- 1Monogastric Science Research Centre, School of Veterinary Medicine and Biosciences, Scotland's Rural College (SRUC), Edinburgh, United Kingdom
- 2Centre for Innovation in Genomics and Microbiome Sciences, School of Medicine and Biosciences, University of West London, London, United Kingdom
- 3Seaweed Generation Ltd., Falmouth, United Kingdom
- 4Faculty of Health and Life Sciences, Biosciences, University of Exeter, Exeter, United Kingdom
- 5UK Agri-Tech Centre, York, United Kingdom
Palmaria palmata (P. palmata), commonly known as Dulse, is a red seaweed with increasing relevance as a potential sustainable feed ingredient for monogastric animals. It provides balanced proteins, essential amino acids, minerals, and diverse bioactive compounds with potential antioxidant and immunomodulatory effects. Unlike conventional protein sources, it can be cultivated without competing for arable land or freshwater, aligning with the goals of a circular economy. However, challenges include compositional variability, seasonal and geographic influences, and risks of contaminants such as heavy metals, iodine, and microbial hazards. Feeding trials in non-ruminants demonstrate that inclusion is feasible without adverse effects and may improve gut health and product quality; however, the evidence remains limited in scope and duration. This review consolidates current knowledge on the nutritional composition, bioactive compounds, safety concerns, processing technologies, and feeding outcomes of P. palmata, highlighting evidence gaps for safe inclusion levels, optimised processing, hazard management, and sustainability evaluation.
1 Introduction
P. palmata (Linnaeus) Weber & Mohr, widely recognised by its common name dulse, is an edible red macroalga (Rhodophyta) with a long history of human and animal use across the North Atlantic (McHugh, 2003; Mouritsen et al., 2013). First described as Fucus palmatus by Carl Linnaeus in 1753 and later reassigned to Palmaria in 1805, it inhabits moderately exposed shores, attaching to rocks or other algae such as Fucus spp. and Laminaria hyperborea (Stévant et al., 2023). Its fronds can grow up to 30–50 cm long, change colour seasonally from deep red in winter to greenish yellow in summer (Sears, 1998). Harvested since at least the 12th century, P. palmata has been consumed across Ireland, Scotland, Iceland, Norway, France, Atlantic Canada, and New England, where it was eaten fresh, dried, or incorporated into breads and stews (McHugh, 2003; Mouritsen et al., 2013). Beyond its use as food, it was also valued in folk medicine and occasionally used as livestock feed. Today, P. palmata is increasingly positioned within the “blue bioeconomy” as a sustainable biomass supporting circular food systems and the Sustainable Development Goals (FAO, 2018; Parodi et al., 2018; Duarte et al., 2020).
Interest in its application as feedstuff for monogastric animals has grown as part of broader efforts to identify sustainable, circular feed ingredients that can reduce dependence on imported protein sources (Makkar et al., 2016; Øverland et al., 2019). Using locally available P. palmata could enhance feed security, diversify raw material supply chains, and lower the environmental footprint of feed production. This advantage stems from its ability to grow in coastal environments without competing for arable land or freshwater, making it particularly attractive within circular economy frameworks. However, integrating P. palmata into practical feeding strategies also presents challenges, including variability in composition due to seasonality and geography, the presence of undesirable substances such as heavy metals and iodine, and the need for effective processing methods to ensure feed safety and nutrient bioavailability (Holdt and Kraan, 2011; Moroney et al., 2014; Cherry et al., 2019; Stévant et al., 2023).
The scientific literature on P. palmata spans nutritional composition, processing techniques, safety hazards, and its potential impacts on animal performance and health (Holdt and Kraan, 2011; Stévant et al., 2023). However, the existing evidence is fragmented across disciplines, as most compositional studies (e.g., Beacham et al., 2019) are directed towards biorefinery or bioenergy,applications, with limited investigations assessing its nutritional relevance in monogastric farm animals (Karimi, 2015; Kulshreshtha et al., 2014). These challenges make it difficult to draw firm conclusions on safe inclusion levels or consistent effects on productivity and health outcomes. There is therefore a need to consolidate available knowledge and identify research priorities that will guide future use of P. palmata in monogastric feeding systems.
The objective of this review is to synthesise current knowledge on P. palmata with a specific emphasis on its nutritional composition, bioactive compounds, processing and safety considerations, and outcomes from feeding trials in monogastric animals. Special attention is given to regulatory aspects, hazard management, and sustainability implications. By integrating these themes, this review highlights both the opportunities and the research priorities required to support the safe and effective use of P. palmata in monogastric nutrition.
1.1 Literature search strategy
A structured literature search was conducted to identify relevant studies on P. palmata and its application in monogastric nutrition. The search was carried out using major scientific databases, including Web of Science, Scopus, PubMed, and Google Scholar. Keywords and combinations included “Palmaria palmata,” “dulse,” “red seaweed,” “poultry nutrition,” “monogastric feed,” “seaweed safety,” and “seaweed processing.” Publications from 1980 onwards were considered, with priority given to peer-reviewed articles but also including authoritative reports from regulatory and international organisations.
Inclusion criteria required original data or substantive discussion of nutritional composition, bioactive compounds, processing technologies, safety hazards, regulatory frameworks, or animal feeding trials. Studies on other red seaweeds were considered when direct evidence for P. palmata was lacking, providing contextual comparison. Exclusion criteria included studies not available in English and those lacking relevance to animal nutrition or feed safety.
This approach ensured comprehensive coverage of nutritional, functional, safety, and sustainability aspects, allowing for integration of findings across disciplines relevant to P. palmata in monogastric feeding systems.
2 Harvesting, processing, and quality determinants
2.1 Harvesting
The quality of red seaweeds such as P. palmata is strongly influenced by both harvesting method and timing. Traditionally, P. palmata and other seaweeds have been hand-collected during low tide from intertidal zones or gathered as drift material, a practice still common in coastal communities in Ireland, Iceland, and Atlantic Canada (McHugh, 2003; Mouritsen et al., 2013). Hand harvesting maintains frond integrity and reduces contamination by sand, shells, or epiphytes. Mechanical methods, including rakes, dredges, or cutters, have been trialled to increase yields but can damage thalli, introduce debris, and compromise natural regrowth (Mac Monagail et al., 2017).
Cultivation is increasingly recognised as a viable strategy for ensuring continuous, year-round biomass supply and enhancing the consistency of biomass quality. Successful cultivation has been demonstrated in land-based tanks from spores (Le Gall et al., 2004) and in open-water systems using seeded ropes or nets (Stévant et al., 2023). Cultivated production enables greater control over harvest timing and nutritional profile. However, large-scale cultivation remains limited, with wild-harvested material continuing to be the main source available for feed applications. This review therefore, focuses primarily on wild-harvested P. palmata, reflecting its current relevance and widespread availability.
2.2 Postharvest handling, drying, and processing strategies
Fresh P. palmata is highly perishable due to its high moisture content (~80%), making immediate postharvest treatment essential to preserve quality for feed use. Cold storage at ~4°C can maintain colour, texture, and microbial stability for up to two weeks (Liot et al., 1993), providing a short-term option for local usage, but that is impractical for long-distance supply chains. Blast freezing at –20 to –40°C effectively halts enzymatic activity, preserves pigments and proteins, and allows flexibility in downstream processing (Fleurence, 1999; Dumay et al., 2013). Although energy-intensive, freezing is widely used to secure biomass of consistent quality for research and specialised feed applications.
Blanching, which involves briefly immersing seaweed in hot fresh water or seawater (30–95°C for 1–2 min), is a common stabilisation method used to inactivate endogenous enzymes, reduce iodine content, lower microbial loads, and preserve colour (Lüning and Mortensen, 2015; Stévant et al., 2017). While highly effective for food and feed safety, blanching may cause nutrient leaching if conditions are not carefully controlled (Nielsen et al., 2020; Akomea-Frempong et al., 2021).
Drying remains the most common strategy for preserving P. palmata for long-term use. Sun drying is an inexpensive and widely practised method. However, it is highly weather-dependent, difficult to standardise and carries risks of oxidation and microbial contamination (Chan et al., 1997; Fernandes and Rodrigues, 2007), making it challenging to comply with standards such as HACCP and food safety regulations. Solar dryers provide a more hygienic and controlled alternative, although their effectiveness still depends heavily on local climatic conditions (Fudholi et al., 2014a; 2014b). Convective hot-air drying (25–80°C) allows greater process control and can improve protein extractability, but higher temperatures may degrade antioxidants, polyunsaturated fatty acids, and vitamin C (Wong and Cheung, 2001a, b; Gupta et al., 2011). Freeze-drying (<– 40°C under vacuum) offers the best preservation of colour, amino acids, and other labile compounds, yet its high cost and low scalability limit its practicality for large-scale feed production (Chan et al., 1997; Nowak and Jakubczyk, 2020).
Fermentation is also being explored as a value-adding postharvest strategy. Lactic acid fermentation can lower sodium and heavy metal contents while improving digestibility, antioxidant capacity, and the release of bioactive peptides (Marrion et al., 2003; Dumay et al., 2013; Bruhn et al., 2019). Overall, each stabilisation method presents trade-offs in terms of cost, nutrient preservation, microbial safety, and scalability. Their comparative advantages and limitations for monogastric feed applications are summarised in Table 1, which outlines the relevance of these processes for maintaining nutrient quality, ensuring feed safety, and enabling the practical inclusion of P. palmata in monogastric diets.
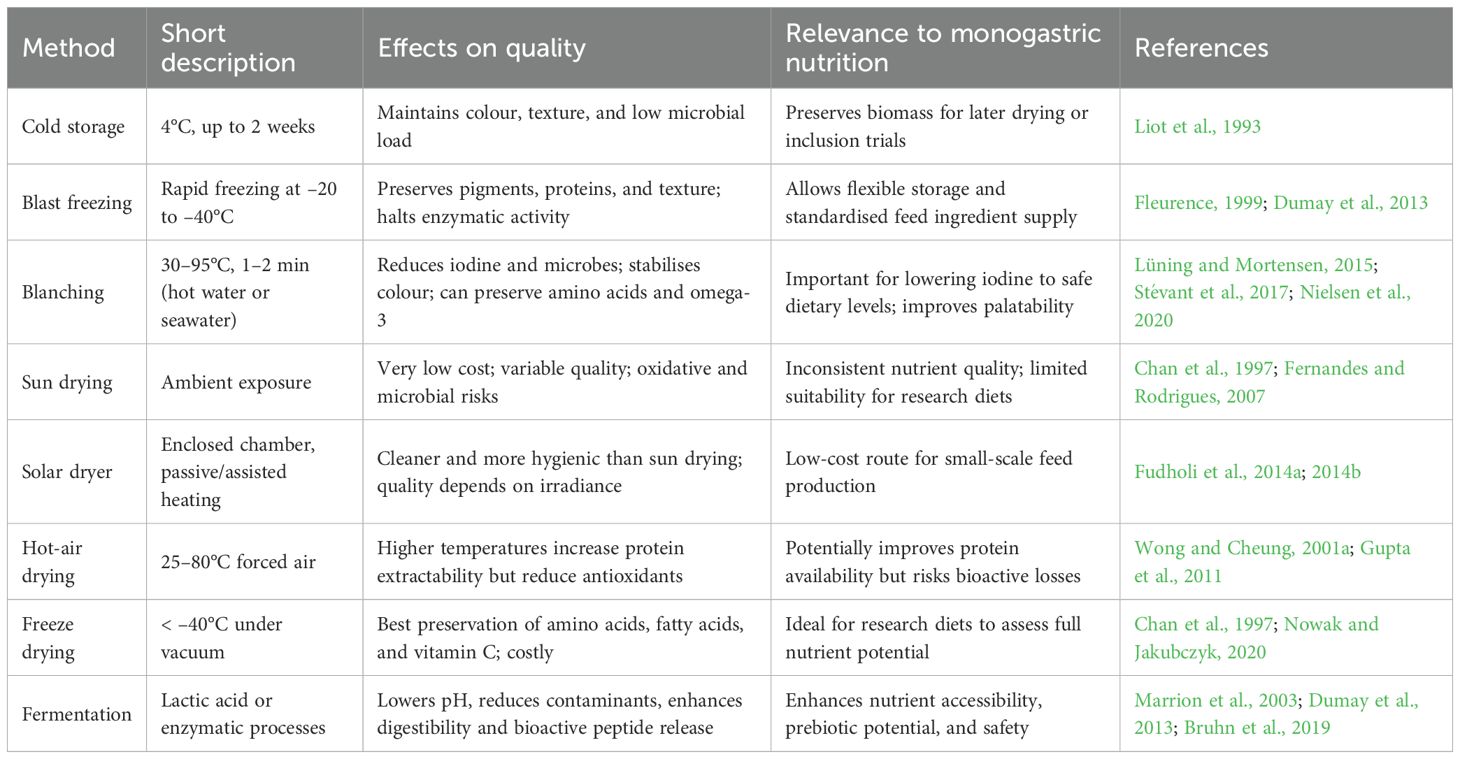
Table 1. Postharvest stabilisation and processing methods for P. palmata: effects on nutrient retention, quality, and relevance for monogastric feed.
3 Nutritional profile of Palmaria palmata
3.1 Proximate composition
The proximate composition of P. palmata varies considerably depending on its state and processing method. Fresh wild fronds typically contain high moisture (78–90%), corresponding to only 10–22% dry matter (DM), with clear seasonal and geographic variation (Rødde et al., 2004). Reviews report moisture contents as high as 88% in fresh material, leaving ~12% DM, with moisture levels typically peaking in winter and spring (Stévant et al., 2023). Once dried, however, P. palmata becomes considerably more stable. Commercial dried products contain ~9.7% moisture (i.e., ~90.3% DM) when analysed using gravimetric methods (Campos et al., 2022). Moisture content is also influenced by the degree of drying. For example, semi-dried products used for short-term storage contained ~20% moisture (~80% DM), whereas fully dried products in the same study reached ~6% moisture, corresponding to ~94% DM (Stévant et al., 2020a, b).
These findings highlight the strong influence of harvest season, processing, and storage on reported nutrient values. On a dry matter basis, P. palmata is a nutrient-dense red macroalga with crude protein typically ranging from 8–35% DW, placing it between cereals and conventional protein-rich feedstuffs. In a UK-based study, Beacham et al. (2019) reported P. palmata to contain approximately 9–18% protein, ~1% lipid, high carbohydrate, and 18–30% ash, supporting its characterisation as a moderate-protein, mineral-rich red alga. Table 2 provides a comparative overview of the proximate composition of dried P. palmata alongside soyabean meal, maize, and wheat, highlighting its intermediate protein content, relatively high fibre fraction, and distinctive ash levels.
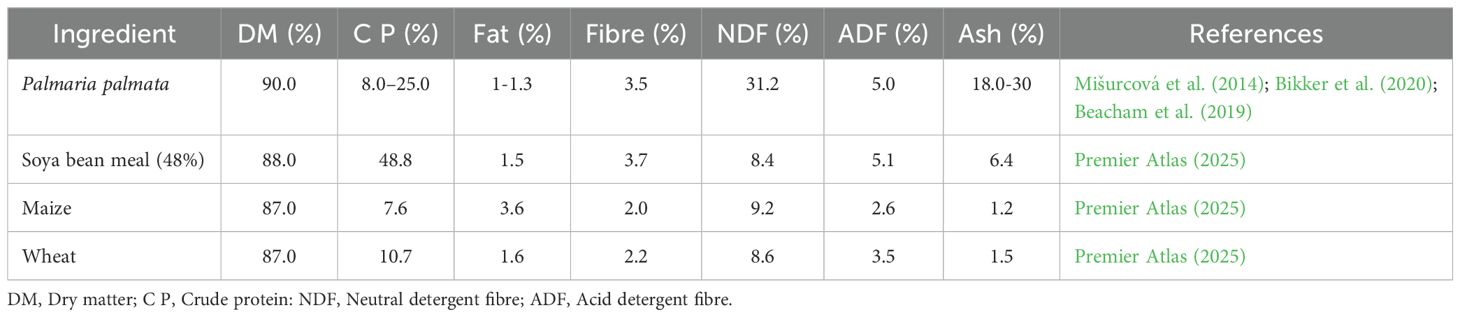
Table 2. Proximate composition of dried P. palmata compared with soya bean meal and cereal ingredients (% DM basis).
3.2 Protein and amino acid composition
The crude protein (CP) content of P. palmata ranges from 8–25% DM, depending on season, origin, and post-harvest handling (Fleurence, 1999; Holdt and Kraan, 2011; Mišurcová et al., 2014). CP content typically peaks in winter and early spring (up to ~21.9% but declines to ~11.9% in summer and autumn, reflecting a physiological trade-off between nitrogen assimilation and carbohydrate storage during periods of rapid growth (Morgan et al., 1980; Fleurence et al., 1995; Galland-Irmouli et al., 1999). Some studies have reported values as high as 35% (Moroney et al., 2014), although such variation may also be explained by variation in environmental factors, analytical methodology, and the inclusion or exclusion of non-protein nitrogen in earlier estimates. Compared with soya bean meal (48% CP), P. palmata contains less overall protein but remains comparable to cereal ingredients such as wheat and maize (Premier Atlas, 2025).
A critical consideration when interpreting these values is the nitrogen-to-protein conversion factor. The conventional factor used is 6.25, which assumes that all nitrogen derives from protein, with an average nitrogen content of 16%. This is a reasonable assumption for animal feed but not for seaweeds. In P. palmata, a substantial proportion of nitrogen occurs in non-protein compounds such as nucleotides and other nitrogenous solutes. Thus, for untreated P. palmata, a factor of 4.7 has been recommended (Morgan et al., 1980; Lourenço et al., 2002, 2004), and thus CP levels would be lower than when the standard factor is applied.
Processing can alter the ratio of protein to non-protein nitrogen, thus necessitating even lower factors (2.5–4.1) (Harnedy and FitzGerald, 2013; Stévant et al., 2017; Naseri et al., 2020). Treatments such as washing, blanching, drying, fermentation, or enzymatic hydrolysis can remove soluble nitrogen fractions or change protein solubility, thereby shifting the true protein content relative to total nitrogen. Applying reduced factors in these cases produces a more accurate estimate of the true protein and prevents misleading comparisons with soya bean meal or other conventional ingredients. Thus, careful selection of the nitrogen-to-protein conversion factor, tailored to both species and processing, is critical for generating reliable nutritional data for feed formulations.
Beyond crude protein levels, the amino acid profile of P. palmata is notable for its richness in essential amino acids (EAAs), which often exceed those found in animal feed (Table 3). Lysine (4.6–8.2% of CP) and methionine (1.9–2.7% of CP) are particularly abundant, exceeding levels in soya bean meal (2.95% and 0.63%) or cereals such as maize (0.23% and 0.16%) and wheat (0.32% and 0.17%) (Mišurcová et al., 2014; Galland-Irmouli et al., 1999; Morgan et al., 1980; Mai et al., 1994; Bikker et al., 2020). Branched-chain amino acids are also present at relatively high levels, with isoleucine (3.5–5.3% of CP), leucine (5.5–7.8%), and valine (5.1–6.9%) all exceeding values in soya bean meal (2.24%, 3.71%, 2.32%). Among the non-essential amino acids, aspartic acid (8.5–18.5%) and glutamic acid (6.7–13.0%) dominate, contributing to the characteristic umami flavour of P. palmata (Galland-Irmouli et al., 1999; Mišurcová et al., 2014). Glycine (4.9–13.3%), alanine (6.3–7.8%), and tyrosine (1.3–4.5%) are also present at higher concentrations than in conventional feedstuffs. However, some amino acids, such as cystine, histidine, and tryptophan, tend to occur at lower relative levels, which may represent potential limiting factors when formulating balanced diets.
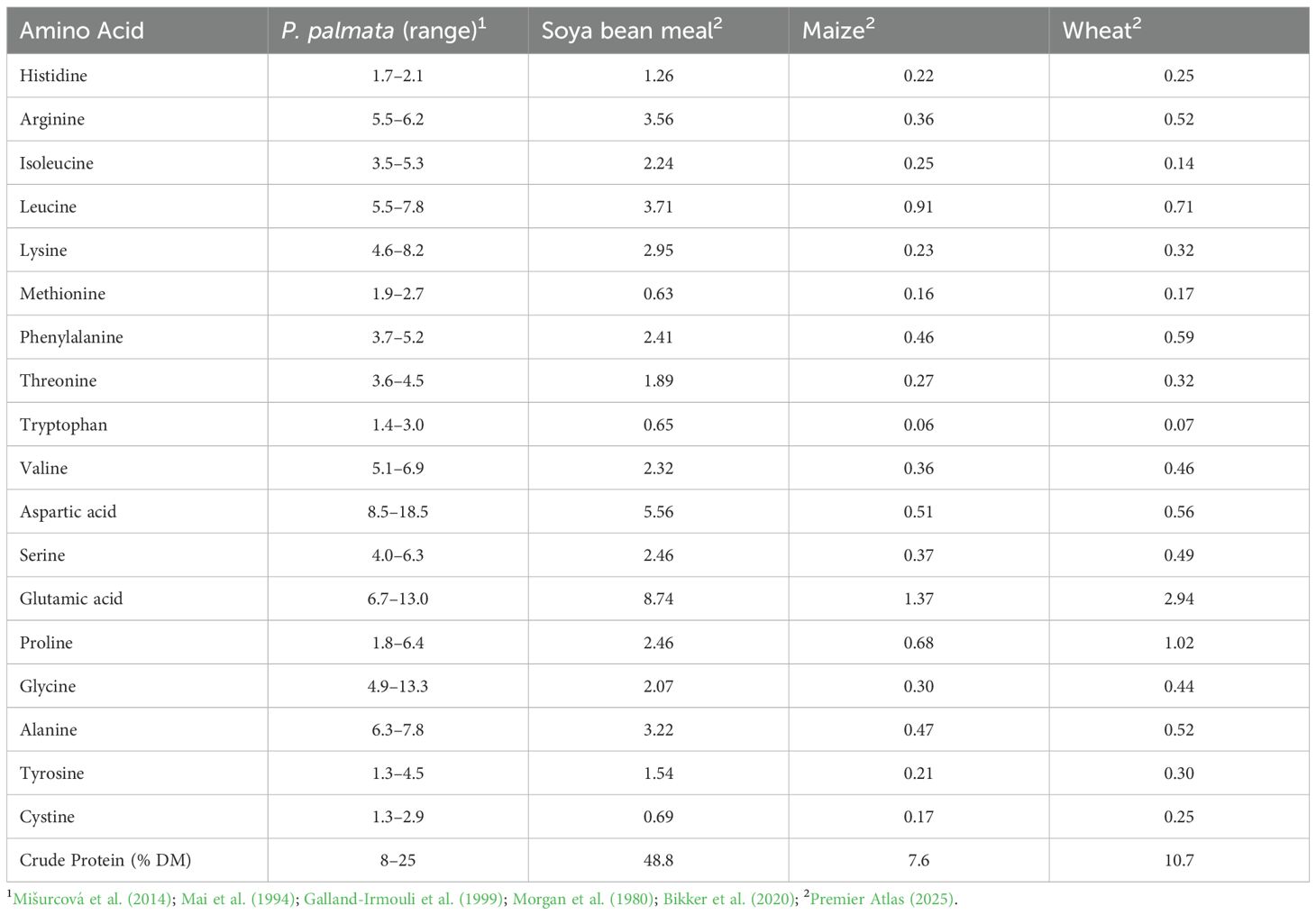
Table 3. Comparative crude protein and amino acid composition of Palmaria palmata, soya bean meal, maize, and wheat (% of CP, DM basis).
Although the CP content of dried P. palmata (8–25% DM) is lower than that of soya bean meal (48.8% DM), its richness in key EAAs makes it a valuable complement to cereal-based monogastric diets where lysine, methionine, and threonine are often limiting (Table 3). In addition, enzymatic hydrolysis, especially when combined with carbohydrase such as xylanase, has been shown to enhance protein solubility and release bioactive peptides with antioxidant, antihypertensive, and antimicrobial properties, further supporting the role of P. palmata as both a protein source and a functional feed ingredient (Harnedy and FitzGerald, 2013).
3.3 Fatty acid composition
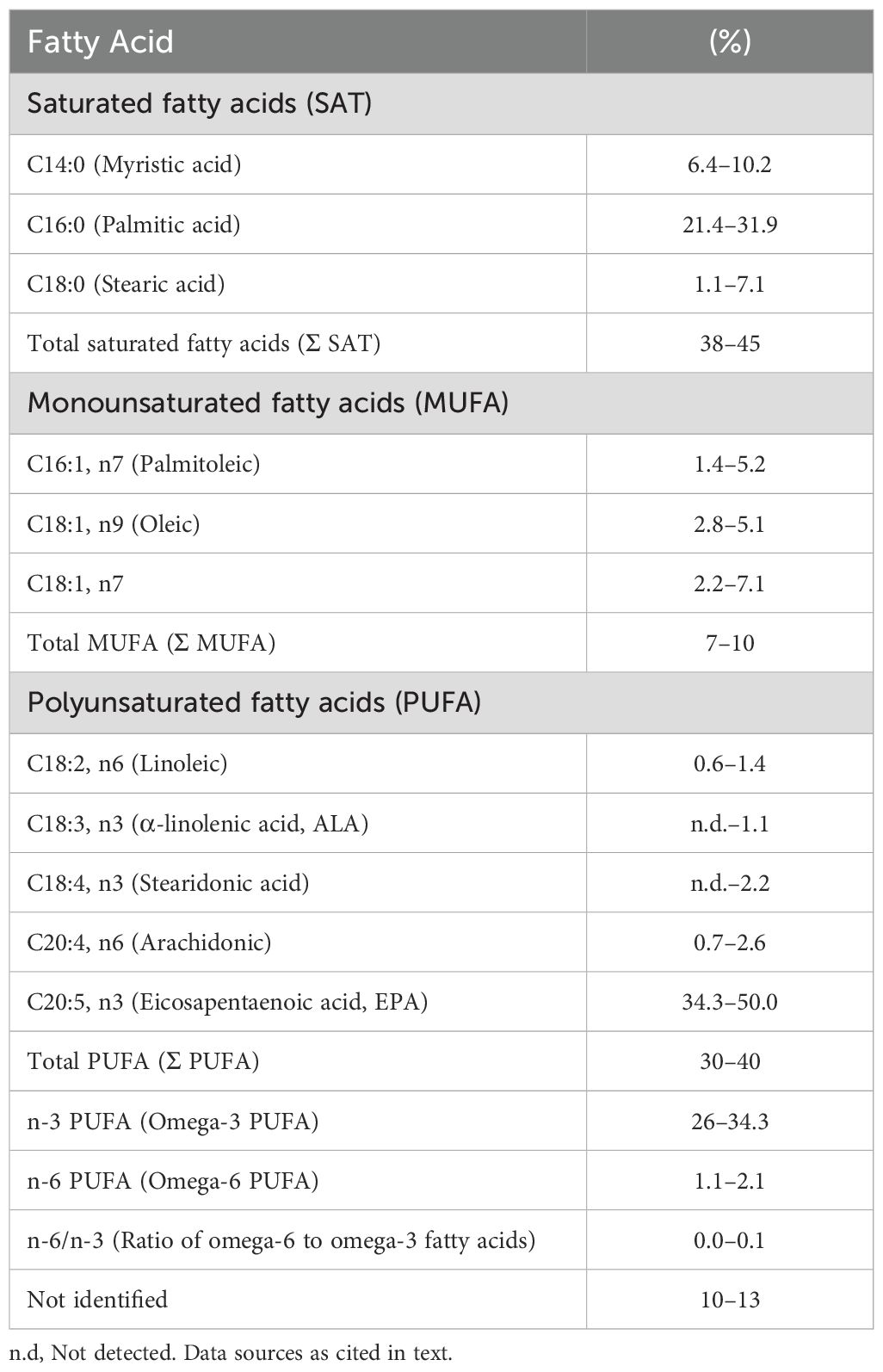
The fatty acid profile of P. palmata is characterised by a relatively high proportion of saturated fatty acids (ΣSAT 38–45% of total fatty acid methyl esters, FAME), with palmitic acid (C16:0, 23–31.9%) and myristic acid (C14:0, 6.4–10.2%) as the main contributors (Mishra et al., 1993; MacArtain et al., 2007; Mæhre et al., 2014). Monounsaturated fatty acids are present at modest levels (ΣMUFA 7–10%), primarily oleic acid (C18:1 n9). Polyunsaturated fatty acids (ΣPUFA 30–40%) are largely accounted for by eicosapentaenoic acid (EPA, C20:5 n3), which alone represents 34.3–47% of total FAME (Fleurence et al., 1994). This composition results in an exceptionally low n-6/n-3 ratio (≤0.1), uncommon among plant-derived feed ingredients.
In contrast, soya bean meal, maize, and wheat are lipid-poor and their fatty acid fraction is mainly linoleic acid (C18:2 n6). P. palmata is therefore notable for its enrichment in long-chain n-3 fatty acids, particularly EPA, highlighting its potential as a complementary n-3 source alongside conventional feedstuffs. Table 4 summarises the reported fatty acid ranges, while Figure 1 illustrates the distribution of major fatty acid classes, both based on data compiled from Mishra et al. (1993); MacArtain et al. (2007), and Mæhre et al. (2014).
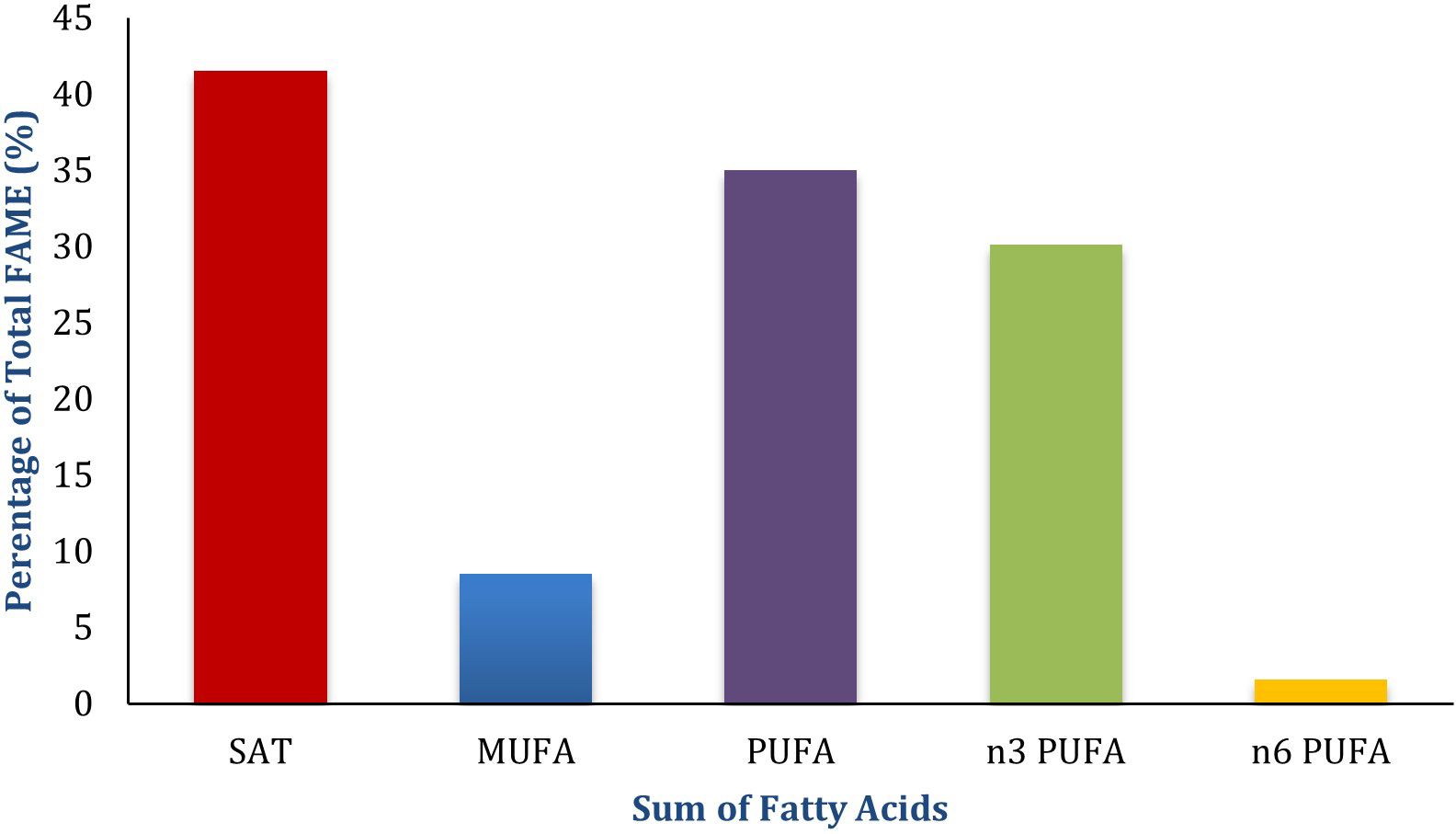
Figure 1. Fatty acid composition of P. palmata (midpoint values of reported ranges, expressed as % of total fatty acid methyl esters, FAME).
3.4 Vitamins and minerals
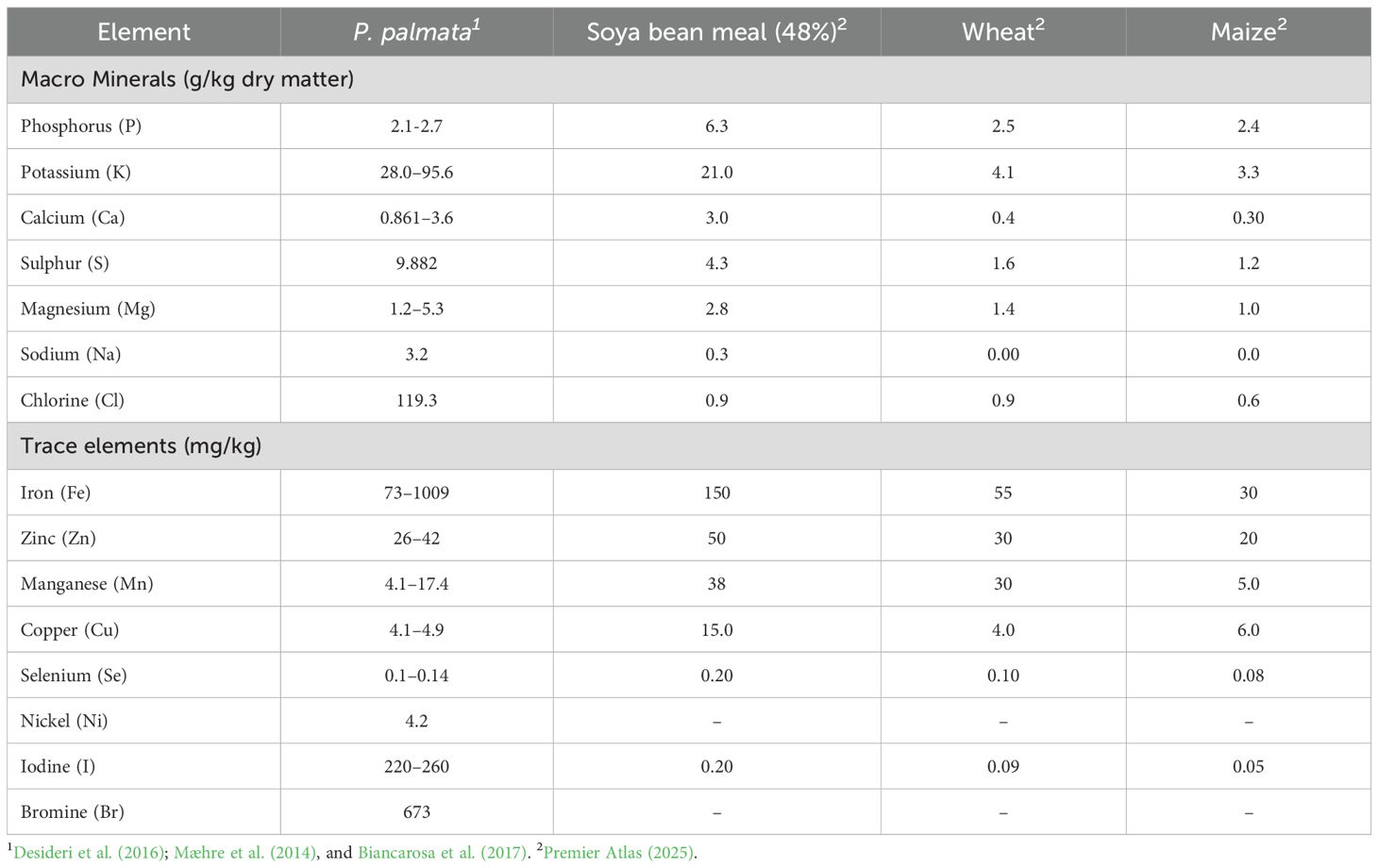
P. palmata provides a dense mineral and vitamin profile, reflected in its ash content, which typically ranges from 12–37% (Desideri et al., 2016; Mæhre et al., 2014; Biancarosa et al., 2018; Beacham et al., 2019). Major minerals include potassium, chloride, magnesium, calcium, phosphorus, sodium, and sulphur (Desideri et al., 2016; Mæhre et al., 2014; Biancarosa et al., 2018; Beacham et al., 2019). Trace elements such as iron, zinc, manganese, copper, nickel, bromine, and selenium are also present, with Beacham et al. (2019) confirming detectable levels of iron, phosphorus, potassium, calcium, and zinc in UK-collected material. Moderate iodine concentrations (220–260 mg/kg) have been reported, which are considerably lower than in brown seaweeds, making P. palmata a comparatively safe iodine source for food and feed applications (Holdt and Kraan, 2011; MacArtain et al., 2007).
In addition to its mineral richness, P. palmata contains significant amounts of riboflavin (B2), niacin (B3), vitamin C, vitamin E, and provitamin A (Kraan, 2013; Mabeau and Fleurence, 1993). Table 5 presents the mineral composition of P. palmata relative to soya bean meal, maize, and wheat, highlighting its superior density of both macro- and micro-minerals compared with animal feed.
4 Bioactive compounds and functional properties
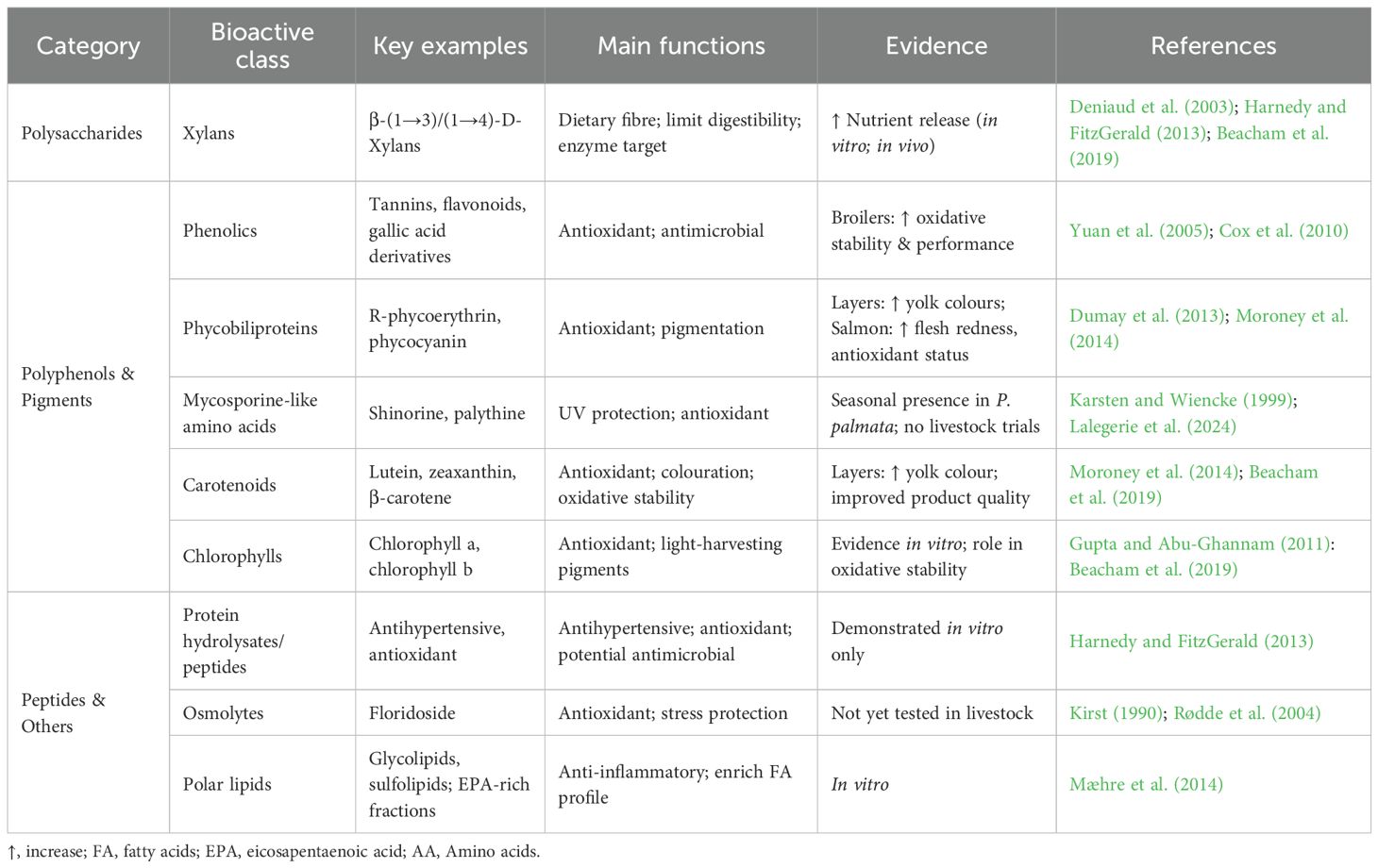
P. palmata contains a wide array of bioactive compounds, including sulphated polysaccharides, phenolics, carotenoids, phycobiliproteins, and peptides, which together underpin antioxidant, antimicrobial, immunomodulatory, and metabolic activities relevant to animal health and product quality (Holdt and Kraan, 2011; Lordan et al., 2011; Harnedy and FitzGerald, 2013; Cherry et al., 2019; Xu et al., 2024). These compounds are increasingly recognised for their potential roles in supporting gut function, modulating the immune system, and enhancing oxidative stability in livestock products. However, their concentration and activity vary considerably depending on season, harvest site, and processing method (Karsten and Wiencke, 1999; Lalegerie et al., 2024).
4.1 Polysaccharides
P. palmata is distinctive among red seaweeds in having xylan-rich cell walls, rather than the carrageenan’s or agars typical of most Rhodophyta (Bjarnadóttir et al., 2018; Premarathna et al., 2024). These walls are reinforced by mixed-linked β-(1→3)/(1→4)-D-Xylans, commonly referred to as rhodymenan, which provide structural rigidity through hydrogen bonding and covalent interactions with charged glycoproteins.
Functionally, these polysaccharides act as dietary fibre, influencing gut health and nutrient utilisation (Deniaud et al., 2003). Although they display only modest antioxidant activity, largely due to associated phenolics rather than the xylan backbone, their primary nutritional significance lies in their role as fermentable fibre with potential prebiotic effects, supporting beneficial microbial activity and short-chain fatty acid (SCFA) production (Harnedy and FitzGerald, 2013; De Jesus Raposo et al., 2016). In animal nutrition, the enzymatic breakdown of Xylans is particularly relevant. Xylanase supplementation in poultry diets has been shown to release xylo-oligosaccharides and increase caecal SCFA concentrations, thereby improving gut fermentation dynamics and growth performance (Craig et al., 2019). This reflects wider commercial practice, as xylanase is already widely used in monogastric diets to enhance energy release from cereal arabinoxylans, with additional evidence that xylo-oligosaccharides can support gut barrier integrity and immune modulation (Craig et al., 2019). However, direct studies on P. palmata xylans remain limited, and most functional inferences are extrapolated from studies on cereal arabinoxylans (e.g., from wheat, maize, and barley) rather than from P. palmata itself. Such findings highlight the potential value of P. palmata Xylans as biofunctional feed components when combined with targeted enzymatic strategies.
Alongside structural polysaccharides, P. palmata also accumulates floridean starch as its principal storage carbohydrate, consistent with other red algae. Floridean starch is an amylopectin-like glucan, consisting of α-(1→4)-linked chains with α-(1→6) branches but lacking amylose, and is deposited in the cytoplasm rather than plastids (Usov, 2011). While less studied in the context of nutrition, floridean starch contributes to the overall carbohydrate pool of P. palmata and represents a potential source of digestible energy in feed applications.
4.2 Polyphenols and pigments
P. palmata contains a range of polyphenolic compounds, including tannins, flavonoids (catechins, flavonol derivatives), and phenolic acids such as gallic acid derivatives and hydroxycinnamic acids, which underpin its antioxidant and antimicrobial activities (Yuan et al., 2005; Gupta and Abu-Ghannam, 2011); Yuan and Macquarrie, 2015). Alongside these, P. palmata is rich in pigments that contribute both to functional bioactivity and product quality. These include carotenoids (β-carotene, lutein, zeaxanthin, violaxanthin, antheraxanthin), chlorophyll-a, and phycobiliproteins (notably R-phycoerythrin and phycocyanin) (Romay et al., 2003; Sekar and Chandramohan, 2008; Beacham et al., 2019).
R-phycoerythrin can represent a substantial fraction of soluble protein, acting as both a natural colourant and an antioxidant (Dumay et al., 2013). Carotenoids and chlorophylls contribute to antioxidant capacity and nutritional value, while phycobiliproteins add both bioactivity and potential for functional food applications. These pigments have demonstrated relevance in animal feeding trials, where they contribute to yolk pigmentation in poultry and flesh coloration in fish such as salmon, as well as enhancing oxidative stability (Moroney et al., 2014, 2015). In addition, P. palmata produces mycosporine-like amino acids (MAAs), such as shinorine and palythine, which function as UV-protective and antioxidant compounds; however, their occurrence is strongly seasonal, and their effects in livestock models have not yet been evaluated (Karsten and Wiencke, 1999; Lalegerie et al., 2024). From an applied perspective, seaweed pigments also offer potential as natural colourants in poultry and aquaculture feeds, reducing reliance on synthetic additives.
Together, phenolics and pigments inhibit lipid peroxidation, scavenge free radicals, and may modulate gut redox balance, supporting their role in maintaining tissue integrity and improving food product quality (Cox et al., 2010; Moroney et al., 2015). When used as seaweed extracts, their recovery rate and stability are strongly influenced by processing. Enzyme-assisted extraction enhances the recovery of phenolics and pigments, whereas high-temperature drying can degrade bioactivity (Harnedy and FitzGerald, 2013; Yuan and Macquarrie, 2015; Subbiah et al., 2023). Nevertheless, the bioavailability and in vivo activity of P. Palmata phenolics and pigments in monogastrics remain underexplored, representing an important research gap.
4.3 Peptides and other bioactive compounds
Enzymatic hydrolysis of P. palmata proteins produces bioactive peptides with demonstrated antioxidant, antihypertensive and antidiabetic activities (Harnedy and FitzGerald, 2011; Harnedy and FitzGerald, 2013). Antimicrobial properties have been reported in other macroalgal species, but evidence specific to P. palmata is limited, and immunomodulatory roles are supported only by preliminary in vitro studies rather than animal trials. The generation and functionality of these peptides are highly dependent on protease selection and hydrolysis conditions, as methods optimising overall protein yield may not necessarily maximise bioactive potential (Harnedy and FitzGerald, 2013; Echave et al, 2022). Although such peptides are often proposed as natural alternatives to antibiotic growth promoters in livestock diets, this application remains largely theoretical, with limited direct evidence from feeding trials. Much of the supporting data instead comes from studies on polysaccharides in related red seaweeds (Cian et al., 2015). Other bioactive molecules present in P. palmata include the osmolyte floridoside, which has antioxidant and stress-protective roles, and polar lipids such as glycolipids and sulfolipids enriched with eicosapentaenoic acid (EPA), which have demonstrated anti-inflammatory and bioactive properties in vitro (Kirst, 1990; Rødde et al., 2004; Mæhre et al., 2014). While promising, these compounds have not yet been assessed in animal feeding trials. Looking ahead, targeted enzymatic hydrolysis and fractionation strategies could enable the production of defined peptide or lipid fractions with functional properties, but scaling these approaches for feed applications remains a challenge. The principal bioactive classes identified in P. palmata, together with their functions and the extent of evidence available from in vitro assays and livestock studies, are summarised in Table 6.
4.4 Derived products and emerging applications in animal nutrition
Beyond direct dietary inclusion, several value-added products derived from Palmaria palmata have been explored for their potential in animal nutrition and other bio-based applications. Through targeted processing and fractionation, P. palmata can yield ingredients with distinct nutritional and functional roles that align with circular bioeconomy principles.
Protein concentrates and hydrolysates, produced through enzymatic extraction, contain approximately 45–50% protein with improved digestibility compared to raw seaweed. These fractions are rich in essential amino acids and bioactive peptides exhibiting amongst others antioxidant properties, making them potential partial substitutes for fish or soybean meal in monogastric feeds (Harnedy and FitzGerald, 2013). The polysaccharide-rich xylan fraction, when included in pig diets at 5%, enhances hindgut fermentation and short-chain fatty acid production without increasing digesta viscosity, suggesting its prebiotic potential (Hoebler et al., 2000; Bobin-Dubigeon et al., 1997).
Further enzymatic extraction and cell-disruption techniques have enabled the recovery of bioactive and nutrient-dense fractions from P. palmata. Protease-based extraction (using Alcalase®, Flavourzyme®, and Formea® Prime) yields protein-rich, water-soluble extracts enriched with amino acids and phenolic compounds that display antioxidant, antidiabetic, and anti-obesity activities, supporting their use as functional feed ingredients (Ghelichi et al., 2025). Similarly, pulsed electric field and enzymatic-assisted fractionation produce a protein-rich pellet suitable for animal feed and a sugar–mineral-rich supernatant that may be valorised in agricultural applications (Maribu et al., 2024).
Moreover, thermolysin-digested water extracts (d-DWE) of P. palmata contain bioactive compounds derived from phycobiliproteins and chlorophyll a with strong anti-inflammatory activity. These extracts suppress proinflammatory mediators such as nitric oxide, interleukin-6, and tumour necrosis factor-α in vitro and reduce inflammation in vivo. A novel peptide (LRDGEIILRY) derived from the phycoerythrin β-chain and chlorophyll degradation products (e.g., pheophorbide a) was identified as a key contributor (Lee et al., 2017; Sakai et al., 2011; Subramoniam et al., 2012). The thermally stable, water-soluble nature of these compounds highlights P. palmata as a promising source of bioactive ingredients suitable for functional feed and nutraceutical formulations.
Beyond food and feed applications, P palmata extracts are also utilised in the cosmetic industry due to their bioactive and antioxidant properties. Commercial products such as P. palmata extract and powder are incorporated into moisturisers, serums, and anti-ageing formulations for their hydrating and anti-inflammatory effects. For example, Densinaria™, a P palmata-derived ingredient, is used in hair-care products to improve strength and volume. These applications illustrate the broad biotechnological potential of P. palmata and support its further valorisation across multiple sectors, including cosmetics, nutraceuticals,food and feed (SpecialChem, 2024; CosmileeEurope, 2025).
4.5 Cross-reactivity and allergic responses to Palmaria palmata
Allergic reactions to edible macroalgae are rare, but the increasing use of red seaweeds in food and feed formulations has drawn attention to their potential to cause hypersensitivity in susceptible individuals. Within this group, species such as P. palmata, Porphyra spp., and Chondrus crispus have been implicated in immunoglobulin E (IgE)-mediated allergic reactions (James et al., 2023). Although reported cases remain infrequent, and biological relevance to monogastric farmed animal application is yet to be established, these examples demonstrate that red algal constituents can act as allergenic triggers through carbohydrate or protein structures capable of eliciting immune recognition.
Carrageenans from P. palmata and other red algae contain alternating galactose residues that include the α-gal epitope Galα1-3Galβ1-4GlcNAc-R (Macher and Galili, 2008). This carbohydrate structure is recognised by anti-Gal antibodies in humans and has been linked to α-gal syndrome, a delayed allergic reaction to mammalian meat (Steinke et al., 2015). Carrageenan exposure has been shown to activate proinflammatory pathways, including Toll-like receptor 4 and NF-κB signalling, leading to cytokine release and intestinal inflammation in human colonic epithelial cells (Borthakur et al., 2007; Tobacman, 2001). Clinical observations have reported hypersensitivity reactions such as urticaria and gastrointestinal distress following carrageenan ingestion (Kular et al., 2018), supporting its potential to act as a food allergen in susceptible individuals.
In addition to polysaccharide-associated epitopes, red seaweed proteins may also trigger immune responses. Porphyra tenera (nori), a red algal species closely related to P. palmata, contains tropomyosins homologous to those found in crustaceans, which are major shellfish allergens associated with extensive IgE cross-reactivity (Motoyama et al., 2007; Yang et al., 2025). This similarity raises the possibility that P. palmata proteins may also cross-react with IgE antibodies in individuals sensitised to shellfish allergens. Furthermore, contamination during wild harvest or aquaculture can introduce small marine invertebrates containing tropomyosin and other allergenic proteins, compounding the risk of allergic reactions (Lopata et al., 2010; Hajeb and Selamat, 2012).
Although direct evidence of P. palmata-specific allergy is limited, available studies collectively indicate that red seaweeds possess both carbohydrate and protein structures capable of eliciting immune responses. Further research is needed to characterise these allergenic determinants and ensure the safe incorporation of red macroalgae into food and feed applications.
5 Regulatory framework and feed safety
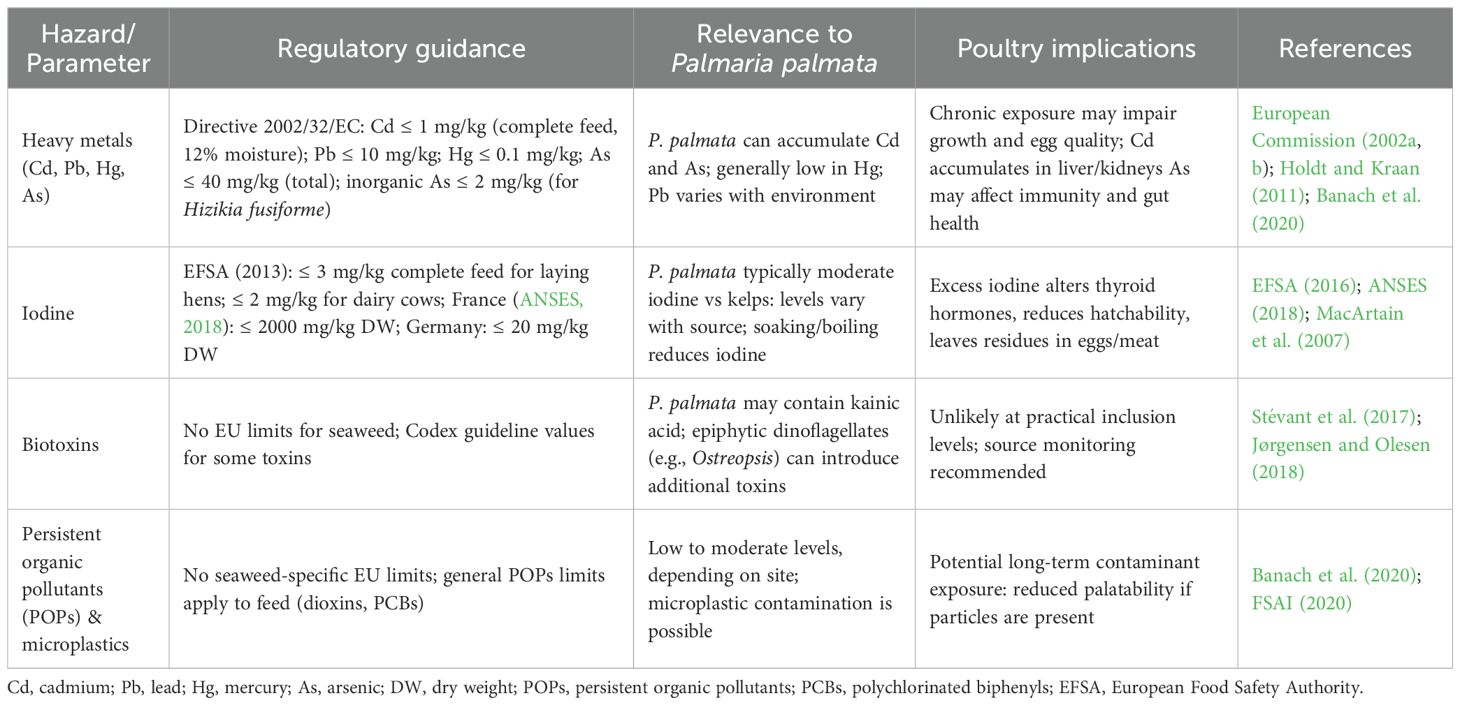
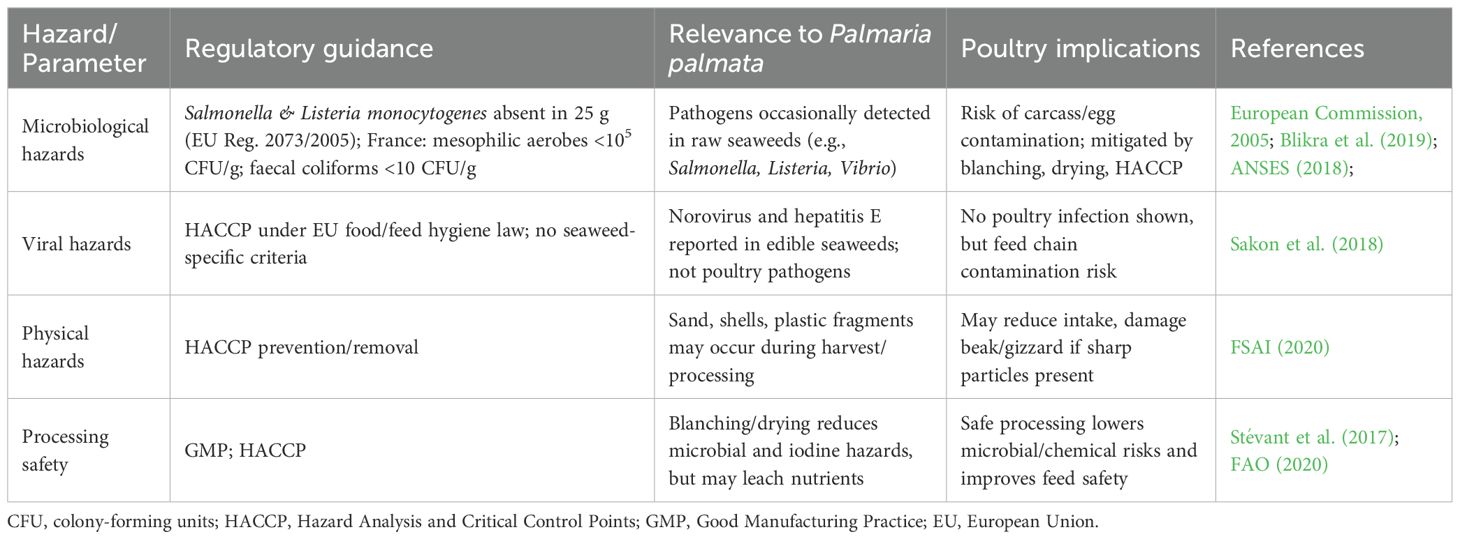
In the EU, seaweeds used as feed materials are regulated under Directive 2002/32/EC (2002a) to control undesirable substances in animal feed. For red seaweeds, such as P. palmata, the most relevant legally controlled chemical hazards are heavy metals and iodine, while microbiological, viral, and physical hazards are managed under general hygiene law and national specifications.
The inclusion of seaweeds in animal feed is formally recognised under Regulation (EC) No 767/2009, provided that products meet established hygiene and safety standards (European Commission, 2009). Directive 2002/32/EC sets maximum levels for undesirable substances, including cadmium, lead, mercury, and arsenic, an important consideration for red seaweeds given their ability to accumulate minerals (European Commission, 2002a; Banach et al., 2020). Tables 7a, b summarise the regulatory and safety considerations for P. palmata as a feed ingredient.
Among these hazards, iodine is a critical factor, as concentrations in P. palmata often exceed food guidance thresholds, which do vary between countries (e.g., 2,000 mg/kg dry weight in France versus 20 mg/kg in Germany). Excess iodine in poultry diets can impair thyroid function, reduce hatchability, and elevate residues in eggs and meat (Teas et al., 2004; Makkar et al., 2016). Biotoxins also warrant attention: P. palmata contains the neurotoxic amino acid kainic acid, with levels ranging from trace amounts to approximately 560 µg/g dry weight (Jørgensen and Olesen, 2018). In addition, epiphytic dinoflagellates such as Ostreopsis can colonise seaweed surfaces and introduce further toxins (Rhodes et al., 2000; Monti et al., 2007).
Microbiological hazards, including Salmonella, Listeria monocytogenes, and Vibrio spp., as well as viral agents such as norovirus and hepatitis E virus, have been reported in minimally processed edible seaweeds (Kusumi et al., 2017; Sakon et al., 2018). Physical contaminants, including microplastics, shell fragments, and sand, are also documented in harvested biomass (FSAI, 2020).
Collectively, these risks emphasise the importance of HACCP-based hygiene management, validated processing methods (e.g., blanching, drying, fermentation), and site-specific monitoring to ensure compliance with EU/UK legislation and safe inclusion of P. palmata in poultry diets.
6 Feeding trials in poultry and aquaculture species
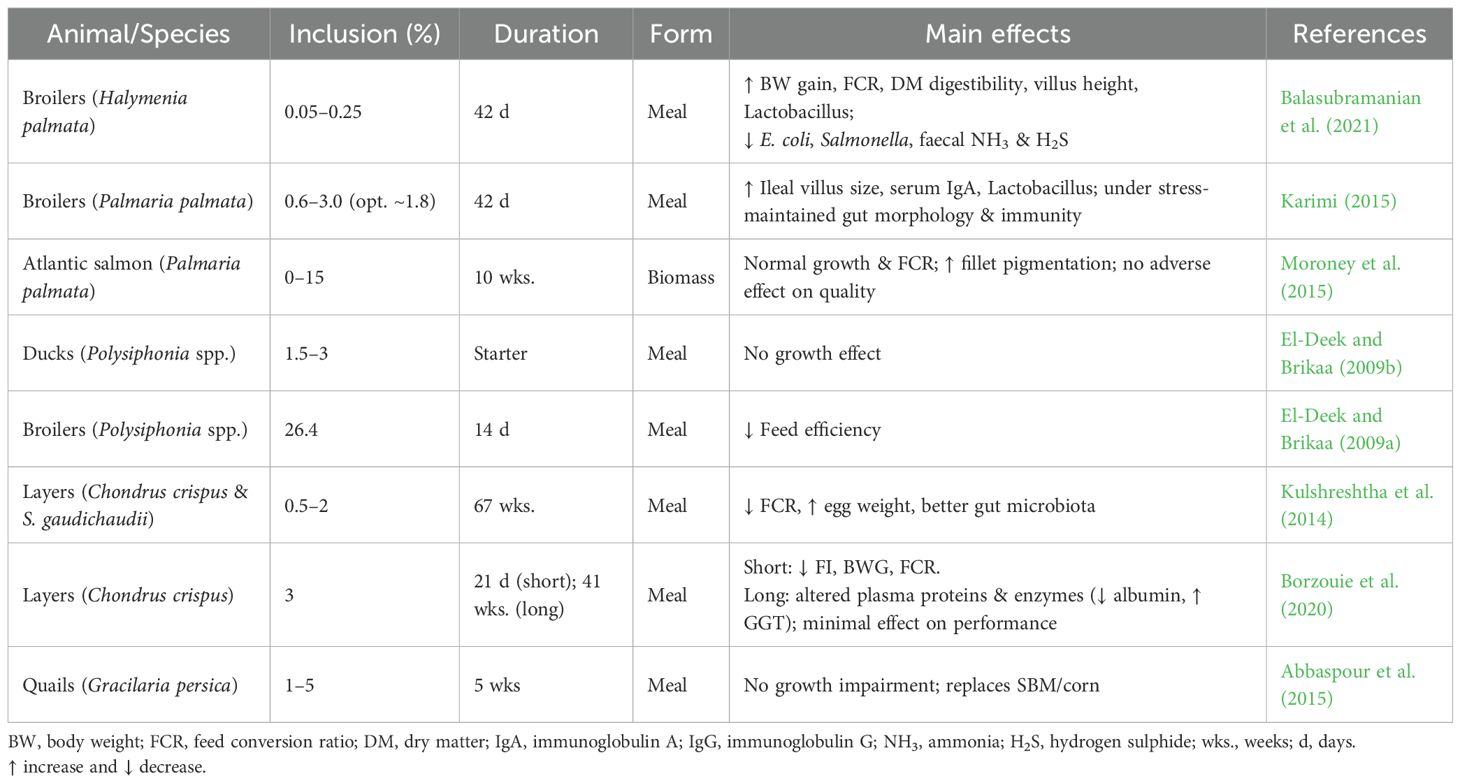
While regulatory frameworks establish safety boundaries, feeding trials provide essential evidence of how seaweeds function as practical feed ingredients by identifying safe upper limits, that is, inclusion levels at which no significant negative impacts on production parameters are observed. Several red seaweeds have been evaluated in poultry with variable but informative outcomes. Across studies, low dietary inclusion levels of red seaweeds appear well tolerated, whereas higher levels can have mixed effects depending on species. For example, Polysiphonia spp. at 1.5–3% of the diet had no effect on duck growth, but a much higher level (26.4%) reduced feed efficiency in broilers (El-Deek and Brikaa, 2009a, b). In quails, supplementation with the red alga Gracilariopsis persica at 1–5% of the diet did not impair growth or egg production but improved egg quality by lowering serum and yolk cholesterol, reducing yolk malondialdehyde concentrations, and enhancing lipid-related indices such as the ratio of high-density lipoprotein to low-density lipoprotein cholesterol (Abbaspour et al., 2015). Similarly, in layers, dietary inclusion of the red seaweeds Chondrus crispus and Sarcodiotheca gaudichaudii at 1–2% improved egg weight and feed efficiency, while also enhancing gut health through increased counts of beneficial lactic acid bacteria and reduced coliforms such as Escherichia coli (Kulshreshtha et al., 2014). Collectively, these examples highlight the broader potential of red seaweeds as functional feed ingredients, though outcomes vary with inclusion level and host species.
Within this broader context, targeted studies using P. palmata remain relatively limited in number but consistently demonstrate nutritional and functional benefits. In broilers, very low inclusion (0.05–0.25%) improved body-weight gain, feed efficiency, dry matter digestibility, gut microbial balance (increase Lactobacillus, decrease E. coli and Salmonella), and reduced emissions of ammonia and hydrogen sulphide (Balasubramanian et al., 2021). At slightly higher levels (0.6–3.0%, optimum ~1.8%), P. palmata enhanced villus morphology and stimulated immune markers such as plasma IgA, supporting gut integrity and systemic immunity (Karimi, 2015). In layers, supplementation with seaweed meal at 2% of the diet improved yolk pigmentation and oxidative stability, although longer-term effects on egg production remain to be confirmed (Borzouie et al., 2020). The study also examined interactions with genetic strain (Lohmann Brown vs. Lohmann LSL-Lite) and heat stress, providing additional insights into context-dependent responses. Under both thermoneutral and heat-stress conditions, seaweed inclusion enhanced yolk colour intensity, reflecting deposition of algal pigments, and reduced yolk lipid peroxidation, suggesting a protective antioxidant effect. However, production parameters such as feed intake, egg output, and feed conversion ratio were not significantly altered. Plasma biochemical profiles showed some modulation, including lower triglyceride concentrations in certain treatment groups, but no adverse changes in liver enzymes or hematological indices were observed. These results suggest that moderate dietary inclusion of seaweed can enhance specific egg quality traits and lipid stability without compromising performance, though further research is needed to establish dose–response relationships, persistence of effects over extended laying cycles, and whether benefits differ across strains or environmental conditions. Evidence from aquaculture reinforces these outcomes, with studies in Atlantic salmon showing that dietary inclusion of dried P. palmata at levels up to 15% sustained normal growth and feed efficiency while enhancing fillet pigmentation through the deposition of red algal pigments (Moroney et al., 2015).
Overall, the available evidence indicates that P. palmata can be safely incorporated at low to moderate levels in poultry diets, improving growth, gut health, immunity, and product quality. Responses are dose-dependent, with inclusion above ~5% likely to reduce palatability and digestibility due to high fibre content (Holdt and Kraan, 2011; Stévant et al., 2017). Table 8 summarises feeding trials with P. palmata and other red seaweeds across monogastric species. Results for P. palmata are consistently positive, showing improvements in performance, gut morphology, immune responses, and product quality at practical inclusion levels. In contrast, outcomes with other red seaweeds are more variable and appear influenced by species, dose, and processing method. Together, these findings highlight the promising but underexplored role of P. palmata in sustainable poultry and monogastric nutrition.
To realise this potential, further well-controlled studies are needed to refine safe and effective inclusion levels, explore interactions with feed enzymes such as xylanase, and assess long-term impacts on performance, food safety, and product quality. Importantly, feeding trial outcomes must also be considered alongside the regulatory and safety concerns outlined in Tables 7a, b, since processing and hazard management will ultimately determine the feasibility of P. palmata as a commercial feed ingredient.
7 Palmaria palmata and the gut microbiome
Research on the impact of P. palmata on the poultry gut microbiota remains limited, with only a handful of controlled feeding trials currently available. Consequently, much of the mechanistic understanding is derived from mammalian and aquaculture models, which nevertheless provide valuable insights into how P. palmata polysaccharides, phenolics, and peptides may influence microbial ecology and host responses.
In mammals, Yousof et al. (2023) demonstrated that daily oral supplementation with an aqueous P. palmata extract at 600 mg/kg body weight in a cuprizone-induced multiple sclerosis (MS) mouse model markedly altered gut microbial communities, as assessed by 16S rRNA gene sequencing. The extract was administered continuously across both demyelination and remyelination phases to maintain exposure to P. palmata-derived bioactives. Supplementation increased the Firmicutes/Bacteroidetes ratio, which is often associated with improved energy harvest and intestinal health, and promoted the growth of beneficial lactic acid bacteria such as Lactobacillus and Bifidobacterium. These taxa are well recognised for their ability to produce short-chain fatty acids (SCFAs), maintain epithelial integrity, and exert systemic anti-inflammatory effects. At the same time, the intervention suppressed potentially pathogenic taxa, including pro-inflammatory Proteobacteria, which are frequently elevated in dysbiosis linked to neuroinflammation.
The magnitude of these microbiome shifts varied with both the age of the animals and stage of disease, with younger mice and those in the early phase of MS showing the most pronounced enrichment of beneficial taxa. This suggests that the prebiotic and immunomodulatory effects of P. palmata may be most effective when applied preventively or during the early onset of inflammatory processes, rather than once dysbiosis and tissue damage are firmly established. Importantly, these microbial changes were accompanied by reductions in neuroinflammatory markers and improvements in clinical outcomes, directly linking modulation of the gut microbiome with systemic and neurological benefits.
However, not all studies have shown significant effects. Pinna et al. (2021) supplemented adult dog diets with a mixed seaweed preparation that included P. palmata at about 1.5% of dietary dry matter. At this inclusion level, no significant effects were detected on faecal microbiota composition, faecal secretory IgA, or apparent nutrient digestibility. The authors suggested that the lack of measurable response likely reflected several interacting factors: the relatively low dietary inclusion, potentially below the threshold required to deliver fermentable polysaccharides to the colon; the stable and resilient nature of the adult canine gut microbiota, which often resists dietary perturbation unless exposed to higher doses or longer feeding periods; and methodological limitations, as the study relied on qPCR targeting a restricted panel of bacterial groups rather than broader high-throughput sequencing approaches. These considerations imply that the absence of observed effects was due to host- and study-specific constraints, rather than an inherent lack of functional potential in P. palmata.
By contrast, aquatic models provide more consistent evidence of microbiome modulation. Gobet et al. (2018) conducted a year-long trial in European abalone (Haliotis tuberculata), in which animals were maintained on monospecific diets composed entirely of P. palmata. This high-level exposure offered the gut microbiota continuous access to P. palmata polysaccharides as the sole carbohydrate substrate. Under these conditions, distinct microbial enrichments were observed, notably of taxa such as Polaribacter and Pseudahrensia, which possess carbohydrate-active enzymes (CAZymes) capable of degrading complex algal polysaccharides. The strong dietary effects in abalone reflect their evolutionary adaptation as specialist macroalgal grazers with gut microbiota highly responsive to seaweed-derived glycans. These findings underscore the contrast between low-inclusion studies in terrestrial omnivores, which may fail to detect microbial shifts, and high-exposure models in herbivorous or algivorous species, which clearly demonstrate P. palmata’s potential as a selective microbial substrate.
8 Challenges and mitigation strategies
Despite its nutritional potential, several challenges constrain the inclusion of P. palmata in animal diets. A major issue is compositional variability, with protein, lipid, and ash contents shifting according to season, harvest site, and processing method (Makkar et al., 2016). Protein, lipid, and ash contents can fluctuate widely, complicating consistent formulation. Mitigation requires batch-specific compositional analyses and adoption of standardised nitrogen-to-protein conversion factors (Morgan et al., 1980; Lourenço et al., 2002, 2004; Holdt and Kraan, 2011).
High ash and mineral content, notably sodium, potassium, and iodine can disrupt electrolyte balance or exceed dietary thresholds (Mæhre et al., 2014; Stévant et al., 2017). Pretreatments such as soaking, blanching, or partial extraction can lower mineral loads, while balanced formulation may offset residual risks (Nielsen et al., 2020; Akomea-Frempong et al., 2021).
Digestibility is another constraint, as the polysaccharide-rich cell wall (cellulose, Xylans, sulphated galactans) resists enzymatic breakdown in monogastrics (Fleurence, 1999; Cherry et al., 2019). Enzyme supplementation (e.g., Carbohydrases, xylanases) or fermentation can enhance nutrient release and bioactive peptide recovery, thereby improving both nutritional value and functional potential (Harnedy and FitzGerald, 2013; Øverland et al., 2019).
Finally, safety concerns remain central. P. palmata may accumulate heavy metals (Cd, Pb, Hg, As) or excess iodine, and can carry physical (shell, microplastics) or microbial (Listeria, Salmonella) hazards (Banach et al., 2020; Blikra et al., 2019; Cho and Rhee, 2020). Mitigation requires HACCP-based controls, careful site selection, validated processing methods, and strict regulatory compliance (ANSES, 2018; FSAI, 2020).
Collectively, these mitigation strategies highlight the importance of factors to consider when integrating P. palmata into monogastric feeding systems through a combination of processing optimisation, analytical monitoring, and targeted feed formulation approaches to ensure both safety and efficacy.
9 Conclusions and research priorities
The evidence reviewed highlights both the potential and the constraints of P. palmata as a feed ingredient. Its balanced amino acid profile, presence of bioactive compounds, and moderate mineral contribution make it a promising partial substitute for conventional proteins. Yet, challenges related to variable composition, fibre-rich cell walls, and risks of mineral or contaminant accumulation limit its current application. Feeding trials generally indicate positive effects on performance, gut health, and product quality, but most are short-term and at limited inclusion levels, making it difficult to define safe upper limits.
To progress from potential to practice, further research should prioritise:
a. Establishing safe, effective inclusion ranges through long-term, multi-dose trials in poultry and pigs.
b. Optimising processing technologies (blanching, drying, fermentation) to stabilise composition, reduce contaminants, and retain bioactive compounds.
c. Assessing nutrient and bioactive transfer into meat and eggs to safeguard food quality and consumer confidence.
d. Undertaking life cycle assessments (LCA) to quantify environmental benefits versus conventional feed proteins.
e. Evaluating the techno-economic feasibility of large-scale cultivation and processing to support industry adoption.
Addressing these priorities will be essential to unlock the role of P. palmata in sustainable poultry and livestock production. With targeted innovation, this underutilised red seaweed could become a valuable component of next generation monogastric feeding strategies.
Author contributions
FK: Conceptualization, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. HM: Writing – review & editing. DS: Resources, Validation, Writing – review & editing. PE: Writing – review & editing. MA: Resources, Validation, Writing – review & editing. FS: Writing – review & editing. MS: Writing – review & editing. JH: Funding acquisition, Project administration, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The work was funded by Innovate UK and the Biotechnology and Biological Sciences Research Council (BBSRC) under the competition “Novel low-emission food production systems: Feasibility studies” (Project No. 1006777, Novel Seaweed Chicken Feed Feasibility). SRUC also receives support from the Scottish Government’s Rural and Environment Science and Analytical Services Division (RESAS).
Acknowledgments
The authors gratefully acknowledge Carol Davies Sala (University of West London) for her assistance in gathering some literature relevant to the gut microbiome section of this review.
Conflict of interest
Authors DS, PE, and MA were employed by the company Seaweed Generation Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbaspour B., Davood S. S., and Mohammadi-Sangcheshmeh A. (2015). Dietary supplementation of Gracilariopsis persica is associated with some quality related sera and egg yolk parameters in laying quails. J. Sci. Food Agric. 95, 643–648. doi: 10.1002/jsfa.6844, PMID: 25061008
Akomea-Frempong S., Skonberg D. I., Camire M. E., and Perry J. J. (2021). Impact of blanching, freezing, and fermentation on physicochemical, microbial, and sensory quality of sugar kelp Saccharina latissima. Foods 10, 2258. doi: 10.3390/foods10102258, PMID: 34681308
ANSES (2018). Opinion of the French Agency for Food, Environmental and Occupational Health and Safety on the risks associated with the consumption of seaweed (Maisons Alfort, France: French Agency for Food, Environmental and Occupational Health & Safety (ANSES). Available online at: https://www.anses.fr/en/system/files/ERCA2020SA0106EN.pdf.
Balasubramanian B., Shanmugam S., Park S., Recharla N., Koo J. S., Andretta I., et al. (2021). Supplemental impact of marine red seaweed Halymenia palmata on growth performance, nutrient digestibility, blood profiles, intestine histomorphology, meat quality, fecal gas emission, and microbial counts in broilers. Animals 11, 1244. doi: 10.3390/ani11051244, PMID: 33925270
Banach J. L., Hoek-Van Den Hil E. F., and Van Der Fels-Klerx H. L. (2020). Food safety hazards in the European seaweed chain. Compr. Rev. Food Sci. Food Saf. 19, 332–364. doi: 10.1111/1541-4337.12523, PMID: 33325177
Beacham T. A., Cole I. S., DeDross L. S., Raikova S., Chuck C. J., Macdonald J., et al. (2019). Analysis of seaweeds from South West England as a biorefinery feedstock. Appl. Sci. 9, 4456. doi: 10.3390/app9204456
Biancarosa I., Espe M., Bruckner C. G., et al. (2017). Amino acid composition, protein content, and nitrogen-to-protein conversion factors of 21 seaweed species from Norwegian waters. Journal of Applied Phycology 29, 1001–1009. doi: 10.1007/s10811-016-0984-3
Biancarosa I., Belghit I., Bruckner C. G., Liland N. S., Waagbø R., Amlund H., et al. (2018). Chemical characterization of twenty one species of marine macroalgae common in Norwegian waters. J. Sci. Food Agric. 98, 2035–2042. doi: 10.1002/jsfa.8798, PMID: 29193189
Bikker P., Stokvis L., Van Krimpen M. M., Van Wikselaar P. G., and Cone J. W. (2020). Evaluation of seaweeds from Northwestern European waters for application in animal nutrition. Anim. Feed Sci. Technol. 263, 114460. doi: 10.1016/j.anifeedsci.2019.114460
Bjarnadóttir M., Aðalbjörnsson B. V., Nilsson A., Slizyte R., Roleda M. Y., Hreggviðsson G.Ó., et al. (2018). Palmaria palmata as an alternative protein source. J. Appl. Phycology 30, 2061–2070. doi: 10.1007/s10811-017-1330-2
Blikra M. J., Løvdal T., Vaka M. R., Roiha I. S., Lunestad B. T., Lindseth C., et al. (2019). Assessment of food quality and microbial safety of brown macroalgae Alaria esculenta and Saccharina latissima. J. Sci. Food Agric. 99, 1198–1206. doi: 10.1002/jsfa.9289, PMID: 30054912
Bobin-Dubigeon C., Hoebler C., Lognone V., Lahaye M., Barry J. L., Gallant D., et al. (1997). Chemical composition and fermentative characteristics of dietary fibres from edible seaweeds. Sci. Des. Aliments 17, 619–639.
Borthakur A., Bhattacharyya S., Dudeja P. K., and Tobacman J. K. (2007). Carrageenan induces interleukin 8 production through a Bcl10 pathway in normal human colonic epithelial cells. Am. J. Physiol. Gastrointestinal Liver Physiol. 292, G829–G838. doi: 10.1152/ajpgi.00380.2006, PMID: 17095757
Borzouie S., Rathgeber B. M., Stupart C. M., MacIsaac J., and MacLaren L. A. (2020). Effects of dietary inclusion of seaweed, heat stress and genetic strain on laying hens. Animals 10, 1570. doi: 10.3390/ani10091570, PMID: 32899340
Bruhn A., Brynning G., Johansen A., Lindblad C., Mathiesen L., Ravn H., et al. (2019). Fermentation of sugar kelp Saccharina latissima. J. Appl. Phycology 31, 3175–3187. doi: 10.1007/s10811-019-01827-4
Campos B. M., Ramalho E., Marmelo I., Noronha J. P., Malfeito-Ferreira M., Mata P., et al. (2022). Edible seaweeds in the Portuguese market. Front. Bioscience Elite Edition 14, 26. doi: 10.31083/j.fbe1404026, PMID: 36575846
Chan J. C.-C., Cheung P. C.-K., and Ang P. O. (1997). Effects of three drying methods on Sargassum hemiphyllum. J. Agric. Food Chem. 45, 3056–3059. doi: 10.1021/jf9609478
Cherry P., O’Hara C., Magee P. J., McSorley E. M., and Allsopp P. J. (2019). Risks and benefits of consuming edible seaweeds. Nutr. Rev. 77, 307–329. doi: 10.1093/nutrit/nuy066, PMID: 30840077
Cho T. J. and Rhee M. S. (2020). Health functionality and quality control of laver Porphyra or Pyropia. Mar. Drugs 18, 14. doi: 10.3390/md18010014, PMID: 31877971
Cian R. E., Drago S. R., De Medina F. S., and Martínez-Augustin O. (2015). Proteins and carbohydrates from red seaweeds and effects on gut function. Mar. Drugs 13, 5358–5383. doi: 10.3390/md13085358, PMID: 26308006
CosmileeEurope (2025).Palmaria palmata extract, ingredient profile. Available online at: https://cosmileeurope.eu/inci/detail/10118/palmaria-palmata-extract (Accessed November 8, 2025).
Cox S., Abu-Ghannam N., and Gupta S. (2010). Antioxidant and antimicrobial activity of six Irish seaweeds. Int. Food Res. J. 17, 205–220.
Craig A. D., Bedford M. R., Hastie P., Khattak F., and Olukosi O. A. (2019). Carbohydrases or prebiotic oligosaccharides in broilers on wheat or barley diets. J. Sci. Food Agric. 99, 3246–3254. doi: 10.1002/jsfa.9537, PMID: 30549054
De Jesus Raposo M. F., De Morais A. M., and De Morais R. M. (2016). Emergent sources of prebiotics. Mar. Drugs 14, 27. doi: 10.3390/md14020027, PMID: 26828501
Deniaud E., Quemener B., Fleurence J., and Lahaye M. (2003). Mixed linked beta 1 to 3 and beta 1 to 4 D xylans from Palmaria palmata cell wall. Int. J. Biol. Macromolecules 33, 9–18. doi: 10.1016/S0141-8130(03)00028-9
Desideri D., Cantaluppi C., Ceccotto F., Meli M. A., Roselli C., and Feduzi L. (2016). Essential and toxic elements in seaweeds for human consumption. J. Toxicol. Environ. Health Part A 79, 112–122. doi: 10.1080/15287394.2015.1113598, PMID: 26817952
Duarte C. M., Wu J., Xiao X., Bruhn A., and Krause-Jensen D. (2020). Can seaweed farming play a role in climate change mitigation. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00572
Dumay J., Clément N., Morançais M., and Fleurence J. (2013). Optimizing hydrolysis of Palmaria palmata for R-phycoerythrin extraction. Bioresource Technol. 131, 21–27. doi: 10.1016/j.biortech.2012.12.146, PMID: 23334315
Echave J., Otero P., Garcia-Oliveira P., Munekata P. E. S., Pateiro M., Lorenzo J. M., Simal-Gandara J., and Prieto M. A. (2022). Seaweed-derived proteins and peptides: promising marine bioactives. Antioxidants 11, 176. doi: 10.3390/antiox11010176, PMID: 35052680
EFSA (2016). Presence of microplastics and nanoplastics in food. EFSA J. 14, e04501. doi: 10.2903/j.efsa.2016.4501, PMID: 40007823
El-Deek A. A. and Brikaa A. M. (2009a). Nutritional and biological evaluation of marine seaweed as a feedstuff and as a pellet binder in poultry diet. Int. J. Poultry Sci. 8, 875–881. doi: 10.3923/ijps.2009.875.881
El-Deek A. A. and Brikaa A. M. (2009b). Different levels of seaweed in duck diets and carcass quality. Int. J. Poultry Sci. 8, 1014–1021. doi: 10.3923/ijps.2009.1014.1021
European Commission (2002a). Directive 2002 to 32 to EC on undesirable substances in animal feed. Off. J. Eur. Union 140, 10–21.
European Commission (2002b). Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Official Journal of the European Communities L31. https://www.legislation.gov.uk/eur/2002/178/pdfs/eur_20020178_adopted_en.pdf (Accessed November 9, 2025).
European Commission (2005). Commission Regulation EC No 2073 to 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 338, 1–26.
European Commission (2009). Regulation EC No 767 to 2009 on the placing on the market and use of feed. Off. J. Eur. Union 229, 1–28.
FAO (2018). The global status of seaweed production, trade and utilization (Rome: FAO). Available online at: https://openknowledge.fao.org/items/a6000671-f73d-4fd0-a681-7a1ef4febb0a.
FAO (2020). The state of world fisheries and aquaculture 2020. Sustainability in action. (Rome). doi: 10.4060/ca9229en
Fernandes F. A. N. and Rodrigues S. (2007). Ultrasound as pre treatment for drying of fruits. J. Food Eng. 82, 261–267. doi: 10.1016/j.jfoodeng.2007.02.032, PMID: 18462985
Fleurence J. (1999). Seaweed proteins and potential uses. Trends Food Sci. Technol. 10, 25–28. doi: 10.1016/S0924-2244(99)00015-1
Fleurence J., Gutbier G., Mabeau S., and Leray C. (1994). Fatty acids from eleven marine macroalgae of the French Brittany coast. J. Appl. Phycology 6, 527–532. doi: 10.1007/BF02182406
Fleurence J., Massiani L., Guyader O., and Mabeau S. (1995). Enzymatic cell wall degradation to improve protein extraction from Chondrus crispus, Gracilaria verrucosa and Palmaria palmata. J. Appl. Phycology 7, 393–397. doi: 10.1007/BF00003999
FSAI (2020). Safety considerations of seaweed and seaweed derived foods on the Irish market (Dublin). Available online at: https://www.fsai.ie (Accessed October 23, 2025).
Fudholi A., Sopian K., Othman M. Y., and Ruslan M. H. (2014b). Energy and exergy analyses of solar drying system of red seaweed. Energy Buildings 68, 121–129. doi: 10.1016/j.enbuild.2013.07.072
Fudholi A., Sopian K., Ruslan M. H., Alghoul M. A., and Sulaiman M. Y. (2014a). Review of solar dryers for agricultural and marine products. Renewable Sustain. Energy Rev. 14, 1–30. doi: 10.1016/j.rser.2009.07.032
Galland-Irmouli A. V., Fleurence J., Lamghari R., Luçon M., Rouxel C., Barbaroux O., et al. (1999). Nutritional value of proteins from Palmaria palmata. J. Nutr. Biochem. 10, 353–359. doi: 10.1016/S0955-2863(99)00014-5, PMID: 15539310
Ghelichi S., Sørensen A.-D. M., Hajfathalian M., Holdt S. L., and Jacobsen C. (2025). Screening enzymatic extracts of Palmaria palmata based on composition and bioactivity. Algal Res. 89, 104048. doi: 10.1016/j.algal.2025.104048
Gobet A., Mest L., Perennou M., Dittami S. M., Caralp C., Coulombet C., et al. (2018). Digestive microbiota of the European abalone Haliotis tuberculata. Microbiome 6, 60. doi: 10.1186/s40168-018-0430-7, PMID: 29587830
Gupta S. and Abu-Ghannam N. (2011). Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods’. Innovative Food Science and Emerging Technologies, 12(4), pp. 600–609. doi: 10.1016/j.ifset.2011.07.004
Gupta S., Cox S., and Abu-Ghannam N. (2011). Drying temperature effects on Irish brown seaweed. LWT Food Sci. Technol. 44, 1266–1272. doi: 10.1016/j.lwt.2010.12.022
Hajeb P. and Selamat J. (2012). A contemporary review of seafood allergy. Clin. Rev. Allergy Immunol. 42, 365–385. doi: 10.1007/s12016-011-8285-0, PMID: 22045217
Harnedy P. A. and FitzGerald R. J. (2011). Bioactive proteins, peptides and amino acids from macroalgae. Journal of Phycology 47 (2), 218–232. doi: 10.1111/j.1529-8817.2011.00969.x, PMID: 27021854
Harnedy P. A. and FitzGerald R. J. (2013). Extraction of protein from Palmaria palmata. LWT Food Sci. Technol. 51, 375–382. doi: 10.1016/j.lwt.2012.09.013
Hoebler C., Guillon F., Darcy-Vrillon B., Vaugelade P., Lahaye M., Worthington E., et al. (2000). Supplementation of pig diet with algal fibre alters digesta characteristics. J. Sci. Food Agric. 80, 1357–1364. doi: 10.1002/1097-0010(200007)80:9<1357::AID-JSFA657>3.0.CO;2-B
Holdt S. L. and Kraan S. (2011). Bioactive compounds in seaweed. J. Appl. Phycology 23, 543–597. doi: 10.1007/s10811-010-9632-5
James C. A., Welham S. J., and Rose P. J. (2023). Edible algae allergenicity, a short report. J. Appl. Phycology 35, 339–352. doi: 10.1007/s10811-022-02880-2
Jørgensen K. and Olesen P. T. (2018). Kainic acid in Palmaria palmata. Food Additives Contaminants Part B 11, 198–200. doi: 10.1080/19393210.2018.1462258, PMID: 29656702
Karimi S. H. (2015). Effects of Palmaria palmata supplemented diets in broilers under normal or stressed conditions MSc Thesis. Halifax, Nova Scotia: Dalhousie University. Available online at: http://hdl.handle.net/10222/64662 (Accessed November 8, 2025).
Karsten U. and Wiencke C. (1999). Formation of UV absorbing mycosporine like amino acids in Palmaria palmata. J. Plant Physiol. 155, 407–415. doi: 10.1016/S0176-1617(99)80124-2
Kirst G. O. (1990). Salinity tolerance in eukaryotic marine algae. Annu. Rev. Plant Physiol. Mol. Biol. 41, 21–53. doi: 10.1146/annurev.pp.41.060190.000321
Kraan S. (2013). “Pigments and minor compounds in algae,” in Functional Ingredients from Algae for Foods and Nutraceuticals. Ed. Domínguez H. (Woodhead Publishing, Cambridge), 205–251. doi: 10.1533/9780857098689.1.205
Kular H., Dean J., and Cook V. (2018). A case of carrageenan allergy in a pediatric patient. Ann. Allergy Asthma Immunol. 121, S119. doi: 10.1016/j.anai.2018.09.395
Kulshreshtha G., Rathgeber B., Stratton G., Thomas N., Evans F., Critchley A., et al. (2014). Red seaweeds in layer hen diets. Poultry Sci. 93, 2991–3001. doi: 10.3382/ps.2014-04200, PMID: 25352682
Kusumi E., Tanimoto T., Hosoda K., Tsubokura M., Hamaki T., Takahashi K., et al. (2017). Multiple norovirus outbreaks due to shredded dried laver seaweed in Japan. Infection Control Hosp. Epidemiol. 38, 885–886. doi: 10.1017/ice.2017.70, PMID: 28438230
Lalegerie F., Stiger-Pouvreau V., and Connan S. (2024). Mycosporine like amino acids in Palmaria palmata, implication of usujirene in photoprotection. Mar. Drugs 22, 121. doi: 10.3390/md22030121, PMID: 38535462
Lee D., Nishizawa M., Shimizu Y., and Saeki H. (2017). Anti inflammatory effects of dulse via simultaneous water extraction of phycobiliproteins and chlorophyll a. Food Res. Int. 100, 514–521. doi: 10.1016/j.foodres.2017.06.040, PMID: 28873715
Le Gall L., Pien S., and Rusig A. M. (2004). Cultivation of Palmaria palmata from isolated spores. Aquaculture 229, 181–191. doi: 10.1016/S0044-8486(03)00390-9
Liot F., Colin A., and Mabeau S. (1993). Microbiology and storage life of fresh edible seaweeds. J. Appl. Phycology 5, 243–247. doi: 10.1007/BF00004025
Lopata A. L., O’Hehir E. E., and Lehrer S. B. (2010). Shellfish allergy. Clin. Exp. Allergy 40, 850–858. doi: 10.1111/j.1365-2222.2010.03513.x, PMID: 20412131
Lordan S., Ross R. P., and Stanton C. (2011). Marine bioactives as functional food ingredients. Mar. Drugs 9, 1056–1100. doi: 10.3390/md9061056, PMID: 21747748
Lourenço S. O., Barbarino E., De-Paula J. C., Pereira L. O. S., and Lanfer Marquez U. M. (2002). Amino acid composition and nitrogen to protein conversion factors for nineteen tropical seaweeds. Phycological Res. 50, 233–241. doi: 10.1046/j.1440-1835.2002.00278.x
Lourenço S. O., Barbarino E., Lavín P. L., Lanfer Marquez U. M., and Aidar E. (2004). Distribution of intracellular nitrogen in marine microalgae and new nitrogen to protein conversion factors. Eur. J. Phycology 39, 17–32. doi: 10.1080/0967026032000157156
Lüning K. and Mortensen L. (2015). ). European aquaculture of sugar kelp for food industries, iodine content and epiphytic animals as major problems. Botanica Marina 58, 449–455. doi: 10.1515/bot-2015-0036
Mabeau S. and Fleurence J. (1993). Seaweed in food products. Trends Food Sci. Technol. 4, 103–107. doi: 10.1016/0924-2244(93)90091-N
MacArtain P., Gill C. I., Brooks M., Campbell R., and Rowland I. R. (2007). Nutritional value of edible seaweeds Nutrition Reviews, 65, 535–543. doi: 10.1301/nr.2007.dec.535-543, PMID: 18236692
Macher B. A. and Galili U. (2008). The Galalpha1,3Galbeta1,4GlcNAc R alpha Gal epitope, a carbohydrate of unique evolution and clinical relevance. Biochim. Biophys. Acta Gen. Subj. 1780, 75–88. doi: 10.1016/j.bbagen.2007.11.003, PMID: 18047841
Mac Monagail M., Cornish L., Morrison L., Araújo R., and Critchley A. T. (2017). Sustainable harvesting of wild seaweed resources. Eur. J. Phycology 52, 371–390. doi: 10.1080/09670262.2017.1365273
Mæhre H. K., Malde M. K., Eilertsen K. E., and Elvevoll E. O. (2014). Protein, lipid and mineral contents in common Norwegian seaweeds for food and feed. J. Sci. Food Agric. 94, 3281–3290. doi: 10.1002/jsfa.6681, PMID: 24700148
Mai K., Mercer J. P., and Donlon J. (1994). Amino acid composition of abalone and six macroalgae species. Aquaculture 128, 115–130. doi: 10.1016/0044-8486(94)90107-4
Makkar H. P. S., Tran G., Heuzé V., Giger-Reverdin S., Lessire M., Lebas F., et al. (2016). Seaweeds for livestock diets. Anim. Feed Sci. Technol. 212, 1–17. doi: 10.1016/j.anifeedsci.2015.09.018
Maribu I., Blikra M. J., Eilertsen K. E., and Elvevold K. (2024). Protein enrichment of Palmaria palmata using pulsed electric field and enzymatic processing. J. Appl. Phycology 36, 3665–3673. doi: 10.1007/s10811-024-03338-3, PMID: 39713085
Marrion O., Fleurence J., Schwertz A., Guéant J. L., Mamelouk L., and Villaume C. (2003). Improving digestibility of proteins of Palmaria palmata by physical processes and fermentation. J. Sci. Food Agric. 83, 850–855. doi: 10.1002/jsfa.1418
Mishra V. K., Temelli F., Ooraikul B., Shacklock P. F., and Craigie J. S. (1993). Lipids of palmaria palmata. Botanica Marina 36, 169–174. doi: 10.1515/botm.1993.36.2.169
Mišurcová L., Buňka F., Vávra Ambrožová J., Machů L., Samek D., and Kráčmar S. (2014). Amino acid composition of algal products and contribution to RDI. Food Chem. 151, 120–125. doi: 10.1016/j.foodchem.2013.11.040, PMID: 24423510
Monti M., Minocci M., Beran A., and Iveša L. (2007). First record of Ostreopsis cf. ovata on macroalgae in the Northern Adriatic Sea. Mar. pollut. Bull. 54, 598–601. doi: 10.1016/j.marpolbul.2007.01.013, PMID: 17368489
Morgan K. C., Wright J. L. C., and Simpson F. J. (1980). Review of chemical constituents of Palmaria palmata. Economic Bot. 34, 27–50. doi: 10.1007/BF02859553
Moroney N. C., O’Grady M. N., O’Doherty J. V., and Kerry J. P. (2014). Seaweed extracts in porcine diets and pork shelf life. Meat Sci. 96, 881–889. doi: 10.1016/j.meatsci.2013.09.019, PMID: 24200561
Moroney N., O’Grady M., Robertson R., Stanton C., O’Doherty J., and Kerry J. (2015). Laminarin and fucoidan extracts from Laminaria digitata in pig diets and pork quality. Meat Sci. 99, 132–141. doi: 10.1016/j.meatsci.2014.08.016, PMID: 25443973
Motoyama K., Suma Y., Ishizaki S., Nagashima Y., and Shiomi K. (2007). Molecular cloning of tropomyosins identified as allergens in six crustacean species. J. Agric. Food Chem. 55, 985–991. doi: 10.1021/jf062798x, PMID: 17263503
Mouritsen O. G., Dawczynski C., Duelund L., Jahreis G., Vetter W., and Schröder M. (2013). On the human consumption of dulse Palmaria palmata. J. Appl. Phycology 25, 1777–1791. doi: 10.1007/s10811-013-0014-7
Naseri A., Marinho G. S., Holdt S. L., Bartela J. M., and Jacobsen C. (2020). Enzyme assisted extraction and characterization of protein from Palmaria palmata. Algal Res. 47, 101849. doi: 10.1016/j.algal.2020.101849
Nielsen C. W., Holdt S. L., Sloth J. J., Marinho G. S., Sæther M., Funderud J., et al. (2020). Reducing iodine in Saccharina latissima by water blanching. Foods 9, 569. doi: 10.3390/foods9050569, PMID: 32375299
Nowak D. and Jakubczyk E. (2020). The freeze drying of foods and the effect on physical properties. Foods 9, 1488. doi: 10.3390/foods9101488, PMID: 33080983
Øverland M., Mydland L. T., and Skrede A. (2019). Marine macroalgae as protein and bioactive sources in feed for monogastric animals. J. Sci. Food Agric. 99, 13–24. doi: 10.1002/jsfa.9143, PMID: 29797494
Parodi A., Leip A., De Boer I. J. M., Slegers P. M., Ziegler F., Temme E. H. M., et al. (2018). The potential of future foods for sustainable and healthy diets. Nat. Sustainability 1, 782–789. doi: 10.1038/s41893-018-0189-7
Pinna C., Vecchiato C. G., Grandi M., Stefanelli C., Zannoni A., and Biagi G. (2021). Seaweed supplementation and fecal microbiota in adult dogs. Animals 11, 2234. doi: 10.3390/ani11082234, PMID: 34438692
Premarathna A. D., Ahmed T. A. E., Kulshreshtha G., Humayun S., Darko C. N. S., Rjabovs V., et al. (2024). Polysaccharides from red seaweeds and effects of extraction methods. Food Hydrocolloids 147, 109307. doi: 10.1016/j.foodhyd.2023.109307
Premier Atlas (2025). Ingredient matrix (Brereton Business Brereton Business Park, The levels, Rugeley, Staffordshire, WS15 1RD, UK: Premium Nutrition). www.premiernutritionatlas.co.uk.
Rhodes L., Adamson J., Suzuki T., Briggs L., and Garthwaite I. (2000). Toxic marine epiphytic dinoflagellates in New Zealand. New Zealand. J. Mar. Freshw. Res. 34, 371–383. doi: 10.1080/00288330.2000.9516939
Rødde R. S., Hagen S., Vårum K. M., Larsen B. A., and Myklestad S. M. (2004). Seasonal and geographical variation in Palmaria palmata composition. Botanica Marina 47, 125–133. doi: 10.1515/BOT.2004.012
Romay C., González R., Ledón N., Remirez D., and Rimbau V. (2003). C-phycocyanin and antioxidant, anti inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 4, 207–216. doi: 10.2174/1389203033487216, PMID: 12769719
Sakai S., Komura Y., Nishimura Y., Sugawara T., and Hirata T. (2011). Inhibition of mast cell degranulation by phycoerythrin and phycoerythrobilin from Porphyra yezoensis. Food Sci. Technol. Res. 17, 171–177. doi: 10.3136/fstr.17.171
Sakon N., Sadamasu K., Shinkai T., Hamajima Y., Yoshitomi H., Matsushima Y., et al. (2018). Foodborne outbreaks linked to norovirus contaminated nori, Japan 2017. Emerging Infect. Dis. 24, 920–923. doi: 10.3201/eid2405.171733, PMID: 29664371
Sears J. R. (1998). NEAS keys to the benthic marine algae of the northeastern coast of North America (Dartmouth, MA: Northeast Algal Society).
Sekar S. and Chandramohan M. (2008). Phycobiliproteins as a commodity. J. Appl. Phycology 20, 113–136. doi: 10.1007/s10811-007-9188-1
SpecialChem (2024).Palmaria palmata extract, INCI page. Available online at: https://www.specialchem.com/cosmetics/inci-ingredients/palmaria-palmata-extract.
Steinke J. W., Platts-Mills T. A., and Commins S. P. (2015). The alpha gal story, lessons from connecting the dots. J. Allergy Clin. Immunol. 135, 589–596. doi: 10.1016/j.jaci.2014.12.1947, PMID: 25747720
Stévant P., Marfaing H., Dumay J., Fleurence J., Sandbakken I., Chapman A., et al. (2023). Concise review of dulse Palmaria palmata. J. Appl. Phycology 35, 3923–3950. doi: 10.1007/s10811-022-02899-5
Stévant P., Marfaing H., Rustad T., Sandbakken I., Fleurence J., and Chapman A. (2017). Nutritional value of Alaria esculenta and Saccharina latissima and effects of short term storage. J. Appl. Phycology 29, 2417–2426. doi: 10.1007/s10811-017-1130-1
Stévant P., Marfaing H., Rustad T., Sandbakken I., Fleurence J., Chapman A., et al. (2020a). Data on sensory characteristics and composition of dulse after dry and semi dry storage. Data Brief 33, 106343. doi: 10.1016/j.dib.2020.106343, PMID: 33024802
Stévant P., Olafsdottir A., Deleris P., Dumay J., Fleurence J., Ingadottir B., et al. (2020b). Semi dry storage as a maturation process for dulse. Algal Res. 51, 102048. doi: 10.1016/j.algal.2020.102048
Subbiah V., Duan X., Agar O. T., Dunshea F. R., Barrow C. J., and Suleria H. A. R. (2023). Drying techniques and phenolic compounds in Australian beach cast brown seaweeds. Algal Res. 72, 103140. doi: 10.1016/j.algal.2023.103140
Subramoniam A., Asha V. V., Nair S. A., Sasidharan S. P., Sureshkumar P. K., Rajendran K. N., et al. (2012). Anti inflammatory activities of chlorophyll a and inhibition of TNF alpha expression. Inflammation 35, 959–966. doi: 10.1007/s10753-011-9399-0, PMID: 22038065
Teas J., Pino S., Critchley A., and Braverman L. E. (2004). Variability of iodine content in edible seaweeds. Thyroid 14, 836–841. doi: 10.1089/thy.2004.14.836, PMID: 15588380
Tobacman J. K. (2001). Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ. Health Perspect. 109, 983–994. doi: 10.1289/ehp.01109983, PMID: 11675262
Usov A. I. (2011). “Polysaccharides of the red algae,” in Advances in Carbohydrate Chemistry and Biochemistry, vol. 65 . Ed. Horton D. (Academic Press), 115–217. doi: 10.1016/B978-0-12-385520-6.00004-2, PMID: 21763512
Wong K. and Cheung P. C. (2001a). Influence of drying treatment on three Sargassum species. J. Appl. Phycology 13, 43–50. doi: 10.1023/A:1008149215156
Wong K. and Cheung P. C. (2001b). Influence of drying treatment on Sargassum species. Protein extractability, in vitro protein digestibility and amino acid profile. J. Appl. Phycology 13, 51–58. doi: 10.1023/A:1008188830177
Xu M., Zhang Y., Wu B., Zhang Y., Qiao M., Singh G., et al. (2024). Critical review of Palmaria palmata and its bioactive compounds in the omics era. Algal Res. 82, 103606. doi: 10.1016/j.algal.2024.103606
Yang Y., Zhang Y., He X., Huan F., Chen J., Liu M., et al. (2025). An overview of seafood allergens and allergenicity elimination processing techniques. Foods 14, 2241. doi: 10.3390/foods14132241, PMID: 40646992
Yousof S. M., Alghamdi B. S., Alqurashi T., Alam M. Z., Tash R., Tanvir I., et al. (2023). Palmaria palmata as a prebiotic in a mouse model of multiple sclerosis. Pharmaceuticals 16, 1355. doi: 10.3390/ph16101355, PMID: 37895826
Yuan Y. V., Carrington M. F., and Walsh N. A. (2005). Extracts from dulse are effective antioxidants and inhibitors of cell proliferation in vitro. Food Chem. Toxicol. 43, 1073–1081. doi: 10.1016/j.fct.2005.02.012, PMID: 15833383
Keywords: Palmaria palmata, red seaweed, dulse, processing, monogastric feed, circular feed ingredient, nutrient composition
Citation: Khattak F, Mkrtchyan HV, Smallman D, Estridge P, Allen MJ, Short F, Sutcliffe M and Houdijk JGM (2025) From coastline to feed trough: unlocking Palmaria palmata feed potential for monogastric farm animals. Front. Anim. Sci. 6:1717400. doi: 10.3389/fanim.2025.1717400
Received: 01 October 2025; Accepted: 31 October 2025;
Published: 19 November 2025.
Edited by:
Ravikanthreddy Poonooru, University of Missouri, United StatesReviewed by:
Babirye Khadijah, Islamic University in Uganda, UgandaJelena Vujetić, University of Novi Sad, Serbia
Copyright © 2025 Khattak, Mkrtchyan, Smallman, Estridge, Allen, Short, Sutcliffe and Houdijk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farina Khattak, ZmFyaW5hLmtoYXR0YWtAc3J1Yy5hYy51aw==
 Farina Khattak
Farina Khattak Hermine V. Mkrtchyan
Hermine V. Mkrtchyan Duncan Smallman3
Duncan Smallman3