- 1School of Animal Science and Technology, Jiangsu Agri-animal Husbandry Vocational College, Taizhou, Jiangsu, China
- 2School of Food Science and Technology, Jiangsu Agri-animal Husbandry Vocational College, Taizhou, Jiangsu, China
- 3Yuxi Veterinary Station of Jiangyan District, Taizhou, Jiangsu, China
There has been increased awareness of the negative effects of antibiotic use in poultry production, necessitating research on sustainable alternatives. Honeycomb polysaccharides (HPs) have attracted considerable attention due to their bioactivity. Therefore, we investigated the effects of HPs on growth and slaughter performance, antioxidant capacity, and immune function in Cyan-Shank Partridge chickens. Male Cyan-Shank Partridge chicks (n = 300; 1-day-old) were randomly assigned to five groups: negative control group (NC, fed a basal diet), three experimental groups (fed basal diet supplemented with 300 (HPs300), 600 (HPs600), or 1,200 mg/kg (HPs1200) of HPs), and chlortetracycline hydrochloride group (positive control group, PC). Compared to NC group, there was a significant increase (p < 0.05) in body weight at 20 days of age, average daily weight gain from day 1 to 20, and average daily feed intake from day 41 to 60 in the group HPs1200. Additionally, leg muscle ratio was significantly higher (p < 0.05) in group HPs1200 than in NC group. Compared with those in NC group, there was a significant decrease (p < 0.05) in serum malondialdehyde and a significant increase (p < 0.05) in serum superoxide dismutase activity in group HPs1200. Moreover, serum immunoglobulin A (IgA), IgM, and IgG levels were significantly higher (p < 0.05) in group HPs1200 than in group NC. Conclusively, HPs addition to feed at 1,200 mg/kg enhances growth performance, slaughter performance, serum antioxidant capacity, and immune function in Cyan-Shank Partridge chickens.
Introduction
Polysaccharides are crucial natural bioactive compounds, with immunomodulatory, antibacterial, antiviral, anti-parasitic, antitumor, and anti-radiation properties. Research has shown that incorporating polysaccharides into feed significantly boosts immune functions in livestock and poultry, promotes the development of immune organs, stimulates antibody production, activates lymphocytes, enhances growth, and reduces mortality rates (Si, 2023; Mao et al., 2022; Liu J et al., 2024). Therefore, active polysaccharides are promising natural green feed additives with the potential to replace antibiotics. Currently, most polysaccharides utilized as feed additives originate from plant sources (Xie et al., 2019; Yang et al., 2019; Xing et al., 2020), with limited studies on the effects of animal-derived polysaccharides.
Honeycomb polysaccharides (HPs), the primary active components, have been isolated from honeycombs. Honeycomb is a by-product discarded in beekeeping production, making it available in large quantities at low prices. Polysaccharide extraction for use as feed additives offers significant advantages. HPs are a complex mixture of both plant- and animal-derived components, with total sugar, protein, uronic acid, and reducing sugar contents of 75.77, 13.82, 0.19, and 4.20%, respectively. HPs consist of mannose, glucosamine sulphate, ribose, rhamnose, galacturonic acid, galactosamine, glucose, galactose, xylose, and arabinose, and are classified as non-toxic (Yin et al., 2018a). Preliminary study has revealed that HPs possess notable antioxidant properties and exert significant immunomodulatory effects in cyclophosphamide-induced immunosuppressed mice (Hou, 2012; Yin et al., 2018b, 2021).
However, the practical applicability and dose-dependent efficacy of HPs as poultry feed additives remain unexplored. The present study employed Cyan-Shank Partridge chickens as an experimental model to investigate the comparative impacts of dietary HPs at varying concentrations (300, 600, 1,200 mg/kg) versus an antibiotic control group on growth performance, carcass yield, antioxidant capacity, and immune function. These findings will contribute to validating HPs as a novel animal-derived polysaccharide additive, thereby providing critical theoretical foundations and technical support for advancing sustainable development in the poultry industry.
Materials and methods
Preparation of HPs
To prepare HPs, bee honeycombs were crushed, stirred, and boiled in distilled water at a ratio of 3:1 (water to honeycomb) for 30 min. After filtering the mixture through a 120-mesh gauze, the residue was subjected to two additional extraction cycles under the same conditions. Filtrates from all the extractions were pooled and concentrated under reduced pressure. Thereafter, the polysaccharides were precipitated by adding 95% ethanol for 6 h, followed by centrifugation at 4000 rpm for 10 min. Finally, the precipitate was collected and dried in an oven at 40°C to yield crude HPs.
Rearing procedures and management
Animal experiments were conducted at the Poultry Production Training Centre of Jiangsu Agri-Animal Husbandry Vocational College. Before the experiment, all equipment (chicken cages, waterers, and feeders) and the entire chicken house were thoroughly disinfected. After a 7-day post-disinfection period, the chicks were introduced into the facility. Notably, the temperature conditions during the experiment were as follows: days 1–3: maintained at 35°C; days 4–7: gradually reduced to 33°C; day 14: decreased to 27°C; day 21: reduced to 23°C and then stabilized at 20–21°C for the rest of the experiment. During the experimental period, the chickens had free access to feed and water. Environmental parameters were dynamically adjusted based on meteorological conditions to maintain optimal humidity (40–60%), lighting (16 h/8 h light/dark photoperiod), and ventilation rates (0.5–1.0 m/s). Health management procedures, including vaccination protocols, were as follows: Marek’s disease vaccine (CVI94 strain) administration on day 1, Newcastle disease vaccine (LaSota strain) on day 7, infectious bronchitis vaccine (H120 strain) on day 14, and infectious bursal disease vaccine (attenuated IBDV) on day 21. All immunizations were administered via intramuscular injection (0.2 mL per chick) under strict aseptic conditions. Disease monitoring was conducted daily based on clinical observations.
Experimental design and grouping
Male Cyan-Shank Partridge chicken chicks (n = 300; 1-day-old) with average body weight of 0.035 kg were randomly divided into five treatment groups with six replicates per group (10 chicks per replicate): Experimental Group 1 (HPs300): basal diet + 300 mg/kg HPs; Experimental Group 2 (HPs600): basal diet + 600 mg/kg HPs; Experimental Group 3 (HPs1200): basal diet + 1,200 mg/kg HPs; Negative control group (NC): basal diet alone; and Positive control group (PC): antibiotic group (basal diet + 250 mg/kg 99% chlortetracycline hydrochloride). Supplementary Table S1 shows the composition and nutritional levels of the basal diet. Notably, the experiment lasted for 60 days.
Determination of slaughter and growth performance
Fasting weights were measured in the morning in each replicate group at 1, 20, 40, and 60 days. Additionally, average daily gain (ADG) was calculated for each group. Feed intake and residual feed were accurately recorded to determine average daily feed intake (ADFI) and feed-to-gain ratio (F/G).
At 60 days of age, one chicken was randomly selected from each replicate, weighed, and slaughtered for performance assessment. Slaughter performance parameters examined included dressed weight, half-eviscerated weight with giblets, eviscerated weight, pectoral muscle weight, leg muscle weight, abdominal fat weight, dressed yield, percentage of half-eviscerated weight with giblets, percentage of eviscerated weight, pectoral muscle ratio, leg muscle ratio, and abdominal fat ratio. All measurements were conducted following the Chinese Agricultural Industry Standard Performance terminology and Measurements for Poultry(NY/T 823-2020).
Determination of serum antioxidant and immune indices
Serum samples were collected from the chickens at the end of the experiment (60 days old) for various biochemical analyses. After fasting for 12 h with ad libitum access to water, blood samples were collected from one randomly selected chicken in each replicate (six chickens per group) via wing vein puncture (5 mL per chicken) and allowed to stand for 10min. Thereafter, the samples were centrifuged at 2500 rpm for 8 min to separate the serum, which was stored at -80°C for subsequent analysis.
Serum malonaldehyde (MDA) and glutathione peroxidase (GSH-Px) levels were measured using the TBA and colorimetric methods, respectively. Additionally, serum catalase (CAT) activity and total protein (TP) content were determined using the ammonium molybdate and Coomassie Brilliant Blue methods, respectively. Moreover, IgA, IgM, and IgG levels were determined via enzyme-linked immunosorbent assay (ELISA) using TECAN M200Pro kits (Nanjing Jiancheng Bioengineering Institute). Additionally, superoxide dismutase (SOD) and albumin (ALB) levels were measured using a Hitachi 3100 biochemical analyzer (Shanghai Coibo Biotechnology Co., Ltd).
Data analysis
Significant differences between groups were determined using one-way analysis of variance with SPSS 26.0, followed by Duncan’s multiple range post-hoc test. Additionally, we conducted orthogonal polynomial trend analysis for HPs dose-response relationships with SPSS. Results are expressed as mean ± standard error of the mean (SEM). Statistical significance was set at p < 0.05.
The growth performance indices ADG, ADFI, and F/G were calculated using the following formulae:
Results
Effect of HPs on growth performance in Cyan-Shank Partridge chickens
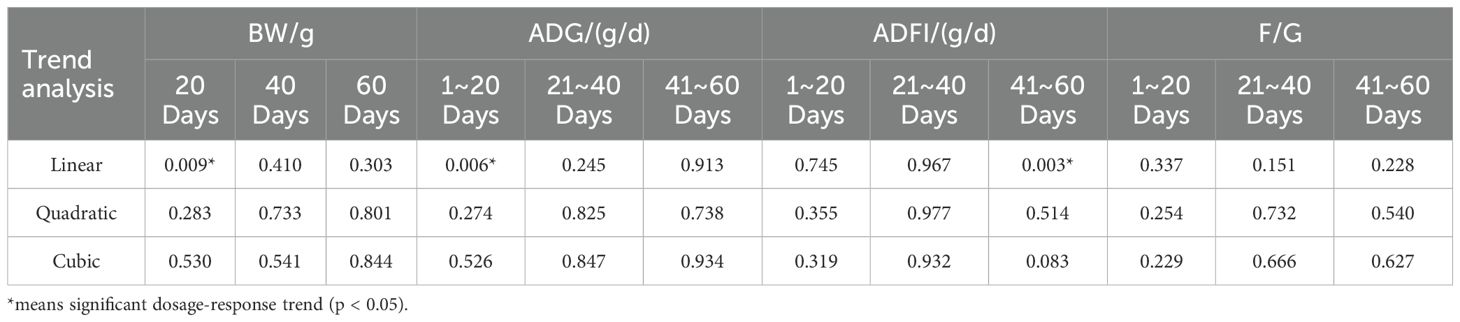
Table 1 shows the growth performance of Cyan-Shank Partridge chickens fed diets supplemented with HPs. Compared with the NC group, supplementation with HPs (HPs300, HPs600, and HPs1200) significantly increased body weight at 20 days and ADG from day 1 to 20 (p < 0.05). Even the HPs1200 group demonstrated a significant increase compared to the PC group. Additionally, ADFI was significantly higher in the HPs1200 group versus NC (p<0.05) during days 41-60, though no significant difference was observed compared to the PC group. The PC group did not exhibit significant differences in the growth performance indicators compared to the NC group in this study.

Table 1. Effect of honeycomb polysaccharides on growth performance in Cyan-Shank Partridge chickens.
Trend analysis (Table 2) of different HPs supplementation levels (NC, HPs300, HPs600, and HPs1200) across growth performance phenotypes revealed that only three indicators exhibited linear significant trends (p < 0.05): body weight at day 20, ADG from days 1-20, and ADFI during days 41-60. No significant trends were observed in other parameters or in quadratic/cubic analyses.
Effects of HPs on slaughtering performance in Cyan-Shank Partridge chickens
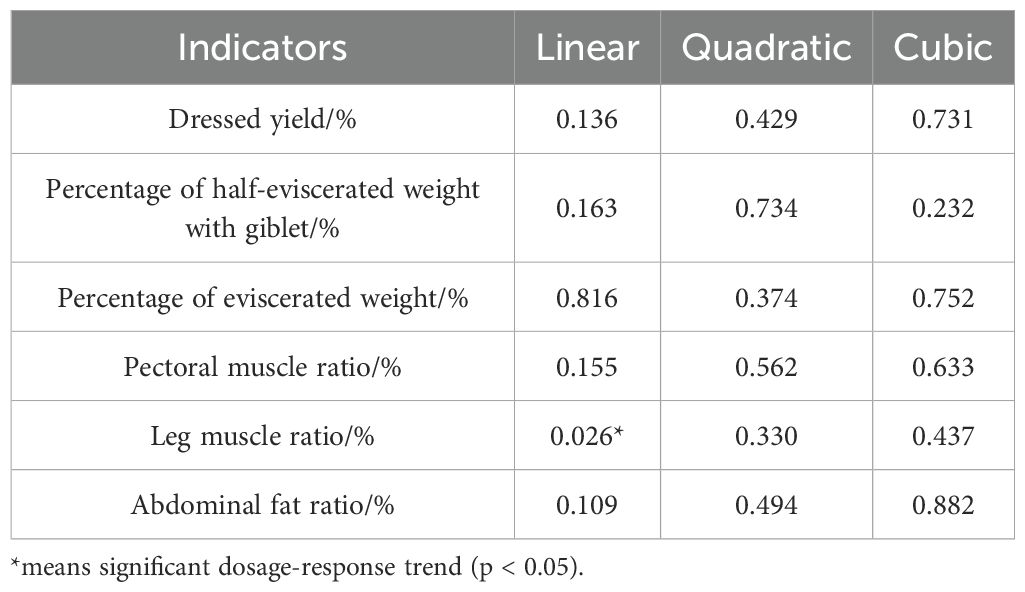
Table 3 shows the slaughter performance of Cyan-Shank Partridge chickens fed diets supplemented with HPs. Although there were no significant differences (p> 0.05) in most slaughter performance parameters between the groups, leg muscle ratio was significantly higher (p< 0.05) in HPs1200 and PC groups than in NC group (Figure 1). Furthermore, trend analysis (Table 4) revealed a significant linear relationship between leg muscle ratio and HPs supplementation dosage (p < 0.05). No significant difference was observed for all the slaughtering performance parameters between HPs groups and the PC group.

Table 3. Effects of honeycomb polysaccharides on slaughtering performance in Cyan-Shank Partridge chickens.

Figure 1. Leg muscle ratio among experimental groups. Different lowercase letters indicate significant differences (p < 0.05), whereas identical letters denote no significant differences (p > 0.05).
Effects of HPs on serum antioxidant indices in Cyan-Shank Partridge chickens
Table 5 shows the serum antioxidant indices of Cyan-Shank Partridge chickens fed diets supplemented with HPs. At 60 days of age, serum MDA levels were significantly lower in both HPs and PC groups compared to the NC group (p < 0.05, Figure 2). Although there were no significant differences (p> 0.05) in serum CAT and GSH-Px activities among the groups, serum SOD activity was significantly higher (p < 0.05, Figure 2) in groups HPs600 and HPs1200 than in NC group. There was also no significant for all the serum antioxidant indices between the HPs and PC groups.
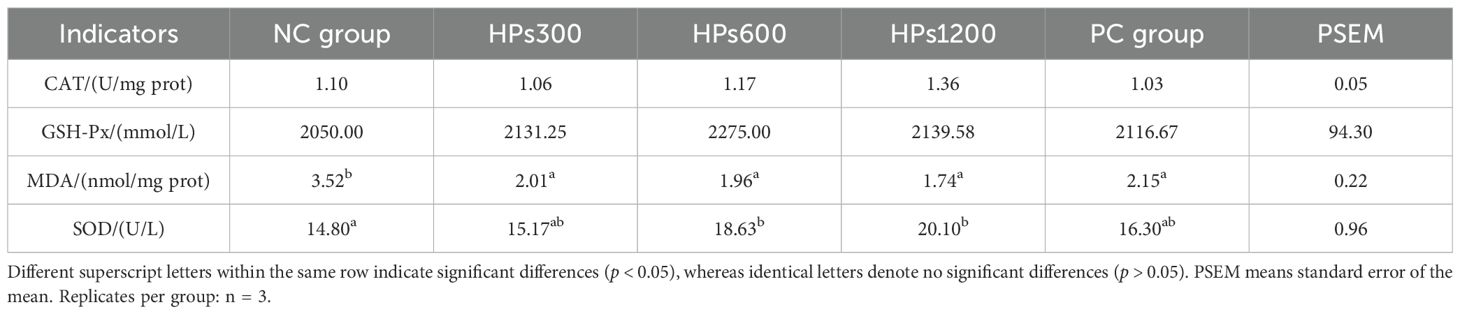
Table 5. Effects of honeycomb polysaccharides on serum antioxidant indices in Cyan-Shank Partridge chickens.
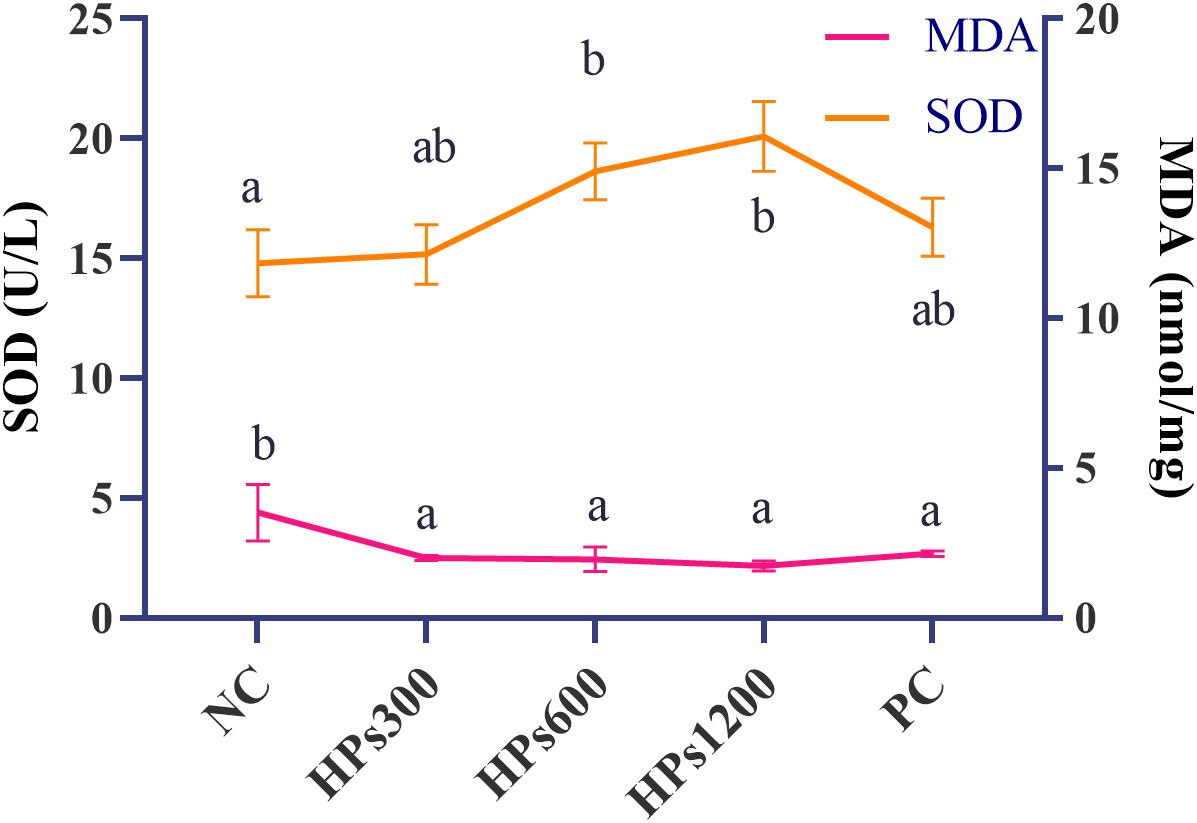
Figure 2. Changes in serum antioxidant indices (MDA and SOD) across experimental groups. Different lowercase letters indicate significant differences (p < 0.05), whereas identical letters denote no significant differences (p > 0.05).
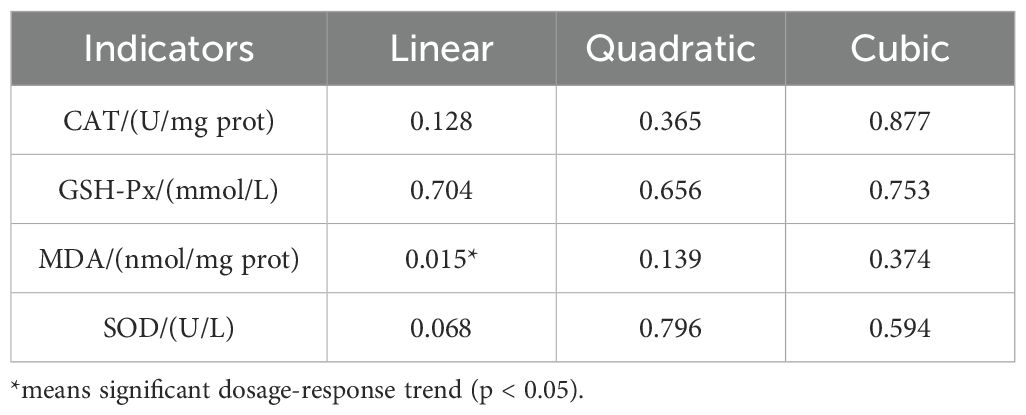
Trend analysis of HPs dosage on serum antioxidant indices demonstrated a significant linear correlation exclusively with MDA levels (p < 0.05), whereas the linear association with SOD activity did not achieve statistical significance (p = 0.068), approaching borderline relevance (Table 6).
Effects of HPs on serum immunological indices in Cyan-Shank Partridge chickens
Table 7 shows the serum immunological indices of chickens fed diets supplemented with HPs for 60 days. Although there were no significant differences (p> 0.05) in serum TP and ALB levels among the groups, serum IgA, IgM, and IgG levels were significantly higher (p< 0.05) in group HPs1200 than in NC group (Figure 3). Notably, there were no significant differences (P> 0.05) in serum IgA, IgM, and IgG levels between groups HPs600 and HPs1200. Additionally, serum IgG level was significantly higher (p< 0.05) in group HPs1200 than in HPs300 and PC groups. However, no significant difference was observed between the NC and PC groups across all measured indices.
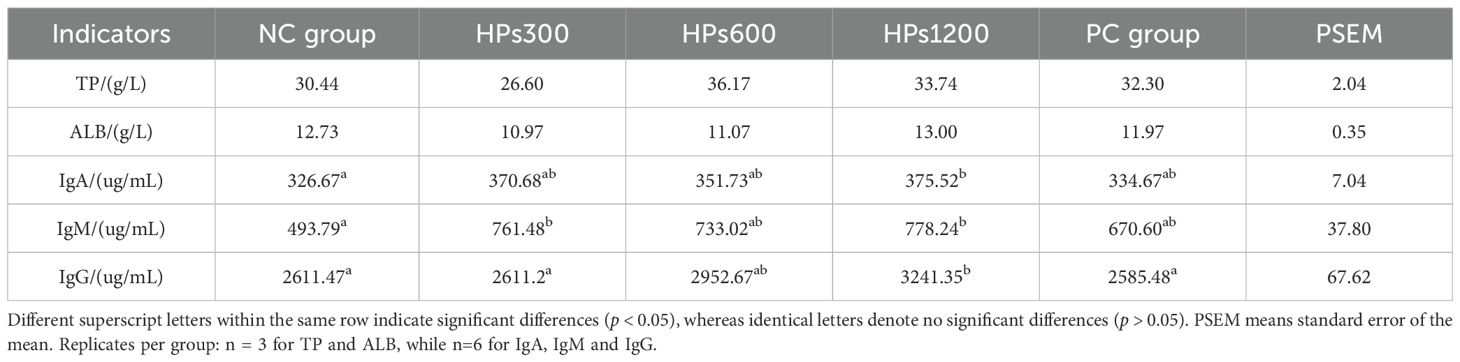
Table 7. Effects of honeycomb polysaccharides on serum immunological indices in Cyan-Shank Partridge chickens.
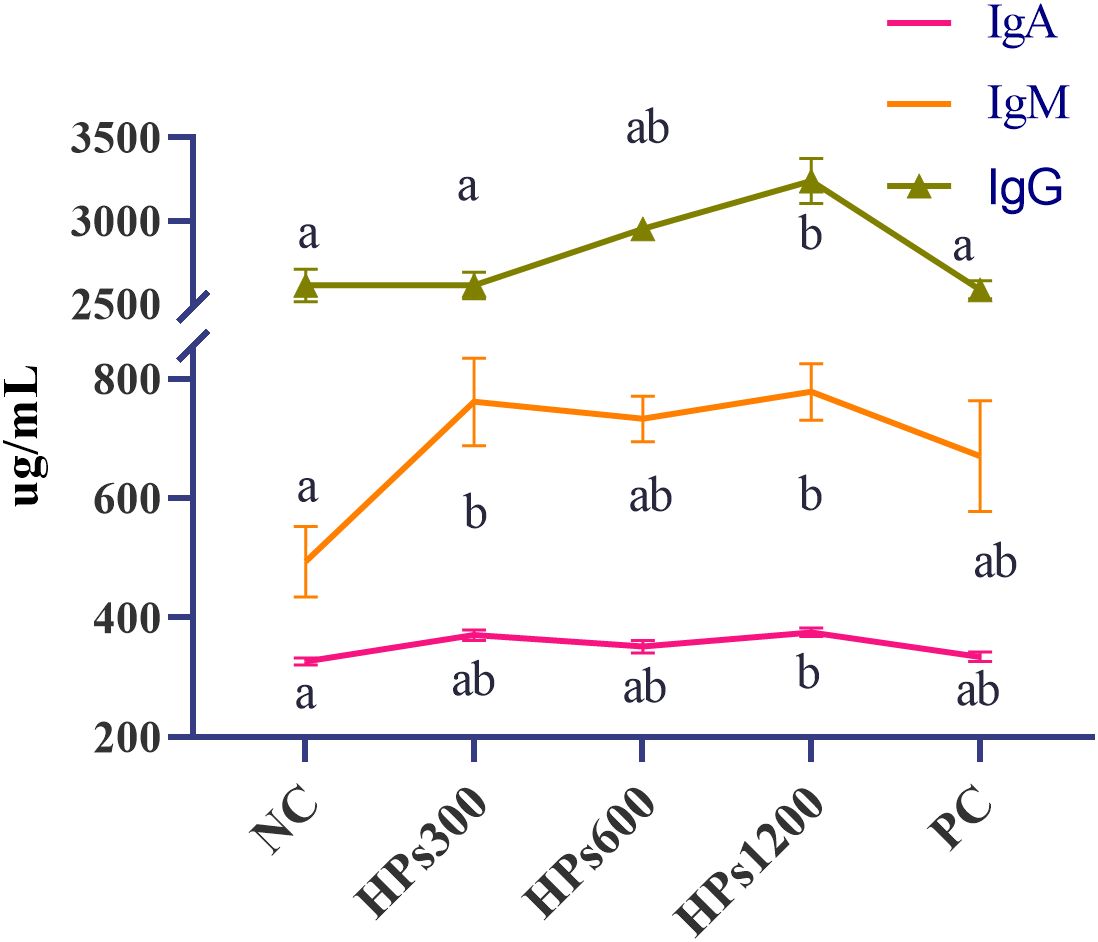
Figure 3. Statistical analysis of significant variations in serum immune markers (IgA, IgM, and IgG) across experimental groups. Different lowercase letters indicate significant differences (p < 0.05), whereas identical letters denote no significant differences (p > 0.05).
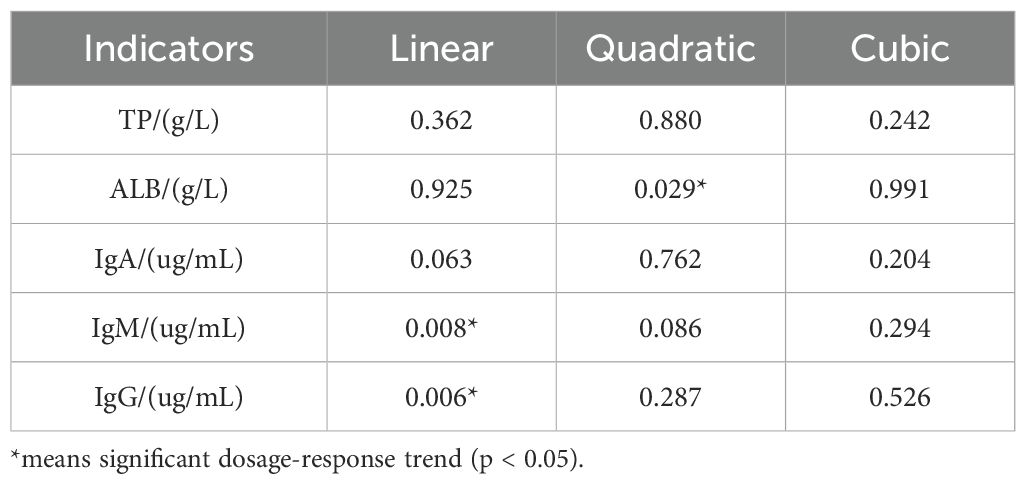
The trend analysis of HPs dosage and changes in immune indicators revealed that only IgM and IgG showed a significant linear relationship with HPs dosage. Although the linear relationship between IgA and dosage did not reach statistical significance, it approached a borderline p-value of 0.063. Notably, ALB demonstrated a significant quadratic relationship with dosage based on quadratic term testing (Table 8).
Discussion
Effects of HPs on the growth and slaughter performance in Cyan-Shank Partridge chickens
Since the implementation of a nationwide ban on antibiotics use in feed production and restricted use in livestock farming in China in 2020, researchers have increasingly explored the use of polysaccharides as feed additives in broiler chickens to enhance disease resistance and improve production performance. For example, Astragalus mongholicus polysaccharides (Liu P et al., 2024), Abrus precatorius (licorice)polysaccharides (Liu Y, 2024), Medicago sativa (alfalfa) polysaccharides (Yang et al., 2017), and Taraxacum (dandelion) polysaccharides (Shan et al., 2023) have been added to feed to improve ADG, feed conversion rate, and slaughter indices. Additionally, Saccharomyces cerevisiae (yeast) polysaccharides (Wang et al., 2020) and Lentinus edodes (shiitake) polysaccharides (Mo et al., 2024) have been shown to improve ADG in broilers.
HPs, an animal-derived polysaccharide isolated from bee combs, have rarely been studied as a poultry feed additive. Our study showed that HPs supplementation in poultry feed significantly enhanced the growth rate of Cyan-Shank Partridge chickens during the early rearing phase (1–20 days) and notably increased ADFI in the later stages compared to the NC group. Even the HPs1200 group demonstrated superior ADG from days 1–20 compared to the positive control (PC) group. Trend analysis revealed significant linear correlations between HPs dosage and three indicators: body weight on day 20, ADG from days 1–20, and ADFI during days 41–60. Collectively, these findings further demonstrate a dose-dependent growth-promoting effect of HPs.
Additionally, high-dose HPs supplementation (HPs1200) and addition of antibiotics (PC) significantly increased (p< 0.05) leg muscle ratio compared with that in the NC group. And the trend analysis also revealed a significant linear relationship between leg muscle ratio and HPs supplementation dosage. Although not statistically significant, HPs supplementation tended to improve the other slaughter performance parameters. Collectively, these findings suggest that HPs supplementation in feed can effectively improve both growth and slaughter performance in Cyan-Shank Partridge chickens.
Effects of HPs on serum antioxidant indices of Cyan-Shank Partridge chickens
Oxidative stress is characterized by the excessive production of reactive oxygen species (ROS; superoxide anions, hydroxyl radicals, and hydrogen peroxide) and reactive nitrogen species (RNS; nitric oxide, nitrogen dioxide, and peroxynitrite), which can competitively deplete the reduced hydrogen required for cellular metabolism, leading to cellular metabolic damage (Sies et al., 2017). Under normal conditions, the balance between oxidation and antioxidant systems in animals maintains homeostasis. Disruption of this equilibrium may result in pathological outcomes such as infection, aging, and tumorigenesis (Poprac et al., 2017) indicators, such as CAT, GSH-Px, MDA, and SOD, are widely used to evaluate antioxidant capacity and overall health status in animals (Blokhina et al., 2003; Payne and Southern, 2005; Zhao and Shen, 2005). Previous studies have demonstrated the potential of polysaccharides to modulate the antioxidant system. For example, Chen et al. (2023) found that Atractylodes polysaccharide supplementation significantly reduced serum MDA levels and increased SOD and GSH-Px activities in Lingnan yellow chickens. Similarly, Yang et al. (2017) reported that Medicago sativa polysaccharide supplementation enhanced GSH-Px activity in 28-day-old broilers and SOD activity in 35-day-old broilers. Zhang et al. (2022) reported that Ziziphus jujuba Mill (jujube) polysaccharide supplementation improved CAT, SOD, GSH-Px, and total antioxidant capacity (T-AOC) activities and decreased serum MDA levels in Gallus gallus, thereby improving egg production. Similarly, Yin et al. (2018b) revealed that HPs significantly elevated serum SOD and CAT activities and reduced MDA levels in immunosuppressed mice.
In the present study, supplementation with HPs at three levels and the antibiotic-supplemented group both significantly decreased serum MDA levels in Cyan-Shank Partridge chickens compared to the NC group; furthermore, trend analysis revealed a significant linear dose-response relationship exclusively between HPs dosage and MDA levels (p < 0.05). Notably, serum SOD activity was significantly higher (p< 0.05) in groups HPs600 and HPs1200 than in NC group. Antioxidant capacity is mediated by enzymes like SOD, which neutralizes reactive oxygen species (ROS) by dismutating superoxide radicals into less harmful molecules (e.g., hydrogen peroxide and oxygen), thereby reducing oxidative stress and cellular damage. In this process, SOD acts as the “core guardian” of antioxidant defense, systematically blocking the generation of malondialdehyde (MDA)—a terminal product of lipid peroxidation—by eliminating its precursor, superoxide radicals. Consequently, we hypothesize that the addition of hydrophilic polymers (HPs) enhances SOD enzymatic activity through molecular interactions that stabilize its active conformation or promote substrate accessibility. This amplified SOD function would accelerate the clearance of oxidative indicators such as ROS and MDA, thereby progressively strengthening the organism’s antioxidant capacity through this dual-action mechanism. Although not statistically significant, other indices, such as GSH-Px and CAT activities, showed an increasing trend following HPs supplementation compared with those in the NC group. There was also no significant for all the serum antioxidant indices between the HPs and PC groups. Collectively, these findings suggest that HPs supplementation, similar to antibiotic supplementation, can enhance antioxidant capacity in Cyan-Shank Partridge chickens by mitigating oxidative stress, thereby promoting overall health.
Effects of HPs on serum immunological indices in Cyan-Shank Partridge chickens
TP is composed of ALB and immunoglobulin, both of which play multiple roles, including maintaining normal colloid osmotic pressure and acid-base balance in blood vessels, transporting various metabolites, and regulating the physiological functions of transported substances. Additionally, these proteins are closely associated with immune functions. Moreover, the three immunoglobulins in the serum, IgA, IgM, and IgG, are the primary antibodies involved in humoral immunity. Notably, serum IgA, IgM, and IgG concentrations reflect the level of humoral immune response in organisms (Wang et al., 2001). Previous studies have demonstrated the immunomodulatory effects of polysaccharides. Liu S et al. (2024) demonstrated that adding different doses of herbal compound polysaccharides to feed significantly increased serum IgG, IgA, and IgM levels in broilers. Similarly, Zhao (2021) found that supplementation with 0.5% Dioscorea polysaccharides significantly elevated serum IgA, IgM, and IgG levels in broiler chickens.
In the present study, there were no significant differences (p> 0.05) in serum TP and ALB levels among the groups. However, high-dose HPs supplementation (HPs1200) significantly increased (p< 0.05) serum IgA, IgM, and IgG levels compared with those in NC group. Notably, the IgG level was significantly higher (p < 0.05) in the HPs1200 group compared to the PC group. Trend analysis revealed that IgM and IgG exhibited significant linear relationships with HPs dosage, whereas ALB demonstrated a significant quadratic relationship with dosage based on quadratic term testing. Collectively, these findings suggest that high-dose HPs supplementation enhances B cell differentiation in plasma cells, thereby promoting immunoglobulin synthesis and secretion and improving immune responses in Cyan-Shank Partridge chickens.
Our study showed that HPs supplementation in poultry diet at 1,200 mg/kg significantly improved growth performance in Cyan-Shank Partridge chickens, as evidenced by enhanced body weight at 20 days of age, ADG during the early rearing phase (1–20 days), and ADFI during the late phase (41–60-day). Additionally, HPs supplementation significantly increased leg muscle ratios. Moreover, HPs supplementation reduced serum MDA levels and elevated SOD activity, indicating improved antioxidant capacity. Furthermore, high-dose HPs supplementation significantly upregulated serum immunoglobulin (IgA, IgM, and IgG) levels, indicating enhanced humoral immunity. Conclusively, these findings indicate that HPs supplementation at 1,200 mg/kg may improve growth performance, slaughter performance, antioxidant capacity, and immune function in Cyan-Shank Partridge chickens.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by The Animal Ethics Committee of Jiangsu Agri-animal Husbandry Vocational College. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HZ: Conceptualization, Methodology, Writing – original draft. LY: Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. JZ: Data curation, Writing – original draft. GY: Data curation, Formal Analysis, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by 2022 Jiangsu Province “Qinglan Project” Academic Leader Program (Project No. Su Jiao Shi Han (2022) No. 29); the Key Project of Jiangsu Agri-Animal Husbandry Vocational College (Project No. NSF2021ZR03); and the Science and Technology Innovation Team Project of Jiangsu Agri-Animal Husbandry Vocational College (Project No. NSF2023TC03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1641431/full#supplementary-material
References
Blokhina O., Virolainen E., and Fagerstedt K. V. (2003). Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 91, 179–194. doi: 10.1093/aob/mcf118
Chen H., Yang S., Lu Z., Qian L., Li B., Li W., et al. (2023). Polysaccharide of Atractylodes macrocephala Koidz alleviates cyclophosphamide induced oxidative stress and apoptosis in liver of Lingnan yellow chickens. Chin. J. Anim. Nutr. 35, 1976–1984. doi: 10.12418/CJAN2023.185
Hou S. (2012). Study on extraction, identification and function of bioactive ingredients extracted from honeybee comb (Harbin-150028, Heilongjiang, China: MSc Dissertation. Harbin University of Commerce).
Liu Y. (2024). Effect of liquorice polysaccharide on the growth, development and economic benefits of broilers. China Feed, 51–54. doi: 10.15906/j.cnki.cn11-2975/s.20241013
Liu J., Wu K., Wang L., Zhang K., Han S., Chen F., et al. (2024). Protective effects of astragalus polysaccharides, saponins and probiotic compounds on intestinal tract of broilers infected with E.coli. Acta Vet Zootech Sin. 55, 2241–2252. doi: 10.11843/j.issn.0366-6964.2024.05.041
Liu S., Yang K., Su S., Li Y., Bi Y., Ye L., et al. (2024). Effects of traditional Chinese medicine compound polysaccharides on growth performance, immune regulation, and intestinal flora of broilers. Feed Res. 47, 59–64. doi: 10.13557/j.cnki.issn1002-2813.2024.19.012
Liu P., Yu P., and Wang X. (2024). The effect of adding astragalus polysaccharides to the diet on the production performance, organ index, slaughtering performance, and muscle quality of broilers. China Feed 08, 25–28. doi: 10.15906/j.cnki.cn11-2975/s.20240807
Mao X., Liang H., Wang H., and Dong N. (2022). Protective effects of plant polysaccharides on animal intestine and its molecular mechanism. China Anim. Husbandry Vet Med. 49, 150–160. doi: 10.16431/j.cnki.1671-7236.2022.01.016
Mo W., Liu L., Gu Z., Zhong Q., He H., Xiong B., et al. (2024). Effects of lentinan on growth performance, immune function and immune effect of avian influenza vaccine in laying hens. China Feed 10, 63–67. doi: 10.15906/j.cnki.cn11-2975/s.20241016
Payne R. L. and Southern L. L. (2005). Changes in glutathione peroxidase and tissue selenium concentrations of broilers after consuming a diet adequate in selenium. Poult Sci. 84, 1268–1276. doi: 10.1093/ps/84.8.1268
Poprac P., Jomova K., Simunkova M., Kollar V., Rhodes C. J., Valko M., et al. (2017). Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 38, 592–607. doi: 10.1016/j.tips.2017.04.005
Shan S., Yu Y., Li J., Cheng Y., Xu N., Liu S., et al. (2023). Effect of dandelion polysaccharide on immunologic function, serum biochemical indexes and growth performance of broiler chicken. Feed Res. 46, 30–35. doi: 10.13557/j.cnki.issn1002-2813.2023.03.007
Si S. (2023). Application of Astragalus polysaccharide in broiler poultry farming. Breeding and Feed, 22(02), 63–65. doi: 10.13300/j.cnki.cn42-1648/s.2023.02.009
Sies H., Berndt C., and Jones D. P. (2017). Oxidative stress. Annu. Rev. Biochem. 86, 715–748. doi: 10.1146/annurev-biochem-061516-045037
Wang M., Liang X., Wang Q., and Gao T. (2020). Effects of yeast polysaccharide on the growth and carcass traits in Ross 308 broiler. Modern Anim. Husbandry 4, 34–36. doi: 10.3969/j.issn.1008-3111.2020.02.008
Wang S., Wang X., and Han W. (2001). Modern immunology of animal Vol. 3 (Ji Lin, China: Jilin Science & Technical Publishing House Co., Ltd), 73–382.
Xie H., Zou Y., Liu L., Yang Y., and He J. (2019). Effects of botanic polysaccharides on intestinal mucosal morphology and intestinal barrier function in weaned piglets. Chin. J. Vet Sci. 39, 150–157. doi: 10.16303/j.cnki.1005-4545.2019.01.27
Xing M., Cao Q., Wang Y., Xiao H., Zhao J., Zhang Q., et al. (2020). Advances in research on the bioactivity of alginate oligosaccharides. Mar. Drugs 18, 144. doi: 10.3390/md18030144
Yang L., Hu R., Xia S., and He J. (2019). Plant polysaccharide: research progress of biological function and its application in animal production. Chin. J. Anim. Nutr. 31, 2534–2543. doi: 10.3969/j.issn.1006?267x.2019.06.012
Yang Y., Yang Y., and Dong X and Tong J. (2017). Effects of alfalfa polysaccharide on growth performance, slaughter performance, meat quality and antioxidant ability in male and female broilers. Chin. J. Anim. Nutr. 29, 488–501. doi: 10.3969/j.issn.1006-267x.2017.02.016
Yin L., Hu J., Zhu Q., Ji T., and Lu G. (2021). Effect of honeybee comb polysaccharide on mRNA expression of spleen Th1/Th2 cytokines and transcription factors in immunosuppressed mice. Heilongjiang Anim. Sci. Vet Med. 19, 118–121. doi: 10.13881/j.cnki.hljxmsy.2021.02.0244
Yin L., Ji T., Zhan X., and Li G. (2018a). Comparison of physicochemical properties and antioxidant activity of polysaccharides from honeybee comb with different molecular weights. Food Res. Dev. 39, 1–7. doi: 10.3969/j.issn.1005-6521.2018.04.001
Yin L., Ji T., Zhan X., and Li G. (2018b). Physicochemical properties of polysaccharides from honey comb and their immunomodulatory and anti-oxidative effects on immunosuppressed mice. Mod Food Sci. Technol. 34, 32–37. doi: 10.13982/j.mfst.1673-9078.2018.2.006
Zhang H., Hou X., Ye M., and Li J. (2022). Effect of jujube polysaccharide on antioxidant function, egg laying performance and egg quality of black-bone chicken. Feed Res. 45, 27–30. doi: 10.13557/j.cnki.issn1002-2813.2022.14.007
Zhao W. (2021). Effects of crude yam polysaccharide on growth performance, antioxidant and immune function of broilers (Xinxiang-453003, Henan, China: Henan Institute of Science and Technology. MSc Dissertation).
Keywords: honeycomb polysaccharides, cyan-shank partridge chicken, growth performance, slaughter performance, antioxidant capacity, immune function, antibiotic alternatives
Citation: Zhang H, Yin L, Zhang J and Yan G (2025) Effects of honeycomb polysaccharides on growth performance, slaughter performance, antioxidant capacity, and immune function in Cyan-Shank Partridge chickens. Front. Anim. Sci. 6:1641431. doi: 10.3389/fanim.2025.1641431
Received: 05 June 2025; Accepted: 15 July 2025;
Published: 13 August 2025.
Edited by:
David L. Harmon, University of Kentucky, United StatesReviewed by:
Ravikanthreddy Poonooru, University of Missouri, United StatesYuwen Dong, University of Pennsylvania Division of Gastroenterology and Hepatology, United States
Copyright © 2025 Zhang, Yin, Zhang and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Yin, bGluZ3lpbl90eHhAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Haibo Zhang1†
Haibo Zhang1† Ling Yin
Ling Yin