- 1Department of Agricultural and Food Sciences (DISTAL), University of Bologna, Bologna, Italy
- 2Department of Agricultural and Food Sciences (DISTAL), University of Bologna, Cesena, Italy
The environmental sustainability of feed ingredients, including water use, plays a central role in reducing the footprint of pig production. Sorghum, due to its nutritional value and tolerance to hydric stress, can represent a valid alternative to corn. This study aims to provide a comprehensive evaluation of sorghum, as a complete replacement for corn in post-weaning piglet diets, uniquely applying an integrated multi-omics approach (microbiota 16S rRNA sequencing and 1H-NMR metabolomics) to investigate effects on growth performance and gut health. At weaning (d0), 522 pigs were assigned to one of 3 diets: Control (CTR), sorghum (SO), and extruded sorghum (EX_SO). At d0, d7 and d28 pigs were weighed, ear and tail lesions were scored and fecal samples collected for microbiota, metabolomics, and dry matter (DM) analysis. Feed intake (FI), behavior measurements (BMs) and environmental gases were recorded on d7, d14 and d28. The diet did not affect the BW (p>0.10), average daily gain (ADG; p>0.10) and FI. At d28, the feed to gain ratio (p=0.09) and the fecal DM (p=0.08) tended to be 4.1% lower and 22% higher respectively in the SO group compared with the CTR. Lesions and BMs did not differ. At d7, Oscillospira (p<0.01), was more abundant in the CTR than EX_SO; 2-Oxogutarate, Acetoin, 2-Methyl 3-Ketovalerate, 3-Methyl 2-Oxovalerate were more abundant in the EX_SO than CTR. At d28, the diet affected the beta-diversity (p=0.05); Olsenella was more abundant in the CTR than in the SO (p<0.01), Olsenella (p=0.01) and Ligilactobacillus (p=0.01) were more abundant, while Anaerovoracaceae_Family XIII UCG-001 (p=0.01) was less abundant in the CTR group than EX_SO; Shuttleworthia (p=0.02) and Syntrophococcus (p<0.01) were more abundant in the SO than in the EX_SO; butyrate, propionate and valerate were more abundant in the EX_SO than CTR (p<0.05). In conclusion, this study demonstrates that the complete replacement of corn with sorghum (12% total inclusion) does not impair post-weaning piglet performance, health and welfare under commercial conditions. The extrusion process promoted the modulation of microbial activity and metabolites in large intestine. Results support sorghum as a sustainable alternative; further studies should assess resistant starch and integrate multi-omics.
1 Introduction
The growing issue of desertification in various regions worldwide poses a significant threat to the sustainability of animal production systems. As water resources become increasingly scarcer, optimizing their use in agriculture and livestock becomes an essential priority (FAO, 2018). In this context, the environmental sustainability of feed ingredients plays a central role in reducing the ecological footprint of animal production, as feed accounts for the largest share of resource consumption (Mottet et al., 2017). In swine nutrition, both the environmental footprint and the blue water consumption are strongly influenced by the type and origin of the raw materials used in feed formulations. Among the cereals, corn is one of the most widely used ones in pig diets, but it is responsible for approximately 21% of total blue water consumption (Sporchia et al., 2021). In response to these concerns, and in alignment with the European Union’s environmental policies, such as the Green Deal and the “Farm to Fork” strategy, there is growing interest in identifying more sustainable alternatives to conventional feed ingredients, including corn (European Commission, 2020). Sorghum (Sorghum vulgare) is a cereal of considerable interest due to its remarkable tolerance of hydric stress and adaptability to arid and semi-arid climates. It offers both environmental and economic advantages (Amelework et al., 2015). Moreover, from a nutritional perspective, sorghum is nutritionally rich in starch (~60–70%) and provides a moderate protein content (~90–115 g/kg), with protein digestibility coefficients that vary by animal species and grain processing but can reach up to 0.84 (Torres Cepeda et al., 1996; Mariscal-Landín et al., 2010). These features suggest that sorghum could be a viable substitute for corn in pig’ diets. Its large inclusion in the pigs’ diet is quite acceptable for growing and finishing pigs (Stein et al., 2016; Benz et al., 2011). However, the inclusion of sorghum in post-weaning diet is uncommon and has produced variable results in terms of performance. While some studies report that replacing corn with sorghum (at levels between 30–61%) can support similar growth rates in weaning piglets (Rodrigues et al., 2016), others indicate a reduction in performance at higher inclusion levels, likely due to of anti-nutritional factors presence the sorghum depending on cultivar (Pan et al., 2021; Thomas et al., 2020). To date, advances in sorghum breeding have produced lower-tannin cultivars (Hodges et al., 2021). Currently, in the European Union, legislation requires that sorghum cultivars contain less than 0.4% tannins to be included as feed ingredients (European Commission, 2000).
Technological treatments, such as extrusion, have been suggested as a way to mitigate the negative effects of antinutritional factors in cultivars that are rich in them. Additionally, the extrusion process is generally recognized for improving digestibility by modifying the starch structure and enhancing feed palatability (Fang et al., 2025; Rodrigues et al., 2016; Liu et al., 2013; Chae et al., 2000). Rodriguez et al. (2020) found that extruded sorghum improved the digestibility of gross energy, digestible and metabolizable energy, starch, and crude protein, although these improvements did not always translate into better growth or feed conversion ratios. However, other studies do not report significant improvements in sorghum digestibility due to the extrusion process (Chae et al., 2000). Consequently, the impact of extrusion on the nutritional value of sorghum remains unclear. An adequately controlled extrusion process, particularly during the cooling phase, disrupts the starch-protein matrix, promotes starch gelatinization, and limits retrogradation, thereby increasing enzymatic access to starch and protein and improving digestibility (Yadav et al., 2022). By contrast, suboptimal cooling (for example, a low target temperature or a slow cooling rate), promotes starch retrogradation. This increases of resistant starch levels, leading to a concomitant reduction in small-intestinal digestibility (Xie et al., 2014).
Taken together, these findings reinforce the feasibility of incorporating sorghum into post-weaning piglet diets as part of a broader strategy aimed at enhancing the sustainability and resilience of swine production. However, inconsistent results across studies (Liu et al., 2013; Chae et al., 2000) and the fragile intestinal health of piglets during the early post-weaning period still restrict the use of sorghum in post-weaning diets.
Moreover, the majority of the studies have focused on growth performance outcomes, with far less attention given to the gut microbiota–metabolite axis, which is increasingly recognized as a key determinant of post-weaning piglet health (Chen et al., 2025). The early post-weaning period is particularly critical, as intestinal immaturity and environmental stressors make piglets more vulnerable to gut dysbiosis, metabolic imbalances, and reduced performance (Zheng et al., 2024). A deeper understanding of how dietary sorghum can influence the microbial communities, its metabolism, and derived metabolites is essential to evaluate its suitability as a sustainable corn substitute.
We hypothesize that sorghum will not negatively impact the performance of piglets compared to corn during the post-weaning phase. Additionally, we hypothesize that the extrusion process will support the intestinal health and well-being of piglets by improving nutrient digestibility and modulating the gut microbiota and its metabolism.
Therefore, the present study aims to evaluate the effects of replacing corn with sorghum, both non-extruded and extruded, in a post-weaning piglet diet on growth performance, gut health using a multi-omics approach and animal welfare.
2 Materials and methods
2.1 Experimental design
The procedures complied with the Italian law pertaining to experimental animals and were approved by the Ethic- Scientific Committee for Experiments on Animals of the University of Bologna (Trial ID 4796, Prot. n. 218339/2024).
The study was performed at a commercial farm located in northern Italy. A total of 522 weaned piglets from a single farrowing batch were enrolled in the study. At weaning (d0), piglets were selected based on body weight (BW: 6.63 ± 1.091 kg), age (28 ± 2.1 days) and litter origin. They were individually identified with numbered ear tags, and allocated to one of the following dietary treatments, with four replicates (pens) per treatment: Control (CTR), Sorghum (SO), or Extruded Sorghum (EX_SO). Allocation was performed using a randomized block design based on initial BW, age, and litter of origin to ensure balanced mean weights, age and same litters representation across groups at the beginning of the study. The pens provided a space allowance of 0.31 ± 0.01 m² per piglet.
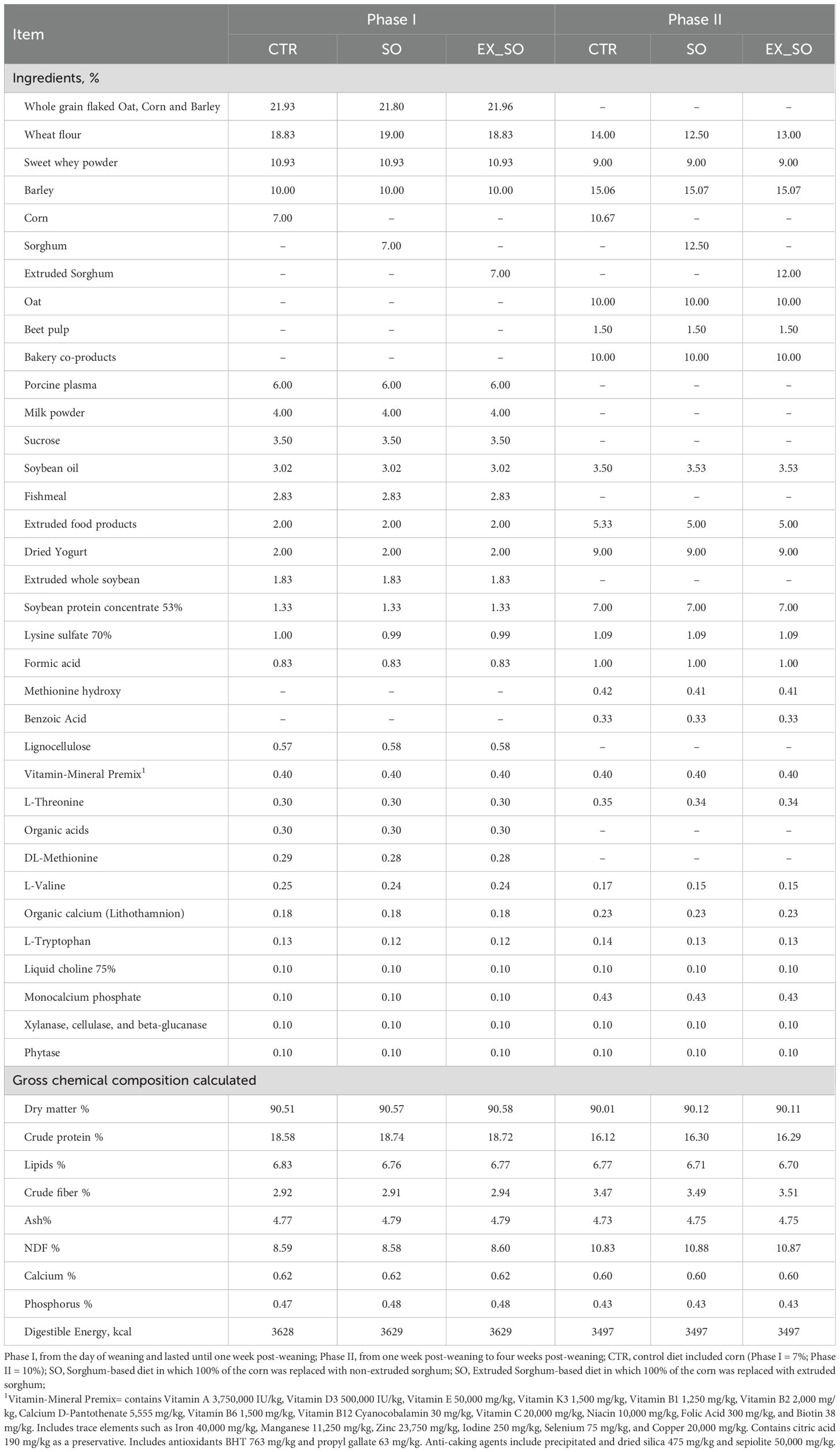
Piglets had a two-phase feeding program: phase I started on the day of weaning (d0) and lasted until one-week post-weaning (d7), while phase II spanned from d7 to four weeks post-weaning (d28). Throughout all feeding phases, diets were provided in pelleted form. The CTR diet included corn (Phase I = 7%; Phase II = 10%). The SO and EX_SO diets replaced 100% of the corn with non-extruded and extruded sorghum, respectively. Table 1 presents the inclusion levels of raw materials and the nutrient composition of experimental diets. Diets were isoenergetic and iso amino acidic (within each phase) and formulated to meet the nutritional requirements of the piglets at the different stages of growth (NRC, 2012). The piglets had ad libitum access to water and feed throughout the entire duration of the experimental trial.
Piglets were weighed individually on days d0, d7 and d28, and the ADG was calculated. On d7, d14 and d28, the amount of feed delivered, and feed residues were recorded to estimate the feed intake (FI) per each pen and the Feed-to-Gain ratio (F:G) were calculated.
Two types of animal-based measures (ABMs) were assessed: behavioral measures (BMs) and ear and tail lesions. Both were adapted from Vitali et al. (2020), Amatucci et al. (2023), Palumbo et al. (2023, 2025), the Welfare Quality® protocol (2009), and the Classyfarm® protocol (2019). The evaluation of BMs was conducted on the same days as FI recordings, whereas the assessment of ear and tail lesions was performed on d7 and d28. In brief, BMs were evaluated in the morning, when the animals were more active, between 10:00 am and 11:00 am. The weaned pigs were observed directly in each pen five times, with a two-minute interval between each scan. Before the initial observation, if needed, the assessor clapped their hands to prompt all animals to stand, and after five minutes, the pigs could be observed. The behaviors recorded included two types of observations: abnormal behaviors (such as suckling) and the following behavior categories defined in Welfare Quality® (2009):
1. Social behavior, divided into “negative social behavior,” which includes aggressive interactions, and “positive social behavior,” which includes actions such as sniffing, nosing, licking, playing, and gently moving away from another animal without any aggression or fighting.
2. Exploratory behavior, divided into “exploring the pen,” which includes sniffing, nosing, and licking the surroundings, and “exploring enrichment material,” which involves playing with straw or other enrichment materials. Animals not displaying social or exploratory behaviors were recorded as “resting” when they were inactive and lying down, or as “other” for activities such as eating or drinking. The frequency of each behavior was then expressed as the percentage (%) of the average of the five observations of the animals exhibiting the behavior over the total of animals in the pen:
Tail lesion scores ranged from 0 to 2: 0 = no injuries; 1 = superficial bite along the tail with no evidence of swelling; 2 = presence of scarring, visible open lesion, swelling, or partial absence of the tail, according to the method proposed by WelfareQuality® (2009). For ear lesions, meaning any visible lesion observed on the ears of each pig, the scoring was as follows: 0 = up to 4 visible lesions; 1 = 5 to 10 visible lesions; 2 = 11 to 15 visible lesions, as described in the Welfare Quality® (2009) method. The results for each pen were expressed as the prevalence of the scores obtained (0, 1, 2), representing the proportion of animals assigned to each category. A lesion score index (LSI) was then calculated applying the following formula:
The LSI index considers both the frequency and severity of the lesions, ranging from 0 to 200 (where 0 represents the absence of lesions and 200 represents all animals with severe lesions).
On d7 and d28, a total of 45 fecal samples per timepoint (15 pigs/group) were collected from the same piglets using rectal swabs stored into a sterile tube. The samples were snap frozen in liquid nitrogen and then stored at -80 °C for subsequent bacterial DNA extraction (V3-V4 16S rRNA for microbiota profiling), metabolomic profile analysis, and dry matter content The concentration of air ammonia (NH3) and carbon dioxide (CO2) gases were assessed on the same days as ABMs were recorded, using a XAM8000 Multigas Detector (Dräger, Lübeck, Germany). The instrument was calibrated prior to each measurement session according to the manufacturer’s specifications to ensure data accuracy. According to the method of Palumbo et al. (2023) and Palumbo et al. (2025), each gas was measured at the pigs’ level (approximately 30 cm above the ground) at three locations per pen: the corner closest to the center of the room, the middle of the pen, and the opposite corner near the external wall. Measurements were taken with the probe held steadily at each location for approximately 10–15 seconds, allowing for stabilization of sensor readings before recording the values.
2.2 Laboratory analysis
2.2.1 Quantification of dry matter in feces
The dry matter content of the frozen fecal samples was determined after freeze-drying for 72 hours. Results were expressed as the percentage (%) of dry matter over the total weight of fresh fecal sample freeze dried.
2.2.2 Proton nuclear magnetic resonance spectroscopy analysis for identifying metabolomic profile
The metabolomic profiles of the feces were prepared for proton nuclear magnetic resonance (1H-NMR) analysis by vortex-mixing 80 mg of each sample with 1mL of deionized water, followed by centrifugation for 10 min at 18,630 g and 4 °C. A total of 700 uL of supernatant were added to 100 uL of D2O solution of 2,2,3,3-d4-3-(trimethylsilyl)-propionic acid sodium salt (TSP) 10mM, used as NMR chemical-shift reference, buffered at pH 7.00 ± 0.02 by means of 1M phosphate buffer. 10 uL of NaN3 (2 mmol/L) was also added to avoid microbial proliferation. Finally, the sample was centrifuged again at the above conditions. 1H-NMR spectra were recorded at 298 K with an AVANCE III spectrometer (Bruker, Milan, Italy) operating at a frequency of 600.13 MHz, following the procedure described by Virdis et al. (2024). The characterization the molecular profile was done in Chenomx software (Chenomx Inc., Canada, ver 8.3) by means of comparisons with Chenomx’s (ver. 10) and HMDB’s (release 2) databases. The quantification of the molecules in absolute terms was done in one reference sample, by relying on TSP as internal standard. Probabilistic quotient normalization (PQN) (Dieterle doi: 10.1021/ac051632c) was employed to adjust any other spectrum towards the reference, to compensate for differences in insoluble solids and water. Each molecule’s concentration was calculated by considering the area of one of its signals, measured by global spectra deconvolution, implemented in MestReNova software (Mestrelab research S.L. Santiago De Compostela (Spain) - ver 14.2.0-26256). This was done after the application of a line broadening of 0.3 and after having adjusted the baseline by Whittaker Smoother procedure.
2.2.3 Fecal microbiota analysis
Total bacterial DNA was isolated and extracted using the FastDNA SPIN kit (MP Biomedicals, Santa Ana, CA, USA). The concentration and purity of the extracted bacterial DNA were evaluated with a NanoDrop spectrophotometer (Fisher Scientific, Schwerte, Germany) by measuring the absorbance ratios at 260/280 and 260/230. The V3–V4 regions of the 16S rRNA gene (~460 bp) were amplified, and amplicons were generated using the universal primers (Takahashi et al., 2014). Amplification was performed using the Platinum™ Taq DNA Polymerase High Fidelity (Thermo Fisher Scientific, Monza, Italy), and the resulting amplicons were sequenced on the Illumina MiSeq platform (300 × 2 bp). Library preparation followed the standard protocol for the MiSeq Reagent Kit V3, and sequencing was conducted using the MiSeq platform (Illumina Inc., San Diego, CA, USA).
2.3 Statistical and bioinformatic analysis
Statistical analyses and bioinformatic analyses were performed out using RStudio (RStudio, PBC, Boston, MA, USA). Prior to model fitting, the assumption of normality for the dependent variable was assessed visually using a quantile–quantile plot (qqnorm) and statistically using the Shapiro–Wilk test. Data on individual piglet performance (BW and ADG), fecal NH3, and dry matter were analyzed using the “lme4” function from the homonymous R package with a linear mixed-effects model and an ANOVA model. The experimental diet (CTR vs. SO vs. EX_SO) and sex were included as fixed factors, while the sow and the experimental pen were included as random factors. Group performance such as FI, F: G ratio, BMs and LSI together with air gases were fitted in a linear regression model (“lm” function) and ANOVA model including the experimental diet as fixed factor. For individual performance, piglet was the experimental unit, while for group performance, the pen was used as experimental unit. Model diagnostics were performed to assess the adequacy of model assumptions. The distribution of residuals was examined visually using a quantile-quantile (Q–Q) plot and an overlaid reference line (qqnorm and qqline), to evaluate the assumption of normality. In addition, residual versus fitted value plots, obtained via the “plot” function, were inspected to verify the assumption of homoscedasticity and to detect any potential model misspecification or influential data points. Comparisons between dietary groups were tested using a post-hoc test (Tukey test).
The statistical analysis of the metabolomic data consisted of two parts. First, the metabolomic datasets were analyzed using a multivariate approach with MetaboAnalyst 6.0. Subsequently, the most relevant metabolites were analyzed using an ANOVA model (Virdis et al., 2024). In the multivariate analysis, the data were normalized using sum and log transformation to account for systematic differences between samples. After the normalization data were analyzed using partial least squares-discriminant analysis (PLS-DA) on MetaboAnalyst 6.0, the variable importance projection (VIP) scores were determined for diet and timepoint. Metabolites with a VIP score > 1.8 were analyzed using a linear mixed effects model and ANOVA model in which the fixed and random factors were the same as those utilized for individual piglet performance (BW and ADG), and fecal dry matter.
Regarding microbiota profile, amplicon sequence variants (ASVs) were generated using DADA2 1.14.0 (Callahan et al., 2016), for the taxonomic assignment, the Silva database release 138.1 (Quast et al., 2013) was used as a reference. Briefly, primers were trimmed to a consistent length: forward reads were truncated at position 290, and reverse reads were truncated at position 220 to remove low-quality sequences. The statistical analysis of alpha diversity indices (Shannon, Simpson, and Chao1), beta diversity, and taxonomic composition were performed the “phyloseq” (McMurdie and Holmes, 2013), “Vegan” (Dixon, 2003), “Adonis” (Martinez Arbizu, 2020) and “DESeq2” (Love et al., 2017) packages. Alpha diversity indices were analyzed using an ANOVA model incorporating the same fixed and random factors as those used in the individual performance analysis. A Euclidean distance matrix was computed from the variance stabilizing -transformed abundance table and used to perform Principal Coordinates Analysis (PCoA) using the “ordinate” function in phyloseq. The beta diversity was calculated and plotted using a non-parametric multidimensional scaling plot; the differences were tested using a PERMANOVA (Adonis.test) model with 999 permutations including the same factors used for alpha diversity, except for pen, which was treated as a fixed effect. Pairwise contrast was made using the pairwise Adonis function. Differences in taxonomic composition between treatments were tested using the “DESeq2” package, aggregating data at the genera level. For data normalization and to reduce the dependence of variance on the mean, the variance stabilizing transformation was applied using the “varianceStabilizingTransformation” function from the DESeq2 package. Pairwise comparisons between experimental groups were performed using the “results” function with contrasts specified for each comparison. Differentially abundant genera were identified based on an adjusted p-value (Benjamini–Hochberg false discovery rate, FDR) < 0.05, and results were expressed as log2 fold changes (log2FC) between groups. A correlation analysis was performed between the significantly different bacterial genera and metabolites derived by the pairwise comparisons between groups. Correlations were computed using Spearman’s rank correlation coefficient as implemented in the “microbiome” package. Statistical significance was assessed by two-sided testing, and p-values were corrected for multiple testing using the Benjamini–Hochberg FDR procedure. Results were expressed as least-squares means and standard error of the mean (SEM). A difference was declared significant when p-value (p) ≤ 0.05 and tendency when 0.05 < p ≤ 0.10.
3 Results
3.1 Health, performance and behaviors
Throughout the experimental period, mortality rates were 1.87% in the CTR group, 2.57% in the SO group, and 1.86% in the EX_SO group, showing no difference between the experimental groups.
Piglets fed the CTR diet and those receiving the SO and EX_SO diets exhibited no significant differences in BW at all the timepoints and similar ADG during the periods d0 to d7, d7 to d28, and d0 to d28 (Table 2). Parameters related to FI and F:G did not show any significant variations among the dietary groups, except for a tendency in F:G from day 0 to day 28, where piglets from the SO group tended to have better feed conversion compared to the CTR group (P = 0.09; Table 3).

Table 2. Effects of replacing corn with sorghum or extruded sorghum and sex on individual body weight and average daily gain of pigs from weaning to four weeks post-weaning.
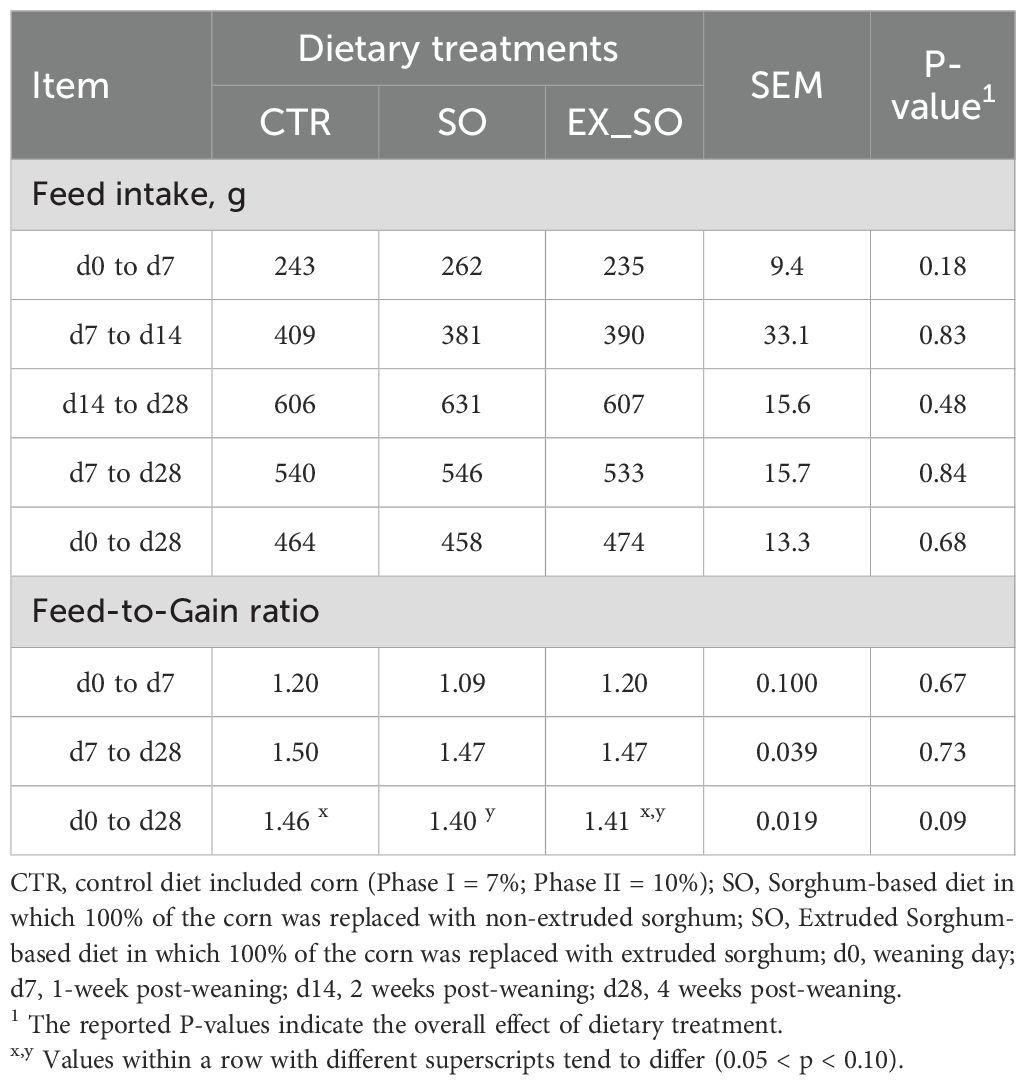
Table 3. Effects of replacing corn with sorghum or extruded sorghum on feed intake and feed to gain per pen of pigs from weaning to four weeks post-weaning.
Regarding BMs, ear and tail LSI, as well as air gas concentrations, the results did not reveal significant differences between piglets fed with CTR diet and those receiving the experimental diets (Tables 4–6, respectively).
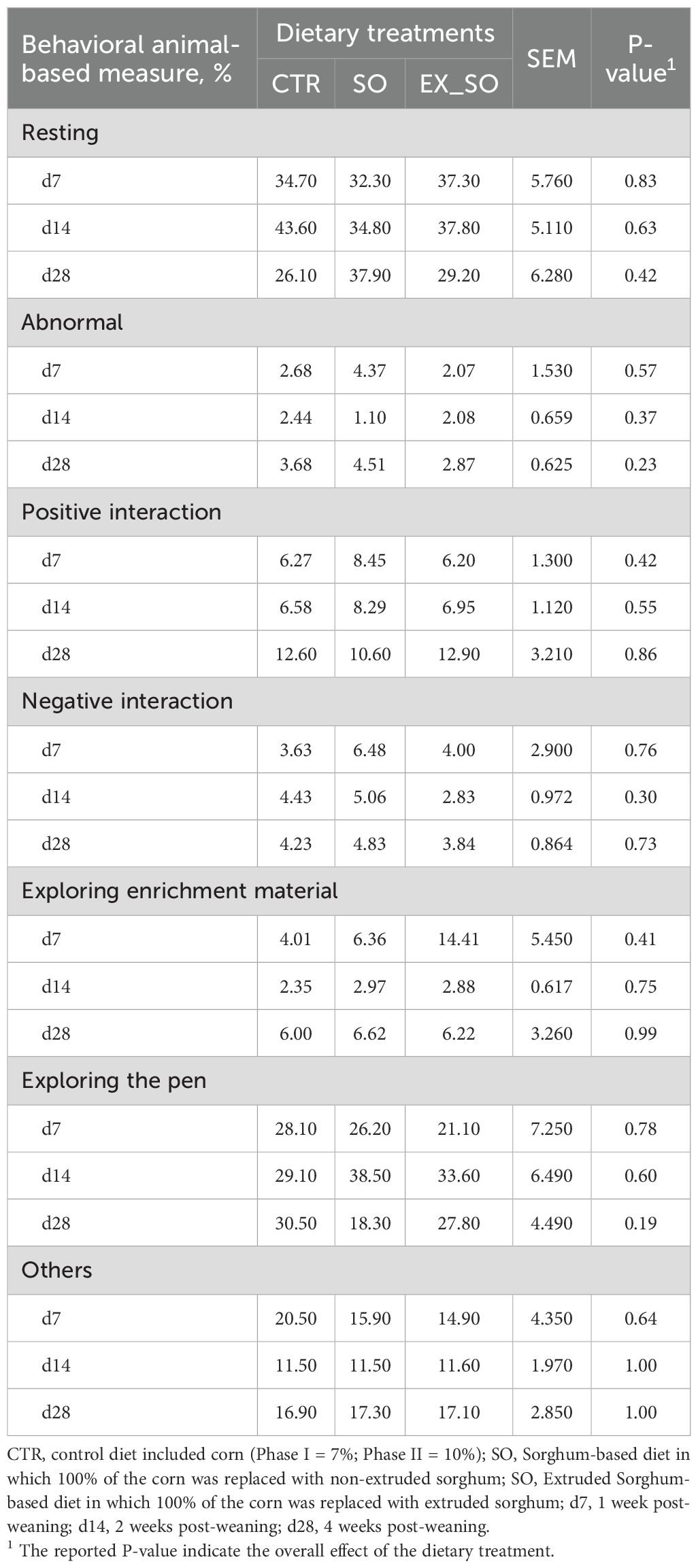
Table 4. Effects of replacing corn with sorghum or extruded sorghum on behavioral animal-based measures per pen of pigs from weaning to one week to four weeks post-weaning.
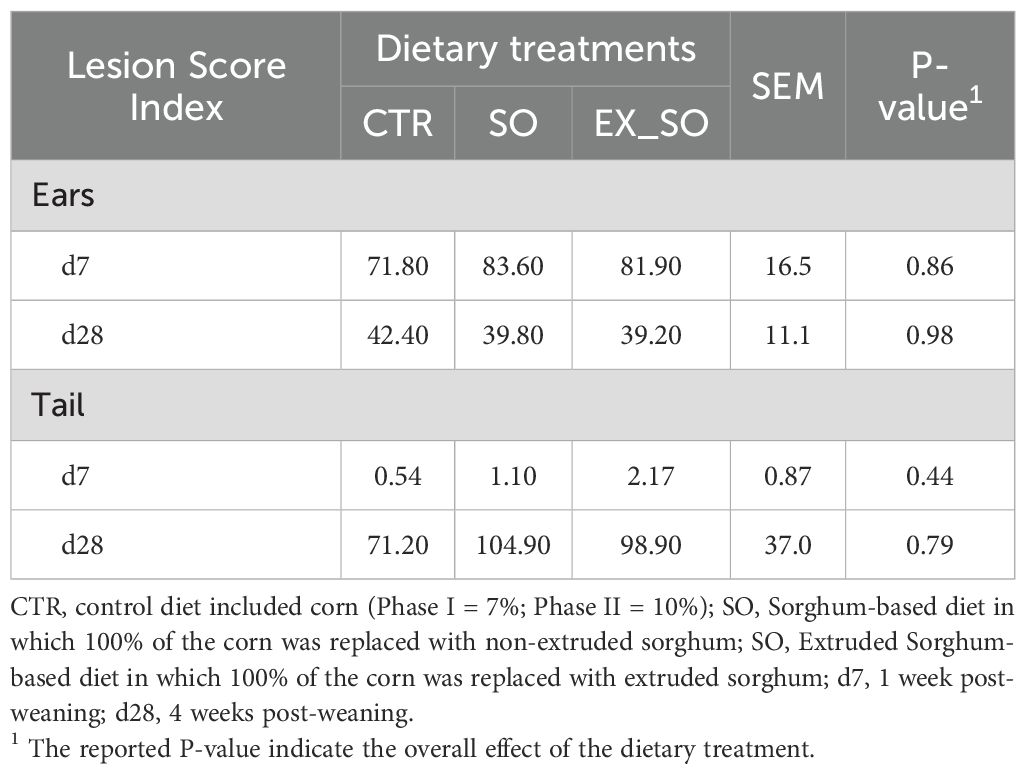
Table 5. Effects of replacing corn with sorghum or extruded sorghum on behavioral animal-based measures per pen of pigs from weaning to four weeks post-weaning.
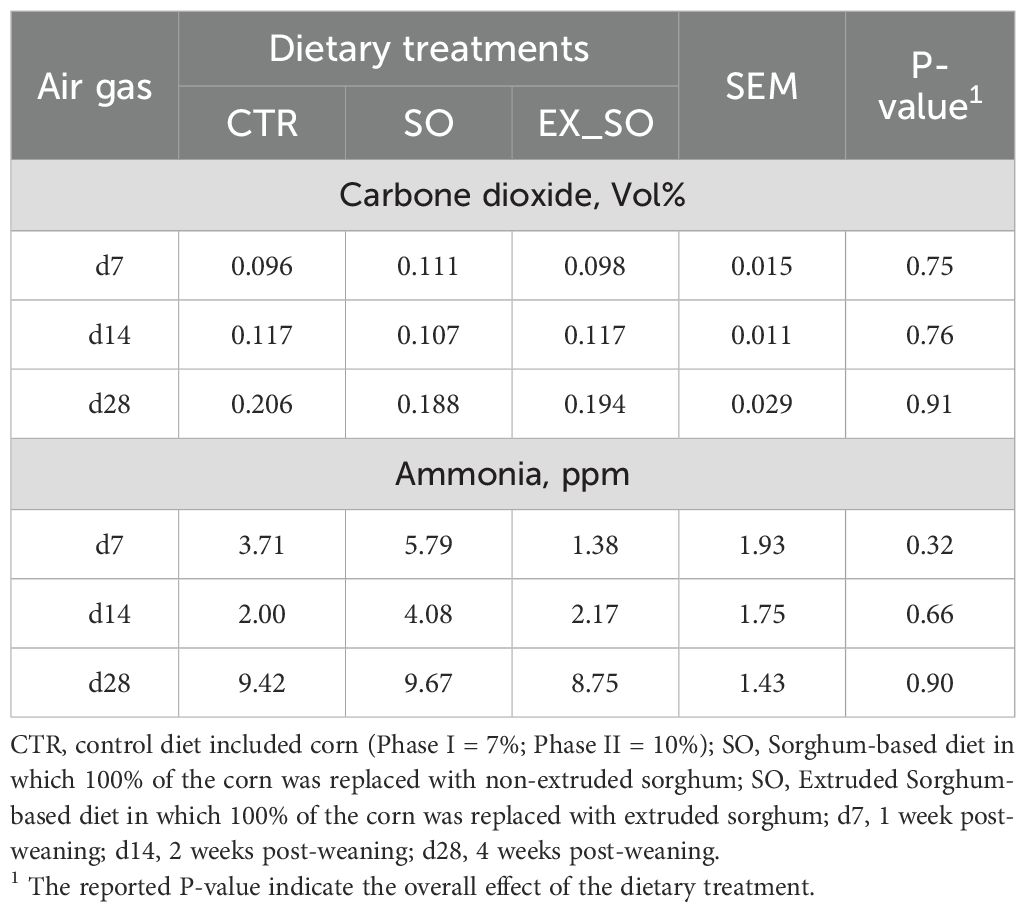
Table 6. Effects of replacing corn with sorghum or extruded sorghum on air carbon dioxide and ammonia per pen of pigs from weaning to four weeks post-weaning.
3.2 Dry matter percentage in the feces
Fecal dry matter content tended to be influenced by diet at d28 (P = 0.08), with a lower tendency in piglets of CTR group compared to SO group (Figure 1). No significant differences were detected on the other sampling timepoints.
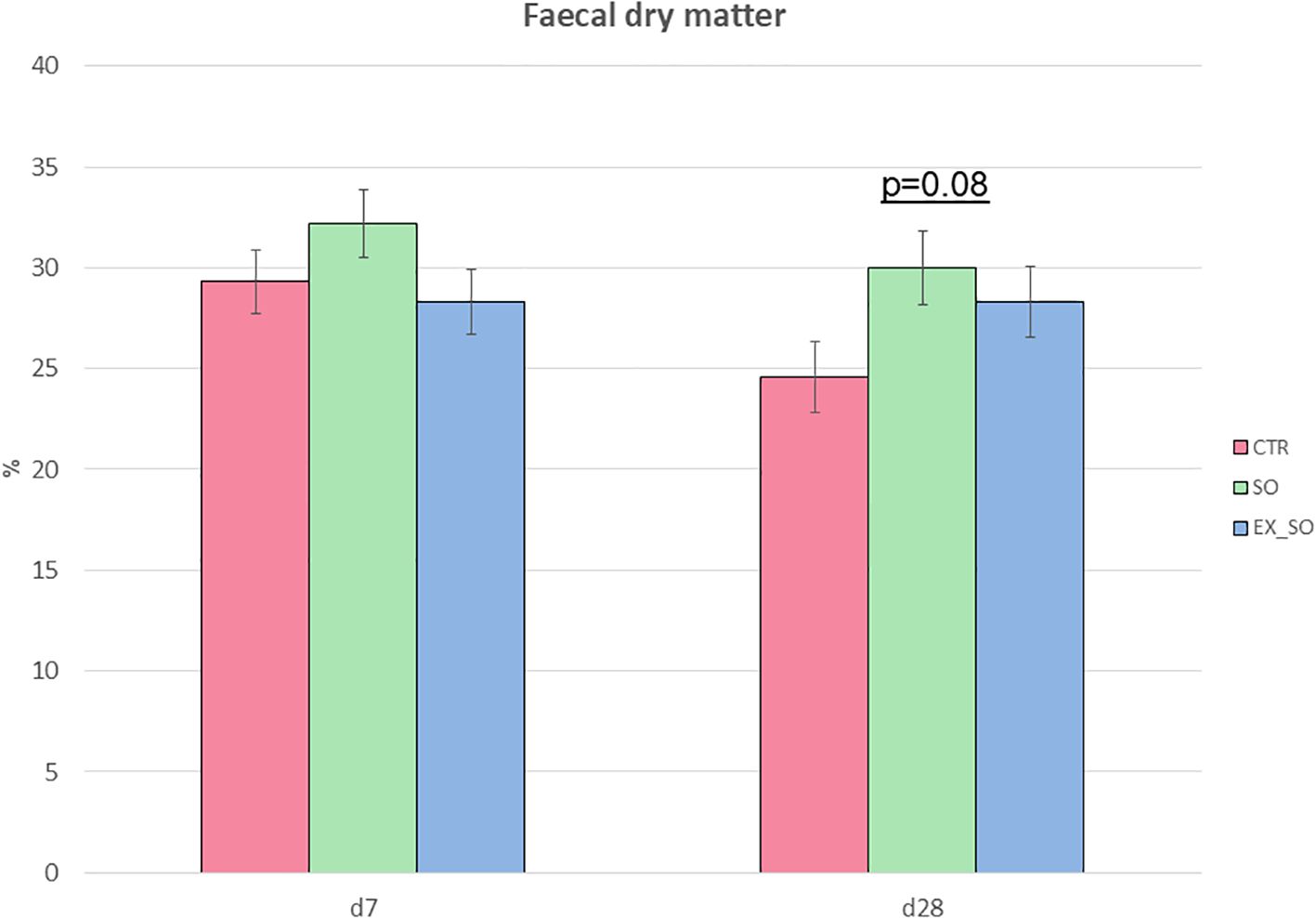
Figure 1. Effects of replacing corn with sorghum or extruded sorghum on fecal dry matter percentage from weaning to one-week post-weaning (d7) and four weeks post-weaning (d28). Fecal samples (45 per timepoint, 15 per experimental group) were collected from the same piglets at 1 and 4 weeks post-weaning. CTR, control diet included corn (Phase I = 7%; Phase II = 10%); SO, Sorghum-based diet in which 100% of the corn was replaced with non-extruded sorghum; SO, Extruded Sorghum-based diet in which 100% of the corn was replaced with extruded sorghum; P, The reported P-value indicates the overall effect of the dietary treatment. d7 = 1 week post-weaning; d28 = 4 weeks post-weaning.
3.3 Metabolomic profile of the feces
Figure 2 presents the results of multivariate analyses (PLS-DA) evaluating the impact of diet on the fecal metabolomic profile at d7 and d28. As shown in the left section of the figure, the samples were partially separated into the three experimental groups (CTR, SO, and EX_SO) at each time point and the most separated group were CTR vs EX_SO by PC1 (10.7% of explained variance at d7; 26.1% of explained variance at d28), while the SO group samples were located in between the two clusters of CTR and EX_SO.
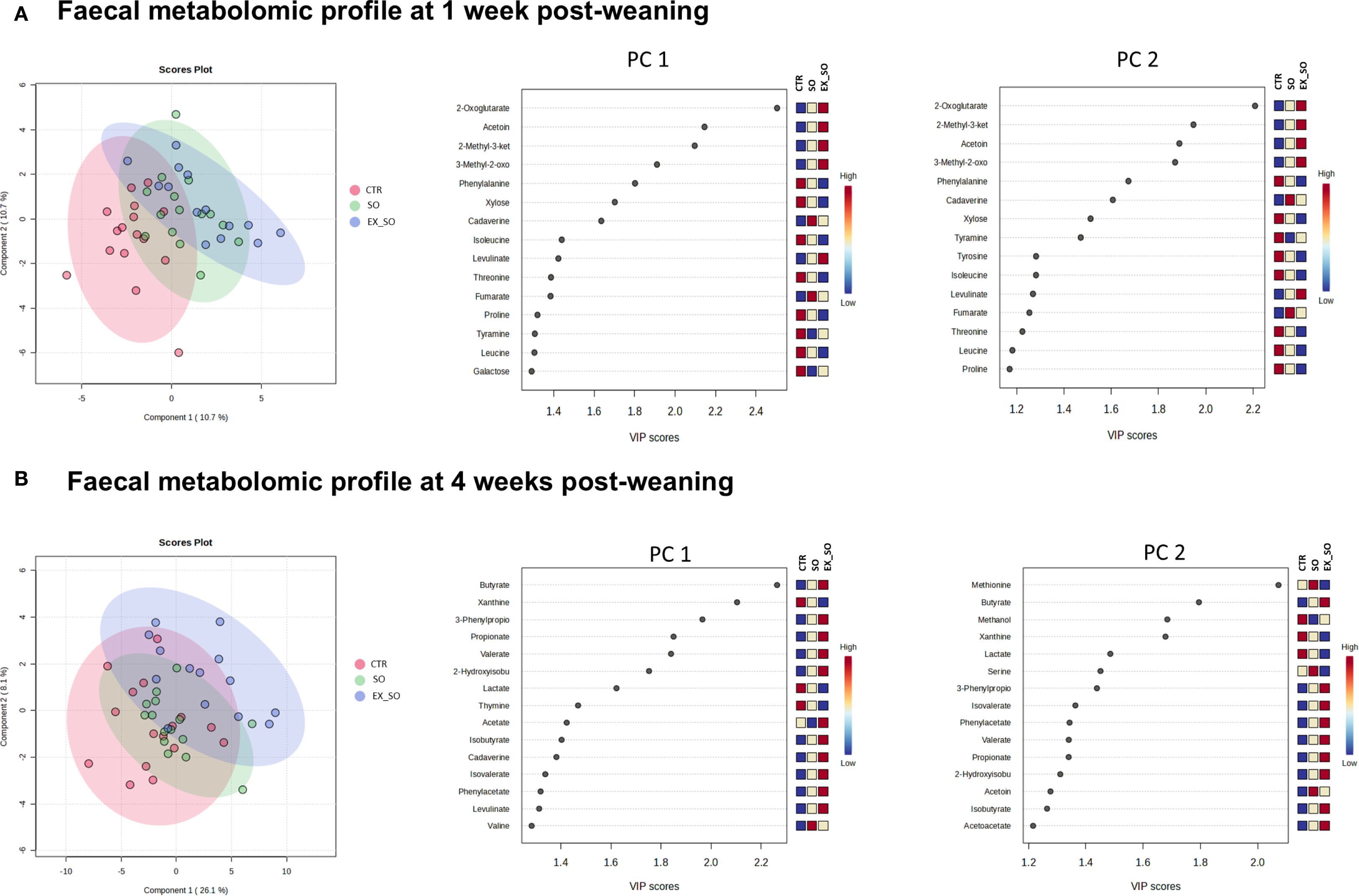
Figure 2. (A, B) Effects of replacing corn with sorghum or extruded sorghum on fecal metabolomic profile from weaning to one-week post-weaning (A) and four weeks post-weaning (B). Fecal samples (45 per timepoint, 15 per experimental group) were collected from the same piglets at 1 and 4 weeks post-weaning. PLS-DA was applied to investigate differences in the metabolome profile. Score plots of the PCs 1 and 2 were reported on the left side. The sources of variation for the most influential metabolites were displayed based on their variable importance projection (VIP) scores. The colored boxes on the right indicate the concentrations of the corresponding metabolite in each experimental group. VIP score > 1.8 Abbreviations: CTR, control diet included corn (Phase I = 7%; Phase II = 10%); SO, Sorghum-based diet in which 100% of the corn was replaced with non-extruded sorghum; SO, Extruded Sorghum-based diet in which 100% of the corn was replaced with extruded sorghum.
On d7, the CTR group was characterized (VIP score > 1.8) by a higher abundance of phenylalanine, while the EX_SO group was characterized by a higher abundance of 2-oxoglutarate, acetoin, 2-methyl-3-keto, and 3-methyl-2-oxo. The ANOVA analysis on d7 revealed that the CTR group had a significantly higher concentration of phenylalanine (p = 0.05; Supplementary Table 1), along with significantly a lower concentration of acetoin (p < 0.01) compared to the EX_SO group. Additionally, the CTR group showed lower levels of 2-oxoglutarate (p < 0.01), 2-methyl-3-keto (p < 0.01) and 3-methyl-2-oxo (p < 0.01) when compared to both the SO and EX_SO groups.
At d28, the CTR group was characterized (VIP score > 1.8) by a higher abundance of xanthine, while the SO group was enriched in methionine. Conversely, the EX_SO group showed increased levels of butyrate, 3-phenylpropionate, propionate, and valerate. The ANOVA model confirmed that the CTR group exhibited a higher concentration of xanthine (p < 0.01) and methionine (p = 0.01), and lower concentrations of butyrate (p < 0.01) compared to the EX_SO group. Conversely, the EX_SO group had higher concentrations of 3-phenylpropionate (p < 0.01), propionate (p < 0.01), and valerate (p < 0.01) compared to both the CTR and SO groups.
3.4 Microbiota profile of the feces
The sequencing process produced 4’516’427 reads. As a preliminary verification, rarefaction curves were plotted (Supplementary Figure 1), showing the number of species in relation to the sample size of the sequence. Overall, all samples reached a plateau, suggesting good sequencing efficiency and indicating that the procedure captured all the taxonomic variability present. At d7, the DADA2 algorithm identified a total of 1,900 amplicon sequence variants, belonging to 15 phyla (mainly Firmicutes 87.29 ± 2.57% and Bacteroidota 8.90 ± 0.64%), 63 families (predominantly Lactobacillaceae 27.2 ± 7.67%, Lachnospiraceae 22.6 ± 1.22%, and Clostridiaceae 11.0 ± 5.65%), and 181 genera (mainly Lactobacillus 21.6 ± 13.64%, Clostridium sensu stricto 1 10.7 ± 6.41%, Blautia 6.9 ± 1.86%, Ruminococcus torques group 4.6 ± 2.76%, Lactobacillaceae_HT002 4.5 ± 2.76%, and Subdoligranulum 4.09 ± 1.61%).
At d28, the DADA2 algorithm identified a total of 2,829 amplicon sequence variants, belonging to 18 phyla (mainly Firmicutes 82.5 ± 1.26% and Bacteroidota 10.0 ± 0.19%), 67 families (predominantly Lachnospiraceae 19.3 ± 0.45%, Clostridiaceae 13.6 ± 4.27%, Lactobacillaceae 13.3 ± 5.40%, and Ruminococcaceae 8.6 ± 0.36%), and 186 genera (mainly Clostridium sensu stricto 1 13.4 ± 4.57%, Lactobacillus 11.9 ± 8.65%, Blautia 5.7 ± 0.86%, Terrisporobacter 4.1 ± 2.36%, Subdoligranulum 3.6 ± 0.69%, Oscillospiraceae UCG-005 3.0 ± 0.31%, Prevotella_9 2.8 ± 0.36%, Faecalibacterium 2.06 ± 0.40%, Agathobacter 1.80 ± 0.42%, and Rikenellaceae RC9 gut group 1.75 ± 0.11%).
Microbiota analysis revealed no significant effects of dietary treatments on alpha diversity indices (Shannon, InvSimpson, Chao1) across the two timepoints (d7 and d28; Figure 3). Beta diversity was also unaffected by the experimental diets at d7 (R² = 0.05, p = 0.11; Figure 4A), while at d28, the dietary treatment showed a significant effect (R² = 0.05, p = 0.05; Figure 4B), with pairwise comparisons indicating a significant difference between the CTR and SO groups (p = 0.03). Taxonomic analysis at genus level revealed that at d7, the genus Oscillospira (log2FC: 4.39, p -adj < 0.01; Figure 5) was more abundant in the CTR group compared to the EX_SO group. At d28, Olsenella (log2FC: 2.49, p -adj < 0.01) was more abundant in the CTR group compared to the SO group. Additionally, Olsenella (log2FC: 2.34, p -adj = 0.01) and Ligilactobacillus (log2FC: 3.94, p -adj = 0.01) were more abundant, whereas Anaerovoracaceae_Family XIII UCG-001 (log2FC: -3.73, p -adj = 0.01) was significantly less abundant in the CTR group than in the EX_SO group. Additionally, Shuttleworthia (log2FC: 2.17, p -adj = 0.02) and Syntrophococcus (log2FC: 16.22, p -adj < 0.01) were more abundant in the SO group compared to the EX_SO group.
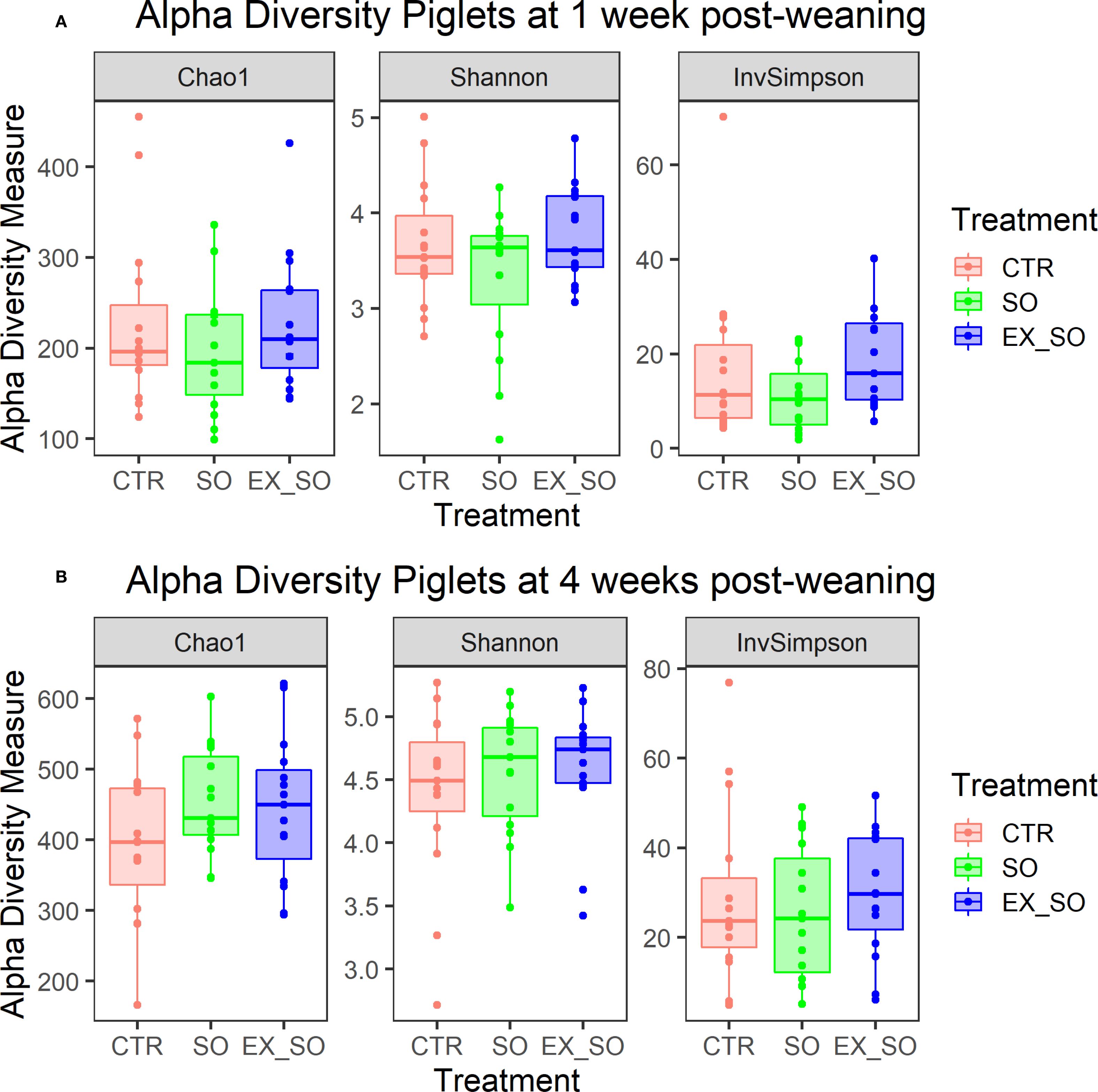
Figure 3. (A, B) Effects of replacing corn with sorghum or extruded sorghum on alpha diversity indices (Chao1, Shannon, InvSimpson) of fecal samples from piglets at one (A) and four weeks post-weaning (B). Fecal samples (45 per timepoint, 15 per experimental group) were collected from the same piglets at 1, and 4 weeks post-weaning. CTR, control diet included corn (Phase I = 7%; Phase II = 10%); SO, Sorghum-based diet in which 100% of the corn was replaced with non-extruded sorghum; SO, Extruded Sorghum-based diet in which 100% of the corn was replaced with extruded sorghum.
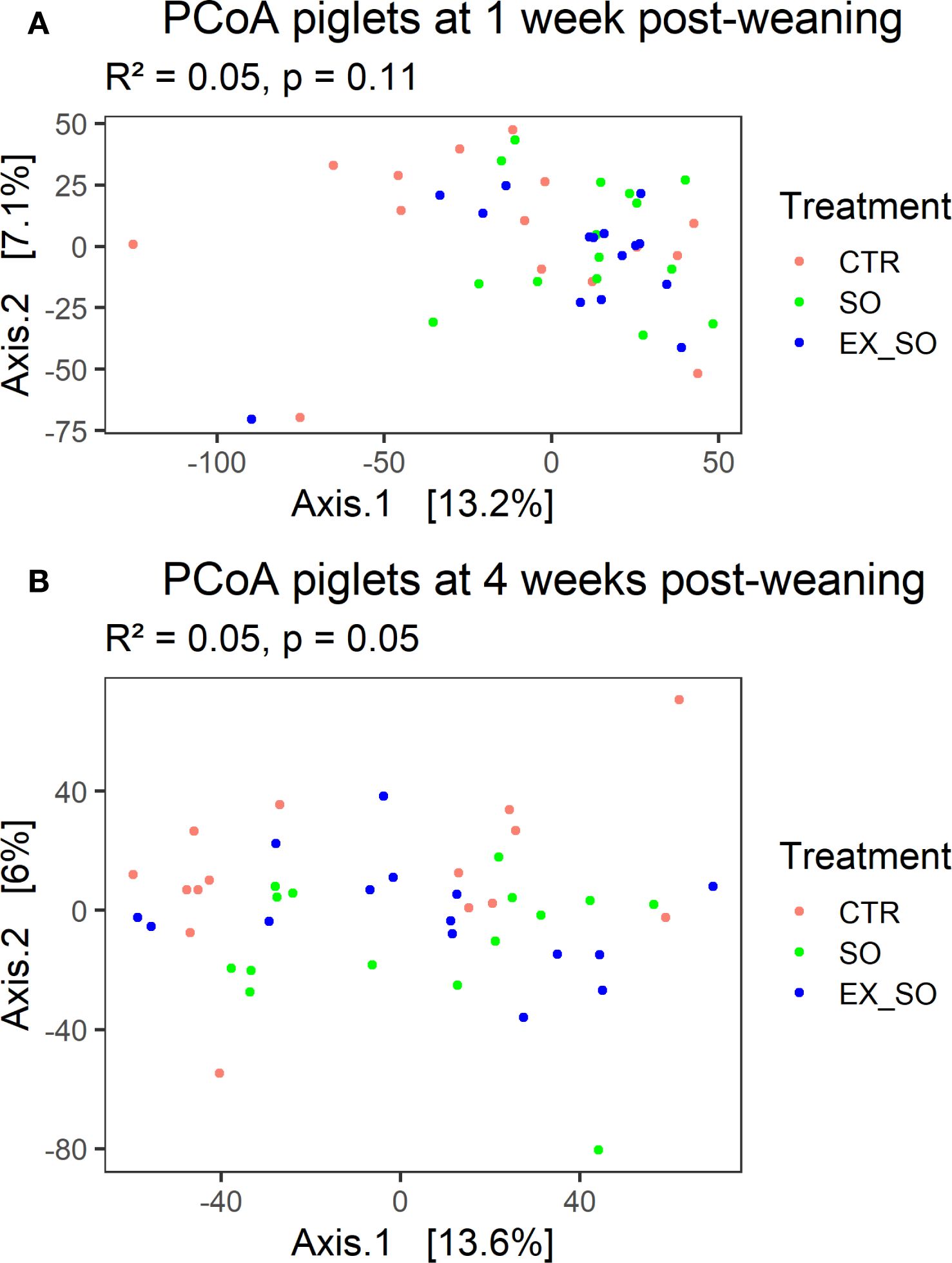
Figure 4. (A, B) Effects of replacing corn with sorghum or extruded sorghum on fecal microbial community composition of weaned pigs at one-week (A) and four weeks post-weaning (B). Fecal samples (45 per timepoint, 15 per experimental group) were collected from the same piglets at 1 and 4 weeks post-weaning. The score plot is showing the results of the Principal Coordinates Analysis based on the Bray-Curtis distance matrix. CTR, control diet included corn (Phase I = 7%; Phase II = 10%); SO, Sorghum-based diet in which 100% of the corn was replaced with non-extruded sorghum; EX_SO, Extruded Sorghum-based diet in which 100% of the corn was replaced with extruded sorghum.
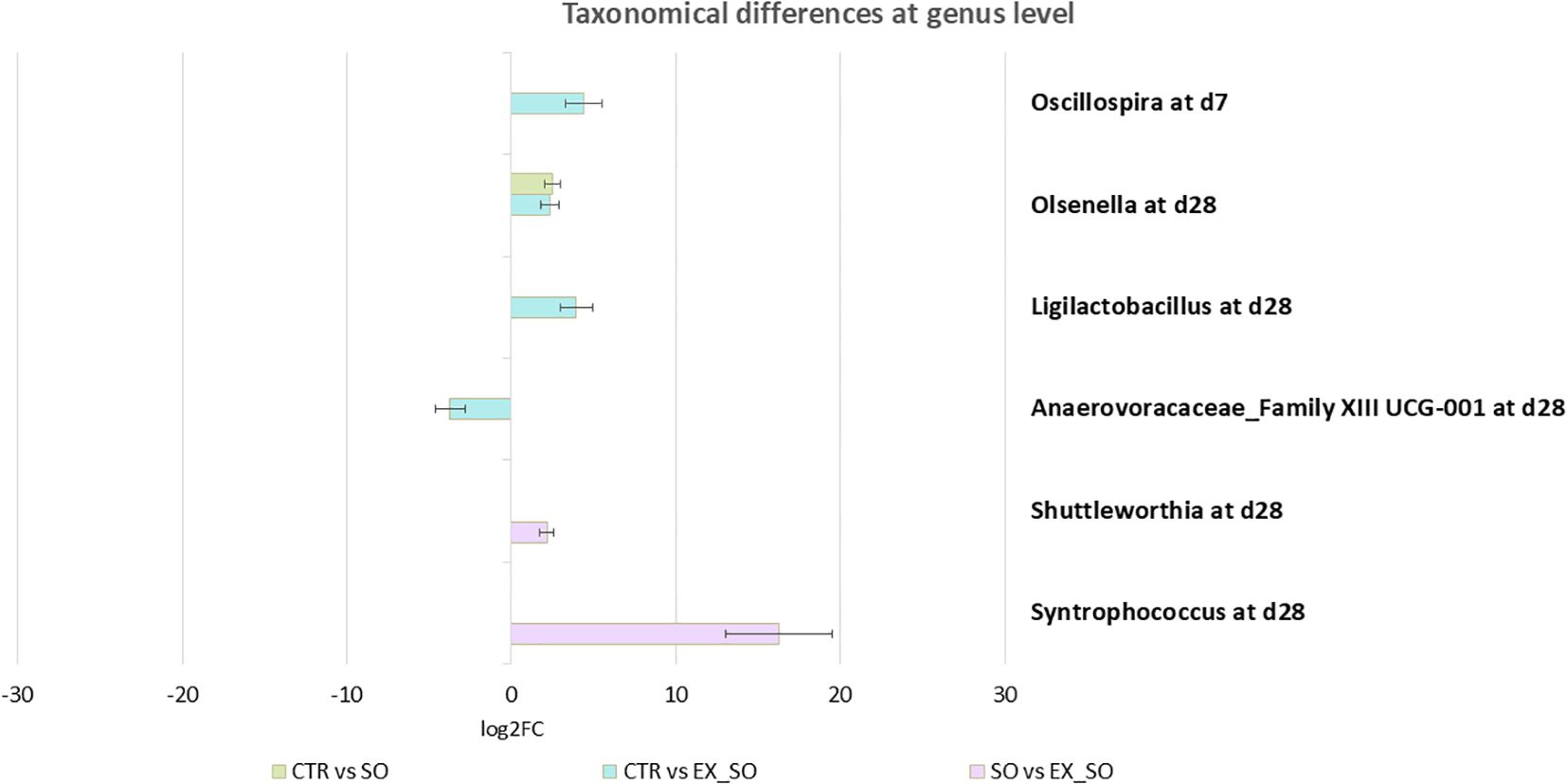
Figure 5. Effects of replacing corn with sorghum or extruded sorghum on fecal taxonomical differences at genus level from weaning to one week post-weaning (d7) and four weeks post-weaning (d28). Fecal samples (45 per timepoint, 15 per experimental group) were collected from the same piglets at 1 and 4 weeks post-weaning. CTR, control diet included corn (Phase I = 7%; Phase II = 10%); SO, Sorghum-based diet in which 100% of the corn was replaced with non-extruded sorghum; EX_SO, Extruded Sorghum-based diet in which 100% of the corn was replaced with extruded sorghum; d7 = 1 week post-weaning; d28 = 4 weeks post-weaning.
3.5 Correlation analysis between metabolites and microbial taxa of the feces
On d7, no significant correlations were detected between bacterial genera and metabolites. Figure 6 shows the significant correlations between bacterial genera and metabolites identified on d28. Ligilactobacillus was positively correlated with xanthine (r = 0.56, p = 0.02) and negatively correlated with 3-phenylpropionate (r = –0.50, p = 0.03). Olsenella showed positive correlations with glutarate (r = 0.54, p = 0.02) and thymine (r = 0.51, p = 0.03). Anaerovoracaceae Family XIII UCG-001 was positively correlated with butyrate (r = 0.51, p = 0.03).
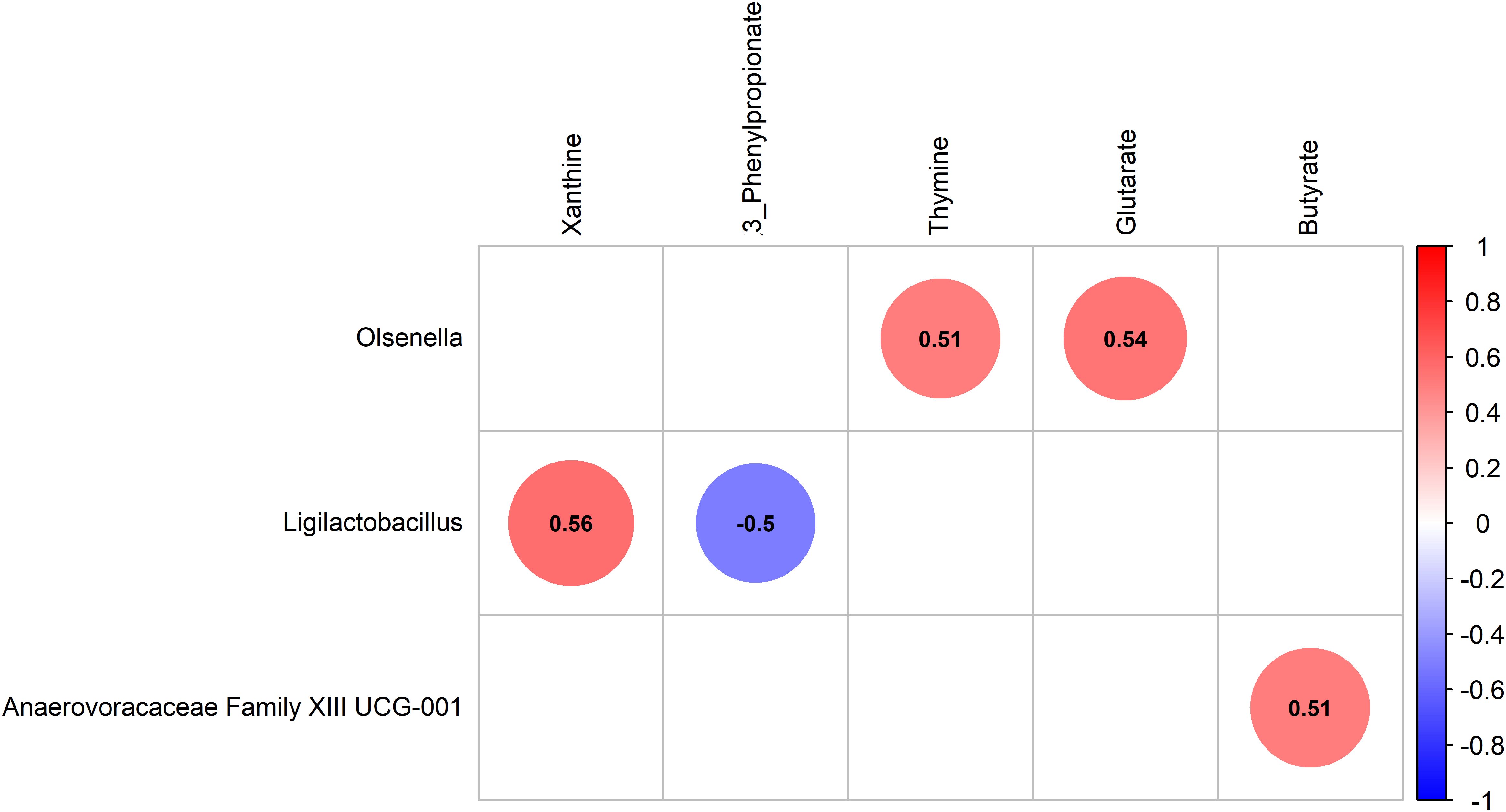
Figure 6. Correlation analysis between differentially abundant fecal bacterial genera and metabolites at 4 weeks post-weaning. Positive associations are represented in red, whereas negative associations are shown in blue, with circle size indicating the magnitude of the Spearman correlation coefficient.
4 Discussion
Complete replacement of corn (up to 10%), in both non−extruded and extruded forms, did not impair growth performance and survivability in post−weaning piglets. These findings align with those of Chae et al. (2000) and Rodrigues et al. (2016) who showed that fully substituting corn with sorghum did not compromise growth during the post−weaning period. In their study, ADG was not affected by dietary treatments, indicating that sorghum supports growth rates comparable to corn. Additionally, Chae et al. (2000) observed that piglets receiving extruded grains, regardless of whether the grains were corn or sorghum, achieved a better F:G ratio and consumed less feed from weaning until 33 days post-weaning. This suggests a modest improvement in efficiency associated with extrusion processing. In our study, extrusion of sorghum did not produce statistically significant improvements of the piglets’ performance. Extrusion process is often associated with improved nutrient availability (Rodrigues et al., 2016; Liu et al., 2013). However, the cooling temperature following extrusion may lead to the retrogradation of sorghum starch resulting in an increase in resistant starch content in EX_SO diet, which in turn, could reduce its digestibility (Xie et al., 2014). In addition, in the present study, a trend for better F:G ratio was observed in the SO group compared with the CTR group considering the total period (d0-d28). This slight effect may be due to the properties of sorghum starch. In fact, starch is found inside the endosperm of cereals, enmeshed in a protein matrix. This matrix is particularly strong in sorghum due to the starch-kafirin (most bunt protein in the matrix) relationship (Haziman et al., 2025). This starch-kafirin complex in sorghum may reduce the velocity of starch digestibility, leading to better nutrient absorption in post-weaning piglets.
In line with this trend, increased fecal dry matter content in SO-fed compared to the CTR-fed piglets at d28 was observed. This may reflect enhanced water reabsorption, potentially indicating more efficient nutrient absorption and a reduced incidence of diarrhea in the SO group (Pedersen et al., 2012; Vente-Spreeuwenberg et al., 2003). However, a study by Pan et al. (2021) reported negative effects of corn replacement with sorghum during the post-weaning phase, including impairments in gut health. The discrepancy between the findings of the present study and those reported by Pan et al. (2021) is likely attributable to differences in the sorghum varieties used, as certain types contain higher levels of anti-nutritional factors, such as tannins, which can negatively impact gut health and nutrient digestibility and performance (Girard and Bee, 2020; Pan et al., 2022). It is indeed important to note that in this study, white sorghum of European origin, characterized by a tannin content of < 0.4% was used, whereas in the study by Pan et al. (2021), the authors used non-European cultivars.
Building upon the previously discussed findings on dry matter, we included metabolomic and fecal microbial analysis to gain deeper insights into the physiological adaptations induced by replacing corn with sorghum post-weaning piglet diet, thereby assessing its impact on gut health.
Notably dietary treatment led to a more impactful effect on microbiota and gut metabolites at d28 than at d7. The lack of significant results on alpha and beta diversity of the microbiota at d7 for the microbiota suggests that microbial community require more time to adapt to a different diet. In contrast, some changes were observed in the metabolome on d7. This discrepancy in the outcomes obtained between the two omics approaches can be attributed to the intrinsic characteristics of the techniques themselves. Specifically, it should be noted that metabolomics is able to capture more rapid fluctuations within the environment, whereas 16S analysis provides information on relative abundances without revealing the actual activity of the microorganisms (Go et al., 2024). Notably, on d 7, fecal phenylalanine levels were higher in CTR piglets compared to EX_SO piglets. The excess phenylalanine in the feces of CTR animals suggests less efficient amino acid utilization or limited microbial degradation of this aromatic amino acid during early post-weaning period (Beaumont et al., 2020). On the other side, the EX_SO group was characterized by an increase in metabolite derived by BCAAs fermentation such as 2-ketoacids (e.g. 2-methyl-3-keto, 3-methyl-2-oxo), suggesting a lack of absorption of BCAAs in the small intestine. Furthermore, the increase in these compounds may also have favored their decarboxylation and thus an increase in pyruvate metabolism, which manifested itself in an increase in acetoin (a by-product of pyruvate fermentation).
The correlation analyses between microbial taxa and metabolites did not reveal meaningful associations at this early stage. Although Oscillospira was more abundant in the CTR group compared to EX_SO, this genus was not correlated with any of the metabolites identified, indicating a lack of functional association at this stage. This genus, is generally associated with fiber fermentation and positive colonic health (Den Besten et al., 2013; Slavin, 2013; Siddiqui and Cresci, 2021; Zhao et al., 2018). Its increase in the CTR group, together with the absence of severe diarrhea and no difference in the dry matter at d7 suggested good gut health of the CTR group, beside the higher concentration in phenylalanine (Tao et al., 2015).
Differences in microbial and metabolomic profile were most evident on d28. Although no significant dietary effects on alpha diversity indices (Shannon, Inverse Simpson, Chao1) were observed, suggesting that the overall richness and evenness of microbial species were maintained across all treatments (Yu et al., 2024), a significant effect of dietary treatment on beta diversity emerged, with distinct microbial community shifts between the groups. Differences in the taxa abundance between groups were especially evident when comparing CTR and EX_SO groups which differs for the abundance in three taxa (Anaerovoracaceae Family XIII UCG-001, Ligilactobacillus and Oscillospira).
Also, for the metabolome, the clustering in the groups seems more evident (and explains a greater variance) at d28 than at d7, and the greatest difference was found between the CTR group and the SO_EX group. The piglets fed the EX_SO diet exhibited significantly elevated levels of short-chain fatty acids (SCFAs) in the feces, notably butyrate, propionate, and valerate. Butyrate serves as the primary energy source for colonocytes, fulfilling up to 70–80% of their energy requirements. It supports gut barrier integrity by acidifying the colonic lumen (lowering pH), thereby inhibiting pathogenic bacteria, and by modulating immune and inflammatory responses through various signaling pathways (Den Besten et al., 2013; Slavin, 2013; Siddiqui and Cresci, 2021). The higher concentration of butyrate observed in EX_SO piglets compared to CTR might suggest an increased butyrogenic bacterial activity, likely through enhanced fermentation of resistant starch components that reach the hindgut (Jha and Berrocoso, 2015). Similarly, propionate production is often linked to bacterial fermentation of indigestible polysaccharides such as resistant starch (Miller and Wolin, 1996). Moreover, the observed increase in propionate levels in EX_SO piglets, compared to both the CTR and SO groups, may indicate an enhanced gut health. This aligns with findings by Jacobson et al. (2018), who demonstrated that propionate produced by Bacteroides species contributes to colonization resistance against pathogenic bacteria such as Salmonella in a murine model. Valerate was also found at higher concentrations in the feces of piglets fed the EX_SO diet compared to both CTR and SO groups. Its production can arise from the fermentation of branched chain amino acids or via the chain elongation of propionate in the gut (Nery et al., 2012; De Smit et al., 2019). The observed increase in butyrate, propionate and valerate suggests enhanced microbial fermentation of a diverse array of substrates in the EX_SO-fed piglets, indicative of a richly fermentative gut environment. Considering the significant more abundant taxa observed on the EX_SO group at d28, it has been found Anaerovoracaceae Family XIII UCG-001, a taxon within the Clostridiales order which is associated with amino acid fermentation (Fang et al., 2012). This increase in Anaerovoracaceae Family XIII UCG-001 could be partially consistent linked with the rise in valerate observed in the EX_SO group. However, this hypothesis was not supported by the correlation analysis. Nevertheless, the correlation analysis showed a positive correlation between Anaerovoracaceae Family XIII UCG-001 and butyrate, suggesting that this taxon contributed to the higher SCFA level detected in EX_SO piglets. However, this taxa is uncultured and known only from sequencing data, identified at best to the family level. This limits biological interpretation and the assignment of specific metabolic functions (Kamble et al., 2020). Overall, the presence of more fermentative metabolites in the feces of SO_EX may be primarily driven by the extrusion process of sorghum that led to an increase in retrograded starch. This form of resistant starch can develop when heat treatments such as extrusion are followed by cooling (Chang et al., 2021). In the EX_SO group, such processing likely induced partial starch gelatinization, with subsequent retrogradation increasing the resistant starch content. This indigestible fraction bypasses enzymatic digestion in the small intestine and undergoes microbial fermentation in the colon, potentially altering bacterial metabolites production (Piecyk et al., 2018; Jha and Berrocoso, 2015). Therefore, the observed variations in SCFAs profile may reflect both the intrinsic properties of the cereal and processing-induced modifications to starch structure and fermentability in the hindgut. However, this remains a hypothesis, as specific analysis on digestibility and resistant starch levels in diets were not performed in the present study. Additionally, the EX_SO group’s feces had elevated 3-phenylpropionate compared to the other experimental diets. 3-phenylpropionate is a microbial metabolite that can derive both from the catabolism of aromatic amino acids such as tyrosine and phenylalanine (Li et al., 2024), and from the microbial degradation of plant phenolics (such as those abundant in sorghum) into benzoic-acid derivatives. It has been associated with greater microbial diversity, and beneficial metabolic profiles in humans (Pallister et al., 2017) and enhanced the intestinal epithelial barrier in mice model (Hu et al., 2023). In our context, an elevated 3-phenylpropionate level suggests that extrusion may have made sorghum’s polyphenols more accessible to gut bacteria, resulting in greater production of this phenolic metabolite. This could be beneficial, as it implies detoxification of potentially anti-nutritional phenolics and generation of metabolites that correlate with improved gut ecosystem function (Salazar Lopez et al., 2016; de Sousa et al., 2019), however these beneficial effects did not reflect into better survivability and performance of EX_SO group.
Notably, CTR feces had higher xanthine and methionine compared to the EX_SO group. The metabolic consequence of elevated methionine and xanthine in the hindgut is noteworthy: microbes can ferment excess methionine and xanthine, producing of sulfur and nitrogen metabolites, that, if excessive, might disrupt colonic health (Wolf et al., 2021; Richardson et al., 2013). However, in CTR piglets, the fact that methionine and xanthine remained in those forms (rather than fully broken down) might imply limited microbial activity on the utilization of those substrates. Interestingly, the CTR group showed significantly higher abundances of Ligilactobacillus genera compared with the SO_EX group. This genus showed a positive correlation with xanthine and a negative correlation with 3-phenylpropionate (which was lower in the CTR fecal samples). Interestingly, some strain of Ligilactobacillus (CECT 30632 and MPac32) were found to be able to produce xanthine form guanosine suggesting that these strains possess purine nucleosidases (Rodríguez et al., 2023). This property makes these strains attractive candidates for use as human probiotics, as they are capable of limiting the excess purines found in rich diets and preventing gout and hyperuricemia (Rodríguez et al., 2023). In the present context, this property could indicate an advantage for purine reutilization and stimulation of the “salvage pathway” driven by Ligilactobacillus in the CTR group.
In addition, lactic acid bacteria such as Ligilactobacillus are generally considered beneficial bacteria for pigs, and some Ligilactobacillus strains are well recognized as beneficial probiotics for pigs (Cuevas-Gómez et al., 2024; Alba et al., 2023). However, in the present study, their enrichment in CTR did not translate into measurable improvements in gut-health. This observation is consistent with findings that the beneficial effects of Ligilactobacillus, particularly regarding immune modulation and pathogen defense, may be more pronounced under challenge conditions. For instance, L. salivarius strains isolated from the porcine gut were shown to modulate innate immune responses and enhance protection during viral-bacterial intestinal co-infection, but such benefits were less apparent in non-challenged animals (Indo et al., 2021).
Looking at the difference between pigs fed the SO diet and EX_SO, the non-extruded sorghum, showed a significantly higher abundance of Shuttleworthia and Syntrophococcus. Both genera are recognized producers of SCFAs, particularly butyrate and valerate (Liu et al., 2021; Doré and Bryant, 1989; Bryant and Kammerer, 1985). Despite that, the metabolomic analysis showed significantly higher fecal levels of butyrate, propionate, and valerate in the EX_SO group. This apparent inconsistency can be explained by several non-mutually exclusive factors. First, there is substantial functional redundancy in the gut microbiota: many different bacterial taxa (including less-recognized butyrogenic members) possess the metabolic pathways to produce butyrate, so community butyrate output can remain high even without expansion of the canonical butyrate-producing genera. Namely, multiple bacterial lineages may compensate functionally, fermenting available substrates into butyrate and masking changes at the taxonomic level (Vital et al., 2017). Secondly, metabolic activity is not always directly reflected by bacterial abundance measured through 16S rRNA sequencing. In the EX_SO piglets, certain butyrate-producing bacteria may have become more metabolically active without a corresponding increase in their population size (Kircher et al., 2022). Methodological constraints of 16S rRNA gene profiling could underline the disparity between metabolomics and microbiota data. Amplicon sequencing provides limited taxonomic resolution (often only to the genus level) and cannot distinguish strain-level or functional gene differences; it also cannot reveal whether detected microbes are actively expressing pathways of interest. As previously noted, metabolomics has the ability to capture more rapid fluctuations than microbiota analysis. Thus, microbial metabolic readouts and community profiles do not always align, and integrative approaches (e.g. metagenomics and metatranscriptomics) may be needed to fully elucidate such discrepancies (Kamble et al., 2020). This latter aspect, alongside digestibility, represents a major constraint in data interpretation, and future metagenomic and physiological analyses are warranted to clarify the underlying mechanisms.
Finally, since it can be hypothesized that dietary changes alter nutrient digestibility, intestinal fermentation, and microbial community and these changes can, in turn, affect the microbiota–gut–brain axis (Rabhi et al., 2020; Trevisi et al., 2021), the piglets’ behavior and lesions were monitored during the study. It is noteworthy that health, welfare and environmental parameters remained unaffected by the dietary modifications in the present study. Specifically, parameters such as BMs, ear and tail LSI, and air gas concentrations did not exhibit significant differences between piglets fed the CTR diet and those fed sorghum-based diets. Similarly, Graziosi et al. (2024) reported that during the fattening period (from 4 to 13 weeks after arrival at the fattening unit), reducing corn by 21% (substituting it with white sorghum) resulted in slight differences in BMs, notably leading to an increase in exploratory behaviors of the pigs, but no significant differences in ear and tail LSI or air gas concentrations. However, it should be emphasized that, as the welfare and behavior of piglets in the post-weaning period can be influenced by several factors (Palumbo et al., 2025), which may differ from the factors influencing the behavior of adult pigs (Palumbo et al., 2023), it is always good practice to monitor these aspects when carrying out a feeding intervention. The lack of difference among diets on behavior, welfare and injury observed in the present study are in line with those observed on performance and suggest that the substitution of corn for sorghum does not compromise the digestion of the diet to such an extent that it may cause discomfort for the animals.
In conclusion, this study demonstrates that the complete replacement of corn with sorghum, till 12% on inclusion, both non-extruded and extruded, does not impair post-weaning piglet performance, health, or welfare under commercial farming conditions. On the contrary, in its non-extruded form, sorghum tends to improve the F:G ratio and increase the fecal dry matter during the first four weeks post-weaning. Furthermore, environmental parameters such as fecal NH3 levels and behavioral indicators remained unaffected, supporting the environmental and welfare compatibility of these alternative feeding strategies. The extrusion process modulated microbial activity and metabolite profiles. Interpretation, however, is limited by the lack of direct measurements of resistant starch and digestibility, and the reliance on 16S rRNA profiling. Future studies should quantify resistant starch levels, measure digestibility, and integrate shotgun metagenomics with metabolomics.
Data availability statement
The raw sequence data are available at NCBI Sequence Read Archive (SRA), accession number PRJNA1289664.
Ethics statement
The procedures complied with the Italian law pertaining to experimental animals and were approved by the Ethic- Scientific Committee for Experiments on Animals of the University of Bologna (Trial ID 4796, Prot. n. 218339/2024). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
FP: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. FC: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. LL: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. AZ: Data curation, Formal analysis, Methodology, Writing – original draft. PT: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. DL: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The present study was funded by SustainableHeavySuis Project (CUP: J33C23002980002) -L.R. n. 17/2022-DGR 165 del 06/02/2023, Emilia Romagna Region, Italy.
Acknowledgments
The authors acknowledge Gruppo Martini for providing the animals enrolled in the trial. A special thank you to Dr. Umberto Rolla and Dr. Fabrizio Conte for their support regarding the trial set-up and diet formulation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor BC declared a past co-authorship with the author DL.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1670477/full#supplementary-material
References
Alba C., Castejón D., Remiro V., Rodríguez J. M., Sobrino O. J., de María J., et al. (2023). Ligilactobacillus salivarius MP100 as an alternative to metaphylactic antimicrobials in swine: the impact on production parameters and meat composition. Animals 13, 1653. doi: 10.3390/ani13101653
Amatucci L., Luise D., Luppi A., Virdis S., Prosperi A., Cirelli A., et al (2023). Evaluation of carcass quality, body and pulmonary lesions detected at the abattoir in growing pigs subjected or not to tail docking. Porcine Health Manag 9, 4. doi: 10.1186/s40813-022-00297-4
Amelework B., Shimelis H., Tongoona P., and Laing M. (2015). Physiological mechanisms of drought tolerance in sorghum, genetic basis and breeding methods: A review. Afr. J. Agric. Res. 10, 3029–3040. doi: 10.5897/AJAR2015.9595
Beaumont M., Cauquil L., Bertide A., Ahn I., Barilly C., Gil L., et al. (2020). Gut microbiota-derived metabolite signature in suckling and weaned piglets. J. Proteome Res. 20, 982–994. doi: 10.1021/acs.jproteome.0c00745
Benz J. M., Tokach M. D., Dritz S. S., Nelssen J. L., DeRouchey J. M., Sulabo R. C., et al. (2011). Effects of increasing choice white grease in corn- and sorghum-based diets on growth performance, carcass characteristics, and fat quality characteristics of finishing pigs. J. Anim. Sci. 89, 773–782. doi: 10.2527/jas.2010-3033
Bryant M. P. and Kammerer J. J. (1985). Fatty and aromatic acid catabolizing bacteria from methanogenic ecosystems. Annu. Tech. Prog. Rep.
Callahan B. J., McMurdie P. J., Rosen M. J., Han A. W., Johnson A. J. A., and Holmes S. P. (2016). DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13, 581-583. doi: 10.1038/nmeth.3869
Chae B. J., Kim Y. G., Han I. K., Kim J. H., Cho W. T., Hancock J. D., et al. (2000). Effects of particle size and extrusion of maize and sorghum on ileal digestibility and growth performance in pigs weaned at 14 and 21 days of age. J. Anim. Feed Sci. 9, 665–679. doi: 10.22358/jafs/68116/2000
Chang Q., Zheng B., Zhang Y., and Zeng H. (2021). A comprehensive review of the factors influencing the formation of retrograded starch. Int. J. Biol. Macromol. 186, 163–173. doi: 10.1016/j.ijbiomac.2021.07.050
Chen J., Malhi K. K., Li X., Xu X., Kang J., Zhao B., et al. (2025). Metasilicate-based alkaline mineral water improves the growth performance of weaned piglets by maintaining gut-liver axis homeostasis through microbiota-mediated secondary bile acid pathway. Anim. Nutr. 20, 95–109. doi: 10.1016/j.aninu.2024.09.003
Cuevas-Gómez I., de Andrés J., Cardenas N., Espinosa-Martos I., and Jiménez E. (2024). Feed supplementation with Ligilactobacillus salivarius PS21603 optimises intestinal morphology and gut microbiota composition in weaned piglets. Benef. Microbes 15, 195–210. doi: 10.1163/18762891-bja00001
Den Besten G., van Eunen K., Groen A. K., Venema K., Reijngoud D. J., and Bakker B. M. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340. doi: 10.1194/jlr.R036012
De Smit S. M., de Leeuw K. D., Buisman C. J., and Strik D. P. (2019). Continuous n-valerate formation from propionate and methanol in an anaerobic chain elongation open-culture bioreactor. Biotechnol. Biofuels 12, 1–16. doi: 10.1186/s13068-019-1468-x
de Sousa A. R., de Castro Moreira M. E., Grancieri M., Toledo R. C. L., de Oliveira Araújo F., Mantovani H. C., et al. (2019). Extruded sorghum improves gut microbiota, reduces inflammation, and oxidative stress in obese rats fed a high-fat diet. J. Funct. Foods 58, 282–291. doi: 10.1016/j.jff.2019.05.009
Dixon P. (2003). VEGAN, a package of R functions for community ecology. J Veg Sci 14, 927-930. doi: 10.1111/j.1654-1103.2003.tb02228.x
Doré J. and Bryant M. P. (1989). Lipid growth requirement and influence of lipid supplement on fatty acid and aldehyde composition of Syntrophococcus sucromutans. Appl. Environ. Microbiol. 55, 927–933. doi: 10.1128/aem.55.4.927-933.1989
European Commission (2000). Communication from the Commission on the characteristics of products to be supplied under the Community food aid programme, (2000/C 312/01). Off. J. Eur. Communities C 312, 1–26.
European Commission (2020). Farm to fork strategy: for a fair, healthy and environmentally-friendly food system. Commun. Commun. Eur. Parliament Council Eur. Econ. Soc Comm. Commun. Regions 381, 1–9.
Fang F., He Y. X., Wang H. Q., Zhang Y. L., Zhong Y. D., Hu X. T., et al. (2025). Impact of eight extruded starchy whole grains on glycemic regulation and fecal microbiota modulation. Food Hydrocoll. 160, 110756. doi: 10.1016/j.foodhyd.2024.110756
Fang M. X., Zhang W. W., Zhang Y. Z., Tan H. Q., Zhang X. Q., Wu M., et al. (2012). Brassicibacter mesophilus gen. nov., sp. nov., a strictly anaerobic bacterium isolated from food industry wastewater. Int. J. Syst. Evol. Microbiol. 62, 3018–3023. doi: 10.1099/ijs.0.034660-0
FAO (2018). The future of food and agriculture: alternative pathways to 2050 (Rome: Food Agric. Organ. United Nations).
Girard M. and Bee G. (2020). Tannins as a potential alternative to antibiotics to prevent coliform diarrhea in weaned pigs. Anim. 14, 95–107. doi: 10.1017/S1751731119002143
Go D., Yeon G. H., Park S. J., Lee Y., Koh H. G., Koo H., et al. (2024). Integration of metabolomics and other omics: from microbes to microbiome. Appl. Microbiol. Biotechnol. 108, 538. doi: 10.1007/s00253-024-13384-z
Graziosi M. V., Luise D., Amarie R. E., Correa F., Elmi A., Virdis S., et al. (2024). A growing-finishing diet formulated to reduce the soybean meal does not compromise the growth performance, health, behaviour and gut health of Italian heavy pigs. Ital. J. Anim. Sci. 23, 1507–1523. doi: 10.1080/1828051X.2024.2409349
Haziman M. L., Ishaq M. I., Qonit M. A. H., Lestari E. G., Susilawati P. N., Widarsih W., et al. (2025). Sorghum starch review: Structural properties, interactions with proteins and polyphenols, and modification of physicochemical properties. Food Chem. 463, 139810. doi: 10.1016/j.foodchem.2024.139810
Hodges H. E., Walker H. J., Cowieson A. J., Falconer R. J., and Cameron D. D. (2021). Latent anti-nutrients and unintentional breeding consequences in Australian Sorghum bicolor varieties. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.625260
Hu J., Chen J., Xu X., Hou Q., Ren J., and Yan X. (2023). Gut microbiota-derived 3-phenylpropionic acid promotes intestinal epithelial barrier function via AhR signaling. Microbiome 11, 102. doi: 10.1186/s40168-023-01551-9
Indo Y., Kitahara S., Tomokiyo M., Araki S., Islam M. A., Zhou B., et al. (2021). Ligilactobacillus salivarius strains isolated from the porcine gut modulate innate immune responses in epithelial cells and improve protection against intestinal viral-bacterial superinfection. Front. Immunol. 12. doi: 10.3389/fimmu.2021.652923
Jacobson A., Lam L., Rajendram M., Tamburini F., Honeycutt J., Pham T., et al. (2018). A gut commensal-produced metabolite mediates colonization resistance to Salmonella infection. Cell Host Microbe 24, 296–307. doi: 10.1016/j.chom.2018.07.002
Jha R. and Berrocoso J. D. (2015). Dietary fiber utilization and its effects on physiological functions and gut health of swine. Anim 9, 1441-1452. doi: 10.1017/S1751731115000919
Kamble A., Sawant S., and Singh H. (2020). 16S ribosomal RNA gene-based metagenomics: A review. Biomed. Res. J. 7, 5–11. doi: 10.4103/BMRJ.BMRJ_4_20
Kircher B., Woltemate S., Gutzki F., Schlüter D., Geffers R., Bähre H., et al. (2022). Predicting butyrate-and propionate-forming bacteria of gut microbiota from sequencing data. Gut Microbes 14, 2149019. doi: 10.1080/19490976.2022.2149019
Li P., Feng X., Ma Z., Yuan Y., Jiang H., Xu G., et al. (2024). Microbiota-derived 3-phenylpropionic acid promotes myotube hypertrophy by Foxo3/NAD+ signaling pathway. Cell Biosci. 14, 62. doi: 10.1186/s13578-024-01244-2
Liu Y. S., Li S., Wang X. F., Xing T., Li J. L., Zhu X. D., et al. (2021). Microbiota populations and short-chain fatty acids production in cecum of immunosuppressed broilers consuming diets containing γ-irradiated Astragalus polysaccharides. Poult. Sci. 100, 273–282. doi: 10.1016/j.psj.2020.09.089
Liu S. Y., Selle P. H., and Cowieson A. J. (2013). Strategies to enhance the performance of pigs and poultry on sorghum-based diets. Anim. Feed Sci. Technol. 181, 1–14. doi: 10.1016/j.anifeedsci.2013.01.008
Love M. I., Anders S., and Huber W. (2017). Analyzing RNA-seq data with DESeq2. R package reference manual.
Mariscal-Landín G., de Souza T. R., and Avalos M. A. (2010). Ileal amino acids digestibility of sorghum in weaned piglets and growing pigs. Anim. 4, 1341–1348. doi: 10.1017/S1751731110000273
Martinez Arbizu P. (2020). pairwiseAdonis: Pairwise multilevel comparison using adonis. R package version 0.4:1.
McMurdie P. J. and Holmes S. (2013). phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217. doi: 10.1371/journal.pone.0061217
Miller T. L. and Wolin M. J. (1996). Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Environ. Microbiol. 62, 1589–1592. doi: 10.1128/aem.62.5.1589-1592.1996
Mottet A., de Haan C., Falcucci A., Tempio G., Opio C., and Gerber P. (2017). Livestock: On our plates or eating at our table? A New Anal. feed/food debate. Glob. Food Secur. 14, 1–8. doi: 10.1016/j.gfs.2017.01.001
National Research Council, Division on Earth, Life Studies, Committee on Nutrient Requirements of Swine (2012). Nutrient requirements of swine (Washington DC: Natl. Acad. Press).
Nery J., Goudez R., Biourge V., Tournier C., Leray V., Martin L., et al. (2012). Influence of dietary protein content and source on colonic fermentative activity in dogs differing in body size and digestive tolerance. J. Anim. Sci. 90, 2570–2580. doi: 10.2527/jas.2011-4112
Pallister T., Jackson M. A., Martin T. C., Zierer J., Jennings A., Mohney R. P., et al. (2017). Hippurate as a metabolomic marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Sci. Rep. 7, 13670. doi: 10.1038/s41598-017-13722-4
Palumbo F., Luise D., Virdis S., Correa F., Bassi P., and Trevisi P. (2023). Relationship between growing pig’s housing conditions, behaviours, lesions and health issues under Italian farming system. Ital. J. Anim. Sci. 22, 1040–1049. doi: 10.1080/1828051X.2023.2268116
Palumbo F., Trevisi P., Dalcanale S., and Luise D. (2025). Implication of environmental and management conditions on welfare and health parameters of post-weaned pigs under intensive farming system. Ital. J. Anim. Sci. 24, 894–904. doi: 10.1080/1828051X.2025.2483943
Pan L., An D., and Zhu W. Y. (2021). Sorghum as a dietary substitute for corn reduces the activities of digestive enzymes and antioxidant enzymes in pigs. Anim. Feed Sci. Technol. 273, 114831. doi: 10.1016/j.anifeedsci.2021.114831
Pan L., Li W., Gu X. M., and Zhu W. Y. (2022). Comparative ileal digestibility of gross energy and amino acids in low and high tannin sorghum fed to growing pigs. Anim. Feed Sci. Technol. 292, 115419. doi: 10.1016/j.anifeedsci.2022.115419
Pedersen K. S., Skrubel R., Stege H., Angen Ø., Ståhl M., and Hjulsager C. K. (2012). Association between average daily gain, faecal dry matter content and concentration of Lawsonia intracellularis in faeces. Acta Vet. Scand. 54, 58. doi: 10.1186/1751-0147-54-58
Piecyk M., Drużyńska B., Ołtarzewska A., Wołosiak R., Worobiej E., and Ostrowska-Ligęza E. (2018). Effect of hydrothermal modifications on properties and digestibility of grass pea starch. Int. J. Biol. Macromol. 118, 2113–2120. doi: 10.1016/j.ijbiomac.2018.07.063
Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41, D590-D596. doi: 10.1093/nar/gks1219
Rabhi N., Thibodeau A., Côté J. C., Devillers N., Laplante B., Fravalo P., et al. (2020). Association between tail-biting and intestinal microbiota composition in pigs. Front. Vet. Sci. 7. doi: 10.3389/fvets.2020.563762
Richardson A. J., McKain N., and Wallace R. J. (2013). Ammonia production by human faecal bacteria, and the enumeration, isolation and characterization of bacteria capable of growth on peptides and amino acids. BMC Microbiol. 13, 6. doi: 10.1186/1471-2180-13-6
Rodrigues E. A., Badiola I., Francesch M., and Torrallardona D. (2016). Effect of cereal extrusion on performance, nutrient digestibility, and cecal fermentation in weanling pigs. J. Anim. Sci. 94, 298–302. doi: 10.2527/jas.2015-9745
Rodríguez J. M., Garranzo M., Segura J., Orgaz B., Arroyo R., Alba C., et al. (2023). A randomized pilot trial assessing the reduction of gout episodes in hyperuricemic patients by oral administration of Ligilactobacillus salivarius CECT 30632, a strain with the ability to degrade purines. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1111652
Rodriguez D. A., Lee S. A., Jones C. K., Htoo J. K., and Stein H. H. (2020). Digestibility of amino acids, fiber, and energy by growing pigs, and concentrations of digestible and metabolizable energy in yellow dent corn, hard red winter wheat, and sorghum may be influenced by extrusion. Anim. Feed Sci. Technol. 268, 114602. doi: 10.1016/j.anifeedsci.2020.114602
Salazar Lopez N. J., Loarca-Piña G., Campos-Vega R., Gaytán Martínez M., Morales Sánchez E., Esquerra-Brauer J. M., et al. (2016). The extrusion process as an alternative for improving the biological potential of sorghum bran: Phenolic compounds and antiradical and anti-inflammatory capacity. Evid. Based Complement. Alternat. Med. 2016, 8387975. doi: 10.1155/2016/8387975
Siddiqui M. T. and Cresci G. A. (2021). The immunomodulatory functions of butyrate. J. Inflamm. Res. 14, 6025–6041. doi: 10.2147/JIR.S300989
Slavin J. (2013). Fiber and prebiotics: mechanisms and health benefits. Nutrients 5, 1417–1435. doi: 10.3390/nu5041417
Sporchia F., Kebreab E., and Caro D. (2021). Assessing the multiple resource use associated with pig feed consumption in the European Union. Sci. Total Environ. 759, 144306. doi: 10.1016/j.scitotenv.2020.144306
Stein H. H., Lagos L. V., and Casas G. A. (2016). Nutritional value of feed ingredients of plant origin fed to pigs. Anim. Feed Sci. Technol. 218, 33–69. doi: 10.1016/j.anifeedsci.2016.05.003
Tao X., Xu Z., and Wan J. (2015). Intestinal microbiota diversity and expression of pattern recognition receptors in newly weaned piglets. Anaerobe 32, 51-56. doi: 10.1016/j.anaerobe.2014.12.005
Thomas L. L., Espinosa C. D., Goodband R. D., Stein H. H., Tokach M. D., Dritz S. S., et al. (2020). Nutritional evaluation of different varieties of sorghum and the effects on nursery pig growth performance. J. Anim. Sci. 98, skaa120. doi: 10.1093/jas/skaa120
Torres Cepeda T. E., MG A. G., and Maiti R. (1996). Relationship between nutritional composition and anatomical parameters in sorghum (Sorghum bicolor L. Moench). Arch. Latinoam. Nutr. 46, 253–259.
Trevisi P., Luise D., Correa F., and Bosi P. (2021). Timely control of gastrointestinal eubiosis: a strategic pillar of pig health. Microorganisms 9, 313. doi: 10.3390/microorganisms9020313
Vente-Spreeuwenberg M. A. M., Verdonk J. M. A. J., Beynen A. C., and Verstegen M. W. A. (2003). Interrelationships between gut morphology and faeces consistency in newly weaned piglets. Anim. Sci. 77, 85–94. doi: 10.1017/S1357729800053686
Virdis S., Luise D., Correa F., Laghi L., Arrigoni N., Amarie R. E., et al. (2024). Productive and metabolomic consequences of arginine supplementation in sows during different gestation periods in two different seasons. J. Anim. Sci. Biotechnol. 15, 121. doi: 10.1186/s40104-024-01079-4
Vital M., Karch A., and Pieper D. H. (2017). Colonic butyrate-producing communities in humans: an overview using omics data. Msystems 2, 00130–00117. doi: 10.1128/msystems.00130-17
Vitali M., Santacroce E., Correa F., Salvarani C., Maramotti F. P., Padalino B., et al. (2020). On-farm welfare assessment protocol for suckling piglets: a pilot study. Animals 10, 1016. doi: 10.3390/ani10061016
Wolf P. G., Cowley E. S., Breister A., Matatov S., Lucio L., Polak P., et al. (2021). Diversity and distribution of sulfur metabolism in the human gut microbiome and its association with colorectal cancer. bioRxiv 2021-07, 450790. doi: 10.1101/2021.07.01.450790
Xie Y. Y., Hu X. P., Jin Z. Y., Xu X. M., and Chen H. Q. (2014). Effect of repeated retrogradation on structural characteristics and in vitro digestibility of waxy potato starch. Food Chem. 163, 219–225. doi: 10.1016/j.foodchem.2014.04.102
Yadav G. P., Dalbhagat C. G., and Mishra H. N. (2022). Effects of extrusion process parameters on cooking characteristics and physicochemical, textural, thermal, pasting, microstructure, and nutritional properties of millet-based extruded products: A review. J. Food Process Eng. 45, e14106. doi: 10.1111/jfpe.14106
Yu S. J., Morris A., Kayal A., Milošević I., Van T. T. H., Bajagai Y. S., et al. (2024). Pioneering gut health improvements in piglets with phytogenic feed additives. Appl. Microbiol. Biotechnol. 108, 142. doi: 10.1007/s00253-023-12925-2
Zhao J., Liu P., Wu Y., Guo P., Liu L., Ma N., et al. (2018). Dietary fiber increases butyrate-producing bacteria and improves the growth performance of weaned piglets. J. Agric. Food Chem. 66, 7995–8004. doi: 10.1021/acs.jafc.8b02545
Keywords: swine nutrition, extrusion, environmental sustainability, microbiota, metabolomics
Citation: Palumbo F, Correa F, Laghi L, Zurru A, Trevisi P and Luise D (2025) Water-sustainable feeding strategies in post-weaning piglets: effects of sorghum-based diets on growth performance and gut health. Front. Anim. Sci. 6:1670477. doi: 10.3389/fanim.2025.1670477
Received: 21 July 2025; Accepted: 12 September 2025;
Published: 16 October 2025.
Edited by:
Bianca Castiglioni, National Research Council (CNR), ItalyReviewed by:
Yuwen Dong, University of Pennsylvania, United StatesZhiqing Li, Hunan Agricultural University, China
Copyright © 2025 Palumbo, Correa, Laghi, Zurru, Trevisi and Luise. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diana Luise, ZGlhbmEubHVpc2UyQHVuaWJvLml0
 Francesco Palumbo
Francesco Palumbo Federico Correa
Federico Correa Luca Laghi
Luca Laghi Antonio Zurru
Antonio Zurru Paolo Trevisi
Paolo Trevisi Diana Luise
Diana Luise