- 1Department of Veterinary Science, University of Turin, Grugliasco, Italy
- 2Department of Food Safety, Nutrition and Veterinary Public Health, Unit of Emerging Zoonoses, Istituto Superiore di Sanità, Rome, Italy
Housing systems strongly influence the welfare, growth performance, and meat quality of farmed rabbits, particularly in local slow-growing breeds requiring specific management. This study assessed the effects of different housing systems on Grigio di Carmagnola rabbits, aiming to identify solutions balancing welfare and productivity. Three housing systems—single cage (S), group colony (G), and mixed pilot (M)—were compared using 300 weaned rabbits evenly distributed among treatments. Due to aggression and high mortality, the G group was excluded at 100 days of age, before reaching the commercial slaughter ages of 120 and 150 days. Growth traits, haematological and intestinal parameters, carcass composition, and meat quality were evaluated. Growth performance did not differ significantly between S and M groups, while slaughter age influenced weight gain, feed conversion, and carcass composition. Lymphocyte counts increased with age, indicating immune maturation. The M group showed higher jejunal villus height, and gastrointestinal muscularis thickness decreased with age. Meat fat content increased with age but was unaffected by housing system. The M system fostered early socialization and reduced post-pubertal aggression. The mixed pilot system provided a balance between welfare and performance, combining social contact with manageable behavior. Appropriate housing strategies tailored to the physiology and growth rate of autochthonous breeds are crucial to maintain productivity, biodiversity, and sustainable local farming. Further research should explore housing designs and enrichment to enhance ethical rabbit production.
1 Introduction
Previous research provides valuable insights into the impact of different housing systems (HS) on growth performance, meat quality, and welfare in rabbits (Mugnai et al., 2014; Ozella et al., 2024; Szendrő et al., 2013; Van Damme et al., 2023). The European Union (EU) is the third-largest rabbit meat producer globally, following China and North Korea (FAO FAOSTAT, 2024). In the EU, commercial rabbit production is classified by housing system, with conventional cages (barren cages) accounting for 85% of total production, enriched cages for 9%, and pen or “park” systems for 6% (European Commission, 2017). The European Food Safety Authority has published a scientific opinion on rabbit health and welfare within the EU (EFSA Panel on Animal Health and Welfare (AHAW), 2020). This document compares various production systems, highlighting a reduction in welfare associated with conventional cages (wired barren cages housing one (unicellular) or two (bicellular) rabbits). Welfare concerns are primarily attributed to restricted movement and the inability to exhibit natural behaviors, including social interactions (EFSA Panel on Animal Health and Welfare (AHAW), 2020). In response to these findings and pressure from the “End the Cage Age” initiative submitted to the European Commission in October 2020, EU legislation is being revised to promote alternative HS. For example, the Italian Ministry of Health’s new guidelines for growing rabbits recommend pen and group farming (Circular No. 1/2021 DGSAF). However, transitioning to modernized production systems imposes financial burdens, particularly on small- and medium-sized farms that breed local, slow-growing rabbits (slaughtered at 120–150 days). These farms face greater challenges than industrial producers, who use fast-growing lines (slaughtered at 70–77 days) with a feed conversion ratio (FCR) of approximately 2.5. By contrast, local breeds often exhibit slower growth rates and higher FCR, increasing production costs. Despite these challenges, preserving local breeds is vital for maintaining biodiversity and safeguarding the unique genetic resources and cultural heritage of typical livestock products (Notter, 1999). These breeds are adapted to local environments, displaying greater resilience, disease resistance, and hardiness. Additionally, they contribute ecosystem services and cultural value, enhancing their appeal as niche products in local culinary traditions (Biodiversity for Food and Agriculture and Ecosystem Services, 2020). Such traits will be crucial for adapting to climate change and ensuring food security for future generations (Leroy et al., 2018).
Group farming presents a complex balance of welfare, productivity, and social dynamics, especially for breeding does. While group housing can replicate the natural social structure of wild rabbits, studies reveal increased stress and aggression, often leading to higher mortality and morbidity rates among kits and does. Elevated corticosterone levels, indicative of stress, and significant kit mortality due to aggressive behaviors such as biting and scratching have been observed in group-housed does (Ozella et al., 2024). Additionally, hierarchy formation within groups can lead to fights, injuries, and further welfare issues (Van Damme et al., 2023). Some research suggests that part-time group housing for fattening rabbits could represent a compromise. This approach may help reduce aggression while maintaining some level of social interaction, although risks of injury and stress remain (Van Damme et al., 2023).
In the Piedmont region of northwestern Italy, the Grigio di Carmagnola rabbit is a native breed characterized by a gray coat with darker shades. This medium- to slow-growing breed reaches a slaughter weight of 3.5–4.5 kg at 120–150 days and, for its characteristic carcass and meat quality, is a Slow Food presidio®. Traditionally reared in single cages, this breed’s welfare may be compromised under modern pen and group farming regulations. Their natural reactivity and pronounced behaviors increase the risk of injuries and stress, especially after reaching sexual maturity (Szendrő et al., 2013). Declining welfare can manifest in physiological disruptions such as intestinal barrier dysfunction (Söderholm and Perdue, 2001) and altered hematological parameters like lymphocyte and neutrophil concentrations (Washington and Van Hoosier, 2012), ultimately affecting growth, carcass traits, and meat quality.
To implement the new EU guidelines effectively, it is crucial to consider the physiological and behavioral traits of each rabbit breed, tailoring housing systems to optimize welfare. Current public and regulatory emphasis on welfare-centric farming must also account for natural behaviors, ensuring an environment conducive to the well-being of farmed animals.
This study focuses on the growth period up to the typical commercial slaughter age (120–150 days) for the Grigio di Carmagnola rabbit and does not extend to long-term reproductive effects. While this scope presents a limitation, it aligns with the study’s primary objective of assessing growth performance and meat quality during this critical phase. Additionally, environmental and management variations were not strictly controlled; however, this enhances the study’s real-world relevance by capturing the diversity of farming practices. Although economic feasibility was not analyzed, the findings offer a valuable basis for future cost-benefit assessments, contributing to a more comprehensive evaluation of sustainable production systems.
This study aims to evaluate the effects of three housing systems—single cage (S), group colony (G), and mixed pilot system (M)—at typical commercial slaughter ages (120 and 150 days) on the welfare, growth performance, hematological parameters, intestinal health, carcass traits, and meat quality of Grigio di Carmagnola rabbits.
2 Materials and methods
2.1 Animals and experimental design
The trial was conducted at the Department of Veterinary Science, University of Turin (Italy), from March to July 2022, with approval from the University’s Bioethics Committee (Protocol no. 0245520).
A total of 300 weaned Grigio di Carmagnola rabbits (35 days old) were randomly assigned to one of three housing systems (HS) and monitored until slaughter. These systems were designed to reflect different living conditions and developmental stages.
In the single-cage system (S), 100 rabbits were housed individually in cages measuring 60 × 25 × 33 cm (1,500 cm²), with one rabbit per cage (one replicate). In the group-farming system (G), 100 rabbits were kept in collective pens, each containing 10 rabbits, with one collective cage as a replicate per housing unit. These enclosures measured 200 × 100 × 100 cm (20,000 cm²), providing more space and allowing social interaction.
The mixed pilot system (M) initially followed the same group-housing conditions as the G system, with 100 rabbits housed in 10 collective cages. At 78 days of age, the rabbits were transferred to single cages. This hybrid approach was designed to assess the impact of both group and individual housing at different stages of development.
All experimental groups were kept in the same artificially ventilated building (McBride, 1988) with an airflow rate of 0.3 m/s. Environmental parameters, including temperature (+15 to +28°C) and relative humidity (60%–75%), were monitored daily. A 12-h light/dark cycle (12L/12D) was maintained throughout the study. Rabbits were provided ad libitum access to feed (CP 16.4%, EE 2.5%, fiber 15.5%, ash 7.6%, minerals – Ca, P, and Na – 2.0%) and water and were checked daily for health status, with any deceased animals promptly removed.
At 100 days of age, the experimental protocol was revised due to increased agonistic and violent behaviors observed in the G group. Following puberty, at around 70–80 days of age, the frequency of aggressive interactions and fights in this group severely impacted rabbit health and welfare, resulting in an increase in injuries. For ethical and welfare reasons, all rabbits in the G group were slaughtered and excluded from the trial at 100 days of age, and the study continued with only the S and M groups.
Finally, two slaughter sessions were performed—one at 120 days and the other at 150 days of age—with 20 rabbits randomly sampled per housing system and slaughter age. The presence of rabbit lesions was recorded as 0 = no skin injuries and 1 = skin injuries due to scratches or deeper lesions caused by bites.
2.2 Growth performance and carcass measurements
Live weight (LW) of rabbits was recorded weekly, and the average daily gain (ADG) was calculated individually. Average daily feed intake (ADFI) and mortality rate were recorded weekly, and the feed conversion ratio (FCR) was calculated as grams of feed consumed per gram of weight gain.
At 120 and 150 days of age, rabbits from the S and M groups were slaughtered in an authorized commercial slaughterhouse following protocols to ensure animal welfare, after fasting for 12 h. Before slaughter, animals were weighed to obtain slaughter weight (SW). Slaughtering and carcass dissection procedures followed the recommendations of the World Rabbit Science Association (WRSA) described by Blasco et al. (2010).
After jugulation, blood samples were collected in 5 mL EDTA tubes and immediately refrigerated at +4°C until analysis. The stunned rabbits were bled, and then the skin, gastrointestinal tract, distal part of the legs, urinary bladder, and genital organs were removed.
The hot carcass (HC), including the head but excluding the liver, kidneys, and intravisceral fat, was weighed. Carcasses were then chilled at 4°C for 24 h. After chilling, the cold carcass (CC) weight was determined by weighing the chilled carcasses, and the weights of the head, liver, kidneys, spleen, heart, and lungs were recorded.
Slaughter yield, expressed as a percentage, was calculated by dividing the CC weight by the SW. Additionally, the proportions of individual organs and carcass parts relative to CC weight were calculated as needed. The dressing-out percentage was determined by dividing HC weight by SW.
2.3 Blood analysis
At each slaughter age, blood samples (2 ml for each rabbit) were collected (on 8 rabbits per group). After collection, samples were immediately sent to the laboratory where they were centrifuged and frozen at -80°C until analysis. Complete blood count was performed on EDTA blood samples with an automated laser analyzer (ADVIA®120 Haematology System, Siemens Diagnostics). Automated differentials were checked by microscopic evaluation of blood smears stained with May Grunwald-Giemsa. On blood samples main hematic parameters (white blood cells (WBC) (103 cells/μL), neutrophils (NEUT) (%), lymphocytes (LYM) (%), monocytes (MONO) (%), eosinophils (EOS) (%), basophils (BASO) (%), large unstained cells (LUC) (%), hemoglobin (HGB) (g/dl), hematocrit (HCT) (%), mean corpuscular volume (MCV) (fL), mean corpuscular hemoglobin (MCH) (pg), mean corpuscular hemoglobin concentration (MCHC) (g/dL), cellular hemoglobin concentration mean (CHCM) (g/dL), mean cellular hemoglobin content CH (pg), red blood cell distribution width (RDW) (%),hemoglobin distribution width (HDW) (g/dL), platelets (PLT) (103 cells/μL), mean platelet volume (MPV) (fL) were determined (Ameri et al., 2011).
2.4 Histological features
At slaughter (120 and 150 days of age), gut segments approximately 5 cm in length were collected from the duodenum, jejunum, and ileum of eight animals per group for histomorphometry. The selected gut segments were flushed with a 0.9% saline solution to remove their contents. Additionally, spleen samples and one portion of the liver (0.5–1.5 g per organ) were collected.
All samples were fixed in 10% buffered formalin, embedded in paraffin wax blocks, sectioned into 5-μm slices, mounted onto glass slides, and stained with hematoxylin and eosin (H&E) for histological analysis. Liver tissue sections were also subjected to periodic acid–Schiff (PAS) staining to characterize cytoplasmic accumulations. Sections were brought to water, immersed in 0.5% periodic acid solution for 20 min, washed in running tap water for 5 min, and immersed in Schiff’s reagent for a further 30 min. Sections were then rinsed in running tap water for 10 min, dehydrated, and mounted. Glycoproteins stained magenta.
One slide per jejunal segment was examined by light microscopy, and each slide was captured using a Nikon DS-Fi1 digital camera (Nikon Corporation, Minato, Tokyo, Japan) coupled to a Zeiss Axiophot microscope (Carl Zeiss, Oberkochen, Germany) with a 2.5× objective lens. The NIS-Elements F software was used for image capture, and morphometric analysis was performed using Image-Pro Plus software (version 6.0, Media Cybernetics, Maryland, USA).
The evaluated morphometric indices were villus height (from the tip of the villus to the crypt), crypt depth (Cd; from the base of the villus to the submucosa), villus width, and the villus height-to-crypt depth ratio (Laudadio et al., 2012). These morphometric analyses were performed on 10 well-oriented and intact villi and 10 crypts chosen from the jejunum. Mucosal and muscular thickness were measured at three standardized points of the gut mucosal and muscular layers per captured field.
In addition, the following histopathological alterations were evaluated: hepatocyte degeneration and inflammation in the liver, and follicular hyperplasia or depletion in the spleen. Gut histopathological findings were separately assessed for the mucosa (inflammatory infiltrates) and submucosa (inflammatory infiltrates and gut-associated lymphoid tissue [GALT] activation) for each gut segment (duodenum, jejunum, and ileum).
All observed histopathological alterations and PAS expression were evaluated using a semiquantitative scoring system as follows: absent (score = 0), mild (score = 1), moderate (score = 2), and severe (score = 3) (Del Piano et al., 2024; Pacorig et al., 2022). The total score of each gut segment was obtained by adding the mucosa and submucosa scores. All slides were evaluated independently by two blinded observers.
2.5 Chemical analyses and hygienic traits of meat
After 24 h at 4°C, the ultimate pH of the Longissimus lumborum was measured using a combined glass-penetrating electrode (Ingold, Mettler Toledo, Greifensee, Switzerland). Afterward, muscles were dissected, emptied, packaged, and stored at −20°C until analysis.
Chemical analysis of meat (dry matter [DM], ash, protein, and fat percentages) was performed by an accredited laboratory (DGRL G065753 del 04.05.2017; GRUPPO MAURIZI srl, Via Pellaro 22, 00178 Rome, Italy), following Rapporti ISTISAN 1996/34, while total protein was determined according to ISO 1871:2009. Meat lipids (samples of about 5 g) were extracted in a homogenizer with 20 mL of 2:1 chloroform–methanol (Folch et al., 1957), followed by filtration through Whatman No. 1 filter paper.
To assess production process hygiene levels, a trained operator sampled 20 rabbit carcasses per group at 120 and 150 days of age using a prehydrated sponge with 10 mL of buffered peptone water. ISO procedures were followed for total mesophilic aerobic bacteria counts (TMABc) and Enterobacteriaceae counts (ISO 4833–1:2013 and ISO 21528–2:2017, respectively).
To evaluate contamination of the left thigh muscle, a sample was aseptically collected to isolate Salmonella spp. (ISO 6579–1:2017), Escherichia coli spp. (ISO 16649–12:2001), and Listeria monocytogenes (ISO 11290–2). Results were expressed as CFU/g.
2.6 Statistical analysis
A two-way analysis of variance (ANOVA) was used to evaluate the effects of housing system, time, and their interactions. Multiple comparisons of means were carried out by calculating the least significant difference using the Duncan test. Statistical analyses were performed using R software, version 3.1.2 (R Core Team, 2014), with a significance level of P < 0.05 considered statistically significant. Differences in mortality and lesion percentages were evaluated using the χ² test, with significance set at P < 0.05.
Statistical analysis was also conducted using RStudio software (2024.12.0 + 467). The normality of data distribution was assessed using the Shapiro–Wilk test, while Levene’s test was used to evaluate the homogeneity of variances. Performance at 100, 120, and 150 days of age, slaughter performance, and hematological parameters were analyzed using a generalized linear mixed model. This model included two fixed factors—housing system and time—and their interaction. Additionally, the age of the animals was included as a covariate to account for its potential impact on growth performance.
Histopathological scores were analyzed using a generalized linear mixed model with a negative binomial distribution. Results were presented as least square means along with the standard error of the mean (SEM). Statistical significance was set at P ≤ 0.05.
3 Results
3.1 Growth and slaughtering performance and carcass traits
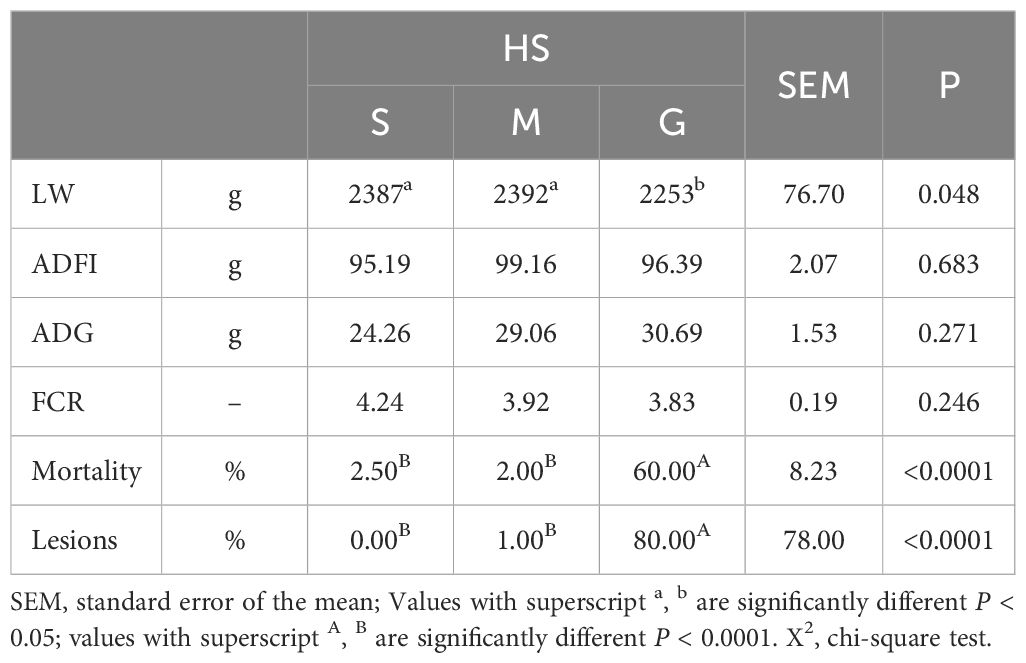
Table 1 presents the productive performance of rabbits at 100 days. The G system showed significantly lower live weight (LW) and the highest mortality and number of injured rabbits (P < 0.05, P < 0.0001, and P < 0.0001, respectively), whereas S and M rabbits reached similar productive performance.
Table 2 presents the productive performance of S and M rabbits that reached the commercial slaughter ages of 120 and 150 days. Slaughter age (SA) showed a significant effect on LW, average daily feed intake (ADFI), and feed conversion ratio (FCR) (P < 0.001, P < 0.001, and P < 0.01, respectively). The increase in body mass was accompanied by a significant rise in ADFI, which increased from 110.4 g at 120 days to 125.9 g at 150 days, reflecting higher nutritional demands as rabbits matured. Furthermore, the FCR also increased significantly, rising from 4.9 at 120 days to 6.5 at 150 days. Notably, no significant differences in average daily weight gain or mortality rate were observed based on housing system or slaughter age.
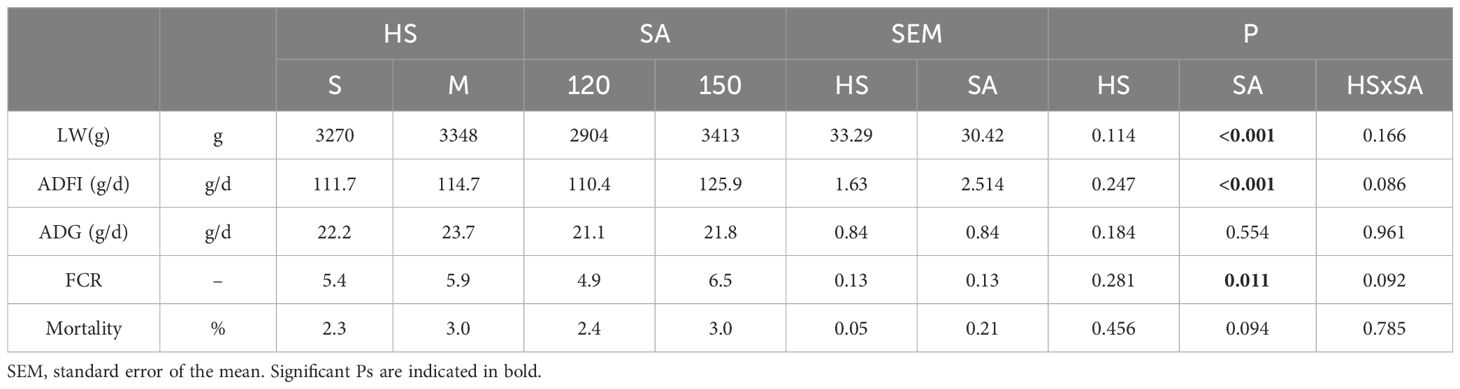
Table 2. Effect of housing system and slaughter age on live weight productive performance of Grigio di Carmagnola rabbits.
Table 3 summarizes the carcass and meat quality traits of Grigio di Carmagnola rabbits. Slaughter age significantly influenced several parameters. Slaughter weight (SW) increased from 3,011 g at 120 days to 3,418 g at 150 days (P < 0.05). Ready-to-cook weight (RTC) rose from 2,186 g to 2,405 g (P < 0.05), and cold carcass weight (CC) increased from 2,158 g to 2,354 g (P < 0.05).
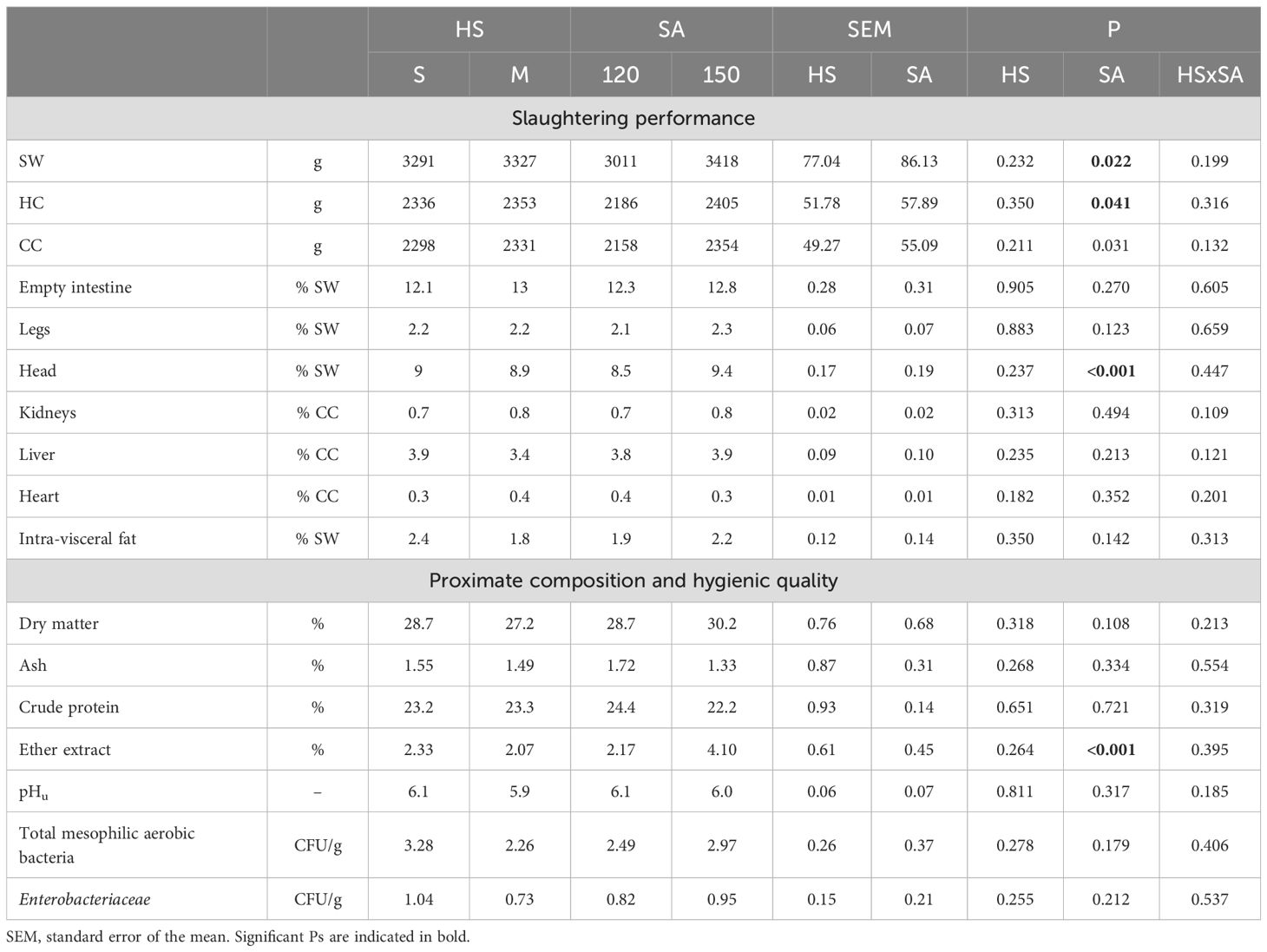
Table 3. Effect of housing system and slaughter age on the slaughtering performance of Grigio di Carmagnola rabbits.
In contrast, carcass yield decreased from 72% to 68% (P < 0.05). Additionally, head proportion showed a marked rise from 8.5% to 9.4% (P < 0.001). Regarding the proximate composition of meat, neither SA nor HS affected most parameters. However, ether extract content in the meat significantly increased, from 2.17% at 120 days to 4.10% at 150 days (P < 0.001). Finally, there were no significant differences in microbiological contamination (total mesophilic aerobic bacteria count and Enterobacteriaceae counts) attributable to either SA or HS.
3.2 Blood parameters
Overall, no notable differences were observed in blood parameters (Table 4), except for the percentage of lymphocytes, which was significantly affected by the housing system (P < 0.05). Rabbits in the M group displayed a higher lymphocyte percentage (54.77%) compared to those in the S group (40.55%). Slaughter age also showed a significant effect on lymphocyte percentage (P = 0.006), which increased with age (48.6% vs 54.7% at 120 and 150 days, respectively).
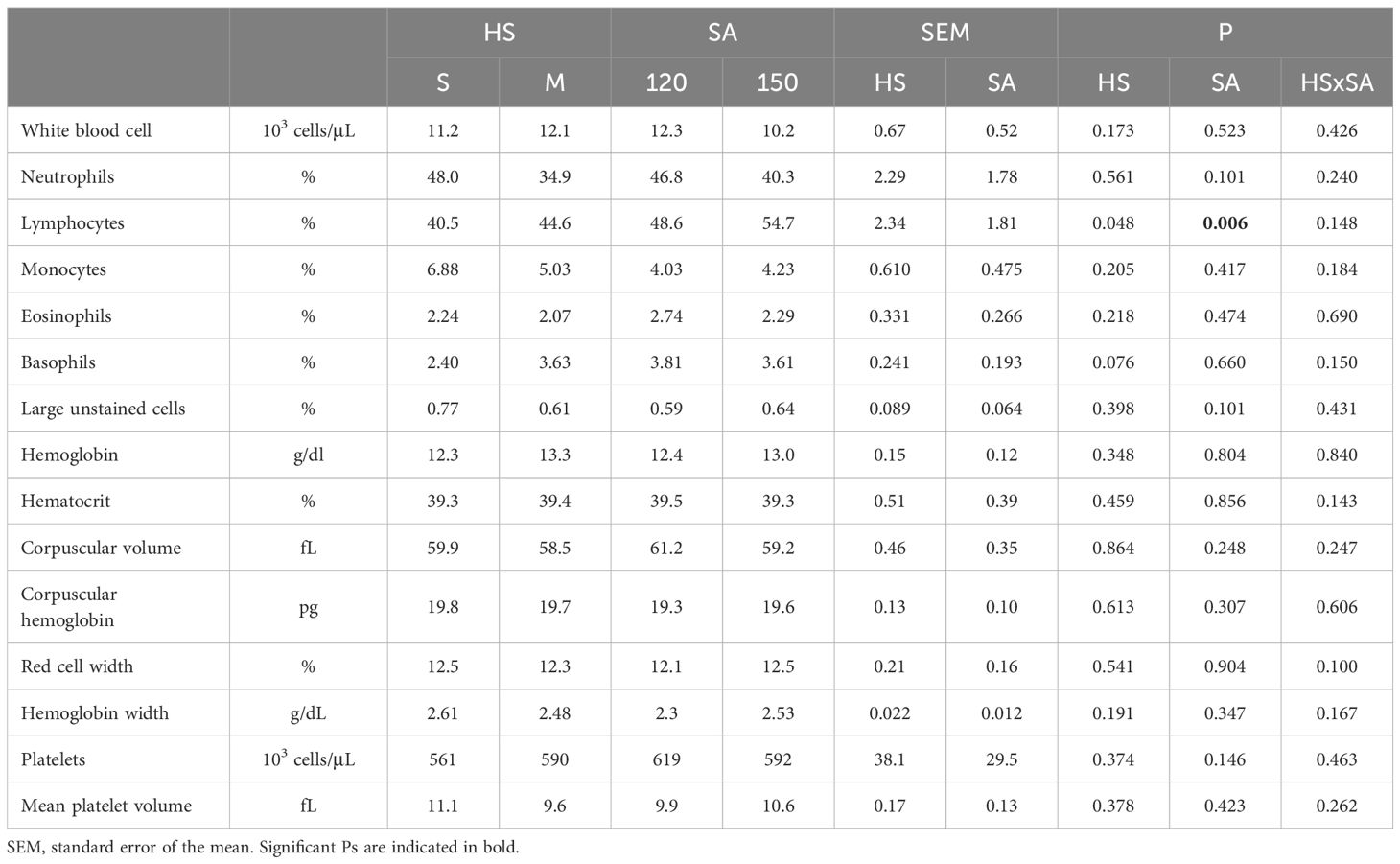
Table 4. Effect of housing system and slaughter age on blood parameters of Grigio di Carmagnola rabbits.
3.3 Histological features
Table 5 presents the morphometric and histopathological features observed in rabbits, showing that most parameters remained unchanged under the tested conditions. However, the housing system exerted a significant effect on jejunal villus height (P < 0.05), with rabbits in the M group exhibiting longer villi (0.32 mm) compared to those in the S group (0.26 mm). The same M group also exhibited higher mucosal weight.
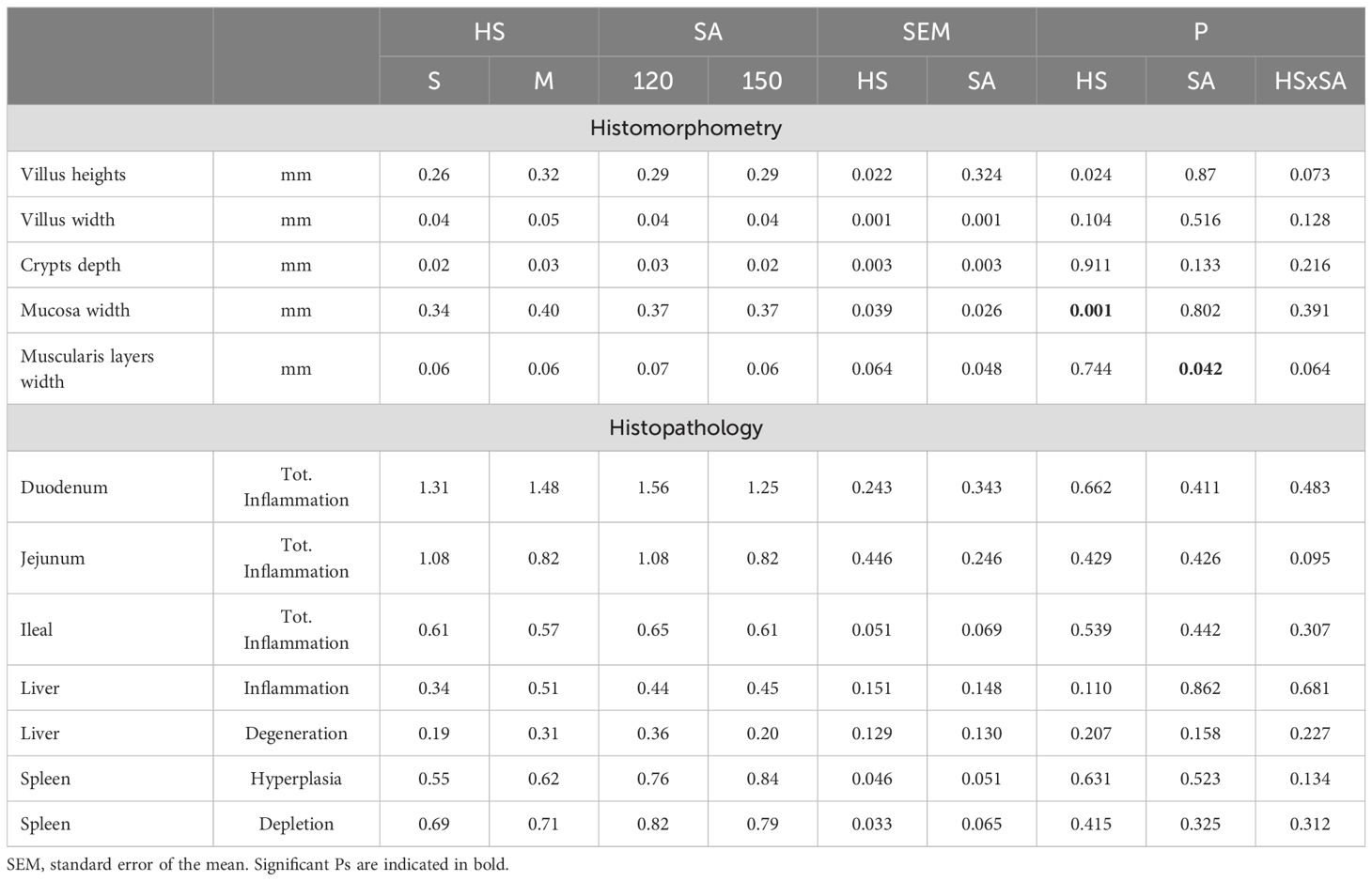
Table 5. Effect of housing system and slaughter age on jejunal histomorphometry and histopathology of all organs in Grigio di Carmagnola rabbits.
Age at slaughter affected (P > 0.05) the width of the muscularis layers, which decreased at older ages. Other histopathological alterations varied from absent or minimal to mild in different intestinal segments, spleen, and liver, but no significant influence of SA was observed (P > 0.05) (Figures 1A–E). The PAS histochemical stain performed on liver sections revealed that glycoproteins were responsible for hepatocyte degeneration, with severity ranging from mild to moderate and no differences among groups (Figure 1F).
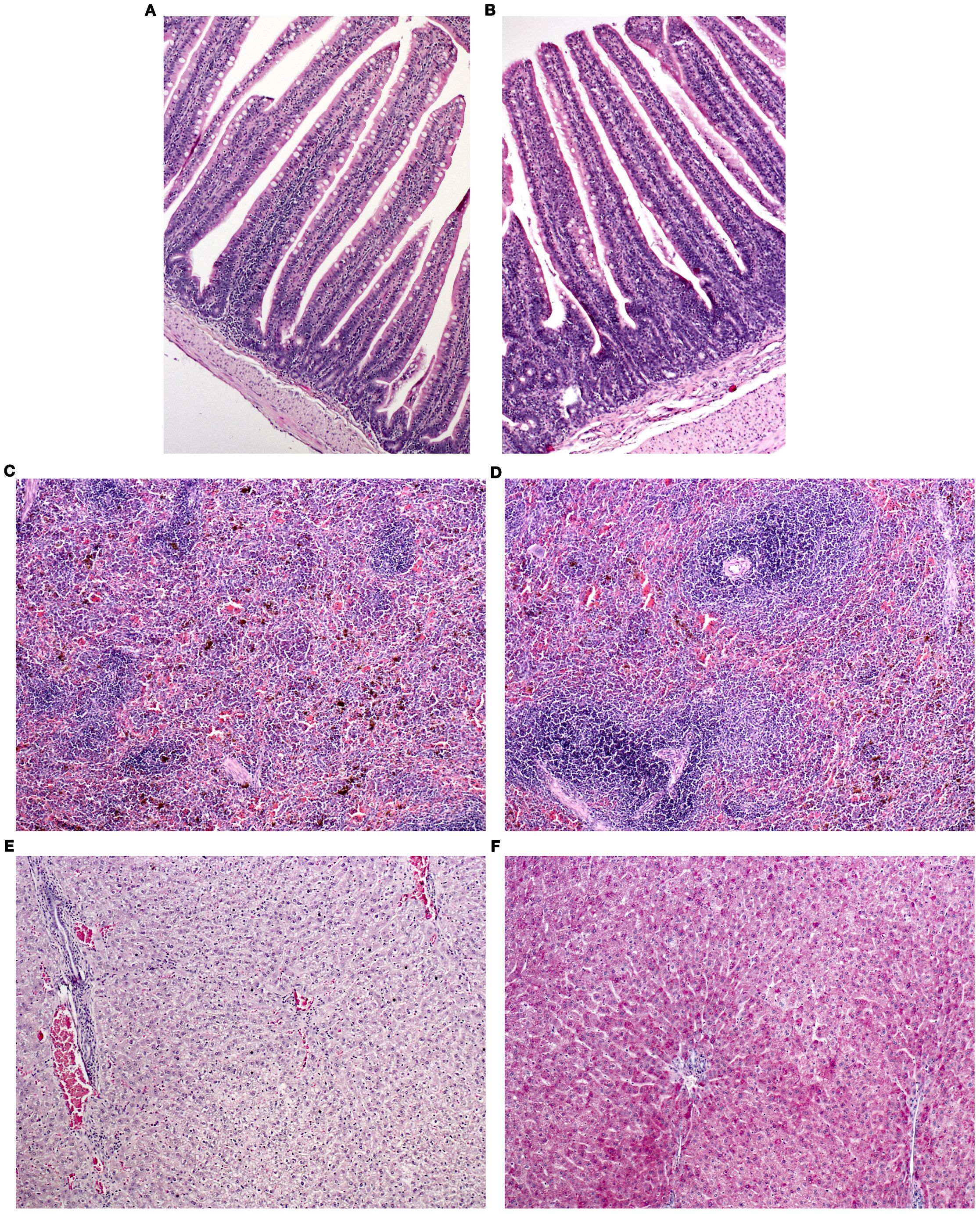
Figure 1. Histopathological features of the organs of Grigio di Carmagnola rabbits. (A) jejunum of a rabbit from the single-cage group (S): mild inflammation in the lamina propria and at the base of the villus (asterisk); (B) jejunum of a rabbit from the mixed group (M): mild inflammation in the lamina propria of the villi (asterisks); (C) spleen of a rabbit from the S group: absence of lesions (score 0); (D) spleen of a rabbit from the M group: mild hyperplasia (score 1) (asterisks); (E) liver of a rabbit from the M group: focal inflammation (score 0.5) and mild, diffuse degeneration (score 1); (F) liver of a rabbit from the group M: mild and multifocal to disseminated glycoprotein accumulation (score 1). (A–E) hematoxylin and eosin stain, 200x; (F) PAS stain, 200x.
4 Discussion
The primary objective of this study was to assess the impact on Grigio di Carmagnola production of adaptation to new regulatory requirements that impose group housing in meat rabbit production. As previously mentioned, the effects of group HS on rabbits after puberty clearly pose challenges to animal health and welfare.
It is known that issues with aggressive behavior leading to harm in rabbit meat production arise toward the end of the fattening stage, which lasts between 60 and 90 days—just prior to puberty—but the incidence of aggression peaks around 80 days. The general explanation for this phenomenon is the rise in sexual maturity (Szendrő et al., 2009; Vervaecke et al., 2010).
To ensure compliance with welfare regulations and maintain an ethical farming approach, the trial was discontinued for the G group due to the rapid escalation of dominance and aggressive behaviors among conspecifics, which exacerbated rabbit mortality and injury (Ozella et al., 2024). Consequently, all animals in the G group had to be excluded from the study at 100 days of age. These modifications in the experimental protocol were necessary and underscore the importance of considering the specific physiology of both rabbit growth and breed (sexual maturity in Grigio di Carmagnola) when implementing new management practices.
On the contrary, social housing systems—often advocated for their potential to promote natural behaviors and enhance welfare in commercial hybrid housing—can also introduce significant risks, including increased aggression and injuries, which negatively affect overall health and productive outcomes in slow-growing rabbits. Group housing in rabbit farming presents a complex interplay of advantages for fast-growing rabbits, particularly concerning animal welfare, health, and productivity when raised until 65 days. Conversely, numerous studies (Ozella et al., 2024), including our findings, have demonstrated that continuous group housing has been linked to heightened aggression, elevated cortisol concentrations, and increased kit mortality rates, all of which indicate a negative impact on welfare and productivity (Pérez-Fuentes et al., 2020; Szendrő et al., 2019; Huang et al., 2021).
These findings highlight the necessity for careful management strategies to mitigate the adverse effects of group housing. However, to maximize potential benefits while minimizing welfare risks, housing systems must be optimized through appropriate environmental modifications and management interventions (Szendrő et al., 2013; Huang et al., 2021; Pérez-Fuentes et al., 2020; Vilardo et al., 2025). A potential compromise between social and individual housing is semi-group housing, in which rabbits are housed together only during specific physiological phases (Munari et al., 2020; Braconnier et al., 2020). This approach has shown promising results in improving productivity while reducing some of the aggression- and stress-related issues observed in continuous group housing systems (Szendrő et al., 2016).
Although our study did not reveal significant differences in growth performance between the single-cage system and the mixed system, a broader analysis of the literature suggests that housing conditions can exert nuanced effects on rabbit growth, welfare, and health. Research indicates that individually housed rabbits generally exhibit higher daily weight gain and final body weight compared to those in group housing. For instance, studies have shown that rabbits housed individually demonstrate superior growth performance, with greater weight gain and final live weight than those in bi-cage or collective housing, despite similar feed conversion rates across different housing types (Xiccato et al., 2013).
In accordance, single-cage systems—though restricting social interactions and movement—generally result in lower stress levels and reduced injury rates, ultimately contributing to improved health and growth outcomes (Pérez-Fuentes et al., 2020; Xiccato et al., 2013). Given these considerations, the lack of observed differences in growth performance between the single-cage and mixed systems in our study is particularly noteworthy. In the mixed system, rabbits initially spent the early growth phase in a group setting before being transferred to individual cages upon reaching the physiological stage of hormonal development and sexual maturity. This approach, though not extensively studied, may offer a viable compromise between welfare and productivity, aligning with the needs of many slow-growing rabbit breeders, such as those of the Grigio di Carmagnola rabbit. Overall, while single-cage systems may offer advantages in terms of growth performance and disease reduction, group housing can enhance behavioral expression, often at the cost of increased health issues.
Growth is a fundamental factor in rabbit meat production. Profit functions and economic weights have been estimated by Armero and Blasco (1992); Prayaga and Eady (2000), and Cartuche et al. (2014). However, the age or slaughter weight of the animal has a significant impact on carcass and meat quality, particularly in terms of fat deposition (Yalçin et al., 2006; Dalle Zotte, 2002). Increases in ether extract content, which indicates fat levels in rabbit meat, are consistently reported with advancing age. Hernandez et al. (2004) observed that older rabbits had significantly higher dissectible and intramuscular fat compared to younger ones, with increases of 0.97% and 0.79%, respectively. These results align with our findings, showing a progressive rise in fat content between slaughter ages of 120 and 150 days.
Fat deposition in rabbits is influenced not only by age and biological growth patterns but also by genetic background and dietary management (Hernández and Gondret, 2006; Xiccato and Trocino, 2010; Cullere and Dalle Zotte, 2018; Trocino et al., 2019). Understanding the interplay of age, diet, and genetics in shaping fat content is essential for optimizing both animal welfare and meat quality. This becomes particularly important when considering traditional livestock systems and local breeds, which often fall outside standardized commercial production.
In such contexts, fat levels are not merely a production trait but also a determinant of culinary identity, influencing taste, texture, and consumer preference (Dalle Zotte, 2002; Paci et al., 2012; Nagy et al., 2016; Alves et al., 2021). Preserving traditional rabbit lines and their associated management practices supports cultural heritage, maintains genetic diversity, and sustains niche markets where lean but flavorful rabbit meat is prized for regional cuisines.
The results of blood analysis in Grigio di Carmagnola rabbits reveal that most hematological parameters remained unchanged with age, except for a notable increase in lymphocytes. This increase is a complex process influenced by the maturation of lymphoid tissues and the differentiation of lymphocyte subsets. The structural evolution of lymph nodes follows a well-defined timeline: differentiation of lymph lobules begins at approximately 10 days, and by 30 days, the lymph nodes are fully organized into functional zones, including distinct T-cell and B-cell–dependent areas (Myroshnychenko, 2020). This maturation process is associated with a progressive increase in lymphocyte counts, particularly CD8+ T cells, which become more prevalent in various organs, while B cells expand in peripheral blood and mesenteric lymph nodes (Jeklova et al., 2007).
Additionally, the morphometric parameters of lymphoid organs, such as the spleen and lymph nodes, exhibit a significant increase in size and mass from birth to 90 days, reflecting the rapid development of the immune system. However, our study—despite the absence of significant lesions in the spleens—observed a more pronounced increase in lymphocytes after 100 days, likely due to the slower growth rate of native rabbit breeds (Myroshnychenko and Lieshchova, 2022). These findings align with similar trends observed in other species, including poultry and pigs, where lymphocyte counts tend to increase with age as part of immune system maturation (Fiorilla et al., 2024; Pomorska-Mól et al., 2011). Overall, the age-related increase in lymphocytes in rabbits corresponds to the continued development and differentiation of lymphoid tissues, leading to the expansion of lymphocyte subsets and the establishment of a more robust immune response as the animals mature.
Stress itself is a crucial factor that can affect intestinal health through stress-induced physiological changes (Ozella et al., 2024; Pérez-Fuentes et al., 2020). Moreover, stress can increase the risk of coccidiosis (Lambertini et al., 2010; Pinheiro et al., 2011). Housing systems that allow greater movement and natural behaviors, such as free-range setups, often lead to reduced growth rates (Dal Bosco et al., 2002). For instance, compared to pen systems, a caged rabbit system usually offers less space for motor activities but increases feed intake, leading to a greater energy requirement for sustaining both growth and movement.
Furthermore, even if the stocking rate is the same as in a caged system, putting more rabbits in a single pen can encourage a greater level of social interaction. This occurs because rabbits compete for resources (such as food and space) and hierarchical status. These variations—such as increased activity and energy requirements, social interactions, and/or competition for feed—can compromise overall rabbit growth, muscle and fat deposition, and consequently carcass characteristics and meat quality (Krunt et al., 2022; Maertens and Van Herck, 2000; Xiccato et al., 2013). These changes may reflect variations in nutrient uptake efficiency, possibly linked to modifications in villus structure (Pinheiro et al., 2011; Krunt et al., 2022).
While direct measurements of intestinal morphometric parameters are lacking, the broader impact of housing conditions on rabbit health and growth suggests that intestinal morphology could be influenced by stress and nutritional dynamics associated with different housing systems. In the present study, M rabbits showed higher values for jejunal villus height, resulting in a greater absorptive surface area, suggesting enhanced absorption capabilities and, consequently, a potential improvement in nutrient absorption and growth. Conversely, the decrease in the muscularis layer width of rabbits could be due both to aging (Pinheiro et al., 2011) and to adaptation to various conditions, including changes in diet, injury, or disease (Shaw et al., 2012).
Only more in-depth investigations assessing the intestinal and systemic inflammatory response or intestinal microbiota may allow a better understanding of how mixed systems can positively influence rabbit gut health.
5 Conclusions
This study highlights the complex interplay between housing systems, welfare, and productivity in slow-growing native rabbits. Group housing, while promoting natural behaviors, led to increased aggression and stress, necessitating the early discontinuation of the trial. In contrast, single-cage housing minimized injuries and stress, supporting better health and growth.
The mixed system, in which rabbits were initially housed in groups before transitioning to single cages, showed no adverse effects on rabbit health and growth performance, suggesting it as a viable compromise between Grigio di Carmagnola rabbit welfare and productivity. Moreover, even though no clear correlation between housing system and intestinal health was observed, the mixed system appeared to be associated with good digestive function.
Finally, the observed age-related increase in fat content and lymphocyte counts reflects natural physiological development. Overall, optimizing housing strategies through transitional systems could enhance welfare while maintaining productivity. Future research should further explore these approaches to ensure sustainable and ethical rabbit farming.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by University’s Bioethics Committee (Protocol no. 0245520). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SS: Writing – review & editing, Investigation, Methodology. MC: Investigation, Writing – review & editing. EF: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. VT: Supervision, Writing – review & editing. DP: Investigation, Writing – review & editing. DI: Investigation, Writing – review & editing. BM: Investigation, Writing – review & editing. PP: Investigation, Writing – review & editing. MP: Investigation, Writing – review & editing. RC: Investigation, Writing – review & editing. FR: Investigation, Writing – review & editing. CB: Investigation, Writing – review & editing. CM: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by PSR 2014-2020. AlPiCoGriPi – Allevamento Pilota del Coniglio Grigio Piemontese: biodiversità, benessere e qualità della carne (Research agreement n. CUPJ69H22000000002); The Department of Veterinary Sciences, Università degli Studi di Torino covered the open access APC.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
S: Single cage group
M: Mixed group
G: Group colony
HS: Housing system
SA: Slaughter age
LW: Live weight
ADFI: Average daily feed intake
ADG: Average daily gain
FCR: Feed conversion ratio
SW: Slaughter weight
HC: Hot carcass weight
CC: Cold carcass weight
pHu: Ultimate pH of the meat
WBC: White blood cells
NEUT: Neutrophils
LYM: Lymphocytes
MONO: Monocytes
EOS: Eosinophils
BASO: Basophils
LUC: Large unstained cells
HGB: Hemoglobin
HCT: Hematocrit
MCV: Mean corpuscular volume
MCH: Mean corpuscular hemoglobin
MCHC: Mean corpuscular hemoglobin concentration
CHCM: Cellular hemoglobin concentration mean
CH: Mean cellular hemoglobin content
RDW: Red blood cell distribution width
HDW: Hemoglobin distribution width
PLT: Platelets
MPV: Mean platelet volume
VH: Villus height
Cd: Crypt depth
GALT: Gut-associated lymphoid tissue
DM: Dry matter
CFU/g: Colony forming units per gram
TMABc: Total mesophilic aerobic bacteria counts
References
Alves S. P., Rafael J., Guedes C. M., and Bessa R. J. B. (2021). Fatty acid composition and nutritional value of rabbit meat as affected by genotype, feeding system, and slaughter age. Anim. Feed Sci Technol. 276, 114948.
Ameri M., Schnaars H. A., Sibley J. R., and Honor D. J. (2011). Stability of hematologic analytes in monkey, rabbit, rat, and mouse blood stored at 4C in EDTA using the ADVIA 120 hematology analyzer. Veterinary Clin. Pathol 40, 188–193. doi: 10.1111/j.1939-165X.2011.00304.x
Armero E. and Blasco A. (1992). Economic weights for rabbit selection. J. Appl. Rabbit Res. 15, 637–642.
Blasco A., Ouhayoun J., and Masoero G. (2010). Harmonization of criteria and terminology in meat rabbit research. World Rabbit Sci 1(1). doi: 10.4995/wrs.1993.189
Braconnier M., Gomez Y., and Gebhardt-Henrich S. G. (2020). Different regrouping schedules in semi group-housed rabbit does: Effects on agonistic behaviour, stress and lesions. Appl. Anim. Behav. Sci 228, 105024. doi: 10.1016/j.applanim.2020.105024
Cartuche L., Pascual M., Gómez E. A., and Blasco A. (2014). Economic weights in rabbit meat production. Animal 8, 1281–1288.
Cullere M. and Dalle Zotte A. (2018). Rabbit meat production and consumption: State of knowledge and future perspectives. Meat Sci 143, 137–146. doi: 10.1016/j.meatsci.2018.04.029
Dal Bosco A., Castellini C., and Mugnai C. (2002). Rearing rabbits on a wire net floor or straw litter: behaviour, growth and meat qualitative traits. Livestock Production Sci 75, 149–156. doi: 10.1016/S0301-6226(01)00307-4
Dalle Zotte A. (2002). Perception of rabbit meat quality and major factors influencing the rabbit carcass and meat quality. Livestock production Sci 75, 11–32. doi: 10.1016/S0301-6226(01)00308-6
Del Piano F., Almroth B. C., Lama A., Piccolo G., Addeo N. F., Paciello O., et al. (2024). Subchronic oral exposure to polystyrene microplastics affects hepatic lipid metabolism, inflammation, and oxidative balance in gilthead seabream (Sparus aurata). Ecotoxicology Environ. Saf. 279, 116455. doi: 10.1016/j.ecoenv.2024.116455
EFSA Panel on Animal Health and Welfare (AHAW), Saxmose Nielsen S., Alvarez J., Bicout D. J., Calistri P., Depner K., et al. (2020). Health and welfare of rabbits farmed in different production systems. EFS2 18(1), e05944. doi: 10.2903/j.efsa.2020.5944
European Commission (2017). Directorate General for Health and Food Safety (Commercial Rabbit Farming in the European Union). Available online at: https://data.europa.eu/doi/10.2772/62174.
FAO FAOSTAT. (2024) Agricultural production statistics 2010–2023. FAOSTAT Analytical Briefs, No. 96. Rome. Available online at: https://www.fao.org/faostat/en/home.
Fiorilla E., Gariglio M., Martinez-Miro S., Rosique C., Madrid J., Montalban A., et al. (2024). Improving sustainability in autochthonous slow-growing chicken farming: exploring new frontiers through the use of alternative dietary proteins. J. Cleaner Production 434, 140041. doi: 10.1016/j.jclepro.2023.140041
Folch J., Lees M., and Stanley G. S. (1957). A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509. doi: 10.1016/S0021-9258(18)64849-5
Hernandez P., Aliaga S., Pla M., and Blasco A. (2004). The effect of selection for growth rate and slaughter age on carcass composition and meat quality in rabbits. J. Anim. Sci 82, 3138–3143. doi: 10.2527/2004.82113138x
Hernandez P. and Gondret F. (2006). “Rabbit meat quality” in Recent Advances in Rabbit Sciences; COST Action; COST: Brussels, Belgium.
Huang Y., Breda J., Savietto D., Debrusse A. M., Combes S., and Fortun-Lamothe L. (2021). Part-time grouping of rabbit does in enriched housing: effects on performances, injury occurrence and enrichment use. Animal 15, 100390. doi: 10.1016/j.animal.2021.100390
Jeklova E., Leva L., and Faldyna M. (2007). Lymphoid organ development in rabbits: major lymphocyte subsets. Dev. Comp. Immunol. 31, 632–644. doi: 10.1016/j.dci.2006.10.002
Krunt O., Zita L., Kraus A., Bureš D., Needham T., and Volek Z. (2022). The effect of housing system on rabbit growth performance, carcass traits, and meat quality characteristics of different muscles. Meat Sci 193, 108953. doi: 10.1016/j.meatsci.2022.108953
Lambertini L., Vignola G., and Zaghini G. (2010). Alternative pen housing system for fattening rabbits: effects of group density and litter. World Rabbit Sci 9(1). doi: 10.4995/wrs.2001.457
Laudadio V., Passantino L., Perillo A., Lopresti G., Passantino A., Khan R. U., et al. (2012). Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poultry Sci 91, 265–270. doi: 10.3382/ps.2011-01675
Leroy G., Baumung R., Boettcher P., Besbes B., From T., and Hoffmann I. (2018). Animal genetic resources diversity and ecosystem services. Global Food Secur. 17, 84–91. doi: 10.1016/j.gfs.2018.04.003
Maertens L. and Van Herck A. (2000). Performance of weaned rabbits raised in pens or in classical cages: First results. World Rabbit Sci 8, 435–440.
Mugnai C., Dal Bosco A., Cardinali R., Rebollar P. G., Moscati L., and Castellini C. (2014). Effect of pasture availability and genotype on welfare, immune function, performance and meat characteristics of growing rabbits. World Rabbit Sci. 22, 29. doi: 10.4995/wrs.2014.1342
Munari C., Mugnai C., Braconnier M., Toscano M. J., and Gebhardt-Henrich S. G. (2020). Effect of different management protocols for grouping does on aggression and dominance hierarchies. Appl. Anim. Behav. Sci 227, 104999. doi: 10.1016/j.applanim.2020.104999
Myroshnychenko I. (2020). Postnatal morphogenesis of lymph node parenchyma compartments in meat rabbit breeds. Theor. Appl. Veterinary Med. 8, 179–184. doi: 10.32819/2020.82025
Myroshnychenko I. and Lieshchova M. (2022). Topography and dynamics of spleen and lymph nodes’ Morphometric parameters in rabbits. Theor. Appl. Veterinary Med. 10, 21–26. doi: 10.32819/2022.10013
Nagy I., Radnai I., Gyovai P., Matics Z., Nagyné-Kiszlinger H., Bíróné-Németh E., et al. (2016). Genetic parameters and inbreeding depression for carcass traits in Pannon White rabbits. Animal 10, 406–411.
Notter D. R. (1999). The importance of genetic diversity in livestock populations of the future. J. Anim. Sci 77, 61. doi: 10.2527/1999.77161x
Ozella L., Sartore S., Macchi E., Manenti I., Mioletti S., Miniscalco B., et al. (2024). Behaviour and welfare assessment of autochthonous slow-growing rabbits: the role of housing systems. PloS One 19, 1–17. doi: 10.1371/journal.pone.0307456
Paci G., Cecchi F., Preziuso G., Ciampolini R., and D’Agata M. (2012). Carcass traits and meat quality of rabbits from a local breed and a commercial hybrid. Ital. J. Anim. Sci 11, e73.
Pacorig V., Galeotti M., and Beraldo P. (2022). Multiparametric Semi-quantitative Scoring System for the histological evaluation of marinefish larval and juvenile quality. Aquac. Rep. 26, 101285. doi: 10.1016/j.aqrep.2022.101285
Pérez-Fuentes S., Muñoz-Silvestre A., Moreno-Grua E., Martínez-Paredes E., Viana D., Selva L., et al. (2020). Effect of different housing systems (Single and group penning) on the health and welfare of commercial female rabbits. Animal 14, 1270–1277. doi: 10.1017/S1751731119003379
Pinheiro V., Outor-Monteiro D., Silva S. R., Silva J. A., and Mourão J. L. (2011). Growth performance, carcass characteristics and meat quality of growing rabbits housed in cages or open-air park. Arch. Anim. Breed. 54, 625–635. doi: 10.5194/aab-54-625-2011
Pomorska-Mól M., And I., and Markowska-Daniel I. (2011). Age-dependent changes in relative and absolute size of lymphocyte subsets in the blood of pigs from birth to slaughter. Bulletin- Veterinary Institute Pulawy 55, 305–310.
Prayaga K. C. and Eady S. J. (2000). Performance and carcass characteristics of rabbit genotypes. Aust. J. Agric. Res. 51, 801–810.
Shaw D., Gohil K., and Basson M. D. (2012). Intestinal mucosal atrophy and adaptation. World J. Gas-troenterol 18, 6357–6375. doi: 10.3748/wjg.v18.i44.6357
Söderholm J. D. and Perdue M. H. II (2001). Stress and intestinal barrier function. Am. J. Physiology-Gastrointestinal Liver Physiol. 280, G7–G13. doi: 10.1152/ajpgi.2001.280.1.G7
Szendrő Z., McNitt J. I., Matics Z., Miko A., and Zsolt G. (2016). Alternative and enriched housing systems for breeding does: A review. World Rabbit Sci 24, 1. doi: 10.4995/wrs.2016.3801
Szendrő Z. S., Mikó A., Odermatt M., Gerencsér Z. S., Radnai I., Dezséry B., et al. (2013). Comparison of performance and welfare of single-caged and group-housed rabbit does. Animal 7, 463–468. doi: 10.1017/S1751731112001760
Szendrő Z., Princz Z., Romvári R., Locsmándi L., Szabó A., Bázár G., et al. (2009). Effect of group size and stocking density on productive, carcass, meat quality and aggression traits of growing rabbits. World Rabbit Sci 17, 153–162.
Szendrő Z., Trocino A., Hoy S., Xiccato G., Villagrá A., and Maertens L. (2019). A review of recent research outcomes on the housing of farmed domestic rabbits: reproducing does. World Rabbit Sci 27, 1–14. doi: 10.4995/wrs.2019.10599
Trocino A., Cullere M., and Dalle Zotte A. (2019). Rabbit production and science: The future. World Rabbit Sci 27, 61–74.
Van Damme L., Ampe B., Delezie E., and Tuyttens F. (2023). Effects of group size and cage enrichment on social behaviour and skin injuries of breeding rabbits housed part-time in group. Animal 17, 100850. doi: 10.1016/j.animal.2023.100850
Vervaecke H., De Bonte L., Maertens L., Tuyttens F., Stevens J. M. G., and Lips D. (2010). Development of hierarchy and rank effects in weaned growing rabbits (Oryctolagus cuniculus). World Rabbit Sci 18, 139–149. doi: 10.4995/wrs.2010.8229
Vilardo V. B., Pinto M. S., Silva A. M. D. C., and Santori R. T. (2025). Behavioral effects of floor pens as environmental enrichment for laboratory rabbits (Oryctolagus cuniculus). Appl. Anim. Behav. Sci 286, 106629. doi: 10.1016/j.applanim.2025.106629
Washington I. M. and Van Hoosier G. (2012). “Clinical Biochemistry and Hematology,” in The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents (Elsevier), 57–116, ISBN: 978-0-12-380920-9.
Xiccato G. and Trocino A. (2010). “Energy and Protein Metabolism and Requirements,” in The Nutrition of the Rabbit (CABI), pp 83–118.
Xiccato G., Trocino A., Majolini D., Tazzoli M., and Zuffellato A. (2013). Housing of growing rabbits in individual, bicellular and collective cages: growth performance, carcass traits and meat quality. Animal 7, 627–632. doi: 10.1017/S175173111200198X
Keywords: autochthonous rabbit, cage system, group housing, performance, meat quality
Citation: Sartore S, Capucchio MT, Fiorilla E, Tafuro V, Pattono D, Ippolito D, Miniscalco B, Ponzio P, Profiti M, Crosetto R, Raspa F, Bianchi C and Mugnai C (2025) Housing systems and slaughter age: key factors in productivity, health, and some meat quality parameters of an autochthonous Grigio di Carmagnola rabbit breed. Front. Anim. Sci. 6:1670505. doi: 10.3389/fanim.2025.1670505
Received: 21 July 2025; Accepted: 02 October 2025;
Published: 22 October 2025.
Edited by:
Esin Ebru Onbasilar, Ankara University, TürkiyeReviewed by:
Iveta Placha, Slovak Academy of Sciences (SAS), SlovakiaBram Brahmantiyo, National Research and Innovation Agency (BRIN), Indonesia
Caterina Losacco, University of Bari Aldo Moro, Italy
Copyright © 2025 Sartore, Capucchio, Fiorilla, Tafuro, Pattono, Ippolito, Miniscalco, Ponzio, Profiti, Crosetto, Raspa, Bianchi and Mugnai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valentina Tafuro, dmFsZW50aW5hLnRhZnVyb0B1bml0by5pdA==
†These authors share first authorship
 Stefano Sartore
Stefano Sartore Maria Teresa Capucchio
Maria Teresa Capucchio Edoardo Fiorilla
Edoardo Fiorilla Valentina Tafuro
Valentina Tafuro Daniele Pattono1
Daniele Pattono1 Dorotea Ippolito
Dorotea Ippolito Patrizia Ponzio
Patrizia Ponzio Federica Raspa
Federica Raspa