- 1ICAR-Central Institute for Research on Buffaloes, Hisar, Haryana, India
- 2Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar, Haryana, India
The present study was designed to investigate the responsiveness to exogenous gonadotrophin on post-partum fertility during breeding and non-breeding seasons in buffaloes. During the breeding season, twenty-two post-partum buffaloes that calved between September and February were included and further divided into two groups: treatment (n = 12) and control (n = 10). Buffaloes in the treatment group received an exogenous GnRH analogue (Receptal; 10 µg buserelin acetate, i.v.) on day 21 post-partum, whereas the control group received no treatment. During the non-breeding season, post-partum buffaloes (n = 20) that calved between April and August were divided into two groups, treatment (n = 10) and control (n = 10), and the treatment group received exogenous GnRH analogue (Receptal; 10 µg buserelin acetate, i.v.) on day 21 post-partum. During breeding season, average pre-treatment LH concentration was similar in both treatment (1.7 ± 0.3 ng/ml, n=8) and control (1.2 ± 0.2 ng/ml, n=8) groups. During non-breeding season, pre-treatment LH concentration was comparable in both treatment (0.8 ± 0.1 ng/ml, n=8) and control (0.9 ± 0.2 ng/ml, n=8) groups. The basal LH level was lower (P < 0.05) during the non-breeding season compared with that during the breeding season (0.88 ± 0.1 ng/ml, n=16 vs 1.44 ± 0.2 ng/ml, n=16). GnRH administration on day 21 post-calving has been found to improve the early resumption of cyclicity during breeding calved (83.3%) and non-breeding calved buffaloes (80%) by day 28 post-partum. Administration of GnRH helped resume early cyclicity in all animals during both seasons. Following GnRH administration, cyclicity resumed in 82% of the treatment group and 45% of the control group on day 28 post-calving. By day 60 post-partum, 95.5% and 75% of buffaloes in the treatment and control groups, respectively, had resumed cyclicity. GnRH administration on day 21 post-calving did not influence conception rate, as overall conception rates within three inseminations were similar in the treatment and control groups during the breeding season (75% vs. 70%). In conclusion, administration of GnRH post-partum aids early resumption of ovarian cyclicity and reduces days open in buffaloes, irrespective of season.
1 Introduction
The post-partum period in buffalo starts with parturition and ends with complete uterine involution, normal estrus expression, and resumption of ovarian cyclicity. Hormonal changes during the peri-parturient period, besides regulating lactogenesis and parturition, have an impact on post-partum reproductive activity (El-Wishy, 2007a). Knowledge of these changes is essential to understand the factors responsible for the initiation of cyclic ovarian activity following parturition. At the time of parturition, progesterone and estradiol concentrations reduce to basal levels, which influence post-partum reproductive activity.
Buffaloes are considered difficult breeders because of their inherent susceptibility to environmental stress, which causes anestrus, delayed age at first calving, and a higher incidence of silent ovulations (El-Wishy, 2007b; Bhat and Dhaliwal, 2023). Post-partum anestrus is usually longer in buffalo than in cattle under similar management conditions (Jainudeen and Hafez, 1993). The delay in the resumption of ovarian cyclicity after parturition is mainly attributed to inadequate nutrition, seasonal effects, genital pathologies, and managemental factors. Environmental factors in buffaloes directly and adversely affect the neuroendocrine axis (Rao and Pandey, 1983). The luteinizing hormone (LH), which plays a significant role in reproduction, is directly under brain control and regulated by ovarian steroids (Phogat et al., 1997a). The peak plasma LH concentration also varies with seasons, with levels higher during the cooler months compared with the non-breeding season (Rao and Pandey, 1983).
Gonadotrophin-releasing hormone (GnRH) occupies a central role in reproductive function in mammals. It is a neuropeptide released in a synchronized, pulsatile manner from neurons terminating at the medial basal hypothalamus–median eminence, an area bathed by the hypothalamo-hypophyseal portal vessels (Hassanein et al., 2024). GnRH moves into the portal vessels and is delivered to gonadotroph cells in the anterior pituitary gland. At the gonadotroph cells, GnRH binds to specific cell-surface receptors, triggering a sequence of events for the synthesis of LH and FSH (Peters et al., 1985).
Several researchers have used GnRH agonists in cattle and buffaloes to improve post-partum fertility. Variable effects of GnRH agonists have been reported on pituitary responsiveness, post-partum ovarian cyclicity, uterine involution, and conception rate (Sharma et al., 2017). Administration of exogenous GnRH analogues causes a steep LH peak concentration within 2 h after treatment, with the duration of the surge lasting 3–5 h (McDougall et al., 1995), resulting in ovulation of the dominant follicle (DF) of 10 mm or more (Crowe et al., 1993). The pattern of LH release is similar in suckled and non-suckled buffaloes, although the quantity of LH is affected by suckling (Singh et al., 2006). GnRH administration around the second week of calving helped induce ovulation (100%) in buffaloes within 45 days post-partum, with a significantly higher conception rate (Abdel-Halim and Helmy, 2014). Resumption of ovarian cyclicity can be enhanced by using different types of hormones in the early post-partum period, with variable results (Kandiel et al., 2013). Faster uterine involution and a higher conception rate in Indian Murrah buffaloes were observed after administration of GnRH or PGF2α two weeks after parturition (Ingawale et al., 2014).
Considering these findings, the present study was designed to investigate the responsiveness to exogenous gonadotrophin on post-partum fertility during breeding and non-breeding seasons in buffaloes in an organized herd.
2 Materials and methods
For this study, 42 post-partum pluriparous healthy Murrah buffaloes were selected that calved normally at term with no post-partum complications. All buffaloes were suckled for milk letdown, and all calves were weaned as per standard managemental practices. According to the season of calving, animals were randomly divided into two groups, comprising 22 animals in the breeding season (September to February; Group 1) and 20 animals in the non-breeding season (April to August; Group 2). All experimental animals were fed routinely as per ICAR (2013) standards with free access to clean drinking water. All procedures were conducted with the approval of the Institutional Animal Ethics Committee of ICAR-CIRB.
2.1 Group 1
Twenty-two post-partum pluriparous Murrah buffaloes (average body weight 445 ± 5 kg; BCS 3–3.5) that calved between September and February were included in this group. During the breeding season, relative humidity ranged between 70% and 80%, and ambient temperature ranged from 11 °C to 39 °C. The animals were further divided into two groups, i.e., treatment (n = 12) and control (n = 10). Buffaloes in the treatment group received an exogenous GnRH analogue (Receptal; 10 µg buserelin acetate, i.v.) (Sharma et al., 2017) on day 21 post-partum, whereas animals in the control group (n = 10) received no treatment.
2.2 Group 2
Post-partum pluriparous Murrah buffaloes (n = 20; average body weight 450 ± 5 kg; BCS 3–3.5) that calved between April and August were included in this group. During the non-breeding season, relative humidity ranged between 25% and 60%, and ambient temperature ranged from 35°C to 47°C. Buffaloes in this group were further divided into two groups, i.e., treatment (n = 10) and control (n = 10). Similar to Group 1, buffaloes in the treatment group received an exogenous GnRH analogue (Receptal; 10 µg buserelin acetate, i.v.) on day 21 post-partum, whereas the remaining buffaloes (n = 10) served as controls and received no treatment. All animals were monitored regularly using per rectal examination, ultrasonographic scanning, and blood sampling as described below.
2.3 Blood sampling
To measure luteinizing hormone (LH) concentration, blood sampling was carried out on day 21 post-partum, when GnRH injection was administered as per the study design. Blood was collected from a minimum of eight buffaloes in each treatment and control group during both the breeding and non-breeding seasons. Samples were collected at 0, 30, 60, 90, 120, and 150 min after GnRH injection. Blood was centrifuged at 1,500 rpm for 15 min to separate plasma. The separated plasma was stored frozen at −80°C in 500 µL aliquots until LH estimation.
2.3.1 Estimation of LH
Plasma LH concentration was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Bioassay Technology Laboratory), and values were expressed as ng/mL. The procedure described by the manufacturer was followed, and a standard curve was prepared using known standards. The coefficients of intra-assay and inter-assay variation were <10% and <12%, respectively.
An ELISA plate pre-coated with bovine NEFA antibody was used for the estimation of LH concentration. NEFA present in the sample was added and bound to the antibodies coated on the wells, after which biotinylated bovine NEFA antibody was added and bound to NEFA in the sample. Subsequently, streptavidin–HRP was added, which bound to the biotinylated NEFA antibody. The detection range of the assay was 0.08–33.34 ng/mL.
2.4 Transrectal ultrasonography
All ultrasound examinations were performed by a single operator using a Hitachi Aloka Color Doppler Prosound F31 ultrasound scanner equipped with an intraoperative electronic convex sector probe (IPX7). Ultrasound examinations were conducted on days 0, 7, 14, 21, 28, 35, 42, 60, and 90 post-partum to evaluate the resumption of ovarian cyclicity and uterine involution. The day of calving was considered day 0 in this study. Pregnancy diagnosis was performed after day 30 post-insemination in inseminated buffaloes.
2.4.1 Uterine involution
This refers to the process by which the uterus returns to its normal size and functional state post-calving. Rectal palpation was started from day 21 post-partum at weekly intervals until complete involution of the uterus. Uterine involution was considered complete when there was little difference between the previously gravid and non-gravid horns, and the entire uterus was palpable within the pelvic cavity (El-Wishy, 2007a).
2.4.2 Onset of ovarian cyclicity
To determine the onset of ovarian cyclicity, ultrasonography was carried out from day 0 onwards until a corpus luteum was detected in any ovary or until day 90 post-partum, whichever occurred earlier.
2.4.3 Days open
Days open were calculated as the time interval between calving and successful conception.
2.4.4 Conception rate
Conception rate was calculated at different artificial inseminations (AI) and post-partum days. All buffaloes under study exhibiting signs of estrus were inseminated as per standard procedures.
2.5 Statistical analysis
Different parameters, viz. LH concentration and days open, were analyzed and compared between the control and treatment groups as well as between the two seasons using two-way ANOVA. The significance between time intervals within each group was assessed using repeated measures ANOVA. Other parameters, viz. resumption of ovarian cyclicity, uterine involution, and conception rates between groups at different time intervals, were analyzed using Fisher’s exact test. Differences with P < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 16.0 for Windows (IBM Corp., Armonk, NY, USA).
3 Results
3.1 LH hormone status
During the breeding season, the average pre-treatment LH concentration was similar in both the treatment (1.7 ± 0.3 ng/mL) and control (1.2 ± 0.2 ng/mL) groups. A sharp rise in LH concentration was observed within 30 min of GnRH injection, peaking (18.3 ± 0.9 ng/mL) at 120 min post-injection in the treatment group (Table 1). However, in the control group, LH values remained static across different time intervals.
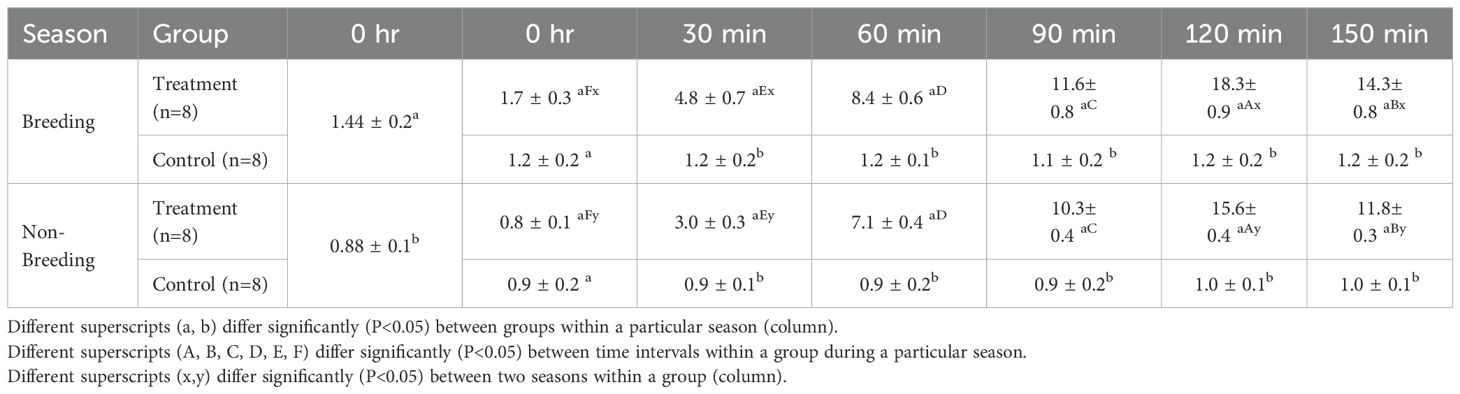
Table 1. Plasma LH concentrations (ng/ml; mean ± SE) at different time intervals following administration of GnRH analogue in post-partum buffaloes during breeding and non-breeding seasons.
During the non-breeding season, pre-treatment LH concentration was comparable in both the treatment (0.8 ± 0.1 ng/mL) and control (0.9 ± 0.2 ng/mL) groups. Similar to the breeding season, a sharp rise in LH values was observed within 30 min of GnRH injection, peaking (15.6 ± 0.4 ng/mL) at 120 min post-injection in the treatment group, whereas LH concentrations in the control group remained static across all time intervals.
The pattern of LH release in response to buserelin acetate treatment was significantly higher (P < 0.05) during both the breeding (18.3 ± 0.9 ng/mL) and non-breeding (15.6 ± 0.4 ng/mL) seasons, with a peak at 120 min post-treatment. LH concentrations in the treated group during the breeding season at 0 h (1.7 ± 0.3 ng/mL), 30 min (4.8 ± 0.7 ng/mL), 120 min (18.3 ± 0.9 ng/mL), and 150 min (14.3 ± 0.8 ng/mL) were higher (P < 0.05) than those in the non-breeding counterparts. However, the magnitude of LH release in the treatment group was significantly lower (P < 0.05) during the non-breeding season at different time intervals compared with the breeding season. The basal LH level was comparable between the breeding and non-breeding seasons.
3.2 Resumption of ovarian cyclicity
Resumption of ovarian cyclicity was assessed by the appearance of the first corpus luteum following calving during regular ultrasound examinations of post-partum buffaloes. It was observed that 10% of buffaloes during the non-breeding season and 20%–25% of buffaloes during the breeding season resumed cyclicity by day 21 post-calving (Table 2). By day 28, 30% of buffaloes that calved during the non-breeding season and 60% that calved during the breeding season resumed cyclicity (Table 3).
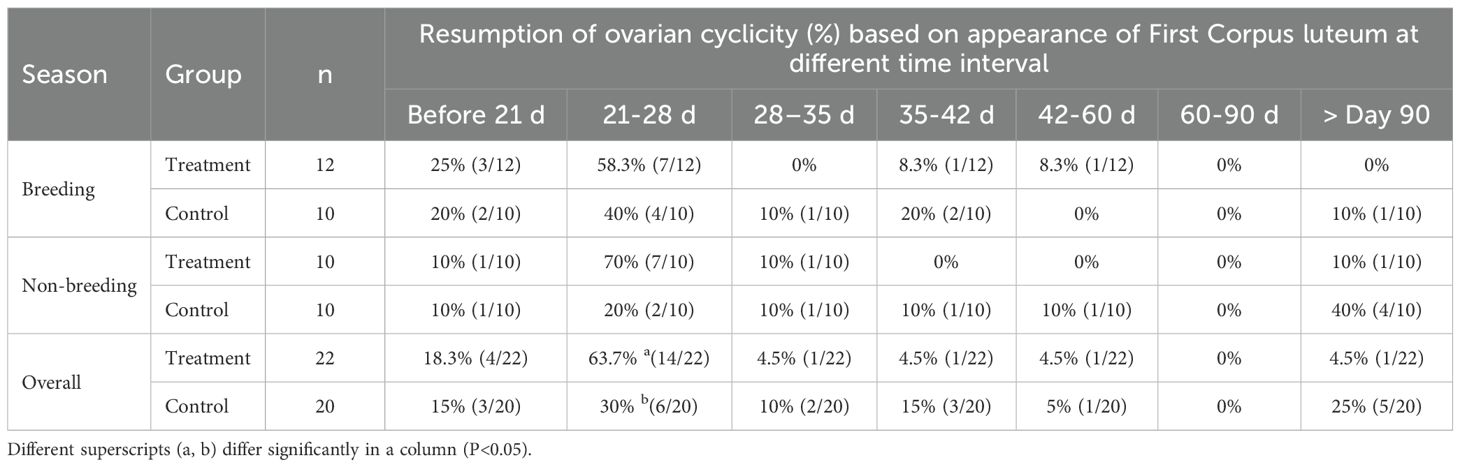
Table 2. Resumption of ovarian cyclicity (%) with respect to post-partum days in treatment and control group during breeding and non-breeding seasons.
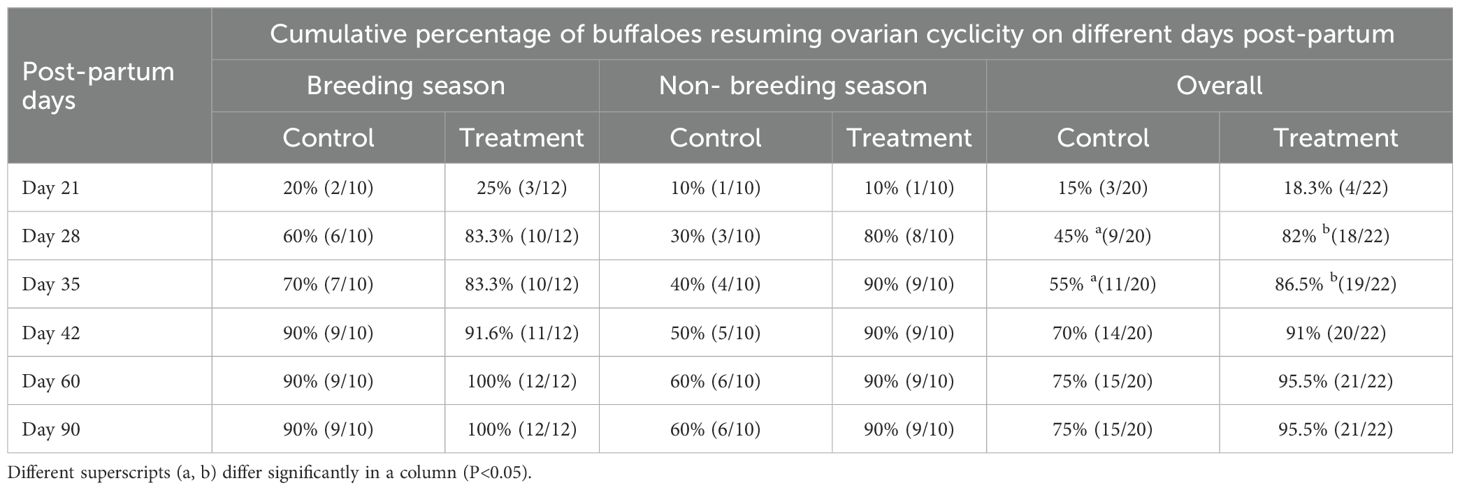
Table 3. Cumulative percentage of buffaloes resuming ovarian cyclicity with respect to post-partum days in treatment and control groups during breeding and non-breeding seasons.
It is evident that by day 28, the majority of buffaloes exhibited their first estrus, as indicated by the appearance of the first corpus luteum. By day 60 post-partum, 90% of buffaloes in the control group during the breeding season and 60% during the non-breeding season had developed a corpus luteum. GnRH administration on day 21 post-calving was found to improve early resumption of cyclicity in buffaloes calving during both the breeding (83.3%) and non-breeding (80%) seasons by day 28 post-partum. Moreover, 10% of buffaloes that calved during the breeding season and 40% that calved during the non-breeding season did not resume cyclicity by day 90 post-partum.
Administration of GnRH on day 21 post-partum facilitated early cyclicity resumption in all animals during both seasons. Irrespective of season, overall 18.3% of buffaloes in the treatment group and 15% in the control group had already resumed cyclicity before administration of GnRH on day 21 post-calving (Table 3). Following GnRH administration, cyclicity resumed in 82% of the treatment group and 45% of the control group by day 28 post-calving. By day 60 post-partum, 95.5% and 75% of buffaloes in the treatment and control groups, respectively, had resumed cyclicity. Beyond day 90 post-partum, 4.5% of buffaloes in the treatment group and 25% in the control group remained anovular.
3.3 Uterine involution
None of the buffaloes that calved during the breeding season had completed uterine involution before day 21 post-partum in either the treatment or control groups. However, by day 28, 58% of buffaloes in the treatment group and 40% in the control group had completed uterine involution. By day 35, uterine involution was complete in all buffaloes in the treatment group and in 70% of buffaloes in the control group. In the control group, all buffaloes completed uterine involution by day 42 post-calving (Table 4). During the non-breeding season, 20% of buffaloes in both the treatment and control groups had already completed uterine involution by day 21 post-partum. On days 28, 35, and 42 post-calving, the cumulative percentages of complete uterine involution were 40%, 70%, and 90%, respectively, in the control group during both the breeding and non-breeding seasons, indicating that uterine involution was not influenced by the season of calving. Similarly, there appeared to be no significant effect of GnRH administration on uterine involution, as by day 42 post-calving, uterine involution was complete in 95% of buffaloes in both the treatment and control groups (Tables 4, 5).
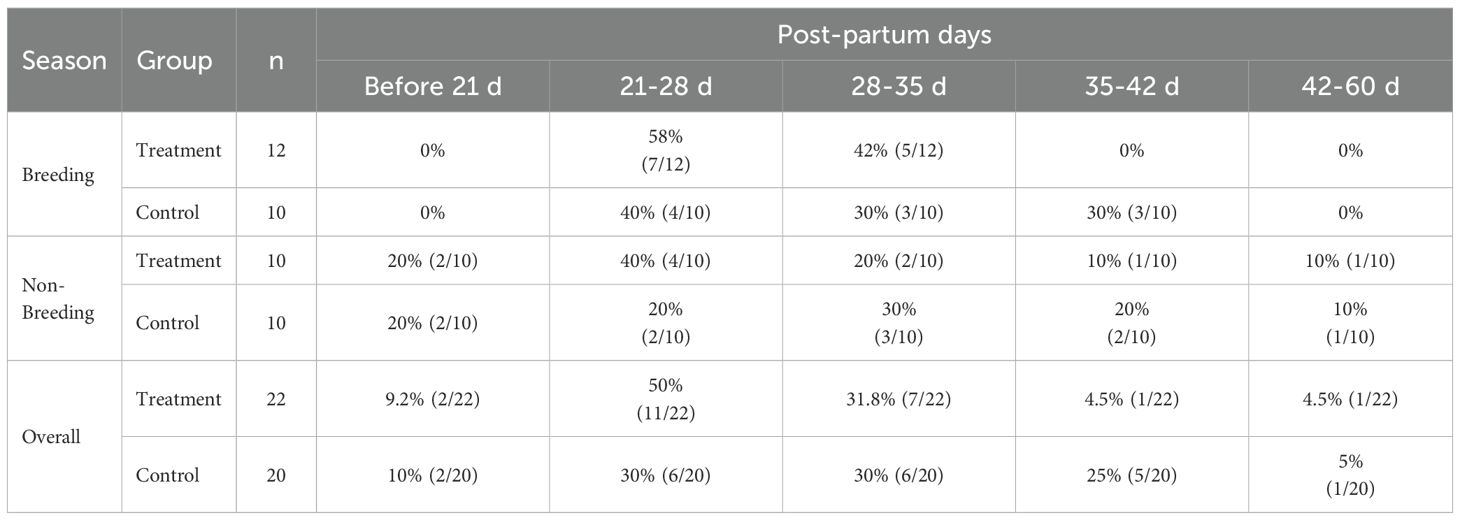
Table 4. Buffaloes completing uterine involution (%) at different post-partum days in treatment and control group during breeding and non-breeding season.
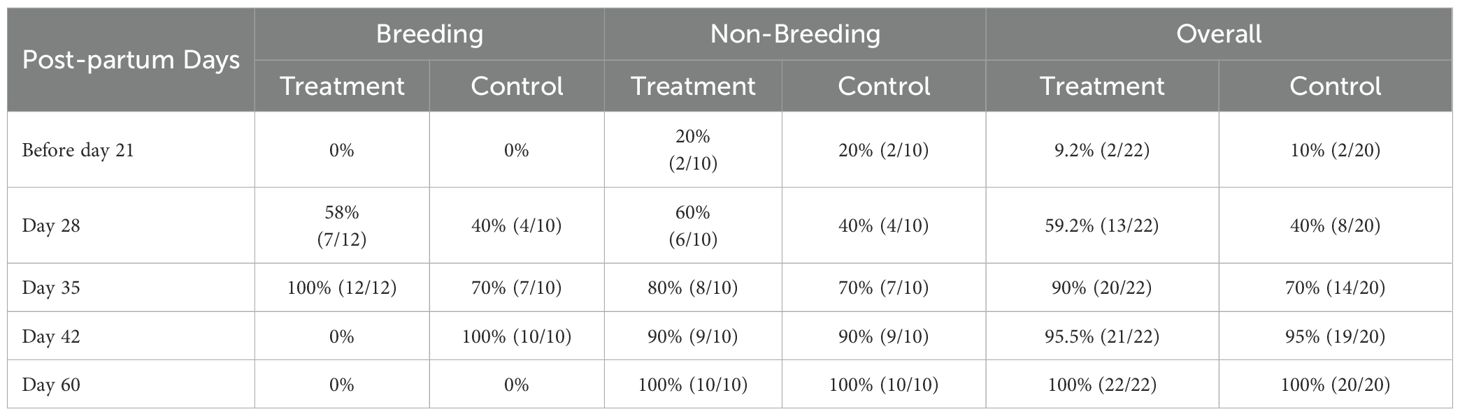
Table 5. Cumulative uterine involution (%) at different days post-partum during breeding and non-breeding season in treatment and control groups.
3.4 Days open
Buffaloes in both treatment and control groups were inseminated according to farm management practices using frozen–thawed semen. No significant difference (P > 0.05) was observed in days open between the treatment (121.9 ± 24.9 days) and control (162.5 ± 47.4 days) groups during the breeding season (Table 6). Similarly, days open did not differ significantly between control groups during the breeding (162.5 ± 47.4 days) and non-breeding (154.1 ± 22.7 days) seasons. However, GnRH administration on day 21 post-partum significantly reduced days open when given during the non-breeding season (90.5 ± 17.4 vs154.1 ± 22.7 days).
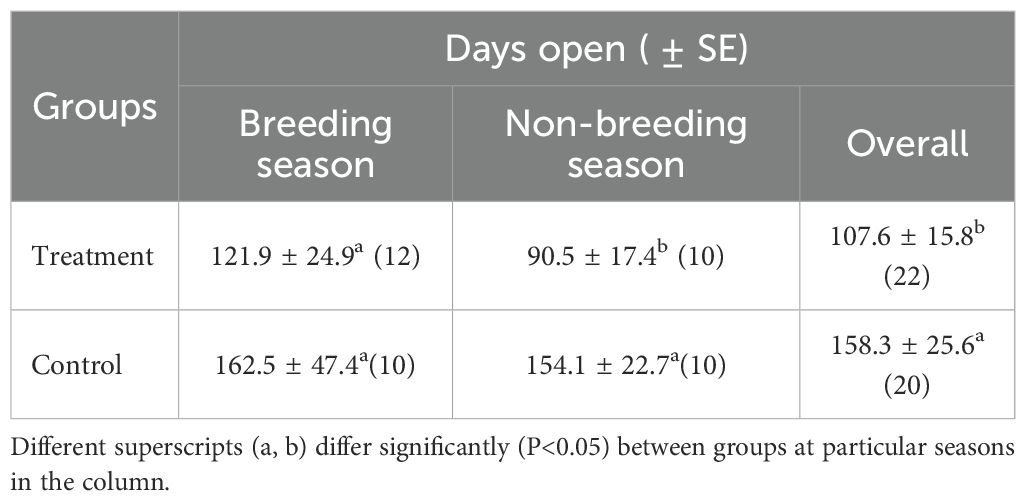
Table 6. Days open (Mean ± SE) during breeding and non-breeding seasons in treatment and control group. .
3.5 Conception rate
Buffaloes that conceived at different time intervals post-calving (<90 days, 90–120 days, and >120 days) were further evaluated. During the breeding season, the majority of buffaloes conceived within 90 days of calving in both the treatment and control groups (Table 7). However, during the non-breeding season, GnRH administration was beneficial, as most buffaloes (60%) conceived within 90 days post-calving, whereas in the control group, the majority (70%) conceived beyond 120 days post-calving (Table 7).
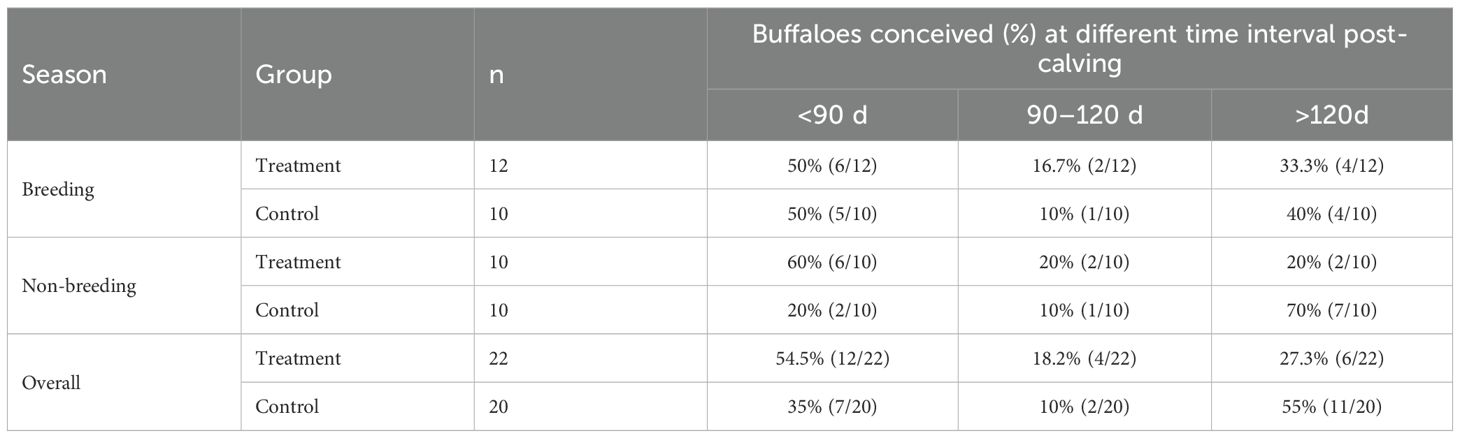
Table 7. Buffaloes conceiving (%) with respect to different post-calving intervals in treatment and control groups during breeding and non-breeding seasons.
GnRH administration on day 21 post-calving did not influence conception rate, as the overall conception rate within three inseminations remained comparable between the treatment and control groups during the breeding season (75% vs. 70%). However, during the non-breeding season, a higher proportion of buffaloes in the treatment group (80%) conceived within three artificial inseminations (AI) compared with only 50% in the control group (Table 8). When overall results were compared irrespective of season, 77.3% of buffaloes in the treatment group and 60% in the control group conceived within the first three inseminations.
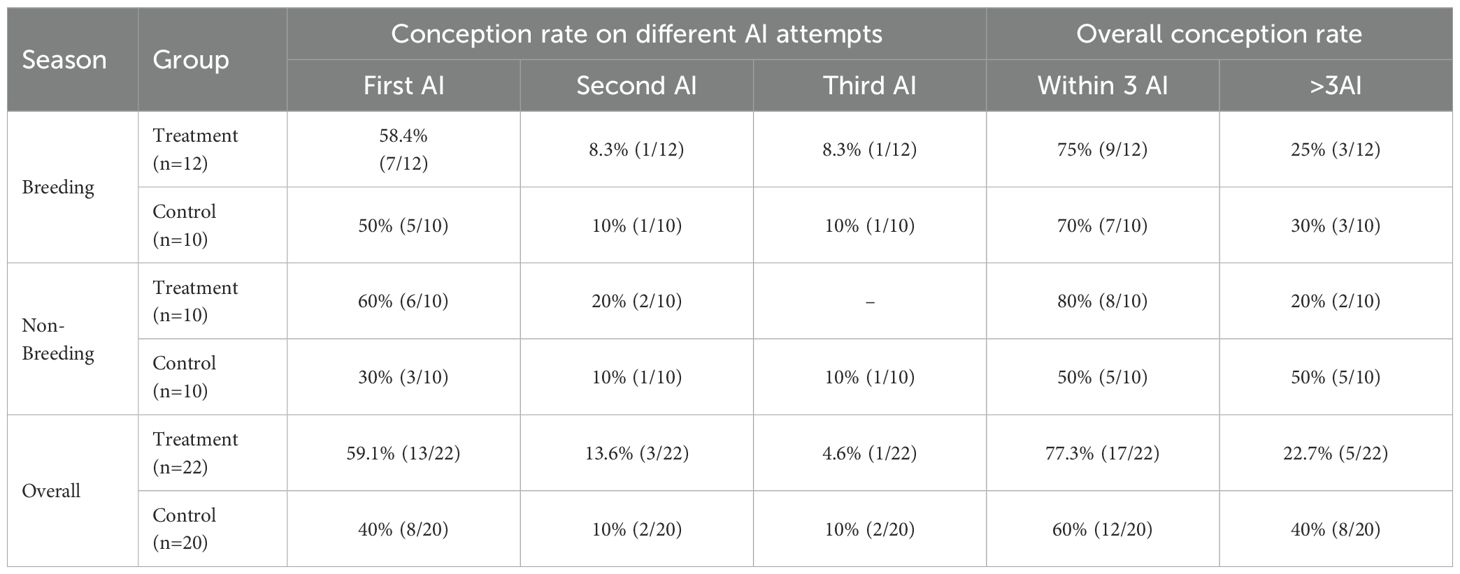
Table 8. Conception rate (%) trends across different AI attempts in treatment and control groups during breeding and non-breeding seasons, AI (artificial insemination).
4 Discussion
4.1 Hormone status
In the present experiment, GnRH analogue (buserelin acetate) administration on day 21 post-partum resulted in an increase in LH concentration that peaked at 120 min post-injection during both breeding and non-breeding seasons, which was in accordance with Fields et al. (2009). In our experiment, average basal LH concentrations and the time interval for attaining peak LH levels following GnRH administration were similar to earlier reports by Singh et al. (2006) and Mehmood et al. (2024). The baseline LH concentrations ranged between 0.72 and 3.0 ng/mL during most of the estrus cycle, and peak values of 20–40 ng/mL have been observed on the day of estrus in cattle and buffaloes (Avenell et al., 1985).
The pituitary gland remains suppressed for GnRH sensitivity during the immediate post-partum period and gradually becomes sensitive to releasing LH in response to GnRH (De Rensis et al., 1993). This sensitivity is recovered by day 14 in cows and by day 20 in buffaloes after calving (Alam and Dobson, 1986). However, the time of appearance of pituitary sensitivity varies according to species and breed (Peters et al., 1981). In addition, suckling induces differences in the time of appearance and intensity of pituitary responsiveness and in the magnitude of LH release. The inhibitory effect of suckling on the resumption of cyclic activity after calving acts more on the LH and GnRH release mechanisms than on their synthesis (Singh et al., 2006).
4.2 Resumption of ovarian cyclicity
Early resumption of post-partum ovarian cyclicity is important for ensuring high reproductive efficiency in dairy animals. During the late gestation period, high levels of placental and ovarian steroids (estrogen and progesterone) exert a negative feedback effect on the hypothalamic–pituitary axis (El-Wishy, 2007b). Resumption of ovarian cyclicity can be assessed by the appearance of the first corpus luteum using regular ultrasound examination of post-partum animals. El-Wishy (2007b) reported that 34%–49% of animals resume cyclicity during the first 90 days post-partum, while 31%–40% remain acyclic for more than 150 days. Barman et al. (2011) reported that within 30 days, 40%–45% of buffaloes resume cyclicity, and by day 60 post-partum, 80%–100% of Murrah buffaloes become regularly cyclic. In Nili-Ravi buffaloes, 25%, 54%, 15%, and 6% of buffaloes resume post-partum cyclicity in <30, 31–60, 61–90, and >90 days post-partum, respectively (Mustafa, 2019).
Our findings that by day 28, 30% of buffaloes that calved during the non-breeding season and 60% that calved during the breeding season resumed cyclicity clearly indicate that season has a great influence on the resumption of ovarian cyclicity. GnRH administration on day 21 post-partum improved the early resumption of cyclicity in buffaloes calving during both the breeding (83.3%) and non-breeding (80%) seasons by day 28 post-partum. Furthermore, 10% of buffaloes that calved during the breeding season and 40% that calved during the non-breeding season did not resume cyclicity by day 90 post-partum. Administration of GnRH on day 21 post-partum facilitated early cyclicity resumption in all animals during both seasons.
The mechanism by which GnRH produces its effect is by inducing a pulsatile release of LH, which results in ovulation of the dominant follicle present at the time of treatment (Rajamahendran et al., 1998; Mehmood et al., 2024). The ovulation induction rate in response to a single injection, multiple injections, or continuous infusion of exogenous GnRH varies from 10% to 100% in post-partum cows depending upon the size of the dominant follicle at the time of injection (Crowe et al., 1993). Dominant follicles of 10 mm or more in size usually ovulate between 24 and 48 h, with a steep LH peak concentration within 2 h after treatment and a surge duration of 3–5 h (Stevenson and Call, 1988; McDougall et al., 1995).
Days open were lower and overall conception rates were significantly higher in the treatment group than in the control group during the non-breeding season but were non-significant during the breeding season, similar to the findings of Abdel-Halim and Helmy (2014). Maintenance of an appropriate calving interval requires rapid uterine involution accompanied by the return of normal cyclic activity, which is a prerequisite for reducing days open and improving conception rates (Nanda et al., 2003). Early resumption of cyclicity and uterine involution can be enhanced by administration of PGF2α, GnRH analogues, or other uterine ecbolics in cattle and buffaloes (Nanda et al., 2003; Abdel-Halim and Helmy, 2014; Garcia-Ispierto et al., 2019). Our findings were similar to the above observations and confirmed that the use of GnRH analogue helps in achieving early ovarian cyclicity, thereby promoting early breeding, reducing days open, and improving overall conception rates in the treatment group compared with the control group.
4.3 Uterine involution
Maintenance of an appropriate calving interval requires rapid involution of the uterus accompanied by the return of normal cyclic activity (El-Wishy, 2007a). Uterine involution has been reported to be completed in buffaloes within 27–74 days of calving (Lohan et al., 2000). Post-partum administration of PGF2α or other uterine ecbolics enhances uterine contractility and hastens uterine involution in buffaloes (Nanda et al., 2003). Sheshappa et al. (2002) found that cows receiving GnRH only on day 14 post-partum had a shorter uterine involution period than controls, but those receiving sequential injections of GnRH on day 14 and PGF2α on days 22–26 post-partum had a shorter involution period than those receiving GnRH analogue only.
In our experiment, 20% of buffaloes during the non-breeding season and none during the breeding season had completed uterine involution before day 21 post-partum. The majority of buffaloes (>70%) completed uterine involution by day 35 post-partum in the control group. Administration of GnRH on day 21 post-partum helped in early uterine involution, as 91% of buffaloes completed uterine involution by day 35 post-partum. Contrary to our findings, Abdel-Halim and Helmy (2014) did not find a significant difference in uterine involution days between the treatment group (100 µg GnRH; Receptal administered on days 12–14 post-partum) and the control group.
GnRH improved reproductive efficiency by reducing the interval from calving to conception and decreasing the number of services per conception. During the non-breeding season, however, 20% of buffaloes in both the treatment and control groups completed uterine involution by day 21 post-partum. On days 28, 35, and 42 post-calving, the cumulative percentages of complete uterine involution were 40%, 70%, and 90%, respectively, in the control group during both breeding and non-breeding seasons, indicating that uterine involution was not influenced by season of calving. Similarly, there appeared to be no significant effect of GnRH administration on uterine involution, as by day 42 post-calving, uterine involution was complete in 95% of buffaloes in both the treatment and control groups. In contrast, Kumar et al. (2010) found that administration of GnRH–PGF2α in the early post-partum period improved uterine involution in crossbred cows. More research on these lines needs to be carried out in the future.
4.4 Days open and conception rate
GnRH administration on day 21 post-partum was found to significantly reduce days open when given during the non-breeding season, while no significant difference was observed in days open between treatment and control groups during the breeding season. During the non-breeding season, GnRH administration was beneficial, as the majority of buffaloes (60%) conceived within 90 days post-calving. Administration of GnRH during the non-breeding season could assist heat-stressed follicles by providing greater potential resistance to adverse environmental conditions and enhancing the fertilizing capacity of oocytes (Gallab et al., 2022).
Abdel-Halim and Helmy (2014) reported a higher conception rate in the buserelin acetate–treated group compared with the control group (55.8 ± 2.6) during the early post-partum period of buffalo cows. GnRH treatment aids in the early post-partum period by stimulating ovulation in buffaloes, thereby enhancing post-partum behavioral estrus, shortening the number of days open, decreasing the number of services per conception, and improving conception rate (Lamb et al., 2010; Adam et al., 2021; Gallab et al., 2022). More investigations are warranted on these lines.
5 Conclusion
From the study, it is evident that the use of GnRH in the early post-partum period elicits a predictable increase in fertility that can be reliably applied to buffalo herds. We found its beneficial effects on reproductive parameters such as the resumption of cyclicity and reduction in days open during both the breeding and non-breeding seasons. Nonetheless, more research on these lines is warranted to devise suitable reproductive management strategies for improving post-partum fertility in buffaloes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Institutional Animal Ethics Committee of ICAR-CIRB. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
RK: Methodology, Validation, Formal Analysis, Data curation, Visualization, Writing – review & editing, Writing – original draft, Investigation. JP: Visualization, Data curation, Validation, Formal Analysis, Conceptualization, Writing – review & editing, Supervision, Writing – original draft, Investigation. RS: Conceptualization, Resources, Project administration, Funding acquisition, Writing – review & editing, Data curation, Methodology, Writing – original draft, Supervision, Visualization, Investigation. SP: Methodology, Investigation, Visualization, Data curation, Writing – review & editing, Writing – original draft, Formal Analysis, Validation. YB: Data curation, Writing – original draft, Methodology, Visualization, Validation, Writing – review & editing, Formal Analysis. JA: Data curation, Writing – original draft, Conceptualization, Methodology, Visualization, Validation, Writing – review & editing, Formal Analysis.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The authors thank the Director, ICAR-CIRB, Hisar for supporting this research work. The funding of this research work under AICRP on Nutritional and Physiological approaches for enhancing reproductive performance in animals by Indian Council of Agricultural Research, New Delhi, is duly acknowledged.
Acknowledgments
Research was supported by the lndian Council of Agricultural Research, Department of Agricultural Research and Education, Government of lndia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Halim B. R. and Helmy N. A. (2014). Follicular dynamics, ovulation and reproductive hormones following GnRH pre-treatment in post-partum suckled dairy buffalo-cows. Assiut Vet. Med. J.60 141), 112–119.
Alam M. G. S. and Dobson H. (1986). Effect of various veterinary procedures on plasma concentrations of Cortisol, luteinising hormone and prostaglandin F2α metabolite in the cow. Vet. Rec. 118, 7–10.
Avenell J. A., Saepudin Y., and Fletcher I. C. (1985). Concentrations of LH and progesterone in the peripheral plasma of Swamp buffalo cow (Bubalus bubalis) around the time of estrus. J. Reprod. Fertil. 74, 419–424. doi: 10.1530/jrf.0.0740419
Barman P., Yadav M. C., Kumar H., and Meur S. K. (2011). Effect of bull exposure on ovarian cyclicity in post-partum buffaloes. Buffalo Bull. 30, 240–249.
Bhat G. R. and Dhaliwal G. S. (2023). Estrus and ovulation synchrony of buffaloes (Bubalus bubalis): a review. Buffalo Bull. 42, 239–261. doi: 10.56825/bufbu.2023.4222415
Crowe M. A., Goulding D., Baguisi A., Boland M. P., and Roche J. F. (1993). Induced ovulation of the first post-partum dominant follicle in beef suckler cows using a GnRH analogue. J. Reprod. Fertil. 99, 551–555. doi: 10.1530/jrf.0.0990551
De Rensis F., Hunter M. G., and Foxcroft G. R. (1993). Suckling-induced inhibition of luteinizing hormone secretion and follicular development in the early post-partum sow. Biol. Reprod. 48, 964–969. doi: 10.1095/biolreprod48.5.964
El-Wishy A. B. (2007a). The postpartum buffalo: I. Endocrinological changes and uterine involution. Anim. Reprod. Sci. 97, 201–215. doi: 10.1016/j.anireprosci.2006.03.004
El-Wishy A. B. (2007b). The post-partum buffalo II.Acyclicity and anestrous. Anim. Reprod. Sci. 97, 216–236. doi: 10.1016/j.anireprosci.2006.03.003
Fields S. D., Perry B. L., and Perry G. A. (2009). Effects of GnRH treatment on initiation of pulses of LH, LH release, and subsequent concentrations of progesterone. Dom. Anim. Endocrin. 37, 189–195. doi: 10.1016/j.domaniend.2009.04.006
Gallab R. S., Hassanein E. M., Rashad A. M. A., and El-Shereif A. A. (2022). Maximizing the reproductive performances of anestrus dairy buffalo cows using GnRH analogue-loaded chitosan nanoparticles during the low breeding season. Anim. Reprod. Sci. 244, 107044. doi: 10.1016/j.anireprosci.2022.107044
Garcia-Ispierto I., De Rensis F., Pérez-Salas J. A., Nunes J. M., Pradés B., Serrano-Pérez B., et al. (2019). The GnRH analogue dephereline given in a fixed-time AI protocol improves ovulation and embryo survival in dairy cows. Res. Vet. Sci. 122, 170–174. doi: 10.1016/j.rvsc.2018.11.020
Hassanein E. M., Szelényi Z., and Szenci O. (2024). Gonadotropin-Releasing Hormone (GnRH) and its agonists in bovine reproduction II: Diverse Applications during insemination, Post-insemination, pregnancy, and postpartum periods. Animals 26, 14(11):1575.
Ingawale M. V., Bakshi S. A., Birade H. S., Chinchkar S. R., and Gulavane S. U. (2014). Effect of GnRH and PGF2α administration in early post-partum period on fertility potential of buffaloes. Buffalo Bull. 33, 228–232.
Jainudeen M. R. and Hafez E. S. E. (1993). Cattle and buffalo. E.S.E. Hafez (Ed.), Reproduction in Farm Animals (6th ed.), Lea and Febiger, Philadelphia, USA (1993), pp. 315–329.
Kandiel M. M., Gad B. A., Sosa G. A., and El-Azab A. I. (2013). Follicular dynamics and uterine status after synchronization of ovulation in early post-parturient Egyptian buffaloes. Buffalo Bull. 32, 165–181.
Kumar S., Chandra R., Haque N., Toppo S., and Rahman H. (2010). Effect of GnRH and PGF2a on uterine involution and post-partum fertility in crossbred cows. Indian J. Anim. Sci. 80, 1175–1178.
Lohan I. S., Malik R. K., Saini M. S., Dhanda O. P., Singh B., and Singh B. (2000). Uterine and ovarian changes during the early post-partum period in Murrah buffaloes. Buffalo Bull. 19, 20–23.
McDougall S., Williamson N. B., and Macmillan K. L. (1995). GnRH induces ovulation of a dominant follicle in primiparous dairy cows undergoing anovulatory follicle turnover. Anim. Reprod. Sci. 39, 205–214. doi: 10.1016/0378-4320(95)01385-D
Mehmood M. U., Chishti G. A., Waseem M., Azam B., Naseer Z., Saadullah M., et al. (2024). Preovulatory follicular dynamics and ovulatory events following the use of GnRH 84 h after medroxyprogesterone acetate sponge removal in postpartum buffaloes. J. Reprod. Dev. 70, 349–355. doi: 10.1262/jrd.2024-040
Mustafa H. J. (2019). Effect of modulation of follicular wave pattern and subclinical endometritis on reproductive performance in nili-ravi buffalo. ICAR Indian Veterinary Research Institute, Izatnagar, Uttar Pradesh.
Nanda A. S., Barr P. S., and Prabhakar S. (2003). Enhancing the reproductive performance in dairy buffaloes: major constrains and achievement. Reprod. Suppl. 61, 27–36.
Peters A. R., Lamming G. E., and Fisher M. W. (1981). A comparison of plasma LH concentrations of milked and suckling post-partum cows. J. Reprod. Fertil. 62, 567–573. doi: 10.1530/jrf.0.0620567
Peters A. R., Pimentel M. G., and Lamming G. E. (1985). Hormone responses to exogenous GnRH pulses in post-partum dairy cows. J. Reprod. Fert. 75, 557–565. doi: 10.1530/jrf.0.0750557
Phogat J. B., Smith R. F., and Dobson H. (1997a). The influence of stress on neuroendocrine control of the hypothalamic-pituitary-ovarian axis-a review. Vet. Bul. 67, 551–567.
Rajamahendran R., Ambrose J. D., Schmitt E. J., Thatcher M. J., and Thatcher W. W. (1998). Effect of Buserelin injection and deslorelin (GnRH-Agonist) implants on plasma progesterone, LH, accessory CL formation, follicle and Corpus luteum dynamics inHolstein cows. Theriogenology 50, 1141–1155. doi: 10.1016/S0093-691X(98)00215-5
Rao L. V. and Pandey R. S. (1983). Seasonal variations in oestradiol- 17b and luteinizing hormone in the blood of buffalo cows (Bubalus bubalis). J. Endocrinol. 98, 251–255. doi: 10.1677/joe.0.0980251
Sharma R. K., Phulia S. K., Jerome A., and Singh I. (2017). Ovsynch Plus protocol improves ovarian response in anovular Murrah buffaloes in low-breeding season. Reprod. Domest. Anim. 52, 1030–1035. doi: 10.1111/rda.13020
Sheshappa H., Honnappagol S. H., Dhabale R. B., and Tandle M. K. (2002). Efficacy of GnRH and PGF2 alpha in augmenting reproductive performance of post-partum dairy cows. Indian J. Anim. Reprod. 23, 75–76.
Singh A. K., Brar P. S., Nanda A. S., and Prakash B. S. (2006). Effect of suckling on basal and GnRH induced LH release in post-partum dairy buffaloes. Anim. Reprod. Sci. 95, 244–250. doi: 10.1016/j.anireprosci.2005.10.004
Keywords: buffalo, GnRH, breeding, season, fertility
Citation: Kumar R, Phogat JB, Sharma RK, Phulia SK, Bangar Y and Andonissamy J (2025) Responsiveness to exogenous gonadotrophin on post-partum fertility during breeding and non-breeding seasons in buffaloes. Front. Anim. Sci. 6:1608654. doi: 10.3389/fanim.2025.1608654
Received: 09 April 2025; Accepted: 06 October 2025;
Published: 29 October 2025.
Edited by:
Mohan Mondal, ICAR-National Dairy Research Institute, IndiaReviewed by:
Yendraliza Yendraliza, State Islamic University of Sultan Syarif Kasim Riau, IndonesiaMuhammad Qamer Shahid, University of Veterinary and Animal Sciences, Pakistan
Copyright © 2025 Kumar, Phogat, Sharma, Phulia, Bangar and Andonissamy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rakesh Kumar Sharma, UmFrZXNoLlNoYXJtYS1pY2FyQGljYXIub3JnLmlu; Jerome Andonissamy, amVyb21lLmEtaWNhckBpY2FyLm9yZy5pbg==
 Rajesh Kumar1
Rajesh Kumar1 S. K. Phulia
S. K. Phulia Jerome Andonissamy
Jerome Andonissamy