- 1Department of Animal Science, Tshwane University of Technology, Pretoria, South Africa
- 2Agricultural Research Council, Germplasm, Conservation, Reproductive Biotechnologies, Pretoria, South Africa
A significant amount of long-chain polyunsaturated fatty acids, such as arachidonic acid (ARA) and docosatetraenoic acid (DHA) (C20:4n-6 and C22:4n-6, respectively), are present in rooster semen. The ARA is a form of omega-6 fatty acid with a vital part in several biological procedures such as cell membrane structure and sperm cell signaling pathways. The DHA is a major polyunsaturated, crucial for the rooster semen quality, thus linked to the male reproductive efficiency. Despite the DHA link to rooster semen quality and fertility, it has been found to undergo a serious decrease as roosters age. Moreover, the frozen-thawed rooster semen survival is still low, spurring innovative strategies to improve frozen-thawed rooster semen, sperm cell damage leading to the negative impact on motility, viability, and membrane integrity. These challenges sparked interest in using long-chain polyunsaturated fatty acids, particularly omega n-3 and omega n-6, to increase spermatozoa quality and reproductive efficiency. Noteworthy, chickens cannot synthesize omega n-3 and omega-n-6 de novo, necessitating their dietary supplementation. In rooster sperm, omega-6 polyunsaturated fatty acids are natural components that are vital for achieving an optimal fertility rate. However, the dietary supplementation of these long-chain polyunsaturated fatty acids alone has been reported to result in lipid peroxidation and sperm susceptibility to reactive oxygen species, necessitating the addition of a natural antioxidants. Although previous studies have shown that both fresh and preserved semen have improved semen parameters and a good fertility rate when antioxidants are supplemented to the diet, there have been conflicting results after adding antioxidants and long-chain polyunsaturated fatty acids (LCPUFAs) to the diet. Therefore, this review’s goal is to postulate the understanding of the role of LCPUFA precursors as antioxidants, their challenges, and perspectives on the improvement of rooster semen quality. Enhancing rooster semen quality supports better fertility and hatchability in poultry, contributing to sustainable food production systems and ensuring affordable protein sources for communities, thereby addressing Sustainable Development Goals (SDGs) particularly on zero hunger and food security.
1 Introduction
Agenda 2063 of the African Union and the Sustainable Development Goals (SDGs) of the United Nations aim to address food security and end poverty in all its forms (Gumede, 2021; Sithole et al., 2025). In addition to being essential for farmers’ survival in terms of food production and income generation, chickens provide an affordable means of obtaining vital protein sources, like eggs and meat for household consumption in order to maintain a balanced diet (Sithole et al., 2025). Moreover, smallholder farmers favor chickens because they are comparatively cheap to raise, need small space, and require fewer inputs. This alone can address the SDGs 1 and 2, which emphasize poverty elimination in all its forms (including securing balanced nutrition) and zero hunger (Street, 2023). Practically, achieving and eliminating poverty in all its forms remain the greatest challenge facing humankind despite significant interventions (Gumede, 2021). The ongoing population growth projected to reach 9 billion in 2050 will result in stagnation, inability to end poverty, and demand a doubling of food production (Adeleke and Babalola, 2020). This happens at a time when the world is highly affected by climate change, causing heat stress to animals, thus jeopardizing the production of animal products (Ngcobo et al., 2025).
The global temperature has been increasing by 0.2 to 0.3˚C a decade since 1975 (Ngcobo et al., 2025). This global temperature rise has had a negative effect on the agriculture sector, thus jeopardizing its growth (Oke et al., 2024) and threatening food production. Provided that the poultry industry has been recognized as one of the crucial sectors in the livestock industry with the quickest growth rate, it is promising to improve nutrition and food security through its cheap products, such as eggs and meat, which are consumed by the majority worldwide (Oke et al., 2024). However, there are challenges in this sector, such as the lower male-to-female ratio in the flock, where males appear to be more crucial for flock fertility than females, highlighting the importance of improving male fertility (Ommati et al., 2013). Despite the recent interest in the application of long-chain polyunsaturated fatty acids (LCPUFAs) in rooster diets to improve their fertility (Alagawany et al., 2019; Cartoni Mancinelli et al., 2022), this area is still not well understood with regards to rooster semen quality. There is still an undescribed challenge of semen quality decline post-preservation (Silyukova et al., 2022), lowering the hatching rate (Maapola et al., 2023).
Poor post-thawed semen quality and hatching rate sparked an interest in the use of LCPUFAs, specifically omega n-3 (ω3) and omega n-6 (ω6), to advance reproductive efficiency through improving frozen-thawed spermatozoa. The LCPUFAs, particularly ω3 and ω6 precursors such as alpha-linolenic acid (ALA) and linoleic acid (LA), respectively, have been utilized to advance semen quality and reproductive efficiency of mammals (Ngcobo et al., 2021). The main distinction between ω3 and ω6 LCPUFAs is that ω3 has a double bond between the third and fourth carbons in its carbon chain, while the ω6 has a double bond between the 6th carbon atom (Balić et al., 2020). The presence of these LCPUFAs in the cellular membrane is vital for maintaining the soundness of the lipid bilayer (Sithole et al., 2025). Therefore, supplementing roosters’ diets with ω3 polyunsaturated sources is thought to improve semen quality and fertility, especially from 39 to 47 weeks of age, as these fatty acids are minor natural constituents of chicken sperm (Alagawany et al., 2019). On the other hand, it is known that peroxidative damage is the primary cause of motility loss during rooster semen storage because it affects sperm morphology and decreases sperm motility (Long and Kramer, 2003), necessitating the enrichment of antioxidants to stabilize the production of reactive oxygen species (ROS) (Qamar et al., 2023). Sperms typically have a low antioxidant capacity; thus, enzymatic and non-enzymatic antioxidants in the seminal plasma shields the sperm by hunting ROS (Ommati et al., 2013). Furthermore, the sperm’s antioxidant system functions to primarily protect the sperm’s integrity and function by inhibiting apoptosis and the production of ROS (Qamar et al., 2023). This strikes a balance between the production of antioxidants and the development required to ensure that cells are functioning properly. Antioxidants are required and crucial for protecting sperm membranes from peroxidative damage (Partyka and Niżański, 2021). Despite some research done with a focus on the use of LCPUFAs on improving semen quality, very few studies are related to the cryopreservation of rooster semen. Moreover, there are mixed outcomes after the enhancement of diets with LCPUFAs, with some studies reporting negative effect of feeding LCPUFAs on semen quality and fertility. Subsequently, this review aims to postulate the understanding of the role of LCPUFA precursors and antioxidants, their challenges, and perspectives on the improvement of rooster semen quality.
2 An overview of chicken physiology
Roosters have a special reproductive system with their testicles located inside, below the dorsal abdomen and shaped in an oval or bean shape (Mfoundou et al., 2022). Their sperm remain viable inside the body and at the body temperature, unlike mammalian spermatozoa (Rutllant and Khamas, 2024). They are characterized by having semen that is milky white and highly concentrated in comparison to mammalian semen (Mfoundou et al., 2022; Rutllant and Khamas, 2024). Nevertheless, it is well known that improved roosters’ reproductive success depends on early testicular development (Du et al., 2021). Therefore, the development and maturing of Sertoli and Leydig cells from 2 to 15 weeks is the crucial stage in early development of rooster testicles (Mucksová et al., 2009). By aiding pluripotent primordial germ cells in developing into spermatogonia, these cells sustain the germinal cells throughout the chickens’ lives (Feng et al., 2015) hence, the time frame from 10 to 15 weeks is the fundamental stage for later testicular development with the help from steroids hormones (Feng et al., 2015).
Serum levels of hormones, including testosterone (T), follicle-stimulating hormone (FSH), gonadotropin-releasing hormone (GnRH), and luteinizing hormone (LH) on day 35 significantly increased in chickens (Feng et al., 2015). This is attributed to the fact that in the rooster testes, the FSH, LH, and testosterone determine both spermatogenesis and steroidogenesis (Vizcarra et al., 2010). Follicle-stimulating hormone and LH control spermatogenesis through cyclic adenosine 3, 5’-monophosphate (cAMP) (Huang et al., 2023) while LH further binds receptors in the membrane of Leydig cells and triggers the secretion of testosterone. Generally, the mechanism of hormones in chickens is that FSH activates spermatogenesis by binding to its receptors on the Sertoli cells’ membranes (Santi et al., 2020; Shah et al., 2021). As a result, in rooster breeders, testicular function is linked to FSH concentrations, which then triggers a strong correlation between FSH and testis weight (Feng et al., 2015). On the other hand, the maturation of spermatozoa and the initiation of spermatogenesis depend on FSH (Oduwole et al., 2018). The testosterone levels, on the other hand, determine the development of testicles and how roosters behave (Feng et al., 2015) while acting on the Sertoli and peritubular cells of the seminiferous tubules and stimulating spermatogenesis.
2.1 Significance of the unique physiology of chickens and fertilization
The reproductive physiology of chickens is developed to an extent that they have one ovary that is working, as compared to mammals with two working ovaries (Rutllant and Khamas, 2024; Sithole et al., 2025). Additionally, the right ovary halts developing when the female hatches, but the left one keeps maturing, leaving the female with just the left ovary for the remainder of her reproductive life. Noteworthy, in poultry, fertilization happens internally, with the sperm meeting the egg within the chicken’s reproductive tract, while with mammals, fertilization can be either internal or external depending on the species (Gwatkin, 2012). This makes it impossible to preserve chicken embryos in vitro. Hence currently, cryopreservation of semen (despite its challenges) is the sole feasible and less costly in vitro method for preserving chicken germplasm ex situ (Maapola et al., 2023; Sithole et al., 2025).
3 An overview of the long-chain polyunsaturated fatty acids composition
The LCPUFAs include the ω6 and ω3 fatty acid family and contain about 20- and 22-carbon chains within their carbon molecule (Abedi and Sahari, 2014). As shown in Figure 1, the primary precursor for ω6 LCPUFA is linoleic acid (LA), while ω3 is synthesized by the alpha-linolenic acid (ALA) (Miles et al., 2021). Owing to the absence of Δ-12 and Δ-15 desaturase enzymes, which are necessary for forming carbon-carbon double bonds outside the Δ-12 and Δ-15 carbons, these precursors cannot be produced de novo (Figure 1) in livestock, such as chickens, hence, they should be supplemented in the diet (Ngcobo et al., 2021). Among many sources of ω3 and ω6, additives such as seeds, nuts, vegetable oils and vegetable oil-based spreads are the richest source, however, eicosapentaenoic acid (EPA) and DHA ω3 can be found in seafood, particularly fatty or oily fish, and supplements like fish oils, while ARA ω6 can be located in meat and eggs (Miles et al., 2021). Despite all these knowledge, there are documented drawbacks to fish oil use, which includes the presence of heavy metal contamination and antibiotics in fish sources (Sidhu, 2003), as well as smells and odors that are undesirable, issues with stability, high cost, and challenges in purifying because of the small levels of DHA and EPA fatty acids (Abedi and Sahari, 2014). Moreover, there is a reported competition between humans and animals for fish, and human fishing activities often remove fish at unsustainable rates, which may lead to the extinction of fish due to overfishing activities (Department of Forestry, Fisheries and the Environment (DFFE) et al., 2023).
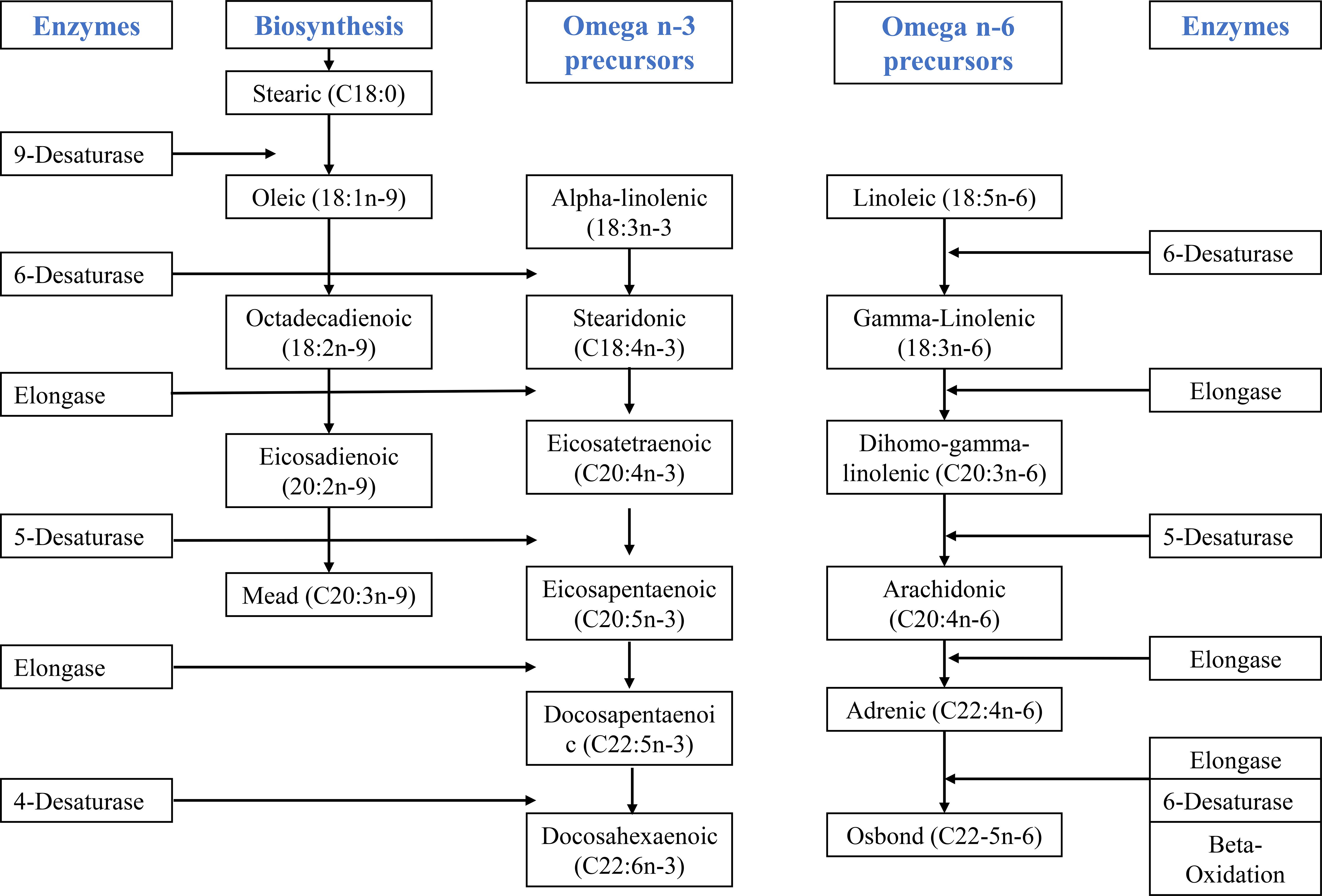
Figure 1. Mechanism of the biosynthesis of long-chain polyunsaturated fatty acids from precursors of critical fatty acids. Adapted from (Ngcobo et al., 2021) and modified.
3.1 Sources of long-chain polyunsaturated fatty acids
3.1.1 Microbial sources
Microorganisms such as bacteria, fungi, algae, mosses, and protozoa can manufacture diverse polyunsaturated fatty acids (PUFAs) through aerobic and anaerobic pathways (Abedi and Sahari, 2014). Microorganisms provide superior substitutes for the production of PUFAs (Huang et al., 2023). Most notably, microalgae and oleaginous fungi, such as Schizochytrium sp, Yarrowia lipolytica, and Mortierella alpina, have been successfully utilized as primary sources of DHA, EPA, and ARA, respectively. The Moritella marina bacteria, Thraustochytrium spp, and Entomophthora spp fungi, and other species are from the Thraustochytriales, including Thraustochytrium aureum, Thraustochytrium roseum, and Thraustochytrium sp. ATCC 20892, which is a family that is known to be microorganisms that renders extreme levels of DHA (Wu et al., 2005). The microorganisms known as microalgae, on the other hand, are powered by light and generate useful metabolites including PUFAs, antioxidants, and antimicrobials (Guedes et al., 2011; Abedi and Sahari, 2014). As a substitute for more expensive plant and animal sources, they have been viewed as vectors for the commercial production of oils and fats; their benefits over fish oils include their easy refining, low danger of chemical contamination, and lack of unpleasant odor (Guedes et al., 2011).
3.1.2 Aquatic sources
The key aquatic species that have been proven to have LCPUFAs include fishes, shrimps, prawns, crabs, shellfish, and algae (Abedi and Sahari, 2014). The ω3 LCPUFAs can be acquired from fatty fish, such as salmon, tuna, mackerel, anchovies, capelin, Atlantic cod, Atlantic herring, Atlantic mackerel, Atlantic menhaden, salmonids, sardines, shark (liver), herring, sardines, and fish oil (Hoppenbrouwers et al., 2019). These sources are particularly rich in long-chain ω3 PUFAs, including EPA and DHA, which are not readily converted from plant-based ALA in the body, and are often a good source of other essential nutrients like iodine and selenium, which are not as readily available in many plant-based foods (Marsol-Vall et al., 2022).
3.1.3 Animal sources
The majority of essential fatty acids originates from milk, dairy products, lamb, beef, poultry, eggs, and pork (Woods and Fearon, 2009). These are mostly guided by the composition of the diets, the digestive system of the animal, and by the biosynthesis mechanisms within the animal (Woods and Fearon, 2009; Abedi and Sahari, 2014). Animal sources offer several benefits (higher bioavailability of EPA and DHA, and the presence of fat-soluble vitamins) over plant sources when it comes to PUFAs, especially ω3s. They provide pre-formed EPA and DHA, which are readily usable by the body, unlike plant sources, which primarily provide ALA, which requires conversion to EPA and DHA (Mahaffey et al., 2011). Nevertheless, a lot of research has reported negative and positive effects of animal sources on fertility and other factors. For example, milk with a high concentration of unsaturated fatty acids, particularly PUFAs, was found to be more prone to oxidation and the development of off-flavors (Woods and Fearon, 2009). On the other hand, egg yolk serves as a great source of PUFAs, especially DHA, which contains 0.1% EPA, 0.7% DHA, and 0.8% ALA (Abedi and Sahari, 2014).
3.1.4 Plant sources
Plant-based sources of LCPUFAs for chickens include various seeds, such as oilseeds, nuts, seeds, and certain algae (Nguyen et al., 2018). Moreover, most of these seeds are acknowledged to be rich in ALA, a precursor for the ω3 LCPUFAs EPA and DHA, which are beneficial for animal health and further utilized for the withdrawal of oils rich in ω3 PUFAs (Rizzo et al., 2023). Amongst these sources, flaxseed has been outstanding, consisting of high ALA concentrations with a total fatty acid percentage ranging from 39-60% (Goyal et al., 2014; Zamani Ghaleshahi et al., 2020). It is considered as a good source of ALA, which can be converted to other beneficial fatty acids in chickens (Kartikasari et al., 2012). Moreover, incorporating flaxseed in chickens’ diet helps in supporting their immune systems, making them more resistant to common chicken diseases since flaxseed oil contains anti-inflammatory properties of ω3 fatty acids help in reducing the risks associated with inflammation (Lee et al., 2021). Black raspberry seed oil is another source of LCPUFAs, comprising 35% ALA of the overall fats and 98-99% of unsaturated fatty acids; however, with slightly lower ratios of ω6 to ω3 at 1:6:1 (Parry and Yu, 2004). Boysenberry seed oil is another plant-based source comprising a high percentage (19.5%) of ALA and a ratio of ω6 to ω3 fatty acid of 2:8:1. It has a very high PUFA concentration of 73.3% and more than 91% of the seed oil made up of all unsaturated fatty acids (Yu et al., 2020).
4 Role of long-chain polyunsaturated fatty acids in improving fertility rates and semen quality of roosters
Dietary supplementation with LCPUFAs, particularly ω3 such as DHA and EPA, can influence rooster semen quality (Yuan et al., 2023) and has many advantages on semen quality as shown in Figure 2. Moreover, adding ω3 to the diet is reported to influence chicken’s immunity, resulting in the production of chicken products that are good for consumers’ health (Mousa et al., 2017). This has triggered numerous studies in chickens to focus on the functional action of various LCPUFA varieties and their dietary levels on the mechanism of lipids. The use of LCPUFAs in chicken diets has been confirmed to significantly decrease cholesterol and overall lipid content in the blood (Alagawany et al., 2019). Fatty acids have several effects, such as improving sperm cell quality through improving fertility, quantity, and overall semen quality (Ngcobo et al., 2024), hence, several studies reported good results on the use of PUFAs (Al-Daraji et al., 2010; Eslami et al., 2016; Badwy et al., 2024). Moreover, a study by (Bongalhardo et al., 2009) reported that adding animal sources such as fish oil to rooster diets influenced fertility through lowering fatty acid ratio (ω3:ω6) in sperm membranes, which may modify the physical properties of the membrane or induce resistance to peroxidative damage. Furthermore, a diet enriched with a medium ratio of ω3: ω6 fatty acids influenced DHA and ω-3 PUFAs while reducing DHA and ARA in rooster sperm (Al-Daraji et al., 2010). However, some studies have indicated that high doses of unsaturated fatty acids, which are examples of high doses of LCPUFAs, are harmful to health, raise the amount of oxidative stress, and reduce the production of testosterone crucial enzymes, all of which will have a negative impact on semen quality (Yuan et al., 2023).
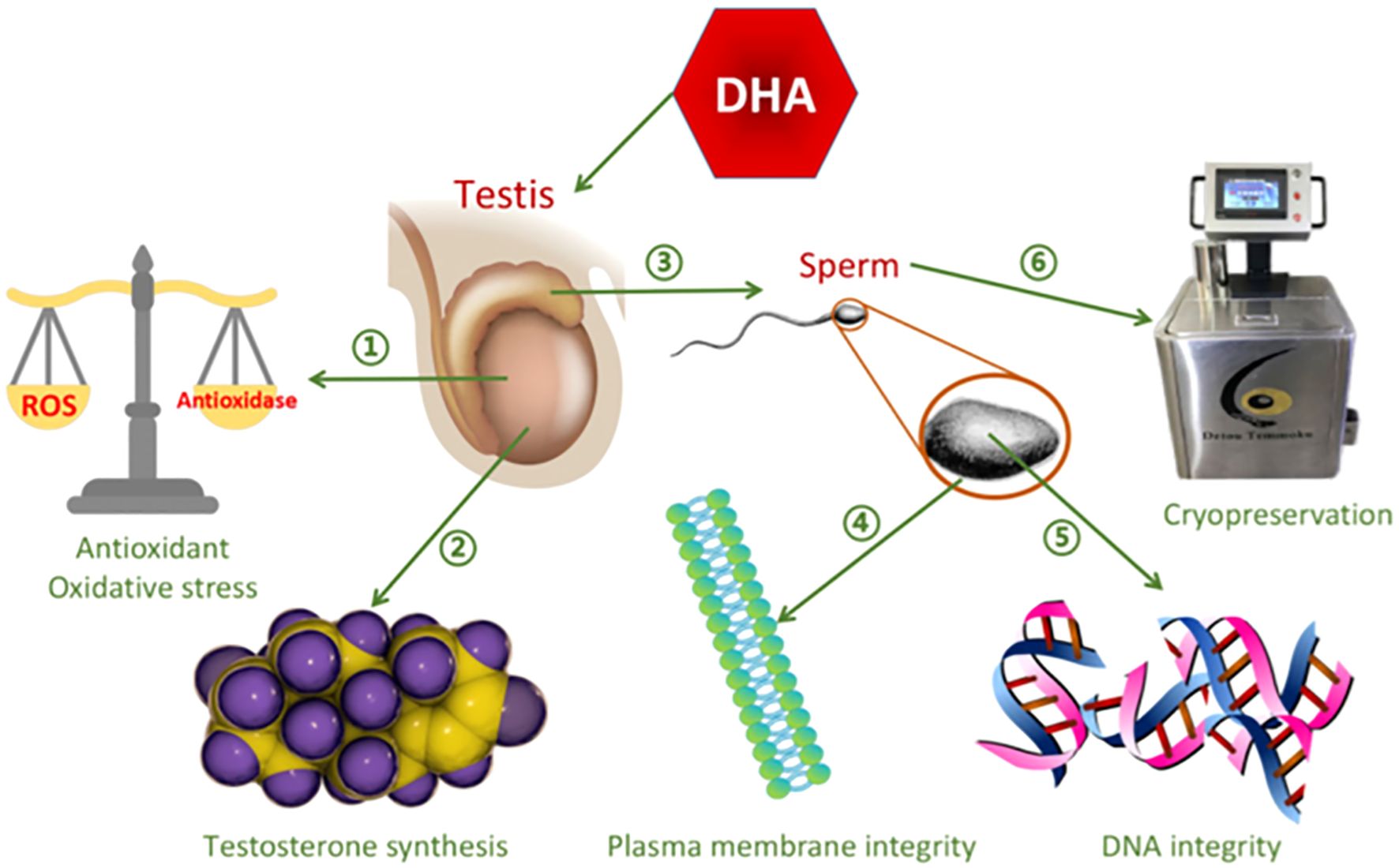
Figure 2. The functions of DHA on semen quality: 1. Improves antioxidant capacity and decrease oxidative stress 2. Enhance testosterone synthesis 3. Enhance sperm maturation 4. Improves plasma membrane integrity of the sperm 5. Improves integrity of the DNA 6. Prevent sperms from damage caused by cryopreservation (Yuan et al., 2023).
Lipids are an essential element of the sperm which aids not only in the energy metabolism of sperm but also in various roles linked with fertility (Zaniboni et al., 2006). Rooster sperm contain high levels of LCPUFAs, in which the primary LCPUFAs are AA and DHA (C20:4n-6 and C22:4n-6, respectively, which are positively correlated with fertility rate (Abbaspour et al., 2020). Their multiple double bonds enhance membrane flexibility and support signal transduction pathways crucial for sperm motility and viability. However, this same structural feature makes LCPUFAs highly vulnerable to reactive oxygen species (ROS), predisposing spermatozoa to lipid peroxidation, DNA fragmentation, and reduced functionality during storage and cryopreservation (Wang et al., 2025). Reactive oxygen species are by-products of normal cellular metabolism and play dual roles in sperm physiology (Chandimali et al., 2025). At physiological levels, ROS are necessary for capacitation, hyperactivation, and acrosome reaction (Dutta et al., 2019). It has been proven that the leading fatty acids in sperm of roosters are ω6. Many studies (see Table 1) have investigated the effect of dietary enrichment of LCPUFAs from diverse sources on semen quality and fertility of roosters. For instance (Zanussi et al., 2019), reported that supplementing with flaxseed at 2% improved overall semen fertility (control-85.4% and flax- 91.67%) by enhancing semen performance and fertility potential. According to (Badwy et al., 2024), 1% PSO improved semen variables such as volume, sperm quality, sperm morphology, and velocities. Comparable results were observed by (Qi et al., 2019) where the inclusion of 2% flaxseed was used, and the conclusion was that the 2% group had an improved semen quality (control- 70.83% and 2%flaxseed- 82.29%) compared to the other treatment group. These benefits are attributed to enrichment of DHA and eicosapentaenoic acid (EPA) in sperm membranes, which optimize membrane stability and protect against premature acrosome reaction. However (Abbaspour et al., 2020), reported that whole flaxseed supplementation at 2% was unable to modify the fatty acid composition of the sperm or enhance the quality of the sperm from aged broiler breeder roosters. A documented belief about the effect of dietary ω3 fatty acids on sperm is that they can alter the quantity and composition of PUFA in the head and tail of sperm (Esmaeili et al., 2015). Furthermore, it is clear that ω3 PUFAs have a role in many sperm physiological processes, and that dietary ω3 fatty acids enhance certain sperm traits, such as sperm motility (Feng et al., 2015; Abbaspour et al., 2020). Notably, a study by (Feng et al., 2015) used ω3 and ω6 additives (soya bean oil and flaxseed oil, respectively) and reported an increase in the spermatogonia development and germ cell layers of roosters.
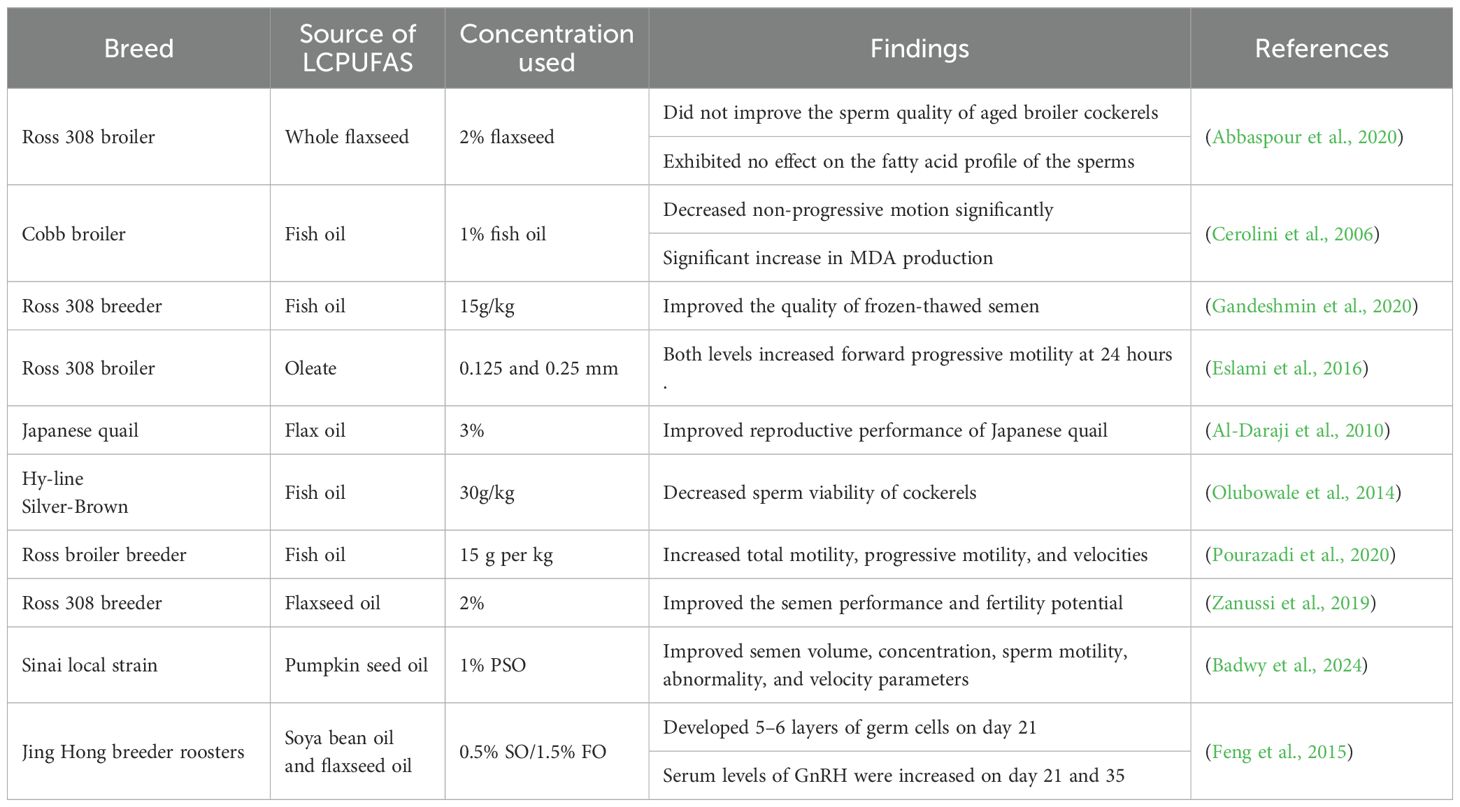
Table 1. Effect of long-chain polyunsaturated fatty acids on the reproductive performance of cockerels fed different supplementation at different levels.
Despite these promising results, some studies have reported negative results such as that high levels of LCPUFA supplementation increase oxidative stress, elevate malondialdehyde (MDA) production (a marker of lipid peroxidation), and even reduce testosterone synthesis (Table 1). These detrimental effects appear to be dose-dependent and are more pronounced when antioxidant supplementation is absent. For instance (Cerolini et al., 2006) reported that dietary supplementation with 1% fish oil significantly increased MDA production (lipid peroxidation) (0.002 mg/109). The ability of LCPUFAs to shield the sperm from chemical (oxidative) and physical (cryopreservation) harm has been outlined in earlier research (Hudson and Wilson, 2003). Moreover, the contradiction in most research outcomes vary with the source of LCPUFAs, the age and physiological status of the chickens, and genetic background (Kartikasari et al., 2012). For instance, younger roosters respond more positively to supplementation compared to older breeders with semen quality that is already declining (Authaida et al., 2023). Furthermore, experimental design factors such as duration of feeding, type of extender used for semen storage, and housing conditions also influence results, contributing to the apparent inconsistencies across the literature (Arif et al., 2025; Halawa et al., 2025).
Noteworthy, the incorporation of LCPUFAs into sperm membranes modifies membrane lipid composition and biophysical properties (Catala, 2015). Higher levels of DHA have been associated with increased membrane fluidity, improved mitochondrial activity, and enhanced motility. In addition, LCPUFAs modulate hormonal signaling pathways and regulate the expression of genes linked to steroidogenesis and spermatogenesis (Mora et al., 2023). Nevertheless, according to (Sithole et al., 2025) if delivered in high concentrations, the membrane’s polyunsaturated fatty acids make the sperm to be very vulnerable to reactive oxygen species (ROS), resulting in lipid peroxidation (see Figure 3). This then necessitates the addition of antioxidants such as vitamin E and ascorbic acid to maintain a stability between the generation of ROS and the readily available defensive antioxidants (Qamar et al., 2023).
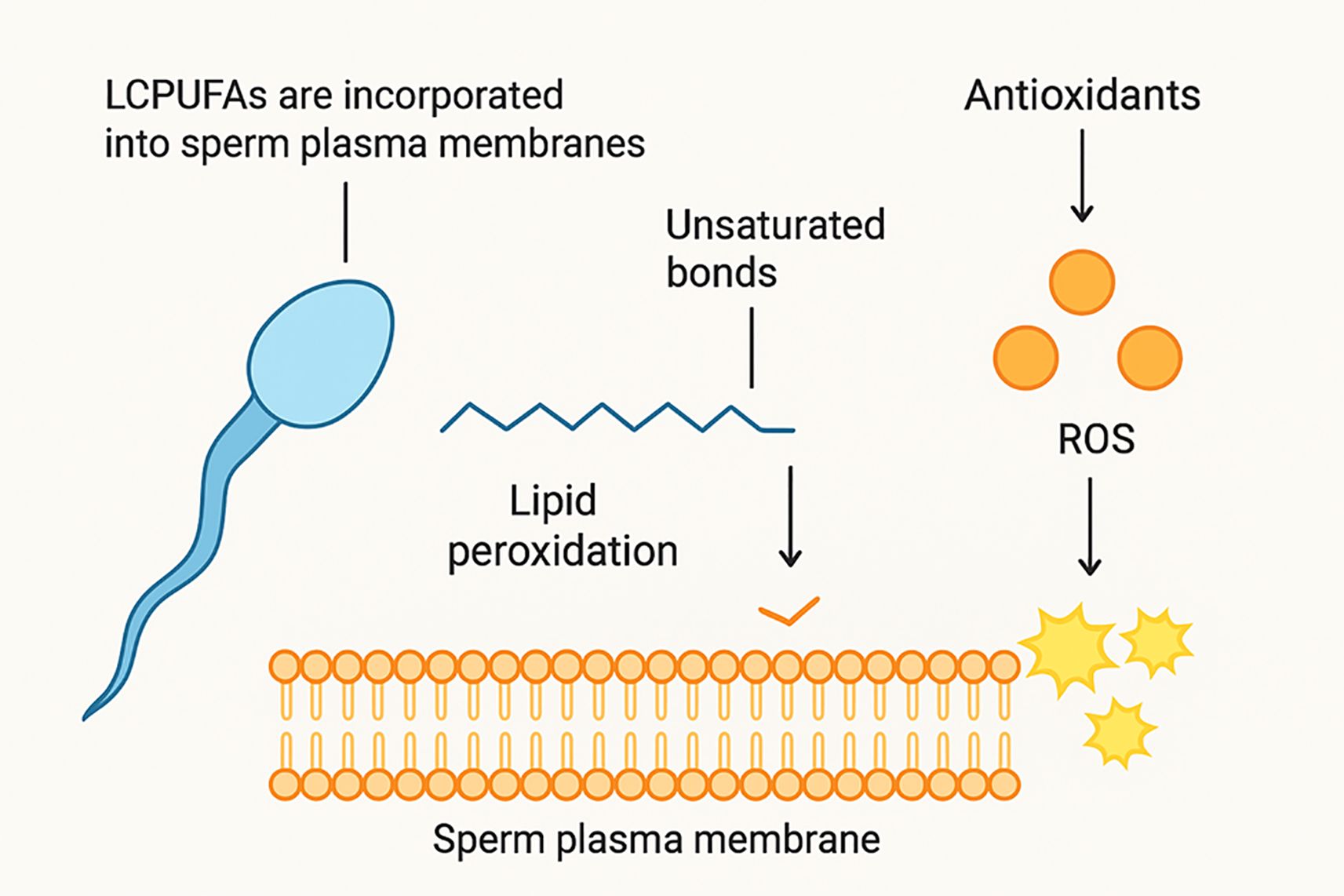
Figure 3. Mechanisms by which LCPUFAs and antioxidants affect the quality of cockerel semen (Wang et al., 2025).
The principal element of the sperm’s antioxidant system is responsible for protecting the membrane from ROS and preventing apoptosis so to preserve the integrity and function of the sperm. Provided that the survival of sperm cell post the freeze-thaw procedure is reported to be still poor, cryopreservation of rooster semen remains a tough challenge (Maapola et al., 2023). Thus, using frozen-thawed semen for AI leads to decreased fertilization rate. An attempt to research the impact of a diet enhanced with LCPUFAs on the improvement of the sperm subsequent to cryopreservation has been made by minor studies in roosters. For instance (Gandeshmin et al., 2020), reported that enriching the diet of roosters using 15 g fish oil per kg diet increased the quality of frozen-thawed semen.
The evidence provided indicates that LCPUFAs are indispensable for optimal sperm function, however, their benefits are conditional. Supplementation improves semen quality when provided in moderate doses and in combination with sufficient antioxidant support. Conversely, excessive or unbalanced supplementation increases susceptibility to oxidative damage, counteracting potential gains (Chandimali et al., 2025). Thus, the key challenge is not whether LCPUFAs are beneficial, but rather how to optimize their levels, sources, and combinations with antioxidants for reliable improvements in fertility and semen preservation.
5 Antioxidants role on the fertility preservation of sperm in roosters
Antioxidants are substances or enzymes that can eliminate, hunt, or defuse ROS and their effects on sperm quality (Jena et al., 2023). Antioxidants are there to preserve the structure and function of cells by defending the plasma membrane from ROS (Jena et al., 2023).They prevent deoxyribonucleic acid (DNA) fragmentation and early sperm maturation, lessen cryodamage, enhance sperm quality, and protect sperm from ROS generated by abnormal sperm or leukocytes. Additionally, they protect the integrity of the acrosome by stopping the premature acrosome response while operating by disrupting the oxidative chain reaction, which lowers oxidative stress (Qamar et al., 2023). Reactive oxygen species may be delivered physiologically and at low amounts to help with sperm maturation, capacitation, and acrosome response (Wang et al., 2025). They further help in striking a balance between ROS production on the physiological and pathological levels (Figure 4). Ironically, elevated ROS levels harm nuclear DNA and sperm membrane lipids, resulting in infertility and subfertility (Qamar et al., 2023). This is where the significance of a tightly regulated balance between ROS production and antioxidant defenses becomes evident. A variety of antioxidants can be used to treat cases of infertility and subfertility in order to alter the level of balance.
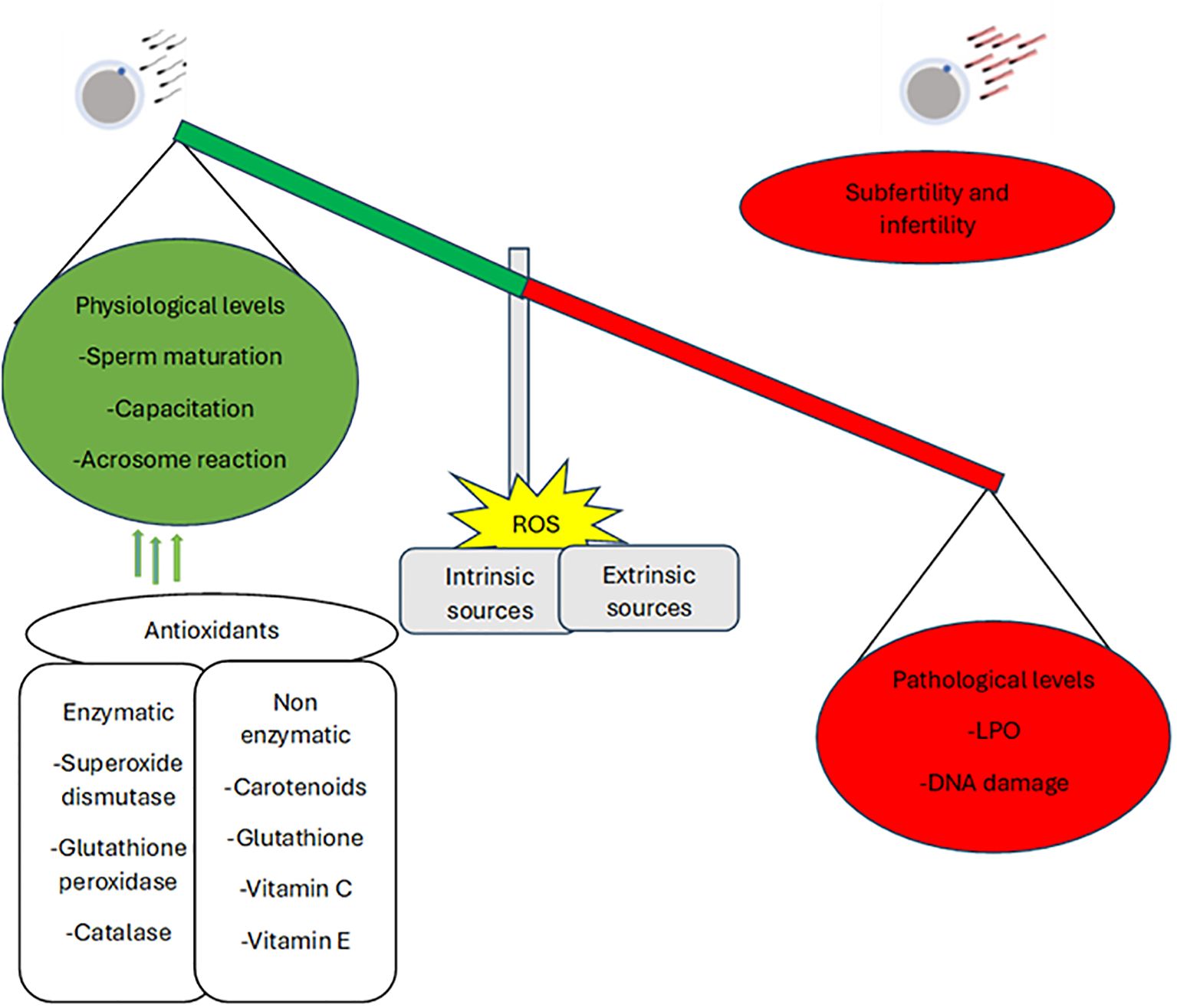
Figure 4. The impacts and balance of ROS on sperm fertilizing ability (Qamar et al., 2023).
The antioxidant capacity of the sperm is lowered throughout the process of spermatogenesis as it loses the majority of its cytoplasmic contents; therefore, the antioxidant capacity of the seminal plasma is what determines how well the sperm is protected against ROS (Qamar et al., 2023; Antinozzi et al., 2025). Seminal plasma serves as the primary line of defense against extracellular ROS because it is composed of a variety of enzymatic and non-enzymatic antioxidant molecules, such as carotenoids (vitamin A), catalase (CAT), GSH, coenzyme Q 10 (CoQ10), glutathione reductase (GSH) glutathione peroxidase (GPx), pyruvate, superoxide dismutase (SOD), vitamin C, vitamin E, taurine, hypotaurine, and uric acid (Saleh and Hcld, 2002). The antioxidant system of the body is influenced by the dietary intake of antioxidants, minerals, and vitamins (Agarwal et al., 2005). The use of antioxidants to lessen the impact of ROS overproduction, whether right into the semen extenders or through dietary supplements, has been thoroughly studied and documented in the literature (Qamar et al., 2023). Dietary antioxidants often need treatment strategies that are more consistent and last longer in order to improve male fertility. Therefore, the dosage determines the impact of each antioxidant.
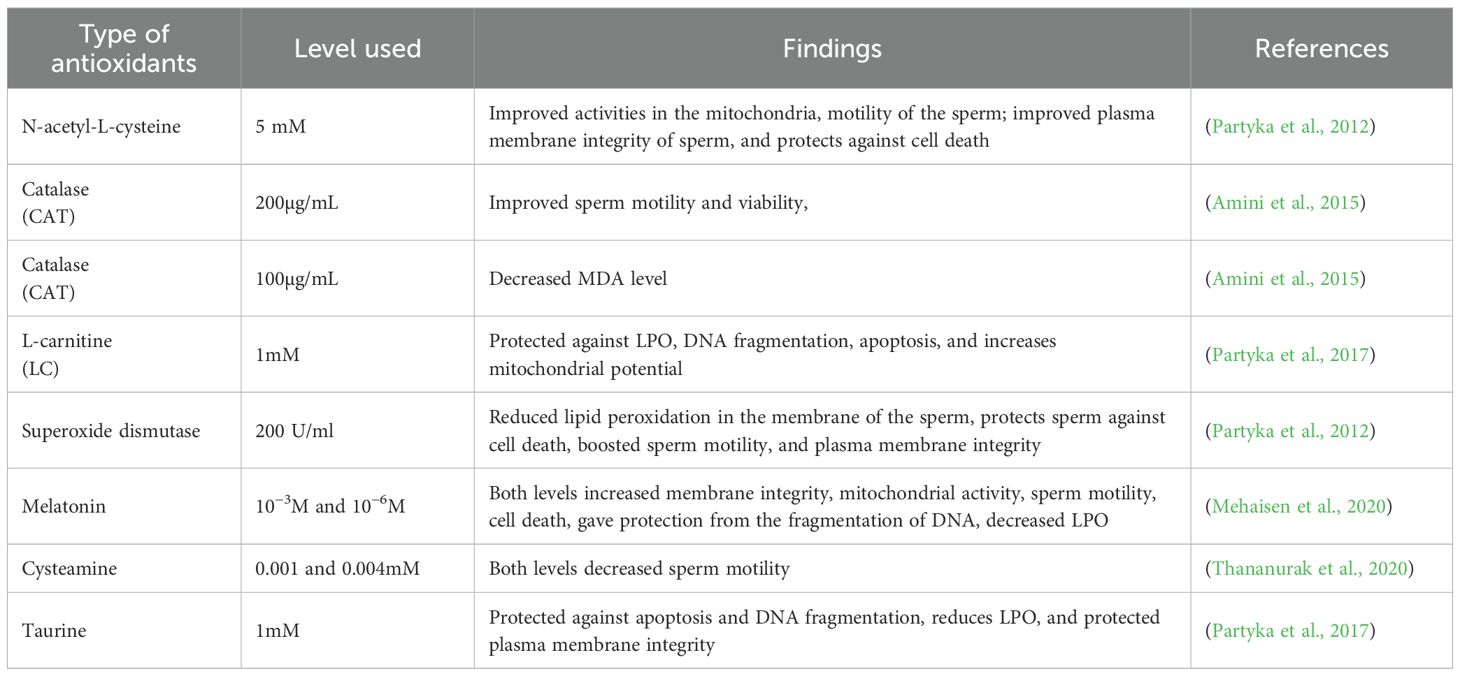
Table 2 below depicts the effect of antioxidants on the reproductive performance of roosters fed different supplementations, investigated by different studies. The positive effects of antioxidants inclusion to improve semen quality are evident from the above results. For instance, vitamin E inclusion at 150mg vE/kg increased semen volume (0.71% to 0.94%), number of sperm cells (1.42% to 2.52%), and percentage live sperm (86.8% to 96.9%) and reduced sperm abnormality (from 11.0% to 5.6%); decreased morphological defect rates of chicken spermatozoa; and improved the semen performance and fertility potential. When roosters were exposed to heat stress, the characteristics of semen quality improved; increased sperm count and motility, decreased the percentage of dead sperm, and improved the antioxidative status of seminal plasma. In contrast, the addition of antioxidants like selenium, selenomethionine, and glutathione increased the stimulating effect on GSH-Px activity in seminal plasma. Moreover, 300 mg/kg VC (84.0 ng/dL) and 200 mg/kg VE (91.2 ng/dL) were reported to enhance serum testosterone and sperm motility remarkably while also contributing to alleviating the oxidative stress caused by dexamethasone (Min et al., 2016). A study by (Attia et al., 2020) reported that 200 mg/kg ascorbic acid improved semen quality, fertility, seminal plasma, and blood biochemistry and hematology of heat-stressed roosters.
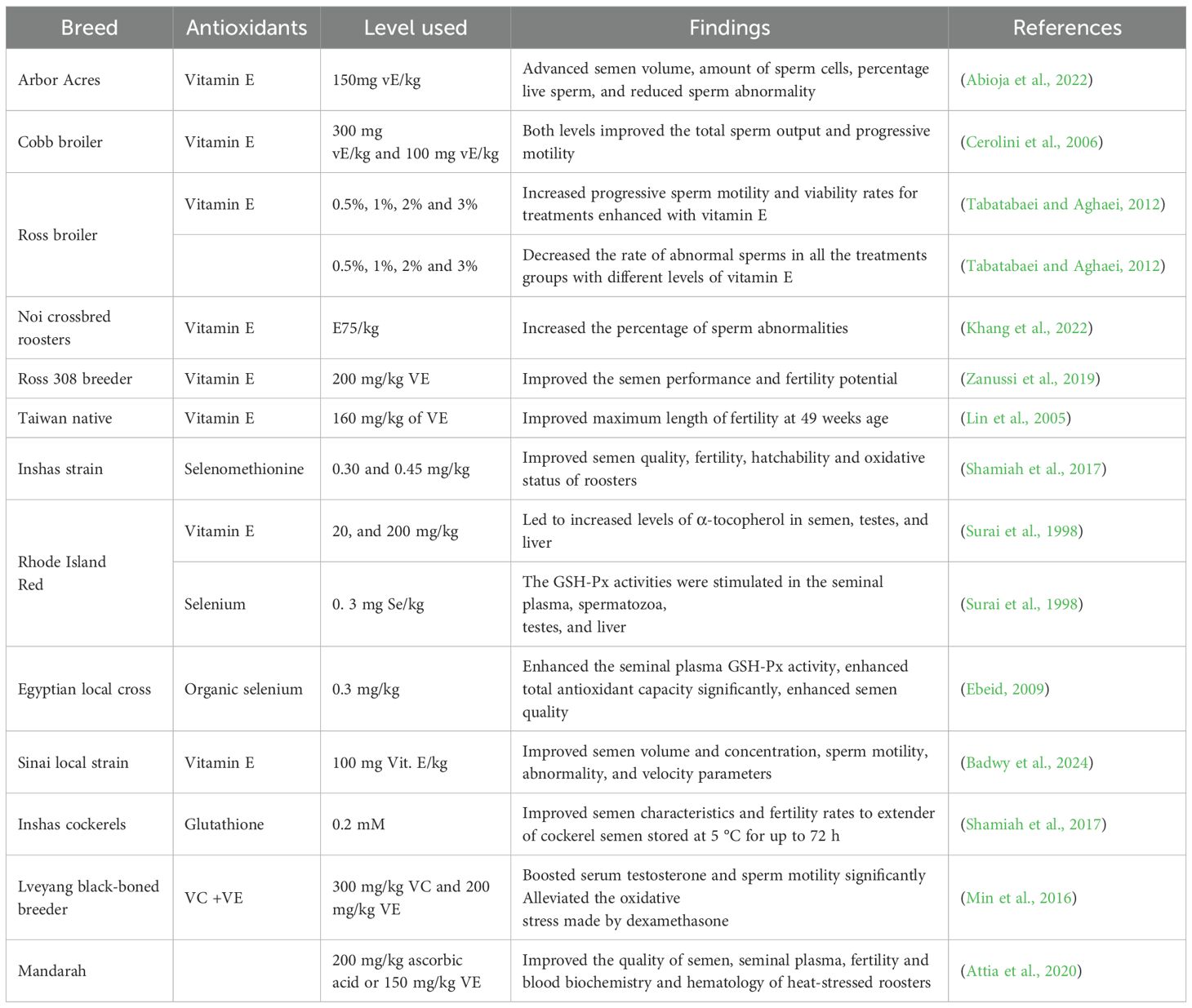
Table 2. Effect of antioxidants on the reproductive performance of cockerels fed different supplementations.
Contrary to the strong evidence for beneficial effects, in some studies, high doses of antioxidants such as excessive vitamin E or cysteamine have reduced motility and impaired sperm function. This paradox may be explained by the “antioxidant paradox,” where excessive scavenging of ROS disrupts their physiological role in capacitation and acrosome reaction (Dutta et al., 2022). Moreover, variations in experimental design such as antioxidant form, administration procedure, treatment duration, environmental stressors such as temperature, housing, and genetic background of the chickens contribute to divergent results. Another factor is the interaction between antioxidants and LCPUFAs. While antioxidants are essential for preventing peroxidation of PUFA-enriched membranes, inadequate antioxidant support during LCPUFA supplementation amplifies oxidative stress (Kodali et al., 2020). Thus, contradictory findings often arise when LCPUFAs are fed without appropriate antioxidant balancing. Antioxidants act at multiple levels to protect spermatozoa, this may include membrane protection (vitamin E, being lipid-soluble, localizes within sperm membranes and prevents peroxidative chain reactions initiated by ROS); enzymatic defense (enzymes such as SOD catalyze the dismutation of superoxide anions into hydrogen peroxide, which is subsequently neutralized by CAT and GPx); DNA protection (water-soluble antioxidants like vitamin C scavenge ROS in the seminal plasma, reducing oxidative DNA damage); mitochondrial function (by limiting ROS accumulation, antioxidants help maintain mitochondrial activity, thereby sustaining ATP production and motility) (Kowalczyk, 2022; Qamar et al., 2023).
5.1 Antioxidants in semen cryopreservation
Though rooster semen is considered to have high activity of antioxidants, this cannot be enough to completely scavenge the detrimental effect of peroxidative sperm injury during semen cryopreservation (Partyka et al., 2012). There is no doubt that cryopreservation of semen enhances lipid peroxidation, leading to susceptibility of rooster semen to lipid peroxidation as the antioxidant activity decreases (Partyka et al., 2012; Fleming and Thomson, 2025). Numerous studies have shown that antioxidant-supplemented diluents offer significant defense against ROS and the development of lipid peroxidation in the semen (Baumber et al., 2000). However, minor percentage of these studies were addressing cryopreservation of rooster semen. Noteworthy previous studies addressed the impact of supplementing antioxidants in the diet on the quality of rooster semen (Bréque et al., 2003). Numerous studies have also used non-enzyme and enzyme antioxidants to improve semen quality before cryopreservation. For instance, in Table 3, antioxidants at different levels improved semen quality parameters like mitochondrial activity, sperm motility, and enhanced the functionality of seminal plasma, decreased MDA levels; however, 0.001- and 0.004-mM levels of cysteamine decreased sperm motility. That could be attributed to the fact that cysteamine leads to hydrogen peroxide production and oxidative stress, which declines glutathione peroxidase activity at high concentrations (Salimi et al., 2024).
Collectively, antioxidants are indispensable for protecting spermatozoa against oxidative stress and ensuring functional competence, particularly during storage and cryopreservation (Kaltsas, 2023). However, their effects are dose- and context-dependent, for instance, moderate supplementation enhances fertility, while excessive levels may disrupt normal physiological ROS signaling (Manful et al., 2025). Importantly, antioxidants should not be considered in isolation but as part of an integrated nutritional strategy with LCPUFAs. Optimizing their ratios, delivery methods, and timing of administration is likely to yield the most reliable improvements in semen quality and fertility.
6 Future prospects and or research directions into improving rooster semen quality
6.1 Optimizing LCPUFA supplementation for enhancing reproductive performance in roosters
The balance between ω3 and ω6 fatty acids in the diet is vital (Jeong et al., 2024). Therefore, future research is needed to focus and continue to explore optimal ratios for enhancing reproductive performance in roosters, as an imbalance can negatively impact semen quality (Clément et al., 2012). Moreover, adding antioxidants to semen extenders is one of the strategies currently practiced, and thus, future studies should explore the novel and more effective natural and synthetic antioxidants to further alleviate oxidative damage during storage and improve post-storage semen quality.
6.2 Synergistic effects of LCPUFAs with other nutrients to improve protection and fertility
While there is evidence out there supporting a beneficial interaction between LCPUFAs and antioxidants, particularly in reducing oxidative stress and inflammation, the synergistic effect of these two is still evolving and not fully established across all scenarios (Ngcobo et al., 2021; Mishra et al., 2023). Prospects research should focus on ideal combinations and dosage of LCPUFAs and several antioxidants to improve protection and fertility (Mishra et al., 2023). Future studies should again focus on determining the utmost effective and practical methods for administering dietary versus extender supplementation for ideal results (Bailey et al., 2019). Overall, a focus on modifying diets with precise LCPUFAs and antioxidant profiles for different rooster breeds, ages, and production systems should be tested (Cartoni Mancinelli et al., 2022). This should stress on combining nutritional strategies with ARTs and cryopreservation to maximize their efficacy. Moreover, deep insights into understanding the molecular pathways by which LCPUFAs and antioxidants influence spermatogenesis, sperm function, and fertility will lead to more targeted and effective options (Sengupta et al., 2024; Wang et al., 2025).
6.3 Targeted delivery of antioxidants via nanotechnology
Nanotechnology offers a promising approach for the targeted delivery of antioxidants, while enhancing their efficacy and reducing potential side effects (Falchi et al., 2018; Saadeldin et al., 2020). Thus, targeted delivery of antioxidants using nanotechnology in rooster semen can significantly improve semen quality by giving protection to the sperm from oxidative damage, especially during cryopreservation (Falchi et al., 2018). For instance, inclusion of Nano-Se to semen extender enhanced the post-thawing quality and oxidative variables of rooster semen (Safa et al., 2016). Thus, prospect studies should be geared towards using nanoparticles to engineer and transport antioxidants such as vitamin E, selenium, or quercetin directly to sperm cells or their mitochondria to enhance their effectiveness on protection compared to traditional methods (Tiwari et al., 2022).
6.4 Mycotoxin challenges
Mycotoxins are defined as secondary metabolites that are produced by molds that contaminate a wide variety of plants and feed (Olariu et al., 2025). Their control in fields such as agriculture is considered one of the challenges because of their several effects on chickens (Murugesan et al., 2015). Hence, future studies aiming at addressing mycotoxins and rooster reproduction should pay focus on emerging innovative diagnostic tools for early detection, discovering novel strategies to mitigate mycotoxin contamination in feed, and investigating the long-term effects of mycotoxin exposure on rooster reproductive performance (Olariu et al., 2025). This can be tailored in a way of understanding the epigenetic changes induced by mycotoxins and their transgenerational impact on fertility and reproductive success (Bilska et al., 2018). Moreover, future research can contribute to focusing on the role of LCPUFAs and antioxidants in reducing the detrimental effects of mycotoxins on rooster reproduction, improving flock health, and fostering sustainability of the poultry industry (Gómez-Osorio et al., 2024).
6.5 Use of omics technologies to improve rooster reproductive performance
By offering greater view of the molecular mechanism underpinning reproduction, the application of omics technologies such as transcriptomics, proteomics, metabolomics, and genomics offers significant opportunities to improve chicken reproductive efficiency (Zhou et al., 2025). In addition to helping in identifying the metabolic pathways essential for fertility, these tools can be used to identify genes associated with reproductive parameters such as fertility, comprehend the patterns of gene expression at numerous stages of reproduction, and offer insight into the locating proteins involved in reproductive processes (Panner Selvam et al., 2019; Wadood, 2024). Furthermore, researchers’ understanding of the critical processes governing fertility, hatchability, and inclusive reproductive efficiency in chicken, could be greatly enhanced by these technologies (Wadood, 2024). These technologies can then be employed to gain a complete view of the molecular changes in rooster semen in response to LCPUFA and antioxidant supplementation. These may further assist in identifying novel biomarkers for semen quality and fertility.
6.6 Influence of gut microbiome on rooster reproductive performance
Microbiome is defined as the sum of microorganisms related to an organism and its interaction (Moszak et al., 2020). The gut microbiome in chickens plays a significant role in reproductive performance, with evidence suggesting that manipulating the gut microbiota using probiotics, prebiotics, and other interventions can improve overall reproductive health (Aruwa et al., 2021; Naeem and Bourassa, 2025). Thus, understanding the intricate relation between the gut microbiome and chicken reproduction will assist researchers and poultry producers to develop strategies to improve poultry health and enhance productivity (Naeem and Bourassa, 2025). Therefore, upcoming prospects should focus on exploring the possible relationship between LCPUFAs and antioxidants, the gut microbiome of roosters, and their subsequent impact on nutrient absorption and overall reproductive health (Yue et al., 2024).
7 Conclusion
This review highlights the dual role of LCPUFAs and antioxidants in shaping rooster semen quality. LCPUFAs are essential components of sperm membranes, enhancing fluidity, signaling, and fertilization potential, yet their susceptibility to oxidative damage makes them a double-edged sword. Antioxidants, both dietary and extender-based, provide critical protection against ROS and lipid peroxidation, but their effectiveness depends on precise dosing and physiological context. A critical synthesis of the literature reveals that contradictory findings in semen quality outcomes largely stem from imbalances: excessive LCPUFA supplementation without sufficient antioxidants leads to oxidative stress, while overly high antioxidant doses can suppress the physiological ROS signaling required for capacitation and acrosome reaction. The most consistent improvements occur when LCPUFAs and antioxidants are provided together in optimized ratios, underscoring their interdependence. Despite encouraging evidence, several gaps remain. Few studies have systematically tested optimal LCPUFA-to-antioxidant ratios, considered genetic variation among breeds, or explored advanced delivery systems such as nanotechnology. Moreover, most existing data are limited to short-term experiments, leaving long-term reproductive outcomes and implications for genetic resource conservation underexplored. The effective use of LCPUFAs and antioxidants should be viewed not as isolated strategies but as integrated nutritional interventions. Optimizing their synergy has the potential to improve semen quality, enhance fertility, and increase the success of cryopreservation in poultry breeding programs. Such advances will strengthen genetic conservation efforts and contribute to global food security by supporting more sustainable poultry production systems.
Author contributions
SS: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. KN: Funding acquisition, Resources, Supervision, Validation, Visualization, Writing – review & editing. MM: Funding acquisition, Resources, Supervision, Validation, Visualization, Writing – review & editing. JN: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the Agricultural Research Council, and Tshwane University of Technology (TUT) (Grant no. API012403000057).
Acknowledgments
The authors would like to acknowledge the following institutions: the Tshwane University of Technology (TUT), and the Agricultural Research Council.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbaspour B., Sharifi S. D., Ghazanfari S., Mohammadi-Sangcheshmeh A., and Honarbakhsh S. (2020). Effect of dietary supplementation of whole flaxseed on sperm traits and sperm fatty acid profile in aged broiler breeder roosters. Reprod. Domest. Anim. 55, 594–603. doi: 10.1111/rda.13658
Abedi E. and Sahari M. A. (2014). Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci Nutr. 2, 443–463. doi: 10.1002/fsn3.121
Abioja M. O., Apuu S., Daramola J. O., Wheto M., and Akinjute O. F. (2022). Semen quality and sperm characteristics in broiler breeder cockerels fed vitamin E during hot season. Acta Sci. Anim. Sci. 45, e56848. doi: 10.4025/actascianimsci.v44i1.56848
Adeleke B. S. and Babalola O. O. (2020). Oilseed crop sunflower (Helianthus annuus) as a source of food: Nutritional and health benefits. Food Sci Nutr. 8, 4666–4684. doi: 10.1002/fsn3.1783
Agarwal A., Gupta S., and Sharma R. K. (2005). Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 3-28. doi: 10.1186/1477-7827-3-28
Alagawany M., Elnesr S. S., Farag M. R., Abd El-Hack M. E., Khafaga A. F., Taha A. E., et al. (2019). Omega-3 and omega-6 fatty acids in poultry nutrition: effect on production performance and health. Animals 9, 573. doi: 10.3390/ani9080573
Al-Daraji H. J., Al-Mashada H. A., Al-Hayani W. K., Al-Hassani A. S., and Mirza H. A. (2010). Effect of n-3 and n-6 Fatty Acid Supplemented Diets on Semen Quality in Japanese Quail (Coturnix coturnix japonica). Int. J. Poultry Sci 9, 656–663. doi: 10.3923/ijps.2010.656.663
Amini M. R., Kohram H., Zare-Shahaneh A., Zhandi M., Sharideh H., and Nabi M. M. (2015). The effects of different levels of catalase and superoxide dismutase in modified Beltsville extender on rooster post-thawed sperm quality. Cryobiology 70, 226–232. doi: 10.1016/j.cryobiol.2015.03.001
Antinozzi C., Di Luigi L., Sireno L., Caporossi D., Dimauro I., and Sgrò P. (2025). Protective role of physical activity and antioxidant systems during spermatogenesis. Biomolecules 15, 478. doi: 10.3390/biom15040478
Arif A., Zahoor N., Tang J., Tang M., Dong L., Khan S. Z., et al. (2025). Cryopreservation strategies for poultry semen: A comprehensive review of techniques and applications. Veterinary Sci. 12, 145. doi: 10.3390/vetsci12020145
Aruwa C. E., Pillay C., Nyaga M. M., and Sabiu S. (2021). Poultry gut health – microbiome functions, environmental impacts, microbiome engineering and advancements in characterization technologies. J. Anim. Sci. Biotechnol. 12, 119. doi: 10.1186/s40104-021-00640-9
Attia A., Abou-Shehema B. M., Abdellah A. A., and Asmaa Sh. E.-N. (2020). EFFECT OF ASCORBIC ACID AND/OR ALPHA-TOCOPHEROL FORTIFICATION ON SEMEN QUALITY, METABOLIC PROFILE, ANTIOXIDANTS STATUS, AND DNA OF ROOSTERS EXPOSED TO HEAT STRESS. THE JAPS 30, 325–335. doi: 10.36899/JAPS.2020.2.0051
Authaida S., Ratchamak R., Boonkum W., and Chankitisakul V. (2023). Increasing sperm production and improving cryosurvival of semen in aged Thai native roosters as affected by selenium supplementation. Anim. Biosci. 36, 1647–1654. doi: 10.5713/ab.23.0079
Badwy M., El-Hadad E., and El-Weshahy O. (2024). Semen quality, sperm variables, blood profile, immunity, and antioxidant capacity of Sinai cockers fed diet supplemented with vitamin E or/and pumpkin seed oil. J. Anim. Poultry Production 0, 77–88. doi: 10.21608/jappmu.2024.278455.1113
Bailey R. L., Dodd K. W., Gahche J. J., Dwyer J. T., Cowan A. E., Jun S., et al. (2019). Best practices for dietary supplement assessment and estimation of total usual nutrient intakes in population-level research and monitoring. J. Nutr. 149, 181–197. doi: 10.1093/jn/nxy264
Balić A., Vlašić D., Žužul K., Marinović B., and Bukvić Mokos Z. (2020). Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. IJMS 21, 741. doi: 10.3390/ijms21030741
Baumber J., Ball B. A., Gravance C. G., Medina V., and Davies-Morel M. C. G. (2000). The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J. Androl. 21, 895–902. doi: 10.1002/j.1939-4640.2000.tb03420.x
Bilska K., Stuper-Szablewska K., Kulik T., Buśko M., Załuski D., Jurczak S., et al. (2018). Changes in phenylpropanoid and trichothecene production by fusarium culmorum and F. graminearum Sensu Stricto via Exposure to Flavonoids Toxins 10, 110. doi: 10.3390/toxins10030110
Bongalhardo D. C., Leeson S., and Buhr M. M. (2009). Dietary lipids differentially affect membranes from different areas of rooster sperm. Poultry Sci 88, 1060–1069. doi: 10.3382/ps.2008-00392
Bréque C., Surai P., and Brillard J. (2003). Roles of antioxidants on prolonged storage of avian spermatozoa in vivo and in vitro. Mol. Reprod. Devel. 66, 314–323. doi: 10.1002/mrd.10347
Cartoni Mancinelli A., Mattioli S., Twining C., Dal Bosco A., Donoghue A. M., Arsi K., et al. (2022). Poultry meat and eggs as an alternative source of n-3 long-chain polyunsaturated fatty acids for human nutrition. Nutrients 14, 1969. doi: 10.3390/nu14091969
Catala A. (2015). Lipid peroxidation modifies the assembly of biological membranes “The Lipid Whisker Model. Front. Physiol. 5. doi: 10.3389/fphys.2014.00520
Cerolini S., Zaniboni L., Maldjian A., and Gliozzi T. (2006). Effect of docosahexaenoic acid and α-tocopherol enrichment in chicken sperm on semen quality, sperm lipid composition and susceptibility to peroxidation. Theriogenology 66, 877–886. doi: 10.1016/j.theriogenology.2006.02.022
Chandimali N., Bak S. G., Park E. H., Lim H.-J., Won Y.-S., Kim E.-K., et al. (2025). Free radicals and their impact on health and antioxidant defenses: a review. Cell Death Discov. 11, 19. doi: 10.1038/s41420-024-02278-8
Clément C., Witschi U., and Kreuzer M. (2012). The potential influence of plant-based feed supplements on sperm quantity and quality in livestock: A review. Anim. Reprod. Sci 132, 1–10. doi: 10.1016/j.anireprosci.2012.04.002
Department of Forestry, Fisheries and the Environment (DFFE), van der Lingen C., and Dudley S. (2023). Status of the South African Marine Fishery Resources 2023 (Pretoria: Department of Forestry, Fisheries and the Environment). doi: 10.15493/DFFE.10000006
Du X., Qin F., Amevor F. K., Zhu Q., Shu G., Li D., et al. (2021). Rearing system influences the testicular development, semen quality and spermatogenic cell apoptosis of layer roosters. Poultry Sci 100, 101158. doi: 10.1016/j.psj.2021.101158
Dutta S., Majzoub A., and Agarwal A. (2019). Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 17, 87–97. doi: 10.1080/2090598X.2019.1599624
Dutta S., Sengupta P., Roychoudhury S., Chakravarthi S., Wang C. W., and Slama P. (2022). Antioxidant paradox in male infertility: ‘A blind eye’ on inflammation. Antioxidants 11, 167. doi: 10.3390/antiox11010167
Ebeid T. A. (2009). Organic selenium enhances the antioxidative status and quality of cockerel semen under high ambient temperature. Br. Poultry Sci 50, 641–647. doi: 10.1080/00071660903303415
Eslami M., Ghaniei A., and Mirzaei Rad H. (2016). Effect of the rooster semen enrichment with oleic acid on the quality of semen during chilled storage. Poultry Sci 95, 1418–1424. doi: 10.3382/ps/pew041
Esmaeili V., Shahverdi A. H., Moghadasian M. H., and Alizadeh A. R. (2015). Dietary fatty acids affect semen quality: a review. Andrology 3, 450–461. doi: 10.1111/andr.12024
Falchi L., Khalil W. A., Hassan M., and Marei W. F. A. (2018). Perspectives of nanotechnology in male fertility and sperm function. Int. J. Veterinary Sci Med. 6, 265–269. doi: 10.1016/j.ijvsm.2018.09.001
Feng Y., Ding Y., Liu J., Tian Y., Yang Y., Guan S., et al. (2015). Effects of dietary omega-3/omega-6 fatty acid ratios on reproduction in the young breeder rooster. BMC Vet. Res. 11, 73. doi: 10.1186/s12917-015-0394-9
Fleming S. D. and Thomson L. K. (2025). The oxidative stress of human sperm cryopreservation. Antioxidants 14, 402. doi: 10.3390/antiox14040402
Gandeshmin A. P., Sharafi M., and Alizadeh A. (2020). Enhancement of rooster semen freezing ability with the use of dietary sources of omega-3 and omega-6 fatty acids. Anim. Feed Sci Technol. 268, 114598. doi: 10.1016/j.anifeedsci.2020.114598
Gómez-Osorio L.-M., Vasiljevic M., Raj J., Chaparro-Gutierréz J. J., and López-Osorio S. (2024). Mycotoxins and coccidiosis in poultry – co-occurrence, interaction, and effects. Front. Vet. Sci. 11. doi: 10.3389/fvets.2024.1387856
Goyal A., Sharma V., Upadhyay N., Gill S., and Sihag M. (2014). Flax and flaxseed oil: an ancient medicine & modern functional food. J. Food Sci. Technol. 51, 1633–1653. doi: 10.1007/s13197-013-1247-9
Guedes A. C., Amaro H. M., Barbosa C. R., Pereira R. D., and Malcata F. X. (2011). Fatty acid composition of several wild microalgae and cyanobacteria, with a focus on eicosapentaenoic, docosahexaenoic and α-linolenic acids for eventual dietary uses. Food Res. Int. 44, 2721–2729. doi: 10.1016/j.foodres.2011.05.020
Gumede V. (2021). “Poverty in South Africa,” in The Oxford Handbook of the South African Economy. Eds. Oqubay A., Tregenna F., and Valodia I. (Mbombela: Oxford University Press), 157–174. doi: 10.1093/oxfordhb/9780192894199.013.8
Gwatkin R. (2012). Fertilization Mechanisms in Man and Mammals (Springer Science & Business Media), 163.
Halawa W., Khnissi S., Bensouf I., Bejaoui B., Chalouati H., Salman M., et al. (2025). Impact of semen extenders, storage duration, and insemination timing on semen quality and reproductive performance in Palestinian Assaf sheep. Vet. World 18, 808–818. doi: 10.14202/vetworld.2025.808-818
Hoppenbrouwers T., Cvejić Hogervorst J. H., Garssen J., Wichers H. J., and Willemsen L. E. M. (2019). Long chain polyunsaturated fatty acids (LCPUFAs) in the prevention of food allergy. Front. Immunol. 10. doi: 10.3389/fimmu.2019.01118
Huang P.-W., Yan C.-X., Sun X.-M., and Huang H. (2023). Economical downstream processing of microbial polyunsaturated fatty acids. Trends Biotechnol. 41, 857–859. doi: 10.1016/j.tibtech.2023.01.001
Hudson B. P. and Wilson J. L. (2003). Effects of dietary menhaden oil on fertility and sperm quality of broiler breeder males. J. Appl. Poultry Res. 12, 341–347. doi: 10.1093/japr/12.3.341
Jena A. B., Samal R. R., Bhol N. K., and Duttaroy A. K. (2023). Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 162, 114606. doi: 10.1016/j.biopha.2023.114606
Jeong H. Y., Moon Y. S., and Cho K. K. (2024). ω-6 and ω-3 polyunsaturated fatty acids: inflammation, obesity and foods of animal resources. Food Sci. Anim. Resour. 44, 988–1010. doi: 10.5851/kosfa.2024.e65
Kaltsas A. (2023). Oxidative stress and male infertility: the protective role of antioxidants. Medicina 59, 1769. doi: 10.3390/medicina59101769
Kartikasari L. R., Hughes R. J., Geier M. S., Makrides M., and Gibson R. A. (2012). Dietary alpha-linolenic acid enhances omega-3 long chain polyunsaturated fatty acid levels in chicken tissues. Prostaglandins Leukotrienes Essential Fatty Acids 87, 103–109. doi: 10.1016/j.plefa.2012.07.005
Khang N. T. K., Suong N. T. M., and Phuong L. T. (2022). Effects of vitamin E supplement and semen collection time on sperm quality of locally Noi crossbred cocks. MSJ 4, 1–8. doi: 10.31893/multiscience.2022018
Kodali S. T., Kauffman P., Kotha S. R., Yenigalla A., Veeraraghavan R., Pannu S. R., et al. (2020). “Oxidative lipidomics: analysis of oxidized lipids and lipid peroxidation in biological systems with relevance to health and disease,” in Measuring oxidants and oxidative stress in biological systems. Eds. Berliner L. J. and Parinandi N. L. (Springer, Cham (CH). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK566435/.
Kowalczyk A. (2022). The role of the natural antioxidant mechanism in sperm cells. Reprod. Sci. 29, 1387–1394. doi: 10.1007/s43032-021-00795-w
Lee S.-M., Kim H. K., Lee H.-B., Kwon O.-D., Lee E.-B., Bok J.-D., et al. (2021). Effects of flaxseed supplementation on omega-6 to omega-3 fatty acid ratio, lipid mediator profile, proinflammatory cytokines and stress indices in laying hens. J. Appl. Anim. Res. 49, 460–471. doi: 10.1080/09712119.2021.2000416
Lin Y. F., Chang S. J., Yang J. R., Lee Y. P., and Hsu A. L. (2005). Effects of supplemental vitamin E during the mature period on the reproduction performance of Taiwan Native Chicken cockerels. Br. Poultry Sci 46, 366–373. doi: 10.1080/00071660500098186
Long J. and Kramer M. (2003). Effect of vitamin E on lipid peroxidation and fertility after artificial insemination with liquid-stored Turkey semen. Poultry Sci 82, 1802–1807. doi: 10.1093/ps/82.11.1802
Maapola R. R., Nephawe K. A., Nedambale T. L., Nedambale T. L., and Ramukhithi F. V. (2023). Evaluation of Japanese quail egg-yolk extender in cryopreservation of cockerel semen. Am. J. Anim. Veterinary Sci. 18, 74–80. doi: 10.3844/ajavsp.2023.74.80
Mahaffey K. R., Sunderland E. M., Chan H. M., Choi A. L., Grandjean P., Mariën K., et al. (2011). Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption: Nutrition Reviews©. Nutr. Rev. 69, 493–508. doi: 10.1111/j.1753-4887.2011.00415.x
Manful C. F., Fordjour E., Subramaniam D., Sey A. A., Abbey L., and Thomas R. (2025). Antioxidants and reactive oxygen species: shaping human health and disease outcomes. IJMS 26, 7520. doi: 10.3390/ijms26157520
Marsol-Vall A., Aitta E., Guo Z., and Yang B. (2022). Green technologies for production of oils rich in n-3 polyunsaturated fatty acids from aquatic sources. Crit. Rev. Food Sci Nutr. 62, 2942–2962. doi: 10.1080/10408398.2020.1861426
Mehaisen G. M. K., Partyka A., Ligocka Z., and Niżański W. (2020). Cryoprotective effect of melatonin supplementation on post-thawed rooster sperm quality. Anim. Reprod. Sci 212, 106238. doi: 10.1016/j.anireprosci.2019.106238
Mfoundou J. D. L., Guo Y., Yan Z., and Wang X. (2022). Morpho-histology and morphometry of chicken testes and seminiferous tubules among yellow-feathered broilers of different ages. Veterinary Sci. 9, 485. doi: 10.3390/vetsci9090485
Miles E. A., Childs C. E., and Calder P. C. (2021). Long-chain polyunsaturated fatty acids (LCPUFAs) and the developing immune system: A narrative review. Nutrients 13, 247. doi: 10.3390/nu13010247
Min Y., Sun T., Niu Z., and Liu F. (2016). Vitamin C and vitamin E supplementation alleviates oxidative stress induced by dexamethasone and improves fertility of breeder roosters. Anim. Reprod. Sci 171, 1–6. doi: 10.1016/j.anireprosci.2016.04.005
Mishra S. K., Belur P. D., Chandrasekar V., and Iyyaswami R. (2023). Designing of A synergistic mixture of natural antioxidants through statistical approaches for enhancing the oxidative stability of sardine oil. Curr. Res. Nutr. Food Sci. 11, 1166–1176. doi: 10.12944/CRNFSJ.11.3.21
Mora I., Pérez-Santamaria A., Tortajada-Pérez J., Vázquez-Manrique R. P., Arola L., and Puiggròs F. (2023). Structured docosahexaenoic acid (DHA) enhances motility and promotes the antioxidant capacity of aged C. elegans. Cells 12, 1932. doi: 10.3390/cells12151932
Moszak M., Szulińska M., and Bogdański P. (2020). You are what you eat—The relationship between diet, microbiota, and metabolic disorders—A review. Nutrients 12, 1096. doi: 10.3390/nu12041096
Mousa S. A., Abdel-Rahe S. M., Abdel-Rahe H. A., and Sadeek A. L. S. (2017). Effect of dietary fat sources and antioxidant types on growth performance and carcass quality of Japanese quails. Int. J. Poultry Sci 16, 443–450. doi: 10.3923/ijps.2017.443.450
Mucksová J., Brillard J. P., Hejnar J., Poplštein M., Kalina J., Bakst M., et al. (2009). Identification of various testicular cell populations in pubertal and adult cockerels. Anim. Reprod. Sci 114, 415–422. doi: 10.1016/j.anireprosci.2008.10.016
Murugesan G. R., Ledoux D. R., Naehrer K., Berthiller F., Applegate T. J., Grenier B., et al. (2015). Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poultry Sci 94, 1298–1315. doi: 10.3382/ps/pev075
Naeem M. and Bourassa D. (2025). Probiotics in poultry: unlocking productivity through microbiome modulation and gut health. Microorganisms 13, 257. doi: 10.3390/microorganisms13020257
Ngcobo J. N., Egerszegi I., and Nephawe K. A. (2025). Recent advances in understanding the impact of environmental heat stress on sheep production and reproductive performance: A subtropical climate perspective. Climate 13, 130. doi: 10.3390/cli13060130
Ngcobo J. N., Nedambale T. L., Nephawe K. A., Sithole S. M., Chokoe T. C., and Ramukhithi F. V. (2024). Dietary supplementing South African indigenous rams with flaxseed oil and ascorbic acid improves cryopreserved semen quality and in vitro fertility. Trop. Anim. Health Prod. 56, 200. doi: 10.1007/s11250-024-04057-0
Ngcobo J. N., Ramukhithi F. V., Nephawe K. A., Mpofu T. J., Chokoe T. C., and Nedambale T. L. (2021). Flaxseed oil as a source of omega n-3 fatty acids to improve semen quality from livestock animals: A review. Animals 11, 3395. doi: 10.3390/ani11123395
Nguyen D. V., Malau-Aduli B. S., Cavalieri J., Nichols P. D., and Malau-Aduli A. E. O. (2018). Supplementation with plant-derived oils rich in omega-3 polyunsaturated fatty acids for lamb production. Veterinary Anim. Sci 6, 29–40. doi: 10.1016/j.vas.2018.08.001
Oduwole O. O., Peltoketo H., and Huhtaniemi I. T. (2018). Role of follicle-stimulating hormone in spermatogenesis. Front. Endocrinol. 9. doi: 10.3389/fendo.2018.00763
Oke O. E., Akosile O. A., Oni A. I., Opowoye I. O., Ishola C. A., Adebiyi J. O., et al. (2024). Oxidative stress in poultry production. Poultry Sci 103, 104003. doi: 10.1016/j.psj.2024.104003
Olariu R. M., Fiţ N. I., Bouari C. M., and Nadăş G. C. (2025). Mycotoxins in broiler production: impacts on growth, immunity, vaccine efficacy, and food safety. Toxins 17, 261. doi: 10.3390/toxins17060261
Olubowale O. S., Greyling J. P. C., deWitt F. H., Hugo A., and Raito M. B. (2014). Effect of dietary lipid sources on the quality of Hy-line silver-brown cockerels. JAPS: J Anim. Health Plant Sci. 24 (4)
Ommati M. M., Zamiri M. J., Akhlaghi A., Atashi H., Jafarzadeh M. R., Rezvani M. R., et al. (2013). Seminal characteristics, sperm fatty acids, and blood biochemical attributes in breeder roosters orally administered with sage (Salvia officinalis) extract. Anim. Prod. Sci. 53, 548. doi: 10.1071/AN12257
Panner Selvam M. K., Baskaran S., and Agarwal A. (2019). “Proteomics of reproduction: Prospects and perspectives,” in Advances in Clinical Chemistry (US: Elsevier), 217–243. doi: 10.1016/bs.acc.2019.04.005
Parry J. and Yu L. (2004). Fatty acid content and antioxidant properties of cold-pressed black raspberry seed oil and meal. J. Food Sci 69, FCT189-FCT193. doi: 10.1111/j.1365-2621.2004.tb13356.x
Partyka A., Łukaszewicz E., and Niżański W. (2012). Effect of cryopreservation on sperm parameters, lipid peroxidation and antioxidant enzymes activity in fowl semen. Theriogenology 77, 1497–1504. doi: 10.1016/j.theriogenology.2011.11.006
Partyka A. and Niżański W. (2021). Supplementation of avian semen extenders with antioxidants to improve semen quality—Is it an effective strategy? Antioxidants 10, 1927. doi: 10.3390/antiox10121927
Partyka A., Rodak O., Bajzert J., Kochan J., and Niżański W. (2017). The effect of L-carnitine, hypotaurine, and taurine supplementation on the quality of cryopreserved chicken semen. BioMed. Res. Int. 2017, 1–8. doi: 10.1155/2017/7279341
Pourazadi L., Sharafi M., Torshizi M. A. K., Shahverdi A., and Alizadeh A. (2020). Peroxisome proliferator-activated receptors (PPARs) as a mediator of dietary fatty acids affects reproductive performance in broiler breeder roosters. Theriogenology 158, 331–338. doi: 10.1016/j.theriogenology.2020.09.020
Qamar A. Y., Naveed M. I., Raza S., Fang X., Roy P. K., Bang S., et al. (2023). Role of antioxidants in fertility preservation of sperm — A narrative review. Anim. Biosci. 36, 385–403. doi: 10.5713/ab.22.0325
Qi X., Shang M., Chen C., Chen Y., Hua J., Sheng X., et al. (2019). Dietary supplementation with linseed oil improves semen quality, reproductive hormone, gene and protein expression related to testosterone synthesis in aging layer breeder roosters. Theriogenology 131, 9–15. doi: 10.1016/j.theriogenology.2019.03.016
Rizzo G., Baroni L., and Lombardo M. (2023). Promising sources of plant-derived polyunsaturated fatty acids: A narrative review. Int. J. Environ. Res. Public Health 20, 1683. doi: 10.3390/ijerph20031683
Rutllant J. and Khamas W. (2024). “Reproductive System,” in Anatomy and Histology of the Domestic Chicken (John Wiley & Sons, Ltd), 93–108. doi: 10.1002/9781119841739.ch7
Saadeldin I. M., Khalil W. A., Alharbi M. G., and Lee S. H. (2020). The current trends in using nanoparticles, liposomes, and exosomes for semen cryopreservation. Animals 10, 2281. doi: 10.3390/ani10122281
Safa S., Moghaddam G., Jozani R. J., Daghigh Kia H., and Janmohammadi H. (2016). Effect of vitamin E and selenium nanoparticles on post-thaw variables and oxidative status of rooster semen. Anim. Reprod. Sci 174, 100–106. doi: 10.1016/j.anireprosci.2016.09.011
Saleh R. A. and Hcld A. A. (2002). Oxidative stress and male infertility: from research bench to clinical practice. J. Androl. 23, 737–752. doi: 10.1002/j.1939-4640.2002.tb02324.x
Salimi T., Hajarian H., Karamishabankareh H., and Soltani L. (2024). Effects of sodium selenite, cysteamine, bacterially synthesized Se-NPs, and cysteamine loaded on Se-NPs on ram sperm cryopreservation. Sci. Rep. 14, 852. doi: 10.1038/s41598-023-50221-1
Santi D., Crépieux P., Reiter E., Spaggiari G., Brigante G., Casarini L., et al. (2020). Follicle-stimulating hormone (FSH) action on spermatogenesis: A focus on physiological and therapeutic roles. JCM 9, 1014. doi: 10.3390/jcm9041014
Sengupta P., Pinggera G., Calogero A. E., and Agarwal A. (2024). Oxidative stress affects sperm health and fertility—Time to apply facts learned at the bench to help the patient: Lessons for busy clinicians. Reprod. Med. Biol. 23, e12598. doi: 10.1002/rmb2.12598
Shah W., Khan R., Shah B., Khan A., Dil S., Liu W., et al. (2021). The molecular mechanism of sex hormones on sertoli cell development and proliferation. Front. Endocrinol. 12. doi: 10.3389/fendo.2021.648141
Shamiah S., Abd El-Karim R. E., Eshera A. A. M., Fouda S. F., and Zaghloul H. K. (2017). Effects of dietary selenomethione supplementation on semen quality, fertility and antioxidant status of cockerels. Egyptian J. Nutr. Feeds 20, 227–236. doi: 10.21608/ejnf.2017.104119
Sidhu K. S. (2003). Health benefits and potential risks related to consumption of fish or fish oil. Regul. Toxicol. Pharmacol. 38, 336–344. doi: 10.1016/j.yrtph.2003.07.002
Silyukova Y., Fedorova E., and Stanishevskaya O. (2022). Influence of technological stages of preparation of rooster semen for short-term and long-term storage on its quality characteristics. CIMB 44, 5531–5542. doi: 10.3390/cimb44110374
Sithole S. M., Nephawe K. A., Mpofu T. J., Mtileni B., Mphaphathi M. L., and Ngcobo J. N. (2025). Extinction status, challenges, and conservation approaches of South African indigenous and village chickens: A systematic review. Agriculture 15, 216. doi: 10.3390/agriculture15020216
Surai P., Kostjuk I., Wishart G., Macpherson A., Speake B., Noble R., et al. (1998). Effect of vitamin E and selenium supplementation of cockerel diets on glutathione peroxidase activity and lipid peroxidation susceptibility in sperm, testes, and liver. Biol. Trace Elem Res. 64, 119–132. doi: 10.1007/BF02783329
Tabatabaei S. and Aghaei A. (2012). Effect of l-carnitine on sperm quality during liquid storage of chicken semen. Comp. Clin. Pathol. 21, 711–717. doi: 10.1007/s00580-010-1163-6
Thananurak P., Chuaychu-noo N., Thélie A., Phasuk Y., Vongpralub T., and Blesbois E. (2020). Different concentrations of cysteamine, ergothioneine, and serine modulate quality and fertilizing ability of cryopreserved chicken sperm. Poultry Sci 99, 1185–1198. doi: 10.1016/j.psj.2019.10.040
Tiwari S., Dewry R. K., Srivastava R., Nath S., and Mohanty T. K. (2022). Targeted antioxidant delivery modulates mitochondrial functions, ameliorates oxidative stress and preserve sperm quality during cryopreservation. Theriogenology 179, 22–31. doi: 10.1016/j.theriogenology.2021.11.013
Vizcarra J. A., Kirby J. D., and Kreider D. L. (2010). Testis development and gonadotropin secretion in broiler breeder males. Poultry Sci 89, 328–334. doi: 10.3382/ps.2009-00286
Wadood A. A. (2024). The omics revolution in understanding chicken reproduction: A comprehensive review (Marylang, US: aithor.com). Available online at: https://aithor.com/paper-summary/the-omics-revolution-in-understanding-chicken-reproduction-a-comprehensive-review.
Wang Y., Fu X., and Li H. (2025). Mechanisms of oxidative stress-induced sperm dysfunction. Front. Endocrinol. 16. doi: 10.3389/fendo.2025.1520835
Woods V. B. and Fearon A. M. (2009). Dietary sources of unsaturated fatty acids for animals and their transfer into meat, milk and eggs: A review. Livestock Sci 126, 1–20. doi: 10.1016/j.livsci.2009.07.002
Wu S.-T., Yu S.-T., and Lin L.-P. (2005). Effect of culture conditions on docosahexaenoic acid production by Schizochytrium sp. S31. Process Biochem. 40, 3103–3108. doi: 10.1016/j.procbio.2005.03.007
Yu L., Choe U., Li Y., and Zhang Y. (2020). “Oils from Fruit, Spice, and Herb Seeds,” in Bailey’s Industrial Oil and Fat Products. Ed. Shahidi F. (Wiley), 1–35. doi: 10.1002/047167849X.bio060.pub2
Yuan C., Wang J., and Lu W. (2023). Regulation of semen quality by fatty acids in diets, extender, and semen. Front. Vet. Sci. 10. doi: 10.3389/fvets.2023.1119153
Yue Y., Luasiri P., Li J., Laosam P., and Sangsawad P. (2024). Research advancements on the diversity and host interaction of gut microbiota in chickens. Front. Vet. Sci. 11. doi: 10.3389/fvets.2024.1492545
Zamani Ghaleshahi A., Ezzatpanah H., Rajabzadeh Gh, and Ghavami M. (2020). Comparison and analysis characteristics of flax, perilla and basil seed oils cultivated in Iran. J. Food Sci. Technol. 57, 1258–1268. doi: 10.1007/s13197-019-04158-x
Zaniboni L., Rizzi R., and Cerolini S. (2006). Combined effect of DHA and α-tocopherol enrichment on sperm quality and fertility in the Turkey. Theriogenology 65, 1813–1827. doi: 10.1016/j.theriogenology.2005.10.013
Zanussi H. P., Shariatmadari F., Sharafi M., and Ahmadi H. (2019). Dietary supplementation with flaxseed oil as source of Omega-3 fatty acids improves seminal quality and reproductive performance in aged broiler breeder roosters. Theriogenology 130, 41–48. doi: 10.1016/j.theriogenology.2019.02.030
Keywords: omega n-3, omega n-6, reactive oxygen species, fertility, sperm, SDGs
Citation: Sithole SM, Nephawe KA, Mphaphathi ML and Ngcobo JN (2025) Understanding the role of long-chain polyunsaturated fatty acid and antioxidants in enhancing rooster semen quality: a comprehensive review. Front. Anim. Sci. 6:1669519. doi: 10.3389/fanim.2025.1669519
Received: 19 July 2025; Accepted: 04 September 2025;
Published: 18 September 2025.
Edited by:
Muhammet Rasit Ugur, IVF Michigan Fertility Centers, United StatesReviewed by:
Tlou Christopher Kujoana, University of South Africa, South AfricaMilan Maletic, University of Belgrade, Serbia
Copyright © 2025 Sithole, Nephawe, Mphaphathi and Ngcobo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sindisiwe Mbali Sithole, c2l0aG9sZXNtQGFyYy5hZ3JpYy56YQ==
 Sindisiwe Mbali Sithole
Sindisiwe Mbali Sithole Khathutshelo Agree Nephawe
Khathutshelo Agree Nephawe Masindi Lottus Mphaphathi2
Masindi Lottus Mphaphathi2 Jabulani Nkululeko Ngcobo
Jabulani Nkululeko Ngcobo