- 1School of Agriculture and Food Sustainability, The University of Queensland, Gatton, QLD, Australia
- 2Department of Animal Behavior and Management, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt
- 3School of Life and Environmental Sciences, Faculty of Science, The University of Sydney, Sydney, NSW, Australia
There is growing interest in identifying alternative sustainable feed ingredients for ruminant diets. This study evaluated four processed pongamia seedcake (PSC) meals—three produced using a proprietary extraction method and one by traditional hexane extraction—in two separate in vitro experiments. Experiment 1 assessed PSC as a partial replacement for conventional protein meals (canola and cottonseed) at total inclusion levels of 0, 4, or 8% DM. Experiment 2 evaluated PSC as a partial replacement for an energy source (barley grain) at final inclusion levels of 0, 12, 15, or 30% DM. There was no interaction between level × processing type (P ≥ 0.10) in either experiment. In Experiment 1, neither the type nor the level of PSC affected methane production (mL/g digested DM). Replacement of canola or cottonseed meal with PSC reduced (P < 0.01) in vitro dry matter digestibility (IVDMD) by an average of 7% across all PSC types and inclusion levels compared with the control. However, total volatile fatty acid (VFA) concentrations were not affected by PSC type or level. In Experiment 2, inclusion of PSC in a barley-based diet increased (P < 0.01) methane production (mL/g digested DM) by up to 20.8% compared with the control. IVDMD was not affected by PSC inclusion up to 15% DM (P > 0.05). Processing type had varying effects: PSC4 (hexane-extracted) had 5.5% greater IVDMD (P = 0.02) than PSC1 and 3.8% greater than PSC2 but was similar to PSC3—all three produced using the novel extraction methods. These findings suggest that PSC processed with novel extraction methods has potential as a feed ingredient, but its application requires careful consideration. While it can replace conventional protein meals without reducing total VFA production, this may occur at the cost of reduced diet digestibility. Furthermore, when PSC replaces barley grain, methane production may increase. The suitability of PSC therefore depends on the extraction method, inclusion level, and the dietary component it replaces.
1 Introduction
The increasing global demand for livestock products, particularly from the ruminant sector, has intensified the need for more sustainable and efficient feeding strategies. Ruminant production is a major contributor to global food security, providing approximately 45% of the world’s animal-derived protein supply (Mottet et al., 2017). However, this sector faces several significant challenges, among which escalating feed costs are the most critical. Feed can account for 60% or more of total production expenses, with protein sources often representing over 15% of feed costs (Parisi et al., 2020; Greenwood, 2021). In addition to economic pressures, the use of conventional protein sources such as soybean meal (44%–48% crude protein; CP), canola meal (36%–40% CP), and cottonseed meal (40%–44% CP) is increasingly scrutinized due to their environmental footprint. These ingredients are linked to high greenhouse gas emissions and extensive land use and are considered to compete with the human food supply (Kim et al., 2019; Pexas et al., 2023). As a result, there is growing interest in identifying alternative, cost-effective, and environmentally sustainable protein sources that do not compromise animal productivity or health.
Pongamia (Millettia pinnata), a leguminous tree native to South and Southeast Asia and Australia, has gained attention as a potential source of protein-rich feed (Murphy et al., 2012). Pongamia trees can produce up to 30 kg of seed annually, with an oil content reaching 40% of the seed’s weight, primarily composed of oleic acid (47%–60%) (Usharani et al., 2019). Following oil extraction, approximately two-thirds of the seed remains as a by-product known as pongamia seedcake (PSC), which contains 28%–34% CP depending on the extraction method (Dutta et al., 2012).
Earlier extraction methods left residual oil in the PSC containing high concentrations of furanoflavonoids, particularly karanjin (~3,700 ppm) and pongamol (~1,674 ppm) (Housman et al., 2020). These compounds imparted a bitter taste and negatively affected feed intake and rumen fermentation (Gupta et al., 1981; Vinay and Kanya, 2008; Dutta et al., 2012). The processing methods used for the PSC in this study reduced these concentrations to below 165 ppm, a level hypothesized to be below the threshold for adverse effects on rumen microbes or animal intake. Therefore, the present in vitro study was conducted to evaluate three PSCs extracted using novel methods, each differing in karanjin and pongamol content, compared with the traditional hexane extraction method (PSC types), as alternative protein concentrates in cattle feedlot diets. The study assessed the impact of PSC inclusion at varying levels as partial replacements for canola meal or cottonseed meal (protein sources) or barley grain (energy source) on in vitro gas production, digestibility, and rumen fermentation characteristics. We hypothesized that PSC inclusion would not negatively affect rumen fermentation. Regarding methane emissions, we posited a complex outcome: while the residual oil in PSC could potentially reduce methane production, fermentation of its unique fiber components could counteract this effect. Therefore, the net impact on methane production was investigated.
2 Materials and methods
Animals used in this study were housed and cared for at the University of Queensland’s Gatton Campus, Queensland Animal Science Precinct, Gatton, Australia. All animal care and sampling procedures were conducted in compliance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and approved by the University of Queensland Production Animal Ethics Committee (approval number 2022/AE000541).
2.1 Experimental diets
Canola meal, cottonseed meal, barley hay, barley grain, and four expeller-pressed, solvent-extracted, defatted PSC meals (Terviva Inc., CA, United States) were used as substrates for the in vitro incubations. The test meal (PSC) was manufactured using a proprietary extraction process to meet strict product specifications (Terviva Inc., Alameda, CA, USA). Briefly, beans were harvested, dried immediately after harvest, and stored at ambient temperature in a commercial storage facility. The beans were then pressed to separate oil from solids (presscake). The presscake was extracted using either a proprietary solvent extraction process (PSC1–PSC3) or hexane extraction (PSC4) as an industry-standard control. Residual solvent was evaporated from the resulting meal, and the meal was dried to specification. Karanjin and pongamol concentrations were quantified in each meal by high-performance liquid chromatography using purified karanjin and pongamol as reference standards (Marone et al., 2022). Concentrations for each meal are presented in Table 1. The feed ingredients were dried at 60°C for 48 h and then ground using a cutting mill to pass through a 1-mm sieve. The chemical compositions of the feed ingredients used in the in vitro incubations are presented in Table 1.
2.2 Donor animals and rumen fluid collection
Three fistulated Holstein steers approximately 3 years old, with an average body weight of 581 kg, were used to collect rumen fluid 3 h after the morning feed on three separate occasions (once per run). Animals were fed at the maintenance level on a diet of Rhodes grass hay.
Rumen fluid samples were collected from the dorsal, anterior ventral, medium ventral, posterior dorsal, and posterior ventral regions of the rumen. The collected rumen fluid was strained through four layers of surgical gauze, placed into a pre-warmed thermos flask (39°C), and immediately transferred to the laboratory.
2.3 Experimental design and in vitro incubation
Following the method described by Meale et al. (2012), this in vitro batch culture fermentation was conducted using two experimental designs to evaluate the replacement of protein sources (canola meal or cottonseed meal) or an energy source (barley grain) with increasing levels of four PSCs.
In Experiment 1, the basal diet contained 12% protein meal (canola or cottonseed), 80% barley grain, and 8% hay. The protein meal was replaced with PSC at rates of 0%, 33%, or 67%, resulting in final dietary PSC inclusion levels of 0%, 4%, and 8% DM, respectively.
In Experiment 2, the basal diet consisted of 92% barley grain and 8% hay. The barley grain was replaced with the four PSC types at inclusion levels of 0%, 12%, 15%, and 30% of dietary DM. All diets were formulated to be isonitrogenous and isocaloric, given the small inclusion levels of PSC in the overall diet. All treatments were incubated in rumen fluid in triplicate within each experimental run. The entire experiment was repeated in three independent runs conducted on consecutive weeks, using freshly collected rumen fluid for each run.
The basal diet (0.5 g DM substrate) was weighed and placed into pre-weighed ANKOM filter bags (F57; ANKOM Technology, Macedon, NY, USA) with a pore size of 25 µm. The bags were heat-sealed and placed in 50-mL amber serum bottles. Under continuous carbon dioxide (CO2) flushing, each bottle received 25 mL of a 1:2 mixture of rumen fluid and buffer. The modified McDougall’s buffer used in this study was prepared according to Czerkawski and Breckenridge (1977). Bottles were then sealed with rubber stoppers and incubated in a Ratek OM25 digital shaking incubator (Ratek Instruments Pty Ltd., Victoria, Australia) at 120 oscillations per minute and 39°C for 24 h.
2.4 Incubation medium sampling and analysis
Samples of the headspace gas at 6 h and 24 h were collected and transferred into 12-mL evacuated exetainers for the determination of methane proportions by gas chromatography (Chaves et al., 2006). The remaining gas volume at both time points was measured using a water-displacement apparatus (Fedorah and Hrudey, 1983).
After 24 h of incubation, bottles were opened and placed on ice, and pH was measured immediately using a pH meter. A subsample (1.5 mL) of the fermentation medium was transferred into an Eppendorf tube containing 300 µL of metaphosphoric acid (20% w/w) and stored at −20°C until analysis for volatile fatty acids (VFA) by gas chromatography, following the specifications and procedure of Forwood et al. (2019).
ANKOM bags were removed from the bottles, rinsed, washed with tap water until the effluent was clear, and dried at 60°C for 48 h to determine in vitro dry-matter digestibility (IVDMD). IVDMD was calculated as the proportion of dry matter (DM) that disappeared from the initial DM weight placed into the bag after determining the weight of the undigested residue.
2.5 Chemical analysis
The chemical composition of the feed ingredients used in this study was determined by Dairy One Laboratory (Ithaca, NY, USA) following the standard procedures of the AOAC (1995). DM, organic matter (OM), and ether extract (EE) were measured according to methods 930.15, 942.05, and 920.39, respectively.
Neutral-detergent fiber (aNDF) was analyzed using the procedure of Mertens (2002), and acid-detergent fiber (ADF) and acid-detergent lignin (ADL) were analyzed following AOAC (1995; method 973.18) as adapted for the ANKOM200 fiber analyzer (ANKOM Technology Corp., Macedon, NY, USA). NDF and ADF values were expressed inclusive of residual ash. Sodium sulfite and amylase were used for NDF analyses.
Crude protein (CP) was estimated from nitrogen (nitrogen × 6.25) according to the Kjeldahl method (984.13). Non-fiber carbohydrate (NFC) was calculated using the formula listed in Equation 1:
Total digestible nutrients (TDN) were calculated according to Weiss et al. (1992). Rumen-degradable protein (RDP) was determined by Streptomyces griseus (SGP) enzymatic digestion (Cornell University, 1990; Coblentz et al., 1999), and rumen-undegradable protein (RUP) was calculated as total protein minus the degradable fraction.
2.6 Statistical analysis
Data were screened for normality using the PROC UNIVARIATE procedure of SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC, USA), with no observations removed. Normally distributed data were analyzed using the MIXED procedure in SAS. Each diet (cottonseed meal, canola meal, and barley grain) was considered separately.
The model included level, type, and their interaction (level × type) as fixed effects, while the three experimental runs and the run × treatment interaction were considered random effects. Results are presented as least-squares means (LSMEANS/DIFF), with statistical significance declared at P ≤ 0.05 and trends at 0.05 < P ≤ 0.10.
3 Results
3.1 Experiment 1
The interaction between PSC type and level of inclusion, as well as PSC type alone, had no effect on any of the parameters examined (Tables 2, 3; P ≥ 0.10) when used as a partial replacement for cottonseed meal or canola meal.
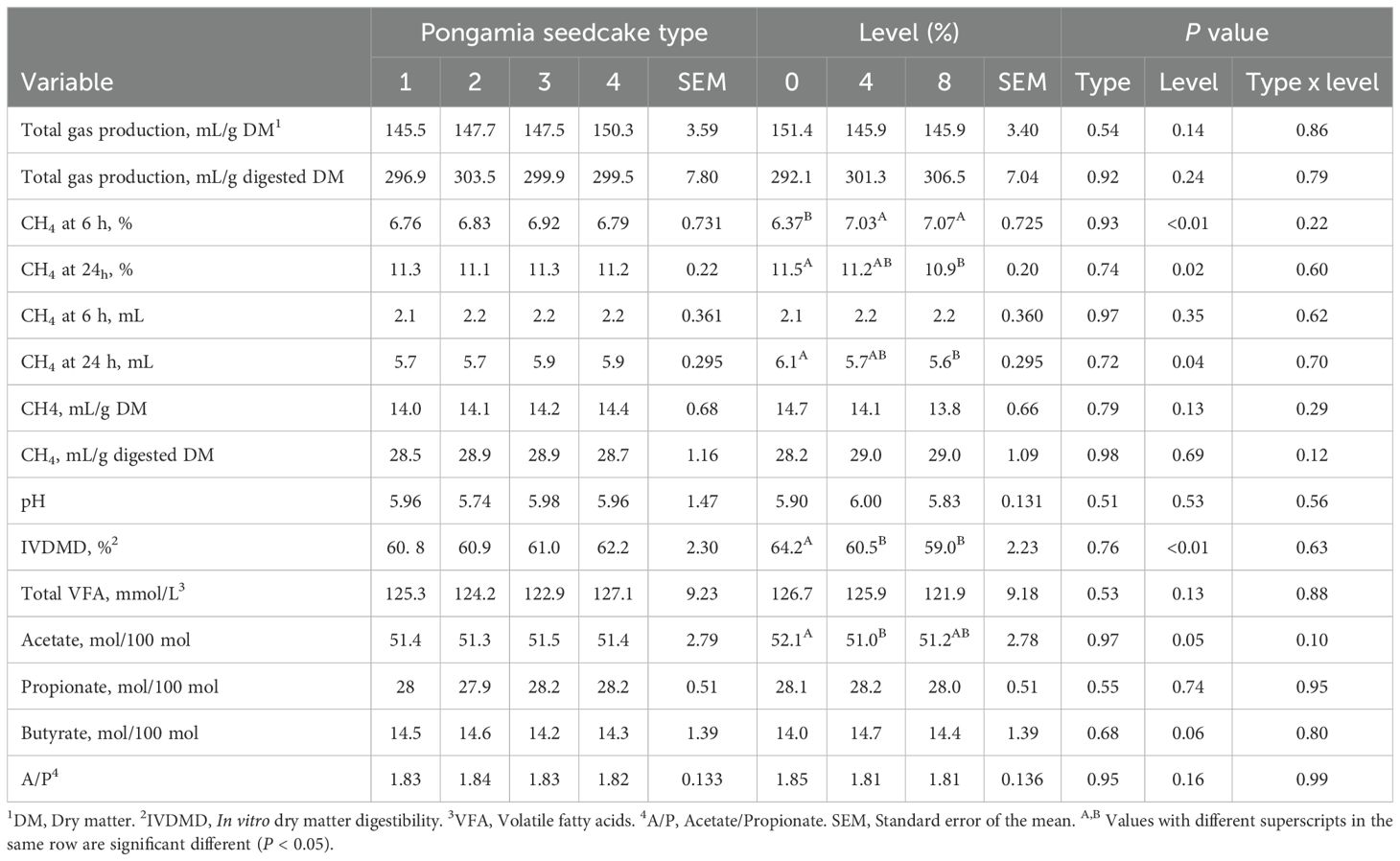
Table 2. Effect of replacing canola meal with pongamia seedcake (PSC) at different inclusion levels in a feedlot diet on in vitro fermentation characteristics (n=9).
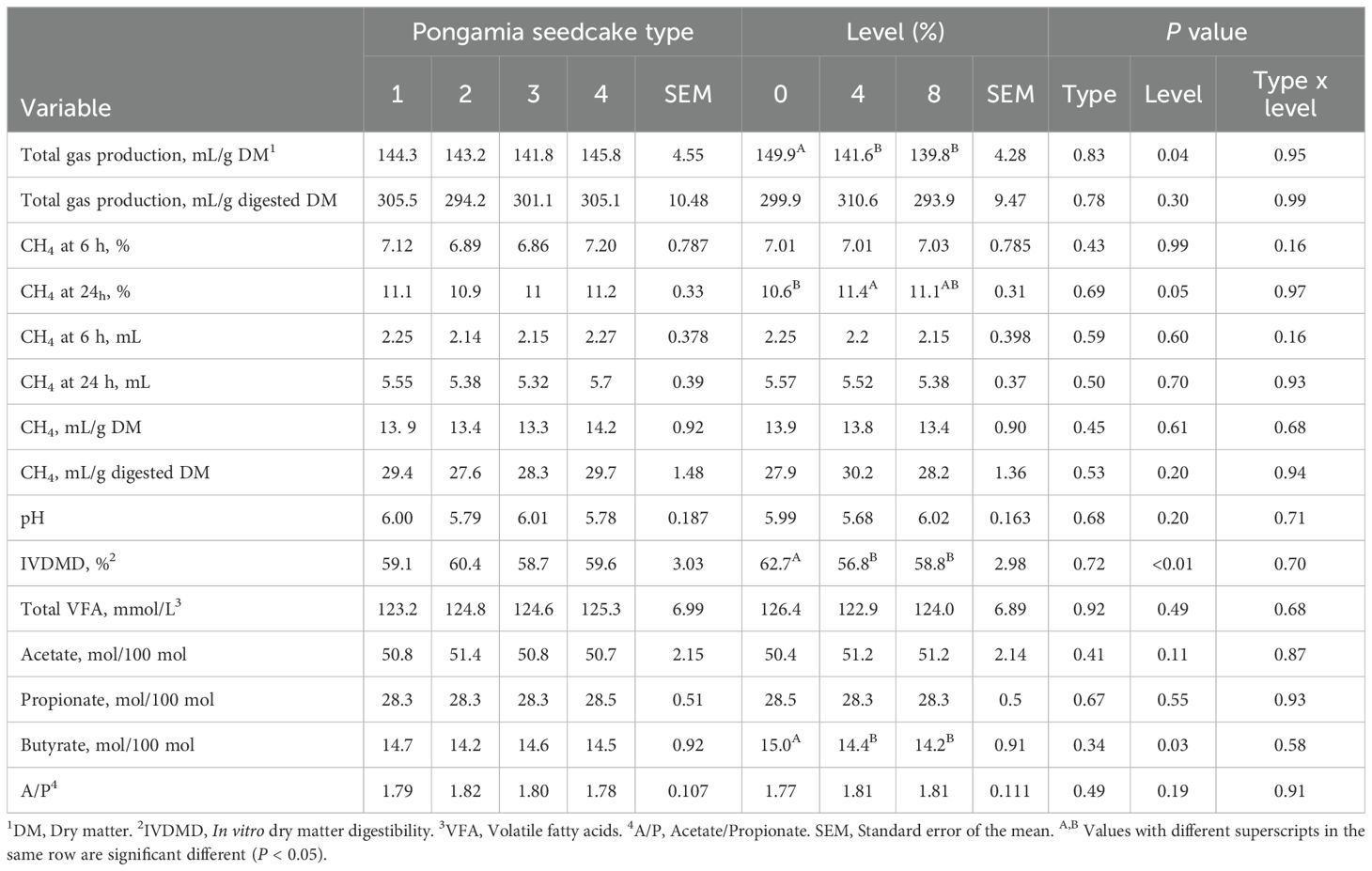
Table 3. Effect of replacing cottonseed meal with pongamia seedcake (PSC) at different inclusion levels in a feedlot diet on in vitro fermentation characteristics (n=9).
Partial replacement of canola meal with PSC, regardless of level, did not affect total gas production (mL/g DM or mL/g digested DM; P ≥ 0.14; Table 2) compared with the control. Methane concentration (%) at 6 h was, on average, 10.7% higher (P = 0.01) in diets containing PSC, whereas at 24 h, CH4 (% and mL) was 5.2% lower (P = 0.02) in canola meal diets containing 8% PSC compared with the control. Inclusion level of PSC did not affect CH4 production (mL/g digested DM; P = 0.69) or pH (P = 0.53). Canola meal diets containing 4% and 8% PSC had, on average, 6.9% lower IVDMD (P < 0.01) than the control, but were similar to each other. Total VFA concentration (mM) and the percentage of propionate in total VFA were not affected by PSC level (P ≥ 0.13). Acetate, expressed as a percentage of total VFA, was 2.1% lower in canola meal diets containing 4% PSC (P = 0.05), whereas butyrate percentage tended to increase in diets containing PSC, regardless of level (P = 0.06; Table 2).
Partial replacement of cottonseed meal with PSC, regardless of level, decreased total gas production (mL/g DM; P ≤ 0.04; Table 3) but was similar to the control when corrected for digested DM (mL gas/g digested DM; P = 0.30). Methane concentration (%) at 6 h was not affected by PSC level (P ≥ 0.99). However, at 24 h, cottonseed meal diets containing 4% PSC had 7.5% higher CH4 (%, P = 0.05), compared with the control. Methane production (mL/g DM or mL/g digested DM) was not affected by PSC level (P ≥ 0.20). Similarly, pH was consistent across treatments, regardless of PSC level (P ≥ 0.20).
Replacement of cottonseed meal with PSC at 4% and 8% DM decreased IVDMD (P < 0.01) by an average of 7.8% compared with the control, with no difference between the two inclusion levels. Total VFA concentration (mM) and the percentages of acetate and propionate in total VFA were not influenced by PSC level (P ≥ 0.11). Butyrate, as a percentage of total VFA, was lower in cottonseed meal diets containing 4% and 8% PSC compared with the control (P = 0.03; Table 3).
3.2 Experiment 2
The interaction between PSC type and level of inclusion had no effect on any parameters tested (P ≥ 0.37; Table 4) when PSC replaced barley grain. However, inclusion of PSC as a partial replacement for barley grain, regardless of level, increased total gas production (mL/g DM or mL/g digested DM; P ≤ 0.01; Table 4) compared with the control.
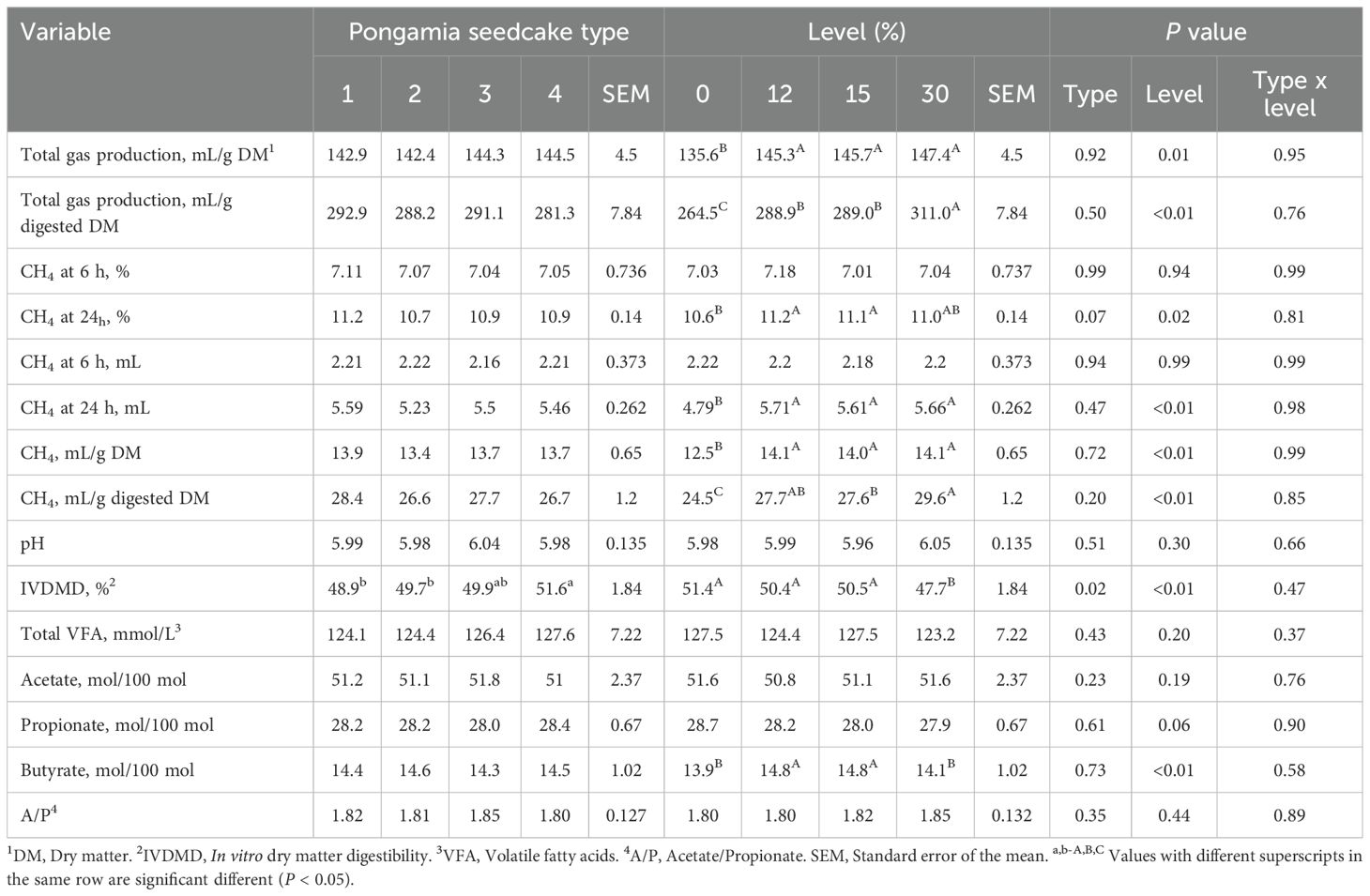
Table 4. Effect of replacing barley grain with pongamia seedcake (PSC) at different inclusion levels in a feedlot diet on in vitro fermentation characteristics (n=9).
After 24 h of incubation, diets containing 12% and 15% PSC resulted in 5.7% and 4.7% higher CH4 concentrations (%; P = 0.02), respectively, compared with the control. Methane production (mL/g digested DM) was up to 20.8% greater in diets where PSC replaced barley grain (P < 0.01) than in the control.
pH was similar across treatments, regardless of PSC type or level (P ≥ 0.30). The type of PSC used to replace barley grain affected IVDMD: PSC4 showed 5.5% greater IVDMD than PSC1 and 3.8% greater than PSC2 but was similar to PSC3. IVDMD was comparable in barley grain diets containing up to 15% PSC but was 7.2% lower (P < 0.01) in diets containing 30% PSC compared with the control.
Neither PSC type nor level affected total VFA concentration (mM) or the percentage of acetate in total VFA (P ≥ 0.19). The percentage of propionate in total VFA tended to decrease with increasing PSC levels (P = 0.06). Butyrate percentage increased by 6.5% in diets containing 12% and 15% PSC (P < 0.01; Table 4) but was similar in diets containing 30% PSC compared with the control.
4 Discussion
Processing methods significantly influence the chemical composition of PSC, particularly its protein and fat contents (Soren and Sastry, 2009; Soren et al., 2017), and resulting concentrations of anti-nutritional compounds, which ranged from 94 ppm to 424 ppm total karanjin and pongamol in the current trial compared with previous reports of approximately 3,700 ppm karanjin and 1,674 ppm pongamol at ~1674 ppm (Housman et al., 2020). The PSC types assessed here were rich in protein (35%–37%), slightly higher than the levels reported in previous studies (22%–30%) (Natanam et al., 1989a; Kumar et al., 2007; Nagalakshmi et al., 2011; Housman et al., 2020). The protein content of the PSC types was lower than that of canola meal (43.3%) but considerably higher than that of cottonseed meal (23.3%) and barley grain (11.3%), indicating that PSC could serve as a viable protein source in ruminant diets. The proportion of degradable protein in PSC was notably high (76%–85%), exceeding that of canola (53%) and cottonseed meal (46%). This is beneficial for microbial protein synthesis in the rumen because protein degradation produces ammonia-nitrogen, a vital precursor for amino acid and microbial protein synthesis (Bach et al., 2005). All PSC types showed low levels of fiber (NDF, ADF, and ADL), indicating that PSC could provide a highly digestible NFC fraction, with PSC4 reaching up to 50%, which supports rumen microbial efficiency and energy supply (Villalba et al., 2021). These characteristics suggest that PSC can serve as an energy-dense protein source suitable for feedlot diets.
VFA are the primary energy source for ruminants and serve as key indicators of microbial fermentation efficiency (Owens and Basalan, 2016). Inclusion of the PSC types at all levels tested, partially replacing canola meal, cottonseed meal, or barley grain, had no effect on total VFA concentration, despite a decrease in IVDMD when replacing protein sources. This suggests that the four PSC types provided sufficient fermentable substrates to support rumen microbial activity and maintain end-product formation—and, in the case of protein sources, potentially increased microbial efficiency, as less digested substrate still yielded a similar amount of VFA. This interpretation is further supported by the similar total gas production (mL/g DM) observed in treatments containing canola meal and cottonseed meal, while an increase was noted in the barley grain–based diets. Similar findings were reported by Housman et al. (2020), who observed no decline in total VFA concentration when PSC was used as a protein supplement in forage-based diets, and by Soren et al. (2011), who demonstrated that replacing 50% of soybean meal protein with lime-treated, solvent-extracted PSC did not compromise rumen fermentation in growing lambs. The decline in IVDMD when replacing protein sources could be attributed to the presence of anti-nutritional factors such as tannins and protease inhibitors (e.g., trypsin and chymotrypsin), which are known to interfere with protein digestion (Natanam et al., 1989b; Rattansi and Dikshit, 1997). This interpretation is supported by earlier findings by Srivastava et al. (1990) and Singh et al. (2006), who reported significant reductions in protein digestibility in goat kids and male lambs, respectively, when fed various forms of PSC. Conversely, other studies have shown no negative effects on nutrient digestibility when processed PSC was used to partially replace soybean meal (Soren and Sastry, 2009) or when included up to 12% as the sole protein supplement in lamb diets (Nagalakshmi et al., 2011), suggesting that the impact of PSC on digestibility may depend on its inclusion level, processing method, and the composition of the basal diet.
Interestingly, in the barley grain–based diet, IVDMD remained unaffected at PSC inclusion levels of 12% and 15%, declining significantly only at 30%. Because barley grain contains less protein than canola or cottonseed meal, the influence of PSC’s anti-nutritional factors on protein digestibility may have been diluted, allowing a greater proportion of the diet to ferment efficiently. Among the four PSC types, PSC4 resulted in the greatest IVDMD in barley-based diets, which could be attributed to its relatively lower fat content and consequently reduced levels of anti-nutritional factors.
It is well established that increased dietary fat can impair rumen microbial activity and fiber degradation (Patra, 2013; Enjalbert et al., 2017). Furthermore, PSC4 had the highest NFC content, which may have contributed to a more balanced nutrient profile and improved fermentability. The observed variation in IVDMD and butyrate production across PSC types and dietary treatments may reflect interactions between the chemical composition of PSC and the basal diet matrix.
While several feeding strategies have been shown to reduce CH4 emissions due to the presence of bioactive phytochemicals such as tannins, saponins, and polyphenols (Jafari et al., 2020; Ku-Vera et al., 2020; Lambo et al., 2024), or due to lipid content (Ahmed et al., 2021; Arndt et al., 2022), the PSC types evaluated in this study particularly PSC1, PSC2, and PSC3, which had higher fat content did not reduce CH4 production. This lack of CH4 mitigation could indicate that, although the fat content was relatively high in PSC1 specifically, its absolute inclusion level in the diet at 8% was low in the case of canola and cottonseed meals, when compared to the control (2.5% and 3.0% vs. 1.9%, respectively). This amount may not have been sufficient to exert a notable anti-methanogenic effect compared to the control. Previous studies have shown that the efficacy of dietary fats in suppressing methanogenesis depends on fat type, saturation level, and inclusion rate (Patra, 2013). Moreover, the secondary metabolites present in PSC may not have reached concentrations sufficient to inhibit methanogenic archaea or associated microorganisms under the tested conditions.
Interestingly, in Experiment 1, an increase in the percentage of methane was observed with 4% inclusion of PSC as a replacement for cottonseed meal. However, this was offset by a corresponding reduction in gas production, resulting in a net neutral effect on methane output, as reflected in methane expressed in milliliters and in methane per gram of digested DM. In Experiment 2, methane production increased at all PSC inclusion levels. Although the exact reason for this is unclear, it is possible that the specific type of non-fiber carbohydrate (NFC) in PSC was fermented in a way that produced more hydrogen, a precursor of methane. However, no change in propionate concentration was observed, and hydrogen could not be quantified in the current study.
Similarly, PSC and its components may have influenced protozoal populations, which are associated with methanogens; however, microbial populations were not assessed here. To the best of our knowledge, this is the first study to investigate the potential of PSC to mitigate enteric CH4 emissions. Nevertheless, we acknowledge that rumen fluid used in this study was sourced from forage-fed animals; thus, the rumen microbiome may not have been fully adapted to the high-grain diets examined. This factor may have influenced the findings, and further research is needed to validate these results.
Finally, this study reports only in vitro results, which, although valuable for screening dietary effects, are not fully representative of in vivo conditions due to the complex nature of the rumen environment. Future studies using live animals are therefore required to confirm these findings.
5 Conclusion
Findings from this in vitro study indicate that the impact of PSC on ruminal fermentation parameters is context dependent, varying with both the dietary background and PSC inclusion strategy. In Experiment 1, substituting canola or cottonseed meals with PSC did not influence CH4 production or total VFA concentrations, although increasing PSC levels led to a significant reduction in IVDMD. In contrast, Experiment 2 showed that incorporating PSC in place of barley grain significantly increased CH4 production, while among the PSC types tested, PSC4 (hexane extracted) demonstrated the most favorable digestibility profile when replacing barley grain.
Importantly, inclusion of PSC at levels up to 15% in a barley grain–based diet did not negatively affect either IVDMD or total VFA concentrations. Overall, PSC shows potential as an alternative feed ingredient, but its application involves clear trade-offs. While it can serve as a partial protein or energy source without depressing total VFA production, this may occur at the cost of reduced diet digestibility (when replacing protein meals) or increased methane emissions (when replacing grain).
It is important to note that these findings were derived from an in vitro fermentation system. Therefore, further in vivo research is essential to confirm these effects on nutrient digestibility, animal performance, and enteric methane emissions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by University of Queensland Production Animal Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
EA: Investigation, Writing – review & editing, Visualization, Writing – original draft. MN: Writing – review & editing, Writing – original draft, Investigation. AV: Formal Analysis, Visualization, Resources, Writing – review & editing, Writing – original draft, Conceptualization. SM: Project administration, Writing – review & editing, Formal Analysis, Methodology, Investigation, Conceptualization, Resources, Writing – original draft, Supervision, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was funded as an ARC Linkage Project (LP220100090) by the Australian Research Council, Terviva Inc., Australian Agricultural Company Ltd., Meat and Livestock Australia Ltd., Santos Ltd. Australia Pacific Lng Pty. Ltd, Troforte Innovations Pty. Ltd., and Arrow Energy Pty Ltd.
Conflict of interest
The authors declare that this study received funding from Terviva Inc., Australian Agricultural Company Ltd., Meat and Livestock Australia Ltd., Santos Ltd. Australia Pacific Lng Pty. Ltd, Troforte Innovations Pty. Ltd., and Arrow Energy Pty Ltd. Terviva Inc. provided information regarding the manufacturing of the pongamia meal. No other funder was involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed E., Fukuma N., Hanada M., and Nishida T. (2021). Insects as novel ruminant feed and a potential mitigation strategy for methane emissions. Animals 11, 2648. doi: 10.3390/ani11092648
AOAC (1995). Official methods of analysis. 16th ed (Arlington, VA: Association of Official Analytical Chemists).
Arndt C., Hristov A. N., Price W. J., McClelland S. C., Pelaez A. M., Cueva S. F., et al. (2022). Full adoption of the most effective strategies to mitigate methane emissions by ruminants can help meet the 1.5°C target by 2030 but not 2050. Proc. Natl. Acad. Sci. U. S. A. 119, e2111294119. doi: 10.1073/pnas.2111294119
Bach A., Calsamiglia S., and Stern M. D. (2005). Nitrogen metabolism in the rumen. J. Dairy Sci. 88 Suppl 1, E9–21. doi: 10.3168/jds.S0022-0302(05)73133-7
Chaves A. V., Thompson L. C., Iwaasa A. D., Scott S. L., Olson M. E., Benchaar C., et al. (2006). Effect of pasture type (alfalfa vs. grass) on methane and carbon dioxide production by yearling beef heifers. Can. J. Anim. Sci. 86, 409–418. doi: 10.4141/A05-081
Coblentz W. K., Abdelgadir I. E. O., Cochran R. C., Fick W. H., Olson K. C., and Turner J. E. (1999). Degradability of forage proteins by in situ and in vitro enzymatic methods. J. Dairy Sci. 82, 343–354. doi: 10.3168/jds.S0022-0302(99)75241-0
Czerkawski J. W. and Breckenridge G. (1977). Design and development of a long-term rumen simulation technique (Rusitec). Br. J. Nutr. 38, 371–384. doi: 10.1079/BJN19770102
Dutta N., Panda A. K., and Kamra D. N. (Eds.) (2012). “Use of Pongamia glabra (karanj) and Azadirachta indica (neem) seed cakes for feeding livestock,” in Biofuel co-products as livestock feed - opportunities and challenges (Rome, Italy: FAO), 379–402.
Enjalbert F., Combes S., Zened A., and Meynadier A. (2017). Rumen microbiota and dietary fat: a mutual shaping. J. Appl. Microbiol. 123, 782–797. doi: 10.1111/jam.13501
Fedorah P. M. and Hrudey S. E. (1983). A simple apparatus for measuring gas production by methanogenic cultures in serum bottles. Environ. Sci. Technol. Lett. 4, 425–432. doi: 10.1080/09593338309384228
Forwood D. L., Hooker K., Caro E., Huo Y., Holman D. B., Meale S. J., et al. (2019). Crop sorghum ensiled with unsalable vegetables increases silage microbial diversity. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.02599
Greenwood P. L. (2021). Review: An overview of beef production from pasture and feedlot globally, as demand for beef and the need for sustainable practices increase. Animal 15 Suppl 1, 100295. doi: 10.1016/j.animal.2021.100295
Gupta B. S., Srivastava J. P., Tripathi A. K., Verma A. K., and Thakur S. (1981). Biological evaluation of karanj (Pongamia glabra) cake. IJAH 20, 70–75.
Housman L. E., Sawyer J. E., Cox J. R., Redmon L. A., and Wickersham T. A. (2020). Expeller-pressed and solvent-extracted Pongamia seedcake as a protein supplement for cattle consuming a basal diet of forage. Anim. Feed Sci. Technol. 266, 114521. doi: 10.1016/j.anifeedsci.2020.114521
Jafari S., Meng G. Y., Rajion M. A., and Ebrahimi M. (2020). The use of plant by-products as non-conventional feedstuff for livestock feeding with reference to rumen methanogenesis. Agrofor. Syst. 94, 1491–1500. doi: 10.1007/s10457-019-00426-z
Kim S. W., Less J. F., Wang L., Yan T., Kiron V., Kaushik S. J., et al. (2019). Meeting global feed protein demand: challenge, opportunity, and strategy. Annu. Rev. Anim. Biosci. 7, 221–243. doi: 10.1146/annurev-animal-030117-014838
Kumar R., Kamra D. N., Agarwal N., and Chaudhary L. C. (2007). In vitro methanogenesis and fermentation of feeds containing oil seed cakes with rumen liquor of buffalo. Asian Australas. J. Anim. Sci. 20, 1196–1200. doi: 10.5713/ajas.2007.1196
Ku-Vera J. C., Jiménez-Ocampo R., Valencia-Salazar S. S., Montoya-Flores M. D., Molina-Botero I. C., Arango J., et al. (2020). Role of secondary plant metabolites on enteric methane mitigation in ruminants. Front. Vet. Sci. 7. doi: 10.3389/fvets.2020.00584
Lambo M. T., Ma H., Liu R., Dai B., Zhang Y., and Li Y. (2024). Review: Mechanism, effectiveness, and the prospects of medicinal plants and their bioactive compounds in lowering ruminants’ enteric methane emission. Animal 18, 101134. doi: 10.1016/j.animal.2024.101134
Marone P. A., Olson J., Matulka R., Bauter M., and Astwood J. D. (2022). Safety and toxicologic evaluation of Edible Pongamia Oil: A novel food ingredient. Food Chem. Toxicol. 166. doi: 10.1016/j.fct.2022.113213
Meale S. J., Chaves A. V., Baah J., and McAllister T. A. (2012). Methane production of different forages in in vitro ruminal fermentation. Asian Australas. J. Anim. Sci. 25, 86–91. doi: 10.5713/ajas.2011.11249
Mertens D. R. (2002). Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: collaborative study. J. AOAC Int. 85, 1217–1240. doi: 10.1093/jaoac/85.6.1217
Mottet A., Haan C., Falcucci A., Tempio G., Opio C., and Gerber P. (2017). Livestock: On our plates or eating at our table? A new analysis of the feed/food debate. Glob. Food. Sec. 14, 1–8. doi: 10.1016/j.gfs.2017.01.001
Murphy H. T., O’Connell D. A., Seaton G., Raison R. J., Rodriguez L. C., Braid A. L., et al. (2012). A common view of the opportunities, challenges, and research actions for pongamia in Australia. Bioenerg. Res. 5, 778–800. doi: 10.1007/s12155-012-9190-6
Nagalakshmi D., Dhanalakshmi K., and Himabindu D. (2011). Replacement of groundnut cake with sunflower and karanj seed cakes on performance, nutrient utilisation, immune response and carcass characteristics in Nellore lambs. Small Ruminant Res. 97, 12–20. doi: 10.1016/j.smallrumres.2011.02.003
Natanam R., Kadirvel R., and Chandrasekaran D. (1989a). Chemical composition of karanja (Pongamia glabra Vent) kernel and cake as animal feed. Indian J. Anim. Nutr. 6, 270–273.
Natanam R., Kadirvel R., and Viswanathan K. (1989b). The effect of karanja (Pongamia glabra Vent) cake on the performance of White Leghorn pullets. Anim. Feed Sci. Technol. 27, 89–93. doi: 10.1016/0377-8401(89)90132-6
Owens F. N. and Basalan M. (2016). “Ruminal fermentation,” in Rumenology. Eds. Millen D. D., de Beni Arrigoni M., and Lauritano Pacheco R. D. (Springer International Publishing, Cham), 63–102.
Parisi G., Tulli F., Fortina R., Marino R., Bani P., Dalle Zotte A., et al. (2020). Protein hunger of the feed sector: the alternatives offered by the plant world. Ital. J. Anim. Sci. 19, 1204–1225. doi: 10.1080/1828051X.2020.1827993
Pennsylvania State University (1990) Proceedings. Cornell Nutrition Conference for Feed Manufacturers publisher: Cornell University, NY, USA. 81–88.
Patra A. K. (2013). The effect of dietary fats on methane emissions, and its other effects on digestibility, rumen fermentation and lactation performance in cattle: A meta-analysis. Livest. Sci. 155, 244–254. doi: 10.1016/j.livsci.2013.05.023
Pexas G., Doherty B., and Kyriazakis I. (2023). The future of protein sources in livestock feeds: implications for sustainability and food safety. Front. Sustain. Food Syst. 7. doi: 10.3389/fsufs.2023.1188467
Rattansi R. and Dikshit M. (1997). Protease inhibitors and in vitro digestibility of karanja (Pongamia glabra) oil seed residue. A comparative study of various treatments. J. Am. Oil Chem. Soc 74, 1161–1164. doi: 10.1007/s11746-997-0040-1
Singh P., Sastry V., Garg A. K., Sharma A. K., Singh G. R., and Agrawal D. K. (2006). Effect of long term feeding of expeller pressed and solvent extracted karanj (Pongamia pinnata) seed cake on the performance of lambs. Anim. Feed Sci. Technol. 126, 157–167. doi: 10.1016/j.anifeedsci.2005.05.025
Soren N. M. and Sastry V. (2009). Replacement of soybean meal with processed karanj (Pongamia glabra) cake on the balances of karanjin and nutrients, as well as microbial protein synthesis in growing lamb. Anim. Feed Sci. Technol. 149, 16–29. doi: 10.1016/j.anifeedsci.2008.04.011
Soren N. M., Sastry V. R. B., and Saha S. K. (2011). Blood biochemical profile, immune response and rumen fermentation pattern ingrowing lambs fed processed karanj (Pongamia glabra) cake based diets. Indian J. Anim. Sci. 80.
Soren N. M., Sharma A. K., and Sastry V. R. B. (2017). Biochemical and histopathological changes in sheep fed different detoxified karanj (Pongamia glabra) seed cake as partial protein supplements. Anim. Nutr. 3, 164–170. doi: 10.1016/j.aninu.2017.04.002
Srivastava J. P., Gupta B. S., Thakur S., and Verma A. K. (1990). Utilization of deoiled karanj (Pongamia glabra) cake in kid grower rations. Indian J. Anim. Nutr. 7, 15–20.
Usharani K. V., Naik D., and Manjunatha R. L. (2019). Pongamia pinnata (L.): Composition and advantages in agriculture: A review. J. Pharmacogn. Phytochem. 8, 2181–2187.
Villalba J. J., Ates S., and MacAdam J. W. (2021). Non-fiber carbohydrates in forages and their influence on beef production systems. Front. Sustain. Food Syst. 5. doi: 10.3389/fsufs.2021.566338
Vinay B. J. and Kanya T. S. (2008). Effect of detoxification on the functional and nutritional quality of proteins of karanja seed meal. Food Chem. 106, 77–84. doi: 10.1016/j.foodchem.2007.05.048
Keywords: alternative feed, digestibility, methane, rumen fermentation, ruminants
Citation: Ahmed E, Ni M, Chaves AV and Meale SJ (2025) An in vitro evaluation of fat-extracted pongamia seedcake in barley-based feedlot diets. Front. Anim. Sci. 6:1685542. doi: 10.3389/fanim.2025.1685542
Received: 14 August 2025; Accepted: 08 October 2025;
Published: 28 October 2025.
Edited by:
Amir Mahboubi Soufiani, University of Borås, SwedenReviewed by:
Sladana Rakita, University of Novi Sad, SerbiaMelanie Brede, University of Veterinary Medicine Hannover, Germany
Milad Parchami, University of Borås, Sweden
Copyright © 2025 Ahmed, Ni, Chaves and Meale. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah J. Meale, cy5tZWFsZUB1cS5lZHUuYXU=
 Eslam Ahmed1,2
Eslam Ahmed1,2 Alex V. Chaves
Alex V. Chaves Sarah J. Meale
Sarah J. Meale