- Department of Animal Nutrition, Faculty of Veterinary Medicine, University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca, Romania
The increasing prevalence of obesity in domestic cats is closely linked to the emergence of diabetes mellitus, highlighting the need for early diagnostic tools. Obese cats typically show elevated leptin and reduced adiponectin levels—markers associated with early insulin resistance. Leptin resistance and compensatory hyperinsulinemia further reflect early metabolic dysfunction. These endocrine changes mirror those seen in humans and dogs, suggesting shared mechanisms across species. Incorporating these biomarkers into clinical practice could aid early risk stratification and preventive care. However, standardized reference ranges and cost-effective assays are still needed. This review assesses the predictive value of adiponectin, leptin, and insulin in identifying diabetes risk in obese cats.
1 Introduction
Obesity is an increasingly common and serious health concern in domestic cats, with reported prevalence ranging from 11.5% to 63% worldwide (Antakyalioglu et al., 2024; Tarkošová et al., 2016). This condition contributes to a range of comorbidities, most notably osteoarthritis and diabetes mellitus (DM), both of which significantly impair quality of life and longevity. Metabolic dysfunction associated with excess adipose tissue, particularly adipokine imbalance marked by increased leptin and decreased adiponectin, plays a central role in the development of insulin resistance and related disorders (Stenberg et al., 2023; Araújo et al., 2024).
Among the biomarkers associated with metabolic regulation, the leptin-to-adiponectin ratio (LAR) has gained attention as a sensitive indicator of insulin resistance and metabolic imbalance across species, including felines (Öhlund et al., 2018). Leptin, secreted in proportion to fat mass, regulates energy balance by signaling satiety to the hypothalamus, while adiponectin enhances insulin sensitivity and exerts anti-inflammatory effects (O’Neill et al., 2016; Nelson and Reusch, 2014). In obese cats, elevated leptin levels and reduced adiponectin concentrations result in a higher LAR, reflecting diminished leptin responsiveness and an inflammatory state that predisposes to diabetes mellitus (O’Neill et al., 2016; Araújo et al., 2024).
Beyond metabolic implications, obesity can also exacerbate orthopedic and cardiovascular complications, including limited mobility, hypertension, and increased heart rate (Sandøe et al., 2014; Clark and Hoenig, 2021; Souza et al., 2020; Rodan and Ellis, 2013). These multifaceted effects emphasize the need for integrated weight-management strategies and owner education regarding the consequences of feline obesity (Vitor et al., 2024; Klotsman et al., 2021).
This review critically examines the diagnostic and predictive potential of three key biomarkers: insulin, leptin, and adiponectin, in the context of feline obesity and diabetes. By synthesizing current evidence on their interplay and clinical utility, we aim to inform the development of early detection strategies and targeted interventions to reduce diabetes risk in feline patients.
1.1 Search strategy
A comprehensive narrative literature search was performed using the following databases: PubMed, Scopus, Web of Science, VetMed Resource, and PubMed Central. The search targeted open access publications published between 2000 and 2024, focusing on peer-reviewed studies relevant to feline obesity and metabolic health. The following keyword combinations were used: feline obesity, cat nutrition, leptin, adiponectin, energy balance, high-protein diet, feline metabolic health, body composition, inflammation markers, and dietary management. Only articles involving domestic cats were included, although comparative data from other species were considered when they offered translational value. Studies were selected based on their relevance, clarity of methodology, and direct contribution to understanding the physiological, nutritional, and endocrine mechanisms involved in feline obesity and diabetes risk. No formal quality scoring tools were applied, as this was not a systematic review.
2 Obesity in feline patients: epidemiology and health implications
2.1 Prevalence of obesity in cats
The prevalence of overweight and obese cats has increased dramatically, according to several studies. Depending on the population and geographic area, estimates range from 11.5% to 63% of cats being overweight or obese (Tarkošová et al., 2016). This pattern is indicative of a larger epidemic where weight growth has been influenced by environmental and lifestyle variables, much like what has been seen in human populations. Studies conducted in the UK have shown that between 40% and 52% of pet cats are obese, especially indoor cats who are less active (Courcier et al., 2010; Courcier et al., 2012). Reduced physical activity, higher calorie intake, and shifting owner conceptions of the ideal body condition have all been linked to the rising trend of obesity in cats (Phillips et al., 2017; Courcier et al., 2010; Mori et al., 2016). In particular, owners frequently ignore excess weight because they find larger cats charming or attractive, which can cause those pets to gain more weight (Mori et al., 2016).
Furthermore, it has been proposed that the COVID-19 pandemic made the problem of feline obesity worse, with reports showing a rise in weight-related problems when pet owners changed their feeding schedules and spent more time at home (Machado et al., 2022). Cat obesity rates were shown to have increased as a result of this modification and fewer exercise chances during lockdowns, which highlights the necessity of focused weight management programs at veterinary clinics (Machado et al., 2022).
The growing frequency of obesity in cats has notable consequences since being overweight is connected to several health problems, including respiratory diseases, osteoarthritis, and diabetes mellitus (Loftus and Wakshlag, 2014). This underlines how urgently veterinary practitioners must provide obesity control and preventive strategies, including nutrition advice, exercise recommendations and owner education.
2.2 Causes of obesity in cats
Genetic predisposition greatly affects the course of obesity in cats. Studies indicate that variations in their endocrine and metabolic systems might lead to some breeds to be susceptible to obesity. Heritability factors linked to body weight and fat deposition show this genetic influence, suggesting that obesity may be partially inherited (Wallis and Raffan, 2020; Bairqdar et al., 2023). The importance of genetics in obesity susceptibility has been highlighted by the discovery that certain genetic variants are connected to body fat distribution and metabolism (Bairqdar et al., 2023; Hastuti, 2022). Some cats can also be genetically inclined to insulin resistance, which could aggravate weight gain, and the development of diseases related to obesity (Wallis and Raffan, 2020).
One of the main causes of feline obesity is environmental factors. Compared to their outdoor counterparts, cats who live indoors have fewer opportunities for physical activity, which frequently results in inactive lifestyles. Indoor cats are more likely to be obese because they participate less in exercise-promoting natural hunting activities (Stenberg et al., 2023; Arena et al., 2021). Moreover, a cat’s degree of activity and therefore its body weight may be influenced by elements such the number of pets and the daily human interaction time (Teng et al., 2020; Godfrey et al., 2024). Factors include housing space, the accessibility of interesting toys, and opportunities for environmental enrichment also affect a cat’s overall activity and metabolism (Lawson et al., 2019).
Cat obesity is directly caused by diet, which includes feeding habits in addition to the type and amount of food consumed. Ad libitum (free feeding) cats frequently eat too many calories, which causes them to gain weight (Rollins and Murphy, 2019). It has also been demonstrated that body weight and fat formation are influenced by the macronutrient composition of their food, particularly the proportion of proteins to carbs (Li and Pan, 2020; Goloni et al., 2024). Changed gut microbiota patterns observed in obese cats could make weight control efforts more difficult by influencing how energy is drawn from diet (Kieler et al., 2015; Ma et al., 2022). The link between certain dietary practices, such as high starch consumption, and challenges controlling weight in neutered cats (Hoenig et al., 2003; Goloni et al., 2024; Saavedra et al., 2024) underlines the need for post-surgical food management in avoiding obesity. Overeating and treat distribution can also cause too much calorie intake, which increases the likelihood of obesity (Lackey et al., 2016; Lawson et al., 2019).
2.3 Consequences of obesity
Cat obesity has a major negative effect on their health, leading to a number of issues that shorten their lifespan and negatively impact on their general well-being.
2.3.1 Insulin resistance
The increased risk of diabetes mellitus is one of the most serious effects of obesity. Chronically high blood glucose levels are the result of insulin resistance, which is caused by excess body fat and impairs the body’s ability to respond to insulin (Hoelmkjaer et al., 2016). Research shows that cats who are obese are far more likely to develop type 2 diabetes, and their insulin sensitivity decreases by 30% for every kilogram of body weight (Kocabağlı et al., 2017; Clark and Hoenig, 2021). These animals’ metabolic dysfunction is characterized by a combination of insulin resistance and glucotoxicity, which, if left untreated, can result in serious diabetes consequences (Lewitt et al., 2016; Clark and Hoenig, 2021; Martin and Wood, 2010).
Many studies have shown that overweight cats have higher fasting insulin levels, implying this issue (Vitor et al., 2024; Appleton et al., 2000b; Zapata et al., 2017; Hoelmkjaer et al., 2016). As a result, these cats could struggle to keep appropriate glucose homeostasis, which could influence their diabetes mellitus risk, a condition defined by consistently elevated blood sugar levels caused by insulin resistance. Recent research has revealed the processes behind the link between obesity and insulin resistance, especially in cats. For instance, Hoenig et al. found that, particularly in older populations, overweight cats show significant changes in postprandial glucose metabolism and decreased insulin sensitivity (Hoenig et al., 2011a). This is corroborated by Vitor et al., who found that in obese cats, higher blood glucose levels are correlated with higher body condition scores (Vitor et al., 2024; Lewitt et al., 2016). The dysregulation of glucose metabolism is exacerbated by changes in the secretion of adipokines, such as leptin and adiponectin, which are implicated in insulin signaling (Araújo et al., 2024; Takashima et al., 2016).
This interaction highlights how complicated cat metabolism is, especially when it comes to obesity and food composition. Being genuine carnivores, cats naturally consume a diet low in carbs and rich in protein. Due to unnatural nutritional constraints, insulin dynamics may be negatively altered by diets high in carbs, which can result in a reduction in insulin sensitivity (Brearley et al., 2006; Hoenig et al., 2007).
2.3.2 Liver dysfunction
Furthermore, liver illness, especially hepatic lipidosis, which can happen when excessive fat buildup overtakes liver function, is closely associated with obesity in cats (Tarkošová et al., 2016). Because the liver cannot process fat effectively, fat increases and causes hepatic dysfunction, this condition is significantly more common in overweight and obese cats. Liver disease can have serious effects, including increased death rates and multi-organ dysfunction in cats (Tarkošová et al., 2016; Bjørnvad and Hoelmkjaer, 2014).
2.3.3 Joint disease
Reduced mobility and joint issues are also associated with obesity. Cats who are overweight are more likely to develop degenerative joint disease and lameness because of the increased stress on their joints (Maniaki et al., 2021; Bjørnvad and Hoelmkjaer, 2014). This disease is particularly worrisome since it produces persistent pain as well as decreases a cat’s activity level, hence aggravating obesity by decreasing exercise (Bjørnvad and Hoelmkjaer, 2014). Additionally, because obesity alters body composition and fat distribution, research has suggested a potential link between obesity and a higher prevalence of dermatological conditions and lower urinary tract issues (Tarkošová et al., 2016).
3 Relevance of adipokines
Important fat metabolism regulators in the context of obesity, adipokines are bioactive chemicals secreted by adipose tissue that control several metabolic processes. Among these peptides, leptin and adiponectin influence insulin sensitivity, inflammation, energy expenditure, and appetite control (Arner, 2005; Blüher and Schwarz, 2014; Blüher, 2012; Park and Shimokawa, 2024; Gupta et al., 2024). Dysregulation of adipokine secretion in obese people relates to metabolic disorders, such insulin resistance and type 2 diabetes, thereby emphasizing their clinical value (Abed et al., 2023; Armstrong et al., 2011; Andrade-Oliveira et al., 2015).
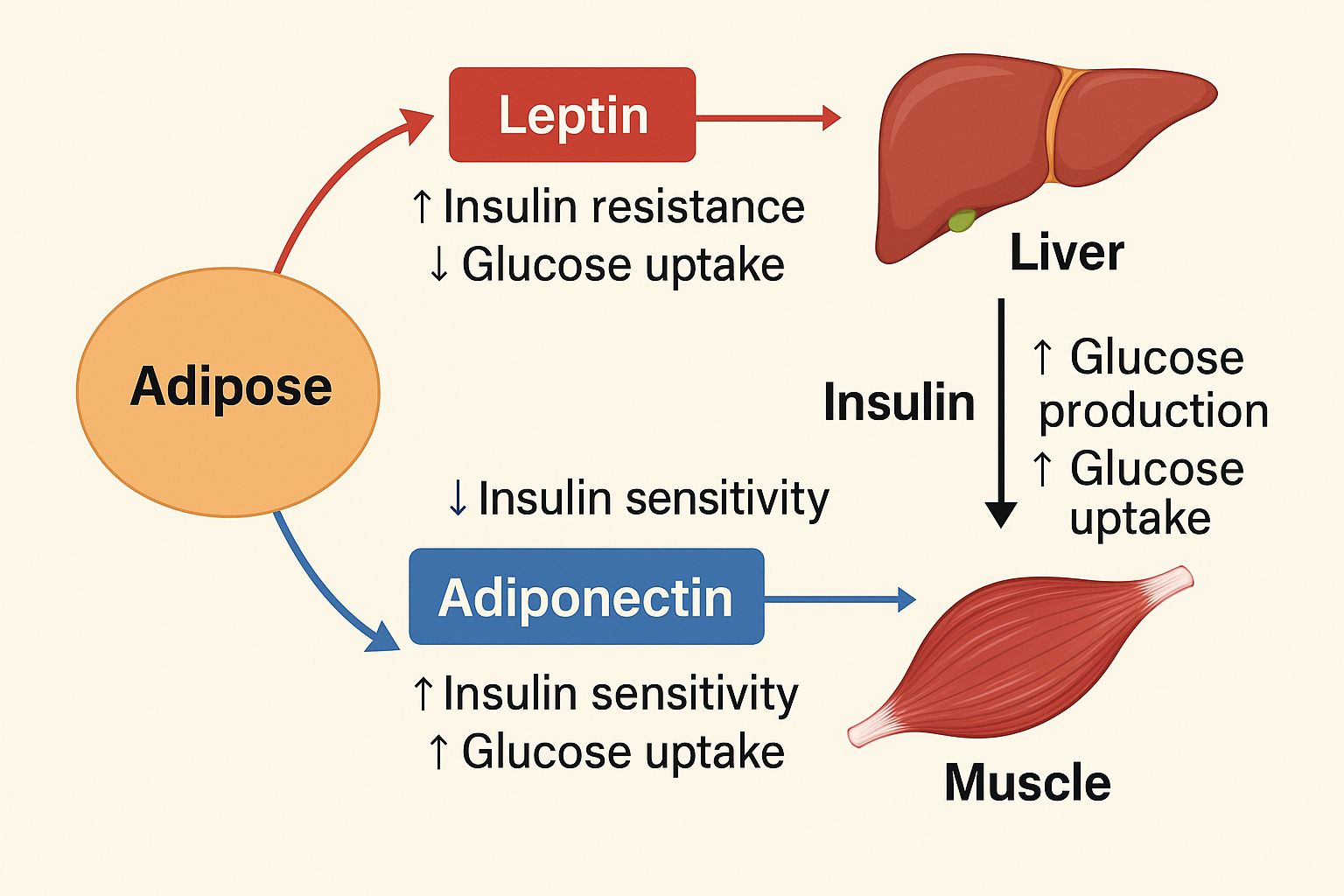
Leptin regulates energy expenditure and appetite. Obese cats, on the other hand, often exhibit leptin resistance, characterized by greater serum levels but lesser responsiveness, which helps to preserve weight (Appleton et al., 2002). Studies reveal that obese cats have reduced adiponectin levels; its decline links with obesity and may aggravate insulin resistance and inflammatory responses, typical of obesity-related issues (Ishioka et al., 2009; Zapata et al., 2017). Changes in these signaling molecules synthesis in cats have been linked to the emergence of obesity-related conditions such diabetes and heart disease (Ishiooka et al., 2009; Okada et al., 2019; Kim et al., 2023; Corvera, 2021). Additionally, adipokines systemic impact is demonstrated by the inter-organ communication they facilitate, which impacts not just adipose tissue but also muscle, liver, and vascular functions (Korek and Krauss, 2015; Dongre, 2021; Gupta et al., 2024). The underlying mechanism is illustrated in Figure 1, which provides a detailed explanation of its components and how they interact. Given the striking similarity of this trend to findings in people, cats are a great model for research on metabolic diseases connected to obesity (Velde et al., 2013).
3.1 Adipokines: key regulators of fat metabolism
3.1.1 Leptin
Leptin is a vital adipokine that is mostly secreted by adipose tissue and is necessary for regulating energy balance, metabolism, and appetite (Lee et al., 2007). It is essential for maintaining body weight homeostasis, and it is more prevalent in obese individuals, including cats (Takashima et al., 2016). Because it informs the hypothalamus about the condition of energy storage, leptin plays a crucial role in reducing appetite and raising energy expenditure (Zhao et al., 2019).
Because of the expansion of fat tissue, leptin levels usually increase when a cat is obese; it has also been shown to be positively related with body weight, body condition score, and body fat percentage (Vester et al., 2011). These relationships show leptin’s dual role as metabolic control and a sign of obesity. According to one study, overweight cats have greater leptin levels and acquire a kind of leptin resistance comparable to that seen in overweight humans (Lee et al., 2007; Appleton et al., 2000b). Hyperleptinemia is a condition in which high amounts of circulating leptin do not sufficiently control weight or decrease hunger (Sharma and Sharma, 2018). Apart from its connection to energy metabolism, leptin exhibits pro-inflammatory properties that may aggravate chronic inflammation caused by obesity (Booth et al., 2016; Araújo et al., 2024). Increased production of pro-inflammatory cytokines by macrophages and other immune cells infiltrating adipose tissue in obese individuals can worsen insulin resistance and metabolic dysfunction (Araújo et al., 2024). Furthermore, because of its interactions with other hormonal pathways, particularly its role in controlling insulin sensitivity, leptin is seen as a key factor in the evolution of obesity-related comorbidities such diabetes mellitus (Takashima et al., 2016).
3.1.2 Adiponectin
Adiponectin is an important adipokine that regulates insulin sensitivity and metabolic health. It also has a big impact on feline obesity. Adiponectin, which is mostly secreted by adipose tissue, comes in a variety of forms (Frank and Walsh, 2017; Hoenig et al., 2011a; Takashima et al., 2016).
Studies revealing that adiponectin levels directly correlate with metabolic health in lean cats and obese cats have far lower circulating levels of adiponectin than cats with a lower body condition score (Tvarijonaviciute et al., 2012; Öhlund et al., 2015; Hoenig et al., 2013). Insulin resistance, a risk factor for diabetes mellitus, can result from this decreased adiponectin secretion (Öhlund et al., 2015; Hoenig et al., 2013).
On the other hand, reduced adiponectin levels in obesity are associated with higher inflammatory marker levels and impaired lipid metabolism, which exacerbates insulin resistance (Hoenig et al., 2013; Stenberg et al., 2023). Interestingly, research indicates that overweight cats who lose weight have more adiponectin, suggesting that weight loss efforts could help to correct certain metabolic anomalies (Takashima et al., 2016; Hoenig et al., 2013).
3.1.3 Insulin
Like other species, insulin is necessary for energy homeostasis and glucose metabolism in cats. In cats, insulin sensitivity and glucose tolerance are significantly influenced by body weight and obesity; these two parameters are intimately linked to insulin secretion and action. The dysregulation of insulin is a critical factor in the development of metabolic disorders in obese cats, as it is a critical regulator of glucose metabolism (Strage et al., 2021). In these animals, an increase in adiposity is closely linked to a decrease in insulin sensitivity, which results in a compensatory increase in pancreatic insulin secretion. This hyperinsulinemia frequently serves as an early indicator of metabolic dysfunction, occurring prior to the development of overt hyperglycemia and clinical diabetes mellitus (Clark and Hoenig, 2021; Appleton et al., 2000b).
Research has particularly revealed that every kilo of body weight correlates to a notable drop in insulin sensitivity (Clark and Hoenig, 2021). Emphasizing that hyperinsulinemia may function as an early diagnostic indicator of the progression to diabetic conditions, obese cats have been observed to exhibit elevated plasma insulin concentrations in comparison to their ideal-weight counterparts (Appleton et al., 2000b).
Establishing precise reference ranges for hyperinsulinemia in cats remains challenging, as reported values vary considerably depending on the analytical method, physiological state, and study population (Strage et al., 2021). According to this study, baseline insulin concentrations in healthy cats ranged approximately from 2 to 18 μU/mL, depending on analytical method and physiological status (Appleton et al., 2001).
Importantly, there is no standardized threshold currently accepted for defining hyperinsulinemia in feline patients. Most authors agree that insulin values should be interpreted in light of the animal’s clinical picture, including body condition, glucose levels, and insulin-to-glucose ratios, rather than relying solely on an isolated numeric cutoff.
Gene expression research on fat cats has also exposed the molecular processes underlying insulin dysregulation. Changes in the expression of genes linked to insulin signaling and glucose metabolism in both adipose and skeletal muscle tissues have been found by Stenberg et al. (2023). These results imply that alterations at the transcriptional level brought on by obesity could help explain higher insulin secretion and compromised insulin function.
3.2 Exploration of additional adipokines: resistin, chemerin, omentin, and CTRP-12
In cats, adipokines such resistin, chemerin, omentin, and CTRP-12 are crucial for controlling metabolic processes. Numerous research has examined their existence in adipose tissue and their functional relevance, providing insight into their effects on obesity and associated metabolic problems in cats.
3.2.1 Resistin
Takashima et al. molecularly cloned feline resistin and showed that it was expressed in both normal and obese cats. Resistin is known to be linked to insulin resistance in a variety of animals (Quijada et al., 2013- abstract). Although the precise routes are less known than for other species, their research showed that feline resistin levels positively linked with obesity, indicating a role in the development of insulin resistance (Takashima et al., 2016).
3.2.2 Chemerin
The involvement of another adipokine, chemerin, inflammation and metabolic control has been studied. Chemerin plays a role in immune cell recruitment and adds to the low-grade inflammation that is frequently observed in obese individuals (Recinella et al., 2020; Sierawska and Niedźwiedzka-Rystwej, 2022; Kirichenko et al., 2022). Although the precise role of chemerin in adipose tissue in cats is still unclear, new research indicates it might be a useful indicator of metabolic disorders associated with obesity (Velde et al., 2013).
3.2.3 Omentin
Because of its insulin-sensitizing qualities, omentin may be used therapeutically to treat obesity and other metabolic diseases in cats (Appleton et al., 2000b). Omentin’s anti-inflammatory properties were emphasized by Mazaki-Tovi et al., 2019a, who also discovered a negative correlation between its concentrations and body fat percentage, indicating a preventive function against the metabolic problems associated with obesity.
3.2.4 CTRP-12
Although it belongs to a family of adipokines important in metabolic control, CTRP-12 is less well-studied in cats than in humans and rodents. Although further research is required to validate these results, some research indicates that CTRP-12 may improve lipid metabolism and insulin sensitivity, which could help manage obesity in cats (Luo et al., 2023).
3.3 Adipokine dysregulation in obesity
One important element worsening a number of obesity-related illnesses in cats is adipokine dysregulation. Adipose tissue growth causes an imbalance in the secretion of several adipokines, which results in metabolic inefficiency and a persistent state of low-grade systemic inflammation. The health of cats is significantly impacted by this dysregulation, especially when it comes to developing diseases like insulin resistance, diabetes mellitus, and joint issues (Sierawska and Niedźwiedzka-Rystwej, 2022; Araújo et al., 2024; Appleton et al., 2000b).
While preventive adipokines like adiponectin are frequently decreased in obese cats, pro-inflammatory adipokines like leptin, tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) are usually secreted in greater amounts (Takashima et al., 2016; Dong et al., 2018). Despite being designed to indicate fullness and control energy balance, elevated leptin levels in obesity often result in leptin resistance, a condition in which the hypothalamus loses its sensitivity to the appetite-suppressing effects of leptin. Because too much leptin stimulates inflammatory pathways that impede the action of insulin, this phenomenon not only feeds the cycle of overeating and weight gain, but also plays a role in the development of insulin resistance (Banks and Farrell, 2003; Gerdes et al., 2011; Lynch et al., 2017; Sierawska and Niedźwiedzka-Rystwej, 2022; Kotal et al., 2023).
On the other hand, adiponectin has been linked to decreased inflammation and shows insulin-sensitizing qualities. Obese cats who have low adiponectin levels are more likely to develop metabolic diseases. An obvious illustration of how adipokine dysregulation might contribute to the development of diabetes in cats is its deficit, which has been connected to insulin resistance and hyperglycemia (Dong et al., 2018; Sierawska and Niedźwiedzka-Rystwej, 2022; Deng and Scherer, 2010).
Nevertheless, pro-inflammatory adipokines like TNF-α and IL-6 can worsen insulin resistance and adipose tissue dysfunction, creating a vicious cycle that worsens metabolic disorders (Yu et al., 2024; Zhang, 2010). Adipokines can mediate inflammatory processes that directly affect joint health, such as osteoarthritis, which is common in obese cats and is exacerbated by persistent low-grade inflammation (Moreno-Aliaga et al., 2010).
Moreover, because obesity, inflammation and altered adipokine profiles have been related to increased cardiovascular risk, dysregulated adipokine levels can affect cardiovascular health (Lynch et al., 2017; Dadej et al., 2022; Chantemèle et al., 2011). The correlation between adipokine dysregulation and systemic health problems in cats is best illustrated by the increase in chronic illnesses linked to obesity.
3.3.1 Insulin dosing and resistance in obese cats
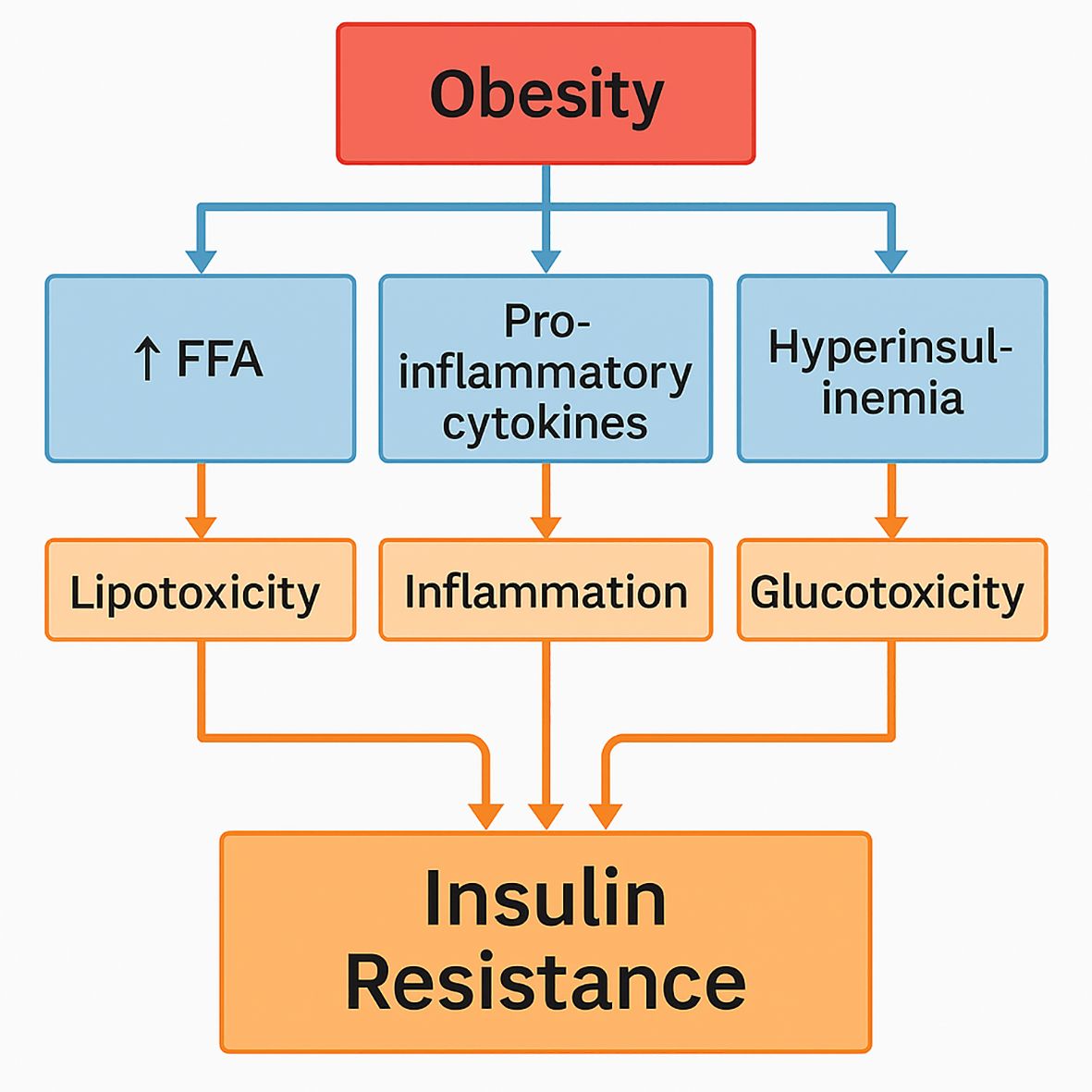
The intricate and multiple routes by which excess body fat causes insulin resistance and the onset of type 2 diabetes in cats represent the interactions among adipose tissue, adipokines, inflammation, and glucose metabolism. Many metabolic changes caused by increasing body fat, particularly visceral fat, promote insulin resistance, a condition defined by a reduced capacity of insulin-responsive tissues to react to the effects of insulin (Cook and Evans, 2021). Obesity is related to changes in the release of adipokines, which are signaling proteins produced by adipose tissue. While insulin-sensitizing adipokines like adiponectin are lower, pro-inflammatory adipokines such as leptin and TNF-alpha are markedly raised in obese cats (Hoenig et al., 2013; Araújo et al., 2024). A persistent low-grade inflammatory state brought on by excessive inflammatory cytokine levels that harm insulin signaling pathways and disrupt insulin’s normal metabolic functions causes increased insulin resistance and compromised glucose uptake in peripheral tissues (Zini et al., 2008). Hyperglycemia is exacerbated as a result of the liver and muscle tissues losing their sensitivity to insulin and in order to maintain normal glucose levels in the face of chronic insulin resistance, the pancreatic beta cells must produce more insulin. Insulin secretion capacities may be further compromised over time by beta-cell fatigue and malfunction brought on by the increased demand for insulin synthesis (Abdullah et al., 2010; Osto et al., 2013; Zini et al., 2008). Long-term exposure to insulin-resistant hyperglycemia in cats can cause glucotoxicity, which damages beta cells and reduces their capacity to produce insulin efficiently, the mechanism is illustrated in Figure 2 (Zini et al., 2008; Osto et al., 2013). Because of decreased glucose clearance associated with insulin resistance, postprandial glucose levels in obese cats stay noticeably higher for longer periods of time (Hoenig et al., 2011a; Appleton et al., 2000b).
These metabolic abnormalities point to a general decrease in glucose tolerance, which accelerates the development of type 2 diabetes. Inflammation connected to obesity disrupts insulin signaling and raises oxidative stress in tissues. High reactive oxygen species (ROS) damaging insulin signaling pathways can help to build a feedback loop maintaining metabolic inefficiency and insulin resistance (Clark and Hoenig, 2021; Zagayko et al., 2019).
The development and worsening of obesity in cats are largely caused by lifestyle factors, particularly a sedentary lifestyle and the consumption of high-calorie, nutrient-poor diets (Kocabağlı et al., 2017; Öhlund et al., 2015).
3.3.2 Species differences in adipose tissue
Dogs and cats have quite different adipose tissues in terms of composition, function, and the type of adipokines they release, which affect inflammation and metabolism. Adipocytes in dogs and cats react differently to nutritional elements and inflammatory stimuli, which can result in significant differences in the properties of their adipose tissue (Osto and Lutz, 2015).
According to research, inflammatory triggers such as lipopolysaccharides (LPS) and tumor necrosis factor-alpha (TNFα) cause adipocytes in dogs to release pro-inflammatory adipokines. For example, Ryan et al. pointed out that canine adipose cells produce inflammatory adipokines such leptin and interleukin-6 (IL-6), which may play a significant part in inflammation linked to obesity (Ryan et al., 2010). Additionally, Mazaki-Tovi et al. (2018) found that dietary fatty acids may alter the release of IL-6 and adiponectin in canine adipose tissue, suggesting that food has a direct impact on inflammatory responses and adipokine profiles. Essentially, dogs have a reactive adipose tissue phenotype, which may be a factor in disorders linked to obesity.
In contrast, the situation regarding inflammation and adipokine expression in feline adipose tissue is different. Although research shows that high-fat diets mostly increase oxidative stress rather than inflammation in their adipose depots, cats still tend to develop obesity-associated adipose tissue inflammation (Ampem et al., 2015; Osto and Lutz, 2015). Their metabolic responses may be impacted by this divergence; notable variations have been observed in the cytokine composition of adipose tissue, especially in the expression pathways that control insulin sensitivity. According to Stenberg et al., the metabolic changes in feline adipose tissue might be the result of a special evolutionary trajectory in which cats, as obligate carnivores, have different energy-storage systems than dogs (Stenberg et al., 2023).
Of their investigation of the gene expression profiles of feline adipocytes, Riedel et al. discovered unique renin-angiotensin system regulation patterns that are less noticeable in canine adipose tissues (Riedel et al., 2015). Adipokines may play different roles in regulating inflammation and energy metabolism in diverse species, as suggested by these variations. According to James et al. (2017), some adaptive responses of cats to energy mobilization and storage during times of excess or fasting have consequences for understanding how susceptible they are to metabolic diseases in comparison to dogs.
Additionally, the two species differ in the functional capacities of mesenchymal stem cells generated from adipose tissue. According to characterization studies, mesenchymal stem cells from adipose tissue in both species can differentiate into distinct lineages, but their properties and efficiency may vary depending on the tissue source. To further clarify the unique biological traits originating from their adipose tissues, Rashid et al. (2021) for instance, described the differences between the mesenchymal stem cells of dogs and cats with regard to their capacity for proliferation and differentiation (Rashid et al., 2021).
4 Potential therapeutic approaches
4.1 Dietary interventions
In both human and feline populations, dietary therapies can enhance insulin sensitivity and have a considerable impact on adipokine levels. Diets that are high in protein and low in carbohydrates have been found to have a significant impact on metabolic health, improving insulin sensitivity and altering adipokine secretion.
4.1.1 High-protein diets
Research suggests that a high-protein diet can lower pro-inflammatory adipokines like TNF-alpha and IL-6 while raising healthy adipokines like adiponectin. High-protein diets have been shown to raise serum levels of adiponectin, which have insulin-sensitizing properties, in cats (Berman et al., 2021; Öhlund et al., 2021). Adiponectin is essential for increasing insulin sensitivity because it increases muscle glucose absorption and encourages fatty acid oxidation. Diets heavy in protein have also been connected to better glycemic control since higher protein consumption raises postprandial blood glucose levels (Grant et al., 2020; Clark and Hoenig, 2021).
Although high-protein diets are often linked to concerns about renal stress, certain studies indicate they may offer advantages in particular contexts, for instance, in helping cats preserve muscle mass during the early stages of chronic kidney disease (Hall et al., 2019). These findings emphasize that the impact of dietary protein on kidney function is not uniform and must be evaluated in relation to each cat’s medical profile. Notably, Kubo et al. (2023) found no harmful renal effects in healthy subjects consuming high-protein diets, suggesting that cats without kidney compromise may better tolerate elevated protein intake (Grant et al., 2020).
Given protein’s fundamental role in feline health, adjusting intake based on individual health status is essential, especially in aging cats or those with existing renal issues. The interplay between protein metabolism, phosphorus levels, and kidney response reinforces the need for tailored nutritional plans that take into account each cat’s unique vulnerabilities.
4.1.2 Low-carbohydrate diets
Studies show that cats’ glucose metabolism may be negatively impacted by high-carb diets. According to a study, cats who consume high-carb diets nonetheless have a less ideal metabolic state even though they do not cause β-cell malfunction like some other animals do. According to Hewson-Hughes et al. (2011), a high carbohydrate content was particularly linked to increased postprandial glucose and insulin levels, which started debates on the relevance of dietary composition in controlling cat diabetes (Verbrugghe and Hesta, 2017; Hoenig et al., 2011a; Laflamme et al., 2022).
Compared to conventional high-carb diets, low-carb diets can frequently result in more noticeable improvements in insulin sensitivity. In order to diminish insulin resistance, these diets are associated with lower serum insulin levels and less body fat accumulation. According to research, meals high in protein and low in carbohydrates may increase insulin signaling pathways, which in turn may improve glucose metabolism, better lipid profiles and a lower incidence of fatty liver disease, which is frequently a result of metabolic disturbances linked to obesity (Asaro et al., 2017; Mazaki-Tovi et al., 2013; Tricò et al., 2021; Doostvandi, 2016). These dietary changes can help in reestablishing the proper balance of adipokines by lowering pro-inflammatory cytokines and raising levels of anti-inflammatory adipokines.
High-protein, low-carbohydrate diets have been associated with improvements in insulin sensitivity and metabolic regulation in obese cats (Coradini et al., 2011). These dietary approaches influence adipokine profiles, often resulting in reduced leptin levels and increased adiponectin concentrations, thereby contributing to a lower leptin–adiponectin ratio (LAR), a marker increasingly recognized for its association with insulin resistance (Appleton et al., 2000b; Mazaki-Tovi et al., 2019a). Since a high LAR has been linked to metabolic dysfunction and inflammation, nutritional strategies that restore adipokine balance may enhance metabolic outcomes. This effect supports the potential use of LAR not only as a biomarker of metabolic status, but also as a target for monitoring response to dietary interventions (Stenberg et al., 2023).
4.2 Implications for insulin resistance in clinical practice
Cats with obesity and insulin resistance may benefit from diets high in protein and low in carbohydrates. In addition to addressing the metabolic abnormalities frequently observed in obese cats, these dietary patterns also help normalize adipokine levels and improve insulin sensitivity, which can improve general health (Mabuza et al., 2018; Clark and Hoenig, 2021).
5 Clinical implications and future direction
5.1 Clinical applications
A promising approach to treating obesity-related conditions and enhancing cats’ metabolic health is to incorporate adipokine and insulin dosing techniques into veterinary practice. Veterinarians now have new therapeutic options thanks to the growing knowledge of adipokines’ physiological functions and their pharmaceutical modulation. Adipokines are important for controlling insulin sensitivity, inflammation, and energy metabolism. Treating diseases like obesity and insulin resistance in cats may benefit from therapeutic modulation of these molecules. For example, dietary therapies or supplements that raise circulation levels of adiponectin may help obese cats lose weight and improve their metabolic function (Würfel et al., 2023).
In order to address obesity and related metabolic diseases, veterinarians can use dietary changes to affect adipokine levels. Low-carb, high-protein diets may improve insulin sensitivity by lowering inflammation brought on by obesity and raising adiponectin levels (Nicholson et al., 2018). Optimizing food intake and adipokine production can be achieved by creating customized nutrition programs based on each feline’s unique metabolic profile (e.g. body fat percentage, body mass index, ideal body weight). Treatments aimed at increasing insulin sensitivity or weight reduction via dietary modifications, for example, might be good for cats with high leptin but low adiponectin (Mazaki-Tovi et al., 2019b).
Adipokine levels might be included in clinical practice to enhance diagnostic capabilities. Regular assessments of it can enable doctors to assess the effectiveness of weight-loss programs or treatments and modify treatment plans accordingly.
5.2 Predictive potential and clinical implications
The potential of combined hormone profiling to predict the onset of diabetes and guide early clinical interventions is substantial. The start and development of insulin resistance and type 2 diabetes mellitus have been linked to the dysregulation of major metabolic hormones, including insulin, leptin, and adiponectin, in both human and animal models. Recent human research indicates that a state of hyperinsulinemia typically precedes overt hyperglycemia, with shifts in circulating hormone levels functioning as early indicators of metabolic dysfunction (Gottlieb et al., 2022).
Increased insulin production in response to decreased insulin sensitivity in peripheral tissues is thought to cause this early hyperinsulinemia, which is further aggravated by related adipokine profile alterations. The leptin/adiponectin ratio, for instance, has been linked to a higher risk of diabetes and compromised insulin sensitivity (Gottlieb et al., 2022). Feline studies have shown similar endocrine tendencies. Studies on domestic cats has shown that there are correlations between circulating adipokine concentrations and indices of adiposity and insulin resistance (Appleton et al., 2000b). The combined use of adiponectin, leptin, and insulin profiling appears to offer a more sensitive approach for detecting subclinical metabolic disturbances, despite the fact that the majority of clinical investigations in veterinary medicine have historically relied on measuring fasting glucose or fructosamine levels. This understanding highlights the potential predictive value of combined hormone measurements in evaluating the risk of developing diabetes in obese feline patients (Appleton et al., 2000b).
Comparative research with humans and dog models—where biomarker assays are more readily available and well standardized—further support the notion that early hormone changes are essential in the onset of metabolic disease. In spite of the promising nature of combined hormone profiling, its clinical adoption in veterinary medicine is currently limited due to several challenges. The availability and standardization of assays continue to pose significant obstacles. While human and canine studies are facilitated by well-established reference ranges and cost-effective assays, equivalent standardized methodologies for cats are still in the process of being developed (Sasaki et al., 2001). Routine clinical implementation is further complicated by the relatively high cost of sensitive multi-assays and the absence of generally recognized reference intervals for feline adiponectin, leptin, and insulin. As a result, while early hormonal changes can offer valuable insights into the progression of metabolic dysfunction, their predictive potential must be considered in the context of practical factors, including assay cost, reproducibility, and diagnostic robustness in a variety of clinical settings (Gottlieb et al., 2022; Appleton et al., 2000b).
Overcoming these analytical limitations requires targeted efforts. One key strategy is the establishment of collaborative inter-laboratory studies aimed at validating feline-specific adipokine and insulin assays. Such collaborations would allow for cross-platform comparisons, improved reproducibility, and standardization of reference ranges. Furthermore, the development and commercial availability of species-specific reagents, particularly for feline leptin and adiponectin, would enhance both clinical and research applications. Finally, harmonizing pre-analytical variables, such as fasting duration, sample type, and storage conditions should be prioritized to improve assay consistency across studies.
5.3 Current challenges and opportunities
Improving treatment strategies depends on our understanding of insulin management and adipokine regulation in feline patients. Even if literature is growing, there are still several subjects that require further study to completely grasp the underlying mechanisms of these therapies and enhance their clinical use in the management of obesity and related disorders. Despite the fact that adipokines are known to be important mediators of metabolic diseases, little is known about the precise processes by which they interact, especially when it comes to feline obesity. Adipokines including chemerin, vaspin, and visfatin require further investigation into their functions in insulin signaling pathways and inflammation (Araújo et al., 2024; Helfer and Wu, 2018).
Knowing how various adipokines interact and affect one another may help us better understand how these adipokines work together to affect insulin resistance and obesity-related health problems in cats. Most of the current research focuses on cross-sectional analyses of adipokine levels in obese versus non-obese cats, which leaves us with a limited understanding of how dietary interventions or weight loss affect adipokine profiles over time (Araújo et al., 2024; Appleton et al., 2000b). Longitudinal studies tracking adipokine levels in individual cats during the course of food changes or weight-management programs could expose critical time periods for potential therapeutics. While studies have identified several adipokines that could help with metabolic disorders, further investigation is required to clarify their exact roles and effects in cat patients. To effectively validate adipokines and insulin as early predictive biomarkers, longitudinal studies should be designed to follow overweight but non-diabetic cats over time. A recommended model would include baseline metabolic profiling: leptin, adiponectin, insulin and glucose, followed by follow-ups every 3–6 months over a 2–3-year period. These cohorts should account for age, diet, activity level, and neuter status. Notably, biosample archiving and the inclusion of consistent assay methodologies across timepoints will be essential to allow both intra- and inter-study comparison. Such designs would help map the trajectory of metabolic deterioration and identify inflection points predictive of diabetes onset. Further study on the functions of new adipokines such CTRP12 and omentin would help to clarify, for instance, their insulin-sensitizing actions and general impact on controlling diabetes and obesity in cats (Wei et al., 2012; Elhafez et al., 2020). Targeting these adipokines pharmacologically may lead to novel therapy options.
Although dietary changes are known to influence insulin sensitivity and adipokine levels, additional controlled clinical studies are needed to exactly assess the impact of various dietary treatments on cat adipokine profiles (Araújo et al., 2024; Appleton et al., 2000b). Finding the ideal macronutrient compositions that produce the best results for metabolic health should be the main goal of future study.
Practical methods for incorporating adipokine measurements into routine veterinary practice are still unknown, despite the growing interest in using these measurements as biomarkers for conditions linked to obesity (Würfel et al., 2023; Sierawska and Niedźwiedzka-Rystwej, 2022).
6 Translational relevance and limitations
The findings presented in this paper underscore the importance of dietary strategies, metabolic pathways, and adipokine activity in both the development and management of obesity in cats. While parallels drawn from human and canine studies provide useful context and contribute to the broader understanding of these mechanisms, direct application is limited by species-specific differences, particularly in nutritional requirements, insulin responsiveness, and adipose tissue behavior. Given the unique metabolic traits of felines, clinical implementation must be approached with caution. Further cat-specific research is essential to validate these translational insights and refine therapeutic options.
7 Conclusion
In summary, this review illustrates that adiponectin, leptin, and insulin are promising early biomarkers for the prediction of metabolic dysregulation in obese feline patients. Diminished insulin sensitivity and evident hyperglycemia have been consistently linked to decreased levels of adiponectin, elevated leptin concentrations, and compensatory hyperinsulinemia, thereby functioning as early indicators of impending diabetes mellitus. The clinical relevance of combined hormone profiling for tailored risk assessment and preventive intervention in feline practice is emphasized by the synthesis of current evidence and the parallels derived from human and canine studies. Nevertheless, there are still substantial obstacles to overcome, such as the necessity of establishing species-specific reference ranges and the development of cost-effective, standardized assays. In order to promote early therapeutic strategies and ultimately enhance clinical outcomes in obese cats, future research should concentrate on the longitudinal validation of these biomarkers, the refinement of assay methodologies, and the integration of these measures into routine preventive screening protocols.
Author contributions
LC: Writing – review & editing, Writing – original draft. SD: Conceptualization, Supervision, Writing – review & editing. AM: Methodology, Supervision, Validation, Project administration, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdullah A., Stoelwinder J., Shortreed S., Wolfe R., Stevenson C., Walls H., et al. (2010). The duration of obesity and the risk of type 2 diabetes. Public Health Nutr. 14, 119–126. doi: 10.1017/s1368980010001813
Abed B., Courcier E. A., O’Higgins R., Mellor D. J., and Yam P. S. (2023). Partial vs. complete weight reduction protocols in obese cats: A non-randomized observational cohort study. Front. Vet. Sci. 10, 1211543. doi: 10.3389/fvets.2023.1211543
Ampem G., Azegrouz H., Bacsadi Á., Balogh L., Schmidt S., and Thuróczy J. (2015). Adipose tissue macrophages in non-rodent mammals: A comparative study. Cell Tissue Res. 363, 461–478. doi: 10.1007/s00441-015-2253-1
Andrade‑Oliveira V., Câmara N. O. S., and Moraes‑Vieira P. M. (2015). Adipokines as drug targets in diabetes and underlying disturbances. J. Diabetes Res. 2015, 681612. doi: 10.1155/2015/681612
Antakyalioglu B., Özturan Y., Parlatır Y., Akın İ., and Ural K. (2024). Intragastric botulinum toxin-a injection: A novel approach to successfully manage feline obesity as an alternative technique to conventional treatment. Vet. Rec. Case Rep. 13. doi: 10.1002/vrc2.1030
Appleton D. J., Rand J., and Sunvold G. (2000a). Plasma leptin concentrations in cats: Reference range, effect of weight gain and relationship with adiposity as measured by dual energy x-ray absorptiometry. J. Feline Med. Surg. 2, 191–199. doi: 10.1053/jfms.2000.0103
Appleton D. J., Rand J. S., and Sunvold G. D. (2000b). Plasma leptin concentrations are independently associated with fat mass and body condition in cats. J. Am. Vet. Med. Assoc. 217 (5), 695–700. doi: 10.2460/javma.2000.217.695
Appleton D. J., Rand J. S., and Sunvold G. D. (2001). Determination of reference values for glucose tolerance, insulin tolerance, and insulin sensitivity tests in clinically normal cats. American Journal of Veterinary Research 624, 630–636.
Appleton D. J., Rand J., and Sunvold G. (2002). Plasma leptin concentrations are independently associated with insulin sensitivity in lean and overweight cats. J. Feline Med. Surg. 4, 83–93. doi: 10.1053/jfms.2002.0166
Araújo S., Martins P., Pereira T., Sampaio T., Menezes R., Costa M., et al. (2024). Evidence of obesity-induced inflammatory changes in client-owned cats. Vet. World 17 (8), 1695–1703. doi: 10.14202/vetworld.2024.1685-1692
Arner P. (2005). Insulin resistance in type 2 diabetes – Role of the adipokines. Curr. Mol. Med. 5, 333–339. doi: 10.2174/1566524053766022
Arena L., Menchetti L., Diverio S., Guardini G., Gazzano A., and Mariti C. (2021). Overweight in Domestic Cats Living in Urban Areas of Italy: Risk Factors for an Emerging Welfare Issue. Animals 11, 2246. doi: 10.3390/ani11082246
Armstrong M. J., Mottershead T. A., Ronksley P. E., Sigal R. J., Campbell T. S., and Hemmelgarn B. R. (2011). Motivational interviewing to improve weight loss in overweight and/or obese patients: A systematic review and meta‑analysis of randomized controlled trials. Obesity Reviews 12 (9), 709–723. doi: 10.1111/j.1467-789X.2011.00892.x
Asaro N., Guevara M., Berendt K., Zijlstra R., and Shoveller A. (2017). Digestibility is similar between commercial diets that provide ingredients with different perceived glycemic responses and the inaccuracy of using the modified Atwater calculation to calculate metabolizable energy. Vet. Sci. 4, 54. doi: 10.3390/vetsci4040054
Bairqdar A., Ivanoshchuk D. E., and Shakhtshneider E. (2023). Functionally Significant Variants in Genes Associated with Abdominal Obesity: A Review. Genes (MDPI). doi: 10.3390/genesxxxxxxx
Banks W. and Farrell C. (2003). Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. AJP. Endocrinol. Metab. 285, E10–E15. doi: 10.1152/ajpendo.00468.2002
Berman C., Lobetti R., Zini E., Fosgate G., and Schoeman J. (2021). Influence of high-protein and high-carbohydrate diets on serum lipid and fructosamine concentrations in healthy cats. J. Feline Med. Surg. 24, 759–769. doi: 10.1177/1098612x211047062
Bjørnvad C. and Hoelmkjaer K. (2014). Management of obesity in cats. Vet. Med.: Res. Rep. 97, 97–107. doi: 10.2147/vmrr.s40869
Blüher M. (2012). Clinical relevance of adipokines. Diabetes Metab. J. 36, 317. doi: 10.4093/dmj.2012.36.5.317
Blüher S. and Schwarz P. (2014). Metabolically healthy obesity from childhood to adulthood — Does weight status alone matter? Metabolism 63 (9), 1478–1489. doi: 10.1016/j.metabol.2014.06.009
Booth A., Magnuson A., Fouts J., and Foster M. (2016). Adipose tissue: An endocrine organ playing a role in metabolic regulation. Hormone Mol. Biol. Clin. Invest. 26, 25–42. doi: 10.1515/hmbci-2015-0073
Brearley M., Polton G., Littler R., and Niessen S. (2006). Coarse fractionated radiation therapy for pituitary tumours in cats: A retrospective study of 12 cases. Vet. Comp. Oncol. 4, 209–217. doi: 10.1111/j.1476-5829.2006.00108.x
Chantemèle E., Mintz J., Rainey W., and Stepp D. (2011). Impact of leptin-mediated sympatho-activation on cardiovascular function in obese mice. Hypertension 58, 271–279. doi: 10.1161/hypertensionaha.110.168427
Clark M. and Hoenig M. (2021). Feline comorbidities: Pathophysiology and management of the obese diabetic cat. J. Feline Med. Surg. 23, 639–648. doi: 10.1177/1098612X211021540
Cook A. and Evans J. (2021). Feline comorbidities: Recognition, diagnosis and management of the cushingoid diabetic. J. Feline Med. Surg. 23, 4–16. doi: 10.1177/1098612x20979507
Coradini M., Rand J., Morton J., and Rawlings J. (2011). Effects of two commercially available feline diets on glucose and insulin concentrations, insulin sensitivity and energetic efficiency of weight gain. Br. J. Nutr. 106, S64–S77. doi: 10.1017/s0007114511005046
Corvera S. (2021). Cellular heterogeneity in adipose tissues. Annu. Rev. Physiol. 83, 257–278. doi: 10.1146/annurev-physiol-031620-095446
Courcier E. A., Mellor D. J., Pendlebury E., Evans C., and Yam P. S. (2012). An investigation into the epidemiology of feline obesity in Great Britain: results of a cross‑sectional study of 47 companion animal practices. Vet. Rec. 171 (22), 560. doi: 10.1136/vr.100953
Courcier E. A., O’Higgins R., Mellor D. J., and Yam P. S. (2010). Prevalence and risk factors for feline obesity in a first opinion practice in Glasgow, Scotland. J. Feline Med. Surg. 12 (10), 746–753. doi: 10.1016/j.jfms.2010.05.011
Dadej D., Szczepanek-Parulska E., Wrotkowska E., and Ruchała M. (2022). Cushing’s syndrome is associated with altered adipokine profile. Front. Endocrinol. 13. doi: 10.3389/fendo.2022.1032329
Deng Y. and Scherer P. (2010). Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann. New York Acad. Sci. 1212, 1–6. doi: 10.1111/j.1749-6632.2010.05875.x
Dong Z., Gong H., Chen Y., Wu H., Wu J., Deng Y., et al. (2018). LH-21, a peripheral cannabinoid receptor 1 antagonist, exerts favorable metabolic modulation including antihypertensive effect in KK-Ay mice by regulating inflammatory cytokines and adipokines on adipose tissue. Front. Endocrinol. 9. doi: 10.3389/fendo.2018.00167
Dongre U. (2021). Adipokines in insulin resistance: Current updates. Biosci. Biotechnol. Res. Asia 18, 357–366. doi: 10.13005/bbra/2922
Doostvandi T. (2016). The association between dietary patterns and insulin resistance: A systematic review. Int. J. Nutr. Food Sci. 5, 14. doi: 10.11648/j.ijnfs.s.2016050102.13
Elhafez Z., Mohamed M., Elmouttaleb A., and Elatif D. (2020). Evaluation of serum omentin-1 and apelin levels and their association with insulin resistance and obesity in Egyptian women with polycystic ovary syndrome. Int. Res. J. Pharm. 11, 32–40. doi: 10.7897/2230-8407.110216
Frank N. and Walsh D. (2017). Repeatability of oral sugar test results, glucagon-like peptide-1 measurements, and serum high-molecular-weight adiponectin concentrations in horses. J. Vet. Internal Med. 31, 1178–1187. doi: 10.1111/jvim.14725
Gerdes S., Osadtschy S., Rostami-Yazdi M., Buhles N., Weichenthal M., and Mrowietz U. (2011). Leptin, adiponectin, visfatin and retinol-binding protein-4 – mediators of comorbidities in patients with psoriasis? Exp. Dermatol. 21, 43–47. doi: 10.1111/j.1600-0625.2011.01402.x
Godfrey H., Morrow S., Abood S. K., and Verbrugghe A. (2024). Identifying the target population and preventative strategies to combat feline obesity. J. Feline Med. Surg. 26 (2). doi: 10.1177/1098612X241228042
Goloni C., Pacheco L. G., Luis L. W., Theodoro S. S., Scarpim L. B., Dalpubel D., et al. (2024). High starch intake favours bodyweight control in neutered and spayed cats living in homes fed ad libitum. Br. J. Nutr. 131 (10), 1786–1802. doi: 10.1017/S0007114524000333
Gottlieb S., Rand J. S., Ishioka K., Dias D. A., Boughton B. A., Roessner U., et al. (2022). Measures of insulin sensitivity, leptin, and adiponectin concentrations in cats in diabetic remission compared to healthy control cats. Front. Vet. Sci. 9, 905929. doi: 10.3389/fvets.2022.905929
Grant C., Shoveller A., Blois S., Bakovic M., Monteith G., and Verbrugghe A. (2020). Dietary intake of amino acids and vitamins compared to NRC requirements in obese cats undergoing energy restriction for weight loss. BMC Vet. Res. 16, 1–12. doi: 10.1186/s12917-020-02649-0
Gupta J., Sharma Y., Wahi N., and Kumar K. (2024). Unique characteristics of adipocytes in metabolic health: Insights and implications. Acta Biol. Slovenica 67, 80–91. doi: 10.14720/abs.67.3.18726
Hall J. A., Yerramilli M., Obare E., Yerramilli M., Yu S., and Jewell D. E. (2019). Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J. Vet. Internal Med. 28, 1676–1683. doi: 10.1111/jvim.12442
Hastuti P. (2022). Obesity and the role of genetic polymorphism: A review of genes as the risk of obesity. J. Med. Sci. (Berkala Ilmu Kedokteran) 54 (2). doi: 10.19106/JMedSci005402202209
Helfer G. and Wu Q. (2018). Chemerin: A multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 238, R79–R94. doi: 10.1530/joe-18-0174
Hewson-Hughes A., Gilham M., Upton S., Colyer A., Butterwick R., and Miller A. (2011). The effect of dietary starch level on postprandial glucose and insulin concentrations in cats and dogs. Br. J. Nutr. 106, S105–S109. doi: 10.1017/s0007114511001887
Hoelmkjaer K., Albrechtsen N., Holst J., Cronin A., Nielsen D., Mandrup-Poulsen T., et al. (2016). A placebo-controlled study on the effects of the glucagon-like peptide-1 mimetic, exenatide, insulin secretion, body composition and adipokines in obese, client-owned cats. PloS One 11, e0154727. doi: 10.1371/journal.pone.0154727
Hoenig M., Jordan E., Glushka J., Kley S., Patil A., Waldron M., et al. (2011a). Effect of macronutrients, age, and obesity on 6- and 24-h postprandial glucose metabolism in cats. AJP Regul. Integr. Comp. Physiol. 301, R1798–R1807. doi: 10.1152/ajpregu.00342.2011
Hoenig M., Pach N., Thomaseth K., Lê A., Schaeffer D., and Ferguson D. (2013). Cats differ from other species in their cytokine and antioxidant enzyme response when developing obesity. Obesity 21. doi: 10.1002/oby.20306
Hoenig M., Thomaseth K., Waldron M., and Ferguson D. (2007). Insulin sensitivity, fat distribution, and adipocytokine response to different diets in lean and obese cats before and after weight loss. AJP Regul. Integr. Comp. Physiol. 292, R227–R234. doi: 10.1152/ajpregu.00313.2006
Hoenig M., Wilkins C., Holson J., and Ferguson D. (2003). Effects of obesity on lipid profiles in neutered male and female cats. Am. J. Vet. Res. 23 (7), 639–648. doi: 10.2460/ajvr.2003.64.299
Ishioka K., Omachi A., Sasaki N., Kimura K., and Saito M. (2009). Feline adiponectin: Molecular structures and plasma concentrations in obese cats. J. Vet. Med. Sci. 71, 189–194. doi: 10.1292/jvms.71.189
James A., Zhang X., Crisan M., Hardy W., Liang P., Meyers C., et al. (2017). Isolation and characterization of canine perivascular stem/stromal cells for bone tissue engineering. PloS One 12, e0177308. doi: 10.1371/journal.pone.0177308
Kieler I., Mølbak L., Hansen L., Hermann-Bank M., and Bjørnvad C. (2015). Overweight and the feline gut microbiome – A pilot study. J. Anim. Physiol. Anim. Nutr. 100, 478–484. doi: 10.1111/jpn.12409
Kim J., Oh C., and Kim H. (2023). The interplay of adipokines and pancreatic beta cells in metabolic regulation and diabetes. Biomedicines 11, 2589. doi: 10.3390/biomedicines11092589
Kirichenko T., Markina Y., Bogatyreva A., Tolstik T., Varaeva Y., and Стародубова А. (2022). The role of adipokines in inflammatory mechanisms of obesity. Int. J. Mol. Sci. 23, 14982. doi: 10.3390/ijms232314982
Klotsman M., Adin C., Anderson W., and Gilor C. (2021). Safety, tolerability, and proof-of-concept study of OKV-119, a novel exenatide long-term drug delivery system, in healthy cats. Front. Vet. Sci. 8. doi: 10.3389/fvets.2021.661546
Kocabağlı N., Kutay H., Dokuzeylül B., Süer İ., and Apt M. (2017). The analysis of computer data regarding obesity and associated diseases in cats examined at private veterinary practices. Acta Scientiae Veterinariae 45, 5. doi: 10.22456/1679-9216.80775
Korek E. and Krauss H. (2015). New adipokines with potential significance in the pathogenesis of obesity and metabolic disorders. Advances in Hygiene and Experimental Medicine 69, 799–810. doi: 10.5604/17322693.1161415
Kotal S., Mukherjee P., Chakraborty D., and Tewari S. (2023). A review on role of different adipokines in gestational diabetes. J. Adv. Zool. 44, 2109–2113. doi: 10.17762/jaz.v44is6.2794
Kubo S., Imano H., Muraki I., Kitamura A., Noda H., Cui R., et al. (2023). Total protein intake and subsequent risk of chronic kidney disease: The circulatory risk in communities study. Environ. Health Prev. Med. 28, Article 32. doi: 10.1265/ehpm.22-00247
Lackey D., Lazaro R., Li P., Johnson A., Hernández-Carretero A., Weber N., et al. (2016). The role of dietary fat in obesity-induced insulin resistance. AJP Endocrinol. Metab. 311, E989–E997. doi: 10.1152/ajpendo.00323.2016
Laflamme D., Backus R., Forrester S., and Hoenig M. (2022). Evidence does not support the controversy regarding carbohydrates in feline diets. J. Am. Vet. Med. Assoc. 260, 506–513. doi: 10.2460/javma.21.06.0291
Lawson M. L., Evans K., Mellor D. J., and Yam P. S. (2019). Owner and Cat-Related Risk Factors for Feline Overweight or Obesity. Front. Vet. Sci. 6, 266. doi: 10.3389/fvets.2019.00266
Lee M., Wang Y., Ricci M., Sullivan S., Russell C., and Fried S. (2007). Acute and chronic regulation of leptin synthesis, storage, and secretion by insulin and dexamethasone in human adipose tissue. AJP Endocrinol. Metab. 292, E858–E864. doi: 10.1152/ajpendo.00439.2006
Lewitt M., Strage E., and Church D. (2016). An individual approach to feline diabetes care: A case report and literature review. Acta Veterinaria Scand. 12 (3), 210–225. doi: 10.1186/s13028-016-0245-0
Li Q. and Pan Y. (2020). Differential responses to dietary protein and carbohydrate ratio on gut microbiome in obese vs. lean cats. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.591462
Loftus J. and Wakshlag J. (2014). Canine and feline obesity: A review of pathophysiology, epidemiology, and clinical management. Vet. Med. Res. Rep. 6, 49–60. doi: 10.2147/vmrr.s40868
Luo J., He Z., Li Q., Lv M., Cai Y., Ke W., et al. (2023). Adipokines in atherosclerosis: Unraveling complex roles. Front. Cardiovasc. Med. 10. doi: 10.3389/fcvm.2023.1235953
Lynch M., Ahern T., Sweeney C., Malara A., Tobin A., O’Shea D., et al. (2017). Adipokines, psoriasis, systemic inflammation, and endothelial dysfunction. Int. J. Dermatol. 56, 1103–1118. doi: 10.1111/ijd.13699
Ma X., Brinker E., Graff E. C., Cao W., Gross A. L., Johnson A. K., et al. (2022). Whole‑Genome Shotgun Metagenomic Sequencing Reveals Distinct Gut Microbiome Signatures of Obese Cats. Microbiol. Spectr. 10 (3). doi: 10.1128/spectrum.00837-22
Mabuza L., Gamede M., Maikoo S., Booysen I., Ngubane P., and Khathi A. (2018). Effects of a ruthenium Schiff base complex on glucose homeostasis in diet-induced pre-diabetic rats. Molecules 23, 1721. doi: 10.3390/molecules23071721
Machado B. S., Bruno C. E., Silva D. I., Barth J. C., Santos L. P., Alves M. S., et al. (2022). An overweight/obesity survey among dogs and cats attended at a veterinary teaching hospital during the second year of the COVID-19 pandemic. Arq. Bras. Med. Vet. Zootec. 74 (6), 999–1006. doi: 10.1590/1678-4162-12696
Maniaki E., Murrell J., Langley-Hobbs S., and Blackwell E. (2021). Associations between early neutering, obesity, outdoor access, trauma and feline degenerative joint disease. J. Feline Med. Surg. 23, 965–975. doi: 10.1177/1098612x21991456
Martin L. J. M. and Wood P. R. (2010). Influence of a high‑protein diet on energy balance in obese cats. J. Anim. Physiol. Anim. Nutr. 94 (5), e350–e357. doi: 10.1111/j.1439‑0396.2010.01062.x
Mazaki-Tovi M., Abood S., Segev G., and Schenck P. (2013). Alterations in adipokines in feline hepatic lipidosis. J. Vet. Internal Med. 27, 242–249. doi: 10.1111/jvim.12055
Mazaki-Tovi M., Aroch I., Tavori U., and Bruchim Y. (2019b). Adipokine secretion in feline primary adipose tissue culture in response to dietary fatty acids. BMC Vet. Res. 15, 324. doi: 10.1186/s12917-019-2065-8
Mazaki-Tovi M., Bolin S., and Schenck P. (2018). Dietary fatty acids differentially regulate secretion of adiponectin and interleukin-6 in primary canine adipose tissue culture. Lipids 53, 205–216. doi: 10.1002/lipd.12021
Mazaki-Tovi M., Bolin S., and Schenck P. (2019). Adipokines secretion in feline primary adipose tissue culture in response to dietary fatty acids. BMC Vet. Res. 15, 2065. doi: 10.1186/s12917-019-2065-8
Moreno-Aliaga M., Lorente-Cebrián S., and Martínéz J. (2010). Regulation of adipokine secretion by n-3 fatty acids. Proc. Nutr. Soc. 69, 324–332. doi: 10.1017/s0029665110001801
Mori N., Iwasaki E., Okada Y., Kawasumi K., and Arai T. (2016). Overall prevalence of feline overweight/obesity in Japan as determined from a cross-sectional sample pool of healthy veterinary clinic-visiting cats in Japan. Turkish J. Vet. Anim. Sci. 40, 304–312. doi: 10.3906/vet-1502-31
Nelson R. W. and Reusch C. E. (2014). Animal models of disease: classification and etiology of diabetes in dogs and cats. J. Endocrinol. 222, T1–T9. doi: 10.1530/JOE-14-0200
Nicholson T., Church C., Baker D., and Jones S. (2018). The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J. Inflammation 80 (6), 981–987. doi: 10.1186/s12950-018-0185-8
O’Neill S., Bohl M., Gregersen S., Hermansen K., and O’Neill C. (2016). Leptin, adiponectin, and insulin resistance: the role of low-grade inflammation in type 2 diabetes. Mediators Inflammation 2016, 1–11. doi: 10.1155/2016/2734765
Öhlund M., Fall T., Holst B., Hansson-Hamlin H., Bonnett B., and Egenvall A. (2015). Incidence of diabetes mellitus in insured Swedish cats in relation to age, breed and sex. J. Vet. Internal Med. 29, 1342–1347. doi: 10.1111/jvim.13584
Öhlund M., Müllner E., Moazzami A., Hermansson U., Pettersson A., Anderson F., et al. (2021). Differences in metabolic profiles between the Burmese, the Maine Coon and the Birman cat—Three breeds with varying risk for diabetes mellitus. PloS One 16, e0249322. doi: 10.1371/journal.pone.0249322
Öhlund M., Palmgren M., and Holst B. (2018). Overweight in adult cats: A cross-sectional study. Acta Veterinaria Scand. 60. doi: 10.1186/s13028-018-0359-7
Okada Y., Ueno H., Mizorogi T., Ohara K., Kawasumi K., and Arai T. (2019). Diagnostic criteria for obesity disease in cats. Frontiers in Veterinary Science 6, Article 284. doi: 10.3389/fvets.2019.00284
Osto M. and Lutz T. (2015). Translational value of animal models of obesity—Focus on dogs and cats. Eur. J. Pharmacol. 759, 240–252. doi: 10.1016/j.ejphar.2015.03.036
Osto M., Zini E., Reusch C., and Lutz T. (2013). Diabetes from humans to cats. Gen. Comp. Endocrinol. 182, 48–53. doi: 10.1016/j.ygcen.2012.11.019
Park S. and Shimokawa I. (2024). Influence of Adipokines on Metabolic Dysfunction and Aging. Biomedicines 12 (4), 873. doi: 10.3390/biomedicines12040873
Phillips A. M., Coe J. B., Rock M. J., and Adams C. L. (2017). Feline obesity in veterinary medicine: insights from a thematic analysis of communication in practice. Front. Vet. Sci. 4, 117. doi: 10.3389/fvets.2017.00117
Quijada E., Dean D., and Marcucci M. (2013). Resistin expression study used to develop a simplified mouse model of adipokine expression. FASEB J. 27 (S1), Supplement 563.3. doi: 10.1096/fasebj.27.1_supplement.563.3
Rashid U., Yousaf A., Yaqoob M., Saba E., Moaeen-ud-Din M., Waseem S., et al. (2021). Characterization and differentiation potential of mesenchymal stem cells isolated from multiple canine adipose tissue sources. BMC Vet. Res. 17, Article 3100. doi: 10.1186/s12917-021-03100-8
Recinella L., Orlando G., Ferrante C., Chiavaroli A., Brunetti L., and Leone S. (2020). Adipokines: New potential therapeutic target for obesity and metabolic, rheumatic, and cardiovascular diseases. Front. Physiol. 11. doi: 10.3389/fphys.2020.578966
Riedel J., Badewien-Rentzsch B., Kohn B., Hoeke L., and Einspanier R. (2015). Characterization of key genes of the renin–angiotensin system in mature feline adipocytes and during in vitro adipogenesis. J. Anim. Physiol. Anim. Nutr. 100, 1139–1148. doi: 10.1111/jpn.12392
Rodan I. and Ellis S. (2013). Framework for a healthy feline environment. J. Feline Med. Surg. 15, 173–173. doi: 10.1177/1098612x13477536
Rollins A. W. and Murphy M. (2019). Nutritional assessment in the cat: Practical recommendations for better screening of nutritional status. J. Feline Med. Surg. 21 (8), 442–448. doi: 10.1177/1098612X19843213
Ryan V., German A., Wood I., Hunter L., Morris P., and Trayhurn P. (2010). Adipokine expression and secretion by canine adipocytes: Stimulation of inflammatory adipokine production by LPS and TNFα. Pflügers Archiv Eur. J. Physiol. 460, 603–616. doi: 10.1007/s00424-010-0845-x
Saavedra C., Pérez C., Oyarzún C., and Torres‑Arévalo Á. (2024). Overweight and obesity in domestic cats: epidemiological risk factors and associated pathologies. J. Feline Med. Surg. 26. doi: 10.1177/1098612X241285519
Sandøe P., Palmer C., Corr S., Astrup A., and Bjørnvad C. (2014). Canine and feline obesity: A One Health perspective. Vet. Rec. 175, 610–616. doi: 10.1136/vr.g7521
Sasaki N., Shibata H., Honjoh T., Kimura K., Saito M., and Ohishi I. (2001). cDNA cloning of feline leptin and its mRNA expression in adipose tissue. J. Vet. Med. Sci. 63, 1115–1120. doi: 10.1292/jvms.63.1115
Sharma V. and Sharma N. (2018). Leptin signaling—Leptin resistance in obesity and its correlation with body mass index. Natl. J. Physiol. Pharm. Pharmacol. 8 (6), 817–819. doi: 10.5455/njppp.2018.8.0102230012018
Sierawska O. and Niedźwiedzka-Rystwej P. (2022). Adipokines as potential biomarkers for type 2 diabetes mellitus in cats. Front. Immunol. 13. doi: 10.3389/fimmu.2022.950049
Souza F., Golino D., Bonatelli S., Alfonso A., Mamprim M., Balieiro J., et al. (2020). Effect of obesity on ecocardiographic parameters and vertebral heart size (VHS) in cats. Semina Ciências Agrárias 41, 493–504. doi: 10.5433/1679-0359.2020v41n2p493
Stenberg K., Novotny G., Lutz T., Mandrup-Poulsen T., and Bjørnvad C. (2023). Obesity-induced changes in gene expression in feline adipose and skeletal muscle tissue. J. Anim. Physiol. Anim. Nutr. 107, 1262–1278. doi: 10.1111/jpn.13802
Strage E., Ley C., Forkman J., Öhlund M., Stadig S., Bergh A., et al. (2021). Homeostasis model assessment, serum insulin and their relation to body fat in cats. BMC Vet. Res. 17, 1–10. doi: 10.1186/s12917-020-02729-1
Takashima S., Nishii N., Kato A., Matsubara T., Shibata S., and Kitagawa H. (2016). Molecular cloning of feline resistin and the expression of resistin, leptin and adiponectin in the adipose tissue of normal and obese cats. J. Vet. Med. Sci. 78, 23–28. doi: 10.1292/jvms.15-0233
Tarkošová D., Story M., Rand J., and Svoboda M. (2016). Feline obesity—Prevalence, risk factors, pathogenesis, associated conditions and assessment: A review. Vet. Med. 61, 295–307. doi: 10.17221/145/2015-vetmed
Teng K. T., McGreevy P. D., Toribio J.‑A. L. M. L., and Dhand N. K. (2020). Positive attitudes towards feline obesity are strongly associated with ownership of obese cats. PLoS One 15 (6), e0234190. doi: 10.1371/journal.pone.0234190
Tricò D., Moriconi D., Berta R., Baldi S., Quiñones-Galvan A., Guiducci L., et al. (2021). Effects of low-carbohydrate versus Mediterranean diets on weight loss, glucose metabolism, insulin kinetics and β-cell function in morbidly obese individuals. Nutrients 13, 1345. doi: 10.3390/nu13041345
Tvarijonaviciute A., German A., Martínez-Subiela S., Tecles F., and Cerón J. (2012). Analytical performance of commercially-available assays for feline insulin-like growth factor 1 (IGF-1), adiponectin and ghrelin measurements. J. Feline Med. Surg. 14, 138–146. doi: 10.1177/1098612x11432236
Velde H., Janssens G., Rooster H., Polis I., Peters I., Ducatelle R., et al. (2013). The cat as a model for human obesity: Insights into depot-specific inflammation associated with feline obesity. Br. J. Nutr. 110, 1326–1335. doi: 10.1017/S0007114513000226
Verbrugghe A. and Hesta M. (2017). Cats and carbohydrates: the carnivore fantasy? Vet. Sci. 4, 55. doi: 10.3390/vetsci4040055
Vester B., Belsito K., and Swanson K. (2011). Serum metabolites, ghrelin and leptin are modified by age and/or diet in weanling kittens fed either a high- or moderate-protein diet. Anim. Sci. J. 83, 426–433. doi: 10.1111/j.1740-0929.2011.00974.x
Vitor R., Oliveira J., Navarro A., Lima A., Oliveira G., Munhoz A., et al. (2024). Body condition scores in cats and associations with systolic blood pressure, glucose homeostasis, and systemic inflammation. Vet. Sci. 11, 151. doi: 10.3390/vetsci11040151
Wallis N. and Raffan E. (2020). The genetic basis of obesity and related metabolic diseases in humans and companion animals. Genes 11 (11), 1378. doi: 10.3390/genes11111378
Wei Z., Peterson J., Lei X., Cebotaru L., Wolfgang M., Baldeviano G., et al. (2012). C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J. Biol. Chem. 287, 10301–10315. doi: 10.1074/jbc.m111.303651
Würfel M., Blüher M., Stümvoll M., Ebert T., Kovács P., Tönjes A., et al. (2023). Adipokines as clinically relevant therapeutic targets in obesity. Biomedicines 11, 1427. doi: 10.3390/biomedicines11051427
Yu Y., Wang L., Zhong N., Wen D., and Liu L. (2024). Multifaced roles of adipokines in endothelial cell function. Front. Endocrinol. 15, e1490143. doi: 10.3389/fendo.2024.1490143
Zagayko A., Briukhanova T., Lytkin D., Kravchenko G., and Fylymonenko V. (2019). “Prospects for using the natural antioxidant compounds in the obesity treatment.” In Obesity - Open Access. (IntechOpen). doi: 10.5772/intechopen.83421
Zapata R., Meachem M., Cardoso N., Mehain S., McMillan C., Snead E., et al. (2017). Differential circulating concentrations of adipokines, glucagon and adropin in a clinical population of lean, overweight and diabetic cats. BMC Vet. Res. 13 (1), 1–9. doi: 10.1186/s12917-017-1011-x
Zhang H. (2010). Emerging role of adipokines as mediators in atherosclerosis. World J. Cardiol. 2, 370. doi: 10.4330/wjc.v2.i11.370
Zhao S., Zhu Y., Schultz R., Li N., He Z., Zhang Z., et al. (2019). Partial leptin reduction as an insulin sensitization and weight loss strategy. Cell Metab. 30, 706–719.e6. doi: 10.1016/j.cmet.2019.08.005
Keywords: feline obesity, adiponectin, leptin, insulin, diabetes mellitus, adipokines
Citation: Cernat L, Daina S and Macri A (2025) From fat to facts: the potential of adipokine and insulin dosing in obese feline patients. Front. Anim. Sci. 6:1637154. doi: 10.3389/fanim.2025.1637154
Received: 28 May 2025; Accepted: 11 July 2025;
Published: 18 August 2025.
Edited by:
Assar Ali Shah, Jiangsu University, ChinaReviewed by:
Ravikanthreddy Poonooru, University of Missouri, United StatesSara Frazzini, University of Milan, Italy
Copyright © 2025 Cernat, Daina and Macri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sorana Daina, c29yYW5hLm1hdGVpQHVzYW12Y2x1ai5ybw==
 Laura Cernat
Laura Cernat Sorana Daina
Sorana Daina Adrian Macri
Adrian Macri