- 1CSIRO, Australian Centre for Disease Preparedness, Geelong, VIC, Australia
- 2Melbourne Veterinary School, Faculty of Science, University of Melbourne, Werribee, VIC, Australia
- 3CSIRO, Health and Biosecurity, Geelong, VIC, Australia
Effective manual or chemical restraint of pigs for blood collection is crucial when conducting infectious disease research under high biocontainment laboratory conditions; however, these methods can introduce stress and physiological disruption. We aimed to establish proof-of-concept for the use of jugular vein vascular access ports (VAPs) for repeated, conscious blood collection in both individually and group housed pigs, trained using a positive reinforcement training regime. In study 1, pigs were housed individually, with three pigs implanted with VAPs. An additional control pig was not implanted with VAPs and was anaesthetised for all blood collections. Daily assessments of behaviour were conducted and saliva collected for corticosterone analysis. In study 2, pigs were housed in a group, with three pigs implanted with VAPs. Three control pigs were not implanted with VAPs and were anaesthetised for all blood collections. To assess the physiological impacts of both blood collection methods, heart rate variability, pulse rate and rectal temperature were assessed during a blood collection event. Across both studies 31 of 33 VAP blood collections were successful, with the remaining two collections successful using light sedation. An initial time and cost investment was required for VAP implantation, but the time required to collect blood via VAPs was halved compared to anaesthetising pigs for blood collection. One pig died during VAP surgery from anaesthesia-related complications. Pigs implanted with VAPs displayed low negative and high positive behaviour scores, low salivary corticosterone levels, and maintained steady clinical parameters during a blood collection event, whilst anaesthetised pigs displayed reduced HRV and temperature and increased pulse rate. This study provides proof-of-concept and recommendations for using VAPs in trained pigs under high biocontainment, as a welfare-positive method of conscious, repeated blood collection.
1 Introduction
Research into improved management and welfare of laboratory pigs is lacking, despite the importance of pigs as models for medical and agricultural research (Marchant-Forde and Herskin, 2018). The size and strength of pigs means that advancing best practice methodology for laboratory pig welfare and management has unique difficulties that are not as commonly encountered with smaller, more traditional laboratory species (Swindle et al., 2012). Despite these challenges, progressing best practice methods is needed for research institutes to maintain their societal licence to use animals (Bailey, 2018). For studies requiring blood samples, pigs typically require restraint to ensure successful blood collection and reduce the risk of injury to personnel. Restraint can be achieved via the use of anaesthesia, snares, or Panepinto-like slings (O’Malley et al., 2022). The requirement to work under high biocontainment for infectious and exotic disease research can further exacerbate the challenges associated with blood collection in pigs. These studies are typically undertaken with stringent animal handling, sample collection, and equipment-use protocols to maintain staff safety and the biocontainment of experimental pathogens (Copps, 2005). Therefore, novel methods of pig management are required.
The use of positive reinforcement training has been successfully employed for the voluntary collection of swabs and measurement of rectal temperature of conscious pigs, negating the need for manual restraint or anaesthesia (Layton et al., 2025). Further, pigs have been trained to walk into a Panepinto-like sling to be restrained for direct venous blood collection via the jugular or ear vein. However, this typically requires a time investment of 30–60 minutes of training per day over multiple weeks, which is prohibitive for many short-term studies (Yang et al., 2021). Positive reinforcement techniques can be combined with cannulation, particularly of the ear vein, for blood collection without repeated venepuncture. Externalised catheters have the benefit of being able to be implanted without an invasive surgical procedure, with blood being collected using the provision of rewards with and without light restraint. However, the presence of externalised hardware components can be problematic for use in group-housed pigs, externalised catheters typically require flushing and maintenance multiple times per day to maintain patency, and duration of patency is typically limited (Elane et al., 2024; Lombardo et al., 2010).Snares are a commonly utilised alternative method for successful and rapid pig restraint for direct venous blood collection, but the repeated use of snares causes fear-based aversion that amplifies stress and makes snaring more difficult over time (Rushen et al., 1993). As the induction of stress in laboratory pigs is problematic for both welfare and scientific integrity, stress-inducing animal management techniques and procedures should be minimised wherever possible (Layton et al., 2023). The use of anaesthesia is another option that can facilitate blood collection when repeat sampling events are required. Chemical restraint of pigs can reduce stress by removing or reducing the need for heavy manual restraint, and is important for protecting pig welfare during potentially stressful or painful procedures (Costea et al., 2023). However, anaesthesia can introduce a number of physiological and immunological disruptions, and should therefore be used judiciously (Layton et al., 2023).
Compared to repeated blood collection using snares or chemical restraint, vascular access ports (VAPs) can offer an alternative means of repeated blood collection. VAPs consist of a catheter line inserted into a vein (most commonly the jugular vein) that connects to a subcutaneous port, allowing blood samples to be drawn without the need for direct venous entry. Whilst a surgical procedure under general anaesthesia is required for initial implantation, the cumulative risk and welfare burden is arguably less for studies in which repeated use of snares and anaesthesia would otherwise be required (Bernal et al., 2022). As VAPs are entirely subcutaneous, the risk of damage from co-housed pigs is also less compared to more traditional externalised indwelling catheters, thereby reducing the risk of infection and improving patency (Chuang et al., 2005). VAPs may therefore be a more suitable and practical option for use in group-housed laboratory pigs, however their use under high biocontainment conditions for infectious disease research has not been investigated.
This study aimed to establish proof-of-concept for using VAPs for conscious, voluntary and unrestrained blood collection in trained pigs, housed both individually and in a group, under high biocontainment conditions. We hypothesised that using VAPs in pigs trained with a previously described positive reinforcement training (PRT) regime would reduce the consequences of repeated blood sampling when compared with sample collection requiring general anaesthesia, and that VAPs would facilitate successful conscious blood collection in both individual and group housing scenarios. VAP patency and the number of successful unrestrained blood collections was assessed in individually and group housed pigs, in addition to the cumulative time investment of blood collection via VAPs compared to repeat anaesthesia in a standard infectious disease pig study. Welfare and physiological measures were also evaluated, to determine the welfare impacts to pigs of VAP implantation and use.
2 Materials and methods
2.1 Ethics statement
Animal studies were approved by the Australian Centre for Disease Preparedness (ACDP) Animal Ethics Committee (permit numbers 2054 and 22008). All procedures were conducted per the guidelines of the National Health and Medical Research Council as described in the Australian code for the care and use of animals for scientific purposes, 8th edition (NHMRC, 2013).
2.2 Study design
Two independent studies were conducted to assess the implantation and use of VAPs for conscious blood collection in individually housed (study 1) and group housed (study 2) pigs within a high biocontainment facility. Both studies were performed in 6-week-old Landrace cross female pigs sourced from the same local commercial piggery (Geelong Victoria). Pigs were housed within the microbiologically secure animal facility at the ACDP under Biosafety Containment Level 3 conditions. In Study 1, individually housed pigs (n=3) underwent 21 days of PRT prior to VAP surgery (day 0), followed by blood collections three times per week for 19 days (n=8 total blood collections per pig). An additional pig that did not undergo PRT or VAP surgery was anaesthetised for all blood collections and was included as an observational comparison to VAP pigs to represent standard pig management procedures. In study 2, group housed pigs either underwent daily PRT for 14 days followed by VAP surgery (n=3) or did not undergo PRT or VAP surgery (n=3), followed by blood collections three times per week for 10 days (n=4 blood collections per pig). Figure 1 presents study 1 and 2 timelines and variables measured in each study (Figure 1).
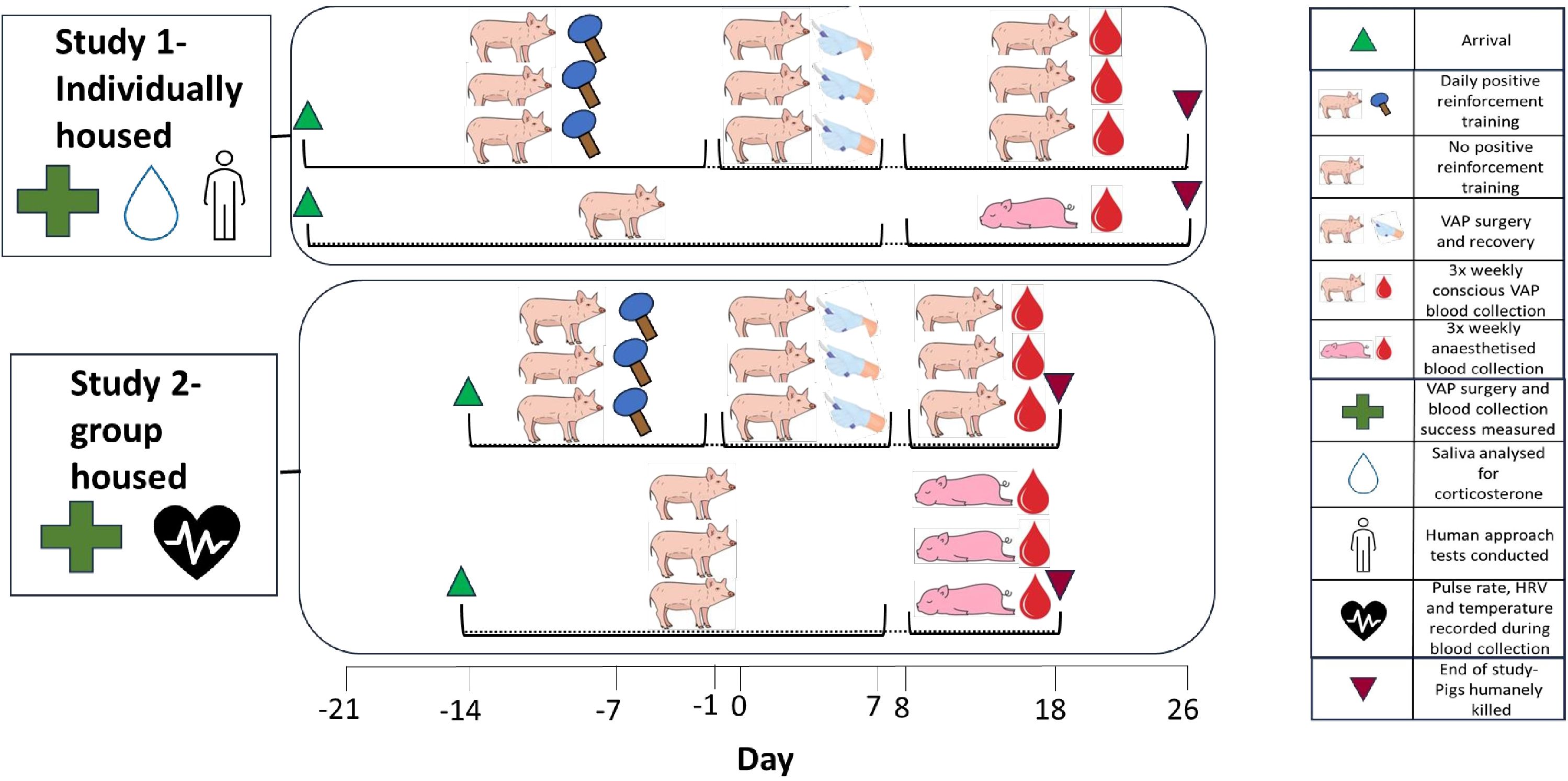
Figure 1. Study 1 and 2 timelines and variables measured for assessment of jugular vein vascular access ports under high biocontainment conditions.
2.3 Animal housing, husbandry and daily routine
Individually housed pigs were maintained in pens measuring 1.9 m x 2.96 m, within sight, sound and smell of one another. Group housed pigs (n=6) were maintained in a pen measuring 7 m x 2.96 m. Each pig pen was furnished with a plastic bed containing straw and a rubber mat, in addition to enrichment items (plastic balls, chains and rubber hosing) that were rotated daily. Barastoc™ pig grower pellets (Ridley Corporation, Melbourne, Australia) were available ad libitum. Room temperature was maintained at 22°C, and lights maintained on an 8-hour light/16-hour dark cycle. All daily husbandry was conducted prior to the initiation of PRT and blood collection. For individually housed pigs a human approach test (HAT) and saliva collection occurred daily, immediately following husbandry activities, from the day after arrival.
2.4 Vascular access port surgery and maintenance
External jugular vein vascular access ports were surgically implanted as described by Chuang et al. (2005) and Swindle et al. (2005) (Figure 2a). Pigs were anaesthetised intramuscularly with tiletamine/zolazepam 2.2 mg/kg (Virbac, Carros, France) combined with acepromazine 2 mg/kg (Boehringer Ingelheim, Germany), intubated and maintained on isoflurane in oxygen between 1.2–3%, 1–2 L/min O2 (Santa Cruz Animal Health, Dallas, USA). A maximum dose of 2.5mg/kg of bupivacaine (Marcain® 5mg/ml, Zoetis, Parsippany, USA) was administered subcutaneously at incision sites 10 minutes prior to surgery. Buprenorphine 0.05mg/kg (Zoetis, Parsippany, USA) and meloxicam 0.2mg/kg (Troy Animal Healthcare, Glendenning, Australia) were administered intramuscularly during recovery. Oral amoxycillin/clavulanic acid at 25 mg/kg (Zoetis, Parsippany, USA) was administered for 6 days, commencing 12 hours prior to surgery. A second dose of oral meloxicam at 0.2 mg/kg was provided 24 hours post-surgery. Pigs remained anaesthetised for approximately 2 hours, with a surgery time of approximately 90 minutes.
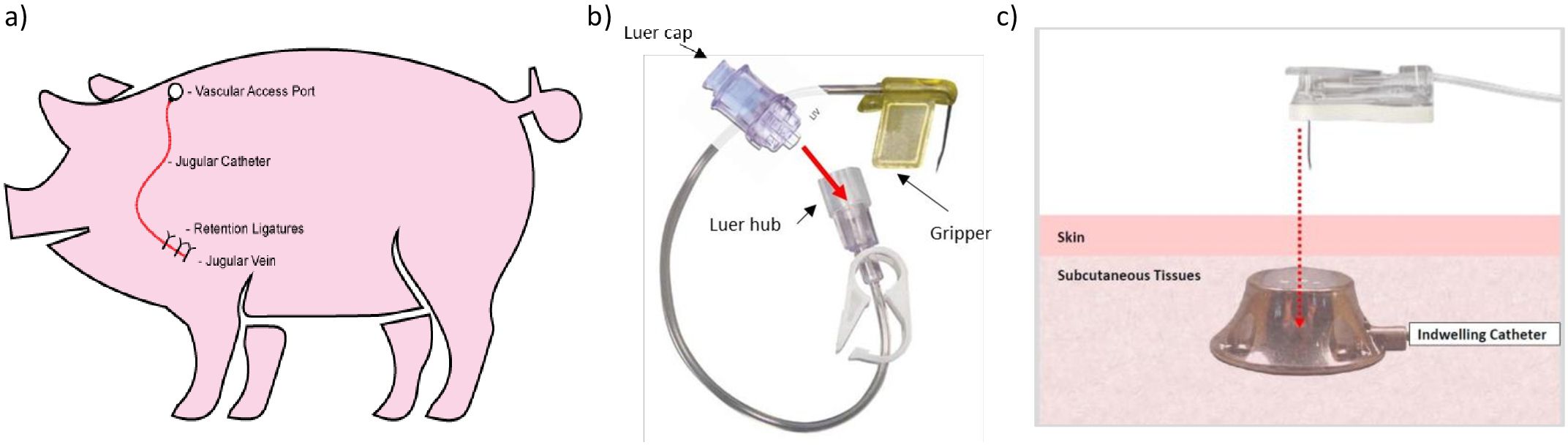
Figure 2. External jugular vein vascular access port implantation and use in pigs. Schematic of surgical implantation of an external jugular vein vascular access port for repeated conscious blood collection (a), prepared Huber needle and extension line (b) and vascular access port needle insertion technique (c).
Following surgery, group-housed pigs were maintained in individual recovery pens (1.9 m x 2.96 m) for 7 days in the same room within sight, sound and smell of other pigs. Pigs were then reintroduced to the group pen following this recovery period.
2.5 Human approach test for behavioural assessment
From the day after arrival a human approach test (HAT) was conducted daily for all individually housed pigs. After husbandry, video recording commenced from outside of the pen. The operator entered the pen and conducted a three-minute human approach test as described by Hemsworth et al. (1981) by standing silently in the pen corner. The operator moved their legs up and down slowly and rhythmically to discourage mouthing behaviour by the pigs during the three-minute HAT. A behavioural ethogram was utilised to assess pig behaviour during the daily HAT, with behaviours classified as either positive or negative and graded as described previously to assign both a positive and negative behaviour score (Layton et al., 2025). Daily positive behaviour scores were retrospectively categorised as low (<100), moderate (101–299) or high (300–500), based on a postulated maximum achievable positive behaviour score of 500. Daily negative behaviour scores were retrospectively categorised as low (<30), moderate (31–100) or high (101–170), based on a postulated maximum achievable negative behaviour score of 170. Positive behaviours were observed to more commonly occur in tandem (tail wagging, grunts, exploratory contact with operator) compared to negative behaviours (hard bites to operator, bar biting, squeals); therefore, positive behaviour score categories and limits were higher than those of negative behaviour scores.
2.6 Saliva collection for corticosterone analysis
Saliva was collected daily from individually housed pigs by allowing pigs to voluntarily chew on Sarstedt Salivette™ swabs for cortisol (Numbrecht, Germany). Salivette swabs were centrifuged within 12h of collection for 2 minutes at 1000 xg at 4°C, then saliva was stored at −80°C. Samples were thawed to room temperature prior to analysis, then analysed using 96-well corticosterone ELISA kits (ENZO Life Sciences, New York, USA) according to manufacturer’s instruction, as described by Layton et al. (2025).
2.7 Positive reinforcement training
Positive reinforcement training was conducted as described by Layton et al. (2025). Briefly, in Phase 1 pigs were trained to touch a wooden paddle with their snout, in phase 2 pigs were trained to enter a pig sling crate (Panepinto, Colorado, USA) and in phase 3 pigs were trained to remain stationary for the collection of blood via the VAP, either standing within the sling crate or within-pen.
2.8 Blood collection
Prior to VAP blood collection, skin directly over the VAP was cleaned with 70% ethanol, and topical anaesthetic cream was applied (lidocaine 25mg/g plus prilocaine 25mg/g) and secured with a Tegaderm™ patch (3M, North Ryde, NSW). The patch was removed after 40 minutes and then skin re-cleaned with 70% ethanol. A non-coring VAP Huber needle (22g, 1”) was attached to a line, hub and Luer valve (SAI Infusion Technologies, Lake Villa, Illinois) and primed with sterile saline. The Luer cap was then secured to the Luer valve (Figure 2b). The Huber needle and primed extension line was held by the ‘gripper’ (flexible plastic wings at the base of the needle) and inserted at a 90 degree angle (Figure 2c). Once the needle was inserted into the port, the heparinised saline locking solution (SAI Infusion Technologies, Lake Villa, Illinois) was withdrawn from the VAP line using a 10ml syringe containing 5ml sterile saline. A blood sample was then drawn from the line using an empty 10ml syringe, followed by flushing with 10ml of sterile saline to clear the VAP line of blood. The line was then injected with heparinised saline to completely fill the VAP line, and the needle removed. PRT commenced prior to needle insertion and concluded once blood sampling was complete and the needle was removed. Light sedation using intramuscular xylazine at 0.5 mg/kg (Troy Animal Healthcare, Glendenning, Australia) was administered when signs of stress on two needle insertion attempts were displayed (repeatedly moving away from operators, reacting to needle insertion or repeated startled vocalisations). Blood collection re-commenced once sedation took effect (~5 minutes after administration). For blood collection from pigs not undergoing training or VAP surgery, pigs were anaesthetised with Zoletil (zolazepam/tiletamine) 4 mg/kg and xylazine 2 mg/kg intramuscular. Blood was collected via the cranial vena cava using a 20 g needle and vacutainer.
2.9 Measurement of clinical parameters during a blood collection event
Pigs were fitted with modified PetPace collars (PetPace LLC, Burlington, Massachusetts, USA) for the collection of pulse rate and heart rate variability during a blood collection event. Collars were first modified for safe use in pigs by placing a single layer of gauze over the rubber signal amplifying cones on the inner surface of the collar, which was then wrapped in two layers of self-adhesive bandage. Collars were fitted to pigs 15 minutes prior to VAP blood collection (n=3) or administration of anaesthetic (n=3), and readings automatically collected every 5 minutes. Collars remained on pigs for a total of 180 minutes, which included baseline readings, anaesthetic administration (where relevant), blood collection, and recovery. Rectal temperatures were collected via a lubricated rectal thermometer at 20-minute intervals.
2.10 Statistical analysis
Differences in clinical parameters from baseline and between anaesthetised and VAP sampled pigs during a sample collection event were analysed with 2-way ANOVA with Tukey’s multiple comparisons. All data was analysed using GraphPad Prism (version 9.1.2, La Jolla, California, USA).
3 Results
VAPs were surgically implanted in three individually housed and three group-housed pigs, to investigate their use for conscious blood collection under high biocontainment conditions. VAP blood collections were successful in conscious individually and group housed pigs in 31 of 33 attempts. The remaining two blood collection events were successful after the administration of light sedation to one individually housed pig. When pigs were placed back into group-housing 7 days after surgery, no damage to the VAP sites from biting or chewing by co-housed pigs occurred. Pigs engaged readily with PRT and allowed blood collection to occur, touching a paddle to receive treats throughout the procedure. Blood collection via VAP in pigs trained with PRT was deemed safe and practical to conduct under biosafety level 3 high biocontainment conditions, requiring two or three staff. If pigs moved during blood collection (typically from excitement in anticipation of PRT and treats) the operator collecting the blood moved with the pig whilst continuing to draw blood, ensuring the extension line remained slack to avoid pulling on the line and needle. Anaesthetic administration, surgery time and recovery anaesthesia took approximately two hours per pig. The total labour time to collect a VAP blood sample was approximately seven minutes per pig, consisting of two minutes to clean the skin over the VAP and apply local anaesthetic cream, and five minutes to collect a blood sample and flush and lock the VAP line. An additional 40 minutes was required for contact time for local anaesthetic cream; however, as cream was applied prior to commencing daily husbandry, other in-room tasks were completed whilst waiting for local anaesthesia to take effect. Consequently, when blood collection was required from multiple VAP pigs, anaesthetic cream could be applied and blood samples collected from pigs consecutively, allowing three pigs to have blood collected in 55 minutes. The greater the number of pigs having blood collected via VAP, the greater the efficiency. For example, the total time for 10 pigs between commencing local anaesthetic cream application and locking the last VAP line would be approximately 90 minutes. One pig in the individually housed group died under anaesthetic after breathing ceased due to suspected anaesthesia-related complications. Malignant hyperthermia was investigated by assessing muscle tissue histologically and was determined not to be the cause of death, but no other cause was determined. For all animals, VAP patency was maintained with no complications for the study duration (between 18 and 28 days from VAP implantation to study end), and no additional maintenance of the VAPs was required between blood collections.
In comparison, all attempts for blood collection under general anaesthetic were successful in individually and group housed pigs (13 of 13). The total time to collect a blood sample from an anaesthetised pig was approximately 80 minutes per animal, requiring two or three staff. This included 5 minutes for anaesthetic administration and reaching adequate anaesthetic depth, 5 minutes for blood collection, and an additional 70 minutes for each pig to recover to standing. The total time to collect blood from three group-housed anaesthetised pigs (from general anaesthetic injection of the first pig to recovery to standing of the last pig) was approximately 105 minutes. When extrapolating to 10 pigs anaesthetised in consecutive pairs, the total time from administering anaesthetic to the first pig to the last pig recovering to standing would be 180 minutes. No pigs experienced complications during or after general anaesthesia, and integrity of the cranial vena cava was maintained throughout the studies.
The behaviour of individually housed pigs (VAP n=2, anaesthetised n=1) was measured daily via a human approach test, both prior to the commencement of and during the 3x weekly sample collection phase (Figure 3). The behaviour of pigs in the period between arrival and VAP surgery (during the positive reinforcement training phase) is presented previously (Layton et al., 2025). VAP pigs displayed moderate and high positive behaviour scores and low negative behaviour scores, during both the period of VAP surgery and recovery and during the period of regular conscious blood collections. In contrast to VAP sampled pigs, the anaesthetised pig displayed low and moderate positive behaviour scores, both in the periods prior to and during regular anaesthetised blood collections. During the 7 days prior to blood collections this pig displayed low and moderate negative behaviour scores, and low, moderate and high negative behaviour scores during anaesthetised blood collections (Figure 3).
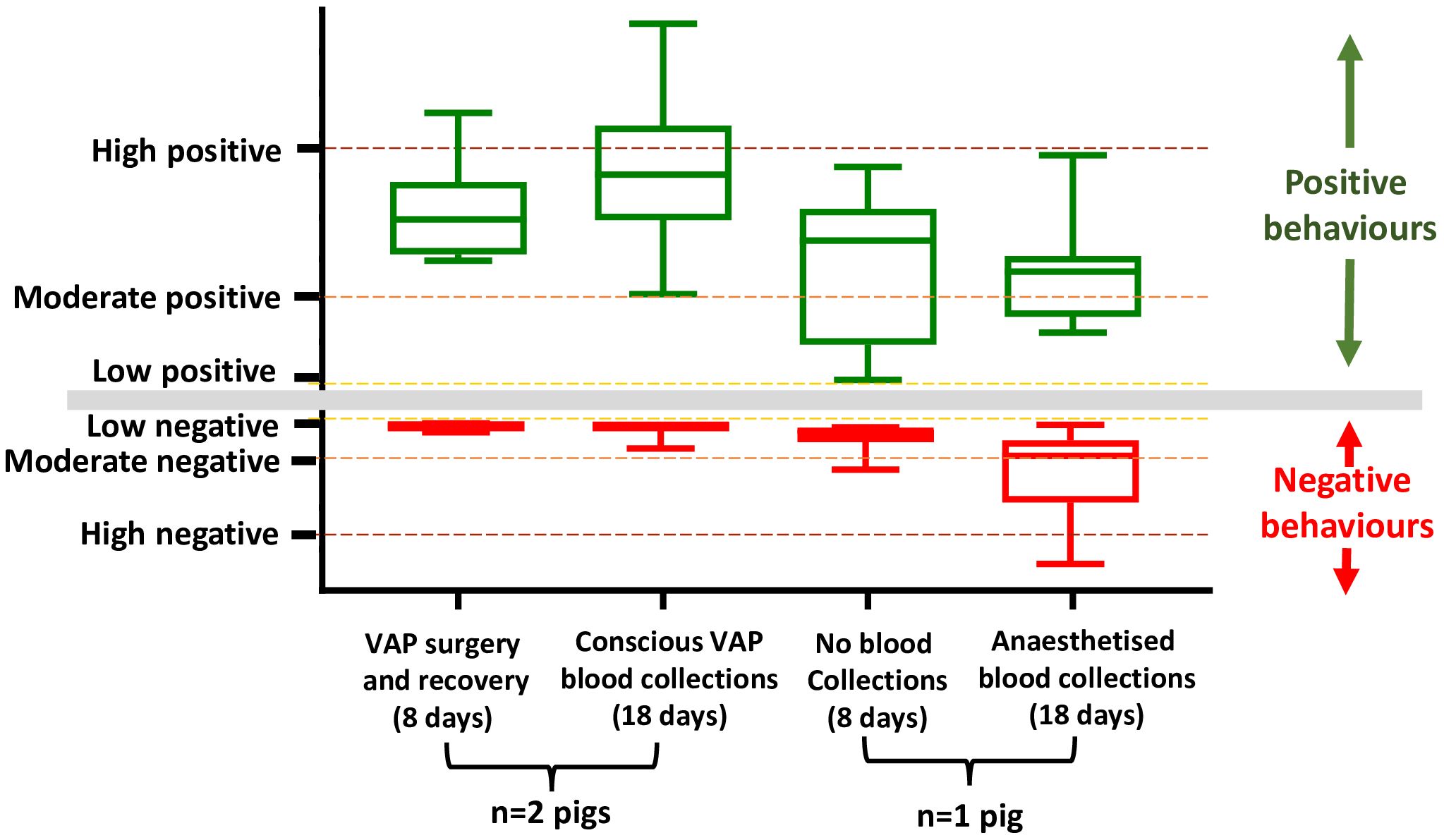
Figure 3. Pigs undergoing VAP surgery and conscious VAP blood collections display moderate-to-high positive and low negative behaviour scores. Pigs (n=2) were surgically implanted with VAPs on day 0 followed by 7 days for surgical healing and recovery (‘VAP surgery and recovery’). Blood was subsequently collected conscious from the same pigs via VAP 3x weekly for 19 days (‘Conscious VAP blood collections’). A single pig was not implanted with a VAP and underwent 8 days without blood collections (‘No blood collections’), followed by the same pig undergoing 3x weekly blood collections under anaesthesia for 19 days (‘anaesthetised blood collections’). Pigs were assessed daily using both a negative behaviour score and positive behaviour score immediately prior to sample collection events. Positive behaviour scores were categorised as low (<100 points), moderate (101–300 points) or high (>300 points). Negative behaviour scores were categorised as low (<30 points), moderate (31–100 points) or high (>100 points). Boxes display the mean and interquartile range; whiskers display the range.
Saliva was also collected daily from individually housed pigs for the measurement of salivary corticosterone, as an inference of stress. Daily salivary corticosterone concentrations of all pigs remained below the published limit for stressed pigs (4000 pg/ml) throughout the study, with the exception of the anaesthetised pig during the blood collection phase (Supplementary Figure 1). During the final week of blood collections, this pig displayed a single instance of corticosterone levels indicative of high stress (6,243 pg/ml) (Supplementary Figure 1).
The heart rate variability (HRV) of group-housed pigs undergoing conscious VAP (n=3) or anaesthetised (n=3) blood collection was measured every 20 minutes for 180 minutes, as an inference of stress and physiological disruption (Figure 4). Mean HRV at each timepoint (measured as vasovagal tonus index) of VAP sampled pigs remained between 11.18 and 11.65 prior to, during and after blood collection. There were no significant changes at any timepoints from baseline (p>0.05). In contrast, pigs anaesthetised for blood collection dropped from a mean HRV of 11.25 immediately prior to anaesthetic administration, to 8.67 at 60 minutes after anaesthetic administration (Figure 4). The HRV of anaesthetised pigs was significantly lower at 20 minutes compared to baseline (p<0.05) (Figure 4). Additionally, the HRV of pigs anaesthetised for blood collection was significantly lower than conscious VAP sampled pigs at 20 and 40 minutes (p<0.001) and 60 minutes (p<0001) after anaesthetic administration (Figure 4). Furthermore, rectal temperatures (39.3–39.8°C) and pulse rates (50–88 bpm) of all conscious VAP pigs remained within normal range throughout the 180-minute period (Supplementary Figure 2). In contrast, the rectal temperatures of pigs anaesthetised for blood collection dropped below the normal lower limit of 38°C at 80, 100 and 120 minutes after anaesthetic administration, and pulse rates rose above the normal upper limit of 90 bpm at the same timepoints (Supplementary Figure 2).
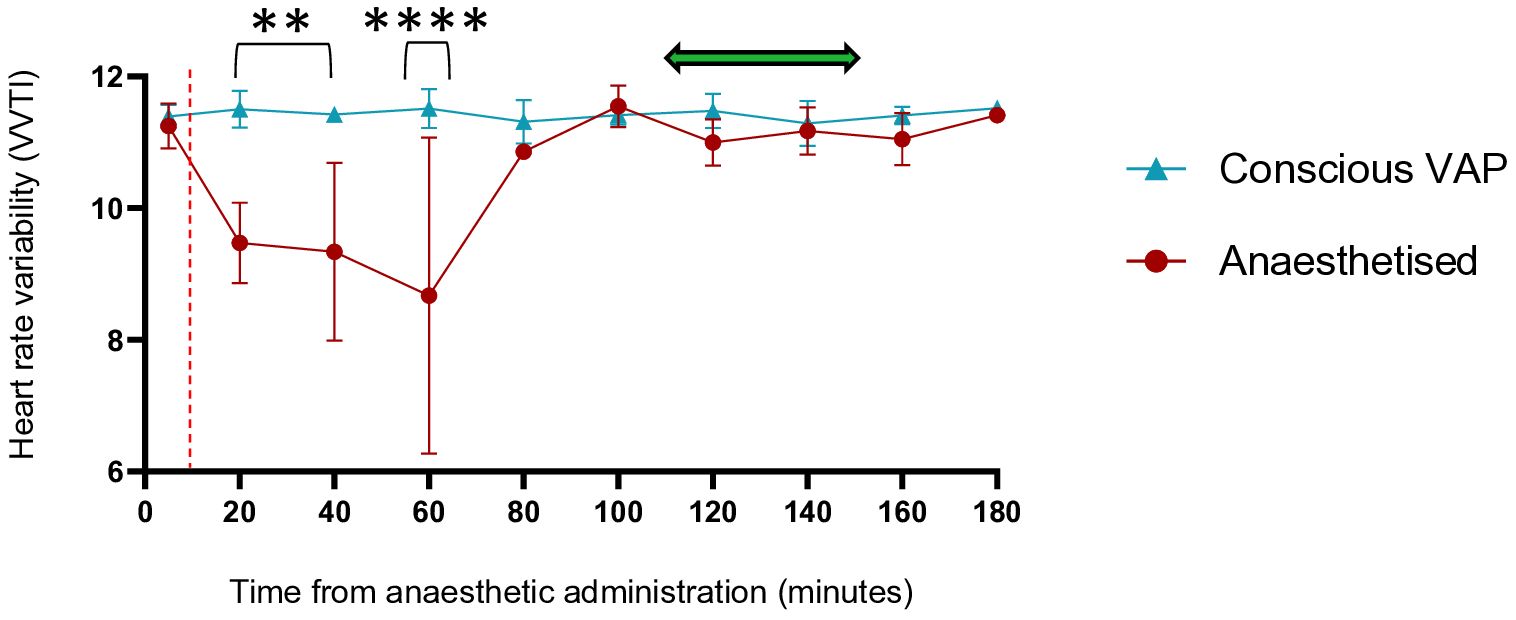
Figure 4. Heart rate variability remains stable in pigs sampled conscious via VAPs. Heart rate variability was measured as vasovagal tonus index (VVTI) via PetPace collar monitors. Conscious VAP pigs (n=3) had blood collected from a jugular vein vascular access port whilst standing unrestrained, anaesthetised pigs (n=3) had blood collected from the cranial vena cava. Red vertical line indicates the time of blood collection. ? = Time period of anaesthetic recovery to consistent standing of anaesthetised pigs. Comparisons at each timepoint were made between conscious VAP and anaesthetised pigs using 2-way ANOVA with multiple comparisons, P<0.0001 ****, p<0.001 **. Data points represent the median, error bars represent the range.
4 Discussion
The present study provides proof-of-concept for the use of VAPs for conscious, voluntary blood collection in both individually and group housed pigs under high biocontainment laboratory conditions. Of the five pigs that recovered successfully from the VAP surgical procedure, 31 of 33 blood collections were successful using positive reinforcement training techniques, without the use of any physical or chemical restraint. The successful use of VAPs in the present study reflects previous literature. Chuang et al. (2005) compared the use of indwelling catheters and jugular vein vascular access ports for repeated blood sampling in group housed pigs on-farm. The authors implanted pigs with either indwelling catheters or VAPs, followed by serial blood sampling. They found that whilst pigs implanted with indwelling catheters had a high rate of infection and thrombolytic complications, VAPs remained patent with no complications for the duration of the study. Blood draws in both groups were successful, but pigs implanted with VAPs required restraint in a sling to allow for successful blood draws (Chuang et al., 2005). By utilising the rapid PRT regime described by Layton et al. (2025) in conjunction with VAPs, the present study presents a novel method of collecting repeated blood from laboratory-housed pigs without the need for anaesthesia, snaring or specialised sling restraint equipment.
It is important to note that VAP implantation requires an additional time and cost investment (approximately 90 minutes per pig, in addition to the time required to prepare for surgery). This resource investment should be considered in the context of each study, as implanting pigs with VAPs would likely not be justified for studies with a low number of blood collection events. This is particularly relevant for studies where repeated blood collections are required for less than 5–7 days; whilst singly housing pigs to facilitate the use of externalised catheters may be a suitable option for studies of shorter duration, for longer studies the patency of externalised catheters is unlikely to be successfully maintained. Studies with a higher number of blood collections are likely to benefit from using VAPs instead of repeated anaesthesia, due to VAP blood collection taking half the time compared to anaesthetising pigs. As the number of blood collections increase, the time and labour costs of VAP surgery is increasingly justified. Whilst these reductions in time and labour cost for longer studies involving multiple blood collections is a benefit of VAP implantation in pigs, an additional consideration in the decision to use VAPs is the risk of surgical complications. Whilst no complications occurred in the 13 instances that pigs were anaesthetised for blood collections, one of six pigs that underwent VAP implantation died during surgery. The pig was unable to be successfully intubated at the beginning of surgery, and as such inhalational anaesthesia was provided with a firm fitted face mask; therefore, the ability to control anaesthetic depth, particularly when complications developed, was reduced. Endotracheal intubation is a notoriously difficult procedure in pigs, due to the unique and complex pig endotracheal anatomy. Whilst techniques such as adequate anaesthesia depth and intubating pigs in ventral recumbency have been shown to improve successful outcomes, it remains a technically challenging procedure (Theisen et al., 2009). The risk of surgical complications related to VAP surgery is therefore a risk and limitation that needs to be considered within the context of the research being conducted.
Optimised operator technique and equipment in the present study was a crucial component of successful VAP surgery and blood collection. When collecting blood from the VAP, inserting the needle firmly at a 90° angle was important for ensuring the needle entered the port and did not slip into adjacent tissue. One individually housed pig underwent two previous blood collection attempts where the needle missed the port (due to not advancing the needle firmly and rapidly) and entered the adjacent tissue, before the needle was successfully placed into the VAP. On the subsequent two blood collections, this pig required light sedation to facilitate successful VAP blood collection. Entry into the tissue adjacent to the VAP was a possible reason for the pig becoming needle-shy and requiring light sedation, as the topical local anaesthesia would likely not have penetrated to the depth of the needle (Wahlgren and Quiding, 2000). For operators unfamiliar with the use of VAPs for blood collection, the use of a VAP non-animal model or VAP-implanted cadavers is recommended prior to commencing VAP blood collection in conscious pigs.
In addition to the practical benefits of PRT-facilitated unrestrained VAP blood collections, VAP pigs displayed low negative behaviour scores, and moderate and high positive behaviour scores, both during VAP surgical recovery and whilst undergoing regular blood collections. Such behavioural indicators, including aversion to humans, have been shown to correspond with pain and stress levels in pigs. This was demonstrated in a study by Tallet et al. (2019), who assessed the behaviour of pigs submitted to tail docking with a cautery iron, sham docking, or no docking. The authors reported a higher incidence of adverse and avoidance behaviours toward a novel motionless human in pigs tail docked with a cautery iron, compared to pigs that underwent sham docking or no docking. As a result, the authors concluded that these behaviours indicated greater stress and pain in pigs tail docked with a cautery iron (Tallet et al., 2019). The lack of negative behaviours and maintenance of positive behaviours of individually housed VAP pigs in the present study therefore suggests that VAP surgery and use did not negatively impact the welfare of pigs, although larger study numbers are required to confirm this. The higher negative behaviour scores displayed by the anaesthetised pig during the blood collection period was in contrast to the behaviour of VAP pigs, however due to the low number of pigs this is an observation, and no definitive conclusions can be drawn. As behaviour assessment alone does not provide a robust picture of welfare state, additional indicators of welfare were also measured.
Salivary corticosterone is another minimally-invasive means of assessing stress in pigs; therefore, saliva was collected from individually housed pigs in the present study in addition to HATs. Corticosterone levels of VAP pigs were indicative of pigs that were not experiencing high levels of stress, as defined in a study by Rey-Salgueiro et al. (2018). In this study, the authors assessed salivary cortisol and corticosterone levels in pigs during transport to, and lairage at, an abattoir pre-slaughter. They found that salivary cortisol and corticosterone levels were highest after transport followed by 4 hours of lairage at the abattoir, with 4000 pg/ml corticosterone indicative of high stress in pigs (Rey-Salgueiro et al., 2018). In the present study, VAP sampled pigs remained under this salivary corticosterone level for the duration of VAP surgery, recovery and regular blood collections, suggesting that pigs were not highly stressed by these procedures. In contrast, the pig that was anaesthetised for blood collections displayed one instance of high salivary corticosterone (above 6000 pg/ml) during the period of regular blood collections. However, as only one pig was anaesthetised, this is observational only and further research with a greater number of pigs is required.
In addition to the assessment of behaviour and corticosterone levels in individually housed pigs, HRV during a blood collection event was measured in VAP (n=3) and anaesthetised pigs (n=3) as an additional measure of stress and physiological state. Whilst VAP pigs maintained steady HRV throughout the assessment period, the HRV of anaesthetised pigs dropped significantly from baseline at the 20 minute timepoint. Additionally, the HRV of anaesthetised pigs was significantly lower than that of VAP pigs at multiple timepoints throughout the assessment period. HRV is the fluctuation in the length of heart beat intervals, and is a means of quantifying the ability of the heart to respond to physiological and environmental stressors and stimuli (Kim et al., 2018). A higher HRV correlates with improved autonomic nervous system regulation, whilst a lower HRV is an indicator of psychological stress (Endukuru and Tripathi, 2016). General anaesthesia is also known to affect HRV, due to its impacts on the autonomic nervous system. A study by Zhan et al. (2021) assessed HRV outputs against different levels of consciousness in anaesthetised human patients. The authors found that as the depth of anaesthesia increased, HRV decreased (Zhan et al., 2021). This correlation is therefore consistent with the reduction in HRV observed in anaesthetised pigs in the present study. As pigs that had blood collected via VAP did not receive anaesthesia, any changes to HRV throughout the collection event could be reasonably attributed to stress and psychological state. The consistency of HRV readings from conscious sampled VAP pigs therefore demonstrated no detection of stress measured via HRV, and indicates that pigs remained relaxed during the VAP blood collection procedure. This preliminary finding supports further investigation of VAPs as a low-stress method for conscious blood collection in trained pigs.
Rectal temperature and pulse rates were also collected from group housed VAP and anaesthetised pigs during the same sample collection and recovery event. Pigs that were sampled consciously via VAPs maintained steady temperature and pulse rates within normal limits throughout the 180-minute period. In contrast, pigs that were anaesthetised for blood collection displayed a significant decrease in temperature (to below the lower normal limit) and a significant increase (to above the normal upper limit) in pulse rate at the 80, 100 and 120 minute timepoints. However, rectal temperature and pulse rates remained within normal limits up until 80 minutes post-anaesthetic administration. This is consistent with a study by Lee et al. (2010), who assessed the rectal temperature and pulse rate of pigs undergoing the same anaesthesia regime as used in the present study. The authors assessed pigs for 70 minutes after anaesthetic administration, and did not observe heart rates above 100 beats per minute. Additionally, drops in rectal temperatures relative to baseline were observed, but were not statistically significant and remained above 38°C (Lee et al., 2010). This supports the results of the present study, and suggests that short anaesthesia events are less likely to significantly disrupt the maintenance of temperature and heart rate in pigs. Where pigs would remain anaesthetised for longer than 70 minutes, the use of VAPs for conscious blood collection is likely to improve the maintenance of temperature and heart rate within normal limits.
The results from this preliminary study indicate that conscious blood collection using VAPs and PRT can provide a welfare-positive option for repeated blood collection in research pigs, including under biosafety level 3 high biocontainment conditions. VAPs can provide an option for repeated blood samples over a longer duration (>7 days) and in a group-housing scenario, where externalised catheters for conscious blood collection are unlikely to maintain patency and can be damaged by co-housed pigs. Whilst VAP implantation requires an initial time and cost investment and is therefore not justified for all studies, time and labour cost savings at each blood collection event compared to using general anaesthesia make VAPs a suitable option for studies requiring a greater number of blood collections. Whilst eliminating anaesthesia for repeated blood collections can reduce variability in study data, care must be taken to control for variables that may be introduced by VAP implantation surgery. This can be achieved by adequately controlling surgical pain and inflammation by utilising multimodal anaesthesia and analgesia during and post-surgery, optimising sterile technique to prevent surgical infection, and allowing sufficient time for surgical healing prior to a study commencing. Optimal surgery recovery time should be determined by the rate of recovery and individual study objectives, but a minimum of 7 days is recommended. Consideration should also be given to the welfare impacts of an invasive surgical procedure, and potential impacts on the statistical power of a study if a pig dies or is deemed not fit due to surgical complications. For these reasons, VAPs will not be the most suitable method of blood collection for every study; however, when VAPs are used, intubating pigs for surgery to maintain optimal surgical anaesthetic control and oxygen delivery is vital to reduce these risks. Additionally, suitable surgical expertise, monitoring equipment and surgical monitoring protocols are crucial for effective surgical risk mitigation. The use of non-animal models and cadavers for training prior to VAP surgery and use in live pigs is also recommended to optimise study and welfare outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Australian Centre for Disease Preparedness Animal Ethics Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
RL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. DB: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Visualization, Writing – review & editing. PM: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing. AF: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing. DL: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing. MB: Conceptualization, Methodology, Resources, Visualization, Writing – review & editing. TA: Data curation, Investigation, Methodology, Resources, Visualization, Writing – review & editing. ES: Data curation, Investigation, Methodology, Resources, Visualization, Writing – review & editing. SR: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Visualization, Writing – review & editing. GT: Data curation, Investigation, Methodology, Visualization, Writing – review & editing. DW: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Visualization, Writing – review & editing. KS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding support was provided through an Australian Government Research Training Program Scholarship and an Annual Performance and Investment Review grant provided by the CSIRO, grant number 225256.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1573822/full#supplementary-material
References
Bailey J. (2018). Does the stress of laboratory life and experimentation on animals adversely affect research data? A critical review. Alternat to Lab. Anim. 46, 291–305. doi: 10.1177/026119291804600501
Bernal J., Adrian S., Burkart H., and Laffins M. (2022). Guideline for vascular access port use and maintenance in large animals for biomedical research. Surgeries 3, 219–228. doi: 10.3390/surgeries3030024
Chuang M. S., Orvieto M. A., Laven B. A., Gerber G. S., Wardrip C., Ritch C., et al. (2005). Comparison of external catheters with subcutaneous vascular access ports for chronic vascular access in a porcine model. J. Am. Assoc. Lab. Anim. Sci. 44, 24–27.
Copps J. (2005). Issues related to the use of animals in biocontainment research facilities. ILAR J. 46, 34–43. doi: 10.1093/ilar.46.1.34
Costea R., Ene I., and Pavel R. (2023). Pig sedation and anesthesia for medical research. Animals 13 (24), 3807. doi: 10.3390/ani13243807
Elane G. L., Bauck A. G., Hobbs K. J., King A., Fields C., Ziegler A., et al. (2024). Review of venipuncture and intravenous catheterization techniques in pigs. J. Am. Vet Med. Assoc. 262, 1388–1396. doi: 10.2460/javma.24.03.0169
Endukuru C. K. and Tripathi S. (2016). Evaluation of cardiac responses to stress in healthy individuals-a non-invasive evaluation by heart rate variability and stroop test. Int. J. Sci. Res. 5, 286–289.
Hemsworth P., Brand A., and Willems P. (1981). The behavioural response of sows to the presence of human beings and its relation to productivity. Livestock Prod Sci. 8, 67–74. doi: 10.1016/0301-6226(81)90031-2
Kim H. G., Cheon E. J., Bai D. S., Lee Y. H., and Koo B. H. (2018). Stress and heart rate variability: a meta-analysis and review of the literature. Psychiatry Invest. 15, 235. doi: 10.30773/pi.2017.08.17
Layton R., Beggs D., Fisher A., Mansell P., Riddell S., Layton D., et al. (2025). A positive-reinforcement training regimen for refined sample collection in laboratory pigs. Animals 15, 471. doi: 10.3390/ani15040471
Layton R., Layton D., Beggs D., Fisher A., Mansell P., and Stanger K. J. (2023). The impact of stress and anaesthesia on animal models of infectious disease. Front. Vet Sci. 10. doi: 10.3389/fvets.2023.1086003
Lee J. Y., Jee H. C., Jeong S. M., Park C. S., and Kim M. C. (2010). Comparison of anaesthetic and cardiorespiratory effects of xylazine or medetomidine in combination with tiletamine/zolazepam in pigs. Vet Record 167, 245–249. doi: 10.1136/vr.c3611
Lombardo C., Damiano G., Cassata G., Palumbo V. D., Cacciabaudo F., Spinelii G., et al. (2010). Surgical vascular access in the porcine model for long-term repeated blood sampling. Acta BioMed. 81, 101–103.
Marchant-Forde J. N. and Herskin M. S. (2018). “Pigs as laboratory animals,” in Advances in Pig Welfare (Sawston, Cambridge: Woodhead Publishing), 445–475. doi: 10.1016/B978-0-08-101012-9.00015-0
O’Malley C. I., Hubley R., Tambadou H., and Turner P. V. (2022). Refining restraint techniques for research pigs through habituation. Front. Vet Sci. 9. doi: 10.3389/fvets.2022.1016414
NHMRC. (2013). Australian Code for the Care and Use of Animals for Scientific Purposes (Canberra, Australia: National Health and Medical Research Council), ISBN: ISBN 1864965975.
Rey-Salgueiro L., Martinez-Carballo E., Fajardo P., Chapela M. J., Espiñeira M., and Simal-Gandara J. (2018). Meat quality in relation to swine well-being after transport and during lairage at the slaughterhouse. Meat Sci. 142, 38–43. doi: 10.1016/j.meatsci.2018.04.005
Rushen J., Schwarze N., Ladewig J., and Foxcroft G. (1993). Opioid modulation of the effects of repeated stress on ACTH, cortisol, prolactin, and growth hormone in pigs. Physiology & Behavior. 53 (5), 923–928. doi: 10.1016/0031-9384(93)90270-P
Swindle M. M., Makin A., Herron A. J., Clubb F. J. Jr., and Frazier K. S. (2012). Swine as models in biomedical research and toxicology testing. Vet Pathol. 49, 344–356. doi: 10.1177/0300985811402846
Swindle M. M., Nolan T., Jacobson A., Wolf P., Dalton M. J., and Smith A. C. (2005). Vascular access port (VAP) usage in large animal species. J. Am. Assoc. Lab. Anim. Sci. 44, 7–17.
Tallet C., Rakotomahandry M., Herlemont S., and Prunier A. (2019). Evidence of pain, stress, and fear of humans during tail docking and the next four weeks in piglets (Sus scrofa domesticus). Front. Vet Sci. 6. doi: 10.3389/fvets.2019.00462
Theisen M. M., Maas M., Hartlage M. G., Ploner F., Niehues S. M., Van Aken H. K., et al. (2009). Ventral recumbency is crucial for fast and safe orotracheal intubation in laboratory swine. Lab. Anim. 43, 96–101. doi: 10.1258/la.2008.008044
Wahlgren C. F. and Quiding H. (2000). Depth of cutaneous analgesia after application of a eutectic mixture of the local anesthetics lidocaine and prilocaine (EMLA cream). J. Am. Acad. Dermatol. 42, 584–588. doi: 10.1067/mjd.2000.104303
Yang H. Y., Galang K. G., Gallegos A., Ma B. W., and Isseroff R. R. (2021). Sling training with positive reinforcement to facilitate porcine wound studies. JID Innov 1, 100016. doi: 10.1016/j.xjidi.2021.100016
Keywords: swine, welfare, anaesthesia, restraint, stress, VAP
Citation: Layton R, Beggs D, Mansell P, Fisher A, Layton D, Boyd M, Allen T, Soldani E, Riddell S, Taylor G, Williams DT and Stanger KJ (2025) Jugular vein vascular access ports for serial pig blood sampling in high biocontainment infectious disease studies. Front. Anim. Sci. 6:1573822. doi: 10.3389/fanim.2025.1573822
Received: 09 February 2025; Accepted: 30 April 2025;
Published: 22 May 2025.
Edited by:
Pavan Kumar, Guru Angad Dev Veterinary and Animal Sciences University, IndiaReviewed by:
Malan Strbenc, University of Ljubljana, SloveniaUbedullah Kaka, Putra Malaysia University, Malaysia
Harvie P. Portugaliza, Visayas State University, Philippines
Copyright © 2025 Layton, Beggs, Mansell, Fisher, Layton, Boyd, Allen, Soldani, Riddell, Taylor, Williams and Stanger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel Layton, UmFjaGVsLkxheXRvbkBjc2lyby5hdQ==
 Rachel Layton
Rachel Layton David Beggs
David Beggs Peter Mansell
Peter Mansell Andrew Fisher
Andrew Fisher Daniel Layton
Daniel Layton Matthew Boyd1
Matthew Boyd1 David T. Williams
David T. Williams Kelly J. Stanger
Kelly J. Stanger