- 1Department of Agricultural and Food Sciences (DISTAL), University of Bologna, Bologna, Italy
- 2Department of Agriculture, Food and Environment, University of Pisa, Pisa, Italy
- 3Department of Medical Veterinary Sciences (DIMEVET), University of Bologna, Ozzano dell’Emilia, Italy
- 4R&D Department, Animine, Annecy, Annecy, France
- 5R&D Department, Cargill Incorporated, Elk River, MN, United States
Zinc (Zn) is an essential trace element for piglets, especially during the challenging post-weaning period. This study investigated the effects of two dietary Zn sources, namely zinc sulfate (ZnSO4) and a porous zinc oxide (pZnO), used at the European-authorized dietary level on piglets of differing birth body weights (BBWs): low (LBBW <1 kg) and normal (NBBW >1 kg). At weaning (25 days, d0), 64 piglets were assigned to four groups based on BBW and Zn source and fed diets that reached a total Zn level of 150 mg/kg. Body weight was monitored weekly until d21. On d9 and d21, 32 piglets were slaughtered for gut histology, immunohistochemistry, gene expression, blood markers, pH measurement, microbiota, and short-chain fatty acid (SCFA) analysis. The NBBW group had higher BW throughout the study (P<0.01), confirming BBW as a key factor influencing growth and physiological maturity. The pZnO group tended to have a higher average daily gain in the periods d0-d9 and d9-d14 (P<0.10). The pZnO reduced jejunum pH at d21 (P = 0.02). The interaction between diet and BBW influenced the nuclear factor kappa B subunit 2 (NFKB2) expression at d9 (P = 0.03), with LBBW piglets fed ZnSO4 showing higher expression. At d21, the interaction between diet and BBW affected the villus height (P = 0.05) and the absorptive mucosal surface (P = 0.02), which were higher in the NBBW group than in the LBBW group fed ZnSO4, while no difference was observed between the NBBW and LBBW groups fed the pZnO. Differences in microbiota beta diversity were associated with BBW (P = 0.07 at d9; P = 0.03 at d21), and taxa abundance varied with Zn source and BBW. Overall, the results demonstrate that the pZnO positively influenced gut health and performance in weaned piglets, particularly in the LBBW group. The differential response to Zn sources according to BBW suggests that tailored mineral strategies could help mitigate the effects of weaning stress in vulnerable piglets.
1 Introduction
Currently, genetic selection is leading to an increased spread of genetic lines of high-prolific sows; as a consequence of the high-prolific sows’ genetic selection, there has been an increase in piglets born with low birth body weight (LBBW) (Beaulieu et al., 2010). The term “LBBW” refers to piglets with a birth weight of less than 1.1 kg (Li et al., 2018) and is often associated with high neonatal mortality, delayed postnatal growth and development (Quiniou et al., 2002; Wellington et al., 2021), lower BW at the end of the nursing and during the weaning period (Rodrigues et al., 2020), and abnormal intestinal morphology (Chen et al., 2021; Gondret et al., 2013). Furthermore, LBBW piglets are also associated with increased gastrointestinal tract (GIT) impairment due to the severe immaturity of the intestinal gut mucosa (Michiels et al., 2013), as indicated by the lower expression of the inhibitor of the apoptosis gene, which codes for intestinal development (Ayuso et al., 2021), compromised intestinal permeability (Tao et al., 2019), and low expression of tight junction proteins such as Claudin 4 and Occludin 1 (Ayuso et al., 2021).
Among nutrients, zinc (Zn) is an important trace element that is involved in the synthesis of over 300 enzymes, including intestinal brush border enzymes. It functions as a structural and functional element in protein molecules, DNA replication, and the reverse transcription process (Ciesinski et al., 2018). Furthermore, it is involved in metabolic pathways and molecular synthesis and in controlling gene expression, and is linked to gut health and immune function in animals (Ciesinski et al., 2018). Zn has been shown to reinforce the gut barrier by maintaining tight junction proteins, thereby reducing permeability and preventing the entry of potentially pathogenic bacteria into the bloodstream (Ortega and Szabó, 2021). Additionally, Zn plays a crucial role in intestinal development by promoting enterocyte proliferation, supporting villus height, and enhancing the enzymatic activity essential for nutrient absorption (Wan and Zhang, 2022). Zn also exerts significant anti-inflammatory effects by modulating cytokine expression and reducing oxidative stress in the gut epithelium, which is especially crucial for LBBW piglets, who are more prone to intestinal inflammation (Oh et al., 2021). According to recent European Union (EU) regulations, piglets’ diets can contain up to 150 mg/kg of Zn, which is higher than the recommendation of the National Research Council (NRC) (NRC, 2012) (60 to 100 mg/kg). Currently, in Europe, the level of Zn in the raw material used to formulate common post-weaning diets is approximately 35/45 mg/kg (Revy et al., 2003). Therefore, it is possible to add Zn from other sources to the post-weaning diets to reach a total of 150 mg/kg. Zinc oxide (ZnO) was one of the main Zn forms used to support the gut health of piglets; however, the literature is mainly based on high doses of ZnO, which are currently forbidden in the EU, while research on the effects of European-authorized doses of Zn on restoring small intestine impairment is still lacking.
Moreover, Zn is available in different forms, characterized by different solubility and/or bioavailability, subsequently impacting its physiological role and the coverage of piglets’ requirements. In Europe, Zn sulfate (ZnSO4) has been used as the primary Zn nutritional source in feed for a long time due to its high water solubility (Diao et al., 2021; Villagómez-Estrada et al., 2020). In fact, binding Zn to the sulfate group leads to an increase in its bioavailability (Bonetti et al., 2021).
More recently, different sources of Zn, such as ZnSO4, have been tested with promising results on gut health and in alleviating weaning disorders in piglets (Long et al., 2017). In particular, the use of a low dose (500 mg/kg) of porous ZnO (pZnO) has been demonstrated to be as effective as the pharmacological dose of ZnO in sustaining gut function in weaning pigs (Long et al., 2017). This form of porous Zn is characterized by small aggregates and agglomerated particles (Cardoso et al., 2021) and has higher solubility than other ZnO sources (Wang et al., 2019), despite being a non-water-soluble source. Indeed, the dissolution kinetics of porous Zn may follow a mixed reaction–diffusion mechanism, including a dissolution occurring on the internal surface of the pores and a diffusion of Zn²+ ions through the pores into the surrounding medium. As a result, the release rate of porous Zn is slower than that of ZnSO4. These differences between pZnO and ZnSO4 may also influence the expression of intestinal Zn transporters (Ma et al., 2021). In particular, two Zn transporter families have been detected: solute carrier (SLC) belonging to family 30 (zinc transporter, ZnT) and family 39 (ZIP). ZIP and ZnT differ in their direction of Zn transport, position, and their mechanistic transport (Yin et al., 2022). ZIP family transporters promote Zn2+ influx into the cytosol from the subcellular compartments or the extracellular space (Kambe and Wagatsuma, 2023). In contrast, ZnT transporters decrease Zn2+ from the cytosol in two possible ways: by facilitating Zn uptake in subcellular compartments or by excreting it into the circulation (Rutter et al., 2016). Since ZnSO4 releases Zn²+ ions rapidly, which can saturate intestinal transporters (from the ZIP family), leading to the risk that part of the Zn may be lost or precipitate in the presence of anti-nutrients (e.g., phytates, fibers). In contrast, pZnO, characterized by a more gradual and sustained release of Zn²+ ions, can enhance intestinal absorption along the entire small intestine and prevent the local saturation of transporters, making absorption more efficient over time (Ma et al., 2021).
Based on current knowledge, since the LBBW pigs suffer a greater negative impact at weaning, characterized by a lower BW at weaning; less intestinal development, especially of the small intestine; and a lower maturity of the immune system than NBBW piglets, we hypothesized that the administration of a porous form of ZnO could benefit their development, reducing the physiological gap between LBBW and NBBW piglets. To the authors’ knowledge, this is the first study aiming to compare two different sources of Zn in piglets differing in their birth body weight (BBW). Following our preliminary results (Negrini et al., 2022, 2023), the aim of the present study was to compare the effects of ZnSO4 or a pZnO, which differ in their solubility, dissolution kinetics, and intestinal absorption, on the gut health status of LBBW and NBBW piglets from weaning until 3 weeks post-weaning.
2 Materials and methods
2.1 Experimental design
The in vivo trial was approved by the Ethics Committee for Experiments on Animals at the University of Bologna, Italy and by the Italian Ministry of Health (Authorization n. 287/2021 PR, issued in compliance with art. 31 of the D.lgs. 26/2014) and complied with the Animal Research Reporting of In Vivo Experiments guidelines (Percie du Sert et al., 2020).
A total of 64 piglets (Large white × Landrace) were selected from multiparous sows (5 ± 1.5 parity order) reared on an Italian commercial farm. Healthy piglets from each litter were weighed at birth and were categorized as LBBW when the BBW was <1 kg (0.92 ± 0.09 kg; n=32) or NBBW when the BBW was >1 kg (1.37 ± 0.09 kg, n=32). The piglets were then monitored and weighed 3 days after birth (LBBW: 1.23 ± 0.00014 kg; NBBW: 1.74 ± 0.00021 g) and again at weaning (25 days of age; LBBW: 6.284,52 ± 0.75 kg and NBBW: 7.777,50 ± 0.77 g). No creep feed was provided during the suckling phase.
At weaning (d0), the piglets were transported to the experimental unit at the University of Bologna and arranged in a 2 x 2 factorial design that included BBW (LBBW or NBBW) and diet (ZnSO4 or pZnO) (HiZox, Animine, Annecy, France) as factors. Therefore, the piglets were divided into four experimental groups (8 replicates of 2 piglets/group) balanced for litter of origin (both BBW classes were represented in each litter) and BW (within each BBW class): 1) LBBW piglets receiving a diet with ZnSO4; 2) LBBW piglets receiving a diet with pZnO; 3) NBBW piglets receiving a diet with ZnSO4; and 4) NBBW piglets receiving a diet with pZnO.
To prepare the two diets, the basal diet was initially produced in a single batch without Zn addition. After production, Zn concentrations were determined in the basal diet. Then, the basal diet was divided into two sub-batches. Each sub-batch was integrated with one of the two Zn sources to reach approximately 150 mg/kg of total Zn using a small mixer.
Humidity, crude protein, crude fat, and crude fiber in the diet were analyzed following the methods approved by the European (2009). The zinc content of the diets was analyzed using inductively coupled plasma mass spectrometry ICP/MS (Wilschefski and Baxter, 2019). The final Zn levels of the two diets, obtained after supplementation with HiZnO and ZnSO4, respectively, fall within the EU authorized range, considering the analytical error of the analysis. Calcium and phosphorus concentration analyses were carried out using inductively coupled plasma optical emission spectroscopy (ICP-OES, AOAC Official Method 984.27; AOAC Int., 2007). Metabolizable and Net energy (ME and NE, respectively) were estimated using the InraPorc software (Version 1.8.0.0) (van Milgen et al., 2008) based on the Noblet et al. (1994).
The estimated and analyzed composition values of the basal diet are reported in Table 1.
During the study, the piglets were fed ad libitum with continuous access to water. The piglets were kept in pens with a slatted floor containing enrichment material consisting of metal chain and cotton rope. The room temperature was kept at 30°C at the beginning of the trial and then gradually decreased to 26°C by the end of the trial (day 21). In addition, infrared lamps were used during the first 10 post-weaning days. Temperature and humidity were recorded daily.
2.2 Measurements and sample collection
Piglets were weighed individually on d0 and then every week until d21 post-weaning (end of the trial). Feed intake (FI), general health status, and fecal consistency (5-point scale: 1 hard feces – 5 watery feces) were recorded daily. The piglets were considered to have diarrhea when the fecal score was > 3 (Correa et al., 2022). The piglets (one piglet/replicate) were slaughtered at two time points: d9 (total of 32 piglets; acute post-weaning phase) and d21 (total of 32 piglets; post-weaning recovery phase). At slaughter, the piglets were sedated and euthanized as previously described by Trevisi et al. (2023).
At d9 and d21, the piglets were fasted for 12 hours in order to reach the basal physiological level of Zn in the blood circulation and to avoid confounding factors regarding the determination of Zn in the target tissues. Blood samples were collected at d9 (from all the piglets) and d21 (from the remaining piglets) in 10 mL tubes with a clot activator (Vacutest Kima Padova, Italy). After 2 hours of clotting, the blood samples were centrifuged at 2,000 x g at room temperature for 10 minutes to obtain the serum. The serum was then stored at –80°C until the analyses for reactive oxygen metabolite (ROM), haptoglobin (Hp), and Zn concentrations.
At slaughter, the intestinal content from the distal jejunum, cecum, and colon was immediately sampled and processed for pH determination (Vio, Giorgio Bormac S.r.l., Carpi, MO, Italy). For microbiota profile characterization and short-chain fatty acid (SCFA) analyses, a sample of the colon content was collected in a sterile tube, immediately frozen in liquid nitrogen, and then stored at -80°C. Two samples of the distal jejunal mucosa were collected from each piglet. One was gently scraped, promptly frozen in liquid nitrogen, and then stored at -80°C for gene expression analyses, and the second one was fixed in formalin for immunohistochemical and morphological analyses. Finally, a liver sample (from the same lobe) was collected, frozen in liquid nitrogen, and then stored at -80°C for Zn concentration analyses.
2.3 Blood and liver analysis
ROM concentration was determined colorimetrically from the serum samples using the d-ROMs test kit (Diacron International Sr1, Grosseto, Italy). Regarding the automatic analysis of the ROMs (Brambilla et al., 2002), the analyses were carried out as described by Correa et al. (2022). The concentrations of Hp were determined in the serum samples using the Tridelta Phase Hp Kit (Tridelta Development Ltd., Maynooth, Co. Kildare, Ireland) as previously described by Trevisi et al. (2017). Zn concentrations in the serum and liver were analyzed using inductively coupled plasma optical emission spectrometry (ICP-OES, Method 985.01 A, B, and C; 135 AOAC Int., 2007) after wet ash sample preparation (Method 975.03 B(b); AOAC Int., 2007).
2.4 Gene expression analysis in the jejunum
Total RNA was isolated from the jejunal mucosal samples of the piglets and transformed into complementary DNA (cDNA) using the method described by Luise et al. (2023). Duplex real-time PCR was carried out for the following specific genes: IAP (intestinal alkaline phosphatase) as an intestinal development marker; CLAUD4 (Claudin-4) for its role in the regulation of the tight junction pathway; NFKB2 (Nuclear Factor Kappa B Subunit 2), to assess the activation of inflammatory pathways; GPX2 (Glutathione Peroxidase 2), as a marker of oxidation status; SLC39A4 (Solute Carrier Family 39 Member 4), and SLC30A7 (Solute Carrier Family 30 Member 7) as Zn transporters. Each reaction consisted of 2 µL of cDNA and an 8 µL mix containing probe assays (Supplementary Table S1), along with 2X TaqMan Mastermix. The assays were carried out in triplicate as previously described by Trevisi et al. (2023). Target gene expression was presented as a fold change, calculated using the formula 2-ΔΔCt.
2.5 Morphological and immunohistochemical analysis of the jejunum mucosa
Samples from the jejunum were preserved in 10% buffered formalin for 48 hours and subsequently embedded in paraffin for the morphological assessments. Sections of the paraffin were then stained with hematoxylin and eosin. Measurements of the height and width of 30 villi and the width and depth of 30 crypts were taken from each sample. Only villi and crypts aligned perpendicularly to the muscularis mucosae were selected for the morphometric study. Using a conventional microscope linked to a digital camera and computer with cytometry software (Byk Gulden, Milan, Italy), the sections were examined at a lower magnification. Villus height was defined as the distance from the base of the crypt to its top, while crypt depth was measured from its base to the level of the crypt opening. The mucosal-to-serosal amplification ratio (M), indicating the absorptive mucosal area in the jejunum, was computed as described by Correa et al. (2022).
The samples were observed using a Nikon Eclipse Ni microscope, and the images were captured with a Nikon DS-Fi2 digital camera using NIS Elements software BR 4.20.01 (Nikon Instruments Europe BV, Amsterdam, The Netherlands). A 20X objective lens was utilized for morphometric assessments.
Regarding the immunohistochemical procedure, the avidin-biotin peroxidase complex (ABC) technique was utilized. The paraffin sections initially underwent deparaffinization and rehydration. Antigenic sites were exposed by heating the slides in sodium citrate buffer (pH 6.0) using a microwave. To inhibit endogenous peroxidase, the sections were treated with a 1% methanol peroxidase solution for 30 minutes at room temperature. Subsequently, a 30-minute incubation in 0.01 M phosphate-buffered saline containing 10% goat or horse serum was carried out to block non-specific antibody binding. The slides were then left overnight at 4°C with the following antibodies: polyclonal goat anti-porcine immunoglobulin A (IgA) (1:2000; Novus Biologicals, NB724), polyclonal rabbit anti-CD3 serum (1:1500; Sigma Aldrich, C7930) and monoclonal mouse anti-Claudin-4 (1:300; ThermoFisher -Invitrogen, 32-9400, clone 3E2C1). After rinsing, the slides were incubated at RT for 1 hour with biotin-conjugated secondary antibodies [(goat anti-rabbit IgG, horse anti-goat IgG, and goat anti-mouse IgG, each diluted 1:200) (Vector)], followed by treatment with the ABC complex (Vector Elite Kit, Vector Laboratories). Visualization of the immune reactions was achieved using a 3,3’-diaminobenzidine chromogen solution (Vector DAB Kit, Vector Laboratories), and Toluidine blue was used to counterstain the sections. Figure 1 shows a representative image of porcine jejunal mucosa stained with anti-IgA, CD3, and Claudin-4 antibodies.
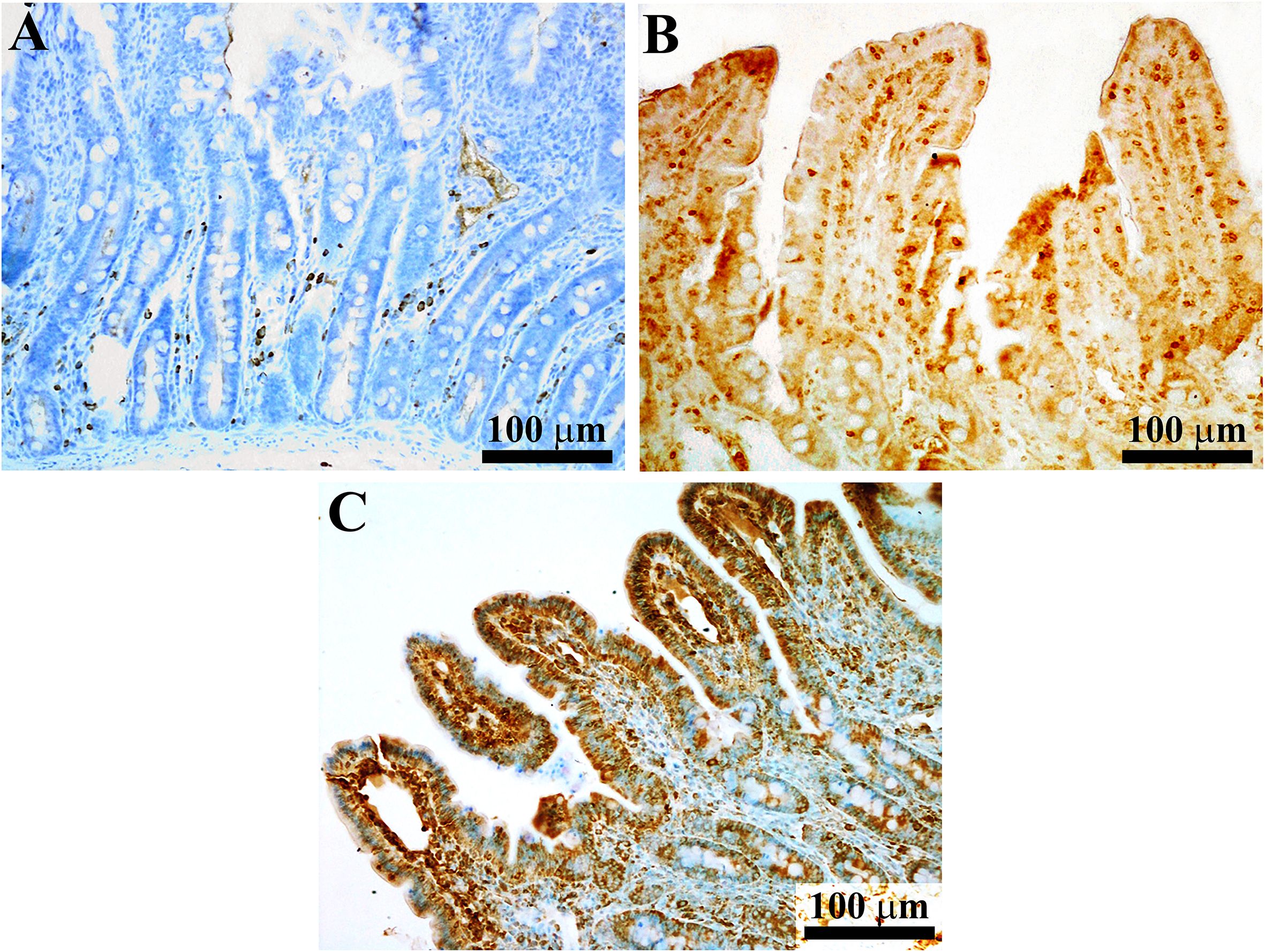
Figure 1. Representative images of porcine jejunal mucosa stained with anti-IgA, CD3, and Claudin antibodies. In image (A) the immunoreactive (IR) gAproducing cells are mainly located in the connective tissue (lamina propria) surrounding the intestinal glands, rather than in the connective tissue of the villus. T lymphocytes [(CD3-IR cells, image (B)] are located in both the epithelial layer (above the basement membrane) and the lamina propria. Intraepithelial T lymphocytes are mixed with enterocytes along the villus axis. Claudin 4 immunoreactivity involves the enterocyte profile, especially in the villi (less so in the intestinal glands) (C). The images in (A, C) were counterstained with toluidine blue.
Quantitative assessment of the IgA cells followed the protocol described by Bianco et al. (2014). The IgA-positive cell counts were carried out in two functional regions of the intestinal lamina propria: 1) along the villus axis and 2) interspersed within the crypts. The IgA-positive cell density was quantified and expressed as the number of immunoreactive IgA cells per 4,000 μm2. The lamina propria region was manually outlined to exclude significant blood vessels and epithelial cells.
The distribution and count of jejunal intra-epithelial lymphocytes, T lymphocytes along the villi, and the lamina propria between the crypts were assessed in three distinct areas: the first within the enterocytes above the epithelial basal lamina, the second within the lamina propria along the villus axis, and the third within the lamina propria interspersed with the crypts, as previously defined by Vega-López et al. (2001).
For Claudin-4 immunoreactivity assessment, 10 villi from each sample were examined. The sections were graded based on the presence, distribution, and intensity of Claudin-4, using the following scores: 1: light/limited staining, 2: moderate staining, and 3: intense staining, as detailed by Correa et al. (2022). The analyses involved 10 villi and 10 crypts per piglet.
2.6 Bacterial DNA extraction and bioinformatic analysis
The DNA extraction was carried out on 62 samples in total. The bacterial DNA present in the colon content was isolated using the FastDNA SPIN Kit for Soil (MP Biomedicals, Santa Ana, CA, USA), following the instructions provided. To assess DNA concentration and purity, a spectrophotometric analysis was carried out using the NanoDrop instrument (Thermo Fisher Scientific Inc., Waltham, Massachusetts, United States).
The V3-V4 regions of the 16S rRNA gene (~460 bp) were targeted for amplification. The amplification process utilized universal primers Pro341F and Pro805R as detailed by Takahashi et al. (2014). Platinum™ Taq DNA Polymerase High Fidelity (Thermo Fisher Scientific Inc., Waltham, Massachusetts, United States) was used. The Illumina MiSeq platform was utilized for sequencing with a 300x2bp configuration. Library preparation adhered to the standard MiSeq Reagent Kit V3 protocol, and sequencing took place on the MiSeq instrument (Illumina Inc., San Diego, CA, USA).
For bioinformatic analyses, the DADA2 pipeline (Callahan et al., 2016) was used, and the Silva database (Quast et al., 2013) (version 138.1) was used for the taxonomic assignments. After primer removal, both forward and reverse reads were trimmed at positions 290 and 250, respectively, based on the average quality scores. Default settings for the other parameters within the DADA2 analysis were retained.
2.7 Short-chain fatty acids and lactic acid concentrations in colon content
Examination of SCFAs (including acetate, propionate, isobutyrate, butyrate, valerate, and isovalerate) and lactic acid in the colon content samples was conducted using HPLC, as previously reported by Trevisi et al. (2023). Quantitative analysis was carried out based on an external calibration curve using standard solutions (Sandri et al., 2017).
2.8 Statistical analyses
R version 3.6 was used to carry out the statistical analyses, with the “car” 3.1-1 (Fox and Weisberg, 2019), “lm4” 1.1-31 (Bates et al., 2015), and “lsmeans” 2.30–0 packages (Lenth, 2021).
The four groups were arranged based on a 2 x 2 factorial design, taking into consideration the BBW class and diet. Prior to initiating the statistical assessments, the data distributions were examined. Parameters including BW, average daily gain (ADG), fecal score, intestinal content pH, ROMs, Hp, gene expression, morphological metrics, SCFAs, and lactic acid underwent analysis using a linear mixed model. This model included BBW class, diet, and their interaction as fixed factors, with the litter as a random factor. For the FI and the gain to feed (G:F) ratio, the pen was considered the experimental unit. These parameters were analyzed using a linear model with diet and BBW class as fixed factors. The difference between the groups was tested using Tukey’s post hoc test.
For the microbiota analysis, the vegan 2.6 (Dixon, 2003), phyloseq 1.38.0 (McMurdie and Holmes, 2011), and microbiomeMarker 1.0.2 (Cao et al., 2022) R packages were used for statistical analyses on alpha diversity, beta diversity, and taxonomic composition. For alpha diversity, the Chao1, Shannon, and Simpson indices were calculated, and differences among the groups were analyzed using a linear model. Diet, BBW class, their interaction, and sequencing depth were included in the model. The beta diversity was calculated utilizing the Bray–Curtis distance matrix, visualized via a non-metric multidimensional scaling (NMDS) plot. The effects of BBW class and diet were tested using a non-parametric PERMANOVA model (Adonis test) with 999 permutations. Differential abundance analysis concerning various taxa was carried out using Linear discriminant analysis Effect Size (LefSe) (Segata et al., 2011), integrated within the microbiomeMarker package (v 1.0.2). Data were aggregated at the genus level, with a cutoff value set at 3, and a P.adj < 0.05 was considered significant for the LefSe analysis (Correa et al., 2022).
The results were considered significant at P < 0.05, while P ≥ 0.05 and P < 0.10 were considered tendencies (Correa et al., 2022).
3 Results
3.1 Health and performance
Two piglets out of 64 were excluded from the study due to reduced FI during the first week post-weaning and the consequent loss in BW (one in the ZnSO4 group and one in the pZnO group from the LBBW class). No effect of diet and BBW class was observed on the fecal score. Overall, the piglets were healthy since the average fecal score was below 3 (cutoff for diarrhea).
Table 2 reports the effects of the diet and the BBW class on growth performance. The interaction between diet and BBW class was never significant. The BW was not affected by diet at d7, d9, and d21, while at d14, the piglets in the pZnO group tended to be heavier than the piglets in the ZnSO4 group (P = 0.09). The BBW class significantly influenced the BW of the piglets; the NBBW piglets were heavier than the LBBW piglets at all the time points (P < 0.001).
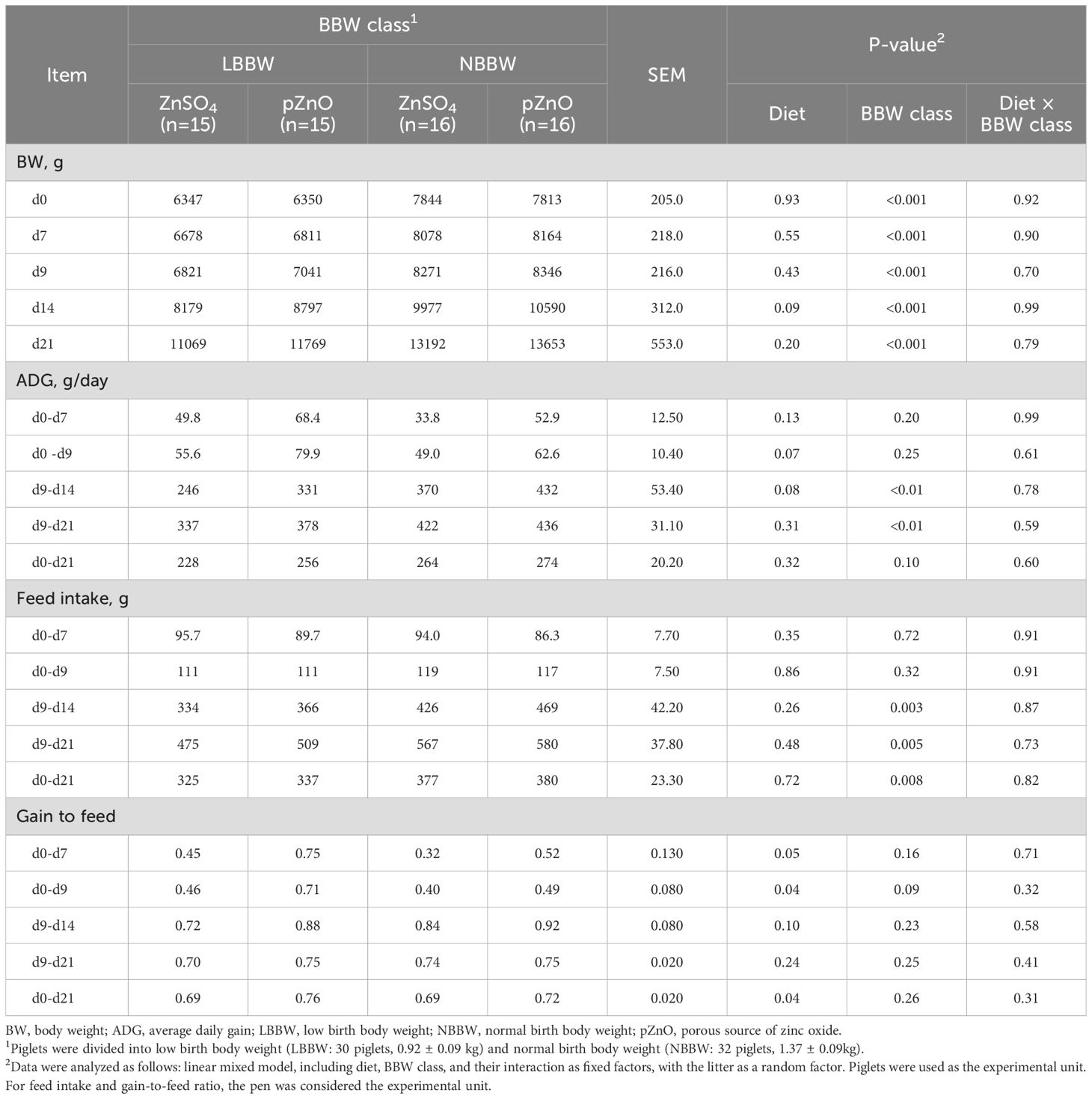
Table 2. The effects of zinc administration, body weight class, and their interaction on the performance outcomes of post-weaning piglets.
The ADG was not affected by diet in the periods from d0 to d7, from d9 to d21, and from d0 to d21, while it tended to be higher in the pZnO group compared with the ZnSO4 group when considering the periods from d0 to d9 (P = 0.07) and from d9 to d14 (P = 0.08). No differences were observed in the ADG in the periods from d0 to d7, from d0 to d9, and from d0 to d21 for the BBW, while it affected the ADG in the periods from d9 to d14 (P < 0.01) and from d9 to d21 (P = 0.01); the NBBW piglets had a higher ADG compared with the LBBW piglets (P < 0.01).
The FI was never affected by diet or by the interaction between diet and BBW in any of the periods analyzed, while the BBW class influenced piglet FI from d9 to d14, from d9 to d21, and from d0 to d21, resulting in a higher FI in the NBBW group (P < 0.01).
The G:F ratio was affected by diet considering the periods from d0 to d7 (P = 0.05), from d0 to d9 (P = 0.04), and from d0 to d21 (P = 0.04), resulting in a higher G:F ratio for the pZnO group as compared with the ZnSO4 group. The BBW class never affected the G:F ratio except for a tendency from d0 to d9 (P = 0.09) when the LBBW piglets had a higher G:F ratio as compared to the NBBW piglets.
3.2 Blood and liver parameters
The effects of diet, BBW class, and their interaction on Zn concentrations in serum and liver, and on Hp and ROM concentrations are reported in Table 3. The effects of interaction between diet and BBW class, diet, and BBW class were never significant.
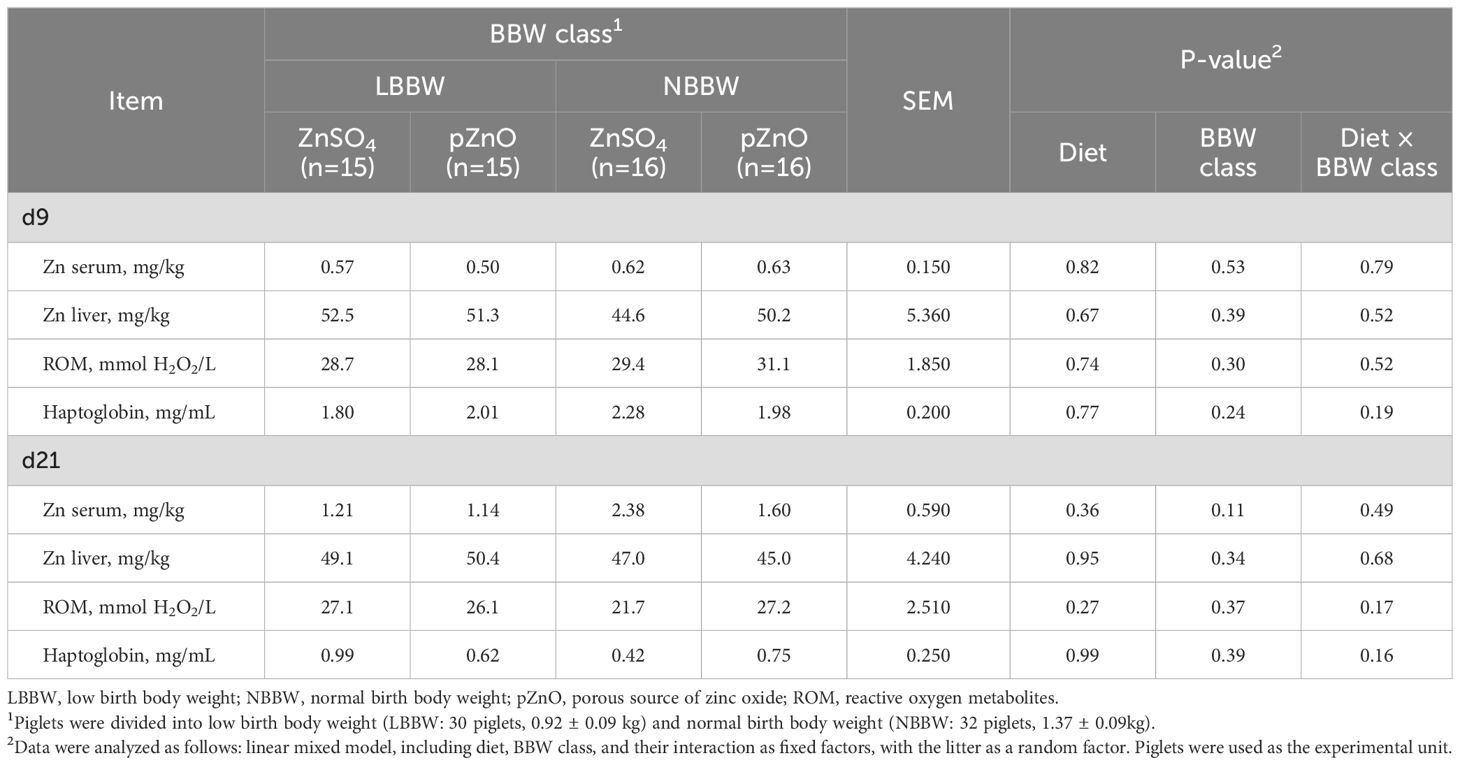
Table 3. The effects of zinc administration, body weight category, and their interaction on zinc concentrations in the serum and liver and the reactive oxygen metabolite and haptoglobin concentrations of post-weaning piglets at d9 and d21.
3.3 Gene expression in the jejunum mucosa
Table 4 reports the effects of diet, BBW class, and their interaction on the gene expression in the jejunal mucosa at d9 and d21. The expression of NFKB-2 was significantly affected by the interaction between diet and BBW class (P = 0.03) at d9. The expression of NFKB-2 was also affected by diet (P = 0.02) and BBW class (P = 0.005); the pairwise contrasts showed that the LBBW group tended to have a higher NFKB-2 than the NBBW group but only in the ZnSO4 group (P = 0.09). The other genes were not affected by diet, BBW class, or their interaction at d9. At d21, the interaction between diet and BBW class did not affect the expression of any gene. Diet tended to affect the SLC39A4 expression, resulting in higher expression in the ZnSO4 group than in the pZnO group (P = 0.09). The other genes were not affected by diet at d21.
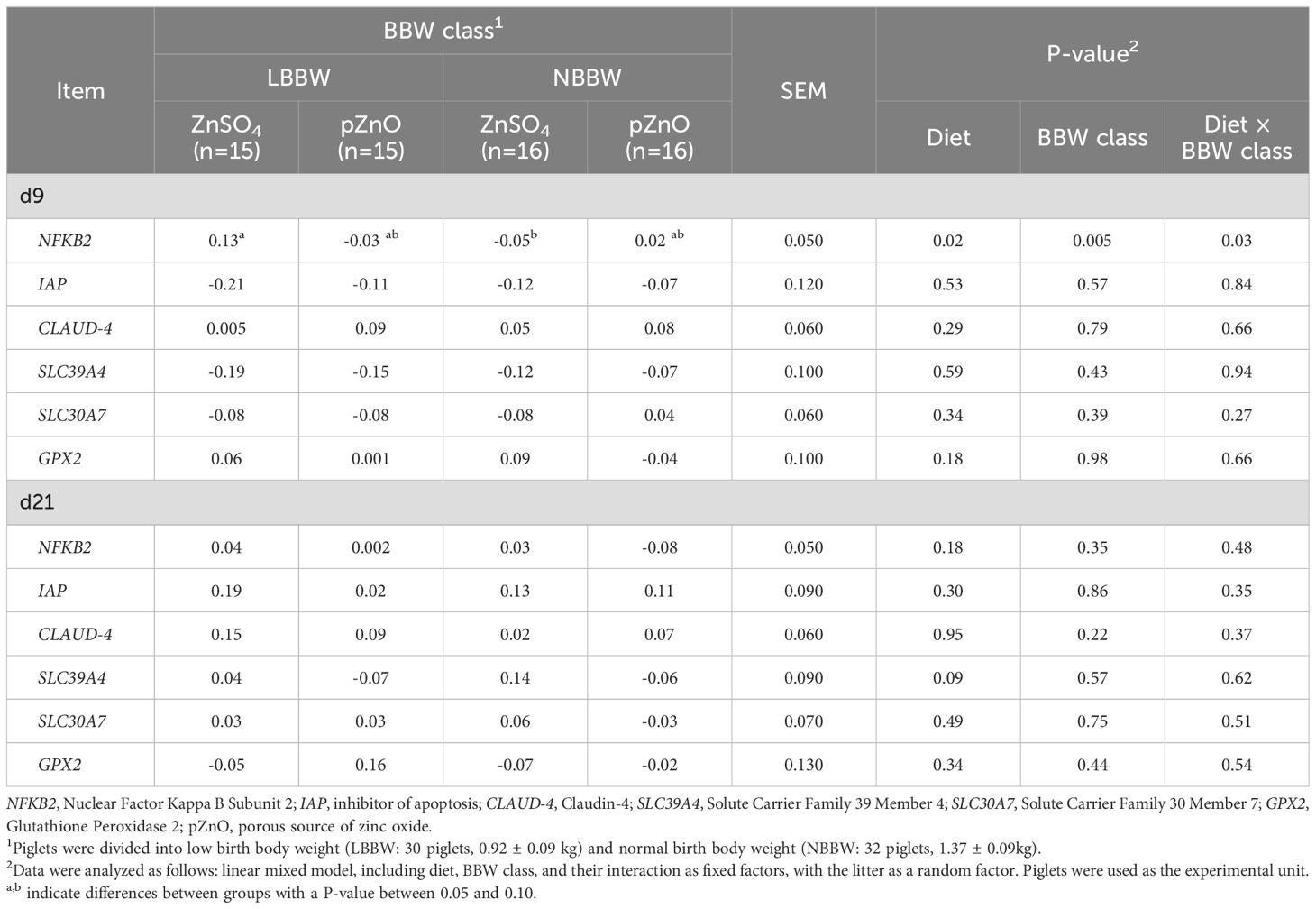
Table 4. The effects of zinc administration, body weight class, and their interaction on gene expression in the jejunum mucosa of post-weaning piglets at d9 and d21.
3.4 Intestinal morphology and immunological parameters regarding the jejunum mucosa
Table 5 reports the effects of diet, BBW class, and their interaction on the distal jejunum morphology and immunohistochemical parameters at d9 and d21. At d9, the interaction between diet and BBW class tended to affect the crypt depth (P = 0.08) and the number of T lymphocytes in the villi (P = 0.050). The T lymphocytes in the villi were lower in the LBBW piglets fed pZnO than in all the other groups. At d9, the diet almost entirely did not affect the morphological and immunological parameters, with the only exception of the number of T lymphocytes in the crypts (P = 0.0005), which was higher in the ZnSo4 group than in the pZNO group. The BBW class tended to affect the villus width (P = 0.08) at d9, resulting in wider villi in the NBBW than in the LBBW piglets. The BBW class tended to affect the IgA cell count in the villi at d9 (P = 0.059) and significantly affected the IgA in the crypts (P = 0.003); the NBBW piglets had higher values in both the villi and the crypts as compared to the LBBW piglets. The BBW class also affected the number of T lymphocytes in the crypts (P = 0.016), which was higher in the LBBW piglets.
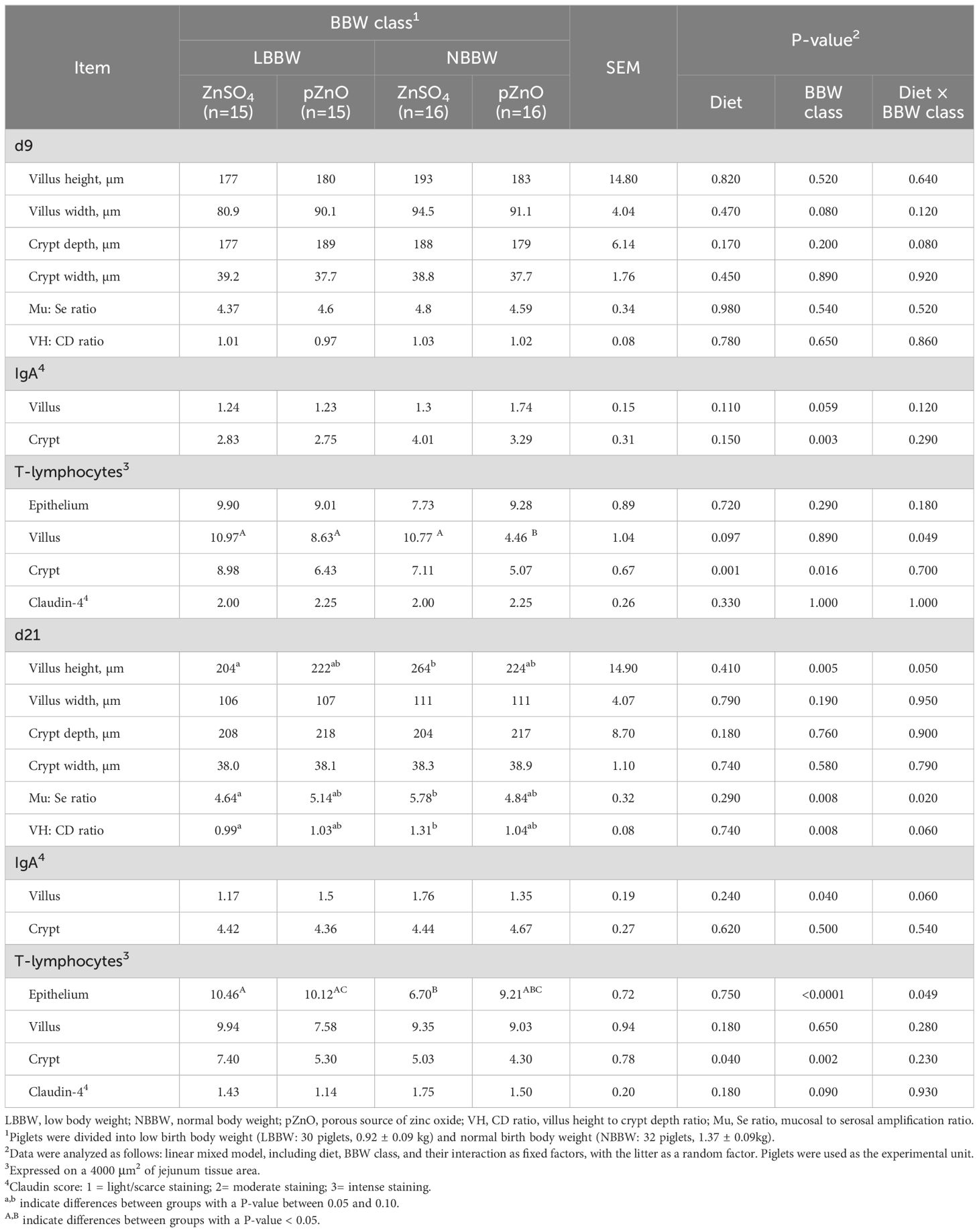
Table 5. The effects of zinc administration, body weight category, and their interaction on the distal jejunum morphology, IgA positive cells, T lymphocytes, and Claudin-4 evaluation in post-weaning piglets at d9 and d21.
At d21, the interaction between diet and BBW class tended to influence the villus height (P = 0.05) and the height-to-crypt-depth ratio (VH: CD) (P = 0.068), which were also affected by the BBW class (P = 0.005 and P = 0.008, respectively); the LBBW piglets had lower villus heights and a lower VH: CD compared to the NBBW piglets, and the LBBW piglets in the ZnSO4 group tended to have lower villus height and lower VH: CD than the NBBW piglets in the ZnSO4 group (P = 0.06). The absorptive mucosal surface was affected by the interaction between diet and BBW class (P = 0.02) and by the BBW class (P = 0.008); it was higher in NBBW piglets than the LBBW piglets, and when examining the differences among the groups, it tended to be higher in the NBBW piglets than in the LNNB piglets fed ZnSO4 (P = 0.08), while no differences were observed between the NBBW and LBBW piglets fed pZnO. The interaction between diet and BBW class tended to influence the IgA in the villi (P = 0.06), which were also affected by BBW class (P = 0.04); the NBBW piglets had higher IgA than the LBBW piglets. The interaction between diet and BBW class affected the number of T lymphocytes in the epithelium (P = 0.049), which was also affected by the BBW class (P <0.001); the LBBW piglets had a higher number of epithelial T lymphocytes. However, the LBBW piglets fed pZnO had a comparable number of epithelial T lymphocytes as the NBBW piglets fed pZnO. Finally, diet (P = 0.04) and BBW class (P = 0.002) influenced the number of T lymphocytes in the crypt, which was higher in the ZnSo4 groups than the pZnO groups and in the LBBW piglets compared to the NBBW piglets. BBW tended to influence the Claudin-4 score, which was higher in the NBBW group (P = 0.09).
3.5 pH values of the gut content
The effects of diet, BBW class, and their interaction on the pH values of the jejunum, cecum, and colon contents are reported in Table 6. The interaction between diet and BBW class was never significant. Diet and BBW class did not influence the pH values at d9. At d21, the pH of the jejunum contents was lower in the piglets in the pZnO group compared with those in the ZnSO4 group (P = 0.02). The BBW class did not influence the pH of the gut contents at d21.
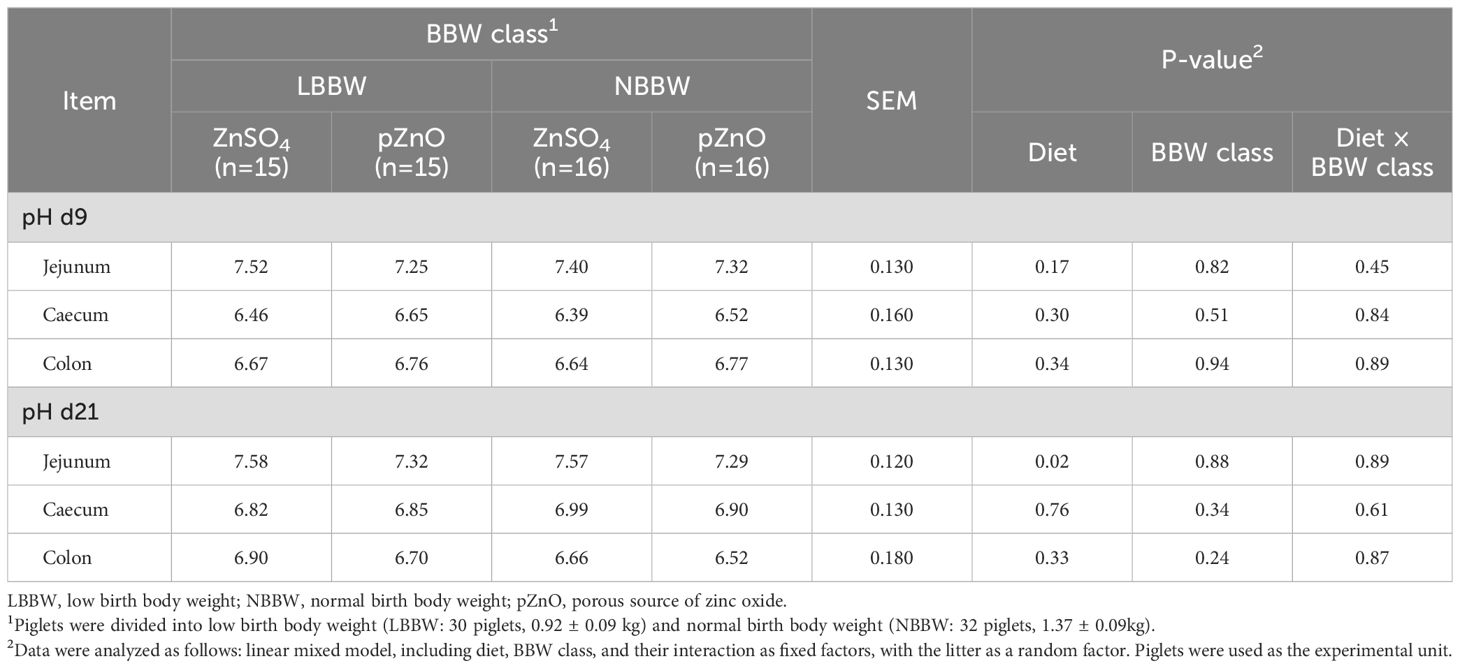
Table 6. The effects of zinc administration, body weight category, and their interaction on the pH of the jejunum, cecum, and colon content in post-weaning piglets at d9 and d21.
3.6 Concentrations of short-chain fatty acids and lactic acid in the colon content
Table 7 reports the effects of diet, BBW class, and their interaction on the SCFA and lactic acid concentrations in the colon content at d9 and d21. At d9, the interaction between diet and BBW class was never significant. The isovalerate tended to be influenced by diet, resulting in a higher value in the ZnSO4 group (P = 0.06). The BBW class significantly affected the acetate (P = 0.03) and isobutyrate (P = 0.01) concentrations, and tended to influence valerate concentration (P = 0.09); acetate and valerate concentrations were higher in the LBBW piglets, while isobutyrate concentration was higher in the NBBW piglets.
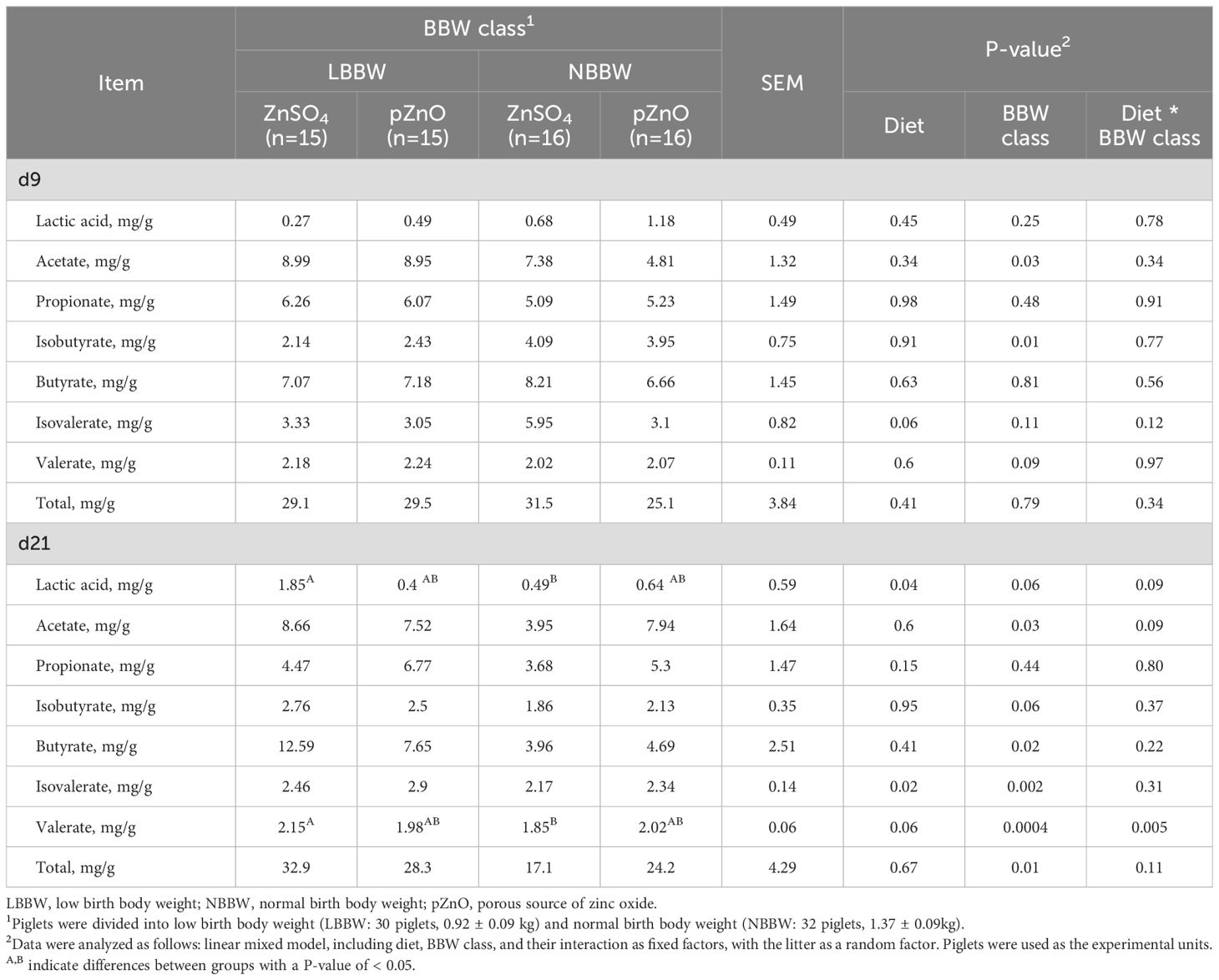
Table 7. The effects of zinc administration, body weight category, and their interaction on SCFA and lactate concentrations in the colon content of post-weaning piglets at d9 and d21.
At d21, the interaction between diet and BBW class tended to affect the lactic acid and acetate concentrations (P = 0.09). Lactic acid also tended to be affected by BBW class (P = 0.06) and it was affected by diet (P = 0.04). The LBBW piglets fed ZnSO4 had higher lactic acid values than the NBBW piglets fed ZnSO4. Acetate concentrations were also affected by the BBW class and were higher in the LBBW piglets (P = 0.03). The interaction between diet and BBW class also affected the level of valerate (P = 0.005), which was also affected by BBW class (P = 0.004) and tended to be affected by the diet (P = 0.06). Valerate concentration was higher in the LBBW piglets compared to the NBBW piglets only in the ZnSO4 group (P = 0.02). Diet also affected the isovalerate concentration (P = 0.02), with higher values in the piglets that received pZnO. BBW class also affected the butyrate concentration (P = 0.02) and the total SCFA value (P = 0.01), and tended to affect the isobutyrate concentration (P = 0.06), which were all higher in the LBBW group than in the NBBW group.
3.7 Colon microbial profile
A total of 2,728,354 raw reads were obtained, which were attributed to a total of 6803 amplicon sequence variants (ASVs). The ASVs were associated with 21 phyla, 89 families, and 225 genera. The predominant phyla comprised Firmicutes at 57 ± 0.11%, Bacteroidota at 31 ± 0.10%, Spirochaetota at 6 ± 0.05%, and Proteobacteria at 2 ± 0.02%. Among the most prevalent families were Prevotellaceae at 20 ± 0.09%, Lachnospiraceae at 17 ± 0.06%, Spirochaetaceae at 6 ± 0.05%, and Oscillospiraceae at 6 ± 0.03%. Notably, the most abundant genera included Prevotella at 12 ± 0.08%, Lactobacillus at 8 ± 0.12%, and Treponema at 5 ± 0.05%.
The alpha and beta diversity results are shown in Figures 2A, B and 3A, B, respectively. Alpha diversity was not affected by diet, BBW class, or their interaction. Regarding the beta diversity, the Adonis test showed that the BBW class tended to affect the microbial composition at d9 (R2 = 0.04, P = 0.07) and significantly affected the bacterial composition at d21 (R2 = 0.05, P = 0.03). Figures 4A and B show the results of the LefSe analyses. At d9, the LBBW piglets fed ZnSO4 were characterized by a greater abundance of the Lachnospiraceae XPB1014 group (LDA_score=3.51, P.adj=0.03), Monoglobus (LDA_score=3.35, P.adj=0.01), and Shuttleworthia (LDA_score=3.20, P.adj=0.03); the NBBW piglets fed ZnSO4 were characterized by a greater abundance of Phascolarctobacterium (LDA_score=3.85, P.adj=0.04); and the LBBW piglets fed pZnO were characterized by a greater abundance of Intestinimonas (LDA_score=3.13, P.adj=0.03). At d21, the piglets in the NBBW group fed ZnSO4 were characterized by a greater abundance of Succinivibrio (LDA_score=3.95, P.adj=0.02), the LBBW piglets fed ZnSO4 were characterized by a greater abundance of Streptococcus (LDA_score=3.97, P.adj=0.03) and Peptococcus (LDA_score=3.15, P.adj=0.01), and the piglets in the NBBW group fed pZnO were characterized by a greater abundance of Ruminococcus gauvreauii (LDA_score=4.09, P.adj=0.01).
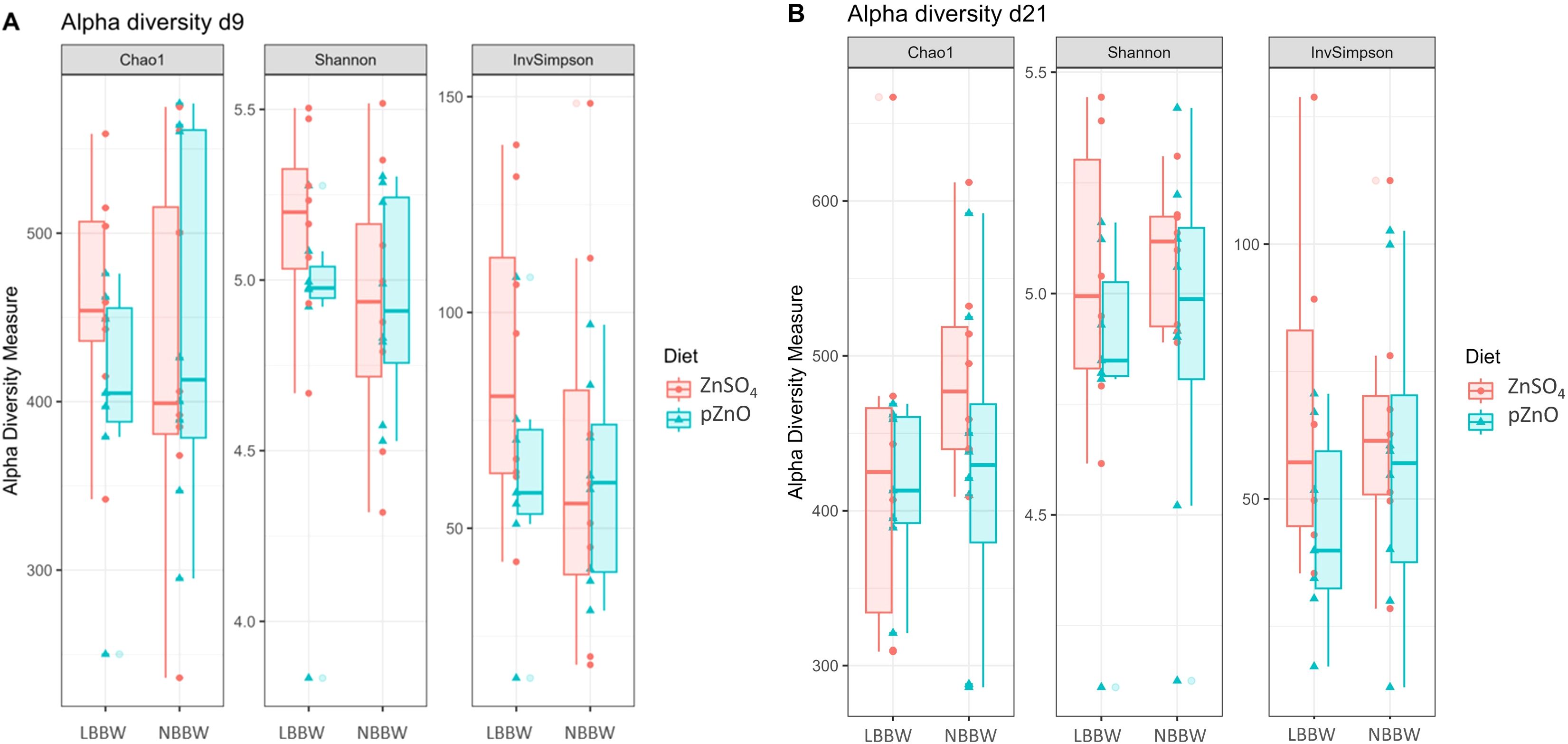
Figure 2. (A, B) The effects of birth body weight category and Zn source on the alpha diversity indices in the colon content samples from post-weaning piglets at d9 and d21. LBBW, low birth body weight; NBBW, normal birth body weight; pZnO, porous source of zinc oxide. The Chao1, Shannon, and Simpson indices were calculated, and differences among the groups were analyzed using a linear model. Diet, BBW class, their interaction, and sequencing depth were included in the model. Piglets were used as the experimental unit.
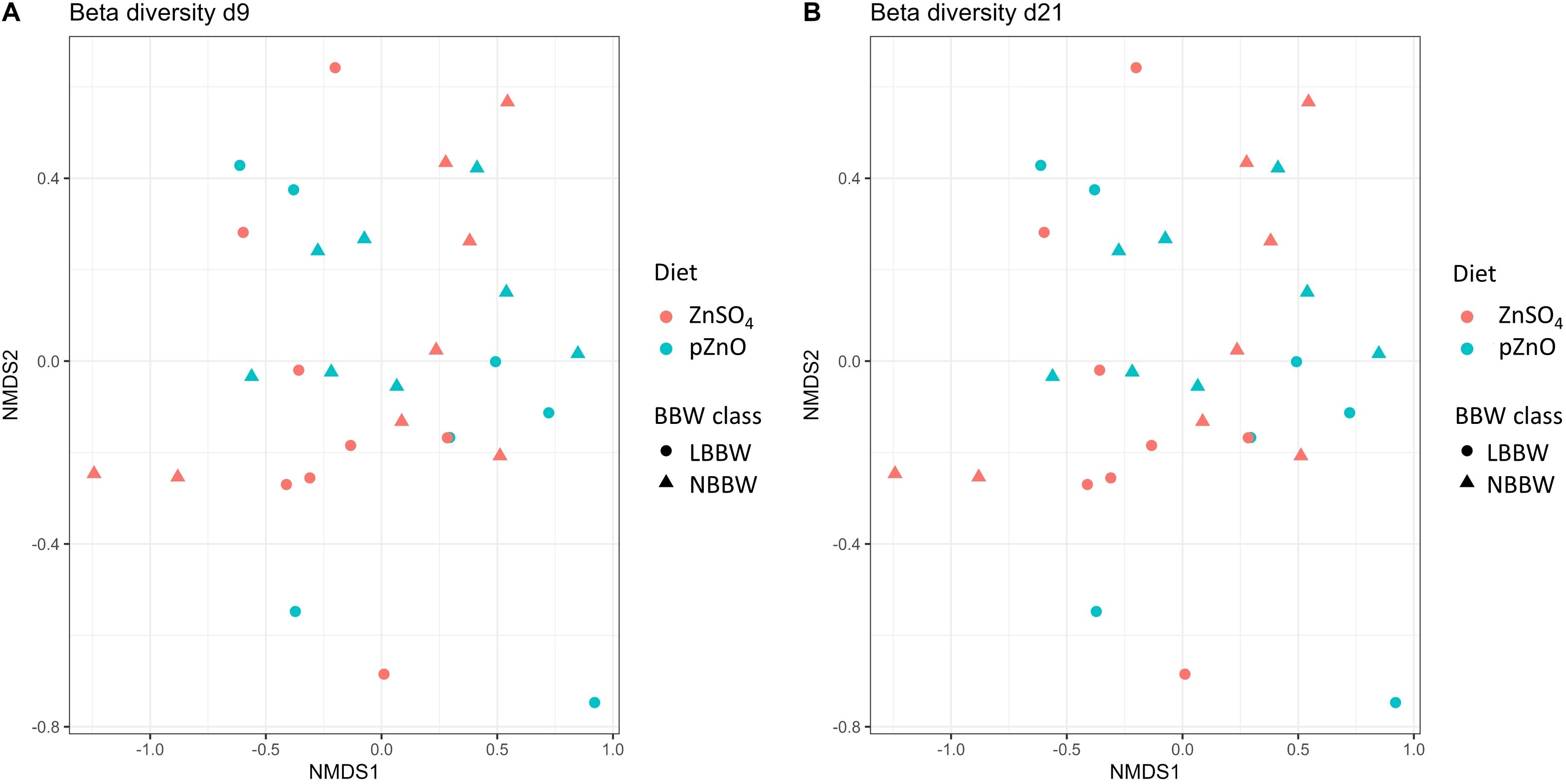
Figure 3. (A, B) The effects of birth body weight category and Zn source on beta diversity in the colon content samples from post-weaning piglets at d9 and d21. LBBW, low birth body weight; NBBW, normal birth body weight; pZnO, porous source of zinc oxide. Beta diversity was calculated utilizing the Bray–Curtis distance matrix, visualized via a non-metric multidimensional scaling (NMDS) plot. The effects of BBW class and diet were tested using a non-parametric PERMANOVA model (Adonis test) with 999 permutations. Piglets were used as the experimental unit.
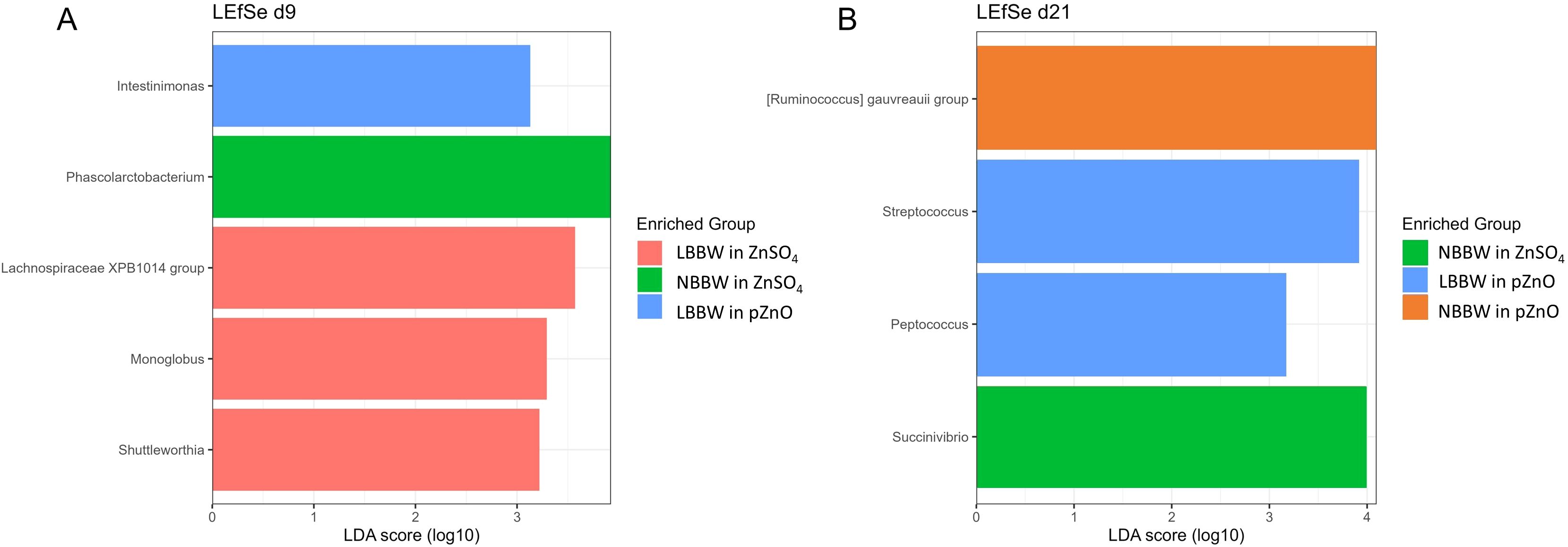
Figure 4. (A, B) Linear discriminant analysis effect size (LEfSe) plots of the biomarker taxa for each experimental group at the genus level in the colon content at d9 and d21. LBBW, low birth body weight; NBBW, normal birth body weight; pZnO, porous source of zinc oxide. Differential abundance analysis concerning various taxa was carried out using LefSe, integrated within the microbiomeMarker package (v 1.0.2). Data were aggregated at the genus level, with a cut-off value set at 3 and a P.adj < 0.05 considered significant for the LefSe analysis. Piglets were used as the experimental unit.
4 Discussion
This study highlighted the effects of a porous source of ZnO in maintaining gut integrity and sustaining the feed efficiency in post-weaning piglets born with a low or a normal birth BW.
BBW was confirmed to be a key factor in intestinal maturation (Collins et al., 2017), as evidenced by the enhanced ADG and FI outcomes in the NBBW piglets compared to the LBBW piglets. The BW of the LBBW and NBBW piglets remained well differentiated on the day of weaning and during the entire experimental period.
Regardless of the effects of BBW, the Zn source did not affect the FI of the animals; however, the piglets receiving the pZnO had a better G:F ratio in the early post-weaning period, which is a positive indicator of the intestinal health status (Chalvon-Demersay et al., 2021). This result could have been linked to the physical characteristics of the pZn source tested (Peng et al., 2019). In fact, the pZnO tested has a high porosity and small aggregated and agglomerated particles (Long et al., 2017) compared to ZnSO4, which is characterized by high solubility. Therefore, the pZnO, characterized by a higher surface area, could have a more local effect on the gut mucosa, supporting gut health in both NBBW and LBBW pigs.
As defined by Chalvon-Demersay et al. (2021), gut health is composed of four interconnected pillars, namely, immune fitness, gut morphology, oxidative balance, and microbiota profile and functions. Focusing first on immune fitness, mucosal immunity is characterized by inductive sites, represented mainly by the mucosal-associated lymphoid tissue (MALT) and the effector sites, such as lymphocytes, plasma cells, macrophages, dendritic cells, and mast cells located in the lamina propria, where the cell-mediated and humoral immunity take place (Bianco et al., 2014). Furthermore, the primary intestinal line of defense based on the adaptive mucosal immune system consists of the polymeric immunoglobulins, the most abundant of which are the secretory IgA (IgAs) cells produced by the plasma cells located in the lamina propria along the gut (Trevisi et al., 2013). Our results showed a higher IgA cell count in the crypts of the lamina propria than in the villi of the lamina propria, as previously described by Allen and Porter (1973). The results highlight how BBW was the most significant factor affecting the immune maturation of the animals, as the higher values of IgA cells in the LBBW piglets at d21 compared to the NBBW piglets were an important indicator. Additionally, pZnO was able to reduce the T lymphocyte counts observed in the crypts at day 9, which may indicate lower mucosal immune activation. As immune responses are energetically demanding, decreased immune stimulation allows more metabolic resources to be diverted toward growth processes (Serrano-Jara et al., 2022). This immunological downregulation in both LBBW and NBBW piglets may thus explain the improved feed efficiency associated with the pZnO source. Moreover, at d9, a higher T lymphocyte count was also coupled with a higher gene expression of NFKB2 in the LBBW piglets fed ZnSO4. The NFKB2 gene encodes for a protein involved in the activation of inflammatory pathways (Liu et al., 2017) and is associated with the activation of T lymphocytes (Beinke and Ley, 2004). The elevated T lymphocyte levels in the LBBW pigs fed ZnSO4 persisted until day 21 in the villi, indicating that this soluble Zn source may not be able to support gut discomfort post-weaning in LBBW pigs, which experience a higher discomfort compared to NBBW pigs, confirming the observation by Muns et al. (2016) and reinforcing the model’s validity in the present study.
At 21 days post-weaning, the NBBW piglets fed ZnSO4 showed greater villus height, a higher VH: CD ratio, and a larger absorptive mucosal surface compared to the LBBW piglets fed the same diet. However, these differences were not observed between the NBBW and LBBW piglets fed pZnO. This suggests that pZnO may help in supporting mucosal recovery in LBBW pigs from weaning-induced inflammation, an effect not seen with ZnSO4. These results further support the hypothesis that pZnO can support gut integrity in LBBW piglets.
Regarding the blood parameters, circulating concentrations of Hp and ROMs were analyzed to provide a direct measurement of the health and oxidative status of the piglets. The lack of effect on the Hp concentration, even though a higher immune system activation was observed in the LBBW piglets fed ZnSO4, could be explained by the timing of the sampling. In fact, Hp is an early marker of inflammation compared to the mucosal parameters measured (Saco and Bassols, 2023).
In the present study, microbial composition was mainly influenced by the BBW of the piglets. This relationship between BBW and gut microbiota has also been reported in previous studies (Kiros et al., 2019; Luise et al., 2021; Pluske et al., 2018), suggesting that the interplay between the bacteria composition and the host could be affected by the physiological and intestinal development of the piglets. For an in-depth discussion of the effects of BBW on gut microbiota, we refer to our previous work (Trevisi et al., 2023). Regarding the effects of the pZnO source on the gut ecosystem, it was able to reduce jejunal pH, which has been associated with an increase in the production of SCFAs derived from the bacterial fermentation of the carbohydrate. However, due to the 12 hours of fasting prior to the sacrifice, it was not possible to analyze SCFA concentrations in the jejunal content since there was not enough. However, the present study showed a significant effect of the pZnO source on the cecum SCFA profile, especially at d21 post-weaning. In contrast to the result reported by Michiels et al. (2013), in the present study, the Zn source did not affect the acetate concentration in the cecum. However, the pZnO group had a higher concentration of isovalerate, which is a primary metabolite of the protein fermentation (Bekebrede et al., 2022), and a lower concentration of lactate compared to the piglets fed ZnSO4. Although the results regarding the SCFAs may indicate a reduction in digestive capacity in favor of proteolytic fermentation for the pZnO group, the present outcomes regarding the G:F ratio and the morphological parameters did not show a detrimental effect on the gut functionality of the piglets in the pZnO group, which were more efficient. The difference in lactate concentration is of interest. Indeed, the ability of ZnO to reduce the abundance of Lactobacillus spp. is well known (Starke et al., 2014; Wei et al., 2020), although this effect has only been studied in pigs supplemented with high dietary ZnO concentrations. A possible explanation for these results could be the modulatory effect of the ZnO source tested on the gut microbial profile, even when administered at lower doses. Although the present findings showed that the Zn source did not profoundly affect the alpha and beta diversity indices, some differences in the taxa abundance were observed. Currently, the knowledge available regarding the pig gut microbiota is not sufficient to strongly support a conclusion regarding the effects of a few taxa on a complex ecosystem, such as that of the GIT. However, generating data to provide knowledge regarding this complex topic is important. In the present study, the taxa abundance was influenced by the Zn source, BBW class, and time. At d9, the LBBW group fed pZnO had a greater abundance of Intestinimonas, which has the potential of producing SCFAs from both amino acids and sugars (Bui et al., 2016). The LBBW piglets fed ZnSO4 were characterized by a higher relative abundance of Lachnospiraceae, known for their ability to degrade starch and plant-derived matrices (Sun et al., 2021), and Shuttleworthia, obligate anaerobes that are capable of producing butyrate as a major product (Helm et al., 2021). The NBBW piglets fed ZnSO4 were characterized by a higher relative abundance of Phascolarctobacterium, which is known to produce propionate (Watanabe et al., 2012). At d21, the piglets in the NBBW group fed ZnSO4 had a greater relative abundance of the Succinivibrio genus, which is capable of degrading complex carbohydrates (Zhao et al., 2020). Meanwhile, the LBBW piglets fed pZnO were characterized by a higher relative abundance of Streptococcus, which can produce branched-chain fatty acids (isobutyrate and isovalerate) as a final product; this result supported the higher isovalerate concentration observed at d21 in the pZnO group. Finally, the NBBW group fed pZnO had a higher relative abundance of Ruminococcus gauvreauii, which is positively related to the production of SCFAs, mainly to the production of acetate as an end fermentation product (Toya et al., 2020).
The discrepancies observed between the results of quantified SCFAs and the expected SCFA production based on discriminated taxa may have been due to the limitation of the method used to characterize the microbiota, which was based on DNA and lacked the ability to identify the real active microbes in the gut (Ramos Meyers et al., 2022).
In the present study, a slight effect of the Zn source was also observed in the expression of the SLC39A4 gene at d21. This gene encodes zinc transporter protein 4 (ZIP4), which is needed for the uptake of Zn in the intestine (Martin et al., 2013). In particular, the piglets fed ZnSO4 showed a modest increase in the expression of this gene. This could have been related to the fasting period in association with the higher solubility of ZnSO4. In addition, due to the involvement of Zn in several physiological processes, fasting could have exacerbated the expression of the molecular mechanisms that increase Zn intestinal uptake. A recent study by Yin et al. (2022) suggested that the SLC39A4 protein could also be able to self-regulate when the physiological Zn level is satisfied. This mechanism could explain the decreased expression of the SLC39A4 in the intestinal mucosa of the pZnO piglets. This source of ZnO could, indeed, have led to a faster fulfilment of the physiological Zn requirements compared to ZnSO4.
Moreover, no difference in Zn concentrations was observed in the liver and serum of the piglets after 12 hours of fasting. This result suggests that the physiological Zn level was maintained with both Zn sources. This is further supported by the study of Brugger et al. (2014), in which an average of 28 mg/kg in total was quantified in the liver of piglets receiving a Zn-deficient diet. These values were lower compared to those observed in our study (50 mg/kg on a total basis).
In conclusion, the results of the study supported the long-term impact of BBW on piglet physiological maturity, as evidenced by its influence on weaning weight, performance outcomes, and gut development. Building on this evidence, the inclusion of 120 mg/kg of a porous form of ZnO in weaned pigs could be considered a valid strategy to help mitigate weaning stress, especially in LBBW piglets. This Zn source and dosage were able to both meet the nutritional requirements and exert a beneficial effect on the gut microbial ecosystem, thereby supporting gut immune system activation. Thus, the development of nutritional strategies that fulfill mineral requirements while promoting post-weaning recovery through local effects on the intestinal mucosa in LBBW piglets appears to be achievable.
Data availability statement
Raw reads are publicly available in the Sequence Read Archive (SRA) under the accession number PRJNA1021401.
Ethics statement
The in vivo trial was approved by the Ethics Committee for Experiments on Animals of the University of Bologna, Italy and by the Italian Ministry of Health (Authorization n. 287/2021 PR released in compliance with art. 31 of the D.lgs. 26/2014) and complied with the Animal Research Reporting of In Vivo Experiments guidelines (Percie du Sert et al., 2020). The studies were conducted in accordance with the local legislation and institutional requirements. The study involved the use of farm animals, for which ethical approval was duly requested and obtained from the Italian Animal Welfare Committee. The animals were legally purchased by the University of Bologna and reared at the university’s experimental facility.
Author contributions
CN: Data curation, Methodology, Writing – original draft, Investigation, Formal analysis. DL: Investigation, Writing – review & editing, Supervision, Data curation, Formal analysis, Writing – original draft, Methodology. FC: Writing – review & editing, Formal analysis, Software, Data curation, Methodology. SV: Writing – review & editing, Formal analysis, Methodology. AS: Writing – review & editing, Formal analysis, Methodology. AB: Formal analysis, Writing – review & editing, Methodology. NM: Writing – review & editing, Formal analysis. AM: Writing – review & editing, Formal analysis. MM: Methodology, Writing – review & editing, Formal analysis. PT: Supervision, Writing – review & editing, Funding acquisition, Investigation, Conceptualization, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was carried out in collaboration with Animine (France) and within the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) – MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4 – D.D. 1032 17/06/2022, CN00000022).
Acknowledgments
The authors would like to thank Dr. Archetti and the Istituto Zooprofilattico Sperimentale della Lombardia and Emilia Romagna for their help in analyzing the blood samples and the feed’s zinc concentrations. The authors would also like to thank Dr. Thomas Valentini and the Amadori company for the feed analyses.
Conflict of interest
AM is an employee of Animine Annecy, France. Author NM was employed by the company Cargill Incorporated.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
This manuscript reflects only the authors’ views and opinions; neither the European Union nor the European Commission can be considered responsible for them.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1614280/full#supplementary-material
References
(2009). Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed (Text with EEA relevance).
Allen W. D. and Porter P. (1973). The relative distribution of IgM and IgA cells in intestinal mucosa and lymphoid tissues of the young unweaned pig and their significance in ontogenesis of secretory immunity. Immunol. 24, 493–501.
Ayuso M., Irwin R., Walsh C., Van Cruchten S., and Van Ginneken C. (2021). Low birth weight female piglets show altered intestinal development, gene expression, and epigenetic changes at key developmental loci. FASEB J. 35, e21522. doi: 10.1096/fj.202002587R
Bates D., Mächler M., Bolker B., and Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Software 67, 1–48. doi: 10.18637/jss.v067.i01
Beaulieu A. D., Aalhus J. L., Williams N. H., and Patience J. F. (2010). Impact of piglet birth weight, birth order, and litter size on subsequent growth performance, carcass quality, muscle composition, and eating quality of pork. J. Anim. Sci. 88, 2767–2778. doi: 10.2527/jas.2009-2222
Beinke S. and Ley S. C. (2004). Functions of NF-κB1 and NF-κB2 in immune cell biology. Biochem. J. 382, 393–409. doi: 10.1042/BJ20040544
Bekebrede A. F., Noorman L., Keijer J., de Boer V. C. J., and Gerrits W. J. J. (2022). Functional metabolic capacity of pig colonocytes is differentially modulated by fermentable fibre and poorly digestible protein. Animal 16, 100625. doi: 10.1016/j.animal.2022.100625
Bianco C., Felice V., Panarese S., Marrocco R., Ostanello F., Brunetti B., et al. (2014). Quantitative immunohistochemical assessment of IgA, IgM, IgG and antigen-specific immunoglobulin secreting plasma cells in pig small intestinal lamina propria. Vet. Immunol. Immunopathol. 160, 281–287. doi: 10.1016/j.vetimm.2014.05.014
Bonetti A., Tugnoli B., Piva A., and Grilli E. (2021). Towards zero zinc oxide: feeding strategies to manage post-weaning diarrhea in piglets. Animals 11, 642. doi: 10.3390/ani11030642
Brambilla G., Civitareale C., Ballerini A., Fiori M., Amadori M., Archetti L. I., et al. (2002). Response to oxidative stress as a welfare parameter in swine. Redox Rep. 7, 159–163. doi: 10.1179/135100002125000406
Brugger D., Buffler M., and Windisch W. (2014). Development of an experimental model to assess the bioavailability of zinc in practical piglet diets. Arch. Anim. Nutr. doi: 10.1080/1745039X.2014.898392
Bui T. P. N., Shetty S. A., Lagkouvardos I., Ritari J., Chamlagain B., Douillard F. P., et al. (2016). Comparative genomics and physiology of the butyrate-producing bacterium Intestinimonas butyriciproducens. Environ. Microbiol. Rep. 8, 1024–1037. doi: 10.1111/1758-2229.12483
Callahan B. J., McMurdie P. J., Rosen M. J., Han A. W., Johnson A. J. A., and Holmes S. P. (2016). DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Cao Y., Dong Q., Wang D., Zhang P., Liu Y., and Niu C. (2022). microbiomeMarker: an R/Bioconductor package for microbiome marker identification and visualization. Bioinform. 38, 4027–4029. doi: 10.1093/bioinformatics/btac438
Cardoso D., Narcy A., Durosoy S., Bordes C., and Chevalier Y. (2021). Dissolution kinetics of zinc oxide and its relationship with physicochemical characteristics. Powder Technol. 378, 746–759. doi: 10.1016/j.powtec.2020.10.049
Chalvon-Demersay T., Luise D., Le Floc’h N., Tesseraud S., Lambert W., Bosi P., et al. (2021). Functional amino acids in pigs and chickens: implication for gut health. Front. Vet. Sci. 8. doi: 10.3389/fvets.2021.663727
Chen J., Song Y., Chen D., Yu B., He J., Mao X., et al. (2021). Low birth weight disturbs the intestinal redox status and mitochondrial morphology and functions in newborn piglets. Anim. (Basel) 11, 2561. doi: 10.3390/ani11092561
Ciesinski L., Guenther S., Pieper R., Kalisch M., Bednorz C., and Wieler L. H. (2018). High dietary zinc feeding promotes persistence of multi-resistant E. coli swine gut. PloS One 13, e0191660. doi: 10.1371/journal.pone.0191660
Collins C. L., Pluske J. R., Morrison R. S., McDonald T. N., Smits R. J., Henman D. J., et al. (2017). Post-weaning and whole-of-life performance of pigs is determined by live weight at weaning and the complexity of the diet fed after weaning. Anim. Nutr. 3, 372–379. doi: 10.1016/j.aninu.2017.01.001
Correa F., Luise D., Amatucci L., Palumbo F., Virdis S., Negrini C., et al. (2022). Effect of an Escherichia coli F4/F18 bivalent oral live vaccine on gut health and performance of healthy weaned pigs. Animal 16, 100654. doi: 10.1016/j.animal.2022.100654
Diao H., Yan J., Li S., Kuang S., Wei X., Zhou M., et al. (2021). Effects of dietary zinc sources on growth performance and gut health of weaned piglets. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.771617
Dixon P. (2003). VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14, 927–930. doi: 10.1111/j.1654-1103.2003.tb02228.x
Fox J. and Weisberg S. (2019). An R Companion to Applied Regression. 3rd ed. (Thousand Oaks CA: Sage).
Gondret F., Père M.-C., Tacher S., Daré S., Trefeu C., Le Huërou-Luron I., et al. (2013). Spontaneous intra-uterine growth restriction modulates the endocrine status and the developmental expression of genes in porcine fetal and neonatal adipose tissue. Gen. Comp. Endocrinol. 194, 208–216. doi: 10.1016/j.ygcen.2013.09.018
Helm E. T., Gabler N. K., and Burrough E. R. (2021). Highly fermentable fiber alters fecal microbiota and mitigates swine dysentery induced by brachyspira hyodysenteriae. Animals 11, 396. doi: 10.3390/ani11020396
Kambe T. and Wagatsuma T. (2023). Metalation and activation of Zn2+ enzymes via early secretory pathway-resident ZNT proteins. Biophys. Rev. 4, 041302. doi: 10.1063/5.0176048
Kiros T. G., Luise D., Derakhshani H., Petri R., Trevisi P., D’Inca R., et al. (2019). Effect of live yeast Saccharomyces cerevisiae supplementation on the performance and cecum microbial profile of suckling piglets. PloS One 14, e0219557. doi: 10.1371/journal.pone.0219557
Lenth R. V. (2021). Emmeans: estimated marginal means, aka least-squares means. Comprehensive R Archive Network (CRAN).
Li N., Huang S., Jiang L., Wang W., Li T., Zuo B., et al. (2018). Differences in the gut microbiota establishment and metabolome characteristics between low- and normal-birth-weight piglets during early-life. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.01798
Liu T., Zhang L., Joo D., and Sun S.-C. (2017). NF-κB signaling in inflammation. Sig Transduct Target Ther. 2, 1–9. doi: 10.1038/sigtrans.2017.23
Long L., Chen J., Zhang Y., Liang X., Ni H., Zhang B., et al. (2017). Comparison of porous and nano zinc oxide for replacing high-dose dietary regular zinc oxide in weaning piglets. PloS One 12, e0182550. doi: 10.1371/journal.pone.0182550
Luise D., Correa F., Stefanelli C., Simongiovanni A., Chalvon-Demersay T., Zini M., et al. (2023). Productive and physiological implications of top-dress addition of branched-chain amino acids and arginine on lactating sows and offspring. J. Anim. Sci. Biotechnol. 14, 40. doi: 10.1186/s40104-022-00819-8
Luise D., Sciellour M. L., Buchet A., Resmond R., Clement C., Rossignol M.-N., et al. (2021). The fecal microbiota of piglets during weaning transition and its association with piglet growth across various farm environments. PloS One 16, e0250655. doi: 10.1371/journal.pone.0250655
Ma X., Qian M., Yang Z., Xu T., and Han X. (2021). Effects of zinc sources and levels on growth performance, zinc status, expressions of zinc transporters, and zinc bioavailability in weaned piglets. Animals 11, 2515. doi: 10.3390/ani11092515
Martin L., Lodemann U., Bondzio A., Gefeller E.-M., Vahjen W., Aschenbach J. R., et al. (2013). A high amount of dietary zinc changes the expression of zinc transporters and metallothionein in jejunal epithelial cells in vitro and in vivo but does not prevent zinc accumulation in jejunal tissue of piglets. J. Nutr. 143, 1205–1210. doi: 10.3945/jn.113.177881
McMurdie P. J. and Holmes S. (2011). Phyloseq: a bioconductor package for handling and analysis of high-throughput phylogenetic sequence data, in: Biocomputing 2012. World Sci., 235–246. doi: 10.1142/9789814366496_0023
Michiels J., Vos M. D., Missotten J., Ovyn A., Smet S. D., and Ginneken C. V. (2013). Maturation of digestive function is retarded and plasma antioxidant capacity lowered in fully weaned low birth weight piglets. Br. J. Nutr. 109, 65–75. doi: 10.1017/S0007114512000670
Muns R., Nuntapaitoon M., and Tummaruk P. (2016). Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 184, 46–57. doi: 10.1016/j.livsci.2015.11.025
Negrini C., Luise D., Correa F., Amatucci L., Virdis S., Romeo A., et al. (2022). “Growth performance and gut health of low and normal birth weight piglets fed different zinc sources,” in Abstract retrieved from Proceedings of the Book of Abstracts of the 73rd Annual Meeting of the European Federation of Animal Science(Porto, Portugal), 198–198.
Negrini C., Luise D., Correa F., Amatucci L., Virdis S., Roméo A., et al. (2023). “Implication of authorized level of Zn provided from different sources on the performance and health of low and normal birth weight piglets post-weaning,” in Abstract retrieved from Proceedings of the Book of Abstract of the Italian Association of Animal Science (ASPA)(Bari, Italy), 347–347.
Noblet J., Fortune H., Shi X. S., and Dubois S. (1994). Prediction of net energy value of feeds for growing pigs1. J. Anim. Sci. 72, 344–354. doi: 10.2527/1994.722344x
Oh H.-J., Park Y.-J., Cho J. H., Song M.-H., Gu B.-H., Yun W., et al (2021). Changes in diarrhea score, nutrient digestibility, zinc utilization, intestinal immune profiles, and fecal microbiome in weaned piglets by different forms of zinc. Animals 11, 1356. doi: 10.3390/ani11051356
Ortega A. D. S. V. and Szabó C. (2021). Adverse effects of heat stress on the intestinal integrity and function of pigs and the mitigation capacity of dietary antioxidants: a review. Animals 11, 1135. doi: 10.3390/ani11041135
Peng P., Chen J., Yao K., Yin Y., Long L., and Fang R. (2019). The effects of dietary supplementation with porous zinc oxide on growth performance, intestinal microbiota, morphology, and permeability in weaned piglets. Anim. Sci. J. 90, 1220–1228. doi: 10.1111/asj.13228
Percie du Sert N., Ahluwalia A., Alam S., Avey M. T., Baker M., Browne W. J., et al. (2020). Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 18, e3000411. doi: 10.1371/journal.pbio.3000411
Pluske J. R., Turpin D. L., and Kim J. C. (2018). Gastrointestinal tract (gut) health in the young pig. Anim. Nutr. 4, 187–196. doi: 10.1016/j.aninu.2017.12.004
Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Quiniou N., Dagorn J., and Gaudré D. (2002). Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. Peri- Post-Natal Mortality Pig 78, 63–70. doi: 10.1016/S0301-6226(02)00181-1
Ramos Meyers G., Samouda H., and Bohn T. (2022). Short chain fatty acid metabolism in relation to gut microbiota and genetic variability. Nutrients 14, 5361. doi: 10.3390/nu14245361
Revy P. S., Jondreville C., Dourmad J. Y., and Nys Y. (2003). Le zinc dans l’alimentation du porc : oligo-élément essentiel et risque potentiel pour l’environnement. INRAE Productions Animales 16, 3–18. doi: 10.20870/productions-animales.2003.16.1.3639
Rodrigues L. A., Wellington M. O., Sands J. M., Weber L. P., Olver T. D., Ferguson D. P., et al. (2020). Characterization of a swine model of birth weight and neonatal nutrient restriction. Curr. Dev. Nutr. 4, nzaa116. doi: 10.1093/cdn/nzaa116
Rutter G. A., Chabosseau P., Bellomo E. A., Maret W., Mitchell R. K., Hodson D. J., et al. (2016). Intracellular zinc in insulin secretion and action: a determinant of diabetes risk? Proc. Nutr. Soc 75, 61–72. doi: 10.1017/S0029665115003237
Saco Y. and Bassols A. (2023). Acute phase proteins in cattle and swine: A review. Vet. Clin. Pathol. 52, 50–63. doi: 10.1111/vcp.13220
Sandri M., Dal Monego S., Conte G., Sgorlon S., and Stefanon B. (2017). Raw meat based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet. Res. 13, 65. doi: 10.1186/s12917-017-0981-z
Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60. doi: 10.1186/gb-2011-12-6-r60
Serrano-Jara D., Rivera-Gomis J., Tornel J. A., Bernabé A., Martínez-Conesa C., Navarro J. A., et al. (2022). Effects of dietary supplementation with purple garlic powder and oregano essential oil on intestinal health in post-weaning piglets from commercial farms. Vet. Res. Commun. 47.2, 901–909. doi: 10.1007/s11259-022-10053-2
Starke I. C., Pieper R., Neumann K., Zentek J., and Vahjen W. (2014). The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol. Ecol. 87, 416–427. doi: 10.1111/1574-6941.12233
Sun J., Wang K., Xu B., Peng X., Chai B., Nong S., et al. (2021). Use of hydrolyzed chinese gallnut tannic acid in weaned piglets as an alternative to zinc oxide: overview on the gut microbiota. Animals 11, 2000. doi: 10.3390/ani11072000
Takahashi S., Tomita J., Nishioka K., Hisada T., and Nishijima M. (2014). Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PloS One 9, e105592. doi: 10.1371/journal.pone.0105592
Tao S., Bai Y., Li T., Li N., and Wang J. (2019). Original low birth weight deteriorates the hindgut epithelial barrier function in pigs at the growing stage. FASEB J. 33, 9897–9912. doi: 10.1096/fj.201900204RR
Toya T., Corban M. T., Marrietta E., Horwath I. E., Lerman L. O., Murray J. A., et al. (2020). Coronary artery disease is associated with an altered gut microbiome composition. PloS One 15, e0227147. doi: 10.1371/journal.pone.0227147
Trevisi P., Gandolfi G., Priori D., Messori S., Colombo M., Mazzoni M., et al. (2013). Age-related expression of the polymeric immunoglobulin receptor (pIgR) in the gastric mucosa of young pigs. PloS One 8, e81473. doi: 10.1371/journal.pone.0081473
Trevisi P., Latorre R., Priori D., Luise D., Archetti I., Mazzoni M., et al. (2017). Effect of feed supplementation with live yeast on the intestinal transcriptome profile of weaning pigs orally challenged with Escherichia coli F4. Animal 11, 33–44. doi: 10.1017/S1751731116001178
Trevisi P., Negrini C., Correa F., Virdis S., Laghi L., Marcello M., et al. (2023). Insight into the long-term impact of birth weight on intestinal development, microbial settlement, and the metabolism of weaned piglets. J. Anim. Sci. 101, skad395. doi: 10.1093/jas/skad395
van Milgen J., Valancogne A., Dubois S., Dourmad J.-Y., Sève B., and Noblet J. (2008). InraPorc: A model and decision support tool for the nutrition of growing pigs. Anim. Feed Sci. Technol Math. Models that Predict Effects Feed Characteristics Anim. Perform. 143, 387–405. doi: 10.1016/j.anifeedsci.2007.05.020
Vega-López M. A., Arenas-Contreras G., Bailey M., González-Pozos S., Stokes C. R., Ortega M. G., et al. (2001). Development of lntraepithelial cells in the porcine small intestine. Dev. Immunol. 8, 147–158. doi: 10.1155/2001/25301
Villagómez-Estrada S., Pérez J. F., Darwich L., Vidal A., van Kuijk S., Melo-Durán D., et al. (2020). Effects of copper and zinc sources and inclusion levels of copper on weanling pig performance and intestinal microbiota. J. Anim. Sci. 98, skaa117. doi: 10.1093/jas/skaa117
Wan Y. and Zhang B. (2022). The impact of zinc and zinc homeostasis on the intestinal mucosal barrier and intestinal diseases. Biomolecules 12, 900. doi: 10.3390/biom12070900
Wang W., Van Noten N., Degroote J., Romeo A., Vermeir P., and Michiels J. (2019). Effect of zinc oxide sources and dosages on gut microbiota and integrity of weaned piglets. J. Anim. Physiol. Anim. Nutr. 103, 231–241. doi: 10.1111/jpn.12999
Watanabe Y., Nagai F., and Morotomi M. (2012). Characterization of Phascolarctobacterium succinatutens sp. nov., an Asaccharolytic, Succinate-Utilizing Bacterium Isolated from Human Feces. Appl. Environ. Microbiol. 78, 511–518. doi: 10.1128/AEM.06035-11
Wei X., Tsai T., Knapp J., Bottoms K., Deng F., Story R., et al. (2020). ZnO modulates swine gut microbiota and improves growth performance of nursery pigs when combined with peptide cocktail. Microorganisms 8, 146. doi: 10.3390/microorganisms8020146
Wellington M. O., Rodrigues L. A., Li Q., Dong B., Panisson J. C., Yang C., et al. (2021). Birth weight and nutrient restriction affect jejunal enzyme activity and gene markers for nutrient transport and intestinal function in piglets. Anim. (Basel) 11, 2672. doi: 10.3390/ani11092672
Wilschefski S. C. and Baxter M. R. (2019). Inductively coupled plasma mass spectrometry: introduction to analytical aspects. Clin. Biochem. Rev. 40, 115–133. doi: 10.33176/AACB-19-00024
Yin S., Duan M., Fang B., Zhao G., Leng X., and Zhang T. (2022). Zinc homeostasis and regulation: Zinc transmembrane transport through transporters. Crit. Rev. Food Sci. Nutr. 0, 1–11. doi: 10.1080/10408398.2022.2048292
Keywords: gut maturation, immune system activation, microbiota, mineral nutrition, weaning
Citation: Negrini C, Luise D, Correa F, Virdis S, Serra A, Bonaldo A, Manzke N, Monteiro A, Mazzoni M and Trevisi P (2025) Effects of birth body weight and zinc source on growth, gut health, and immune response in weaned piglets. Front. Anim. Sci. 6:1614280. doi: 10.3389/fanim.2025.1614280
Received: 18 April 2025; Accepted: 10 June 2025;
Published: 11 July 2025.
Edited by:
Todd Riley Callaway, University of Georgia, United StatesReviewed by:
Margaret Bryer, University of Wisconsin-Madison, United StatesShiyi Tian, Nanjing Agricultural University, China
Copyright © 2025 Negrini, Luise, Correa, Virdis, Serra, Bonaldo, Manzke, Monteiro, Mazzoni and Trevisi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paolo Trevisi, cGFvbG8udHJldmlzaUB1bmliby5pdA==
 Clara Negrini
Clara Negrini Diana Luise
Diana Luise Federico Correa
Federico Correa Sara Virdis
Sara Virdis Andrea Serra
Andrea Serra Alessio Bonaldo
Alessio Bonaldo Naiana Manzke4,5
Naiana Manzke4,5 Alessandra Monteiro
Alessandra Monteiro Maurizio Mazzoni
Maurizio Mazzoni Paolo Trevisi
Paolo Trevisi