- 1Department of Animal Nutrition and Forage Production, Faculty of AgriSciences, Mendel University in Brno, Brno, Czechia
- 2Agrovyzkum Rapotin Ltd., Zemedelska, Sumperk, Czechia
Enteric methane emissions from ruminant livestock represent a major contributor to agricultural greenhouse gases and reflect an energetic inefficiency in ruminant metabolism. This review critically evaluates current mitigation strategies aimed at reducing CH4 production in ruminants, with an emphasis on practical applicability, biological mechanisms, and integration into sustainable dairy production systems. Nutritional interventions—including tannins, saponins, essential oils, garlic compounds, seaweed (e.g., Asparagopsis), probiotics, and chemical inhibitors such as 3-nitrooxypropanol (3-NOP)—are discussed in the context of their effects on rumen microbiota, fermentation patterns, and animal productivity. Biological strategies such as archaeal-targeted vaccines, bacteriophage therapy, and microbiome engineering remain largely experimental but represent promising future directions. Genetic selection for low-emission phenotypes and improved manure management are also explored as complementary approaches to reduce emissions. Although some additives have achieved CH4 reductions of 30–50% in vivo, results vary depending on diet, dose, delivery matrix, and duration. Notably, the long-term effects on productivity, nutrient utilization, and product quality remain underexplored. Integrated strategies combining dietary, genetic, and management interventions tailored to specific production systems are likely necessary to achieve meaningful, sustained reductions in ruminant CH4 emissions.
1 Introduction
Due to its role as a potent greenhouse gas (GHG), methane (CH4) production in ruminants is an increasingly critical topic in scientific literature, particularly in intensive dairy farming (Króliczewska et al., 2023). Atmospheric concentrations of CH4, a potent GHG, have risen dramatically since pre-industrial times, increasing by approximately 150% since the year 1750 (Pachauri et al., 2014). Methane is a colorless, odorless, and flammable gas that constitutes the primary component of natural gas (Candelaresi and Spazzafumo, 2021). Although it naturally occurs in the atmosphere at low concentrations, enteric CH4—mainly produced via microbial fermentation in the gastrointestinal tract of ruminants (i.e., cattle, sheep, and goats)—represents a significant source of agricultural GHG emissions (Thacharodi et al., 2024). This biologically produced CH4 is mostly released via eructation (belching) (Morgavi et al., 2023) and contributes both to global warming and to energy inefficiency, as it accounts for a 6–10% loss of gross dietary energy (Castelán-Ortega et al., 2014). Globally, the livestock sector contributes approximately 14.5% of total anthropogenic GHG emissions, with enteric fermentation alone accounting for nearly 40% of agricultural GHG (FAO, 2017). Among livestock-related emissions, enteric CH4 represents the dominant source, contributing up to 88% of CH4 emissions from the sector (Arndt et al., 2022). Since CH4 has a significantly higher global warming potential than carbon dioxide (CO2) (Mar et al., 2022), the livestock farming sector presents a key opportunity for reducing emissions while also improving production efficiency.
Within the rumen, a complex and diverse microbiome—including bacteria, protozoa, and fungi—ferments ingested feed to produce volatile fatty acids (VFA) such as acetate, propionate, and butyrate, which are primary energy sources for the host animal (Matthews et al., 2019). During fermentation, metabolic cofactors like NADH, NADPH, and FADH are re-oxidized, resulting in the production of molecular hydrogen (H2). Methanogenic archaea then utilize this H2 to reduce CO2 to CH4, thereby preventing the accumulation of metabolic H2 but at the cost of significant energy loss—energy that could otherwise contribute to productive functions such as milk synthesis (Castelán-Ortega et al., 2014). Methane production in the rumen is influenced by several factors, including feed composition, chewing behavior, salivation, and gastrointestinal motility (Snelling and John, 2017).
Microbial CH4 emissions of anthropogenic origin are predominantly associated with three primary sources: livestock production (115 Tg CH4 yr−1), landfills and waste management (68 Tg CH4 yr−1), and rice cultivation (30 Tg CH4 yr−1). Within the livestock sector, enteric fermentation represents the principal emission pathway, contributing approximately 85% of total CH4 emissions from this category, equivalent to 98 Tg CH4 yr−1 (Saunois et al., 2019). Cattle are the leading source of enteric CH4 emissions globally, a consequence of their substantial global population (~1.5 billion animals), extensive rumen volume, and specific digestive physiology (Malik et al., 2021).
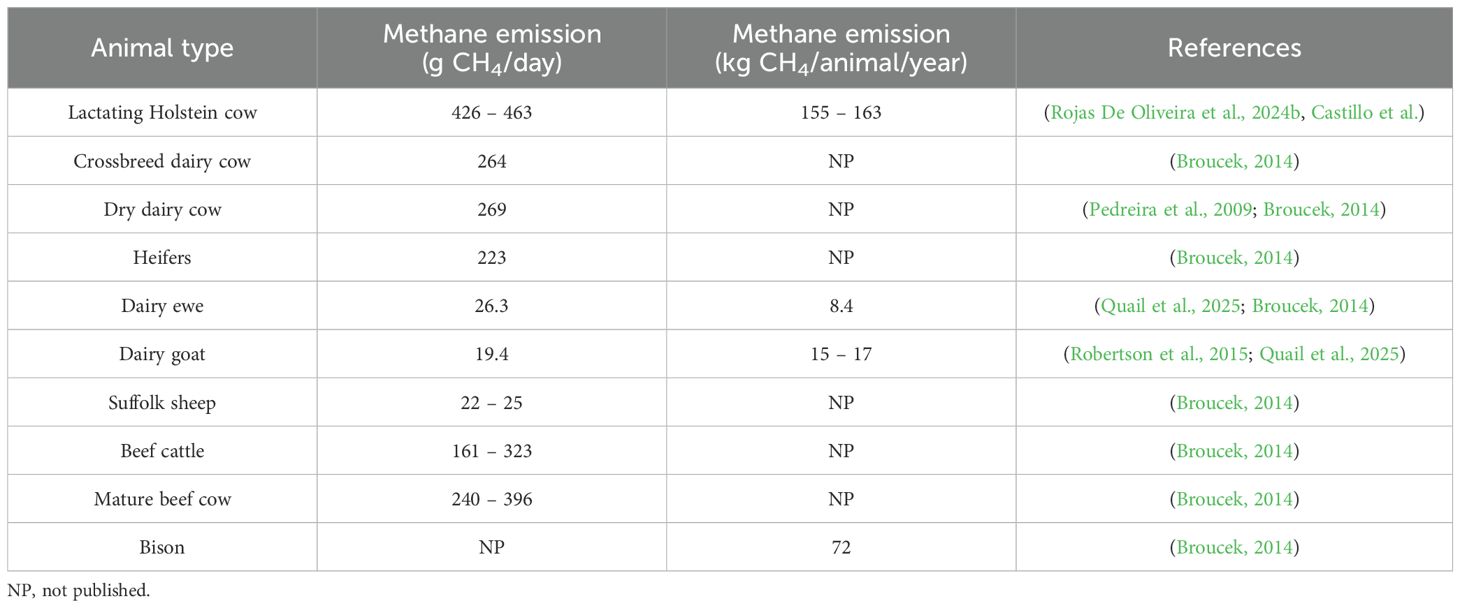
Estimated CH4 emissions vary widely among livestock species and production stages (Starsmore et al., 2024b). Among dairy breeds, Holsteins generate more CH4 than crossbreds, while heifers on fertilized pastures produce more methane (around 223 g CH4/day) than those grazing on unfertilized pastures (around 179 g CH4/day). Various factors, including fecal consistency, digestible material content, climate, and exposure duration, influence CH4 emissions from manure. On dairy farms, annual CH4 emissions from manure storage and pens can reach 120 kg per cow (Kide et al., 2017; Cezimbra et al., 2021).
Table 1 summarizes typical daily and annual CH4 emissions for dairy cows, sheep, beef cattle, and other ruminants, highlighting differences based on physiological status, breed, and management system.
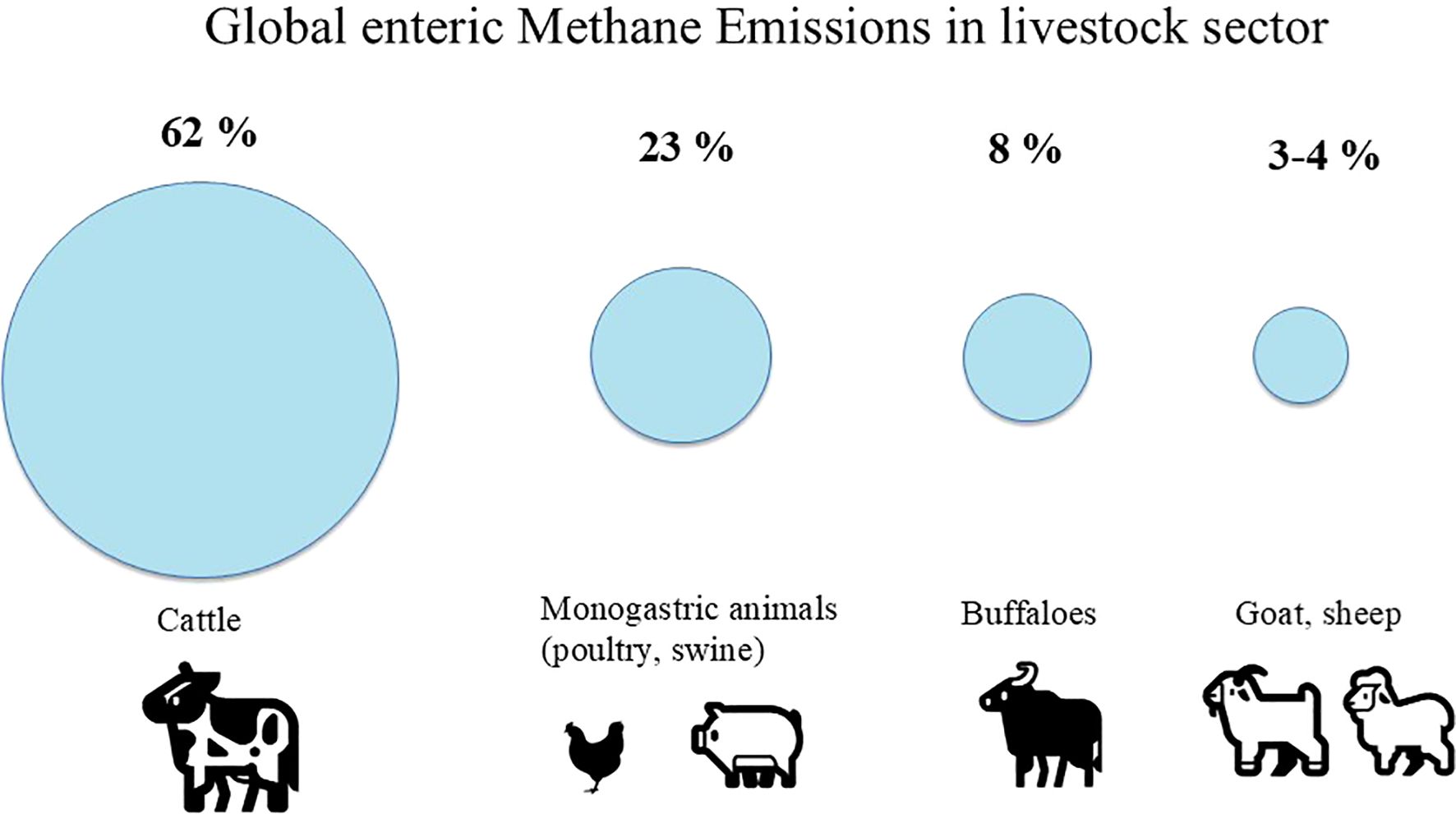
A recent study by Evangelista et al. (2024) examining trends in livestock-related methane emissions reported that cattle contribute the largest share, accounting for approximately 62% of total emissions. This is followed by buffaloes (8%), goats (4%), sheep (3%), and monogastric species such as pigs and poultry, which together account for 23% of emissions (Figure 1).
Mitigating methane production in dairy cows presents a dual opportunity: reducing environmental impact while enhancing milk production, yield, and composition. This synergistic effect underscores the importance of advancing research on effective mitigation strategies in dairy farming. The development of CH4 mitigation strategies is crucial, considering increasing regulatory pressures to reduce agriculture’s contribution to climate change (Reisinger et al., 2021).
Various strategies have been proposed, including feed additives that inhibit methane-producing microbes, breeding programs selected for low-methane cattle (Króliczewska et al., 2023), and precision monitoring systems that enable individualized intervention. Studies highlight the potential of biologically active compounds such as algae extracts, tannin preparations, and 3-Nitrooxypropanol (3-NOP) (Pepeta et al., 2024), and essential oils (EOs) in modifying the rumen microbiome and reducing enteric CH4 production (Belanche et al., 2025).
The goal of this review is to evaluate current research findings and present viable strategies that balance enteric CH4 reduction with economic feasibility and productive efficiency in dairy systems. Specifically, the review aims to (i) synthesize current evidence on the magnitude and variability of CH4 emissions across dairy production contexts; (ii) assess the efficacy of leading mitigation strategies—including dietary interventions such as macroalgae (e.g., Asparagopsis taxiformis), tannin-rich extracts, essential oils, probiotics, and synthetic inhibitors like 3-nitrooxypropanol (3-NOP); and (iii) evaluate the potential trade-offs and co-benefits of these approaches in relation to rumen fermentation, nitrogen metabolism, animal performance, and environmental sustainability. Special emphasis is placed on the impact of these compounds on microbial activity and fermentation dynamics. Mitigation techniques are categorized based on mode of action, active ingredient, dosage, application period, observable effects, and supporting literature. By integrating and critically appraising recent findings, this review provides a comprehensive framework to inform future research priorities, evidence-based policymaking, and practical implementation of CH4 mitigation strategies in modern dairy production.
2 Animal management and breeding strategies
Effective management strategies are essential for reducing GHG emissions from livestock systems. Such reductions are not only critical for improving the environmental sustainability of farming but also provide a benchmark for comparing and evaluating the relative effectiveness of different mitigation practices. By quantifying GHG reductions under alternative management strategies, researchers and policymakers can identify the most impactful interventions and prioritize their implementation at both farm and national levels (Zhang et al., 2024b). Additionally, from an economic perspective, management adjustments represent a cost-effective approach that not only mitigates direct enteric CH4 emissions from cattle but also enhances soil quality and grassland biodiversity, thereby improving the overall CH4 balance and sustainability of the production system (FAO, 2016).
An overview of the principal animal management and breeding strategies to mitigate enteric CH4 emissions, together with their mechanisms, evidence maturity, and limitations, is summarized in Table 2.
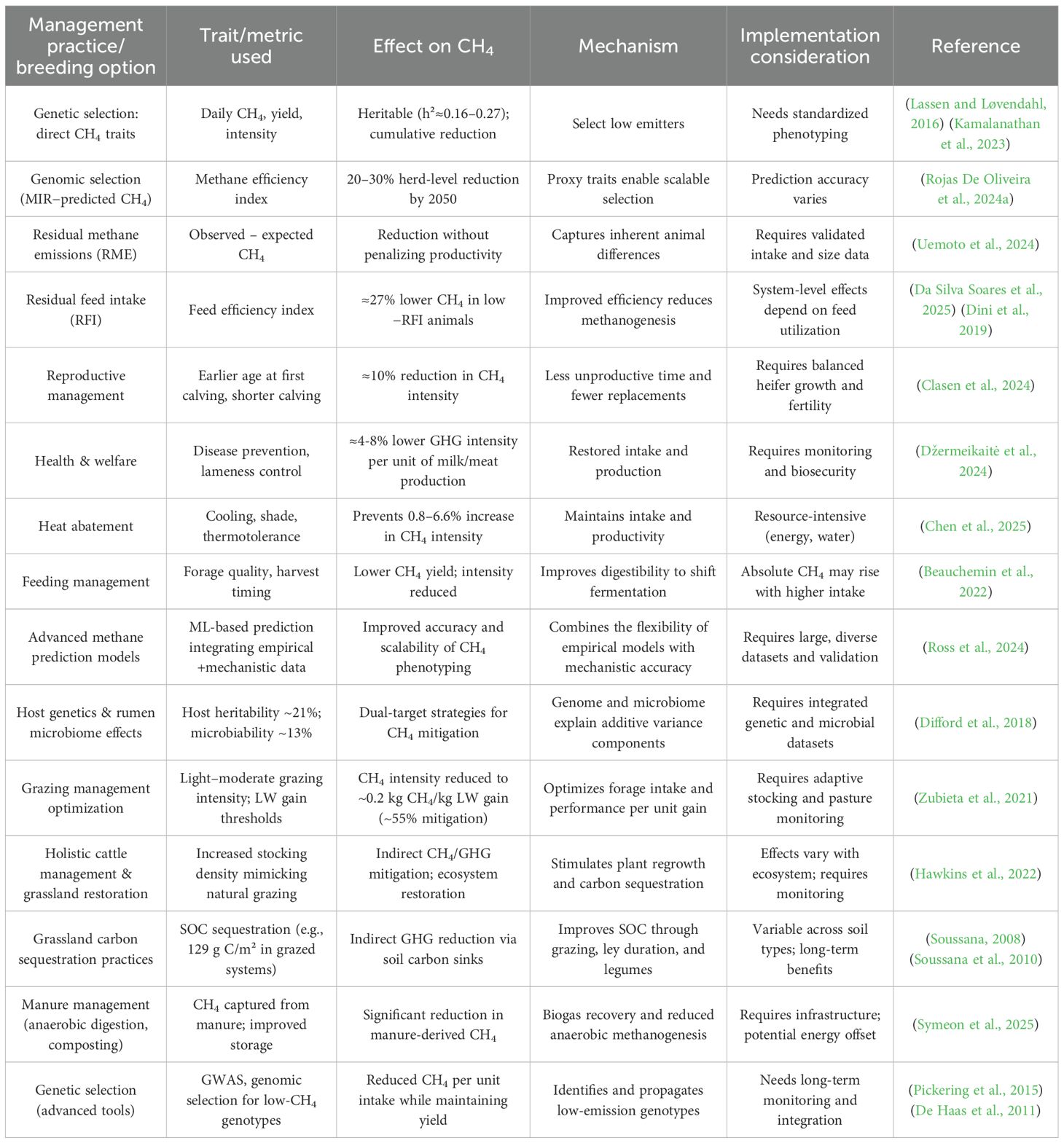
Table 2. Animal management and breeding strategies for reducing enteric methane production in ruminants.
Grazing management offers considerable potential. Zubieta et al. (2021) demonstrated that optimizing herbage intake and live weight (LW) gain under light-to-moderate grazing intensities can reduce CH4 intensity to approximately 0.2 kg CH4/kg LW gain, representing a 55% mitigation potential for pasture-based systems. Holistic cattle management strategies, such as increasing stocking density, may replicate historic grazing patterns of large wild herbivores, thereby restoring grasslands, preventing desertification, and indirectly lowering GHG emissions (Wyffels et al., 2013; Hawkins et al., 2022).
Grasslands also act as carbon sinks. Average sequestration rates of 5 ± 30 g C/m² annually have been reported, though values vary widely depending on soil type, grazing system, and management (Soussana et al., 2010; Bārdule et al., 2024).
Several management practices can reduce carbon losses and enhance sequestration, including: (i) minimizing soil disturbances such as tillage and grassland-to-cropland conversion, (ii) improving nutrient-poor permanent grasslands, (iii) adopting light rather than heavy grazing, (iv) extending the duration of grass leys, and (v) incorporating grass-legume mixtures or converting grass leys into permanent grasslands (Soussana, 2008). Additionally, manure management is a critical area of mitigation.
Technologies such as anaerobic digestion capture CH4 from manure and convert it into biogas, while composting and improved storage (e.g., frequent removal and aeration) reduce CH4 release during storage (Montes et al., 2013). Breeding and genetic selection present long-term, cumulative opportunities for CH4 mitigation. Selecting cattle with lower residual feed intake (RFI) enhances feed efficiency and is associated with reduced CH4 emissions per unit of feed consumed (Manzanilla-Pech et al., 2021). Studies have confirmed a strong association between RFI and methane production: efficient animals with low RFI typically consume less feed than expected for their body weight and growth rate, resulting in lower CH4 output (Nkrumah et al., 2006; Hegarty et al., 2007).
However, in dairy cattle, early lactation physiology complicates the use of RFI because cows in negative energy balance require high feed intake to prevent metabolic and fertility problems, which may increase herd-level CH4 intensity if not properly managed (Garnsworthy, 2004).
Evidence from quantitative genetics confirms that methane-related traits are heritable (h² = 0.12–0.3), enabling genetic improvement (Lassen and Løvendahl, 2016; Pszczola et al., 2019; Kamalanathan et al., 2023). Traditional measurement methods, such as respiration chambers, are accurate but impractical at scale. In contrast, GreenFeed systems, in-parlor sniffers, and milk mid-infrared (MIR) prediction models now enable scalable phenotyping, paving the way for genomic selection (Lassen and Løvendahl, 2016; Rojas De Oliveira et al., 2024b). For example, research on Canadian Holsteins has led to the development of a national genomic evaluation for CH4 efficiency using MIR-predicted data, which is expected to reduce herd-level methane emissions by 20–30% by 2050 without compromising milk yield (Rojas De Oliveira et al., 2024a). In another research, the sniffer method has been reported as a reliable approach for identifying Holstein cows with lower CH4 emissions. It can therefore serve as an indicator trait for genetic selection (Uemoto et al., 2024).
Residual methane emissions (RME), defined as the deviation between observed and expected methane output after adjusting for intake and body size, have emerged as promising breeding objectives because they capture inherent animal variation independent of productivity (Starsmore et al., 2024a). Smith et al. (2022) reported that RME is strongly associated with rumen microbiota composition, supporting its use as a robust phenotype for identifying inherently low-emission animals. Complementary host–microbiome studies indicate that both host genetics and microbial composition independently explain CH4 variation, suggesting synergistic opportunities for genetic and microbial interventions (Wallace et al., 2002; Difford et al., 2018). These findings further emphasize the potential of manipulating the rumen microbiota as a strategy to mitigate enteric CH4 production.
Emerging approaches include machine learning models, which integrate empirical and mechanistic data to improve CH4 prediction and phenotyping (Ross et al., 2024). Advanced genetic tools, such as genome-wide association studies (GWAS) and genomic selection, are being applied to identify low-emission genotypes, with the potential to breed animals that maintain production while reducing CH4 emissions (Pickering et al., 2015; Manzanilla-Pech et al., 2021). However, the realization of genetic gain is inherently slow, often requiring decades, and possible trade-offs with other traits (e.g., fertility, robustness, or feed efficiency) must be carefully monitored to ensure long-term sustainability (De Haas et al., 2011; Pickering et al., 2015; Gatenby, 2021). Given these limitations, genetic strategies should not be viewed in isolation but rather as part of an integrated mitigation framework. While genetic improvement provides permanent, cumulative reductions in CH4 emissions, the rate of progress is slow and dependent on long-term breeding programs. In contrast, management interventions—such as dietary modification, manure treatment, and optimized grazing—offer more immediate reductions in GHG. A combined approach, aligning rapid management-based gains with sustained genetic progress, is therefore essential to achieve both short-term emission reduction targets and long-term climate goals (Beauchemin et al., 2022).
3 Biological strategies
3.1 Bioaugmentation with homoacetogenic bacteria
One of the promising biological approaches is bioaugmentation with homoacetogenic bacteria (homoacetogens), which compete with methanogens for H2 in the rumen, thereby reducing CH4 emissions (Ungerfeld, 2020).
During ruminal fermentation, H2 and CO2 serve as the primary substrates for methanogens; methanogenesis acts as the main H2 sink, keeping dissolved H2 levels low (1–10 Pa), which is essential for maintaining efficient fermentation pathways (Kohn and Boston, 2000; Mackie et al., 2023; Fregulia et al., 2024).
Homoacetogens convert H2 and CO2 into acetate via the Wood–Ljungdahl pathway, offering an alternative electron sink to methanogenesis (Danielsson et al., 2012). However, the effectiveness of this approach depends on several factors, including rumen pH, substrate availability, and the ability of homoacetogens to establish and outcompete methanogens in the complex rumen ecosystem (Gagen et al., 2010).
According to Karekar et al. (2022) homoacetogens exhibit a versatile metabolism that is suitable for diverse substrates and can act as a carbon sink by converting CO2 into bioproducts, potentially improving efficiency by diverting H2 away from methanogenesis. However, their competitive advantage in mature rumen systems appears limited, as methanogens overwhelmingly dominate H2 utilization and suppress homoacetogenic activity. Experimental approaches that integrate methanogenesis inhibition—such as the use of 2-bromoethanesulfonic acid (BES)—with microbial bioaugmentation strategies have demonstrated promising potential for mitigating enteric CH4 production. For instance, in the study by Murali et al. (2021) BES treatment increased headspace H2 and reduced acetate; subsequent bioaugmentation with Acetitomaculum ruminis and Acetobacterium woodii restored acetate levels by 45% and 70%, respectively. Similarly, Stefanini Lopes and Ahring (2023) demonstrated that combining a kangaroo-derived homoacetogenic consortium with almond-shell biochar improved acetic acid production in vitro, albeit temporarily, highlighting transient benefits and the need for stabilization strategies.
Although homoacetogenesis is energetically less favorable than methanogenesis (Conrad, 2023) its competitiveness can be enhanced through strategies such as supplementing substrates like glucose, glycerol, and xylose, along with H2 and CO2, to leverage its mixotrophic advantages (Tsapekos et al., 2022). To enhance the viability of homoacetogenesis, strategies such as co-supplementation with acetogenesis stimulants (e.g., fumarate, malate, or nitrate) and optimizing feeding regimens have been explored (Morgavi et al., 2010). Additionally, genetic screening of ruminant microbiomes has identified novel homoacetogenic strains with greater resilience to rumen conditions, offering potential for further development (Henderson et al., 2015).
Additional measures include the introduction of acetogenesis stimulants, such as yeast cultures, maintaining a lower ruminal pH, and identifying novel acetogen strains capable of thriving at low H2 thresholds and increasing their densities in the rumen (Yang et al., 2015).
Propionate-producing bacteria, along with nitrate- and nitrite-reducing, and sulfate-reducing bacteria, have thermodynamic advantages over methanogens in utilizing H2 as an electron donor (Lan and Yang, 2019). However, their low abundance or the absence of necessary substrates in the rumen limits their activity (Choudhury et al., 2022). Enhancing the propionate-producing pathway can be achieved by supplementing animals with propionate precursors such as fumarate and malate or introducing functionally complementary propionate-producing bacterial consortia as additives (Jeong et al., 2024). Given the low natural concentrations of nitrate and sulfate in the rumen, using these compounds as additives could stimulate the growth of nitrate- and sulfate-reducing bacteria. However, toxic by-products such as nitrite and hydrogen sulfide (H2S) must be carefully managed (Latham et al., 2016). Strategies to mitigate toxicity risks include combining sulfate-reducing bacteria (SRB) with nitrate-reducing, sulfur-oxidizing bacteria or employing SRB strains capable of utilizing H2S or nitrite (Greene et al., 2003).
Exploring microbes that compete with methanogens and redirect H2 away from methanogenesis presents a promising strategy for reducing CH4 emissions in the rumen (Lan and Yang, 2019). Despite its potential, bioaugmentation with homoacetogenic bacteria faces challenges, including the need for long-term microbial stability in the rumen and variations in host responses across different animal species. Large-scale field trials are necessary to evaluate the long-term feasibility and effectiveness of this approach under commercial farming conditions (Wallace, 2004). Future research should focus on strain selection, microbial adaptation strategies, and possible synergies with other methane mitigation technologies to improve implementation (Martin et al., 2010).
3.2 The use of bacteriophages
Bacteriophages (phages), traditionally applied in phage therapy to treat bacterial infections such as enteric diseases, sepsis, and chronic infections (Lin et al., 2017), are gaining attention for broader roles, including food preservation, microbiome modulation, and even environmental applications like climate change mitigation (Elois et al., 2023). Recently, phage therapy has been proposed as a novel strategy to target methanogenic archaea in the rumen to reduce enteric CH4 production (Lobo and Faciola, 2021). By selectively lysing methanogens, phages may suppress methane formation without significantly disturbing other rumen microbial populations (Morkhade et al., 2020).
The conceptual appeal of phage-based CH4 mitigation lies in its specificity, ecological safety, and potential to bypass some of the limitations associated with chemical inhibitors or vaccines. However, this strategy remains in its infancy, and several critical challenges must be addressed.
To date, only a limited number of studies have investigated the isolation and characterization of archaeal phages that target rumen methanogens. For example, Ouwerkerk et al. (2011) initiated the development of a phage library specifically targeting the dominant methanogenic archaea in Australian livestock systems. However, experimental evidence on the in vivo efficacy of such phages remains limited. The effectiveness of archaeaphage therapy mainly relies on the ability to identify highly specific phages that can infect predominant methanogenic species—such as Methanobrevibacter ruminantium and Methanobacterium spp.—without disrupting beneficial rumen microbial functions (Lobo and Faciola, 2021). Despite their potential, the identification of archaeal phages remains limited, underscoring a substantial knowledge gap in our understanding of phage-host interactions within methanogenic communities. Among fully sequenced microbial genomes, six archaeal phages have been described, including Methanobacterium phage psi M1, Methanobacterium phage psi M2 (a variant of M1), and Methanobacterium phage psi M100, all of which belong to the Siphoviridae phage family. These phages demonstrate the capacity to infect key rumen methanogens such as Methanobacterium spp., a dominant archaeal genus in the rumen. Moreover, members of the Siphoviridae family have shown infectivity toward Methanobacterium, Methanobrevibacter, and Methanococcus species (Mcallister and Newbold, 2008). Leahy et al. (2013) presented the complete genome sequence of the rumen methanogen Methanobrevibacter ruminantium M1, offering critical insights into its metabolic and cellular pathways. A prophage identified in M. ruminantium encodes 69 phage-related proteins, including the lytic enzyme PeiR from prophage φMru, which shows potential as a biocontrol agent against ruminal methanogens. A novel approach was proposed, utilizing viral enzyme-loaded nanoparticles that effectively lyse not only the original methanogen host strain but also a diverse range of ruminal methanogen species in pure in vitro cultures, resulting in significant CH4 reductions of up to 97% (Altermann et al., 2018). However, this broad-spectrum activity raises concerns about potential disruption to the natural rumen microbial ecosystem.
Rumen phage populations are highly diverse and individualized, with concentrations ranging from 107 to 109 particles per milliliter (Swain et al., 1996). This high diversity, coupled with host-specific microbial interactions, raises concerns about the stability, persistence, and consistent efficacy of introduced phages within the rumen ecosystem. To date, no study has comprehensively identified the phage taxa present in the rumen and their specific archaeal hosts, nor has it assessed their interactions with the methanogen community at a large scale. These knowledge gaps underscore a critical barrier to the development of phage-based CH4 mitigation strategies in ruminants, highlighting the need for advanced metagenomic and host-linkage studies to inform future applications.
3.3 Use of antimethanogenic vaccines
One proposed strategy to mitigate CH4 emissions is the development of vaccines targeting methanogenic archaea in the rumen. These vaccines aim to elicit an immune response that reduces methanogen populations, thereby lowering methane production without adversely affecting essential microbial communities in the rumen (Wedlock et al., 2013). Developing an effective methane-reducing vaccine requires identifying immunogenic proteins unique to methanogens to ensure a robust immune response while maintaining overall gut health (Baca-González et al., 2020). Research indicates that vaccines targeting key methanogen species can significantly alter rumen archaeal populations, leading to a measurable reduction in methane emissions (Williams et al., 2009). However, long-term efficacy remains a critical challenge, as the rumen microbiome is highly dynamic and capable of adapting to immune pressures over time (Wedlock et al., 2010).
In vivo (Wright et al., 2004; Zhang et al., 2015), and in vitro (Cook et al., 2008) studies evaluating antimethanogenic vaccines have reported variable and often time-dependent effects on enteric CH4 production. Notably, the lack of a consistent reduction in CH4 emissions—despite increased methanogen-specific antibody titers and observed shifts in archaeal community composition—suggests that vaccine formulations may lack broad-spectrum efficacy against the diverse rumen methanogen populations (Williams et al., 2009). Moreover, population-level differences in immune responses across species and breeds introduce high inter-animal variability, complicating the predictability and scalability of vaccine interventions (Buddle et al., 2011). One of the major limitations in the development of antimethanogenic vaccines is the challenge of identifying antigens that are both conserved and immunogenic across the diverse array of methanogenic archaea present in the rumen. Methanogens exhibit high variability in surface structures and protein epitopes (Reeve, 1992), which complicates the formulation of a broadly protective vaccine. In addition, variation in host immune response—driven by genetic background, physiological status, and rumen microbiota composition—leads to inconsistent antibody production and limited uniformity in microbial suppression. Some animals exhibit high antibody titers with negligible impact on archaeal populations or methane output, while others respond poorly to vaccination protocols. These issues have been reported in both dairy and sheep trials and represent key barriers to reliable implementation (Wedlock et al., 2013; Subharat et al., 2016). Another source of variation is animal age, as it is well-known that young animals are more susceptible to infectious diseases than adults (Watson et al., 1994). Moreover, the durability of the immune response and the potential for microbial adaptation or vaccine escape remain unresolved. Further research is needed to identify robust antigen targets and optimize delivery systems that can consistently elicit long-term methane mitigation across diverse ruminant populations.
Despite these constraints, vaccination remains a promising and potentially cost-effective approach for mitigating methane emissions. It offers practical advantages, particularly for grazing systems with limited access to feed additives. However, successful implementation will require optimized antigen discovery, improved delivery systems (e.g., oral or slow-release formulations), and robust field trials to assess long-term impacts on CH4 emissions, animal performance, and microbial ecology (Baca-González et al., 2020).
The advantages and challenges of biological strategies for reducing methane emissions from ruminants are presented through a SWOT analysis, which is presented in Table 3.
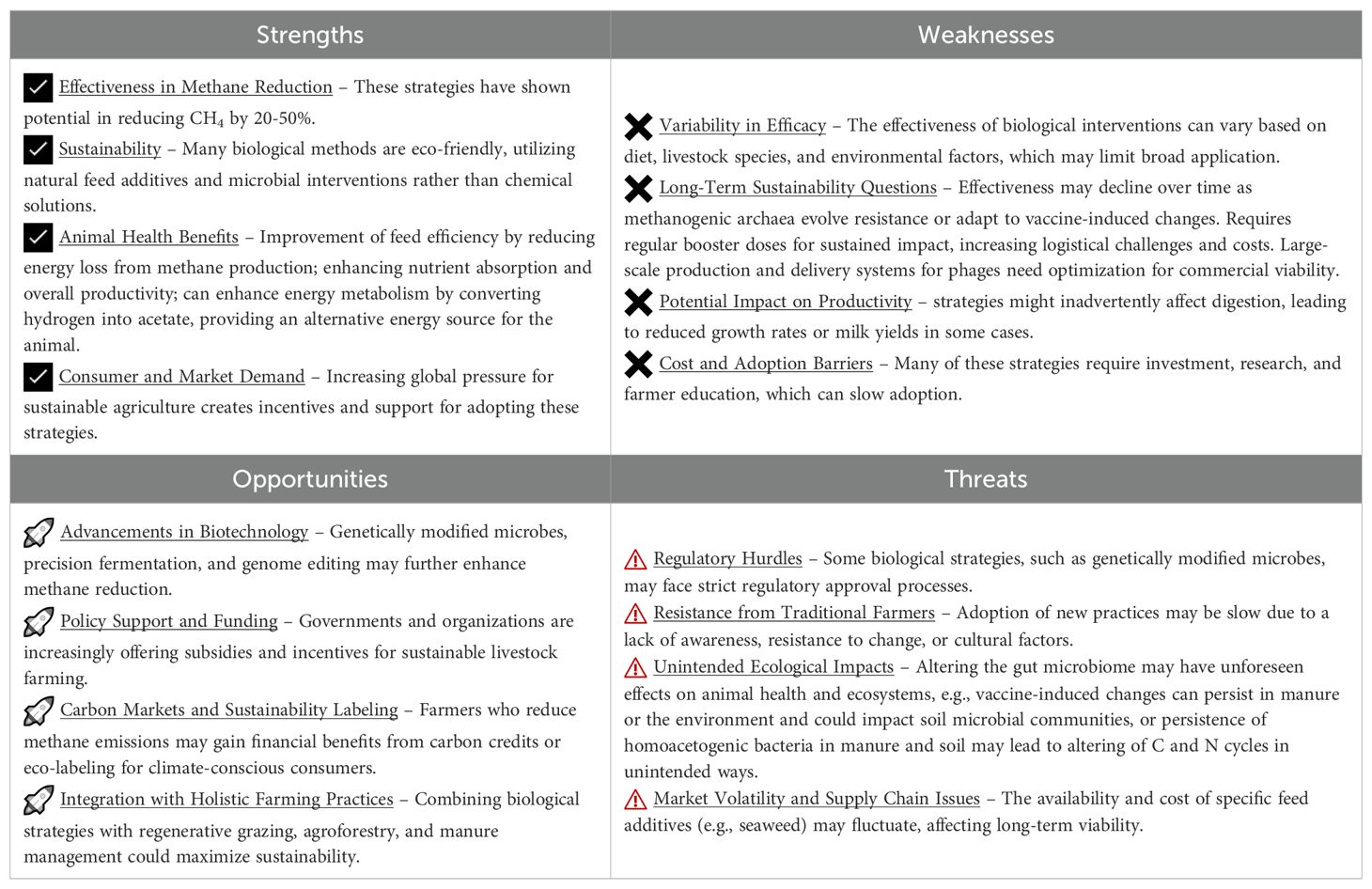
Table 3. SWOT analysis of biological strategies for reducing methane emissions in ruminant livestock.
4 Nutritional strategies
Enteric methane (CH4) represents both an energetic loss and a significant contributor to agricultural greenhouse gas emissions, produced predominantly via ruminal microbial fermentation and closely associated with dry matter intake (DMI) (Hornbuckle and Tennant, 1997; Dressler et al., 2024).
Nutritional strategies to mitigate CH4 emissions primarily focus on redirecting hydrogen (H2) toward alternative sinks and improving carbohydrate fermentability. Increasing the digestibility of non-structural carbohydrates (starch, sugars) shifts rumen fermentation toward propionate—the main competing H2 sink—thereby lowering CH4 yield, whereas structural carbohydrates favor acetate production and methanogenesis (Morgavi et al., 2010; Beauchemin et al., 2022). Key interventions include starch processing (e.g., steam-flaking, fine grinding), which enhances ruminal starch availability and reduces CH4 emissions relative to whole grain; controlled use of rapidly fermentable sugars, with variable effects; and improvements in fiber digestibility through particle size reduction or exogenous fibrolytic enzymes (Johnson et al., 1994; Tavendale et al., 2005; Beauchemin and Mcginn, 2006; Mcallister and Newbold, 2008; Benchaar et al., 2014).
Forage selection also plays a critical role: replacing grass or legume silages with corn silage, which has higher non-fiber carbohydrate (NFC) content, consistently reduces CH4 yield and intensity. Similarly, high-sugar grasses and energy-dense roughages can further mitigate emissions (Soteriades et al., 2018; Sun et al., 2022). Research by Hristov (2024) suggests that the type of roughage in the diet influences CH4 production. When comparing corn silage with legume silage, methane emissions were either unchanged or slightly reduced with corn silage. Furthermore, replacing grass silages with corn silage resulted in a 9–16% reduction in CH4 yield and a 6% decrease in CH4 intensity. In total mixed rations (TMR) with a higher proportion of grass silage, methane reductions were more modest, typically reaching up to 4%. These findings highlight the potential of corn silage as a viable approach for reducing CH4 emissions in ruminant diets.
Complementary feed additives such as 3−nitrooxypropanol (3−NOP) and bromoform−rich red seaweed extracts have demonstrated enteric CH4 reductions in the range of ~30–50%, with red seaweed (e.g., Asparagopsis spp.) occasionally delivering up to ~80% in experimental settings (3−NOP: ~30–45%; Asparagopsis average ~37%, maxima ~98%) (De Bhowmick and Hayes, 2023; Romero et al., 2023; Hristov, 2024; Meo-Filho et al., 2024). While integrated nutritional strategies, especially when combined with manure-management technologies, hold theoretical potential for aggregate reductions approaching ~60%, empirical data from combined enteric-plus-manure mitigation rarely reach this level under current commercial conditions (Hristov, 2024).
These cumulative findings underscore the critical role of diet composition and additive strategies in reducing enteric methane emissions, setting the stage for emerging approaches—such as algal supplementation—that offer targeted biochemical mechanisms and potentially greater mitigation efficacy under specific production contexts.
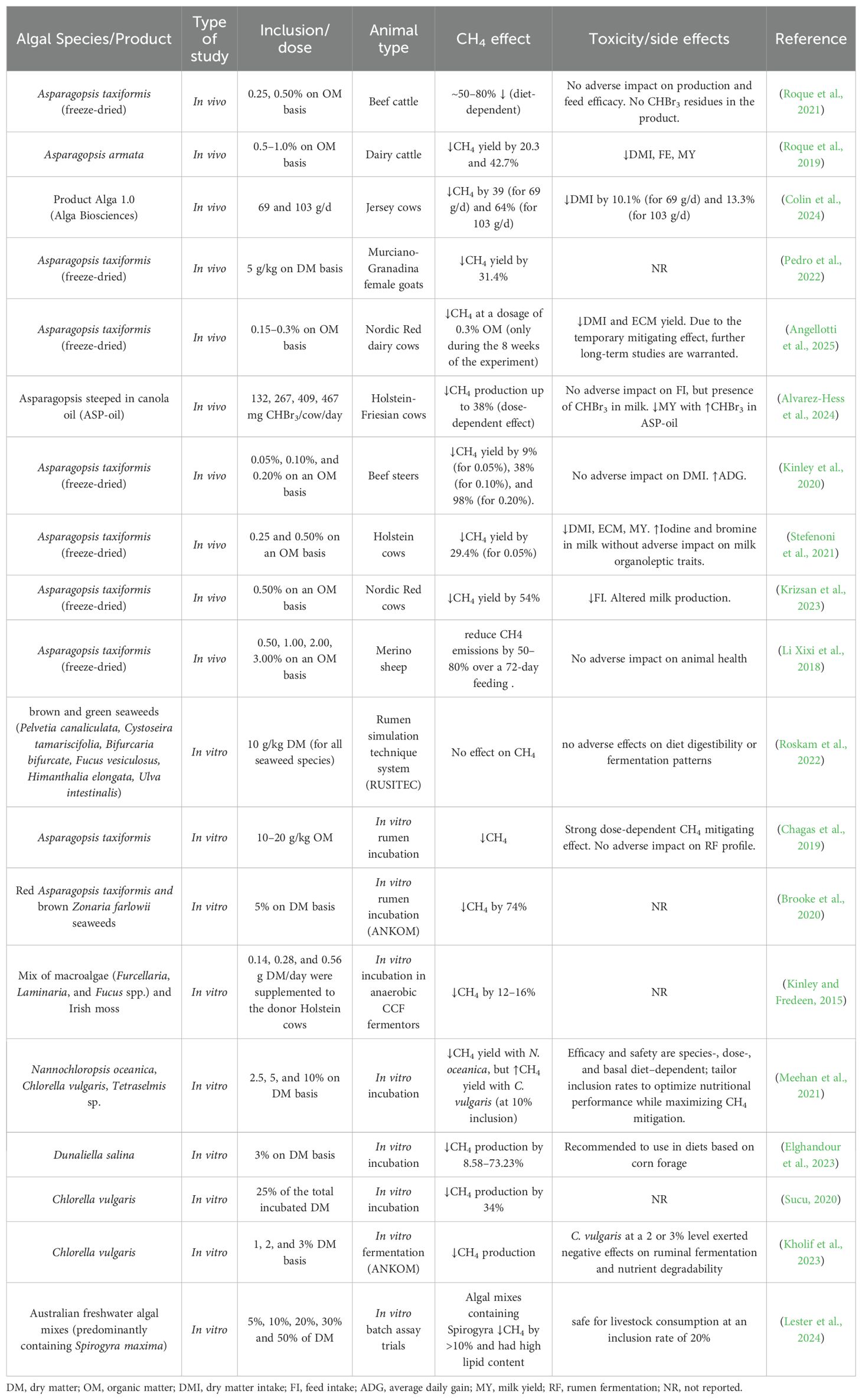
4.1 Algae
Algal biomass is increasingly positioned as a sustainable, circular feed ingredient with the potential to lower the carbon footprint of ruminant production. Beyond serving as a high-quality nutrient source, specific macro- and microalgal taxa contain bioactive compounds that modulate rumen microbiology and hydrogen sinks, thereby holding high potential for enteric CH4 mitigation. Recent reviews highlight both the promise and the practical constraints (supply, processing costs, and standardization) associated with scaling algae for livestock systems (De Bhowmick and Hayes, 2023; Wanapat et al., 2024).
The summary report of the literature analysis on the effects of supplementing ruminant diets with probiotic bacteria is presented in Table 4.
Among seaweeds, red macroalgae of the genus Asparagopsis remain the most potent enteric CH4 mitigation option in vivo. Multiple trials in beef cattle have demonstrated substantial reductions when A. taxiformis is included at low dietary levels, with reported decreases often exceeding 50% and, in some cases, approaching 80%, depending on the diet composition and inclusion rate. The primary mechanism involves the inhibition of the methyl-coenzyme M reductase (MCR) pathway by halogenated methane analogs—especially bromoform (CHBr3)—which suppresses the terminal step of methanogenesis (Thorsteinsson et al., 2023; Kelly et al., 2025).
Efficacy varies with species, dose, basal diet, and type of supplement used in the study (freeze-dried biomass vs. stabilized actives) (Alvarez-Hess et al., 2024). In a finishing-diet research study, a proprietary bromoform-containing algae product (“Alga 1.0”) fed at 69 or 103 g/d reduced methane yield by 39% and 64%, respectively, without affecting digestibility but decreasing DMI by ~10–13%. These data underscore the trade-off between mitigation and intake that may emerge at higher effective doses (Colin et al., 2024).
Safety and residue outcomes are an active area of research. Transfer of CHBr3 to milk and urine has been detected under certain conditions in dairy cows fed Asparagopsis. However, tissue accumulation was not observed, and excretion appeared transient in that study. Additionally, some trials with Asparagopsis armata at 0.5–1.0% of dietary OM in dairy cows reduced CH4 yield but also lowered DMI, highlighting the need for careful dosing and monitoring of animal performance and product quality (including iodine/halogen load) (Muizelaar et al., 2021). Similarly, in dairy cows, supplementation with A. taxiformis at 0.3% of dietary OM reduced enteric CH4 emissions by ~30% during the first 8 weeks, with no sustained effect from week 9 to 12. This inclusion level also led to reductions in DMI (~7%) and ECM (~2%), shifts in VFA profiles (↓acetate; ↑propionate, butyrate, valerate), and elevated concentrations of bromine and iodine in milk (5-fold and 9-fold higher than controls, respectively), highlighting the need for long-term evaluation of efficacy, safety, and product integrity (Angellotti et al., 2025).
By contrast, brown and green seaweeds generally lack halomethanes at higher levels; their antimethanogenic potential is less consistent and often modest. For example, bromoform-free brown/green species included at 10 g/kg diet DM did not reduce CH4 in RUSITEC tests, whereas metabolomics indicate these taxa contain phenolics (e.g., phlorotannins) and other sulfated compounds that could influence fermentation. Species, season, and geography contribute to pronounced chemical variability (Nørskov et al., 2021; Roskam et al., 2022).
Several microalgae and cyanobacteria have shown methane-mitigating potential—though, to date, none match Asparagopsis in vivo. In vitro study comparing Chlorella vulgaris, Tetraselmis spp., and Nannochloropsis oceanica found the lowest CH4 yield with N. oceanica at 10% of incubated DM, likely linked to its high n-3 PUFA content (Meehan et al., 2021). Likewise, Dunaliella salina, when used as an additive with maize forages, lowered biogas/CH4 kinetics without compromising fermentation characteristics (Elghandour et al., 2023).
In vivo findings are mixed and context-dependent. Some studies report that Chlorella can increase methanogenic archaea and protozoa in goats, whereas others (including associative feeding strategies with low-level Chlorella) suggest potential to improve fermentation while decreasing CH4 (Tsiplakou et al., 2017; Kholif et al., 2023). Cyanobacteria Spirulina (Arthrospira spp.) is widely used as a protein/antioxidant supplement. Across small-ruminant studies, Spirulina supplementation has been shown to modulate the rumen microbiome, but it yields inconsistent methane responses. In lactating goats, ≈1% of diet DM—especially when combined with live yeast—lowered Methanobrevibacter prevalence and predicted CH4, though effects were small-scale (Emara Rabee et al., 2025). In ewes, graded doses of methanogen inhibitors shifted community structure without reducing total methanogens, and Methanobrevibacter tended to increase at the highest inclusion rate (Christodoulou et al., 2023). In lambs, ~3% (fresh-weight basis) of the altered microbiota did not produce consistent enteric CH4 outcomes (Wang et al., 2024b). Collectively, Spirulina may influence archaeal ecology at low inclusion rates, yet robust, controlled trials are needed to clarify its effects on CH4 emissions.
Microalgal feed supplements appear to modulate rumen fermentation and H2 disposal pathways (e.g., favoring propionate or microbial lipid sinks); however, the magnitude of CH4 suppression is typically lower than that achieved with Asparagopsis.
Collectively, the literature supports algae as a diverse toolbox for enteric methane abatement. Asparagopsis (via bromoform) delivers the most considerable and reproducible reductions—especially in high-concentrate systems—while brown/green macroalgae and microalgae offer nutritional value and modest, formulation-dependent CH4 mitigation. Critical research gaps include: (1) scalable, cost-efficient cultivation and processing for consistent bioactive content; (2) long-term animal health and product-quality surveillance (residues, iodine/halogens); (3) delivery formats that sustain efficacy without depressing intake; and (4) robust performance data in pasture-based and dairy systems.
4.2 Biochar supplementation for enteric methane mitigation
Biochar (BH) has garnered increasing interest as a potential CH4 mitigation agent in ruminant nutrition due to its high surface area, porosity, and adsorptive capacity, which may modulate rumen fermentation and microbial dynamics. Proposed mechanisms include altering microbial habitats, reducing hydrogen availability for methanogenesis, and promoting the proliferation of alternative hydrogen-utilizing microbes (Leng et al., 2013; Saenab et al., 2018). However, evidence for its effectiveness remains inconsistent across studies (Winders et al., 2019; Sperber et al., 2022).
In a recent two-phase study in beef cattle, supplementation with tailored (“fit-for-purpose”) biochars yielded modest reductions in CH4 emissions (8.8–12.9%) under controlled pen conditions. Still, no effect was observed under grazing systems, highlighting a disconnect between controlled trials and practical field application (Martinez-Fernandez et al., 2024). Similarly, in dairy cattle, a Latin square trial revealed that neither biochar nor biochar–urea blends affected CH4 emissions or productive performance (Terler et al., 2023), while supplementation at 1% DM in lactating Holsteins also yielded no benefits (Dittmann et al., 2024). A study in lambs found no favorable effects on CH4 production or growth, both in vitro and in vivo (Lind et al., 2024). Additionally, mineral-enriched biochar failed to elicit any changes in CH4 or rumen fermentation in Holstein steers (Ni et al., 2024). By contrast, an in vivo study in ewes reported improved feed efficiency and reduced CH4 emissions with biochar supplementation (Burezq and Khalil, 2025), indicating that host species, diet type, and biochar formulation may all influence response. This inconsistency likely stems from differences in pyrolysis conditions, feedstock type, particle size, and chemical composition of the biochar used. Smaller particle sizes and acidic pH have been associated with greater CH4 mitigation (Zhou et al., 2017; Osman et al., 2022), while the presence of phenolic compounds may exert additional antimicrobial effects. A recent quantitative review confirmed the modest average efficacy of biochar across studies but emphasized the substantial heterogeneity and lack of dose–response consistency, calling for standardization in biochar production and application protocols (Pepeta et al., 2024).
Overall, while biochar shows mechanistic potential as a CH4 mitigation tool, primarily through indirect modulation of ruminal hydrogen metabolism, current in vivo evidence does not yet support its broad implementation in commercial livestock systems. Future work should focus on defining optimal biochar types, inclusion levels, and diet contexts, as well as the possible synergistic effects with other mitigation agents.
4.3 Garlic
Garlic (Allium sativum) and its organosulfur compounds—such as allicin, diallyl sulfide, diallyl disulfide, and allyl mercaptan – have attracted attention as natural feed additives for mitigating enteric CH4 emissions in ruminants. These compounds exhibit antimicrobial activity against methanogenic archaea and rumen protozoa and have been shown to alter fermentation profiles by promoting propionate production, thereby redirecting H2 away from methanogenesis (Shang et al., 2019; Sari et al., 2022).
However, the efficacy of garlic-based interventions appears highly variable. It is influenced by multiple factors, including the specific compound used, its concentration and stability, the delivery matrix (e.g., oil, extract, powder), and interactions with the basal diet (Kamel et al., 2008; Sari et al., 2022).
Recent in vivo evidence supports the methane-reducing potential of garlic-derived products under controlled and grazing conditions. In a respiration chamber study with mid-lactation dairy cows, supplementation with a garlic–citrus extract over 18 days reduced CH4 production (−10.3%), intensity (−11.7%), and tended to lower CH4 yield (−9.7%) without affecting dry matter intake or milk yield. Propionate concentrations increased, while Methanobrevibacter abundance declined. Similarly, under grazing conditions, daily supplementation of 33 g/cow of GCE over 12 weeks improved DMI and ECM yield. This led to an 8.39% reduction in milk GHG intensity, as determined by a life cycle assessment, although CH4 was not directly quantified in the study (Khurana et al., 2024).
Meta-analyses and recent reviews have emphasized the heterogeneity in response to garlic supplementation, highlighting formulation sensitivity as a key factor influencing efficacy (Shang et al., 2019; Sari et al., 2022a, Ding et al., 2023; Martin and Chaudhry, 2024). Several studies demonstrated that garlic products provide a range of biological benefits to ruminants (Ogbuewu et al., 2019; Yang et al., 2021). While garlic-based products offer a promising natural approach to CH4 mitigation, especially at practical inclusion levels that do not compromise intake or animal performance, their persistence and repeatability under commercial conditions remain uncertain.
In summary, garlic and its bioactive constituents have demonstrated potential for mitigating CH4 through both direct inhibition of methanogens and fermentation shifts that favor propionate production. However, the success of such strategies depends heavily on compound selection, dosing, delivery method, and dietary context. Long-term, multi-period in vivo studies are needed to confirm sustained efficacy, evaluate adaptation, and guide the development of commercially viable formulations.
4.4 Tannins
Tannins—classified as condensed (CT) or hydrolyzable (HT) based on their chemical structure—are among the most widely studied plant secondary compounds for enteric CH4 mitigation in ruminants. Their antimethanogenic effects are attributed to multiple mechanisms, including suppression of protozoa and associated methanogens, shifts in VFAs production (typically characterized by reduced acetate and increased propionate), and complexation with dietary proteins and carbohydrates, which can reduce H2 availability for methanogenesis (Patra and Saxena, 2011; Goel and Makkar, 2012). The extent of mitigation depends heavily on the type of tannin, the botanical source, the inclusion rate, and the adaptation period.
A comprehensive meta-analysis by Jayanegara et al. (2012) covering both in vitro and in vivo data confirmed an apparent, dose-dependent reduction in CH4 emissions, particularly with CT sources. More recently, a systematic review by Cardoso-Gutierrez et al. (2021) focused on tropical forages and reported consistent CH4 suppression across multiple studies. However, the magnitude of reduction was highly variable and linked to the specific plant species and dosage employed. Goel and Makkar (2012) highlighted that CT mitigates CH4 primarily via indirect mechanisms, such as reducing fiber digestion and thus limiting H2 availability. In contrast, HT appear to exert more direct antimethanogenic effects by inhibiting the growth and activity of methanogens and hydrogen-producing microbes. Animal-level studies further demonstrate the complex and dose-dependent impacts of tannin supplementation on CH4 mitigation and animal productivity. In dairy goats, stepwise inclusion of quebracho-derived condensed tannins (CT; 0–6% of diet DM) elicited non-linear responses, with milk yield peaking at approximately 4% CT, beyond which diet digestibility declined and effects on methane emissions became inconsistent (Battelli et al., 2024). Similarly, dietary inclusion of hydrolyzable tannins (HT) has been associated with improvements in milk yield and udder health, further supporting their utility in dairy systems (Ali et al., 2017). In an earlier in vivo study, Beauchemin et al. (2007a) reported a 14% reduction in CH4 emissions following dietary supplementation with Quebracho tannin extract, accompanied by a shift in VFA production toward propionate, a competitive H2 sink. Comparable results were observed by Grainger et al. (2009) who supplemented condensed tannins from Lotus pedunculatus and reported up to 29% CH4 reduction without adverse effects on dry matter intake or animal productivity.
In vitro investigations support the potential of forage-derived tannins. For example, purified CT extracts from Hedysarum coronarium (sulla) and Lotus corniculatus (big trefoil) decreased CH4 production by up to ~15% at inclusion rates of 30 g/kg DM. However, gas production and fermentation efficiency were negatively affected at the highest levels (Verma et al., 2023). These findings underscore the importance of optimizing tannin inclusion levels to mitigate undesirable effects on rumen fermentation and animal productivity.
In summary, tannins represent a viable strategy for mitigating enteric CH4 emissions in ruminants, particularly when their use is aligned with dietary context and production objectives. Low-to-moderate inclusion levels (<3–4% of diet DM) have been shown to reduce CH4 output without adversely affecting animal performance; however, higher doses may impair nutrient digestibility and feed efficiency. Effective formulation requires careful consideration of tannin type (condensed vs. hydrolyzable), bioactivity, and interactions with the basal diet to ensure sustained mitigation and production efficiency.
In addition, key knowledge gaps remain regarding the mechanisms by which tannins reduce methanogenesis, including their effects on nutrient utilization, direct inhibition of methanogens, suppression of protozoa, and modulation of hydrogen sinks within the rumen environment. Addressing these uncertainties through targeted in vivo research will be essential to optimizing tannin-based strategies for practical application.
4.5 Saponins
Saponins—diverse glycosides abundant in legumes and tropical plants—are recognized for their antiprotozoal and antimicrobial properties (Patra and Saxena, 2009; Goel and Makkar, 2012). By suppressing rumen protozoa—key partners of methanogenic archaea—saponins diminish hydrogen transfer to methanogens, thereby reducing CH4 formation. They also act directly against methanogens, shifting fermentation toward propionate production —a competitive hydrogen sink (Hristov et al., 2013; Pen et al., 2006; Patra and Saxena, 2009; Firkins and Mitchell, 2023). Commercial saponin sources such as Yucca schidigera and Quillaja saponaria are well-characterized: QS contains ~10% triterpenoid saponins across 20+ structures, while YS offers ~4.4% steroidal saponins spanning 28 variants (Kholif, 2023). Other promising sources include Sapindus saponaria, which exhibits potent antiprotozoal activity (Hu et al., 2018), and fenugreek (Trigonella foenum-graecum), notable for its high saponin content (~4.63 g per 10 g) and potential antimethanogenic action (Singh and Garg, 2006; Visuvanathan et al., 2022).
In vitro, S. saponaria fruit extracts (100 mg/g) significantly decreased CH4 without impairing fermentation. At the same time, inclusion of its seed pericarp reduced protozoa and improved weight gain in sheep, though CH4 was not measured (Navas-Camacho et al., 2001; Hess et al., 2003). Fenugreek extracts also inhibited total gas and CH4 production and shifted VFAs toward propionate in vitro (Dey, 2015; Niu et al., 2021), while improving nitrogen utilization without affecting intake or digestibility (Wina et al., 2005).
Although saponins exhibit considerable potential to reduce enteric methane emissions across a range of inclusion levels, thereby supporting environmentally sustainable ruminant nutrition (Ridla et al., 2021). Evidence suggests that their effects may not be consistently sustained over time. Several long-term in vitro studies have indicated that the methane-suppressing effects of certain saponin extracts on rumen microbial fermentation may be transient rather than permanent (Wang et al., 1998; Cardozo et al., 2004). This attenuation may be partly explained by microbial adaptation, as rumen microbes can adjust to repeated exposure to bioactive compounds such as saponins (Makkar and Becker, 1997; Wallace et al., 2002).
However, in vivo responses to saponin supplementation remain inconsistent. For instance, supplementation of whole-plant Yucca schidigera or Quillaja saponaria at 10 g/kg DM failed to reduce CH4 emissions in lactating dairy cows (Holtshausen et al., 2009), while lower-dose inclusion in sheep yielded only numerical reductions (Pen et al., 2007). Similarly, in dairy goats, supplementation with fenugreek seeds at 0.1 kg/d had no significant impact on milk yield or health status (El-Tarabany et al., 2018; Akbağ et al., 2022). By contrast, substantial CH4 reductions of 28%, 35.8%, and 47.9% were observed in sheep supplemented with tea seed saponins at 5, 10, and 20 g/kg DM, respectively (Zhang et al., 2021), highlighting the role of the botanical source and dose in determining efficacy. Beyond ruminant systems, low-level inclusion of fenugreek (0.04%) has demonstrated benefits in aquaculture species—improving growth, antioxidant capacity, and immune function (Yu et al., 2019; Abdel-Wareth et al., 2021; Yang et al., 2022; Paneru et al., 2022), indicating the broader applicability of saponins across animal production systems. A recent meta-analysis encompassing 66 in vivo treatments (up to 40 g/kg DM) revealed no adverse effects on feed intake; however, the effects on productivity and fermentation were highly variable and dependent on the plant source, animal species, and dietary context (Yanza et al., 2024).
These findings underscore the need for additional long-term, species-specific studies to better understand the persistence of saponin-induced CH4 mitigation and to refine supplementation strategies for practical livestock systems.
The summary report of the analysis of literature data on the effects of supplementation of ruminant diets with garlic, tannins, or saponins is shown in Table 5.
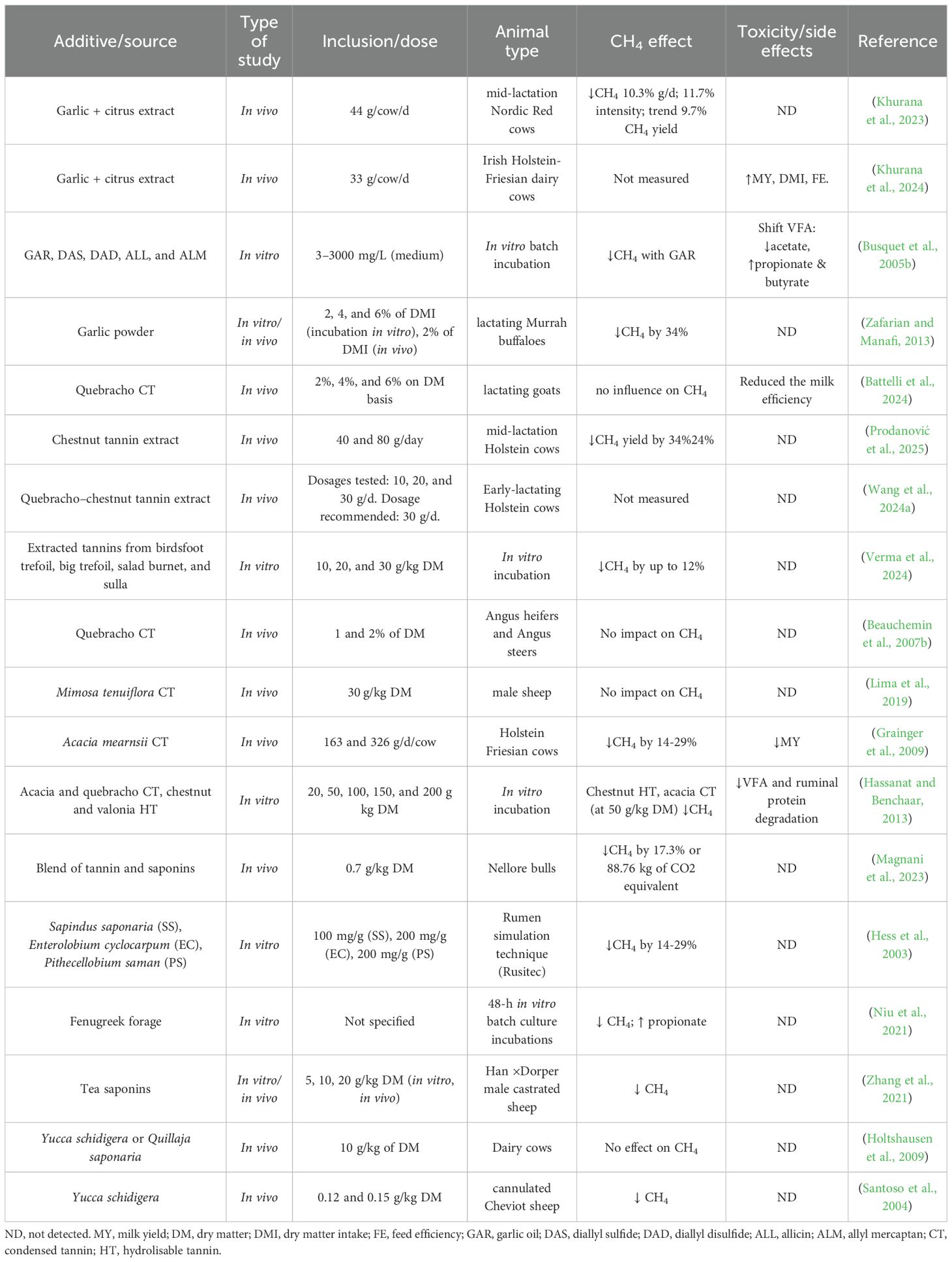
Table 5. Observations from different articles reporting effects of garlic, tannins, and saponins on enteric CH4 mitigation.
4.6 Essential oils as natural methane mitigation agents
Essential oils (EOs) are plant-derived volatile compounds with antimicrobial properties that have been explored as natural feed additives to mitigate enteric CH4 emissions in ruminants. Their effects are attributed to the modulation of rumen microbial communities, the inhibition of methanogens and protozoa, and alterations in fermentation profiles (Castillejos et al., 2005; Calsamiglia et al., 2007; Patra and Yu, 2012). Compounds such as thymol, eugenol, carvacrol, cinnamaldehyde, and flavonoids (e.g., naringin, hesperidin) have demonstrated methane-reducing potential in both in vitro and in vivo systems (Busquet et al., 2005a; Patra and Yu, 2015; Yu et al., 2024).
In vitro studies report CH4 reductions ranging from 10% to 91%, depending on EO type, dose, and microbial sensitivity (Busquet et al., 2006; Cobellis et al., 2016). For example, garlic oil constituents—diallyl disulfide and allyl mercaptan—reduced CH4 production by up to 74% in batch cultures (Busquet et al., 2005a), high-carvacrol oregano oil reduced methane by 22% at 1000 mg/L, although with concurrent suppression of VFA production and feed digestion (Benchaar and Hassanat, 2024).
Similarly, citrus flavonoids (naringin and hesperidin, each at 10 g/kg DM) or citrus flavonoid extract (20 g/kg DM) significantly reduced CH4 and ammonia concentrations in vitro, alongside declines in archaea Methanobrevibacter spp. and protozoa Isotricha spp. populations (Yu et al., 2024). The authors suggest that flavonoids may possess synergistic effects in mitigating ruminal CH4 and have the potential to enhance N utilization. Using the rumen simulation technique (RUSITEC), Soliva et al. (2011) reported a 91% reduction in daily CH4 emissions, accompanied by a decrease in protozoal counts and an increase in total bacterial populations, highlighting the strong methane-mitigating potential of the garlic oil under controlled in vitro conditions. In another in vitro study, five essential oils—clove, eucalyptus, garlic, oregano, and peppermint – reduced CH4 production by 34.4%, 17.6%, 42.3%, 87.0%, and 25.7%, respectively, at 1.0 g/L, with oregano oil showing the most significant CH4 inhibition (Patra and Yu, 2012).
In vivo, results have been inconsistent. Agolin® Ruminant (a commercial EOs blend) reduced CH4 emissions by 8.8%, improved milk yield by 4.1%, and enhanced feed efficiency by 4.4% in lactating dairy cows (Belanche et al., 2020). A carbon footprint modelling study confirmed a 6% reduction in GHG emissions across several feeding strategies (Becker et al., 2023). However, Benchaar and Hassanat (2025) found no effect of the same blend (1 g/day) on lactational performance or CH4 output in dairy cows. Castro-Montoya et al. (2015) reported a 15% CH4 reduction after 6 weeks of supplementation with 0.2 g/d of Agolin® Ruminant in dairy cows. Interestingly, no significant changes were seen in beef heifers supplemented with the same dose.
Conversely, several studies have reported inconsistent or non-significant effects of essential oil supplementation on CH4 mitigation and animal performance. For example, an EOs blend of cresols, thymol, limonene, vanillin, eugenol, and salicylates (1.2 g/day) did not confer any measurable benefits in mid-lactation Holstein dairy cows in terms of CH4 mitigation, lactational performance, or rumen fermentation parameters (Joch et al., 2019). Likewise, Jiménez-Ocampo et al. (2021) demonstrated CH4 reductions with 1.5 g/kg DMI of naringin and chitosan in in vivo trials. However, in situ tests using the same doses (1.5–3.0 g/kg DMI) showed no significant changes in CH4 or nutrient use. Supplementation with eucalyptus and anise oils at 0.5 g/animal/day in sheep had no significant effect on methane production (Wang et al., 2018). An in vitro experiment using rumen inoculum from Daragh ewes demonstrated that sage, pine, and clove EOs at 300–900 mg/L led to dose-dependent CH4 suppression and improved the ruminal fatty acid profile (Bokharaeian et al., 2023).
These contrasting findings underscore the complexity of host–additive interactions and suggest that the delivery method, dosage, and microbial adaptation may have a significant influence on experimental results.
Recommended effective doses for CH4 mitigation typically range from 20 to 1000 mg/L in vitro and 500 to 1000 mg/day in vivo. However, high doses may impair fibre digestion and reduce feed intake (Cobellis et al., 2016; Joch et al., 2019). Long-term exposure to EOs may induce microbial adaptation, reducing their effectiveness over time.
Thus, EOs supplementation should be approached with caution—strategies such as encapsulation, rotational use, or combination with other phytochemicals are recommended to sustain efficacy while minimizing adverse effects (Benchaar and Greathead, 2011; Patra and Yu, 2015).
4.7 Probiotics
Ezema (2013) described probiotics as live, non-pathogenic, and non-toxic microorganisms that, when administered in appropriate amounts, confer beneficial effects on the host animal. Their mechanism of action includes improving feed digestibility, enhancing beneficial microbial populations, competing with methanogens for substrates (e.g., hydrogen), and modulating ruminal fermentation pathways (Uyeno et al., 2015). In ruminant nutrition, commonly used probiotics—also referred to as direct-fed microbials—include yeast species such as Saccharomyces cerevisiae, as well as bacterial genera including Bacillus, Bifidobacterium, Enterococcus, Lactobacillus, Propionibacterium, Megasphaera elsdenii, and Prevotella bryantii (Seo et al., 2010).
The summary report of the literature analysis on the effects of supplementing ruminant diets with probiotic bacteria is presented in Table 6.
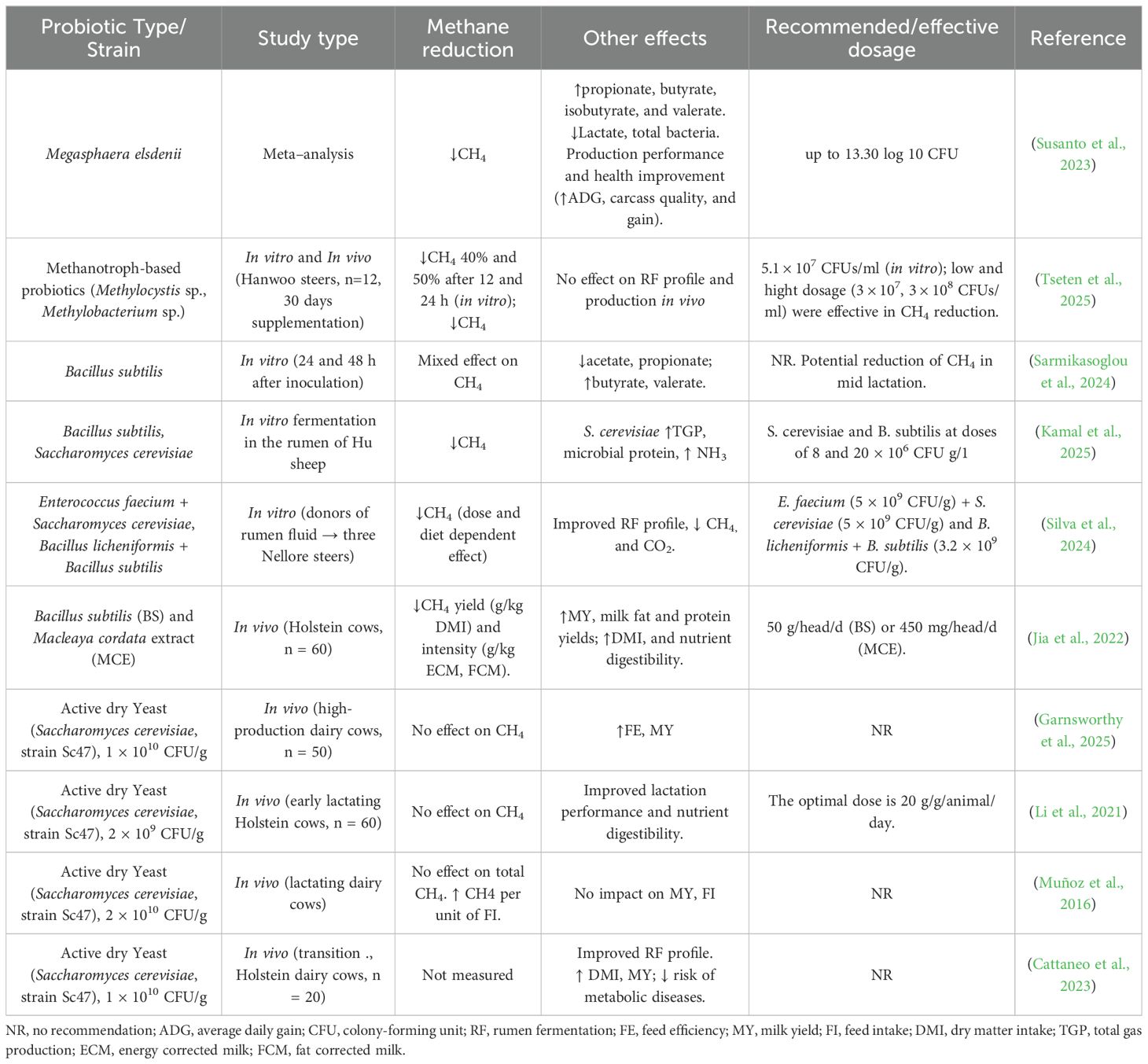
Table 6. Observations from different articles reporting effects of bacterial and yeast probiotics on enteric CH4 and rumen functions.
Bacterial probiotics have been shown to improve rumen function, enhance dry matter intake, feed efficiency, and weight gain in ruminants (Elghandour et al., 2015). They may also inhibit pathogenic microbes, modulate gut microbiota, and stimulate the immune system via bacteriocin production (Khan et al., 2016). Additionally, their supplementation has been associated with increased milk yield, fat-corrected milk, and milk fat content (Elghandour et al., 2015; Khan et al., 2016).
Studies of Bacillus subtilis supplementation in cattle have reported improvements in digestibility, performance, milk production, reductions in somatic cell counts, reductions in CH4 emissions, and stimulation of proteolytic and amylolytic bacterial growth (Sun et al., 2013; Jia et al., 2022). The inclusion of B. subtilis under in vitro conditions has demonstrated potential for reducing ruminal methane production when supplemented in mid-lactation dairy cow diets, suggesting its promise as a methane mitigation additive (Sarmikasoglou et al., 2024). In young Holstein calves, dietary supplementation with a probiotic mixture (L. plantarum, Pediococcus acidilactici, Pediococcus pentosaceus, and B. subtilis) has been shown to enhance health status and decrease the need for medicinal treatments (Wang et al., 2022).
M. elsdenii, a lactic acid-utilizing bacterium, has also been investigated for its probiotic potential. Its capacity to metabolize lactate into VFAs such as butyrate and propionate supports pH stability and reduces lactate accumulation, which can limit methanogenic activity (Carberry et al., 2012; Cabral and Weimer, 2024). A recent meta−analysis by Susanto et al. (2023) integrating 32 studies (136 data points) found that M. elsdenii inclusion significantly reduced CH4 emissions (p < 0.05), while simultaneously improving fermentation profiles (e.g., increased propionate, butyrate, isobutyrate, valerate; decreased lactic acid and acetate proportion) and enhancing livestock performance (e.g., average daily gain, body condition score, carcass traits).
Yeast-based probiotics have emerged as a potential strategy for mitigating enteric CH4 emissions in ruminants. Although supplementation with live yeast, particularly Saccharomyces cerevisiae, is known to stimulate cellulolytic bacterial populations, potentially increasing H2 production—a key substrate for methanogenesis—it may also simultaneously enhance the proliferation of alternative H2-utilizing microorganisms. This dual microbial modulation may lead to a net reduction in CH4 production by diverting metabolic H2 flux away from methanogens and toward competing fermentation pathways, such as propionate or acetogenesis. Such mechanisms suggest that yeast probiotics could play a supportive role in reducing CH4 emissions while improving overall rumen function and fermentation efficiency (Newbold and Rode, 2006; Chaucheyras-Durand et al., 2008; Newbold et al., 1996; Fonty and Chaucheyras-Durand, 2006). In several in vitro studies, the addition of S. cerevisiae has been shown to decrease CH4 production (Bayat et al., 2015; Kamal et al., 2025).
While direct anti-methanogenic effects of yeast are less pronounced, their supportive role in maintaining rumen health and competitive microbial dynamics can indirectly contribute to CH4 mitigation. Additionally, S. cerevisiae can improve feed intake, nutrient digestibility, rumen ecology, and growth performance (Khalouei et al., 2020; Phesatcha et al., 2021), and milk production in dairy cows (Majdoub-Mathlouthi et al., 2009; Moallem et al., 2009; Maamouri et al., 2014; Bayat et al., 2015; Rossow et al., 2018; Perdomo et al., 2020; Cattaneo et al., 2023). It can also reduce oxidative stress and improve dairy cattle performance under heat-stress conditions (Perdomo et al., 2020; Benedetti et al., 2024). Despite promising results, the application of probiotics in ruminants for CH4 mitigation remains limited compared to chemical inhibitors or feed formulation strategies. In addition, the effectiveness of probiotics is often inconsistent due to variations in strain specificity, dosage, delivery method, dietary context, and host microbiome composition. Long-term, large-scale in vivo studies under commercial conditions are necessary to validate their efficacy in CH4 reduction and assess potential interactions with other mitigation strategies.
Nonetheless, probiotics—particularly when used in synergistic combinations or in conjunction with complementary additives—represent a sustainable and biologically integrated strategy for mitigating methane. In addition to their environmental benefits, probiotics contribute to enhanced rumen health, improved nutrient utilization, and increased overall animal productivity.
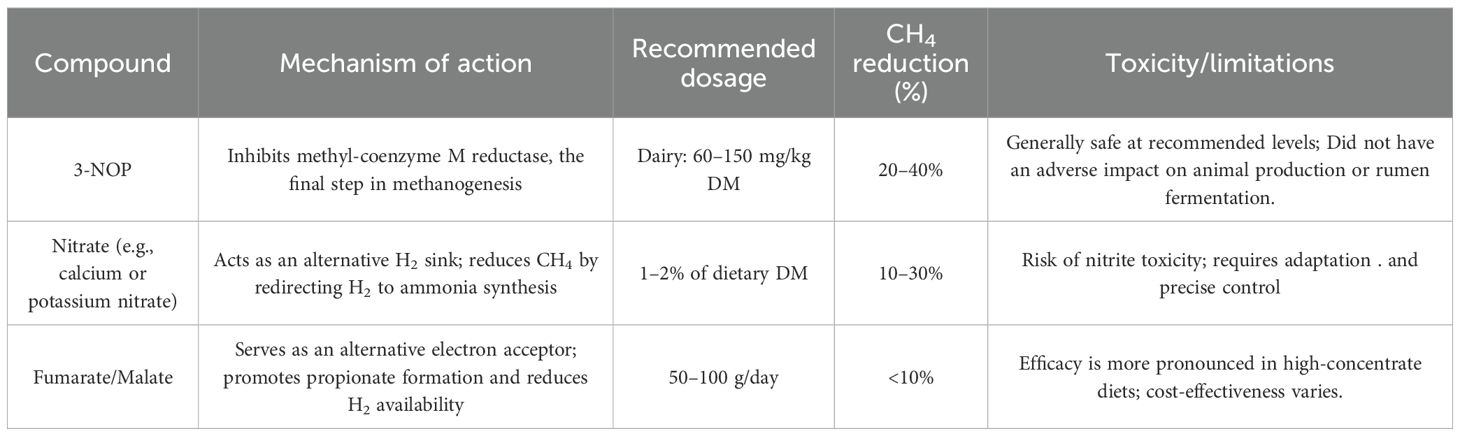
5 Chemical compounds
Chemical compounds have emerged as effective feed additives to mitigate enteric CH4 emissions in ruminants. These compounds typically function by inhibiting methanogenic archaea, redirecting H2 utilization to alternative pathways, or modifying rumen fermentation profiles. Among the most extensively studied are 3-nitrooxypropanol (3-NOP), nitrate salts, and organic acids like fumarate and malate. Each exhibits unique mechanisms of action and variable efficacy depending on diet composition, animal species, and dosage.
Recommended dosages and toxicity of chemical compounds reducing enteric methane are represented in Table 7.
3-NOP is widely recognized for its ability to selectively inhibit methyl-coenzyme M reductase (MCR), a key enzyme in the methanogenesis process. This compound shares structural similarity with methyl-coenzyme M. The practical use of 3-NOP remains under evaluation, primarily due to safety considerations (Yu et al., 2021; Pitta et al., 2022; Hristov et al., 2015; Yu et al., 2021b).
In both dairy and beef cattle, 3-NOP has consistently demonstrated CH4 reductions ranging from 20% to 40% without adversely affecting feed intake, nutrient digestibility, or animal productivity (Dijkstra et al., 2018; Romero-Perez et al., 2014; Kebreab et al., 2023). While productivity effects are generally modest, they tend to be favorable—several studies have reported improvements in milk composition, particularly in fat and protein content, in dairy cattle, as well as enhanced feed conversion efficiency in beef cattle (Melgar et al., 2020; Yu et al., 2021).
Commercially available as Bovaer®, 3-NOP has received regulatory approval in over 65 countries, including the EU, US, and Brazil (Elanco, 2024). The European Food Safety Authority (EFSA) recommends a maximum dose of 100 mg/kg DM or 88 mg of 3-NOP per kilogram of complete feed (Bampidis et al., 2021). However, several studies report on enhanced CH4 mitigation at higher doses. For instance, a recent study demonstrated that supplementing dairy cattle with 3-NOP at an average dose of 123 mg/kg DM resulted in a significant mean reduction in enteric methane emissions of 39.0 ± 5.4% (Dijkstra et al., 2018). Similarly, Alemu et al. (2021) observed that supplementing corn-based finishing diets with 3-NOP at 100, 125, and 150 mg/kg DM significantly reduced CH4 yield in a commercial feedlot setting, with the 125 mg/kg DM dose yielding a 76% reduction, highlighting its efficacy as a methane mitigation strategy in beef production systems. A recent meta-analysis by Kebreab et al. (2023) further confirmed a dose-dependent response, with significantly greater methane reductions achieved at inclusion rates exceeding 100 mg/kg DM. It is essential to note that while current regulatory recommendations are specific to dairy cattle, the application of 3-NOP in other ruminant species, such as beef cattle, requires further research to validate efficacy, optimal dosage, and safety. Dijkstra et al. (2018) reported that 3-Nitrooxypropanol has more substantial antimethanogenic effects in dairy cattle than in beef cattle.
The nutrient composition of the diet significantly influences the efficacy of 3-NOP diet (Almeida et al., 2023). Diets with higher concentrations of neutral detergent fiber (NDF) and crude fat tend to reduce their methane-mitigating potential. In contrast, increased starch content enhances their effectiveness in lowering CH4 yield and intensity (Kebreab et al., 2023; Zhang et al., 2024a).
A short-term study in lactating dairy cows by Van Gastelen et al. (2022) confirmed that both 3-NOP dose and diet composition are critical determinants of efficacy. Cows receiving 60 or 80 mg 3-NOP/kg DM across three different diets exhibited significantly greater CH4 mitigation when fed a corn silage-based diet compared to a grass silage-based one. Importantly, 3-NOP had no adverse effects on dry matter intake, milk yield, milk composition, or feed efficiency. Similar findings were reported in another study by Van Gastelen et al. (2020), which found that supplementation with 60 mg 3-NOP/kg DM did not affect production or intake parameters.
In contrast, results from a longer-term study by Van Gastelen et al. (2024) suggested that diet composition may have an even greater effect on the efficacy of 3-NOP than the duration of supplementation following its initial introduction. Schilde et al. (2021) reported a synergistic reduction in CH4 emissions when 3-NOP was combined with a high-concentrate, low-fiber (CFP) diet. At the same time, the mitigating effect of 3-NOP declined over time when added to a high-forage ration. These findings underscore the need for further long-term research to clarify the persistent impact of 3-NOP on CH4 emissions and to better understand how dietary variability influences its mitigation potential.
Another class of methane-reducing compounds includes nitrate salts, such as calcium nitrate or potassium nitrate (Yang et al., 2016). Nitrate serves as an alternative H2 sink in the rumen, competing with carbon dioxide for hydrogen and by redirecting the reductive potential toward ammonia synthesis (Datta et al., 2017). While nitrate can reduce CH4 emissions by 10–30%, its application is limited by the potential risk of nitrite accumulation and toxicity, requiring careful management of dosage and adaptation periods (Yang et al., 2016). To mitigate the risk of nitrite toxicity associated with nitrate supplementation, several strategies have been proposed, including the use of sulfur-based additives, inoculation with nitrite-reducing bacteria (Latham et al., 2019; Zhao and Zhao, 2022), and gradual acclimation of animals to dietary nitrate (Lee and Beauchemin, 2014). These approaches aim to enhance the safety of nitrate application while preserving its potential for mitigating methane.
Fumarate and malate, organic acids involved in the tricarboxylic acid (TCA) cycle, have also been evaluated for their ability to reduce CH4. These compounds function as alternative electron acceptors, promoting propionate formation over acetate and butyrate, thereby reducing hydrogen availability for methanogenesis (Asanuma et al., 1999). However, the efficacy of fumarate and malate appears to be dose-dependent and is often more pronounced in high-concentrate diets, with CH4 reductions typically below 10% (Morgavi et al., 2010).
Despite their demonstrated efficacy in controlled trials, the large-scale application of chemical compounds in methane mitigation must consider factors such as cost, safety, consumer acceptance, and regulatory approval. Nonetheless, these compounds—particularly 3-NOP—represent important tools in the development of low-emission livestock systems.
The advantages and challenges of nutritional strategies for reducing methane emissions from ruminants are presented through a SWOT analysis, as shown in Table 8.
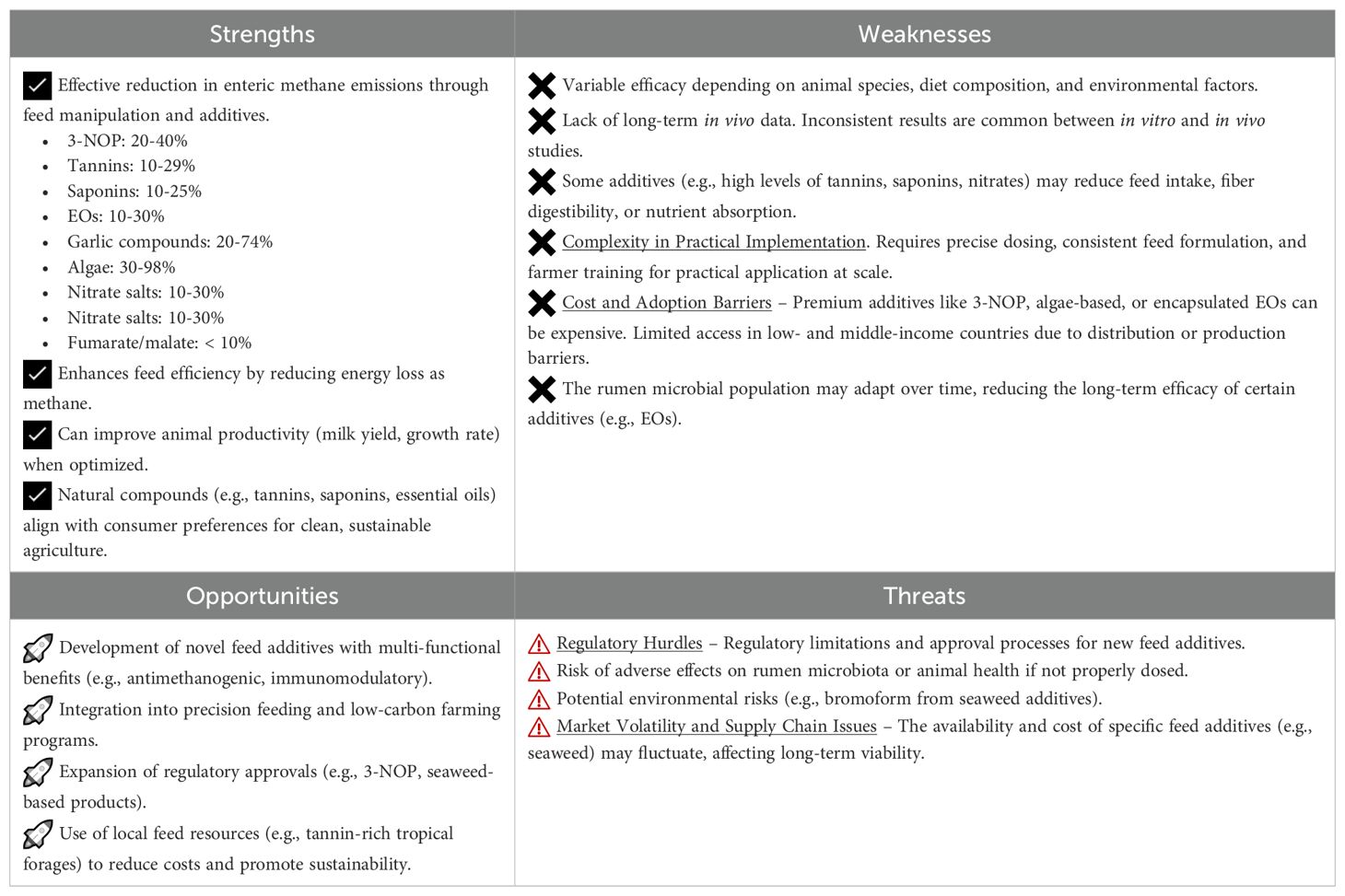
Table 8. SWOT analysis of nutritional strategies for reducing methane emissions in ruminant livestock.
6 Conclusions
Reducing enteric methane emissions in ruminants requires the strategic application of validated nutritional, botanical, and management interventions. Among currently available tools, 3-nitrooxypropanol (3-NOP) offers the most consistent and repeatable reductions in CH4 emissions under both research and commercial conditions. Products derived from Asparagopsis spp. can achieve greater absolute mitigation—often exceeding 50%—but require careful management of inclusion rates, potential impacts on dry matter intake and milk composition, and regulatory concerns related to bromoform and iodine residues.
Botanical additives such as garlic, tannins, and saponins hold additional promise by modulating the rumen microbiota and suppressing methanogens and protozoa. However, their efficacy is highly dependent on the delivery matrix, dose, ruminant species, and background diet. Notably, higher inclusion levels—particularly of condensed tannins—can impair fiber digestibility and animal performance, necessitating diet-specific optimization and formulation limits to avoid negative trade-offs.
In parallel, management-based strategies such as improving forage quality, selecting silages with higher non-fiber carbohydrate (NFC) content, and refining grazing intensity offer additional avenues for reducing CH4 yield and intensity. These approaches can enhance overall nutrient use efficiency and complement additive-based interventions at the farm level.
Collectively, these findings underscore the importance of integrating proven feed additives with targeted dietary formulation and forage management to achieve sustained, cost-effective methane mitigation in ruminant systems.
Author contributions
SM: Investigation, Writing – review & editing, Formal Analysis, Writing – original draft, Conceptualization, Data curation, Methodology, Software. SH: Conceptualization, Methodology, Writing – review & editing, Writing – original draft. PH: Project administration, Methodology, Writing – review & editing, Writing – original draft, Formal Analysis.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Project No. SS06020190 “Development of an anti-methanogenic feed supplement to mitigate the environmental impact of livestock farming” is co-financed with the state support of the Technology Agency of the Czech Republic as part of the Program Environment for Life 6. This project was funded under the National Recovery Plan, part of the European Recovery and Resilience Instrument. This study was supported by the Ministry of Agriculture of the Czech Republic, institutional support MZE-RO1223.
Conflict of interest
Author SM was employed by company Agrovyzkum Rapotin Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Wareth A. A. A., Elkhateeb F. S. O., Ismail Z. S. H., Ghazalah A. A., and Lohakare J. (2021). Combined effects of fenugreek seeds and probiotics on growth performance, nutrient digestibility, carcass criteria, and serum hormones in growing rabbits. Livestock Sci 251, 104616. doi: 10.1016/j.livsci.2021.104616
Akbağ H. I., Savaş T., and Karagül Yüceer Y. (2022). The effect of fenugreek seed (Trigonella foenum-graecum) supplementation on the performance and milk yield characteristics of dairy goats. Arch. Anim. Breed 65, 385–395. doi: 10.5194/aab-65-385-2022
Ali M., Mehboob H., Mirza M., Raza H., and Osredkar M. (2017). Effect of hydrolysable tannin supplementation on production performance of dairy crossbred cows. JAPS: J. Anim. Plant Sci. 27.
Almeida A. K., Cowley F., Mcmeniman J. P., Karagiannis A., Walker N., Tamassia L. F. M., et al. (2023). Effect of 3-nitrooxypropanol on enteric methane emissions of feedlot cattle fed with a tempered barley-based diet with canola oil. J. Anim. Sci. 101. doi: 10.1093/jas/skad237
Altermann E., Schofield L. R., Ronimus R. S., Beattie A. K., and Reilly K. (2018). Inhibition of rumen methanogens by a novel archaeal lytic enzyme displayed on tailored bionanoparticles. Front. Microbiol. 9, 2378. doi: 10.3389/fmicb.2018.02378
Alvarez-Hess P. S., Jacobs J. L., Kinley R. D., Roque B. M., Neachtain A. S. O., Chandra S., et al. (2024). Effects of a range of effective inclusion levels of Asparagopsis armata steeped in oil on enteric methane emissions of dairy cows. Anim. Feed Sci Technol. 310, 115932. doi: 10.1016/j.anifeedsci.2024.115932
Angellotti M., Lindberg M., Ramin M., Krizsan S. J., and Danielsson R. (2025). Asparagopsis taxiformis supplementation to mitigate enteric methane emissions in dairy cows-Effects on performance and metabolism. J. Dairy Sci. 108, 2503–2516. doi: 10.3168/jds.2024-25258
Arndt C., Hristov A. N., Price W. J., Mcclelland S. C., Pelaez A. M., Cueva S. F., et al. (2022). Full adoption of the most effective strategies to mitigate methane emissions by ruminants can help meet the 1.5 C target by 2030 but not 2050. Proc. Natl. Acad. Sci. 119, e2111294119. doi: 10.1073/pnas.2111294119
Asanuma N., Iwamoto M., and Hino T. (1999). Effect of the addition of fumarate on methane production by ruminal microorganisms in vitro. J. Dairy Sci 82, 780–787. doi: 10.3168/jds.S0022-0302(99)75296-3
Baca-González V., Asensio-Calavia P., González-Acosta S., Pérez de la Lastra J. M., and Morales de la Nuez A. (2020). Are vaccines the solution for methane emissions from ruminants? A systematic review. Vaccines 8, 460. doi: 10.3390/vaccines8030460
Bampidis V., Azimonti G., Bastos M. D. L., Christensen H., Dusemund B., Fašmon Durjava M., et al. (2021). Safety and efficacy of a feed additive consisting of 3-nitrooxypropanol (Bovaer® 10) for ruminants for milk production and reproduction (DSM Nutritional Products Ltd). Efsa J. 19, e06905. doi: 10.2903/j.efsa.2021.6905
Bārdule A., Laiho R., Jauhiainen J., Soosaar K., Lazdiņš A., Armolaitis K., et al. (2024). Annual net CO 2 fluxes from drained organic soils used for agriculture in the hemiboreal region of Europe. EGUsphere 2024, 1–29. doi: 10.5194/egusphere-2024-2523
Battelli M., Colombini S., Crovetto G. M., Galassi G., Abeni F., Petrera F., et al. (2024). Condensed tannins fed to dairy goats: Effects on digestibility, milk production, blood parameters, methane emission, and energy and nitrogen balances. J. Dairy Sci. 107, 3614–3630. doi: 10.3168/jds.2023-24076
Bayat A., Kairenius P., Stefański T., Leskinen H., Comtet-Marre S., Forano E., et al. (2015). Effect of camelina oil or live yeasts (Saccharomyces cerevisiae) on ruminal methane production, rumen fermentation, and milk fatty acid composition in lactating cows fed grass silage diets. J. dairy Sci 98, 3166–3181. doi: 10.3168/jds.2014-7976
Beauchemin K. and Mcginn S. (2006). Methane emissions from beef cattle: Effects of fumaric acid, essential oil, and canola oil. J. Anim. Sci 84, 1489–1496. doi: 10.2527/2006.8461489x
Beauchemin K., Mcginn S., Martinez T., and Mcallister T. (2007a). Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J. Anim. Sci 85, 1990–1996. doi: 10.2527/jas.2006-686
Beauchemin K. A., Mcginn S. M., Martinez T. F., and Mcallister T. A. (2007b). Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle1. J. Anim. Sci 85, 1990–1996. doi: 10.2527/jas.2006-686
Beauchemin K. A., Ungerfeld E. M., Abdalla A. L., Alvarez C., Arndt C., Becquet P., et al. (2022). Invited review: Current enteric methane mitigation options. J. Dairy Sci 105, 9297–9326. doi: 10.3168/jds.2022-22091
Becker F., Spengler K., Reinicke F., and Heider-Van Diepen C. (2023). Impact of essential oils on methane emissions, milk yield, and feed efficiency and resulting influence on the carbon footprint of dairy production systems. Environ. Sci. pollut. Res. Int. 30, 48824–48836. doi: 10.1007/s11356-023-26129-8
Belanche A., Bannink A., Dijkstra J., Durmic Z., Garcia F., Santos F. G., et al. (2025). Feed additives for methane mitigation: A guideline to uncover the mode of action of antimethanogenic feed additives for ruminants. J. Dairy Sci 108, 375–394. doi: 10.3168/jds.2024-25046
Belanche A., Newbold C. J., Morgavi D. P., Bach A., Zweifel B., and Yáñez-Ruiz D. R. (2020). A meta-analysis describing the effects of the essential oils blend agolin ruminant on performance, rumen fermentation and methane emissions in dairy cows. Animals 10, 620. doi: 10.3390/ani10040620
Benchaar C. and Greathead H. (2011). Essential oils and opportunities to mitigate enteric methane emissions from ruminants. Anim. Feed Sci Technol., 166–167. doi: 10.1016/j.anifeedsci.2011.04.024
Benchaar C. and Hassanat F. (2024). Assessing the effects of high-carvacrol oregano oil on rumen microbial fermentation, gas production, and methane production in vitro. Can. J. Anim. Sci. 105, 1-6. doi: 10.1139/cjas-2024-0083
Benchaar C. and Hassanat F. (2025). Diet supplementation with a mixture of essential oils: Effects on enteric methane emissions, apparent total-tract nutrient digestibility, nitrogen utilization, and lactational performance. J. Dairy Sci. 108 (4), 3560-3572. doi: 10.3168/jds.2024-25447
Benchaar C., Hassanat F., Gervais R., Chouinard P. Y., Petit H. V., and Massé D. I. (2014). Methane production, digestion, ruminal fermentation, nitrogen balance, and milk production of cows fed corn silage- or barley silage-based diets. J. Dairy Sci 97, 961–974. doi: 10.3168/jds.2013-7122
Benedetti L., Cattaneo L., Vercesi A., Trevisi E., and Piccioli-Cappelli F. (2024). Effects of live saccharomyces cerevisiae yeast administration in periparturient dairy cows. Anim. (Basel) 14. doi: 10.3390/ani14030472
Bokharaeian M., Ghoorchi T., Toghdory A., and Esfahani I. (2023). The dose-dependent role of sage, clove, and pine essential oils in modulating ruminal fermentation and biohydrogenation of polyunsaturated fatty acids: A promising strategy to reduce methane emissions and enhance the nutritional profile of ruminant products. Appl. Sci. 13, 11605. doi: 10.3390/app132011605
Brooke C. G., Roque B. M., Shaw C., Najafi N., Gonzalez M., Pfefferlen A., et al. (2020). Methane reduction potential of two pacific coast macroalgae during in vitro ruminant fermentation. Front. Mar. Sci 7, 561. doi: 10.3389/fmars.2020.00561
Broucek J. (2014). Production of methane emissions from ruminant husbandry: a review. J. Environ. Prot. 5, 1482. doi: 10.4236/jep.2014.515141
Buddle B. M., Denis M., Attwood G. T., Altermann E., Janssen P. H., Ronimus R. S., et al. (2011). Strategies to reduce methane emissions from farmed ruminants grazing on pasture. Veterinary J. 188, 11–17. doi: 10.1016/j.tvjl.2010.02.019
Burezq H. A. and Khalil F. (2025). Investigating the impact of biochar on methane gas emissions and its effect on enteric fermentation. Kuwait J. Sci 52, 100332. doi: 10.1016/j.kjs.2024.100332
Busquet M., Calsamiglia S., Ferret A., Cardozo P. W., and Kamel C. (2005a). Effects of cinnamaldehyde and garlic oil on rumen microbial fermentation in a dual flow continuous culture. J. Dairy Sci. 88, 2508–2516. doi: 10.3168/jds.S0022-0302(05)72928-3
Busquet M., Calsamiglia S., Ferret A., Carro M. D., and Kamel C. (2005b). Effect of garlic oil and four of its compounds on rumen microbial fermentation. J. Dairy Sci 88, 4393–4404. doi: 10.3168/jds.S0022-0302(05)73126-X
Busquet M., Calsamiglia S., Ferret A., and Kamel C. (2006). Plant extracts affect in vitro rumen microbial fermentation. J. Dairy Sci 89, 761–771. doi: 10.3168/jds.S0022-0302(06)72137-3
Cabral L. D. S. and Weimer P. J. (2024). Megasphaera elsdenii: its role in ruminant nutrition and its potential industrial application for organic acid biosynthesis. Microorganisms 12. doi: 10.3390/microorganisms12010219
Calsamiglia S., Busquet M., Cardozo P. W., Castillejos L., and Ferret A. (2007). Invited review: essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci 90, 2580–2595. doi: 10.3168/jds.2006-644
Candelaresi D. and Spazzafumo G. (2021). “1 - Introduction: the power-to-fuel concept,” in Power to Fuel. Ed. Spazzafumo G. (UK: Academic Press). doi: 10.1016/B978-0-12-822813-5.00005-9
Carberry C. A., Kenny D. A., Han S., Mccabe M. S., and Waters S. M. (2012). Effect of phenotypic residual feed intake and dietary forage content on the rumen microbial community of beef cattle. Appl. Environ. Microbiol. 78, 4949–4958. doi: 10.1128/AEM.07759-11
Cardoso-Gutierrez E., Aranda-Aguirre E., Robles-Jimenez L. E., Castelán-Ortega O. A., Chay-Canul A. J., Foggi G., et al. (2021). Effect of tannins from tropical plants on methane production from ruminants: A systematic review. Veterinary Anim. Sci 14, 100214. doi: 10.1016/j.vas.2021.100214
Cardozo P., Calsamiglia S., Ferret A., and Kamel C. (2004). Effects of natural plant extracts on ruminal protein degradation and fermentation profiles in continuous culture. J. Anim. Sci 82, 3230–3236. doi: 10.2527/2004.82113230x
Castelán-Ortega O. A., Ku-Vera C., and Estrada-Flores J. G. (2014). Modeling methane emissions and methane inventories for cattle production systems in Mexico. Atmósfera 27, 185–191. doi: 10.1016/S0187-6236(14)71109-9
Castillejos L., Calsamiglia S., Ferret A., and Losa R. (2005). Effects of a specific blend of essential oil compounds and the type of diet on rumen microbial fermentation and nutrient flow from a continuous culture system. Anim. Feed Sci Technol. 119, 29–41. doi: 10.1016/j.anifeedsci.2004.12.008
Castillo C., Sami Mshary G., Viana J., Muiño R., and Hernández J.. (2024). “Enteric Methane Emissions Factors in High-Producing Dairy Cows,” in José and Muiño, Rodrigo and Hernández, Joaquín, Enteric Methane Emissions Factors in High-Producing Dairy Cows, Amsteerdam, Netherlands. doi: 10.2139/ssrn.5007199
Castro-Montoya J., Peiren N., Cone J. W., Zweifel B., Fievez V., and De Campeneere S. (2015). In vivo and in vitro effects of a blend of essential oils on rumen methane mitigation. Livestock Sci 180, 134–142. doi: 10.1016/j.livsci.2015.08.010
Cattaneo L., Lopreiato V., Piccioli-Cappelli F., Trevisi E., and Minuti A. (2023). Effect of supplementing live Saccharomyces cerevisiae yeast on performance, rumen function, and metabolism during the transition period in Holstein dairy cows. J. Dairy Sci 106, 4353–4365. doi: 10.3168/jds.2022-23046
Cezimbra I. M., De Albuquerque Nunes P. A., De Souza Filho W., Tischler M. R., Genro T. C. M., Bayer C., et al. (2021). Potential of grazing management to improve beef cattle production and mitigate methane emissions in native grasslands of the Pampa biome. Sci Total Environ. 780, 146582. doi: 10.1016/j.scitotenv.2021.146582
Chagas J. C., Ramin M., and Krizsan S. J. (2019). In vitro evaluation of different dietary methane mitigation strategies. Animals 9, 1120. doi: 10.3390/ani9121120
Chaucheyras-Durand F., Walker N., and Bach A. (2008). Effects of active dry yeasts on the rumen microbial ecosystem: Past, present and future. Anim. Feed Sci Technol. 145, 5–26. doi: 10.1016/j.anifeedsci.2007.04.019
Chen L., Thorup V. M., and Østergaard S. (2025). Modeling the effects of heat stress on production and enteric methane emission in high-yielding dairy herds. J. Dairy Sci 108, 3956–3964. doi: 10.3168/jds.2024-25460
Choudhury P. K., Jena R., Tomar S. K., and Puniya A. K. (2022). Reducing enteric methanogenesis through alternate hydrogen sinks in the rumen. Methane 1, 320–341. doi: 10.3390/methane1040024
Christodoulou C., Mavrommatis A., Loukovitis D., Symeon G., Dotas V., Kotsampasi B., et al. (2023). Effect of spirulina dietary supplementation in modifying the rumen microbiota of ewes. Animals 13, 740. doi: 10.3390/ani13040740
Clasen J. B., Fikse W. F., Ramin M., and Lindberg M. (2024). Effects of herd management decisions on dairy cow longevity, farm profitability, and emissions of enteric methane - a simulation study of milk and beef production. Animal 18, 101051. doi: 10.1016/j.animal.2023.101051
Cobellis G., Trabalza-Marinucci M., and Yu Z. (2016). Critical evaluation of essential oils as rumen modifiers in ruminant nutrition: A review. Sci Total Environ. 545-546, 556–568. doi: 10.1016/j.scitotenv.2015.12.103
Colin R. L., Sperber J. L., Buse K. K., Kononoff P. J., Watson A. K., and Erickson G. E. (2024). Effect of an algae feed additive on reducing enteric methane emissions from cattle. Transl. Anim. Sci. 8, txae109. doi: 10.1093/tas/txae109
Conrad R. (2023). Complexity of temperature dependence in methanogenic microbial environments. Front. Microbiol. 14, 1232946. doi: 10.3389/fmicb.2023.1232946
Cook S., Maiti P., Chaves A., Benchaar C., Beauchemin K., and Mcallister T. (2008). Avian (IgY) anti-methanogen antibodies for reducing ruminal methane production: in vitro assessment of their effects. Aust. J. Exp. Agric. 48, 260–264. doi: 10.1071/EA07249
Danielsson R., Schnürer A., Arthurson V., and Bertilsson J. (2012). Methanogenic population and CH4 production in Swedish dairy cows fed different levels of forage. Appl. Environ. Microbiol. 78, 6172–6179. doi: 10.1128/AEM.00675-12
Da Silva Soares T. L., De Paula Soares Valente J., Santos F. L. C., Kelles K. R., Da Silva Soares T., and Mercadante M. E. Z. (2025). A systematic review and meta-analysis: relationship between residual feed intake and traits related to methane emissions in cattle. Trop. Anim. Health Prod 57, 171. doi: 10.1007/s11250-025-04423-6
Datta M., Jha P., and Arumbaka S. (2017). Effects of nitrate supplementation on nutrition, performance and methane mitigation in ruminants: A review. Int. J. Livestock Res. 1. doi: 10.5455/ijlr.20170624054734
De Bhowmick G. and Hayes M. (2023). Potential of seaweeds to mitigate production of greenhouse gases during production of ruminant proteins. Glob Chall 7, 2200145. doi: 10.1002/gch2.202200145
De Haas Y., Windig J. J., Calus M. P. L., Dijkstra J., De Haan M., Bannink A., et al. (2011). Genetic parameters for predicted methane production and potential for reducing enteric emissions through genomic selection. J. Dairy Sci 94, 6122–6134. doi: 10.3168/jds.2011-4439
Dey A. (2015). Effect of fenugreek leaf extract (Trigonella foenum-graecum L.) on in vitro methanogenesis and fermentation of wheat straw-based diet (Triticum aestivum L.) fed to buffaloes. Sri Lanka J. Food Agric. 1. doi: 10.4038/sljfa.v1i1.2
Difford G. F., Plichta D. R., Løvendahl P., Lassen J., Noel S. J., Højberg O., et al. (2018). Host genetics and the rumen microbiome jointly associate with methane emissions in dairy cows. PloS Genet. 14, e1007580. doi: 10.1371/journal.pgen.1007580
Dijkstra J., Bannink A., France J., Kebreab E., and Van Gastelen S. (2018). Short communication: Antimethanogenic effects of 3-nitrooxypropanol depend on supplementation dose, dietary fiber content, and cattle type. J. Dairy Sci 101, 9041–9047. doi: 10.3168/jds.2018-14456
Ding H., Ao C., and Zhang X. (2023). Potential use of garlic products in ruminant feeding: A review. Anim. Nutr. 14, 343–355. doi: 10.1016/j.aninu.2023.04.011
Dini Y., Cajarville C., Gere J. I., Fernandez S., Fraga M., Pravia M. I., et al. (2019). Association between residual feed intake and enteric methane emissions in Hereford steers. Transl. Anim. Sci. 3, 239–246. doi: 10.1093/tas/txy111
Dittmann M. T., Baki C., Terranova M., Amelchanka S. L., Dubois S., Wiget A., et al. (2024). The effect of biochar supplementation on feed utilization, milk production and methane emission in lactating dairy cows. Anim. Feed Sci Technol. 318, 116127. doi: 10.1016/j.anifeedsci.2024.116127
Dressler E. A., Bormann J. M., Weaber R. L., and Rolf M. M. (2024). Use of methane production data for genetic prediction in beef cattle: A review. Trans. Anim. Sci 8, txae014. doi: 10.1093/tas/txae014
Džermeikaitė K., Krištolaitytė J., and Antanaitis R. (2024). Relationship between dairy cow health and intensity of greenhouse gas emissions. Anim. (Basel) 14. doi: 10.3390/ani14060829
Elanco (2024). Elanco announces FDA has completed review of Bovaer®, first-in-class methane-reducing feed ingredient for U.S. dairy industry. Available online at: https://www.elanco.com/en-us/insights/elanco-announces-fda-has-completed-review-of-bovaer-first-in-class-methane-reducing-feed-ingredient-for-u-s-dairy-industry:Elanco (Accessed March 13, 2025).
Elghandour M., Maggiolino A., Alvarado-Ramírez E. R., Hernández-Meléndez J., Rivas-Cacerese R. R., Hernández-Ruiz P. E., et al. (2023). Marine microalgae as a nutritive tool to mitigate ruminal greenhouse gas production: in vitro fermentation characteristics of fresh and ensiled maize (Zea mays L.) forage. Vet. Sci. 10. doi: 10.3390/vetsci10090556
Elghandour M. M., Salem A. Z., Castañeda J. S. M., Camacho L. M., Kholif A. E., and Chagoyán J. C. V. (2015). Direct-fed microbes: A tool for improving the utilization of low quality roughages in ruminants. J. Integr. Agric. 14, 526–533. doi: 10.1016/S2095-3119(14)60834-0
Elois M. A., Silva R. D., Pilati G. V. T., Rodríguez-Lázaro D., and Fongaro G. (2023). Bacteriophages as biotechnological tools. Viruses 15. doi: 10.3390/v15020349
El-Tarabany A. A., Teama F. E. I., Atta M. A., and El-Tarabany M. S. (2018). Impact of dietary fenugreek seeds on lactational performance and blood biochemical and hematological parameters of dairy goats under hot summer conditions. Mljekarstvo 68, 214–223. doi: 10.15567/mljekarstvo.2018.0306
Emara Rabee A., Ghandour M. M. M., Sallam A. M., Raef O., Elwakeel E. A., Sabra E. A., et al. (2025). Milk yield, rumen fermentation, and microbiota of Shami goats fed diets supplemented with spirulina and yeast. AMB Express 15, 108. doi: 10.1186/s13568-025-01916-3
Evangelista C., Milanesi M., Pietrucci D., Chillemi G., and Bernabucci U. (2024). Enteric methane emission in livestock sector: bibliometric research from 1986 to 2024 with text mining and topic analysis approach by machine learning algorithms. Anim. (Basel) 14. doi: 10.3390/ani14213158
Ezema C. (2013). Probiotics in animal production: A review. J. Veterinary Med. Anim. Health 5, 308–316. doi: 10.12691/jaem-7-1-3
FAO (2016). “Climate change, Agriculture, and Food security,” in The State of Food and Agriculture 2016 (Rome, Italy: FAO).
FAO (2017). “Livestock solutions for climate change,” in Food and Agriculture Organization of the United Nations (Rome, Italy: FAO). Available online at: https://openknowledge.fao.org/items/2985e4e2-3c37-4e7c-aa7c-3655de93d53c.
Firkins J. L. and Mitchell K. E. (2023). Invited review: Rumen modifiers in today’s dairy rations. J. Dairy Sci 106, 3053–3071. doi: 10.3168/jds.2022-22644
Fonty G. and Chaucheyras-Durand F. (2006). Effects and modes of action of live yeasts in the rumen. Biologia 61, 741–750. doi: 10.2478/s11756-006-0151-4
Fregulia P., Dias R. J. P., Campos M. M., Tomich T. R., Pereira L. G. R., and Neves A. L. A. (2024). Composition of the rumen microbiome and its association with methane yield in dairy cattle raised in tropical conditions. Mol. Biol. Rep. 51, 447. doi: 10.1007/s11033-024-09381-0
Gagen E. J., Denman S. E., Padmanabha J., Zadbuke S., Al Jassim R., Morrison M., et al. (2010). Functional gene analysis suggests different acetogen populations in the bovine rumen and tammar wallaby forestomach. Appl. Environ. Microbiol. 76, 7785–7795. doi: 10.1128/AEM.01679-10
Garnsworthy P. C. (2004). The environmental impact of fertility in dairy cows: a modelling approach to predict methane and ammonia emissions. Anim. Feed Sci Technol. 112, 211–223. doi: 10.1016/j.anifeedsci.2003.10.011
Garnsworthy P. C., Saunders N., Goodman J. R., Algherair I. H., and Ambrose J. D. (2025). Effects of live yeast on milk yield, feed efficiency, methane emissions and fertility of high-yielding dairy cows. animal 19, 101379. doi: 10.1016/j.animal.2024.101379
Gatenby J. (2021). Urgent steps must be taken to reduce methane emissions. New Rep. Says. Available online at: https://www.york.ac.uk/news-and-events/news/2021/research/reduce-methane-report/ (Accessed March 10, 2025).
Goel G. and Makkar H. P. (2012). Methane mitigation from ruminants using tannins and saponins. Trop. Anim. Health production 44, 729–739. doi: 10.1007/s11250-011-9966-2
Grainger C., Clarke T., Auldist M., Beauchemin K., Mcginn S., Waghorn G., et al. (2009). Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Can. J. Anim. Sci 89, 241–251. doi: 10.4141/CJAS08110
Greene E., Hubert C., Nemati M., Jenneman G., and Voordouw G. (2003). Nitrite reductase activity of sulphate-reducing bacteria prevents their inhibition by nitrate-reducing, sulphide-oxidizing bacteria. Environ. Microbiol. 5, 607–617. doi: 10.1046/j.1462-2920.2003.00446.x
Hassanat F. and Benchaar C. (2013). Assessment of the effect of condensed (acacia and quebracho) and hydrolysable (chestnut and valonea) tannins on rumen fermentation and methane production in vitro. J. Sci Food Agric. 93, 332–339. doi: 10.1002/jsfa.5763
Hawkins H.-J., Venter Z.-S., and Cramer M. D. (2022). A holistic view of Holistic Management: What do farm-scale, carbon, and social studies tell us? Agriculture Ecosyst. Environ. 323, 107702. doi: 10.1016/j.agee.2021.107702
Hegarty R. S., Goopy J. P., Herd R. M., and Mccorkell B. (2007). Cattle selected for lower residual feed intake have reduced daily methane production1,2. J. Anim. Sci 85, 1479–1486. doi: 10.2527/jas.2006-236
Henderson G., Cox F., Ganesh S., Jonker A., Young W., and Janssen P. H. (2015). Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 5, 14567. doi: 10.1038/srep14567
Hess H. D., Kreuzer M., Dıíaz T. E., Lascano C. E., Carulla J. E., Soliva C. R., et al. (2003). Saponin rich tropical fruits affect fermentation and methanogenesis in faunated and defaunated rumen fluid. Anim. Feed Sci Technol. 109, 79–94. doi: 10.1016/S0377-8401(03)00212-8
Holtshausen L., Chaves A., Beauchemin K., Mcginn S., Mcallister T., Odongo N., et al. (2009). Feeding saponin-containing Yucca schidigera and Quillaja saponaria to decrease enteric methane production in dairy cows. J. Dairy Sci 92, 2809–2821. doi: 10.3168/jds.2008-1843
Hornbuckle W. E. and Tennant B. C. (1997). “Gastrointestinal function,” in Clinical biochemistry of domestic animals. (UK: Academic Press), 367-406.
Hristov A. N. (2024). Invited review: Advances in nutrition and feed additives to mitigate enteric methane emissions. J. Dairy Sci 107, 4129–4146. doi: 10.3168/jds.2023-24440
Hristov A. N., Oh J., Firkins J. L., Dijkstra J., Kebreab E., Waghorn G., et al. (2013). Special topics–Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J. Anim. Sci. 91, 5045–5069. doi: 10.2527/jas.2013-6583
Hristov A. N., Oh J., Giallongo F., Frederick T. W., Harper M. T., Weeks H. L., et al. (2015). An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production. Proc. Natl. Acad. Sci. U.S.A. 112, 10663–10668. doi: 10.1073/pnas.1504124112
Hu Q., Chen Y. Y., Jiao Q. Y., Khan A., Li F., Han D. F., et al. (2018). Triterpenoid saponins from the pulp of Sapindus mukorossi and their antifungal activities. Phytochemistry 147, 1–8. doi: 10.1016/j.phytochem.2017.12.004
Jayanegara A., Leiber F., and Kreuzer M. (2012). Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. 96, 365–375. doi: 10.1111/j.1439-0396.2011.01172.x
Jeong J., Yu C., Kang R., Kim M., and Park T. (2024). Application of propionate-producing bacterial consortium in ruminal methanogenesis inhibited environment with bromoethanesulfonate as a methanogen direct inhibitor. Front. Veterinary Sci 11, 1422474. doi: 10.3389/fvets.2024.1422474
Jia P., Tu Y., Liu Z., Li F., Yan T., Ma S., et al. (2022). Diets supplementation with Bacillus subtilis and Macleaya cordata extract improve production performance and the metabolism of energy and nitrogen, while reduce enteric methane emissions in dairy cows. Anim. Feed Sci Technol. 294, 115481. doi: 10.1016/j.anifeedsci.2022.115481
Jiménez-Ocampo R., Montoya-Flores M. D., Herrera-Torres E., Pámanes-Carrasco G., Arceo-Castillo J. I., Valencia-Salazar S. S., et al. (2021). Effect of chitosan and naringin on enteric methane emissions in crossbred heifers fed tropical grass. Anim. (Basel) 11. doi: 10.3390/ani11061599
Joch M., Kudrna V., Hakl J., Božik M., Homolka P., Illek J., et al. (2019). In vitro and in vivo potential of a blend of essential oil compounds to improve rumen fermentation and performance of dairy cows. Anim. Feed Sci Technol. 251, 176–186. doi: 10.1016/j.anifeedsci.2019.03.009
Johnson K., Huyler M., Westberg H., Lamb B., and Zimmerman P. (1994). Measurement of methane emissions from ruminant livestock using a sulfur hexafluoride tracer technique. Environ. Sci Technol. 28, 359–362. doi: 10.1021/es00051a025
Kamal M., Linlin K., Gao J., Xinrui Z., Xinming C., Haibo W., et al. (2025). Effects of Saccharomyces cerevisiae and Bacillus subtilis on in vitro fermentation in the rumen of Hu sheep. J. Sci. Food Agric. 105, 498–506. doi: 10.1002/jsfa.13848
Kamalanathan S., Houlahan K., Miglior F., Chud T. C. S., Seymour D. J., Hailemariam D., et al. (2023). Genetic analysis of methane emission traits in holstein dairy cattle. Anim. (Basel) 13. doi: 10.3390/ani13081308
Kamel C., Greathead H. M. R., Tejido M. L., Ranilla M. J., and Carro M. D. (2008). Effects of allicin and diallyl disulfide on in vitro rumen fermentation of a mixed diet. Anim. Feed Sci Technol. 145, 351–363. doi: 10.1016/j.anifeedsci.2007.05.050
Karekar S., Stefanini R., and Ahring B. (2022). Homo-acetogens: their metabolism and competitive relationship with hydrogenotrophic methanogens. Microorganisms 10, 397. doi: 10.3390/microorganisms10020397
Kebreab E., Bannink A., Pressman E. M., Walker N., Karagiannis A., Van Gastelen S., et al. (2023). A meta-analysis of effects of 3-nitrooxypropanol on methane production, yield, and intensity in dairy cattle. J. Dairy Sci 106, 927–936. doi: 10.3168/jds.2022-22211
Kelly L., Pressman E. M., Ramirez-Agudelo J. F., Chernavsky H., Hess P. A., Jacques S., et al. (2025). The effect of Rumin8 Investigational Veterinary Product-a bromoform based feed additive-on enteric methane emissions, animal production parameters, and the rumen environment in feedlot cattle. Transl. Anim. Sci. 9, txaf028. doi: 10.1093/tas/txaf028
Khalouei H., Seranatne V., Fehr K., Guo J., Yoon I., Khafipour E., et al. (2020). Effects of Saccharomyces cerevisiae fermentation products and subacute ruminal acidosis on feed intake, fermentation, and nutrient digestibilities in lactating dairy cows. Can. J. Anim. Sci 101, 143–157. doi: 10.1139/cjas-2020-0018
Khan R. U., Naz S., Dhama K., Karthik K., Tiwari R., Abdelrahman M. M., et al. (2016). Direct-fed microbial: beneficial applications, modes of action and prospects as a safe tool for enhancing ruminant production and safeguarding health. International Journal of Pharmacology Faisalabad, Pakistan: Asian Network for Scientific Information 12 (3), 220–231. doi: 10.3923/ijp.2016.220.231
Kholif A. E. (2023). A review of effect of saponins on ruminal fermentation, health and performance of ruminants. Vet. Sci. 10. doi: 10.3390/vetsci10070450
Kholif A. E., Gouda G. A., Morsy T. A., Matloup O. H., Sallam S. M., and Patra A. K. (2023). Associative effects between Chlorella vulgaris microalgae and Moringa oleifera leaf silage used at different levels decreased in vitro ruminal greenhouse gas production and altered ruminal fermentation. Environ. Sci. pollut. Res. Int. 30, 6001–6020. doi: 10.1007/s11356-022-22559-y
Khurana R., Brand T., Tapio I., and Bayat A.-R. (2023). Effect of a garlic and citrus extract supplement on performance, rumen fermentation, methane production, and rumen microbiome of dairy cows. J. Dairy Sci 106, 4608–4621. doi: 10.3168/jds.2022-22838
Khurana R., Salami S. A., Poblete R. B., Fischer A., Cofré L. A., Bustos V., et al. (2024). Effect of a garlic and citrus extract supplement on the lactation performance and carbon footprint of dairy cows under grazing conditions in Chile. Animals 14, 165. doi: 10.3390/ani14010165
Kide W., Burte R., Desai B., and Bharambe V. (2017). Impact of rumen methanogenesis on climate change: A review. J. Agroecology Natural Resource Manage. 4, 2394–2786.
Kinley R. D. and Fredeen A. H. (2015). In vitro evaluation of feeding North Atlantic stormtoss seaweeds on ruminal digestion. J. Appl. Phycology 27, 2387–2393. doi: 10.1007/s10811-014-0487-z
Kinley R. D., Martinez-Fernandez G., Matthews M. K., De Nys R., Magnusson M., and Tomkins N. W. (2020). Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J. Cleaner Production 259, 120836. doi: 10.1016/j.jclepro.2020.120836
Kohn R. and Boston R. (2000). “The role of thermodynamics in controlling rumen metabolism,” in Modelling nutrient utilization in farm animals. (Cabi Wallingford UK: Cabi). 11–24.
Krizsan S. J., Ramin M., Chagas J. C., Halmemies-Beauchet-Filleau A., Singh A., Schnürer A., et al. (2023). Effects on rumen microbiome and milk quality of dairy cows fed a grass silage-based diet supplemented with the macroalga Asparagopsis taxiformis. Front. Anim. Sci 4, 1112969. doi: 10.3389/fanim.2023.1112969
Króliczewska B., Pecka-Kiełb E., and Bujok J. (2023). Strategies used to reduce methane emissions from ruminants: Controversies and issues. Agriculture 13, 602. doi: 10.3390/agriculture13030602
Lan W. and Yang C. (2019). Ruminal methane production: Associated microorganisms and the potential of applying hydrogen-utilizing bacteria for mitigation. Sci Total Environ. 654, 1270–1283. doi: 10.1016/j.scitotenv.2018.11.180
Lassen J. and Løvendahl P. (2016). Heritability estimates for enteric methane emissions from Holstein cattle measured using noninvasive methods. J. Dairy Sci. 99, 1959–1967. doi: 10.3168/jds.2015-10012
Latham E. A., Anderson R. C., Pinchak W. E., and Nisbet D. J. (2016). Insights on alterations to the rumen ecosystem by nitrate and nitrocompounds. Front. Microbiol. 7, 228. doi: 10.3389/fmicb.2016.00228
Latham E. A., Pinchak W. E., Trachsel J., Allen H. K., Callaway T. R., Nisbet D. J., et al. (2019). Paenibacillus 79R4, a potential rumen probiotic to enhance nitrite detoxification and methane mitigation in nitrate-treated ruminants. Sci Total Environ. 671, 324–328. doi: 10.1016/j.scitotenv.2019.03.390
Leahy S. C., Kelly W. J., Li D., Li Y., Altermann E., Lambie S., et al. (2013). The complete genome sequence of Methanobrevibacter sp. AbM4. Standards genomic Sci. 8, 215–227. doi: 10.4056/sigs.3977691
Lee C. and Beauchemin K. A. (2014). A review of feeding supplementary nitrate to ruminant animals: nitrate toxicity, methane emissions, and production performance. Can. J. Anim. Sci 94, 557–570. doi: 10.4141/cjas-2014-069
Leng R., Inthapanya S., and Preston T. (2013). All biochars are not equal in lowering methane production in in vitro rumen incubations. Livest. Res. Rural Dev. 12, 12.
Lester R. E., Macqueen A., Armstrong E. K., Dodemaide D. T., Dwyer G. K., Mock T. S., et al. (2024). Can freshwater plants and algae act as an effective feed supplement to reduce methane emissions from ruminant livestock? Sci Total Environ. 914, 169296. doi: 10.1016/j.scitotenv.2023.169296
Li Y., Shen Y., Niu J., Guo Y., Pauline M., Zhao X., et al. (2021). Effect of active dry yeast on lactation performance, methane production, and ruminal fermentation patterns in early-lactating Holstein cows. J. Dairy Sci 104, 381–390. doi: 10.3168/jds.2020-18594
Lima P., Apdini T., Freire A., Santana A., Moura L., Nascimento J., et al. (2019). Dietary supplementation with tannin and soybean oil on intake, digestibility, feeding behavior, ruminal protozoa and methane emission in sheep. Anim. Feed Sci Technol. 249, 10–17. doi: 10.1016/j.anifeedsci.2019.01.017
Lin D. M., Koskella B., and Lin H. C. (2017). Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. gastrointestinal Pharmacol. Ther. 8, 162. doi: 10.4292/wjgpt.v8.i3.162
Lind V., Sizmaz Ö., Demirtas A., Sudagidan M., Weldon S., Budai A., et al. (2024). Biochar effect on sheep feed intake, growth rate and ruminant in vitro and in vivo methane production. animal 18, 101195. doi: 10.1016/j.animal.2024.101195
Li Xixi L. X., Norman H., Kinley R., Laurence M., Wilmot M., Bender H., et al. (2018). Asparagopsis taxiformis decreases enteric methane production from sheep. Animal Production Science, 58 (4), 681-688. doi: 10.1071/AN15883
Lobo R. R. and Faciola A. P. (2021). Ruminal phages - A review. Front. Microbiol. 12, 763416. doi: 10.3389/fmicb.2021.763416
Maamouri O., Selmi H., and M’hamdi N. (2014). Effects of yeast (Saccharomyces cerevisiae) feed supplement on milk production and its composition in Tunisian Holstein Friesian cows. Sci. Agric. Bohem 45, 170–174. doi: 10.2478/sab-2014-0104
Mackie R. I., Kim H., Kim N. K., and Cann I. (2023). Hydrogen production and hydrogen utilization in the rumen: key to mitigating enteric methane production. Anim. bioscience 37, 323. doi: 10.5713/ab.23.0294
Magnani E., Silva T. H., Sakamoto L., Manella M. Q., Dias F. M. G. N., Mercadante M. E., et al. (2023). Tannin-based product in feedlot diet as a strategy to reduce enteric methane emissions of Nellore cattle finished under tropical conditions. Trans. Anim. Sci 7. doi: 10.1093/tas/txad048
Majdoub-Mathlouthi L., Kraiem K., and Larbier M. (2009). Effects of feeding Saccharomyces cerevisiae Sc 47 to dairy cows on milk yield and milk components, in Tunisian conditions. Livestock Res. Rural Dev. 21, 187.
Makkar H. P. and Becker K. (1997). Degradation of quillaja saponins by mixed culture of rumen microbes. Lett. Appl. Microbiol. 25, 243–245. doi: 10.1046/j.1472-765X.1997.00207.x
Malik P., Trivedi S., Mohapatra A., Kolte A., Sejian V., Bhatta R., et al. (2021). Comparison of enteric methane yield and diversity of ruminal methanogens in cattle and buffaloes fed on the same diet. PloS One 16, e0256048. doi: 10.1371/journal.pone.0256048
Manzanilla-Pech C., Løvendahl P., Gordo D. M., Difford G., Pryce J., Schenkel F., et al. (2021). Breeding for reduced methane emission and feed-efficient Holstein cows: An international response. J. Dairy Sci 104, 8983–9001. doi: 10.3168/jds.2020-19889
Mar K. A., Unger C., Walderdorff L., and Butler T. (2022). Beyond CO2 equivalence: The impacts of methane on climate, ecosystems, and health. Environ. Sci Policy 134, 127–136. doi: 10.1016/j.envsci.2022.03.027
Martin R. and Chaudhry A. (2024). The effects of garlic as a feed additive on ruminal fermentability and ruminant performance: A meta-analysis. J. Agric. Food Res. 18, 101531. doi: 10.1016/j.jafr.2024.101531
Martin C., Morgavi D. P., and Doreau M. (2010). Methane mitigation in ruminants: from microbe to the farm scale. Animal 4, 351–365. doi: 10.1017/S1751731109990620
Martinez-Fernandez G., Kinley R. D., Smith W. J. M., Simington J., Joseph S., Tahery S., et al. (2024). Effect of fit-for-purpose biochars on rumen fermentation, microbial communities, and methane production in cattle. Front. Microbiol. 15, 1463817. doi: 10.3389/fmicb.2024.1463817
Matthews C., Crispie F., Lewis E., Reid M., O’toole P. W., and Cotter P. D. (2019). The rumen microbiome: a crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 10, 115–132. doi: 10.1080/19490976.2018.1505176
Mcallister T. and Newbold C. (2008). Redirecting rumen fermentation to reduce methanogenesis. Aust. J. Exp. Agric. 48, 7–13. doi: 10.1071/EA07218
Meehan D. J., Cabrita A. R. J., Silva J. L., Fonseca A. J. M., and Maia M. R. G. (2021). Effects of Chlorella vulgaris, Nannochloropsis oceanica and Tetraselmis sp. supplementation levels on in vitro rumen fermentation. Algal Res. 56, 102284. doi: 10.1016/j.algal.2021.102284
Melgar A., Harper M. T., Oh J., Giallongo F., Young M. E., Ott T. L., et al. (2020). Effects of 3-nitrooxypropanol on rumen fermentation, lactational performance, and resumption of ovarian cyclicity in dairy cows. J. Dairy Sci. 103, 410–432. doi: 10.3168/jds.2019-17085
Meo-Filho P., Ramirez-Agudelo J. F., and Kebreab E. (2024). Mitigating methane emissions in grazing beef cattle with a seaweed-based feed additive: Implications for climate-smart agriculture. Proc. Natl. Acad. Sci. 121, e2410863121. doi: 10.1073/pnas.2410863121
Moallem U., Lehrer H., Livshitz L., Zachut M., and Yakoby S. (2009). The effects of live yeast supplementation to dairy cows during the hot season on production, feed efficiency, and digestibility. J. Dairy Sci 92, 343–351. doi: 10.3168/jds.2007-0839
Montes F., Meinen R., Dell C., Rotz A., Hristov A. N., Oh J., et al. (2013). SPECIAL TOPICS—Mitigation of methane and nitrous oxide emissions from animal operations: II. A review of manure management mitigation options. J. Anim. Sci 91, 5070–5094. doi: 10.2527/jas.2013-6584
Morgavi D., Cantalapiedra-Hijar G., Eugène M., Martin C., Noziere P., Popova M., et al. (2023). Reducing enteric methane emissions improves energy metabolism in livestock: is the tenet right? animal 17, 100830. doi: 10.1016/j.animal.2023.100830
Morgavi D., Forano E., Martin C., and Newbold C. J. (2010). Microbial ecosystem and methanogenesis in ruminants. animal 4, 1024–1036. doi: 10.1017/S1751731110000546
Morkhade S., Bansod A., Kolaskar A., and Thakare S. (2020). A complete review on: methanogens methane producers of rumen and abatement strategies-biotechnology and microbiological strategies review. Int. J. Vet. Sci. Anim. Husb 5, 11–17.
Muizelaar W., Groot M., Van Duinkerken G., Peters R., and Dijkstra J. (2021). Safety and transfer study: transfer of bromoform present in asparagopsis taxiformis to milk and urine of lactating dairy cows. Foods 10, 584. doi: 10.3390/foods10030584
Muñoz C., Wills D., and Yan T. (2016). Effects of dietary active dried yeast (Saccharomyces cerevisiae) supply at two levels of concentrate on energy and nitrogen utilisation and methane emissions of lactating dairy cows. Anim. Production Sci 57, 656–664. doi: 10.1071/AN15356
Murali N., Srinivas K., and Ahring B. K. (2021). Increasing the production of volatile fatty acids from corn stover using bioaugmentation of a mixed rumen culture with homoacetogenic bacteria. Microorganisms 9, 337. doi: 10.3390/microorganisms9020337
Navas-Camacho A., Cortes J., and Gutierrez E. (2001). “Dietary supplementation with saponins to improve rumen function and animal performance in the tropics,” in International Symposium on Silvopastoral Systems, 2nd Congress on Agroforestry and Livestock Production in Latin America (Turrialba (Costa Rica): CATIE), 380–385.
Newbold C. J. and Rode L. (2006). “Dietary additives to control methanogenesis in the rumen,” in International congress series (Amsterdam, Netherlands: Elsevier), 138–147. doi: 10.1016/j.ics.2006.03.047
Newbold C. J., Wallace R., and Mcintosh F. (1996). Mode of action of the yeast Saccharomyces cerevisiae as a feed additive for ruminants. Br. J. Nutr. 76, 249–261. doi: 10.1079/BJN19960029
Ni M., Parra M. C., Chaves A. V., and Meale S. J. (2024). Effect of enriched biochar on methane emissions, rumen microbial structure and rumen fermentation characteristics in Holstein steers. Livestock Sci 289, 105590. doi: 10.1016/j.livsci.2024.105590
Niu H., Xu Z., Yang H. E., Mcallister T. A., Acharya S., and Wang Y. (2021). In vitro ruminal fermentation of fenugreek (Trigonella foenum-graecum L.) produced less methane than that of alfalfa (Medicago sativa). Anim. Biosci. 34, 584–593. doi: 10.5713/ajas.20.0114
Nkrumah J. D., Okine E. K., Mathison G. W., Schmid K., Li C., Basarab J. A., et al. (2006). Relationships of feedlot feed efficiency, performance, and feeding behavior with metabolic rate, methane production, and energy partitioning in beef cattle. J. Anim. Sci. 84, 145–153. doi: 10.2527/2006.841145x
Nørskov N. P., Bruhn A., Cole A., and Nielsen M. O. (2021). Targeted and untargeted metabolic profiling to discover bioactive compounds in seaweeds and hemp using gas and liquid chromatography-mass spectrometry. Metabolites 11. doi: 10.3390/metabo11050259
Ogbuewu I. P., Okoro V. M., Mbajiorgu E. F., and Mbajiorgu C. A. (2019). Beneficial effects of garlic in livestock and poultry nutrition: A review. Agric. Res. 8, 411–426. doi: 10.1007/s40003-018-0390-y
Osman A. I., Fawzy S., Farghali M., El-Azazy M., Elgarahy A. M., Fahim R. A., et al. (2022). Biochar for agronomy, animal farming, anaerobic digestion, composting, water treatment, soil remediation, construction, energy storage, and carbon sequestration: a review. Environ. Chem. Lett. 20, 2385–2485. doi: 10.1007/s10311-022-01424-x
Ouwerkerk D., Gilbert R. A., and Klieve A. (2011). Archaeaphage therapy to control rumen methanogens (Final report, Project code B.CCH.1007). North Sydney, NSW, Australia: Meat & Livestock Australia Limited.
Pachauri R. K., Allen M. R., Barros V. R., Broome J., Cramer W., Christ R., et al. (2014). “Climate change 2014: synthesis report,” in Contribution of Working Groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change (Geneva, Switzerland: Ipcc).
Paneru D., Tellez-Isaias G., Romano N., Lohakare G., Bottje W. G., and Lohakare J. (2022). Effect of graded levels of fenugreek (Trigonella foenum-graecum L.) seeds on the growth performance, hematological parameters, and intestinal histomorphology of broiler chickens. Vet. Sci. 9. doi: 10.3390/vetsci9050207
Patra A. and Saxena J. (2009). The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutr. Res. Rev. 22, 204–219. doi: 10.1017/S0954422409990163
Patra A. K. and Saxena J. (2011). Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci Food Agric. 91, 24–37. doi: 10.1002/jsfa.4152
Patra A. K. and Yu Z. (2012). Effects of essential oils on methane production and fermentation by, and abundance and diversity of, rumen microbial populations. Appl. Environ. Microbiol. 78, 4271–4280. doi: 10.1128/AEM.00309-12
Patra A. K. and Yu Z. (2015). Essential oils affect populations of some rumen bacteria in vitro as revealed by microarray (RumenBactArray) analysis. Front. Microbiol. 6, 297. doi: 10.3389/fmicb.2015.00297
Pedreira M. D. S., Primavesi O., Lima M. A., Frighetto R., Oliveira S. G. D., and Berchielli T. T. (2009). Ruminal methane emission by dairy cattle in Southeast Brazil. Scientia Agricola 66, 742–750. doi: 10.1590/S0103-90162009000600004
Pedro R., Rongcai H., Elisabeth J., Juan M., A Ignacio M.-G., Emilio M., et al. (2022). In vivo study of combining asparagopsis taxiformis and phloroglucinol to reduce methaneProduction and improve rumen fermentation efficiency in goats.In Program & Abstracts of the 8th International Greenhouse Gas & Animal Agriculture Conference (GGAA 2022), Orlando, Florida, USA (p. 239). Gainesville, FL, USA: University of Florida, IFAS Office of Conferences & Institutes. Available online at: https://conference.ifas.ufl.edu/ggaa/documents/GGAA-2022-Program-Abstracts-Online.pdf. https://hal.inrae.fr/hal-04184024v1.
Pen B., Sar C., Mwenya B., Kuwaki K., Morikawa R., and Takahashi J. (2006). Effects of Yucca schidigera and Quillaja saponaria extracts on in vitro ruminal fermentation and methane emission. Anim. Feed Sci Technol. 129, 175–186. doi: 10.1016/j.anifeedsci.2006.01.002
Pen B., Takaura K., Yamaguchi S., Asa R., and Takahashi J. (2007). Effects of Yucca schidigera and Quillaja saponaria with or without β 1–4 galacto-oligosaccharides on ruminal fermentation, methane production and nitrogen utilization in sheep. Anim. Feed Sci Technol. 138, 75–88. doi: 10.1016/j.anifeedsci.2006.11.018
Pepeta B. N., Hassen A., and Tesfamariam E. H. (2024). Quantifying the impact of different dietary rumen modulating strategies on enteric methane emission and productivity in ruminant livestock: a meta-analysis. Animals 14, 763. doi: 10.3390/ani14050763
Perdomo M. C., Marsola R. S., Favoreto M. G., Adesogan A., Staples C. R., and Santos J. E. P. (2020). Effects of feeding live yeast at 2 dosages on performance and feeding behavior of dairy cows under heat stress. J. Dairy Sci 103, 325–339. doi: 10.3168/jds.2019-17303
Phesatcha K., Phesatcha B., Chunwijitra K., Wanapat M., and Cherdthong A. (2021). Changed rumen fermentation, blood parameters, and microbial population in fattening steers receiving a high concentrate diet with saccharomyces cerevisiae improve growth performance. Vet. Sci. 8. doi: 10.3390/vetsci8120294
Pickering N. K., Oddy V., Basarab J., Cammack K., Hayes B., Hegarty R., et al. (2015). Animal board invited review: genetic possibilities to reduce enteric methane emissions from ruminants. animal 9, 1431–1440. doi: 10.1017/S1751731115000968
Pitta D. W., Indugu N., Melgar A., Hristov A., Challa K., Vecchiarelli B., et al. (2022). The effect of 3-nitrooxypropanol, a potent methane inhibitor, on ruminal microbial gene expression profiles in dairy cows. Microbiome 10, 146. doi: 10.1186/s40168-022-01341-9
Prodanović R., Bošnjaković D., Djordjevic A., Simeunović P., Arsić S., Mitrović A., et al. (2025). Effects of chestnut tannin extract on enteric methane emissions, blood metabolites and lactation performance in mid-lactation cows. Anim. (Basel) 15. doi: 10.3390/ani15152238
Pszczola M., Calus M. P. L., and Strabel T. (2019). Short communication: Genetic correlations between methane and milk production, conformation, and functional traits. J. Dairy Sci 102, 5342–5346. doi: 10.3168/jds.2018-16066
Quail M. R., Davies I. G., Moorby J. M., and Fraser M. D. (2025). Comparative intake, digestibility and enteric methane emissions by growing lambs and goat kids fed a medium digestibility grass nuts diet. animal 19, 101489. doi: 10.1016/j.animal.2025.101489
Reeve J. N. (1992). Molecular biology of methanogens. Annu. Rev. Microbiol. 46, 165–191. doi: 10.1146/annurev.mi.46.100192.001121
Reisinger A., Clark H., Cowie A. L., Emmet-Booth J., Gonzalez Fischer C., Herrero M., et al. (2021). How necessary and feasible are reductions of methane emissions from livestock to support stringent temperature goals? Philos. Trans. R. Soc. A 379, 20200452. doi: 10.1098/rsta.2020.0452
Ridla M., Laconi E., Nahrowi N., and Jayanegara A. (2021). Effects of saponin on enteric methane emission and nutrient digestibility of ruminants: An in vivo meta-analysis. IOP Conf. Series: Earth Environ. Sci 788, 012028. doi: 10.1088/1755-1315/788/1/012028
Robertson K., Symes W., and Garnham M. (2015). Carbon footprint of dairy goat milk production in New Zealand. J. Dairy Sci 98, 4279–4293. doi: 10.3168/jds.2014-9104
Rojas De Oliveira H., Sweett H., Narayana S., Fleming A., Shadpour S., Malchiodi F., et al. (2024a). Development of genomic evaluation for methane efficiency in Canadian Holsteins. JDS Commun. 5, 756–760. doi: 10.3168/jdsc.2023-0431
Rojas De Oliveira H., Sweett H., Narayana S., Fleming A., Shadpour S., Malchiodi F., et al. (2024b). Development of genomic evaluation for methane efficiency in Canadian Holsteins* *Presented as part of the Joint CSAS (Canadian Society of Animal Science) and ADSA Production, Management, & the Environment Symposium: Mitigation Strategies to Achieve Dairy Net Zero at the 2023 ADSA Annual Meeting, June 2023. JDS Commun. 5, 756–760. doi: 10.3168/jdsc.2023-0431
Romero P., Belanche A., Jiménez E., Hueso R., Ramos-Morales E., Salwen J. K., et al. (2023). Rumen microbial degradation of bromoform from red seaweed (Asparagopsis taxiformis) and the impact on rumen fermentation and methanogenic archaea. J. Anim. Sci Biotechnol. 14, 133. doi: 10.1186/s40104-023-00935-z
Romero-Perez A., Okine E. K., Mcginn S. M., Guan L. L., Oba M., Duval S. M., et al. (2014). The potential of 3-nitrooxypropanol to lower enteric methane emissions from beef cattle. J. Anim. Sci. 92, 4682–4693. doi: 10.2527/jas.2014-7573
Roque B. M., Salwen J. K., Kinley R., and Kebreab E. (2019). Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J. Cleaner Production 234, 132–138. doi: 10.1016/j.jclepro.2019.06.193
Roque B. M., Venegas M., Kinley R. D., De Nys R., Duarte T. L., Yang X., et al. (2021). Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PloS One 16, e0247820. doi: 10.1371/journal.pone.0247820
Roskam E., Kirwan S. F., Kenny D. A., O’donnell C., O’flaherty V., Hayes M., et al. (2022). Effect of brown and green seaweeds on diet digestibility, ruminal fermentation patterns and enteric methane emissions using the rumen simulation technique. Front. Anim. Sci 3, 1021631. doi: 10.3389/fanim.2022.1021631
Ross S., Wang H., Zheng H., Yan T., and Shirali M. (2024). Approaches for predicting dairy cattle methane emissions: from traditional methods to machine learning. J. Anim. Sci. 102. doi: 10.1093/jas/skae219
Rossow H. A., Riordan T., and Riordan A. (2018). Effects of addition of a live yeast product on dairy cattle performance. J. Appl. Anim. Res. 46, 159–163. doi: 10.1080/09712119.2017.1281810
Saenab A., Wiryawan K., Retnani Y., and Wina E. (2018). Manipulation of rumen fermentation by bioindustrial products of cashew nut shell (Anacardium occidentale) to reduce methane production. Media Peternakan, 40, 94-100. doi: 10.14334/jitv.v23i2.1821
Santoso B., Mwenya B., Sar C., Gamo Y., Kobayashi T., Morikawa R., et al. (2004). Effects of supplementing galacto-oligosaccharides, Yucca schidigera or nisin on rumen methanogenesis, nitrogen and energy metabolism in sheep. Livestock Production Sci 91, 209–217. doi: 10.1016/j.livprodsci.2004.08.004
Sari N. F., Ray P., Rymer C., Kliem K. E., and Stergiadis S. (2022). Garlic and its bioactive compounds: Implications for methane emissions and ruminant nutrition. Animals 12, 2998. doi: 10.3390/ani12212998
Sarmikasoglou E., Sumadong P., Dagaew G., Johnson M. L., Vinyard J. R., Salas-Solis G., et al. (2024). Effects of Bacillus subtilis on in vitro ruminal fermentation and methane production. Transl. Anim. Sci. 8, txae054. doi: 10.1093/tas/txae054
Saunois M., Stavert A. R., Poulter B., Bousquet P., Canadell J. G., Jackson R. B., et al. (2019). The global methane budget 2000–2017. Earth System Sci Data Discussions 2019, 1–136. doi: 10.5194/essd-2019-128
Schilde M., Von Soosten D., Hüther L., Meyer U., Zeyner A., and Dänicke S. (2021). Effects of 3-nitrooxypropanol and varying concentrate feed proportions in the ration on methane emission, rumen fermentation and performance of periparturient dairy cows. Arch. Anim. Nutr. 75, 79–104. doi: 10.1080/1745039X.2021.1877986
Seo J. K., Kim S.-W., Kim M. H., Upadhaya S. D., Kam D. K., and Ha J. K. (2010). Direct-fed microbials for ruminant animals. Asian-Australasian J. Anim. Sci. 23, 1657–1667. doi: 10.5713/ajas.2010.r.08
Shang A., Cao S.-Y., Xu X.-Y., Gan R.-Y., Tang G.-Y., Corke H., et al. (2019). Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 8, 246. doi: 10.3390/foods8070246
Silva T. H., Amâncio B. R., Magnani E., Meurer G. W., Reolon H. G., Timm T. G., et al. (2024). Evaluation of direct-fed microbials on in vitro ruminal fermentation, gas production kinetic, and greenhouse gas emissions in different ruminants’ diet. Front. Anim. Sci 5, 2024. doi: 10.3389/fanim.2024.1320075
Singh V. and Garg A. N. (2006). Availability of essential trace elements in Indian cereals, vegetables and spices using INAA and the contribution of spices to daily dietary intake. Food Chem. 94, 81–89. doi: 10.1016/j.foodchem.2004.10.053
Smith P. E., Kelly A. K., Kenny D. A., and Waters S. M. (2022). Differences in the composition of the rumen microbiota of finishing beef cattle divergently ranked for residual methane emissions. Front. Microbiol. 13, 855565. doi: 10.3389/fmicb.2022.855565
Snelling J. and John R. (2017). The ruminal microbiome associated with methane emissions from ruminant livestock. J Animal Sci Biotechnol. 8, 289–299. doi: 10.1186/s40104-017-0141-0
Soliva C. R., Amelchanka S. L., Duval S. M., and Kreuzer M. (2011). Ruminal methane inhibition potential of various pure compounds in comparison with garlic oil as determined with a rumen simulation technique (Rusitec). Br. J. Nutr. 106, 114–122. doi: 10.1017/S0007114510005684
Soteriades A. D., Gonzalez-Mejia A. M., Styles D., Foskolos A., Moorby J. M., and Gibbons J. M. (2018). Effects of high-sugar grasses and improved manure management on the environmental footprint of milk production at the farm level. J. Cleaner Production 202, 1241–1252. doi: 10.1016/j.jclepro.2018.08.206
Soussana J.-F. (2008). The role of the carbon cycle for the greenhouse gas balance of grasslands and of livestock production systems. Livestock Global Climate Change 12.
Soussana J.-F., Tallec T., and Blanfort V. (2010). Mitigating the greenhouse gas balance of ruminant production systems through carbon sequestration in grasslands. animal 4, 334–350. doi: 10.1017/S1751731109990784
Sperber J., Troyer B., Erickson G. E., and Watson A. K. (2022). Evaluation of the effects of pine-sourced biochar on cattle performance and methane and carbon dioxide production from growing and finishing steers. Trans. Anim. Sci 6, txac152. doi: 10.1093/tas/txac152
Starsmore K., Lahart B., Villalobos-Lopez N., Egan M., Herron J., Burke J., et al. (2024a). Residual methane emissions in grazing lactating dairy cows. New Z. J. Agric. Res. 67, 285–295. doi: 10.1080/00288233.2023.2277239
Starsmore K., Lopez-Villalobos N., Shalloo L., Egan M., Burke J., and Lahart B. (2024b). Animal factors that affect enteric methane production measured using the GreenFeed monitoring system in grazing dairy cows. J. Dairy Sci 107, 2930–2940. doi: 10.3168/jds.2023-23915
Stefanini Lopes R. and Ahring B. (2023). Enhancing acetic acid production in in vitro rumen cultures by addition of a homoacetogenic consortia from a kangaroo: unravelling the impact of inhibition of methanogens and effect of almond biochar on rumen fermentations. Fermentation 9, 885. doi: 10.3390/fermentation9100885
Stefenoni H. A., Räisänen S. E., Cueva S. F., Wasson D. E., Lage C. F. A., Melgar A., et al. (2021). Effects of the macroalga Asparagopsis taxiformis and oregano leaves on methane emission, rumen fermentation, and lactational performance of dairy cows. J. Dairy Sci 104, 4157–4173. doi: 10.3168/jds.2020-19686
Subharat S., Shu D., Zheng T., Buddle B. M., Kaneko K., Hook S., et al. (2016). Vaccination of sheep with a methanogen protein provides insight into levels of antibody in saliva needed to target ruminal methanogens. PloS One 11, e0159861. doi: 10.1371/journal.pone.0159861
Sucu E. (2020). Effects of microalgae species on in vitro rumen fermentation pattern and methane production. Ann. Anim. Sci 20, 207–218. doi: 10.2478/aoas-2019-0061
Sun X., Cheng L., Jonker A., Munidasa S., and Pacheco D. (2022). A review: plant carbohydrate types-the potential impact on ruminant methane emissions. Front. Vet. Sci. 9, 880115. doi: 10.3389/fvets.2022.880115
Sun P., Wang J., and Deng L. (2013). Effects of Bacillus subtilis natto on milk production, rumen fermentation and ruminal microbiome of dairy cows. Animal 7, 216–222. doi: 10.1017/S1751731112001188
Susanto I., Wiryawan K. G., Suharti S., Retnani Y., Zahera R., and Jayanegara A. (2023). Evaluation of Megasphaera elsdenii supplementation on rumen fermentation, production performance, carcass traits and health of ruminants: a meta-analysis. Anim. Biosci. 36, 879–890. doi: 10.5713/ab.22.0258
Swain R. A., Nolan J. V., and Klieve A. V. (1996). Natural variability and diurnal fluctuations within the bacteriophage population of the rumen. Appl. Environ. Microbiol. 62, 994–997. doi: 10.1128/aem.62.3.994-997.1996
Symeon G. K., Akamati K., Dotas V., Karatosidi D., Bizelis I., and Laliotis G. P. (2025). Manure management as a potential mitigation tool to eliminate greenhouse gas emissions in livestock systems. Sustainability 17, 586. doi: 10.3390/su17020586
Tavendale M. H., Meagher L. P., Pacheco D., Walker N., Attwood G. T., and Sivakumaran S. (2005). Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis. Anim. Feed Sci Technol. 123, 403–419. doi: 10.1016/j.anifeedsci.2005.04.037
Terler G., Winter M., Mandl M., Sweeney J., and Steinwidder A. (2023). Effect of biochar or biochar and urea supplementation on feed intake, milk yield, feed conversion and methane production of dairy cows. Czech J. Anim. Sci 68. doi: 10.17221/38/2023-CJAS
Thacharodi A., Hassan S., Ahmed Z. H. T., Singh P., Maqbool M., Meenatchi R., et al. (2024). The ruminant gut microbiome vs enteric methane emission: The essential microbes may help to mitigate the global methane crisis. Environ. Res. 261, 119661. doi: 10.1016/j.envres.2024.119661
Thorsteinsson M., Weisbjerg M., Lund P., Bruhn A., Hellwing A., and Nielsen M. (2023). Effects of dietary inclusion of 3 Nordic brown macroalgae on enteric methane emission and productivity of dairy cows. J. dairy Sci 106, 6921–6937. doi: 10.3168/jds.2023-23437
Tsapekos P., Alvarado-Morales M., and Angelidaki I. (2022). H2 competition between homoacetogenic bacteria and methanogenic archaea during biomethanation from a combined experimental-modelling approach. J. Environ. Chem. Eng. 10, 107281. doi: 10.1016/j.jece.2022.107281
Tseten T., Sanjorjo R. A., Son J.-W., Baik K. S., Berdos J. I., Kim S.-H., et al. (2025). Reduction of enteric methane emission using methanotroph-based probiotics in Hanwoo steers. Anim. Microbiome 7, 19. doi: 10.1186/s42523-025-00385-0
Tsiplakou E., Abdullah M. A., Skliros D., Chatzikonstantinou M., Flemetakis E., Labrou N., et al. (2017). The effect of dietary Chlorella vulgaris supplementation on micro-organism community, enzyme activities and fatty acid profile in the rumen liquid of goats. J. Anim. Physiol. Anim. Nutr. (Berl) 101, 275–283. doi: 10.1111/jpn.12521
Uemoto Y., Tomaru T., Masuda M., Uchisawa K., Hashiba K., Nishikawa Y., et al. (2024). Exploring indicators of genetic selection using the sniffer method to reduce methane emissions from Holstein cows. Anim. Biosci. 37, 173–183. doi: 10.5713/ab.23.0120
Ungerfeld E. M. (2020). Metabolic hydrogen flows in rumen fermentation: principles and possibilities of interventions. Front. Microbiol. 11, 589. doi: 10.3389/fmicb.2020.00589
Uyeno Y., Shigemori S., and Shimosato T. (2015). Effect of probiotics/prebiotics on cattle health and productivity. Microbes Environ. 30, 126–132. doi: 10.1264/jsme2.ME14176
Van Gastelen S., Burgers E. E. A., Dijkstra J., De Mol R., Muizelaar W., Walker N., et al. (2024). Long-term effects of 3-nitrooxypropanol on methane emission and milk production characteristics in Holstein-Friesian dairy cows. J. Dairy Sci. 107, 5556–5573. doi: 10.3168/jds.2023-24198
Van Gastelen S., Dijkstra J., Binnendijk G., Duval S. M., Heck J. M. L., Kindermann M., et al. (2020). 3-Nitrooxypropanol decreases methane emissions and increases hydrogen emissions of early lactation dairy cows, with associated changes in nutrient digestibility and energy metabolism. J. Dairy Sci. 103, 8074–8093. doi: 10.3168/jds.2019-17936
Van Gastelen S., Dijkstra J., Heck J. M. L., Kindermann M., Klop A., De Mol R., et al. (2022). Methane mitigation potential of 3-nitrooxypropanol in lactating cows is influenced by basal diet composition. J. Dairy Sci. 105, 4064–4082. doi: 10.3168/jds.2021-20782
Verma S., Akpensuen T. T., Wolffram S., Salminen J. P., Taube F., Blank R., et al. (2024). Investigating the efficacy of purified tannin extracts from underutilized temperate forages in reducing enteric methane emissions in vitro. Sci. Rep. 14, 12578. doi: 10.1038/s41598-024-63434-9
Verma S., Akpensuen T., Wolffram S., Salminen J.-P., Taube F., Blank R., et al. (2023). Effect of different condensed and hydrolysable tannin-rich extracts on methane production in vitro. In Kadžiulienė Ž., Jaškūnė K., Norkevičienė E., Toleikienė M., and Šarūnaitė L. (Eds.), Grassland Science in Europe, Vol. 28: The future role of ley-farming in cropping systems. Proceedings of the 22nd Symposium of the European Grassland Federation, Vilnius, Lithuania, 11–14 June 2023 (pp. 267–269). Akademija, Kėdainiai District, Lithuania: Lithuanian Research Centre for Agriculture and Forestry (LAMMC). (Editing & production: Wageningen Academic Publishers, Wageningen, The Netherlands.). doi: 10.1038/s41598-022-14424-2
Visuvanathan T., Than L. T. L., Stanslas J., Chew S. Y., and Vellasamy S. (2022). Revisiting trigonella foenum-graecum L.: pharmacology and therapeutic potentialities. Plants (Basel) 11. doi: 10.3390/plants11111450
Wallace R. J. (2004). Antimicrobial properties of plant secondary metabolites. Proc. Nutr. Soc. 63, 621–629. doi: 10.1079/PNS2004393
Wallace R. J., Mcewan N. R., Mcintosh F. M., Teferedegne B., and Newbold C. J. (2002). Natural products as manipulators of rumen fermentation. Asian-Australasian J. Anim. Sci. 15, 1458–1468. doi: 10.5713/ajas.2002.1458
Wanapat M., Prachumchai R., Dagaew G., Matra M., Phupaboon S., Sommai S., et al. (2024). Potential use of seaweed as a dietary supplement to mitigate enteric methane emission in ruminants. Sci Total Environ. 931, 173015. doi: 10.1016/j.scitotenv.2024.173015
Wang B., Jia M., Fang L., Jiang L., and Li Y. (2018). Effects of eucalyptus oil and anise oil supplementation on rumen fermentation characteristics, methane emission, and digestibility in sheep. J. Anim. Sci 96, 3460–3470. doi: 10.1093/jas/sky216
Wang M., Li Y., Ren S., Shen Y., Chen P., Cui Q., et al. (2024a). Effects of quebracho–chestnut tannin extract supplementation on production performance, nitrogen partitioning, and rumen fermentation patterns in early-lactating Holstein cows. Anim. Feed Sci Technol. 315, 116043. doi: 10.1016/j.anifeedsci.2024.116043
Wang Z., Liu X., Zhao M., Ma W., Wang Y., Jia Y., et al. (2024b). Effect of spirulina on the rumen microbiota and serum biochemical parameters of lambs. Microorganisms 12, 2473. doi: 10.3390/microorganisms12122473
Wang Y., Mcallister T., Newbold C. J., Rode L., Cheeke P., and Cheng K. (1998). Effects of Yucca schidigera extract on fermentation and degradation of steroidal saponins in the rumen simulation technique (RUSITEC). Anim. feed Sci Technol. 74, 143–153. doi: 10.1016/S0377-8401(98)00137-0
Wang H., Yu Z., Gao Z., Li Q., Qiu X., Wu F., et al. (2022). Effects of compound probiotics on growth performance, rumen fermentation, blood parameters, and health status of neonatal Holstein calves. J. Dairy Sci 105, 2190–2200. doi: 10.3168/jds.2021-20721
Watson D., Colditz I., Andrew M., Gill H., and Altmann K. (1994). Age-dependent immune response in Merino sheep. Res. Veterinary Sci 57, 152–158. doi: 10.1016/0034-5288(94)90051-5
Wedlock D., Janssen P., Leahy S., Shu D., and Buddle B. (2013). Progress in the development of vaccines against rumen methanogens. animal 7, 244–252. doi: 10.1017/S1751731113000682
Wedlock D., Pedersen G., Denis M., Dey D., Janssen P., and Buddle B. (2010). Development of a vaccine to mitigate greenhouse gas emissions in agriculture: vaccination of sheep with methanogen fractions induces antibodies that block methane production in vitro. New Z. Veterinary J. 58, 29–36. doi: 10.1080/00480169.2010.65058
Williams Y. J., Popovski S., Rea S. M., Skillman L. C., Toovey A. F., Northwood K. S., et al. (2009). A vaccine against rumen methanogens can alter the composition of archaeal populations. Appl. Environ. Microbiol. 75, 1860–1866. doi: 10.1128/AEM.02453-08
Wina E., Muetzel S., and Becker K. (2005). The impact of saponins or saponin-containing plant materials on ruminant production–a review. J. Agric. Food Chem. 53, 8093–8105. doi: 10.1021/jf048053d
Winders T. M., Jolly-Breithaupt M. L., Wilson H. C., Macdonald J. C., Erickson G. E., and Watson A. K. (2019). Evaluation of the effects of biochar on diet digestibility and methane production from growing and finishing steers. Transl. Anim. Sci. 3, 775–783. doi: 10.1093/tas/txz027
Wright A. D. G., Kennedy P., O’neill C. J., Toovey A. F., Popovski S., Rea S. M., et al. (2004). Reducing methane emissions in sheep by immunization against rumen methanogens. Vaccine 22, 3976–3985. doi: 10.1016/j.vaccine.2004.03.053
Wyffels S. A., Delcurto T., and Clark A. (2013). Influence of beef cattle stocking density on utilization of vegetative communities in a late-spring early-summer native bunchgrass prairie. Proc. West. Sect. Am. Soc Anim. Sci., 332–335. doi: 10.5296/jas.v8i4.17462
Yang L., Chen L., Zheng K., Ma Y.-J., He R.-X., Arowolo M. A., et al. (2022). Effects of fenugreek seed extracts on growth performance and intestinal health of broilers. Poultry Sci 101, 101939. doi: 10.1016/j.psj.2022.101939
Yang C.-L., Guan L.-L., Liu J.-X., and Wang J.-K. (2015). Rumen fermentation and acetogen population changes in response to an exogenous acetogen TWA4 strain and Saccharomyces cerevisiae fermentation product. J. Zhejiang University. Sci. B 16, 709. doi: 10.1631/jzus.B1500013
Yang C., Rooke J. A., Cabeza I., and Wallace R. J. (2016). Nitrate and inhibition of ruminal methanogenesis: microbial ecology, obstacles, and opportunities for lowering methane emissions from ruminant livestock. Front. Microbiol. 7, 132. doi: 10.3389/fmicb.2016.00132
Yang Y., Wang H., and Lv S. (2021). Effects of different ratio of garlic skin on serum biochemistry, anti-oxidative status, and immunity of Yimeng black goats. Feed Res. 8, 1–5. doi: 10.3390/foods14111911
Yanza Y. R., Irawan A., Jayanegara A., Ramadhani F., Respati A. N., Fitri A., et al. (2024). Saponin extracts utilization as dietary additive in ruminant nutrition: A meta-analysis of in vivo studies. Anim. (Basel) 14. doi: 10.3390/ani14081231
Yu G., Beauchemin K. A., and Dong R. (2021). A review of 3-nitrooxypropanol for enteric methane mitigation from ruminant livestock. Animals 11, 3540. doi: 10.3390/ani11123540
Yu H., Liang H., Ren M., Ji K., Yang Q., Ge X., et al. (2019). Effects of dietary fenugreek seed extracts on growth performance, plasma biochemical parameters, lipid metabolism, Nrf2 antioxidant capacity and immune response of juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 94, 211–219. doi: 10.1016/j.fsi.2019.09.018
Yu S., Zhao Y., Li L., Zhao H., Liu M., and Jiang L. (2024). Flavonoids from citrus peel display potential synergistic effects on inhibiting rumen methanogenesis and ammoniagenesis: a microbiome perspective. Environ. Sci pollut. Res. 31, 1–16. doi: 10.1007/s11356-024-32509-5
Zafarian R. and Manafi M. (2013). Effect of garlic powder on methane production, rumen fermentation and milk production of buffaloes.
Zhang L., Huang X., Xue B., Peng Q., Wang Z., Yan T., et al. (2015). Immunization against rumen methanogenesis by vaccination with a new recombinant protein. PloS One 10, e0140086. doi: 10.1371/journal.pone.0140086
Zhang C., Jiang X., Wu S., Zhang J., Wang Y., Li Z., et al. (2024a). Dietary fat and carbohydrate-balancing the lactation performance and methane emissions in the dairy cow industry: A meta-analysis. Anim. Nutr. 17, 347–357. doi: 10.1016/j.aninu.2024.02.004
Zhang F., Li B., Ban Z., Liang H., Li L., Zhao W., et al. (2021). Evaluation of origanum oil, hydrolysable tannins and tea saponin in mitigating ruminant methane: In vitro and in vivo methods. J. Anim. Physiol. Anim. Nutr. 105, 630–638. doi: 10.1111/jpn.13501
Zhang Z., Macedo I., Linquist B. A., Sander B. O., and Pittelkow C. M. (2024b). Opportunities for mitigating net system greenhouse gas emissions in Southeast Asian rice production: A systematic review. Agriculture Ecosyst. Environ. 361, 108812. doi: 10.1016/j.agee.2023.108812
Zhao Y. and Zhao G. (2022). Decreasing ruminal methane production through enhancing the sulfate reduction pathway. Anim. Nutr. 9, 320–326. doi: 10.1016/j.aninu.2022.01.006
Zhou G.-W., Yang X.-R., Marshall C. W., Li H., Zheng B.-X., Yan Y., et al. (2017). Biochar addition increases the rates of dissimilatory iron reduction and methanogenesis in ferrihydrite enrichments. Front. Microbiol. 8, 589. doi: 10.3389/fmicb.2017.00589
Keywords: methane, GHG, ruminant livestock, methanogenesis, mitigation strategies, feed additives
Citation: Malyugina S, Holik S and Horky P (2025) Mitigation strategies for methane emissions in ruminant livestock: a comprehensive review of current approaches and future perspectives. Front. Anim. Sci. 6:1610376. doi: 10.3389/fanim.2025.1610376
Received: 11 April 2025; Accepted: 08 September 2025;
Published: 25 September 2025.
Edited by:
Suban Foiklang, Maejo University, ThailandReviewed by:
Ravikanthreddy Poonooru, University of Missouri, United StatesChaichana Suriyapha, Khon Kaen University, Thailand
Hossam M. Ebeid, National Research Centre, Egypt
Copyright © 2025 Malyugina, Holik and Horky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pavel Horky, cGF2ZWwuaG9ya3lAbWVuZGVsdS5jeg==; Svetlana Malyugina, c3ZldGxhbmEubWFseXVnaW5hQG1lbmRlbHUuY3o=
 Svetlana Malyugina
Svetlana Malyugina Simon Holik1
Simon Holik1 Pavel Horky
Pavel Horky