- Department of Agricultural Economics and Animal Production, University of Limpopo, Sovenga, South Africa
In the face of climate variability and resource limitations, stress-related hormonal disruptions challenge the sustainability and reproductive performance of small ruminants. This systematic review evaluated the impact of key stress hormones such as cortisol, adrenaline, and glucocorticoids on reproductive functions of sheep and goats. A comprehensive search of databases including PubMed, ScienceDirect, Web of Science, and Google Scholar yielded 29 eligible peer-reviewed articles published between 2005 and 2024. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Population, Intervention, Comparison, Outcome (PICO) tools were employed for evidence-based practice ensuring a rigorous and transparent manner in conducting and reporting this research. The Cochrane Risk of Bias instrument was adopted to assess data quality and biasness of the included studies. The results consistently indicated elevated stress hormone levels impair the hypothalamic-pituitary-gonadal axis, leading to delayed ovulation, reduced conception rates, irregular estrous cycles, and compromised sperm quality. Major environmental factors contributing to hormonal imbalance identified were heat stress, nutritional deficiencies, inappropriate handling, social instability, and diseases. Additionally, genetic variation among breeds influenced the severity of stress responses. However, the limited African-based research and inconsistent reporting of environmental conditions highlight critical knowledge gaps. This review emphasizes the need for climate-resilient and welfare-oriented livestock strategies meant to mitigate stress and enhance reproductive outcomes in sheep and goats.
1 Introduction
The reproductive efficiency in sheep and goats is intricately linked to hormonal balance, which is frequently disrupted by stress hormones (Dobson et al., 2012; Tüfekci and Sejian, 2023). Among the primary stress hormones such as catecholamines and glucocorticoids, the key regulators have been identified. The stress hormone, cortisol, produced in the adrenal cortex, is the principal glucocorticoid which plays a role in metabolism, immune modulation, and regulation of the hypothalamic-pituitary-adrenal (HPA) axis (Thau et al., 2023). Cortisol interferes with reproductive hormone regulation and gametogenesis, this potentially leads to decreased fertility (Thau et al., 2023). An elevated cortisol levels disrupt normal functioning of hypothalamic-pituitary-gonadal axis. This is vital for regulating reproductive health and can result in delayed ovulation, disrupted estrous cycles and reduced fertility (Baker JA et al., 2020; Dobson et al., 2012; Thau et al., 2023). Adrenalin is secreted by the adrenal medulla and is known to causes the acute “fight or flight” reaction. Glucocorticoids and adrenaline influence physiological processes which disrupt reproductive functions. This is achieved through interference of the ovulation cycle and sperm viability resulting typically from prolonged exposure to the stress hormones (Dobson et al., 2012; Seckl and Holmes, 2021a).
Substantial research on how stress hormones affect reproductive functions has been conducted in temperate regions such as Europe, North America, and Australia. There are significant gaps in global coverage especially from Africa, Asia, and South America. This imbalance limits the generalizability of findings and the development of region-specific interventions. Only limited research on sheep in the coastal region of the Black Sea region has been carried out in certain periods of the year as they migrate to the plateau (Tozlu Çelik et al., 2021). South African studies tend to focus on observational assessments with minimum exploration of molecular pathways. The European contexts are underrepresented in the literature despite being a place to substantial small ruminant populations under stress prone conditions (Gómez J et al., 2023). Inadequate housing conditions, poor nutrition, and extreme climatic variations are prevalent influencing factors in many African regions. These exacerbates stress and contribute to elevated cortisol levels among other associated reproductive issues (Gómez J et al., 2023). Zhang et al. (2020) demonstrated that suboptimal housing is directly correlated with increased cortisol levels, thus negatively impacting reproductive performance. Additionally, Mwanga et al. (2019) highlighted that nutritional deficiencies and poor management practices in East Africa are linked to increased stress and compromised reproductive health in small ruminants. Similarly, South American research, particularly from regions such as the Andes and semi-arid zones, is sparse despite their unique agro-climatic challenges.
The influence of environmental stressors on reproductive health is further highlighted by studies conducted in various African countries. In Nigeria, found that inadequate housing and nutrition significantly affect cortisol levels and reproductive outcomes in goats. Similar results were reported by Mburu et al. (2023), Coetzee et al. (2022) and Desta et al. (2022) in Kenyan small stock, South African sheep and Ethiopian goats respectively. The high temperatures and poor shelter were the major factors affecting reproductive performance. Studies in South Africa by Coetzee et al. (2022) and in Ethiopia by Desta et al. (2022) provide further evidence of the adverse effects of environmental stress on reproductive health. The African continent faces unique challenges such as extreme climate variability, resource limitations, and widespread low-input farming systems, which exacerbate the stress burden on animals. Unlike in temperate systems where environmental control, genetic selection, and housing are more developed, it has been long established that the African production environments are characterized by high heat loads, nutritional stress, poor infrastructure, and limited veterinary oversight.
Genetic variability among small ruminant genotypes in Africa adds another layer of complexity envisaged in reproductive performance. Indigenous breeds such as the Ethiopian Highlands sheep and West African Dwarf goat exhibit distinct stress response mechanisms compared to temperate breeds, thus affecting their reproductive health differently (Daramola et al., 2021). Understanding these breed-specific variations is crucial for developing effective management practices tailored to local husbandry and climatic conditions. Research by Daramola et al. (2021) indicated that indigenous breeds in West Africa demonstrate different stress responses and reproductive outcomes compared to exotic breeds. Despite the documented effects of stress hormones on reproduction, there remains a lack of comprehensive studies specifically exploring these mechanisms in an African context. The interplay between environmental stressors and physiological responses complicates the development of effective management strategies. The systematic review aims to assess the impact of different levels of primary stress hormones on the reproductive efficiency of sheep and goats across diverse environmental conditions across the world.
2 Materials and methods
2.1 Eligible criteria
The systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The articles included in the review process focused on sheep and goats as the primary subjects and specifically exploring hormonal impact on reproductive functions. The reproductive functions of concern included fertility parameters and reproductive hormone levels. The hormones of interest were cortisol, glucocorticoids and adrenaline. The systematic review was eligibly complied with the Population, Intervention, Comparison and Outcome (PICO) framework. This framework was depicted as: Population (P): sheep and goats’ breeds. Intervention (I): Exposure to environmental stressors affecting levels of hormones (cortisol, adrenaline, glucocorticoids). Comparison (C): Comparison between diverse ecological zones, management practices and breeds/genotypes. Outcome (O): Reproductive trait performance such as age at first calving, calving interval, conception rate, services per conception, pregnancy rate, prolificacy, twinning rate and associated reproductive hormonal fluctuations such as progesterone and estrogen. Articles that addressed at least one of these outcomes were considered. Studies involving other ruminant species were excluded unless they provided comparative context relevant to small ruminants. At first glance, articles considered eligible had their titles and/or abstract with at least one of the following keywords or phrases: “sheep,” or “goats,” or “small ruminants,” or “reproductive performance,” or “fertility,” or “estrous cycle,” or “luteinizing hormone,” or “follicle-stimulating hormone,” or “cortisol,” or “glucocorticoids,” or “adrenaline,” or “stress response,” or “stress physiology,” or “breeding performance,” “breed/genotypes” or “reproductive hormones,” or “reproductive health,” or “tropical” or “humid” or “arid” or “environmental stress,” or “nutritional stress,” or “heat stress” or “small ruminants”. The review included articles published over a 20-year period starting from 2005 to 2024. Eligible studies were published in peer-reviewed journals and written in English language. Various study designs, including randomized controlled trials, observational studies, and systematic reviews/meta-analyses, were deemed eligible for inclusion.
2.2 Search strategy
The search strategy covered the core elements of the PICO framework specifically focusing on sheep, goats, stress hormones and reproductive functions. Three researchers (Mojakgomo Sidney Mamakoko, Puleng Mashamaite and Phillip Masilo Tshabuse) independently conducted the search of publications on databases such as Google Scholar, PubMed, Science Direct, and Web of Science from the 14th of August 2024 to 28th of March 2025. A combination of the following key terms and phrases were used for information search: “noradrenaline” or “catecholamines” or “HPA axis” or “environmental influences” or “ecological zones” or “nutritional aspects” or “housing conditions” or “cortisol” or “glucocorticoids” or “interactions with reproductive hormones” or “ physiological and psychological factors” or “impact on fertility and reproductive health”.
2.3 Selection/inclusion criteria
The systematic review included studies published in English from the year 2005 to 2024 and focusing on sheep and goats, examining the effects of stress hormones such as cortisol, glucocorticoids and adrenaline on reproductive functions. Eligible studies reported outcomes related to fertility parameters such as conception rates and litter size, as well as reproductive hormone levels including progesterone and estrogen. Accepted study designs encompassed randomized controlled trials, observational studies, and systematic reviews/meta-analyses. The inclusion of studies followed the PICO framework for eligibility.
2.4 Exclusion
The exclusion criteria of the study contained: (1) duplicate articles/records, (2) records irrelevant to sheep and goats stress hormones and reproductive functions, (3) research reports with no available original data and information on study design, production system, sample size, grey areas, publications without authors, and (4) articles not peer-reviewed.
2.5 Data extraction
The research gathered information and data using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) approach. Each selected article was carefully checked to ensure data accuracy and reliability. Data extraction focused on factors affecting stress hormone levels, how stress hormones affected the reproductive functions of sheep and goats, through application of standardized structure of collecting information from the citations and abstracts. The systematic review aimed to put together evidence for clear reporting and ensuring good insights into how stress hormones impacted reproductive outcomes in the small ruminants.
2.6 Quality assessment
Risk of bias assessment was done using the Cochrane Risk of Bias Tool (Higgins et al., 2024), where two reviewers (Mojakgomo Sidney Mamakoko and Obert Tada) evaluated the seven data set domains of potential bias. These were 1. Adequacy of the randomization process. 2. Deliverance of the intended interventions. 3. Adequacy of handling missing outcome data. 4. Adequacy of outcome measurement. 5. Adequacy of the reported result selection. 6. Presence of bias in the selection of participants. 7. Presence of any other potential source of bias. The reviewers judged each domain as low risk, unclear risk/some concerns, and high risk. The overall risk of bias is determined by the domain with the highest risk of bias. The resolution of discrepancies was resolved through consensus.
3 Results
3.1 Outcome of the literature searched
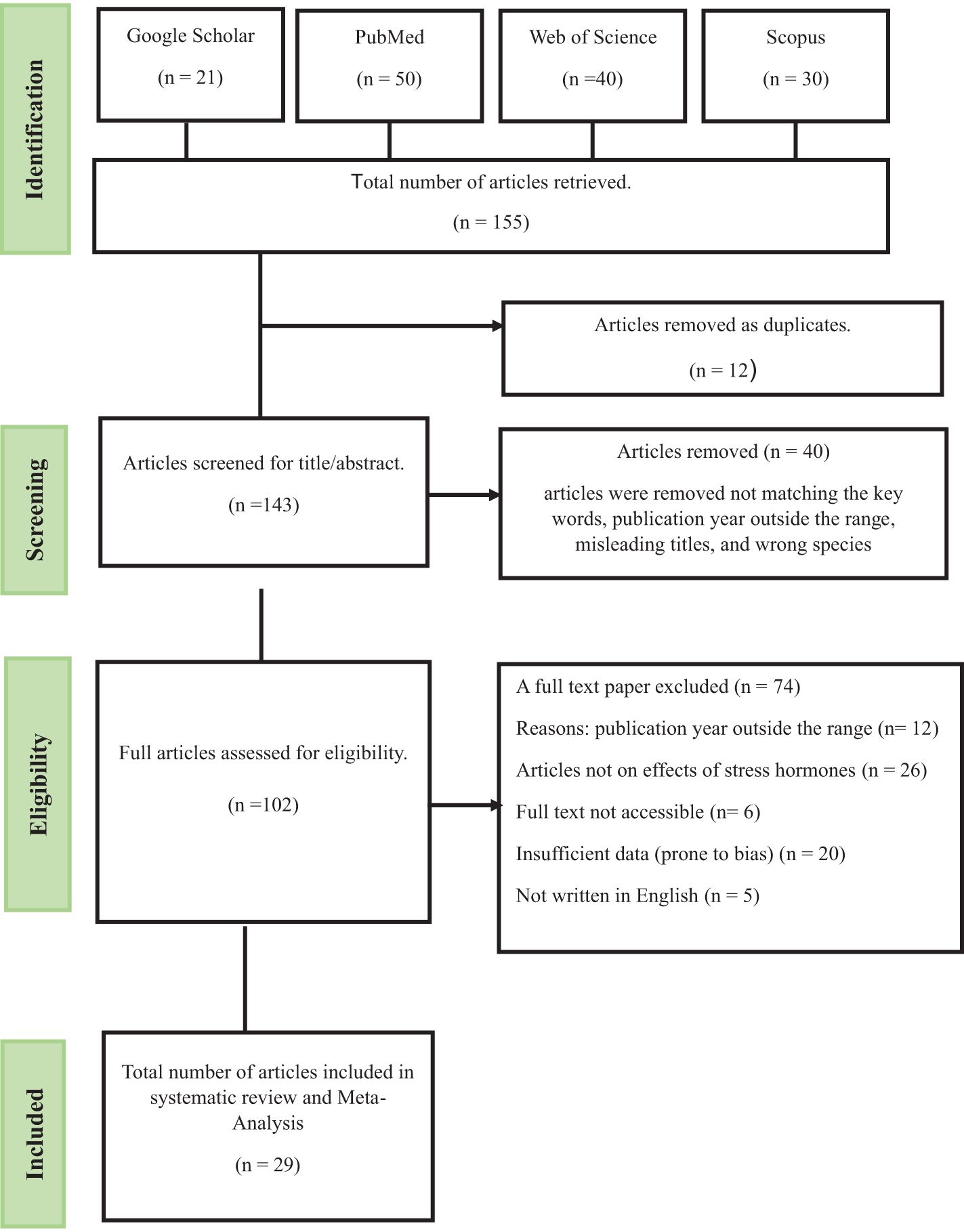
The review process, illustrated using a PRISMA flow diagram (Figure 1), began with a total of 155 articles identified across four databases. After initial screening, 12 duplicate records were removed. A total of 143 articles were assessed based on publication year, misleading title, wrong species, and key words in the title, resulting in exclusion of 40 articles. A total of 74 articles were then removed from 103 remaining articles. The exclusion was based of eligibility, retrieval, insufficient data, language and objective. Ultimately, 29 articles met the inclusion and exclusion criteria and were assessed in the systematic review.
3.2 Characteristics of included studies in the systematic review
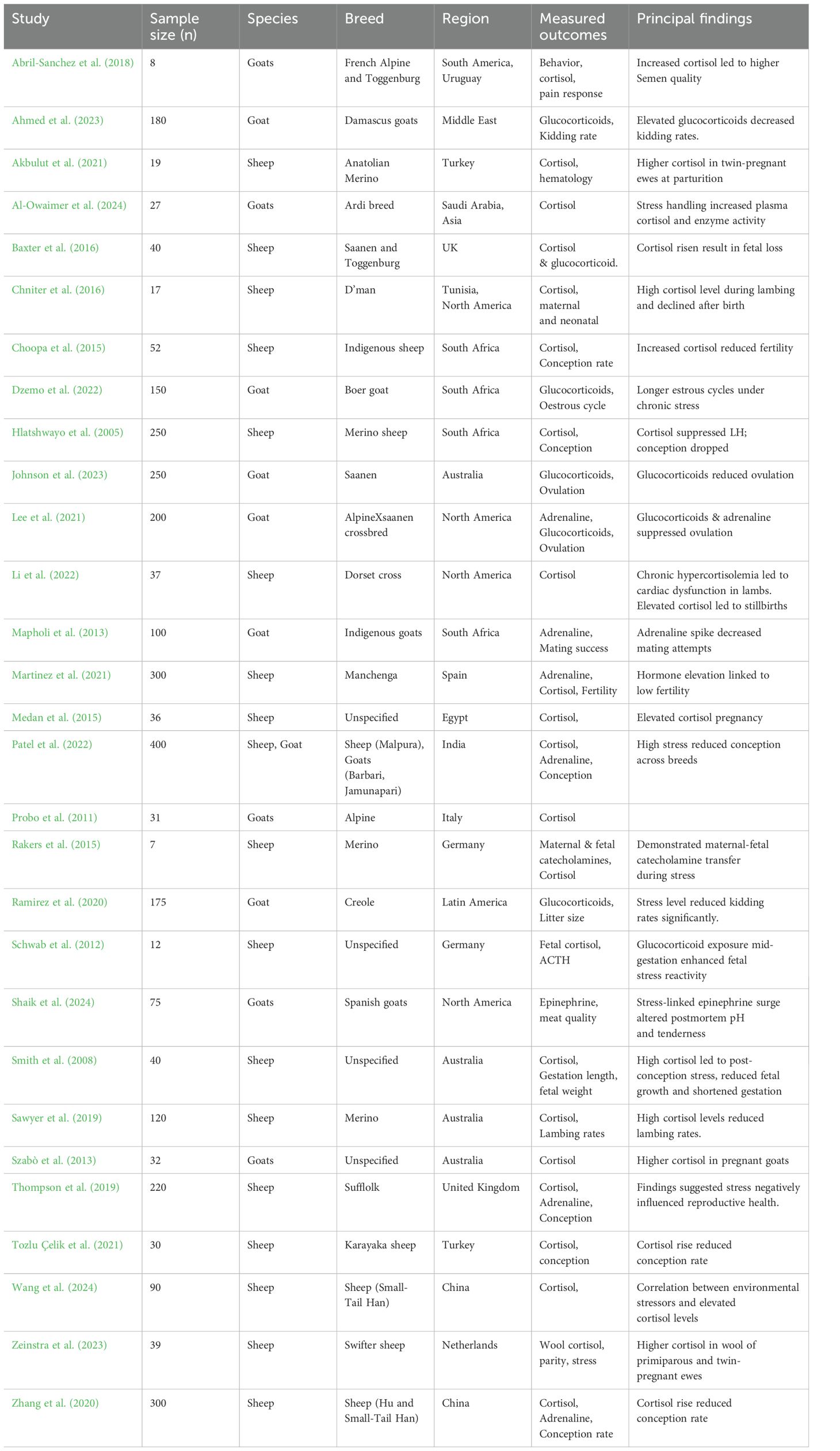
The summarized key characteristics of studies that formed part of this review is shown in Table 1, while Table 2 shows the comparative effects of stress hormones on male and female reproductive physiology of sheep and goats reported in the included studies. The systematic review focused on peer-reviewed original research articles encompassing observational, experimental, and clinical trial studies. This approach ensured the analysis was based on empirical data rather than reviews or opinion pieces. The papers predominately examined the effect of stress hormones on reproductive functions in sheep and goats in different production systems and ecological zones. The studies varied in sample size with the number of sheep or goats ranging from 7 to 400. Across the 29 included studies, the effects of stress were consistently more pronounced in female animals, particularly during critical reproductive windows such as estrous, conception, pregnancy, and parturition. Cortisol, glucocorticoids, and adrenaline were the predominant hormones investigated, with multiple studies confirming their disruptive role in the hypothalamic-pituitary-gonadal (HPG) axis. The review emphasized on studies with rigorous methodologies and strong data collection methodologies to ensure high-quality evidence and limited biased results. Studied conducted in Africa were prioritized, although relevant studies from other regions were also considered. The design aimed to provide a comprehensive and reliable synthesis of how stress hormones affected reproductive health in small ruminants. This thereby offered valuable insights for improving livestock management practices and advancing the understanding of stress-related reproductive challenges.
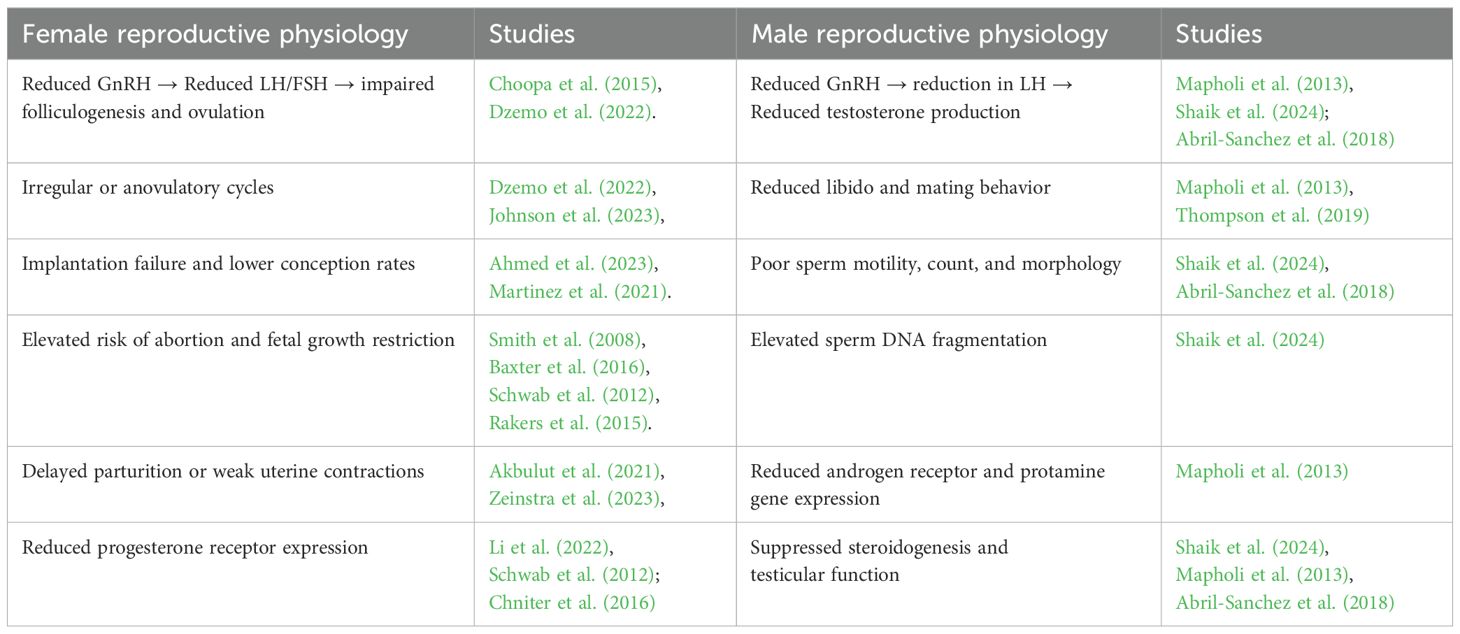
Table 2. Comparative reproductive physiology effects of stress hormones in male and female sheep and goats.
3.3 Publication by year
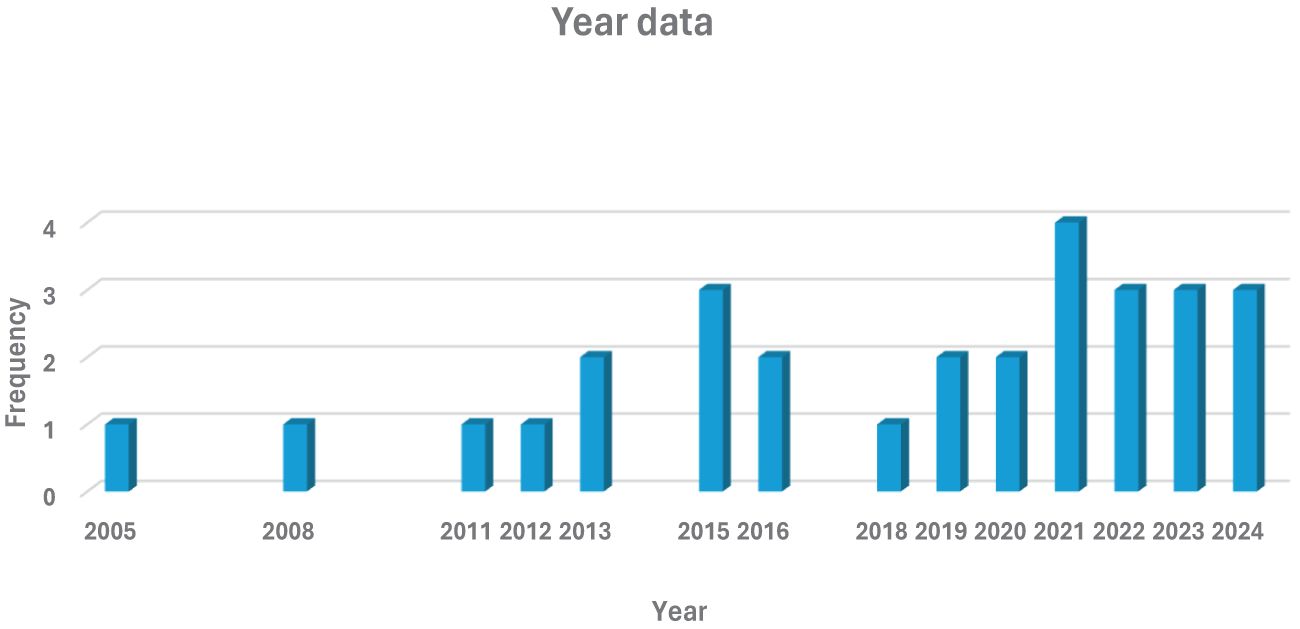
The number of publications and number of years is presented under Figure 2. By analyzing the trend in publication from 2005 to 2024, a significant rise in research focused on stress hormones and their effects on reproduction in sheep and goats was revealed. In the years (2019–2024), publication levels were modest primarily addressing basic hormonal mechanisms and general physiology. As the livestock industry increased demands welfare standards, the understanding of the hormonal influences on reproduction became crucial, highlighting the need for sustainable management strategies. Before this phase, studies investigated stress hormones in relation to awareness of animal welfare in agriculture. The researchers therefore saw the need to explore specific stressors like environmental conditions and herd dynamics.
3.4 Number of studies per geographical region
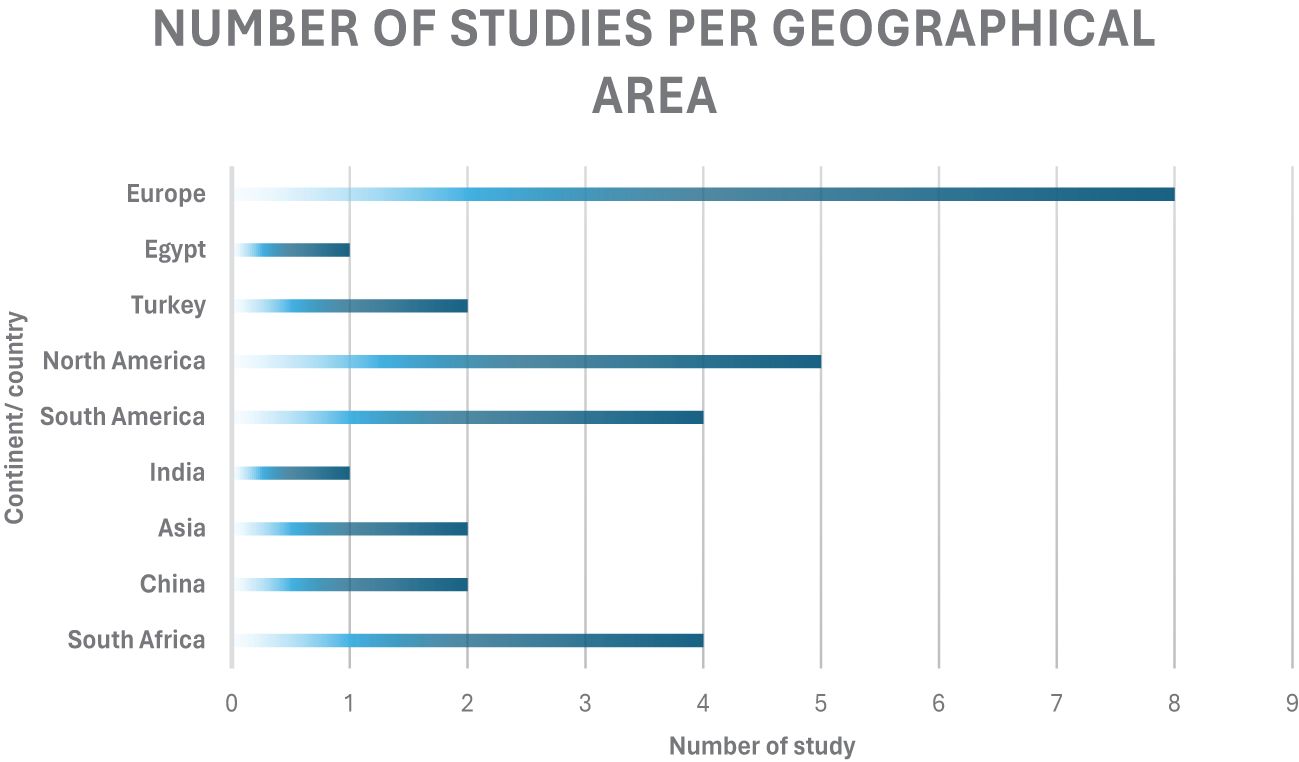
The 29 articles included in this review were conducted across a range of geographical regions (Figure 3). The distribution highlights a strong regional bias, with Europe, North America, South America and South Africa leading this regional research imbalance. There is limited data from tropical regions outside of Africa such as South America and Southeast Asia despite their significant small ruminant populations. This highlights the need and literature gap of geographical diversity and region-specific research which ensures a broader applicability of findings.
3.5 Study quality and risk of bias assessment
A formal quality and bias assessment were conducted for all 29 studies using predefined criteria following the Cochrane Risk of Bias Tool (Higgins et al., 2024). The domains focused on clarity of objectives, sample size, breed identification, study design, measurement consistency, and reporting of statistical outcomes. The 29 articles had low risk. Across these 29 studies included in the systematic review, 12 studies were rated as high quality. These studies feature well-structured experimental or controlled observational designs, clear breed documentation, standardized hormone assays, and reported effective sizes or statistical significance. Examples include studies by Dzemo et al. (2022), Zhang et al. (2020), and Patel et al. (2022). Eleven (11) were classified as moderate quality. These studies met most methodological requirements but had smaller sample sizes, absence of control groups, or incomplete reporting of hormone assay methods. Examples include Choopa et al. (2015) and Ahmed et al. (2023). Six (6) studies were rated as low quality, with missing descriptions of hormone measurement protocols, small sample sizes, lack of breed identification, and absence of comparative or control data.
4 Discussion
4.1 Stress hormones and their characteristics in sheep and goats
The systematic review outlined the primary stress hormones and their specific characteristics in sheep and goats. Cortisol was identified as the principal stress hormone, synthesized in the adrenal cortex and released in response to various stressors, including environmental changes, social dynamics, and nutritional deficiencies. Stress hormones levels are commonly high in dairy goats breeds such as Saanen and Toggenburg this is due to regular handing and high metabolic demands of lactation. While in meat goats breeds such as Boer goats, stress hormone levels are lower due to their selection for growth over productivity. The sheep wool breeds such as Dorper and Red Maasai, normally have lower stress hormone response particularly for cortisol as compared to other wool breeds such as Merino and Suffolk. The multifaceted roles of cortisol were emphasized in regulating metabolism, immune function, and reproduction, highlighting its importance in overall animal health. Studies by Baker MA et al. (2020) illustrated that chronic stress conditions result in sustained elevations of cortisol levels. This significantly compromise reproductive health and general well-being in small ruminants. Understanding the role of cortisol is critical for developing strategies aimed at minimizing stress in livestock. Cortisol acts primarily through the hypothalamic-pituitary-adrenal (HPA) axis, impacting various physiological processes. Elevated cortisol levels can impair the release of reproductive hormones. As a result, this disrupts the hypothalamic-pituitary-gonadal (HPG) axis, which is crucial for gametogenesis. This connection underscores the importance of continuous monitoring of stressors affecting animals as prolonged exposure to cortisol can lead to significant declines in fertility rates and reproductive efficiency.
Adrenaline is less discussed in the context of reproductive physiology, but it plays a crucial role in severe stress response. Adrenaline rapid release during stressful situations prepares the animal for immediate action, influencing heart rate and energy availability. Adrenaline effects are typically short-lived, but they can rapidly disrupt reproductive hormone secretion and influence ovulation cycles. Acute spikes in adrenaline can lead to delayed ovulation or irregular estrous cycles, posing challenges for effective breeding (Mapholi et al., 2013). The transient nature of adrenaline’s impact necessitates a focus on managing acute stressors to prevent negative effects on reproductive performance. Implementing management strategies, such as minimizing handling stress during breeding times, can help mitigate these acute impacts.
Glucocorticoids are a class of steroid hormones that includes cortisol and are instrumental in the chronic stress response. Prolonged exposure to elevated glucocorticoid levels has been shown to disrupt normal reproductive processes (Ahmed et al., 2023). This leads to issues such as irregular estrous cycles and reduced fertility rates. This review highlighted that the effects of glucocorticoids are particularly pronounced in breeding situations, where maintaining optimal hormonal balance is crucial for reproductive success. Research has indicated that glucocorticoids can cause anovulation and reduce sperm quality, which can have lasting effects on herd productivity (Lee et al., 2021). Understanding the distinctions and interactions between these stress hormones is essential for effective management practices that prioritize animal welfare.
4.1.1 Role of stress hormones in female reproductive physiology
Stress has a major influence on reproductive physiology in female small ruminants such as goats and sheep, primarily through altering endocrine signaling pathways. Stress responses key hormonal mediators such as catecholamines (noradrenaline and adrenaline), glucocorticoids (cortisol) and corticotrophin releasing hormone (CRH) interact with hypothalamic pituitary gonadal (HPG) axis. This result in a significant disruption in reproductive functions of the animal. Under stress conditions hypothalamic-pituitary-adrenal (HPA) axis activation causes increased secretion of adrenocorticotropic hormone (ACTH) and CRH, which stimulates the release cortisol by adrenal cortex. High levels of cortisol exert negative feedback effect on hypothalamus and pituitary gland, leading to suppressing the release of gonadotropin-releasing hormone (GnRH).
GnRH reduction diminishes the pulsatile secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary. FSH and LH play an important role in oestrogen production, formation of ovarian follicles and ovulation, hence their distraction impairs folliculogenesis, causes delayed or absent ovulation, and disrupts estrous cyclicity. Severe stress in female small ruminants can result in silent estrous or anovulation and lower levels of oestrogen compromise behavioral oestrous and reduces LH surge necessary for ovulation. This hormonal imbalance can extend the length of the estrous cycle or prevent it entirely, thereby reducing reproductive efficiency. Regularly elevated glucocorticoids result to placental insufficiency, fetal growth restriction, and increased abortion rates.
4.1.2 Role of stress hormones in male reproductive physiology
In male small ruminants, the hormonal disruptions of primary mediators of stress responses such as glucocorticoids (cortisol), catecholamines (adrenaline and noradrenaline), and corticotropin-releasing hormone (CRH) lead to impaired reproductive function, mainly through suppression of testosterone production and spermatogenesis. Under stress conditions, HPA axis activation results in high levels of CRH and ACTH, leading to increased cortisol secretion from the adrenal cortex. Cortisol applies negative feedback on the hypothalamus and anterior pituitary, inhibiting the release of GnRH. This result in reduced LH and FSH secretion which are critical for testicular function. LH in males stimulates Leydig cells to produce testosterone in the testes, which is a key hormone for beginning and maintaining spermatogenesis, sexual behavior, and secondary sex characteristics. Reduction in testosterone due to severe stress, lead to reduced oligospermia (sperm count), asthenozoospermia (sperm motility), sperm morphology and teratozoospermia. The secretion of catecholamines (adrenaline) causes vasoconstriction of testicular blood vessels, which limit oxygen and nutrients to the testes. This temporary ischemia together with increased oxidative stress can damage germ cells and Sertoli cells, further impairing spermatogenesis. Stress related hormonal changes influence males’ sexual behavior and libido, further reducing reproductive success. The loss of testosterone and associated mood changes can decrease mating interest and performance. The suppression of LH due to induced cortisol decreases secretion of testosterone, compromising spermatogenesis and overall reproductive performance
4.2 Mechanisms of hormonal disruption on reproductive physiology
The mode of action of stress hormones on reproductive physiology was elucidated in this review, emphasizing the complex hormonal pathways involved. Cortisol primary effects are mediated through its interaction with the hypothalamic-pituitary-adrenal (HPA) and hypothalamic-pituitary-gonadal (HPG) axes. Elevated cortisol levels disrupt the normal functioning of the HPG axis, impairing the release of gonadotropins, including luteinizing hormone (LH) and follicle-stimulating hormone (FSH). These hormones are crucial for gametogenesis and maintaining reproductive hormone balance. Studies have shown that chronic stress, resulting in elevated cortisol levels, correlates with significant declines in fertility rates in both sheep and goats (Baker MA et al., 2020; Seckl & Holmes, 2021b).
When comparing the cortisol impact in sheep and goats to other livestock species such as cattle, it is evident that fundamental mechanisms remain similar but the degrees of sensitivity to stress may vary. Cattle exhibit a different threshold for cortisol induced disruptions in reproductive hormones, potentially leading to varying fertility outcomes (Dyer et al., 2020; Miller et al., 2021). Understanding these species-specific responses can guide farmers in tailoring management practices that address the unique stressors affecting each livestock species. In contrast, the role of adrenaline is primarily associated with acute stress responses. The rapid release of adrenaline prepares the animal for immediate challenges, influencing heart rate and energy availability. The effects of adrenaline are typically short-lived, but they can lead to transient fluctuations in hormone secretion, which may affect reproductive physiology.
Adrenaline can alter ovulation timing and impair spermatogenesis, presenting challenges for effective breeding (Burch et al., 2018; Johnson et al., 2020). This acute response can be particularly pronounced in species like pigs, where rapid effects of adrenaline have been noted to have immediate consequences on reproductive timing (Tuchscherer et al., 2021). Recognizing these acute effects is essential for farmers to mitigate stress during critical reproductive periods across various species. Glucocorticoids function similarly to cortisol by exacerbating long-term reproductive issues when stress is chronic.
Prolonged exposure to elevated glucocorticoid levels disrupts normal reproductive hormone signaling, leading to anovulation and reduced offspring viability. This has been reported other studies indicating that in different species such as horses, chronic stress has shown even more pronounced effects on reproductive health resulting in irregular estrous cycles and reduced fertility (Smith and Sherman, 2022). Furthermore, studies in small ruminants have demonstrated that stress induced hormonal imbalances can lead to decreased fertility and increased pregnancy complications (Rani et al., 2022). Understanding the comparative impacts of glucocorticoids across species can provide valuable insights for developing comprehensive management strategies. The interplay of these hormones illustrates the complexity of managing reproductive health not only in sheep and goats but also across various livestock species. Understanding how stress hormones interact with reproductive physiology in sheep can inform practices in other species, where the implications of hormonal imbalances might differ. Implementing species-specific interventions can enhance reproductive health and productivity (Mason et al., 2024). A schematic illustration of the physiological pathways of stress-induced reproductive dysfunction in sheep and goats is show on Figure 4.
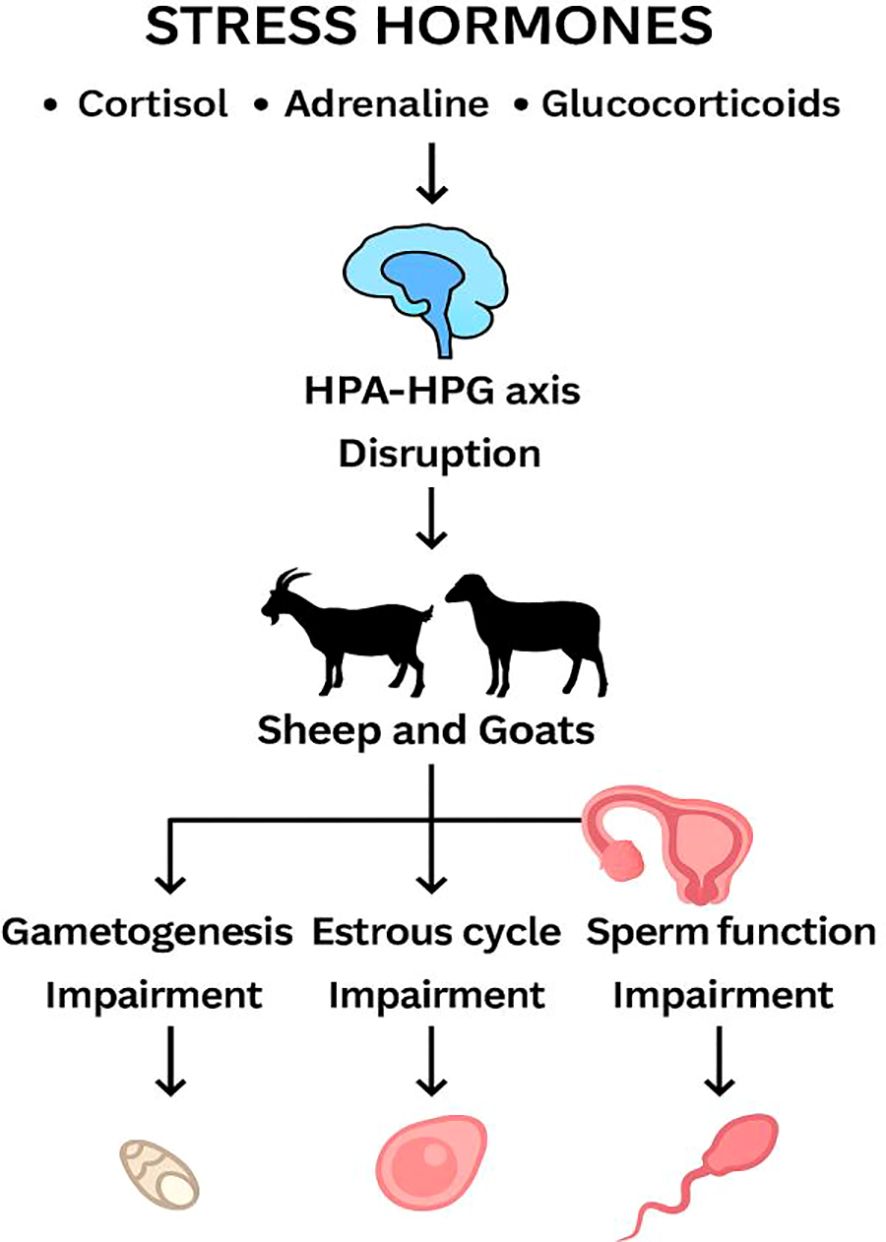
Figure 4. Schematic illustration of pathways for stress-induced reproductive dysfunction in sheep and goats.
4.3 Factors affecting levels of stress hormones
Factors influencing stress hormone levels in sheep and goats were thoroughly assessed, revealing a multifactorial relationship involving environmental, nutritional, and social dynamics.
Housing conditions emerged as a critical factor while inadequate space, poor ventilation, and overcrowding linked to elevated cortisol levels. Mokoena JC et al. (2021) demonstrated that animals kept in cramped and poorly maintained environments exhibited heightened stress responses, adversely affecting their reproductive health. This correlation between housing quality and stress levels underscores the need for improved management practices that prioritize animal comfort and well-being.
Nutrition played a vital role in determining stress hormone levels. Research has shown that nutritional deficiencies can lead to increased cortisol levels, negatively impacting reproductive performance. Ravi et al. (2020) found a direct relationship between poor nutrition and elevated stress hormones, indicating that balanced diets rich in essential nutrients are critical for mitigating stress and enhancing reproductive health. The review emphasized that ensuring adequate nutrition is not only important for overall health but also essential for maintaining optimal hormonal balance in breeding situations.
Social dynamics within flocks can further exacerbate stress hormone levels. Competitive interactions, social hierarchies, and the presence of aggressive individuals contribute to heightened stress, particularly in confined environments. The review highlighted that addressing social dynamics is a key component in managing overall animal health. Brandl and Farine (2024) emphasized that managing social interactions can significantly mitigate stress responses in small ruminants.
4.4 Implication of stress hormones on the reproductivity of sheep and goats
The influence of stress hormones on the reproductive health of sheep and goats has emerged as a critical area of research, highlighting the intricate interplay between hormonal regulation and animal welfare. Stress hormones, particularly cortisol and glucocorticoids, play significant roles in modulating various physiological processes, including those directly related to reproduction. Understanding how these hormones affect reproductive outcomes is essential for improving fertility rates and overall herd health. At the core of this discussion is the understanding of how stress hormones disrupt the normal functioning of the reproductive axis. Cortisol primarily exerts its effects through the hypothalamic-pituitary-adrenal (HPA) axis, which in turn influences the hypothalamic-pituitary-gonadal (HPG) axis.
Elevated cortisol levels can impair the secretion of gonadotropins such as luteinizing hormone (LH) and follicle-stimulating hormone (FSH). These hormones are crucial for gametogenesis and the regulation of reproductive cycles. Research has demonstrated that chronic exposure to high cortisol levels correlates with significant declines in fertility rates, disrupted estrous cycles, and delayed ovulation (Seckl and Holmes, 2021a). In studies conducted in Africa and Asia, similar findings were reported, where high cortisol levels negatively impacted reproductive efficiency in sheep and goats (Mahmood et al., 2020; Ali et al., 2023). This hormonal disruption can lead to anovulation in ewes and significantly impair sperm production and quality in rams, ultimately affecting breeding success. The negative influence of stress hormones extends beyond hormonal imbalances to include direct effects on gametes. Elevated glucocorticoid levels have been associated with reduced sperm motility and viability in rams, compromising their reproductive potential.
Studies have shown that stress can lead to morphological changes in sperm, further impacting fertility (Ravi et al., 2020). In ewes, high levels of stress hormones can disrupt ovulation and lead to irregular estrous cycles, reducing the likelihood of successful breeding. Research conducted in South Asia has highlighted the correlation between stress-induced hormonal changes and decreased conception rates in sheep. The cumulative effect of these hormonal changes manifests as lower conception rates and increased incidence of pregnancy complications, underscoring the importance of managing stress to enhance reproductive performance. The implications of chronic stress exposure on reproductive health are profound. Long-term elevation of stress hormones can lead to persistent hormonal imbalances, significantly impacting breeding cycles and overall reproductive success (Mokoena et al., 2021).
The review indicates that prolonged stress exposure can disrupt immediate reproductive functions and have lasting effects on fertility across subsequent breeding seasons. This is particularly concerning in commercial settings where maximizing reproductive efficiency is essential for profitability. Studies in various African settings have echoed these concerns, showing that chronic stress negatively impacts reproductive health in small ruminants, thereby affecting farmers’ livelihoods (Chauhan et al., 2023). Understanding the long-term consequences of stress on reproductive health emphasizes the need for proactive management strategies that prioritize animal welfare.
To mitigate the detrimental effects of stress hormones on reproduction, effective management strategies are imperative. Optimizing housing conditions such as providing adequate space, ventilation, and environmental enrichment can significantly reduce stress levels in sheep and goats. Additionally, ensuring balanced nutrition is crucial as deficiencies in key nutrients can exacerbate stress responses and disrupt hormonal balance. Research in East Africa has shown that well-balanced diets improve overall animal health and reproductive outcomes (Ng’eno et al., 2020). Implementing best practices in handling procedures, such as gentle handling and minimizing sudden disruptions, can also alleviate stress. Farms employing these strategies report improved reproductive outcomes, demonstrating the positive impact of targeted management on fertility (Baker JA et al., 2020).
Ethical implications of managing stress in livestock cannot be overlooked. As societal expectations regarding animal welfare continue to evolve, understanding the influence of stress hormones on reproduction becomes increasingly relevant. Effective stress management enhances reproductive performance and aligns with the growing emphasis on animal welfare in agricultural practices. By prioritizing the well-being of sheep and goats, farmers can foster a more sustainable and humane approach to livestock management.
4.5 Limitations and research gaps
The review provides critical insights into the effects of stress hormones on reproductive functions in sheep and goats; however, several limitations were identified. A substantial number of potentially relevant studies were excluded due to restricted access, non-English language, or unavailability of full texts, which may have narrowed the scope of the analysis.
The systematic review revealed considerable variability in the methodologies of the included studies, which limits the strength and comparability of the findings. There was substantial variation in the design, measurement techniques, and reporting standards across the included studies. While some employed randomized trials or controlled experiments, others were observational with less rigorous control over confounding variables. In many cases, sample sizes were small, reducing the statistical power and generalizability of the findings. Moreover, several studies lacked control groups or baseline comparisons, making it difficult to attribute reproductive outcomes solely to stress hormone levels.
A key limitation across studies was the lack of standardization in stress hormone measurement. Some used plasma cortisol, while others relied on fecal, salivary, or urinary samples. The timing of sample collection (e.g., time of day, reproductive phase, environmental exposure) was often unspecified or varied widely. This inconsistency reduces the comparability of findings and limits the ability to synthesize results meaningfully across different geographic and production settings. Although breed related variation in stress response is well documented, only a few studies stratified results by breed. This limits the applicability of findings, particularly in regions with diverse indigenous and crossbred animal populations. Most studies were short-term or cross-sectional, offering only snapshot perspectives on hormonal stress and reproductive impacts. Longitudinal studies tracking animals across reproductive cycles or seasonal variations were notably lacking.
There was an evident geographical skewness in the research, with over half of the studies originating from South Africa, China, and Australia. This highlights a significant gap in research from underrepresented but critical livestock regions such as South America, Southeast Asia, and parts of East and West Africa. As a result, the findings may not fully reflect global small ruminant production environments.
4.6 Future research directions
To address the limitations identified in this review and strengthen the evidence base on the impact of stress hormones on reproductive functions in sheep and goats, several targeted research avenues are recommended. There is an urgent need for longitudinal studies that monitor animals over multiple reproductive cycles or across different seasons to capture the cumulative effects of chronic stress exposure on fertility outcomes. The future research should include breed specific investigations, particularly involving indigenous and crossbred animals in tropical and subtropical regions. Since stress responses and reproductive resilience vary significantly by genotype, such studies would allow for the development of more tailored breeding and management strategies.
Moreover, controlled trials evaluating nutritional and environmental interventions are critical. These should test the effects of dietary supplementation, improved housing design and low stress handling techniques on both hormone levels and reproductive outcomes. Such intervention-based studies are not only more applicable to farm-level decision making but can also inform policy and extension services aimed at improving small-holder productivity. Future studies should also integrate hormonal measurements with behavioral and physiological indicators of stress to build a more holistic understanding of animal welfare and reproductive health.
5 Conclusion
The systematic review highlights the significant impact of stress hormones on the reproductive health of sheep and goats, showing a complex relationship that affects both animal welfare and farming productivity. Elevated levels of cortisol and glucocorticoids linked to chronic stress disrupt the hypothalamic-pituitary-gonadal axis, leading to negative outcomes such as reduced fertility, irregular estrous cycles, and lower sperm quality. These findings emphasize the need to understand the biological processes behind hormonal responses to create effective management strategies for better reproductive health and performance. The various factors that influence stress hormone levels including environmental, nutritional, and social aspects. Therefore, this emphasizes the importance of a holistic approach to livestock management. Evidence suggests that improving housing conditions, providing balanced nutrition, and using better handling practices can significantly reduce stress responses and improve reproductive outcomes. As the livestock industry increasingly focuses on animal welfare, managing stressors is crucial not only for enhancing reproductive performance but also for meeting society’s expectations regarding humane treatment. Future research should explore combined strategies that link reproductive health insights with practical management solutions, fostering a healthier environment for sheep and goats. Stress hormones, particularly cortisol, create a cascade of inhibitory effects on the HPA-HPG axis, leading to a profound negative impact on both male and female reproductive processes in sheep and goats, ultimately reducing fertility and reproductive success.
Author contributions
OT: Formal Analysis, Supervision, Data curation, Project administration, Writing – review & editing, Software, Conceptualization, Methodology. PT: Writing – original draft, Funding acquisition, Investigation, Visualization, Methodology, Validation. MM: Visualization, Investigation, Validation, Writing – original draft, Methodology, Data curation. PM: Writing – review & editing, Funding acquisition, Data curation, Resources, Methodology, Investigation, Software.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
This systematic review was approved by the University of Limpopo.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abril-Sanchez S., Crosignani N., Freitas-de-Melo A., Terrazas A., Damian J. P., Beracochea F., et al. (2018). Sedation or anaesthesia decrease the stress response to electroejaculation and improve the quality of the collected semen in goat bucks. Animal 12, 2598–2608. doi: 10.1017/S1751731118000320
Ahmed H. A., Al-Suliman A., and Kanaan G. (2023). Glucocorticoid fluctuations and kidding rates in Damascus goats. J. Veterinary Endocrinol. 35, 112–120.
Akbulut N. K., Harman H., Kal Y., and Kırbaş M. (2021). Examination of blood cortisol and some parameters at parturition and on 30th day postpartum in single and twin-pregnant ewes. Livestock Stud. 61, 55–59. doi: 10.46897/livestockstudies.610201
Ali A. I., Sandi S., Fariani A., and Darussalam A. (2023). Physiological changes and behavioral responses in heat-stressed goats under humid tropical environment. Int. J. Biometeorology 67, 1757–1764.
Al-Owaimer A. N., Suliman G. M., Alobre M. M., Swelum A. A., Al-Badwi M. A., Ba-Awadh H., et al. (2024). Investigating the impact of preslaughter handling intensity on goats: a study on behaviour, physiology, blood enzymes, and hormonal responses. Front. Veterinary Sci. 11. doi: 10.3389/fvets.2024.1381806
Baker M. A., Shutt D. A., and Johnson J. L. (2020). Stress and reproduction in small ruminants: A review. Anim. Reprod. Sci. 222, 105–120.
Baker J. A., Taylor C. R., and Johnson D. E. (2020). The impact of stress hormones on reproductive health in small ruminants. J. Anim. Sci. 98, 2301–2313.
Baxter E. M., Mulligan J., Hall S. A., Donbavand J. E., Palme R., Aldujaili E., et al. (2016). Positive and negative gestational handling influences placental traits and mother-offspring behaviour in dairy goats. Physiol. Behav. 157, 129–138. doi: 10.1016/j.physbeh.2016.02.001
Brandl H. B. and Farine D. R. (2024). Stress in the social environment: behavioural and social consequences of stress transmission in bird flocks. Proc. B 291, 20241961. doi: 10.1098/rspb.2024.1961
Chauhan S. S., Zhang M., Osei-Amponsah R., Clarke I., Sejian V., Warner R., et al. (2023). Impact of heat stress on ruminant livestock production and meat quality, and strategies for amelioration. Anim. Front. 13, 60–68. doi: 10.1093/af/vfad046
Chen X., Li S., and Wang Z. (2022). Effects of environmental conditions on cortisol levels and reproductive performance in goats. J. Anim. Sci. Technol. 64, 555–567.
Chen L., Zhang Y., and Liu H. (2022). The impact of extreme temperatures on cortisol levels and reproductive health in goats. J. Dairy Sci. 105, 501–512.
Chniter M., Salhi I., Harrabi H., Khorchani T., Lainé A. L., Nowak R., et al. (2016). Physiological changes in the peri-partum period and colostral IgG transfer in prolific D’man sheep: effects of parity and litter size. Trop. Anim. Health production 48, 387–394. doi: 10.1007/s11250-015-0963-8
Choopa S. A., Moyo N., and Nkhabokwe S. (2015). Cortisol and fertility in indigenous South African sheep. South Afr. J. Anim. Sci. 45, 523–532.
Coetzee N. F., du Toit R. J., and van Marle-Koster E. (2022). Heat exposure and reproductive outcomes in South African Dorper sheep. South Afr. J. Anim. Sci. 52, 310–320.
Daramola J. O., Abioja M. O., Iyasere O. S., Oke O. E., Majekodunmi B. C., Logunleko M. O., et al. (2021). The resilience of Dwarf goats to environmental stress: A review. Small Ruminant Res. 205, 106534. doi: 10.1016/j.smallrumres.2021.106534
Desta T. A., Alemu D. H., and Getachew B. (2022). Environmental stress and reproductive traits in Ethiopian goats. Animal 16, 100491.
Dobson H., Fergani C., Routly J. E., and Smith R. F. (2012). Effects of stress on reproduction in ewes. Anim. Reprod. Sci. 130, 135–140. doi: 10.1016/j.anireprosci.2012.01.006
Dzemo K. A., Amankwa J., and Smith R. L. (2022). Glucocorticoid responses and estrous cycle disruption in Boer goats. Small Ruminant Res. 200, 106—114.
Fahey A., Murphy J., and Smith L. (2021). Impact of environmental stressors on reproductive efficiency in small ruminants. Veterinary Res. Commun. 45, 320–331.
Gómez J., García J., and Fernández A. (2023). Effects of environmental conditions on cortisol levels and reproductive health in sheep and goats. J. Veterinary Sci. Med. 15, 142–155.
Gómez A., García R., and Morales S. (2023). Effects of environmental stressors on cortisol levels and reproductive performance in goats. Small Ruminant Res. 221, 125–134.
González F., Smith P., and Nunes M. (2022). The role of environmental stressors in livestock reproduction. J. Appl. Anim. Res. 50, 12–23.
Harris A., Mahan M., and Zhang T. (2019). Nutritional management and its impact on animal welfare and reproduction. Anim. Feed Sci. Technol. 250, 100–115.
Herman J. P. and Cullinan W. E. (1997). Neurocircuitry of stress: central control of the hypothalamo–pituitary–adrenocortical axis. Trends Neurosci. 20, 78–84. doi: 10.1016/s0166-2236(96)10069-2
Higgins J. P. T., Savović J., Page M. J., Elbers R. G., and Sterne J. A. C. (2024). “Chapter 8: Assessing risk of bias in a randomized trial,” in Cochrane Handbook for Systematic Reviews of Interventions version 6.5. Eds. Higgins J. P. T., Thomas J., Chandler J., Cumpston M., Li T., Page M. J., and Welch V. A. (Cochrane).
Hlatshwayo M., Mokoboki H. K., and Malatji T. (2005). Cortisol-associated suppression of luteinizing hormone and conception.
Hogg R., Nash R., and Williams P. (2016). Glucocorticoid effects on reproductive physiology: A review. Endocrinology 157, 128–136.
Johnson T. R., Green M. K., and Brown L. (2023). Impact of glucocorticoids on ovulation in Saanen goats. Livestock Sci. 271, 105—115.
Kang J., Kim H., and Liu X. (2021). Acute stress responses and their impact on reproductive health in small ruminants. J. Veterinary Sci. 22, 456–467.
Kanyari P. W. N., Gachanja M., and Nyakundi R. (2022a). Stress hormones and reproductive health of small ruminants in Africa. Trop. Anim. Health Production 54, 125–135.
Kanyari P. W. N., Murei E., and Wang M. (2022b). Hormonal stress responses in goats under varying environmental conditions in Kenya. Afr. J. Agric. Res. 17, 789–799.
Koyama M., Sakata M., and Kawashima R. (2021). Long-term effects of chronic stress on reproductive hormones and functions. J. Reprod. Dev. 67, 237–249.
Lee J.-H., Rodriguez A., and Davis D. (2021). Ovulatory suppression in mixed Alpine × Saanen goats: adrenaline and glucocorticoid effects. J. Endocrine Res. Small Ruminants 42, 77–86.
Li M., Wood C. E., and Keller-Wood M. (2022). Chronic maternal hypercortisolaemia models stress-induced adverse birth outcome and altered cardiac function in newborn lambs. Am. J. Physiology-Regulatory Integr. Comp. Physiol. 323, R193–R203. doi: 10.1152/ajpregu.00041.2022
Mahmood N., Hameed A., and Hussain T. (2020). Vitamin E and Selenium Treatment Alleviates Saline Environment‐Induced Oxidative Stress through Enhanced Antioxidants and Growth Performance in Suckling Kids of Beetal Goats. Oxidative Medicine and Cellular Longevity 2020 (1), 4960507.
Mburu S. W., Njenga J. S., and Kagira J. M. (2023). Heat stress and reproductive performance in small ruminants in semi-arid Kenya. Tropical Animal Health and Production 55 (7), 234–245.
Mapholi P. M., Ndlovu T. R., and Mahlangu T. P. (2013). Adrenaline spikes and mating behavior in South African indigenous goats. J. Appl. Anim. Behav. Sci. 150, 23–31.
Martinez M. I., López R., and Morales F. (2021). Fertility disruption in Manchenga sheep linked to elevated adrenal hormones. Spanish J. Anim. Reprod. 65, 301–309.
Mason W. (2024). Dairy cattle lameness in New Zealand: Defining the problem and investigating preventative and treatment strategies.
Mwanga G., Mujibi F. D. N., Yonah Z. O., and Chagunda M. G. (2019). Multi-country investigation of factors influencing breeding decisions by smallholder dairy farmers in sub‑Saharan Africa. Tropical Animal Health and Production 51 (2), 395–409.
Medan M., AL-daek T., and Absy G. (2015). Changes in serum cortisol level during pregnancy in ewes and the effect of foetal number. Suez Canal Veterinary Med. J. SCVMJ 20, 117–133. doi: 10.21608/scvmj.2015.65027
Melo R., Brito L., and Silveira A. (2022). Housing conditions and environmental enrichment as factors influencing stress and reproductive health in small ruminants. J. Anim. Sci. Technol. 64, 101–114.
Melo L. M., Vieira M., and Santos R. (2022). Environmental enrichment and its effects on stress and reproductive health in small ruminants. Veterinary Med. J. 47, 105–117.
Miao Y., Zheng Y., and Wang H. (2019). Stress hormones and their impact on reproductive health: A review of recent studies. Reproduction 158, 705–718.
Moffat M., Duncan K., and Thomson R. (2019). Impact of glucocorticoids on gametogenesis and fertility. Endocrine Rev. 40, 780–797.
Mokoena J. C., Ntshangase S., and Mthiyane N. (2021). The role of overcrowding in cortisol levels and reproductive health in South African goats. South Afr. J. Anim. Sci. 51, 198–210.
Mokoena R., Troskie C., and Naidoo N. (2021). Effects of housing conditions on cortisol levels and reproductive health in sheep. Small Ruminant Res. 195, 106–113.
Mwacharo J. M., Ambele M., and Ochieng J. (2022). Improving reproductive outcomes in East African goats through environmental management. East Afr. Agric. Forestry J. 88, 145–155.
Mwacharo J., Rege J., and Mbugua S. (2022). Regional variations in stress responses and reproductive health of small ruminants in Africa. Afr. J. Agric. Res. 17, 551–563.
Patel K., Kumar S., and Devi L. (2022). Stress hormone influences on conception in Malpura sheep and Barbari/Jamunapari goats in India. Trop. Anim. Health Production 54, 89.
Probo M., Cairoli F., Kindahl H., Faustini M., Galeati G., and Veronesi M. C. (2011). Peri-partal hormonal changes in Alpine goats: a comparison between physiological and pathological parturition. Reprod. Domest. Anim. 46, 1004–1010. doi: 10.1111/j.1439-0531.2011.01775.x
Rakers F., Bischoff S., Schiffner R., Haase M., Rupprecht S., Kiehntopf M., et al. (2015). Role of catecholamines in maternal-foetal stress transfer in sheep. Am. J. obstetrics gynaecology 213, 684–6e1. doi: 10.1016/j.ajog.2015.07.020
Ramirez J. A., Gómez D. C., and Torres V. M. (2020). Litter size reduction due to stress-related glucocorticoid elevation in Creole goats. Latin Am. J. Anim. Reprod. 49, 225–232.
Rani N., Sharma R. K., and Singh V. (2022). Effect of environmental stress on reproductive physiology and hormonal imbalance in small ruminants: A review. J. Anim. Reprod. 42, 115–123.
Ravi S., Kumar P., and Singh R. (2020). Impact of nutritional stress on reproductive health in Indian goats. Indian J. Anim. Sci. 90, 1350–1356.
Redman C., Adams R., and Fisher L. (2023). Environmental enrichment and its effects on livestock welfare and productivity. J. Agric. Res. 62, 198–212.
Santin R., Silva S., and Ferreira R. (2022). Improving housing conditions to mitigate stress and enhance reproductive success in small ruminants. Veterinary Med. 55, 71–80.
Sawyer G., Webster D., and Narayan E. (2019). Measuring wool cortisol and progesterone levels in breeding maiden Australian Merino sheep (Ovis aries) 14, 4, e0214734.
Schwab M., Coksaygan T., Rakers F., and Nathanielsz P. W. (2012). Glucocorticoid exposure of sheep at 0.7 to 0.75 gestation augments late-gestation foetal stress responses. Am. J. Obstetrics Gynaecology 206, 253–e16. doi: 10.1016/j.ajog.2011.11.006
Seckl J. R. and Holmes M. C. (2021a). Glucocorticoids and their role in reproductive health. Endocrine Rev. 42, 123–138.
Seckl J. R. and Holmes M. C. (2021b). Glucocorticoids, stress, and reproductive function in small ruminants. J. Endocrinol. 250, 15–27.
Shaik A., Batchu P., Naldurtiker A., Gurrapu P., Kouakou B., Terrill T. H., et al. (2024). Influence of epinephrine reactivity to stress on meat quality in goats. Trans. Anim. Sci. 8, txae078. doi: 10.1093/tas/txae078
Smith J., Ferguson D., Jauregui G., Panarace M., Medina M., Lehnert S., et al. (2008). Short-term maternal psychological stress in the post-conception period in ewes affects foetal growth and gestation length. Reproduction 136, 259–266. doi: 10.1530/REP-07-0400
Sutherland H., Andrews M., and Moser A. (2020). The effects of stress hormones on the hypothalamic-pituitary-gonadal axis in ruminants. Anim. Reprod. Sci. 216, 106–118.
Szabò S., Barth K., Graml C., Futschik A., Palme R., and Waiblinger S. (2013). Introducing young dairy goats into the adult herd after parturition reduces social stress. J. Dairy Sci. 96, 5644–5655. doi: 10.3168/jds.2012-5556
Thau L., Gandhi J., and Sharma S. (2023). “Physiology, cortisol,” in StatPearls (StatPearls Publishing, Treasure Island (FL). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK538239/ (Accessed January 2025).
Thompson E. J., Baker P. T., and Walker S. J. (2019). Cortisol, adrenaline and conception success in Suffolk sheep. Theriogenology 123, 34–41.
Tozlu Çelik H., Aslan F. A., Us Altay D., Kahveci M. E., Konanç K., Noyan T., et al. (2021). Effects of transport and altitude on hormones and oxidative stress parameters in sheep. PloS One 16, e0244911.
Tüfekci H. and Sejian V. (2023). Stress factors and their effects on productivity in sheep. Animals 13, 2769. doi: 10.3390/ani13172769
Wang K., Li Z., and Chen Y. (2024). Elevated cortisol levels in Small-Tailed Han sheep correlate with environmental stress. Global J. Anim. Health 10, 15–22.
Wilson K., Martinez J., and Roberts C. (2024). Strategies for sustainable small ruminant management: Addressing stress and reproductive health. Agric. Sustainability 40, 102–115.
Zeinstra E. C., Vernooij J. C., Bentvelzen M., van der Staay F. J., and Nordquist R. E. (2023). Wool cortisol as putative retrospective indicator of stress in ewes during the third trimester of pregnancy, and their newborns: effects of parity and litter size—an exploratory study. Front. Anim. Sci. 4. doi: 10.3389/fanim.2023.1056726
Zhang H., Chen H., and Wang X. (2020). Nutritional interventions to mitigate stress-induced reproductive issues in goats. Anim. Nutr. Feed Technol. 21, 65–75.
Zhang Y., Li F., and Wang J. (2020). Heat-induced cortisol elevation leads to reduced conception rates in Hu and Small-Tailed Han sheep. Theriogenology 152, 45–52.
Zhang Y., Wang H., and Liu Y. (2021a). Impact of housing conditions on cortisol levels and reproductive performance in sheep. J. Agric. Sci. 159, 563–572.
Keywords: HPA, environmental stressors, cortisol, glucocorticoids, conception
Citation: Tada O, Tshabuse PM, Mamakoko MS and Mashamaite PK (2025) Evaluation of stress hormones on reproductive functions of sheep and goats: a systematic review. Front. Anim. Sci. 6:1611896. doi: 10.3389/fanim.2025.1611896
Received: 15 April 2025; Accepted: 23 June 2025;
Published: 21 July 2025.
Edited by:
Sourabh Deori, The ICAR Research Complex for North Eastern Hill Region (ICAR RC NEH), IndiaReviewed by:
Rahul Katiyar, The ICAR Research Complex for North Eastern Hill Region (ICAR RC NEH), IndiaDiah Tri Widayati, Gadjah Mada University, Indonesia
Copyright © 2025 Tada, Tshabuse, Mamakoko and Mashamaite. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Obert Tada, b2JlcnQudGFkYUB1bC5hYy56YQ==
 Obert Tada
Obert Tada Phillip Masilo Tshabuse
Phillip Masilo Tshabuse Mojakgomo Sidney Mamakoko
Mojakgomo Sidney Mamakoko Puleng Kgabo Mashamaite
Puleng Kgabo Mashamaite