- Department of Poultry Science, University of Arkansas, Fayetteville, AR, United States
Understanding how poultry perceive and interpret their environment is essential to enhancing their welfare. Animal welfare science relies on measures of the behavioral and physiological components of affective states (positive and negative) as welfare indicators. There has been growing interest in using the judgment bias test (JBT) to study birds’ affective states by assessing their responses to ambiguous cues. The objective of this study was to investigate the affective state of two chicken breeds with different evolutionary histories: the Red Junglefowl (RJF), the primary ancestor of modern chickens, and the Athens Canadian Random Bred (ACRB), a rustic domesticated breed, using the JBT at two ages. Another objective was to explore the effect of the JBT on the fear and stress responses of the chickens that participated in the JBT compared to those that did not (NJBT) on days (D) 35 and 63. Fear was evaluated using the tonic immobility test, and stress was measured noninvasively from thermal images of the eye and beak. Chickens successfully discriminated between positive (POS) and neutral (NEU) JBT cues, showing shorter latencies to approach the POS cue (P < 0.0001). While there were no breed differences on D29 of the JBT, RJF chickens exhibited shorter latencies to approach cues than ACRB on D60 (P < 0.001). Independent of testing at both ages, RJF had a longer duration of tonic immobility than ACRB (P < 0.01), indicating higher fearfulness. While the JBT did not affect D35 tonic immobility, JBT chickens had longer tonic immobility durations than NJBT on D63 (P < 0.05). Chickens that participated in the JBT had lower eye and beak minimum surface temperatures than NJBT (P < 0.05), indicating that the JBT may have increased stress post-testing. These findings highlight the influence of domestication on the affective states and the importance of considering fear and stress in measuring the affective states of chickens.
Introduction
Cognitive behavioral tests can be used to understand the affective states of animals when evaluating their welfare (Fraser, 2008). Affective states, referring to an individual’s moods and emotions (Paul et al., 2005), can have positive or negative valence and can be measured by observing changes in behavior, physiology, and cognition (Mendl et al., 2010; Kremer et al., 2020). The assessment of these states has become a primary research focus due to the growing interest in improving animal welfare by reducing negative and increasing positive affective states (Boissy et al., 2007). However, quantifying the subjective component of affective states remains challenging (Boissy and Lee, 2014; Mendl and Paul, 2004).
Ethologists use the judgment bias test (JBT) to explore how affective states impact cognition. Animals trained to discriminate between positive and negative cues are presented with ambiguous cues between known positive and negative stimuli (Harding et al., 2004). These cues can vary, involving spatial (Lindqvist et al., 2007), visual (Salmeto et al., 2011), auditory (Murphy et al., 2013), olfactory (Boleij et al., 2012), tactile (Brydges and Hall, 2017), or multimodal stimuli (Bethell, 2015). The JBT is used to evaluate how animals perceive ambiguous stimuli based on their affective state (Mendl and Paul, 2004; Mendl et al., 2010; Roelofs et al., 2016), with animals in a negative affective state interpreting ambiguous cues pessimistically and those in a positive state interpreting them optimistically. An animal’s approach or avoidance behavior in response to ambiguous cues is interpreted as their affective state (Mendl et al., 2009; Deakin et al., 2016; Košťál et al., 2020).
The JBT has been used to evaluate how affective states influence the decision-making process in domestic chickens (Salmeto et al., 2011). Various experimental designs have used JBTs to assess the affective states of chickens, examining the effects of environmental conditions (Deakin et al., 2016; Zidar et al., 2018; Ross et al., 2019; Anderson et al., 2021), genetic selection for feather pecking behavior (Pichová et al., 2021), reward cycle disruption (Seehuus et al., 2013), corticosterone injections (Iyasere et al., 2017), pharmacological interventions using an anxiety-depression model (Hymel and Sufka, 2012), and acute stress (Hernandez et al., 2015). Understanding the affective state of animals is a critical component of their welfare. However, the testing process itself involves acclimating animals to a novel environment and subjecting them to multiple phases of daily handling for training and testing, which are stressors that can compromise their welfare (Roelofs et al., 2016; Browning, 2022). Fear and stress are fundamental aspects of animal behavior that significantly affect their response to stimuli and environments (Boissy, 1995).
Fear, an adaptive mechanism crucial for survival, is a short-term emotional response that motivates flight from, freezing in, or fighting a perceived imminent danger or threat (Steimer, 2002). When fear becomes excessive or chronic, it may adversely affect welfare and productivity (Jones, 1986). Ethologists assess fearfulness through behavioral assays, such as tonic immobility, that expose chickens to threatening stimuli and monitor their responses. Tonic immobility is a common method to test fear in chickens (Forkman et al., 2007) because it is a natural, temporary paralysis behavior expressed under threat (Miyatake et al., 2009). Previous studies have found that different breeds exhibit diverse fear and stress responses, indicating that genetic factors affect the perception of fearful stimuli and stressors (Albentosa et al., 2003; Abe et al., 2013; Ferrante et al., 2016; Peixoto et al., 2020). Birds exhibit individual differences in fear and stress responses, with birds showing higher fearfulness having greater stress responses (Cockrem, 2007).
Stress has the potential to adversely affect cognitive functions, resulting in impairments in decision-making and problem-solving abilities (Zidar et al., 2018). Affective states in chickens can be assessed through physiological responses, such as changes in body temperature that can be detected using infrared thermography (Boissy et al., 2007; Moe et al., 2017). The stress response in chickens begins with the activation of the sympathetic nervous system, triggering the release of catecholamines and stimulating the hypothalamic-pituitary-adrenal axis to release glucocorticoids (Carsia, 2015; Ouyang et al., 2021). This process causes peripheral vasoconstriction, redirecting blood flow from the extremities and surface of the skin to vital organs to conserve heat (Bolton and Bowman, 1969). Consequently, the core body temperature increases, resulting in the surface temperature of the skin decreasing as part of this physiological adjustment (Edgar et al., 2013). Stress induces these temperature changes and can negatively impact the cognitive function of chickens (Zidar et al., 2018; Campderrich et al., 2019). These physiological indicators provide insights into affective states.
The objective of this study was to investigate the affective state of two chicken breeds with different evolutionary histories: the Red Junglefowl, the primary ancestor of modern chickens (Gyles et al., 1966), and the Athens Canadian Random Bred, a rustic domesticated breed representing a broiler from the 1950s (Hess, 1962), using the JBT at two ages. A second objective was to explore the effect of the JBT on the fear and stress responses of the chickens that participated in the JBT compared to those that did not. We hypothesized that the Red Junglefowl chickens would respond more pessimistically to the JBT and exhibit heightened fear and stress responses compared to the Athens Canadian Random Bred chickens.
Materials and methods
Animals and facilities
This experiment was carried out at the University of Arkansas Poultry Research Farm. All methods were approved by the University of Arkansas Division of Agriculture Institutional Animal Care and Use Committee. On day-of-hatch, 160 Red Junglefowl (RJF; N = 80) and Athens Canadian Random Bred chickens (ACRB; N = 80) were vent sexed and wing tagged for individual identification, yielding 40 males and 40 females of each breed. Chickens from each breed were housed separately in two pens (3.05 m × 4.57 m) with clean wood shavings for a space allowance of 0.17m2 per bird within the same facility (6.17 m x 6.17 m). Feed and water were provided ad libitum; each pen had four tube feeders and a nipple water line. Chickens were kept under a photoperiod of 23 hours of light (L) and 1 hour of dark (D) at chick placement, and dark hours were increased until the photoperiod was 16L:8D from day 14 until the end of the study. The room temperature was set at 32.2°C at chick placement and was incrementally decreased to 20°C on day 28 until the end of the study. Chickens were monitored twice daily to ensure good health and welfare.
Judgment bias test
Bird selection
Not all chickens participated in the judgment bias test. At hatch, chicks were weighed (g), and 15 males and 15 females from each breed were selected according to average body weight within the median range (RJF = 32 g; ACRB = 41 g) on day (D) 0 for a total of 60 chicks selected for testing. To facilitate visual identification of chickens selected for testing from the population, they were marked with non-toxic livestock spray (All-weather, LA-CO Industries, Inc., IL, USA) and were re-marked as necessary throughout the study. All birds were monitored twice daily, and no behavioral or physical indicators of aggression were observed.
Test arena
The judgment bias test (JBT) was carried out in a separate identical facility (617 cm x 617 cm) adjacent to the housing facility, in an arena made from plywood (91 cm × 61 cm × 61 cm) and a black plastic start box (35 cm × 35 cm × 35 cm) with sliding doors on the front and top (Supplementary Figure S1). The temperature and lighting in the testing facility were the same as in the home pen.
Selected chickens participated in the JBT at two ages, from D2-29 (JBT 1) and D55-60 (JBT 2). Chickens were tested in groups of males and females by the same observer, with each group exclusively comprising individuals of the same breed, and the breed groups alternated between each test. There were 5 phases of the JBT: 1) acclimation, 2) training, 3) reinforcement, 4) discrimination, and 5) the judgment bias test. The positive reward (POS) was a white cardboard tray with dried mealworms, and the neutral (NEU) reward was a black cardboard tray (containing nothing). The days, order, number of chickens tested, sex per trial, number of trials, and trial durations for each phase are detailed in Supplementary Table S1. The number and percentage of chickens that met the pass criteria in each phase of the JBT are in Table 1. Chickens were acclimated and trained in randomly selected pairs of males and females during subsequent test phases to reduce the stress of a novel environment, handling, and social isolation (Gjøen et al., 2023). When an odd number of chickens remained on a testing day, a naïve chicken of the opposite sex that was not previously tested was added to maintain the pairing structure to control for the social aspect in every phase of the JBT.
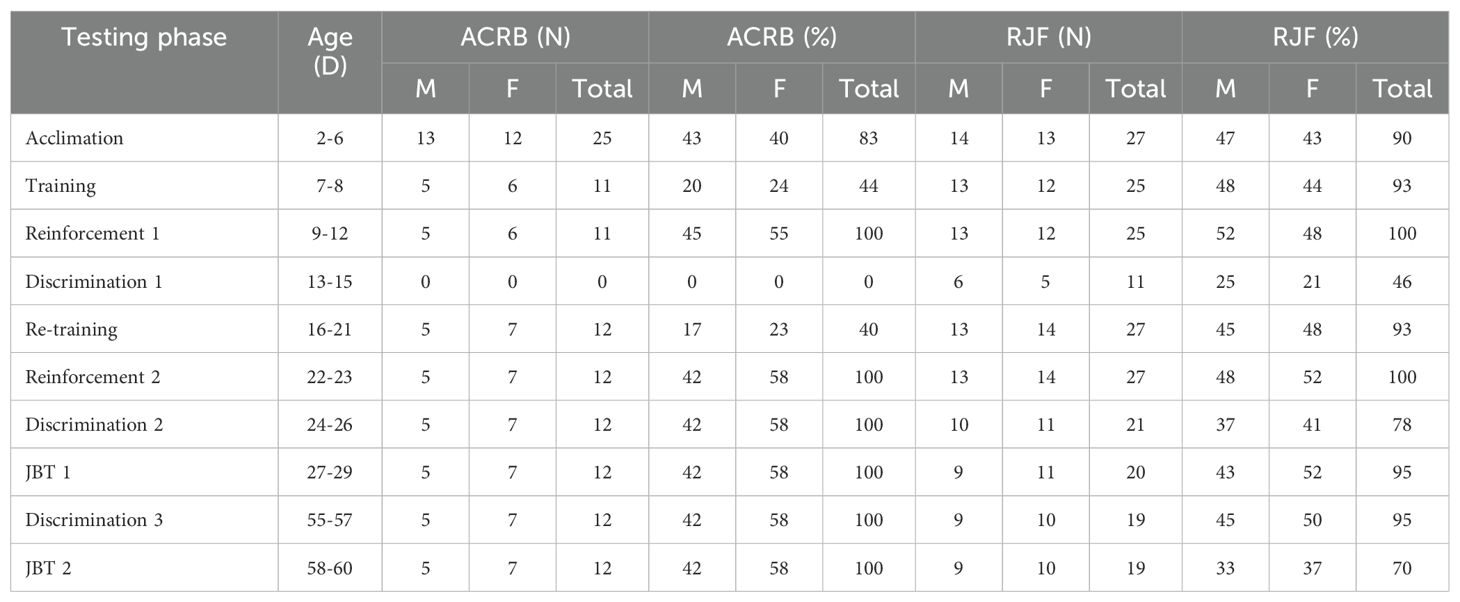
Table 1. The testing ages (D), number (N), and percentage (%) of male (M) and female (F) Red Junglefowl (RJF) and Athens Canadian Random Bred (ACRB) chickens that met the pass criteria in each phase of the judgment bias test (JBT).
Acclimation
First, chickens were familiarized with the test arena, the positive (POS) cue, and the reward in the Acclimation phase from D2-6. Due to human error, the RJF were acclimated for 3 more days than ACRB. On each test day, chickens were transported to the test facility from their home pen in a small plastic bucket containing litter. The test arena contained four white cardboard trays (5 cm × 5 cm) filled with three dried mealworms placed in the center and live mealworms randomly scattered throughout the test arena. To ensure acclimation and association of the test arena with the POS cue, live mealworms were provided because their movement is highly stimulating for chickens. Chickens were placed into the top opening of the start box, and after 10 s, the front door was opened for chickens to access the test arena freely while the observer was out of the chicken’s line of sight. The number of birds, trials, and trial durations varied until they were standardized to a male and female pair for two trials lasting 120 s on D6. Chickens passed the acclimation phase and proceeded to the training phase once they consumed mealworms from at least 1 white cardboard tray and those that did not were excluded from the subsequent phases. Fourteen RJF males, 13 RJF females, 13 ACRB males, and 12 ACRB females passed and proceeded to the training phase.
Training
Next, chickens were trained to associate the POS cue with the reward, which was a white cardboard tray containing 6 dried mealworms placed in the right corner on the opposite end from the start box in the test arena in the training phase on D7-8. Chickens participated in 3 consecutive trials for 120 s per trial. The observer recorded each chicken’s latency (s) to approach and consume mealworms from the white cardboard tray. Chickens passed the training phase and proceeded to the reinforcement phase if they consumed at least 1 mealworm from the white cardboard tray during at least 3 out of the 6 (50%) trials over the 2 days. Thirteen RJF males, 12 RJF females, 5 ACRB males, and 6 ACRB females passed and proceeded to the reinforcement phase.
Reinforcement
After the training phase, chickens participated in the reinforcement phase on D9-12. In the reinforcement phase, chickens were presented with the POS cue with dried mealworms positioned at the top right corner that was partially covered with white filter paper. This phase aimed to teach the chickens to investigate and displace the partially covered white cardboard tray to access dried mealworms underneath (Iyasere et al., 2017). Chickens participated in 3 trials with a maximum duration of 120 s for each consecutive trial. The observer recorded each chicken’s latency (s) to approach and consume mealworms from the POS cardboard tray. Chickens passed the reinforcement phase and continued to the discrimination phase if they successfully approached or consumed mealworms in at least 4 out of the 12 (33%) trials across D9-12. Thirteen RJF males, 12 RJF females, 5 ACRB males, and 6 ACRB females passed and proceeded to the discrimination phase.
Discrimination
After the reinforcement phase, chickens participated in the discrimination phase on D13-15. One female RJF mortality was recorded prior to testing. In the discrimination phase, chickens were introduced to the neutral cue (NEU, unrewarded), which was an empty black cardboard tray (5 cm x 5 cm) with no mealworms positioned in the left corner of the test arena. Cue order followed the methods described in Anderson et al. (2021). Chickens were presented with either the POS or NEU cue in an alternating order (Supplementary Table S2). Chickens participated in 6 trials with a maximum duration of 60 s for each consecutive trial, and the observer recorded the latency of the chickens to approach each cue. Chickens passed the discrimination phase if they consumed mealworms from the POS cue in 7 out of the 9 trials (77%), and they also must not have approached the NEU cue in more than 2 out of the 9 trials (22%). Six RJF males and 5 RJF females passed, but no ACRB males or females passed.
Re-training
Since no ACRB chickens passed the discrimination phase on D15, the decision was made to return to the training phase of the JBT on D16-21 (15 males and 15 females from each breed). The re-training followed the same procedures in the training phase, except the pass criteria. Chickens passed if they consumed mealworms from the white cardboard tray in 9 out of the 18 trials (50%) over the six days of re-training. Thirteen RJF males, 14 RJF females, 5 ACRB males, and 7 ACRB females passed and proceeded to reinforcement 2.
Reinforcement 2
After the re-training phase, chickens participated in the reinforcement 2 phase on D22-23. The procedures and pass criteria were the same as described in the reinforcement phase. Thirteen RJF males, 14 RJF females, 5 ACRB males, and 7 ACRB females passed and proceeded to discrimination 2.
Discrimination 2
After the second reinforcement phase, chickens participated in the discrimination 2 phase on D24-26. Chickens were tested as described previously. Ten RJF males, 11 RJF females, 5 ACRB males, and 7 ACRB females passed and proceeded to the JBT.
Judgment bias test
After the second discrimination phase, chickens participated in the first judgment bias test (JBT 1), which is the final phase of the JBT on D27-29. One male RJF mortality was recorded prior to testing. Chickens were presented with single cues, either POS, NEU, or novel ambiguous cues. The ambiguous cues were near neutral (NNEU), middle (MID), and near positive (NPOS) cues that were 75%, 50%, and 25% grey 5 cm x 5 cm cardboard trays, respectively. Ambiguous cues were placed at different locations along the wall of the arena opposite the start box. The NPOS cue was located 12 cm from the POS cue location (left corner), MID cue was in the center, and the NNEU cue was located 12 cm from the NEU cue location (right corner). The order of cue presentation is detailed in Supplementary Table S3, following the methods described by Anderson et al. (2021). Chickens participated in 9 trials with a maximum duration of 30 s for each consecutive trial. The observer recorded the latency of the chickens to approach each cue.
Discrimination 3 and judgment bias test 2
For the second judgment bias test (JBT 2), chickens were tested beginning with the discrimination 3 phase from D55-57. One female RJF mortality was recorded prior to testing. Nine RJF males, 10 RJF females, 5 ACRB males, and 7 ACRB females passed discrimination 3 and proceeded to the JBT 2 phase between D58-60. JBT procedures followed the same protocol as previously described.
Stress and fear measures
To compare the levels of stress and fear between the two breeds of chickens that were tested and untested in the JBT, 5 males and 5 females that made it to the final phase of the JBT, as well as 5 males and 5 females untested in any phase of the JBT (NJBT), were randomly selected from each breed (N = 20 RJF, 20 ACRB, 40 chickens in total). The JBT 1 ended on D29, and chickens had not been handled in the six days before the measures were collected on D35. On D35, stress and fear measures were collected. First, each chicken was removed from their home pen, and a thermal image of their head was taken. Next, fear was measured with the tonic immobility test. When the tonic immobility test ended, the chickens were weighed and returned to their home pen. Similarly, JBT 2 ended on D60, and chickens had not been handled for three days before collecting the same measures in the same order on D63. The same measures from the same chickens were collected at both ages.
Eye and beak thermal images
After each chicken was removed from the home pen, an infrared camera captured a thermal image of the chicken’s head at a focal distance of 330 cm. To minimize handling-related stress, images from each chicken were taken within 60 s of removal from the home pen. All images were taken by the same trained personnel to ensure consistency. Due to technical issues, different thermal cameras were used on D35 and D63. On D35, an infrared camera (FLIR ONE Pro LT, Wilsonville, OR, USA) was used with an emissivity of 0.95 and an atmospheric temperature of 20°C. Images were uploaded on a computer and analyzed using software (FLIR Thermal Studio Starter v.2.0), and an ellipse tool was used to measure the pixels representing the surface temperature of the eye region, and the minimum temperature (°C) was recorded. On D63, an infrared camera (Ti480P, Fluke Corporation, Everette, WA, USA) with a background temperature of 22°C, emissivity of 0.95, and transmission of 100% was used. Thermal images were uploaded on a computer and analyzed using Fluke software (SmartView Classic v4.4). An ellipse tool was used to measure the pixels representing the surface temperature of the eye and beak regions, and the minimum temperature was recorded. The minimum surface temperature was selected for analysis as it was more sensitive than the average or maximum temperatures recorded (Edgar et al., 2013). The thermal camera used to take images on D63 had better resolution, so the beak area could be measured.
Tonic immobility test
The tonic immobility test was conducted in the hallway outside of the home pen from 0800 to 1100 h on D35 & D63). Each chicken was placed into a wooden V-shaped cradle by a handler, carefully placing the bird on its back in the cradle and restrained for 15 s with one hand gently covering the head and the other over the chicken’s sternum to induce tonic immobility. The handler lifted their hands from the chicken and moved out of the chicken’s line of sight. Induction was successful if the chicken did not attempt to right itself for 10 s after removing the hand. If the chicken attempted to right itself before this time, induction was re-attempted by the handler, with a maximum of 2 additional attempts. Chickens participated in the tonic immobility test for a maximum of 600 s, then were returned to the home pen. Fearfulness was recorded as the latency (s) the chicken spent in tonic immobility before righting itself, with a longer latency indicating greater fearfulness (Gallup, 1979).
Statistical analysis
Statistical analysis of the judgment bias test data was performed using R version 4.4.1 to determine the main effects of breed (RJF, ACRB), sex (male, female), test (JBT, NJBT), and their interactions. Survival analyses were performed to calculate the Cox Hazard Ratio (HR), along with 95% confidence intervals [upper limit (UL), lower limit (LL)] and p-values using the “survival” and “survminer” packages in R to determine the probability of reaching the learning criterion for training, re-training, discrimination 1, discrimination 2 and discrimination 3. To assess the impact of breed, sex, and test on latency to approach each cue during the judgment bias test, a linear mixed-effects model was fitted using the lmer function from the “lme4” package in R. Tonic immobility duration (s) was square root transformed to meet the assumptions of normality and homoscedasticity and the model included fixed effects for breed, sex, and test, and their interactions. Random intercepts were included for individual (ID) nested within pairs, for trials of individuals for the discrimination phases, and for ID for the judgment bias test phases to account for repeated measures and paired observations, respectively. When interactions were significant, post hoc pairwise comparisons of estimated marginal means (‘emmeans’, R package) were carried out with Holm’s adjustment for multiple comparisons. To evaluate the effects of sex, breed, test on the duration of tonic immobility, another lmer mixed-effect model was fit using the log-transformed response duration to transform the data. This model also included the fixed effects of sex, breed, test, and their interactions. An ANOVA was conducted using the aov function in R to evaluate the effects of breed, sex, and their interaction on body weight.
An ANOVA was used in JMP Pro version 17 software for the main effects of breed, sex, test, and their interaction on D35 and D63 eye and D63 beak minimum surface temperatures. Tukey’s Honest Significant Difference (HSD) test was applied to separate the means where significant differences were detected. Results are reported as estimated mean ± the standard error of the estimated mean, with statistical significance determined at a p-value < 0.05.
Results
Judgment bias test
The number and percentage of chickens from both breeds that progressed to each phase of the judgment bias test (JBT) are shown in Table 1.
Training
The hazard ratio analysis revealed a significant effect of breed on the probability of chickens passing training (HR = 3.01, 95% CI = 1.12, 8.11, P < 0.05) but not re-training. Specifically, the Kaplan-Meier survival curve showed that by the 4th trial, 75% of RJF passed, with 80% RJF passing by the 6th trial. At the same time, the ACRB took more trials to pass, with only 50% passing by the end of the 6th trial (Figure 1a). At re-training, while not statistically significant, RJF showed fewer trials to pass, with all (100%) RJF compared with the 90% ACRB passing by the 12th trial (Figure 1b).
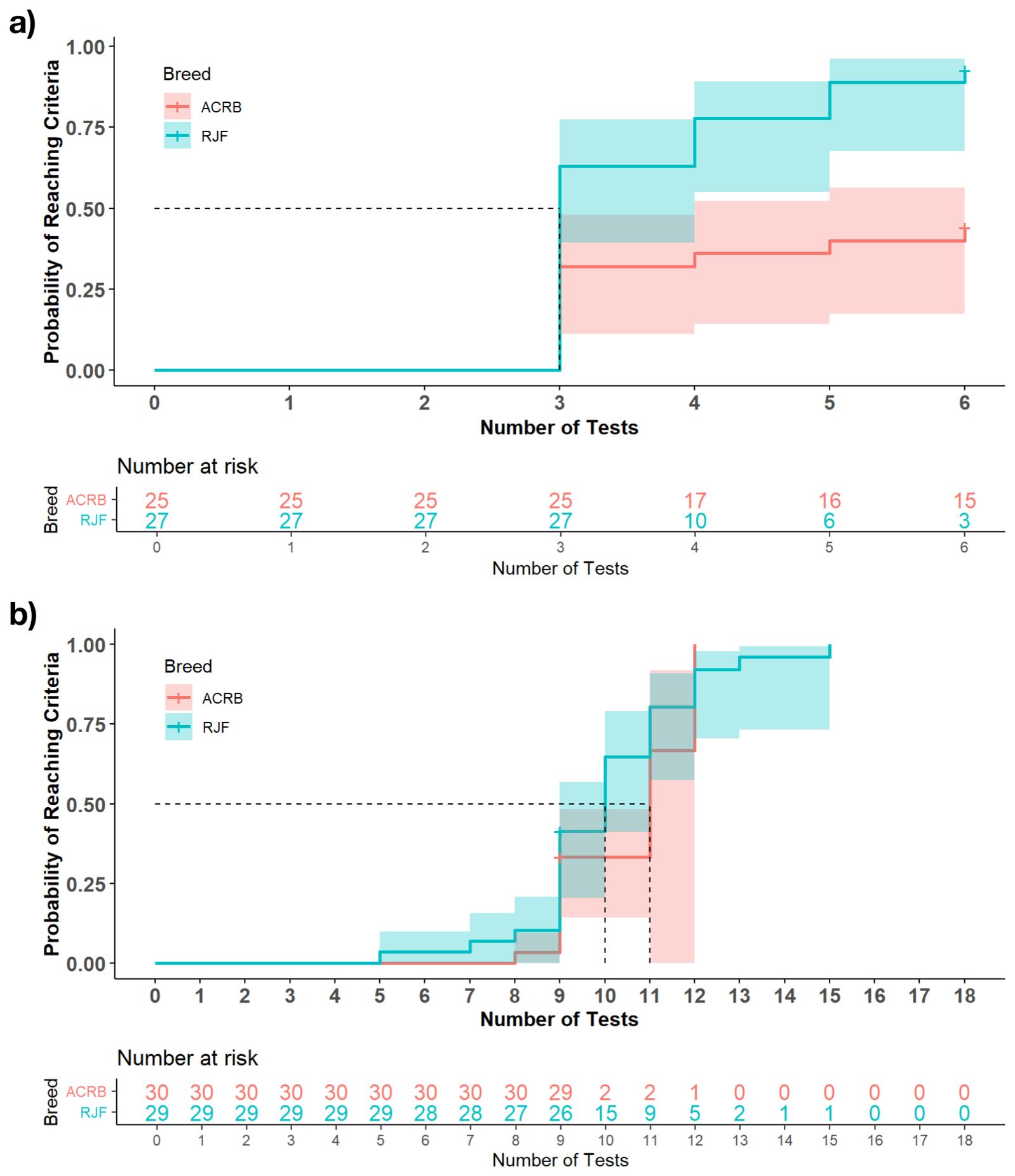
Figure 1. The Kaplan-Meier survival curve of the probability of chickens completing the training phase at (a) Training 1 and (b) Training 2 of the judgment bias test. The dotted lines represent the number of tests taken for 50% of the population to reach the pass criteria. The number at risk for each breed represents the number of individuals who had not failed or met the pass criteria after each repeated training trial.
Discrimination
In discrimination 1, chickens had a shorter (P < 0.0001) latency to approach the POS (22 ± 3 s) compared to NUE (58 ± 5 s) (Table 2). RJF had a shorter (P < 0.01) latency (33 ± 3 s) to approach any cue compared to ACRB (44 ± 4 s), and RJF had a shorter (P < 0.0001) latency to approach the POS cue (16 ± 3 s) than ACRB (29 ± 4 s).
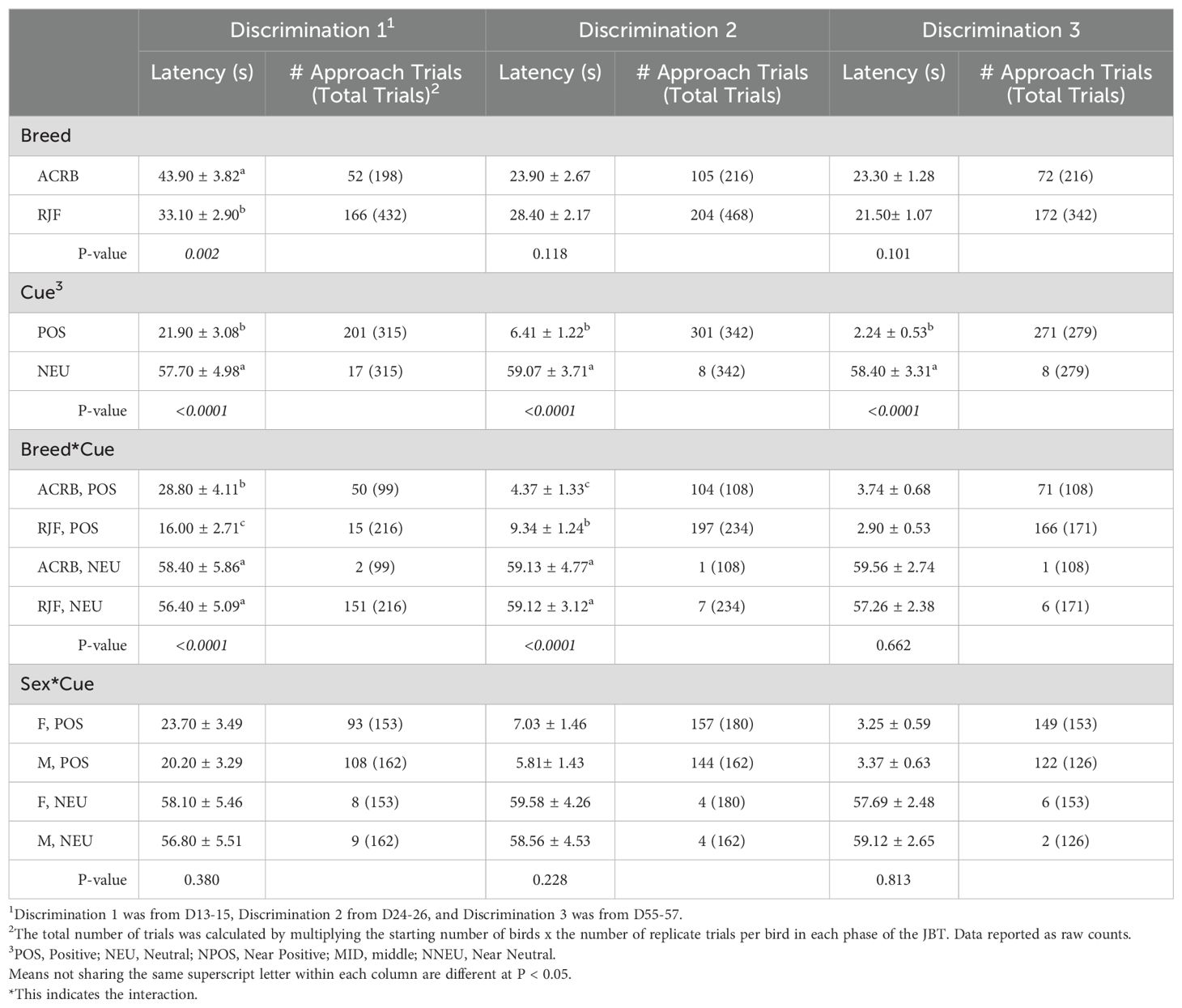
Table 2. Breed, sex, and cue main effects and their 2-way interactions on the mean (± SE) latency to approach (s) cues of Red Jungle Fowl (RJF) and Athens Canadian Random Bred (ACRB) chickens in the Discrimination phases of the judgment bias test.
In discrimination 2, chickens had a shorter (P < 0.0001) latency to approach the POS (6 ± 1 s) compared to the NEU (59 ± 4 s), and ACRB approached the POS cue faster (4 ± 1 s, P < 0.0001) than RJF (9 ± 1 s; Table 2). In discrimination 3, chickens had a shorter (P < 0.0001) latency to approach the POS (3 ± 0.5 s) compared to the NUE (58 ± 2 s) cue (Table 2).
Results of the hazard ratio analyses showed no effects of breed, sex, or breed and sex interaction on the probability of the chickens passing discrimination 1, discrimination 2, or discrimination 3. While there was no statistical difference, at discrimination 1, the Kaplan-Meier survival curve showed the probability of RJF passing increased notably after the 7th trial, eventually reaching 60% by the 9th trial. In contrast, ACRB showed no increase in the probability of passing reaching 0% by the 9th trial (Figure 2a). Similarly, at discrimination 2 (Figure 2b) and discrimination 3 (Figure 2c), both breeds showed an increase in the probability of passing after the 7th trial.
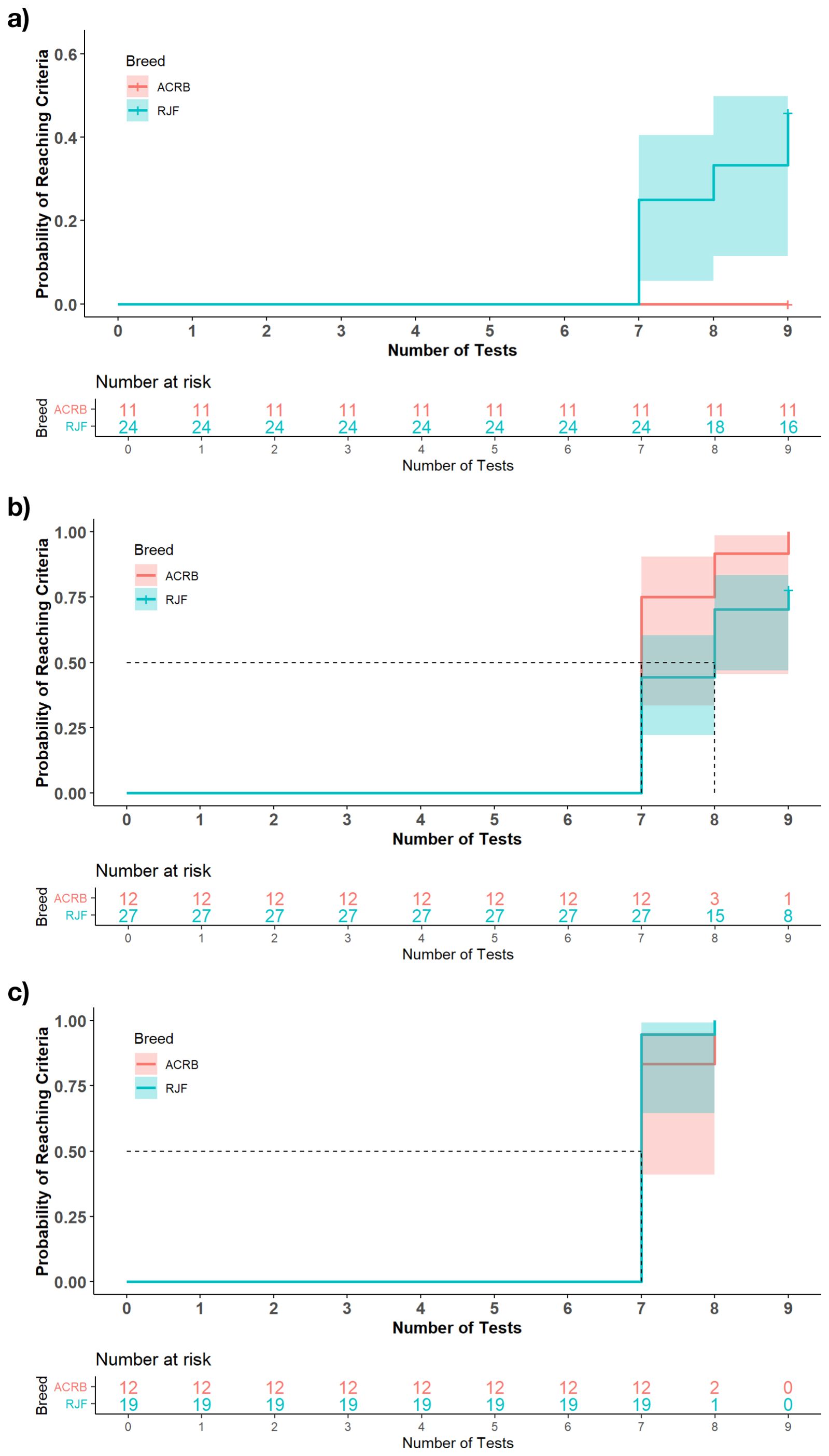
Figure 2. The Kaplan-Meier survival curve of the probability of chickens completing the discrimination phase at (a) Discrimination 1, (b) Discrimination 2, and (c) Discrimination 3 of the judgment bias test. The dotted lines represent the number of tests taken for 50% of the population to reach the criteria. The number at risk for each breed represents the number of individuals who had not failed or met the pass criteria after each repeated training trial.
Judgment bias
On D29 of the first JBT (JBT 1), there were no significant differences between ACRB and RJF. However, there was a significant effect of cue on latency to approach cue types, with chickens having a shorter (P < 0.0001) latency to approach the POS (2 ± 0.1 s) followed by MID (23 ± 0.6 s), NPOS (26 ± 0.6 s), NNEU (28 ± 0.6 s), then the NUE cue (30 ± 0.4 s) (Table 3).
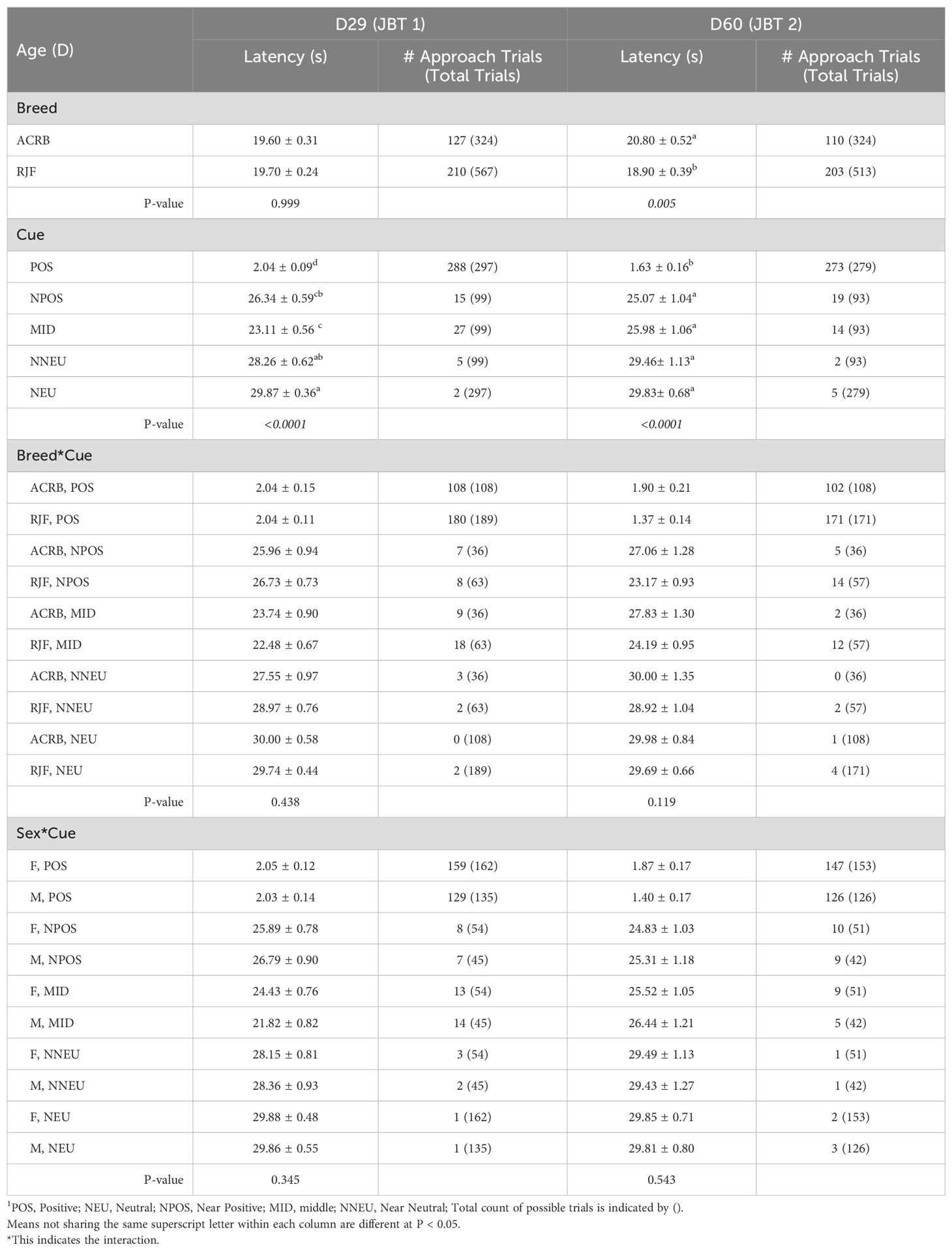
Table 3. Breed, sex, and cue main effects and their 2-way interactions on the mean (± SE) latency to approach (s) the cues of male (M) and female (F) Red Jungle Fowl (RJF) and Athens Canadian Random Bred (ACRB) chickens on D29 and D60 in judgment bias test.
On D60 of the second JBT (JBT 2), the main effects of breed and cue were significant. Chickens had a shorter (P < 0.001) latency to approach the POS (2 ± 0.2 s), followed by the NPOS (25 ± 1 s), MID (26 ± 1 s), NNEU (29 ± 1 s), and NEU (30 ± 1 s) cues, and RJF had a shorter (P < 0.01) latency (19 ± 0.4 s) to approach all cues compared to ACRB (21 ± 0.5 s) (Table 3).
Fear and stress measures
Eye and beak surface temperature
Chickens that did not participate in the JBT (NJBT) had a higher (P < 0.05) minimum eye temperature (32.1 ± 0.46°C) compared to JBT chickens (30.8 ± 0.46°C) on D35 (Figure 3a). NJBT chickens had higher (P < 0.01) minimum eye and beak temperatures (35.1 ± 0.19°C and 34.4 ± 0.32°C, respectively) compared to JBT chickens (34.0 ± 0.19°C and 32.1 ± 0.32°C, respectively) on D63 (Figure 3b). The interaction between test and breed showed that NJBT RJF had a higher (P < 0.05) eye minimum temperature (32.7 ± 0.65°C) compared to JBT RJF (29.8 ± 0.65°C) on D35 (Figure 4a), but this effect was not seen on D63. Unlike D35, an interaction effect was found between the test and breed on D63, where NJBT ACRB had a higher (P < 0.05) beak minimum temperature (35.0 ± 0.46°C) compared to JBT ACRB (31.4 ± 0.46°C) (Figure 4b).
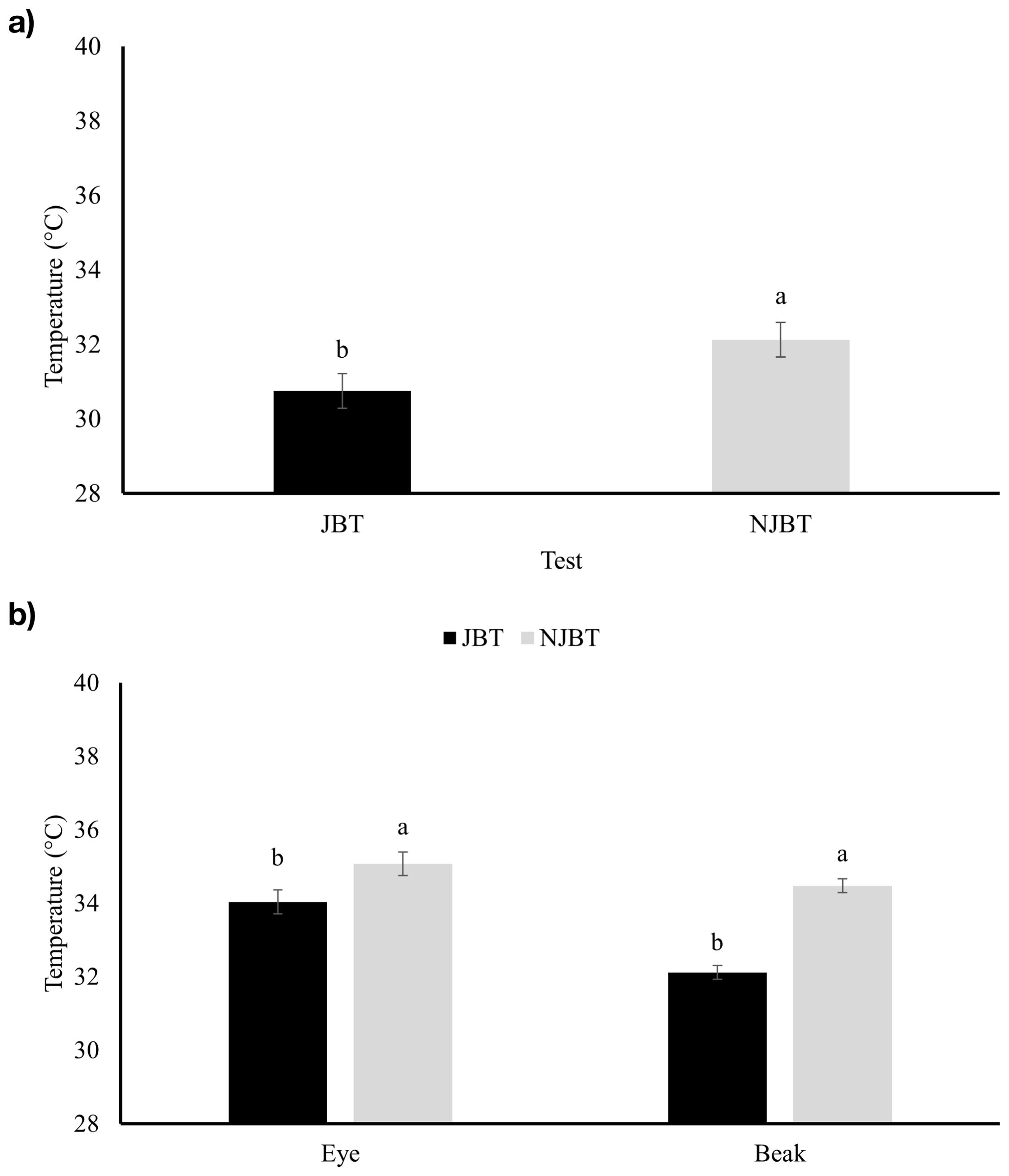
Figure 3. Minimum eye and beak surface temperature (°C) of Red Junglefowl (RJF) and Athens Canadian Random Bred (ACRB) chickens that were tested (JBT) and untested (NJBT) in the judgment bias test on (a) D35 and (b) D63. Different letters indicate significant differences (Tukey’s Honest Significant Difference test) at p < 0.05.
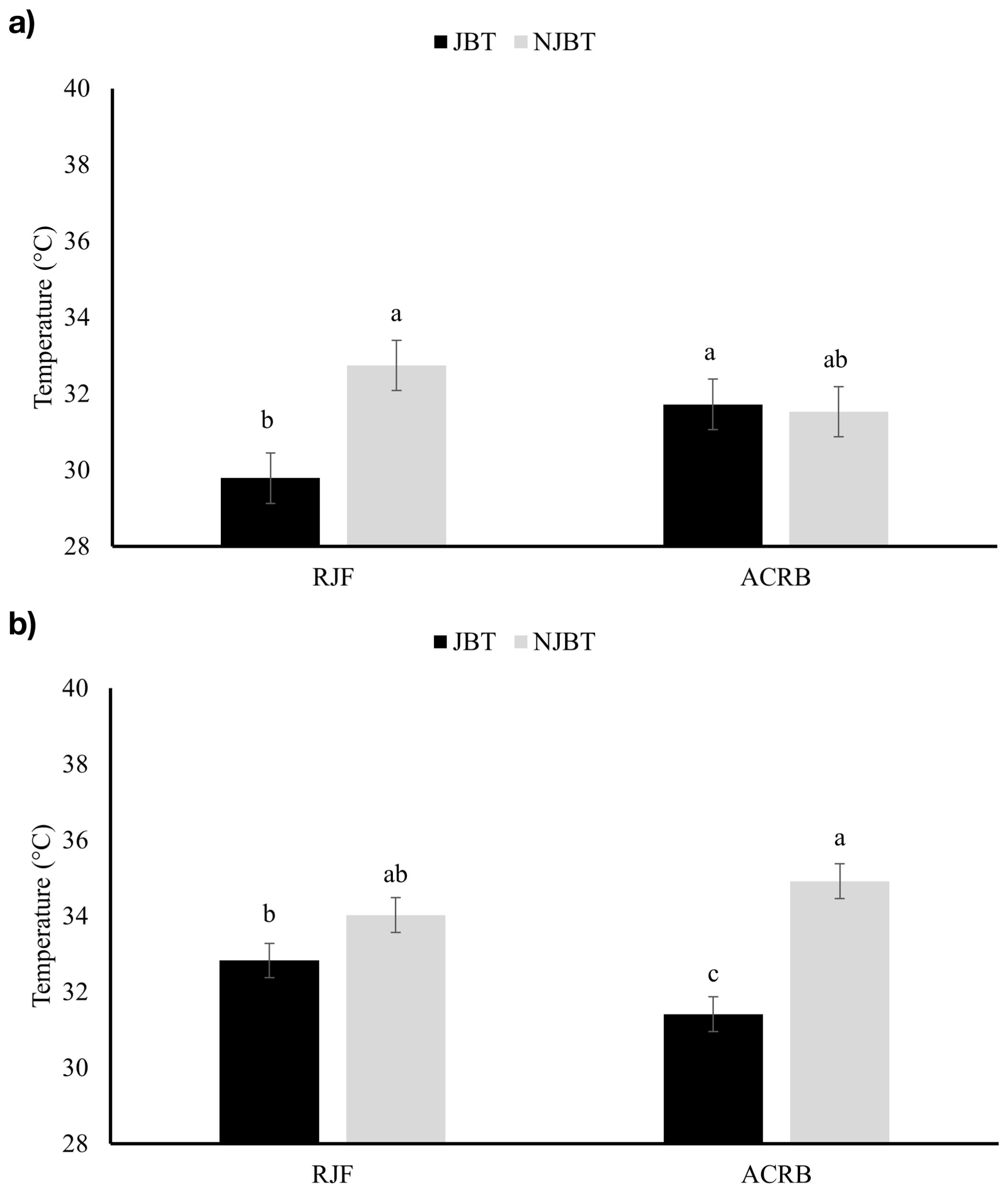
Figure 4. Minimum eye surface temperature (°C) on (a) D35 and (b) minimum beak surface temperature (°C) on D63 of Red Junglefowl (RJF) and Athens Canadian Random Bred (ACRB) chickens that were tested (JBT) and untested (NJBT) in the judgment bias test.
Tonic immobility
The main effects of test and the interaction of sex and test significantly affected tonic immobility on D35, while the main effects of test, breed, and sex, as well as the interaction between sex and test, were significant for D63. On D35, RJF had a longer (P < 0.01) duration (77 ± 15 s) compared to ACRB (37 ± 7 s), and NJBT males had a longer (P < 0.01) duration (93 ± 25 s) than NJBT females (29 ± 8 s) (Table 4). On D63, JBT chickens had a longer (P < 0.05) duration (129 ± 25 s) than NJBT chickens (61 s ± 11.80). Similar to D35, RJF had a longer (P < 0.01) duration than ACRB (129 ± 25 s) and NJBT males had a longer (P = 0.005) duration than NJBT females on D63 (Table 4).
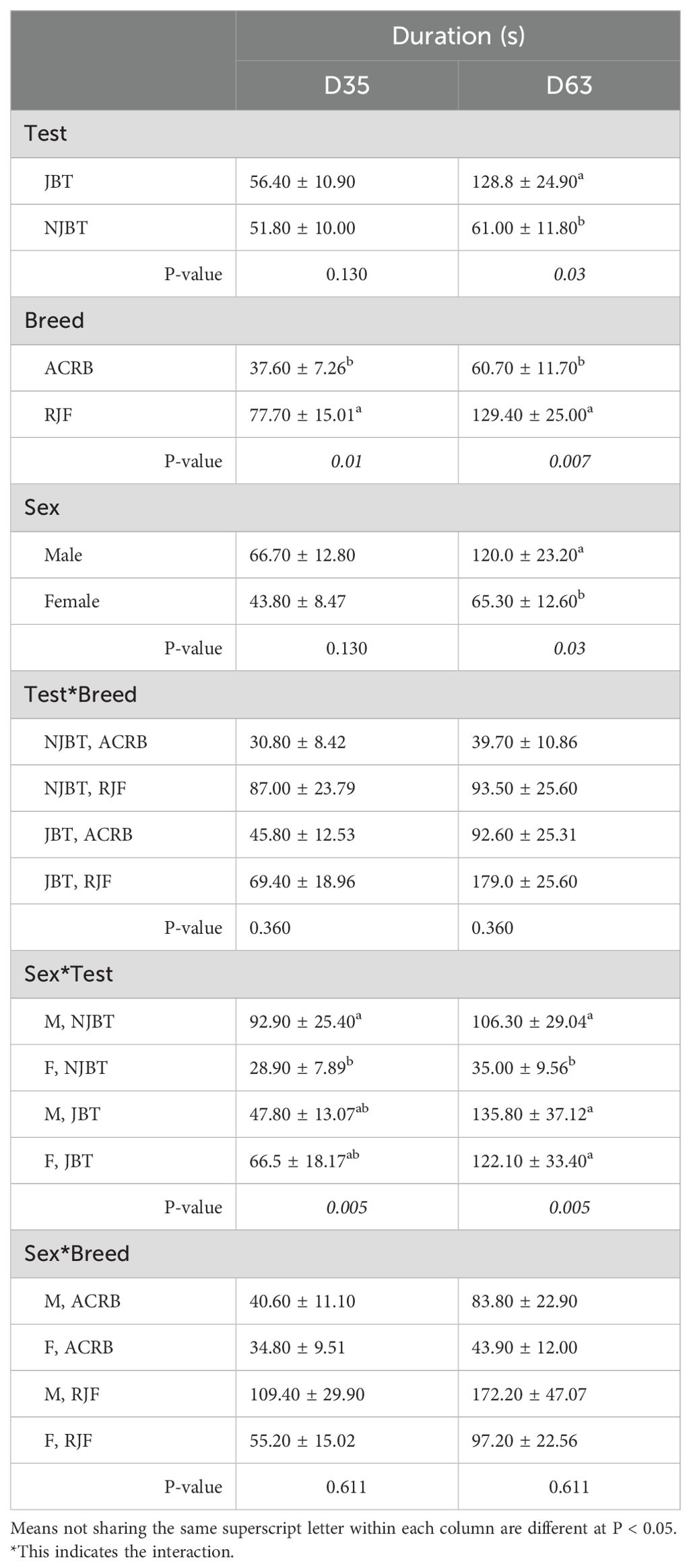
Table 4. Test, breed, and sex main effects and their 2-way interactions on the mean (± SE) duration (s) of tonic immobility of male (M) and female (F) Red Jungle Fowl (RJF) and Athens Canadian Random Bred (ACRB) chickens that were tested (JBT) or untested (NJBT) in the JBT on D35 and D60.
Body weight
Body weight was significantly affected by breed, sex, and their interaction at D35 and by breed and sex on D63. At D35, ACRB had a greater (P < 0.01) body weight (478 ± 10 g) compared to RJF (439 ± 10 g). Similarly, at D63, ACRB had a greater body weight (P < 0.01, 1137 ± 24 g) than RJF (1040 ± 24 g). Males had a greater (P < 0.01) body weight than female birds at both D35 and D63. The interaction between breed and sex on D35 showed that female ACRB had a lower body weight (391 ± 14 g) than male ACRB and both RJF sexes (Table 5).
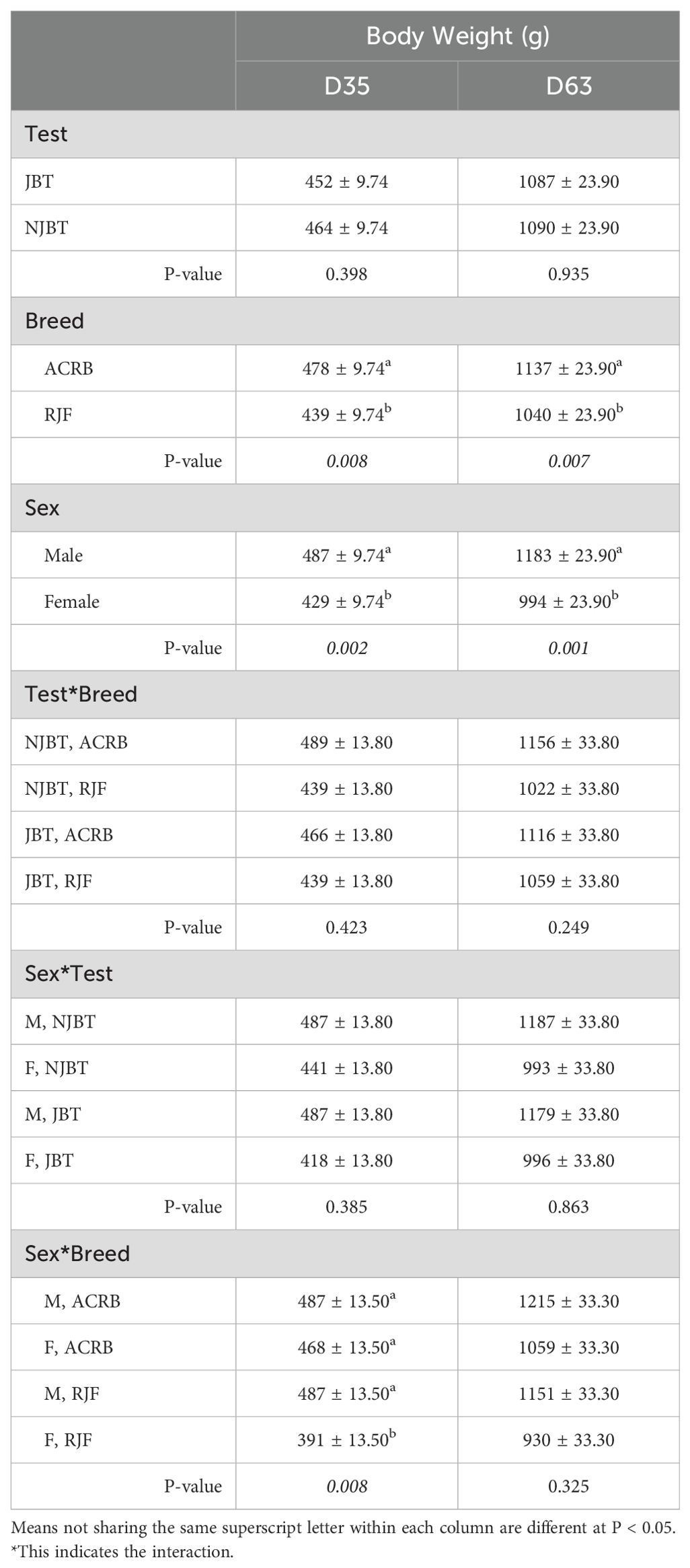
Table 5. Significant breed and sex main effects and their two-way interaction on mean body weight (g) on D35 and D63 of male and female Red Jungle Fowl (RJF) and Athens Canadian Random Bred (ACRB) chickens that were tested (JBT) or untested (NJBT) in the JBT.
Discussion
The approach of combining cognitive assessments with fear and stress measures in this study provides a holistic view into the affective states of chickens. This methodology allows for a deeper understanding of how underlying affective states can affect cognitive, fear, and stress responses, and how these can vary between different breeds of chickens. To our knowledge, this study is the first to examine the difference between the Red Junglefowl and the Athens Canadian Random Bred Chickens at the same ages that were reared in the same housing conditions. The stress and fear measures collected in the current study differ from previous studies (Zidar et al., 2018; Anderson et al., 2021) in that an untested baseline population was maintained, which enabled us to observe the inherent differences between breeds, rather than introducing additional variables such as stress-inducing factors or enriched environments to investigate behavioral and physiological responses. This approach offers a distinctive viewpoint by highlighting the breed-specific responses.
Discrimination and judgment bias
The results of the discrimination training showed that chickens successfully learned to approach the positive cue with a shorter latency compared to the neutral cue at all the phases of discrimination learning. These findings align with a study conducted by Pichová et al. (2021), which investigated the cognitive differences between laying hens selectively bred for high and low pecking behavior. At the end of the discrimination training, 36 out of 40 hens successfully discriminated between the positive and negative-colored feeders in their study. The latency to approach the positive cue across the discrimination phases reflects reinforcement learning principles, where chickens learned to associate positive cues with rewards (Zentall et al., 2014). In the current study, chickens consistently approached the positive cue faster than the neutral cue during the three discrimination phases. The results of the current study demonstrate that chickens were able to discriminate between the positive cue, which consistently contained the reward (mealworms), and the neutral stimulus, which remained unrewarded throughout the discrimination learning phase.
No ACRB and eleven RJF passed the first discrimination phase. Further, ACRBs that approached the positive cue did so with a longer latency than RJF. A potential explanation for why the ACRB did not successfully pass the first discrimination phase could have been the lack of a punitive consequence for approaching the neutral cue. In discrimination learning, pairing positive reinforcement with the rewarded cue and a form of punishment or negative feedback for the negative cue enhances learning by creating a clear distinction between stimuli. By not incorporating a punitive consequence for approaching the neutral cue during this study, the ACRB may not have experienced sufficient motivation to avoid the neutral cue and focus exclusively on the positive cue. In contrast, Seehuus et al. (2013) investigated the impact of disrupting the feed reward cycle in laying hen chicks to infer their affective state using a spatial JBT. They reported that the use of a negative (unpalatable rice soaked in quinine) enhanced discrimination learning. Incorporating mild negative reinforcement could have enhanced ACRB discrimination learning outcomes in the present study.
Nevertheless, ACRB chickens seemed to learn at a slower rate than RJF. RJF initially approached the positive cue faster than ACRB during the first discrimination phase, but the ACRB were faster than RJF at approaching the positive cue during the second discrimination phase. By the third discrimination phase, the latencies for both breeds to approach the positive cue were faster, and there was no difference between breeds, indicating they both remembered and discriminated between positive and neutral cues. Inconsistencies exist in similar research. Interestingly, Svensson and Lindahl (2023) investigated the cognitive learning capabilities between White Leghorn chickens and RJF in associative and spatial learning tests. The authors reported that the White Leghorns were initially faster learners than the RJF in an associative learning test. They concluded that domestication has increased boldness and exploration and the risk-aversive behavior of the RJF may serve as an adaptive trait for predator avoidance in the wild. Similar to our results, the results from previous studies report that domestication impairs spatial cognition, with RJF having heightened spatial learning abilities than White Leghorn hens (Lindqvist et al., 2002, 2007; Lindqvist and Jensen, 2009). Lindqvist et al. (2007) reported that both male and female RJF outperformed WL in navigating to a food reward during a spatial learning test, suggesting superior spatial learning abilities. Further investigations by Lindqvist and Jensen (2009) examined contra-freeloading behavior, the tendency to work for food when the same food is freely available, and spatial learning in both breeds. The authors found that RJF exhibited more contra freeloading behavior and better spatial learning performance than WL. These results indicate that domestication may have altered cognition and stress susceptibility, which can also affect fearfulness in the domesticated phenotype.
Both breeds of chickens consistently exhibited shorter latencies to approach the positive cue during the JBT. This rapid approach to the ambiguous cues highlights the effectiveness of the test as a measure of learning and memory in chickens by the association of the positive cue with positive reinforcement (mealworms). Although we did not find breed differences in their latencies to approach the ambiguous cues during the JBT, RJF had a slightly shorter latency to approach cues when they were older, which indicates they may have better memory or a more proactive coping style (Lindroth, 2020) compared to ACRB. This suggests that RJF were more proficient at remembering and associating the cues with previously learned outcomes or it may reflect their natural behavioral traits, such as an increased inclination to explore (Koolhaas et al., 1999; Lindqvist and Jensen, 2009; Lindroth, 2020), which may have been preserved through minimal domestication.
Another possible reason why chickens approached ambiguous cues with similar latencies might be related to their experiences during training and testing sessions. Repeated testing is known to induce anticipation, as reported by previous studies (Wichman et al., 2012; Anderson et al., 2021), where chickens develop an expectation of future events based on past experiences. In the current study, although there were no significant differences between breed and cue interactions, the responses observed during the second phase of the JBT may reflect the effects of prior reinforcement and learning. For instance, the RJF, which exhibited more consistent responses, may have developed a stronger anticipation of favorable outcomes of the JBT than the ACRB. This suggests that differences in previous learning rates during reinforcement at younger ages could influence how chickens approach ambiguous cues at older ages.
Stress
Traditional physiological methods for assessing stress in chickens often rely on measuring stress hormones, such as corticosterone, which necessitates handling and restraint for blood sampling (Mormede et al., 2007; Weimer et al., 2018). While reliable, these methods can induce stress and alter the affective and physiological states of the chickens due to restraint and venipuncture. Restraint and pain can confound the results when the objective is to assess baseline stress levels or reactions to repeated handling (Bortolotti et al., 2008; Alm et al., 2014). To reduce the confounds of blood collection, our study used thermography, a validated non-invasive technique to measure stress responses in chickens (Moe et al., 2017; Weimer et al., 2021), aligning with the growing emphasis on reducing animal distress in research settings.
Chickens that had participated in the JBT showed significantly lower eye surface temperatures than untested chickens at both ages, suggesting they likely had higher residual levels of stress in response to handling for thermal image capture. This was particularly evident at the older age, when the eye surface temperature of JBT chickens was 1.04°C lower than JBT, and 2.36°C for the beak. The magnitude of the difference between eye and beak surface temperatures indicates that the surface temperature of the beak region may be a more reliable measure of stress than the eye (Weimer et al., 2021). The beak region is highly innervated by the sympathetic nervous system, making it sensitive to temperature fluctuations due to changes in blood flow (Kuenzel, 2007). As a result, monitoring beak temperature may provide a valuable indicator of affective states in chickens, reflecting their emotional and physiological responses (Iqbal and Moss, 2021).
Despite repeated gentle human handling and relocation during the testing facility, JBT chickens showed higher stress levels, which may indicate an inability to acclimate to human interaction over time. Studies by Edgar et al. (2013) and Herborn et al. (2015) have shown that repeatedly handling chickens induced significant stress, evidenced by a rapid decrease in surface and eye temperatures. This suggests that the processes involved in cognitive testing procedures may be inherently stressful, potentially exceeding the capacity of chickens to habituate to the handling and movement involved in the test. Our findings further support these observations, particularly highlighting the distinct differences in facial surface temperatures between chickens that participated in the JBT and those untested.
On the other hand, the JBT could have had heightened emotional arousal due to test anticipation for the positive reward (mealworm). Moe et al. (2012) demonstrated that arousal in anticipation of a reward resulted in a measurable decrease in peripheral temperature, reflecting an emotional and physiological response to positive expectations. This suggests that the test elicits an arousal response, influencing both physiological states and optimistic or pessimistic decision-making. Interestingly, while breeds did not differ in eye minimum temperatures at the younger age, the interaction between breed and test conditions did. Specifically, at the younger age, RJF chickens that participated in the JBT displayed lower eye minimum temperatures than untested RJF. However, the reciprocal effect was found in beak minimum surface temperatures at the older age, where JBT-tested ACRB chickens had lower beak minimum temperatures than untested ACRB. The absence of a similar pattern between the stress response and cognition indicates that behavioral and physiological stress responses are independent traits and suggests that individual variation in these responses can be dependent on individual personality (Koolhaas et al., 1999; Lindroth, 2020).
Fear
Chickens that participated in the JBT exhibited longer durations of tonic immobility than untested chickens at the older, but not younger age in the current study. This may indicate that older chickens may be more fearful of repeated testing and handling during the JBT (Jones and Waddington, 1992). Neuhauser et al. (2023) investigated the effects of cognitive judgment bias test training on the tonic immobility of tested and untested laying hens. The authors concluded that such training did not significantly influence fear responses, challenging earlier assumptions about the relationship between cognitive tests and affective states in animals (Zidar et al., 2018). The observed contradiction between our findings and those of Neuhauser et al. (2023) may be attributed to breed and age-specific variations observed in the RJF and ACRB chickens or variations in the JBT experimental design.
Animals experiencing increased negative affective states, such as increased fearfulness (Hicks and Patrick, 2006; Forkman et al., 2007) and stress (Kozak et al., 2019) are more likely to remain longer in tonic immobility. The longer tonic immobility durations observed in males compared to females at the older age suggest that sex influences fear responses. The effects of sex on tonic immobility are inconsistent. For example, Jones and Faure (1981) reported no significant sex differences across chicken strains, while others have reported that males exhibit longer tonic immobility durations (Janczak et al., 2007; Archer, 2018) and increased fearfulness with age in males, but not females (Nakasai et al, 2013) These variations suggest that sex differences in tonic immobility depend on age, hormonal development, strain, and environmental conditions. Testosterone, which is more prominent in males, may increase fear-related behaviors (Archer, 1976). Understanding the effect of sex is crucial for interpreting tonic immobility data in behavioral studies.
In the current study, RJF chickens had a longer tonic immobility duration compared to ACRB at both ages, suggesting that RJF may possess a greater innate fear response which serves as an essential evolutionary function enhancing their survival rates to effectively avoid predators in the wild (Jones, 1996). Domestication attenuates the frequency and intensity of fear-related behaviors in animals (Agnvall et al., 2012). Previous research confirms breed-specific differences in the fear response of chickens. Campler et al. (2009) reported that the RJF showed a higher fear response compared to White Leghorns. This trend is consistent with our findings, where the RJF displayed higher fear levels in the tonic immobility test than the ACRB. Additionally, a study conducted by Gjøen et al. (2023) compared the risk-taking effect of domestication of RJF and White Leghorns on behavior in social and non-social environments and reported that RJF chickens exhibited more fear of a novel object than White Leghorn chickens. This result aligns with the present findings of this study, as observed in the longer tonic immobility duration in the RJF.
Conclusion
Our study combined a cognitive assessment with fear and stress measures of Red Junglefowl and Athens Canadian Random Bred chickens to provide a comprehensive understanding of the effect of domestication on the welfare of chickens. Both breeds successfully learned to discriminate between positive and neutral cues, but not ambiguous cues, in the judgment bias test. Participation increased the stress response at both ages and the fear response at the older age. We hypothesized that the Red Junglefowl chickens would respond more pessimistically to the judgment bias test and exhibit heightened fear and stress responses compared to the Athens Canadian Random Bred chickens. Our hypothesis was only partially supported, as Red Junglefowl chickens tended to approach cues more quickly and exhibited longer durations of tonic immobility, suggesting a proactive affective state and greater fearfulness than Athens Canadian Random Bred. These findings provide evidence that the link between domestication and physiological and behavioral responses remains nebulous and highlight the importance of considering fear and stress in measuring the affective states of animals.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by University of Arkansas Division of Agriculture Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
VO: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. RW: Writing – review & editing, Data curation, Formal analysis. SO: Resources, Writing – review & editing. SW: Resources, Visualization, Funding acquisition, Validation, Methodology, Project administration, Writing – review & editing, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors would like to acknowledge the Center for Food Animal Wellbeing for funding.
Acknowledgments
Special thanks are extended to graduate and undergraduate students for their invaluable help during the study: Angela Perretti, Jaelen Cherry, Pablo Escovar, Elle Johnson, Karen Rivera Pitty, and Rovin Caballero.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1573847/full#supplementary-material
References
Abe H., Nagao K., Nakamura A., and Inoue-Murayama M. (2013). Differences in responses to repeated fear-relevant stimuli between Nagoya and White Leghorn chicks. Behav. Processes 99, 95–99. doi: 10.1016/j.beproc.2013.07.004
Agnvall B., Jöngren M., Strandberg E., and Jensen P. (2012). Heritability and genetic correlations of fear-related behaviour in Red Junglefowl–possible implications for early domestication. PloS One 7, e35162. doi: 10.1371/journal.pone.0035162
Albentosa M. J., Kjaer J. B., and Nicol C. J. (2003). Strain and age differences in behaviour, fear response and pecking tendency in laying hens. Br. Poultry Sci. 44, 333–344. doi: 10.1080/00071660310001598085
Alm M., Holm L., Tauson R., and Wall H. (2014). Corticosterone metabolites in laying hen droppings—Effects of fiber enrichment, genotype, and daily variations. Poultry Sci. 93, 2615–2621. doi: 10.3382/ps.2014-04193
Anderson M. G., Campbell A. M., Crump A., Arnott G., and Jacobs L. (2021). Environmental complexity positively impacts affective states of broiler chickens. Sci. Rep. 11, 16966. doi: 10.1038/s41598-021-95280-4
Archer J. (1976). Testosterone and fear behavior in male chicks. Physiol. Behav. 17, 561–564. doi: 10.1016/0031-9384(76)90151-7
Archer G. (2018). Sex, genetics and test type affect the responses of chickens to fear testing. Int. Poultry Sci. 17, 320–326. doi: 10.3923/ijps.2018.320.326
Bethell E. J. (2015). A “how-to” guide for designing judgment bias studies to assess captive animal welfare. J. Appl. Anim. Welfare Science 18(sup1) 18 (sup1), S18-S42. doi: 10.1080/10888705.2015.1075833
Boissy A. and Lee C. (2014). How assessing relationships between emotions and cognition can improve farm animal welfare. Rev. Sci. Technol. 33, 103–110. doi: 10.20506/rst.issue.33.1.55
Boissy A., Manteuffel G., Jensen M. B., Moe R. O., Spruijt B., Keeling L. J., et al. (2007). Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 92, 375–397. doi: 10.1016/j.physbeh.2007.02.003
Boleij H., van’t Klooster J., Lavrijsen M., Kirchhoff S., Arndt S. S., and Ohl F. (2012). A test to identify judgement bias in mice. Behav. Brain Res. 233, 45–54. doi: 10.1016/j.bbr.2012.04.039
Bolton T. B. and Bowman W. C. (1969). Adrenoreceptors in the cardiovascular system of the domestic fowl. Eur. J. Pharmacol. 5, 121–132. doi: 10.1016/0014-2999(69)90020-X
Bortolotti G. R., Marchant T. A., Blas J., and German T. (2008). Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Funct. Ecol. 22, 494–500. doi: 10.1111/j.1365-2435.2008.01387.x
Browning H. (2022). Assessing measures of animal welfare. Biol. Philosophy 37, 36. doi: 10.1007/s10539-022-09862-1
Brydges N. M. and Hall L. (2017). A shortened protocol for assessing cognitive bias in rats. J. Neurosci. Methods 286, 1–5. doi: 10.1016/j.jneumeth.2017.05.015
Campderrich I., Nazar F. N., Wichman A., Marin R. H., Estevez I., and Keeling L. J. (2019). Environmental complexity: A buffer against stress in the domestic chick. PloS One 14, e0210270. doi: 10.1371/journal.pone.0210270
Campler M., Jöngren M., and Jensen P. (2009). Fearfulness in red junglefowl and domesticated White Leghorn chickens. Behav. Processes 81, 39–43. doi: 10.1016/j.beproc.2008.12.018
Carsia R. (2015). “Adrenals,” in Sturkie’s Avian Physiology, 6th ed. Ed. Scanes C. G. (Academic Press, New York), 577–615.
Cockrem J. F. (2007). Stress, corticosterone responses and avian personalities. J. Ornithology 148, 169–178. doi: 10.1007/s10336-007-0175-8
Deakin A., Browne W. J., Hodge J. J., Paul E. S., and Mendl M. (2016). A screen-peck task for investigating cognitive bias in laying hens. PloS One 11, e0158222. doi: 10.1371/journal.pone.0158222
Edgar J. L., Nicol C. J., Pugh C. A., and Paul E. S. (2013). Surface temperature changes in response to handling in domestic chickens. Physiol. Behav. 119, 195–200. doi: 10.1016/j.physbeh.2013.06.020
Ferrante V., Mugnai C., Ferrari L., Marelli S. P., Spagnoli E., and Lolli S. (2016). Stress and reactivity in three Italian chicken breeds. Ital. J. Anim. Sci. 15, 303–309. doi: 10.1080/1828051X.2016.1185978
Forkman B., Boissy A., Meunier-Salaün M. C., Canali E., and Jones R. B. (2007). A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 92, 340–374. doi: 10.1016/j.physbeh.2007.03.016
Fraser D. (2008). Understanding animal welfare. Acta Vet Scand 50 (Suppl 1), S1. doi: 10.1186/1751-0147-50-S1-S1
Gallup G. G. (1979). Tonic immobility as a measure of fear in domestic fowl. Anim. Behav. 27, 316–317. doi: 10.1016/0003-3472(79)90159-3
Gjøen J., Jean-Joseph H., Kotrschal K., and Jensen P. (2023). Domestication and social environment modulate fear responses in young chickens. Behav. Processes 210, 104906. doi: 10.1016/j.beproc.2023.104906
Gyles N. R., Miley J. L., and Brown C. J. (1966). The response of resistant and susceptible strains of chickens and their F1 and F2 crosses to subcutaneous inoculations with Rous Sarcoma Virus. Poultry Sci. 46, 465–472. doi: 10.3382/ps.0460465
Harding E. J., Paul E. S., and Mendl M. (2004). Cognitive bias and affective state. Nature 427, 312. doi: 10.1038/427312a
Herborn K. A., Graves J. L., Jerem P., Evans N. P., Nager R., McCafferty D. J., et al. (2015). Skin temperature reveals the intensity of acute stress. Physiol. Behav. 152, 225–230. doi: 10.1016/j.physbeh.2015.09.032
Hernandez C. E., Hinch G., Lea J., Ferguson D., and Lee C. (2015). Acute stress enhances sensitivity to a highly attractive food reward without affecting judgement bias in laying hens. Appl. Anim. Behav. Sci. 163, 135–143. doi: 10.1016/j.applanim.2014.12.002
Hess C. W. (1962). Randombred populations of the southern regional poultry breeding project1. World’s Poultry Sci. J. 18, 147–152. doi: 10.1079/WPS19620019
Hicks B. M. and Patrick C. J. (2006). Psychopathy and negative emotionality: analyses of suppressor effects reveal distinct relations with emotional distress, fearfulness, and anger-hostility. J. Abnormal Psychol. 115, 276. doi: 10.1037/0021-843X.115.2.276
Hymel K. A. and Sufka K. J. (2012). Pharmacological reversal of cognitive bias in the chick anxiety-depression model. Neuropharmacology 62, 161–166. doi: 10.1016/j.neuropharm.2011.06.009
Iqbal A. and Moss A. F. (2021). Key tweaks to the chicken’s beak: the versatile use of the beak by avian species and potential approaches for improvements in poultry production. Animal 15, 100119. doi: 10.1016/j.animal.2020.100119
Iyasere O. S., Beard A. P., Guy J. H., and Bateson M. (2017). Elevated levels of the stress hormone, corticosterone, cause ‘pessimistic’ judgment bias in broiler chickens. Sci. Rep. 7, 6860. doi: 10.1038/s41598-017-07040-y
Janczak A. M., Heikkilä M., Valros A., Torjesen P., Andersen I. L., and Bakken M. (2007). Effects of embryonic corticosterone exposure and post-hatch handling on Tonic Immobility and willingness to compete in chicks. Appl. Anim. Behav. Sci. 107, 275–286. doi: 10.1016/j.applanim.2006.10.002
Jones R. B. (1986). The tonic immobility reaction of the domestic fowl: a review. World’s Poultry Sci. J. 42, 82–96. doi: 10.1079/WPS19860008
Jones R. B. (1996). Fear and adaptability in poultry: insights, implications and imperatives. World’s Poultry Sci. J. 52, 131–174. doi: 10.1079/WPS19960013
Jones R. B. and Faure J. M. (1981). Sex and strain comparisons of Tonic Immobility (“righting time”) in the domestic fowl and the effects of various methods of induction. Behav. Processes 6, 47–55. doi: 10.1016/0376-6357(81)90015-2
Jones R. B. and Waddington D. (1992). Modification of fear in domestic chicks, Gallus gallus domesticus, via regular handling and early environmental enrichment. Anim. Behav. 43, 1021–1033. doi: 10.1016/S0003-3472(06)80015-1
Koolhaas J. M., Korte S. M., De Boer S. F., van der Vegt B. J., Van Reenen C. G., Hopster H., et al. (1999). Coping styles in animals: Current status in behavior and stress-physiology. Neurosci. Biobehavioral Rev. 23, 925–935. doi: 10.1016/S0149-7634(99)00026-3
Košťál Ľ., Skalná Z., and Pichová K. (2020). Use of cognitive bias as a welfare tool in poultry. J. Anim. Sci. 98, S63–S79. doi: 10.1093/jas/skaa039
Kozak A., Rozempolska-Rucińska I., Kasperek K., and Bownik A. (2019). Level of stress in relation to emotional reactivity of hens. Ital. J. Anim. Sci. 18, 1252–1258. doi: 10.1080/1828051X.2019.1642150
Kremer L., Holkenborg S. K., Reimert I., Bolhuis J. E., and Webb L. E. (2020). The nuts and bolts of animal emotion. Neurosci. Biobehavioral Rev. 113, 273–286. doi: 10.1016/j.neubiorev.2020.01.028
Kuenzel W. J. (2007). Neurobiological basis of sensory perception: welfare implications of beak trimming. Poultry Sci. 86, 1273–1282. doi: 10.1093/ps/86.6.1273
Lindahl L. (2023). Domestication’s Effect on Associative and Spatial Learning in Chickens (Gallus gallus) (Linköping University in Sweden: Linköping University, Department of Physics, Chemistry and Biology). Bachelor’s thesis.
Lindqvist C., Janczak A. M., Nätt D., Baranowska I., Lindqvist N., Wichman A., et al. (2007). Transmission of stress-induced learning impairment and associated brain gene expression from parents to offspring in chickens. PloS One 2, e364. doi: 10.1371/journal.pone.0000364
Lindqvist C. and Jensen P. (2009). Domestication and stress effects on contrafreeloading and spatial learning performance in red jungle fowl (Gallus gallus) and White Leghorn layers. Behav. Processes 81, 80–84. doi: 10.1016/j.beproc.2009.02.005
Lindqvist C., Schütz K., and Jensen P. (2002). Red jungle fowl have more contrafreeloading than white leghorn layers: Effect of food deprivation and consequences for information gain. Behaviour 139, 1195–1209. doi: 10.1163/15685390260437335
Lindroth L. F. (2020). The relationship between corticosterone and personality in red junglefowl (Linköping University in Sweden: Linköping University, Department of Physics, Chemistry and Biology). Bachelor’s thesis.
Mendl M., Burman O. H., Parker R. M., and Paul E. S. (2009). Cognitive bias as an indicator of animal emotion and welfare: Emerging evidence and underlying mechanisms. Appl. Anim. Behav. Sci. 118, 161–181. doi: 10.1016/j.applanim.2009.02.023
Mendl M., Burman O. H., and Paul E. S. (2010). An integrative and functional framework for the study of animal emotion and mood. Proc. R. Soc. B: Biol. Sci. 277, 2895–2904. doi: 10.1098/rspb.2010.0303
Mendl M. and Paul E. S. (2004). Consciousness, emotion and animal welfare: insights from cognitive science. Anim. Welfare 13, S17–S25. doi: 10.1017/S0962728600014330
Miyatake T., Nakayama S., Nishi Y., and Nakajima S. (2009). Tonically immobilized selfish prey can survive by sacrificing others. Proc. R. Soc. Biol. Science. 276, 2763–2767. doi: 10.1098/rspb.2009.0558
Moe R. O., Bohlin J., Flø A., Vasdal G., and Stubsjøen S. M. (2017). Hot chicks, cold feet. Physiol. Behav. 179, 42–48. doi: 10.1016/j.physbeh.2017.05.025
Moe R. O., Stubsjøen S. M., Bohlin J., Flø A., and Bakken M. (2012). Peripheral temperature drop in response to anticipation and consumption of a signaled palatable reward in laying hens (Gallus domesticus). Physiol. Behav. 106, 527–533. doi: 10.1016/j.physbeh.2012.03.032
Mormede P., Andanson S., Auperin B., Beerda B., Guemene D., Malmkvist J., et al. (2007). Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 92, 317–339. doi: 10.1016/j.physbeh.2006.12.003
Murphy E., Nordquist R. E., and van der Staay F. J. (2013). Responses of conventional pigs and Göttingen miniature pigs in an active choice judgement bias task. Appl. Anim. Behav. Sci. 148, 64–76. doi: 10.1016/j.applanim.2013.07.011
Nakasai E., Tanizawa H., Takawaki M., Yanagita K., Kawakami S. I., Oka T., et al. (2013). Age-dependent change of Tonic Immobility response in chicks of a native Japanese chicken breed, Tosa-Jidori. J. Poultry Sci. 50, 321–325. doi: 10.2141/jpsa.0130018
Neuhauser J., Hintze S., Rault J. L., Smith S., and Sirovnik J. (2023). Training for a cognitive judgement bias task does not affect fear or telomere shortening in laying hens. Appl. Anim. Behav. Sci. 265, 105996. doi: 10.1016/j.applanim.2023.105996
Ouyang J. Q., Macaballug P., Chen H., Hodach K., Tang S., and Francis J. S. (2021). Infrared thermography is an effective, noninvasive measure of HPA activation. Stress 24, 584–589. doi: 10.1080/10253890.2020.1868431
Paul E. S., Harding E. J., and Mendl M. (2005). Measuring emotional processes in animals: the utility of a cognitive approach. Neurosci. Biobehavioral Rev. 29, 469–491. doi: 10.1016/j.neubiorev.2005.01.002
Peixoto M. R., Karrow N. A., Newman A., and Widowski T. M. (2020). Effects of maternal stress on measures of anxiety and fearfulness in different strains of laying hens. Front. Veterinary Sci. 7, 128. doi: 10.3389/fvets.2020.00128
Pichová K., Košťál Ľ., de Haan T. I., van der Eijk J. A., and Rodenburg T. B. (2021). High and low feather pecking selection lines of laying hens differ in response to a judgment bias test. Appl. Anim. Behav. Sci. 238, 105305. doi: 10.1016/j.applanim.2021.105305
Roelofs S., Boleij H., Nordquist R. E., and van der Staay F. J. (2016). Making decisions under ambiguity: judgment bias tasks for assessing emotional state in animals. Front. Behav. Neurosci. 10, 119. doi: 10.3389/fnbeh.2016.00119
Ross M., Garland A., Harlander-Matauschek A., Kitchenham L., and Mason G. (2019). Welfare-improving enrichments greatly reduce hens’ startle responses, despite little change in judgment bias. Sci. Rep. 9, 11881. doi: 10.1038/s41598-019-48351-6
Salmeto A. L., Hymel K. A., Carpenter E. C., Brilot B. O., Bateson M., and Sufka K. J. (2011). Cognitive bias in the chick anxiety–depression model. Brain Res. 1373, 124–130. doi: 10.1016/j.brainres.2010.12.007
Seehuus B., Mendl M., Keeling L. J., and Blokhuis H. (2013). Disrupting motivational sequences in chicks: Are there affective consequences? Appl. Anim. Behav. Sci. 148, 85–92. doi: 10.1016/j.applanim.2013.07.008
Steimer T. (2002). The biology of fear- and anxiety-related behaviors. Dialogues Clin. Neurosci. 4, 231–249. doi: 10.31887/DCNS.2002.4.3/tsteimer
Weimer S. L., Wideman R. F., Scanes C. G., Mauromoustakos A., Christensen K. D., and Vizzier-Thaxton Y. (2018). An evaluation of methods for measuring stress in broiler chickens. Poultry Sci. 97, 3381–3389. doi: 10.3382/ps/pey204
Weimer S. L., Wideman R. F., Scanes C. G., Mauromoustakos A., Christensen K. D., and Vizzier-Thaxton Y. (2021). Impact of experimentally induced bacterial chondronecrosis with osteomyelitis (BCO) lameness on health, stress, and leg health parameters in broilers. Poultry Sci. 100, 101457. doi: 10.1016/j.psj.2021.101457
Wichman A., Keeling L. J., and Forkman B. (2012). Cognitive bias and anticipatory behaviour of laying hens housed in basic and enriched pens. Appl. Anim. Behav. Sci. 140, 62–69. doi: 10.1016/j.applanim.2012.05.006
Zentall T. R., Wasserman E. A., and Urcuioli P. J. (2014). Associative concept learning in animals. J. Exp. Anal. Behav. 101, 130–151. doi: 10.1002/jeab.v101.1
Keywords: chicken, affective state, domestication, fear, stress, Red Junglefowl
Citation: Oyeniran VJ, Whittle RH, Orlowski SK and Weimer SL (2025) Judgment bias, fear, and stress responses of Red Junglefowl and Athens Canadian Random Bred chickens. Front. Anim. Sci. 6:1573847. doi: 10.3389/fanim.2025.1573847
Received: 10 February 2025; Accepted: 28 April 2025;
Published: 02 June 2025.
Edited by:
Ruth C. Newberry, Norwegian University of Life Sciences, NorwayReviewed by:
T. Bas Rodenburg, Utrecht University, NetherlandsKatarína Pichová, Slovak Academy of Sciences (SAS), Slovakia
Copyright © 2025 Oyeniran, Whittle, Orlowski and Weimer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shawna L. Weimer, c3dlaW1lckB1YXJrLmVkdQ==
 Victor J. Oyeniran
Victor J. Oyeniran Sara K. Orlowski
Sara K. Orlowski Shawna L. Weimer
Shawna L. Weimer