- 1College of Agronomy and Life Sciences, Kunming University, Kunming, China
- 2Yunnan Provincial Key Laboratory of Animal Nutrition and Feed Science, Faculty of Animal Science and Technology, Yunnan Agricultural University, Kunming, China
- 3Yunnan Mudao Biotechnology Co., Ltd., Kunming, China
- 4Kunming Kingmed Center for Clinical Laboratory Co., Ltd., Kunming, China
Iron deficiency in sows has been demonstrated to have a detrimental effect on porcine fetal growth and development, as well as on the reproductive performance of sows. The placental barrier of sows restricts the transportation of inorganic iron to the fetus, resulting in iron deficiency anemia in neonatal piglets and consequently leading to slow growth. The purpose of this study is to explore the effect of heme Fe on iron metabolism in pregnant sows. Ninety-six multiparous Landrace × Yorkshire (LY) sows (weight 235 ± 15kg) with similar litter size and feeding management were randomly divided into four treatment groups: control group (supplemented with 400 mg/kg), iron deficiency group (with no added FeSO4), heme Fe group (supplemented with 140 mg/kg), and glycine Fe group (supplemented with 470 mg/kg). Iron supplementation lasted from the second trimester (day 30) to day 114 before delivery. In this study, the production performance of sows, the iron content in sow placentas, and in the livers, spleens, placenta and colostrum of newborn piglets, as well as the hemoglobin(HGB) level, the iron regulation parameters in the serum of newborn piglets and the iron regulation genes in the livers and placentas were measured. The results showed that: (1) The number of live births and the average birth weight of piglets in the heme Fe group were 14.8% and 6.33% higher than those in the control group, respectively(P < 0.01). Compared with FeSO4 and glycine Fe, heme Fe improved the production performance of sows. (2) In the heme Fe group, the iron content in colostrum was significantly higher than in the control group (1.27-fold) and glycine Fe group (0.45-fold), while the iron content in the livers of newborn piglets increased by 30.38% and 14.61% compared to the control and glycine Fe groups, respectively (P < 0.01). These results suggest that heme Fe significantly facilitates iron transport in sows, particularly enhancing its deposition in colostrum and neonatal livers. This effect may be attributed to the upregulated expression of heme oxygenase 1(HO-1) gene in the placenta, which enhances the uptake and transport of heme Fe, thereby increasing fetal iron acquisition.(3) In the liver and placentas of sows in the deficiency group, the expression of hepcidin was decreased, while the expressions of transferrin receptor 1 (tfr1), feline leukemia virus subgroup C receptor 1(Flvcr1) and transferrin were increased (P < 0.01). In addition, the gene expression level of HO-1 in the heme Fe group of liver was significantly higher compared to that in the control group (1.85-fold), the iron deficiency group (2.99-fold), and the iron glycinate group (1.67-fold). In conclusion, maternal heme Fe supplements have a significant impact on iron storage in neonatal piglets and are helpful for preventing iron deficiency in newborn piglets.
1 Introduction
The source and level of dietary iron in pregnant sows are of significance for the development of the embryo and the growth of the fetus. Nutrients necessary for fetal growth, including trace elements, are exclusively derived from the mother (Hostetler et al., 2003). As the iron demands of late pregnant sows and lactating sows increase, the iron demands of sows in these stages of pregnancy and lactation also increase rapidly (Mahan and Shields, 1998; Mahan et al., 2009). At present, ferrous sulfate is the most common dietary iron supplement used (Wu et al., 2024). However, it has been demonstrated that supplementation with organic iron is more effective than supplementation with iron salts and can improve iron absorption and the nutritional status of animals (Pineda and Ashmead, 2001). The provision of organic iron to pregnant sows has been demonstrated to enhance birth and weaning weights of piglets, reduce stillbirth and early neonatal mortality, and improve the iron nutritional status of piglets (Liu et al., 2024). Layrisse et al. (2000) reported that glycine Fe could increase the utilization rate of iron in rats and humans (Layrisse et al., 2000). Furthermore, ferrous fumarate has been incorporated into infant foods with the aim of preventing and treating iron deficiency (Hurrell, 2010).
The manifestation of iron deficiency anemia (IDA) in piglets is characterized by a decrease in hemoglobin (HGB) levels below 70 g/L (Rydal et al., 2021), while subclinical anemia is evident when HGB levels range between 70 and 80 g/L. The treatment of iron deficiency symptoms in piglets typically involves the administration of iron injections (Szudzik et al., 2018). However, there are several reports that suggested intramuscular iron injection could have negative effects on piglets, including increasing acute poisoning rate, reducing macrophage activity and phagocytosis, stimulating bacterial growth, and increasing risk of polymyositis (Knight et al., 1983).
In animals, the metabolism of iron is of significance in order to prevent a depletion or an excess of iron. The regulation of iron metabolism is a complex process that is orchestrated by a variety of proteins, including divalent metal transporter and hepcidin (Nemeth and Ganz, 2021). Hepcidin modulates iron metabolism in pregnant animals, thereby directly impacting fetal iron storage (Chibanda et al., 2023). In the uteroplacental syncytiotrophoblasts, hepcidin plays a critical role in regulating the release of iron to the fetus (O’Brien, 2022). HGB synthesis in the body is associated with elevated heme requirements. The Feline Leukemia Virus subgroup C receptor-related protein 1 (Flvcr1) functions as a regulatory mechanism, modulating the body’s response to heme toxicity (Tahara et al., 2004).
The National Research Council (NRC, 2012) (National Research Council (NRC), 2012) recommendations stipulate that the dietary requirement for iron in sows is 80 mg/kg. However, due to the placental barrier effect in sows, piglets primarily acquire iron from colostrum post-partum. However, the low levels of iron in colostrum, in conjunction with insufficient additional iron supplementation, frequently results in neonatal piglets experiencing iron deficiency. In order to explore whether supplementation with heme Fe in sow diets could improve the reproductive performance of sows and the iron nutritional status of their offspring, a study was conducted which the effects of control, iron deficiency, heme Fe, and glycine Fe on the reproductive performance of sows and iron nutrition status of piglets were compared.
2 Materials and methods
2.1 Animal ethics
The experiment was conducted at the Jiangchuan Pig Farm in Yunnan Province, China. All animal procedures strictly adhered to the Guide for the Care and Use of Laboratory Animals (National Research Council, US) and were approved by the Institutional Animal Care and Use Committee (IACUC) of Kunming University (Protocol No. 2023058).
2.2 Animals and experimental treatments
The control group was fed a diet consisting of corn and soybeans, with FeSO4 supplementation, while the iron deficiency group was fed the same food, but without FeSO4 supplementation. One week after artificial insemination (Duroc (D) × LY (Landrace × Yorkshire)), 96 cross-bred sows (LY) were randomly allocated to the iron deficiency group (without FeSO4), the control group (supplemented with 400 mg/kg of FeSO4), the heme Fe group (supplemented with 140 mg/kg of Heme Fe), and the glycine Fe group (supplemented with 470 mg/kg of glycine Fe). All sows were multiparous (having farrowed twice) with a body weight of 235 ± 15kg. Iron supplementation lasted from the second trimester (day 30) to day 114 before delivery. The experiment consisted of 24 replicates per group. From each group, 8 sows were selected for subsequent experiments, and one piglet was chosen from each selected sow. This process ultimately led to the selection of eight piglets from the initial pool of 24 replicates for subsequent blood collection and sample procurement via slaughter. All sows were housed in individual gestation stalls (2.1 × 0.60 × 0.97 m) with half-slatted concrete floors. Individual feeders and drinkers made of stainless steel were used. Sows were transferred to farrowing pens (2.1 × 3.0 m, stainless steel stall and plastic floor) one week before the predicted farrowing date. Maintenance of optimal thermal conditions (17–25°C) and humidity (70%–80%) during gestation and lactation was facilitated by the implementation of warm-air blowers, wetted-pads, and air-exhaust fans. Sows were provided with a restricted amount of feed, which was entirely consumed, thereby ensuring consistent intake per sow. During the early (0–30 d, 2 kg/time), middle (31–84 d, 2.5 kg/time) and late gestation periods (85–114 d, 3 kg/time), sows were fed twice daily at 09:00 and 16:00 hours.
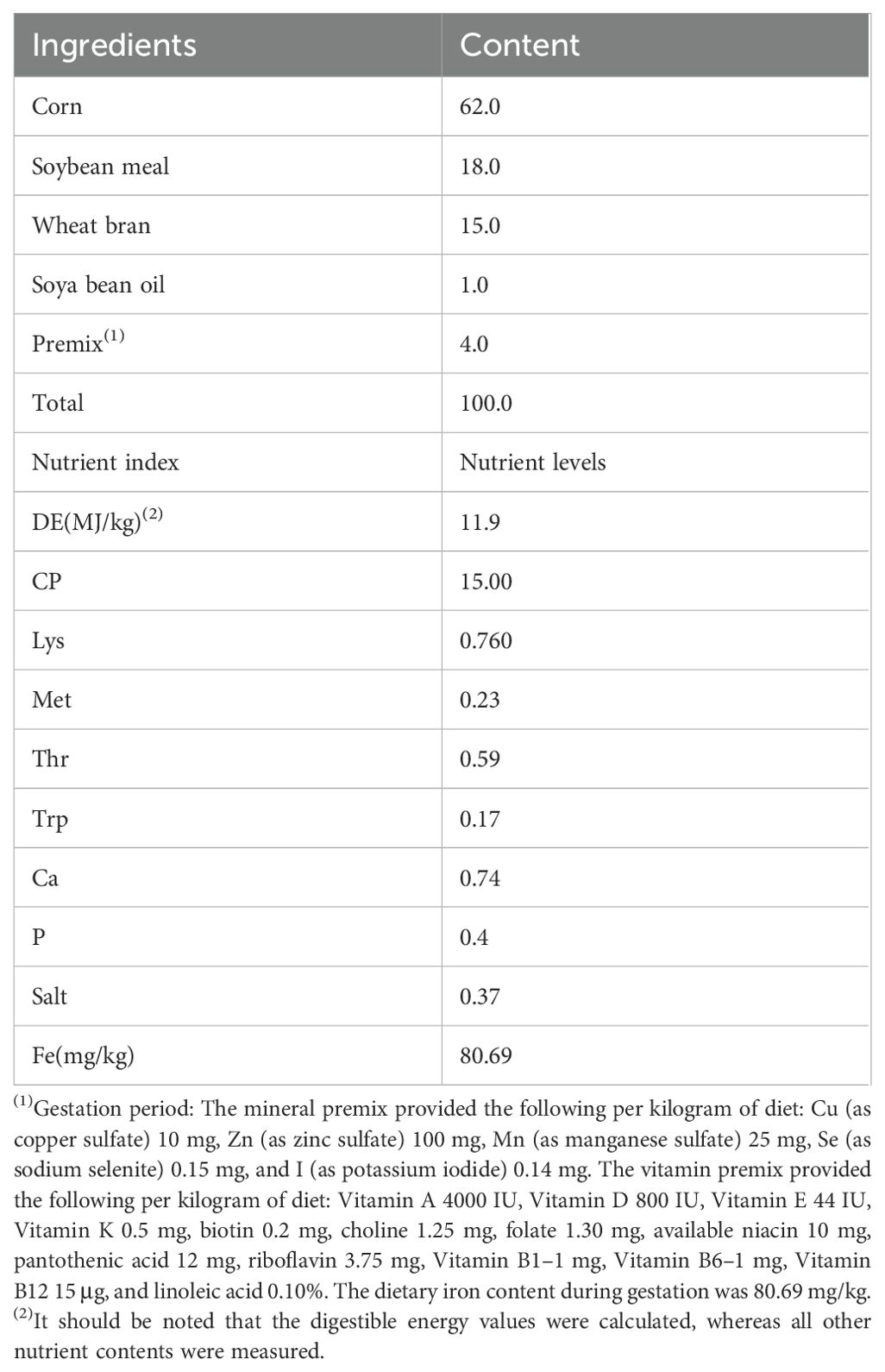
The basal diet was formulated based on the nutritional requirements recommended by the NRC (2012) (National Research Council (NRC), 2012) and the actual production characteristics of LY pregnant sows. In accordance with the objectives and requirements of the experimental design, the experimental diets were supplemented as follows: a control group (FeSO4), a Heme Fe group, a glycine Fe group, and an iron-deficiency group. The composition and nutritional levels of the basal diet are presented in Table 1.
2.3 Sample collection
The selection of the pigs was conducted at random to ensure the inclusion of a single newborn piglet of average weight. Immediately following parturition, the piglets were weighed individually. Subsequently, blood samples were collected from sows and piglets immediately following parturition. One male and one female piglet per litter were euthanized for sampling at birth. The blood samples were subjected to a centrifugation process at 4°C at 1000 g for 10 min, after which the serum was collected and stored at –20°C until further analysis. The liver, spleen, placenta and colostrum samples were collected within 20 minutes of the animals’ demise, snap-frozen in liquid nitrogen and stored at -80°C until further analysis. The selected piglets (n = 8 per treatment group) were not provided with colostrum. During slaughter, 5 ml of whole blood was collected for the detection of HGB content. An additional 10 ml of whole blood was taken from the anterior vena cava to prepare serum samples.
2.4 Determination of HGB and serum hepcidin, transferrin, total iron-binding capacity, serum iron and serum hemopexin concentrations
HGB was measured using a BC-1800 automated blood cell analyzer (Shenzhen Meili Biomedical Electronics Co., Ltd.). Serum hepcidin concentrations and total iron binding capacity (TIBC) were determined using an ELISA kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Serum iron and transferrin were determined by the colorimetric method (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Serum hemopexin was determined using an ELISA kit (Abcam, USA).
2.5 Determination of iron content in neonatal piglets
Neonatal piglets not fed colostrum were weighed and slaughtered within 2 h of birth. Their livers, spleens and placentas were collected and quickly sectioned after removal of adipose tissue. The pieces of tissue were frozen in liquid nitrogen and stored at -20°C. Placentas were collected from the sows, as were colostrum samples. Colostrum was collected within 2 h after farrowing. Colostrum was collected from the nipples of the sows by squeezing directly into 5 ml cryogenic vials and stored at -20°C.Tissue samples (0.5 mg each of liver, spleen and placenta and 3 ml of colostrum) were weighed on an analytical balance. The non-heme Fe content of liver, spleen and placenta was determined as described by Brain et al (Brain et al., 2006). Frozen lung, liver and brain samples were thawed and aliquots weighed. Samples (50-100 μg) were acid hydrolyzed in 2 ml of a mixture of equal volumes of 6-N-hydrochloric acid and 20% trichloroacetic acid at 65°C for 20 h. After cooling to room temperature, the clear yellow solution was transferred to a test tube and a color reagent (0.1% sulphonated bathophenanthroline mixed with 1% thioglycolic acid and distilled water in a 1:25:25 ratio) was added. After incubation for 10 minutes, the optical density was measured at 540 nm. A standard curve was generated using an iron standard solution (VWR). Non-heme Fe in tissue was calculated from the standard curve and expressed as μg/g wet tissue.
2.6 Real-time PCR for mRNA quantification
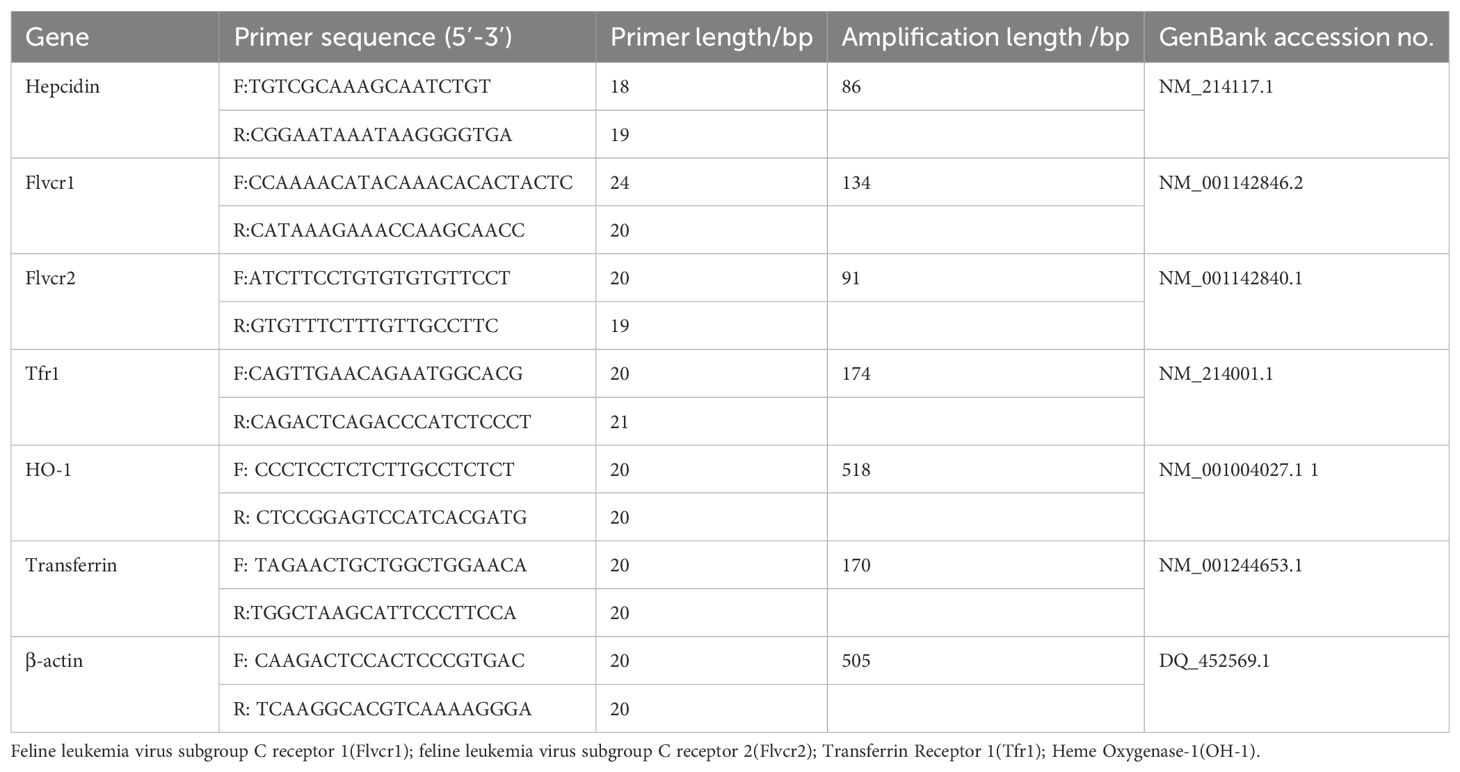
Total RNA was isolated from placenta and liver samples using TRIzol reagent (Invitrogen). RNA samples (2 μg) were treated with DNase and reverse transcribed into complementary DNA (cDNA) using random hexamer primers (Promega). An amount of 2 μl of diluted cDNA (1:25) was used for real-time PCR performed on Mx3000P (Stratagene). β-Actin was chosen as a reference gene for normalization of mRNA expression levels, as its expression was not affected by maternal dietary treatment. Real-time PCR data were analyzed using the 2-ΔΔCt method. mRNA levels are expressed as fold change relative to the mean of the control group. Primers for real-time PCR were synthesized by Generay Biotech Co., Ltd. Primer information is shown in Table 2.
2.7 Statistical analysis
Data from independent experiments are presented as mean ± SD. All statistical analyses were two-tailed with 95% confidence intervals (CI). Results were analyzed by Mann-Whitney U, one-way ANOVA and Tukey’s test using Prism 6 (Graphpad Software) and SPSS (SPSS Inc, USA). Differences were considered significant at p < 0.05.
3 Results
3.1 Effect of different sources of maternal dietary iron on reproductive performance of sows
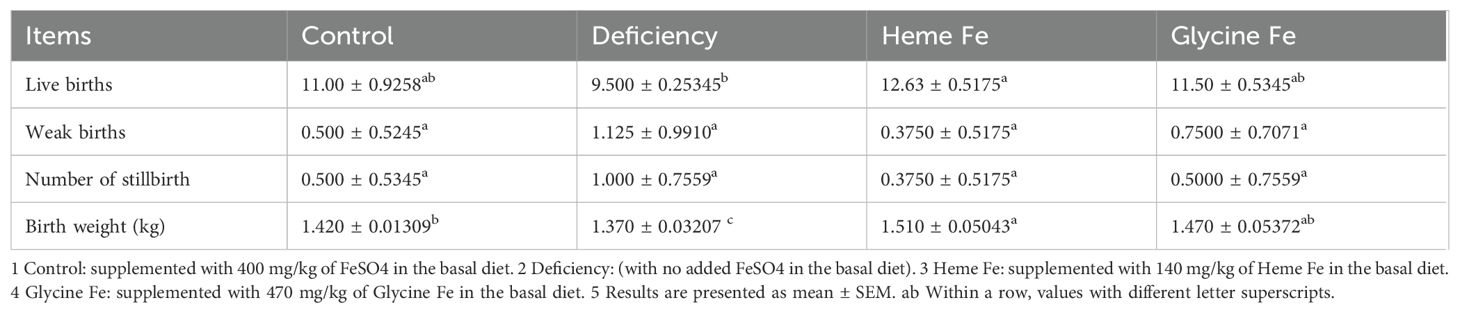
The number of live births in sows from the heme Fe group was significantly higher than that in sows from the iron deficiency group (P < 0.05). Although not statistically significant, the number of live births in sows from the glycine Fe group was also higher than that in sows from the iron deficiency group. The mean birth weight of piglets in the heme Fe group was significantly greater than that in both the control and iron deficiency groups (P < 0.05). Additionally, the mean birth weight in the glycine Fe group was significantly higher than that in the iron deficiency group (P < 0.05) (Table 3).
3.2 Effect of maternal heme Fe diet on serum parameters related to iron metabolism in piglet
As shown in Figure 1, serum hepcidin levels were significantly higher in piglets from sows in the heme Fe group compared to those from sows in the control and iron deficient groups. Additionally, serum hepcidin levels were significantly elevated in the glycine Fe group relative to the iron-deficient group (P < 0.01). Serum transferrin and TIBC levels were significantly higher in iron-deficient piglets than in those from the control, glycine Fe, and heme Fe groups (P < 0.01). The serum iron concentration in piglets from the heme Fe group was significantly higher than that in the control and glycine Fe groups, with respective increases of 11.5% and 10.7% (P < 0.01),. Furthermore, serum hemopexin levels in sows from the heme Fe group were significantly higher than those in the control and iron-deficient groups (P < 0.01).
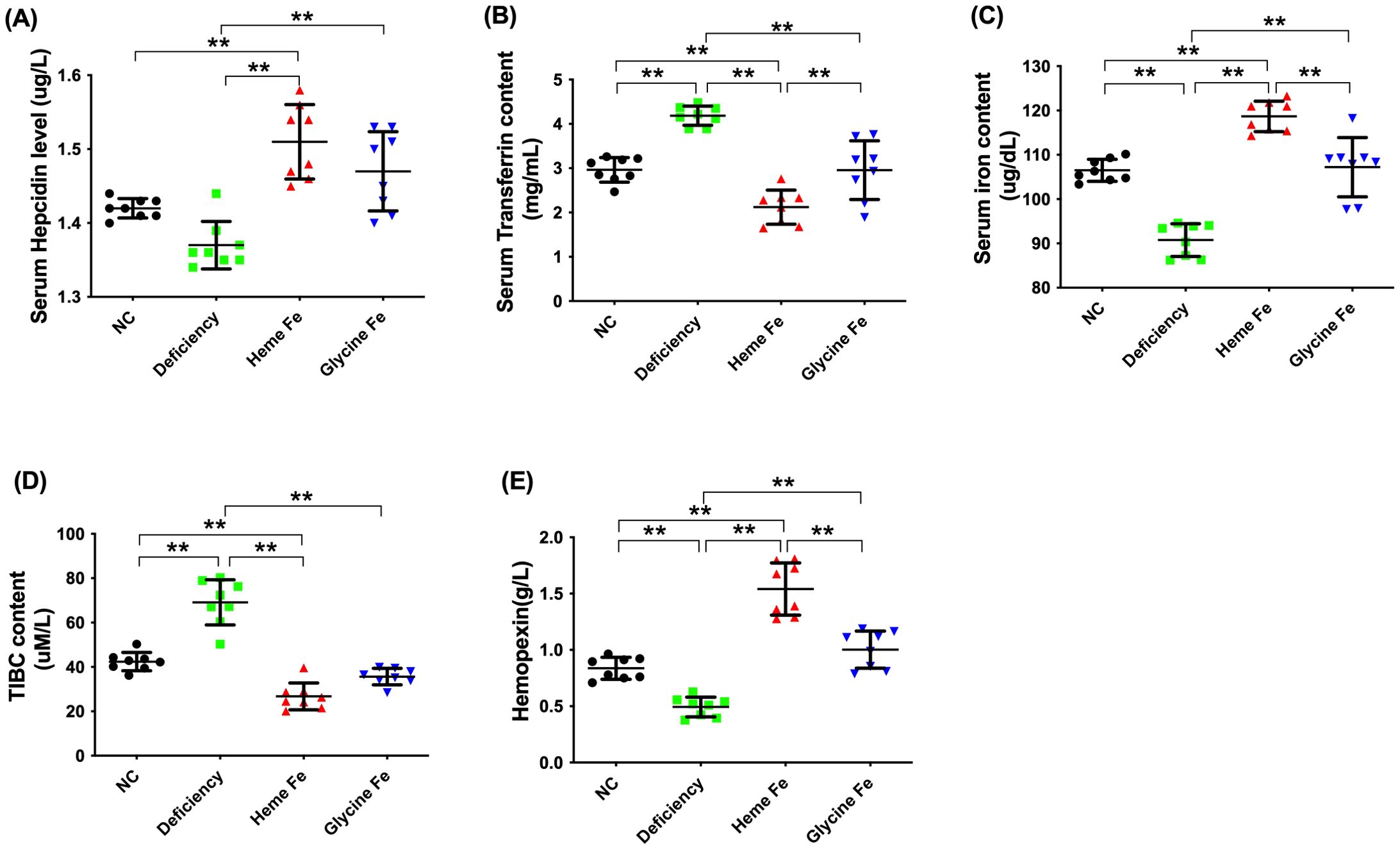
Figure 1. Serum iron parameters of the neonatal piglets were measured. The effects of adding heme Fe and glycine Fe on serum hepcidin (A), transferrin (B), iron concentration (C), TIBC (D), and hemopexin (E) in neonatal piglets were studied. Data represent mean ± SD of eight independent experiments, **p < 0.01, by one-way ANOVA and Tukey's test.
3.3 Effect of maternal heme Fe diet on HGB levels in sows and piglets
As depicted in Figure 2, the mean HGB levels of sows in the control, heme Fe, and glycine Fe groups were 122 g/L, 119 g/L, and 120 g/L, respectively. These values were significantly higher than the normal physiological range. In contrast, the HGB level of sows in the iron-deficient group was 88 g/L, indicating borderline anemia. The mean HGB content of newborn piglets in the heme Fe group was 121 g/L, which was significantly higher than that in the control group (96 g/L), iron-deficient group (65 g/L), and glycine-iron group (92 g/L) (P < 0.01). The lowest HGB levels were observed in the iron-deficient group. This may be attributed to the fact that piglets in the heme Fe group received a greater amount of iron from their mothers for hemoglobin synthesis. Additionally, the HGB levels of piglets in the glycine Fe group were significantly higher than those in the iron deficient group (P < 0.01). However, no significant difference was observed compared to the control group.
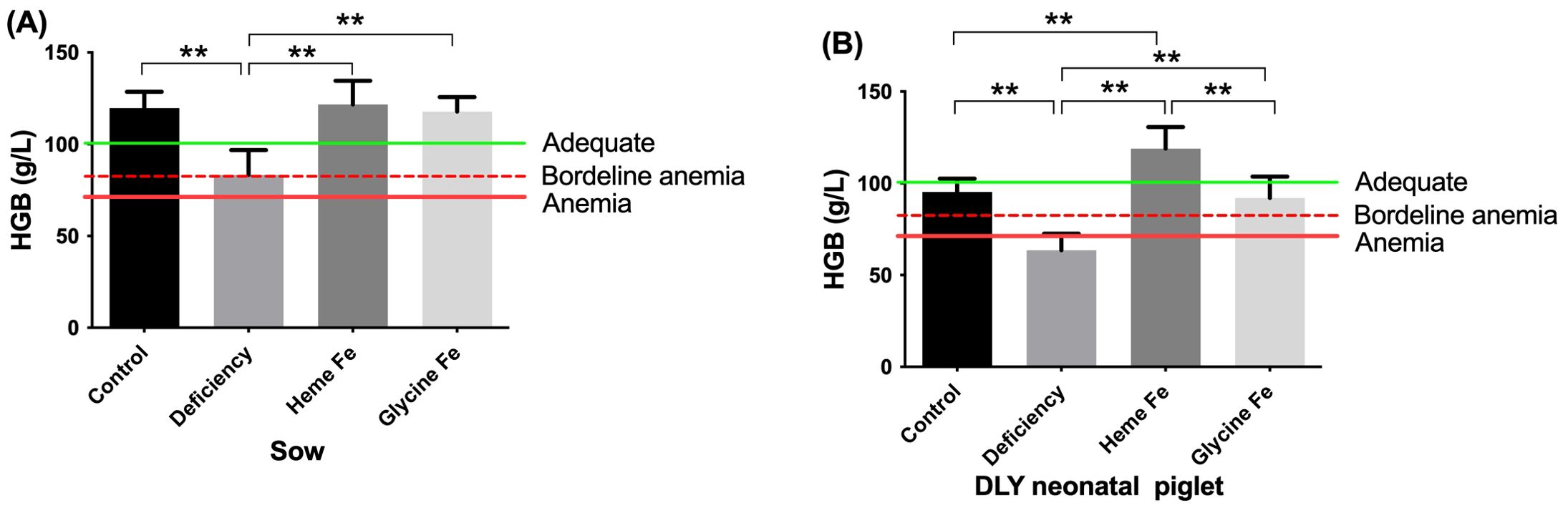
Figure 2. The effect of adding heme Fe and glycine Fe on hemoglobin levels in sows (A) and neonatal piglets (B) were studied. Data represent mean ± SD of eight independent experiments, **p < 0.01, by one-way ANOVA and Tukey's test.
3.4 Effect of maternal dietary heme Fe content on colostrum and tissue iron in piglets
As shown in Figure 3, the liver iron content in piglets from the heme Fe group was significantly higher than that in the control group, the iron deficiency group, and the glycine-iron group (increases of 30.38%, 100.9%, and 14.61%, respectively) (Figure 3A). The findings in piglet spleens were consistent with the trends observed in the liver (P < 0.01 each) (Figure 3B). Furthermore, placental heme Fe content in sows did not differ significantly from that in the iron deficiency group but was significantly lower than in the glycine Fe and control groups (Figure 3C). This may be attributed to a greater proportion of iron being transported across the placenta, thereby enhancing fetal liver and spleen iron content. Additionally, colostrum iron content was higher in both the heme Fe and glycine-iron groups compared to the control and iron-deficient groups, likely due to more efficient passage of organic iron across the mammary barrier (P < 0.01 each) (Figure 3D).
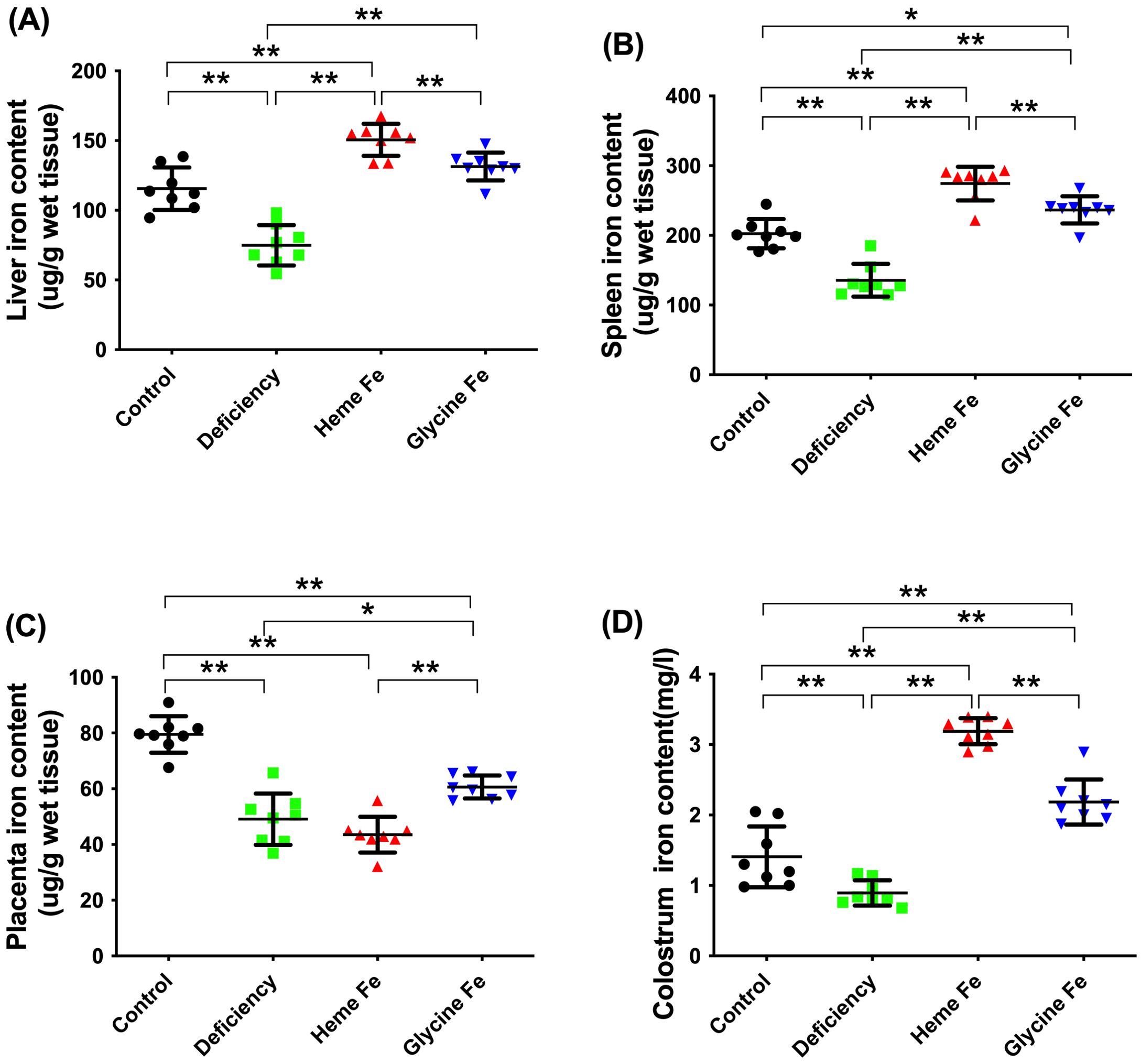
Figure 3. Heme Fe increased the iron storage content in tissues of neonatal piglets. The effects of adding heme Fe and glycine Fe on liver (A), spleen (B) in neonatal piglets and placenta (C), colostrum (D) (μg· g wet tissue) were studied. Data represent mean ± SD of eight independent experiments, *p < 0.05, **p < 0.01, by one-way ANOVA and Tukey's test. .
3.5 Effect of maternal heme Fe diet on expression of iron metabolism related genes in maternal liver and placenta of neonatal piglets
The data presented in Figure 4 indicate that the hepatic hepcidin and Heme Oxygenase-1 (HO-1) mRNA expression levels in neonatal piglets from sows without iron supplementation were significantly lower than those observed in piglets from the control, heme Fe, and glycine Fe groups (P < 0.01 for each comparison). Additionally, liver Tfr1 expression in vivo was significantly higher in piglets from sows in the iron deficiency group compared to those from the control, heme Fe, and glycine Fe groups (P < 0.01 for each comparison). Furthermore, Flvcr1 mRNA expression levels in the livers of neonatal piglets from sows in the iron deficiency group were significantly elevated compared to those in the control, heme Fe, and glycine Fe groups (P < 0.01 for each comparison). Finally, transferrin gene expression levels in neonates from the iron deficiency group were significantly higher than those in the heme and control groups (P < 0.05 and P < 0.01, respectively).
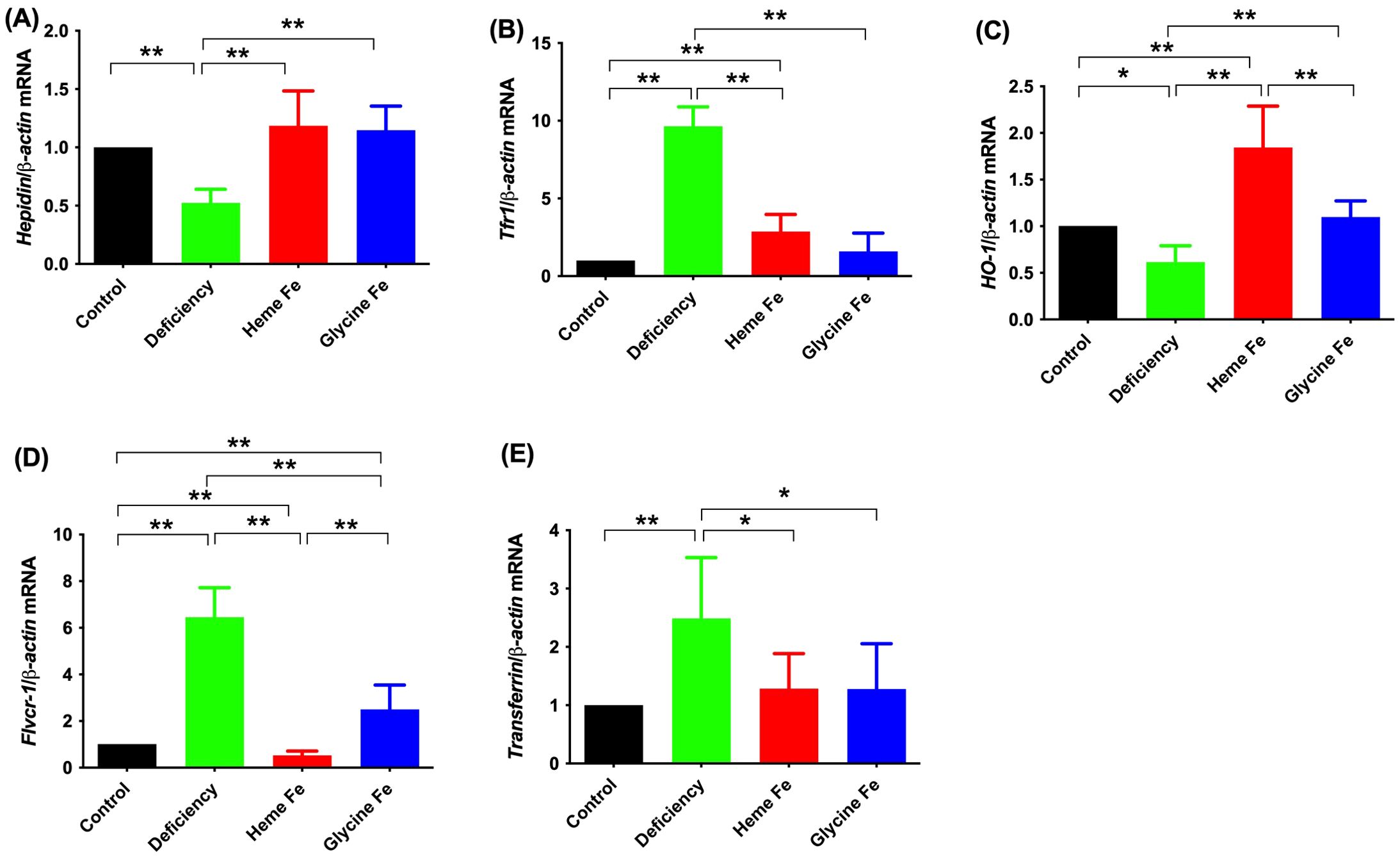
Figure 4. The effects of adding heme Fe and glycine Fe on hepcidin (A), tfr1 (B), HO-1 (C), Flvcr1 (D) and transferrin (E) in neonatal piglet liver was determined by real-time PCR. Data represent mean ± SD of six independent experiments, *p < 0.05, **p < 0.01, by one-way ANOVA and Tukey's test.
As shown in Figure 5, placental hepcidin expression in sows was significantly lower in the iron deficiency and heme Fe groups compared to the control group (P < 0.01). Additionally, hepcidin expression was significantly higher in the glycine Fe group than in the heme Fe group (P < 0.01). Placental expression of Flvcr1 was significantly higher in the heme Fe group compared to the control and glycine Fe groups (P < 0.01 for both). Placental Flvcr2 expression in sows from the iron deficiency and heme Fe groups was significantly higher than that in the control group (P < 0.01 for each). Conversely, Flvcr2 expression in the glycine Fe group was significantly lower than in the control group (P < 0.01). Furthermore, Flvcr2 expression in the heme Fe group was significantly lower than that in the iron deficiency group (P < 0.01). Placental Tfr1 expression in the iron deficiency group was significantly higher than in the control group, while Tfr1 expression in the heme Fe group was significantly lower than in the iron deficiency group (P < 0.01 for each). HO-1 mRNA expression in the placenta of sows in the heme Fe group was significantly higher than in the other groups (P < 0.01).
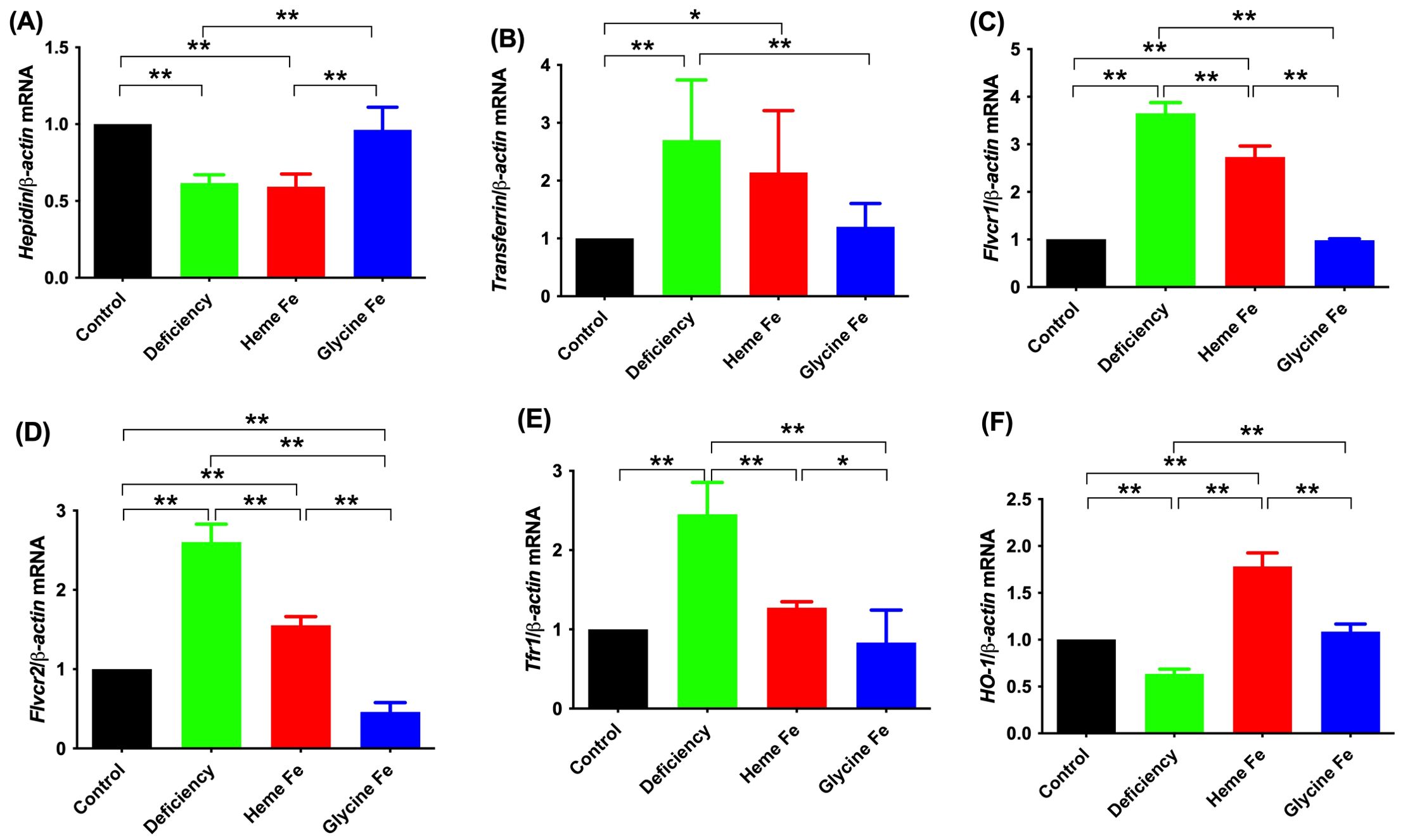
Figure 5. The effects of adding heme Fe and glycine Fe on hepcidin (A), transferrin (B), Flvcr1 (C), Flvcr2 (D) and Tfr1 (E) and HO-1 (F) in neonatal piglets placental was determined by real-time PCR. Data represent mean ± SD of six independent experiments, *p < 0.05, **p < 0.01, by one-way ANOVA and Tukey's test.
4 Discussion
4.1 Heme Fe can improve the reproductive performance in sows
The dietary iron requirement for pregnant and lactating sows is 80 mg/d (NRC, 2012) (National Research Council (NRC), 2012). To date, the NRC has not made recommendations for the iron requirements of neonatal piglets. Due to difficulties in studying the placental barrier and fetal iron nutrition in sows, the iron requirements of fetal and neonatal piglets have not been established.
Here, the dietary iron content of the sows in the iron-deficient group was 80.69 mg/kg (atomic absorption spectroscopy). Assuming an iron utilization rate of approximately 15-30%, the daily dietary iron content of sows is 12–24 mg/kg. This is well below the NRC recommendation and results in severe iron deficiency in pregnant sows. Taken together, the results of this study show that iron deficiency results in reduced litter size, live litter size and birth weight. These results suggest that iron deficiency affects normal growth and development of the pig fetus and may increase early embryonic mortality. We found that the reproductive performance of sows was significantly affected by supplementation with different sources of dietary iron. Supplementation with 140 mg/kg of heme Fe led to piglets being 10.18%, 5.96%, and 3.07% heavier at birth compared to those in the iron-deficient, control (FeSO4), and glycine Fe groups, respectively. In addition, the live litter size of the heme Fe group was 18.42% higher than that of the iron-deficient group. These findings are in agreement with the results reported by E. Merlot et al., who demonstrated that providing organic iron to pregnant sows can enhance neonatal and weaning weights of piglets, decrease stillbirth rates and early neonatal mortality, and improve the iron nutritional status of piglets (Merlot et al., 2024). Furthermore, Layrisse et al. reported that glycine Fe could increase iron utilization efficiency in rats and humans (Layrisse et al., 2000).The findings of this study demonstrate that glycine Fe can effectively enhance the performance of sows, albeit to a lesser extent compared to the heme Fe group. The identical effect was observed in the control group supplemented with FeSO4. Dietary supplementation with 140 mg/kg of heme Fe for pregnant sows significantly promotes fetal growth and development, increases litter size and birth weight. In summary, this supplementation strategy enhances the reproductive performance of sows and improves the growth potential of piglets.
4.2 Effect of heme Fe on serum iron parameters of neonatal piglets
HGB levels are generally used to detect anemia in piglets. However, serum hepcidin levels also provide an important reference point reflecting iron nutritional status. Ganz et al. reported that the serum hepcidin level of a healthy fetus was 90.7 ng/ml (Ganz et al., 2008). Jing Wang et al. reported that serum iron and ferritin levels were significantly elevated in chronic hepatitis patients, suggesting that serum hepcidin levels are closely related to ferritin (Wang et al., 2016). The results of this study show that feeding heme Fe to sows significantly increased serum hepcidin levels in their neonatal piglets. Conversely, iron deficiency in sows significantly decreased liver iron content in their piglets. In fact, the iron-deficient group produced piglets with the lowest serum hepcidin levels and significantly higher serum transferrin and TIBC levels than piglets in the other groups. Supplementation of heme Fe in the sows’ diets resulted in significantly higher serum iron levels in their piglets than in the glycine Fe, control and iron-deficient groups. Serum iron and TIBC are important markers of iron deficiency/overload (Kasvosve and Delanghe, 2002). Serum iron is an important marker of iron entry or exit and has been used to qualitatively measure iron bioavailability. TIBC is used to assess the total amount of transferrin-bound iron (Soldin et al., 2004). Serum hepcidin, transferrin, serum iron and TIBC are sensitive indices for the detection of iron content in organisms. Our investigation of serum iron parameters in neonatal piglets shows that feeding heme Fe to sows may increase iron transport across the placenta to fetal pigs. Serum hemopexin of sows in the Heme Fe group was significantly higher than that in the control and iron deficiency groups, which was consistent with OH-1 expression in the liver and placenta.
4.3 Feeding sows heme Fe increases HGB content in sows and neonatal piglets
HGB is a reliable index that reflects the anemia and iron nutritional status of an animal and is an important indicator of iron deficiency, bioavailability and iron requirement. In this study, the level of iron deficiency observed in the heme-Fe and glycine-Fe groups of sows was not significantly different, and iron deficiency significantly reduced HGB levels in the sow. Therefore, the iron status of the sow indirectly reflects the HGB level. Neonatal piglets from heme Fe supplemented sows had higher HGB levels than those in the control group. The neonatal piglets of sows in the glycine Fe group were borderline anemic, and the neonatal piglets of sows in the iron deficiency group were severely anemic. Taken together, these results showed that while different sources of iron can meet the HGB requirements of the sow, they cannot meet the HGB requirements of the piglets.
4.4 Heme Fe increases the iron content in colostrum and in the tissues of neonatal piglet
Wang et al. reported that the supplementation of sow diets with organoiron complexes failed to effectively prevent iron deficiency anemia in suckling piglets (Wang et al., 2014). In contrast, our study demonstrates that incorporating heme Fe into the sow’s diet significantly enhances liver iron storage in neonatal piglets, achieving levels 1.38 times higher than those observed in the control group. This improvement is accompanied by an increase in colostrum iron content. Colostrum iron concentration in sows is influenced by multiple factors, including nutritional intake, health status, and environmental management (Sun et al., 2023). In this experiment, the health and feeding management of the sows were standardized. Heme Fe supplementation in sows led to a significantly higher colostrum iron content compared to the control, iron deficient, and glycine Fe groups. This is likely attributable to enhanced heme Fe absorption in sows and the efficient transfer of heme Fe across the mammary gland barrier. Furthermore, during pregnancy, the fetus relies entirely on maternal nutrient supply, including trace elements such as iron, which must pass through the placenta. Our results indicate that the placental iron content in the heme Fe group was significantly lower than that in the control and glycine Fe groups.
4.5 Iron regulatory gene expressions in liver of neonatal piglets
Hepcidin regulates iron requirements in organisms through the regulation of iron metabolism-related proteins. Pigeon et al. reported that elevated iron levels in tissues or organisms may promote hepcidin synthesis (Pigeon et al., 2001). Robert Staroń et al. reported that in iron-deficient and iron-overloaded piglets, urinary hepcidin-25 concentrations correlated strongly with hepatic hepcidin mRNA abundance, plasma hepcidin-25 levels, iron transferrin saturation and non-heme liver iron levels (Staroń et al., 2015). The results of this experiment demonstrated that the gene expression of hepcidin was lowest in the iron deficiency group, suggesting that the body was in a state of iron deficiency. In contrast, the gene expression of hepcidin in both the heme Fe and glycine Fe groups was comparable, indicating that these two iron sources may effectively address iron deficiency.
Liver iron overload can result in the downregulation of Tfr1 and transferrin expression while upregulating ferritin expression (Barisani and Conte, 2002; Tolosano, 2015). Our results demonstrated that heme Fe supplementation leads to a reduction in Tfr1 and transferrin expression in the liver. Moreover, the supplementation with glycine Fe and FeSO4 exhibited comparable effects. In circulation, transferrin binds ferric iron (Fe3+) in a soluble and nontoxic form to deliver iron to the bone marrow and other tissues. Parrow et al. recently reported that mice harboring mutations in transferrin, which prevent iron binding at either lobe, exhibit hepatocellular iron overload and decreased liver expression of the iron-regulatory hormone hepcidin (Parrow et al., 2019). Therefore, the iron-deficient group exhibits a lower iron content, leading to an increase in transferrin levels and a decrease in ferrimodulin expression, thereby mobilizing more iron into circulation.
Flvcr1 mRNA expression levels in the liver of piglets from iron deficiency group sows were significantly higher than those in piglets from the Heme Fe and glycine Fe groups. Flvcr1 is a heme export protein and maternal heme metabolism contributes to normal fetal development (Quigley et al., 2004; Watanabe et al., 2004). In human tissues, Flvcr1 is highly expressed in the placenta, uterus, duodenum, liver, and cultured macrophages, suggesting that Flvcr1 can prevent heme toxicity and facilitate Heme Fe transport (Keel et al., 2008). The results of this experiment suggest that Flvcr1 gene expression is upregulated under conditions of iron deficiency in the body.
HO-1 mRNA expression in the liver of neonatal piglets from sows without iron supplementation was significantly lower than that of control, Heme Fe, and glycine Fe group piglets. Chang Cao reported that expression of HO-1 mRNA is strongly induced when heme is transported into cells or when heme turnover increases (Cao and O’Brien, 2013), which is consistent with the results presented here. In addition, the gene expression of OH-1 was significantly elevated in both the glycine Fe and FeSO4 groups compared to the iron deficiency group. Thus, HO-1 may indirectly influence the utilization efficiency of glycinate Fe and FeSO4 by modulating iron metabolic pathways (Suttner and Dennery, 2999).
4.6 Expression of iron regulatory genes in the placenta of sows
The placenta is the interface between the fetus and the mother and can restrict toxins entering the fetus (Al-Saleh et al., 2011). The absorption and transport of placental iron are regulated by a variety of proteins. In obese people, iron storage is decreased and Hepcidin expression is downregulated (Cepeda-Lopez et al., 2016). Hepcidin expression is closely correlated with serum iron levels and is inhibited by pregnancy (Finkenstedt et al., 2012). Maternal Hepcidin is essential for placental iron transport from the mother to the fetus (Tiker et al., 2006). Fetal Hepcidin balance is important for iron transport through the placenta. The results of this experiment demonstrated that hepcidin expression was significantly lower in the placentas of both the iron-deficient and heme Fe group compared to other groups. This suggests that the body may enhance iron transfer from mother to fetus under these conditions. Conversely, hepcidin expression was relatively higher in the iron glycine and FeSO4 groups.
Transferrin and Tfr1 expression levels in the placenta of iron-deficient sows were significantly higher than those in other groups. We hypothesize that the increased expression of transferrin in the placenta may enhance iron transport to the fetus, although further research is required to confirm this hypothesis.
Flvcr1 and Flvcr2 expression was highest in the placenta of iron deficiency and heme Fe group sows and was significantly higher than that in the control group. Jaacks reported that the metabolism of placental heme Fe results in increased placental Flvcr1 and Heme receptor expression (Jaacks et al., 2011). These findings reveal the transport mechanism of heme Fe in placenta, and that the observed high placental Flvcr1 expression was related to the increased iron transfer from mother to fetus.
Once maternal heme Fe supplementation is absorbed into the enterocyte, heme Fe is either catabolized by HO-1 into ferrous iron and subsequently incorporated into the labile iron pool as inorganic Fe, or it may be exported intact into circulation via the heme export protein Flvcr1 (Cao and O’Brien, 2013). The findings of this experiment demonstrated that HO-1 mRNA expression was significantly elevated in the placental tissue of sows in the heme Fe group compared to other groups, suggesting a greater transport of iron sources from the sow to the fetus.
Yutian Pu suggested that iron supplementation promotes the development of the intestine by improving its morphology, which maintains its mucosal integrity and enhances the expression of immuno-associated factors (Pu et al., 2018). Our results demonstrated that dietary supplementation with heme Fe, glycine Fe, and FeSO4 could enhance the birth weight and liver iron concentration of newborn piglets, effectively promote fetal growth and development, and alleviate anemia in newborn piglets. Notably, heme Fe exhibited superior efficacy compared to the other supplements. As the functional iron regulatory gene in piglets, downregulation of Hepcidin expression in pregnant sows would significantly upregulate transferrin, Tfr1, and Flvcr1 iron regulatory proteins, promoting the transfer of iron from mother to fetus. Our results also show that neonatal piglets of heme Fe group sows had the highest liver iron storage level and the sows had the highest colostrum iron content. The liver iron content (154.83 mg/kg) in piglets of the heme Fe group sows was 38.5% higher than in piglets of control group sows and 240.68% higher than in piglets of iron deficiency group sows. The iron content in the colostrum of heme Fe group sows was 2.8-fold higher than that in the control group sows, and more than four times higher than that in the iron deficiency group sows. Studies have reported that sows supplemented with cassava polysaccharide iron can enhance their reproductive capacity, improve colostrum composition, and promote the growth performance of suckling piglets (Deng et al., 2023). Comparable outcomes were achieved using heme Fe in this experiment. In addition, Zhang et al. reported that lactoferrin supplementation during pregnancy significantly enhanced iron storage in the heart, liver, spleen, and lungs of piglets compared to glycine Fe (Zhang et al., 2022). Our findings indicate that glycine Fe can also improve piglet iron storage; however, its efficacy is greater than that of heme Fe. Similarly, the efficacy of heme Fe was significantly greater than that of FeSO4. It was inferred that the heme Fe transporter in the placenta of sows might be different from the transporters of other iron sources, which are more efficient. Hepcidin might act through downregulating placental iron absorption-related proteins at the transcription level. Determining the mechanism by which regulation of Hepcidin-mediated placental iron intake and transfer to the fetal liver occurs requires additional studies.
5 Conclusion
Dietary supplementation with heme Fe, glycine Fe and FeSO4 in pregnant sows can enhance litter size and birth weight of piglets, increase the iron content in the liver and spleen of newborn piglets, elevate the iron concentration in sow colostrum, and significantly boost the HGB levels in piglets. This establishes that iron supplementation, regardless of the source, improves these outcomes compared to no supplementation. However, the heme Fe group exhibited a more pronounced effect.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal studies were approved and conducted in strict accordance with the ethicalrequirements of the Institutional Animal Care and Use Committee of Kunming University, China. The licence number is Kmu2023058. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
ML: Funding acquisition, Writing – original draft, Writing – review & editing. MZ: Data curation, Writing – review & editing. CZ: Formal Analysis, Writing – review & editing. QJ: Data curation, Writing – original draft. XW: Investigation, Writing – review & editing. YD: Software, Writing – review & editing. KC: Formal Analysis, Writing – review & editing. FJ: Writing – review & editing. SH: Writing – review & editing. RG: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Yunnan Province Basic Research Special Project General Project (No. 202401AT070033); Joint Special Project of Local Colleges and Universities in Yunnan General Project (No. 202301BA070001-009). Scientific Research Projects for Talent Introduction in Kunming University (No. YJL23006, No. YJL24015). The Innovation and Entrepreneurship Training Program for College Students (No.S202411393031).
Conflict of interest
Author QJ was employed by the company Yunnan Mudao Biotechnology Co., Ltd. Author FJ was employed by the company Kunming Kingmed Center for Clinical Laboratory Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Saleh I., Shinwari N., Mashhour A., Mohamed Gel D., and Rabah A. (2011). Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women. Int. J. Hyg. Environ. Health 214, 79–101. doi: 10.1016/j.ijheh.2010.10.001
Barisani D. and Conte D. (2002). Transferrin receptor 1 (TfR1) and putative stimulator of Fe transport (SFT) expression in iron deficiency and overload: an overview. Blood Cells Mol. Dis. 29, 498–505. doi: 10.1006/bcmd.2002.0588
Brain J. D., Heilig E., Donaghey T. C., Knutson M. D., Wessling-Resnick M., and Molina R. M. (2006). Effects of iron status on transpulmonary transport and tissue distribution of Mn and Fe. Am. J. Respir. Cell Mol. Biol. 34, 330–337. doi: 10.1165/rcmb.2005-0101OC
Cao C. and O’Brien K. O. (2013). Pregnancy and iron homeostasis: an update. Nutr. Rev. 71, 35–51. doi: 10.1111/j.1753-4887.2012.00550.x
Cepeda-Lopez A. C., Allende-Labastida J., Melse-Boonstra A., Osendarp S. J., Herter-Aeberli I., Moretti D., et al. (2016). The effects of fat loss after bariatric surgery on inflammation, serum hepcidin, and iron absorption: a prospective 6-mo iron stable isotope study. Am. J. Clin. Nutr. 104, 1030–1038. doi: 10.3945/ajcn.115.115592
Chibanda Y., Brookes M., Churchill D., Al-Hassi H., and Ferritin T. (2023). Hepcidin and cytokines link in the diagnoses of iron deficiency anaemia during pregnancy: A review. Int. J. Mol. Sci. 24, 13323. doi: 10.3390/ijms241713323
Deng S., Fang C., Zhuo R., Jiang Q., Song Y., Yang K., et al. (2023). Maternal supplementary tapioca polysaccharide iron improves the growth performance of piglets by regulating the active components of colostrum and cord blood. Animals 13, 2492. doi: 10.3390/ani13152492
Finkenstedt A., Widschwendter A., Brasse-Lagnel C. G., Theurl I., Hubalek M., Dieplinger H., et al. (2012). Hepcidin is correlated to soluble hemojuvelin but not to increased GDF15 during pregnancy. Blood Cells Mol. Dis. 48, 233–237. doi: 10.1016/j.bcmd.2012.02.001
Ganz T., Olbina G., Girelli D., Nemeth E., and Westerman M. (2008). Immunoassay for human serum hepcidin. Blood 112, 4292–4297. doi: 10.1182/blood-2008-02-139915
Hostetler C. E., Kincaid R. L., and Mirando M. A. (2003). The role of essential trace elements in embryonic and fetal development in livestock. Vet. J. 166, 125–139. doi: 10.1016/S1090-0233(02)00310-6
Hurrell R. (2010). Use of ferrous fumarate to fortify foods for infants and young children. Nutr. Rev. 68, 522–530. doi: 10.1111/j.1753-4887.2010.00312.x
Jaacks L. M., Young M. F., Essley B. V., McNanley T. J., Cooper E. M., Pressman E. K., et al. (2011). Placental expression of the heme transporter, feline leukemia virus subgroup C receptor, is related to maternal iron status in pregnant adolescents. J. Nutr. 141, 1267–1272. doi: 10.3945/jn.110.135798
Kasvosve I. and Delanghe J. (2002). Total iron binding capacity and transferrin concentration in the assessment of iron status. Clin Chem Lab Med. 2002, 1014–1018.
Keel S. B., Doty R.T., Yang Z., Quigley J.G., Chen J., Knoblaugh S., et al. (2008). A heme export protein is required for red blood cell differentiation and iron homeostasis. Science 319, 825–828. doi: 10.1126/science.1151133
Knight C., Klasing K., and Forsyth D. (1983). E. coli growth in serum of iron dextran-supplemented pigs. J. Anim. Sci. 57, 387–395. doi: 10.2527/jas1983.572387x
Layrisse M., et al. (2000). Iron bioavailability in humans from breakfasts enriched with iron bis-glycine chelate, phytates and polyphenols. J. Nutr. 130, 2195–2199. doi: 10.1093/jn/130.9.2195
Liu H. W., Gao L. M., Liu G. Y., Tai W. J., Xie C. Y., and Wu X. (2024). Effects of maternal dietary enteromorpha prolifera polysaccharide iron supplement on mineral elements and iron level of neonatal piglets. Biol. Trace Elem. Res. 202, 2588–2597. doi: 10.1007/s12011-023-03874-y
Mahan D. C. and Shields R. G. Jr. (1998). Macro- and micromineral composition of pigs from birth to 145 kilograms of body weight. J. Anim. Sci. 76, 506–512. doi: 10.2527/1998.762506x
Mahan D. C., Watts M. R., and St-Pierre N. (2009). Macro- and micromineral composition of fetal pigs and their accretion rates during fetal development. J. Anim. Sci. 87, 2823–2832. doi: 10.2527/jas.2008-1266
Merlot E., Clouard C., Resmond R., Robert C., Ferchaud S., and Prunier A. (2024). Effects of natural oral alternatives to parental iron supplementation on haematological and health-related blood parameters of organic piglets. Animal 18, 101194. doi: 10.1016/j.animal.2024.101194
National Research Council (NRC) (2012). Nutrient requirements of swine: eleventh revised edition (Washington DC: The National Academies Press). Available at: https://books.google.hu/books?hl=en&lr=&id=myQeL_v_i7sC&oi=fnd&pg=PP1&ots=tF6AQb1obZ&sig=HHx6nnpFRbd5ZZ9twl_vLRl45go&redir_esc=yv=onepage&q&f=fa (Accessed August 2012).
Nemeth E. and Ganz T. (2021). Hepcidin-ferroportin interaction controls systemic iron homeostasis. Int. J. Mol. Sci. 22, 6493. doi: 10.3390/ijms22126493
O’Brien K. O. (2022). Maternal, fetal and placental regulation of placental iron trafficking. Placenta 125, 47–53. doi: 10.1016/j.placenta.2021.12.018
Parrow N. L., Li Y., Feola M., Guerra A., Casu C., Prasad P., et al. (2019). Lobe specificity of iron binding to transferrin modulates murine erythropoiesis and iron homeostasis. Blood 134, 1373–1384. doi: 10.1182/blood.2018893099
Pigeon C., Ilyin G., Courselaud B., Leroyer P., Turlin B., Brissot P., et al. (2001). A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 276, 7811–7819. doi: 10.1074/jbc.M008923200
Pineda O. and Ashmead H. D. (2001). Effectiveness of treatment of iron-deficiency anemia in infants and young children with ferrous bis-glycinate chelate. Nutrition 17, 381–384. doi: 10.1016/S0899-9007(01)00519-6
Pu Y., Li S., Xiong H., Zhang X., Wang Y., and Du H. (2018). Iron promotes intestinal development in neonatal piglets. Nutrients 10, 726. doi: 10.3390/nu10060726
Quigley J. G., Yang Z., Worthington M. T., Phillips J. D., Sabo K. M., Sabath D. E., et al. (2004). Identification of a human heme exporter that is essential for erythropoiesis. Cell 118, 757–766. doi: 10.1016/j.cell.2004.08.014
Rydal M. P., Bhattarai S., and Nielsen J. P. (2021). An experimental model for iron deficiency anemia in sows and offspring induced by blood removal during gestation. Animals 11, 2848. doi: 10.3390/ani11102848
Soldin O. P., Bierbower L. H., Choi J. J., Choi J. J., Thompson-Hoffman S., and Soldin S. J. (2004). Serum iron, ferritin, transferrin, total iron binding capacity, hs-CRP, LDL cholesterol and magnesium in children; new reference intervals using the Dade Dimension Clinical Chemistry System. Clin. Chim. Acta 342, 211–217. doi: 10.1016/j.cccn.2004.01.002
Staroń R., Van Swelm R. P., Lipiński P., Gajowiak A., Lenartowicz M., Bednarz A., et al. (2015). Urinary hepcidin levels in iron-deficient and iron-supplemented piglets correlate with hepcidin hepatic mRNA and serum levels and with body iron status. PloS One 10, e0136695. doi: 10.1371/journal.pone.0136695
Sun C., Song R., Zhou J., Jia Y., and Lu J. (2023). Fermented bamboo fiber improves productive performance by regulating gut microbiota and inhibiting chronic inflammation of sows and piglets during late gestation and lactation. Microbiol. Spectr. 11, e0408422. doi: 10.1128/spectrum.04084-22
Suttner D. M. and Dennery P. A. (2999). Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 13, 1800–1809.
Szudzik M., Starzyński R.R., Jończy A., Mazgaj R., Lenartowicz M., and Lipiński P. (2018). Iron supplementation in suckling piglets: an ostensibly easy therapy of neonatal iron deficiency anemia. Pharmaceuticals 11, 128. doi: 10.3390/ph11040128
Tahara T., Sun J., Nakanishi K., Yamamoto M., Mori H., Saito T., et al. (2004). Heme positively regulates the expression of beta-globin at the locus control region via the transcriptional factor Bach1 in erythroid cells. J. Biol. Chem. 279, 5480–5487. doi: 10.1074/jbc.M302733200
Tiker F., Celik B., Tarcan A., Kilicdag H., Ozbek N., and Gurakan B. (2006). Serum pro-hepcidin levels and relationships with iron parameters in healthy preterm and term newborns. Pediatr. Hematol. Oncol. 23, 293–297. doi: 10.1080/08880010600629213
Tolosano E. (2015). Increasing serum transferrin to reduce tissue iron overload due to ineffective erythropoiesis. Haematologica 100, 565–566. doi: 10.3324/haematol.2015.124966
Wang J., Li D., Che L., Lin Y., Fang Z., Xu S., et al. (2014). Influence of organic iron complex on sow reproductive performance and iron status of nursing pigs. Livestock Sci. 160, 89–96. doi: 10.1016/j.livsci.2013.11.024
Wang J., Dong A., Liu G., Anderson G. J., Hu T. Y., Shi J., et al. (2016). Correlation of serum hepcidin levels with disease progression in hepatitis B virus-related disease assessed by nanopore film based assay. Sci. Rep. 6, 34252. doi: 10.1038/srep34252
Watanabe S., Akagi R., Mori M., Tsuchiya T., and Sassa S. (2004). Marked developmental changes in heme oxygenase-1 (HO-1) expression in the mouse placenta: correlation between HO-1 expression and placental development. Placenta 25, 387–395. doi: 10.1016/j.placenta.2003.10.012
Wu Y., Li Y., Miao Y., Wei H., Luo H., Ren C., et al. (2024). Source and level of dietary iron influence semen quality by affecting inflammation, oxidative stress and iron utilization levels in boars. J. Anim. Sci. Biotechnol. 15, 93. doi: 10.1186/s40104-024-01032-5
Keywords: anemia, heme Fe, hepcidin, piglet, sow
Citation: Li M, Zhao M, Zhang C, Ji Q, Wang X, Du Y, Chen K, Ji F, Huang S and Guo R (2025) Effects of maternal dietary heme Fe supplementation on liver iron levels and expression of iron regulatory genes in newborn piglets. Front. Anim. Sci. 6:1569306. doi: 10.3389/fanim.2025.1569306
Received: 31 January 2025; Accepted: 23 April 2025;
Published: 19 May 2025.
Edited by:
Crystal L. Levesque, South Dakota State University, United StatesReviewed by:
Jorge Perez Palencia, South Dakota State University, United StatesMiaomiao Han, Shanxi Agricultural University, China
Copyright © 2025 Li, Zhao, Zhang, Ji, Wang, Du, Chen, Ji, Huang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meiquan Li, bGltZWlxdWFuMjAxMEAxNjMuY29t; Rongfu Guo, cm9uZ2Z1Z0AxNjMuY29t
 Meiquan Li
Meiquan Li Meiwei Zhao1
Meiwei Zhao1 Chunyong Zhang
Chunyong Zhang Rongfu Guo
Rongfu Guo