- 1College of Animal Science and Technology, Henan University of Animal Husbandry and Economy, Zhengzhou, Henan, China
- 2Henan Engineering Research Center on Animal Healthy Environment and Intelligent Equipment, Zhengzhou, Henan, China
- 3Institute of Agricultural Bio-Environmental Engineering, College of Biosystem Engineering and Food Science, Zhejiang University, Hangzhou, China
Introduction: This study investigated the effects of slightly acidic electrolyzed water (SAEW) on the gut morphology and microbiota structure in early weaned piglets.
Methods: A total of 144 healthy Large White × Landrace × Duroc weaned piglets (21 days old, sex in half, each weighing 7.0 ± 0.5 kg) were randomly assigned into three groups. Each group included four replicates, with each replicate comprising 12 piglets. The weaned piglets in the control (CON) group drank only disinfected tap water. Those in experimental groups I and II were given SAEW with available chlorine concentrations (ACCs) of 0.3 and 0.6 mg/L, respectively. The formal experimental period was 15 days.
Results: The results showed that the weight and the length of the intestines of the piglets in the SAEW I and II groups significantly increased compared with those in the CON group. In the jejunum, the villus height of the SAEW II group significantly increased by 13.1% compared with the CON group (p < 0.05). In the ileum, the villus heights of the SAEW I and II groups significantly increased compared with the CON group (p < 0.05). The villus height/crypt depth ratio of the two experimental groups significantly increased compared with that of the CON group (p < 0.05). No significant changes were observed in the gut microbial diversity. However, the relative abundance (RA) of Bacteroidetes, Proteobacteria, and Actinobacteria increased compared with the CON group (p < 0.05). The RA of Firmicutes in the duodenum, jejunum, ileum, and cecum of piglets significantly decreased in the SAEW I and II groups. At the genus level, the RA of Lactobacillus decreased (p < 0.05), while that of Streptococcus, Prevotella, and Eubacterium increased (p < 0.05).
Discussion: In conclusion, drinking SAEW significantly improves the intestinal development and the morphological structure of piglets and significantly promotes the colonization of the Streptococcus, Prevotella, and Eubacterium flora in the intestinal tract, but reduces the abundance of Lactobacillus in the small intestine. This might be related to the alteration of the intestinal pH value by SAEW. The application effects of more SAEW concentrations require further experimental research.
1 Introduction
Gastrointestinal tract health is a common term that has received increasing attention worldwide. In the past, gastrointestinal tract health was defined as the “avoidance/prevention/absence of disease so that the animals are able to exhibit physiological characteristics in response to endogenous and exogenous stressors” (Kogut and Arsenault, 2016). In recent years, more and more scientists have defined gastrointestinal tract health as being more than just the avoidance/prevention/absence of gastrointestinal tract disease, which further improves our understanding of the entire problem (Koeleman, 2018; Vincent, 2021; Celi et al., 2017; Pluske et al., 2018). The weaning of piglets is a key link to determining the profitability of pig farming and is also a stage in which intestinal health issues are common. The low feed intake after weaning (Hansen et al., 2022), including the lack of luminal nutrition (Jayaraman and Nyachoti, 2017), and other weaning-associated factors can alter the structures and functions of the gastrointestinal tract (Song et al., 2025; Li et al., 2018; Bonetti et al., 2021). Altogether, the immediate post-weaning period not only leads to remarkable functional and structural alterations to the small intestine of pigs (Choudhury et al., 2021a, Choudhury et al., 2021b) but also causes gut inflammation, disrupts the microbiota, alters the crypt–villus structure, and affects the gastrointestinal tract barrier integrity (Meng et al., 2022; Wei et al., 2021; Degroote et al., 2020; Wang et al., 2022). Therefore, it is crucial to improve the intestinal health of piglets and prevent the occurrence of intestinal diseases.
The use of antibiotics in intensive pig production has been an important part of industrial technology for animal food production (Rahman et al., 2022; Lekagul et al., 2019). The addition of antibiotics to piglet feed and drinking water can improve intestinal health, promote intestinal flora development, and improve piglet survival. However, both targeted and non-targeted bacteria may develop resistance to these additives, thus resulting in a potential hazard to human health due to the consumption of enteropathogen-contaminated pork products (Mesfin et al., 2024; Ngangom et al., 2019). Therefore, natural product options, including slightly acidic electrolyzed water (SAEW), have been proposed for pig production.
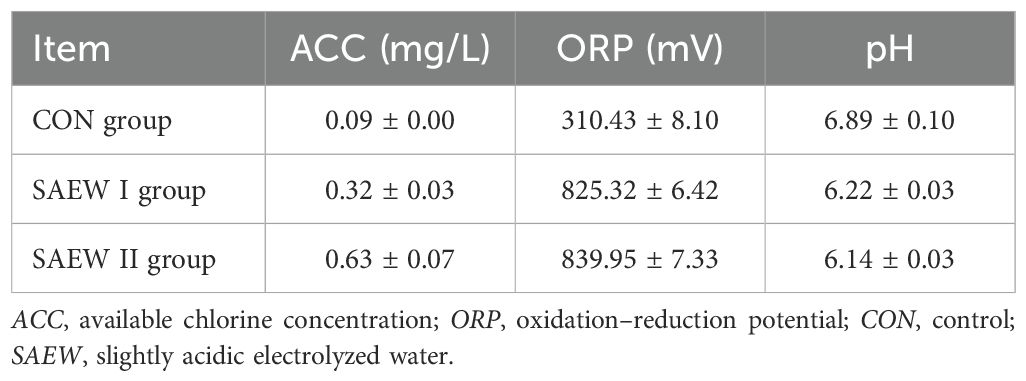
In electrolyzed water production equipment using an electrolysis chamber in the absence of a separating membrane, SAEW with a pH of 5.0–6.5 and an oxidation–reduction potential (ORP) of 800–900 mV is generated by HCl electrolysis or in combination with NaCl (Nan et al., 2019). Against Escherichia coli, the disinfection efficacy of HOCl, a form of free available chlorine, is 80-fold greater than that of ClO− (Ampiaw et al., 2021). It is a promising approach for the food industry due to its wide-spectrum antimicrobial activity, high disinfection efficacy, cost effectiveness, and easy production (Afari and Hung, 2018). SAEW has been widely used in medical disinfection (Yan et al., 2021; Farah and Al-Haj Ali, 2021; Gutiérrez−García et al., 2022), food preservation (Liao et al., 2020; Bing et al., 2022; Lan et al., 2021), and animal husbandry disinfection (Shi et al., 2020; Zang et al., 2019; Li et al., 2019). In recent years, there have been occasional reports on the use of SAEW as an additive for animal drinking water. Inagaki et al. (2021) suggested that SAEW at 5 ppm total residual chlorine is an effective and a safe alternative to sterile water for use as drinking water in laboratory animal facilities. Xiaoxia et al. (2023) provided proof that the addition of 15 and 30 mg/L SAEW to the drinking water of piglets could effectively enhance their production performance and increase the abundance of Bacteroidetes and Firmicutes in the intestinal tract. However, SAEW is a chlorine-containing disinfectant, and the stimulating effect of drinking high-concentration SAEW on the gastrointestinal mucosa cannot be neglected. Ji et al. (2020a, 2020b) reported that the addition of 0.3, 0.5, 0.7, and 1.0 mg/L of SAEW to the drinking water of broilers could effectively improve production performance, reduce abnormal behaviors, improve the immune function, and decrease the intestinal E. coli and Salmonella populations. However, its specific effects on the intestinal morphology and microbiota of piglets remain unclear.
In this study, different concentrations of SAEW were added into the drinking water of early weaned piglets to 1) assess the effect of drinking SAEW on the intestinal pathological morphology of weaned piglets; ii) evaluate the impact of drinking SAEW on the intestinal flora structure of weaned piglets; and iii) examine the feasibility and safety of using SAEW as a drinking water for weaned piglets.
2 Materials and methods
2.1 Experimental design and animals
The study protocols were in compliance with the Animal Husbandry and Economics’ Institutional Animal Care and Use Committee of Henan University (no. HNUAHE488). The institutional safety procedure was followed.
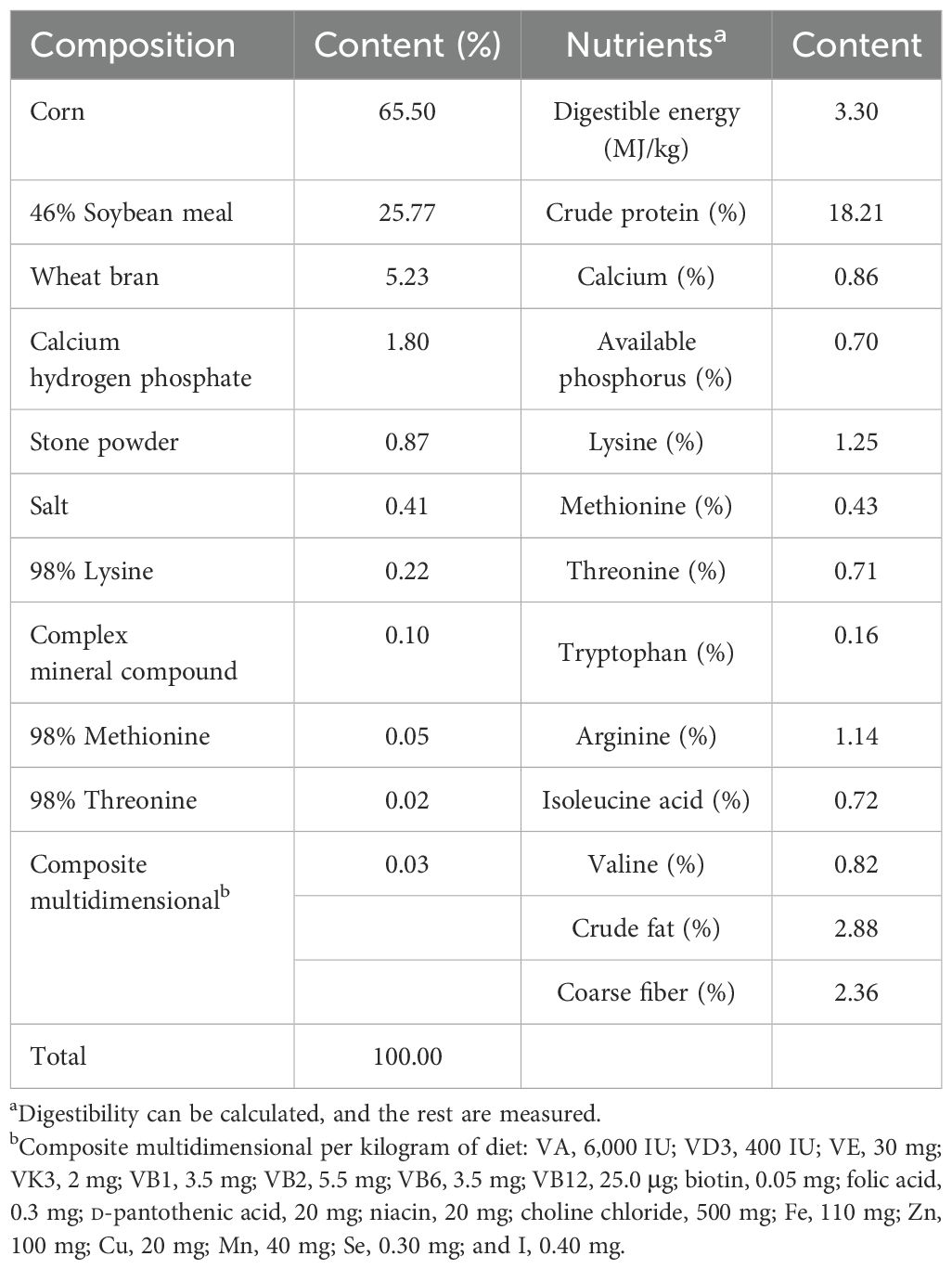
A total of 144 healthy Large White × Landrace × Duroc weaned piglets (21 days old, 72 females and 72 males, each weighing 7.0 ± 0.5 kg) were randomly assigned into three groups. Each group included four replicates, with each replicate comprising 12 piglets (six females and six males). The piglets in the control (CON) group drank only disinfected tap water. Two experimental groups (groups I and II) were given SAEW with available chlorine concentrations (ACCs) of 0.3 and 0.6 mg/L, respectively. The physical and chemical features of these two groups and the CON group are shown in Table 1. A non-membrane generator (Harmony-II, Ruiande Biosafety Technology, Beijing, China) was used to prepare the storage solution for SAEW, and 1.0 g/L NaCl solution containing 100 μg/L HCl was electrolyzed. To prepare the above-mentioned ACCs, the obtained SAEW was diluted in sterile deionized water. The ACCs of SAEW were determined using a digital display chlorine tester (Shanghai Jiahua Test Equipment Co., Ltd., Shanghai, China). The ORP and the pH were measured using an electrode-type tester (Shanghai Kangyi Technology Co., Ltd., Shanghai, China). The formal experiment period lasted for 15 days. The weaned piglets were bred conventionally according to the farm’s standard procedures. All weaned piglets had unlimited access to food and drinking water. The automatic drinking water system for animals was used. The SAEW in the drinking system was replaced once every 7 days. The piglet feed was purchased from Henan Twins Feed Co., Ltd. The basic diet was prepared according to the Nutrient Requirements of Swine from the National Research Council (National Research Council, 2012). Table 2 shows the nutrient levels and the composition of the basic diet.
2.2 Growth performance
The food and water intakes of each group were recorded daily, while the body weight (BW) of each piglet was measured weekly. The average daily feed intake (ADFI), the average daily gain (ADG), and the weight gain-to-feed intake ratio (F/G) were calculated using the group records of feed intake and the individual records of BW.
2.3 Sample collection and preservation
On day 15 of the experiment period, two piglets were selected from each repetition, and a total of eight piglets (four females and four males) were selected from each group. These piglets were fasted for 12 h, weighed, and then anesthetized by intramuscular injection of 4% pentobarbital (PEA). After full anesthesia, the piglets were quickly killed by bloodletting. To collect the 20-cm middle section of the intestine from the colon (mid-spiral colon), ileum (50 cm upstream from the ileocaecal valve), and jejunum (1.5 m from the duodenal–jejunal flexure), the gastrointestinal tract was isolated from the abdominal cavity of each piglet and immediately dissected within 25 min. Under aseptic conditions, the luminal content was collected from the intestinal tissue segments, snap-frozen in liquid nitrogen, and kept at −80°C until subsequent analysis. Representative segments were sampled from the duodenum, jejunum, ileum, and cecum for histological analysis. These bowel segments were fixed in 10% formalin prior to use. The same samples were taken from all animals.
2.4 Macroscopic parameters of the intestinal organs
After scarification, the length and the weight of the intestinal organs were measured. The lengths of the large intestine (colon plus caecum) and the small intestine (duodenum plus jejunum plus ileum), as well as the weights of the large intestine, small intestine, and stomach (empty and full), were recorded for each piglet. After removal of digesta, the empty weight of the intestinal segments was assessed by gently squeezing the intestine. Subsequently, the intestine was rinsed in saline solution, with the excess rinsing fluids removed using paper towels. Used for hematoxylin–eosin (H&E) staining and transmission electron microscopy.
2.5 Morphometric histological measurements
The gut samples were taken out from 10% formalin after fixation for 24 h. The samples were trimmed and flushed with water overnight, followed by dehydration and embedding in paraffin blocks. A FPMRC-HIS-3368AM rotary microtome (Shanghai Optical Instrument No. 5 Factory Co., Ltd., Shanghai, China) was used to cut 5-µm sections, followed by deparaffinization, hydration, and staining with H&E. The stained sections were analyzed and examined using a CSOIF 4XC20BD bright–dark field inverted metallurgical microscope (Shanghai Optical Instrument No. 5 Factory Co., Ltd., Shanghai, China). Five sites were randomly selected in the field of vision, and the images (×5 magnification) were processed using an Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA). The morphometric intestinal parameters crypt depth and villus length (in micrometers) were determined on 40 crypt and villus specimens (n = 5 intestinal sections per piglet, n = 8 piglets per group). Finally, the villus height-to-crypt depth (V/C) ratios were measured.
2.6 Scanning electron microscopy examination
The specimens (1 cm × 1 cm) were cut longitudinally from the duodenum, jejunum, ileum, cecum, and colon. After fixing with 4% glutaraldehyde and post-fixing with 1% osmium tetroxide in 0.1 M sodium cacodylate buffer (pH 7.2) at 4°C for 60 min, the specimens were dehydrated through graded ethanol concentrations, followed by critical point drying. The specimens were attached to aluminum stubs with their internal surface facing upward and then covered with carbon tabs. After sputtering with gold, the specimens were examined using Nova Nano SEM 450 (FEI Company, Hillsboro, NE, USA).
2.7 Microbiota metataxonomic analysis
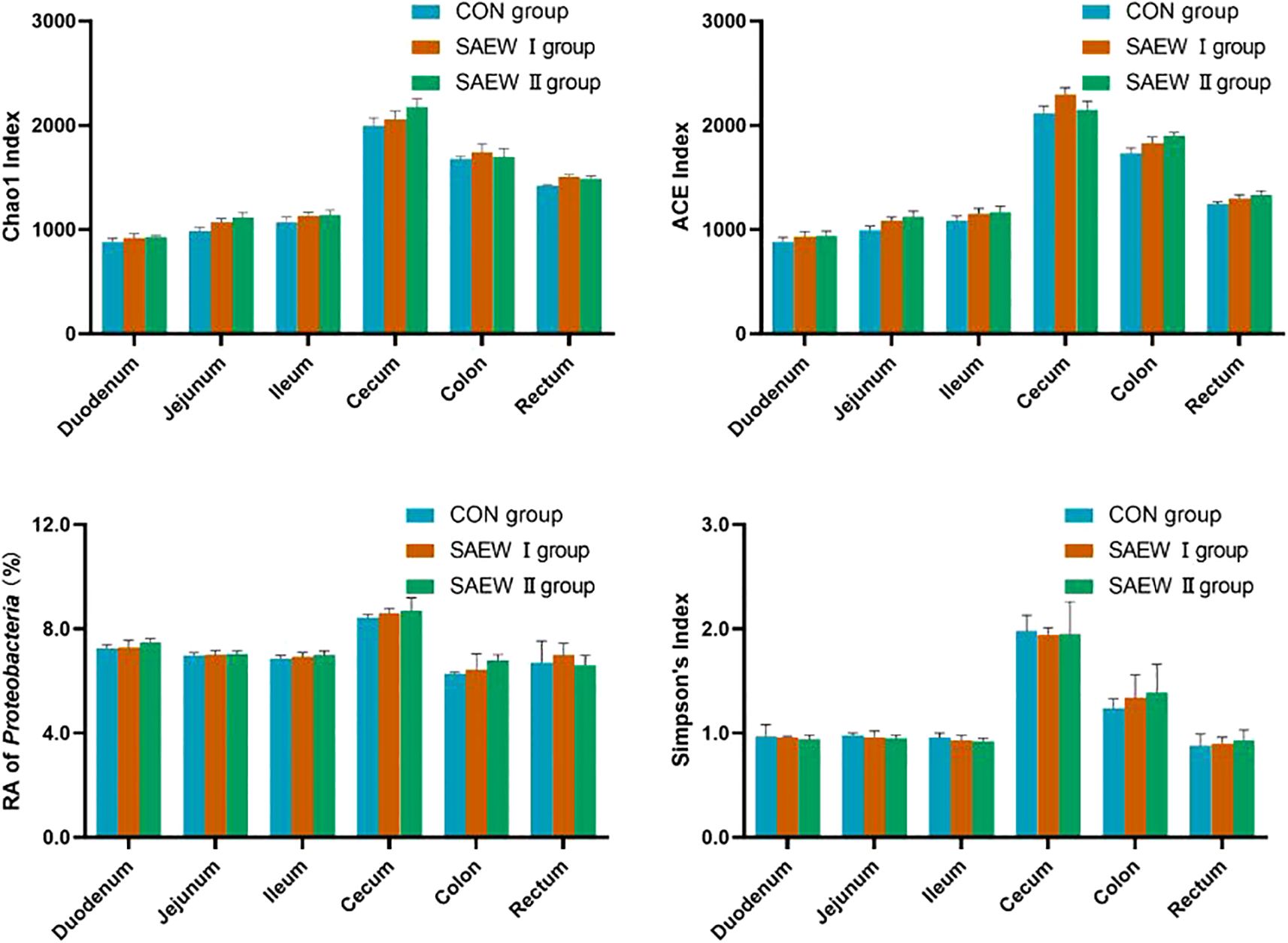
The middle part of the duodenum, jejunum, ileum, cecum, colon, and rectum was selected. For each intestinal segment, five sampling points were selected. A stainless steel-tipped sampling spoon was used to collect approximately 500 mg of content (wet weight), which was placed in the same sterile centrifuge tube. This was mixed well to ensure that the sample is uniform for the subsequent extraction of microbial DNA. Total genomic DNA extraction was conducted with the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) based on the repeated bead beating method (Bo et al., 2021). The purity and yield of the DNA extracts were examined with DYCP-1 Gel Electrophoresis (Tuohe Inc., Beijing, China) and Tnano-700 Ultra Micro-Spectrophotometer (Tuohe Inc., Beijing, China). The microbial communities in the gut contents were analyzed using the 16S rRNA V3–V4 (338F: 5′-ACTCCTACGGGAGGCAGCA-3′ and 806R: 5′-GGACTACVSGGGTATCTAAT-3′) region. Sequencing was undertaken by Beijing Novogene Bioinformatics Technology Co., Ltd. and was performed using the Illumina HiSeq 2000 Sequencing System (BaseClear BV, Leiden, Netherlands). The FASTAQ sequence files were obtained for BaseClear in-house filtering and quality control. SILVA database version 132 was used for binning the sequences into operational taxonomic units (OTUs) at 97% identity threshold (Quast et al., 2012). Taxonomic data were obtained, and the RA of the intestinal microbiota was estimated at the genus and phylum levels across the samples. Multiple sequence alignment was carried out using the PyNAST program and the GreenUene database to obtain the phylogenetic relationships between all representative OTU sequences. Rarefaction of the OTUs was conducted to obtain the sequencing depth (11,000 reads) for each sample. Indicators of species diversity (alpha diversity) were examined based on the evenness (Shannon) and microbial species richness (Chao 1 bias-corrected) indicators. The data from all samples were normalized. The QIIME software was used to compare the α diversity indìces of the gut microbiota and to conduct multi-sample analysis [principal coordinates analysis and unweighted pair group method with arithmetic mean (UPGMA) clustering analysis].
2.8 Data processing and analysis
The results are presented as the mean ± standard deviation. The Shapiro–Wilk method was used to test the normality. One-way ANOVA was performed using Origin (v9.0; Origin Lab Cor., Northampton, MA, USA) to compare the gut morphology and diversity and the RA of the gut microbiota. Multiple comparisons were conducted using Duncan’s test and the least significant difference (LSD) test. The Bonferroni method was used for correction. A p < 0.05 was deemed statistically significant.
3 Results
3.1 Effect of SAEW on growth performance
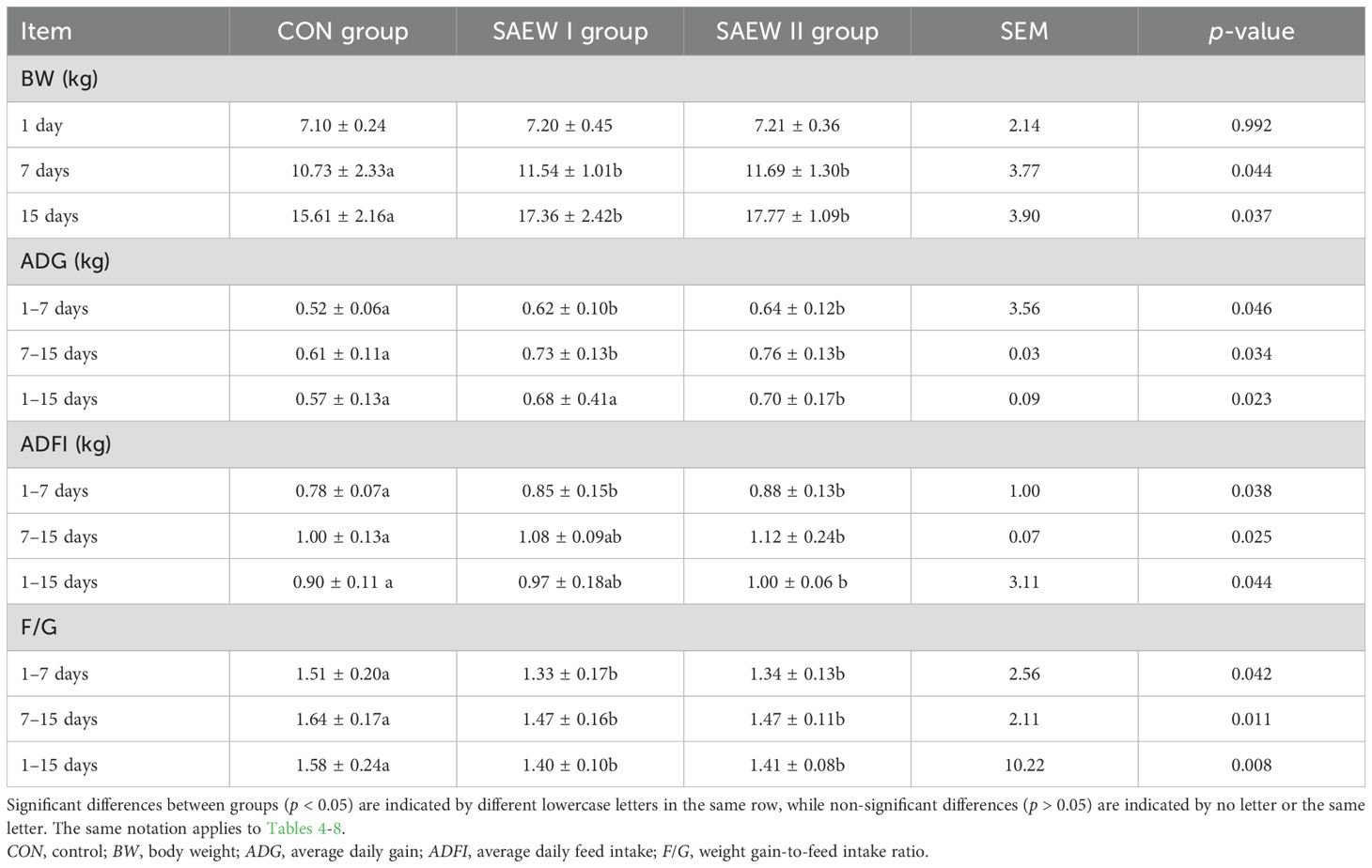
The effect of SAEW on piglet performance is shown in Table 3. On day 1, BW showed no significant difference between the three groups (p = 0.992). On days 7 and 15, the BWs in the SAEW I and II groups significant increased compared with the CON group (p < 0.05). The ADG and ADFI of the SAEW II group increased from day 1 to day 15, and there were significant differences compared with the CON group (p < 0.05). The F/G ratios in the SAEW I and II groups decreased from day 1 to day 15 compared with the CON group (p < 0.05).
3.2 Macroscopic measurements of the intestinal organs
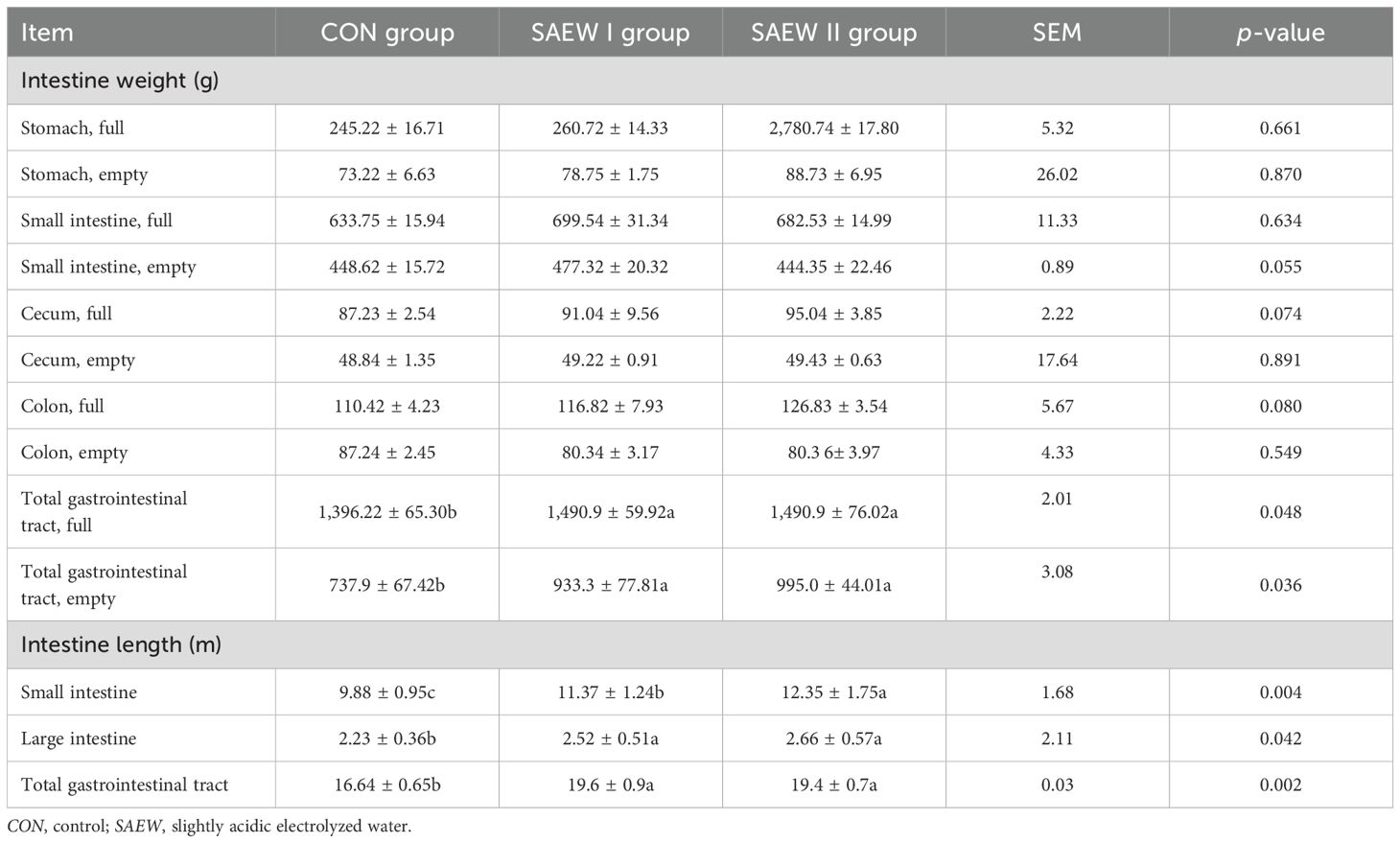
The length and the weight of the intestinal organs were measured to determine the effect of drinking SAEW on the gastrointestinal development of weaned piglets (Table 4). However, the piglets in the SAEW I and II groups had a heavier total gastrointestinal tract (full and empty) compared with those in the CON group (p = 0.048 and p = 0.036, respectively). The total gastrointestinal tract (p = 0.002), small intestine (p = 0.004), and large intestine (p = 0.042) tended to be larger in the SAEW I and II groups compared with the CON group.
3.3 Histological and SEM observations
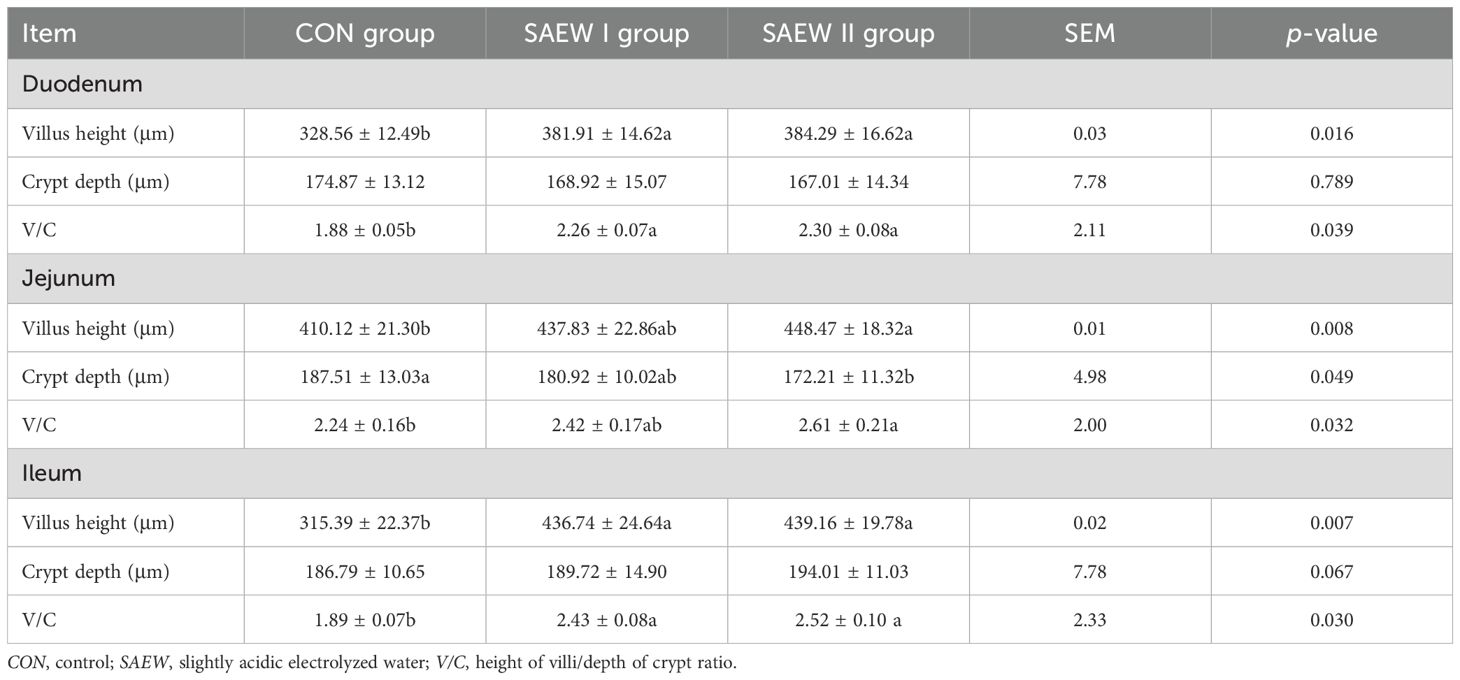
The duodenum, ileum, and jejunum are parts of the small intestine that can absorb carbohydrates and proteins. There are abundant mucosal folds and villi, as well as a few glands in a normal duodenum, jejunum, and cecum. In the duodenum of the CON group, the intestinal wall became thin with villous atrophy (shortened and thin villi), the number of lymph cells in the lamina propria were markedly reduced, and patches of microvilli fell off (Figure 1). However, the duodenum of the piglets in the SAEW I and II groups had healthy and thick villi, with abundant lymph cells in the lamina propria and intact microvilli (Figure 1). Well-formed Peyer’s patches were found in the ileum and jejunal submucosa, and abundant cells were observed in the lamina propria in the SAEW I and II groups. Peyer’s patches were obvious in the submucosa of the ileum and jejunum, but the epithelial cells sloughed off and the villi were atrophied in the CON group (Figure 1). The histological data of the intestines in the three groups are demonstrated in Table 5. The findings showed that the villus height of the duodenum and ileum in the SAEW I and II groups was significantly higher than that in the CON group (p < 0.05). The jejunum villus height in the SAEW II group was significantly higher than that in the CON group (p < 0.05), while the crypt depth was significantly lower than that in the CON group (p < 0.05). There was no significant difference in the jejunum villus height and crypt depth between the SAEW I and CON groups (p > 0.05). The V/C values of the duodenum and ileum in the SAEW I and II groups were significantly higher than those in the CON group (p < 0.05).
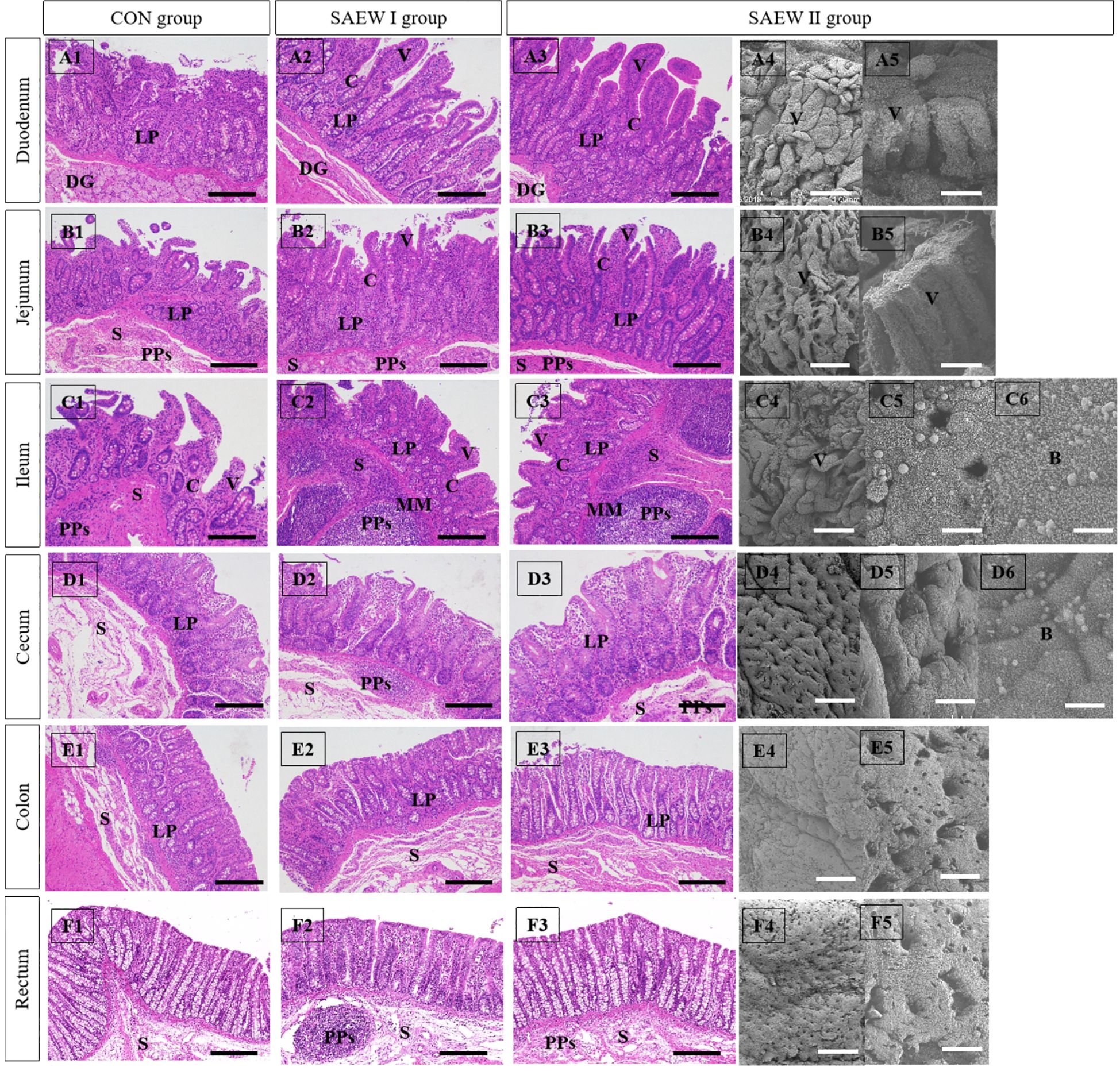
Figure 1. Effects of slightly acidic electrolyzed water (SAEW) on the histological structures of the small intestinal mucosa. B, bacteria; C, crypts; DG, duodenal gland; LP, lamina propria; MM, muscularis mucosa; PPs, Peyer’s patches; S, submucosa; V, villi. Scale bars: 200 μm in (A1–A3, B1–B3, C1–C3), and (D1–D3); 300 μm in (E1–E3, F1–F3, A4, B4, C4, D4, E4, F4); 30 μm in (A54, B5, C5, D5, E5, F5); and 10 μm in (C6) and (D6). The number of histological sections analyzed for each piglet was 6.
The cecum, colon, and rectum are parts of the large intestine that can absorb inorganic salts and water and decompose cellulose via fermentation. Unlike the small intestine, the large intestine does not have villi and mucosal folds; hence, its mucosal surface is smooth. The mucosa of the large intestine is also rich in glands. In the SAEW I and II groups, Peyer’s patches were well formed in the submucosa of the colon and cecum, and abundant lymphocytes were observed in the lamina propria. In the CON group, Peyer’s patches were atrophied in the submucosa of the cecum and colon, and there was congestion in the lamina propria (Figures 1).
3.4 Effects of SAEW on the gut microbiota of piglets
3.4.1 OTU sequences and statistics
OTU clustering was performed at a 98% similarity threshold. From 72 samples in the three groups, 14,916 distinct OTUs were obtained, including 4,442, 5,689, and 4,820 OTUs in the CON, SAEW I, and SAEW II groups, respectively. It can be seen from Figure 2 that, as the number of OTU increases, the curves of all samples tend to be flat, indicating that the actual sequencing volume is sufficient to cover the composition of the bacterial community species and can truly reflect the relative proportion relationship among the various bacterial species in the community.
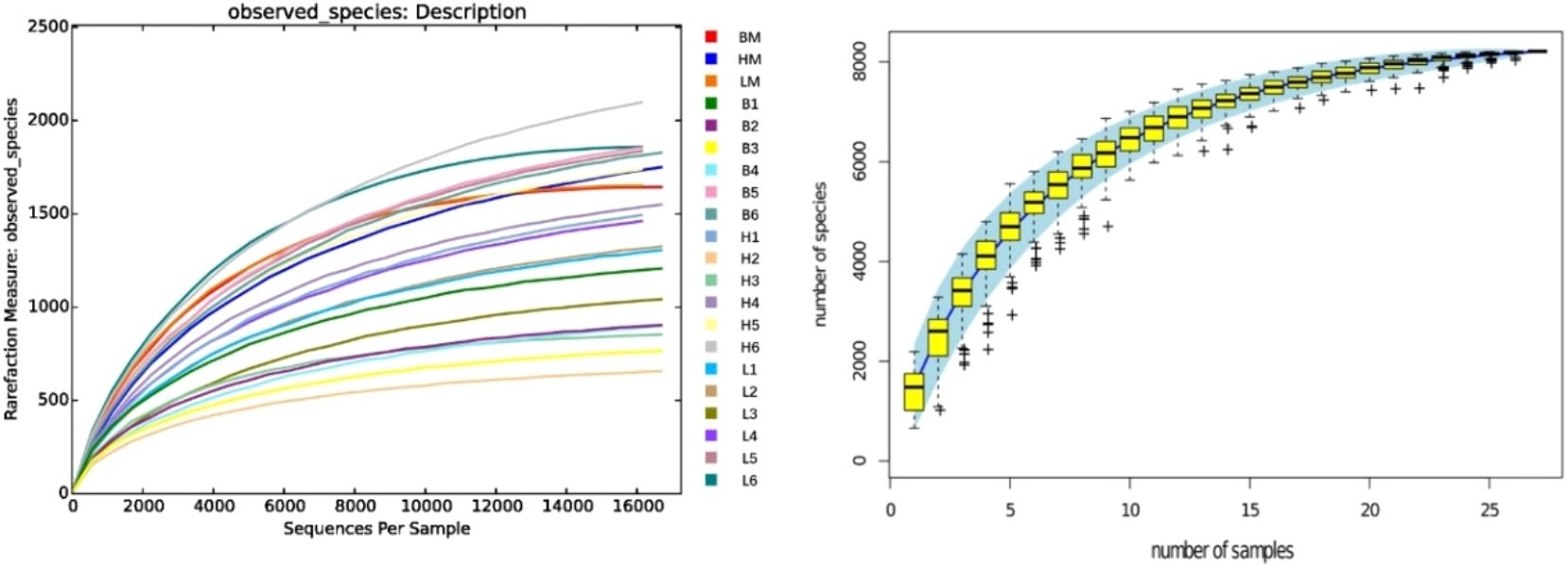
Figure 2. Rarefaction curve and species accumulation curves. B, control (CON) group; H, slightly acidic electrolyzed water (SAEW) II group; L, SAEW I group; BM, CON group, all intestinal mixed sample; HM, SAEW II group, all intestinal mixed sample; LM, SAEW I group, all intestinal mixed sample; 1, duodenum; 2, jejunum; 3, ileum; 4, cecum; 5, colon; 6, rectum.
3.4.2 Analysis of alpha diversity
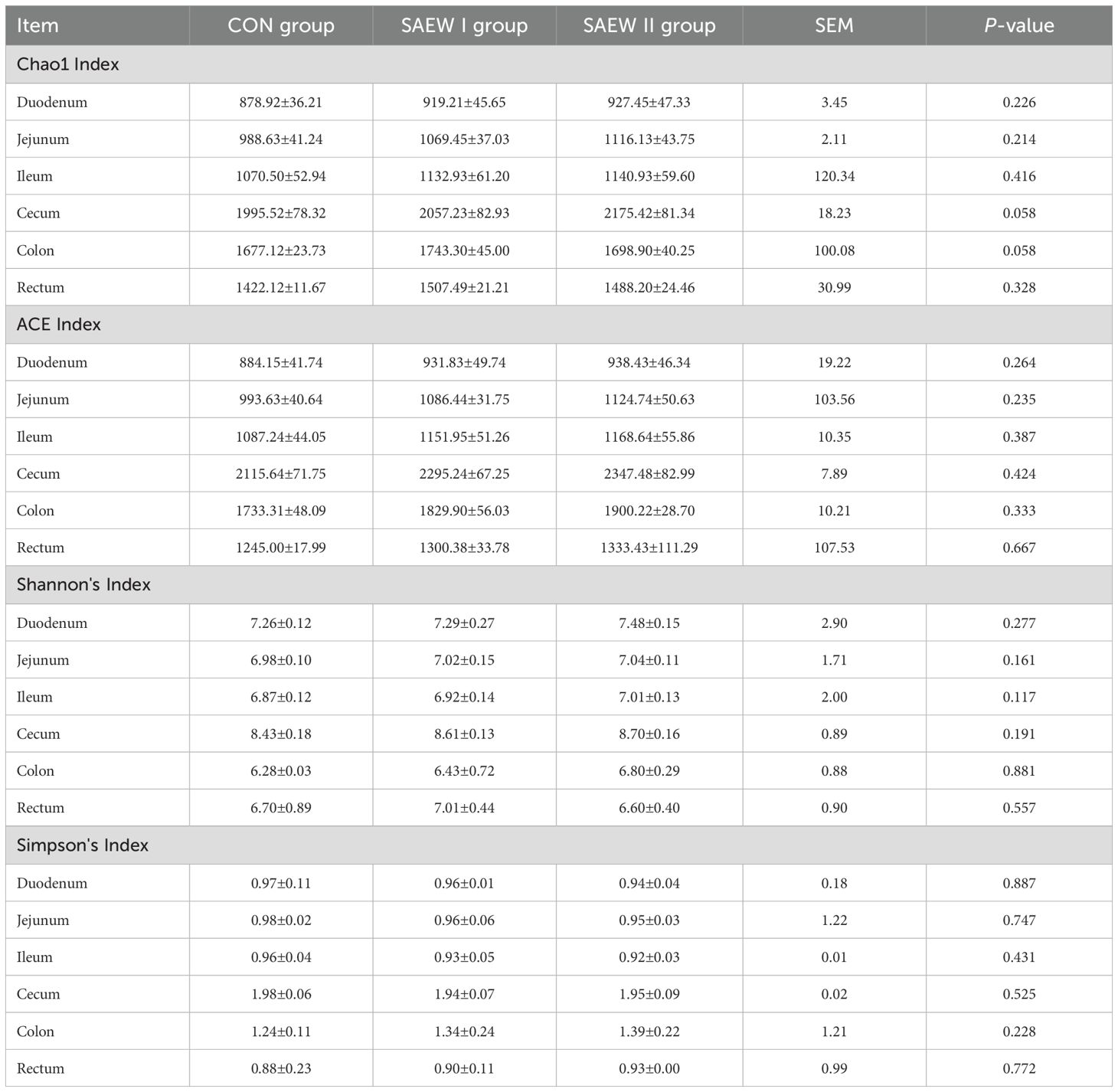
The analysis of the alpha diversity metrics for the different samples at a 98% similarity threshold is shown in Figure 3. The three groups did not differ significantly in terms of the Shannon, ACE, Chao1, and Simpson indices of the microbial communities in the colon, cecum, duodenum, ileum, jejunum, and rectum. Compared with the CON group, the two SAEW groups showed an increasing trend in microbial diversity, but there was no significant difference (p > 0.05).
3.4.3 Microbial composition of the intestine in different treatments at the phylum level
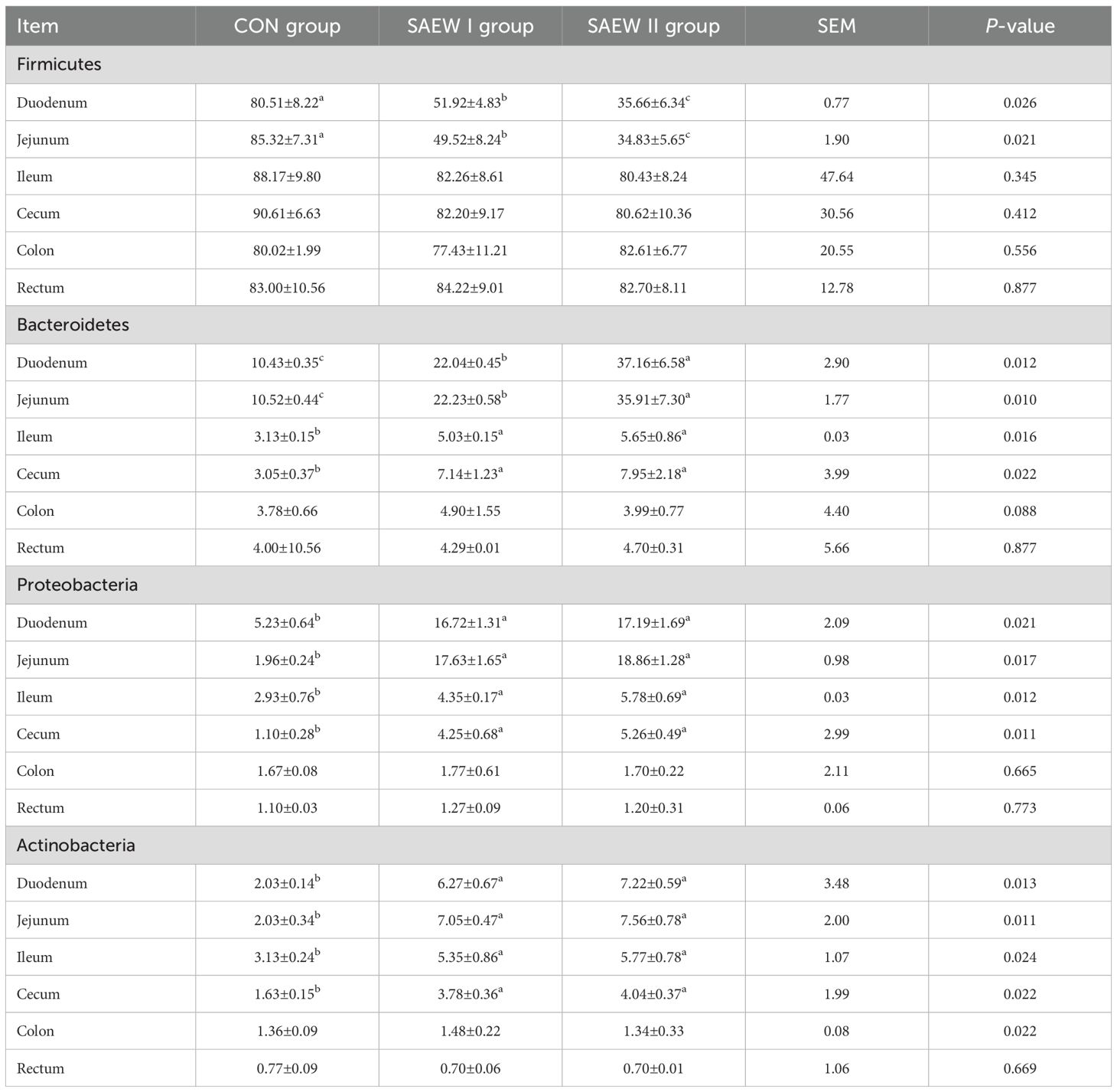
At the phylum level, Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria were the shared predominant bacteria in the gut in all three groups (RA > 1%). The abundance of the shared bacterial phyla in the gut across the groups is shown in Figure 4. The RA of Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria in the duodenum and jejunum changed significantly in the SAEW I and II groups compared with that in the CON group (p < 0.05). Compared with the CON group, the RA of Firmicutes in the duodenum and jejunum of the SAEW I group was significantly reduced by 35.5% and 41.9%, respectively (p < 0.05). In the duodenum, jejunum, ileum, and cecum, the RA of Bacteroidetes increased significantly by 111.5%, 111.4%, 61.3%, and 136.7%, respectively (p < 0.05), that of Proteobacteria increased significantly by 221.1%, 826.3%, 55.1%, and 281.8%, respectively (p < 0.05), and that of Actinobacteria increased significantly by 210%, 250%, 64.5%, and 131.3%, respectively (p < 0.05). In the SAEW II group, the RA of Firmicutes in the duodenum and jejunum was significantly reduced by 55.8% and 59.2%, respectively (p < 0.05). In the duodenum, jejunum, ileum, and cecum, the RA of Bacteroidetes increased significantly by 256.7%, 241.9%, 80.6%, and 163.3%, respectively (p < 0.05), that of Proteobacteria increased significantly by 228.8%, 889.5%, 96.6%, and 372.7%, respectively (p < 0.05), and that of Actinobacteria increased significantly by 260.0%, 275.0%, 83.9%, and 150.0%, respectively (p < 0.05). There was no significant change in the flora of the colon and rectum among the three treatment groups (p > 0.05).
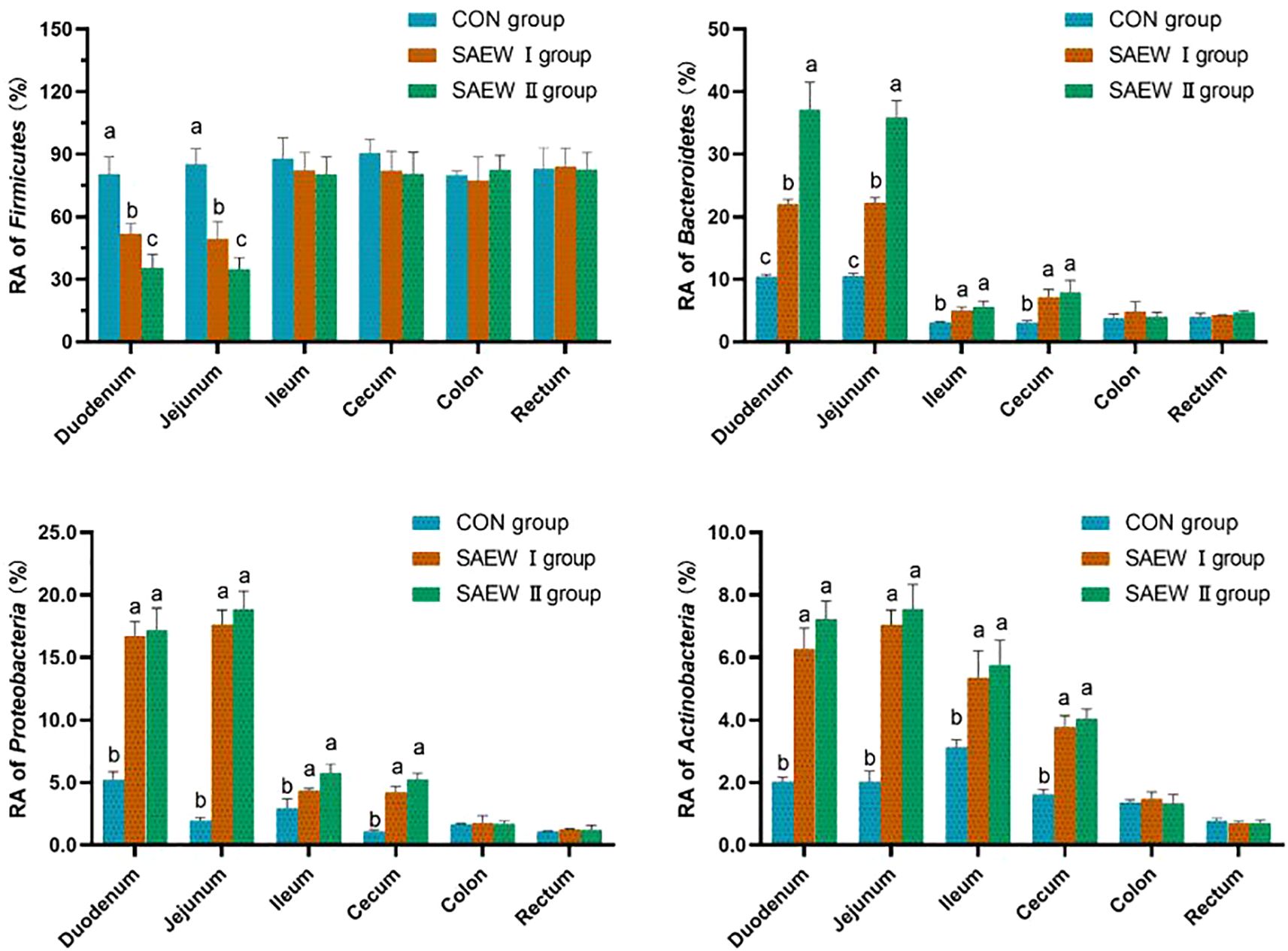
Figure 4. Relative abundance (RA) of the shared bacterial phyla in the gut across the three groups. a represents p>0.05, b represents p<0.05, and c represents p<0.01.
3.3.4 Microbial composition of the gut in different treatment groups at the genus level
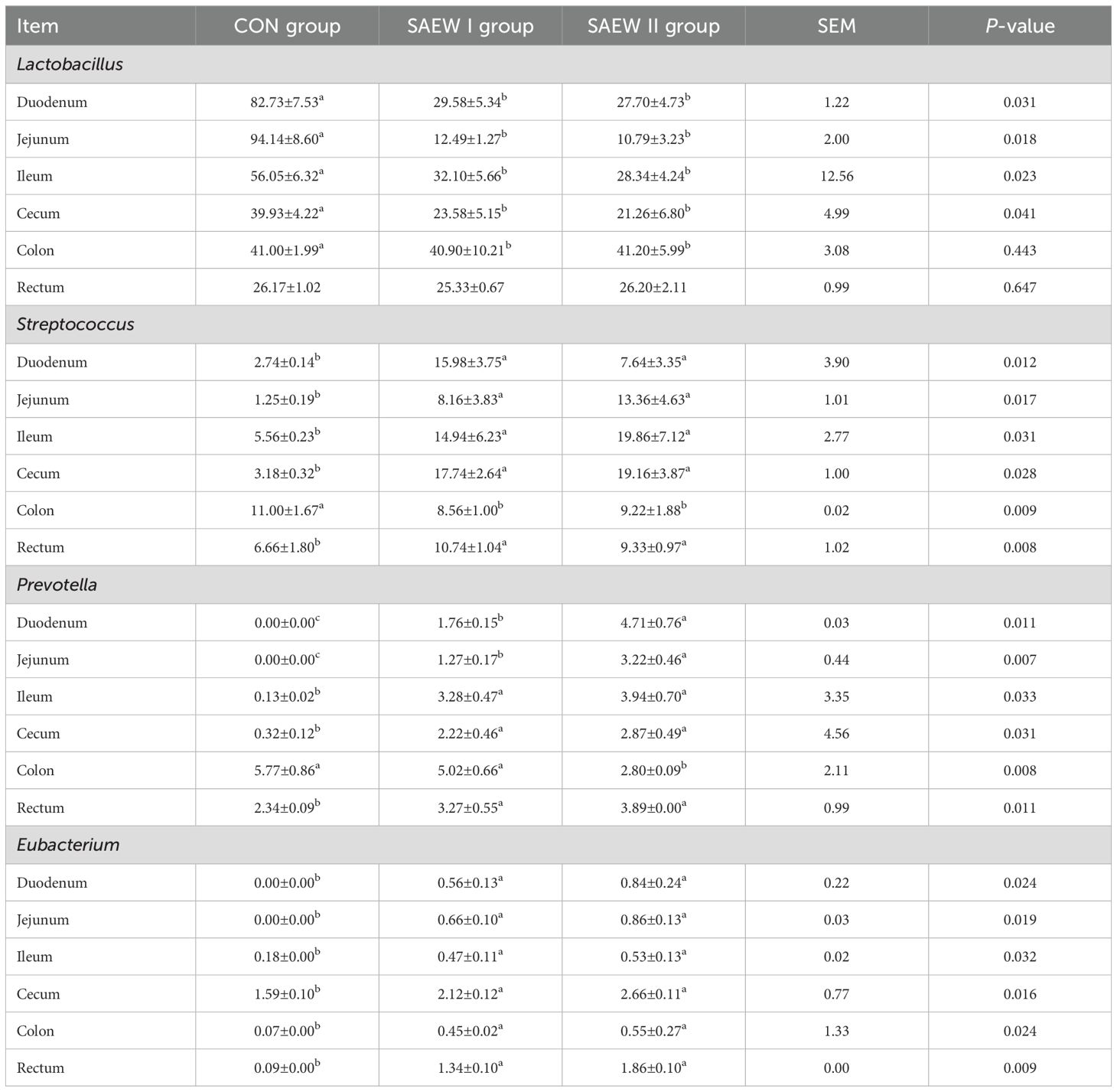
At the genus level, except for the unidentified bacteria, the genera with higher RA were Lactobacillus, Streptococcus, Prevotella, and Eubacterium. The RA of the shared bacterial genera in the gut across the three groups is shown in Figure 5. The RA of Lactobacillus in the duodenum, jejunum, ileum, and cecum was reduced (p < 0.05), while that of Streptococcus, Prevotella, and Eubacterium increased (p < 0.05) in the SAEW I and II groups compared with that in the CON group.
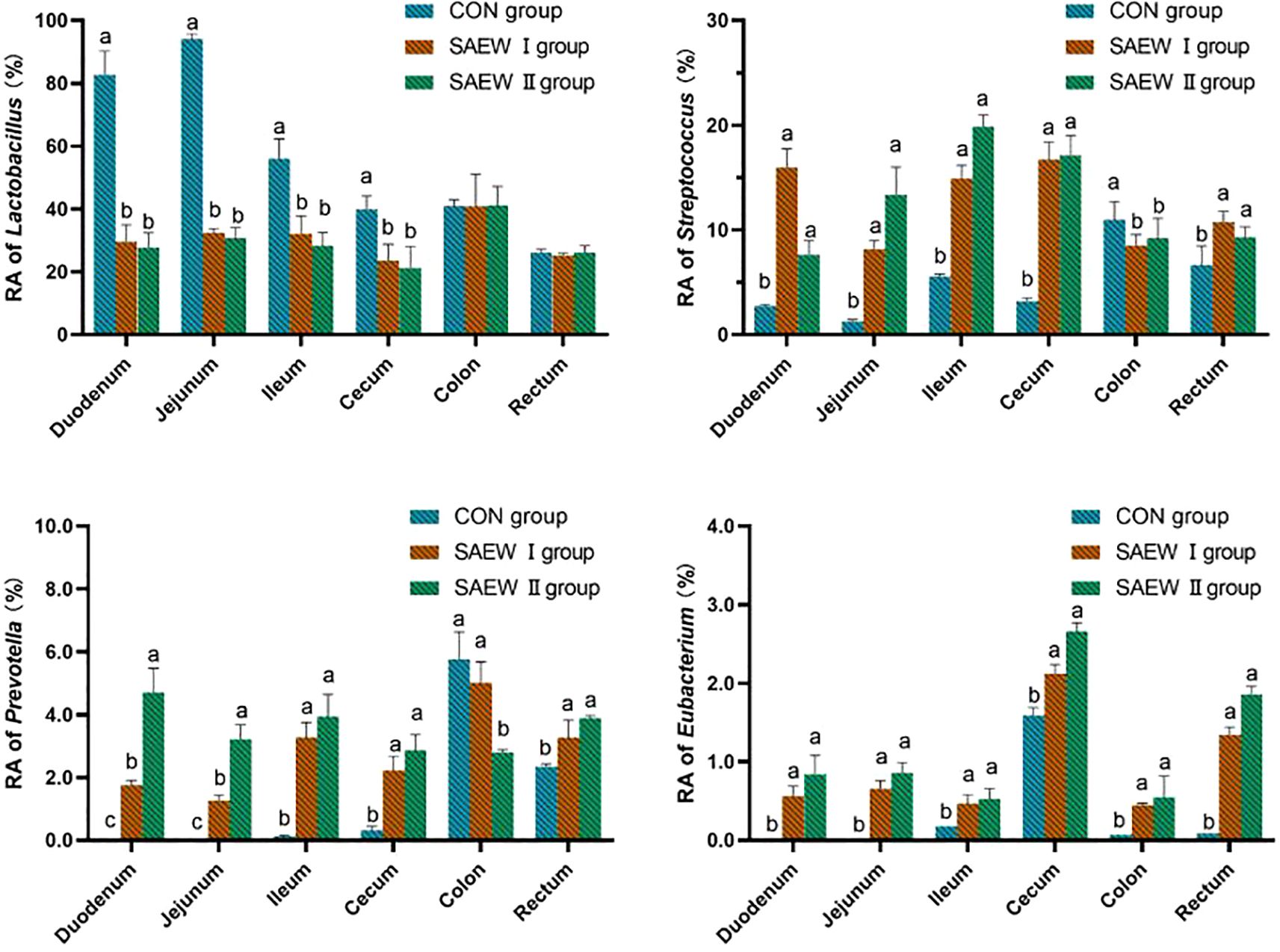
Figure 5. Relative abundance (RA) of the shared bacterial genera in the gut across the groups. a represents p>0.05, b represents p<0.05, and c represents p<0.01.
3.3.5 Comparative analysis of multiple samples
Principal component analysis (PCA) and non-metric multidimensional scaling (NMS) can reflect the size of differences between samples. As can be seen from Figure 6, the samples of the CON, SAEW I, and SAEW II groups were obviously separated, indicating that there were significant differences in the bacterial community structure among the three groups; however, indeed, the bacterial community structure of the SAEW I group was relatively different from that of the CON group. The bacterial community structure of the SAEW II group was significantly different from that of the CON group.
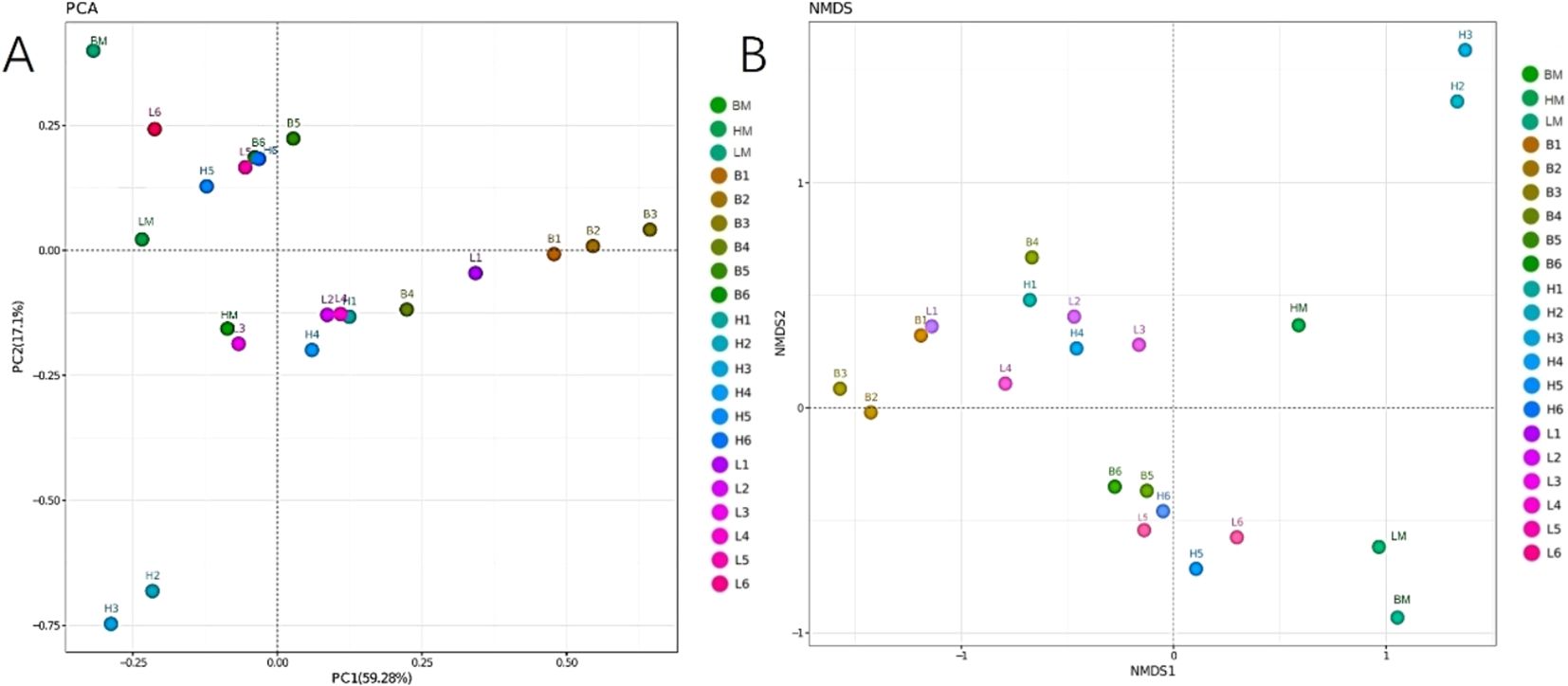
Figure 6. Principal component analysis (PCA) (A) and non-metric multidimensional scaling (NMS) (B) of the effects of drinking slightly acidic electrolyzed water (SAEW) on the different intestinal segments of weaned piglets. B, control (CON) group; H, SAEW II group; L, SAEW I group; BM, CON group, all intestinal mixed sample; HM, SAEW II group, all intestinal mixed sample; LM, SAEW I group, all intestinal mixed sample; 1, duodenum; 2, jejunum; 3, ileum; 4, cecum; 5, colon; 6, rectum.
4 Discussion
4.1 Effects of SAEW on the growth performance and intestinal development of piglets
The results showed that SAEW had a positive impact on the production performance and intestinal development of piglets. The ADG and ADFI were significantly increased, the feed-to-weight ratio was significantly decreased, and the gastrointestinal weight and length were also significantly increased. These results are consistent with Xiaoxia et al. (2023), which indicates that SAEW has the effect of promoting gastrointestinal development and improving the gastrointestinal health of piglets, thereby promoting the digestion and absorption of nutrients, providing sufficient energy and nutrition for the growth of piglets, and subsequently promoting weight gain. This may also be related to the change in the intestinal flora structure of the piglets after drinking SAEW.
4.2 Effects of SAEW on the gut morphology of piglets
During the weaning period, the piglets often experience different types of stresses (Ma et al., 2021), which may cause morphological alterations in the small intestine (Zheng et al., 2021; Yu et al., 2017). The integrity of the intestinal barrier is closely associated with its structure, which is primarily involved in the digestion and absorption of nutrients (Wojnicki et al., 2019). Normal gut morphology is an important guarantee for the maintenance of gut health and functions in piglets. Previous studies have shown that the appropriate addition of acidifiers in animal feeds conferred the following benefits: a reduction in the gastrointestinal pH, maintenance of a favorable digestive capacity of the digestive system, a reduction in the damage caused by feeds to piglet gut, inhibition of the proliferation of harmful microorganisms in the gut, an elevation in the villus height, and a reduction in the crypt depth (Cheng et al., 2021; Lee et al., 2021). The gastrointestinal pH in piglets is a crucial factor for the regulation of digestive enzyme secretion and activity. Many digestive enzymes are more active under acidic conditions, particularly pepsin. An acidic environment in the gastrointestinal tract can improve the digestive capacity of piglets for feed, reduce the damage caused by physical friction to the intestinal villi, inhibit microbial growth, and promote gut development (Cai, 2019). In the experiment, it was observed that after piglets drank SAEW, the average pH value of the gastric contents decreased. The pH value in the piglets of the CON group was 3.91 ± 0.01, while that in the SAEW groups was 3.75 ± 0.03. This also confirms another aspect that drinking SAEW is beneficial to the improvement of the digestive ability of the piglet stomach.
This study also showed that SAEW could improve the villus height while reducing the crypt depth in piglets. The improvement effect was particularly pronounced in the duodenum. The results showed that piglets drinking 0.6 mg/L SAEW had an average daily weight gain of 14% and a decrease in the feed consumption/weight gain ratio of 4%. SAEW improves the villous height in the intestine. The increase of villus height in the intestine significantly increases its absorption area, indicating that animals can more fully contact and absorb nutrients in the feed, thus improving both feed utilization and the economic benefits of breeding.
4.3 Effects of SAEW on the gut microbiota of piglets
The gut microbiota maintains a dynamic balance in the gut of animals (Choudhury et al., 2021b). The gut microbes live in symbiosis with host animals. They are closely associated with the immunity, nutrition, metabolism, and physiological conditions of the hosts (Deepak et al., 2022). Changes in the abundance and diversity of the bacterial communities have a significant impact on the gut homeostasis. Xiaoxia et al. (2023) reported that SAEW dramatically affected the gut microbial diversity in weaned piglets, although it tended to reduce the gut microbiota abundance. The present study showed that SAEW had no significant impact on the gut microbial diversity in piglets, but altered the RA of microbes to a certain degree.
At the phylum level, the bacterial composition analysis indicated that Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria were the predominant phyla in the piglet gut. This result is in agreement with other findings (Guangli et al., 2020; Zheng et al., 2024). The RA of Firmicutes was reduced, while those of Bacteroidetes, Proteobacteria, and Actinobacteria greatly increased in the SAEW I and II groups compared with the CON group. The RA of Firmicutes and Bacteroidetes could affect energy intake and fat metabolism. The greater the ratio of the RA between the two species, the higher is the level of fat deposition (Nong, 2021). It was found that the ratio of RA between the Firmicutes and Bacteroidetes species in piglets decreased after drinking SAEW, indicating that SAEW possibly reduced the fat deposition in piglets. In addition, the Bacteroidetes species play an essential role in strengthening the gut barrier function of animals (Shin et al., 2024; Wang, 2021). The RA of Bacteroidetes species increased, potentially enhancing the defense capabilities of the intestinal tract and preventing pathogen invasion. While the α diversity index remains unchanged, alterations in these specific bacteria might improve intestinal health by influencing the tract metabolism and immune regulation, among other functions. Most of the Proteobacteria species are involved in protein fermentation (Xu et al., 2019). Actinobacteria species exist in lower abundance in the gut of animals. Nevertheless, the Bifidobacterium species belonging to the phylum Actinobacteria play a non-negligible role in the health of animals (Cao, 2012). Altogether, our results indicate that SAEW can boost the gut health of piglets.
The microbial composition analysis at the genus level showed that the RA of Lactobacillus in the gut was markedly reduced in the SAEW I and II groups compared with that in the CON group.
This is probably due to the low concentration of hypochlorous acid (HClO) in SAEW having a nonspecific inhibitory effect on beneficial bacteria such as lactic acid bacteria. Lactobacillus generally prefers a weakly acidic to neutral environment. It was observed that the average intestinal pH value of the piglets in the SAEW groups (5.92 ± 0.10) was lower than that of the CON group (6.13 ± 0.10) in the experiment. This indicates that SAEW has a strong redox potential and acidity, which may disrupt the cell membrane structure of lactic acid bacteria, interfere with their normal metabolic pathways, and lead to a decrease in cell viability. In addition, there are interactions among the microorganisms in the intestinal tract of piglets. SAEW treatment increased the abundance of Prevotella in the intestine, disrupting the original microbial balance and thus inhibiting the growth and reproduction of lactic acid bacteria. This might become a limitation for the application of SAEW in livestock and poultry drinking water. SAEW increased the RA of Streptococcus, Prevotella, and Eubacterium in the piglet gut, consistent with the findings of Xiaoxia et al. (2023). Streptococcus is an important protein-degrading bacterium in the gut that is capable of breaking down proteins into smaller molecules, which are easier for the gut to absorb. This helps piglets better digest and utilize the protein nutrients in their feed, meeting their growth and development needs. There is an interaction among Prevotella, Bacteroides, and Clostridium species, which collectively promote the degradation of cellulose and hemicellulose. After piglets consumed water containing SAEW, the RA of these bacterial populations increased, indicating an enhanced ability to break down cellulose-like substances in the feed. This improves the digestibility and utilization rate of the feed, providing more absorbable nutrients to piglets, promoting the nutritional digestion process in the gut, and maintaining the normal physiological functions of the gut. In a healthy gut microenvironment, various bacterial communities both restrain and collaborate with each other, maintaining a dynamic balance. After SAEW treatment, these three genera inhibited the growth and reproduction of harmful microorganisms through competition for nutrients and the production of antimicrobial substances. When the populations of Streptococcus, Prevotella, and Bacteroides increase, they occupy more ecological niches, which reduces the survival space for harmful bacteria, thus maintaining the stability of the gut microbiota and ensuring gut health. This fact agrees well with the finding that SAEW could regulate the abundance of gut bacteria and promote nutrient digestion and absorption in the gut.
5 Conclusions
SAEW improved the gut morphology, promoted gut morphology development, regulated the abundance of the gut microbiota, and boosted the gut health of piglets. SAEW with an ACC of 0.6 mg/L achieved a better effect under the experimental conditions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Henan University of Animal Husbandry and Economics’ Institutional Animal Care and Use Committee (protocol number HNUAHE 488), and institutional safety procedures were followed. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ZFS: Resources, Funding acquisition, Formal Analysis, Conceptualization, Writing – original draft. LX: Resources, Data curation, Funding acquisition, Writing – original draft. RW: Formal Analysis, Investigation, Writing – original draft. ZYS: Methodology, Investigation, Writing – original draft, Software. ZX: Validation, Visualization, Formal Analysis, Writing – original draft. SG: Writing – original draft, Project administration, Methodology, Data curation. XL: Visualization, Writing – original draft, Formal Analysis, Supervision. YZ: Writing – original draft, Project administration, Validation, Investigation. ZY: Writing – original draft, Formal Analysis, Supervision, Resources, Conceptualization. PC: Project administration, Writing – original draft, Methodology, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the National Key R&D Sub-project of China (2023YFD1300804-2) and the 2025 Henan Provincial High-level Talent Internationalization Training Project (GCC2025033).
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Afari G. K. and Hung Y. C. (2018). A meta-analysis on the effectiveness of electrolyzed water treatments in reducing foodborne pathogens on different foods. Food Control. 93, 150–164. doi: 10.1016/j.foodcont.2018.06.009
Ampiaw R. E., Yaqub M., and Lee W. (2021). Electrolyzed water as a disinfectant: A systematic review of factors affecting the production and efficiency of hypochlorous acid. J. Water Process Eng. 43, 102228. doi: 10.1016/j.jwpe.2021.102228
Bing S., Zang Y., Li Y., Zhang B., Mo Q., Zhao X., et al. (2022). A combined approach using slightly acidic electrolyzed water and tea polyphenols to inhibit lipid oxidation and ensure microbiological safety during beef preservation. Meat Science. 183, 108643. doi: 10.1016/j.meatsci.2021.108643
Bischoff S.C. (2011). 'Gut health': a new objective in medicine? BMC Medicine. 9 (1), 1-14. doi: 10.1186/1741-7015-9-24
Bo Z., Matthew B., Carlos , Chaitanya A. D., Nicolai Stanislas V., Lora Vet H., et al. (2021). Impact of bead-beating intensity on the genus- and species-level characterization of the gut microbiome using amplicon and complete 16S rRNA gene sequencing. Front. Cell. Infection Microbiol. 11, 678522 doi: 10.3389/FCIMB.2021.678522
Bonetti A., Tugnoli B., Piva A., and Grilli E. (2021). Towards zero zinc oxide: feeding strategies to manage post-weaning diarrhea in piglets. Animals. 11, 642. doi: 10.3390/ani11030642
Cai Y. (2019). Effects of Two Acidulants on Growth Performance and Intestinal Health of Weaned Piglets (Changsha, China: Hunan Agricultural University), 7–9.
Cao Y. R. (2012). Diversity and Biological Activity of Actinomycetes in Animal Fece (Yangling, China: Northwest A&F University), 23–25.
Celi P., Cowieson A. J., Fru-Nji F., Steinert R. E., Kluenter A. M., and Verlhac V. (2017). Gastrointestinal functionality in animal nutrition and health: new opportunities for sustainable animal production. Anim. Feed Sci. Technology. 234, 88–100. doi: 10.1016/j.anifeedsci.2017.09.012
Cheng Y., Zhou H., Yu C., Zhang P., Lu Z., Wang Y., et al. (2021). Effects of different types of acidulants on growth performance and immune function of weaned piglets. Chin. J. Anim. Nutrition. 33, 2575–2584. doi: 10.3969/j.issn.1006-267x.2021.05.018
Choudhury R., Middelkoop A., Boekhorst J., Gerrits W. J. J., Kemp B., Bolhuis J. E., et al. (2021b). Early life feeding accelerates gut microbiome maturation and suppresses acute post-weaning stress in piglets. Environ. Microbiology. 23, 7201–7213. doi: 10.1111/1462-2920.15791
Choudhury R., Middelkoop A., De Souza J. G., Van Veen L. A., Gerrits W. J. J., Kemp B., et al. (2021a). Impact of early-life feeding on local intestinal microbiota and digestive system development in piglets. Sci. Reports. 11, 1–16. doi: 10.1038/s41598-021-83756-2
Deepak S., Dolan E., Coyte K. C., McLaughlin J., Brass A., Hancock L., et al. (2022). Understanding the development and function of the gut microbiota in health and inflammation. Front. Gastroenterol. 13 (e1), e13-e211. doi: 10.1136/FLGASTRO-2022-102119
Degroote J., Vergauwen H., Wang W., Van Ginneken C., De Smet S., and Michiels J. (2020). Changes of the glutathione redox system during the weaning transition in piglets, in relation to small intestinal morphology and barrier function. J. Anim. Sci. Biotechnol. 11, 1–17. doi: 10.1186/s40104-020-00440-7
Farah R. F. I. and Al-Haj Ali S. N. (2021). Electrolyzed water generated on-site as a promising disinfectant in the dental office during the COVID-19 pandemic. Front. Public Health 9. doi: 10.3389/fpubh.2021.629142
Guangli Y., Chuanxin S., Shuhong Z., Yan L., Zhiqiang L., Fengyi G., et al. (2020). Characterization of the bacterial microbiota composition and evolution at different intestinal tract in wild pigs (Sus scrofa ussuricus). PeerJ 8, e9124. doi: 10.7717/Peerj.9124
Gutiérrez−García R., La Cerda−Angeles D., Juan C., Cabrera−Licona A., Delgado−Enciso I., Mervitch−Sigal N., et al. (2022). Nasopharyngeal and oropharyngeal rinses with neutral electrolyzed water prevents COVID−19 in front−line health professionals: A randomized, open−label, controlled trial in a general hospital in Mexico City. Biomed. Rep. 16, 1–8. doi: 10.3892/br.2021.1494
Hansen S. V., Nrskov N. P., Nrgaard J. V., Woyengo T. A., Poulsen H. D., Nielsen T. S., et al. (2022). Determination of the optimal level of dietary zinc for newly weaned pigs: A dose-response study. Anim. (2076-2615) 12, 1552. doi: 10.3390/ani12121552
Inagaki H., Shibata Y., Obata T., Kawagoe M., Ikeda K., Sato M., et al. (2021). Effects of slightly acidic electrolysed drinking water on mice. Lab. Animals. 45, 283–285. doi: 10.1258/la.2011.010122
Jayaraman B. and Nyachoti C. M. (2017). Husbandry practices and gut health outcomes in weaned piglets: A review. Anim. Nutrition. 3, 205–211. doi: 10.1016/j.aninu.2017.06.002
Ji Z., Hui X., Shi Z., Xi L., and Ning L. (2020b). Effects of drinking slightly acidic electrolyzed water on intestinal microbes and immune function of broilers. J. Northwest A&F Univ. 48, 27–32. doi: 10.13207/j.cnki.jnwafu.2020.04.004
Ji Z., Hui X., Shi Z., Xi L., and Zhai F. (2020a). Effects of drinking slightly acidic electrolyzed water on growth performance and behavior of Ross 308 broilers. Chin. J. Anim. Husbandry. 56, 123–126. doi: 10.19578/j.cnki.ahfs.2020.03-04.006
Kogut M. H. and Arsenault R. J. (2016). Gut health: The new paradigm in food animal production. Front. Veterinary Science. 3. doi: 10.3389/fvets.2016.00071
Lan W., Lang A., Zhou D., and Xie J. (2021). Combined effects of ultrasound and slightly acidic electrolyzed water on quality of sea bass (Lateolabrax Japonicus) fillets during refrigerated storage. Ultrasonics sonochemistry. 81, 105854. doi: 10.1016/j.ultsonch.2021.105854
Lee J., Kim J. W., Hall H., and Nyachoti C. M. (2021). Effect of dietary organic acids supplementation on growth performance, nutrient digestibility, and gut morphology in weaned pigs. Can. J. Anim. Science. 102, 255–265. doi: 10.1139/cjas-2021-0080
Lekagul A., Tangcharoensathien V., and Yeung S. (2019). Patterns of antibiotic use in global pig production: a systematic review. Veterinary Anim. Science. 7, 100058. doi: 10.1016/j.vas.2019.100058
Li Y., Guo Y., Wen Z., Jiang X., Ma X., and Han X. (2018). Weaning stress perturbs gut microbiome and its metabolic profile in piglets. Sci. Reports. 8, 1–12. doi: 10.1038/s41598-018-33649-8
Li Z., Li B., Zheng W., Tu J., Zheng H., and Wang Y. (2019). Optimization of a wet scrubber with electrolyzed water spray—Part II: Airborne culturable bacteria removal. J. Air Waste Manage. Association. 69, 603–610. doi: 10.1080/10962247.2019.1567622
Liao X., Xiang Q., Cullen P. J., Su Y., Chen S., Ye X., et al. (2020). Plasma-activated water (PAW) and slightly acidic electrolyzed water (SAEW) as beef thawing media for enhancing microbiological safety. Lwt. 117, 108649. doi: 10.1016/j.lwt.2019.108649
Ma W., Mao P., Zhu Y Guo L., Zhang S., Wang Z., and Zhao F. (2021). Standardized ileal digestible tryptophan to lysine ratios affect performance and regulate intestinal mRNA expression of amino acid transporters in weaning pigs fed a low crude protein diet. Anim. Feed Sci. Technol. 275, 114857. doi: 10.1016/j.anifeedsci.2021.114857
Meng Q., Zhang Y., Li J., Shi B., Ma Q., and Shan A. (2022). Lycopene affects intestinal barrier function and the Gut Microbiota in weaned piglets via antioxidant signaling regulation. J. Nutr. 152.11, 2396-2408. doi: 10.1093/jn/nxac208
Mesfin M. Y., Mitiku A. B., and Admasu T. H. (2024). Veterinary drug residues in food products of animal origin and their public health consequences: A review. Veterinary Med. Sci. 10, e70049. doi: 10.1002/vms3.70049
Nan S., Huang X., Wang S., Ye Z., and Zhu S. (2019). Investigation on microbicidal potential and action mechanism for botrytis cinerea of slightly acidic electrolyzed water. Trans. Chin. Soc. Agric. Machinery. 50 (01), 354-358+382. doi: 10.6041/j.issn.1000-1298.2019.01.040
National Research Council (2012). Nutrient requirements of swine: Eleventh Revised Edition (The United States of America: National Academies Press).
Ngangom B. L., Tamunjoh S. S. A., and Boyom F. F. (2019). Antibiotic residues in food animals: Public health concern. Acta Ecologica Sinica. 39, 411–415. doi: 10.1016/j.chnaes.2018.10.004
Nong Q. Y. (2021). Effects of Dietary PUFA Composition on Growth Performance, Meat Quality, Intestinal Flora and Its Metabolites in Heigai Pigs (Hangzhou, China: Zhejiang University), 45–52.
Pluske J. R., Turpin D. L., and Kim J. C. (2018). Gastrointestinal tract (gut) health in the young pig. Anim. Nutrition. 4, 187–196. doi: 10.1016/j.aninu.2017.12.004
Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. (2012). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Rahman M. R. T., Fliss I., and Biron E. (2022). Insights in the development and uses of alternatives to antibiotic growth promoters in poultry and swine production. Antibiotics (2079-6382) 11 (6), 766. doi: 10.3390/antibiotics11060766
Shi Z., Zheng W., Liu T., Hui X., and Xi L. (2020). Minimum inhibitory concentration of slightly acidic electrolyzed water and its bactericidal activity on the surfaces of broiler houses. Int. J. Agric. Biol. 23, 63–67. doi: 10.17957/IJAB/15.1258
Shin J. H., Tillotson G., Mackenzie T. N., Warren C. A., Wexler H. M., Goldstein E. J. C., et al. (2024). Bacteroides and related species: The keystone taxa of the human gut microbiota. Anaerobe 85 (000), 102819 doi: 10.1016/j.anaerobe.2024.102819
Song D., Lee J., Yoo Y., Oh H., Chang S., An J., et al. (2025). Effects of probiotics on growth performance, intestinal morphology, intestinal microbiota weaning pig challenged with Escherichia coli and Salmonella enterica. J. Anim. Sci. Technol. 67, 106–136. doi: 10.5187/jast.2023.e119
Vincent B. Y. (2021). Re: The role of the microbiome in human health and disease: an introduction for clinicians. BMJ. 356. doi: 10.1136/bmj.j831
Wang C. (2021). The Effect of Intestinal Bacteroides Species Structure on the Development of Ulcerative Colitis (Wuxi,China: Jiangnan University), 3–4.
Wang M., Wang L., Tan X., Wang L., Xiong X., Wang Y., et al. (2022). The developmental changes in intestinal epithelial cell proliferation, differentiation, and shedding in weaning piglets. Anim. Nutrition. 9, 214–222. doi: 10.1016/j.aninu.2021.11.006
Wei X., Tsai T., Howe S., and Zhao J. (2021). Weaning induced gut dysfunction and nutritional interventions in nursery pigs: A partial review. Animals. 11, 1279. doi: 10.3390/ani11051279
Wojnicki S. J., Morris A., Smith B. N., Maddox C. W., and Dilger R. N. (2019). Immunomodulatory effects of whole yeast cells and capsicum in weanling pigs challenged with pathogenic Escherichia coli. J. Anim. Sci. 97, 1784–1795. doi: 10.1093/jas/skz063
Xiaoxia H., Dan X., Dongmei J., Li Z., Linyuan S., Mailin G., et al. (2023). Effect of slightly acidic electrolyzed water on growth, diarrhea and intestinal bacteria of newly weaned piglets. Genes 14, 1398. doi: 10.3390/genes14071398
Xu E., Yang H., Liu X. T., Ren M. M., Shen L. L., Lv W. T., et al. (2019). Study on the microbiota structure and content of short-chain fatty acids in different parts of the intestinal tract of Yorkshire pigs. Chin. J. Anim. Nutrition. 31, 4509–4518. doi: 10.3969/j.issn.1006-267x.2019.10.014
Yan P., Daliri E. B. M., and Oh D. H. (2021). New clinical applications of electrolyzed water: a review. Microorganisms. 9, 136. doi: 10.3390/microorganisms9010136
Yu M., Mu C., Yang Y., Zhang C., Su Y., Huang Z., et al. (2017). Increases in circulating amino acids with in-feed antibiotics correlated with gene expression of intestinal amino acid transporters in piglets. Amino Acids 49, 1587–1599. doi: 10.1007/s00726-017-2451-0
Zang Y. T., Bing S. H., Li Y. J., Shu D. Q., Huang A. M., Wu H. X., et al. (2019). Efficacy of slightly acidic electrolyzed water on the microbial safety and shelf life of shelled eggs. Poultry Science. 98, 5932–5939. doi: 10.3382/ps/pez373
Zheng L., Duarte M. E., Sevarolli Loftus A., and Kim S. W. (2021). Intestinal health of pigs upon weaning: Challenges and nutritional intervention. Front. Veterinary Sci. 8. doi: 10.3389/fvets.2021.628258
Keywords: slightly acidic electrolyzed water, early weaned piglets, gut morphology, gut microbiota, growth performance
Citation: Shi Z, Xi L, Wei R, Shi Z, Xu Z, Gao S, Liang X, Zhang Y, Ye Z and Cheng P (2025) Effect of slightly acidic electrolyzed water in drinking water on the intestinal morphology and gut microbiota in early weaned piglets. Front. Anim. Sci. 6:1593059. doi: 10.3389/fanim.2025.1593059
Received: 13 March 2025; Accepted: 28 April 2025;
Published: 29 May 2025.
Edited by:
Assar Ali Shah, Jiangsu University, ChinaReviewed by:
Ravikanthreddy Poonooru, University of Missouri, United StatesGuadalupe Martínez, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina
Copyright © 2025 Shi, Xi, Wei, Shi, Xu, Gao, Liang, Zhang, Ye and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Xi, eGlsZWlAaG51YWhlLmVkdS5jbg==
 Zhifang Shi
Zhifang Shi Lei Xi1,2*
Lei Xi1,2* Zhengyang Shi
Zhengyang Shi Zhangying Ye
Zhangying Ye