- 1College of Bioscience and Biotechnology, Hunan Agricultural University, Changsha, Hunan, China
- 2National Engineering Laboratory for Pollution Control and Waste Utilization in Livestock and Poultry Production, Hunan Provincial Key Laboratory of Animal Nutritional Physiology and Metabolic Process, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, Hunan, China
Introduction: Gluconeogenesis is the primary pathway for ruminants to obtain energy. Enhancement of gluconeogenesis can significantly improve the growth performance of ruminants. Inulin, a prebiotic, has capabilities such as fostering a healthier gut microbiota and modulating metabolism. However, the application of inulin in ruminant feed is still very limited.
Methods: Eighteen healthy Xiangdong black goats (body weight 9.0 ± 0.19 kg) were randomly divided into two groups: the control group and the inulin group, with nine goats in each group. The inulin group used 18.9% inulin instead of normal corn. The total experimental period was 28 days, with 7 days for adaptation before the formal experiment.
Results: Inulin supplementation significantly increased (P < 0.05) phosphoenolpyruvate carboxyl kinase (PEPCK) and glucose-6-phosphatase (G6Pase), as well as the expression of forkhead box protein O1 (FoxO1) in goat livers. At the same time, the serum insulin levels were significantly reduced (P < 0.05). Analysis of rumen microbes and rumen VFA levels revealed that the abundance levels of short-chain fatty acid-producing bacteria (Lachnospiracea, Blautia, Prevotella-1, and Pseudobutyrivibrio) and propionic acid concentration were significantly higher (P < 0.05) in the inulin group. Liver metabolites were analyzed via LC-MS, and increased levels of metabolites associated with the tricarboxylic acid (TCA) cycle and amino acid metabolism were observed following inulin administration.
Discussion: Inulin promotes the process of gluconeogenesis in goat liver by regulating the two key pathways of rumen microorganisms and liver metabolites to increase gluconeogenesis substrates.
1 Introduction
Glucose plays a pivotal role in cell synthesis and function, serving as an essential energy source for maintaining animal physiological metabolism. Microorganisms in the rumen of ruminants can break down feed and produce metabolites such as volatile fatty acids (VFAs), proteins, and vitamins. These metabolites are transported from the rumen to the liver through the gut–liver axis to synthesize glucose via gluconeogenesis in hepatocytes (Wang et al., 2023; Yang et al., 2024). Enhanced gluconeogenesis can bolster feed utilization efficiency (Oliver, 1975) and foster the growth and development of ruminants (Hammon et al., 2013). A deficiency in glucose and precursors for gluconeogenesis during the perinatal phase can culminate in conditions like ovine pregnancy toxemia (Schlumbohm and Harmeyer, 2004). To improve the production performance of ruminants, the key is to improve the utilization rate of feed. The main method is to increase glucose production, that is, to adjust the gluconeogenesis process.
The regulation of gluconeogenesis involves many aspects, including the substrates (e.g., lactic acid, propionic acid, and amino acid), the activities of related enzymes (e.g., PEPCK and G6Pase), and the hormones (e.g., insulin and glucagon) (Bougouin et al., 2018; Caton et al., 2010; Dashty, 2013). The influence of short-chain fatty acid (SCFA) content on gluconeogenesis is the most critical. SCFAs are important gluconeogenesis substrates in ruminants, accounting for 60% of the carbon source required for gluconeogenesis (Larsen et al., 2013). Studies have shown that SCFAs can reach the liver through portal vein circulation in sheep, participate in hepatic gluconeogenesis, and regulate enzyme activity and gene expression related to energy metabolism (Yang et al., 2024). The experimental results of Zhang et al. also showed that injecting propionic acid into cattle can increase serum insulin levels and the mRNA expression levels of key gluconeogenesis enzymes PCK1 and G6PC (Zhang et al., 2015).
Inulin is a naturally occurring fructose-type plant polysaccharide, and its basic unit is β-D-fructopyran, which forms a stable chemical structure through β-(1-2)-glycosidic bonds. As a dietary fiber, inulin has many biological functions, such as enhancing immunity, improving growth performance, promoting intestinal digestion and absorption, and improving metabolic disorders in animals (Tawfick et al., 2022). Inulin is widely used in the diets of humans (Chambers et al., 2019), rats (Zhang et al., 2018), and sows (Li et al., 2020) to modulate gut microbiota, increase insulin sensitivity, and improve glucose metabolism. In ruminant studies, inulin has been shown to improve the gastrointestinal flora of dairy cows, increase the VFA levels in ruminal fluid, and improve immunity and lactation performance (Wang et al., 2021b, 2022). Another study indicated that dietary inulin supplementation enhances the body weight and feed efficiency of beef cattle and changes the rumen fermentation mode from acetic acid fermentation to propionic acid fermentation (Tian et al., 2019). Our previous study also indicated that inulin as a feed additive can effectively improve the intestinal immunity and feed efficiency of goats and promote the growth of ruminants (Yuan et al., 2022).
The regulatory effect of inulin on glucose metabolism in monogastric animals has sparked great interest in its use as a feed additive to improve the efficiency and growth performance of ruminants by regulating metabolism. Previous studies have not investigated inulin’s role in hepatic gluconeogenesis using a systems biology approach. Therefore, this study builds on the premise that inulin regulates rumen microbiota. The molecular mechanism of inulin regulating gluconeogenesis in ruminants can be elucidated by analyzing the gene expression of ruminal microorganisms, ruminal epithelial transporters, liver metabolites, and gluconeogenesis-related enzymes. The aim of this study is to provide a theoretical basis for inulin as a feed additive to improve ruminant glucose metabolism and enhance feed efficiency.
2 Materials and methods
2.1 Animal ethics statement
The methods employed for conducting animal experiments were carried out in accordance with the guidelines accepted by the Animal Care Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, China (Permit No. ISACAS-2019-0620). This study was carried out in compliance with the ARRIVE guidelines (http://www.nc3rs.org.uk/page.asp?id=1357) for reporting animal experiments. All procedures involving animal care and euthanasia of experimental animals followed the guidelines of the American Veterinary Medical Association (AVMA) (Andrews et al., 1993) to minimize pain during animal execution. All experimental schemes have obtained recognition and approval from the Institute of Subtropical Agriculture, the Chinese Academy of Sciences, Changsha, China.
2.2 Experimental design and sample collection
Eighteen healthy Xiangdong black female goats (9.0 ± 0.19 kg body weight and approximately 2 months old) were randomly divided into two groups: the control group and the inulin group, with nine goats in each group. The total experimental period was 28 days, with 7 days for adaptation before the formal experiment. During the trial period, goats could drink freely and were fed at 8:00 and 18:00, each feeding consisting of 180 g of feed. The dosage of inulin was added based on previously published experimental data (Yuan et al., 2023). The composition and ingredients of the experimental diets are shown in Appendix Table 1. The contents of dry matter (DM), crude protein (CP), ether extract (EE), and ash in the diets were analyzed according to AOAC (1995). Acid detergent fiber (ADF) and neutral detergent fiber (NDF) were measured according to the methods of Van Soest et al. (1991) by a Fibretherm Fiber Analyzer (Gerhardt, Bonn, Germany).
All the goats were euthanized on the 29th day of the experiment. The euthanasia method for goats refers to the physical methods in AVMA (Andrews et al., 1993). Blood was collected intravenously from each goat before being slaughtered, and serum was obtained by centrifugation at 3,000 rpm for 15 min and stored at −80°C. The longissimus muscle, liver, and ruminal epithelium of the goats were washed with 0.09% sterile physiological saline, and slices of tissue samples were frozen quickly in liquid nitrogen and stored at −80°C for subsequent analysis. The rumen content was filtered through four layers of cheesecloth and the rumen fluid was obtained. The rumen fluid was divided into two parts. A sample of 15 mL of rumen fluid was collected and immediately frozen in liquid nitrogen for subsequent microbial analysis. Another 2.5 mL of sample of the rumen fluid was centrifuged at 15,000×g for 10 min at 4°C, and then 1.5 mL of supernatant solution was collected and added with 0.15 mL of metaphosphoric acid, homogenized, and stored at −20°C for subsequent determination of VFA.
2.3 Determination of plasma insulin, glucagon, and glucose
Insulin and glucagon concentrations were determined by commercial goat insulin and glucagon ELISA kits (Cusabio Biotech Co., Ltd., Hubei, China) and analyzed using a microplate reader (TECAN, Austria). Blood glucose content was measured by the assay kit (Beijing Leadman Biochemistry Company Limited, Beijing, China) and analyzed using an automatic biochemical analyzer (Hitachi 7600, Hitachi Ltd., Tokyo, Japan).
2.4 Determination of VFAs in ruminal fluid
VFA concentrations were analyzed by a gas chromatograph (Agilent 7890, Palo Alto, CA, USA) according to the procedure described by Zhang and Zhang (2019).
2.5 Glycogen content and enzyme activities
The glycogen content of muscle and liver samples and the activities of hexokinase, phosphoenolpyruvate carboxyl kinase, glucose-6-phosphatase, and glucose-6-phosphate dehydrogenase were measured with kits purchased from Suzhou Keming Biotechnology Co., Ltd., Suzhou, China.
2.6 Gene expression of rumen epithelium and hepatic tissues
RNA was extracted from tissue samples using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNA concentration and quality were determined using an ND-1000 UV–vis spectrophotometer (NanoDrop Ltd., Wilmington, DE, USA) and by both 1% agarose gel electrophoresis and the 260/280 absorbance ratio using an ND1000 spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA) (Wang et al., 2020a). cDNA was synthesized using an Evo M-MLV RT Kit with gDNA Clean for qPCR II Kit (Hunan Accurate Biotech Co., Ltd., Changsha, China) according to the manufacturer’s instructions and stored at −20°C until analysis. Relative mRNA expression of the gene was determined using a LightCycler480 system (Roche, Basel, Switzerland) with SYBR® Green Premix Pro Taq HS qPCR (Hunan Accurate Biotech Co., Ltd., Changsha, China). The target and internal reference (β-actin) gene primer sequences are detailed in Appendix Table 2. Quantitative polymerase chain reaction (qPCR) parameters were as follows: denaturation at 95°C for 30 s, followed by 40 cycles of denaturation at 95°C for 5 s, and annealing at 60°C for 20 s. The specificity of the PCR reaction was confirmed by melt curve analysis. The efficiency of PCR amplification for each gene was checked with the sample dilution. The 2−△△Ct method was used to calculate the relative expression ratios of the targeted genes with the control group used as the calibrator.
2.7 Analysis of bacterial community
One milliliter of rumen fluid was centrifuged at 14,000 rpm, the supernatant was discarded, and then bacterial DNA was extracted. The specific primers with barcodes were synthesized according to the 16S rRNA gene full-length primers 27F (AGRGTTTGATYNTGGCTAG) and 1492R (TASGGHTACCTTGTTASGACTT). PCR amplification was performed, and the products were purified, quantified, and homogenized to form the sequence library (SMRT Bell). The built library was checked first, and the qualified library was sequenced by PacBio Sequel. The data in PacBio Sequel were in bam format. The CCS file was exported using the SMRT Link analysis software, and the data of different samples were identified according to barcode sequences and converted into FASTQ format. The Lima V1.7.0 software was used to identify CCS by barcode and obtain barcode-CCS sequence data. Barcode-CCS was filtered by the software independently developed by the Biomaker company. UCHIME V8.1 software was used to identify and remove chimeric sequences, and the Optimization-CCS sequence was obtained (Edgar et al., 2011).
2.8 Bioinformatic analysis
Bioinformatics analysis of this study was carried out with the assistance of BMK Cloud (Biomarker Technologies Co., Ltd., Beijing, China). All sequences in the current study were stored in the sequence read archive (SRA) of the NCBI database under accession number PRJNA704487. Sequences were clustered at a 97% similarity level (USEARCH, version 10.0), and OTU was filtered with 0.005% of the number of all sequences sequenced as a threshold (Bokulich et al., 2013)Alpha diversity refers to diversity within a specific region or ecosystem. The common indicators for measuring bacterial abundance include Chao (Chao1 richness estimator) and ACE (ACE richness estimator). Indicators to measure community diversity include the Shannon–Wiener diversity index, Simpson diversity index, etc. The smaller the Simpson index, the higher the diversity of the community. The higher the Shannon index, the higher the diversity of the community (Schloss et al., 2009)Principal coordinate analysis (PCoA) involves sorting of eigenvalues and eigenvectors, selecting the top principal components. The PCoA distance matrix can be found in the main coordinates. Differences between individuals or groups can be observed through PCoA. LEfSe (species analysis with significant differences between groups) (called biomarker analysis) used linear discriminant analysis (LDA) to estimate the impact of the abundance of each component (species) on the effect of differences. The analysis was aimed at finding species with significant differences in abundance between groups.
2.9 Metabolite analysis
Six liver tissues (20 mg) were randomly selected from each of the control group and the inulin group and thawed at 4°C. One thousand microliters of tissue extract [75% (9:1 methanol:chloroform): 25% H2O) was added to the tissues and milled in a tissue grinder using magnetic beads. The milling parameters were set to 50 Hz for 60 s. The supernatant of the tissue homogenate was extracted by centrifugation in a high-speed centrifuge (12,000 rpm, 4°C), taken off, set aside, and frozen at −80°C.
Before the sample was analyzed by LCMS, 200 µL of redissolved liquid (50% acetonitrile solution was prepared with 2-chloro-l-phenylalanine) was added to dissolve the frozen sample. Subsequently, 10 µL of supernatant from each of the different groups was extracted and mixed to form a quality control (QC) sample. Samples were chromatographically examined using the Ultra High-Performance Liquid Phase System (Thermo Fisher Scientific, USA). The following liquid chromatography conditions were used: ACQUITY UPLC ® HSS T3 column (Waters, Milford, MA, USA) with a specification of 2.1 mm × 100 mm, 1.8 µm. The parameter was set to a flow rate of 0.3 mL/min and a column temperature of 40°C. Electrospray ionization (ESI) in positive and negative ionization modes was used for chromatographic separation. In LC-ESI (+)-MS mode, the mobile phases were 0.1% formic acid in acetonitrile (v/v) and 0.1% formic acid in water (v/v). In LC-ESI (−)-MS mode, the mobile phases were acetonitrile and ammonium formate (5 mM). The separation procedure method followed the steps described by Zelena et al. (2009). Mass spectrometry was performed using an Orbitrap Exploris 120 (Thermo Fisher Scientific, USA). The conditions for mass spectrometry detection were in accordance with the report of Want et al. (2013).
The mass spectrometry data obtained were input into the ProteoWizard software package’s msconvert tool (v3.0.8789) and stored in mzXML format. The R XCMS software package was used for peak detection, peak filtration, and peak alignment to obtain the quantitative list of substances. The obtained data were compared with the HMDB public metabolite database (http://www.hmdb.ca/). Multivariate statistical analysis was performed using the R language package, including the principal component analysis (PCA), partial least squares-discriminant analysis (PLS-DA), and orthogonal partial least squares discriminant analysis (OPLS-DA). The Kruskal–Wallis H test results and the variable importance in the projection (VIP) obtained in the OPLS-DA model were used to screen for significantly different metabolites. Significantly different metabolites were defined as VIP >1, P <0.05, and fold change (FC) >1.5 or <0.67. Different metabolites were analyzed by the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (MetaboAnalyst 3.0, http://www.metaboanalyst.ca/).
2.10 Statistical analysis
All data were presented as the mean ± standard error of the mean (SEM). Statistical significance was assessed by the independent sample t-test using SPSS (SPSS version 21.0 for Windows; SPSS Inc., Chicago, IL, USA) software packages. Differences were considered significant when P <0.05, and P <0.01 indicated a very significant difference. Pearson correlations were analyzed in GraphPad Prism version 5.01 (GraphPad Software, La Jolla, CA).
3 Results
3.1 Resource identification initiative
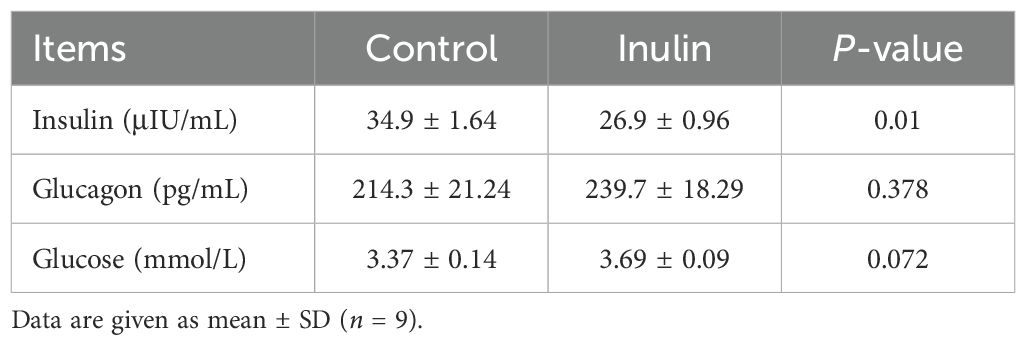
The plasma glucagon and glucose concentration remained consistent across the two groups. Notably, the plasma insulin concentration decreased significantly (P < 0.05) in the inulin group compared to the control group (Table 1).
3.2 VFA contents in the ruminal fluid
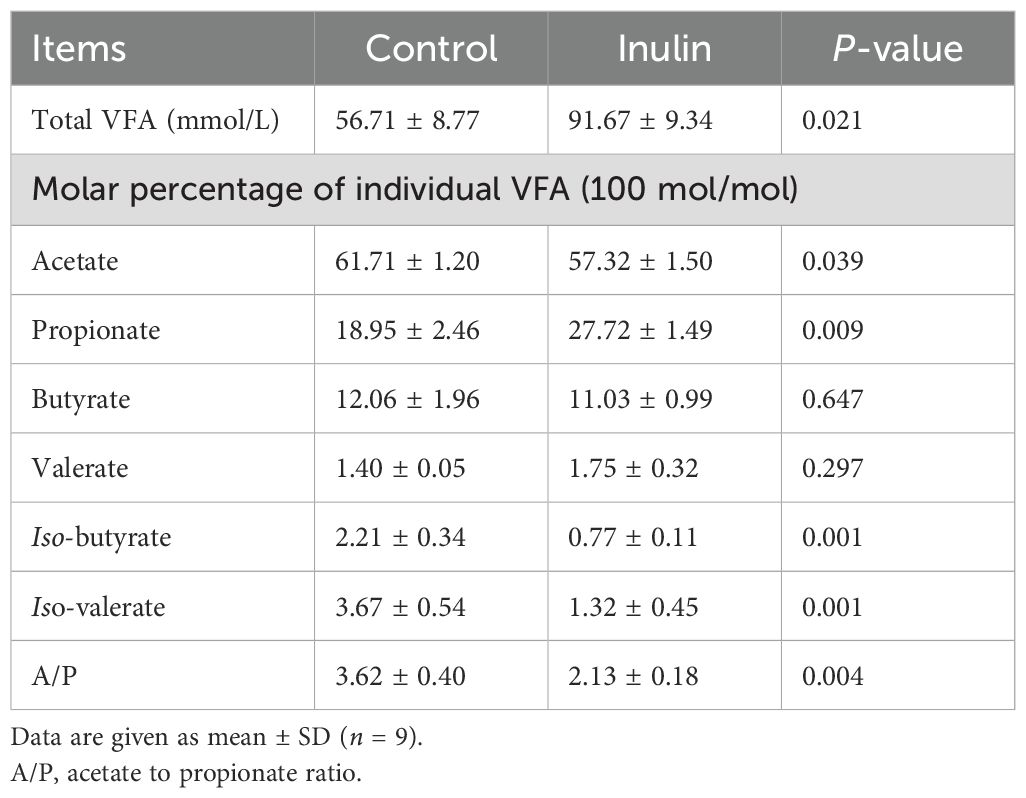
The total VFA content and the molar proportion of propionate in the ruminal fluid were increased significantly (P < 0.05) in the inulin group compared to the control group. Meanwhile, the molar proportions of acetate, iso-butyrate, and iso-valerate and the acetate to propionate ratio were decreased significantly (P < 0.05) in the inulin group compared to the control group. At the same time, the ratio of acetate to propionate in the inulin group was decreased significantly (P < 0.05). However, both groups had similar (P > 0.05) molar proportion of butyric acid to valeric acid (Table 2).
3.3 Bacterial community diversity in the ruminal fluid
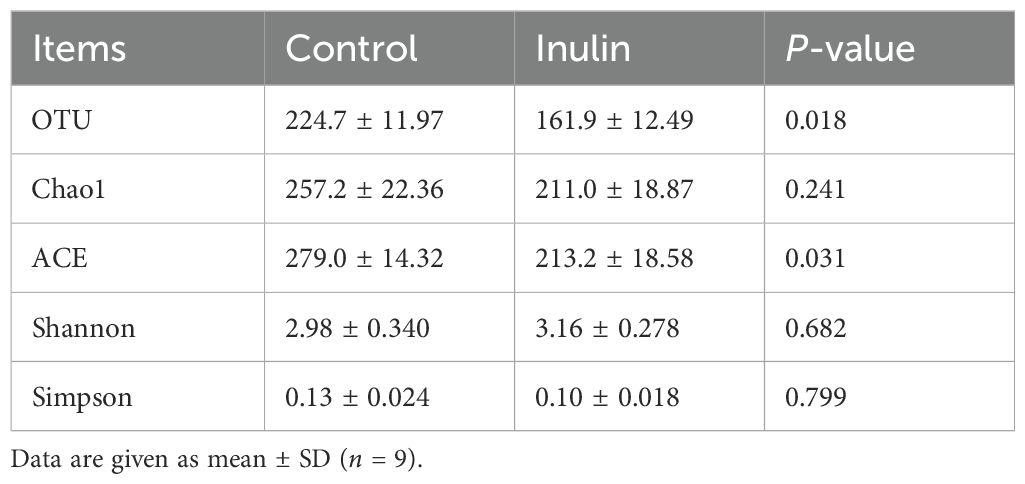
The OTU and ACE indices in the inulin group were significantly lower (P < 0.05) than those in the control group. However, the Chao1, Shannon, and Simpson indices showcased consistency between the two groups (Table 3). PCoA showed distinctly clustered microbiota compositions for each group, with PCoA1 and PCoA2 accounting for 13.61% and 29.24% of the total variation, respectively (Figure 1a).
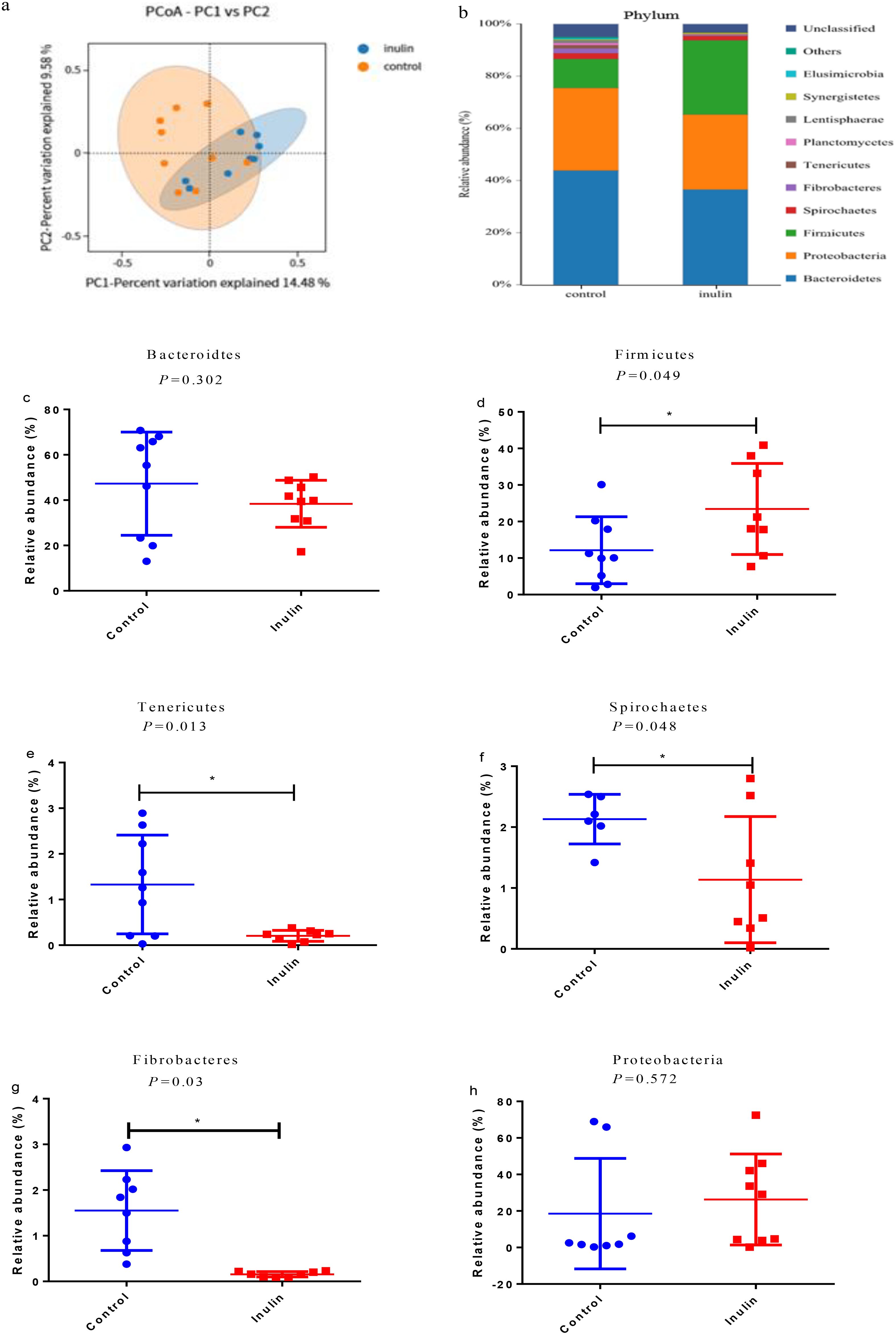
Figure 1. (a) Principal component analysis (PCoA). The red circles represent the samples of the control group, and the blue ones represent the samples of the inulin group. (b) Relative abundance of microbiota at the phylum level in the ruminal fluid of goats. (c–h) Relative abundance of the major phyla in the ruminal fluid of goats. ∗P < 0.05.
At the phylum level, Bacteroidetes, Proteobacteria, and Firmicutes were the dominant bacterial phyla in the ruminal fluid of goats, followed by Spirochaetes, Fibrobacteres, and Tenericutes (Figure 1b). There were significant differences (P < 0.05) in the relative abundances of Firmicutes, Tenericutes, Spirochaetes, and Fibrobacteres between the two groups. Their relative abundance levels in the control group were 12.16%, 1.32%, 2.13%, and 1.55%, respectively. However, their relative abundance levels in the inulin group were 23.43%, 0.20%, 1.14%, and 0.15%, respectively. In addition, the relative abundance of Firmicutes was increased significantly (P < 0.05), while the relative abundance levels of Tenericutes, Spirochaetes, and Fibrobacteres were decreased significantly (P < 0.05) in the inulin group compared with the control group (Figures 1c–h).
As shown in Figure 2a, Rikenellaceae_RC9_gut_group, Prevotella_1, Succiniclasticum, Anacrocella, and Succiniclasticum were dominant in both groups. There were significant differences (P < 0.05) in the relative abundance levels of Quinella, CAG-873, Prevotella_1, Anaeroplasma, and Treponema_2. Their relative abundance levels in the control group were 0.08%, 0.29%, 3.3%, 0.08%, and 1.04%, respectively. However, their relative abundance levels in the inulin group were 7.35%, 4.22%, 9.34%, 1.67%, and 0.19%, respectively (Figures 2b–f). LEfSe analysis shows that the relative abundance levels of Quinella, CAG-873, Prevotella_1, Lachnospiracea, and Blautia were increased significantly (P < 0.05), and the relative abundance levels of Anaeroplasma and Treponema_2 were decreased significantly (P < 0.05) in the inulin group compared to the control group (Figures 2g, h).
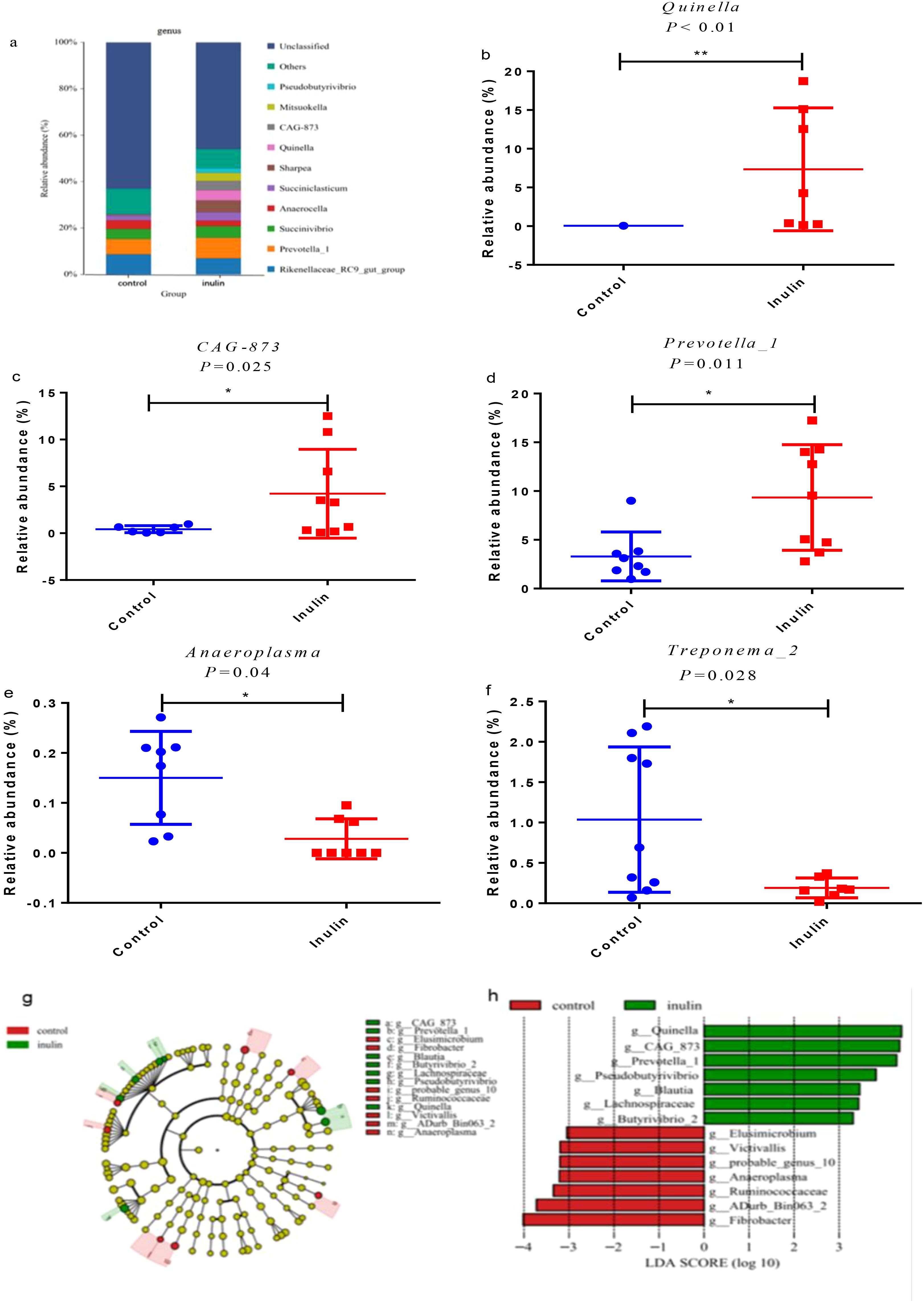
Figure 2. Ruminal bacteria change between the two groups at the genus level in the ruminal fluid of goats. (a) Relative abundance of microbiota at the genus level in the ruminal fluid of goats. (b–f) Significantly different microbiota. (g) Linear discriminant analysis effect size (LEfSe) cladogram shows the taxonomic differences in abundance (from phylum to genus level) between the two groups. (h) Microbiota with significantly different LDA scores that are greater than predicted. The threshold is 3.0. The length of the histogram represents the LDA score, which compares the impact of significant differences between species. ∗P < 0.05, ∗∗P < 0.01.
3.4 Metabolomic analysis of the liver
The PCA analysis showed a clear separation in metabolite profiles between the two groups (Figures 3a, b). A total of 1,794 metabolites were detected in the liver of both groups. Compared with the control group, 228 differential metabolites were upregulated and 66 differential metabolites were downregulated in the inulin group (P < 0.05) (Figure 3c). The volcano plot showed the top 20 metabolites with the most significant differences between the two groups (Figure 3d). KEGG enrichment analysis illustrated that dietary inulin supplementation influenced signals including linoleic acid metabolism; cholesterol metabolism; citrate cycle; cortisol synthesis and secretion; riboflavin signaling pathway; mTOR signaling pathway; glucagon signaling pathway; alanine, aspartate, and glutamate metabolism; and Rap1 signaling pathway (Figure 4a). After dietary supplementation with inulin, the contents of arachidonic acid, FA 18_2, 9-Oxo-ODE, FA18_2; O, and 8(R)-hydroperoxylinoleic acid were increased significantly, and linoleic acid metabolism was upregulated. Cholesterol and taurocholic acid were increased in the inulin group, and the cholesterol metabolism was upregulated. The content of oxoglutaric acid, which is part of the tricarboxylic acid cycle and glucagon signaling pathway, was significantly decreased in the inulin group, while the contents of malic acid and citric acid were increased. The contents of citric acid, L-arginine, and 5-phosphoribosylamine were increased, which were enriched in D-amino acid metabolism (Figure 4b and Table 4).
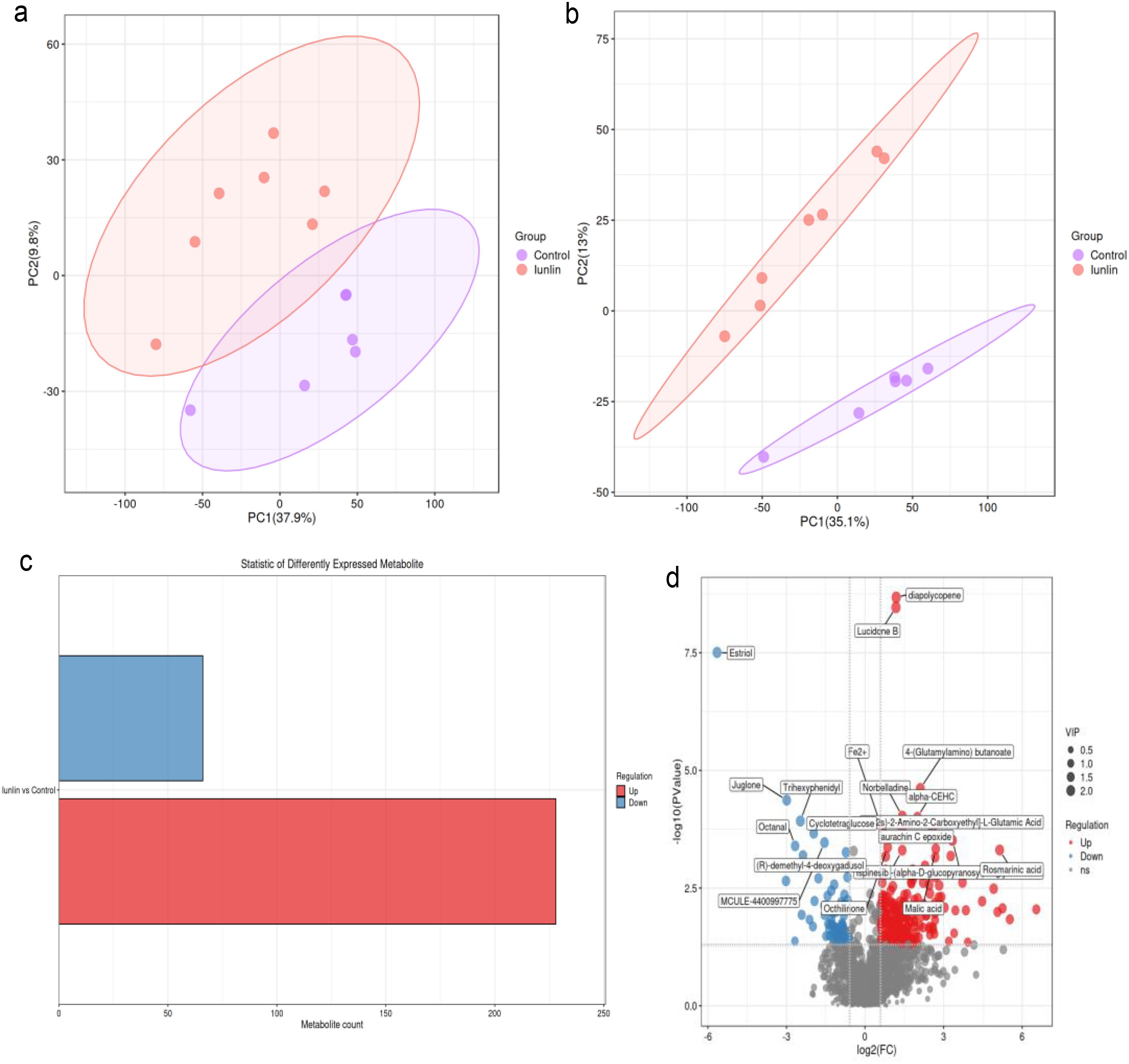
Figure 3. Analysis of liver metabolites in the control group (red) and the inulin group (purple). Principal component analysis (PCA) analysis diagram in positive ion mode (a) and negative ion mode (b). Statistical histogram of differential metabolites: the abscissa represents the number of differential metabolites. Red means upregulation of differential metabolites and blue means downregulation (c). Volcano plot showing the top 20 differential metabolites with the lowest P-values. Differential metabolites that exhibited upregulated expression and downregulated expression are represented by red dots and blue dots, respectively (d).
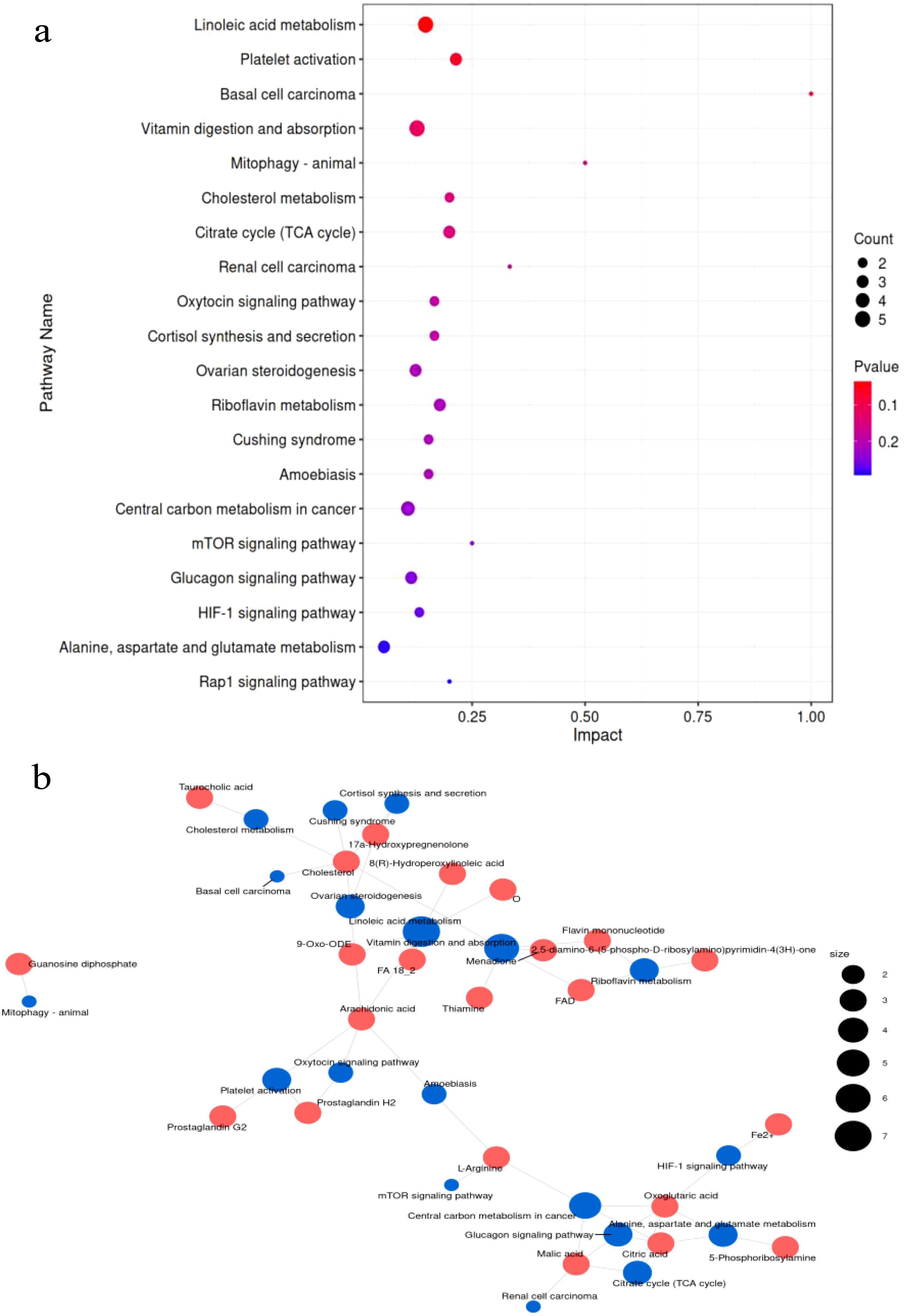
Figure 4. (a) KEGG pathway enrichment analysis of differential metabolites in the livers of goats. The abscissa is the impact value in metabolic pathways. The dot size indicates the corresponding number of metabolites on the KEGG pathway. (b) The relationship between differential metabolites and the metabolic pathway network. Red dots represent different metabolites. Blue dots represent the metabolic pathway. The size of the dots represents the number of different metabolites.
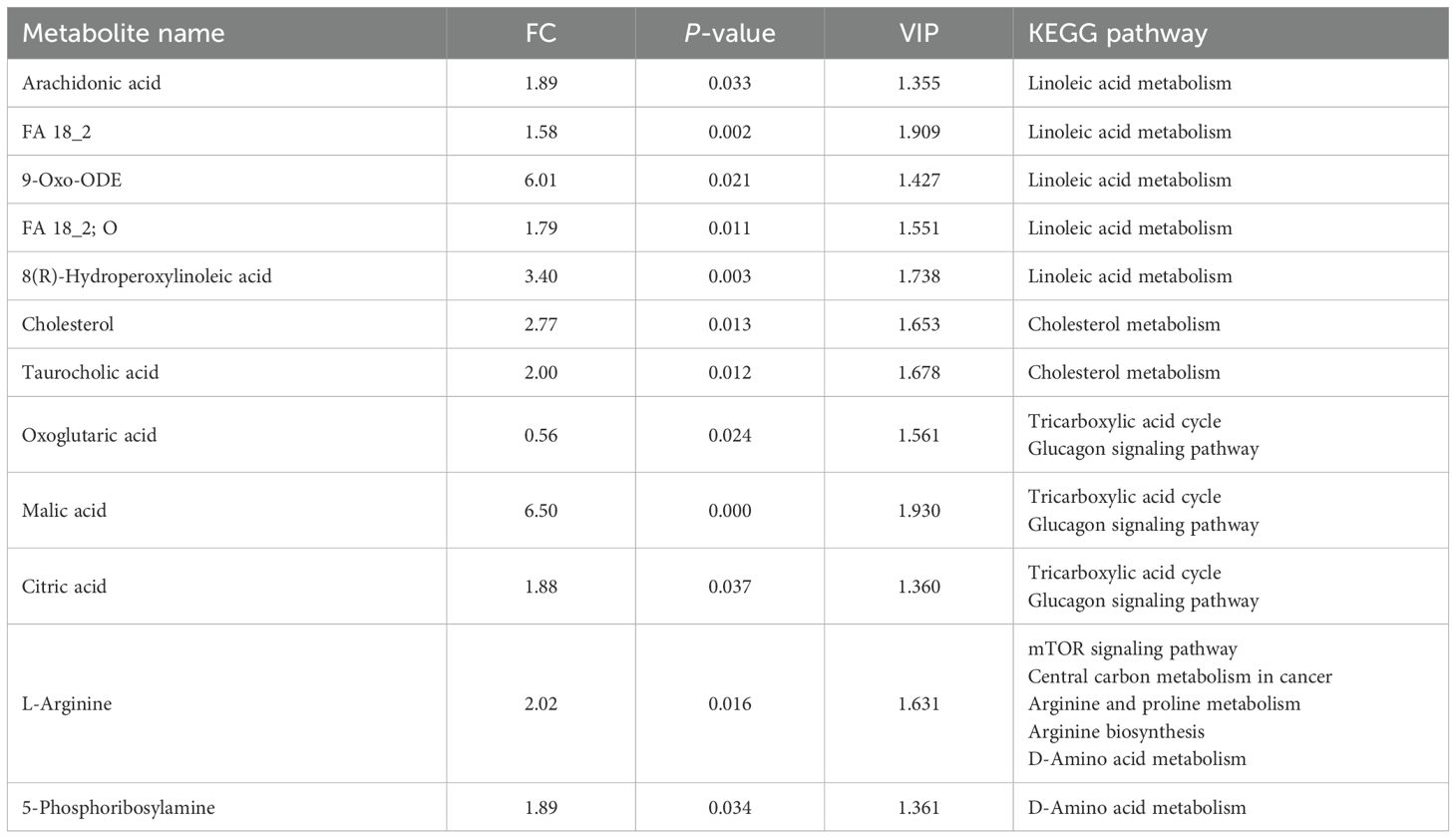
Table 4. Metabolic pathway enrichment analysis of significantly differentially abundant metabolites in the livers of goats.
3.5 Correlation analysis of differential rumen microorganisms, liver metabolites, and VFAs
CAG-873 and Prevotella_1 displayed a positive correlation with FA 18_2, 9-Oxo-ODE, 8(R)-hydroperoxylinoleic acid, L-arginine, 5-phosphoribosylamine, cholesterol, and malic acid. Anaeroplasma was negatively correlated with arachidonic acid, FA 18_2, 9-Oxo-ODE, 8(R)-hydroperoxylinoleic acid, cholesterol, malic acid, taurocholic acid, and citric acid. Analysis of the correlation between liver metabolites and VFAs showed that propionate and TVFA displayed positive correlations with arachidonic acid, FA 18_2, 9-Oxo-ODE, 8(R)-hydroperoxylinoleic acid, cholesterol, malic acid, taurocholic acid, and citric acid. Acetate, iso-butyrate, and iso-valerate demonstrated a negative correlation with arachidonic acid, FA 18_2, 9-Oxo-ODE, 8(R)-hydroperoxylinoleic acid, cholesterol, malic acid, taurocholic acid, and citric acid. Furthermore, CAG-873 and Prevotella_1 demonstrated positive correlations with valerate, propionate, and TVFA (Figure 5).
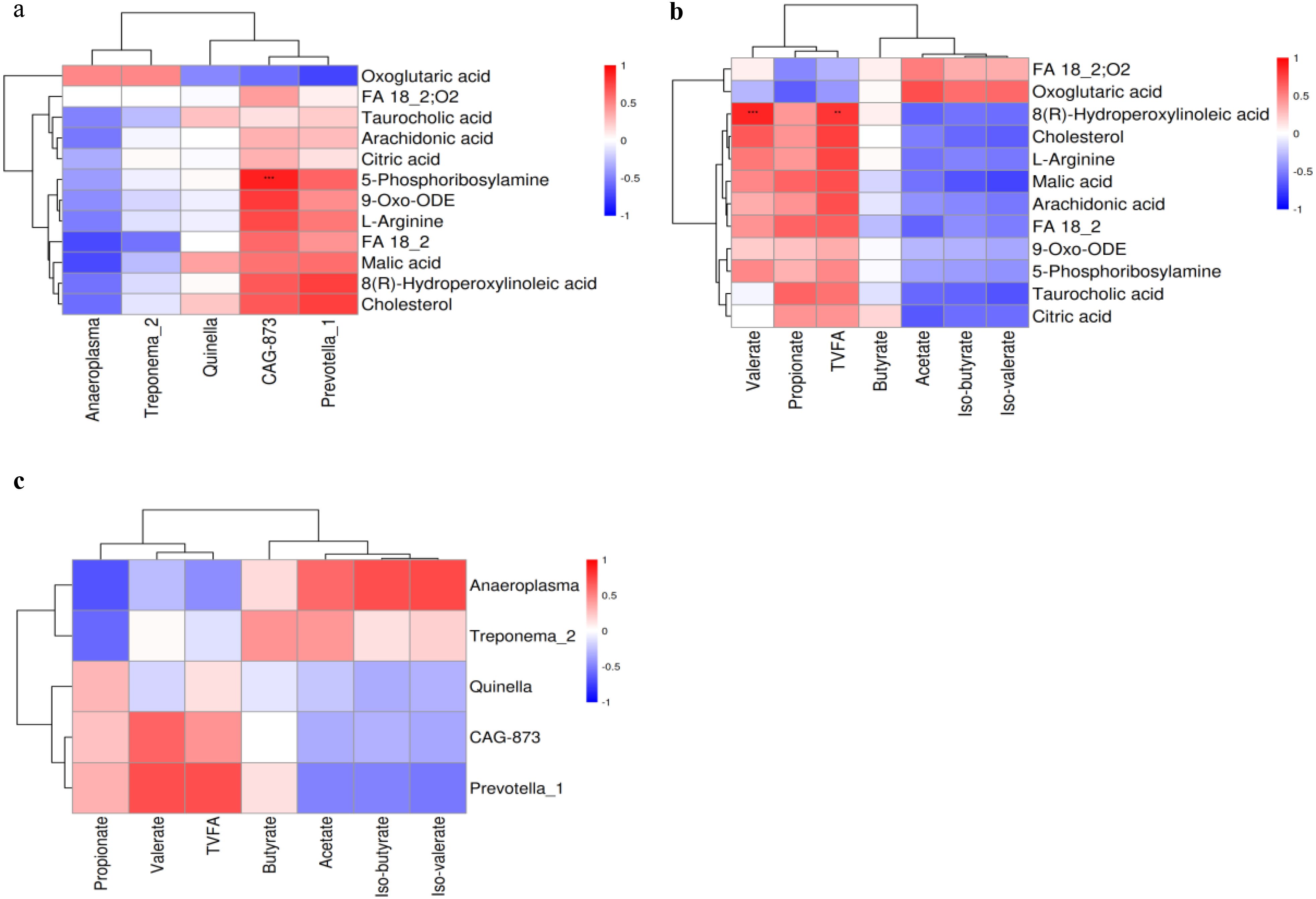
Figure 5. Spearman analysis of differential rumen microbes, VFAs, and differential liver metabolites. (a) Differential rumen microbes and liver metabolites. (b) Differential liver metabolites and VFAs. (c) Differential rumen microbes and VFAs. Red indicates a positive correlation, and blue indicates a negative correlation.
3.6 Glycogen content and enzyme activities
The enzymatic activities of glucose-6-phosphate dehydrogenase (G6PDH), glucose-6-phosphatase (G6Pase), and phosphoenolpyruvate carboxyl kinase (PEPCK) were significantly higher (P < 0.05) in the inulin group than in the control group (Figures 6a, c, d). However, the activity of hexokinase (HK) was significantly decreased (P < 0.05) in the inulin group in comparison with the control group (Figure 6b). The glycogen content of the liver was decreased in the inulin group compared to the control group (P < 0.05) (Figure 6e), while the glycogen content in the muscle remained comparable between the two groups (Figure 6f).
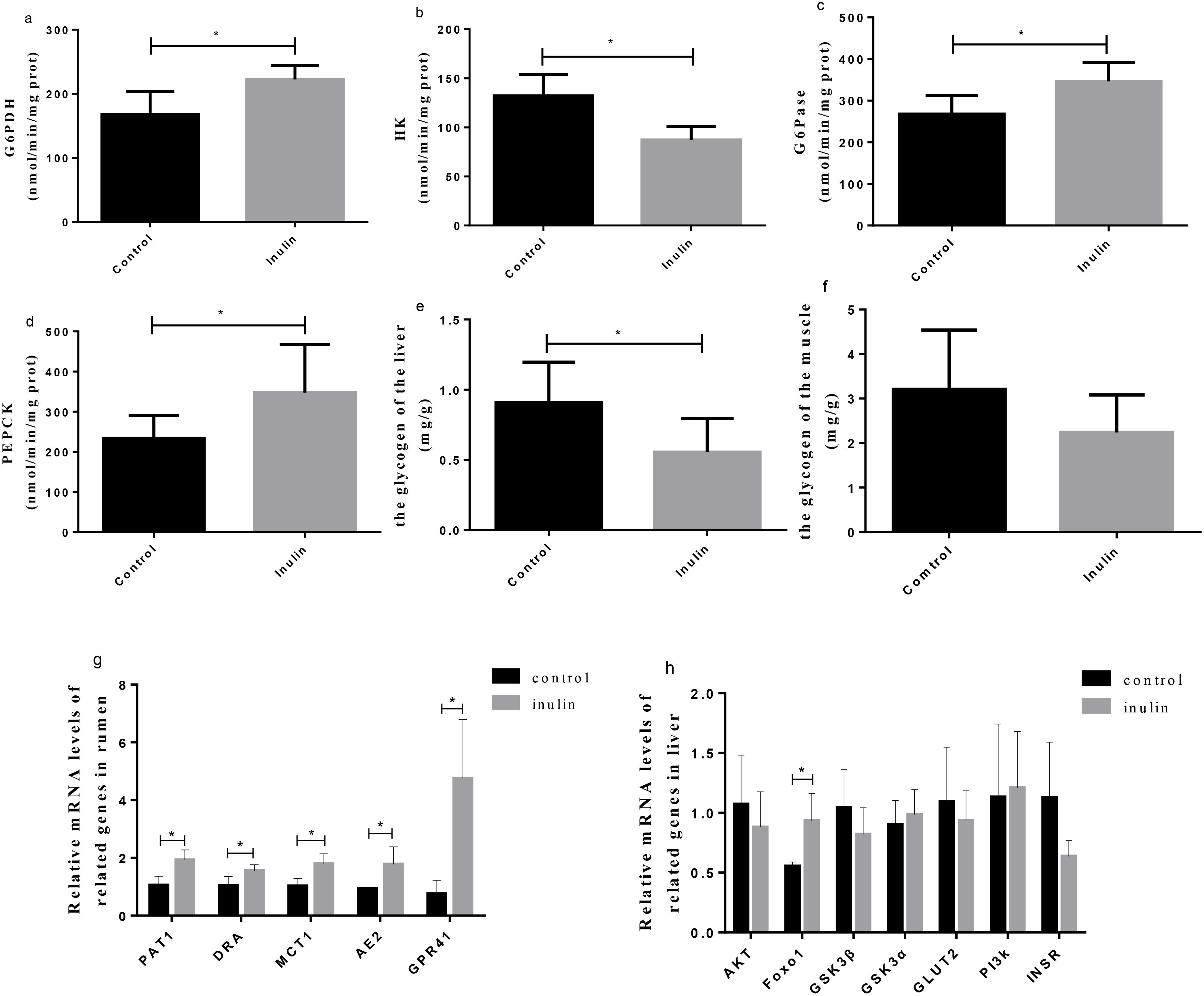
Figure 6. The activities of enzymes related to carbohydrate metabolism (a–d), the content of glycogen in the liver (e) and muscle (f) of goats, and the gene expression in the ruminal epitheliums (g) and livers (h) of goats. G6PDH, glucose-6-phosphate dehydrogenase; G6Pase, glucose-6-phosphatase; HK, hexokinase; PEPCK, phosphoenolpyruvate carboxyl kinase; PAT1, putative anion transporter 1; DRA, adenoma; MCT1, monocarboxylate transporter 1; AE2, anion exchanger 2; GPR41, G-protein-coupled receptor 41; AKT, protein kinase B; Foxo1, forkhead transcription factor o1; GSK3β, glycogen synthase kinase 3 beta; GSK3α, glycogen synthase kinase 3 alpha; GLUT2, glucose transporter 2; PI3K, phosphoinositide-3 kinase; INSR, insulin receptor. *P < 0.05.
3.7 Gene expression
The relative mRNA expression levels of PAT1, DRA, MCT1, AE2, and GPR41 in the ruminal epithelium and that of FoxO1 in the liver in the inulin group were significantly upregulated (P < 0.05) compared to the control group (Figure 6g). The fold change relative mRNA expression levels of PAT1, DRA, MCT1, AE2, GPR41, and FoxO1 in the control and inulin group were 0.53, 0.66, 0.56, 0.53, 0.13, and 0.59, respectively. Nonetheless, the relative mRNA expression levels of AKT, GSK3β, GSK3a, GLUT2, PI3K, and INSR in the liver showed no significant differences between the two groups (Figure 6h).
4 Discussion
As a feed additive, inulin can effectively improve the feed conversion rate and weight gain of animals, thereby enhancing production efficiency. This phenomenon has attracted extensive attention (Grand et al., 2013; Li et al., 2019). In our previous study (data have been published), the average daily weight gain of goats in the inulin group was increased (Yuan et al., 2022). These results demonstrate the potential of inulin as a feed additive in improving the growth performance of goats. However, the specific mechanism by which inulin improves the growth performance of ruminants remains unclear. Adequate energy is essential to improve the growth performance of ruminants. Studies have shown a positive correlation between blood glucose levels and average daily weight gain (Wang et al., 2020b). We found that goat rumen microbiota, liver metabolites, and hormones were significantly altered after dietary inulin supplementation. These results confirm that dietary inulin supplementation has an impact on goat energy metabolism.
Inulin is a dietary fiber that regulates glucose metabolism (Hughes et al., 2022). Miao et al. reported that 3.33 and 1.67 g/kg/day of inulin supplementation significantly reduced blood glucose and insulin levels in gestational diabetes mellitus mice (Miao et al., 2021). Similar results were reported by Zhang et al. (2021), where blood glucose and insulin levels in high-fat mice were significantly reduced when supplemented with 2 g/kg of inulin. The addition of inulin in this study did not cause changes in blood glucose but reduced blood insulin levels. Our results are different, probably because, first of all, the high-fat diet in the above study led to changes in the health status of the animals, such as insulin resistance and reduced glucose tolerance. However, Zhang et al. did not show any changes in blood glucose and insulin levels after feeding normal feed with added inulin (Zhang et al., 2021). Secondly, the above studies are all aimed at monogastric animals, but ruminants can degrade inulin by rumen microorganisms and produce a large amount of gluconeogenetic precursors, such as propionic acid, lactic acid, and amino acids (Zhao et al., 2014; Wang et al., 2021b). These SCFAs are transported into the bloodstream through a transporter protein in the rumen epithelium and then into the liver as a gluconeogenesis substrate (Dijkstra, 1994). Hepatic gluconeogenesis is the most important pathway for ruminants to obtain glucose (Wang et al., 2023), and the decrease in insulin promotes gluconeogenesis (Hatting et al., 2018). Insulin promotes glycogen synthesis, which reduces blood sugar levels, and the liver is more sensitive to insulin signaling (Lawrence and Roach, 1997; Zhao et al., 2016). This might explain the significant increase in liver glycogen in the inulin group, but no change in muscle glycogen.
As the main digestive organ of ruminants, the function of the rumen is to convert the feed into VFAs through the microbial fermentation process (Hoover and Miller, 1991). Different diets can cause changes in VFA content (Wanapat et al., 2015). Zhao et al. used in-vitro fermentation to simulate the rumen environment and found that the content of TVFA, propionic acid, and butyric acid in the inulin group increased compared to the starch group, but the molar percentage of acetic acid and the A/P ratio decreased (Zhao et al., 2014). In addition, it was also observed that after adding inulin to the diet, the content of TVFA and the ratio of propionic acid and butyric acid in the rumen of dairy cows were increased, and the ratio of A/P was decreased (Wang et al., 2021b). In the present study, we found that in the inulin group, the content of TVFA and the ratio of propionic acid were higher, and the ratio of acetic acid and A/P decreased, which means that rumen fermentation mode changed from acetic acid to propionic acid. Propionic acid is an important substrate for gluconeogenesis and provides more than 70% of the carbon source for glucose production. Cui et al. have shown that increasing propionic acid content in the sheep rumen enhanced energy conversion efficiency and promoted weight gain in ruminants (Cui et al., 2023). GPR41 is a member of the G-protein-coupled receptor family and is expressed in animal gastrointestinal tissue. As a receptor for VFAs, GPR41 can bind to propionic acid and regulate insulin secretion to regulate energy metabolism (Kimura et al., 2020). Previous studies have shown that increased concentrations of propionic acid and butyric acid in the intestines of mice fed a dietary fiber diet, as well as increased expression of GPR41, promoted gluconeogenesis (De Vadder et al., 2014). The reduction in goat insulin levels observed in the inulin group in this study might be related to increased propionic acid concentration and increased expression of GPR41. The rumen epithelium contains a VFA transport system, and more than 75% of rumen VFAs enter the liver through the rumen epithelium transport system to provide energy for ruminants (Na and Guan, 2022). The rumen epithelial transport system is regulated by transporters (e.g., MCT1, AE2, DRA, PAT1). MCT1 is located in the basal layer of rumen epithelium and mediates the electroneutral transport of SCFA (Sivaprakasam et al., 2011). DRA, AE2, and PAT1 are mainly distributed outside the basement membrane and are responsible for exchanging SCFA− and HCO3− inside and outside the cell (Wang et al., 2021a; Yu, 2021; Husain et al., 2025). By adjusting the diet to increase the mRNA expression levels of these SCFA transporters, the VFAs’ absorption capacity can be enhanced. Consequently, the increased expression of SCFA transporters in goats in the inulin group suggests that inulin not only promoted the production of large quantities of propionic acid in the goat rumen but also enhanced the overall absorption capacity of SCFA, allowing more propionic acid to enter the liver, providing a sufficient substrate for gluconeogenesis.
Different diets often lead to changes in the composition of rumen bacteria, and VFA concentrations are closely related to rumen bacteria. The PCoA analysis suggested that inulin addition altered the microbial composition. This result was similar to the report that dietary supplementation of inulin altered the composition of rumen microorganisms in beef steers (Louis et al., 2007). The reason for this result might be that inulin could be fermented and metabolized by rumen microorganisms (Tian et al., 2019; Zhao et al., 2014). Furthermore, the decreased OTU and ACE indexes in the inulin group and the similar Chao1, Shannon, and Simpson indices between the two groups indicated that dietary inulin supplementation decreased microbial diversity and did not change the richness of rumen microorganisms in the ruminal fluid of goats. At the phylum level, the increased abundance of Firmicutes was noted in the inulin group. Firmicutes can regulate the absorption of carbohydrates (Huang et al., 2024). Moreover, at the genus level, we observed higher abundance levels of Lachnospiracea, Blautia, Prevotella-1, and Pseudobotyrivibrio in the inulin group compared with the control group in this study. Lachnospiracea, which belongs to the phylum Firmicutes, can degrade fructose and cellobiose to promote propionic acid production in the rumen (Engels et al., 2016). Blautia is a probiotic that can generate large amounts of SCFA (Liu et al., 2021). Similarly, adding resistant starch to the diet of high-fat mice increased the abundance of Blautia in the intestine (Yang et al., 2024). Meanwhile, a similar result was observed by Sun et al., where inulin addition could stimulate the growth of Preverella-1 in human intestines. Prevotella-1 can participate in the metabolism and utilization of plant polysaccharides and can promote propionic acid fermentation in the bovine rumen (Sun et al., 2020; Wu et al., 2022). Pseudobotyrivibrio is positively correlated with propionic acid production in high-fat mice (Henning et al., 2018). Therefore, the higher abundance levels of Lachnospiracea, Blautia, Prevotella-1, and Pseudobotyrivibrio were consistent with the increased propionic acid production in the inulin group in this study. It was likely that inulin supplementation promoted the production of propionic acid by increasing the abundance levels of propionic acid-producing bacteria.
Changes in the gut microbiome can cause changes in the metabolic characteristics of the liver. This is due to the transport of intestinal microbiota metabolites (including carbohydrates, VFAs, and amino acids) into the liver through the gut–hepatic axial portal vein system, thereby affecting metabolic processes (Bauer et al., 2022). Studies report that as populations of VFA and amino acid-producing bacteria increase in the ileum of mice with non-alcoholic fatty liver disease, the concentrations of metabolites such as alanine, aspartate, and glucose also increase in the liver (Qiu et al., 2023). This suggests that dysregulation of intestinal flora may influence hepatic metabolic pathways. We applied metabolomics to analyze the effect of dietary inulin addition on the metabolic profiles in the livers of goats. The 95% confidence complete interval separation for PCA score maps was observed in the livers of goats between the two groups, suggesting that dietary inulin supplementation altered the metabolic profiles of the livers. The contents of arachidonic acid, FA 18_2, 9-Oxo-ODE, FA 18_2;O, and 8(R)-hydroperoxylinoleic acid in the inulin group increased significantly, and linoleic acid metabolism was significantly upregulated. Studies have reported that a significant increase in arachidonic acid content was found in sheep fed high-fiber diets, because Prevotella can activate the activity of phospholipase A2 to stimulate arachidonic acid production (Wu et al., 2020). Linoleic acid, also known as unsaturated fatty acid, can increase NR4A1 expression (de Vera et al., 2016), which promotes gluconeogenesis through the AKT–FOXO1–PEPCK/G6Pase pathway (Miao et al., 2019). We observed a notable elevation in L-arginine and 5-phosphoribosylamine levels in the hepatic tissues of goats subjected to inulin supplementation, accompanied by significant alterations in amino acid metabolic pathways. Dietary inulin supplementation has been shown to improve the rumen microbiota of dairy cows and increase the degradation of microbial proteins, which are converted into amino acids to improve amino acid metabolism (Wang et al., 2021). This might explain why amino acid metabolism was enhanced in this study. Arginine, a non-essential amino acid, is converted to glutamine by arginine succinyltransferase. 5-Phosphoribosylamine is produced from glutamine catalyzed by ribose phosphate pyrophosphokinase. Glutamine can enter the TCA cycle to yield oxaloacetic acid as a substrate for gluconeogenesis (Simões et al., 2024). Furthermore, the supplementation of arginine in the rumen enhanced the expression of gluconeogenetic genes (PEPCK and G6Pase) in cows (Souza Simões et al., 2024). The process of gluconeogenesis is the conversion of propionic acid, glycogenic amino acid, lactic acid, glycerol, and other substances into pyruvate as the initial reaction of gluconeogenesis. Pyruvate generates oxaloacetic acid through pyruvate carboxylase. However, since the mitochondria cannot transport oxaloacetic acid, oxaloacetic acid can only be transported into the cytoplasm through the tricarboxylic acid cycle into malic acid (Chandel, 2021). Increased propionic acid and glycoamino acid contents in the inulin group may be an important reason for higher levels of malic acid and citric acid than in the control group. The above results showed that inulin supplementation caused significant changes in goat rumen microbiota, which in turn caused changes in goat liver metabolites. Inulin supplementation improved linoleic acid metabolism, amino acid metabolism, and the TCA cycle and provided sufficient substrates for liver gluconeogenesis, offering strong evidence that inulin supplementation could improve gluconeogenesis in ruminants.
PEPCK and G6Pase are rate-limiting enzymes that regulate gluconeogenesis, and their activity reflects the situation of gluconeogenesis in animals (Agca et al., 2002). G6PDH is the main rate-limiting enzyme in the pentose phosphate pathway. Gluconeogenesis can provide energy for the pentose phosphate pathway (Allmann et al., 2013; Liang et al., 2017). HK, a rate-limiting enzyme for glycolysis, oversees glucose decomposition to produce pyruvate, essentially countering gluconeogenesis (De Jesus et al., 2022). The increase in PEPCK and G6Pase expression and the decrease in HK expression in the inulin group proved that inulin promoted gluconeogenesis while inhibiting glycolysis. In addition, we found that FoxO1 mRNA expression in goat liver increased in the inulin group. FoxO1 is a key gene that regulates gluconeogenesis. Earlier studies have shown that constitutively active FoxO1-expressing transgenic mice manifested upregulated expression of genes linked to gluconeogenesis and downregulated glycolysis-related genes (Zhang et al., 2006). In contrast, FoxO1 gene knockout mice exhibited reduced hepatic gluconeogenesis (Kamagate et al., 2010). Kawasoe et al. (2022) have reported a higher expression level of FoxO1 in the liver of mice fed with a diet containing 5% inulin, which was consistent with our results. The effect of FoxO1 on promoting gluconeogenesis is achieved by increasing the activities of PEPCK and G6Pase (Kamagate et al., 2010). The present study investigated the effects of dietary inulin supplementation on gluconeogenesis-related enzyme activity and gene expression. We speculate that upregulation of gluconeogenesis-related gene enzyme activity may be related to the alteration of insulin secretion and metabolites by inulin in goats. It has been reported that insulin can downregulate the expression of FoxO1 by activating the PI3K/AKT signaling pathway (Zhang et al., 2019). The maternal insulin infusion significantly reduced the expression of FoxO1 and PEPCK/G6Pase in the liver of hypoglycemic fetuses (Thorn et al., 2012). We speculate that the upregulation of gluconeogenesis-related gene enzyme activity may be related to hormone metabolism, TCA cycle, linoleic acid metabolism, and amino acid metabolism regulation. Although the addition of 18.9% inulin in this study showed a positive regulatory effect on rumen microorganisms and liver metabolism in goats, excessive inulin addition may lead to a decrease in the regulatory efficiency of ruminant microbiota and production performance, which may be related to the degradation efficiency of inulin in the rumen (Wang et al., 2021b). Given the current paucity of systematic research on optimal inulin additive levels in ruminant feed, future studies should further establish the dose–response relationship for inulin supplementation. This will help optimize ruminant feed formulations.
5 Conclusions
Inulin supplementation can reduce serum insulin levels and improve gluconeogenesis enzyme activity (PEPCK, G6Pase). It indicates that the addition of inulin to the diet promotes goat gluconeogenesis. In-depth investigation through omics analysis found that the promotion of inulin on goat gluconeogenesis was achieved by increasing gluconeogenetic substrates. Specifically, inulin altered the composition of goat rumen microorganisms and enhanced the gene expression of rumen epithelial transporters. The goat liver metabolome showed that inulin addition increased the levels of TCA intermediates and amino acids. This study confirms the efficacy of inulin as a feed additive for enhancing ruminant glucose metabolism, providing a foundation for future research on optimizing ruminant feed efficiency through inulin supplementation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA1248192.
Ethics statement
The animal studies were approved by the Animal Care Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, China. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
DZ: Data curation, Writing – review & editing, Methodology, Conceptualization, Formal analysis, Validation, Writing – original draft. YH: Validation, Formal analysis, Investigation, Writing – original draft. YL: Methodology, Validation, Investigation, Supervision, Writing – review & editing. CZ: Writing – review & editing, Supervision, Project administration, Resources. ZT: Visualization, Resources, Project administration, Writing – review & editing, Supervision. JK: Conceptualization, Supervision, Data curation, Writing – review & editing, Resources, Formal analysis. ZW: Conceptualization, Investigation, Visualization, Writing – review & editing, Supervision, Formal analysis, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors acknowledge the financial support received from the National Natural Science Foundation of China (Grant Nos. 32372914, 32302783) and the Natural Science Foundation of Guangxi Province (2024GXNSFAA010330).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1614869/full#supplementary-material
Abbreviations
VFAs, volatile fatty acids; PEPCK, phosphoenolpyruvic acid carboxylase; G6Pase, glucose-6-phosphatase; FoxO1, forkhead box protein O1; TVFA, total volatile fatty acid; TAC, tricarboxylic acid cycle; SCFA, short-chain fatty acid; PCoA, principal component analysis; G6PDH, glucose-6-phosphate dehydrogenase; HK, hexokinase; PAT1, putative anion transporter 1; DRA, adenoma; MCT1, monocarboxylate transporter 1; AE2, anion exchanger 2; GPR41, G-protein-coupled receptor 41; AKT, protein kinase B; GSK, glycogen synthase kinase; GLUT2, glucose transporter 2; PI3K, phosphoinositide-3 kinase; INSR, insulin receptor; A/P, Acetate to propionate ratio.
References
Agca C., Greenfield R. B., Hartwell J. R., and Donkin S. S. (2002). Cloning and characterization of bovine cytosolic and mitochondrial PEPCK during transition to lactation. Physiol. Genomics 11, 53–63. doi: 10.1152/physiolgenomics.00108.2001
Allmann S., Morand P., Ebikeme C., Gales L., Biran M., Hubert J., et al. (2013). Cytosolic NADPH homeostasis in glucose-starved procyclic Trypanosoma brucei relies on Malic enzyme and the pentose phosphate pathway fed by gluconeogenic flux. J. Biol. Chem. 288, 18494–18505. doi: 10.1074/jbc.M113.462978
Andrews E. J., Bennett B. T., Clark J. D., Houpt K. A., Pascoe P. J., Robinson G. W., et al. (1993). 1993 report of the AVMA panel on euthanasia. J. Am. Veterinary Med. Assoc. 202, 229–249. doi: 10.2460/javma.1993.202.02.229
Bauer K. C., Littlejohn P. T., Ayala V., Creus-Cuadros A., and Finlay B. (2022). Nonalcoholic fatty liver disease and the Gut-Liver Axis: Exploring an undernutrition perspective. Gastroenterology. 162, 1858–1875.e1852. doi: 10.1053/j.gastro.2022.01.058
Bokulich N. A., Subramanian S., Faith J. J., Gevers D., Gordon J. I., Knight R., et al. (2013). Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 10, 57–59. doi: 10.1038/nmeth.2276
Bougouin A., Ferlay A., Doreau M., and Martin C. (2018). Effects of carbohydrate type or bicarbonate addition to grass silage-based diets on enteric methane emissions and milk fatty acid composition in dairy cows. J. dairy science. 101, 6085–6097. doi: 10.3168/jds.2017-14041
Caton P. W., Nayuni N. K., Kieswich J., Khan N. Q., Yaqoob M. M., and Corder R. (2010). Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J. Endocrinol. 205, 97–106. doi: 10.1677/joe-09-0345
Chambers E. S., Byrne C. S., Morrison D. J., Murphy K. G., Preston T., Tedford C., et al. (2019). Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: a randomised cross-over trial. Gut. 68, 1430–1438. doi: 10.1136/gutjnl-2019-318424
Chandel N. S. (2021). Carbohydrate metabolism. Cold Spring Harb. Perspect. Biol. 17 (6), 13. doi: 10.1101/cshperspect.a040568
Cui X., Wang Z., Guo P., Li F., Chang S., Yan T., et al. (2023). Shift of feeding strategies from grazing to different forage feeds reshapes the rumen microbiota to improve the ability of tibetan sheep (Ovis aries) to adapt to the Cold Season. Microbiol. Spectr. 11, e0281622. doi: 10.1128/spectrum.02816-22
Dashty M. (2013). A quick look at biochemistry: carbohydrate metabolism. Clin. Biochem. 46, 1339–1352. doi: 10.1016/j.clinbiochem.2013.04.027
De Jesus A., Keyhani-Nejad F., Pusec C. M., Goodman L., Geier J. A., Stoolman J. S., et al. (2022). Hexokinase 1 cellular localization regulates the metabolic fate of glucose. Mol. Cell. 82, 1261–1277.e1269. doi: 10.1016/j.molcel.2022.02.028
De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A., et al. (2014). Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 156, 84–96. doi: 10.1016/j.cell.2013.12.016
de Vera I. M., Giri P. K., Munoz-Tello P., Brust R., Fuhrmann J., Matta-Camacho E., et al. (2016). Identification of a binding site for unsaturated fatty acids in the orphan nuclear receptor Nurr1. ACS Chem. Biol. 11, 1795–1799. doi: 10.1021/acschembio.6b00037
Dijkstra J. (1994). Production and absorption of volatile fatty acids in the rumen. Livestock Production Science. 39, 61–69. doi: 10.1016/0301-6226(94)90154-6
Edgar R. C., Haas B. J., Clemente J. C., Quince C., and Knight R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 27, 2194–2200. doi: 10.1093/bioinformatics/btr381
Engels C., Ruscheweyh H. J., Beerenwinkel N., Lacroix C., and Schwab C. (2016). The common gut microbe eubacterium hallii also contributes to intestinal propionate formation. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.00713
Grand E., Respondek F., Martineau C., Detilleux J., and Bertrand G. (2013). Effects of short-chain fructooligosaccharides on growth performance of preruminant veal calves. J. Dairy Sci. 96, 1094–1101. doi: 10.3168/jds.2011-4949
Hammon H. M., Steinhoff-Wagner J., Flor J., Schönhusen U., and Metges C. C. (2013). Lactation Biology Symposium: role of colostrum and colostrum components on glucose metabolism in neonatal calves. J. Anim. Sci. 91, 685–695. doi: 10.2527/jas.2012-5758
Hatting M., Tavares C. D. J., Sharabi K., Rines A. K., and Puigserver P. (2018). Insulin regulation of gluconeogenesis. Ann. N Y Acad. Sci. 1411, 21–35. doi: 10.1111/nyas.13435
Henning S. M., Yang J., Hsu M., Lee R. P., Grojean E. M., Ly A., et al. (2018). Decaffeinated green and black tea polyphenols decrease weight gain and alter microbiome populations and function in diet-induced obese mice. Eur. J. Nutr. 57, 2759–2769. doi: 10.1007/s00394-017-1542-8
Hoover W. H. and Miller T. K. (1991). Rumen digestive physiology and microbial ecology. Vet. Clin. North Am. Food Anim. Pract. 7, 311–325. doi: 10.1016/s0749-0720(15)30801-x
Huang W., Hua Y., Wang F., Xu J., Yuan L., Jing Z., et al. (2024). Dietary betaine and/or TMAO affect hepatic lipid accumulation and glycometabolism of Megalobrama amblycephala exposed to a high-carbohydrate diet. Fish Physiol. Biochem. 50, 59–75. doi: 10.1007/s10695-022-01160-7
Hughes R. L., Alvarado D. A., Swanson K. S., and Holscher H. D. (2022). The prebiotic potential of Inulin-Type fructans: a systematic review. Adv. Nutr. 13, 492–529. doi: 10.1093/advances/nmab119
Husain N., Kumar A., Anbazhagan A. N., Gill R. K., and Dudeja P. K. (2025). Intestinal luminal anion transporters and their interplay with gut microbiome and inflammation. Am. J. Physiol. Cell Physiol. 328, C1455–c1472. doi: 10.1152/ajpcell.00026.2025
Kamagate A., Kim D. H., Zhang T., Slusher S., Gramignoli R., Strom S. C., et al. (2010). FoxO1 links hepatic insulin action to endoplasmic reticulum stress. Endocrinology. 151, 3521–3535. doi: 10.1210/en.2009-1306
Kawasoe J., Uchida Y., Kawamoto H., Miyauchi T., Watanabe T., Saga K., et al. (2022). Propionic acid, induced in gut by an inulin diet, suppresses Inflammation and ameliorates liver ischemia and reperfusion injury in mice. Front. Immunol. 13. doi: 10.3389/fimmu.2022.862503
Kimura I., Ichimura A., Ohue-Kitano R., and Igarashi M. (2020). Free Fatty Acid receptors in health and disease. Physiol. Rev. 100, 171–210. doi: 10.1152/physrev.00041.2018
Larsen M. and Kristensen N. B. (2013). Precursors for liver gluconeogenesis in periparturient dairy cows. Animal. 7, 1640–1650. doi: 10.1017/s1751731113001171
Lawrence J. C. Jr. and Roach P. J. (1997). New insights into the role and mechanism of glycogen synthase activation by insulin. Diabetes. 46, 541–547. doi: 10.2337/diab.46.4.541
Li B., Schroyen M., Leblois J., Beckers Y., Bindelle J., and Everaert N. (2019). The use of inulin and wheat bran only during the starter period or during the entire rearing life of broilers: effects on growth performance, small intestinal maturation, and cecal microbial colonization until slaughter age. Poult Sci. 98, 4058–4065. doi: 10.3382/ps/pez088
Liang H., Habte-Tsion H. M., Ge X., Ren M., Xie J., Miao L., et al. (2017). Dietary arginine affects the insulin signaling pathway, glucose metabolism and lipogenesis in juvenile blunt snout bream Megalobrama amblycephala. Sci. Rep. 7, 7864. doi: 10.1038/s41598-017-06104-3
Liu X., Mao B., Gu J., Wu J., Cui S., Wang G., et al. (2021). Blautia-a new functional genus with potential probiotic properties? Gut Microbes 13, 1–21. doi: 10.1080/19490976.2021.1875796
Louis P., Scott K. P., Duncan S. H., and Flint H. J. (2007). Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 102, 1197–1208. doi: 10.1111/j.1365-2672.2007.03322.x
Miao M., Dai Y., Rui C., Fan Y., Wang X., Fan C., et al. (2021). Dietary supplementation of inulin alleviates metabolism disorders in gestational diabetes mellitus mice via RENT/AKT/IRS/GLUT4 pathway. Diabetol. Metab. Syndr. 13, 150. doi: 10.1186/s13098-021-00768-8
Miao L., Yang Y., Liu Y., Lai L., Wang L., Zhan Y., et al. (2019). Glycerol kinase interacts with nuclear receptor NR4A1 and regulates glucose metabolism in the liver. FASEB J. 33, 6736–6747. doi: 10.1096/fj.201800945RR
Na S. W. and Guan L. L. (2022). Understanding the role of rumen epithelial host-microbe interactions in cattle feed efficiency. Anim. Nutr. 10, 41–53. doi: 10.1016/j.aninu.2022.04.002
Oliver W. M. (1975). Effect of monensin on gains of steers grazed on coastal Bermudagrass. J. Anim. Science. 41, 999–1001. doi: 10.2527/jas1975.414999x
Qiu J., Chen L., Zhang L., Xu F., Zhang C., Ren G., et al. (2023). Xie Zhuo Tiao Zhi formula modulates intestinal microbiota and liver purine metabolism to suppress hepatic steatosis and pyroptosis in NAFLD therapy. Phytomedicine. 121, 155111. doi: 10.1016/j.phymed.2023.155111
Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/aem.01541-09
Schlumbohm C. and Harmeyer J. (2004). Hyperketonemia impairs glucose metabolism in pregnant and nonpregnant ewes. J. Dairy Sci. 87, 350–358. doi: 10.3168/jds.S0022-0302(04)73174-4
Sivaprakasam S., Bhutia Y. D., Yang S., and Ganapathy V. (2011). Short-chain fatty acid transporters: role in colonic homeostasis. Compr. Physiol. 8, 299–314. doi: 10.1002/cphy.c170014
Souza Simões B., Nehme Marinho M., Lobo R. R., Adeoti T. M., Perdomo M. C., Sekito L., et al. (2024). Effects of supplementing rumen-protected arginine on performance of transition cows. J. Dairy Sci. 107, 10945–10963. doi: 10.3168/jds.2024-25562
Sun Q., Zhu L., Li Y., Cui Y., Jiang S., Tao N., et al. (2020). A novel inulin-type fructan from Asparagus cochinchinensis and its beneficial impact on human intestinal microbiota. Carbohydr Polym. 247, 116761. doi: 10.1016/j.carbpol.2020.116761
Tawfick M. M., Xie H., Zhao C., Shao P., and Farag M. A. (2022). Inulin fructans in diet: Role in gut homeostasis, immunity, health outcomes and potential therapeutics. Int. J. Biol. Macromol. 208, 948–961. doi: 10.1016/j.ijbiomac.2022.03.218
Thorn S. R., Sekar S. M., Lavezzi J. R., O’Meara M. C., Brown L. D., Hay W. W. Jr., et al. (2012). A physiological increase in insulin suppresses gluconeogenic gene activation in fetal sheep with sustained hypoglycemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R861–R869. doi: 10.1152/ajpregu.00331.2012
Tian K., Liu J., Sun Y., Wu Y., Chen J., Zhang R., et al. (2019). Effects of dietary supplementation of inulin on rumen fermentation and bacterial microbiota, inflammatory response and growth performance in finishing beef steers fed high or low-concentrate diet. Anim. Feed Sci. Technology. 258, 114299. doi: 10.1016/j.anifeedsci.2019.114299
Van Soest P. J., Robertson J. B., and Lewis B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Wanapat M., Cherdthong A., Phesatcha K., and Kang S. (2015). Dietary sources and their effects on animal production and environmental sustainability. Anim. Nutr. 1, 96–103. doi: 10.1016/j.aninu.2015.07.004
Wang H., An J., Jin H., He S., Liao C., Wang J., et al. (2021a). Roles of Cl(−)/HCO(3)(−) anion exchanger 2 in the physiology and pathophysiology of the digestive system (Review). Mol. Med. Rep. 24. doi: 10.3892/mmr.2021.12130
Wang D., Chen L., Tang G., Yu J., Chen J., Li Z., et al. (2023). Multi-omics revealed the long-term effect of ruminal keystone bacteria and the microbial metabolome on lactation performance in adult dairy goats. Microbiome 11, 215. doi: 10.1186/s40168-023-01652-5
Wang Y., Han X., Tan Z., Kang J., and Wang Z. (2020a). Rumen-protected glucose stimulates the insulin-like growth factor system and mTOR/AKT pathway in the endometrium of early postpartum dairy cows. Anim. (Basel). 10(2), 357. doi: 10.3390/ani10020357
Wang Y., Nan X., Zhao Y., Jiang L., Wang H., Zhang F., et al. (2021b). Dietary supplementation of inulin ameliorates subclinical mastitis via regulation of rumen microbial community and metabolites in dairy cows. Microbiol. Spectr. 9, e00105-21. doi: 10.1128/Spectrum.00105-21
Wang Y., Nan X., Zhao Y., Jiang L., Wang H., Zhang F., et al. (2022). Changes in the profile of fecal microbiota and metabolites as well as serum metabolites and proteome sfter dietary inulin supplementation in dairy cows with subclinical mastitis. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.809139
Wang Y., Wang Q., Dai C., Li J., Huang P., Li Y., et al. (2020b). Effects of dietary energy on growth performance, carcass characteristics, serum biochemical index, and meat quality of female Hu lambs. Anim. Nutr. 6, 499–506. doi: 10.1016/j.aninu.2020.05.008
Want E. J., Masson P., Michopoulos F., Wilson I. D., Theodoridis G., Plumb R. S., et al. (2013). Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 8, 17–32. doi: 10.1038/nprot.2012.135
Wu Q., Chen H., Zhang F., Wang W., Xiong F., Liu Y., et al. (2022). Cysteamine supplementation in vitro remarkably promoted rumen fermentation efficiency towards propionate production via prevotella enrichment and enhancing antioxidant capacity. Antioxidants (Basel). 11(11), 2233. doi: 10.3390/antiox11112233
Wu J., Yang D., Gong H., Qi Y., Sun H., Liu Y., et al. (2020). Multiple omics analysis reveals that high fiber diets promote gluconeogenesis and inhibit glycolysis in muscle. BMC Genomics 21, 660. doi: 10.1186/s12864-020-07048-1
Yang W., Sha Y., Chen X., Liu X., Wang F., Wang J., et al. (2024). Effects of the interaction between rumen microbiota density-VFAs-hepatic gluconeogenesis on the adaptability of tibetan sheep to plateau. Int. J. Mol. Sci. 25(12), 6726. doi: 10.3390/ijms25126726
Yu Q. (2021). Slc26a3 (DRA) in the gut: expression, function, regulation, role in infectious diarrhea and inflammatory bowel disease. Inflammation Bowel Dis. 27, 575–584. doi: 10.1093/ibd/izaa256
Yuan C., Wang S., Gebeyew K., Yang X., Tang S., Zhou C., et al. (2022). A low-carbon high inulin diet improves intestinal mucosal barrier function and immunity against infectious diseases in goats. Front. Vet. Sci. 9. doi: 10.3389/fvets.2022.1098651
Zelena E., Dunn W. B., Broadhurst D., Francis-McIntyre S., Carroll K. M., Begley P., et al. (2009). Development of a robust and repeatable UPLC-MS method for the long-term metabolomic study of human serum. Anal. Chem. 81, 1357–1364. doi: 10.1021/ac8019366
Zhang Q., Koser S. L., Bequette B. J., and Donkin S. S. (2015). Effect of propionate on mRNA expression of key genes for gluconeogenesis in liver of dairy cattle. J. Dairy Sci. 98, 8698–8709. doi: 10.3168/jds.2015-9590
Zhang X., Medrano R. F., Wang M., Beauchemin K. A., Ma Z., Wang R., et al. (2019). Effects of urea plus nitrate pretreated rice straw and corn oil supplementation on fiber digestibility, nitrogen balance, rumen fermentation, microbiota and methane emissions in goats. J. Anim. Sci. Biotechnol. 10, 6. doi: 10.1186/s40104-019-0312-2
Zhang W., Patil S., Chauhan B., Guo S., Powell D. R., Le J., et al. (2006). FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J. Biol. Chem. 281, 10105–10117. doi: 10.1074/jbc.M600272200
Zhang Q., Xiao X., Zheng J., Li M., Yu M., Ping F., et al. (2021). Improvement in glucose metabolism in adult male offspring of maternal mice fed diets supplemented with inulin via regulation of the hepatic long noncoding RNA profile. FASEB J. 35, e22003. doi: 10.1096/fj.202100355RRR
Zhang Q., Yu H., Xiao X., Hu L., Xin F., and Yu X. (2018). Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats. PeerJ. 6, e4446. doi: 10.7717/peerj.4446
Zhang M. and Zhang X. (2019). The role of PI3K/AKT/FOXO signaling in psoriasis. Arch. Dermatol. Res. 311, 83–91. doi: 10.1007/s00403-018-1879-8
Zhao X. H., Gong J. M., Zhou S., Liu C. J., and Qu M. R. (2014). The effect of starch, inulin, and degradable protein on ruminal fermentation and microbial growth in rumen simulation technique. Ital. J. Anim. Science. 13, 3121. doi: 10.4081/ijas.2014.3121
Keywords: inulin, gluconeogenesis, ruminal microorganism, volatile fatty acid, metabolite
Citation: Zhu D, Hu Y, Liu Y, Zhou C, Tan Z, Kang J and Wang Z (2025) Multi-omics revealed the promoting effect of dietary inulin supplementation on hepatic gluconeogenesis in goats. Front. Anim. Sci. 6:1614869. doi: 10.3389/fanim.2025.1614869
Received: 20 April 2025; Accepted: 10 June 2025;
Published: 14 July 2025.
Edited by:
Bianca Castiglioni, National Research Council (CNR), ItalyReviewed by:
Rangsun Charoensook, Naresuan University, ThailandTiecheng Wu, Gansu Agricultural University, China
Copyright © 2025 Zhu, Hu, Liu, Zhou, Tan, Kang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhe Kang, a2FuZ2poQGlzYS5hYy5jbg==; Zheng Wang, emhlbmd3QGh1bmF1LmVkdS5jbg==
 Dan Zhu
Dan Zhu Yun Hu1,2
Yun Hu1,2 Yong Liu
Yong Liu Chuanshe Zhou
Chuanshe Zhou Zhiliang Tan
Zhiliang Tan Jinhe Kang
Jinhe Kang Zheng Wang
Zheng Wang