- 1USDA-ARS, U.S. National Poultry Research Center, Athens, GA, United States
- 2Department of Poultry Science, University of Georgia, Athens, GA, United States
The woody breast (WB) condition impairs the quality of chicken breast meat, causing a financial loss for the poultry industry. Recent studies suggest that the mitochondria may play a role in muscle and gut health by regulating inflammatory mediators. However, there is limited information available on gut and pancreas health as they relate to WB. Ribonucleotide reductase (RNR), subunit RRM2, is involved in DNA synthesis and mitochondrial function. Inhibition of RRM2 increases gastrointestinal disturbances and apoptosis in the pancreas by disturbing inflammatory mediators and mitochondrial homeostasis. This study aimed to investigate the links between RRM2, pancreas, and gut health in broilers exhibiting WB. Sixty eight-week-old male broilers were used to collect breast muscle, duodenum, and pancreas from 10 broilers exhibiting severe WB and 10 normal (N). Gene expression was measured using qRT-PCR with SYBR reagents. Commercial biochemical assays were used to measure mitochondrial function. To determine if RNR controls mitochondrial functions, an RNR inhibitor was tested in vitro on avian muscle-derived cells. Data were analyzed using Prism and t-tests. In tissues, RRM2 expression was lower in the muscle (P = 0.03), pancreas, and duodenum (both P = 0.04) vs. N. The expression levels of cytochrome b (Cytb) and mitochondrial uncoupling protein 3 (UCP3) were reduced in the muscle (P = 0.05 and P = 0.002) and pancreas (P = 0.009 and P = 0.002) for WB, while only Cytb was reduced in the duodenum (P = 0.04). Total mitochondrial protein and mitochondrial complex V activity were reduced for WB in the muscle (P = 0.0006 and P = 0.004), pancreas (P = 0.01 and P = 0.002), and duodenum (P = 0.001 and P = 0.002), respectively. Broilers with WB had increased malondialdehyde only in the pancreas (P = 0.01). Reduced GSH/GSSG ratios in the muscle (P = 0.01), pancreas (P < 0.05), and duodenum (P = 0.01) of WB broilers indicate more reactive oxygen species (ROS) in the tissues. In vitro, cells treated with an RNR inhibitor had reduced expression of RRM2 (P < 0.0001), Cytb (P = 0.0006), and UCP3 (P = 0.002) as well as reduced mitochondrial complex V activity (P = 0.001) and total mitochondrial proteins (P = 0.01), indicating that RNR may regulate mitochondrial functions. In conclusion, reduced RRM2 expression may potentially reduce RNR function in the muscle, duodenum, and pancreas of broilers with WB and alter tissue function by increasing ROS production, mitochondrial abnormalities, and oxidative damage while reducing energy production.
1 Introduction
Woody breast (WB) refers to a muscle condition resulting in broiler breast meat with abnormal texture and inferior fresh meat quality. Although WB meat is not a health risk to consumers, it has caused significant negative economic impacts on the poultry industry (Barbut, 2020). Although the specific causes of WB are unknown, fast growth rates and genetic selection of broilers have been suggested as potential factors (Kuttappan et al., 2016; Che et al., 2022). Several studies showed that mitochondrial abnormalities potentially play an important role in WB (Hisasaga, 2021; Shakeri et al., 2024) by increasing reactive oxygen species (ROS) in tissues, leading to severe oxidative stress, which in turn causes muscle damage and decreased ATP production (Wang et al., 2023). To date, there is no definitive solution to the WB problem. A few available findings suggest that dietary additives such as minerals may reduce the incidence of severe WB (Cauble et al., 2020; Kuttappan et al., 2021). Therefore, a healthier gut is hypothesized to potentially play a role in reducing WB incidence (Jia et al., 2022). Broilers with a healthier gut may absorb more nutrients and antioxidants (Obianwuna et al., 2023), which can help minimize oxidative stress. Among the three intestinal sections, the duodenum plays a crucial role in gut health as it is the primary site where food is mixed with digestive enzymes from the pancreas, initiating the process of nutrient absorption. The duodenum absorbs most of the minerals and vitamins (Basile et al., 2023), and the pancreas secretes digestive enzymes into the duodenum (Ravindran and Abdollahi, 2021). Therefore, alterations in the duodenum and pancreas could have a significant effect on mineral and vitamin absorption (Jia et al., 2022), which could possibly influence WB development in broilers.
Ribonucleotide reductase (RNR) is an enzyme involved in inflammatory pathways (Jones and Kounatidis, 2017) and mitochondrial function (Bourdon et al., 2007) and has been hypothesized to play a role in WB (Shakeri et al., 2023; Shakeri et al., 2024). RNR, subunit RRM2, is essential for the synthesis and replication of DNA and mitochondrial DNA (mtDNA) (Bourdon et al., 2007). Alterations in the expression of RRM2 are associated with higher ROS production, lower mtDNA content, and decreased levels of mitochondrial proteins, which impair ATP production in tissues (Shakeri et al., 2022). Previous studies in vitro have shown that RNR is essential for DNA synthesis and mtDNA function (Shadel, 2008; Salguero et al., 2012), while inhibition of RNR activity, such as with hydroxyurea, can lead to DNA replication arrest by depleting dNTP pools (Koç et al., 2004). However, there are no available data to show the effects of an RNR inhibitor such as hydroxyurea on the muscle cells of broiler chickens, and also how alterations in RNR activity may impact the mitochondrial function of muscle cells of broiler chickens. Therefore, testing the RNR inhibitor in vitro should be the first step before testing it in vivo. Our previous reports demonstrated that WB meat exhibited reduced RRM2 expression and increased mitochondrial abnormalities and oxidative stress (Shakeri et al., 2023; Shakeri et al., 2024). There is currently no available research examining the connection between RNR activity, gut health, and the WB condition. Therefore, the aim of this study was to investigate the role of RRM2 in duodenum health and mitochondrial function in broilers exhibiting WB. Furthermore, we also treated embryo fibroblasts with an RNR inhibitor to investigate its effects at the cellular level vs. WB. The current data are the first part of the experiment, testing the effects of RNR inhibitors in vitro before testing them in vivo. Since there have been no prior studies on the effects of RNR activity on meat quality, future research must explore various doses of the inhibitor in live birds to gain a clearer understanding of RNR’s impact.
2 Materials and methods
2.1 Animals and sampling
Sixty 8-week-old Ross male broilers were obtained from a local research farm (University of Georgia, Athens, GA). Broilers were weighed individually and euthanized by cervical dislocation according to an approved institutional animal use protocol from the University of Georgia (A2022 04-029-Y3-A1). From the 60 carcasses, 20 were selected (10 severe WB and 10 normal) based on postmortem visual and tactile evaluations of the breast muscles by two trained experts. Scoring was from 1 to 3 for woody breast and white striping, with 1 considered normal and 3 considered severe. Scoring was from 0 to 2 for hemorrhage, with 0 considered normal and 2 considered severe. Breast fillets were deboned and scored for WB, white striping, and petechial hemorrhages according to a previously published paper (Shakeri et al., 2024). Breast muscle (Pectoralis major), duodenum, and pancreas tissue samples were collected <1 h postmortem. The collected tissues were immediately flash frozen in liquid nitrogen and stored at −80°C or fixed in tissue freezing medium for histological analysis.
2.2 Mitochondria extraction
Tissue samples (100 mg) were rinsed with PBS and homogenized in 10 mL of mitochondria isolation buffer (210 mM of sucrose, 2 mM of EGTA, 40 mM of NaCl, and 30 mM of HEPES, pH 7.4) using a tissue grind pestle. The homogenized samples were centrifuged for 10 min at 900×g and 4°C. The supernatant was collected and centrifuged at 10,000×g for 10 min at 4°C. The obtained pellet was resuspended in 500 µL of resuspension buffer (10 mM of Tris and 1 mM of EDTA, pH 7.4) and used for the analysis.
2.3 Biochemical assays
Commercial kits were used to measure mitochondrial complex V activity (My BioScience, Cat. No.: MBS9719098, San Diego, CA, USA), total protein assay (Bio-Rad, Cat. No.: #5000001, USA), TBARS (thiobarbituric acid reactive substances) or MDA (Cayman, Cat. No.: 10009055, Ann Arbor, MI, USA), and GSH/GSSG ratio (glutathione/glutathione) (Invitrogen, Cat. No.: EIAGSHC, Waltham, MA, USA) based on protocols provided by the manufacturers. Each assay was performed with duplicate runs for all samples.
For complex V activity, 100 mg of tissue was mixed with the provided reagents, homogenized on ice, and then centrifuged at 600×g for 5 min at 4°C. The obtained supernatant was centrifuged at 11,000×g for 10 min at 4°C. The obtained pellet was suspended in a provided reagent by the manufacturer for the assay. Enzymatic activity was prepared with the reagents provided, and absorbance was read at 660 nm.
For TBARS, 25 mg of tissue was homogenized in RIPA buffer and centrifuged at 6,000×g for 10 min at 4°C to obtain the supernatant. Both samples and standards were prepared using SDS solution and color reagents after boiling them for 1 h. The standards were prepared based on the manufacturer’s protocol. All samples and standards were loaded onto a plate and read at 530 nm.
For GSH/GSSG, 50 mg of tissue was homogenized with 5%SSA (provided by the manufacturer) and centrifuged at 14,000×g for 10 min at 4°C. Both GSH and GSSG standards were prepared based on the protocol. All standards and samples were mixed with detection reagents, and absorbance was read at 405 nm.
Mitochondria extracted from tissues were used to measure mitochondrial complex V activity and total mitochondrial protein, whereas homogenized tissues (provided protocol by the manufacturer) were used to measure MDA and GSH/GSSG ratio.
2.4 Cell culture
An immortalized cell line (Kim et al., 2001) was used to assess the effects of the RNR inhibitor (hydroxyurea, Thermo Fisher Scientific) on cells. Hydroxyurea is a cell apoptosis inducer that inhibits DNA synthesis. Cells were cultured to confluence (~3–4 × 107 cells) in T75 flasks with growth medium [DMEM (Gibco 10566016, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco A3160402), and 1% penicillin–streptomycin [Gibco 15140148]) at 37°C in a humidified incubator (5% carbon dioxide) before using them for the experiment. Triplicates of the control and treated cultures were prepared and collected for analysis. Briefly, cells at 95%–100% confluence were treated with hydroxyurea (15 mg/mL final concentration in GM) for 24 h. The dosage of hydroxyurea was determined based on findings from previous studies conducted on mice and humans (Kinney et al., 1999; Geetha, 2018). Cells were then washed with D-PBS (Gibco) and trypsinized (0.25% Trypsin EDTA, Millipore Sigma, Rockville, MD) to detach cells from the culture flask. Fresh GM was added to deactivate trypsin and resuspend cells. Cell suspensions were then washed with D-PBS by centrifugation at 1,000 rpm for 8 min, and the cell pellets were recovered for the assays.
2.5 Quantitative real-time PCR
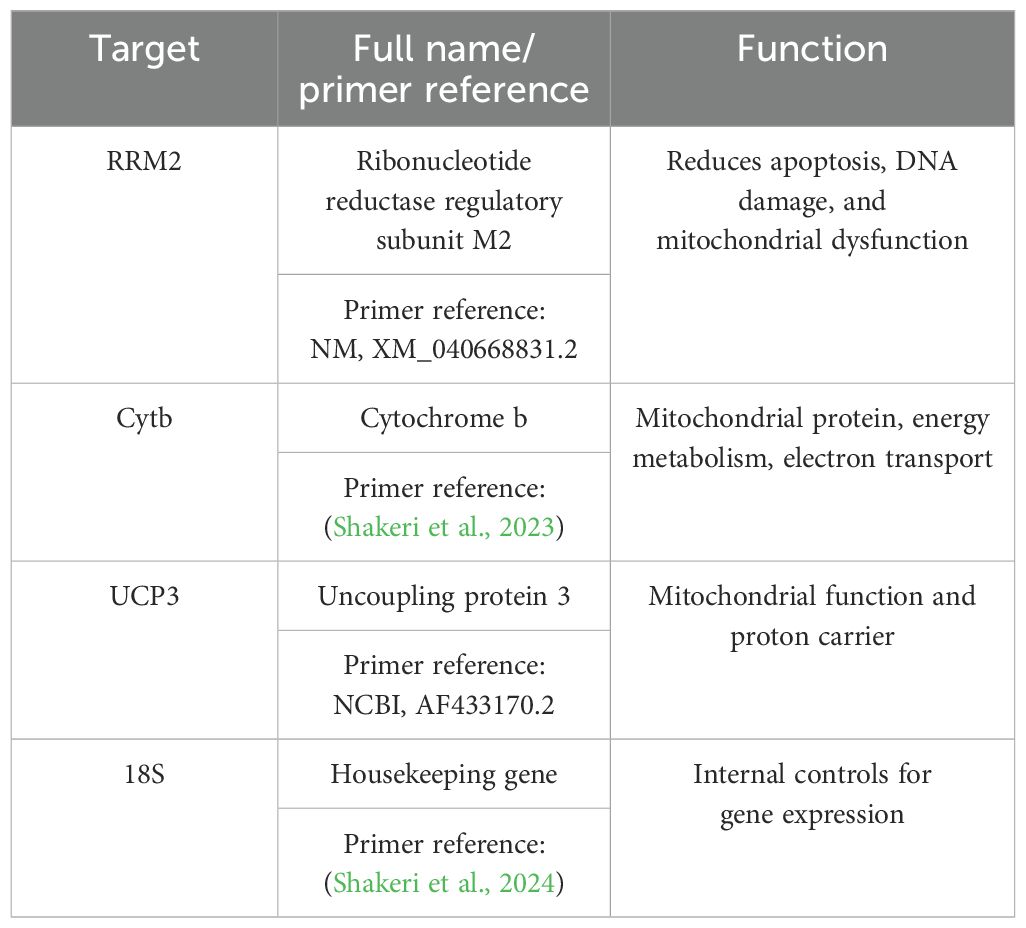
Total RNA was extracted from frozen tissues using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) and an RNeasy Mini Kit (Qiagen, Germantown, MD, USA). Total DNA was extracted from frozen tissues using a commercial QIAamp genomic DNA kit (Qiagen). Quantitative real-time PCR was performed with SYBR Green to measure gene expression used in this study. RNA, random hexamers, dNTPs, and free water were mixed (master mix 1), and then the mixture was heated at 65°C for 5 min and placed on ice for 2 min. Subsequently, 10 µL of Master Mix 2 (1× RT buffer, 25 mM of MgCl2, 0.1 of DTT, RNaseOUT, and SuperScript III) was added to Master Mix 1. The thermocycler program was as follows: 65°C for 5 min, 4°C for 2 min, 25°C for 10 min, 50°C for 50 min, 85°C for 5 min, and 37°C for 20 min. 18S was used to normalize the data, and fold changes were obtained using the 2−ΔΔCt method. All genes used and their function are listed in Table 1.
2.6 Histology
Duodenal tissues were embedded in freezing medium (Triangle Biomedical Sciences, USA). The fixed tissues were cut (10 µm) using a cryostat and then stained with H&E using a standard protocol. All images were obtained (×10 magnification) using a light microscope (Olympus CX33, Japan). The number of lesions in N and WB was used to calculate the difference (Gurcan et al., 2009). One slide per sample was prepared for the analysis (a total of 20).
2.7 Statistical analysis
Data were analyzed using the t-test method (GraphPad Prism, version 10.2.2). t-test analysis was used to show differences between N vs. WB samples and between non-treated vs. treated cells with RNR inhibitor for 24 h. Results were considered significant at P <0.05 and trending toward significant at P = 0.05–0.1. Mean values are given as mean ± SE.
3 Results
3.1 Body weight and breast myopathy scores
Broiler weight and breast myopathy score data are presented in Table 2. WB broilers were heavier vs. N (N vs. WB, 3,831 vs. 4,228 g, P = 0.0002). Breast fillets from the WB group had greater white striping and petechial hemorrhage scores (1.60 vs. 1.10, P = 0.0006 and 1.30 vs. 0.60, P = 0.05, respectively) vs. N fillets.
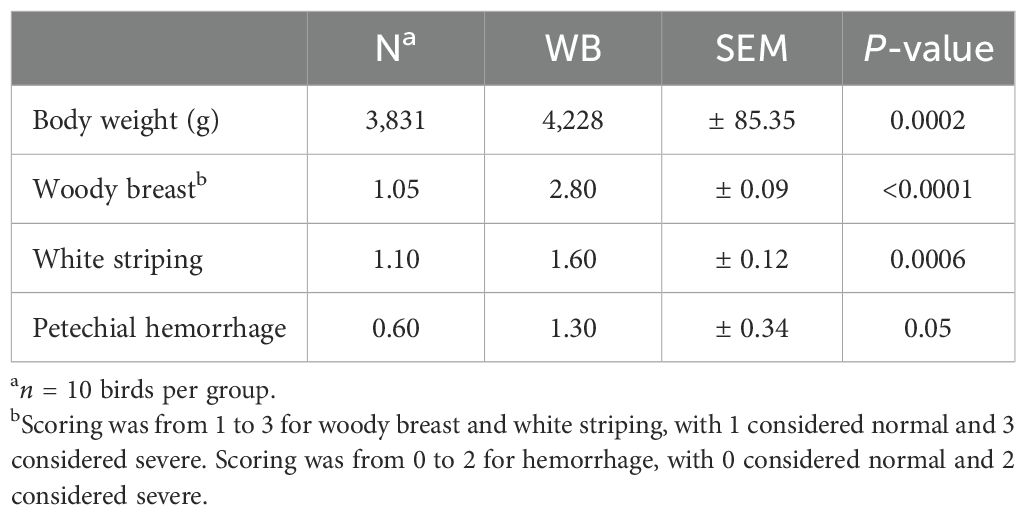
Table 2. Broiler body weight, woody breast, white striping, and petechial hemorrhage scores for normal (N) and woody breast (WB) meat.
3.2 Quantitative real-time PCR
Differences (N vs. WB) in the expression levels of RRM2, Cytb, and UCP3 are presented in the figures for muscle (Figures 1A–C), duodenum (Figures 1A1, B1, C1), and pancreas (Figures 1A2, B2, C2). RRM2 and Cytb expression levels were reduced for WB in the muscle (1.18 vs. 0.48, P = 0.03 and 1.09 vs. 0.67, P = 0.05), duodenum (1.14 vs. 0.60, P = 0.04 and 1.04 vs. 0.65, P = 0.04), and pancreas (1.16 vs. 0.57, P = 0.04 and 1.05 vs. 0.62, P = 0.009), respectively. UCP3 expression was reduced significantly for WB in the muscle (1.13 vs. 0.30, P = 0.002) and was reduced in the pancreas as well (1.19 vs. 0.76, P = 0.15) but not statistically significant. After 24 h, the cells treated with RNR inhibitor in vitro exhibited reduced expression of RRM2 (1.01 vs. 0.18, P < 0.0001), Cytb (1.01 vs. 0.42, P = 0.0006), and UCP3 (1.00 vs. 0.60, P = 0.002) (Figures 2A1, B1, C1) compared to non-treated cells. There were no significant differences between the cells before using the inhibitor (Figures 2A–C).
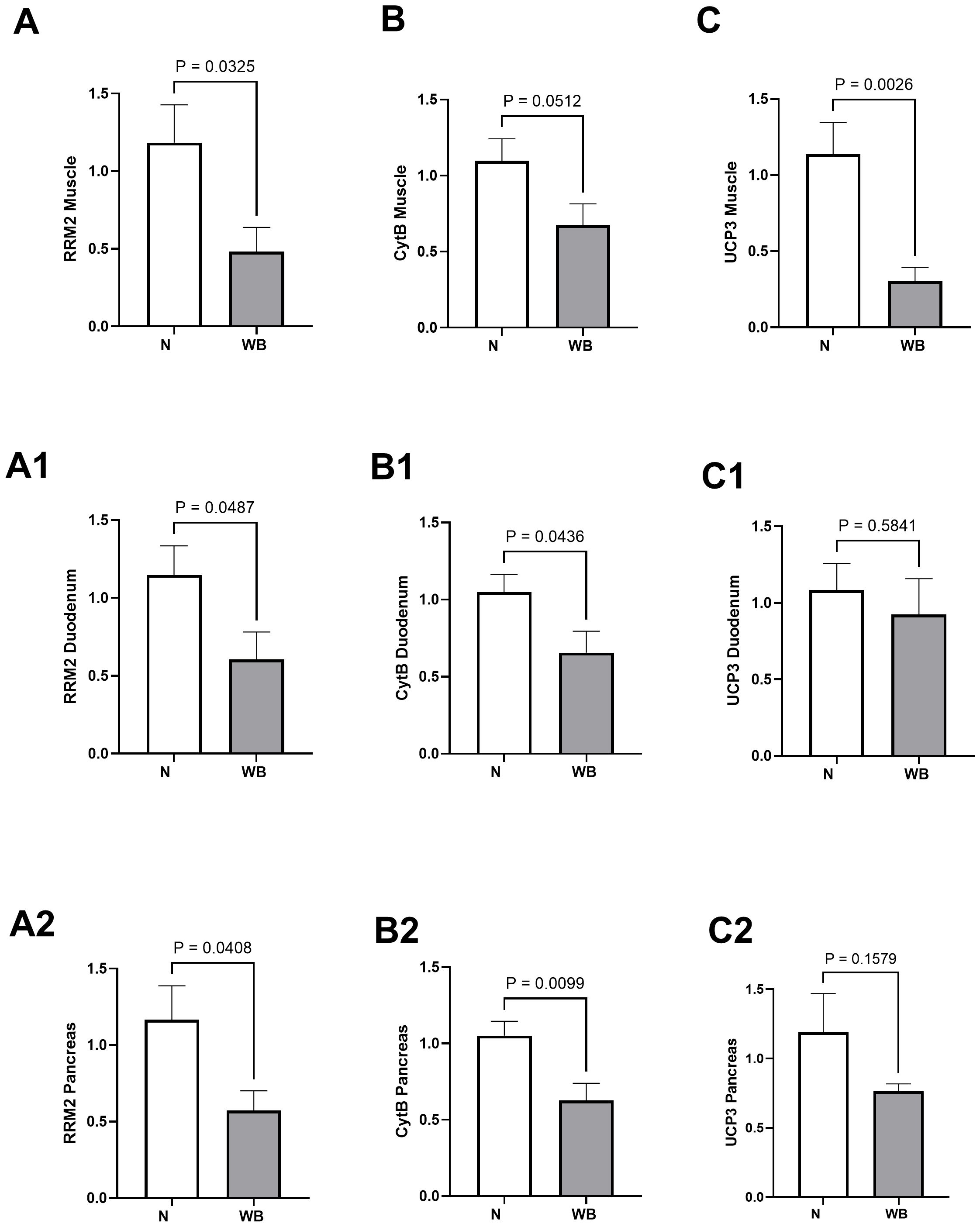
Figure 1. Expression (fold change) of genes related to mitochondrial function in the muscle, duodenum, and pancreas of normal (N) and woody breast (WB) broilers. In tissues, ribonucleotide reductase regulatory subunit M2 (RRM2, A-A2) and cytochrome b (Cytb, B-B2) were reduced in the muscle, duodenum, and pancreas, while uncoupling protein 3 (UCP3, C-C2) was only reduced in the muscle. Results were considered significant at P < 0.05 and trending toward significance at P = 0.05–0.1. Mean values are given as mean ± SE.
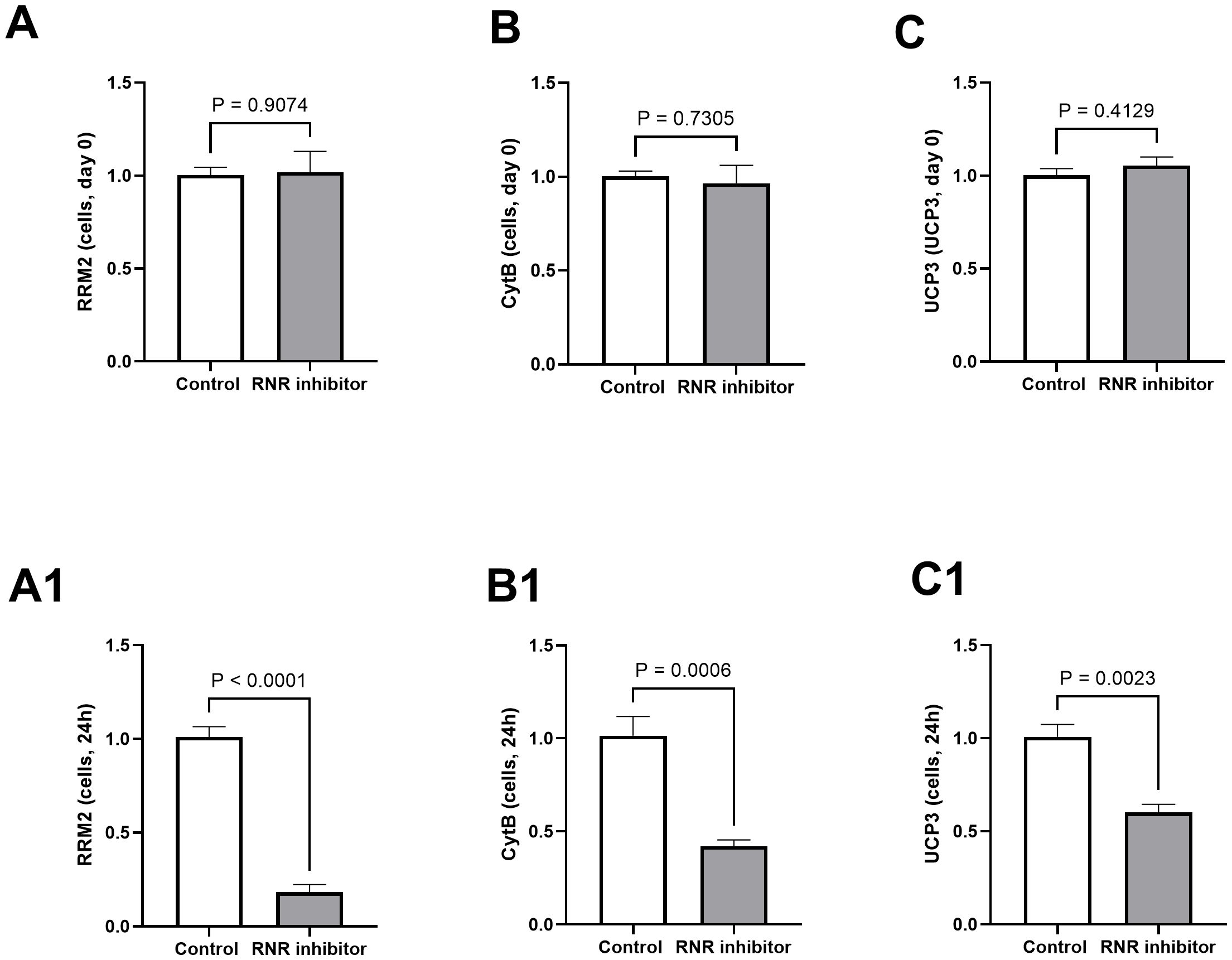
Figure 2. Expression (fold change) of genes related to mitochondrial function in cells before and after treating them with ribonucleotide reductase (RNR) inhibitor. In vitro, ribonucleotide reductase regulatory subunit M2 (RRM2, A, A1), cytochrome b (Cytb, B, B1), and uncoupling protein 3 (UCP3, C, C1) were reduced for cells treated with ribonucleotide reductase inhibitor for 24 h. Results were considered significant at P < 0.05 and trending toward significance at P = 0.05–0.1. Mean values are given as mean ± SE.
3.3 Biochemical assays
Data are presented in Table 3. Total mitochondrial protein and mitochondrial complex V activity were reduced for WB in the muscle (0.75 vs. 0.69 mg/mL, P = 0.0006 and 278.4 vs. 176.9 mM, P = 0.004), duodenum (0.24 vs. 0.14 mg/mL, P = 0.001 and 474.6 vs. 391.9 mM, P = 0.002), and pancreas (0.48 vs. 0.35 mg/mL, P = 0.01 and 446.8 vs. 441.6 mM, P = 0.002). GSH/GSSG ratio was reduced for WB in the muscle (783.4 vs. 550.7 μM, P = 0.01), duodenum (126.5 vs. 114.1 μM, P = 0.01), and pancreas (94.5 vs. 88.6 μM, P = 0.04). MDA increased for WB in the pancreas (4.94 vs. 5.12 nmol/mg, P = 0.01) and statistically insignificantly increased in the muscle and duodenum (5.22 vs. 5.48 nmol/mg, P = 0.18 and 4.58 vs. 5.39 nmol/mg, P = 0.07).
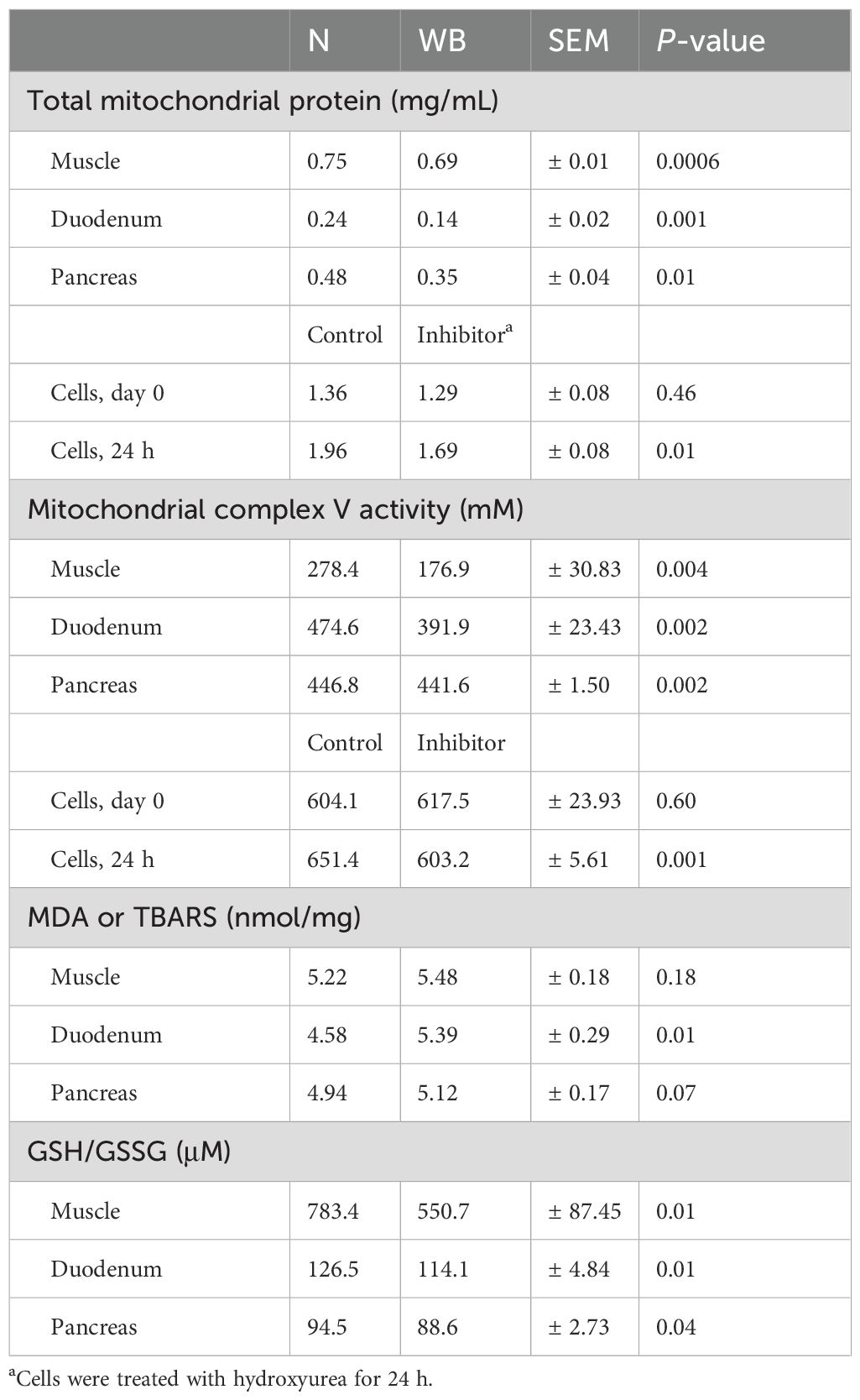
Table 3. Total mitochondrial protein, mitochondrial complex V activity, thiobarbituric acid reactive substances (TBARS), and glutathione/glutathione (GSH/GSSG) ratio in normal (N) and woody breast (WB) broilers or cells after 24 h treatment with RNR inhibitor.
In vitro, cells treated with RNR inhibitor exhibited reduced total mitochondrial protein (1.96 vs. 1.69 mg/mL, P = 0.01) and reduced mitochondria complex V activity (651.4 vs. 603.2 mM, P = 0.001) compared to non-treated cells.
3.4 Histology
The number of moderate or severe lesions in duodenum images of WB was higher vs. N (1.1 vs. 1.7, P = 0.02) (Figure 3).
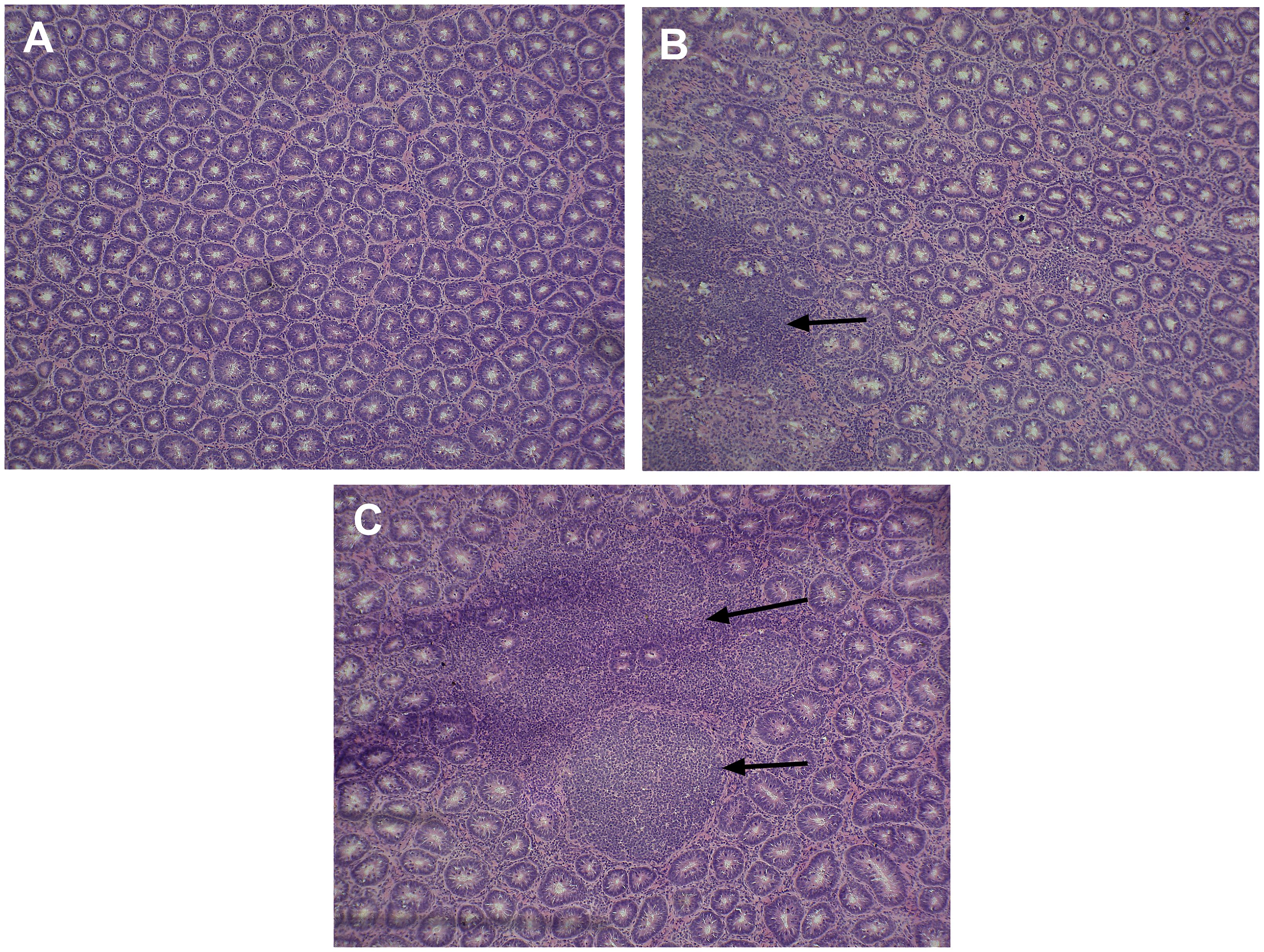
Figure 3. Differences between lesions (indicated by arrows) in the duodenum of normal (A) vs. two different grades of lesions in woody breast broilers (B, C) prepared by using hematoxylin and eosin stain. (A) Normal; (B) less infiltration; (C) moderate or severe infiltration.
4 Discussion
RRM2 is essential for the synthesis and replication of DNA and mitochondrial DNA and is potentially involved in WB myopathy by controlling mitochondrial function and ROS production. Lowered expression of RRM2 in the muscle, duodenum, and pancreas leads to impaired mitochondrial function. Dysfunctional mitochondria lead to excessive production of ROS, resulting in increased oxidative damage to the tissues (Figure 4). The duodenum plays a major role in nutrient absorption, which is important for meat quality, while the pancreas produces and releases digestive enzymes into the duodenum to aid in digestion. Therefore, alterations in the duodenum and pancreas could have a significant effect on mineral and vitamin absorption, which possibly increases WB rate in broilers. Intestine health significantly impacts meat quality by impacting digestion, nutrient absorption, and overall animal health, which in turn affects the composition and characteristics of the meat produced (Chen et al., 2022).
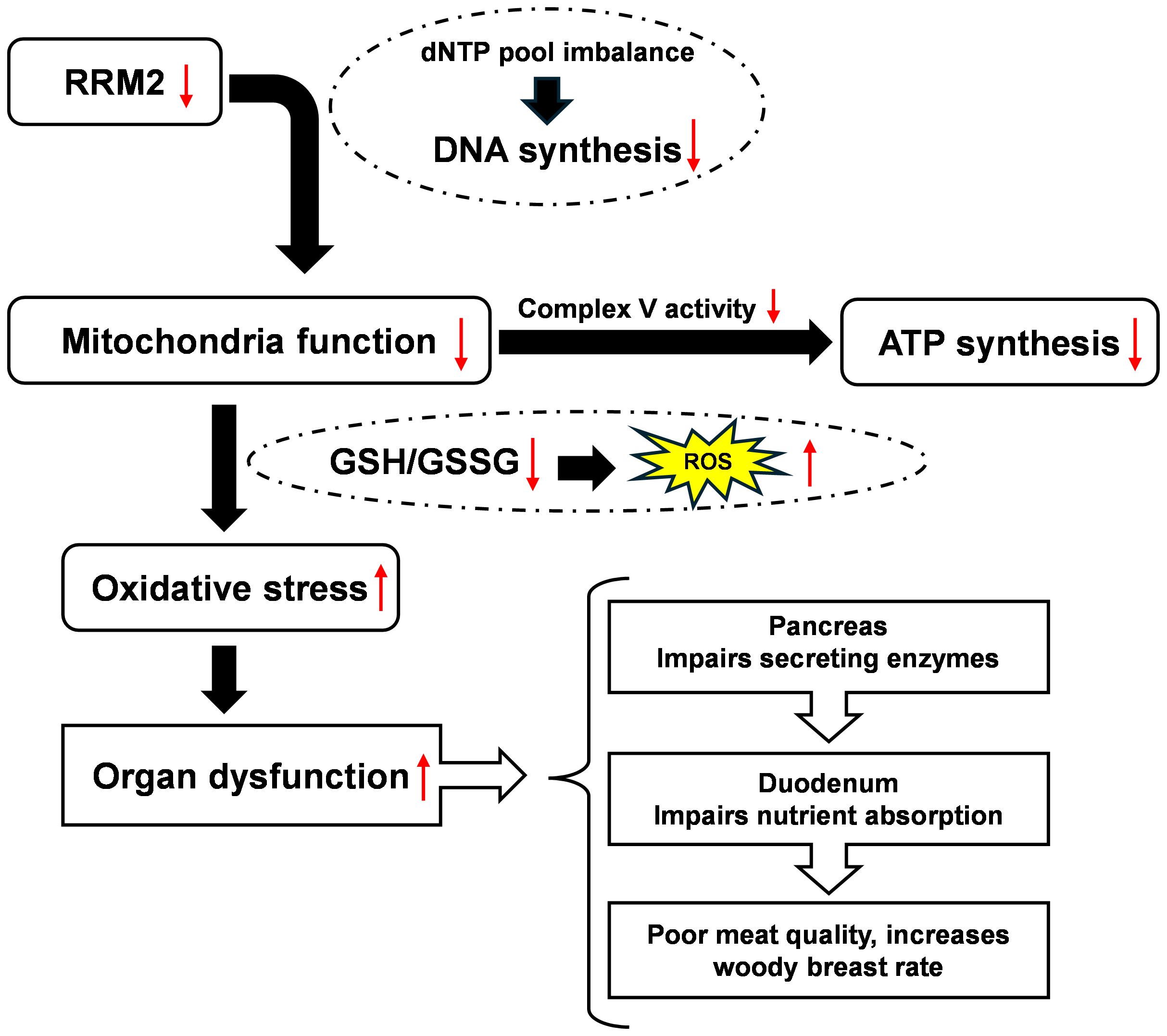
Figure 4. Reduction in the expression of ribonucleotide reductase, subunit RRM2, in the muscle, duodenum, and pancreas impairs mitochondrial function, which could potentially impact gut health and meat quality by increasing reactive oxygen species (ROS) production. Glutathione/glutathione (GSH/GSSG) ratio is an indicator of ROS level in tissues (the higher the ratio, the healthier the tissue).
In this study, we found that RRM2 expression was reduced in broilers with WB as well as in the cells treated with an RNR inhibitor. RRM2 expression reduction has been shown to impact ATP production pathways through altering DNA health and mitochondrial function (Mah et al., 2015; Brown et al., 2023) by increasing ROS and oxidative damage (Cho et al., 2015; Shakeri et al., 2022). The mitochondria are organelles where cells generate ATP and reuse excessive ROS for other vital pathways; when the mitochondria do not function properly, ROS levels increase as ATP production decreases, leading to tissue damage (Wang et al., 2023; Shakeri et al., 2025). Furthermore, higher oxidative damage may increase lesions in tissues (Figure 3).
The expression levels of Cytb and UCP3 were reduced in the muscle, duodenum, and pancreas of broilers with WB and the cells treated with the RNR inhibitor, indicating a disruption in the normal function of the mitochondria. Cytb, a protein within the mitochondria, is encoded by mtDNA and plays a vital role in ATP production (Ahmad et al., 2018). UCP3 is a mitochondrial protein involved in energy metabolism and protecting against oxidative stress (Schrauwen, 2004) and is mainly expressed in skeletal muscle (Toime and Brand, 2010). The data also showed a reduction in mitochondrial complex V activity and total mitochondrial protein in broilers with WB and the cells treated with the RNR inhibitor. Mitochondrial complex V, also known as ATP synthase, is a crucial enzyme in the inner mitochondrial membrane that synthesizes ATP during oxidative phosphorylation (Jonckheere et al., 2012). Low total mitochondrial proteins often lead to mitochondrial disorders and impaired ATP production (Reverter et al., 2017).
Mitochondrial dysfunction is a primary driver of higher ROS in tissues (Bhatti et al., 2017). One of the best ways to determine ROS level is to measure the GSH/GSSG ratio. The ratio, representing the balance between oxidized glutathione, is a key indicator of cellular redox status and a marker of oxidative stress, with a lower ratio indicating increased ROS (Zitka et al., 2012). In fact, when ROS increases, GSH converts to GSSG to neutralize the ROS, leading to a decrease in the GSH/GSSG ratio (Zitka et al., 2012).
The current results showed that treating chicken muscle cells with the RNR inhibitor potentially increased mitochondrial dysfunction and oxidative damage, as observed in the WB. This supports our hypothesis that reducing RRM2 expression potentially increases ROS levels and oxidative damage in muscle tissue, which may contribute to WB occurrence. After confirming the inhibitor’s effects (similar data as WB) in vitro, the next experiment will examine the hypothesis in vivo to observe the differences. Testing the inhibitor in vitro was necessary, as it had not been examined in broiler chicken cells before.
In conclusion, the reduction of RRM2 in WB broilers seemed to correlate with impaired mitochondrial function and increased ROS production, leading to increased oxidative damage to the duodenum and pancreas. Higher levels of oxidative damage can contribute to gut damage and impaired gut function, which can, in turn, disrupt normal absorption of nutrients of minerals or other essential nutrients, resulting in increased incidence and severity of the WB myopathy. Therefore, the study suggests that overexpressing RRM2 may reduce mitochondrial dysfunction and ROS production and consequently decrease WB occurrence.
Data availability statement
The data presented in the study are deposited in the Figshare repository, accession number 10.6084/m9.figshare.29646647. Further inquiries can be directed to the corresponding author(s).
Ethics statement
The animal study was conducted according to an approved institutional animal use protocol from the University of Georgia (A2022 04-029-Y3-A1). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MS: Methodology, Writing – review & editing, Software, Writing – original draft, Investigation, Validation, Formal analysis, Conceptualization, Data curation. EZ: Investigation, Writing – review & editing. CH: Investigation, Writing – review & editing. JC: Investigation, Writing – review & editing. HN: Writing – review & editing, Investigation. WK: Methodology, Writing – review & editing. BK: Writing – review & editing, Methodology. BB: Methodology, Funding acquisition, Writing – review & editing, Supervision, Validation, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported in part by an appointment to the Agricultural Research Service (ARS) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the US Department of Energy (DOE) and the USDA. ORISE is managed by Oak Ridge Associated University (ORAU) under DOE contract No. DE-SC0014664. All opinions expressed in this paper are the authors’ and do not necessarily reflect the policies and views of USDA, DOE, or ORAU/ORISE.
Acknowledgments
We would like to thank our lab technicians and Dr. Hong Zhuang and Dr. Marites Sales for their support and for providing laboratory resources for the project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer.
References
Barbut S. (2020). “Understanding the woody breast syndrome and other myopathies in modern broiler chickens,” in Proceedings of the Australian Poultry Science Symposium. University of Sydney, Australia 99–102.
Basile E. J., Launico M. V., and Sheer A. J. (2023). Physiology, Nutrient Absorption Vol. 2023 (Treasure Island (FL: StatPearls Publishing).
Bhatti J. S., Bhatti G. K., and Reddy P. H. (2017). Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA)-Mol. Basis. Dis. 1863, 1066–1077. doi: 10.1016/j.bbadis.2016.11.010
Bourdon A., Minai L., Serre V., Jais J.-P., Sarzi E., Aubert S., et al. (2007). Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat. Genet. 39, 776–780. doi: 10.1038/ng2040
Brown A., Pan Q., Fan L., Indersie E., Tian C., Timchenko N., et al. (2023). Ribonucleotide reductase subunit switching in hepatoblastoma drug response and relapse. Commun. Biol. 6, 249. doi: 10.1038/s42003-023-04630-7
Cauble R. N., Greene E. S., Orlowski S., Walk C., Bedford M., Apple J., et al. (2020). Research Note: Dietary phytase reduces broiler woody breast severity via potential modulation of breast muscle fatty acid profiles. Poult. Sci. 99, 4009–4015. doi: 10.1016/j.psj.2020.05.005
Che S., Wang C., Iverson M., Varga C., Barbut S., Bienzle D., et al. (2022). Characteristics of broiler chicken breast myopathies (spaghetti meat, woody breast, white striping) in Ontario, Canada. Poult. Sci. 101, 101747. doi: 10.1016/j.psj.2022.101747
Chen B., Li D., Leng D., Kui H., Bai X., and Wang T. (2022). Gut microbiota and meat quality. Front. Microbiol. 13, 951726. doi: 10.3389/fmicb.2022.951726
Cho E.-C., Kuo M.-L., Cheng J.-h., Cheng Y.-C., Hsieh Y.-C., Liu Y.-R., et al. (2015). RRM2B-mediated regulation of mitochondrial activity and inflammation under oxidative stress. Mediators Inflamm. 2015, 287345. doi: 10.1155/2015/287345
Geetha M. (2018). Evaluation of efficacy of Carica papaya leaf extracts to increase platelet count in hydroxyurea induced thrombocytopenia in Albino rats. Int. J. Basic. Clin. Pharmacol. 7, 173.
Gurcan M. N., Boucheron L. E., Can A., Madabhushi A., Rajpoot N. M., and Yener B. (2009). Histopathological image analysis: A review. IEEE Rev. Biomed. Eng. 2, 147–171. doi: 10.1109/RBME.2009.2034865
Hisasaga C. Jr (2021). Assessing the Accuracy of a Woody Breast Identification Technique and Evaluating the Association between Mitochondria Values to Woody Breast Severity (Fresno: California State University).
Jia L., Hsu C.-Y., Zhang X., Li X., Schilling M. W., Peebles E. D., et al. (2022). Effects of dietary bacitracin or Bacillus subtilis on the woody breast myopathy-associated gut microbiome of Eimeria spp. challenged and unchallenged broilers. Poult. Sci. 101, 101960. doi: 10.1016/j.psj.2022.101960
Jonckheere A. I., Smeitink J. A., and Rodenburg R. J. (2012). Mitochondrial ATP synthase: architecture, function and pathology. J. Inherit. Metab. Dis. 35, 211–225. doi: 10.1007/s10545-011-9382-9
Jones S. V. and Kounatidis I. (2017). Nuclear factor-kappa B and Alzheimer disease, unifying genetic and environmental risk factors from cell to humans. Front. Immunol. 8, 1805. doi: 10.3389/fimmu.2017.01805
Kim H., You S., Kim I.-J., Foster L. K., Farris J., Ambady S., et al. (2001). Alterations in p53 and E2F-1 function common to immortalized chicken embryo fibroblasts. Oncogene 20, 2671–2682. doi: 10.1038/sj.onc.1204378
Kinney T. R., Helms R. W., O’Branski E. E., Ohene-Frempong K., Wang W., Daeschner C., et al. (1999). Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Blood. J. Am. Soc. Hematol. 94, 1550–1554. doi: 10.1182/blood.V94.5.1550
Koç A., Wheeler L. J., Mathews C. K., and Merrill G. F. (2004). Hydroxyurea arrests DNA replication by a mechanism that preserves basal dNTP pools. J. Biol. Chem. 279, 223–230. doi: 10.1074/jbc.M303952200
Kuttappan V., Hargis B., and Owens C. (2016). White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 95, 2724–2733. doi: 10.3382/ps/pew216
Kuttappan V. A., Manangi M., Bekker M., Chen J., and Vazquez-Anon M. (2021). Nutritional intervention strategies using dietary antioxidants and organic trace minerals to reduce the incidence of wooden breast and other carcass quality defects in broiler birds. Front. Physiol. 12, 663409. doi: 10.3389/fphys.2021.663409
Mah V., Alavi M., Márquez-Garbán D. C., Maresh E. L., Kim S. R., Horvath S., et al. (2015). Ribonucleotide reductase subunit M2 predicts survival in subgroups of patients with non-small cell lung carcinoma: effects of gender and smoking status. PloS One 10, e0127600. doi: 10.1371/journal.pone.0127600
Obianwuna U. E., Agbai Kalu N., Wang J., Zhang H., Qi G., Qiu K., et al. (2023). Recent trends on mitigative effect of probiotics on oxidative-stress-induced gut dysfunction in broilers under necrotic enteritis challenge: a review. Antioxidants 12, 911. doi: 10.3390/antiox12040911
Ravindran V. and Abdollahi M. R. (2021). Nutrition and digestive physiology of the broiler chick: state of the art and outlook. Animals 11, 2795. doi: 10.3390/ani11102795
Reverter A., Okimoto R., Sapp R., Bottje W. G., Hawken R., and Hudson N. J. (2017). Chicken muscle mitochondrial content appears co-ordinately regulated and is associated with performance phenotypes. Biol. Open 6, 50–58. doi: 10.1242/bio.022772
Salguero I., Guarino E., Shepherd M. E., Deegan T. D., Havens C. G., MacNeill S. A., et al. (2012). Ribonucleotide reductase activity is coupled to DNA synthesis via proliferating cell nuclear antigen. Curr. Biol. 22, 720–726. doi: 10.1016/j.cub.2012.02.070
Schrauwen P. (2004). The role of uncoupling protein 3 in fatty acid metabolism: protection against lipotoxicity? Proc. Nutr. Soc. 63, 287–292. doi: 10.1079/PNS2003336
Shadel G. S. (2008). Expression and maintenance of mitochondrial DNA: new insights into human disease pathology. Am. J. Pathol. 172, 1445–1456. doi: 10.2353/ajpath.2008.071163
Shakeri M., Berisha D., Martinson A., Davis J., and Moussavi-Harami F. (2022). Ribonucleotide reductase mediated regulation of mitochondrial activity in the adult heart. Biophys. J. 121, 396a–397a. doi: 10.1016/j.bpj.2021.11.781
Shakeri M., Choi J., Harris C., Buhr R. J., Kong B., Zhuang H., et al. (2024). Reduced ribonucleotide reductase RRM2 subunit expression increases DNA damage and mitochondria dysfunction in woody breast chickens. Am. J. Vet. Res. 1, 1–7. doi: 10.2460/ajvr.23.12.0283
Shakeri M., Kong B., Zhuang H., and Bowker B. (2023). Potential Role of ribonucleotide reductase enzyme in mitochondria function and woody breast condition in broiler chickens. Animals 13, 2038. doi: 10.3390/ani13122038
Shakeri M., Le H. H., and Shakeri M. (2025). Role of mitochondrial function in farm animals’ Health and production. Adv. Anim. Vet. Sci. 13, 1142–1148. doi: 10.17582/journal.aavs/2025/13.5.1142.1148
Toime L. J. and Brand M. D. (2010). Uncoupling protein-3 lowers reactive oxygen species production in isolated mitochondria. Free Radical Biol. Med. 49, 606–611. doi: 10.1016/j.freeradbiomed.2010.05.010
Wang Z., Brannick E., and Abasht B. (2023). Integrative transcriptomic and metabolomic analysis reveals alterations in energy metabolism and mitochondrial functionality in broiler chickens with wooden breast. Sci. Rep. 13, 4747. doi: 10.1038/s41598-023-31429-7
Keywords: gut health, meat quality, mitochondria, ribonucleotide reductase enzyme, woody breast
Citation: Shakeri M, Ziabtchenko E, Harris C, Choi J, Naeini HRR, Kim WK, Kong B and Bowker B (2025) Reduction in ribonucleotide reductase subunit RRM2 associated with alterations in mitochondrial proteins, leading to impaired gut health of woody breast chicken. Front. Anim. Sci. 6:1637145. doi: 10.3389/fanim.2025.1637145
Received: 28 May 2025; Accepted: 27 June 2025;
Published: 12 August 2025.
Edited by:
Petru Alexandru Vlaicu, National Research Development Institute for Animal Biology and Nutrition, RomaniaReviewed by:
Hui Yuan, Northeast Agricultural University, ChinaSaber Y. Adam, Yangzhou University, China
Zuodong Chen, Nanjing Forest Police College, China
Copyright © 2025 Shakeri, Ziabtchenko, Harris, Choi, Naeini, Kim, Kong and Bowker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Majid Shakeri, bWFqaWQuc2hha2VyaUB1c2RhLmdvdg==; bWFqaWQuc2hha2VyaS5waGRAZ21haWwuY29t; Brian Bowker, YnJpYW4uYm93a2VyQHVzZGEuZ292
 Majid Shakeri
Majid Shakeri Elizabeth Ziabtchenko1
Elizabeth Ziabtchenko1 Janghan Choi
Janghan Choi Woo Kyun Kim
Woo Kyun Kim Byungwhi Kong
Byungwhi Kong Brian Bowker
Brian Bowker