- 1Key Laboratory of Animal Epidemic Disease Detection and Prevention in Panxi District, Xichang University, Xichang, China
- 2College of Animal Science, Xichang University, Xichang, China
- 3Shandong New Hope Liuhe Agriculture and Animal Husbandry Technology Co., Ltd., Dezhou, China
- 4MOA Key Laboratory of Animal Virology, Center for Veterinary Sciences, Department of Veterinary Medicine, College of Animal Sciences, Zhejiang University, Hangzhou, China
Porcine circovirus type 3 (PCV3) is a recently identified member of the porcine circovirus family, primarily associated with conditions such as dermatitis and nephropathy syndrome, reproductive failure, and multisystem inflammatory lesions in swine. There has been a significant increase in the prevalence of PCV3 in China, attracting considerable attention. Consequently, there is an urgent need for a highly sensitive, cost-effective, and efficient method for the detection of clinical samples. This study developed a TaqMan-based quantitative real-time PCR (TaqMan-qPCR) assay utilizing specific probes and primers designed based on the PCV3-REP gene. Following the optimization of reaction conditions, sensitivity analysis determined that the detection limit of this method was 7.3 × 100 copies/µL. Specificity analysis demonstrated no cross-reactivity with other common porcine pathogens, underscoring its specificity. Furthermore, the inter- and intra-assay coefficients of variation were both less than 1%, indicating high reproducibility. A total of 2,454 clinical samples were collected and analyzed using the developed method. The findings revealed that the prevalence of PCV3 was highest in testicular fluid samples, with a rate of 71.28% and the lowest detected Cq values among all sample types, indicating a significant likelihood of vertical transmission of PCV3. Additionally, oral fluid samples exhibited the second highest positive rate at 59.83%, highlighting the importance of monitoring infection rates in fattening pig herds from a veterinary perspective. In conclusion, this study successfully developed a highly sensitive and specific TaqMan-qPCR method, which is effective for detecting PCV3 across a variety of clinical samples.
Introduction
Porcine circovirus (PCV), a member of the family Circoviridae and the genus Circovirus, is characterized as a non-enveloped, covalently closed, single-stranded icosahedral DNA virus. Notably, PCV is the smallest known animal DNA virus capable of autonomous replication, comprising four primary types: PCV1, PCV2, PCV3, and PCV4 (Opriessnig et al., 2020). Porcine circovirus type 3 (PCV3), a recently identified virus, was first detected in the United States in 2016 and has since been reported in pig farms globally (Ouyang et al., 2019; Tan et al., 2021). Research indicates that PCV3 infection is linked to dermatitis and nephropathy syndrome, reproductive failure, and multisystem inflammatory lesions in swine (Jiang et al., 2020). Additionally, PCV3 is considered a potential etiological agent of porcine respiratory disease complex (PRDC) and reproductive disorders in certain instances (Kim et al., 2018). The PCV3 genome comprises single-stranded DNA approximately 2.0 kb in length, exhibiting high conservation and specificity, with significant sequence divergence from other known porcine circoviruses, such as PCV2 and PCV4 (Cui et al., 2022). Research conducted by Ye et al. indicated that PCV3 shares approximately 44% nucleotide sequence homology with PCV2 (Ye et al., 2018). In a separate study, Zhang et al. found that the genetic homology between PCV3 and PCV4 is 43.2% (Zhang et al., 2020). The genome of PCV3 includes several critical genes, notably the viral capsid gene (CAP) and the replication-associated gene (REP), which are integral to the virus’s replication and infection mechanisms (Wang et al., 2024). In recent years, there has been a notable increase in the prevalence of PCV3 in China, garnering considerable attention. Reports have documented a 12.2% positivity rate for PCV3 in pig samples across 21 provinces in China (Qi et al., 2019). Furthermore, a separate study identified a positivity rate of 31.18% in central China, underscoring the virus’s widespread dissemination across various regions and pig populations (Xu et al., 2018). In the context of intensified pig farming in China, particularly following the outbreak of African swine fever virus (ASFV), many large-scale farms have established their own testing laboratories to conduct routine surveillance for ASFV and other pathogens, including PCV3. Consequently, the development and implementation of effective laboratory testing methods are crucial for the epidemiological investigation of PCV3.
Currently, the laboratory diagnostic techniques for PCV3 predominantly include in situ hybridization (ISH), immunohistochemistry (IHC), polymerase chain reaction (PCR), and enzyme-linked immunosorbent assay (ELISA) (Tan et al., 2021). While ISH and IHC are effective for detecting viral presence in tissue cells, they are not suitable for field-based clinical monitoring. The ELISA technique, which primarily focuses on the detection of antibodies against PCV3, is relatively expensive and thus not ideal for large-scale studies. Conversely, the quantitative real-time PCR (qPCR) method is currently regarded as the most effective approach for pathogen detection in intensive pig farming environments. It has been successfully employed for the detection of various porcine pathogens, including ASFV (Hu et al., 2024), porcine diarrhea viruses (Ren et al., 2024b), and certain bacterial pathogens (Ren et al., 2024a). In this study, we designed a pair of specific primers and a dual-quenched probe targeting the conserved region of the PCV3-REP gene to develop a fluorescent quantitative PCR method specifically for PCV3 detection. The establishment of this method provides a robust foundation for the rapid diagnosis of PCV3 in clinical settings and for conducting large-scale epidemiological investigations.
Materials and methods
Primers and probes
Using DNAStar software, we analyzed the sequence of the PCV3-REP gene (Accession number: MK656956.1) obtained from GenBank to design a specific pair of primers and a TaqMan probe with Primer Express 3.0. The designed primers are as follows: 5’-GGTGGGATGGTTATAATG-3’ (forward) and 5’-TAGCCACAAAATTAACAAAC-3’ (reverse), while the TaqMan probe is 5’-FAM-CACCCTTAACAGGAACCCTCAGA-BHQ1-3’. These primers and probe target a conserved region within the sequence, resulting in an amplified gene fragment measuring 141 base pairs. The synthesis of both primers and the probe was conducted by Sangon Biotech (Shanghai) Co., Ltd.
Standard plasmid
The pUC57-PCV3 standard plasmid was constructed by synthesizing and cloning gene sequences amplified from the REP gene of the PCV3 genome into the pUC57 vector. The quantification of this standard plasmid was performed using a UV-visible spectrophotometer, with copy numbers determined using a specific formula (Zhao et al., 2023). Subsequently, the plasmids underwent serial 10-fold dilutions, yielding concentrations ranging from 7.3×109 to 7.3×100 copies/μL, and were stored at -20°C for future applications.
Optimization of reaction conditions
The concentrations of primers and probes were systematically optimized using standard plasmid templates within a 20 μL reaction volume, utilizing the AceQ Universal U+Probe Master Mix V2 (Vazyme #Q513). Primer concentrations (10 μM) were varied from 0.2 to 0.8 μL each, while probe concentrations (10 μM) ranged from 0.1 to 0.4 μL each. These variations were tested alongside annealing temperatures (AT) ranging from 55°C to 61°C. The primary aim was to minimize the quantification cycle (Cq) value while maximizing the increase in fluorescence intensity (ΔRn), thereby enhancing both the amplification efficiency and sensitivity of the reaction.
Evaluation of sensitivity and construction of standard curves
Amplification was performed using a standard plasmid template subjected to 10-fold serial dilutions, with concentrations spanning from 7.3×109 to 7.3×100 copies/μL under optimized conditions. The resulting amplification kinetic curves were analyzed, and a standard curve for the established TaqMan-qPCR method was constructed by plotting the logarithm of the copy number of the positive standard plasmid on the x-axis against the Cq values on the y-axis. This analysis enabled the derivation of the standard linear regression equation for the method.
Evaluation of specificity
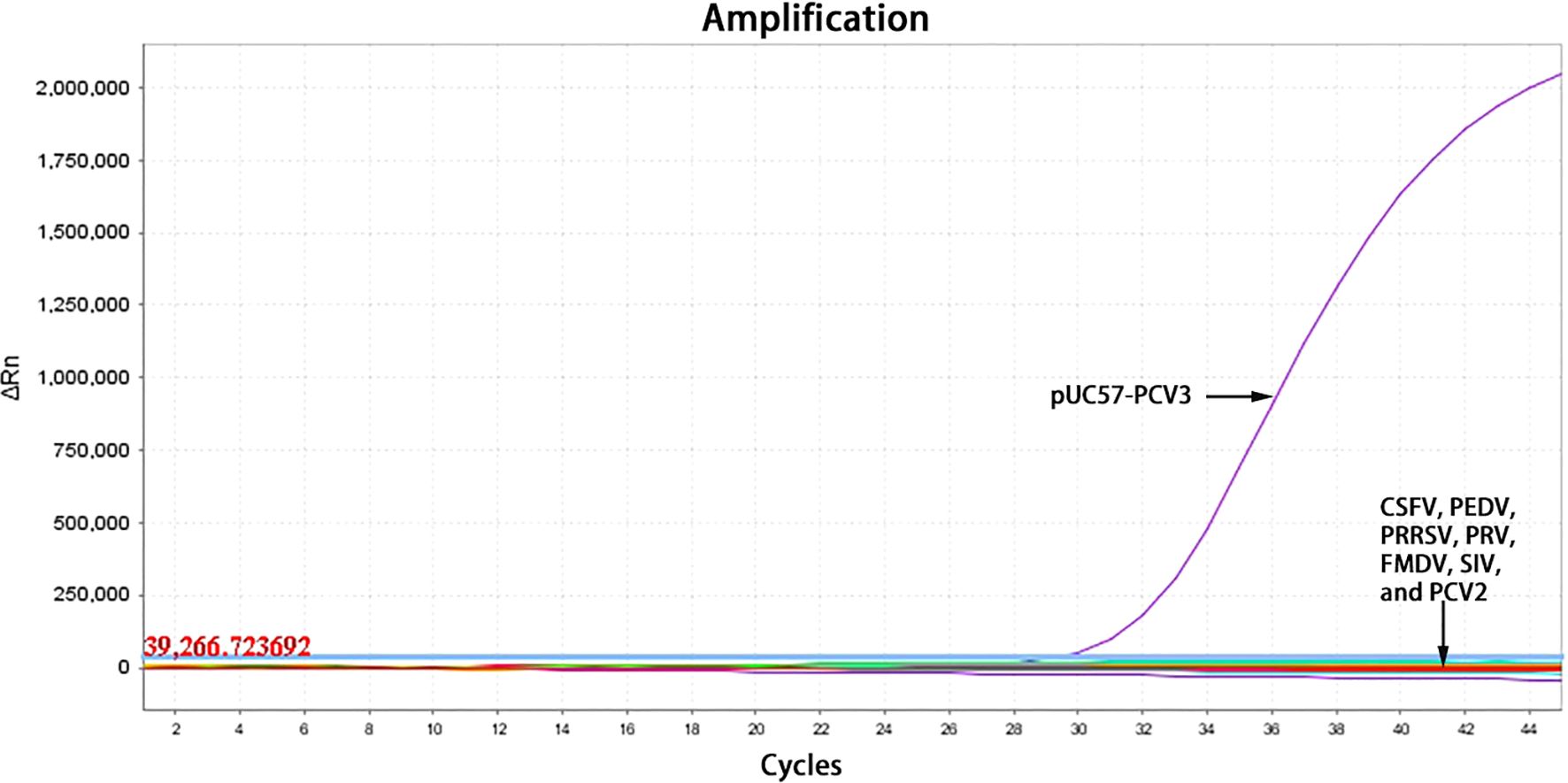
To assess the specificity of the TaqMan-qPCR method, the assay was conducted to detect the cDNA of several viruses, including Classical Swine Fever Virus (CSFV), Porcine Epidemic Diarrhea Virus (PEDV), Porcine Reproductive and Respiratory Syndrome Virus (PRRSV), Pseudorabies Virus (PRV), Foot-and-Mouth Disease Virus (FMDV), Swine Influenza Virus (SIV), and Porcine Circovirus Type 2 (PCV2). The pUC57-PCV3 standard plasmid was employed as the positive control, whereas double-distilled water (ddH2O) served as the negative control.
Evaluation of reproducibility
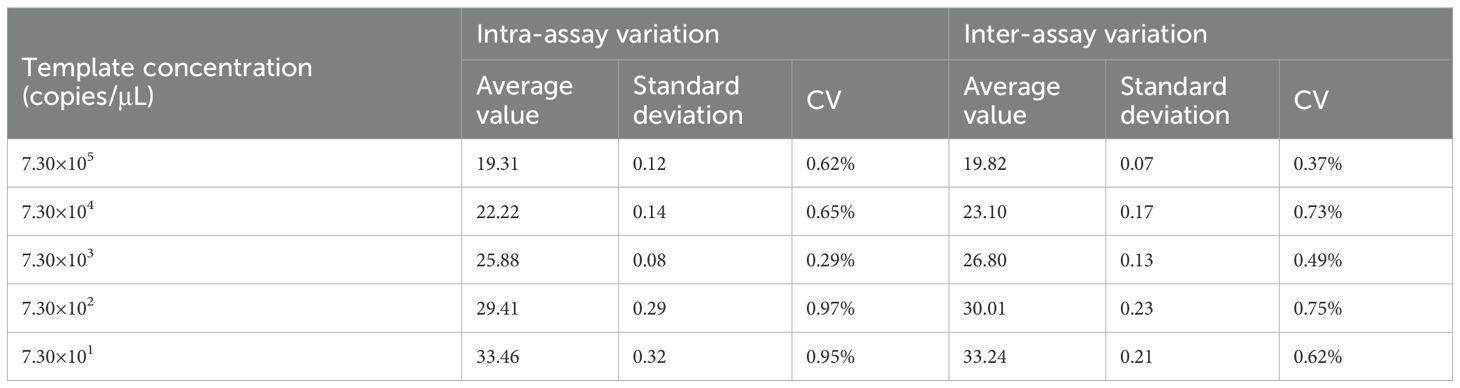
The TaqMan-qPCR method was implemented using pUC57-PCV3 standard plasmids at concentrations ranging from 7.3×105 to 7.3×101 copies/μL as templates, with each concentration evaluated in triplicate under optimized reaction conditions. Each experimental batch included three replicates per dilution level, and the resulting Cq values underwent statistical analysis to determine both intra- and inter-group coefficients of variation. This analysis enabled the evaluation of the method’s reproducibility and stability.
Clinical sample testing
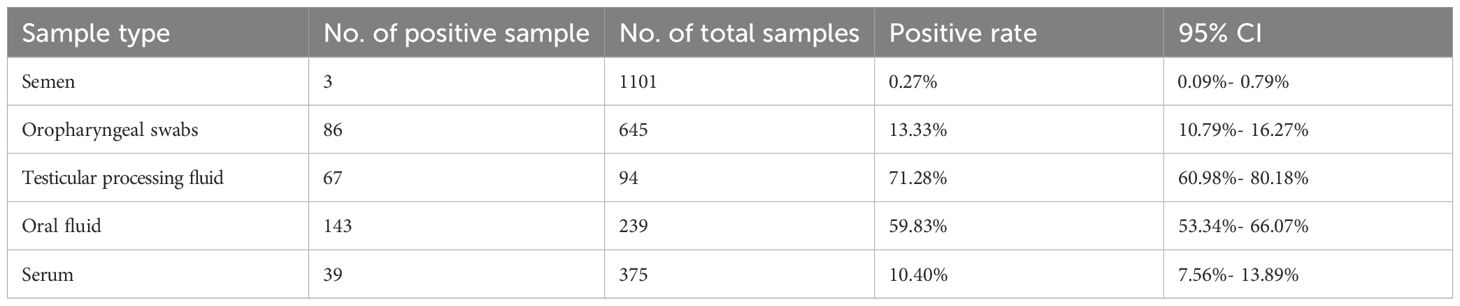
Nine clinical samples stored in our lab were tested simultaneously using both the developed TaqMan-qPCR method and a commercial qPCR kit. The results from the developed method were compared with those from the commercial method. Furthermore, a total of 2,454 clinical samples were collected from pig farms in Sichuan Province by farmers and subsequently sent to our laboratory for analysis. These samples comprised semen (n=1,101), oropharyngeal swabs (n=645), piglet testicular processing fluid (n=94), oral fluid (n=239), and pig serum (n=375). Semen and testicular processing fluid samples were chosen randomly for routine pathogen detection. The other samples were from pigs with respiratory symptoms. Each sample, with a volume of 300 μL, was processed using the NPA-96E automated nucleic acid extractor, developed by Hangzhou Boer Technology Co., Ltd. Post-extraction, qPCR analysis was conducted on 2 μL of the isolated DNA using the TaqMan-qPCR technique. The pUC57-PCV3 standard plasmid was utilized as a positive control, while ddH2O served as a negative control. A Cq value of less than 40 was established to indicate positive results.
Statistical analysis
The positivity rates of PCV3 across the different sample types were expressed as absolute and relative frequencies (%) with a 95% confidence interval (CI), calculated using the Clopper-Pearson method. Cq values of PCV3-positive samples were presented as mean ± standard deviation. Data visualization was conducted using GraphPad Prism 10.0 software, and statistical significance was assessed via the one-way ANOVA, with P<0.05 indicating significant difference.
Results
Optimization of reaction conditions
Initially, the volumes of primers and probes were first optimized at an AT of 60°C. As detailed Supplementary Table S1, the Cq values were minimized with primer volumes of 0.4 μL and probe volumes of 0.2 μL across various template concentrations. Additionally, the AT condition was further optimized. According to Supplementary Table S2, the lowest Cq values were observed at 60°C for template concentrations of 7.30×105, 7.30×103, 7.30×101, and 7.30×100 copies/μL. Consequently, the optimized reaction conditions were as follows: an initial incubation at 37°C for 2 minutes, followed by denaturation at 95°C for 5 minutes. This was succeeded by 40 cycles comprising denaturation at 95°C for 10 seconds and annealing at 60°C for 30 seconds. The optimal reaction volume was determined to be 20 μL, consisting of 10 μL of 2× AceQ Universal U+Probe Master Mix V2, 0.4 μL each of forward and reverse primers (10 μmol/L), 0.2 μL of probe (10 μmol/L), and 2 μL of template, with deionized water added to reach a final volume of 20 μL.
Establishment of the standard curve
The TaqMan-qPCR assay demonstrated proficiency in detecting standard plasmids at concentrations ranging from 7.3×109 to 7.3×100 copies/μL, exhibiting a strong linear correlation. The assay’s minimum detectable concentration was determined to be 7.3×100 copies/μL (Figure 1A). As shown in Figure 1B, the standard curve equation was y = -3.377x + 39.207, with a coefficient of determination (R²) of 0.998 and an efficiency (Eff%) of 99.756%.
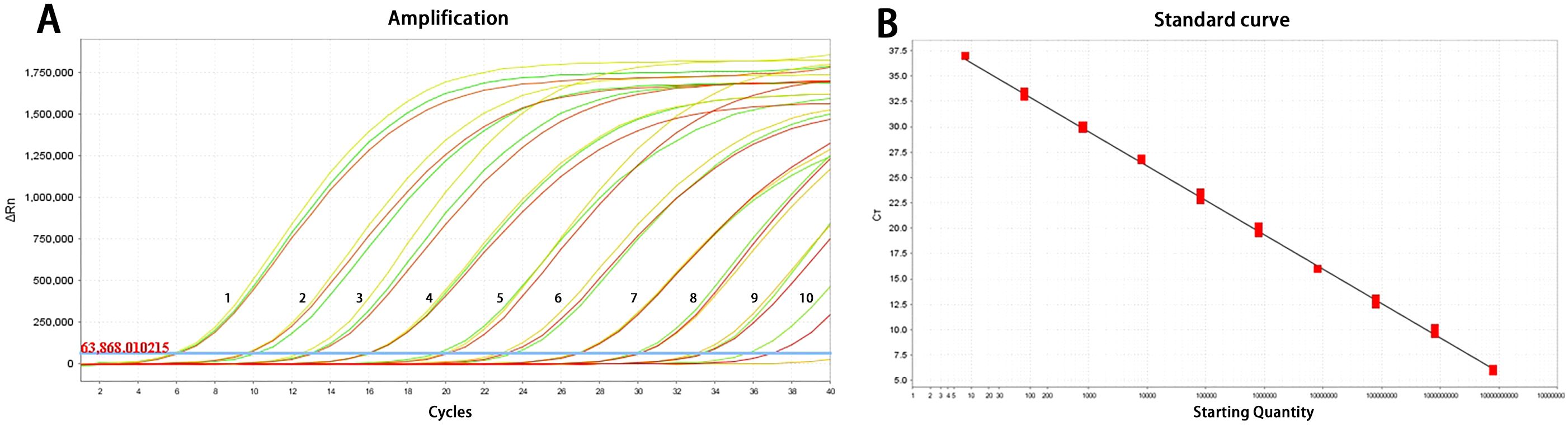
Figure 1. Amplification curve (A) and standard curve (B) of the TaqMan-qPCR method for detection of PCV3. 1-10: 7.3×109 to 7.3×100 copies/μL.
Specificity testing
The optimized reaction protocol was applied to detect nucleic acids from a variety of porcine pathogens, including CSFV, PEDV, PRRSV, PRV, FMDV, SIV, and PCV2. The results indicated that the pUC57-PCV3 standard plasmids produced amplification curves specific to PCV3, while amplification curves were absent for other common porcine pathogens as well as for negative controls (Figure 2). These findings imply that the TaqMan-qPCR assay possesses a high degree of specificity, showing no cross-reactivity with prevalent porcine pathogens.
Repeatability testing
As illustrated in Table 1, the intra-group coefficients of variation ranged from 0.29% to 0.97%, while the inter-group coefficients of variation ranged from 0.37% to 0.75%, indicating the method’s excellent reproducibility.
Clinical sample testing
A comparison was made between the TaqMan-qPCR method and the commercial qPCR kit using nine clinical samples. Supplementary Table S3 showed that the results from the TaqMan-qPCR method were consistent with those from the commercial kit, indicating its effectiveness in detecting PCV3 in clinical samples. Then, we employed the established TaqMan-qPCR methodology to examine the detection rate of PCV3 across various sample types, with the findings detailed in Table 2. The detection rate was highest in piglet testicular processing fluid, at 71.28% (95% CI: 60.98%-80.18%), followed by oral fluid samples at 59.83% (95% CI: 53.34%-66.07%). Notably, the positive detection rates in throat swabs and serum were substantially lower, at 13.33% (95% CI: 10.79%-16.27%) and 10.40% (95% CI: 7.56%-13.89%), respectively. PCV3 was also identified in semen samples, albeit at a minimal rate of 0.27% (95% CI: 0.09%-0.79%), suggesting a potential risk of PCV3 transmission within the boar population. Moreover, as illustrated in Figure 3, the comparative analysis of Cq values across different samples revealed that the Cq values in testicular fluid were significantly lower than those in other sample types (P<0.05), while no significant differences were observed among the other sample types (P>0.05).
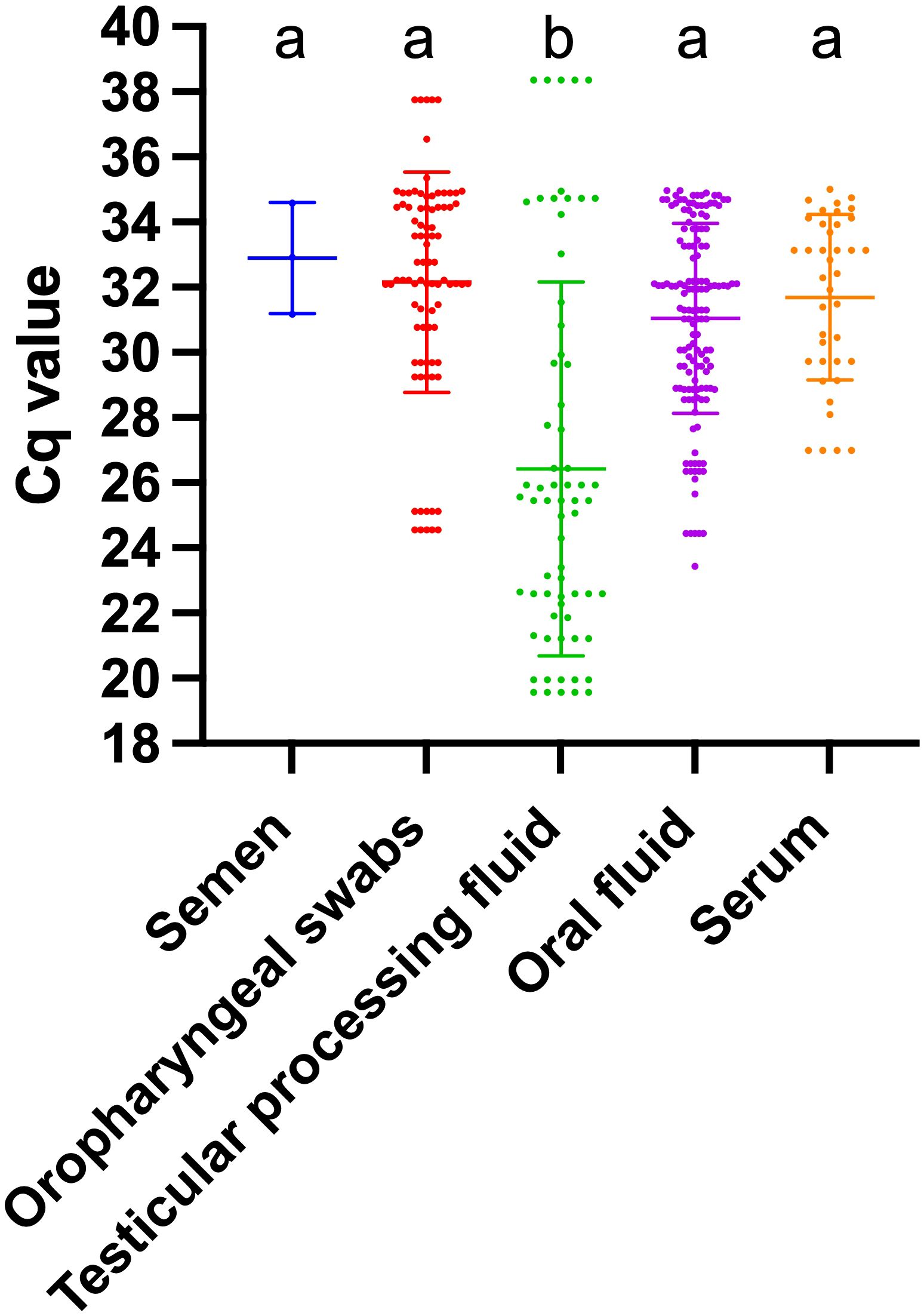
Figure 3. Cq values in different PCV3-positive samples. Different letters indicate significant statistical differences (P<0.05), and the same letter indicates no significant statistical differences (P>0.05).
Discussion
The qPCR method represents a highly sensitive and specific detection technology that offers substantial advantages over traditional PCR methods (Maren et al., 2023). This technique has been effectively utilized in the detection of PCV3. It facilitates real-time monitoring of the PCR amplification process while enabling quantitative analysis of the RNA content of PCV3 in samples. Feng et al. have demonstrated that the sensitivity of qPCR is ten times greater than that of traditional PCR in detecting PCV3 (Feng et al., 2019). Presently, qPCR detection methods for PCV3 are primarily categorized into three types: SYBR Green-based qPCR, TaqMan-qPCR, and multiplex qPCR. The SYBR Green-based qPCR is a widely used fluorescent dye method that is both cost-effective and user-friendly. The established sensitivity of SYBR Green-based qPCR is 61.2 copies/μL (Han et al., 2019), 1.73 × 102 copies/μL (Chen et al., 2018) targeting the REP gene, and 102 copies/μL targeting the CAP gene (Zou et al., 2018), all of which were lower than those of TaqMan-qPCR methods. The TaqMan-based qPCR specific for the PCV3 CAP gene was initially designed by Wang et al., achieving a limit of detection (LOD) of 102 copies/μL (Wang et al., 2017). Recent advancements in TaqMan protocols, with LOD ranging from 10 to 15 copies/μL targeting the REP and CAP genes, have been documented, further demonstrating that the positive detection rate of qPCR protocols significantly surpasses that of conventional PCR methods (Feng et al., 2019; Yuan et al., 2020). In this study, we enhanced the TaqMan-PCR methodology targeting the REP gene of PCV3, achieving a detection sensitivity of 7.3 copies/μL, which surpasses previously reported methods. And the results obtained from clinical samples were consistent with those from a commercial kit, despite the comparison being limited to only nine samples. Furthermore, subsequent PCV3 sequencing results for some clinical samples aligned with the TaqMan-qPCR test outcomes (data not shown), thereby reinforcing the reliability of the established method. The REP gene of PCV3 is regarded as relatively conserved in multiple strains. An examination of the whole genome sequences of PCV3 strains collected in China, alongside a comparison with 34 PCV3 strains documented in the NCBI database, demonstrated that the conservation level of the REP gene exceeded 99% in both genetic and amino acid sequences, surpassing that of the CAP gene (Lv et al., 2023). Additionally, research by Feng et al. revealed that the LOD of the TaqMan-qPCR assay targeting the REP gene was ten times more sensitive than that of the conventional PCR assay targeting the Cap gene (Feng et al., 2019). Multiplex qPCR enables the simultaneous detection of multiple target genes within a single reaction, thereby enhancing detection efficiency and throughput, which is particularly advantageous for rapid screening of diverse pathogens (Rodriguez-Manzano et al., 2019). A duplex qPCR assay for PCV2 and PCV3, with LODs of 2.9 copies and 22.5 copies, respectively, reported a co-infection rate of 27.6% (94/340) (Li et al., 2018). Similarly, a PCV3/PCV4 duplex qPCR, with LODs of 51.7 copies and 67.7 copies for PCV3 and PCV4, respectively, identified a co-infection rate of 17.19% (11/64) (Hou et al., 2021). Nonetheless, the sensitivity of multiplex qPCR is generally lower compared to single-pathogen detection methods. Consequently, in practical applications, multiplex qPCR demonstrates distinct advantages in scenarios requiring the simultaneous detection of multiple pathogens, delivering comprehensive results rapidly (Chen et al., 2021). Nonetheless, for precise and specific detection, single TaqMan-qPCR is a more suitable option, particularly in diagnostic and clinical contexts.
Under clinical conditions, oral fluid samples are typically obtained from fattening pig herds, oropharyngeal swab samples from sows in confinement pens, testicular processing fluid samples from the testes of 3-day-old piglets, and serum samples from all pig herds (Fan et al., 2023). Notably, there are variations in the detection rates of PCV3 across different pig samples. According to literature reports, in 21 Polish pig farms, the detection rate of PCV3 was highest in oral fluids at 37.3%, whereas the detection rates in serum and fecal samples were 9.7% and 15.0%, respectively (Woźniak et al., 2020). Our findings demonstrate a significant detection rate of PCV3 in oral fluid samples, underscoring the veterinary importance of monitoring infection rates in fattening pig herds. In recent years, oral fluid samples have emerged as a more cost-effective and efficient alternative to serum samples for clinical detection purposes (Henao-Diaz et al., 2020). These samples have been utilized in the identification of various swine pathogens, including PRRSV (Decorte et al., 2015) and PCV2 (Woźniak et al., 2019; Fan et al., 2023). However, the unique characteristics of oral fluid samples during collection pose a risk of environmental contamination from sources such as feces and feed. Although existing literature suggests that organic matter does not compromise the sensitivity and specificity of diagnostic results, it may lead to sample turbidity, thereby affecting result accuracy (Henao-Diaz et al., 2020). Enhancing the precision of detection outcomes can be achieved by refining the collection and processing methods for oral fluid samples (Henao-Diaz et al., 2020). Literature suggests that sample contamination may be minimized by preventing the rope from touching the floor, specifically by setting the rope’s bottom at the shoulder height of pigs (White et al., 2014; Pepin et al., 2015). Additionally, Gibert et al. have demonstrated that optimizing the sample centrifugation protocol can enhance the detection of PRRSV nucleic acids (Gibert et al., 2017). Furthermore, these samples are predominantly suitable for pathogen detection at the pen level (Henao-Diaz et al., 2020). Olsen et al. investigated the correlation between the detection rate of oral fluid and the infection rate in pigs within PRRSV-infected populations (Olsen et al., 2013). Nonetheless, further research is required to explore this relationship in the context of PCV3 infection.
The detection of viral presence in testicular fluid is considered a critical indicator of viral vertical transmission, as evidenced in studies on PEDV (Ryu et al., 2021) and Porcine group A rotavirus (Li et al., 2025). In this study, the prevalence of PCV3 in testicular fluid is the highest, with the lowest detected Cq values, suggesting potential vertical transmission of PCV3. Previous research has established that PCV3 can be transmitted from sows to their offspring, resulting in piglets being born with the virus (Vargas-Bermúdez et al., 2021). Furthermore, a study conducted in Hunan Province, China, reported a significantly higher detection rate of PCV3 in sows experiencing reproductive issues compared to healthy sows, further highlighting the critical nature of vertical transmission (Zou et al., 2018). Interestingly, the positive rates of PCV3 in serum or throat swabs are relatively low, which could be attributed to the physiological stage or health status of the pigs tested.
In conclusion, this study developed a highly sensitive and specific TaqMan-qPCR method suitable for detecting PCV3 across various clinical samples, thereby providing an effective tool for monitoring the virus and investigating its epidemiological characteristics.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: Genbank accession number MK656956.1 (https://www.ncbi.nlm.nih.gov/nuccore/MK656956.1).
Ethics statement
The animal study was approved by The committee of experiment operational guidelines and animal welfare of Xichang University (approved permit number: xcc2024015). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ZH: Writing – original draft, Writing – review & editing, Funding acquisition, Project administration. RL: Writing – original draft, Writing – review & editing, Methodology, Software. WX: Writing – review & editing, Methodology. RG: Resources, Writing – review & editing. ML: Resources, Writing – review & editing. ZZ: Methodology, Software, Writing – review & editing. GH: Writing – review & editing, Resources. GY: Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Doctoral Research Initiation Project (YBZ2025004) and the Open project of State Key Laboratory of Animal Biotech Breeding (2025SKLAB6-05), and the Sichuan Science and Technology Program (2025YFHZ0058).
Conflict of interest
Author RL was employed by the company Shandong New Hope Liuhe Agriculture and Animal Husbandry Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1603264/full#supplementary-material
References
Chen G. H., Tang X. Y., Sun Y., Zhou L., Li D., Bai Y., et al. (2018). Development of a SYBR green-based real-time quantitative PCR assay to detect PCV3 in pigs. J. Virol. Methods 251, 129–132. doi: 10.1016/j.jviromet.2017.10.012
Chen N., Xiao Y., Li X., Li S., Xie N., Yan X., et al. (2021). Development and application of a quadruplex real-time PCR assay for differential detection of porcine circoviruses (PCV1 to PCV4) in Jiangsu province of China from 2016 to 2020. Transbound Emerg. Dis. 68, 1615–1624. doi: 10.1111/tbed.13833
Cui Y., Hou L., Pan Y., Feng X., Zhou J., Wang D., et al. (2022). Reconstruction of the evolutionary origin, phylodynamics, and phylogeography of the porcine circovirus type 3. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.898212
Decorte I., Van Campe W., Mostin L., Cay A. B., and De Regge N. (2015). Diagnosis of the Lelystad strain of Porcine reproductive and respiratory syndrome virus infection in individually housed pigs: comparison between serum and oral fluid samples for viral nucleic acid and antibody detection. J. Vet. Diagn. Invest. 27, 47–54. doi: 10.1177/1040638714561252
Fan M., Bian L., Tian X., Hu Z., Wu W., Sun L., et al. (2023). Infection characteristics of porcine circovirus type 2 in different herds from intensive farms in Chin. Front. Vet. Sci. 10. doi: 10.3389/fvets.2023.1187753
Feng C., Wang C., Zhang Y., Du F., Zhang Z., Xiao F., et al. (2019). Establishment of a sensitive TaqMan-based real-time PCR assay for porcine circovirus type 3 and its application in retrospective quarantine of imported boars to China. Vet. Med. Sci. 5, 168–175. doi: 10.1002/vms3.141
Gibert E., Martín-Valls G., and Mateu E. (2017). Comparison of protocols for the analysis of type 1 porcine reproductive and respiratory syndrome virus by RT-PCR using oral fluids. J. Virol. Methods 243, 190–195. doi: 10.1016/j.jviromet.2017.02.010
Han H. Y., Zheng H. H., Zhao Y., Tian R. B., Xu P. L., Hou H. L., et al. (2019). Development of a SYBR green I-based duplex real-time fluorescence quantitative PCR assay for the simultaneous detection of porcine epidemic diarrhea virus and porcine circovirus 3. Mol. Cell Probes 44, 44–50. doi: 10.1016/j.mcp.2019.02.002
Henao-Diaz A., Giménez-Lirola L., Baum D. H., and Zimmerman J. (2020). Guidelines for oral fluid-based surveillance of viral pathogens in swine. Porcine. Health Manag. 6, 28. doi: 10.1186/s40813-020-00168-w
Hou C. Y., Xu T., Zhang L. H., Cui J. T., Zhang Y. H., Li X. S., et al. (2021). Simultaneous detection and differentiation of porcine circovirus 3 and 4 using a SYBR Green І-based duplex quantitative PCR assay. J. Virol. Methods 293, 114152. doi: 10.1016/j.jviromet.2021.114152
Hu Z., Lai R., Tian X., Guan R., and Li X. (2024). A duplex fluorescent quantitative PCR assay to distinguish the genotype I, II and I/II recombinant strains of African swine fever virus in China. Front. Vet. Sci. 11. doi: 10.3389/fvets.2024.1422757
Jiang H., Wei L., Wang D., Wang J., Zhu S., She R., et al. (2020). ITRAQ-based quantitative proteomics reveals the first proteome profiles of piglets infected with porcine circovirus type 3. J. Proteomics 212, 103598. doi: 10.1016/j.jprot.2019.103598
Kim S. H., Park J. Y., Jung J. Y., Kim H. Y., Park Y. R., Lee K. K., et al. (2018). Detection and genetic characterization of porcine circovirus 3 from aborted fetuses and pigs with respiratory disease in Korea. J. Vet. Sci. 19, 721–724. doi: 10.4142/jvs.2018.19.5.721
Li Y., Gao C., Wu L., Qing J., Zhang M., Qiao M., et al. (2025). Isolation and possibility of vertical transmission of G9P[23] and G12P[7] group A rotavirus strains in pigs. Porcine. Health Manage. 11, 32. doi: 10.1186/s40813-025-00445-6
Li X., Qiao M., Sun M., and Tian K. (2018). A duplex real-time PCR assay for the simultaneous detection of porcine circovirus 2 and circovirus 3. Virol. Sin. 33, 181–186. doi: 10.1007/s12250-018-0025-2
Lv W., Cao L., Yang L., Wang N., Li Z., Huang S., et al. (2023). The prevalence and genetic diversity of porcine circoviruses (PCVs) during 2017–2023 in guangdong province, China. Anim. (Basel). 13, 3640. doi: 10.3390/ani13233640
Maren N. A., Duduit J. R., Huang D., Zhao F., Ranney T. G., and Liu W. (2023). Stepwise optimization of real-time RT-PCR analysis. Methods Mol. Biol. 2653, 317–332. doi: 10.1007/978-1-0716-3131-7_20
Olsen C., Wang C., Christopher-Hennings J., Doolittle K., Harmon K. M., Abate S., et al. (2013). Probability of detecting Porcine reproductive and respiratory syndrome virus infection using pen-based swine oral fluid specimens as a function of within-pen prevalence. J. Vet. Diagn. Invest. 25, 328–335. doi: 10.1177/1040638713481471
Opriessnig T., Karuppannan A. K., Castro A., and Xiao C. T. (2020). Porcine circoviruses: current status, knowledge gaps and challenges. Virus Res. 286, 198044. doi: 10.1016/j.virusres.2020.198044
Ouyang T., Niu G., Liu X., Zhang X., Zhang Y., and Ren L. (2019). Recent progress on porcine circovirus type 3. Infect. Genet. Evol. 73, 227–233. doi: 10.1016/j.meegid.2019.05.009
Pepin B., Liu F., Main R., Ramirez A., and Zimmerman J. (2015). Collection of Oral fluid from individually housed sows. J. Swine. Health Product. 23, 35–37. doi: 10.54846/jshap/854
Qi S., Su M., Guo D., Li C., Wei S., Feng L., et al. (2019). Molecular detection and phylogenetic analysis of porcine circovirus type 3 in 21 Provinces of China during 2015-2017. Transbound Emerg. Dis. 66, 1004–1015. doi: 10.1111/tbed.13125
Ren J., Li F., Yu X., Li Y., Li M., Sha Y., et al. (2024a). Development of a TaqMan-based multiplex real-time PCR for simultaneous detection of porcine epidemic diarrhea virus, Brachyspira hyodysenteriae, and Lawsonia intracellularis. Front. Vet. Sci. 11. doi: 10.3389/fvets.2024.1450066
Ren J., Zu C., Li Y., Li M., Gu J., Chen F., et al. (2024b). Establishment and application of a TaqMan-based multiplex real-time PCR for simultaneous detection of three porcine diarrhea viruses. Front. Microbiol. 15. doi: 10.3389/fmicb.2024.1380849
Rodriguez-Manzano J., Moniri A., Malpartida-Cardenas K., Dronavalli J., Davies F., Holmes A., et al. (2019). Simultaneous single-channel multiplexing and quantification of carbapenem-resistant genes using multidimensional standard curves. Anal. Chem. 91, 2013–2020. doi: 10.1021/acs.analchem.8b04412
Ryu J., Kang G. J., Kim O., Park J. Y., and Shin H. J. (2021). Transplacental transmission of porcine epidemic diarrhea virus. Front. Vet. Sci. 8. doi: 10.3389/fvets.2021.802816
Tan C. Y., Lin C. N., and Ooi P. T. (2021). What do we know about porcine circovirus 3 (PCV3) diagnosis so far?: A review. Transbound Emerg. Dis. 68, 2915–2935. doi: 10.1111/tbed.14185
Vargas-Bermúdez D. S., Vargas-Pinto M. A., Mogollón J. D., and Jaime J. (2021). Field infection of a gilt and its litter demonstrates vertical transmission and effect on reproductive failure caused by porcine circovirus type 3 (PCV3). BMC Vet. Res. 17, 150. doi: 10.1186/s12917-021-02862-5
Wang J., Zhang Y., Wang J., Liu L., Pang X., and Yuan W. (2017). Development of a TaqMan-based real-time PCR assay for the specific detection of porcine circovirus 3. J. Virol. Methods 248, 177–180. doi: 10.1016/j.jviromet.2017.07.007
Wang D., Zhao J., Yang X., Ji Y., Yu J., Li Z., et al. (2024). E3 ligase RNF2 inhibits porcine circovirus type 3 replication by targeting its capsid protein for ubiquitination-dependent degradation. J. Virol. 98, e0022324. doi: 10.1128/jvi.00223-24
White D., Rotolo M., and Olsen C. (2014). Recommendations for pen-based oral-fluid collection in growing pigs. J. Swine. Health Product. 22, 138–141. doi: 10.54846/jshap/809
Woźniak A., Miłek D., Matyba P., and Stadejek T. (2019). Real-time PCR detection patterns of porcine circovirus type 2 (PCV2) in polish farms with different statuses of vaccination against PCV2. Viruses 11, 1135. doi: 10.3390/v11121135
Woźniak A., Miłek D., and Stadejek T. (2020). Wide range of the prevalence and viral loads of porcine circovirus type 3 (PCV3) in different clinical materials from 21 polish pig farms. Pathogens 9, 411. doi: 10.3390/pathogens9050411
Xu P. L., Zhang Y., Zhao Y., Zheng H. H., Han H. Y., Zhang H. X., et al. (2018). Detection and phylogenetic analysis of porcine circovirus type 3 in central China. Transbound Emerg. Dis. 65, 1163–1169. doi: 10.1111/tbed.12920
Ye X., Berg M., Fossum C., Wallgren P., and Blomström A.-L. (2018). Detection and genetic characterisation of porcine circovirus 3 from pigs in Sweden. Virus Genes 54, 466–469. doi: 10.1007/s11262-018-1553-4
Yuan L., Liu Y., Chen Y., Gu X., Dong H., Zhang S., et al. (2020). Optimized real-time fluorescence PCR assay for the detection of porcine Circovirus type 3 (PCV3). BMC Vet. Res. 16, 249. doi: 10.1186/s12917-020-02435-y
Zhang H. H., Hu W. Q., Li J. Y., Liu T. N., Zhou J. Y., Opriessnig T., et al. (2020). Novel circovirus species identified in farmed pigs designated as Porcine circovirus 4, Hunan province, China. Transbound Emerg. Dis. 67, 1057–1061. doi: 10.1111/tbed.13446
Zhao L., Wen X. H., Jia C. L., Zhou X. R., Luo S. J., Lv D. H., et al. (2023). Development of a multiplex qRT-PCR assay for detection of classical swine fever virus, African swine fever virus, and Erysipelothrix rhusiopathiae. Front. Vet. Sci. 10. doi: 10.3389/fvets.2023.1183360
Keywords: porcine circovirus type 3, REP gene, TaqMan-qPCR, clinical sample, detection rate
Citation: Hu Z, Lai R, Xu W, Guan R, Li M, Zhang Z, Hao G and Yan G (2025) Development of a TaqMan-based quantitative real-time PCR for the detection of Porcine circovirus type 3. Front. Anim. Sci. 6:1603264. doi: 10.3389/fanim.2025.1603264
Received: 31 March 2025; Accepted: 23 June 2025;
Published: 14 July 2025.
Edited by:
Carlos Tejeda, Austral University of Chile, ChileReviewed by:
Nguyen Dinh-Hung, University of Arizona, United StatesXiaoyan Wu, Shandong Xiehe University, China
Copyright © 2025 Hu, Lai, Xu, Guan, Li, Zhang, Hao and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangwen Yan, eWd3ZGt5QDEyNi5jb20=
†These authors have contributed equally to this work
 Zhiqiang Hu
Zhiqiang Hu Ranran Lai
Ranran Lai Wei Xu
Wei Xu Ran Guan1,2
Ran Guan1,2 Mingxiang Li
Mingxiang Li Guiying Hao
Guiying Hao