Abstract
The objective of this study was to estimate the net requirements for gain (NCug, NFeg; NMng; NZng, NCog, and NCrg) and maintenance (NCum, NFem; NMnm, NZnm, NCom; and NCrm) in males and female hair sheep. Data from six studies, comprising 248 individual records (139 non-castrated males, 75 castrated males, and 34 females), were used to estimate net trace mineral requirements for weight gain. For maintenance requirements, 52 observations (26 intact and 26 castrated males) were analyzed. A meta-analytical approach was employed, incorporating non-linear mixed-effects models with the study treated as a random effect. Model selection was guided by the corrected Akaike information criterion (AICc) and some AICc-derived functions (Akaike difference, model probability, evidence ratio, and number of parameters of the fitted combination), which indicated that heterogeneous variance functions provided a better fit, with minimal model selection uncertainty. The final models selected effectively accounted for variability associated with sex and exhibited high confidence levels (model weights w ≥ 0.9), supporting their adequacy in describing trace mineral requirements. Sex did not affect the intercept and slope of the linear equations, and the net mineral requirements for maintenance were 34.85, 39.63, 6.11, 194, 6.06, and 11.67μg/kg of BW for Cu, Fe, Mn, Zn, Co, and Cr, respectively. The Fe requirements for gain decreased with increasing BW from 10 to 30 kg and average daily gain (ADG) of 150 g/day, ranging from 11.54 – 8.93; 10.58- 6.81; 7.16-2.91 mg for non-castrated males, castrated males, and females, respectively. The estimated dietary requirements for males with a BW of 30 kg and an average daily gain of 150 g were 69.6 mg/day for Fe;76.06 mg/day for Mn, and 2.62 mg/day for Co. Nutritional requirements for growth differ among castrated male, non-castrated male and female sheep for Fe, Mn, Zn and Cr. Thus, this study represents a significant advancement in the recommendation of nutritional requirements for trace elements in sheep breeds raised in tropical conditions.
1 Introduction
Mineral requirements have been studied, given that their precise estimation is essential for optimizing animal nutrition, reducing mineral excretion, and mitigating environmental contamination. The bioavailability of minerals can be modulated by complex interactions within the organism (Lee et al., 2002), which may either enhance or inhibit their absorption and utilization (Underwood and Suttle, 1999). Such interactions can compromise the fulfillment of dietary requirements and contribute to the onset of metabolic disturbances (NRC, 1985).
Microminerals or trace elements are distributed throughout the animal body in small quantities, corresponding to less than 0.3% of the total minerals deposited in the body, but are of great importance for maintaining normal cellular metabolism (Suttle, 2022). Among trace minerals Copper, Mn and Zn are components of enzymes and contribute to increased resistance to infection, play essential physiological roles in ruminants. Zinc is involved in numerous biological processes, including DNA synthesis, gene expression, and cell proliferation. It also plays a pivotal role in bone formation through its structural integration into the enzyme alkaline phosphatase, which is crucial for bone mineralization (Hosnedlová et al., 2007). Iron, in turn, is a fundamental component of the heme group within the protoporphyrin ring of hemoglobin, facilitating the transport of oxygen from the lungs to peripheral tissues as oxyhemoglobin, and the return of carbon dioxide as carboxyhemoglobin via the venous circulation. Furthermore, Fe acts as a cofactor for various enzymes associated with the tricarboxylic acid (Krebs) cycle and participates in oxidative metabolism through its role in catalases and peroxidases (Suttle, 2022). Mn is a required component of manganese superoxide dismutase (MnSOD) for reducing mitochondrial oxidative stress (Li and Yang, 2018). The Iron and manganese minerals prevent the deleterious action of free radicals, being classified as preventive antioxidants.
Deficiencies in these trace minerals may adversely affect growth performance and maintenance of normal cellular metabolism (Zhang et al., 2018), attenuate immune function, and heighten the animal’s vulnerability to infectious and metabolic diseases. In this context, the functional significance of trace minerals in maintaining and promoting animal health has received growing scientific attention (Mortazavi et al., 2025).
Historically, equations to estimate mineral requirements for sheep do not consider the effect of sex (NRC, 2007). However, studies have shown differences between males and females (Vargas et al., 2017; Herbster et al., 2023). These differences may lead to variations in mineral concentrations in tissues and blood between the sexes (AFRC, 1998; Meschy, 2000; NRC, 2007). Knowing the amount of minerals consumed by the animal as well as the amount retained in its body is necessary to determine the need for trace elements for the maintenance and growth of the animals. Adequate mineral nutrition is essential to optimize animal performance because trace minerals play roles in animals such as structural function, constituents of body fluids, acid-base balance, control of osmotic pressure, enzyme cofactors, and the formation of metalloenzymes (McDowell, 2003; Wilson et al., 2016; Suttle, 2022). Additionally, most studies have not considered the effect of sex classes on trace element requirements, which is an imminent need.
Furthermore, when estimating dietary mineral requirements based on each mineral’s absorption coefficient, it assumes minimal urinary mineral excretion. However, not every mineral is absorbed and retained, and has important functions in the body (BR-Corte, 2016). Therefore, determining mineral maintenance requirements by assessing the balance between mineral intake and retention in the animal’s body is more accurate than using the absorption coefficient, as the retention coefficient accurately reflects the proportion of ingested minerals that are effectively retained, providing a more accurate assessment of mineral utilization in sheep.
We hypothesized that the traces elements requirements of hair sheep differ between sex classes. Thus, this study aimed to estimate the net requirements for gain (NCug, NFeg; NMng; NZng, NCog, and NCrg) and maintenance (NCum, NFem; NMnm, NZnm, NCom; and NCrm) in males and female hair sheep.
2 Materials and methods
2.1 Study and data set inclusion criteria
A comprehensive dataset was compiled from six comparative slaughter studies (Cabral et al., 2008; Silva et al., 2016; Pereira et al., 2016, 2017, 2018a, b), incorporating reference data, qualifying variables (e.g., sex, intake level), and essential quantitative measurements. The dataset included detailed information on days on feed, initial and final body weight (BW), empty body weight (EBW), empty body weight gain (EBWG), daily mineral intake, and mineral body composition of Cu, Fe, Mn, Zn, and Co for each animal. Mineral body composition was determined based on the chemical analysis of all body tissues. Additionally, data on daily mineral intake and individual animal records were available, allowing for precise evaluation of mineral retention and metabolism. Description of the studies of database used to estimate net traces element requirements for maintenance and weight gain of hair sheep is shown in Table 1. The dataset was composed of 248 individual observations of hair sheep with three sexes (139 non-castrated, 75 castrated males, and 34 females non-pregnant and non-lactating).
Table 1
| Study | n | Genotype | Sex Class | Age (days) | Trial (days) | BW (kg) | EBW (kg) |
|---|---|---|---|---|---|---|---|
| Cabral et al. (2008) | 24 | Santa Ines | C | 105 | 75 | 17.90 – 24.40 | 13.13 – 22.65 |
| Pereira et al. (2016) | 40 | Morada Nova | NC | 60 | 175 | 10.12 – 31.62 | 6.58 – 27.10 |
| Silva et al. (2016) | 19 | Crossbred | NC | 150 | 58 | 10.64 – 33.60 | 9.77 – 30.10 |
| 17 | C | 7.97 – 31.70 | 7.68 – 29.44 | ||||
| 18 | F | 7.98 – 29.40 | 7.68 – 26.28 | ||||
| Pereira et al. (2017) 1 | 19 | Santa Ines | NC | 60 | 120 | 11.20 – 33.20 | 8.30 – 24.57 |
| 18 | C | 12.60 – 31.56 | 8.50 – 29.30 | ||||
| Pereira et al. (2018a) | 47 | Brazilian Somali | NC | 60 | 105 | 10.90 – 35.74 | 7.95 – 31.18 |
| Pereira et al. (2018b) 1 | 14 | Morada Nova | NC | 120 | 120 | 13.66 – 38.28 | 9.62 – 29.38 |
| 16 | C | 12.54 – 31.25 | 8.66 – 23.08 | ||||
| 16 | F | 12.74 – 26.95 | 8.89 – 21.71 |
Description of the studies of database used to estimate net traces element requirements for maintenance and weight gain of hair sheep.
BW, body weight; EBW, empty body weight; n, number of information.
1Studies used to estimate net maintenance requirements.
The trace elements collected in the data set used to estimate growth requirements varied within each study. For Zn and Fe, data were collected from 224 animals (139 non-castrated males, 51 castrated males, and 34 females) from four studies (Silva et al., 2016; Pereira et al., 2016, 2018a, b). The data for Cu were obtained from 224 animals (139 non-castrated males, 51 castrated males, and 34 females), also from four studies. For Mn, data were gathered from 177 animals (112 non-castrated males, 41 castrated males, and 24 females). Finally, the data for Co and chromium Cr were obtained from 83 animals (33 non-castrated males, 34 castrated males, and 16 females from two studies (Pereira et al., 2017, 2018b).
After confirming that the selected studies met the aforementioned inclusion criteria, a graphical analysis was conducted following the methodology described by St-Pierre (2007). This analysis confirmed the consistency among studies and indicated the absence of outliers.
For maintenance requirements, the data set was formed by two individual studies Pereira et al., 2017, 2018b) containing 52 individual observations of hair sheep with two sex classes (26 non-castrated males and 26 castrated males). The maintenance requirements were estimated based on the mass of trace elements (mg) daily retained in EBW and the intake (mg/day) of each trace element.
The descriptive analyses of the variables used to generate models for growth and maintenance are provided in Tables 2 and 3, respectively. The variables contained in the maintenance and growth data set were body weight (BW), empty body weight (EBW), and mass of each mineral in the EBW. More detailed information about BW and EBW can be seen in Herbster et al. (2023).
Table 2
| Variables | n | Mean | SD | Maximum | Minimum |
|---|---|---|---|---|---|
| Non-castrated male | |||||
| BW (kg) | 139 | 21.56 | 6.54 | 38.28 | 10.12 |
| EBW (kg) | 139 | 17.23 | 5.93 | 31.18 | 6.58 |
| Retention (mg) | |||||
| Cu | 139 | 58.57 | 66.29 | 309.82 | 1.52 |
| Fe | 139 | 1278.85 | 921.33 | 6829.34 | 143.60 |
| Mn | 139 | 24.84 | 24.18 | 153.25 | 0.00 |
| Zn | 139 | 617.05 | 921.33 | 5612.29 | 169.30 |
| Co | 33 | 23.84 | 13.65 | 60.53 | 6.75 |
| Cr | 33 | 31.83 | 18.54 | 82.18 | 18.54 |
| Castrated male | |||||
| BW (kg) | 75 | 21.50 | 5.76 | 31.70 | 7.97 |
| EBW (kg) | 75 | 16.81 | 4.87 | 29.44 | 7.68 |
| Retention (mg) | |||||
| Cu | 51 | 103.56 | 90.31 | 407.57 | 0.90 |
| Fe | 51 | 1738.81 | 904.56 | 3918.77 | 215.07 |
| Mn | 41 | 22.36 | 15.88 | 93.52 | 1.98 |
| Zn | 51 | 993.18 | 904.56 | 3918.77 | 215.07 |
| Co | 34 | 24.65 | 15.41 | 71.14 | 5.88 |
| Cr | 34 | 30.42 | 23.51 | 106.66 | 5.05 |
| Female | |||||
| BW (kg) | 34 | 18.91 | 5.36 | 29.40 | 7.98 |
| EBW (kg) | 34 | 16.03 | 4.99 | 26.28 | 7.68 |
| Retention (mg) | |||||
| Cu | 34 | 128.39 | 83.32 | 328.77 | 30.25 |
| Fe | 34 | 1530.35 | 854.65 | 3216.52 | 96.83 |
| Mn | 34 | 15.74 | 84.08 | 328.77 | 0.00 |
| Zn | 34 | 1087.53 | 854.65 | 3216.52 | 96.83 |
| Co | 34 | 19.83 | 13.46 | 49.66 | 5.67 |
| Cr | 34 | 18.64 | 4.86 | 26.51 | 9.58 |
Descriptive statistics of the data used to estimate net requirements of microminerals for growing in hair sheep.
BW, body weight; EBW, empty body weight; n, number of information; SD, standard deviation.
Table 3
| Variables | n | Mean | SD | Maximum | Minimum |
|---|---|---|---|---|---|
| BW (kg) | 52 | 19.33 | 2.90 | 38.28 | 11.20 |
| EBW (kg) | 52 | 14.24 | 2.42 | 29.38 | 8.30 |
| Intake (µg/day) | |||||
| Cu | 52 | 312.25 | 68.16 | 437.33 | 205.61 |
| Fe | 52 | 3519.4 | 1298.40 | 6489.90 | 1821.80 |
| Mn | 52 | 1755.3 | 538.20 | 2750.90 | 913.70 |
| Zn | 52 | 1303 | 311.70 | 1926.90 | 797.20 |
| Co | 52 | 79.40 | 24.70 | 139.50 | 33.10 |
| Cr | 52 | 28.46 | 17.03 | 59.80 | 3.66 |
| Retention (µg/day) | |||||
| Cu | 52 | 35.76 | 28.90 | 94.26 | -29.34 |
| Fe | 52 | 418.60 | 385.3 | 1734.30 | -262.20 |
| Mn | 52 | 6.70 | 6.90 | 32.70 | -2.23 |
| Zn | 52 | 178.40 | 105.2 | 416.20 | 1.41 |
| Co | 52 | 4.80 | 6.10 | 19.50 | -5.10 |
| Cr | 52 | 10.28 | 8.67 | 36.58 | -1.23 |
Descriptive statistics of the data used to estimate net requirements of microminerals (Cu, Fe, Mn, Zn, Co, and Cr) for maintenance of hair sheep.
BW, body weight; EBW, empty body weight; n, number of information; SD, standard deviation.
2.2 Slaughter procedures
In all studies, similar slaughter procedures were adopted. Before slaughter, the animals were fasted from solids and liquids (water) for 18 hours and then weighed to obtain fasting body weight (FBW). Subsequently, the slaughter was carried out by brain concussion followed by a jugular vein incision until the animals were completely bled out. After that, the animals were skinned and eviscerated. The blood was weighed and sampled.
The gastrointestinal tract (rumen, reticulum, omasum, abomasum, and the small and large intestines) was separated and weighed full. Subsequently, it was emptied, washed, and after draining, weighed empty. The internal organs (liver, kidneys, heart, lungs with trachea, tongue with esophagus, reproductive tract, and spleen), other body parts (carcass, head, skin, blood, and feet), and fats (omental, perirenal, mesenteric, and heart fats) were also weighed. The empty body weight (EBW) was considered as the FBW minus the contents of the gastrointestinal tract, bladder, and gallbladder.
The carcasses were refrigerated at 4 °C for 24 hours and then divided into right and left half-carcasses. Subsequently, the right half-carcasses, non-carcass components (blood, head, paws, internal organs, and the cleaned gastrointestinal tract), and skins were frozen at -20 °C. Then, they are cut separately with the help of a band saw, ground in an industrial cutter, and homogenized. After homogenization, a portion of approximately 500g of each sample was collected and stored in a freezer at - 20 °C for later analysis.
2.3 Chemical analyses
To determine body mineral composition, 500 grams of samples were taken from ground samples of right half-carcasses, non-carcass parts, and hides. The samples were initially pre-dried at 55 °C until reaching a constant weight, and then the dry matter (DM) content was determined (AOAC, 1990; method 967.03). For the studies by Cabral et al. (2008) and Silva et al. (2016), the samples were defatted by successive washings with petroleum ether according to the methodology recommended by Kock and Preston (1979). For other studies (Pereira et al., 2016, 2017, 2018a, b) the samples were defatted through extraction in a Soxhlet apparatus (AOAC, 1990; method 920.39). Subsequently, the fat-free body component samples were ground using a ball mill and analyzed for dry matter (AOAC, 1990; method 967.03).
For the studies by Cabral et al. (2008); Silva et al. (2016), and Pereira et al. (2016) samples of roughage, concentrate, orts, and body components of each study were analyzed for mineral composition through digestion in nitric acid and perchloric acid according to the methodology described by Silva and Queiroz (2002). In the studies by Pereira et al. (2016); Pereira et al. (2017); Pereira et al. (2018a), and Pereira et al. (2018b), the samples of roughage, concentrate, orts, and body components were analyzed for mineral composition through digestion in nitroperchloric acid (Detmann et al., 2012; method INCT-CA M-004/3). Mineral concentrations of Cu, Fe, Mn, Zn, Co, and Cr were determined using inductively coupled plasma-atomic emission spectroscopy with ultrasonic nebulization (Braselton et al., 1997).
2.4 Models for predicting growth and maintenance requirements
The models for predicting the maintenance and retention of trace minerals used, as well as the procedures for fitting and selection of models, are the same as those described in the article by Herbster et al. (2023), a complementary paper on macromineral requirements. Therefore, the models used for maintenance were the linear model (Equation 1) and for trace mineral gain, the allometric models (Equations 2, 3).
The term denotes the retention of the Y-th trace element. The parameters β0 and β1 in represent, respectively, the intercept and the slope of the linear model used for predicting the maintenance requirement of the Y-th trace mineral. The maintenance requirement of the Y-th trace element was estimated when . The retention coefficient at maintenance is given by β1. The intake rate of the Y-th trace mineral (; mg/day) at the maintenance level was estimated as follows: .
The term represents the expected mean retention of the Y-th trace element (, in milligrams (mg), as a function of empty body weight (EBW, kg), denoted by the letter X. The subscript “ “ in Equation 3 denotes the i-th category (i = 1, 2, and 3), which corresponds to the prediction of the requirement of the Y-th trace element for non-castrated, castrated males, and females, respectively. The parameters (Equation 2) and αi (Equation 3) are proportionality parameters that fit the magnitude of the requirement for the Y-th trace mineral for a given value of EBW. The parameters and βi are allometric exponents that describe the requirement for the growth of the Y-th trace mineral scales to EBW.
Combined with these models (Equations 1-3), different variance functions were tested, including homogeneous variance (), assuming homoscedasticity, exponential variance (), and power of the means variance (. Details on how these variance functions were tested can be found in the study by Herbster et al. (2023).
The normality and dispersion of residuals were evaluated, and records with studentized residuals exceeding 2.5 and/or Cook’s distance greater than 1 were identified as influential points (Cook, 1979; Tedeschi, 2006).
The nonlinear mixed models (Equations 1-3) were analyzed using the PROC NLMIXED procedure in SAS (University Edition) with the Newton-Raphson algorithm (tech=NEWRAP) to estimate the maximum likelihood function. Data normality was assumed, i.e., Y~ Normal(), ex ~ Normal(). The selection of the best candidate combination (model and variance function) for predicting each of the trace minerals was carried out using the corrected Akaike information criterion (AICc) (Sugiura, 1978) and some AICc-derived functions for candidate combination (Equations 1-3) and variance function combinations) among those analyzed (Burnham and Anderson, 2002).
The retention of trace minerals per unit of daily weight gain was obtained through the first derivative of Equation 2 or Equation 3 (Vieira et al., 2018), according to the model selected for each trace mineral, as presented in Equation 4 and Equation 5, respectively.
The terms (Equation 4) and (Equation 5) represent the retention in mg of the Y-th () trace mineral per g of EBW. The subscript ‘ ‘ denotes the i-th () category (Equation 5). The predictor variable EBW is represented by the letter , and the parameters , , and , , are the coefficients of the allometric model presented in Equation 2 and Equation 3, respectively.
The linear equations suggested by Herbster et al. (2020) were used to estimate the empty body weight (EBW) and the gain in empty body weight (EBWG), corresponding to body weight (BW) and average daily gain (ADG), respectively. Accordingly, trace element retention predictions were carried out for ADGs of 100, 150, and 200g for each BW of 10, 20, and 30kg. The mean predictions were accompanied by ½ amplitude of the 95% confidence intervals (95% CI; Equation 6). The predicted mean values with their 95% CI were obtained using EBW and EBWG values equivalent to the respective BW and ADG values previously estimated.
95% CI denotes the 95% confidence interval. The term MEAN represents the predicted average value of trace mineral retention (mg) according to Equations 4 and 5 for a given ADG (g) and BW. The confidence interval was estimated using the t-test, with a significance level () of 0.05 and the degrees of freedom () multiplied by the standard error (SE). The degrees of freedom were calculated by subtracting the number of parameters () in the allometric model (Equation 2 or Equation 3), the variance function, and the random parameters from the total number of observations () ().
The dietary requirements for trace elements were calculated as a sum of the net requirements for maintenance and gain divided by the retention coefficient.
3 Results
Following the selection of the optimal combinations between the allometric models and variance functions for each trace element, the corresponding model parameters and variance structures were used to estimate growth mineral requirements, as presented in Table 4. As no significant effect of sex was observed, generalized prediction equations for net concentrations of mineral use for gain (NCug and NCog) were developed.
Table 4
| Micromineral | Models | Variance functions 6 | Random | AICc | Δ | W | ER | Θ |
|---|---|---|---|---|---|---|---|---|
| Cu | Equation 2 | Power of the mean1 | a; b | 2309.7 | 0.0 | 0.60 | 1.00 | 6 |
| Equation 3 | Power of the mean1 | ai; bi | 2311.6 | 1.9 | 0.23 | 2.59 | 14 | |
| Equation 3 | Power of the mean2 | ai; bi | 2313.3 | 3.6 | 0.10 | 6.05 | 16 | |
| Fe | Equation 3 | Power of the mean1 | ai; bi | 3469.0 | 0.0 | 0.68 | 1.00 | 14 |
| Equation 3 | Power of the mean4 | ai; bi | 3471.4 | 2.4 | 0.21 | 3.32 | 18 | |
| Equation 3 | Power of the mean3 | ai; bi | 3473.1 | 4.1 | 0.09 | 7.77 | 16 | |
| Mn | Equation 3 | Power of the mean2 | ai; bi | 1395.4 | 0.0 | 0.93 | 1.00 | 18 |
| Zn | Equation 3 | Power of the mean2 | ai; bi | 3027.4 | 0.0 | 0.59 | 1.00 | 16 |
| Equation 3 | Power of the mean3 | ai; bi | 3028.4 | 1.0 | 0.36 | 1.65 | 16 | |
| Co | Equation 2 | Power of the mean1 | a; b | 577.7 | 0.0 | 0.52 | 1.00 | 4 |
| Equation 2 | Exponential5 | – | 579.6 | 1.9 | 0.20 | 2.59 | 6 | |
| Equation 2 | Exponential5 | – | 580.6 | 2.9 | 0.12 | 4.26 | 4 | |
| Equation 3 | Power of the mean3 | ai; bi | 582.9 | 5.2 | 0.04 | 13.5 | 16 | |
| Equation 3 | Power of the mean3 | ai; bi | 583.1 | 5.4 | 0.03 | 14.9 | 16 | |
| Cr | Equation 3 | Power of the mean1 | ai; bi | 615.2 | 0.0 | 0.69 | 1.00 | 14 |
| Equation 3 | Power of the mean3 | ai; bi | 619.0 | 3.8 | 0.10 | 6.69 | 16 | |
| Equation 3 | Power of the mean2 | ai; bi | 619.4 | 4.2 | 0.08 | 8.17 | 16 | |
| Equation 3 | Power of the mean4 | ai; bi | 619.7 | 4.5 | 0.07 | 9.49 | 18 |
Goodness-of-fit measures used in selecting the best fit among all combinations of allometric model, variance function, and random effects fitted to micromineral growth requirement for hair sheep.
AICc, Akaike’s information criterion corrected for small sample; Δ, Akaike difference; w, model probability; ER, evidence ratio; Θ, number of parameters of the fitted combination.
1 power1: .
2 power2: .
3 power3: .
4 power4: .
5 Exp: .
6 Details of the variance functions are in the text and in Herbster et al. (2023).
The combination of the linear model with exponential variance was the best choice for Mn, Zn, Co, and Cu. However, this combination was unanimous (w > 0.90) only for Mn and Cr. For Fe and Cu, the allometric model with homogeneous variance was the best choice among the candidate models (Table 5).
Table 5
| Microminerals | Variance Function1 | AICc | Δ | w | ER | Θ |
|---|---|---|---|---|---|---|
| Cu | Homogeneous | 485.5 | 0.00 | 0.58 | 1.00 | 3 |
| Exponential | 487.6 | 2.10 | 0.20 | 2.86 | 4 | |
| Power of the mean | 487.4 | 1.90 | 0.22 | 2.59 | 4 | |
| Fe | Homogeneous | 761.2 | 0.00 | 0.49 | 1.00 | 3 |
| Exponential | 762.0 | 0.80 | 0.33 | 1.49 | 4 | |
| Power of the mean | 763.1 | 1.90 | 0.19 | 2.59 | 4 | |
| Mn | Homogeneous | 320.4 | 28.10 | 0.00 | 1.26×106 | 3 |
| Exponential | 292.3 | 0.00 | 0.98 | 1.00 | 4 | |
| Power of the mean | 300.5 | 8.20 | 0.02 | 60.34 | 4 | |
| Zn | Homogeneous | 586.1 | 3.60 | 0.09 | 6.05 | 3 |
| Exponential | 582.5 | 0.00 | 0.53 | 1.00 | 4 | |
| Power of the mean | 583.2 | 0.70 | 0.38 | 1.42 | 4 | |
| Co | Homogeneous | 318.0 | 1.30 | 0.34 | 1.92 | 3 |
| Exponential | 316.7 | 0.00 | 0.66 | 1.00 | 4 | |
| Power of the mean | – | – | – | – | 4 | |
| Cr | Homogeneous | 373.0 | 24.40 | 0.00 | 1.9×106 | 3 |
| Exponential | 348.6 | 0.00 | 1.00 | 1.00 | 4 | |
| Power of the mean | – | – | – | – | 4 |
Goodness-of-fit measures for selecting the best combination between the linear model and the variance functions fitted to predict the micromineral maintenance requirement of hair sheep.
AICc, Akaike’s Information Criterion corrected for small sample; Δ, Akaike difference; w, model probability; ER, evidence ratio; Θ, number of parameters of the fitted combination.
1Description of these variance functions are in the text and in Herbster et al. (2023).
The models for estimating the net requirement for weight gain are presented in Table 6. The net requirements for gain in hair sheep, along with their respective confidence intervals, are summarized in Table 7. Irrespective of sex, the Fe requirements for gain decreased with increasing BW from 10 to 30kg and average daily gain (ADG) of 150 g/day, ranging from 11.54–8.93; 10.58-6.81; 7.16-2.91 mg of Fe for non-castrated males, castrated males, and females, respectively. During growth, non-castrated males showed greater requirements for Mn than castrated males, and females showed greater Mn requirements than castrated males, irrespective of gain. Castrated males had a higher chromium requirement than males and females during growth.
Table 6
| Microminerals | Sex classes | Model | Variance parameters | Net requirements for gain (g/day)1 |
|---|---|---|---|---|
| Cu | General | 10.72 (7.28) EBW 0.7124 (0.1508) | σ= 0.173; ϕ= 1.316 | EBWG × [(7.64) × EBW -0.2876] |
| Fe | Non-castrated male | 151.7 (53.8) EBW 0.8024 (0.1344) | σ= 0.001; ϕ= 1.769 | EBWG × [(121.72) × EBW -0.1976] |
| Castrated male | 219.5 (97.4) EBW 0.6597 (0.1630) | EBWG × [(144.8) × EBW -0.3403] | ||
| Females | 608.3 (313.2) EBW 0.3067 (0.2044) | EBWG × [(186.57) × EBW -0.6933] | ||
| Mn | Non-castrated male | 3.36 (1.57) EBW 1.045 (0.2655) | σ= 0.787; ϕ= 0.8685 | EBWG × [(3.51) × EBW 0.045] |
| Castrated male | 3.73 (1.60) EBW 0.7155 (0.2679) | σ= 0.480; ϕ= 0.8685 | EBWG × [(2.67) × EBW -0.2845] | |
| Female | 3.54 (1.59) EBW 0.8722 (0.2743) | σ= 0.641; ϕ= 0.8685 | EBWG × [(3.09) × EBW -0.1278] | |
| Zn | Non-castrated male | 48.08(10.38) EBW 0.9526 (0.1191) | σ= 0.010; ϕ= 1.525 | EBWG × [(45.8) × EBW -0.0474] |
| Castrated male | 52.98(11.22) EBW 0.9125 (0.1237) | σ= 0.007; ϕ= 1.525 | EBWG × [(48.34) × EBW -0.0875] | |
| Females | 242.83(134.97) EBW0.3559(0.2369) | σ= 0.013; ϕ= 1.525 | EBWG × [(86.42) × EBW -0.6441] | |
| Co | General | 1.37 (0.51) EBW 1.00 (0.147) | σ= 0.011; ϕ= 2.127 | EBWG × [(1.37) × EBW 0.0000] |
| Cr | Non-castrated male | 1.01 (0.26) EBW 1.305 (0.1909) | σ= 0.179; ϕ= 1.161 | EBWG × [(1.32) × EBW 0.3050] |
| Castrated male | 0.84 (0.20) EBW 1.390 (0.1595) | EBWG × [(1.17) × EBW 0.3900] | ||
| Female | 9.95 (16.85) EBW 0.3689 (0.6394) | EBWG × [(3.67) × EBW -0.6311] |
Models for estimating body composition and net requirements for microminerals for gain of hair sheep.
EBW, empty body weight; EBWG, empty body weight gain; σ, standard deviation; ϕ, scaling parameter of the scaling function.
1 The EBWG was estimated according to equation recommended by Herbster et al. (2020).
Table 7
| BW | ADG (g/day) | Sex classes | Cu | Fe | Mn | Zn | Co | Cr |
|---|---|---|---|---|---|---|---|---|
| 10 kg | 100 | Non-castrated male | 7.72 ± 7.52 | 0.347 ± 0.396 | 3.82 ± 3.51 | 0.210 ± 0.572 | ||
| 100 | Castrated male | 7.08 ± 7.92 | 0.144 ± 0.233 | 3.75 ± 3.49 | 0.216 ± 0.643 | |||
| 100 | Female | 4.79 ± 15.99 | 0.222 ± 0.318 | 2.43 ± 5.15 | 0.106 ± 0.220 | |||
| 100 | General | 0.411 ± 0.54 | 0.125 ± 0.014 | |||||
| 150 | Non-castrated male | 11.54 ± 11.24 | 0.519 ± 0.591 | 5.71 ± 5.24 | 0.313 ± 0.854 | |||
| 150 | Castrated male | 10.58 ± 11.84 | 0.216 ± 0.349 | 5.60 ± 5.22 | 0.323 ± 0.961 | |||
| 150 | Female | 7.16 ± 23.9 | 0.332 ± 0.475 | 3.63 ± 7.70 | 0.158 ± 0.329 | |||
| 150 | General | 0.614 ± 0.807 | 0.187 ± 0.021 | |||||
| 200 | Non-castrated male | 15.36 ± 14.96 | 0.690 ± 0.787 | 7.60 ± 6.97 | 0.417 ± 1.137 | |||
| 200 | Castrated male | 14.09 ± 15.76 | 0.287 ± 0.464 | 7.46 ± 6.94 | 0.429 ± 1.278 | |||
| 200 | Female | 9.53 ± 31.81 | 0.442 ± 0.632 | 4.83 ± 10.25 | 0.210 ± 0.437 | |||
| 200 | General | 0.818 ± 1.074 | 0.249 ± 0.027 | |||||
| 20 kg | 100 | Non-castrated male | 6.54 ± 9.39 | 0.360 ± 0.645 | 3.67 ± 4.34 | 0.271 ± 0.885 | ||
| 100 | Castrated male | 5.32 ± 9.31 | 0.114 ± 0.262 | 3.48 ± 4.23 | 0.300 ± 1.065 | |||
| 100 | Female | 2.67 ± 11.52 | 0.200 ± 0.423 | 1.41 ± 3.83 | 0.062 ± 0.148 | |||
| 100 | General | 0.323 ± 0.426 | 0.125 ± 0.042 | |||||
| 150 | Non-castrated male | 9.77 ± 14.04 | 0.539 ± 0.964 | 5.49 ± 6.49 | 0.405 ± 1.323 | |||
| 150 | Castrated Male | 7.95 ± 13.92 | 0.170 ± 0.391 | 5.21 ± 6.33 | 0.448 ± 1.592 | |||
| 150 | Female | 3.99 ± 17.22 | 0.298 ± 0.632 | 2.11 ± 5.73 | 0.093 ± 0.221 | |||
| 150 | General | 0.482 ± 0.637 | 0.187 ± 0.063 | |||||
| 200 | Non-castrated male | 13.01 ± 18.68 | 0.717 ± 1.283 | 7.31 ± 8.64 | 0.539 ± 1.760 | |||
| 200 | Castrated male | 10.57 ± 18.52 | 0.226 ± 0.520 | 6.93 ± 8.42 | 0.597 ± 2.118 | |||
| 200 | Female | 5.31 ± 22.92 | 0.397 ± 0.842 | 2.81 ± 7.62 | 0.123 ± 0.294 | |||
| 200 | General | 0.642 ± 0.848 | 0.249 ± 0.084 | |||||
| 30 kg | 100 | Non-castrated male | 5.98 ± 10.4 | 0.368 ± 0.794 | 3.59 ± 4.80 | 0.311 ± 1.109 | ||
| 100 | Castrated male | 4.55 ± 9.75 | 0.100 ± 0.268 | 3.35 ± 4.61 | 0.358 ± 1.384 | |||
| 100 | Female | 1.95 ± 9.44 | 0.188 ± 0.472 | 1.05 ± 3.20 | 0.047 ± 0.119 | |||
| 100 | General | 0.283 ± 0.390 | 0.125 ± 0.059 | |||||
| 150 | Non-castrated male | 8.93 ± 15.55 | 0.550 ± 1.186 | 5.37 ± 7.18 | 0.465 ± 1.657 | |||
| 150 | Castrated male | 6.81 ± 14.57 | 0.149 ± 0.400 | 5.00 ± 6.89 | 0.535 ± 2.068 | |||
| 150 | Female | 2.91 ± 14.11 | 0.282 ± 0.705 | 1.57 ± 4.79 | 0.070 ± 0.178 | |||
| 150 | General | 0.423 ± 0.582 | 0.187 ± 0.088 | |||||
| 200 | Non-castrated male | 0.563 ± 0.775 | 11.89 ± 20.69 | 0.731 ± 1.578 | 7.15 ± 9.55 | 0.249 ± 0.117 | 0.619 ± 2.206 | |
| 200 | Castrated male | 9.06 ± 19.39 | 0.199 ± 0.532 | 6.66 ± 9.17 | 0.712 ± 2.752 | |||
| 200 | Female | 3.88 ± 18.78 | 0.375 ± 0.938 | 2.09 ± 6.37 | 0.093 ± 0.237 | |||
| 200 | General | 0.563 ± 0.775 | 0.249 ± 0.117 |
Prediction of net trace mineral requirements for growth of hair sheep with different body weights and average daily gains and respective confidence intervals.
BW, body weight; ADG, average daily gain.
The trace element requirements for maintenance were estimated by relationships between minerals retained and mineral intake. No significant effect of sex was observed on the intercepts or slopes of the linear regression models used to estimate the net requirements of Cu, Fe, Mn, Zn, Co, and Cr (Figure 1). The net requirements for maintenance (µg/kg of body weight) and coefficient retention (%) were as follows:34.85µg/kg of BW and 23% for Cu; 39.63 µg/kg of BW and 13,00% for Fe; 6.11 µg/kg of BW and 0,7% for Mn; 194,00 µg/kg of BW and 28.7% for Zn; 6.06 µg/kg of BW and 13.8% for Co; 11.67 µg/kg of BW and 8.6% for Cr, respectively.
Figure 1
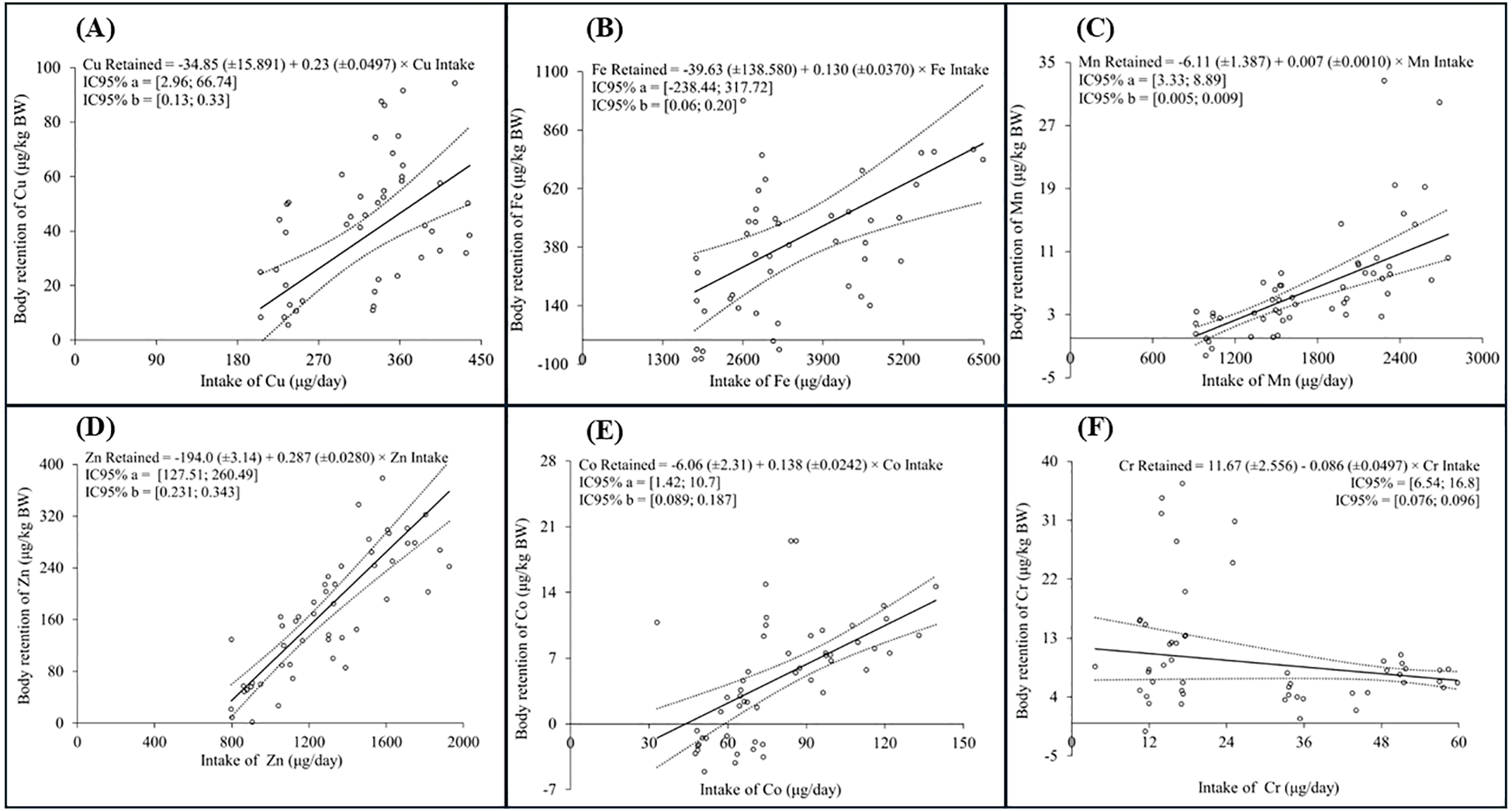
Panels (A–F) show the retention behaviours of the trace minerals copper (Cu), iron (Fe), manganese (Mn), zinc (Zn), cobalt (Co), and chromium (Cr) as a function of their intake. The observed retention values of the trace minerals are represented by circular markers, the solid line represents the predicted mean retention values by the linear model, and the dashed lines are the predicted values of the lower and upper bounds of the 95% confidence interval (95%CI).
The trace element requirements of non-castrated, castrated males and females from 10 to 35kg BW and an ADG of 150g for Fe were (81.94 mg/day; 68.26 and 38.89 mg/day, respectively); Mn (96.58; 43.97 and 62.24 mg/day, respectively); Zn (34.30; 33.30 and 22.82 mg/day, respectively); Cr (7.85; 8.44 and 4.17 mg/day respectively).
The total dietary requirements of this study, as well as those presented by the NRC (2007) and INRA (2018) Committees, are shown in Figure 2. The estimated dietary requirements for males with a body weight (BW) of 30kg and an average daily gain (ADG) of 150g in the present study were 69.6 mg/day for Fe, 76.06 mg/day for Mn, and 2.62 mg/day for Co. These values exceed the recommendations established by the NRC (2007), which suggest 47.62, 17.4, and 0.17 mg/day for Fe, Mn, and Co, respectively. When compared to the INRA (2018), the current results also indicate elevated requirements for Mn and Co, which were reported as 46.87 and 0.28 mg/day, respectively. The estimated dietary requirements for Cu and Zn in hair sheep males were 6.38 and 38.34 mg/day, respectively, exceeding the values recommended by the NRC (2007), which are 4.65 mg/day for Cu and 19.60 mg/day for Zn. In contrast, the Zn requirement determined in the present study was lower than that proposed by INRA (2018), which recommends 46.87 mg/day for animals with similar physiological characteristics.
Figure 2
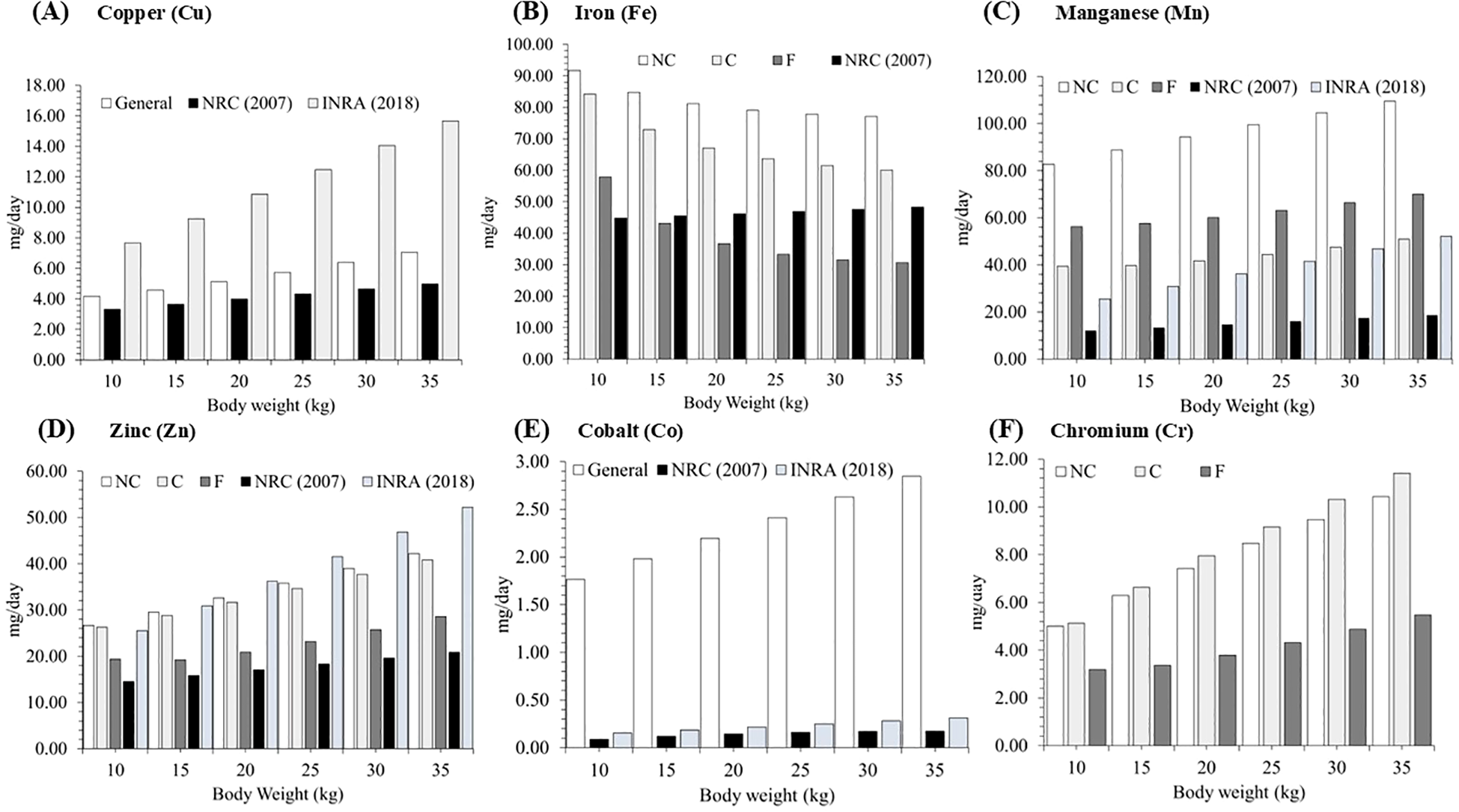
Comparison between the dietary requirements of trace minerals obtained in this study and the recommendations of the NRC (2007) and INRA (2018) considering body weight ranging from 10 to 35 kg and an average daily gain of 150 g/day of non-castrated males (NC), castrated males (C) and females (F).
4 Discussion
A factorial approach has been used to describe trace elements requirements (ARC, 1980). The method entails estimating maintenance, growth, pregnancy, and lactation requirements separately, and subsequently summing them to estimate the total net requirement. The significant advantage of using the factorial method lies in the capability to predict requirements across a diverse range of production circumstances. By dividing the sum of maintenance and production requirements by the absorption coefficient (NRC, 2007) or retention (BR-CORTE, 2016) of the mineral, the dietary requirements can be determined.
In our study, regardless of sex, the sheep exhibited similar requirements for Cu and Co, and the demands increased with increasing body weight. Cu has essential functions in the organism of animals, being necessary for cellular respiration, bone formation, cardiac function, connective tissue development, spinal cord myelination, and keratinization (NASEM, 2016). Based on the present findings, the estimated Cu requirement for maintenance was 0.03485 mg/kg of body weight (BW), with a retention coefficient of 23%. These values are notably higher than those reported by Suttle (2022) and Grace and Clark (1991), who estimated net Cu maintenance requirements at approximately 4 µg/kg BW/day for animals weighing between 20 and 35kg. Cu requirements are peculiar, as they are affected by interactions with Fe, Mo, and S, making it necessary to specify the conditions under which the requirements are applied. Mo in the presence of S markedly reduces the uptake, storage, and utilization of Cu. This occurs through the synthesis of thiomolybdates in the rumen-reticle and formation of insoluble copper in digesta (Suttle and Field, 1983). In our study, sheep weighing 30kg with an ADG of 150g (GPVZ of 136g), the demand corresponds to 1.05 and 63.85 mg Cu/day for the net requirements of maintenance and weight gain. In NRC (2007), using the factorial method, the value of 0.004 mg Cu/kg of BW (4 µg Cu/kg of BW) is adopted, considering the Cu absorption coefficient of 6.0% in lambs, under feedlot conditions. Cu requirements for sheep were first described as 5 mg/kg DM (NRC, 1975) and were later adjusted to 7–11 mg (NRC, 1985). In the ARC (1980), it was suggested that requirements vary from 1 to 8.6 mg of Cu/kg of DM, depending on the physiological state of the animals.
In our study, males and females have similar dietary demands for Co. Co is the precursor of vitamin B12, and is therefore related to energy metabolism, but the amount of dietary Co converted into vitamin B12 varies from 3 to 13% of the Co consumed (Smith, 1987). Studies by Monroe et al. (1952); Looney et al. (1976) with cattle found that 84 to 98% of the Co supplied in the diet was found in the feces, around 5 to 14 days after intake. In this study, the value of 13.8% was estimated as the true retention coefficient, showing that 86.2% of the intake was excreted via feces and urine. Furthermore, the net Co requirements for maintenance in sheep were estimated at 6.06 µg/kg BW. The present study indicates that the net cobalt requirement to support a daily weight gain of 200g in sheep is 2.63 mg/day for an individual with a BW of 30kg. The dietary requirement is relatively higher than the suggestions of INRA (2018), which recommendations of 0.28 mg of Co/day. The NRC (2007) recommends 0.10-0.20 mg Co/kg DM for sheep. CSIRO (2007) suggests a value of 0.11 mg Co/kg DM. According to McDowell (2003), except for P, Co deficiency is one of the most severe mineral deficiencies affecting ruminants in tropical pastures. Co is essential for the synthesis of vitamin B12 by rumen microorganisms (Van Soest, 1994). In the metabolism of ruminants, vitamin B12 is an essential part of multiple enzymes involved in the metabolism of carbohydrates, lipids, some amino acids, and DNA (González-Montaña et al., 2020).
In this study, sex influenced weight gain requirements for Fe, Mn, Zn, and Cr. Around 60% of the Fe in the animal’s body is associated with hemoglobin. The Fe requirement for hair sheep for maintenance is 0.03963 mg of Fe/kg of BW and a retention coefficient of 13%. Sex classes influence the requirements for weight gain, for non-castrated, castrated males, and females weighing 30kg with an ADG of 150g (EBWG of 136). The amount of Fe deposited in the gain and, therefore, the net amount of the mineral required is 8.93, 6.81, and 2.91 mg Fe/day, respectively. The estimates obtained for males by this study are similar to the general recommendation for growing sheep from NRC (2007), of 8.25 mg/day. Fe is one of the most abundant mineral nutrients in the organism and plays a critical role in the synthesis of nucleic acids and proteins, electron transport, cellular respiration, proliferation, and differentiation (Lieu et al., 2001), all of which are intimately related to spermatogenesis and spermatozoa metabolism (Wise et al., 2003).
The net Mn requirement for maintenance was 0.00611 mg/kg of BW and a retention coefficient of 0.7%. For a sheep weighing 30kg BW, the maintenance and dietary Mn requirements are 0.183 and 72.86 mg Mn/day, respectively. In both ARC (1980) and NRC (1985) Systems, values of 10 and 20 mg of Mn/kg of DM are suggested, respectively. Masters et al. (1988) recommended 13 mg Mn/kg DM. The NRC (2007) suggests a requirement for a weight gain of 0.47 mg of Mn/kg BW. Using the prediction equation of this study to estimate the requirement of females with 30kg of BW, with an ADG of 150g (EBWG of 136g), the demand corresponds to 0.28 mg Mn/day, a requirement lower than that of non-castrated males, which require 0.55 mg Mn/day. Males have greater Mn requirements due to testicular growth. Thus, Mn requirements for spermatogenesis and testicular development may be significant. A similar situation occurs with Cu and Mn for females in the reproductive period (Underwood, 1981).
Zinc plays a pivotal role in cellular proliferation, preservation of cellular integrity, and modulation of immune responses, being a key component in the host defense against pathogenic agents (Mortazavi et al., 2025). Since most trace elements, the key role of Zn in animal organism is related to its structural function in some proteins or as an enzyme activator (Suttle, 2022). In this sense, approximately a thousand proteins associated with Zn are recognized in animal organisms. Zinc, an essential component of life in the three domains, follows iron as the second most abundant transition metal ion in living organisms (Cuajungco et al., 2021). Based on the findings of the present study, a net maintenance requirement of 0.194 mg of Zn/kg of body weight (BW) and a retention coefficient of 28% are proposed for sheep, regardless of sex class.
Comparatively, the ARC (1980) established Zn requirements for growing sheep ranging from 24 to 51 mg/kg of dry matter (DM), while the NRC (1985) adopted a value of 20 mg/kg DM. More recently, the NRC (2007) recommended a net maintenance requirement of 0.076 mg Zn/kg BW, with an estimated absorption efficiency of 30% for lambs. This study suggests that non-castrated, castrated males, and females weighing 30kg with an ADG of 150g (EBWG of 136g) demand 5.37, 5.0, and 1.57 mg Zn/day, respectively. Zinc is a component of many metalloenzymes involved in almost every metabolic pathway of the body (Salgueiro et al., 2000). Zinc deficiency alters prostaglandin synthesis, which may affect luteal function (Graham, 1991).
Discussions occur about whether or not chromium is considered an essential element, however, NRC (2007) and NASEM (2016) consider this element as essential. Our study makes an interesting contribution to the requirements for chromium, as it is considered an element, suggesting that the net requirement for maintenance is 0.011 mg Cr/kg BW, and the retention coefficient is 8.6%. For a 30kg sheep, the value of 0.35 mg/day is suggested as a net requirement for maintenance. For dietary requirements, 9.47, 10.31, and 4.88 mg of Cr/day are recommended for non-castrated, castrated, and females, respectively. Although studies on chromium essentiality in sheep are scarce, NRC (2007) states that neither deficiency nor toxicity will likely occur under normal conditions. Therefore, there are no reports of signs of mineral deficiency in animals and humans. However, considering Cr’s relationship with the immune system and its anti-stress effect, some studies have shown reduced morbidity, mortality and increased efficiency in animals recently transported over long distances, both calves and steers that received supplementation with Cr, especially in the organic form (Moonsie-Shageer and Mowat, 1993; Mowat et al., 1993). Likewise, Cr improves the resistance of cattle to diseases, due to its role in stimulating the immune response (Wright et al., 1994). According to the equations suggested in our study for non-castrated, castrated males, and females weighing 30kg with an ADG of 150g (EBWG of 136g), the net amount of the mineral required is 0.46, 0.54 and 0.07 mg of Cr/day, respectively. The net Cr requirement for growing sheep is greater for castrated sheep.
Based on the present study, the trace element requirements for hair sheep were different from those reported by the World Committees. Both the NRC (2007) and INRA (2018) recommendations for trace mineral requirements are based primarily on estimates established by the ARC (1980). In these Committees, trace element requirements were determined using the dose-response methodology and absorption coefficients. In dose-response, the animal’s requirement for a nutrient is given at the maximum response level in the parameters evaluated, such as maximum weight gain, for example. This method may lead to underestimation or overestimation of the estimates, since it is not possible to vary the production levels, since the estimate is based on the maximum response to the productive demand. Furthermore, most studies did not consider the trace elements contained in the feeds that made up the experimental diets.
Another approach that should be considered is the differences between organic and inorganic nutrients in terms of their requirements for maintenance and production result in different metabolic pathways. Organic nutrients, such as protein, for example, after being absorbed, enter the body’s metabolic pool and can be converted to energy, used for tissue synthesis or maintenance; they can be metabolized and deposited; or they can be lost, as end products of metabolism, through normal pathways. In contrast, inorganic ions released during metabolism are not altered, nor do they become unavailable to the tissues. These ions remain available for reformulation of their functional combinations, in the same way as inorganic ions absorbed by the digestive system (Underwood, 1981). This reuse of minerals by the tissues could theoretically be complete, resulting in the absence of maintenance requirements for these nutrients. However, in practice, the mineral conservation processes in the body are not as efficient, resulting in loss of minerals through the kidneys, intestinal mucosa, digestive glands, and skin, thus constituting the mineral requirements for maintenance. The recommendations of various Committees take into account their calculations of endogenous losses through feces. The mineral maintenance requirements encompass not only urinary and fecal losses, but also potential losses through the skin, sweat, and other routes. Regarding the mineral retention coefficients, the values observed for manganese and chromium were relatively lower, which may be attributed to the high concentrations of these minerals in the diet, thereby resulting in increased excretion. can be attributed to endogenous losses associated with variations in voluntary intake, which directly influence maintenance needs. Therefore, the use of the true retention coefficient seems to be more accurate compared to the absorption coefficient for estimating mineral requirements. Thus, while the estimates have been refined, the retention coefficients identified in this study are not definitive and may fluctuate in response to tissue growth. Younger animals typically exhibit higher retention coefficients compared to adults. Therefore, the extent of variation in retention and absorption coefficients concerning growth stages remains to be further elucidated.
Minerals play an important role in animal metabolism, and study contributes to improving our knowledge about recommendations for mineral requirements in hair sheep. However, there exists a need for additional efforts to better understand the effects of genotype and degree of maturity on trace elements requirements, especially requirements for maintenance and growth. Herein, there is a great contribution in providing up-to-date recommendations on trace elements requirements for sexes. Furthermore, the importance of future studies to determine trace elements requirements is highlighted. Finally, there is a need for more in-depth knowledge of the retention coefficients of different minerals. Continuous efforts to improve models for estimating trace elements requirements, together with a better understanding of retention coefficients, will provide greater reliability in the formulation of diets for sheep raised in tropical regions.
5 Conclusion
In conclusion, our study makes a significant contribution by demonstrating the influence of sex on the trace mineral requirements of hair sheep. The requirements for Mn, Co, and Cr increased with increasing body weights, whereas Fe and Zn showed a stable tendency. Our study indicated that the hair sheep trace elements requirements are different from the main nutritional requirements established by NRC (2007) and INRA (2018). Accurate estimation of the mineral requirements of growing sheep necessitates precise knowledge of mineral deposition in the body. It is important to emphasize that trace elements requirements serve as guidelines for the animal’s needs and should be reassessed as new information becomes available. Estimating the various requirements through multiple approaches is advisable. Achieving reasonable agreement between methods means greater confidence in the estimated trace mineral requirements.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
EJ: Writing – review & editing, Formal analysis. ES: Investigation, Writing – review & editing, Methodology. MA: Investigation, Writing – review & editing, Formal analysis, Methodology. CH: Formal analysis, Writing – original draft, Methodology, Investigation. AB: Formal analysis, Methodology, Investigation, Writing – review & editing. MM: Conceptualization, Methodology, Writing – review & editing, Formal analysis. LS: Formal analysis, Methodology, Writing – review & editing. LB: Methodology, Conceptualization, Writing – review & editing, Formal analysis. SS: Writing – review & editing, Methodology, Investigation. RO: Methodology, Conceptualization, Formal analysis, Writing – review & editing. EP: Methodology, Project administration, Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The authors thank the grants provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior– CAPES and Institutos Nacionais de Ciência e Tecnologia INCT– Ciência Animal and Cadeia Produtiva da Carne -INCT- Carne.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
AFRC (Agricultural and Food Research Council) (1998). “ Technical committee on responses to nutrients,” in The nutrition of goats ( CAB International, Wallingford).
2
AOAC (Association of Official Analytical Chemists) (1990). Official Methods of Analysis. 15th ed (Washington, DC: Association of Official Analytical Chemistry).
3
ARC (Agricultural Research Council) (1980). The Nutrient Requirements of Ruminant Livestock (Slough: Commonwealth Agricultural Bureaux).
4
Braselton W. E. Stuart K. J. Mullaney T. P. Herdt T. H. (1997). Biopsy mineral analysis by inductively coupled plasma atomic emission spectroscopy with ultrasonic nebulization. J. Vet. Diagn. Invest.9, 395–400. doi: 10.1177/104063879700900409
5
BR-CORTE (2016). Nutrient Requirements of Zebu and Crossbred Cattle. 3rd ed (Viçosa, MG: Suprema Gráfica Ltda).
6
Burnham K. P. Anderson D. R. (2002). Model Selection and Multimodel Inference: A Practical Information Theoretic Approach (New York: Springer-Verlag).
7
Cabral P. K. A. Azevedo A. M. A. Santos E. M. J. Marques K. B. Gonzaga Neto S. Pereira Filho J. M. (2008). Composição corporal e exigências nutricionais em cálcio e fósforo de cordeiros Santa Inês em pastejo no semi-árido. Acta Sci. Anim. Sci.30, 59–65. doi: 10.4025/actascianimsci.v30i1.3609
8
Cook R. D. (1979). Influential observations in linear regression. J. Am. Stat. Assoc.74, 169–174. doi: 10.2307/2286747
9
CSIRO (Commonwealth Scientific and Industrial Research Organisation) (2007). Nutrient Requirements of Domesticated Ruminants (Melbourne: CSIRO Publishing).
10
Cuajungco M. P. Ramirez M. S. Tolmasky M. E. (2021). Zinc: multidimensional effects on living organisms. Biomedicines9, 208. doi: 10.3390/biomedicines9020208
11
Detmann E. Souza M. A. Valadares Filho S. C. Queiroz A. C. Berchielli T. T. Saliba E. O. S. et al . (2012). Métodos para Análise de Alimentos-INCT (Visconde do Rio Branco: Suprema).
12
González-Montaña J. R. Escalera-Valente F. Alonso A. J. Lomillos J. M. Robles R. Alonso M. E. (2020). Relationship between vitamin B12 and cobalt metabolism in domestic ruminant: an update. Animals10, 1855. doi: 10.3390/ani10101855
13
Grace N. D. Clark R. G. (1991). “ Trace element requirements, diagnosis and prevention of deficiencies in sheep and cattle,” in Physiological aspects of digestion and metabolism in ruminants. Eds. TsudaT.SasakiY.KawashimaR. ( Academic press, San Diego, USA), 321–346.
14
Graham T. W. (1991). Trace element deficiencies in cattle. Vet. Clin. North Am. Food. Anim. Pract.7, 153–215. doi: 10.1016/s0749-0720(15)30816-1
15
Herbster C. J. L. Abreu M. L. C. Brito Neto A. S. Mendes M. S. Silva L. P. Marcondes M. I. et al . (2023). Macromineral requirements for maintenance and growth in male and female hair sheep. Front. Vet. Sci.10. doi: 10.3389/fvets.2023.1032429
16
Herbster C. J. L. Silva L. P. Marcondes M. I. Garcia I. F. F. Oliveira R. L. Cabral L. S. et al . (2020). Weight adjustment equation for hair sheep raised in warm conditions. Animal14, 1718–1723. doi: 10.1017/S1751731120000294
17
Hosnedlová B. Travnicek J. Šoch M. (2007). Current view of the significance of zinc for ruminants: a review. Agric. Trop. Subtrop.40, 57–64.
18
INRA (Institut National de la Recherche Agronomique) (2018). INRA Feeding System for Ruminants (The Netherlands: Wageningen Academic Publishers).
19
Kock S. W. Preston R. L. (1979). Estimation of bovine carcass composition by the urea dilution technique. J. Anim. Sci.48, 319–327. doi: 10.2527/jas1979.482319x
20
Lee S. H. Engle T. E. Hossner K. L. (2002). Effects of dietary copper on the expression of lipogenic genes and metabolic hormones in steers. J. Anim. Sci.80, 1999–2005. doi: 10.2527/2002.8071999x
21
Li L. Yang X. (2018). The essential element manganese, oxidative stress, and metabolic diseases: links and interactions. Oxid. Med. Cell. Longev.218, 7580707. doi: 10.1155/2018/7580707
22
Lieu P. T. Heiskala M. Peterson P. A. Yang Y. (2001). The roles of iron in health and disease. Mol. Asp. Med.22, 1–87. doi: 10.1016/S0098-2997(00)00006-6
23
Looney J. W. Gille G. Preston R. L. Graham E. R. Pfander W. H. (1976). Effects of plant species and cobalt intake upon cobalt utilization and ration digestibility by sheep. J. Anim. Sci.42, 693–698. doi: 10.2527/jas1976.423693x
24
Masters D. G. Paynter D. I. Briegel J. Baker S. K. Purser D. B. (1988). Influence of manganese intake on body, wool and testicular growth of young rams and on the concentration of manganese and the activity of manganese enzymes in tissues. Aust. J. Agric. Res.39, 517–524. doi: 10.1071/AR9880517
25
McDowell L. R. (2003). Minerals in Animal and Human Nutrition (Amsterdam: Elsevier Science).
26
Meschy F. (2000). Recent progress in the assessment of mineral requirements of goats. Livest. Prod. Sci.64, 9–14. doi: 10.1016/S0301-6226(00)00171-8
27
Monroe R. A. Sauberlick H. E. Comar C. L. Hood S. L. (1952). Vitamin B12 biosynthesis after oral and intravenous administration of inorganic Co6o to sheep. Proc. Soc Exp. Bid. Med.80, 250–257. doi: 10.3181/00379727-80-19586
28
Moonsie-Shageer S. Mowat D. N. (1993). Effect of level of supplemental chromium on performance, serum constituents, and immune status of stressed feeder calves. J. Anim. Sci.71, 232–238. doi: 10.2527/1993.711232x
29
Mortazavi M. S. Hajmohammadi M. Buonaiuto G. Colleluori R. Lamanna M. Cavallini D. et al . (2025). The effect of a pre-mix of essential organic minerals on growth, antioxidant indices, and the diarrhea incidence in dairy calves breed in arid climates. Ruminants5, 22. doi: 10.3390/ruminants5020022
30
Mowat D. N. Chang X. Yang W. Z. (1993). Chelated chromium for stressed feeder calves. Can. J. Anim. Sci.73, 49–55. doi: 10.4141/cjas93-004
31
NASEM (National Academies of Sciences, Engineering, and Medicine) (2016). Nutrients Requirements of Beef Cattle (Washington, DC: The National Academic Press).
32
NRC (National Research Council) (1975). Nutrient Requirements of Sheep (Washington, DC: National Academy of Sciences).
33
NRC (National Research Council) (1985). Nutrient Requirements of Sheep (Washington, DC: National Academies Press).
34
NRC (National Research Council) (2007). Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids and New World Camelids (Washington, DC: National Academies Press).
35
Pereira E. S. Carmo A. B. R. Costa M. R. G. F. Medeiros N. A. Oliveira R. L. Pinto A. P. et al . (2016). Mineral requirements of hair sheep in tropical climates. J. Anim. Physiol. Anim. Nutr.100, 1090–1096. doi: 10.1111/jpn.12483
36
Pereira E. S. Lima F. W. R. Campos A. C. N. Carneiro M. S. S. Silva L. P. Pereira M. W. F. et al . (2018a). Net mineral requirements for the growth and maintenance of Somali lambs. Animal13, 112–118. doi: 10.1017/S1751731118000782
37
Pereira E. S. Lima F. W. R. Marcondes M. I. Rodrigues J. P. P. Campos A. C. N. Silva L. P. et al . (2017). Energy and protein requirements of Santa Ines lambs, a breed of hair sheep. Animal11, 2165–2174. doi: 10.1017/S1751731117001185
38
Pereira E. S. Pereira M. W. F. Marcondes M. I. Medeiros A. N. Oliveira R. L. Silva L. P. et al . (2018b). Maintenance and growth requirements in male and female hair lambs. Small Rumin. Res.159, 75–83. doi: 10.1016/j.smallrumres.2017.11.003
39
Salgueiro M. J. Zubillaga M. Lysionek A. Cremaschi G. Goldman C. G. Caro R. (2000). Zinc status and immune system relationship: a review. Biol. Trace. Elem. Res.76, 193–205. doi: 10.1385/BTER:76:3:193
40
Silva D. J. Queiroz A. C. (2002). Análise de Alimentos: Métodos Químicos e Biológicos (Viçosa: Universidade Federal de Viçosa).
41
Silva I. F. Rodrigues R. T. S. Queiroz M.A.Á. Chizzotti M. L. Zanetti M. A. Cunha J. A. et al . (2016). Net requirements of calcium, phosphorus, magnesium, and sulphur for growth of non-descript breed hair lambs of different sex class in the Brazilian semiarid conditions. Trop. Anim. Health Prod.48, 817–822. doi: 10.1007/s11250-016-1035-4
42
Smith R. M. (1987). “ Cobalt,” in Trace Elements in Human and Animal Nutrition ( Academic Press, New York).
43
St-Pierre N. R. (2007). Meta-analyses of experimental data in the animal sciences. R. Bras. Zootec.36, 343–358. doi: 10.1590/S1516-35982007001000031
44
Sugiura N. (1978). Further analysis of the data by Akaike’s information criterion and the finite corrections. Commun. Stat. Theory Methods7, 13–26. doi: 10.1080/03610927808827599
45
Suttle N. F. (2022). The Mineral Nutrition of Livestock. 5th ed (Wallingford: CABI Publishing). doi: 10.1079/9781789240924.0000
46
Suttle N. F. Field A. C. (1983). Effects of dietary supplements of thiomolybdates on copper and molybdenum metabolism in sheep. J. Comp. Pathol.93, 379–389. doi: 10.1016/0021-9975(83)90025-7
47
Tedeschi L. O. (2006). Assessment of the adequacy of mathematical models. Agric. Syst.89, 225–247. doi: 10.1016/j.agsy.2005.11.004
48
Underwood E. J. (1981). The Mineral Nutrition of Livestock. 2nd ed (Slough: Commonwealth Agricultural Bureaux).
49
Underwood E. J. Suttle N. F. (1999). The Mineral Nutrition of Livestock (New York, NY: CABI Publishing). doi: 10.1079/9780851991283.0000
50
Van Soest P. J. (1994). Nutritional Ecology of the Ruminant. 2nd ed (Ithaca: Constock Publishing Associates, 476p).
51
Vargas J. A. C. Almeida A. K. Souza A. P. Fernandes M. H. R. M. Resende K. T. Teixeira I. A. M. A. (2017). Sex effects on macromineral requirements for growth in Saanen goats: a meta-analysis. J. Anim. Sci.95, 4646–4657. doi: 10.2527/jas2017.1825
52
Vieira R. A. M. Júnior N. R. Gomes R. S. Oliveira T. S. Bendia L. C. R. Azevedo F. H. V. (2018). The ontogenetic allometry of body morphology and chemical composition in dairy goat wethers. Animal12, 538–553. doi: 10.1017/S1751731117001884
53
Wilson B. K. Vazquez-Anon M. Step D. L. Moyer K. D. Haviland C. L. Maxwell C. L. (2016). Effect of copper, manganese, and zinc supplementation on the performance, clinical signs, and mineral status of calves following exposure to bovine viral diarrhea virus type 1b and subsequent Mannheimia haemolytica infection. J. Anim. Sci.94, 1123–1140. doi: 10.2527/jas.2015-9503
54
Wise T. Lunstra D. D. Rohrer G. A. Ford J. J. (2003). Relationships of testicular iron and ferritin concentrations with testicular weight and sperm production in boars. J. Anim. Sci.81, 503–511. doi: 10.2527/2003.812503x
55
Wright A. J. Mowat D. N. Mallard B. A. (1994). Supplemental chromium and bovine respiratory disease vaccines for stressed feeder calves. Can. J. Anim. Sci.74, 287–295. doi: 10.4141/cjas94-04
56
Zhang H. Nie H. Wang Z. Wang F. (2018). The net iron, manganese, copper, and zinc requirements for maintenance and growth of Dorper × Hu ewe lambs. Ital. J. Anim. Sci.17, 941–949. doi: 10.1080/1828051X.2018.1431964
Summary
Keywords
micromineral, models, sex classes, sheep, tropical areas
Citation
Justino EdS, Santos EM, Abreu MLC, Herbster CJL, Brito Neto AdS, Marcondes MI, Silva LPd, Bezerra LR, Santos SA, Oliveira RL and Pereira ES (2025) Trace minerals for maintenance and weight gain in male and female hair sheep. Front. Anim. Sci. 6:1624560. doi: 10.3389/fanim.2025.1624560
Received
07 May 2025
Accepted
18 August 2025
Published
17 September 2025
Volume
6 - 2025
Edited by
Adugna Tolera, Hawassa University, Ethiopia
Reviewed by
Giovanni Buonaiuto, University of Bologna, Italy; Melkamu Derseh, International Livestock Research Institute, Ethiopia
Updates
Copyright
© 2025 Justino, Santos, Abreu, Herbster, Brito Neto, Marcondes, Silva, Bezerra, Santos, Oliveira and Pereira.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elzania Sales Pereira, elzania@hotmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.