- 1Department of Animal Science, University of Tennessee, Knoxville, TN, United States
- 2Genomics Center for the Advancement of Agriculture, University of Tennessee Institute of Agriculture, Knoxville, TN, United States
As global demand for animal protein rises, improving feed efficiency in beef cattle has become a central focus for sustainable livestock production. Feed efficiency, often quantified through residual feed intake (RFI), is a complex trait influenced by genetic, physiological, and environmental factors. Among these, skeletal muscle plays a pivotal role due to its central function in growth, metabolism, and nutrient utilization. This mini-review explores the molecular and metabolic mechanisms linking muscle development to feed efficiency, highlighting recent advances in gene expression profiling, hormone signaling, and energy metabolism. Studies have identified key genes and pathways, such as those involved in the electron transport chain and insulin-like growth factor 1 (IGF1) signaling, that contribute to muscle growth and efficient energy use. Additionally, the role of the rumen microbiome in modulating nutrient absorption and its interaction with host muscle metabolism is discussed. Integrating these insights with genomic selection tools provides a promising avenue for enhancing feed efficiency while maintaining production goals. Understanding the biological foundations of muscle development offers valuable opportunities to refine genetic selection and management practices for a more profitable and environmentally sustainable beef industry.
1 Introduction
Beef and buffalo are the third most consumed meat sources globally, with production increasing dramatically from approximately 50 million tonnes per year in 1961 to over 350 million tonnes per year today (Ritchie et al., 2019). While meat serves as a critical protein source for human nutrition, livestock production is often scrutinized for its environmental impact, including greenhouse gas emissions, land use, and resource consumption (González et al., 2020). As the global population continues to grow, projected to nearly double within the next two decades, food production systems must adapt to meet rising demands while maintaining economic viability and minimizing environmental consequences (Scialabba et al., 2013). This necessitates the implementation of innovative strategies and research methodologies to enhance the sustainability of livestock production.
Despite its nutritional value and consumer demand, beef production incurs the highest cost per unit of edible product compared to other livestock species such as poultry, pork, or dairy (Kenny et al., 2018). One of the primary contributors to this high cost is feed, which represents the largest expense in cattle production systems. Over the past several years, the cost of grain and land required for feed production in the United States has increased by 40–60%, a trend that is expected to continue (Lawrence et al., 2008). Furthermore, cattle have a higher feed conversion requirement than other livestock species, meaning they require significantly more feed per unit of body weight gain (Nielsen et al., 2013). Thus, improving feed efficiency, defined as an animal’s ability to convert feed into body mass, has become a focal point of research, as it offers potential benefits for both economic sustainability and resource conservation (Crews, 2005). Enhancing feed efficiency could enable producers to reduce feed costs while optimizing land use and overall productivity.
Feed efficiency is influenced by multiple factors, including genetics, diet composition, and environmental conditions (Khanal et al., 2023). Cattle that exhibit superior feed efficiency require fewer resources to produce muscle, the primary economic and consumer-driven product in beef operations (McKenna et al., 2021). Additionally, improving feed efficiency has broader implications for environmental sustainability, particularly in reducing methane emissions. Methane, a potent greenhouse gas, is a byproduct of microbial fermentation in the rumen, and studies have indicated that cattle with higher feed efficiency may produce lower methane emissions due to differences in rumen microbial activity and metabolic efficiency (Hegarty et al., 2007; Manzanilla-Pech CIV et al., 2022). This relationship underscores the potential for selective breeding and management strategies to mitigate the environmental footprint of beef production while simultaneously enhancing productivity.
Significant progress has been made in understanding the genetic basis of feed efficiency, with numerous studies identifying genetic markers and metabolic pathways associated with variations in efficiency among cattle (Pryce et al., 2014). Research has provided strong evidence linking specific genetic factors to feed utilization, growth rate, and metabolic function (Herd and Arthur, 2009). However, despite these advancements, further investigation is required to fully elucidate the genetic mechanisms underlying feed efficiency and to determine the extent to which environmental factors influence these traits (Crowley et al., 2010; Jiang et al., 2024). Addressing these knowledge gaps will be critical for developing targeted breeding programs and management strategies aimed at optimizing feed efficiency in beef cattle production systems.
2 Measuring feed efficiency
Feed efficiency is a critical phenotype in cattle production, with ongoing advancements in measurement techniques aimed at improving genetic selection for more sustainable and economically viable livestock systems (Archer et al., 1999). Traditionally, feed efficiency has been quantified using feed conversion ratio (FCR), which is used to measure the efficiency with which animals convert feed into body mass, and represents the ratio of weight gain per day to daily dry matter intake (DMI) (Koch et al., 1963; Yi et al., 2018). While FCR has been commonly used in animal agriculture to help producers assess growth performance, optimize feeding practices, and improve overall production efficiency, it is highly correlated with growth rate, meaning animals that grow faster often appear more efficient, even if they consume more feed overall. This makes FCR a poor indicator for genetic selection because it can favor larger or faster-growing animals without truly identifying those that use feed more efficiently, independent of growth (Koch et al., 1963; Arthur et al., 2001; Carstens and Tedeschi, 2006).
The limitations of FCR highlight the value of residual feed intake (RFI), which is phenotypically independent from growth rate, making it a more precise trait for genetic improvement (Carstens and Tedeschi, 2006). RFI is calculated as the difference between an animal’s expected feed intake and its actual feed intake, determined using multiple regression models that account for variations in growth, maintenance, and activity (Koch et al., 1963; Hu et al., 2023). This method incorporates DMI and average daily gain (ADG) to estimate efficiency, where animals with positive RFI consume more than average, while those with negative RFI consume less (Savietto et al., 2014; Kenny et al., 2018). While RFI offers a more refined measure of feed efficiency by adjusting for growth and intake, it does not capture the full range of biological processes that contribute to an animal’s overall efficiency, such as metabolic processes, nutrient absorption, and energy partitioning. Therefore, to fully understand and improve feed efficiency, it is essential to consider additional physiological and genetic factors beyond what RFI captures (Koch et al., 1963; Williams et al., 2011). To ensure these factors are accurately reflected in performance data, the Beef Improvement Federation recommends a standardized testing protocol that includes a 21-day acclimation period followed by a minimum 42-day evaluation phase (contributors, B.G.W). Together, these approaches provide a more comprehensive and reliable framework for evaluating and selecting animals with superior feed efficiency.
Variability in feed intake among cattle is largely driven by differences in energy utilization, which are influenced by physiological states such as lactation, growth stage, and metabolic rate (Allen, 2014). To optimize feed use in beef production systems, it is essential to understand energy metabolism and its role in supporting tissue development and overall animal function (Moe, 1981). Energy derived from the oxidation of feed is partitioned among maintenance, production (e.g., growth, reproduction, lactation), or lost as heat and waste (Patience et al., 2015). As Nielsen (2013) emphasizes, feed efficiency is a multifaceted trait shaped by a combination of age, body weight, genetics, and environmental factors (Nielsen et al., 2013). For instance, growing calves and mature, reproducing cows exhibit distinct feed intake patterns due to varying metabolic demands, digestive efficiency, and activity levels. Although body weight alone offers limited insight into feed efficiency, precise measurements of feed intake are essential for identifying underlying biological variation and informing both genetic selection and management strategies aimed at improving efficiency.
3 Feed efficiency heritability
Genetic selection is an important tool in the beef industry, particularly following the development of artificial insemination (AI), which has revolutionized breeding practices. Historically, livestock breeding focused on maximizing phenotypic traits through the evaluation of offspring performance (García-Ruiz et al., 2016). In modern cattle breeding, advancements in genomics have enabled more precise mapping of the genome to analyze production traits, facilitating the targeted selection of animals for desirable characteristics such as breed, fat deposition, and size. These advancements have significantly enhanced the efficiency and accuracy of genetic selection, allowing producers to make more informed breeding decisions that align with both economic and production goals in the beef industry (Silva Neto et al., 2023). While breeding for feed efficiency has shown potential (Ojo et al., 2024), the genetic underpinnings that govern these complex biological processes remain poorly understood (Tempelman and Lu, 2020). Feed efficiency is a multifactorial trait influenced not only by genetics but also by environmental interactions, making its genetic analysis particularly challenging. Cattle have been selectively bred for traits related to feed efficiency, including end weight, DMI and ADG. However, these traits do not fully capture the complexity of feed efficiency, which is influenced by a range of biological processes such as energy partitioning and metabolic efficiency (Bottje and Carstens, 2012).
Recent research has provided valuable insights into the genetic basis of feed efficiency, with studies indicating that cattle with greater feed efficiency may exhibit altered gene expression related to immune function and hepatic lipid metabolism, two critical systems involved in regulating homeostasis (Salleh et al., 2018; Fonseca et al., 2019). Although Salleh et al. focused on liver gene expression in dairy cattle, their findings suggest potential metabolic similarities in other biological systems, such as muscle tissue, which may also contribute to feed efficiency. Efficient muscle growth relies on the effective utilization of nutrients like amino acids for protein synthesis, directly influencing body weight gain. As bovine genomics research advances, the ability to improve the genetic selection for traits related to muscle metabolism and growth holds great potential for improving nutrient use efficiency in cattle.
Efforts to enhance muscle growth through genetic selection must also account for how animals respond to their environments, as nutrient utilization is shaped not only by genetics but also by external factors. Understanding this gene-environment interaction is critical for improving traits like feed efficiency across diverse production systems. Recent work by Silva Neto et al. (2023) highlighted the relationship between phenotypic gradients and environmental gradients for both DMI and RFI (Silva Neto et al., 2023). As depicted in the adapted Figure 1, based on data from Silva Neto et al. (2023), their study reported moderate heritability estimates for both DMI and RFI, indicating that these traits are influenced by both genetic and environmental factors, with notable interaction between the two. In the adaptation, selected panels from the original figure and data were restructured and re-illustrated to better align with the focus on gene-environment interactions in feed efficiency and to emphasize key differences in how the data were interpreted. These data illustrate that both genetic and environmental influences shape feed efficiency traits like DMI and RFI, reinforcing the importance of accounting for environmental variation in genetic selection strategies.
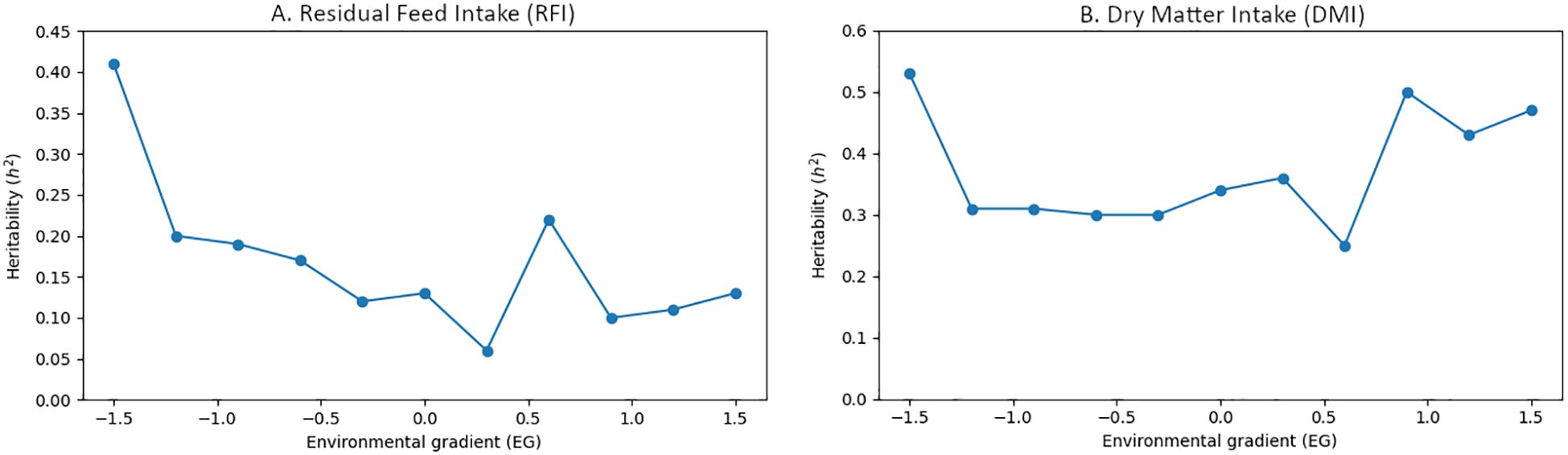
Figure 1. Residual feed intake (RFI) (A) and dry matter intake (DMI) (B) heritability across environmental gradients. Adapted from Silva Neto et al., 2023 (Silva Neto et al., 2023).
4 Molecular and metabolic targets for genetic selection
4.1 Muscle growth and development
Muscle development is the primary economic driver in beef cattle production, as it directly impacts the quality and quantity of the final product. Understanding the underlying mechanisms governing muscle growth, along with the factors that influence feed efficiency, is critical for optimizing production practices. Skeletal muscle is a complex and heterogeneous tissue that functions as an essential organ within the muscular system (Mohammadabadi et al., 2021). Muscle mass plays a crucial role in regulating resting energy expenditure (REE), the energy required for maintaining homeostasis. This energy is primarily derived from carbohydrates, fats, and proteins, and during these metabolic processes, oxygen is consumed, and carbon dioxide is released. While genetics are the primary determinant of muscle growth and development, external factors such as diet, physical activity, and lifestyle also significantly influence muscle mass and efficiency (Westerterp, 2000).
A study by McKenna et al. (2021) explored the molecular basis of muscle growth by analyzing differential gene expression (DEGs) in muscle biopsy samples from heifers and bulls (McKenna et al., 2021). While the number of DEGs found in heifers divergent for RFI was small, all identified genes were associated with the electron transport chain, a crucial component of cellular energy metabolism. This suggests a direct link between RFI and gene expression related to muscle growth and energy utilization. However, the study highlighted the need for further research to strengthen the evidence of these molecular associations and their implications for improving feed efficiency through genetic selection (McKenna et al., 2021).
Investigating early developmental processes such as muscle fiber development offers valuable insight into how genetics and physiology interact to influence growth potential. Muscle fiber development begins early in embryonic stages, and although new muscle fibers are not generated post-embryonically, existing fibers can undergo hypertrophy (Wicks et al., 2019). Nutrition plays a pivotal role in muscle development, with poor nutrition leading to a reduced production of muscle fibers and adipocytes, contributing to the formation of intramuscular fat. These early nutritional influences can significantly affect the progression of myogenesis by altering the number and function of muscle fibers formed during embryonic development, ultimately shaping muscle growth and composition throughout the animal’s life.
Myogenesis, the process of muscle tissue formation, occurs in three main stages that begin during embryonic development (Mohammadabadi et al., 2021). The first stage involves the expression of specific genes and the fusion of myoblasts to form muscle fibers. These myoblasts can either proliferate into additional fibers if adequate growth factors are present or differentiate into myotubes. The second stage involves the alignment of myoblasts, which is essential for proper fiber formation. In the third stage, myoblasts undergo fusion, leading to the formation of multinucleated muscle fibers. During the later stages of myogenesis, satellite cells play a critical role by proliferating and contributing to muscle hypertrophy. Satellite cells are also key in muscle repair and regeneration following injury, as they differentiate into new muscle cells and fibers (Mohammadabadi et al., 2021). Key molecular regulators, such as myogenic regulatory factors (MRFs) and the transcription factors PAX3 and PAX7, play vital roles in myogenesis. MRFs activate genes that promote cell proliferation, while PAX3 regulates their activity, and PAX7 maintains satellite cells in a quiescent state while also activating myoblast formation (Mohammadabadi et al., 2021). Understanding the genetic regulation of these processes is crucial for research aimed at identifying genetic variations associated with muscle growth, which has significant implications for breeding programs targeting muscle mass and efficiency in livestock.
4.2 Growth factor signaling and the role of IGF1
The neuroendocrine system plays a pivotal role in regulating metabolic processes such as growth in mammalian species. This system includes growth hormones that are primarily produced in the hypothalamus and serve to regulate various growth signals throughout the body (Al-Samerria and Radovick, 2021). One such growth hormone is insulin-like growth factor 1 (IGF1), a polypeptide growth factor that is structurally similar to insulin and binds to insulin receptors, influencing a wide range of physiological processes (Laron, 2001). While IGF1 is produced in various tissues, its primary synthesis occurs in the liver, where it is secreted into the bloodstream and transported to target tissues, including skeletal muscle, where it exerts significant effects on growth, protein synthesis, and muscle repair (Al-Samerria and Radovick, 2021).
IGF1 plays a crucial role in regulating cellular growth, promoting protein synthesis, and aiding in muscle regeneration and degradation (Yoshida and Delafontaine, 2020). Figure 2, adapted and based on a study by Ahmad (2020) (Ahmad et al., 2020), illustrates the molecular mechanisms of IGF1 action, particularly in skeletal muscle tissue. The significance of IGF1 is further underscored by studies investigating Laron Syndrome (LS), previously known as Laron dwarfism, a condition caused by mutations in the IGF1 gene. Research summarized by Laron (2001) reveals that individuals with LS experience severe growth deficiencies in multiple tissues, including muscle, skeletal structure, brain, and heart, from birth through adulthood (Laron, 2001). In experimental studies, mice with IGF1 gene knockouts displayed a dramatic reduction in size, approximately half the normal body weight, and exhibited fatal developmental issues, particularly in the diaphragm, emphasizing the essential role of IGF1 in proper growth and development (Laron, 2001).
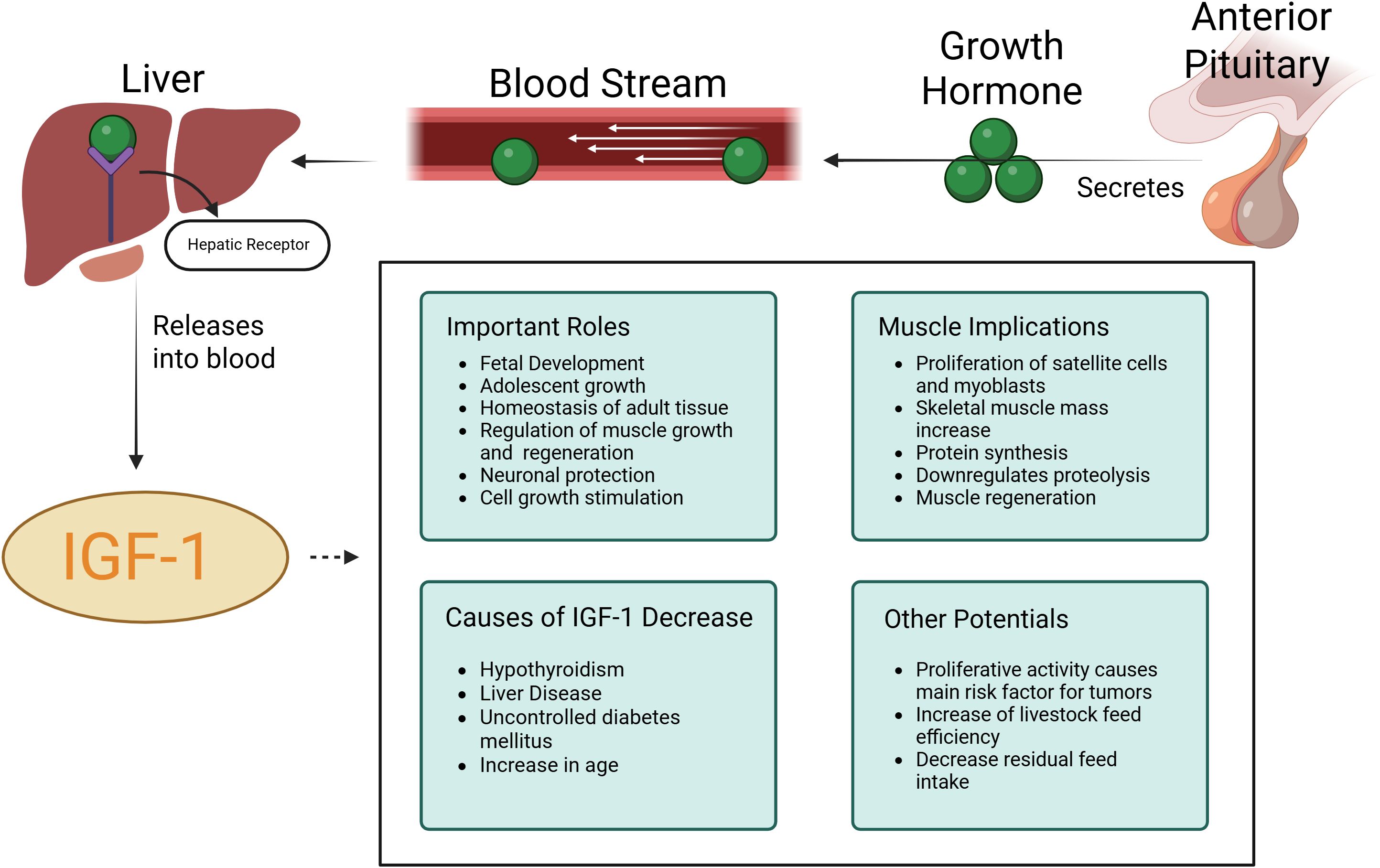
Figure 2. Molecular mechanisms of Insulin-like growth factor 1 (IGF-1) and impacts on muscle development and animal physiology. Adapted from (Ahmad et al., 2020).
In addition to its role in general growth, IGF1 has been implicated in improving feed efficiency in livestock. A study by Montelli et al. (2020) investigated the relationship between leptin and IGF1 in lambs over a 56-day feeding trial (Montelli et al., 2021). The lambs were classified based on their RFI and residual intake and gain (RIG), and it was found that lambs with greater feed efficiency (low RFI and high RIG) exhibited significantly greater levels of both leptin and IGF1 (Montelli et al., 2021). These findings suggest that IGF1 may play a key role in regulating feed efficiency and growth performance, making it an important target for genetic selection in livestock breeding programs aimed at improving muscle mass and feed utilization.
4.3 The rumen microbiome
Ruminants have evolved a highly specialized digestive system that enables them to consume and derive nutrients from plant materials that are typically inedible for humans. This capability arises from their compartmentalized stomachs, which house a diverse community of microorganisms, including bacteria, fungi, protozoa, and methanogens, that play a critical role in breaking down complex feedstuffs (Hackmann and Firkins, 2015). During fermentation in the rumen, microorganisms break down carbohydrates into volatile fatty acids (VFAs), along with gases such as methane and carbon dioxide, and microbial biomass. Many of these fermentation products and byproducts are subsequently absorbed and utilized by the animal to help meet its energy and protein requirements (Hackmann and Firkins, 2015). However, the efficiency of this digestive process is influenced by several factors, such as breed, diet, genomics, and the composition of the rumen microbiome, which in turn can affect nutrient use efficiency and methane emissions (Tapio et al., 2023).
The rumen microbiome has a profound impact on a ruminant’s ability to utilize and store energy, and research has demonstrated its significant role in influencing feed efficiency, particularly through RFI. Clemmons et al. (2019) identified specific microbial and metabolic biomarkers associated with feed efficiency, particularly RFI, in Angus steers (Clemmons et al., 2019). The researchers found that low-RFI steers had distinct microbial and metabolite profiles, including higher serum levels of pantothenate and greater relative abundance of the bacterial class Flavobacteriia. These features were predictive of RFI using machine learning models, suggesting that both Flavobacteriia and pantothenate may serve as useful biomarkers for selecting more feed-efficient animals. Importantly, the study highlighted the complex interaction between microbial communities and host metabolism, offering insight into the biological basis of feed efficiency.
Research in dairy cattle has shown that the composition and functionality of the rumen microbiome are strongly associated with variations in RFI. Tapio et al. (2023) conducted a study with 87 Nordic Red dairy cows in late lactation, ranking them based on their feed efficiency traits (Tapio et al., 2023). The microbial composition of their rumen was sequenced to examine correlations between microbial networks and feed efficiency. The study found that more efficient animals exhibited greater abundances of glycoside hydrolases, enzymes responsible for breaking down sugars, as well as increased bacterial motility and sensing. In contrast, animals with lower feed efficiency exhibited greater activity of glycosyltransferases and other enzymes involved in metabolic pathways. The data revealed that more efficient cows had a microbiome that supported enhanced carbohydrate utilization, as illustrated by the comparison of carbohydrate-active enzyme (CAZy) profiles in high- and low-efficiency groups. These results suggest that cows with greater feed efficiency harbor a microbiome capable of more efficiently processing polysaccharides, whereas less efficient animals expressed enzymes that facilitated carbohydrate degradation rather than utilization. This study provides compelling evidence that the microbiome’s diversity and functional capacity play key roles in optimizing feed efficiency, and that a more specialized microbial community may be linked to improved digestive and metabolic performance.
5 Discussion
Despite significant advancements in the genetic and physiological understanding of feed efficiency in beef cattle, critical research gaps remain, particularly in linking metabolic efficiency to muscle development. Traits like RFI have improved the ability to measure feed efficiency independently of growth rate, but the biological mechanisms driving variation in RFI are still not fully elucidated. Recent studies, such as Clemmons et al. (2019), have identified microbial and serum metabolite biomarkers, namely Flavobacteriia and pantothenate, as potential predictors of feed efficiency (Clemmons et al., 2019), yet further research is needed to validate these findings across diverse populations. At the same time, early life processes such as myogenesis are heavily influenced by both genetic and environmental factors. The interaction between nutrient availability, embryonic muscle development, and long-term growth efficiency remains an area where deeper mechanistic understanding is needed.
Future research should adopt integrative, systems-based approaches that combine genomic, metabolomic, molecular, microbiome, and phenotypic data to improve prediction and selection for both feed efficiency and muscle development. Particular attention should be given to critical developmental windows, such as prenatal and early postnatal stages, where interventions in maternal nutrition or genetic selection could optimize muscle fiber number and hypertrophy potential. Advances in omics technologies and machine learning provide powerful tools to explore these complex interactions, potentially identifying regulatory pathways that control both metabolic efficiency and muscle growth. Additionally, translating this research into practical on-farm tools, such as biomarker-based selection strategies, could accelerate genetic progress and enhance the sustainability of beef production by increasing lean tissue yield while reducing input costs and environmental impact.
As the global population is projected to increase significantly over the next decade, efficient animal production systems will be vital to ensure a sustainable and adequate supply of protein for human consumption. With continued research, beef production can be optimized for both efficiency and sustainability. A deeper understanding of the genetic and physiological mechanisms regulating muscle growth and feed efficiency in cattle will enable the development of more efficient breeding programs. Such advancements could not only meet the growing food demands of the population but also make beef production more economically viable for producers while minimizing its environmental impact.
Author contributions
SA: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. BV: Investigation, Supervision, Writing – original draft, Writing – review & editing. JB: Investigation, Supervision, Writing – original draft, Writing – review & editing. LS: Writing – original draft, Writing – review & editing. PM: Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by the Agriculture and Food Research Initiative grant no. 2020-67015–30832 from the USDA National Institute of Food and Agriculture.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad S. S., Ahmad K., Lee E. J., Lee Y.-H., and Choi I. (2020). Implications of insulin-like growth factor-1 in skeletal muscle and various diseases. Cells 9, 1773. doi: 10.3390/cells9081773
Allen M. S. (2014). Drives and limits to feed intake in ruminants. Anim. Production Sci. 54, 1513–1524. doi: 10.1071/AN14478
Al-Samerria S. and Radovick S. (2021). The role of insulin-like growth factor-1 (IGF-1) in the control of neuroendocrine regulation of growth. Cells 10. doi: 10.3390/cells10102664
Archer J., Richardson E., Herd R., and Arthur P. (1999). Potential for selection to improve efficiency of feed use in beef cattle: a review. Aust. J. Agric. Res. 50, 147–162. doi: 10.1071/A98075
Arthur P., Archer J., Johnston D., Herd R., Richardson E., and Parnell P. (2001). Genetic and phenotypic variance and covariance components for feed intake, feed efficiency, and other postweaning traits in Angus cattle. J. Anim. Sci. 79, 2805–2811. doi: 10.2527/2001.79112805x
Bottje W. G. and Carstens G. E. (2012). Variation in metabolism: biological efficiency of energy production and utilization that affects feed efficiency. Feed efficiency beef industry, 251–273. doi: 10.1002/9781118392331
Carstens G. E. and Tedeschi L. O. (2006). “Defining feed efficiency in beef cattle,” in In proceedings of the proceedings of beef improvement federation 38th annual research symposium and annual meeting (Choctaw, Mississippi: Beef Improvement Federation Conference), 12–21.
Clemmons B. A., Martino C., Powers J. B., Campagna S. R., Voy B. H., Donohoe D. R., et al. (2019). Rumen bacteria and serum metabolites predictive of feed efficiency phenotypes in beef cattle. Sci. Rep. 9, 19265. doi: 10.1038/s41598-019-55978-y
contributors, B.G.W Feed efficiency. Available online at: http://guidelines.beefimprovement.org/index.php?title=Feed_Efficiency&oldid=2735 (Accessed May 21, 2024).
Crews D. H. D. (2005). Genetics of efficient feed utilization and national cattle evaluation: a review. Genet. Mol. Res. 4, 152–165.
Crowley J. J., McGee M., Kenny D. A., Crews D. H. Jr., Evans R. D., and Berry D. P. (2010). Phenotypic and genetic parameters for different measures of feed efficiency in different breeds of Irish performance-tested beef bulls. J. Anim. Sci. 88, 885–894. doi: 10.2527/jas.2009-1852
Fonseca L. D., Eler J. P., Pereira M. A., Rosa A. F., Alexandre P. A., Moncau C. T., et al. (2019). Liver proteomics unravel the metabolic pathways related to feed efficiency in beef cattle. Sci. Rep. 9, 5364. doi: 10.1038/s41598-019-41813-x
García-Ruiz A., Cole J. B., VanRaden P. M., Wiggans G. R., Ruiz-López F. J., and Van Tassell C. P. (2016). Changes in genetic selection differentials and generation intervals in US Holstein dairy cattle as a result of genomic selection. Proc. Natl. Acad. Sci. 113, E3995–E4004. doi: 10.1073/pnas.1519061113
González N., Marquès M., Nadal M., and Domingo J. L. (2020). Meat consumption: Which are the current global risks? A review of recent (2010-2020) evidences. Food Res. Int. 137, 109341. doi: 10.1016/j.foodres.2020.109341
Hackmann T. J. and Firkins J. L. (2015). Maximizing efficiency of rumen microbial protein production. Front. Microbiol. 6. doi: 10.3389/fmicb.2015.00465
Hegarty R. S., Goopy J. P., Herd R. M., and McCorkell B. (2007). Cattle selected for lower residual feed intake have reduced daily methane production1,2. J. Anim. Sci. 85, 1479–1486. doi: 10.2527/jas.2006-236
Herd R. M. and Arthur P. F. (2009). Physiological basis for residual feed intake. J. Anim. Sci. 87, E64–E71. doi: 10.2527/jas.2008-1345
Hu Z., Boschiero C., Li C.-J., Connor E. E., Baldwin R. L., and Liu G. E. (2023). Unraveling the genetic basis of feed efficiency in cattle through integrated DNA methylation and CattleGTEx analysis. Genes 14, 2121. doi: 10.3390/genes14122121
Jiang W., Mooney M. H., and Shirali M. (2024). Unveiling the Genetic Landscape of Feed Efficiency in Holstein Dairy Cows: Insights into Heritability, Genetic Markers, and Pathways via Meta-Analysis. J. Anim. Sci. 102. doi: 10.1093/jas/skae040
Kenny D. A., Fitzsimons C., Waters S. M., and McGee M. (2018). Invited review: Improving feed efficiency of beef cattle – the current state of the art and future challenges. Animal 12, 1815–1826. doi: 10.1017/S1751731118000976
Khanal P., Johnson J., Gouveia G., Ross P., and Deeb N. (2023). Genomic evaluation of feed efficiency in US Holstein heifers. J. Dairy Sci. 106, 6986–6994. doi: 10.3168/jds.2023-23258
Koch R. M., Swiger L. A., Chambers D., and Gregory K. E. (1963). Efficiency of feed use in beef cattle. J. Anim. Sci. 22, 486–494. doi: 10.2527/jas1963.222486x
Laron Z. (2001). Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol. Pathol. 54, 311–316. doi: 10.1136/mp.54.5.311
Lawrence J., Mintert J., Anderson J., and Anderson D. (2008). “Feed grains and livestock: impacts on meat supplies and prices,” in Iowa state university, department of economics, staff general research papers, vol. 23. .
Manzanilla-Pech CIV S. R., Difford G. F., Løvendahl P., and Lassen J. (2022). Selecting for feed efficient cows will help to reduce methane gas emissions. Front Genet. 13, 885932. doi: 10.3389/fgene.2022.885932
McKenna C., Keogh K., Porter R. K., Waters S. M., Cormican P., and Kenny D. A. (2021). An examination of skeletal muscle and hepatic tissue transcriptomes from beef cattle divergent for residual feed intake. Sci. Rep. 11, 8942. doi: 10.1038/s41598-021-87842-3
Moe P. W. (1981). Energy metabolism of dairy cattle. J. Dairy Sci. 64, 1120–1139. doi: 10.3168/jds.S0022-0302(81)82692-6
Mohammadabadi M., Bordbar F., Jensen J., Du M., and Guo W. (2021). Key genes regulating skeletal muscle development and growth in farm animals. Anim. (Basel) 11. doi: 10.3390/ani11030835
Montelli N., Alvarenga T., Almeida A. K., Alvarenga F. A. P., Furusho-Garcia I. F., Greenwood P. L., et al. (2021). Associations of feed efficiency with circulating IGF-1 and leptin, carcass traits and meat quality of lambs. Meat Sci. 173, 108379. doi: 10.1016/j.meatsci.2020.108379
Nielsen M. K., MacNeil M. D., Dekkers J. C. M., Crews D. H., Rathje T. A., Enns R. M., et al. (2013). Review: Life-cycle, total-industry genetic improvement of feed efficiency in beef cattle: Blueprint for the Beef Improvement Federation11The development of this commentary was supported by the Beef Improvement Federation. Prof. Anim. Scientist 29, 559–565. doi: 10.15232/S1080-7446(15)30285-0
Ojo A. O., Mulim H. A., Campos G. S., Junqueira V. S., Lemenager R. P., Schoonmaker J. P., et al. (2024). Exploring feed efficiency in beef cattle: from data collection to genetic and nutritional modeling. Animals 14, 3633. doi: 10.3390/ani14243633
Patience J. F., Rossoni-Serão M. C., and Gutiérrez N. A. A. (2015). review of feed efficiency in swine: biology and application. J. Anim. Sci. Biotechnol. 6, 33. doi: 10.1186/s40104-015-0031-2
Pryce J. E., Wales W. J., de Haas Y., Veerkamp R. F., and Hayes B. J. (2014). Genomic selection for feed efficiency in dairy cattle. Animal 8, 1–10. doi: 10.1017/s1751731113001687
Salleh S. M., Mazzoni G., Løvendahl P., and Kadarmideen H. N. (2018). Gene co-expression networks from RNA sequencing of dairy cattle identifies genes and pathways affecting feed efficiency. BMC Bioinf. 19, 513. doi: 10.1186/s12859-018-2553-z
Savietto D., Berry D., and Friggens N. (2014). Towards an improved estimation of the biological components of residual feed intake in growing cattle. J. Anim. Sci. 92, 467–476. doi: 10.2527/jas.2013-6894
Scialabba N., Jan O., Tostivint C., Turbé A., O’Connor C., Lavelle P., et al. (2013). Food wastage footprint: impacts on natural resources. Summary report.
Silva Neto J. B., Mota L. F. M., Amorim S. T., Peripolli E., Brito L. F., Magnabosco C. U., et al. (2023). Genotype-by-environment interactions for feed efficiency traits in Nellore cattle based on bi-trait reaction norm models. Genet. Selection Evol. 55, 93. doi: 10.1186/s12711-023-00867-2
Tapio M., Fischer D., Mäntysaari P., and Tapio I. (2023). Rumen microbiota predicts feed efficiency of primiparous nordic red dairy cows. Microorganisms 11. doi: 10.3390/microorganisms11051116
Tempelman R. J. and Lu Y. (2020). Symposium review: Genetic relationships between different measures of feed efficiency and the implications for dairy cattle selection indexes. J. Dairy Sci. 103, 5327–5345. doi: 10.3168/jds.2019-17781
Westerterp K. R. (2000). “Control of energy expenditure in humans,” in Endotext. Eds. Feingold K. R., Anawalt B., Blackman M. R., Boyce A., Chrousos G., Corpas E., de Herder W. W., Dhatariya K., Dungan K., Hofland J., et al. (South Dartmouth (MA): MDText.com, Inc.
Wicks J., Beline M., Gomez J. F. M., Luzardo S., Silva S. L., and Gerrard D. (2019). Muscle energy metabolism, growth, and meat quality in beef cattle. Agriculture 9, 195. doi: 10.3390/agriculture9090195
Williams Y., Pryce J., Grainger C., Wales W., Linden N., Porker M., et al. (2011). Variation in residual feed intake in Holstein-Friesian dairy heifers in southern Australia. J. Dairy Sci. 94, 4715–4725. doi: 10.3168/jds.2010-4015
Yi Z., Li X., Luo W., Xu Z., Ji C., Zhang Y., et al. (2018). Feed conversion ratio, residual feed intake and cholecystokinin type A receptor gene polymorphisms are associated with feed intake and average daily gain in a Chinese local chicken population. J. Anim. Sci. Biotechnol. 9, 1–9. doi: 10.1186/s40104-018-0261-1
Keywords: feed efficiency, beef cattle, muscle, physiology, sustainability
Citation: Ascolese SM, Voy BH, Beever JE, Stephens LM and Myer PR (2025) Molecular and metabolic insights into muscle development and feed efficiency in beef cattle. Front. Anim. Sci. 6:1613829. doi: 10.3389/fanim.2025.1613829
Received: 17 April 2025; Accepted: 13 June 2025;
Published: 02 July 2025.
Edited by:
Michael D. Flythe, United States Department of Agriculture, United StatesReviewed by:
Brittany Davis, Agricultural Research Service (USDA), United StatesCopyright © 2025 Ascolese, Voy, Beever, Stephens and Myer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Phillip R. Myer, cG15ZXJAdXRrLmVkdQ==
 Sophia M. Ascolese
Sophia M. Ascolese Brynn H. Voy
Brynn H. Voy Jonathan E. Beever1,2
Jonathan E. Beever1,2 Phillip R. Myer
Phillip R. Myer