- 1Department of Agriculture and Forest Sciences, University of Tuscia, Viterbo, Italy
- 2National Reference Centre for Ovine and Caprine Milk and Dairy Products Quality (C.Re.L.D.O.C.), Istituto Zooprofilattico Sperimentale Lazio e Toscana “Mariano Aleandri”, Roma, Italy
Summer forage scarcity is a major constraint for Mediterranean dairy sheep farming, where high temperatures and drought reduce pasture yield and quality, leading to strong milk seasonality. Teff (Eragrostis tef (Zucc.) Trotter), a C4 annual grass native to Ethiopia, is drought- and heat-tolerant and may represent an alternative to traditional summer forages such as sorghum-sudangrass (Sorghum bicolor (L.) Moench subsp. sudanense). This study evaluated the effects of grazing teff on milk yield and composition in lactating Sarda ewes compared with sorghum-sudangrass under Mediterranean summer conditions. Thirty-two primiparous ewes in late lactation (150 ± 10 DIM) were assigned to two balanced groups (n = 16) and grazed 4 h/day for six weeks (two adaptation, four experimental) on either teff (GT) or sorghum-sudangrass (GS) pastures, supplemented with ad libitum hay and 0.5 kg/day concentrate. Pasture and milk were sampled weekly for chemical and quality analyses. Teff showed higher dry matter (30.8 vs 22.4%), crude protein (14.8 vs 10.6% DM), and ether extract (2.5 vs 1.9% DM), and lower acid detergent fiber (33.1 vs 37.4% DM) and lignin (4.1 vs 5.6% DM) compared with sorghum-sudangrass (P < 0.01). Ewes grazing teff produced slightly less milk (0.86 ± 0.09 vs 0.93 ± 0.11 kg/day; P < 0.05), with lower fat (6.19 vs 6.68%; P < 0.001) but higher lactose content (5.37 vs 5.28%; P < 0.05). Protein (5.60 vs 5.59%), somatic cell count (<200 × 10³ cells/mL), and coagulation traits were unaffected. Overall, both forages adequately supported lactating ewes during the summer. Teff appears promising for water-limited areas due to its heat and drought tolerance and stable nutritional profile, though its slightly lower voluntary intake may limit milk yield. Further studies addressing palatability and intake behavior are warranted.
1 Introduction
Forage scarcity is a major limitation for extensive and semi-extensive livestock systems in regions characterized by hot and dry summer, where high temperatures and water shortage reduce biomass yield and quality, creating seasonal feed gaps for grazing animals (Cooke et al., 2025; Gherbin et al., 2007). In some regions of the world, this structural constraint is expected to be further aggravated by climate change, which may intensify summer heat waves and drought (Sitzia and Ruiz, 2011; Habte et al., 2019).
In Mediterranean-type environments, the impact of this seasonality is particularly pronounced where fresh forage is abundant in spring but very scarce in summer and early autumn. This mismatch contributes to the strong seasonality of milk supply from grazing-based livestock systems, with little or no production in the driest months despite the peak in consumer demand for fresh dairy products (Landau et al., 2005; Todaro et al., 2015). Reducing this gap is crucial to support “out-of-season” milk production and improve the profitability of Mediterranean grazing-based dairy supply chains (Sitzia et al., 2015).
In the case of dairy sheep, several strategies have been explored to mitigate summer forage shortages including grazing on alfalfa (Medicago sativa), irrigated forage crops, the use of crop residues, the use of hay or silage, accelerated lambing schemes, freezing spring milk or curd for later processing (Sitzia et al., 2015), and shifting from local breeds to specialized dairy breeds (e.g., Lacaune) within a more intensive stall-fed systems (Sodi et al., 2024). However, these options are often limited by high investments and costs, reduced Protected Designation of Origin (PDO) cheese quality, or increased management complexity.
To address this issue, the exploitation of warm-season forage species able to maintain productivity under heat and drought stress has been proposed as a promising management strategy to improve limited summer fodder availability (Habte et al., 2019; Billman et al., 2022).
Teff (Eragrostis tef (Zuccagni) Trotter), a warm-season, C4 annual grass native to Ethiopia (D’Andrea, 2008; Miller, 2011), is emerging as a promising option. Teff is known for its high tolerance to drought and heat during summer (Norberg et al., 2009), making it particularly suited to Mediterranean agro-ecosystems. Furthermore, it has been demonstrated to be a fast-growing crop, capable of producing high forage yields with excellent nutritive quality. Comprehensive data on fresh teff are currently lacking, except for those reported by Ruggeri et al. (2024), who characterised the nutritional composition of several genetic lines harvested at different phenological stages. Reported values include: dry matter (DM) 21–34%, organic matter (OM) 88–92% DM, crude protein (CP) 14–16% DM, neutral detergent fibre (aNDFom) 62–67% DM, non-fibrous carbohydrates (NFC) <5–13.9% DM, and gross energy (GE) approximately 4.35 Mcal/kg DM. Teff hay exhibits a favourable nutritive value for ruminants, with a DM content of 91.7 ± 1.2% and an average CP concentration of 14.6 ± 3.3% DM. Fibre fractions are moderate (NDF 56.6 ± 4.2%, ADF 36.0 ± 1.9%), resulting in moderate digestibility (organic matter digestibility 61.8%, dry matter digestibility 58.5 ± 5.1%) and a metabolizable energy content of approximately 8.6 MJ kg⁻¹ DM. The ash content averages 9.4 ± 2.1% DM, and ether extract (EE) 2.3 ± 0.8% DM. Mineral concentrations are well balanced, with calcium 4.7 ± 0.5 g/kg DM, phosphorus 2.6 ± 0.6 g/kg DM, and potassium 18.7 ± 3.1 g/kg DM, values consistent with those typically found in warm-season C4 grasses (Heuzé et al., 2017). Teff can be harvested in less than 45 days after sowing, offering flexibility and rapid turnover for forage production (Miller, 2010). Its agronomic and nutritional characteristics make it a valuable candidate for inclusion into Mediterranean forage systems, particularly as a double cropping option after wheat or barley, helping to bridge the summer forage gap and support resilient, productive sheep farming (Habte et al., 2019; Ruggeri et al., 2024). Although teff hay and straw have been widely tested as alternative feeds for dairy cows (Saylor et al., 2018), beef cattle (Vinyard et al., 2018), horses (Staniar et al., 2010), and sheep (Bonsi et al., 1995), its potential and palatability as grazing fodder in dairy ewe farming remains substantially unexplored.
Sorghum-sudangrass (Sorghum bicolor (L.) Moench subsp. sudanense) (Zhan et al., 2008) is one of the most widely cultivated summer forages in the Mediterranean and other water-limited regions. It is well recognised for its high productivity under heat and drought stress, as well as for its strong regrowth capacity after cutting or grazing. Its narrower leaves and profuse tillering make it particularly suitable for multiple-cut systems, supporting the production of hay, haylage, green chop, and forage for grazing systems (Buxton and O’Kiely, 2003). Several studies have reported improved water-use efficiency of sudangrass under drought conditions (Sarkar and Northup, 2023; Kaplan et al., 2019) and high biomass yields, particularly in some sorghum × sudangrass hybrids (Bleier et al., 2020). Fresh plants shows moderate nutritive value for ruminants, with an average DM content of 20.8 ± 3.3% and CP of 11.0 ± 3.6% DM. Fibre fractions are relatively high (NDF 66.4 ± 7.3%, ADF 36.4 ± 3.6%, CF 30.9 ± 2.2%), leading to moderate digestibility (organic matter digestibility 66.5 ± 5.6%) and a metabolisable energy value of 9.3 MJ/kg DM. Mineral levels are typical of tropical grasses, with calcium 4.6 ± 0.7 g/kg DM and phosphorus 1.5 ± 0.7 g/kg DM (Heuzé and Tran, 2015). As in other sorghum species, sudangrass may accumulate dhurrin—a cyanogenic compound releasing prussic acid—especially under drought stress or excessive fertilisation (Shehab et al., 2020; Holman et al., 2019). Dhurrin levels are highest in young or regrowing plants (Busk and Moller, 2002) and decrease with maturity, so grazing should be delayed until plants reach 40–100 cm in height, as some varieties contain naturally lower concentrations (Hill and Roberts, 2020). Although this crop has been widely adopted by farmers and investigated in dairy cows (Pupo et al., 2022), beef cattle (Arnett et al., 2012), its direct effects on milk yield and composition of dairy sheep under grazing conditions have not yet been evaluated, highlighting a significant gap in the literature.
This study was conducted to evaluate the potential of teff and sorghum-sudangrass pastures as summer forage resources for dairy ewes in Mediterranean environments, with particular focus on their ability to sustain milk yield and composition during periods of seasonal shortage and to support out-of-season milk production. In particular, teff was expected to offer an additional advantage over sorghum-sudangrass, as it combines good forage quality with the absence of dhurrin-related toxicity risk, thereby supporting safer and more sustainable out-of-season milk production.
2 Materials and methods
2.1 Ethical approval
The research and the animal care protocols were in accordance with the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes (European Union (EU), 2010).
2.2 Animals and experimental design
The study lasted a total of 6 weeks (June 18th – July 30th, 2024), including an adaptation period of two-weeks (June 18th – July 1st) followed by a four-weeks experimental phase (July 2nd – 30th). The trial was conducted in a commercial farm in Viterbo, Central Italy (42° 31 ‘05.5”N, 12° 03’ 48.0”E) on 32 healthy, primiparous Sarda dairy ewes that, at the beginning of the adaptation period, were at the late stage of lactation (150 ± 10 days in milk, DIM). Primiparous Sarda ewes were chosen as they are typically the last to lamb in traditional systems without reproductive seasonality control. As a result, they remain in milk during the summer period, coinciding with the growth period of teff and sorghum-sudangrass pastures. The ewes were randomly assigned to two groups of 16 heads each, balanced for individual milk yield (0.93 ± 0.21 kg/day) and body condition score (BCS; 2.7 ± 0.1).
The groups, named GS and GT, were managed under the same housing and milking conditions, but grazed on different pasture types: GS grazed on sorghum-sudangrass (var. Piper; Pacific Seed Company, California, USA) pasture, while GT grazed on teff (Moxie: 37% cv. Tiffany, 63% cv. CW0604; Barenbrug, The Netherlands). Both grasses were grown on-farm without fertilisation or irrigation. Animals were allowed to graze for a total of 4 h per day (09:00-11:00 and 16:00-18:00), scheduled to avoid the hottest hours of the day and to mitigate potential heat stress, with a stocking rate of 2.4 livestock unit (LU) per hectare. At the barn, all ewes received the same basal diet of on-farm produced clover-ryegrass hay (CP: 94 g/kg DM; EE: 9 g/kg DM; ash: 41 g/kg DM; CF: 391 g/kg DM; aNDFom: 574 g/kg DM; ADF: 564 g/kg DM; acid detergent lignin, ADL: 94 g/kg DM), which was offered ad libitum. In addition, each ewe was individually supplied with 0.5 kg/day of commercial pelleted feed (CP: 175 g/kg DM; EE: 50 g/kg DM; ash: 75 g/kg DM; CF: 45 g/kg DM; sodium, Na: 3 g/kg DM) during the two daily milking sessions (06:30 and 18:30). The list of ingredients included in the pelleted feed is provided in the Supplementary Materials (Supplementary Table S1). Tap water was always available.
During the experimental phase, at five weekly sampling points (namely T0, T1, T2, T3, T4), sudangrass and teff samples were collected following a plot-based approach described by Primi et al. (2016), using 1 × 5 m plots where herbaceous biomass was harvested. Fresh biomass was cut at 2 cm above the ground using a double-bladed shear (Benelli et al., 2025). Samples were placed in sealed plastic bags and transported to the laboratory, in a cool box, for proximate analysis.
The BCS was assessed at the T0, T2, and T4, following the method described by Russel et al. (1969). At every time points, individual milk yield (MY, kg/day) was recorded using a sheep and goats Waikato 4.5 L milk meter (Waikato Milking Systems, Hamilton, New Zealand) (accuracy: ± 30 mL/L), and individual samples (50 mL without preservative) were collected during the morning milking for milk composition analysis. At T4, the ewes were milked only once per day, in the morning, as they were entering the dry-off phase.
2.3 Weather conditions
The study area has a Mediterranean climate, characterized by hot, dry summers and cool, rainy winters. The mean annual air temperature is about 14.5°C, while the mean annual precipitation is 755 mm (Ruggeri et al., 2024). Weather data for 2024 (Figure 1) were obtained from the meteorological station of the University of Tuscia (42°25′31.88″ N, 12°4′43.30″ E; Viterbo, Italy), located approximately 10 km from the farm and representing the nearest station with accessible records.
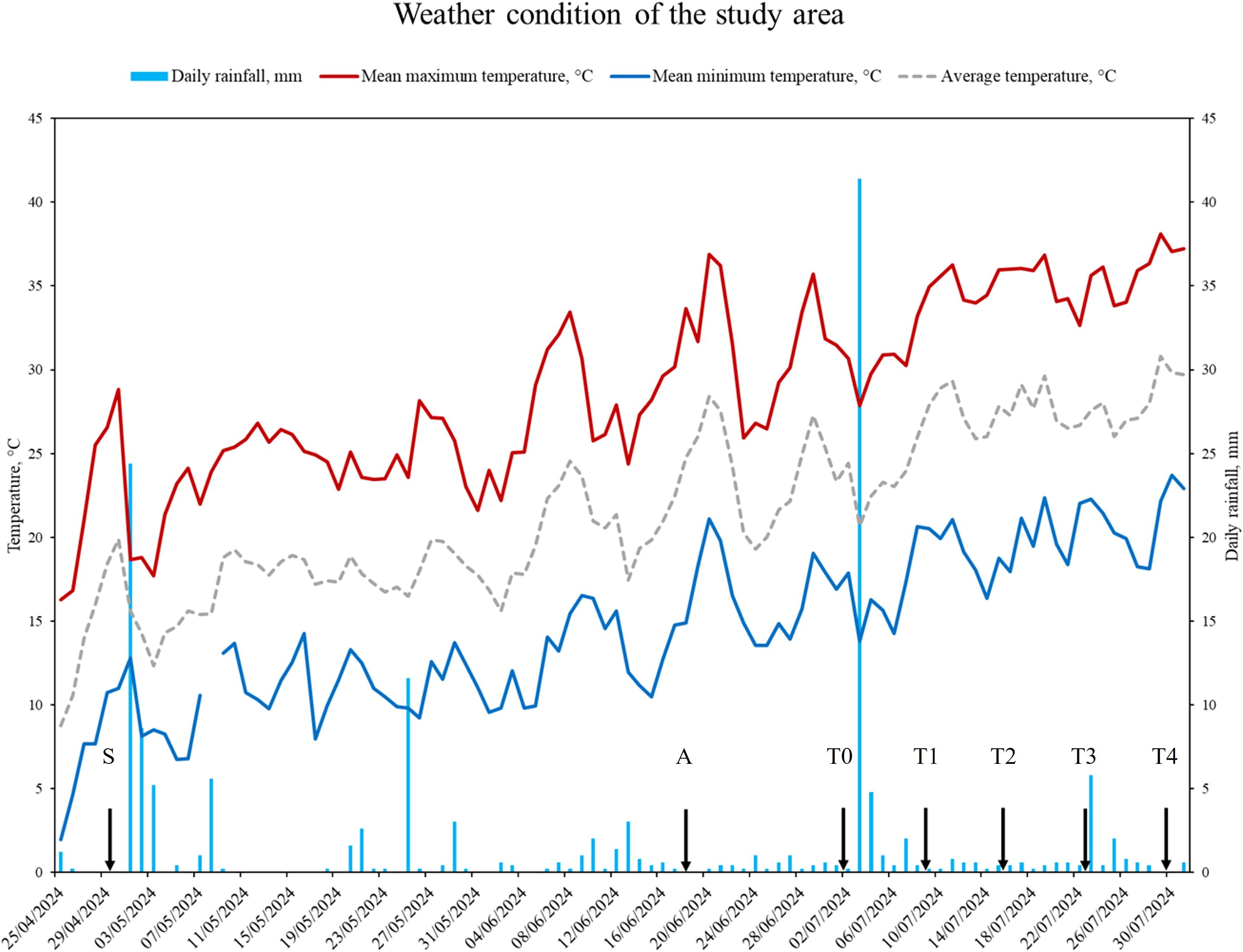
Figure 1. Weather conditions recorded from grass sowing to the end of the trial, including maximum (red line), minimum (blue line), and average (grey, dashed line) air temperature, and cumulative daily rainfall (sky blue bars). Vertical arrows indicate the sowing date (S; 30/04/2024), the adaptation period (A; 18/06/2024 - 01/07/2024), and the sampling times during the trial (T0: 02/07/2024; T1: 09/07/2024; T2: 16/07/2024; T3: 23/07/2024; T4: 30/07/2024).
2.4 Feed analysis and dry matter intake estimation
The DM content of pasture samples was determined by oven-drying at 65°C to a constant weight. Dried samples were then ground to pass through a 1 mm screen and analysed for aNDFom, ADF, ADL (using the filter bag equipment; Ankom Technology Corp., Fairport, NY; Van Soest et al., 1991), CF (AOAC International, 2012; method 962.09), CP (AOAC International, 2000; method 988.05), ash (AOAC International, 2000; method 942.05), and EE (AOAC International, 2005; method 920.39). The non-fibrous carbohydrates (NFC) were calculated as described by the National Research Council (2001) (Equation 1).
Pasture dry matter intake (DMI) was not directly measured in this study. However, in order to obtain a rough estimate consistent with published approaches (Bosco et al., 2021), indicative values of DMI were estimated using the Nutritional Dynamic System Professional software (NDS Pro; R.U.M.&N., Reggio Emilia, Italy), which implements the Cornell Net Carbohydrate and Protein System (CNCPS v6.5/v6.55; Cornell University, New York, USA). The model was parameterised using animal requirements and basic pasture characteristics derived from laboratory analyses and field observations. Estimates were obtained for both the pasture component and the basal diet, and the total DMI under the experimental feeding regime is hereafter referred to as DMI_BD. These values were used only as supportive information to contextualize animal performance and compare forage types and should be interpreted as indicative rather than measured data. For this purpose, the “pasture” tool of NDS Pro was parameterised with data on pasture height, standing biomass, and grazing duration obtained from field observations and plate meter measurements, to provide an indicative estimate of total grass intake under the actual experimental conditions.
2.5 Milk analysis
Milk composition and coagulation analyses were carried out by the National Reference Centre for Ovine and Caprine Milk and Dairy Products Quality (C.Re.L.D.O.C.) at the Istituto Zooprofilattico Sperimentale Lazio e Toscana “M. Aleandri” (Rome, Italy). This laboratory is accredited by ACCREDIA, the Italian Accreditation Body (Laboratory No. 0201A), and operates in compliance with the ISO/IEC 17025:2017 standards of the International Organization for Standardization. Individual milk samples were analysed for fat (FAT, %), protein (PROT, %), lactose (LAC, %), solids-not-fat (SNF, %), frozen point (FP, °C), urea (MU, mg/dL), total casein (CAS, %), citric acid (CA, %), milk conductivity (COND, mS), using a MilkoscanTM 7 device (Foss Analytical A/S, Hillerød, Denmark) calibrated with appropriate milk sheep standards, and somatic cell count (SCC; n/mL) using a Fossomatic™ FC device (Foss Analytical A/S, Hillerød, Denmark). Titratable acidity (TA) was recorded as Soxhlet-Henkel degree (SH°) while pH was measured with a potentiometric method using a pH meter (mod. HQ411D - HACH Company).
RCT (rennet coagulation time; min), k20 (curd firming time; min) and a30 (curd firmness; mm) were measured using a Formagraph LDG 2.0 (Ma.Pe System Srl, Firenze, Italy). For the coagulation trait analysis, sample preparation involved heating 10 mL of milk to 36°C, followed by the addition of calf rennet (200 µL) composed of 75% chymosin and 25% pepsin (175 international milk clotting units/mL; Clerici S.p.A., Cadorago (CO), Italy, saccosystem.com) diluted to a 0.8% (w/v) concentration in distilled water (Zannoni and Annibaldi, 1981). Milk correction for fat (6.5% fat-corrected milk, FCM) and protein (6.5% fat- and 5.8% protein-corrected milk, FPCM) were calculated according to Pulina and Nudda (2004) (Equations 2, 3).
The equations used were:
and
Moreover, somatic cell score (SCS) was calculated using the logarithmic transformation proposed by Wiggans and Shook (1987) as follows (Equation 4):
2.6 Statistical analysis
Pasture-related variables (DM, aNDFom, ADF, ADL, CF, CP, Ash, EE, NFC) and DMI data (DMI_BD), which were not normally distributed according to the Shapiro-Wilk test (P ≤ 0.05), were analysed using the non-parametric Kruskal-Wallis test. Two sets of comparisons were performed: (i) overall differences between the two forage types (teff and sorghum-sudangrass) regardless of sampling time, and (ii) pairwise comparisons between teff and sorghum-sudangrass at each sampling time (T0–T4), to evaluate whether group differences varied across the experimental period.
Milk yield, milk composition, and BCS data were initially checked for normality using the Shapiro-Wilk test. Variables meeting the assumption of normality in raw or log-transformed form (i.e., MY, FAT, FCM, FPCM, PROT, FAT/LAC, FAT/PROT, SNF, SCS, MU, CA, COND, pH, TA) were analysed using a General Mixed Model (Statistica 10; StatSoft Inc., Tulsa, OK, USA). The model included the fixed effects of group (GS, GT), time point (T0–T4), and their interaction.
Animal was included as a random effect, nested within group, to account for repeated measurements and individual variability. Variables that did not meet the normality assumption even after transformation (i.e., LAC, PROT/LAC, SCC, FP, CAS, K20, RCT, a30, and BCS) were analysed using the non-parametric Kruskal-Wallis test (XLSTAT Premium version 2024.2.2; Addinsoft, Paris, France). Significance was set at P ≤ 0.05.
Data from the mixed model are presented as least squares means ± standard error (LSM ± SE; Table 1). Non-parametric variables are reported as mean ± SE in Table 2, while their distributions are provided as medians with interquartile ranges (IQR) in the Supplementary Materials (Supplementary Tables S2 and S3), as appropriate.
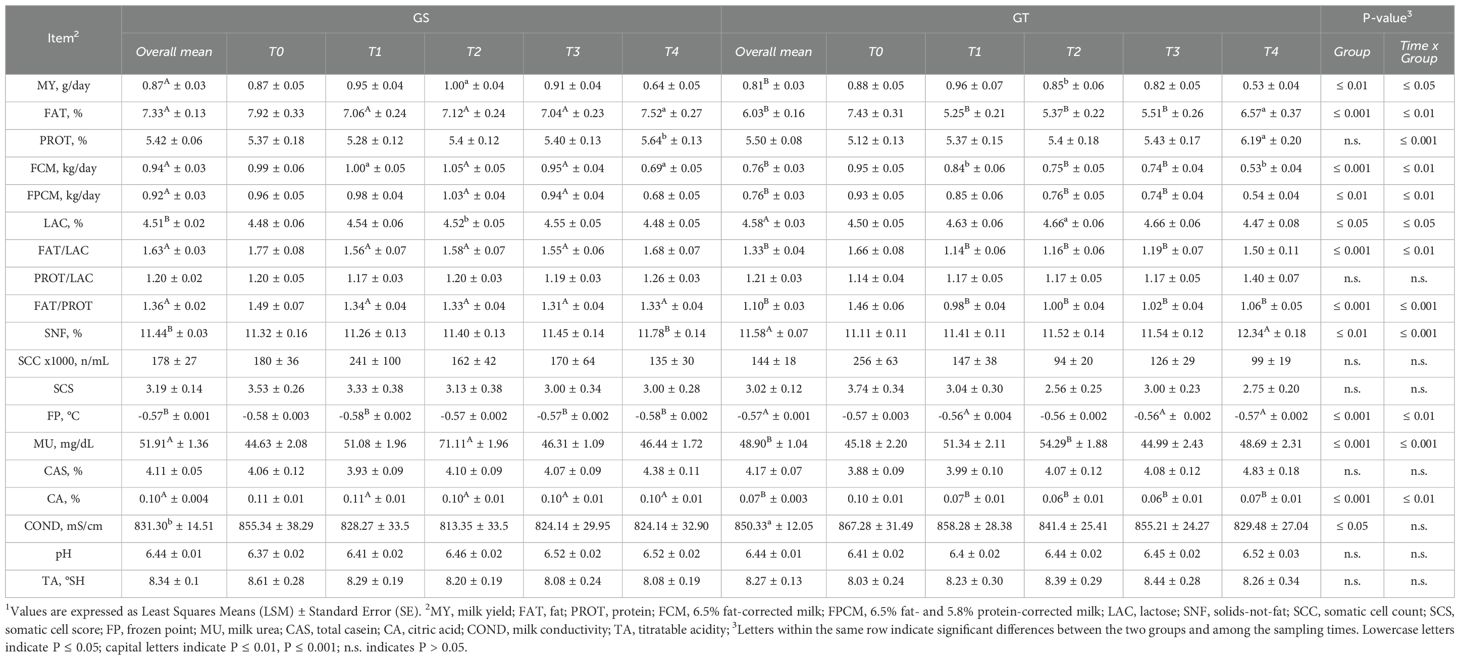
Table 2. Effect of grazing on sorghum-sudangrass (GS) or teff (GT) and sampling period on milk yield and composition1 in dairy ewes.
3 Results
3.1 Forage composition and estimated dry matter intake
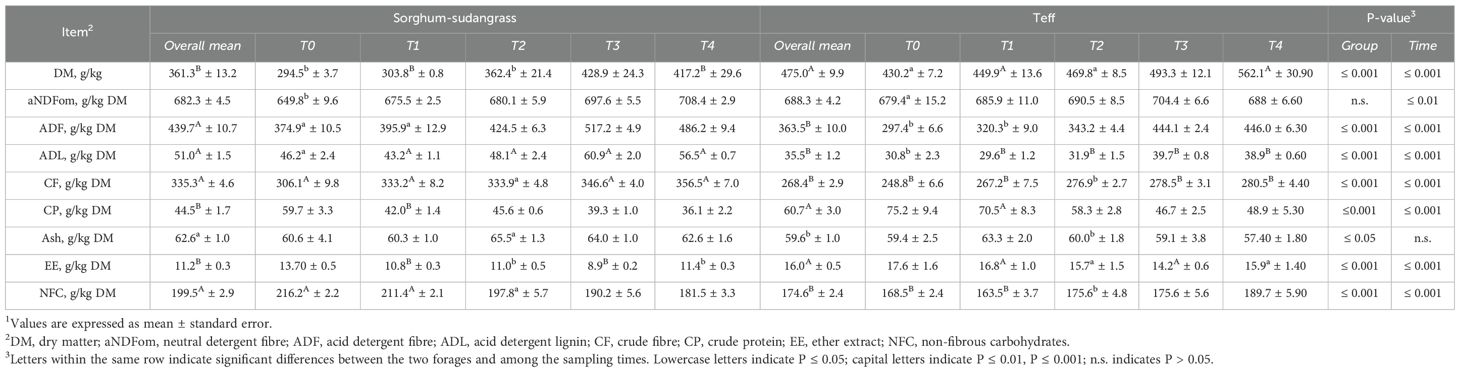
Composition differed significantly between sorghum-sudangrass and teff forages, with marked variations across the five sampling times (Table 1). Dry matter was higher in teff (P ≤ 0.001), showing significant differences from sorghum-sudangrass at all sampling points. Neutral detergent fibre did not differ between groups overall, although differences between the two forages were detected at T0 (P ≤ 0.01). Acid detergent fibre and ADL were significantly lower in teff (P ≤ 0.001; P ≤ 0.001) as was CF (P ≤ 0.001). In contrast, CP and EE were higher in teff (P ≤ 0.01; P ≤ 0.001). The ash content was slightly lower in teff (P ≤ 0.05), with no significant differences across time points. Finally, NFC was significantly lower in teff (P ≤ 0.001). The comparison at each sampling time revealed significant differences between the two forages for DM, ADF, ADL, CP, EE, and NFC (P ≤ 0.001), as well for aNDFom (P ≤ 0.01).
The DMI_BD was consistently lower in animals grazing teff compared with those grazing sorghum-sudangrass (P ≤ 0.001). In GT, DMI_BD remained stable across all sampling times, while in GS gradually decreased from T0 to T4. At each sampling time, the comparison between groups confirmed significant differences (P ≤ 0.001).
3.2 Body Condition Score
The overall BCS evaluation showed no significant differences between groups. At T0, BCS was 2.7 ± 0.15 in GS and 2.7 ± 0.16 in GT. At T2, BCS was 2.5 ± 0.09 in GS and 2.5 ± 0.11 in GT, while at T4 it was 2.5 ± 0.07 and 2.5 ± 0.08, respectively. Significant differences were observed between sampling times within both groups: in GS between T0 and T2 (P ≤ 0.01) and between T0 and T4 (P ≤ 0.05); in GT between T0 and T2 (P ≤ 0.001) and T0 and T4 (P ≤ 0.01). Overall, BCS decreased from T0 to T2 in both groups and then remained stable up to T4.
3.3 Milk
Milk yield was significantly reduced in GT compared with GS (P ≤ 0.01), with a significant time × group interaction (P ≤ 0.05) mainly due to the differences observed at T2. Similarly, both FCM and FPCM were lower in GT, although following a different temporal pattern, as indicated by a significant interaction (P ≤ 0.01). Regarding milk composition (Table 2; Figure 2), FAT content was reduced in GT (P ≤ 0.001), with a marked time × group interaction (P ≤ 0.01). PROT content did not differ between groups, although an interaction effect (P ≤ 0.001) was detected particularly due to the higher value in GT at T4. LAC content was higher in GT compared to GS (P ≤ 0.05), with a time × group interaction (P ≤ 0.05). Consequently, the FAT/LAC ratio was reduced in GT (P ≤ 0.001), with a significant interaction (P ≤ 0.01), while the PROT/LAC ratio remained stable over time and between groups. The FAT/PROT ratio was also lower in GT (P ≤ 0.001), with a significant time × group interaction (P ≤ 0.001). SNF content was higher in GT (P ≤ 0.01), again with a strong interaction effect (P ≤ 0.001). A similar trend was observed for FP, which was on average higher in GT (P ≤ 0.001), with significant differences among sampling times (P ≤ 0.01). No significant differences emerged between groups for SCC, SCS, CAS, pH, TA, or coagulation traits (k20, RCT, a30, Table 3), although RCT was lower in GT at T2 compared with GS (P ≤ 0.05). Moreover, MU was significantly higher in GS (P ≤ 0.001) and increased over time (P ≤ 0.001), particularly at T2. Finally, CA content decreased in GT (P ≤ 0.001), while COND was significantly higher (P ≤ 0.05).
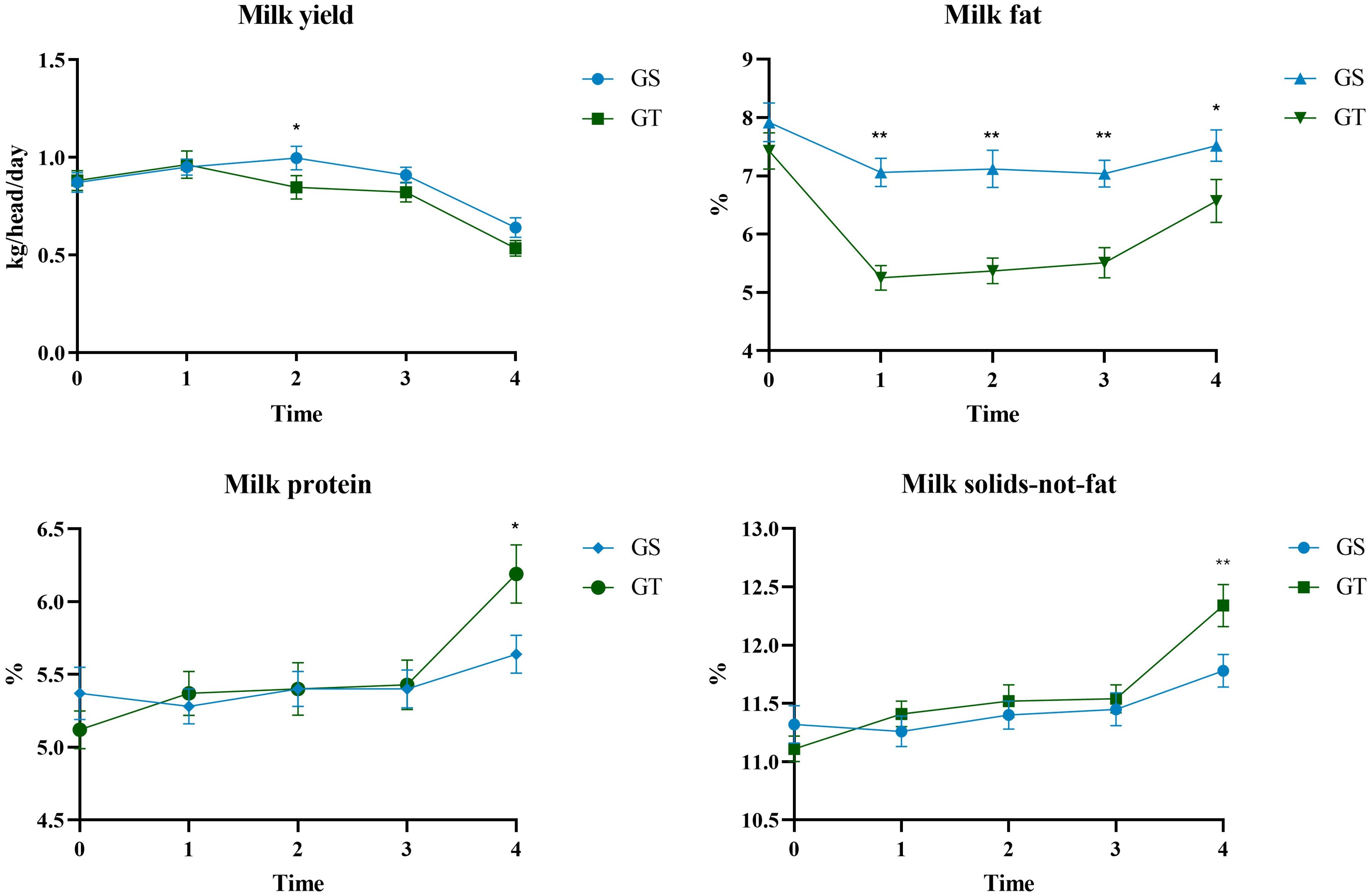
Figure 2. Temporal changes in milk yield, fat, protein and solids-not fat of dairy ewes grazing on sorghum-sudangrass (GS; blue line) and teff (GT; green line). * = P ≤ 0.05; ** = P ≤ 0.01. Error bars represent the standard error mean. Graphs were generated using GraphPad Prism version 8.0.1 (GraphPad Software, Boston, MA, USA).
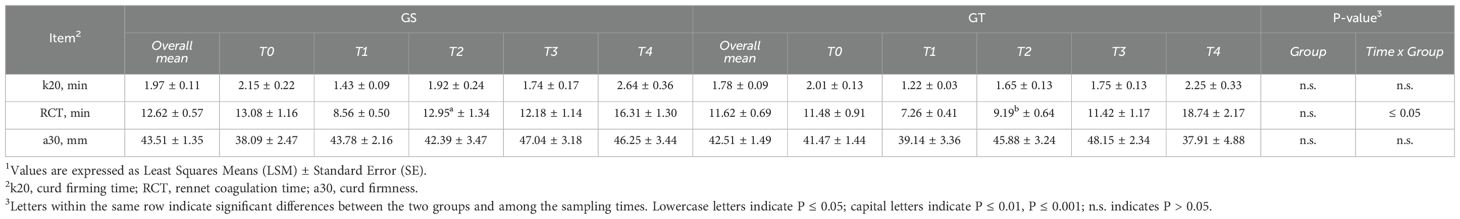
Table 3. Effect of grazing on sorghum-sudangrass (GS) or teff (GT) and sampling period on coagulation traits1 of milk.
4 Discussion
In recent years, teff has emerged as a promising forage crop, while sorghum-sudangrass has confirmed its relevance, particularly in regions characterized by water scarcity in summer. Forage species that can thrive under limited water availability while still providing adequate nutritional value represent valuable alternatives to irrigated crops (Saylor et al., 2018), especially in view of the increasing pressure that climate change is exerting on agro-systems. Rising temperatures and water shortages (Zobeidi et al., 2022) pose major challenges to both crop productivity and the availability of feed resources for livestock. As emphasized by Morand-Fehr et al. (2007), feeding strategies are directly shaped by farmers, and their impact on animal performance is often evident in the short term.
Under the economic sustainability standpoint, in sheep farming, and in livestock production more broadly, animal feeding is the most influential factor affecting farming performance. Feed-related costs typically represent 50-90% of the total expenditure of producing sheep or goat milk, underscoring their central role in the economic sustainability of small ruminant systems (Morand-Fehr et al., 2007). For all these reasons, the adoption of alternative forage species such as teff must be evaluated in terms of their impact on animal productivity, and for their economic feasibility.
Although the present study did not include a formal economic assessment, the evaluation of the economic feasibility of teff compared to more conventional forage crops represents an important aspect for future research, particularly in the context of Mediterranean small ruminant production systems.
Within this context, teff, together with other emerging crops such as quinoa, tritordeum, and chia, has been proposed as a potential alternative particularly suited to Mediterranean conditions (Ruggeri et al., 2024). However, information on the adoption of teff forage in small ruminant production systems, especially under grazing management, remains very limited, particularly in the Mediterranean area. This study represents the first attempt to evaluate the effects on milk performance on lactating Sarda ewes grazing on teff compared to a more common grazing fodder source (sorghum-sudangrass).
In our study, teff exhibited a generally higher DM content compared to sorghum-sudangrass, and with average values exceeding those reported in the literature. Ruggeri et al. (2024) documented a DM range between 21-34%, particularly lower during the heading stage, probably because in their study the crop was irrigated, as DM accumulation increased progressively throughout the plant’s developmental stages. Crude protein content in teff was also found to be higher than that of sorghum-sudangrass; however, it remained lower than the values previously reported. A considerable fraction of CP (41.6 ± 6.4%, unpublished data) in different teff genotypes has been observed to be bound to the aNDFom. According to Miller (2010) and Ruggeri et al. (2024), CP values in teff range between 12-16%, while Uyanik and Carpici (2025) observed broader variability, with CP values ranging from 10 up to 19%. The fibr content of teff, specifically aNDFom, was consistent with the literature findings (Miller, 2010; Ruggeri et al., 2024; Uyanik and Carpici, 2025). Regarding the NFC quota, teff showed lower values than sorghum-sudangrass. However, when compared to previously reported ranges, teff exhibited slightly higher NFC values; Ruggeri et al. (2024) reported an NFC range between 3-12%. The observed discrepancies in nutritional composition between our findings and those in literature are likely due to the use of different teff varieties, agronomic conditions, phenological stage, environmental conditions, and genetic variability which plays a fundamental role in defining the forage quality (Ruggeri et al., 2024; Uyanik and Carpici, 2025).
Similarly to teff, sorghum-sudangrass also showed compositional values that diverged from those reported in the literature, with higher DM and aNDFom contents and lower CP concentrations (Pupo et al., 2022). Reported CP values for sorghum-sudangrass usually range between 11-15% (Miller, 2010; Pupo et al., 2022), whereas in this study they were considerably lower. Fiber fractions were also above the values commonly described in previous works. Such discrepancies can be attributed to the combined influence of agronomic practices and environmental conditions. In fact, both sorghum-sudangrass and teff were grown without irrigation or fertilization during the 2024 season, which was characterized by scarce rainfall and high temperatures (Figure 1). Under these circumstances, sorghum-sudangrass deviated markedly from the nutritional standards reported in the literature, while the teff mix only partially expressed its genetic potential, producing forage of lower nutritional quality than expected. Overall, these findings suggest that the reduction in nutritive value - particularly evident in teff - was the result of the interaction between management practices and climatic stress, rather than an intrinsic limitation of the species.
Although both crops were grown under similar conditions, in this study a more pronounced decline over time in forage quality was observed for sorghum-sudangrass. In comparison, teff maintained relatively more stable nutritional characteristics throughout the growing period. In particular, the accumulation of aNDFom, ADF and ADL were more pronounced in sorghum-sudangrass than in teff.
Although the biomass availability did not represent a limiting factor for both the GT and GS grazing ewes, the indicative estimates of forage intake, as estimated by the NDS Pro, highlighted a difference between the two grazed forages: on average, DMI_BD of teff was 21.7% lower than that of sorghum-sudangrass. This difference could be explained by the forage chemical composition and pasture characteristics, such as leaf morphology, including the presence of hairs and cuticle thickness, leaf size, the physical properties of stems all of which can stimulate, limit, or inhibit animal feeding behaviour (Barre et al., 2006; Decruyenaere et al., 2009). These structural features may potentially limit voluntary DMI and could also affect digestibility. Indeed, previous studies have reported that the in vitro digestibility of teff is approximately 4-5% lower than that of sorghum-sudangrass (Twidwell et al., 2002; Schrenker, 2014). Both intake and digestibility are critical factors influencing the availability of nutrients necessary for milk secretion (Decruyenaere et al., 2009). However, these remain speculative considerations, as no studies in the literature have specifically assessed the effect of grazing on teff in sheep milk production and quality. To the best of our knowledge, existing research has focused on the inclusion of teff hay in total mixed ration (TMR) diets for dairy cows, in which no influence on DMI was reported (Saylor et al., 2018; Wagali et al., 2023). As a result of the lower DM intake, the GT grazing ewes had a lower daily milk yield (-7.37%) compared to the ones grazing on sorghum-sudangrass. Although milk production was initially similar between the two groups, a clear decline in the teff group became evident after three weeks of grazing. A similar, though more pronounced, pattern was observed for FCM, which decreased by 18.4%, with significant differences already apparent by the second sampling point. The marked reduction in FCM is attributable to the lower milk fat content observed in the GT from the second sampling onward. This lower milk fat in the GT persisted throughout the experimental period, with the exception of the final sampling, where differences between the groups, although still present, were smaller, and both groups showed an increase in milk fat content, much more pronounced in the GT, which can be attributed to the single daily milking and the consequent milk concentration effect. Milk protein content remained similar between groups over time, with an increase at the end of the experimentation phase for the same reasons described above for fat content. A significant difference was observed only at the T4 sampling, when milk protein content was higher in the GT than GS ewes, likely due to the greater reduction in milk yield in this group and the resulting concentration effect. Consequently, the FAT/PROT ratio also followed the same trend reported for milk fat.
The reductions observed in MY, FCM, FPCM, and FAT content may be partially explained by the lower DM intake recorded in the GT group, as previously discussed, in agreement with literature reporting a direct relationship between reduced feed intake and decreased milk yield (Pulina et al., 2013). According to Pulina et al. (2005), the energy content of the diet, the amount of protein, and the amount of fibre are the main factors influencing milk yield as well as milk fat and protein content in sheep. Furthermore, a lower intake of NFC, highly digestible carbohydrates that include total starch and sugars, may have contributed, as these compounds represent an essential energy source for ruminants (Ruggeri et al., 2024).
Since GT animals were moved to pasture immediately after milking and received their concentrate portion during milking, based on indicative estimates from the NDS Pro system, they may have consumed less forage while grazing on teff. This could have resulted in an imbalance between concentrate and forage intake in the GT group, which may be consistent with the observed reduction in milk fat content. As noted by Allen (1997), excessive dietary concentration, particularly of fermentable carbohydrates, can reduce ruminal pH, favouring non-cellulolytic over cellulolytic microorganisms (Van Soest, 1994), decreasing acetate and increasing propionate production, which can contribute to lower milk fat content.
Additionally, the lower DMI estimated for the GT group may have resulted in reduced rumination time, thereby producing less saliva with its buffering capacity, which is essential for maintaining a stable rumen environment (Mertens, 1997). It is also possible that teff contains bioactive secondary metabolites, particularly polyphenols and flavonoid derivatives, that could influence rumen microbial populations and fibre fermentation, potentially affecting DMI and milk fat synthesis. However, this remains speculative and warrants further investigation in future studies. Although a reduction in indicative intake was suggested for the GT, BCS did not differ from GS ewes, though it decreased over time in both. It is noteworthy that, despite the similar decrease in BCS, the GT ones showed lower production compared to the GS ones.
To date, no studies have investigated the impact of teff grazing on milk production and quality in sheep. Only a few trials have explored the use of teff in dairy cow diets, primarily through the inclusion of teff hay in TMR. For example, Wagali et al. (2023) reported that replacing more than 11% of the basal TMR with teff hay increased milk yield in dairy cows without altering milk composition, as fat and protein contents remained stable. Similarly, Saylor et al. (2018) included 27.3-29.6% teff hay in the diets of dairy cows and observed no differences in feed intake, milk yield, or milk fat content, but reported higher milk protein concentrations, which they attributed to the greater fermentability of teff-based diets. Although these results are not directly comparable to ours due to the difference of the farming systems, they suggest that teff may be profitably included as a valuable forage component in controlled feeding systems such as TMR feeding. However, under grazing conditions, as observed in our study, sheep may experience reduced intake due to a presumed lower palatability or the chemical and physical characteristics of the teff mix used as pasture, such as the presence of defensive leaf tector trichomes (Favaretto et al., 2015), highlighting the need for further research on the topic.
In the present study, SCC values consistently remained below 200 × 10³ cells/mL, indicating very good udder health status (Paschino et al., 2019), suggesting that the animals were managed under proper husbandry conditions. No significant differences were observed in SCC or SCS between the two grazing groups, as nor their group × time interaction, confirming that grazing on teff in comparison to sorghum-sudangrass did not negatively affect udder health. These findings are in line with previous studies conducted on dairy cows, where the inclusion of teff in the diet did not result in increased SCC or SCS (Saylor et al., 2018; Wagali et al., 2023). Although milk conductivity was slightly lower in the GS, this is not indicative of any health issues but rather reflects a dilution effect due to the higher milk yield observed in this group.
The content of CA in sheep milk is a potential biomarker of mammary gland function and overall metabolic status. Along with established indicators such as SCC, milk electrical conductivity or pH, CA can provide valuable insights into subclinical mastitis and the physiological dynamics of lactation (Oshima and Fuse, 1981). As expected, CA concentrations declined progressively throughout lactation in both experimental groups, reflecting the natural reduction in mammary metabolic activity over time. Beyond its physiological role, the CA content in milk also appears sensitive to dietary influences, particularly in relation to the availability of energetic precursors. In our trial, ewes in GT group consistently exhibited lower milk CA concentrations than those in GS group, despite similar levels at the beginning of the experimental period. This difference suggests that the composition of teff forage may limit the supply of key substrates required for CA synthesis. Citric acid is synthesised in mammary epithelial cells from carbon derived primarily from glucose and acetate and is secreted into milk via exocytosis alongside lactose and milk proteins (Linzell et al., 1976). As an intermediate of the tricarboxylic acid cycle, CA plays a pivotal role in energy metabolism and contributes indirectly to milk fat synthesis by generating NADPH (Garnsworthy et al., 2006). Therefore, the reduced CA concentrations observed in the GT group may indicate suboptimal energy metabolism of the mammary gland, potentially limiting NADPH availability and partially explaining the lower milk fat content compared to the GS group. These findings may contribute to explaining the reduced milk yield and fat content previously observed in the GT, as the lower CA concentrations could reflect a limitation in the energy supply to the mammary gland. The higher lactose concentration observed in GT milk (+1.55%), despite the lower availability of glucogenic substrates, may indicate that lactose synthesis was maintained as a metabolic priority, possibly at the expense of other pathways, as reflected by the lower fat and CA contents in this group.
As previously discussed, teff has a higher total protein content compared to sorghum-sudangrass, which would typically be expected to result in higher MU concentrations (Cannas et al., 1998). However, contrary to this expectation, sheep in the GS exhibited higher MU levels than those in the GT. This difference may be related to variations in the higher rumen degradable protein (RDP) content in sorghum-sudangrass compared to teff, although direct bibliographic evidence for this specific hypothesis is limited. Mikolayunas-Sandrock et al. (2009) demonstrated that the proportions of RDP and rumen undegradable protein (RUP) in the diet significantly influence MU in sheep fed nearly isoenergetic diets. Specifically, sheep receiving 14% RDP and 4% RUP (DM basis) showed higher MU concentrations than those receiving 12% RDP and 4% RUP. Therefore, even though sorghum-sudangrass has a lower total protein content than teff, the observed higher MU concentrations in sorghum-sudangrass could be due to a relatively higher RDP fraction, potentially increasing ammonia production in the rumen and consequently higher MU concentrations in milk. An elevated RDP supply may increase urea excretion and reduce nitrogen utilization efficiency in the animal (Mikolayunas-Sandrock et al., 2009).
Although ewes in the GT showed lower milk yield and fat content, milk rheological properties were generally unaffected, except for RCT at T2. This finding is particularly relevant in sheep production systems, where milk quality is closely associated with cheese-making potential. The preservation of coagulation traits, despite the differences in production and composition, suggests that teff-based diets may still ensure adequate milk processing performance. Such evidence supports the potential suitability of teff as a forage resource in dairy sheep systems, even under challenging environmental conditions.
This study provides novel evidence on the use of teff and sorghum-sudangrass as summer forage resources for dairy sheep under Mediterranean conditions. Both species supported lactating ewes without negative effects on udder health, but differences emerged in terms of intake and milk performance. While teff pastures showed higher dry matter and crude protein contents and a more stable nutritional profile over time indicative estimates suggested slightly lower voluntary dry matter intake compared with sorghum-sudangrass resulted in slightly reduced milk yield and fat concentration, whereas lactose content was higher and protein levels were unaffected.
Overall, the results indicate that both crops can contribute to reducing the seasonal gap in forage supply and may help sustain milk production during summer, thereby supporting out-of-season milk availability in Mediterranean dairy systems. Teff appears promising for water-limited areas, however, on-farm evaluations including direct measurements of pasture DMI are needed to better assess its feeding potential. Its wide genetic variability and the limited selection achieved so far suggest that targeted breeding programs aimed to improving palatability and intake could enhance its role as a valuable summer forage for dairy sheep and other ruminants in hot and dry regions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements. The research and the animal care protocols were in accordance with the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes (European Union (EU), 2010).
Author contributions
RP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. GB: Data curation, Software, Writing – original draft, Writing – review & editing. CE: Data curation, Software, Writing – original draft, Writing – review & editing. CB: Data curation, Investigation, Software, Validation, Writing – review & editing. FN: Data curation, Investigation, Software, Validation, Writing – review & editing. RS: Investigation, Writing – review & editing. UB: Resources, Supervision, Visualization, Writing – review & editing. BR: Methodology, Resources, Supervision, Writing – review & editing. PD: Methodology, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was carried out within the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) -MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4 -D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them. Università degli Studi della Tuscia and Ambito Territoriale di Caccia Roma 2 funded the fellowship of GB.
Acknowledgments
We would like to thank Dr. Michele Ferri, Dr. Francesca Cellitti, and Dr. Irene Bottoni for their assistance in conducting the research described.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1710178/full#supplementary-material
References
Allen M. S. (1997). Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber. J. Dairy Sci. 80, 1447–1462. doi: 10.3168/jds.S0022-0302(97)76074-0
AOAC International (2000). Official Methods of Analysis. 17th ed (Arlington, VA: AOAC International).
AOAC International (2005). Official Methods of Analysis. 18th ed (Gaithersburg, MD: AOAC International).
AOAC International (2012). Official Methods of Analysis. 19th ed (Gaithersburg, MD: AOAC International).
Arnett E. J., Fluharty F. L., Loerch S. C., Zerby H. N., Zinn R. A., and Kuber P. S. (2012). Effects of forage level in feedlot finishing diets on carcass characteristics and palatability of Jersey beef. J. Anim. Sci. 90, 960–972. doi: 10.2527/jas.2011-4027
Barre P., Emile J.-C., Betin M., Surault F., Ghesquière M., and Hazard L. (2006). Morphological characteristics of perennial ryegrass leaves that influence short-term intake in dairy cows. Agron. J. 98, 978–985. doi: 10.2134/agronj2005.0213
Benelli A., Primi R., Evangelista C., Spina R., Milanesi M., Pietrucci D., et al. (2025). Predicting forage nutritional quality with near-infrared spectroscopy. J. Sustain. Agric. 4, e70077. doi: 10.1002/sae2.70077
Billman E. D., de Souza I. A., Smith R. G., Soder K. J., Warren N., and Brito A. F. (2022). Evaluating warm-season annual forages to fill summer forage gaps in short-season climates. Crop Forage Turfgrass Mgmt. 8, e20152. doi: 10.1002/cft2.20152
Bleier J. S., Coblentz W. K., Kalscheur K. F., Panke-Buisse K., and Brink G. E. (2020). Evaluation of warm-season annual forages for forage yield and quality in the north-central United States. Transl. Anim. Sci. 4, txaa145. doi: 10.1093/tas/txaa145
Bonsi M. L. K., Osuji P. O., Tuah A. K., and Umunna N. N. (1995). Intake, digestibility, nitrogen balance and certain rumen characteristics of Ethiopian Menz sheep fed teff straw supplemented with cotton seed cake, dry sesbania, dry leucaena or fresh leucaena. Agrofor. Syst. 31, 243–256. doi: 10.1007/BF00712077
Bosco S., Volpi I., Cappucci A., Mantino A., Ragaglini G., Bonari E., et al. (2021). Innovating feeding strategies in dairy sheep farming can reduce environmental impact of ewe milk. Ital. J. Anim. Sci. 20, 2147–2164. doi: 10.1080/1828051X.2021.2003726
Busk P. K. and Moller B. L. (2002). Dhurrin synthesis in sorghum is regulated at the transcriptional level and induced by nitrogen fertilization in older plants. Plant Physiol. 129, 1222–1231. doi: 10.1104/pp.000687
Buxton D. R. and O’Kiely P. (2003). Preharvest plant factors affecting ensiling. In. Silage Sci. Technol. 42, 199–250. doi: 10.2134/agronmonogr42.c5
Cannas A., Pes A., Mancuso R., Vodret B., and Nudda A. (1998). Effect of dietary energy and protein concentration on the concentration of milk urea nitrogen in dairy ewes. J. Dairy Sci. 81, 499–508. doi: 10.3168/jds.S0022-0302(98)75602-4
Cooke A. S., Machekano H., Gwiriri L. C., Tinsley J. H. I., Silva G. M., Nyamukondiwa C., et al. (2025). The nutritional feed gap: seasonal variations in ruminant nutrition and knowledge gaps in relation to food security in Southern Africa. Food Sec. 17, 73–100. doi: 10.1007/s12571-024-01509-1
D’Andrea A. C. (2008). T’ef (Eragrostis tef) in ancient agricultural systems of highland Ethiopia. Econ. Bot. 62, 547–566. doi: 10.1007/s12231-008-9053-4
Decruyenaere V., Buldgen A., and Stilmant D. (2009). Factors affecting intake by grazing ruminants and related quantification methods: A review. Biotechnol. Agron. Soc Environ. 13, 559–573.
European Union (EU) (2010). Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union L 276, 33–79.
Favaretto A., Santos J., Carneiro C. M., and Scheffer Basso S. M. (2015). The first anatomical and histochemical study of tough lovegrass (Eragrostis plana Nees, Poaceae). Afr. J. Agric. Res. 10, 2940–2947. doi: 10.5897/AJAR2014.9145
Garnsworthy P., Masson L., Lock A., and Mottram T. (2006). Variation of milk citrate with stage of lactation and de novo fatty acid synthesis in dairy cows. J. Dairy Sci. 89, 1604–1612. doi: 10.3168/jds.S0022-0302(06)72227-5
Gherbin P., De Franchi A. S., Monteleone M., and Rivelli A. (2007). Adaptability and productivity of some warm-season pasture species in a Mediterranean environment. Grass Forage Sci. 62, 78–86. doi: 10.1111/j.1365-2494.2007.00566.x
Habte E., Muktar M. S., Negawo A. T., Lee S., Lee K., and Jones C. S. (2019). An overview of teff (Eragrostis tef (Zuccagni) Trotter) as a potential summer forage crop in temperate systems. J. Korean Soc Grassl. Forage Sci. 39, 185–188. doi: 10.5333/KGFS.2019.39.3.185
Heuzé V., Thiollet H., Tran G., and Lebas F. (2017). Tef (Eragrostis tef) hay (Feedipedia, a programme by INRAE, CIRAD, AFZ and FAO). Available online at: https://www.feedipedia.org/node/22768 (Accessed October 20, 2025).
Heuzé V. and Tran G. (2015). Sudan grass (Sorghum × drummondii) (Feedipedia, a programme by INRAE, CIRAD, AFZ and FAO). Available online at: https://www.feedipedia.org/node/375 (Accessed October 20, 2025).
Hill N. S. and Roberts C. A. (2020). “Plant chemistry and antiquality components in forage,” in Forages. The Science of grassland agriculture,, 7th edition, vol. Vol. II . Eds. Moore K. J., Collins M., Nelson C. J., and Redfearn D. D. (Hoboken, NJ, USA: Wiley Blackwell), 633–658. doi: 10.1002/9781119436669
Holman J., Obour A. K., and Mengel D. B. (2019). Nitrogen application effects on forage sorghum production and nitrate concentration. J. Plant Nutr. 20, 2794–2804. doi: 10.1080/01904167.2019.1659321
Kaplan M., Kara K., Unlukara A., Kale H., Buyukkilic Beyzi S., Varol I. S., et al. (2019). Water deficit and nitrogen affects yield and feed value of sorghum-Sudangrass silage. Agric. Water Manage. 218, 30–36. doi: 10.1016/j.agwat.2019.03.021
Landau S., Molle G., Fois N., Friedman S., Barkai D., Decandia M., et al. (2005). Safflower (Carthamus tinctorius L.) as a novel pasture species for dairy sheep in the Mediterranean conditions of Sardinia and Israel. Small Rumin. Res. 59, 239–249. doi: 10.1016/j.smallrumres.2005.05.008
Linzell J. L., Mepham T. B., and Peaker M. (1976). The secretion of citrate into milk. J. Physiol. 260, 739–750. doi: 10.1113/jphysiol.1976.sp011527
Mertens D. R. (1997). Creating a system for meeting the fiber requirements of dairy cows. J. Dairy Sci. 80, 1463–1481. doi: 10.3168/jds.S0022-0302(97)76075-2
Mikolayunas-Sandrock C., Armentano L. E., Thomas D. L., and Berger Y. M. (2009). Effect of protein degradability on milk production of dairy ewes. J. Dairy Sci. 92, 4507–4513. doi: 10.3168/jds.2008-1983
Miller D. (2010). Teff grass: crop overview and forage production guide. 2nd ed.. Cal/West Seed Company, Woodland, CA.
Miller D. R. (2011). Teff grass: crop overview and forage production guide. 3rd ed.. Cal/West Seed Company, Woodland, CA.
Morand-Fehr P., Fedele V., Decandia M., and Le Frileux Y. (2007). Influence of farming and feeding systems on composition and quality of goat and sheep milk. Small Rumin. Res. 68, 20–34. doi: 10.1016/j.smallrumres.2006.09.019
National Research Council (2001). Nutrient Requirements of Dairy Cattle. 7th rev. ed (Washington, DC: National Academies Press). doi: 10.17226/9825
Norberg S., Roseberg R., Charlton B., and Shock C. (2009). Teff: A New Warm Season Grass for Oregon. (Corvallis, OR: Oregon State University Extension Service).
Oshima M. and Fuse H. (1981). Citric acid concentration in subclinical mastitic milk. J. Dairy Res. 48, 387–392. doi: 10.1017/S0022029900021865
Paschino P., Vacca G. M., Dettori M. L., and Pazzola M. (2019). An approach for the estimation of somatic cells’ effect in Sarda sheep milk based on the analysis of milk traits and coagulation properties. Small Rumin. Res. 171, 77–81. doi: 10.1016/j.smallrumres.2018.10.010
Primi R., Filibeck G., Amici A., Bückle C., Cancellieri L., Di Filippo A., et al. (2016). From Landsat to leafhoppers: A multidisciplinary approach for sustainable stocking assessment and ecological monitoring in mountain grasslands. Agric. Ecosyst. Environ. 234, 118–133. doi: 10.1016/j.agee.2016.04.028
Pulina G., Avondo M., Molle G., Francesconi A. H. D., Atzori A. S., and Cannas A. (2013). Models for estimating feed intake in small ruminants. Rev. Bras. Zootec. 42, 675–690. doi: 10.1590/S1516-35982013000900010
Pulina G., Macciotta N., and Nudda A. (2005). Milk composition and feeding in the Italian dairy sheep. Ital. J. Anim. Sci. 4, 5–14. doi: 10.4081/ijas.2005.1s.5
Pulina G. and Nudda A. (2004). “Milk production,” in Dairy Sheep Nutrition. Ed. Pulina G. (CAB International, Wallingford, UK), 1–12.
Pupo M. R., Wallau M. O., and Ferraretto L. F. (2022). Effects of season, variety type, and trait on dry matter yield, nutrient composition, and predicted intake and milk yield of whole-plant sorghum forage. J. Dairy Sci. 105, 5776–5785. doi: 10.3168/jds.2021-21706
Ruggeri R., Rossini F., Ronchi B., Primi R., Stamigna C., and Danieli P. P. (2024). Potential of teff as alternative crop for Mediterranean farming systems: Effect of genotype and mowing time on forage yield and quality. J. Agric. Food Res. 17, 101257. doi: 10.1016/j.jafr.2024.101257
Russel A. J. F., Doney J. M., and Gunn R. G. (1969). Subjective assessment of body fat in live sheep. J. Agric. Sci. 72, 451–454. doi: 10.1017/S0021859600024874
Sarkar R. and Northup B. K. (2023). Simulating water stress in sorghum-Sudangrass forage system with different nitrogen sources and tillage practices. J. Soil Sci. Plant Nutr. 23, 5759–5780. doi: 10.1007/s42729-023-01438-6
Saylor B. A., Min D. H., and Bradford B. J. (2018). Productivity of lactating dairy cows fed diets with teff hay as the sole forage. J. Dairy Sci. 101, 5984–5990. doi: 10.3168/jds.2017-14118
Schrenker D. (2014). Production and economic potential of warm-season annual pastures in rotation with corn-silage for organic dairies. The Pennsylvania State University, University Park, PA.
Shehab A. A., Yao L. H., Wei L. L., Wang D. K., Li Y., Zhang X. F., et al. (2020). The increased hydrocyanic acid in drought-stressed sorghums could be alleviated by plant growth regulators. Crop Pasture Sci. 71, 459–468. doi: 10.1071/CP20057
Sitzia M., Bonanno A., Todaro M., Cannas A., Atzori A. S., Francesconi A. H. D., et al. (2015). Feeding and management techniques to favour summer sheep milk and cheese production in the Mediterranean environment. Small Rumin. Res. 126, 43–58. doi: 10.1016/j.smallrumres.2015.01.021
Sitzia M. and Ruiz F. A. (2011). “Dairy farm management systems,” in Sheep—Encyclopedia of Dairy Sciences, 2nd edn (Elsevier, Oxford, UK), 67–76.
Sodi I., Martini M., Sanjuàn N., Saia S., Altomonte I., Andreucci A., et al. (2024). Massese, Sarda and Lacaune dairy sheep breeds: an environmental impact comparison. Sustainability 16, 4941. doi: 10.3390/su16124941
Staniar W. B., Bussard J. R., Repard N. M., Hall M. H., and Burk A. O. (2010). Voluntary intake and digestibility of teff hay fed to horses. J. Anim. Sci. 88, 3296–3303. doi: 10.2527/jas.2009-2668
Todaro M., Dattena M., Acciaioli A., Bonanno A., Bruni G., Caroprese M., et al. (2015). Aseasonal sheep and goat milk production in the Mediterranean area: Physiological and technical insights. Small Rumin. Res. 126, 59–66. doi: 10.1016/j.smallrumres.2015.01.022
Twidwell E. K. A., Boe A., and Casper D. P. (2002). Teff: A New Annual Forage Grass for South Dakota? SDSU Extension Extra, ExEx 8071 (Brookings, SD: South Dakota State University). Available online at: https://www.nrcs.usda.gov/sites/default/files/2022-10/Teff_grass.pdf (Accessed September 5, 2025).
Uyanik S. E. and Carpici E. B. (2025). Effects of different sowing times and harvesting stages on dry matter yield, quality, and mineral content of teff (Eragrostis teff [Zucc.] Trotter). Agronomy 15, 457. doi: 10.3390/agronomy15020457
Van Soest P. J. (1994). Nutritional Ecology of the Ruminant. 2nd ed (Ithaca, NY: Cornell University Press).
Van Soest P. J., Robertson J. B., and Lewis B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Vinyard J. R., Hall J. B., Sprinkle J. E., and Chibisa G. E. (2018). Effects of maturity at harvest on the nutritive value and ruminal digestion of Eragrostis tef (cv. Moxie) when fed to beef cattle. J. Anim. Sci. 96, 3420–3432. doi: 10.1093/jas/sky202
Wagali P., Ngomuo G., Kilama J., Sabastian C., Ben-Zeev S., Ben-Meir Y. A., et al. (2023). The effect of teff (Eragrostis tef) hay inclusion on feed intake, digestibility, and milk production in dairy cows. Front. Anim. Sci. 4. doi: 10.3389/fanim.2023.1260787
Wiggans G. R. and Shook G. E. (1987). A lactation measure of somatic cell count. J. Dairy Sci. 70, 2666–2672. doi: 10.3168/jds.S0022-0302(87)80337-5
Zannoni M. and Annibaldi S. (1981). Standardization of the renneting ability of milk by Formagraph. Sci. Tecn. Latt.-Cas. 32, 79–94.
Zhan Q. W., Zhang T. Z., Wang B. H., and Li J. Q. (2008). Diversity comparison and phylogenetic relationships of S. bicolor and S. Sudanense as revealed by SSR markers. Plant Sci. 174, 9–16. doi: 10.1016/j.plantsci.2007.09.007
Keywords: grazing management, animal production, annual grass species, milk yield, milk composition, summer grazing, out-of-season production
Citation: Primi R, Bernabucci G, Evangelista C, Boselli C, Napoli F, Spina R, Bernabucci U, Ronchi B and Danieli PP (2025) Milk yield and composition of dairy sheep grazing teff or sorghum-sudangrass under Mediterranean summer conditions. Front. Anim. Sci. 6:1710178. doi: 10.3389/fanim.2025.1710178
Received: 21 September 2025; Accepted: 29 October 2025;
Published: 20 November 2025.
Edited by:
Vincenzo Lopreiato, University of Messina, ItalyReviewed by:
Pichad Khejornsart, Kasetsart University, ThailandPiera Iommelli, University of Naples Federico II, Italy
Copyright © 2025 Primi, Bernabucci, Evangelista, Boselli, Napoli, Spina, Bernabucci, Ronchi and Danieli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Riccardo Primi, cHJpbWlAdW5pdHVzLml0
 Riccardo Primi
Riccardo Primi Gloria Bernabucci
Gloria Bernabucci Chiara Evangelista
Chiara Evangelista Carlo Boselli
Carlo Boselli Francesco Napoli2
Francesco Napoli2 Raffaello Spina
Raffaello Spina Umberto Bernabucci
Umberto Bernabucci Bruno Ronchi
Bruno Ronchi Pier Paolo Danieli
Pier Paolo Danieli