- 1South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Guangzhou, China
- 2Key Laboratory of Efficient Utilization and Processing of Marine Fishery Resources of Hainan Province, Sanya Tropical Fisheries Research Institute, Sanya, China
- 3College of Marine Sciences, Shanghai Ocean University, Shanghai, China
- 4Hebei Key Laboratory of Bohai Sea Fish Germplasm Resources Conservation and Utilization, Beidaihe Central Experiment Station, Chinese Academy of Fishery Sciences, Qinhuangdao, China
Elucidating the gonadal development patterns of organisms holds significant reference value for advancing large-scale breeding and sustainable utilization of species. This study systematically investigated the annual population structure (test diameter-wet weight allometric model), gonadal histology (gametogenesis chronology), and environmental conditions of Tripneustes gratilla. The key findings include the following: 1. Test diameter (60.10–147.89 mm) exhibited a significant positive correlation with wet weight (R² = 73.54%), with both parameters peaking in May–June before declining sharply in September–October, indicating synchronized seasonal morphological changes. 2. Gonadal development displayed a bimodal pattern, with the gonad index (GI) reaching primary (8.26 ± 2.99) and secondary (7.30 ± 3.15) reproductive peaks in September and December, respectively. Compared with males, females presented significantly superior gonadal development during July–August (P < 0.05). 3. Water temperature dynamics were highly synchronized with GI fluctuations, where the July–September warming phase was strongly associated with reproductive peaks, confirming the temperature-driven physiological synchronization of reproductive cycles. These results demonstrate that T. gratilla employs dual survival strategies through seasonal adjustments in morphological-reproductive traits to adapt to environmental fluctuations, providing critical theoretical insights into the reproductive ecology of echinoderms.
1 Introduction
Echinoderms, as a key evolutionary clade of deuterostomes, are distinguished by their unique pentaradial symmetry and calcareous endoskeleton, making them a model group for studying biomineralization, regenerative biology, and marine ecological functions (Carnevali et al., 2024). Among them, members of the class Echinoidea (sea urchins) hold both ecological and economic significance: they maintain coral reef ecosystem balance by grazing on algae (Dang et al., 2020; Qin et al., 2020), while their gonads (the sole edible part) serve as a high-quality protein source, sustaining a global annual aquaculture trade worth USD 2 billion (Rocha et al., 2019). However, climate-driven ocean acidification is causing a global decline in sea urchin populations at a rate of 7.3% per year (Byrne and Hernández, 2020), underscoring the urgent need to elucidate their growth and reproduction mechanisms for sustainable resource utilization.
In China, sea urchin research has long focused on northern cold-water species (e.g., Hemicentrotus pulcherrimus) (Qi et al., 2014; Shang et al., 2024), while the diverse species in the tropical South China Sea harbor unique evolutionary adaptations (Liu M. et al., 2024). Tripneustes gratilla, a keystone ecological species in the Indo-West Pacific warm-water region, exhibits a fragmented, patchy distribution in the South China Sea (limited to the coasts of Hainan, Guangdong, and Taiwan) and holds significant commercial potential (Bronstein et al., 2017; Liu Z. et al., 2024; Valentine and Edgar, 2010). However, recent studies have focused primarily on artificial feed optimization (Dworjanyn et al., 2007) and feeding preferences (Seymour et al., 2013), with critical knowledge gaps related to wild population dynamics (e.g., gonadal developmental plasticity) and the environmental drivers of reproductive cycles remaining. These gaps severely hinder the sustainable exploitation of South China Sea urchin resources. Numerous studies have indicated that sea urchin reproduction is influenced by location (Okamoto et al., 2020), seasonality (Zhao et al., 2024), and food availability (O’Hara and Thórarinsdóttir, 2021), with reproductive cycles and spawning timing directly impacting gonad quality (Rocha et al., 2019). Gonad fullness reflects feeding conditions and prey biomass across temporal or spatial scales but also correlates with test size–weight relationships, gonadal maturity, gut fullness, sex, and lipid content (Azad et al., 2011). Researchers have established gonad developmental staging criteria on the basis of external morphology and internal histological structures. For example, Elmasry et al. (2023) classified Paracentrotus lividus gonadal development into four stages on the basis of external morphology and cellular features in histological sections; Byrne (1990) defined six stages on the basis of gamete morphology and abundance; and Urriago et al. (2016) defined four stages according to germ cell and nutritive phagocyte dynamics. Concurrently, environmental factors such as temperature and light are recognized as key drivers of reproductive cycles and spawning triggers (Zhadan et al., 2017). Temperature, in particular, modulates feeding rates, metabolic activity, nutrient utilization, and energy allocation in marine organisms (Marañón et al., 2018); however, most studies on temperature effects remain confined to laboratory settings (Ding et al., 2019), with limited field-based investigations on gonadal development–temperature linkages. Furthermore, the applicability of existing gonad staging criteria to tropical sea urchins with strategies for continuous reproduction remains debated.
To address these issues, this study focuses on Tripneustes gratilla in the South China Sea. We conducted annual surveys of population structure and habitat conditions, combined with histological analysis of gonadal tissues across seasons. Our objectives were to answer two core questions: (1) How do tropical sea urchins balance energy allocation between growth and reproduction? (2) What are the temperature-driven mechanisms underlying gonadal development in wild populations? The findings provide a theoretical foundation for adaptive management and sustainable utilization of sea urchin resources in the South China Sea.
2 Materials and methods
2.1 Collection and processing of Tripneustes gratilla
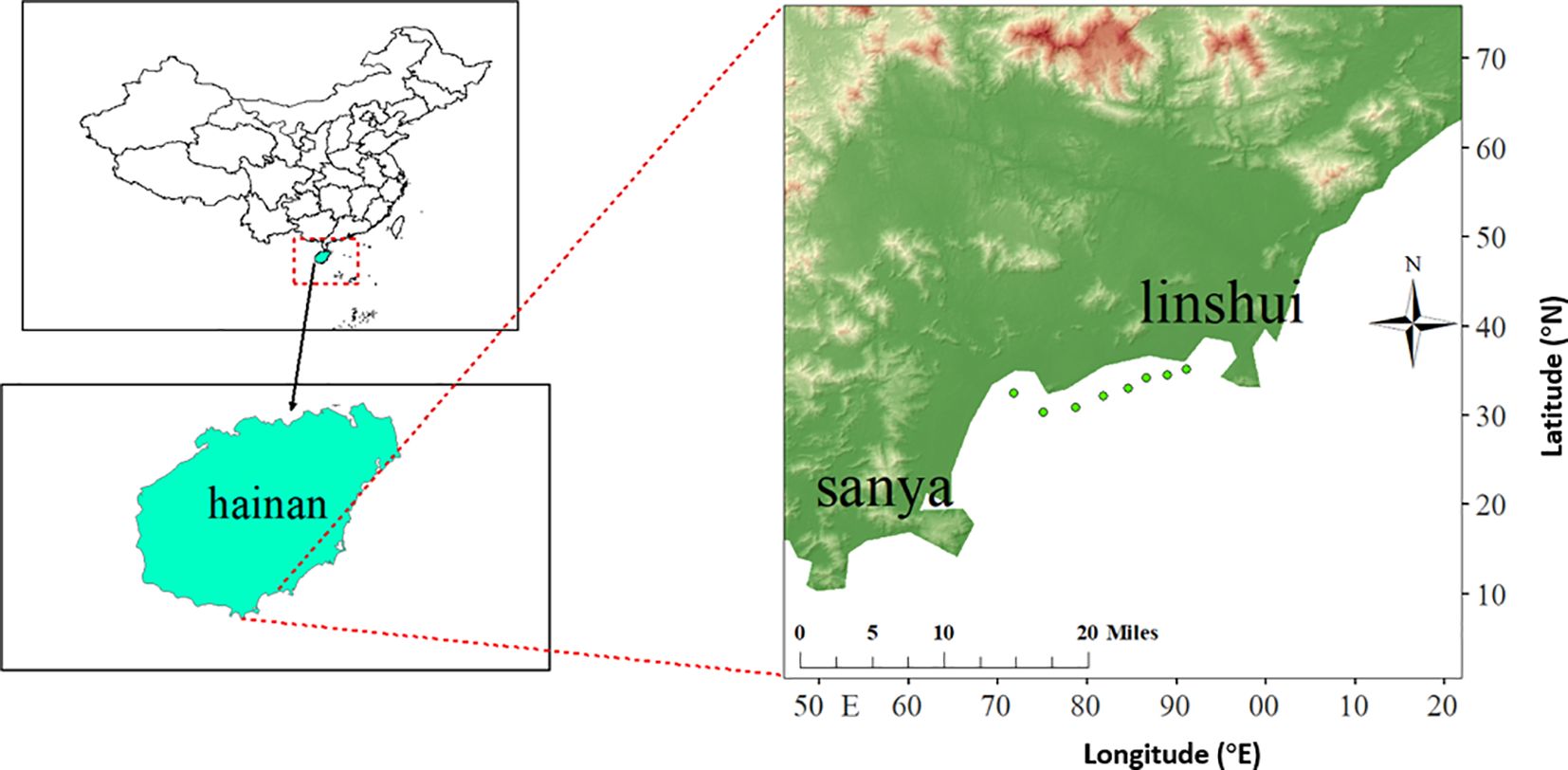
Experimental sea urchins (Tripneustes gratilla) were collected monthly from coastal waters near Xincun Town, Lingshui Li Autonomous County, Hainan Province, China, between July 2022 and June 2023 (Figure 1). A total of 636 individuals were collected. The test diameter (TD, mm) was measured using a precision caliper (± 0.01 mm), and body weight (W, g) was recorded with a laboratory-grade digital balance (± 0.01g). The monthly averages of the water temperature, salinity, and pH were recorded.
The length–weight relationship of the Tripneustes gratilla population was calculated using the following equation:
where W is the body weight (g), L is the test diameter (mm), and a and b are constants. The parameter a represents the growth condition factor, with higher values indicating better environmental conditions (e.g., food availability, hydrology). The parameter b determines the growth pattern: isometric growth occurs when b = 3, whereas allometric growth is indicated if b ≠ 3 (Jisr et al., 2018).
2.2 Histological analysis
Each month, 15–20 active Tripneustes gratilla individuals were selected for gonad sampling. Gonads were dissected using scissors and forceps. Owing to the inability to visually determine sex, no sex identification or recording was performed during sampling. After dissection, the gonads were weighed, and a complete section of each gonad was fixed in 4% paraformaldehyde for 24 hours before being sent to Wuhan Servicebio Biotechnology Co., Ltd., for histological sectioning.
Following standard histological techniques (Delgado and Pérez-Camacho, 2005), fixed gonad tissues were sliced into 1 cm-thick sections, dehydrated through a graded alcohol series, and embedded in paraffin. Sections (5 μm thick) were prepared, mounted on slides with neutral resin, and stained with hematoxylin and eosin (H&E). Gonad developmental stages and sexes were identified using an optical microscope (Olympus Corporation, CX22LEDRFS1, Tokyo, Japan). The gonad stages were classified into five phases according to Fuji’s method: the recovery phase (I), growth phase (II), prematuration phase (III), maturation phase (IV), and postspawning phase (V).
Stage I: Ovarian follicles dominated by oogonia with sparse primary oocytes; testes contained spermatogonia and few dark-stained primary spermatocytes.
Stage II: Oogonia declined with thickened follicle walls and enlarged primary oocytes; testes showed proliferating spermatogonia/spermatocytes without spermatozoa.
Stage III: Ovarian secondary oocytes became pear-shaped, some detaching into lumina; testes exhibited abundant spermatids and early sperm bundles.
Stage IV: Follicle lumens fully occupied by mature eggs or spermatozoa.
Stage V: Postspawning ovaries displayed phagocytic activity and tissue regeneration; testes retained sparse residual spermatozoa.
2.3 Gonad index
The reproductive cycle of Tripneustes gratilla was assessed using the gonad index (GI), which was calculated as follows:
2.4 Data analysis
The raw data were organized in Excel 2019. Normality (Kolmogorov–Smirnov test) and homogeneity of variance (Levene’s test) were assessed using SPSS 27.0 (95% confidence interval). Two-way ANOVA was conducted to analyze the effects of month and sex, followed by Tukey’s HSD post hoc test for multiple comparisons when significant interactions or main effects were detected, with p < 0.05 considered indicative of statistical significance. Pearson correlation analysis (p < 0.01, two-tailed) was used to evaluate the relationships among test diameter, body weight, gonad index, and month. The length–weight relationship parameters were derived via least squares regression in Origin 2022. The growth mode (isometric vs. allometric) was determined by testing (b = 3) using Bailey’s (t)-test. All figures were generated using Origin 2022.
3 Results
3.1 Seasonal variations in the size of Tripneustes gratilla
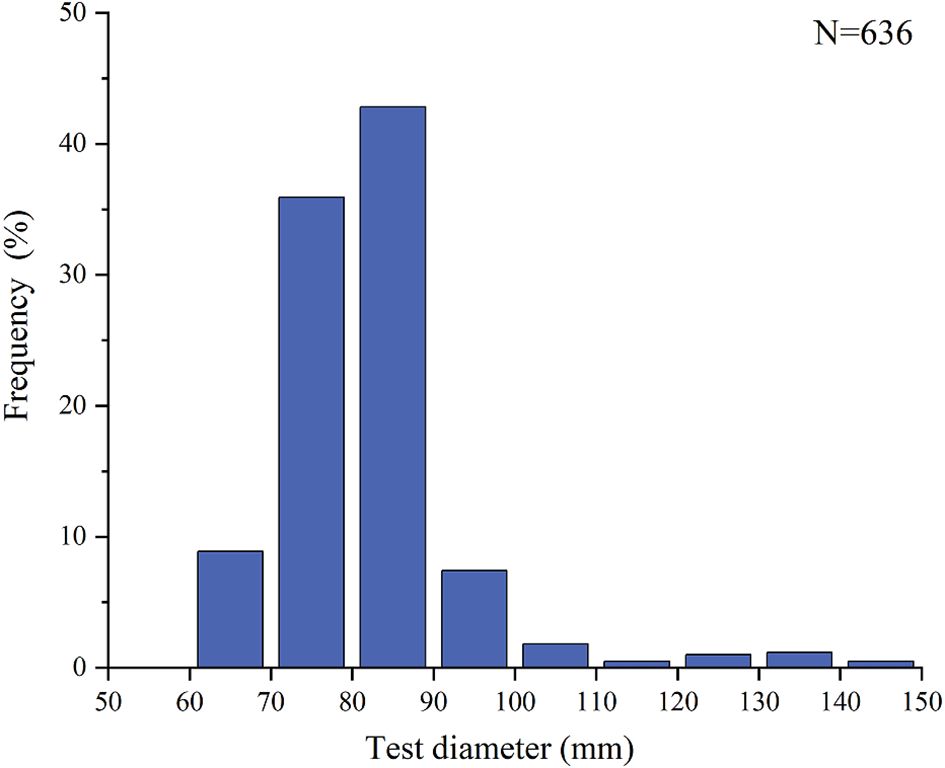
Analysis of the annual data revealed significant monthly fluctuations in the test diameter (TD) and wet weight of Tripneustes gratilla (one-way ANOVA, p < 0.05; Figure 2). Post hoc Tukey’s HSD test showed distinct groupings among months, with values sharing the same lowercase letter indicating no statistically significant difference (p > 0.05). Specifically, higher values for both TD and wet weight were observed from May to June, whereas lower values were recorded in September and October. In this study, the TD of Tripneustes gratilla ranged from 60.10 to 147.89 mm, with a mean TD of 81.96 ± 11.67 mm. Notably, 78.74% of the samples fell within the 70–90 mm TD range (Figure 3), indicating the dominance of individuals within this size class in the population. Scatter plot analysis further demonstrated a significant positive correlation between TD and wet weight (Figure 4). The data points were primarily concentrated within TD values of 70–140 mm and wet weights of 100–500 g, reflecting an increasing trend for wet weight with increasing TD. The fitted curve followed a nonlinear equation (), with a coefficient of determination , indicating that TD explained approximately 73.54% of the variation in wet weight. These results highlight TD as a critical factor influencing wet weight, with a distinct nonlinear relationship between the two variables.

Figure 2. Monthly variations in the body size of Tripneustes gratilla. Data are expressed as mean ± standard deviation. Statistical analysis was performed using two-way ANOVA, followed by Tukey’s HSD post hoc test for multiple comparisons. Different lowercase letters indicate statistically significant differences between months (p < 0.05).

Figure 4. Length–weight (L–W) relationship of Tripneustes gratilla. “Length” (x-axis, Test diameter) represents the shell diameter of Tripneustes gratilla (in millimeters, mm), measured as the maximum linear distance across the test (skeletal shell). “Weight” (y-axis) denotes the wet weight of Tripneustes gratilla (in grams, g), reflecting the total mass of the organism including natural moisture content. Blue scatter points show individual data pairs of shell diameter and wet weight.
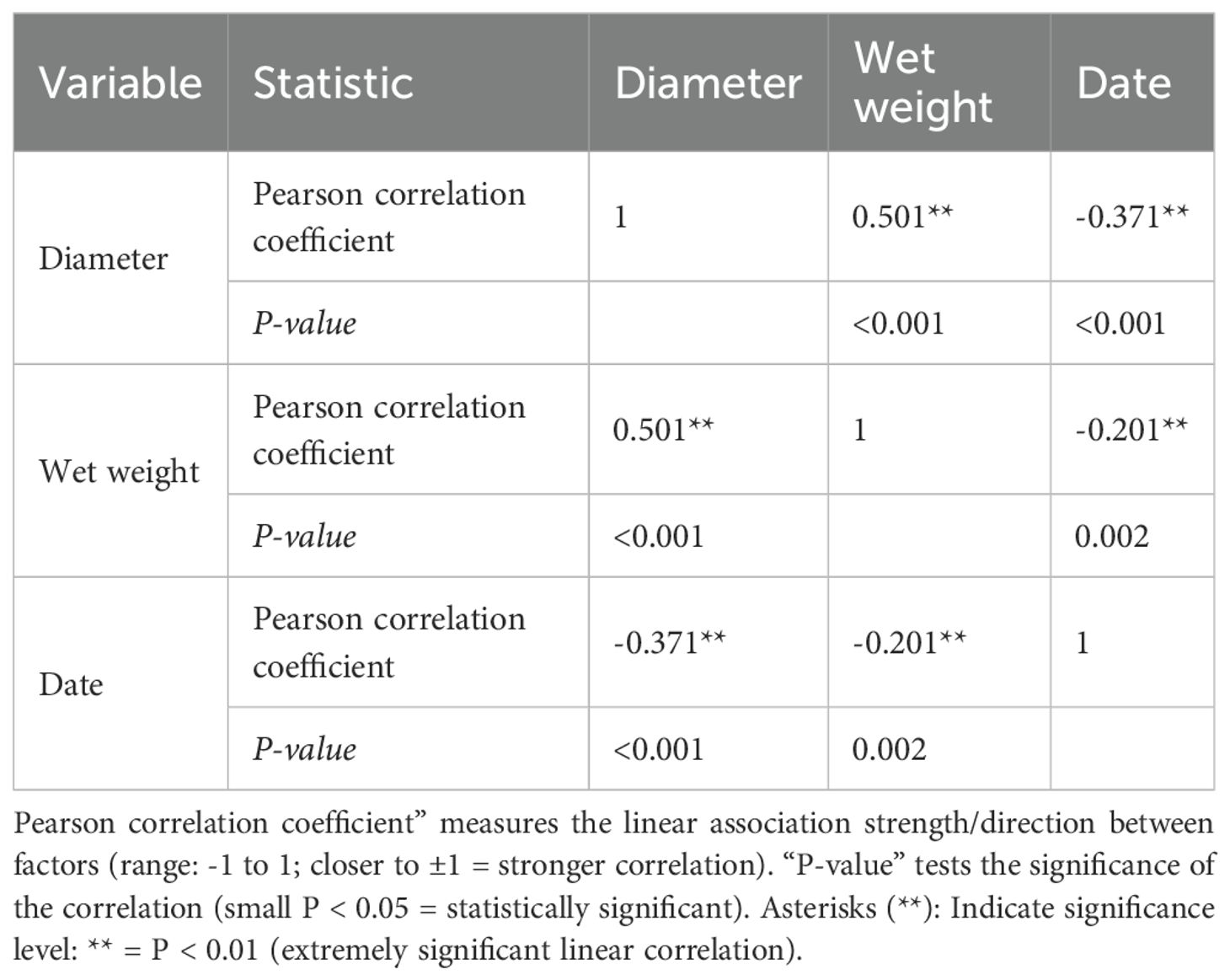
Pearson correlation analysis of factors affecting population dynamics in Tripneustes gratilla (Table 1) revealed a significant negative correlation between TD and month (P < 0.01), a significant positive correlation between TD and wet weight (P < 0.01), and a significant negative correlation between wet weight and month (P < 0.01).
3.2 Seasonal fluctuations in the gonad index and developmental structure of Tripneustes gratilla
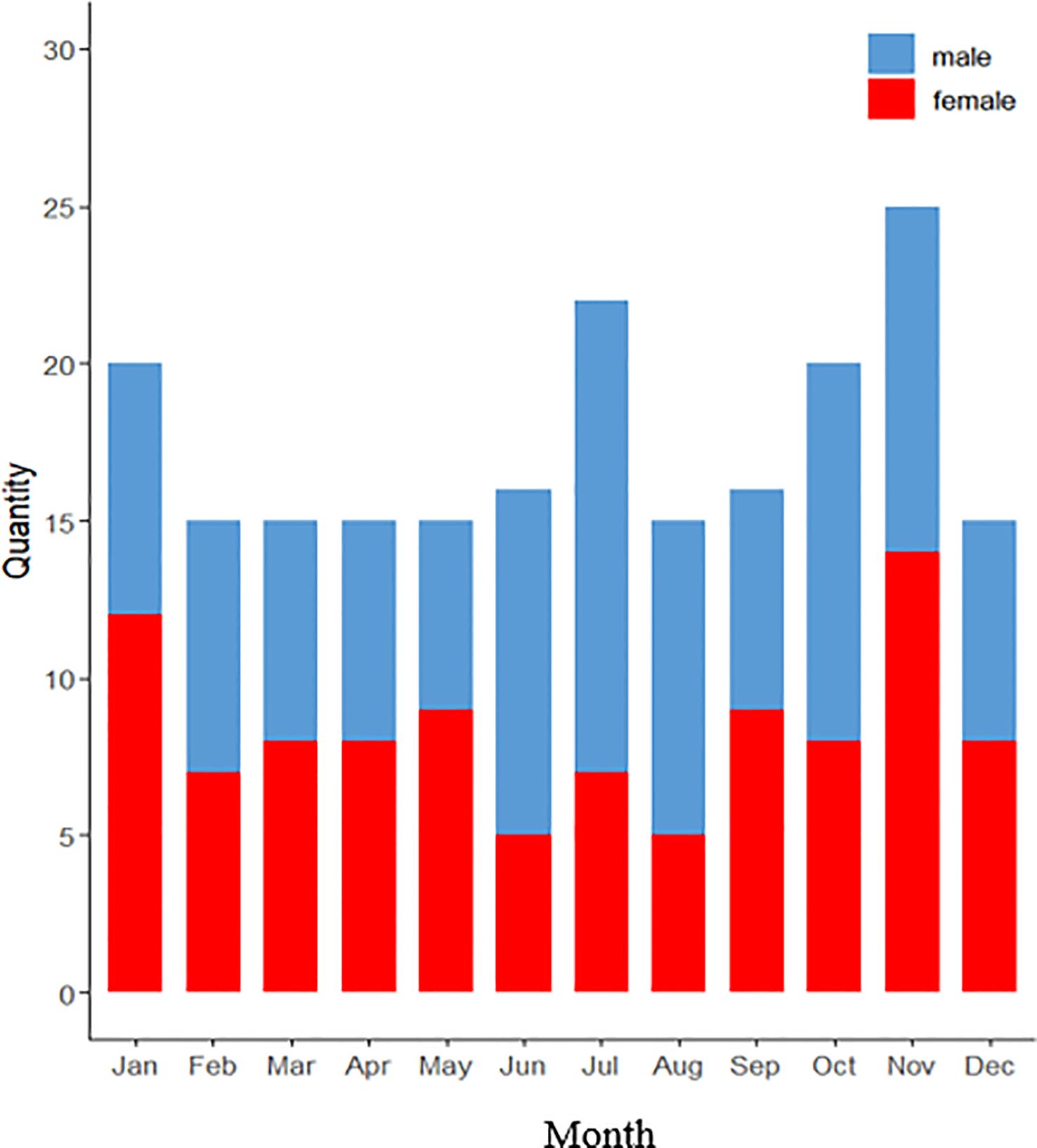
The gonadal development cycle of Tripneustes gratilla can be categorized into five stages (Figure 5). The gonad index (GI) exhibited pronounced cyclical variations, reflecting periodic spawning and recovery processes. Specifically, the reproductive cycle peaks in September (spawning peak), followed by a rapid decline in October and a secondary peak in December (Table 2). The highest monthly average GI values were recorded in September (8.26 ± 2.99) and December (7.30 ± 3.15), whereas the lowest value occurred in June (1.72 ± 0.26). Notably, significant decreases in the GI preceded both peaks. Two-way ANOVA was conducted to assess the effects of month and sex on the GI tract and other biological indices (Table 3). The results indicated that month had a highly significant effect on GI (P < 0.001), whereas sex and the month–gender interaction had no significant effects. Boxplot analysis revealed an increase in the GI from May to August, followed by a decrease from September to December. Compared with males, female sea urchins presented significantly greater GI values in July and August (Figure 6). The female-to-male ratio remained relatively stable across months (Figure 7), although males predominated in most months, with an average ratio of 0.67. Additionally, both the ovarian and testicular maturation stages displayed marked cyclical patterns (Figure 8), with reproductive activity peaking in September, sharply declining in October, and reaching a secondary peak in December.

Table 2. Monthly variations in the test diameter, body weight, gonad weight, and gonadosomatic index (GI) of Tripneustes gratilla..
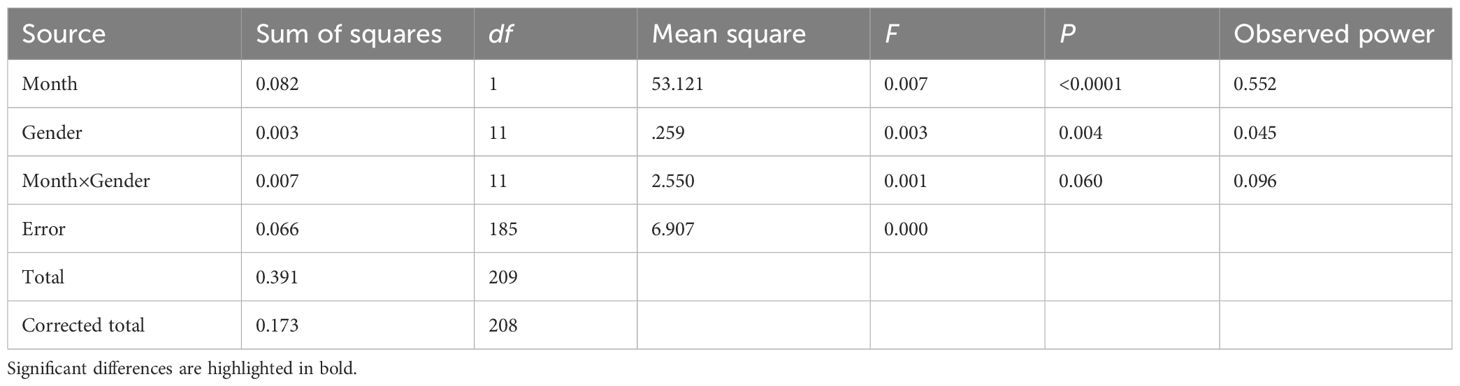
Table 3. ANOVA results comparing the monthly mean gonadosomatic index (GI) by sex in Tripneustes gratilla (α = 0.01).

Figure 6. Annual median gonadosomatic index (GI) by sex (boxplot). The monthly variations, ranges, distribution skewness, and outliers (rhombus symbols) are shown. *p<0.05, **p<0.01, ***p<0.001 (Mann-Whitney U test).

Figure 8. Annual cycle of gonadal development in Tripneustes gratilla: (A) ovary and (B) testis maturation stages (relative frequency).
3.3 Influence of temperature on gonadal development in Tripneustes gratilla
The gonad index (GI) of the sea urchin Tripneustes gratilla exhibits significant seasonal fluctuations throughout the year, reaching its annual peak in September and the lowest value in June. The variation pattern of water temperature aligns with GI trends, showing that GI increases correspondingly with rising water temperatures, particularly during the peak reproductive period (July to September) (Figure 9).

Figure 9. Monthly mean gonadosomatic index (GI, mean ± SE) and seawater temperature (°C) for Tripneustes gratilla.
4 Discussion
4.1 Seasonal variations in test diameter and wet weight and their synergistic effects
This study revealed that the seasonal fluctuations in the body size (test diameter and wet weight) of Tripneustes gratilla not only respond to environmental changes but also reflect dynamic optimization processes involving trade-offs between reproduction and growth (Röpke et al., 2021; Stearns, 1992) and energy allocation strategies (He et al., 2025; Kooijman, 2009). Both the test diameter and wet weight peaked from May to June (Figure 2), coinciding with the critical gametogenesis phase, suggesting that biomass accumulation is primarily allocated to gonadal development (Silva et al., 2013). Prior to the reproductive season, individuals increase their feeding efficiency to allocate resources to somatic growth (test calcification) and reproductive tissue proliferation (Schneider, 2004), with the rigid test serving as a “visible carrier” of energy storage. However, the subsequent decline in biomass (September–October) highlights an ecological paradox: despite higher primary productivity in summer, increased metabolic costs (e.g., elevated respiration rates due to high temperatures) and reproductive expenditure (gamete release) may force sea urchins into an “energy deficit state,” leading to arrested test growth or even calcium resorption (Schneider, 2004). This periodic contraction suggests that T. gratilla may exhibit phenotypic plasticity to adapt to seasonal resource fluctuations, with body size variations reflecting a shift in survival strategy from “maximizing growth” to “maintaining metabolic homeostasis”.
The allometric growth equation for the test diameter and wet weight (y=0.0194x2.048, R²=0.7354) further revealed evolutionary constraints on morpho-functional coupling. An exponent >2 indicates that wet weight increases significantly faster than linear test expansion does, deviating from the geometric expectation of isometric growth (expected exponent ≈ 3) (Boukal et al., 2014), which is likely due to visceral mass and gonadal volume expansion during reproduction (Bennett and Giese, 1955). This result demonstrates that wet weight not only is an indicator of structural growth but also reflects energy storage (e.g., glycogen deposition) and reproductive investment (gamete mass). Notably, the dominance of 70–90 mm individuals in the population (Figure 3) may reflect stabilizing selection: smaller individuals face negative selection due to weaker stress resistance (Himmelman et al., 1984), whereas larger individuals may suffer from higher energy maintenance costs (Tan et al., 2021) or reduced reproductive efficiency (e.g., increased gamete dispersal distance) (Byrne et al., 2003).
From an ecosystem perspective, the strong negative correlation between test diameter and month (P < 0.01) suggests that climate warming may have cascading effects on the population by altering phenological rhythms. If earlier spring warming leads to a mismatch between reproductive preparation and phytoplankton blooms (Jeppesen et al., 2010), the dominance of medium-sized individuals could be disrupted, affecting population age structure stability. This mechanism could be quantitatively predicted using dynamic energy budget (DEB) models (Nisbet et al., 2012) and inform adaptive fisheries management strategies—e.g., coupling closed seasons with real-time monitoring of test diameter–energy status (Liu et al., 2023). In addition to elucidating the physiological drivers of size variation, this study proposes a broader hypothesis: seasonal morphological plasticity in invertebrates may represent a “cryptic adaptive strategy” under environmental fluctuations (Stoks et al., 2014), whose evolutionary significance warrants reevaluation within the framework of energy constraints and life-history trade-offs.
4.2 Bimodal gonadal development and ecological adaptation in Tripneustes gratilla
This study identified a unique bimodal fluctuation in the gonadosomatic index (GI) of T. gratilla, with a primary peak in September and a secondary peak in December, challenging the conventional single-spawning paradigm in sea urchin reproductive ecology (Walker et al., 2001). This bimodality may reflect adaptive evolution under heterogeneous environmental pressures: the September spawning peak aligns with seasonal maxima in environmental factors (e.g., water temperature; Figure 9) to ensure larval hatching during high food availability, whereas the December peak may act as a “reproductive bet-hedging strategy”, enhancing population resilience to unpredictable disturbances through supplemental spawning (Pirotta et al., 2019). The sharp GI declines preceding both peaks (Figure 5) align with the postspawning gonadal atrophy typical of echinoderms (Li and Yang, 2025), but the short interpeak interval (3 months) suggests exceptionally rapid gonadal regeneration, possibly an adaptation to high predation pressure or resource variability.
4.3 Environment–physiology coupling: temperature-driven reproductive synchrony
The strong GI–temperature correlation (Figure 9) underscores water temperature as a key regulator of reproductive cycles. The July–September warming phase coincides with GI surges, likely accelerating gametogenesis via temperature-dependent metabolic rates: warming promotes gonadal cell division and vitellogenesis (James et al., 2007) while increasing sex steroid concentrations (e.g., estradiol (E2) and testosterone (T)) (Yu et al., 2022). However, the December peak (typically a cold period) implies the existence of additional drivers (e.g., photoperiod or nutrient supply) (Shpigel et al., 2004). Although sex had no significant effect on the GI (Table 3), females presented a greater GI than males did in July–August (Figure 6), potentially because of greater energy demands for oogenesis than for spermatogenesis (Hernandez et al., 2020). The male-biased sex ratio (0.67 females/males), a common r-selected trait, may reduce sperm competition costs.
5 Conclusions
In summary, the population structure and size distribution of Tripneustes gratilla in the waters near Xincun Town, Lingshui Li Autonomous County, Hainan Province are influenced by seasonal dynamics and fishing pressure, exhibiting allometric growth. The extended reproductive period (May–December) features two spawning peaks (September and December). Reducing fishing intensity during these critical windows is essential to protect mature individuals and enhance their recruitment. These findings provide a theoretical foundation for artificial breeding (e.g., to select for high-quality gonads) and sustainable wild stock management of T. gratilla.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study protocol was approved by the Laboratory Animal Welfare and Ethics Committee of the South China Sea Fisheries Research Institute (CAFS) (nhdf2025-11). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
CQ: Writing – review & editing, Funding acquisition, Resources, Investigation. ZL: Visualization, Writing – original draft, Formal Analysis, Data curation, Investigation. XZ: Writing – review & editing, Project administration. JZ: Investigation, Writing – review & editing. YG: Investigation, Writing – review & editing. GY: Investigation, Writing – review & editing, Funding acquisition, Resources. ZM: Resources, Funding acquisition, Writing – review & editing, Investigation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by National Key R&D Program of China (2024YFD2401805), the Guangdong Province Basic and Applied Basic Research Fund Project (2023B1515250004), the National Natural Science Foundation of China (32160863).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Azad A. K., Pearce C. M., and McKinley R. S. (2011). Effects of diet and temperature on ingestion, absorption, assimilation, gonad yield, and gonad quality of the purple sea urchin (Strongylocentrotus purpuratus). Aquaculture 317, 187–196. doi: 10.1016/j.aquaculture.2011.03.019
Bennett J. and Giese A. C. (1955). The annual reproductive and nutritional cycles in two western sea urchins. Biol. Bull. 109, 226–237. doi: 10.2307/1538723
Boukal D. S., Dieckmann U., Enberg K., Heino M., and Jørgensen C. (2014). Life-history implications of the allometric scaling of growth. J. Theor. Biol. 359, 199–207. doi: 10.1016/j.jtbi.2014.05.022
Bronstein O., Kroh A., Tautscher B., Liggins L., and Haring E. (2017). Cryptic speciation in pan-tropical sea urchins: A case study of an edge-of-range population of Tripneustes from the Kermadec Islands. Sci. Rep. 7, 5948. doi: 10.1038/s41598-017-06183-2
Byrne M. (1990). Annual reproductive cycles of the commercial sea urchin Paracentrotus lividus from an exposed intertidal and a sheltered subtidal habitat on the west coast of Ireland. Mar. Biol. 104, 275–289. doi: 10.1007/BF01313269
Byrne M. and Hernández J. C. (2020). “Sea urchins in a high CO2 world: Impacts of climate warming and ocean acidification across life history stages,” in Developments in Aquaculture and Fisheries Science. Ed. Lawrence J. M. (Amsterdam, The Netherlands: Elsevier), 281–297. doi: 10.1016/B978-0-12-819570-3.00016-0
Byrne P. G., Simmons L. W., and Roberts J. D. (2003). Sperm competition and the evolution of gamete morphology in frogs. Proc. R. Soc. B: Biol. Sci. 270, 2079–2086. doi: 10.1098/rspb.2003.2433
Carnevali M. D. C., Sugni M., and Bonasoro F. (2024). “Regeneration potential in echinoderms: Revisiting the regeneration concept,” in Frontiers in Invertebrate Physiology: A Collection of Reviews (Apple Academic Press, Oakville).
Dang V. D. H., Cheung P.-Y., Fong C.-L., Mulla A. J., Shiu J.-H., Lin C.-H., et al. (2020). Sea urchins play an increasingly important role for coral resilience across reefs in Taiwan. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.581945
Delgado M. and Pérez-Camacho A. (2005). Histological study of the gonadal development of Ruditapes decussatus (L.) (Mollusca: Bivalvia) and its relationship with available food. Scientia Marina 69, 87–97. doi: 10.3989/scimar.2005.69n187
Ding J., Zheng D., Sun J., Hu F., Yu Y., Zhao C., et al. (2019). Effects of water temperature on survival, behaviors and growth of the sea urchin Mesocentrotus nudus: New insights into the stock enhancement. Aquaculture 519, 734873. doi: 10.1016/j.aquaculture.2019.734873
Dworjanyn S. A., Pirozzi I., and Liu W. (2007). The effect of the addition of algae feeding stimulants to artificial diets for the sea urchin Tripneustes gratilla. Aquaculture 273, 624–633. doi: 10.1016/j.aquaculture.2007.08.023
Elmasry E., El-Sayed A.-F. M., and Abdelrazek F. A. (2023). Gonadal growth and qualitative color assessment of the sea urchin Paracentrotus lividus Lamarck 1816 (Echinodermata: Echinoidae) of the Southeastern Mediterranean Sea. Egyptian J. Aquat. Res. 49, 369–378. doi: 10.1016/j.ejar.2023.08.009
He L., Jiang B., Zheng X., Yuan J., and Lin Z. (2025). Application of a dynamic energy budget model to the blood clam, Tegillarca granosa, reared in culture pond. Aquac. Rep. 40, 102602. doi: 10.1016/j.aqrep.2024.102602
Hernandez E., Vázquez O. A., Torruco A., and Rahman M. S. (2020). Reproductive cycle and gonadal development of the Atlantic sea urchin Arbacia punctulata in the Gulf of Mexico: Changes in nutritive phagocytes in relation to gametogenesis. Mar. Biol. Res. 16, 177–194. doi: 10.1080/17451000.2020.1731758
Himmelman J. H., Guderley H., Vignault G., Drouin G., and Wells P. G. (1984). Response of the sea urchin, Strongylocentrotus droebachiensis, to reduced salinities: Importance of size, acclimation, and interpopulation differences. Can. J. Zool. 62, 1015–1021. doi: 10.1139/z84-144
James P. J., Heath P., and Unwin M. J. (2007). The effects of season, temperature and initial gonad condition on roe enhancement of the sea urchin Evechinus chloroticus. Aquaculture 270, 115–131. doi: 10.1016/j.aquaculture.2007.03.011
Jeppesen E., Meerhoff M., Holmgren K., González-Bergonzoni I., Teixeira-de Mello F., Declerck S. A. J., et al. (2010). Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia 646, 73–90. doi: 10.1007/s10750-010-0171-5
Jisr N., Younes G., Sukhn C., and El-Dakdouki M. H. (2018). Length-weight relationships and relative condition factor of fish inhabiting the marine area of the Eastern Mediterranean city, Tripoli-Lebanon. Egyptian J. Aquat. Res. 44, 299–305. doi: 10.1016/j.ejar.2018.11.004
Kooijman S. A. L. M. (2009). Dynamic Energy Budget Theory for Metabolic Organisation (Cambridge, UK: Cambridge University Press).
Li Z. and Yang Y. (2025). Reproductive physiology and molecular mechanisms underlying testicular development and spermatogenesis in echinoderms: A marine invertebrate deuterostomes. Mol. Reprod. Dev. 92, e70011. doi: 10.1002/mrd.70011
Liu Y., Zhang C., Wei X., Xu B., Xue Y., Ren Y., et al. (2023). Coupling dynamic energy budget and population dynamic models to inform stock enhancement in fisheries management. Fish Fisheries 24, 924–939. doi: 10.1111/faf.12776
Liu M., Lin W., Lin M., Dong L., Liu B., Chen S., et al. (2024). Species diversity and critical habitats of offshore and deep-diving cetaceans in the South China Sea. Biol. Conserv. 299, 110808. doi: 10.1016/j.biocon.2024.110808
Liu Z., Guo Y., Qin C., Mu X., and Zhang J. (2024). High-throughput sequencing analysis revealed a preference for animal-based food in purple sea urchins. Biology 13, 823. doi: 10.3390/biology13080623
Marañón E., Lorenzo M. P., Cermeño P., and Mouriño-Carballido B. (2018). Nutrient limitation suppresses the temperature dependence of phytoplankton metabolic rates’. ISME J. 12, 1836–1845. doi: 10.1038/s41396-018-0105-1
Nisbet R. M., Jusup M., Klanjscek T., and Pecquerie L. (2012). Integrating dynamic energy budget (DEB) theory with traditional bioenergetic models. J. Exp. Biol. 215, 892–902. doi: 10.1242/jeb.059675
O’Hara T. E. and Thórarinsdóttir G. G. (2021). A depth-dependent assessment of annual variability in gonad index, reproductive cycle (gametogenesis) and roe quality of the green sea urchin (Strongylocentrotus droebachiensis) in Breidafjördur, west Iceland. Regional Stud. Mar. Sci. 45, 101846. doi: 10.1016/j.rsma.2021.101846
Okamoto D. K., Schroeter S. C., and Reed D. C. (2020). Effects of ocean climate on spatiotemporal variation in sea urchin settlement and recruitment. Limnol. Oceanogr. 65, 837–850. doi: 10.1002/lno.11440
Pirotta E., Mangel M., Costa D. P., Goldbogen J., Harwood J., Hin V., et al. (2019). Anthropogenic disturbance in a changing environment: Modelling lifetime reproductive success to predict the consequences of multiple stressors on a migratory population. Oikos 128, 1340–1357. doi: 10.1111/oik.06146
Qi Z., Wang J., Mao Y., Zhang J., Jiang Z., and Fang J. (2014). Use of the sea urchin Hemicentrotus pulcherrimus for biological control of fouling in suspended scallop cultivation in Northern China. Aquaculture 420–421, 270–274. doi: 10.1016/j.aquaculture.2013.11.020
Qin C., Chen P., Sarà G., Mo B., Zhang A., and Li X. (2020). Ecological implications of purple sea urchin (Heliocidaris crassispina, Agassiz 1864) enhancement on the coastal benthic food web: Evidence from stable isotope analysis. Mar. Environ. Res. 158, 104957. doi: 10.1016/j.marenvres.2020.104957
Rocha F., Baião L. F., Moutinho S., Reis B., Oliveira A., Arenas F., et al. (2019). The effect of sex, season and gametogenic cycle on gonad yield, biochemical composition and quality traits of Paracentrotus lividus along the North Atlantic coast of Portugal. Sci. Rep. 9, 2994. doi: 10.1038/s41598-019-39912-w
Röpke C., Pires T. H. S., Zuanon J., Freitas C. E. C., Hernandes M. C., Souza F., et al. (2021). Growth–reproduction trade-off and fecundity regulate population stability in Amazon floodplain fishes. Freshw. Biol. 66, 1101–1109. doi: 10.1111/fwb.13702
Schneider J. E. (2004). Energy balance and reproduction. Physiol. Behav. 81, 289–317. doi: 10.1016/j.physbeh.2004.02.007
Seymour S., Paul N. A., Dworjanyn S. A., and de Nys R. (2013). Feeding preference and performance in the tropical sea urchin Tripneustes gratilla. Aquaculture 400–401, 6–13. doi: 10.1016/j.aquaculture.2013.02.030
Shang Z., Jiang Y., Yang F., Wu K., Zheng G., Lin Y., et al. (2024). A homologous series of α-glucans from Hemicentrotus pulcherrimus and their immunomodulatory activity. Int. J. Biol. Macromol. 260, 129657. doi: 10.1016/j.ijbiomac.2024.129657
Shpigel M., McBride S. C., Marciano S., and Lupatsch I. (2004). ‘The effect of photoperiod and temperature on the reproduction of European sea urchin Paracentrotus lividus. Aquaculture 232, 343–355. doi: 10.1016/S0044-8486(03)00539-8
Silva F. F. G., Slotte A., Johannessen A., Kennedy J., and Kjesbu O. S. (2013). Strategies for partition between body growth and reproductive investment in migratory and stationary populations of spring-spawning Atlantic herring (Clupea harengus L.). Fisheries Res. 138, 71–79. doi: 10.1016/j.fishres.2012.07.013
Stoks R., Geerts A. N., and De Meester L. (2014). Evolutionary and plastic responses of freshwater invertebrates to climate change: Realized patterns and future potential. Evol. Appl. 7, 42–55. doi: 10.1111/eva.12108
Tan H., Hirst A. G., Atkinson D., and Kratina P. (2021). Body size and shape responses to warming and resource competition. Funct. Ecol. 35, 1460–1469. doi: 10.1111/1365-2435.13789
Urriago J. D., Wong J. C. Y., Dumont C. P., and Qiu J.-W. (2016). Reproduction of the short-spined sea urchin Heliocidaris crassispina (Echinodermata: Echinoidea) in Hong Kong with a subtropical climate. Regional Stud. Mar. Sci. 8, 445–453. doi: 10.1016/j.rsma.2016.03.005
Valentine J. P. and Edgar G. J. (2010). Impacts of a population outbreak of the urchin Tripneustes gratilla amongst Lord Howe Island coral communities. Coral Reefs 29, 399–410. doi: 10.1007/s00338-010-0610-9
Walker C. W., Unuma T., McGinn N. A., Harrington L. M., and Lesser. M. P. (2001). “Reproduction of sea urchins,” in Developments in Aquaculture and Fisheries Science, vol. 32 . Ed. Lawrence J. M. (Amsterdam, The Netherlands: Elsevier), 5–26. doi: 10.1016/S0167-9309(01)80003-X
Yu J., Li D., Zhu J., Zou Z., Xiao W., Chen B., et al. (2022). Effects of different oxytocin and temperature on reproductive activity in Nile tilapia (Oreochromis niloticus): Based on sex steroid hormone and GtHR gene expression. Fishes 7, 316. doi: 10.3390/fishes7060316
Zhadan P. M., Vaschenko M. A., and Almyashova T. N. (2017). “Effects of environmental factors on reproduction of the sea urchin Strongylocentrotus intermedius,” in Sea Urchin—From Environment to Aquaculture and Biomedicine (Rijeka, Croatia: IntechOpen). doi: 10.5772/intechopen.69511
Keywords: Tripneustes gratilla, gonadal development, reproductive cycle, population characteristics, temperature regulation
Citation: Liu Z, Zhao X, Zhang J, Guo Y, Yu G, Ma Z and Qin C (2025) Temperature modulates bimodal reproduction and energy allocation in the sea urchin Tripneustes gratilla. Front. Anim. Sci. 6:1635191. doi: 10.3389/fanim.2025.1635191
Received: 26 May 2025; Accepted: 28 August 2025;
Published: 23 September 2025.
Edited by:
Ilhan Aydin, Ministry of Agriculture and Forestry of Türkiye, TürkiyeReviewed by:
Andressa Teles, Centro de Investigación Biológica del Noroeste (CIBNOR), MexicoEsin Batir, University of Rome Tor Vergata, Italy
Copyright © 2025 Liu, Zhao, Zhang, Guo, Yu, Ma and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanxin Qin, cWluY3hAc2NzZnJpLmFjLmNu
†These authors share first authorship
 Zerui Liu1,2,3†
Zerui Liu1,2,3† Yu Guo
Yu Guo Zhenhua Ma
Zhenhua Ma Chuanxin Qin
Chuanxin Qin