- 1Department of Veterinary Medicine and Animal Production, University of Napoli Federico II, Naples, Italy
- 2Department of Precision and Regenerative Medicine and Jonian Area, Section of Veterinary Science and Animal Production, University of Bari Aldo Moro, Valenzano, Italy
- 3Department of Veterinary Medicine, University of Bari Aldo Moro, Valenzano, Italy
- 4Institute for the Animal Production Systemin the Mediterranean Environment, National Research Council, Portici, Italy
The aim of the present study was to evaluate the effect of dietary supplementation with fennel seeds powder (FSP) on dairy goats. Twenty goats, homogeneous in terms of body weight (BW), days in milk (DIM), daily milk yield (DMY), and parity, were randomly assigned into two groups (CON: control; FEN: fennel). Both groups were fed alfalfa hay and corn in the barn, with the treated group (FEN) receiving an additional 15 g/head/day of FSP. From findings, no significant differences were detected in milk yield or milk chemical composition, although some fatty acids showed variations between the groups. Additionally, a gene expression analysis was conducted on candidate genes related to milk production, lipid metabolism and the antioxidant system without revealing any differences. However, this field of research appears promising, and further studies are needed to standardize the optimal dosage to achieve positive effects on yield and genomic activation.
1 Introduction
To improve production efficiency, optimize animal health and address sustainability concerns, researchers and farmers are increasingly turning to nutraceuticals. These bioactive compounds derived from natural sources offer potential health benefits (Losacco et al., 2025). They serve as alternative feed additives and positively influence the immune system through antioxidant, anti-inflammatory and antimicrobial effects (Iommelli et al., 2025a) having as a result improved animal health and productivity. Fennel (Foeniculum vulgare Mill) is an aromatic plant belonging to the Apiaceae family, traditionally used in human health care for its medicinal properties (Badgujar et al., 2014). It is valued for its carminative, digestive and diuretic properties (Rather et al., 2016). Its major bioactive components include phenolic compounds, glycosides and volatile organic compounds (VOCs), with trans-anethole, estragole, limonene and fenchone being the most abundant.
In particular, fennel’s galactagogue effect is linked to its high content of trans-anethole, a monoterpene that inhibits dopamine binding to its receptors, acting as an antagonist (Hassanzadeh et al., 2022). This reduces the effect of dopamine on prolactin (PRL) (Benker et al., 1990), allowing PRL to remain active for longer, which directly stimulates milk production by acting on the mammary glands (Lacasse et al., 2012). Although animal studies are scarce, fennel seeds have been tested in dairy ruminants with positive results. In a study, El-Hendawy et al. (2018) tested fennel seed supplementation in Damascus goats and observed improvements in reproductive parameters, milk yield (MY) and milk composition as also observed in Murciana grazing goats in our previous trial (Iommelli et al., 2025b). Recently, Moosavi-Zadeh et al. (2023) investigated the effect of fennel on the diet of dairy cows by assessing milk production characteristics. In both cows and goats, fennel supplementation increased MY, although lactose, protein and fat content showed variable results.
It has already been demonstrated that nutritional factors, including various dietary components such as macronutrients, micronutrients, phytochemicals, bioactive compounds and xenobiotics are able to influence gene regulation (Bonet and Palou, 2020). The increasing use of molecular biology techniques and bioinformatics in research on ruminant nutrition and physiology has significantly deepened our understanding of the regulatory mechanisms underlying key biological processes such as milk production. For example, PUFA have been observed to suppress the expression of the FASN (Simopoulos, 2020) and to exert an influence on SCD expression (Bernard et al., 2009), both genes involved into the regulatory mechanism of milk fat production.
Therefore, the aim of this study was to evaluate the effects of supplementing the diet of lactating goats with fennel seed powder (FSP). The study also hypothesized that the inclusion of FSP in the diet would increase milk production due to the galactagogue properties of fennel and improve nutritional quality due to its high antioxidant content. In addition, gene expression analyses were carried out to identify differences in the expression of genes involved in milk production, antioxidant activity and lipid metabolism.
2 Material and methods
2.1 Experimental design
The feeding trial was performed at Azienda Zootecnica Antonio Amato located in Casaletto Spartano (SA, Italy; 832 m a.s.l., 40°09’ N; 15°37’E) on 20 multiparous lactating Murciana goats according to the Animal Welfare and Good Clinical Practice (Directive 2010/63/EU), lasted 4 weeks and was approved by the local Animal Ethic Committee (protocol number: PG/2019/0070006). Twenty animals, homogeneous for parity (3rd), days in milk (DIM: 60 ± 7.2), body weight (BW: 50 kg ± 2.0 kg) and MY (1922 ± 210 g/day), were randomly allocated into two groups (CON: control and FEN: fennel). All the animals were housed in stall and received 1 kg of alfalfa hay and 700 g/head/day of corn meal in individual pen; in addition, group FEN received 15 g/head/day of fennel seeds powder (FSP, 400-450 µm) (Foeniculum vulgare) which was mixed into the concentrate before been offered to the animals. An adaptation period of 7 days was considered for the treated group for the acclimatization to the new dietary treatment.). The dose was selected according to previous studies in which fennel seeds were used and were able to exert significant positive results on milk productive traits (Iommelli et al., 2025b; El-Hendawy et al., 2018). All the animals had free access to fresh water and feed refusal were daily measured.
2.2 Samples collection
Feed samples were collected at the beginning and at the end of the trial whereas individual MY was daily measured and samples were collected consecutively for the three days at the beginning and at the end of the trial.
2.3 Total phenolic content and antioxidant activity
The total phenolic content (TPC) in fennel seeds samples was determined according to the Folin-Ciocalteu colorimetric method described by Taga et al. (1984). A 0.5 g of powder was dissolved in 10 mL of distilled water and vortexed at 2000 rpm for 5 min (Heathrow Scientific Vortexer, Vernon Hill, IL, USA). The supernatant was immediately collected in a Falcon tube. An aliquot of 250 μL supernatant was mixed with 1250 μL Folin–Ciocalteu reagent and 1000 μL of 7.5% anhydrous sodium carbonate. The mixture was vortexed and incubated in the dark for 120 min. Absorbance was then measured at 760 nm using a UV-VIS spectrophotometer (Hitachi U-2001, Tokyo, Japan). A calibration curve was constructed with gallic acid solutions (0–1000 μg/mL), and results were expressed as mg gallic acid equivalents (GAE)/g. To evaluate the antioxidant activity of the samples, the 2,2 -azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) method was used according to Re et al. (1999), with modifications. Specifically, a 7 mM solution of ABTS in 2.45 mM aqueous potassium persulfate was prepared, and after 16 h of incubation at room temperature in the dark, the solution was diluted with ethanol to obtain an absorbance of 1.000 ± 0.020 at 734 nm. After, 100 µL of the extract according to Wojdyło et al. (2007), was added to 1000 µL of this solution. Measurements of absorbance were taken at 734 nm after 2.5 min of incubation (Hitachi U-2001, Tokyo, Japan). In addition, Trolox was used as the reference and activities were expressed as Trolox equivalents (mg/g TE).
The antioxidant activity of the feed was determined using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay (Sanchez-Moreno et al., 1998). Briefly, 0.1 mL of sample was added to 3.9 mL of DPPH solution (60 μM in methanol) and incubated in the dark for 45 min. Absorbance was measured at 515 nm after 30 min of incubation in the dark at room temperature using a Hitachi U-2001 spectrophotometer (Hitachi, Tokyo, Japan). A calibration curve was prepared with DPPH solutions ranging from 10 to 60 μM. The half-maximal effective concentration (EC50, μg/mL) was defined as the sample concentration required to reduce the initial DPPH absorbance by 50%.
2.4 Volatile organic compounds analysis
The VOCs extraction from fennel seeds samples was performed by solid-phase microextraction (SPME), whereas the GC-MS analysis was carried out with a gas chromatograph (Perkin Elmer, Waltham, MA, USA) coupled with a mass spectrometer (SQ8S; Perkin Elmer, USA). The gas chromatograph was equipped with an Elite-5MS column (Perkin Elmer, USA). A total of 5 g of sample were mixed with 10 ml of saturated sodium chloride solution (360 g/l), and then 10 μl of internal standard solution (4-methyl-2-heptanone; 10 mg/kg in ethanol) was added. The vials were sealed with a polytetrafluoroethylene-silicone septum (Supelco, Bellefonte, PA, USA) and stirred at 60°C; VOCs were extracted from the headspace with a divinylbenzene-carboxen-polydimethylsiloxane SPME fiber (Supelco, USA) with an exposition time of 60 min. After adsorption time, the extracted VOC were thermally desorbed into the gas chromatograph injector splitless mode for 1 min at 250°C. The oven temperature was held at 50°C for 1 min, increased at a rate of 3°C/min up to 200°C and held for 1 min, and then increased from 200°C to 250°C at 100.15°C/min and held for 15 min. Helium was used as a carrier gas at a flow rate of 1 ml/min. The mass spectrometer operated in electronic impact ionization mode at 70 eV, and data were collected in full scan mode. Source and interface temperature were held at 250°C. Compounds were identified by comparing their mass spectra with those contained in the National Institute of Standards and Technology (NIST) 14 library (Gaithersburg, MD, USA). The compounds were considered as correctly identified when their spectra presented a library match factor > 85. The VOCs were expressed on percentage of total volatile compounds detected.
2.5 Chemical composition
2.5.1 Feed
Corn meal, fennel seeds and alfalfa hay were analyzed for dry matter (DM), crude protein (CP), ash, crude fiber (CF) and ether extract (EE) contents, according to AOAC (2012) procedures, and structural carbohydrates were determined as suggested by Van Soest et al. (1991). Feed nutritional value was calculated according to Inra–Nozière et al. (2018).
2.5.2 Milk
Milk samples (150 ml) were analyzed for fat, lactose and protein content by Milko Scan 133B (Foss Matic, Hillerod, Denmark) standardized for goat milk.
2.6 Fatty acids analysis
2.6.1 Feed
Lipid fraction of feed samples was extracted from 15 g of sample using a mixture of chloroform and methanol (2/1 vol/vol) according to Gray et al. (1967) for the analysis of fatty acid (FA) profile. Lipid extract was methylated adding 1 mL of hexane and 0.05 ml of 2 N methanolic KOH. Separation of the methyl esters in forage and cheese samples was performed according to Di Trana et al. (2015). Fatty acid methyl esters (FAME) were identified with reference to the retention time of FA standard mixture of Supelco 37 Component FAME Mix (Supelco, Bellafonte, PA). The content of FA was quantified using internal standards (Supelco, Bellafonte, PA) added during the methylation step. Briefly, 100 mg of lipid extract was mixed with 50 µl of 2 N methanolic KOH and 1 ml of hexane containing the internal standards (20 mg/mL) according to Giorgio et al. (2019). Fatty acid methyl esters were fractionated over a CP-SIL883 column (100 × 0.25 mm i.d., film thickness 0.20 μm fused silica; Varian, Palo Alto, CA, USA) in a Shimadzu (Model 2GC17A) gas chromatograph with a Hewlett-Packard HP 6890 gas system and using flame ionization detection. Helium was used as carrier gas at a constant flow rate of 1.7 ml/min. The oven temperature was programmed as follows: 175°C, held for 4 min; 175–250°C at 3°C/min; and then maintained for 20 min. The injector port and detector temperature were 250°C. Samples (1 μl) were injected with an auto-sampler. Output signals were identified and quantified from the retention times and peak areas of known calibration standards. Composition was expressed as percentages of the total FA. All determinations were carried out in triplicate.
2.6.2 Milk
The FA profile (50 ml of milk) was determined as described by Tudisco et al. (2014). Briefly, milk fat was extracted by using hexane and isopropane (3/2 v/v) (Hara and Radin, 1978). The FAME were prepared by direct transesterification with sulfuric acid and methanol (1:9, v/v) of the lipids (Christie, 1993) and then analyzed in a gas chromatograph (Agilent technologies, model 5890) fitted with an SP-2560 fused silica capillary column (100 m × 0.25 mm i.d. × 0.2 µm film thickness, Supelco, Inc., Bellefonte, PA, USA). Helium was used as carrier gas and set at a constant pressure of 180 kPa, splitting flow of 50 mL/min, and injection volume of 1 µL. The column parameters were: initial temperature of the column maintained at 170°C for 15 min; then, with an increase of 5°C/min, it was brought up to 240°C. The total execution time was 64 min. Fatty acid peaks were identified using pure methyl ester external standards (Larodan Fine Chemicals, AB, Limhamnsgardens Malmo, Sweden). Additional standards for CLA isomers were obtained from Larodan. Chromatogram peak areas were acquired and calculated by Chemstation software (Agilent, technologies) and expressed as g/100g, considering 100g as the sum of the areas of all FAME identified. All determinations were carried out in triplicate. Additionally, FA ratios C14:1/C14:0, C16:1/C16:0, C18:1/C18:0 were measured to evaluate the desaturation activity of stearoyl CoA desaturase (Tudisco et al., 2019a).
2.7 Gene expression analysis
2.7.1 Milk fat sampling and RNA isolation
At two time points (T0 = beginning of the trial and T1 = end of the trial), during the morning milking, the milk samples for gene expression analysis were collected as follows. The udder was washed, dried and disinfected and a total of 50 mL of milk, obtained from the morning milking, were individually collected from each animal in sterilized, RNAse free falcon tubes and immediately centrifugated at 4000 g × 10 minutes at 4°C. One gram of milk fat layer was then scooped out in 15 ml tubes with 3 ml of TRIzol™ LS Reagent (Invitrogen Life Technologies) and vigorously shaken and vortexed for 30 seconds. The samples were immediately transferred in dry ice, transported to the lab and kept at -80°C until further analysis. The RNA extraction was performed following TRIzol™ LS Reagent protocol: after thawing in ice, the samples were vortexed and centrifugated at 10000 g x 10 minutes at 4°C in order to separate the liquid phase, which was transferred into four 2-ml sterilized tubes and added with 200 µL of chloroform. After centrifugation (10000 g ×15 minutes at 4°C) only the upper phase was taken and added in a new tube with isopropanol (500 µL) and left at -20°C over-night for RNA precipitation. After brief vortexing, the samples were centrifugated at 10000 g × 10 minutes at 4°C to obtain a small pellet by removing gently the isopropanol. One step of ethanol washing was performed by adding 1 mL of ethanol (75%) in each tube and completely removing it after a brief centrifugation (7500 g × 5 minutes at 4°C). The pellets obtained were then dissolved in 40 µl of sterilized RNAse free water and the kept at -80°C. Two aliquots of RNA samples were subjected to quantitative and qualitative analysis respectively with NanoDrop (ND-1000 spectrophotometer; NanoDrop, Labtech) to measure the absorbance at 260, 280 and 320 and a 2100 Bioanalyzer (Agilent Technologies) to determine RNA integrity. The mean value of RNA integrity number (RIN) was 4.5 (± 0.8), which is consistent with the results of other studies on milk fat compared to other matrices (Li et al., 2016).
2.7.2 Reverse transcription and real time qPCR
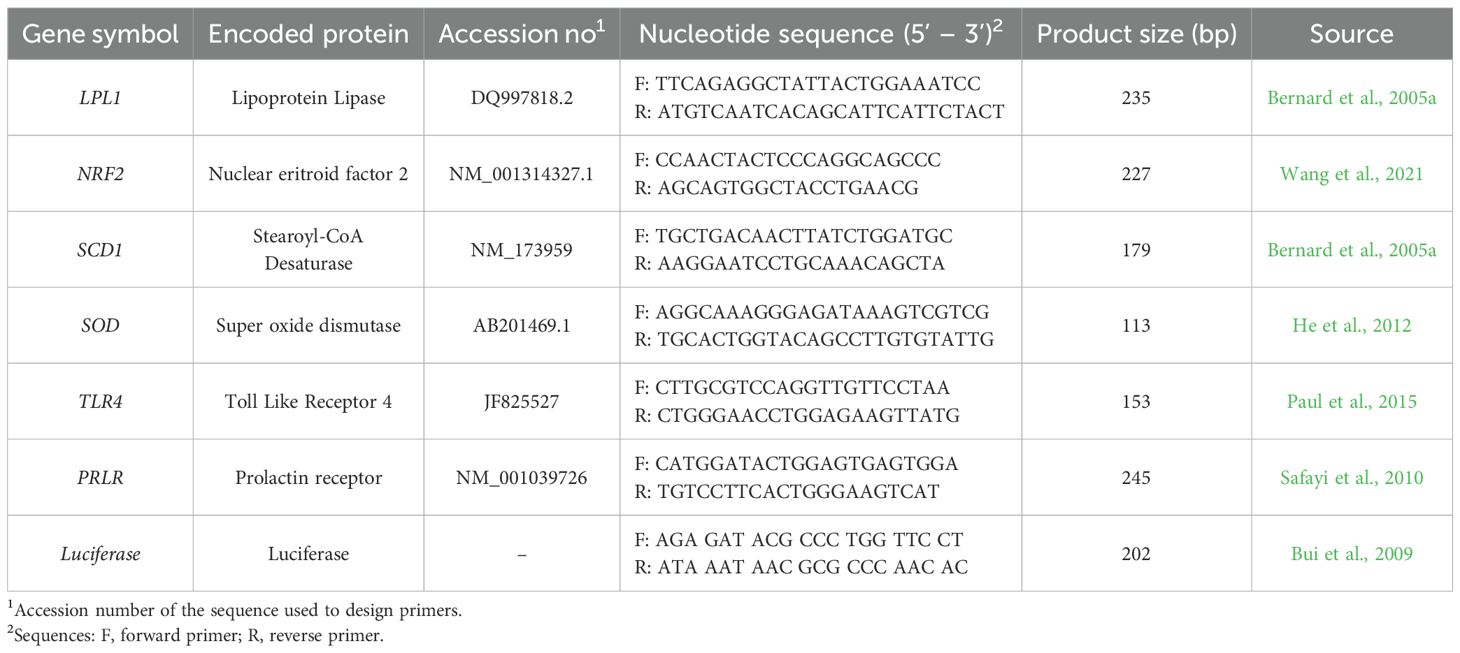
Reverse transcription (RT) was performed from 1 µg of total RNA by using High-Capacity RNA-to-cDNA™ Kit (Applied Biosystems) in a total volume of 20 µL, composed by 6 µL of RNA, 3 µL (2ng/µL) of Luciferase RNA Control (Promega) and 11 µL of Master Mix, according to the manufacturer’s instructions. The complementary DNA (cDNA) obtained was then stored at -20°C for RT-qPCR (Real Time qPCR). Samples of cDNA were finally subjected to RT-qPCR in duplicate with MicroAmp™ Fast Optical 96-Well Reaction Plates (Applied Biosystems) using Power SYBR™ Green PCR Master Mix (Applied Biosystems). The candidate genes (LPL1, NRF2, SCD1, SOD, TLR4, PRLR) were investigated and normalized by adopting Luciferase as reference gene (Jiwaji et al., 2010; Johnson et al., 2005). Each well was filled with 5 µL of cDNA (previously diluted 1:50 with nuclease free water) and 15 µL of Master Mix assembled by 10 µL of Mix SYBR, 2 µL of specific primers (1 µL Forward and 1 µL Reverse) and adjusted to volume of 15 µL with nuclease free water. RT-qPCR reaction was performed using 7300 Real-Time PCR System (Applied Biosystems) set up with the following program: denaturation at 95°C for 10 minutes; 40 cycles of PCR reactions at 95°C for 15 seconds and 60°C for 45 seconds according to the hybridization temperature. The samples were run in duplicate and compared to reference gene to obtain ΔCt (Terzi et al., 2004). Primers were specifically designed on goat reference mRNA sequences and were purchased by Merck (Sigma-Aldrich). Gene primers are reported in Table 1.
2.8 Statistical analysis
Data were analyzed by the one-way ANOVA with SPSS IBM software (V29.0.1.0). Differences were considered statistically significant at P < 0.05. Relative mRNA levels were then calculated for each gene using 2^-ΔCt method. The Ct of reference gene was subtracted from Ct of each gene to obtain ΔCt and then relative mRNA value was calculated by the formula 2^−ΔCt*100. Data were analyzed with two-way ANOVA with SPSS IBM software (V29.0.1.0) for repeated measures considering “treatment” and “period” as fixed factors and “animal” as a random factor.
3 Results
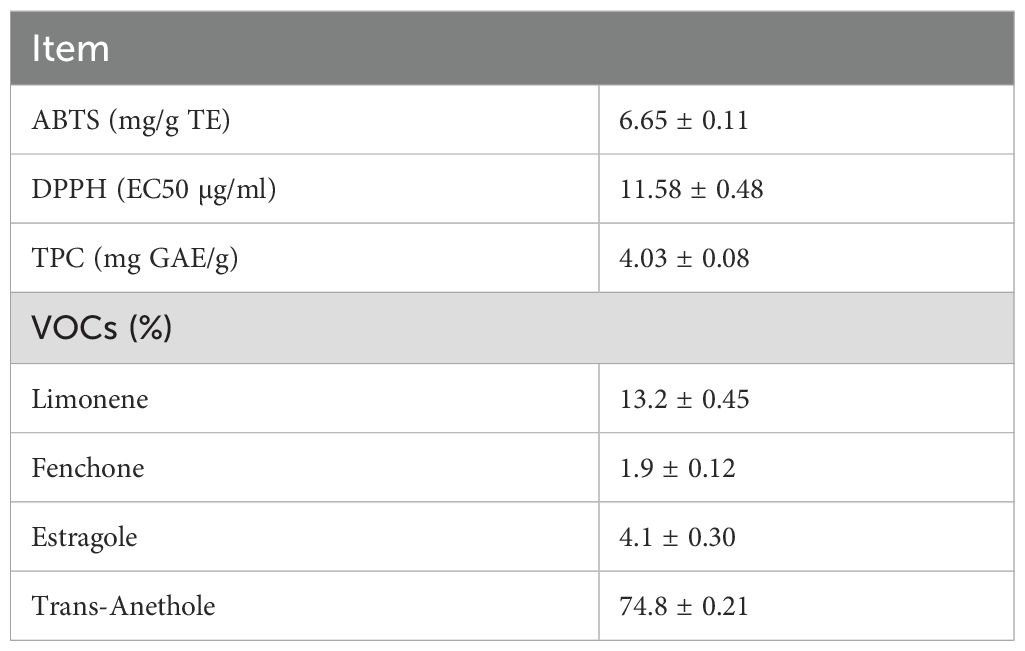
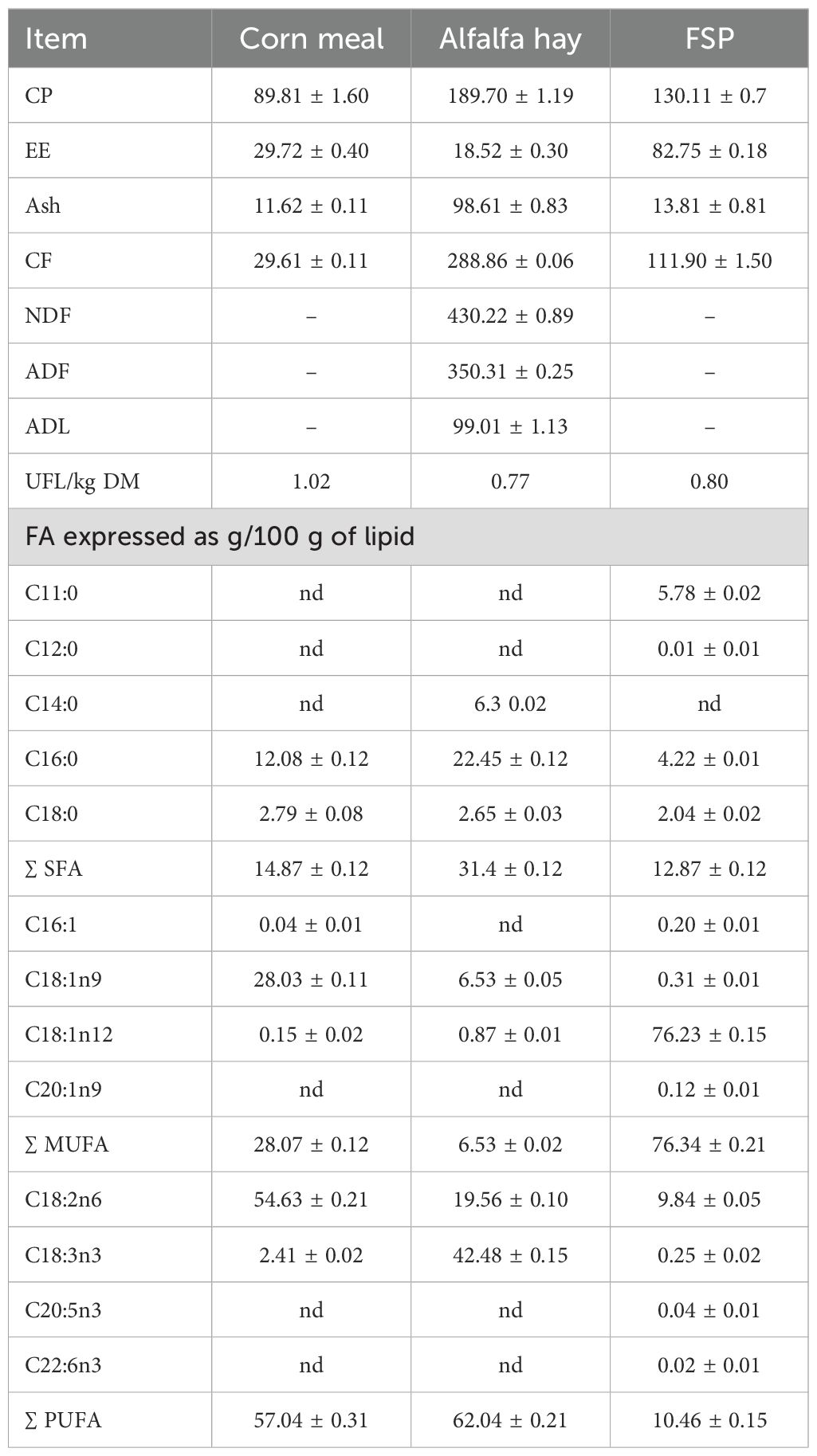
The antioxidant activity, TPC and VOCs composition of FSP are reported in Table 2. In particular, trans-anethole was found to be the most abundant VOC, followed by limonene, estragole and fenchone. Results on feed chemical composition are reported in Table 3. The FSP was characterized by a high lipid content. Corn meal and alfalfa hay had the highest amount of polyunsaturated fatty acids (PUFA). Specifically, corn meal was characterized by the highest amount of C18:2n6 (linoleic acid) whereas alfalfa hay had the highest amount of C18:3n3 (linolenic acid). The key FA of FPS were C18:1n12, characteristic of the family Apiaceae and C18:2n6.
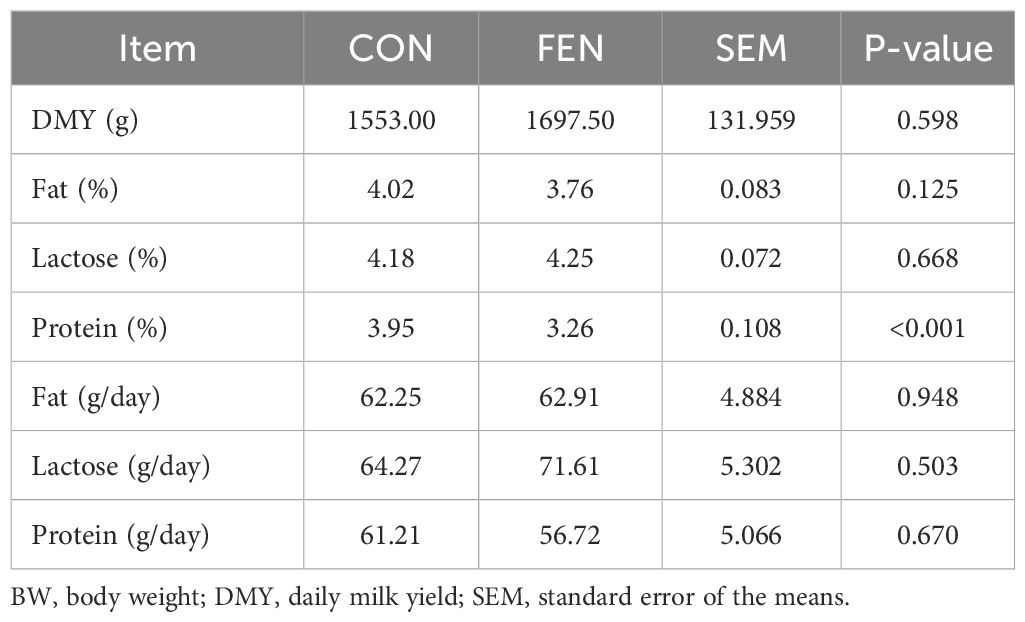
In Table 4 MY and chemical composition are presented. No feed refusal were detected; therefore, the dry matter intake (DMI) was 1460 g/head/day. Group FEN had higher MY even if it was not statistically significant (P > 0.05). No differences were observed for fat and lactose content whereas protein content resulted significantly lower in the supplemented group (P < 0.05). Fat, lactose and protein yield were also measured as g/day and were not affected by the treatment (P > 0.05).
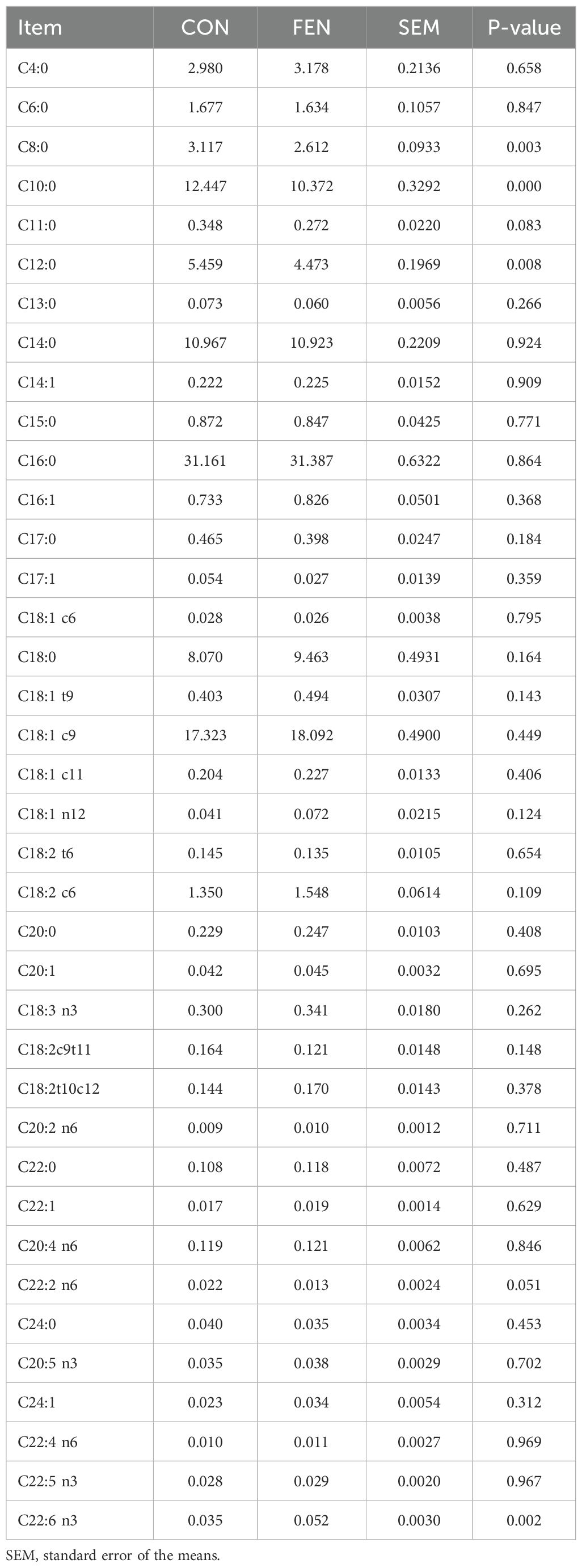
Milk FA profile of the two groups is reported in Table 5. Only few differences were detected between groups. Specifically, among short chain FA, C8:0, C10:0 and C12:0 resulted significantly lower (P < 0.05) in group treated and regarding long chain FA only C22:6 differed between groups, being higher (P < 0.05) in group FEN.
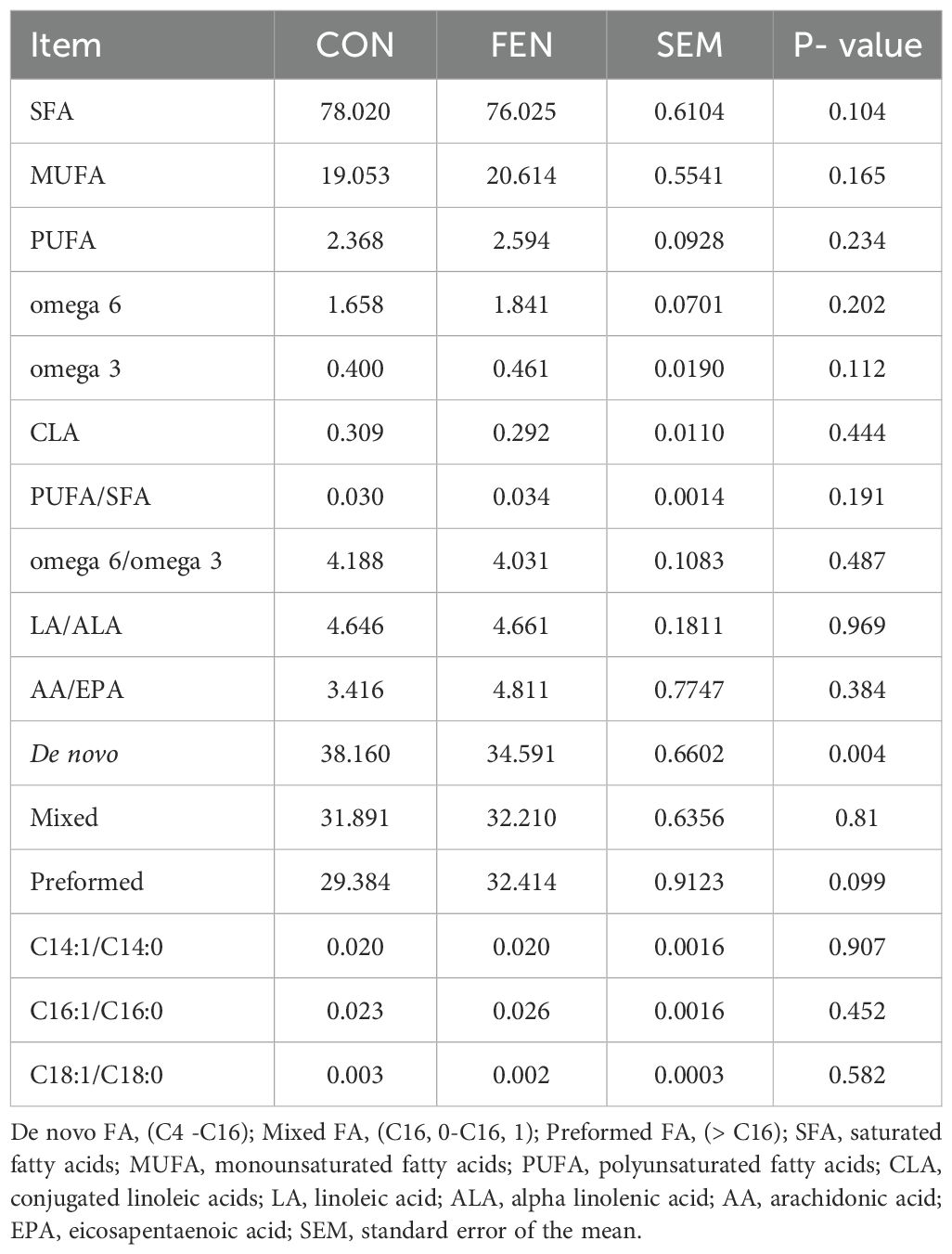
In Table 6 the main FA classes, ratios and indices are depicted. No differences were detected between groups for saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and PUFA content whereas de novo FA (C4:0 to C16:0) resulted significantly lower in group FEN.
In Table 7 the mRNA levels of the candidate genes for the two groups are presented. The expression of the candidate genes was not influenced by the treatment or by the period of sampling. The mRNA levels were found to be the highest for SCD1, followed by LPL and SOD. In contrast, the lowest values were observed for PRLR, NRF2 and TLR4.
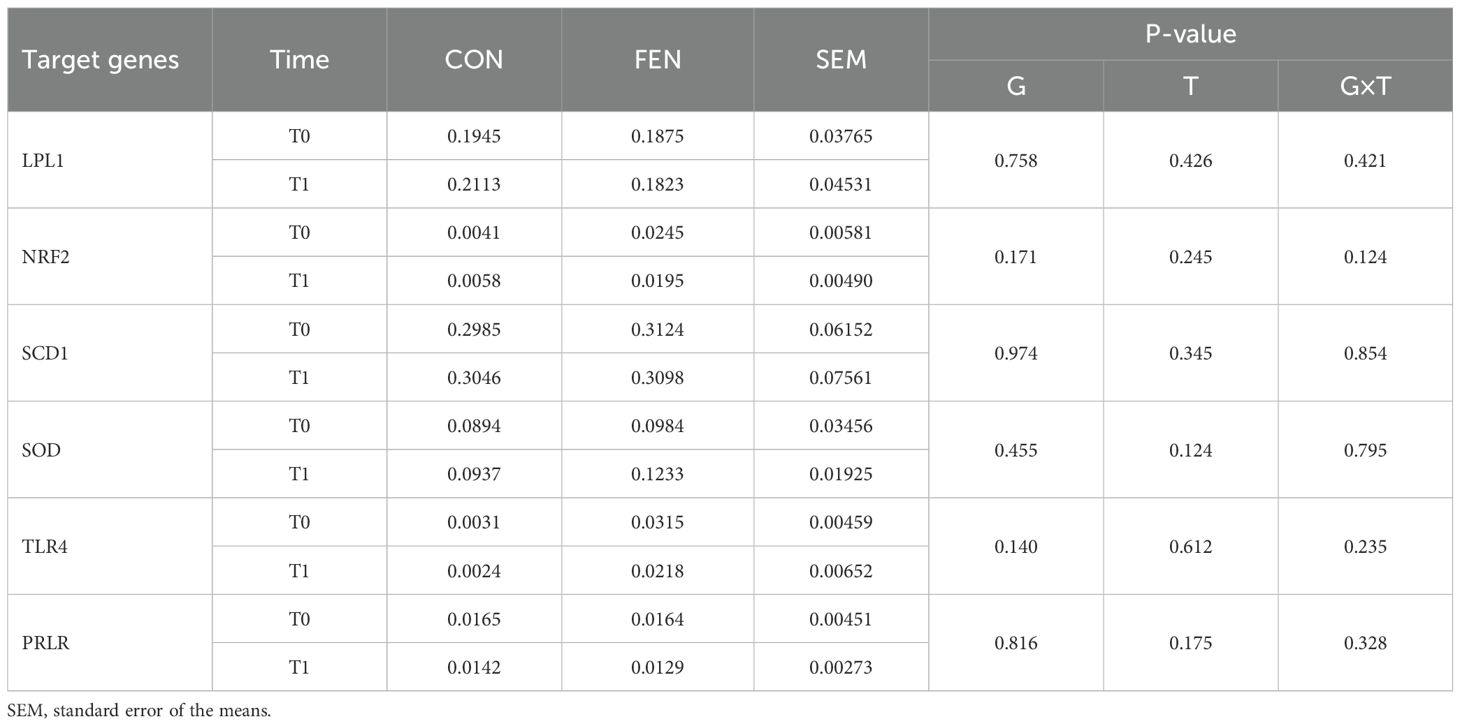
Table 7. Candidate gene expression at T0 (at beginning of the trial) and T1 (at the end of the trial).
4 Discussion
4.1 Diet
The chemical composition of alfalfa hay displayed high crude protein content and nutritive value, as reported by Zicarelli et al. (2022). Additionally, the hay exhibited a considerable concentration of n3 PUFA compared to corn meal and FSP. Indeed, the inclusion in the diet of n3 PUFAs has been demonstrated to increase the levels of beneficial FA in food of animal origin, thereby exerting favorable effects on human health (Trinchese et al., 2019). The results demonstrated that fennel seeds were rich in lipids, in accordance with the observations of Unal et al. (2013). The fennel FA profile was also found to be characterized by a high content of MUFA, primarily represented by petroselinic acid, and a relatively low content of PUFA and SFA, as reported by Najdoska-Bogdanov et al. (2015). Petroselinic acid (C18:1n12) is a distinctive constituent of the Apiaceae plant family, exhibiting particularly high concentrations in fennel oil. A further distinctive feature of the fennel FA profile is the presence of C11:0, which has been demonstrated to possess antimicrobial activity, particularly against bacteria responsible for alimentary diseases, in the form of a monoacylglycerol preparation (Doležalová et al., 2010).
4.2 Milk yield, chemical composition and fatty acid profile
The efficacy of fennel seeds as a galactagogues feeding supplement in several animal species has already been demonstrated (Moosavi-Zadeh et al., 2023; El-Hendawy et al., 2018; Fahim et al., 2022; Iommelli et al., 2025b) with results showing an improvement of performances in terms of MY and chemical composition. Indeed, fennel seeds are a rich source of trans-anethole, a compound that works as an antagonist of dopamine and is therefore capable of increasing PRL release. Furthermore, it has been demonstrated that fennel seeds improve feed efficiency and feed intake in calves and dairy cows (Kargar et al., 2021; Moosavi-Zadeh et al., 2023). In contrast to the findings of El-Hendawy et al. (2018), in the present work MY wasn’t affected by the treatment. Nevertheless, the productive characteristics exhibited by both groups are consistent with those reported for Murciana goats (Ehsaninia et al., 2025). This breed has become increasingly prominent in European countries with a substantial population, wide distribution and with high-quality milk production (Miranda et al., 2019). Furthermore, this breed is characterized by a notable degree of adaptability, attributable to its capacity for extensive grazing and strong resilience (Kaveh Baghbadorani et al., 2024). In the present trial, lower milk protein content was observed in the group that had been supplemented, which contrasts with the findings of El-Hendawy et al. (2018) on Damiati goats. On the opposite, daily protein yield showed no significant alterations, nevertheless, it exhibited a marginal lower concentration in group F when compared to group C. This outcome is likely attributable to the slightly higher milk yield observed in Group F. Although this result was not statistically significant, it enabled group F to maintain its protein yield at a level comparable to that of group C, despite the lower percentage of protein. With regard to milk FA composition, the group receiving FSP supplementation exhibited significantly reduced de novo FA content, particularly in C8:0 (caprylic), C10:0 (capric), and C12:0 (lauric acid) FA. These FA are naturally present in foodstuffs and are particularly abundant in goat milk (Kim and Rhee, 2016). They have been demonstrated to possess antimicrobial properties, specifically against various foodborne pathogens, including Campylobacter jejuni, Cronobacter spp., Helicobacter pylori and S. aureus (Kim and Rhee, 2016). Additionally, capric and caprylic acids are primarily responsible for the distinctive aroma of goat milk (Lo Presti et al., 2023). Petroselinic acid (C:18:1n12) content was found to be similar in the milk of both groups. Research on the metabolism of petroselinic acid remains limited. However, in certain studies incorporating species of Apiaceae into the diet of lactating goats, variations in rumen metabolism have been identified (Kholif et al., 2021; Morsy et al., 2018). This FA has been linked to the rumen metabolism of α-linolenic acid and to mammary desaturation of the resulting isomers (Marín et al., 2015). In addition, rumen biohydrogenation of unsaturated fatty acids suggests a potential modification of dietary petroselinic acid prior to its incorporation into milk fat (Marín et al., 2015). However, the extent to which this modification occurs and its effect on the levels of petroselinic acid in goat’s milk require further research. In the present trial, C22:6 (docosahexaenoic acid, DHA) was also affected by the treatment, exhibiting higher levels in group FEN. The mechanisms by which DHA is incorporated into ruminant milk are complex and vary according to several factors, as comprehensively delineated in the review by Huang et al. (2020). As stated by the authors (Huang et al., 2020), the source of DHA in ruminant milk is a direct transfer of this FA from the diet and an alteration of ALA in hepatocytes. In the present experiment, the presence of these two FA in fennel seeds, albeit in small amounts, may have contributed to the increase of DHA in the milk of group F. However, ruminal biohydrogenation processes have been observed to reduce milk DHA content, as it is converted to a C22:6 isomer that is then hydrogenated to C22:5 fatty acid in the rumen. Nevertheless, the enzymes responsible for this process remain to be fully elucidated (Toral et al., 2018).This FA has significant medical implications, as its dietary presence has been linked to the prevention of numerous human afflictions, including cancer and heart disease (Stillwell and Wassall, 2003).
4.3 Gene expression
Research has demonstrated that mRNA extracted from MFG exhibits a high degree of similarity to mRNA extracted from MEC during the production of milk. This makes MFG a reliable and accessible source for investigating gene expression related to lactation (Brenaut et al., 2012). Nevertheless, several authors (Li et al., 2016; Nichols et al., 2020) found that mRNA isolated from MFG yielded average low RIN values (< 7), as in the present trial. As proposed by Lemay et al. (2013), the observed low RIN of mRNA extracts are intrinsic to the sample type. This is due to the presence of low molecular weight RNA fragments in the mRNA from MFG, resulting from the presence of small amounts of cytoplasmic material from mammary epithelial cells within the cytoplasmic crescents. Nonetheless, this technique has been demonstrated to enable successful gene expression analysis (Brenaut et al., 2012; Jiménez-Montenegro et al., 2025).
Nutrition is a pivotal environmental determinant in modulating gene–environment interactions. Breakthroughs in molecular biology and genomics have enabled increasingly sophisticated investigations into the role of dietary components in regulating gene expression and influencing phenotypic outcomes. For example, PUFA have been observed to suppress the expression of the FASN (Simopoulos, 2020). In addition to nutrients, non-nutritive dietary phytochemicals, such as phenolic compounds, which are present in FSP, have also being investigated for their effects on various aspects of animal metabolism. Nevertheless, the regulation of gene expression in response to dietary changes occurs at multiple levels, including transcription (via transcription factors), post-transcription (such as mRNA stability), translation (e.g., initiation or microRNA activity), and post-translation (e.g., enzyme turnover or activity regulation) (Bernard et al., 2013). However, it is frequently unclear whether the regulatory agents are the nutrients themselves, their metabolites, or the hormones produced in response to dietary shifts. Furthermore, the majority of studies are unable to identify the precise level of regulation involved, as measurements of mRNA, protein levels, and enzyme activity are rarely taken concurrently. No significant differences were observed in the expression of lipogenic genes (LPL1, SCD1) between the experimental groups. In contrast to other lipogenic genes, which are less influenced by dietary factors, SCD is typically more responsive to dietary UFA supplementation, particularly in subcutaneous adipose tissue (Bernard et al., 2005a; Iommelli et al., 2021).
The relationship between SCD expression and milk quality has been the subject of extensive research. Stearoyl-CoA desaturase is responsible for the production of over 75% of the total CLA in milk (Chilliard et al., 2006). Nutritional factors, particularly those containing lipids, have been demonstrated to exert an influence on SCD expression. Tudisco et al. (2019a, 2019b) demonstrated that linseed supplementation reduced SCD activity, a result that was supported by Tsiplakou et al. (2009) and Bernard et al. (2009). Nevertheless, the altered activity of SCD was not correlated with changes in SCD mRNA levels, as observed in the present study. PRLR expression levels were examined due to the galactagogue properties of fennel seeds, which are known to influence prolactin (PRL) levels. Despite this, no significant differences in PRLR expression were found between the experimental groups. The anti-inflammatory properties of fennel, which are commonly observed in medicinal herbs, have been investigated by examining the expression of Toll-like receptor 4 (TLR4), which activates nuclear factor (NF)-κB, a key regulator of numerous genes involved in inflammatory responses (Chang et al., 2015). Liu et al. (2015) demonstrated that high-grain feeding influenced TLR mRNA levels and altered the bacterial composition of the ruminal epithelium in male goats, thereby establishing a connection between the microbial community and TLR expression. The current study revealed no significant differences in TLR4 mRNA levels. The treatment did not affect the expression of genes involved in antioxidant activity, including SOD and NRF2. This finding may be attributed to the relatively brief supplementation period. Accordingly, further investigation is required to ascertain the optimal time of administration and dosage in order to evaluate the impact on mRNA levels of candidate genes.
4 Conclusion
The study demonstrates that FSP supplementation did not significantly affect MY, in contrast to previous findings. However, it has been observed that milk protein content is reduced in the supplemented group. Furthermore, the supplementation has been shown to influence milk FA composition, leading to a decrease in de novo FA such as caprylic, capric, and lauric acids. These acids play a key role in the antimicrobial properties and aroma of goat milk. An increase in DHA levels was observed in the FSP-supplemented group, which may offer health benefits. In terms of genomic results, no significant changes were detected in the expression of lipogenic genes (LPL1, SCD1) or prolactin receptor (PRLR) gene, despite the known galactagogue properties of fennel seeds. The expression levels of TLR4, a gene involved in inflammatory responses, and antioxidant-related genes (SOD, NRF2) remained unchanged. The absence of significant genomic alterations may be attributed to the relatively brief supplementation period. Further studies are required to determine the optimal dosage and duration of FSP supplementation, as well as its effects on different lactation stages or primiparous animals, in order to enhance comprehension of its genomic and productive outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Department of Veterinary Medicine and Animal Production, University of Napoli Federico II, Animal Ethic Committee (protocol number: PG/2019/0070006). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
PI: Investigation, Conceptualization, Writing – original draft. VT: Formal Analysis, Writing – review & editing. EC: Writing – review & editing, Formal Analysis. GB: Formal Analysis, Writing – review & editing. FS: Writing – review & editing, Data curation. MF: Investigation, Writing – review & editing. FI: Funding acquisition, Writing – review & editing. RT: Conceptualization, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
AOAC (2012). Official Methods of Analysis. 19th ed (Arlington, VA: Association of Official Analytical Chemists).
Badgujar S. B., Patel V. V., and Bandivdekar A. H. (2014). Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. BioMed. Res. Int. 2014, 842674. doi: 10.1155/2014/842674
Benker G., Jaspers C., Häusler G., and Reinwein D. (1990). Control of prolactin secretion. Klin. Wochenschr. 68, 1157–1167. doi: 10.1007/BF01815271
Bernard L., Leroux C., Bonnet M., Rouel J., Martin P., and Chilliard Y. (2005a). Expression and nutritional regulation of lipogenic genes in mammary gland and adipose tissues of lactating goats. J. Dairy Res. 72, 250–255. doi: 10.1017/S0022029905000786
Bernard L., Leroux C., and Chilliard Y. (2013). “Expression and nutritional regulation of stearoyl-CoA desaturase genes in the ruminant mammary gland: Relationship with milk fatty acid composition,” in Stearoyl-CoA Desaturase Genes in Lipid Metabolism. Ed. Ntambi J. M. (Springer, New York), 161–193. doi: 10.1007/978-1-4614-7969-7_13
Bernard L., Leroux C., Faulconnier Y., Durand D., Shingfield K. J., and Chilliard Y. (2009). Effect of sunflower-seed oil or linseed oil on milk fatty acid secretion and lipogenic gene expression in goats fed hay-based diets. J. Dairy Res. 76, 241–248. doi: 10.1017/S0022029909003951
Bonet M. L. and Palou A. (2020). “Regulation of gene expression,” in Principles of Nutrigenetics and Nutrigenomics (Academic Press, Cambridge, MA), 17–25.
Brenaut P., Bangera R., Bevilacqua C., Rebours E., Cebo C., and Martin P. (2012). Validation of RNA isolated from milk fat globules to profile mammary epithelial cell expression during lactation and transcriptional response to a bacterial infection. J. Dairy Sci. 95 (10), 6130–6144. doi: 10.3168/jds.2012-5604
Bui L. C., Evsikov A. V., Khan D. R., Archilla C., Peynot N., Hénaut A., et al. (2009). Retrotransposon expression as a defining event of genome reprograming in fertilized and cloned bovine embryos. Reproduction 138, 289–299. doi: 10.1530/REP-09-0042
Chang G., Zhuang S., Seyfert H. M., Zhang K., Xu T., Jin D., et al. (2015). Hepatic TLR4 signaling is activated by LPS from digestive tract during SARA, and epigenetic mechanisms contribute to enforced TLR4 expression. Oncotarget 6, 38578–38589. doi: 10.18632/oncotarget.v6i36
Chilliard Y., Rouel J., and Leroux C. (2006). Goat's alpha-s1 casein genotype influences its milk fatty acid composition and delta-9 desaturation ratios. Anim. Feed Sci. Technol. 131, 474–487. doi: 10.1016/j.anifeedsci.2006.05.025
Christie W. W. (1993). “Preparation of ester derivatives of fatty acids for chromatographic analysis,” in Advances in Lipid Methodology – Two. Ed. Christie W. W. (Oily Press, Dundee, Scotland), 69–111.
Di Trana A., Bonanno A., Cecchini S., Giorgio D., Di Grigoli A., and Claps S. (2015). Effects of Sulla forage (Sulla coronarium L.) on the oxidative status and milk polyphenol content in goats. J. Dairy Sci. 98, 37–46. doi: 10.3168/jds.2014-8414
Doležalová M., Janiš R., Svobodová H., Kašpárková V., Humpolíček P., and Krejčí J. (2010). Antimicrobial properties of 1-monoacylglycerols prepared from undecanoic (C11:0) and undecenoic (C11:1) acid. Eur. J. Lipid Sci. Technol. 112, 1106–1114. doi: 10.1002/ejlt.200900295
Ehsaninia J., Zandi M. B., Taned M., and Bagheripour A. (2025). Estimates of genetic parameters and genetic trends for milk production traits in Murciano-Granadina goats. animal, 101582. doi: 10.1016/j.animal.2025.101582
El-Hendawy N. M., Gabr S. A., El-Ela A. A., Hamad M. E., and Hassbo R. M. (2018). Effect of some antioxidants on productive and reproductive performance of Damascus goats during late pregnancy and lactation stages. Egypt. J. Sheep Goat Sci. 13, 37–47.
Fahim N., Kholif A., and Azzaz H. (2022). Fennel and ginger improved nutrient digestibility and milk yield and quality in early lactating Egyptian buffaloes. Ann. Anim. Sci. 22, 255–270. doi: 10.2478/aoas-2021-0008
Giorgio D., Di Trana A., Di Napoli M. A., Sepe L., Cecchini S., Rossi R., et al. (2019). Comparison of cheeses from goats fed 7 forages based on a new health index. J. Dairy Sci. 102, 6790–6801. doi: 10.3168/jds.2018-15857
Gray I. K., Rumsby M. G., and Hawke J. C. (1967). The variations in linolenic acid and galactolipid levels in Graminaceae species with age of tissue and light environment. Phytochemistry 6, 107–113. doi: 10.1016/0031-9422(67)85014-3
Hara A. and Radin N. S. (1978). Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 90, 420–426. doi: 10.1016/0003-2697(78)90046-5
Hassanzadeh S. A., Abbasi-Maleki S., and Mousavi Z. (2022). Anti-depressive-like effect of monoterpene trans-anethole via monoaminergic pathways. Saudi J. Biol. Sci. 29, 3255–3261. doi: 10.1016/j.sjbs.2022.01.060
He Z. X., Sun Z. H., Tan Z. L., Tang S. X., Zhou C. S., Han X. F., et al. (2012). Effects of maternal protein or energy restriction during late gestation on antioxidant status of plasma and immune tissues in postnatal goats. J. Anim. Sci. 90, 4319–4326. doi: 10.2527/jas.2012-5088
Huang G., Zhang Y., Xu Q., Zheng N., Zhao S., Liu K., et al. (2020). DHA content in milk and biohydrogenation pathway in rumen: a review. PeerJ 8, 10230. doi: 10.7717/peerj.10230
Inra–Nozière P., Sauvant D., and Delaby L. (2018). Feeding System for Ruminants (Wageningen: Wageningen Academic Publishers).
Iommelli P., Infascelli F., Musco N., Grossi M., Ferrara M., Sarubbi F., et al. (2021). Stearoyl-CoA desaturase activity and gene expression in the adipose tissue of buffalo bulls was unaffected by diets with different fat content and fatty acid profile. Agriculture 11, 1209. doi: 10.3390/agriculture11121209
Iommelli P., Musco N., Lombardi P., Spina A. A., Morittu V. M., Sarubbi F., et al. (2025a). Dietary fennel (Foeniculum vulgare Mill) seeds supplementation affects yield, fatty acid composition and flavour profile of milk and cheese in grazing goats. Trop. Anim. Health Prod. 57, 211. doi: 10.1007/s11250-025-04456-x
Iommelli P., Spina A. A., Vastolo A., Infascelli L., Lotito D., Musco N., et al. (2025b). Functional and economic role of some Mediterranean medicinal plants in dairy ruminants’ feeding: A review of the effects of garlic, oregano, and rosemary. Animals 15, 657. doi: 10.3390/ani15050657
Jiménez-Montenegro L., Alfonso L., Soret B., Mendizabal J. A., and Urrutia O. (2025). Preservation of milk in liquid nitrogen during sample collection does not affect the RNA quality for RNA-seq analysis. BMC Genomics 26, 1–14. doi: 10.1186/s12864-025-11707-6
Jiwaji M., Daly R., Pansare K., McLean P., Yang J., Kolch W., et al. (2010). The Renilla luciferase gene as a reference gene for normalization of gene expression in transiently transfected cells. BMC. Mol. Biol. 11, 1–12. doi: 10.1186/1471-2199-11-103
Johnson D. R., Lee P. K., Holmes V. F., and Alvarez-Cohen L. (2005). An internal reference technique for accurately quantifying specific mRNAs by real-time PCR with application to the tceA reductive dehalogenase gene. Appl. Environ. Microbiol. 71, 3866–3871. doi: 10.1128/AEM.71.7.3866-3871.2005
Kargar S., Nowroozinia F., and Kanani M. (2021). Feeding fennel (Foeniculum vulgare) seed as potential appetite stimulant to newborn Holstein dairy calves: Effects on meal pattern, ingestive behavior, oro-sensorial preference, and feed sorting. Anim. Feed Sci. Technol. 278, 115009. doi: 10.1016/j.anifeedsci.2021.115009
Kaveh Baghbadorani M., Kazemi Hasanvand A., Lotfollahzadeh S., Khabazan H., and Hajmohammadi B. (2024). Pseudopregnancy in Murciano−Granadina dairy goats in Iran: prevalence, risk factors and treatment. Discover. Animals 1, 23. doi: 10.1007/s44338-024-00024-z
Kholif A. E., Elazab M. A., Matloup O. H., Olafadehan O. A., and Sallam S. M. A. (2021). Crude coriander oil in the diet of lactating goats enhanced lactational performance, ruminal fermentation, apparent nutrient digestibility, and blood chemistry. Small Ruminant Res. 204, 106522. doi: 10.1016/j.smallrumres.2021.106522
Kim S. A. and Rhee M. S. (2016). Highly enhanced bactericidal effects of medium chain fatty acids (caprylic, capric, and lauric acid) combined with edible plant essential oils (carvacrol, eugenol, β-resorcylic acid, trans-cinnamaldehyde, thymol, and vanillin) against Escherichia coli O157:H7. Food Control 60, 447–454. doi: 10.1016/j.foodcont.2015.08.022
Lacasse P., Lollivier V., Dessauge F., Bruckmaier R. M., Ollier S., and Boutinaud M. (2012). New developments on the galactopoietic role of prolactin in dairy ruminants. Domest. Anim. Endocrinol. 43, 154–160. doi: 10.1016/j.domaniend.2011.12.007
Lemay D. G., Hovey R. C., Hartono S. R., Hinde K., Smilowitz J. T., Ventimiglia F., et al. (2013). Sequencing the transcriptome of milk production: milk trumps mammary tissue. BMC Genomics 14, 1–17. doi: 10.1186/1471-2164-14-872
Li R., Dudemaine P. L., Zhao X., Lei C., and Ibeagha-Awemu E. M. (2016). Comparative analysis of the miRNome of bovine milk fat, whey and cells. PloS One 11, e0154129. doi: 10.1371/journal.pone.0154129
Liu J. H., Bian G. R., Zhu W. Y., and Mao S. Y. (2015). High-grain feeding causes strong shifts in ruminal epithelial bacterial community and expression of Toll-like receptor genes in goats. Front. Microbiol. 6, 167. doi: 10.3389/fmicb.2015.00167
Lo Presti V., Tudisco R., Di Rosa A. R., Musco N., Iommelli P., Infascelli F., et al. (2023). Influence of season on milk fatty acid profile and sensory characteristics of grazing goats in a Mediterranean environment: a sustainable agro-food system. Anim. Prod. Sci. 63 (7), 689–703. doi: 10.1071/AN21538
Losacco C., Pugliese G., Passantino L., Giannico F., Ceci E., Roselli V., et al. (2025) Horehound (Marrubium vulgare L.) as natural dietary feed additive in rabbit: effects on productive traits, antioxidant status, caecal environment, and gut morphology. Front. Anim. Sci. 6, 1658188. doi: 10.3389/fanim.2025.1658188
Marín A. L. M., Gómez-Cortés P., Sánchez N. N., Juárez M., Sigler A. I. G., Blanco F. P., et al. (2015). Associations between major fatty acids in plant oils fed to dairy goats and C18 isomers in milk fat. J. Dairy Res. 82, 152–160. doi: 10.1017/S002202991500014X
Miranda J. C., León J. M., Pieramati C., Gómez M. M., Valdés J., and Barba C. (2019). Estimation of genetic parameters for peak yield, yield and persistency traits in murciano-granadina goats using multi-traits models. Animals 9, 411. doi: 10.3390/ani9070411
Moosavi-Zadeh E., Rahimi A., Rafiee H., Saberipour H., and Bahadoran R. (2023). Effects of fennel (Foeniculum vulgare) seed powder addition during early lactation on performance, milk fatty acid profile, and rumen fermentation parameters of Holstein cows. Front. Anim. Sci. 4. doi: 10.3389/fanim.2023.1097071
Morsy T. A., Kholif A. E., Matloup O. H., Elella A. A., Anele U. Y., and Caton J. S. (2018). Mustard and cumin seeds improve feed utilisation, milk production and milk fatty acids of Damascus goats. J. Dairy Res. 85, 142–151. doi: 10.1017/S0022029918000043
Najdoska-Bogdanov M., Bogdanov J. B., and Stefova M. (2015). Simultaneous determination of essential oil components and fatty acids in fennel using gas chromatography with a polar capillary column. Nat. Prod. Commun. 10, 1934578X1501000933. doi: 10.1177/1934578X1501000933
Nichols K., Bannink A., Van Baal J., and Dijkstra J. (2020). Impact of post-ruminally infused macronutrients on bovine mammary gland expression of genes involved in fatty acid synthesis, energy metabolism, and protein synthesis measured in RNA isolated from milk fat. J. Anim. Sci Biotechnol. 11, 1–12. doi: 10.1186/s40104-020-00456-z
Paul A., Dangi S. S., Gupta M., Singh J., Thakur N., Naskar S., et al. (2015). Expression of TLR genes in Black Bengal goat (Capra hircus) during different seasons. Small Rumin. Res. 124, 17–23. doi: 10.1016/j.smallrumres.2015.01.011
Rather M. A., Dar B. A., Sofi S. N., Bhat B. A., and Qurishi M. A. (2016). Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 9, S1574–S1583. doi: 10.1016/j.arabjc.2012.04.011
Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., and Rice-Evans C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 1231–1237. doi: 10.1016/S0891-5849(98)00315-3
Safayi S., Theil P. K., Hou L., Engbæk M., Nørgaard J. V., Sejrsen K., et al. (2010). Continuous lactation effects on mammary remodeling during late gestation and lactation in dairy goats. J. Dairy Sci. 93, 203–217. doi: 10.3168/jds.2009-2507
Sanchez-Moreno C., Larrauri J. A., and Saura-Calixto F. A. (1998). Procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 76, 270–276. doi: 10.1002/(SICI)1097-0010(199802)76:2<270::AID-JSFA945>3.0.CO;2-9
Simopoulos A. P. (2020). “Impact of nutrigenetics and nutrigenomics on society,” in Principles of Nutrigenetics and Nutrigenomics (London, UK: Academic Press), 549–555.
Stillwell W. and Wassall S. R. (2003). Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem. Phys. Lipids 126, 1–27. doi: 10.1016/S0009-3084(03)00101-4
Taga M., Miller E., and Pratt D. (1984). Chia seeds as a source of natural lipid antioxidants. J. Am. Oil Chem. Soc 6, 928–931. doi: 10.1007/BF02542169
Terzi V., Infascelli F., Tudisco R., Russo G., Stanca A. M., and Faccioli P. (2004). Quantitative detection of Secale cereale by real-time PCR amplification. LWT Food Sci. Technol. 37, 239–246. doi: 10.1016/j.lwt.2003.08.005
Toral P. G., Hervás G., Leskinen H., Shingfield K. J., and Frutos P. (2018). In vitro ruminal biohydrogenation of eicosapentaenoic (EPA), docosapentaenoic (DPA), and docosahexaenoic acid (DHA) in cows and ewes: intermediate metabolites and pathways. J. Dairy Sci 101, 6109–6121. doi: 10.3168/jds.2017-14183
Trinchese G., Cavaliere G., Penna E., De Filippo C., Cimmino F., Catapano A., et al. (2019). Milk from cows fed with high forage/concentrate ratio diet: Beneficial effect on rat skeletal muscle inflammatory state and oxidative stress through modulation of mitochondrial functions and AMPK activity. Front. Physiol. 9, 1969. doi: 10.3389/fphys.2018.01969
Tsiplakou E., Flemetakis E., Kalloniati C., Papadomichelakis G., Katinakis P., and Zervas G. (2009). Sheep and goats differences in CLA and fatty acids milk fat content in relation with mRNA stearoyl-CoA desaturase and lipogenic genes expression in their mammary gland. J. Dairy Res. 76, 392–401. doi: 10.1017/S0022029909990100
Tudisco R., Chiofalo B., Lo Presti V., Morittu V. M., Moniello G., Grossi M., et al. (2019a). Influence of feeding linseed on SCD activity in grazing goat mammary glands. Animals 9, 786. doi: 10.3390/ani9100786
Tudisco R., Grossi M., Calabrò S., Cutrignelli M. I., Musco N., and Addi L. (2014). Influence of pasture on goat milk fatty acids and Stearoyl-CoA desaturase expression in milk somatic cells. Small Rumin. Res. 122, 38–43. doi: 10.1016/j.smallrumres.2014.07.016
Tudisco R., Morittu V. M., Addi L., Moniello G., Grossi M., Musco N., et al. (2019b). Influence of pasture on Stearoyl-CoA Desaturase and miRNA 103 expression in goat milk: Preliminary results. Animals 9, 606. doi: 10.3390/ani9090606
Unal H., Izli N., Kaçar O., and Goksu E. (2013). Physical and nutritional properties of fennel seed. J. Food Agric. Environ. 11, 6–11.
Van Soest P. J., Robertson J. B., and Lewis B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Wang Y., Salem A. Z., Tan Z., Kang J., and Wang Z. (2021). Activation of glucocorticoid receptors is associated with the suppression of antioxidant responses in the liver of goats fed a high-concentrate diet. Ital. J. Anim. Sci. 20, 195–204. doi: 10.1080/1828051X.2021.1873706
Wojdyło A., Oszmiański J., and Czemerys R. (2007). Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 105, 940–949. doi: 10.1016/j.foodchem.2007.04.038
Keywords: fennel seeds, milk production, fatty acid profile, gene expression, dairy goats
Citation: Iommelli P, Tufarelli V, Ceci E, Bozzo G, Sarubbi F, Ferrara M, Infascelli F and Tudisco R (2025) Fennel seed powder supplementation in dairy goat diet: effects on milk productive traits, fatty acid profile and gene expression. Front. Anim. Sci. 6:1635528. doi: 10.3389/fanim.2025.1635528
Received: 26 May 2025; Accepted: 19 September 2025;
Published: 02 October 2025.
Edited by:
Yafeng Huang, Anhui Agricultural University, ChinaReviewed by:
Ravikanthreddy Poonooru, University of Missouri, United StatesIulia Varzaru, National Research Development Institute for Animal Biology and Nutrition, Romania
Copyright © 2025 Iommelli, Tufarelli, Ceci, Bozzo, Sarubbi, Ferrara, Infascelli and Tudisco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vincenzo Tufarelli, dmluY2Vuem8udHVmYXJlbGxpQHVuaWJhLml0
 Piera Iommelli
Piera Iommelli Vincenzo Tufarelli
Vincenzo Tufarelli Edmondo Ceci3
Edmondo Ceci3 Fiorella Sarubbi
Fiorella Sarubbi Federico Infascelli
Federico Infascelli Raffaella Tudisco
Raffaella Tudisco