appl. Genet. 57, 215–224. doi: 10.1007/s13353-015-0325-2
Mulugeta S., Goshu G., and Esatu W. (2020). Growth performance of DZ-white and improved Horro chicken breeds under different agro-ecological zones of Ethiopia. Livest. Sci. 11, 45–53. doi: 10.33259/JLivestSci.2020.45-53
Mupeta B., Wood J., Mandonga F., and Mhlanga J. (Eds.) (2000). A comparison of the performance of village chickens, under improved feed management, with the performance of hybrid chickens in tropical Zimbabwe. Sustaining livestock in challenging dry season environments: Strategies for smallscale livestock farmers (Eds T Smith and SH Godfrey) Proceedings of the third workshop on livestock production programme projects. (Gaborone, Botswana: Southern African Development Community).
Musa H., Chen G., Cheng J., Li B., and Mekki D. (2006). Study on carcass characteristics of chicken breeds raised under the intensive condition. Int. J. Poultry Sci. 5, 530–533.
Mwalusanya N., Katule A., Mutayoba S., Mtambo M., Olsen J., and Minga U. (2002). Productivity of local chickens under village management conditions. Trop. Anim. Health Prod. 34, 405–416. doi: 10.1023/A:1020048327158
Ncobela C. N. and Chimonyo M. (2016). Nutritional quality and amino acid composition of diets consumed by scavenging hens and cocks across seasons. Trop. Anim. Health Prod. 48, 769–777. doi: 10.1007/s11250-016-1025-6
Nkukwana T. (2018). Global poultry production: Current impact and future outlook on the South African poultry industry. South Afr. J. Anim. Sci. 48, 869–884. doi: 10.4314/sajas.v48i5.7
Nurrahmandani M., Sandi S., and Ali A. I. M. (2022). Morphological description of internal organ color and its relationship to body weight of free-range chickens in the palembang landfill environment. BIOVALENTIA: Biol. Res. J. 8, 103–107. doi: 10.24233/biov.8.2.2022.255
Omojola A., Adesehinwa A., Madu H., and Attah S. (2004). Effect of sex and slaughter weight on broiler chicken carcass. J. Food Agri Environ. 2, 61–63.
Osei-Amponsah R., Kayang B. B., and Naazie A. (2012). Age, genotype and sex effects on growth performance of local chickens kept under improved management in Ghana. Trop. Anim. Health Prod. 44, 29–34. doi: 10.1007/s11250-011-0010-3
Ramadan G., Moghaieb R., El-Ghamry A., El-Komy E., Nassar F., Abdou A., et al. (2014). Effect of selection for high live body weight on slaughter performance of broiler breeders. Egyptian Poultry Sci. J. 34, 289–304. doi: 10.21608/epsj.2014.5317
Raphulu T. and Jansen van Rensburg C. (2018). Growth performance and digestive tract development of indigenous scavenging chickens under village management. J. Agric. Rural Dev. Trop. Subtrop. 119 (1), 105–111.
Ravindran V., Wu Y., Thomas D., and Morel P. (2006). Influence of whole wheat feeding on the development of gastrointestinal tract and performance of broiler chickens. Aust. J. Agric. Res. 57, 21–26. doi: 10.1071/AR05098
Schmidt C. J., Persia M., Feierstein E., Kingham B., and Saylor W. (2009). Comparison of a modern broiler line and a heritage line unselected since the 1950s. Poultry Sci. 88, 2610–2619. doi: 10.3382/ps.2009-00055
Sirri F., Castellini C., Roncarati A., Franchini A., and Meluzzi A. (2010). Effect of feeding and genotype on the lipid profile of organic chicken meat. Eur. J. Lipid Sci. Technol. 112, 994–1002. doi: 10.1002/ejlt.200900204
Sonaiya F. (Ed.) (2007). “Smallholder family poultry as a tool to initiate rural development. International Conference Poultry in the Twenty-first Century: avian influenza and beyond,” in Poultry in the Twenty-first Century: Avian Influenza and Beyond (Rome, Italy: Food and Agriculture Organization of the United Nations (FAO)).
Tshovhote N. J. (2015). The characterization of indigenous chickens for utilization, improvement and conservation under traditional production systems: University of the Free State. (South Africa: University of the Free State in Bloemfontein).
Tůmová E. and Chodová D. (2019). Performance and changes in body composition of broiler chickens depending on feeding regime and sex. S. Afr. J. Anim. Sci. 49 (2), 167–176. doi: 10.17221/125/2018-CJAS
Vincek D., Kralik G., Kušec G., Sabo K., and Scitovski R. (2012). Application of growth functions in the prediction of live weight of domestic animals. Cent. Eur. J. Operations Res. 20, 719–733. doi: 10.1007/s10100-011-0199-2
Wallner B. and Machatschke I. H. (2009). Influence of nutrition on aggression. CABI Rev. 2010, 1–10. doi: 10.1079/PAVSNNR20094075
Keywords: consumption, backyard, farming, income, management, protein source, quantity, women
Citation: Tenza T, Mhlongo LC, Ncobela CN and Rani Z (2025) Effect of breed, sex, and age on the body and internal organ weight of chickens for food security in resource-poor communities of KwaZulu-Natal, South Africa. Front. Anim. Sci. 6:1565246. doi: 10.3389/fanim.2025.1565246
Received: 22 January 2025; Accepted: 21 April 2025;
Published: 15 May 2025.
Edited by:
Aristide Maggiolino, University of Bari Aldo Moro, ItalyReviewed by:
Peter Ayodeji Idowu, Tshwane University of Technology, South AfricaNuno Vieira Brito, IUCS - CESPU - Portugal, Portugal
Copyright © 2025 Tenza, Mhlongo, Ncobela and Rani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thando Tenza, dGVuemF0aGFuZG83QGdtYWlsLmNvbQ==
 Thando Tenza
Thando Tenza Lindokuhle Christopher Mhlongo
Lindokuhle Christopher Mhlongo Cyprial Ndumiso Ncobela
Cyprial Ndumiso Ncobela Zikhona Rani1
Zikhona Rani1