- 1Climate & Environment Department, NORCE Norwegian Research Centre, Bergen, Norway
- 2Energy & Technology Department, NORCE Norwegian Research Centre, Bergen, Norway
- 3Department of Biological Sciences, University of Bergen, Bergen, Norway
The spiny dogfish (Squalus acanthias) is a common shark species found along the Norwegian coast. This coast is also utilized for salmon farming, and farmers report incidents of spiny dogfish biting through nets and often entering the fish cages. This causes breaches that allow farmed salmon to escape leading to both financial loss and an ecological risk to endangered wild salmon populations. Unfortunately, the extent and impact of such incidents has not been studied. Here, we conducted survey-based research among the fish farms, unravelling geographical distribution, cause, frequency and impact of spiny dogfish incidents in Norway. We quantified the experiences of fish farmers regarding spiny dogfish incidents and found that most incidents were localized in southern and western Norway during autumn and winter periods. Most spiny dogfish attacks are in groups, primarily targeting the base of fish cages and often attacking the same cage multiple times. These attacks are mostly associated with the presence of dead fish in the cages; the timely removal of carcasses largely mitigates these incidents. However, considering the impact of escapees on wild fish populations, combined with periods of elevated fish mortality within the cages when it is difficult to rapidly remove dead fish, multiple mitigation approaches are necessary. Moreover, spiny dogfish is listed as a vulnerable species and an effective measure in keeping them away from sea cages will reduce their mortality associated with fish farms. Hence, we also discuss suitable shark-deterrents as mitigating measures without harming either the spiny dogfish or the farmed fish.
Introduction
The spiny dogfish (Squalus acanthias) is Norway’s most common shark species and is found along the entire Norwegian coast. Unfortunately, its interaction with aquaculture installations poses both financial and ecological challenges. Fish farmers including salmon farmers operating on the Norwegian coasts, and in particular in Vestland and Rogaland municipalities, have reported spiny dogfish attacks where holes were torn into sea cages housing salmon (Fiskeridirektoratet, 2018). These attacks can lead to the escape of the farmed fish and incur significant expenses to repair the damage to cages and towards capture of escaped salmon. The spiny dogfish are attracted by dead fish that sink to the bottom of the cage, but in addition have been reported to hunt and harm live salmon. Therefore, this is both a financial and a fish welfare challenge. Reducing or eliminating spiny dogfish attacks at aquaculture installations will lead to a reduced number of holes in the cages and reduce the risk of salmon escape incidents. Wild Atlantic salmon is listed as near threatened on the International Union for Conservation of Nature (IUCN) Red List (Darwall, 2022) and Norwegian Red List (Hesthagen et al., 2021). Escaped farmed salmon pose a threat to wild salmon populations through interbreeding with the wild population, transfer of disease and parasites, predation, competition for resource and interference with natural behavior of wild salmon (Forseth et al., 2017). Moreover, there are direct economic benefits for the farming industry to reduce the incidents of holes in the cages, both through reduced repair costs and reduced salmon escape.
The spiny dogfish was on the IUCN Red List as endangered species until 2023 and with signs of improving stock size, it is currently listed as vulnerable (Finucci et al., 2020). Although it is a listed as vulnerable in the Norwegian Red List (Hesthagen et al., 2021), the Norwegian government has recently allowed fishing of spiny dogfish. IUCN recommends fishing quota for spiny dogfish of 22309 tonnes for 2025 and 22594 tonnes for 2026 in the North-East Atlantic and no more than 231 tonnes be fished in 2025 and in 2026 in Norway (ICES, 2022). Nonetheless, considering that spiny dogfish is a slow growing species characterized by late maturity and slow gestation (Ketchen, 1972; Nammack et al., 1985), their stock size remains under pressure. Keeping spiny dogfish away from fish farms could work as a precautionary measure to reduce the mortality of the vulnerable species.
There are limited studies on the subject of shark interaction with fish farms and only scarce information in the scientific literature on the effectiveness of shark deterrents for the spiny dogfish in such settings. Here, using a semi-quantitative method, we have mapped the incidents and impact of spiny dogfish interaction with aquaculture installations in Norway. Furthermore, based on the available literature for related species, and the experience of fish farmers and other stakeholders, we discuss possible sustainable mitigation measures that neither harm the farmed fish or the spiny dogfish.
Methods
Subjects, questionnaire, and procedures
This study was carried out from November 2021 to June 2022. Initial insights into the potential causes and impacts of spiny dogfish interactions with aquaculture were gathered from published literature, newspapers, and communications with fish farmers. Relevant literature, in English and Norwegian, were collected using Google Scholar using a combination of keywords Spiny dogfish AND/OR Aquaculture AND/OR Shark repellent AND/OR shark proof AND/OR Electromagnetic pulse AND/OR Acoustic deterrent AND/OR olfactory deterrent AND/OR visual deterrent. The first 50 upcoming articles published between 1950–2021 was selected. For news reports in Norwegian news media, keywords “pigghå” OR “hai” were used on Google News Norway. Fish farmers included production managers, fish health managers and leaders from affected fish farms and/or fish farms in the affected areas reported in the newspapers. Next, we employed a qualitative study design incorporating Key Informant Questionnaire Interviews and Focused Group Discussions. Based on the initial interviews and discussion, we created five categories of quantifiable questionnaires: these focused on (1) extent of spiny dogfish problem, (2) seasonality of sighting and attacks of spiny dogfish, (3) behavior of spiny dogfish in relation to the fish cages, (4) factors attracting spiny dogfish, (5) awareness, handling and measures to avoid spiny dogfish incidents. A complete list of the questionnaires is found in Supplementary Table 1. Finally, fish farmers, production managers, and operation managers from nine different production areas in Norway were invited to participate in this survey via personal emails and through aquaculture cluster organizations. Fish farms owned by a single company but located in different production areas were considered as separate entities. A total of 34 fish farms completed the electronic questionnaire and were given the option for further contact. Fish farmers who wanted to share their experience with regards to anti-shark measures or specific experiences on behavior of spiny dogfish in relation to their farm, were contacted for follow-up discussions using Microsoft Teams.
Analysis and interpretation
All data analyses were performed on responses from the survey. The study aimed to comprehensively explore the perspectives on ecological and industrial challenges rather than comparing differences among the study participants. The questionnaire featured categorically structured answers with options to provide additional information. The responses were visualized using frequency diagrams. We employed an empirical, data-driven, and inductive approach. The responses from the questionnaire and follow-up interviews were discussed with the project team and verified by cross-referencing the questionnaire comments with interview notes.
Results
Geographical distribution of spiny dogfish incidents
Several news articles including a report from Fiskeridirektoratet (2018) in Norway have reported sporadic incidents of spiny dogfish biting the nets and entering sea cages. To determine the occurrence and impact of such incidents at fish farms in Norway, a survey-based investigation was conducted (Supplementary Table 1). A summary of the distribution of affected fish farms and extent of the incidents is presented in Figure 1A; Supplementary Figure 1. 24 large and small fish farms across all production areas representing different geographical areas and locations in Norway responded to this questionnaire (Figures 1B, C).
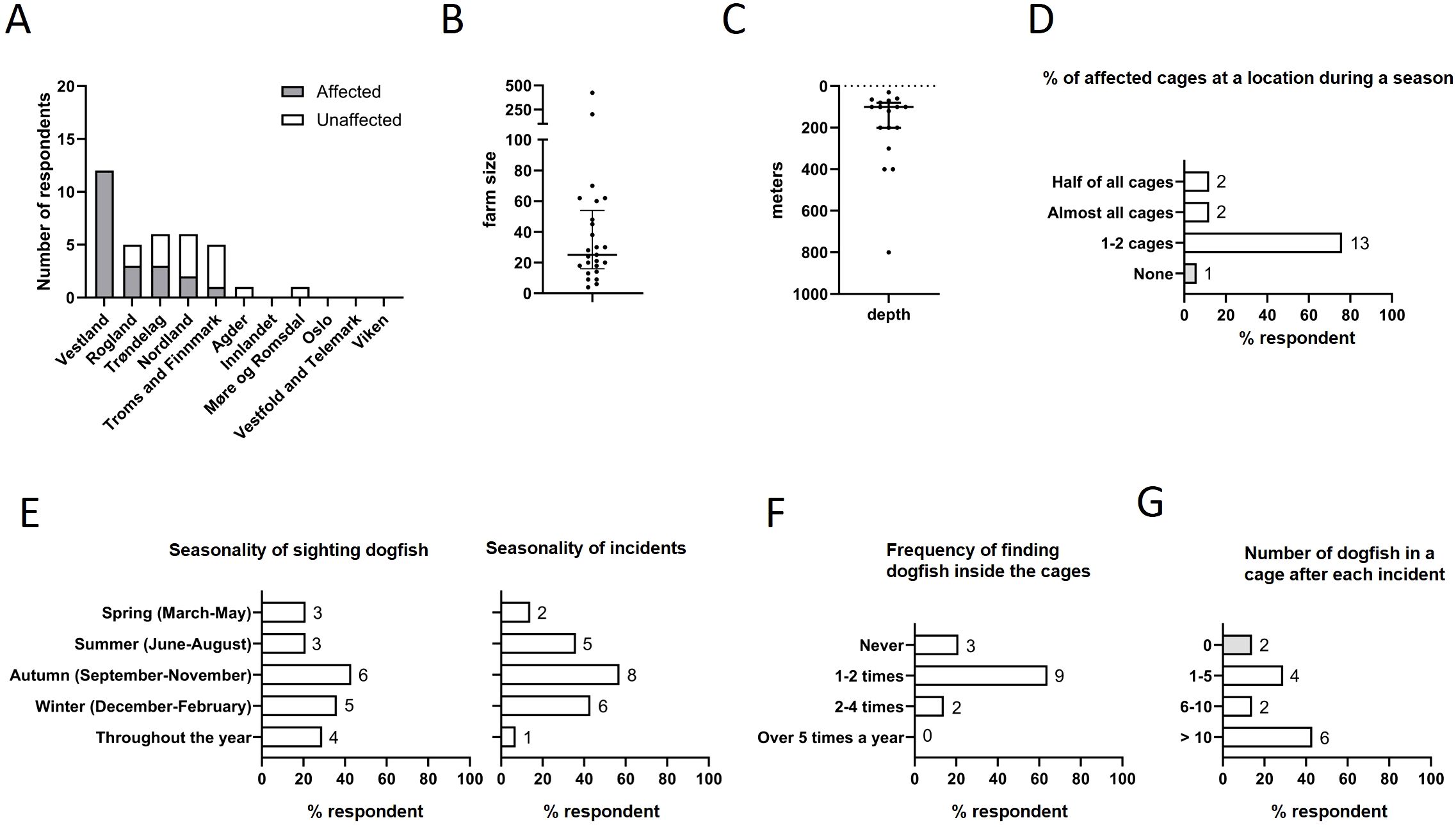
Figure 1. Geographical distribution and seasonality of spiny dogfish interactions with fish farms in Norway. (A) Affected fish farms are largely located in the Vestland, Rogaland, Trøndelag and Nordland regions. (B) Size of fish farms of the respondents. Both small and large fish farms responded to the survey (C) Depth underlying the fish farms varied from relatively shallow depths of 50 meters up to 800 meters. (D) Fraction of affected cages at a site during a season. (E) Seasonality of sighting of dogfish and incidents of dogfish in fish farms. (F) Frequency of finding dogfish in cages. (G) Number of dogfish in cages per incident. For (D–G), numbers to the right of columns indicate the number of respondents.
Out of the 24 respondents, 10 respondents (42%) reported no incidence of spiny dogfish in their fish cages while 14 respondents (58%) reported that they have had such incidents in the last 2–5 years (Figure 1A). The affected farms were predominantly located in Vestland (Bømlafjorden, Hardangerfjorden, Sørfjorden, Sognefjorden, Sunnfjorden, Høgsfjorden, Frøysjøen, Bjorøyosen), Rogaland (Boknafjorden, Vindafjord, Ryfylke, Nedstrandsfjorden, Hjeltefjorden) and Trøndelag (Rørvik, Namsenfjorden, Ånholmen). The reported depth of the water lying beneath fish farms ranged from 50m to 400m with an average depth of 147.1 m (Figure 1C).
All respondents in the Vestland area reported that they had incidents of spiny dogfish in their fish farms. These incidents had varying impacts (Figure 1D). The majority of respondents registering dogfish attacks (10 out of 14, 71%) reported that typically 1–2 cages were attacked during any single incident; 14% (2 of 14) reported that over half of all cages were affected while another 14% (2 of 14) reported that all cages at a location were attacked. Interestingly, one respondent (7%) reported that none of the cages were damaged; however, this farm still had to take measures to avoid spiny dogfish attacking the cages. Some commented that they have taken measures that reduced the number of affected cages.
Seasonality of spiny dogfish incidents
The sighting and incidents of spiny dogfish incidents were reported to be seasonal (Figure 1E). They were most frequently observed around the fish farms during autumn and winter, however in some sites they had been reported to be present throughout the year. The incidents of dogfish attack followed a similar pattern, with autumn and winter months presenting a higher risk. Farmers report that these sharks in most cases created a hole and entered the cages; 64% (9 of 14) reported that dogfish were inside the fish cages 1–2 times a year and 14% (2 of 14) of the respondents reported that dogfish managed to get inside the cages 2–5 times a year; 21% (3 of 14) responded that the dogfish had damaged some of the nets but had never managed to get inside the fish cages (Figures 1F, G).
Characteristics of the spiny dogfish and the affected cages
The reported size for spiny dogfish was between 50–120 cm with 70–80 cm length being the most frequent; sex was not noted. Farmers usually did not perform further investigation on sex typing or physiological status such as pregnancy in the caught spiny dogfish. Almost all farmers report that spiny dogfish made a hole to get in the fish cages (12 of 14, 86%) (Figure 2A). The usual reported size of the hole in the cage was 10–15 cm, but sometimes the holes were larger than 25–40 cm; the holes were located mostly at the bottom (12 of 12, 100%) and middle (3 of 12, 25%) parts of the cage (Figure 2B). Almost all respondents had used nylon netting during the reported period. Age of the netting material varied from 2 months to 5 years. Hence, both new and old cage nets were affected.
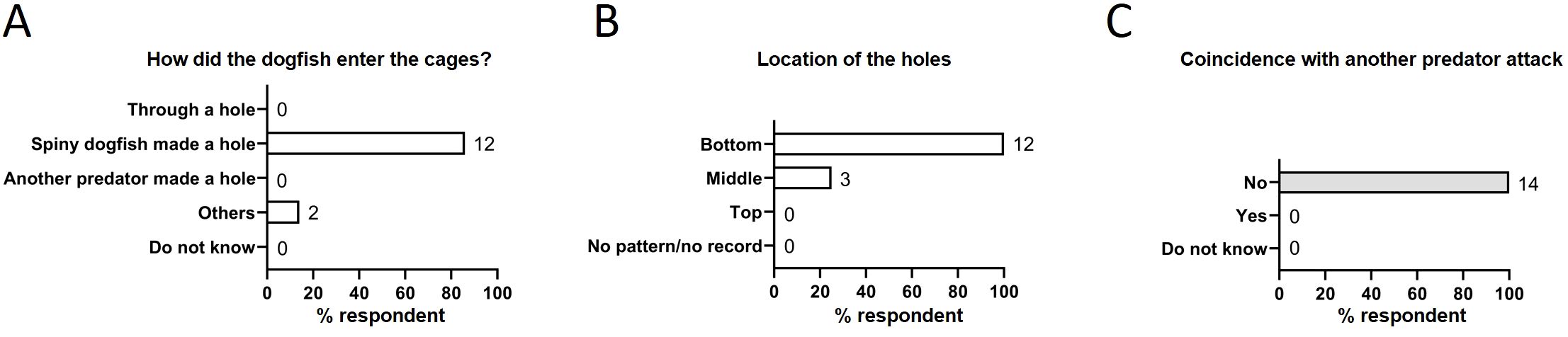
Figure 2. Empirical reports of spiny dogfish attack. (A) How the dogfish got into the cages. (B) location of the holes. (C) Coincidence with other predator attacks. Numbers to the right of columns indicate the number of respondents.
Some farmers have started using high density polyethylene (HDPE) nets and other marketed shark safe nets. Although evidence is not presented here, respondents reported fewer incidents of spiny dogfish getting through these nets. Use of innovative double netting cage design such as a skirt-like design was also reported; this type of netting provides an additional barrier against the sharks and allowed sharks to be released if caught between the inner and outer net. Nonetheless, farmers followed established routines for cleaning dead fish in all these cages and used surveillance techniques to detect damage to the cages.
What attracts spiny dogfish to fish farms
Next, we investigated the attraction cues for spiny dogfish to a specific cage. We asked whether the incidents coincided with another predator attack. All respondents reported that the spiny dogfish incidents did not coincide with another predator incident (Figure 2C). To identify the factors that may attract these sharks to a particular fish farm, we investigated the correlation of dogfish incidents with selected parameters of farm operation. Delousing and sea transfer of smolts are the most stressful operations for farmed salmon and are associated with high mortality. Despite this, majority of respondents (8 of 14, 57%) thought that the spiny dogfish attacks are not related to any farm operation, including delousing (Figure 3A) or transfer of smolts (Figure 3B). Interestingly, these incidents were mostly reported for fish cages with larger fish; 79% respondents (11 of 14) reported that the affected cages had fish of size above 2 kg (Figure 3C). Presence of dead fish in the cages was another factor that correlated with these incidents, with 64% respondents (9 of 14) reporting dead fish present in affected cages (Figure 3D). Once inside, dogfish ate the dead fish (6 of 9, 67%) (Figure 3E). They were generally not aggressive towards live salmon (9 of 14 respondents, 64%), except for one report where dogfish did attack live fish (Figure 3F).
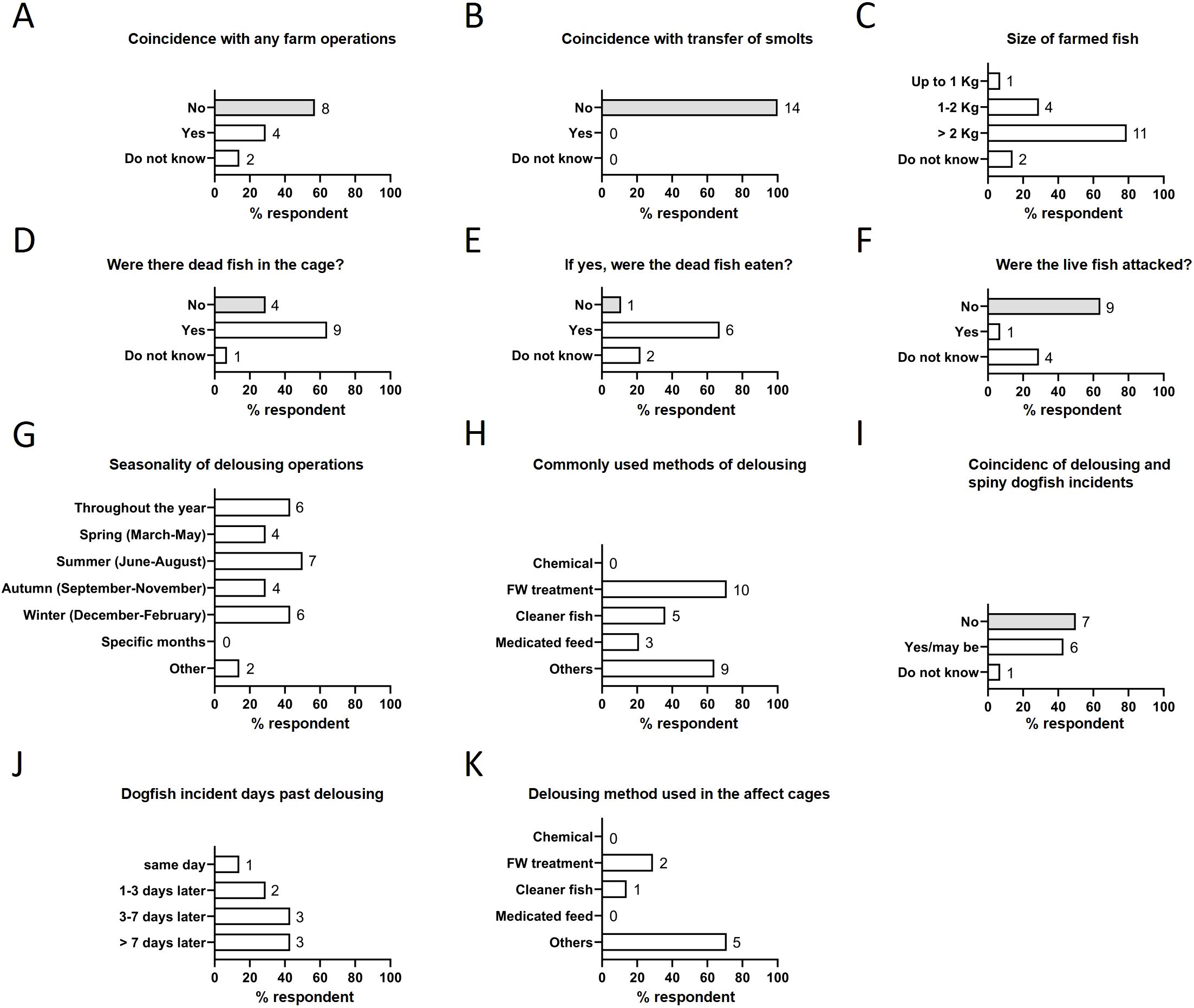
Figure 3. Behavior of spiny dogfish in relation to fish farms and their operations. (A) Farm operations vs dogfish incidents. (B) Transfer of smolts vs dogfish incidents. (C) Size of the farmed fish in the affected cages. (D) Presence of dead fish during dogfish attack. (E) Feeding behavior of dogfish in the cage. (F) Aggression of dogfish in the fish cage. Delousing and dogfish behavior (G-K). (G) Seasonality of delousing. (H) Delousing methods. (I) Opinion on delousing and dogfish incidents. (J) Presence of dogfish after the delousing operation. (K) Delousing methods used in affected cages. Numbers to the right of columns indicate the number of respondents.
Since the delousing operations are associated with stress response and mortality in farmed salmon, we further investigated whether dogfish incidents are directly correlated with delousing operations. In the farms that responded to the questionnaire, delousing operations were not seasonal and dependent on the farm site (Figure 3G). The majority (10 of 14, 71%) of respondents reported using freshwater treatment as a preferred delousing method, respondents also reported using medicated feed, cleaner fish and the thermal methods (Figures 3H, K); there was no observable relation between delousing method and dogfish incidents. Among the respondents, 47% (6 of 14) thought that spiny dogfish incidents correlate with delousing operations (Figure 3I); they reported holes in the cage within 7 days of delousing (Figure 3J). A possible explanation is that the smell from dead fish during the delousing operation attracts spiny dogfish to the location.
Fish farms use various lights in cages for different purposes, including monitoring fish and for controlling maturation. Since the majority of spiny dogfish incidents happened during autumn and winter (Figure 1E), we explored whether light conditions in the farm attracted dogfish to specific cages. Most respondents used anti-maturation lights (12 of 14, 86%) (Figure 4A); submerged lighting was used by all respondents (12 of 12, 100%) (Figure 4B). However, a majority (8 of 12, 67%) reported that dogfish incidents did not coincide with turning on anti-maturation lights in the cages (Figure 4C). Hence no relationship to light conditions was found.
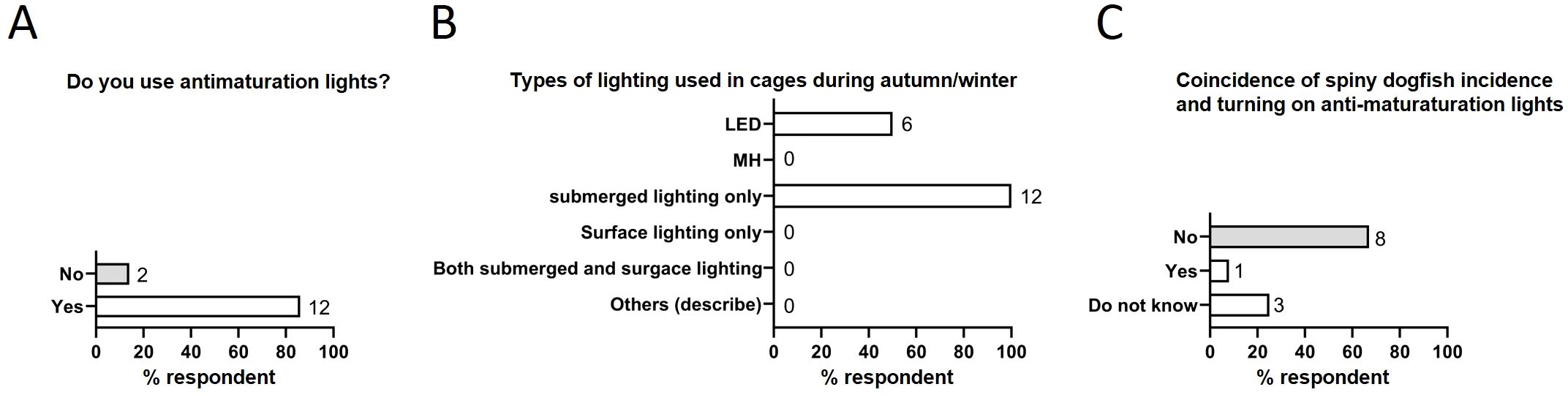
Figure 4. Behavior of spiny dogfish in relation to light conditions in fish cages. (A) Extent of use of anti-maturation light during autumn/winter. (B) Type of lights used in the affected cages. (C) Coincidence of turning on anti-maturation lights and incidence of spiny dogfish. Numbers to the right of columns indicate the number of respondents.
Spiny dogfish associated economic impact
Our study shows that these incidents are seasonal and occur with low frequency. Due to the possible substantial economic impact of damage to nets and cages, farmers take mitigating measures to both avoid and to contain the damage caused by these wild predators. When there is indication of nearby dogfish activity, cages are inspected with divers or remotely operated vehicles (ROV). The self-reported economic cost ranged from 50k to over a million NOK per affected site, with over half of the respondents reporting a typical cost of over 500k NOK per season and to have spent a total of 5–8 million NOK over last 5 years (2017-2022) per affected site. The cost is associated with the repair of damaged nets, surveillance for dogfish presence, handling dogfish after an incident, and losses due to escapees.
Discussion
Here, we present for the first time, a knowledge map on the occurrence and impact of spiny dogfish interaction with salmon farms in Norway. These occurrences are localized to specific geographical areas and are seasonal, typically occurring during November-March. The spiny dogfish are attracted to dead fish at the bottom of cages, often in connection with delousing of operations which can generate high mortality in the treated farmed fish. The dogfish typically attack during darkness and select one or several but not all cages within an installation. Once inside the cages, they are observed to display a calm swimming pattern and typically feed on dead fish. There have been isolated reports of attacks on live salmon within the cages.
Questions remain on stock size, distribution, and behavior of spiny dogfish in relation to the frequency of reported incidents at the fish farms. Our study finds that the incidents are mostly localized to western and southern Norway. A longline capture survey, using baited hooks, has previously reported a similar distribution of spiny dogfish along Norwegian costs during autumn (October-November); high catch rates were observed at depths under 100 meters (Andrade et al., 2024). The reported average size of spiny dogfish were 81.5 cm and 72.3 cm for female and male, respectively. In our study, fish farmers reported a similar size of dogfish found inside the cages. A study by Albert et al. (2019) has investigated the composition of spiny dogfish populations at different regions of the Norwegian coast during 2014-2018. It analyzed landing rate and seasonality in samples collected from gillnets, longlines and trawls and found a similar distribution and seasonality of dogfish as that of geographical and seasonal distribution of spiny dogfish incidents.
The findings here indicate that the dead and decaying fish in the cages act as the main attractant. Attacks are less frequent if dead fish are removed regularly. Good operational housekeeping therefore appears to contribute to mitigation of dogfish-related net damage. A recent telemetry study has observed the vertical movements patterns in female spiny dogfish. An oscillatory diel vertical migration pattern was observed during winter and spring; descents at dawn and ascent at dusk were observed for spiny dogfish (Klöcker et al., 2024). This vertical migration behavior has been linked to foraging activity. Presence of dead fish in the cages during their ascent will present a risk to the fish farms. Due to apparent increased activity during night, Norwegian Fiskeridirektoratet had recommended avoiding delousing operations during darkness (Fiskeridirektoratet, 2018).
Another source of attraction to the cages for both spiny dogfish and other fish species is unconsumed food pellets falling through the net. In (Gaitán-Espitia et al., 2017) the stomach contents of over 100 spiny dogfish, collected from by-catch in Chilean waters in the vicinity of salmon pens, were analyzed. Over half of the specimens had salmon feed pellets as part of their stomach contents, indicating that spiny dogfish are also feeding directly on waste pellets at the pen locations. There is a further possibility that larger wild predatory fish such as spiny dogfish are initially attracted to salmon farms due to aggregations of smaller prey fish feeding on these unconsumed pellets and other waste material.
Many of the wild fish that aggregate at salmon farms stay within 25 m of the cages (Uglem et al., 2014). In Mediterranean open-cage fish farming, where bluefish Pomatomus saltatrix is present as a wild species, there have been incidents where these create holes in the net wall to enter the cage and prey on the live farmed fish (Arechavala-Lopez et al., 2015). Similar occurrences have incidentally been documented for salmon farming; spiny dogfish are likely to have allowed escapes of farmed salmon through holes they created in the net during attempts to prey on dead salmon through the nets (Fiskeridirektoratet, 2018; Moe et al., 2005). One farmer reported that mackerel sturgeons (Thunnus thynnus) were observed chasing mackerel when one such dogfish incident happened. Nonetheless, it was suggested that in most cases spiny dogfish made the holes in the cages.
Destruction of net material by spiny dogfish allows them access to the cages and dead fish and provides farmed salmon an exit through which to escape. Improved netting material with greater resistance to dogfish attacks can provide greater security. Fish farmers currently use several types of netting material including Nylon P6, Econets made from HDPE or PET, Predator X Dyneema®nettings, Sapphire® Garware. These provide some of the best cut resistance in the market and have been used for a long time against shark attacks. Life cycle, fouling and economy must be further analyzed systematically to determine if a larger adoption of such netting material is feasible. Double netting and innovative net/cage design may also provide solutions to cage attack issues. As reported by several fish farmers, a skirt like net that provides a barrier to the fish cage, and which can be easily closed or opened has provided some protection from sharks damaging the fish cages. There is currently an absence of data on the durability of the different net materials and systems used in the cages when challenged with net attack behavior of spiny dogfish. A systematic study on effectiveness of such nets and cage designs is warranted.
An effective deterrent may reduce the attraction of farm sites to dogfish or interfere with their motivation or feeding behavior. Shark deterrents may consist of physical barriers, or target their sensory systems, using visual [e.g. strobe light (Ryan et al., 2018) and bubble curtain as barrier -reviewed in (Hart and Collin, 2015)], auditory [e.g. sound of predators such as Orca or an artificial frequency (Chapuis et al., 2019; Myrberg, 2001; Ryan et al., 2018)], olfactory [e.g. paradaxin’ derived from the moses sole (Pardachirus marmoratus, Soleidae), sodium lauryl sulphate and semiochemical compounds (O’Connell et al., 2014b; Sisneros and Nelson, 2001)] or electromagnetic senses by use of electromagnetic field/stimuli or electromagnetic or magnetic field using rare-earth metals (Courtney et al., 2015; Hart and Collin, 2015; Smith, 1974, 1991; Tallack and Mandelman, 2009). Shark barriers and shark deterrents have previously been developed to keep specific species of sharks away from bathing areas and to offer a degree of personal protection from shark attacks for swimmers, divers and surfers (Blount et al., 2021; Clarke et al., 2024; Huveneers et al., 2013; Kempster et al., 2016; Marcotte and Lowe, 2008; McPhee et al., 2021; O’Connell et al., 2014a). Some of these deterrents have also been tested to keep shark species away from bait and catch in line and net fishing activities (Godin et al., 2013; Howard et al., 2018; Kaimmer and Stoner, 2008; Spaet et al., 2010; Stoner and Kaimmer, 2008; Tallack and Mandelman, 2009). Existing shark deterrents show varying degree of effectiveness depending on species (Riley et al., 2022; Ryan et al., 2018) and geographical area, and none of the measures provide a full deterrent protection (Clarke et al., 2024; Gauthier et al., 2020; McPhee et al., 2021). Adapting the existing deterrents for use on fish farms requires both a basic understanding of the impact of these deterrents on spiny dogfish behavior and physiology, together with testing in the field for their effectiveness. Much of the past research has focused on developing affordable and effective shark deterrents for use in leisure activities and commercial long-line fisheries. Our survey results suggest that there is an equally valid need to reduce interactions between sharks and aquaculture cages. The technology and solutions developed for one area of application could be adapted to help mitigate problems in the aquaculture industry.
A commercially available and effective shark deterrent would need to be specifically aimed at the target species. Previous repellent research has been successful in causing withdrawal response in some species, while not affecting others (Chapuis et al., 2019; Gauthier et al., 2020). Factors such as age, sex, previous experience, and environmental conditions present long-term challenges on the effect of a repellent. Spiny dogfish might easily habituate to cues eliciting withdrawal when repeatedly exposed or ignore unpleasant stimulants if they’re in a feeding frenzy. The response intensities may vary depending on the age, size and geographical location of the shark. Validation of several repellents targeting specific age groups or coastal regions is needed. Ecological impact on the surrounding habitat must be carefully considered when developing such deterrents, together with ensuring that species farmed within aquaculture installations are not adversely affected. Once proven to be effective, the method(s) must be cost effective and technically possible to install in or near sea cages.
Several types of deterrents can be considered for their suitability for use in and around aquaculture installations. Chemical deterrents that have shown promising results as a repellent in the past, such as SDS, needed to be delivered in a magnitude larger than desirable to be effective (Baldridge, 1990; Hart and Collin, 2015). These are difficult to restrict to a specific area in an aquatic environment. The intended recipient might not be the only one affected and it may do more harm to the farmed fish and/or the environment. Auditory stimuli methods provide easy temporal control of stimulus application; however, it may also harm non-target species including farmed and wild fish (Scott et al., 2020). Electro-magnetic stimuli have potentially a controllable short ranging effectiveness (<2 meter). Many marine animals including several species of crustaceans, fish, sea turtles, birds and mammals use geomagnetic cues (Nyqvist et al., 2020; Scott et al., 2020). Even though the intensity from EM deterrents rapidly reduces with distance, the impact of both short and long-term exposure to EM on marine species needs to be investigated. Future use of shark deterrents should be approached carefully to limit their adverse effects on the surrounding environment and non-target species.
Effective management of conflict between natural animal populations and human interests requires careful mapping of behavior and habitat use of the target species. Coastal populations of spiny dogfish appear to maintain a certain level of site association, in contrast, offshore populations appear highly migratory (Huse and Bakketeig, 2018; Olav Aasen, 1962). Facilities situated along the permanent migration routes of dogfish, especially those in fjords where they give birth, are more likely to experience encounters. Our current information on their migration patterns is outdated - the latest tagging experiments were conducted in the 50s and 70s (Huse and Bakketeig, 2018). Knowledge gaps remain concerning the migration pattern and habitat use for the Norwegian populations. Recent and ongoing telemetry studies will provide valuable new knowledge for understanding their behavior in relation to the habitat (Klöcker et al., 2024). This new knowledge should provide farmers with advance warning of when their farms are likely to be exposed to dogfish attacks and which measures are needed to effectively handle the situation. Such advances will benefit future expansion of aquaculture along the Norwegian coast. Although this study has focused on the Norwegian coast, its findings will have relevance in other maritime regions, such as coastal areas of Chile, Canada and the United States (McIntosh et al., 2022), where pen culture is developing in regions shared with spiny dogfish populations (Finucci et al., 2020; Stehlik, 2007).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
PL: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Data curation, Software. AO: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. SB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MB: Investigation, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Norwegian Seafood Research Fund (FHF grant number 901704).
Acknowledgments
We thank Fiona Provan and Naouel Gharbi for administrative support; Stiim Aqua Cluster and Sjømat Norge for help with distribution of questionnaire; the project advisory board for sharing their experience with spiny dogfish. As an obligation to funding agency (FHF), the above research is part of the project final report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/faquc.2025.1539610/full#supplementary-material
Supplementary Figure 1 | Geographical distribution of affected fish farms in Norway. Affected fish farms are largely located in the Vestland, Rogaland, Trøndelag and Nordland regions. Thickness of lines represent percentage of fish farms to total respondent in a particular area. Map sources http://www.norgeskart.no (CC BY 4.0).
SUPPLEMENTARY TABLE 1 | List of survey questionnaire.
References
Albert O. T., Junge C., Myrlund M. K., and Anderson E. (2019). Young mums are rebuilding the spurdog stock (Squalus acanthias L.) in Norwegian waters. ICES J. Mar. Sci. 76, 2193–2204. doi: 10.1093/icesjms/fsz156
Andrade H., Nilsen T., Vollen T., Harbitz A., Junge C., and Albert O. T. (2024). A longline survey for spurdog distribution and life history along the Norwegian coast. Fisheries Manage. Ecol. 31 (2), e12676. doi: 10.1111/fme.12676
Arechavala-Lopez P., Izquierdo-Gomez D., Uglem I., and Sanchez-Jerez P. (2015). Aggregations of bluefish Pomatomus saltatrix (L.) at Mediterranean coastal fish farms: seasonal presence, daily patterns and influence of farming activity. Environ. Biol. Fishes 98, 499–510. doi: 10.1007/s10641-014-0280-5
Baldridge H. D. Jr (1990). Shark repellent: not yet, maybe never. Military Medicine 155 (8), 358–61. doi: 10.1093/milmed/155.8.358
Blount C., Pygas D., Lincoln Smith M. P., McPhee D. P., Bignell C., and Ramsey O. (2021). Effectiveness against white sharks of the Rpela personal shark deterrent device designed for surfers. J. Mar. Sci. Technol. (Taiwan) 29, 582–591. doi: 10.51400/2709-6998.1594
Chapuis L., Collin S. P., Yopak K. E., McCauley R. D., Kempster R. M., Ryan L. A., et al. (2019). The effect of underwater sounds on shark behavior. Sci. Rep. 9 (1), 6924. doi: 10.1038/s41598-019-43078-w
Clarke T. M., Barnett A., Fitzpatrick R., Ryan L. A., Hart N. S., Gauthier A. R. G., et al. (2024). Personal electric deterrents can reduce shark bites from the three species responsible for the most fatal interactions. Sci. Rep. 14 (1), 16307. doi: 10.1038/s41598-024-66679-6
Courtney J., Courtney el, and Courtney M. (2015). Review of magnetic shark deterrents: hypothetical mechanisms and evidence for selectivity. Aquat. Sci. Technol. 3 (1), 70–81. doi: 10.5296/ast.v3i1.6670
Darwall W. R. T. (2022). “Salmo salar. The IUCN red list of threatened species 2023,” in IUCN Red List of Threatened Species. doi: 10.2305/IUCN.UK.2023-1.RLTS.T19855A67373433.en
Finucci B., Cheok J., Chiaramonte G. E., Cotton C. F., Dulvy N. K., Kulka D. W., et al. (2020). “Squalus acanthias. The IUCN red list of threatened species 2020,” in IUCN Red List of Threatened Species. doi: 10.2305/IUCN.UK.2020-3.RLTS.T91209505A124551959.en
Fiskeridirektoratet (2018). Pigghå og fare for rømming. Available online at: https://www.fiskeridir.no/Akvakultur/erfaringsbase-romming/erfaringshendelser/Pigghaa-og-fare-for-roemming (Accessed December 1, 2024).
Forseth T., Barlaup B. T., Finstad B., Fiske P., Gjøsæter H., Falkegård M., et al. (2017). The major threats to Atlantic salmon in Norway. ICES J. Mar. Sci. 74, 1496–1513. doi: 10.1093/icesjms/fsx020
Gaitán-Espitia J. D., Gómez D., Hobday A. J., Daley R., Lamilla J., and Cárdenas L. (2017). Spatial overlap of shark nursery areas and the salmon farming industry influences the trophic ecology of Squalus acanthias on the southern coast of Chile. Ecol. Evol. 7, 3773–3783. doi: 10.1002/ece3.2957
Gauthier A. R. G., Chateauminois E., Hoarau M. G., Gadenne J., Hoarau E., Jaquemet S., et al. (2020). Variable response to electric shark deterrents in bull sharks, Carcharhinus leucas. Sci. Rep. 10 (1), 17869. doi: 10.1038/s41598-020-74799-y
Godin A. C., Wimmer T., Wang J. H., and Worm B. (2013). No effect from rare-earth metal deterrent on shark bycatch in a commercial pelagic longline trial. Fisheries Res. 143, 131–135. doi: 10.1016/j.fishres.2013.01.020
Hart N. S. and Collin S. P. (2015). “Sharks senses and shark repellents,” in Integrative Zoology. 10, 38–64. doi: 10.1111/1749-4877.12095
Hesthagen T., Wienerroither R., Bjelland O., Byrkjedal I., Fiske P., Lynghammar A., et al. (2021). Vurdering av laks Salmo salar for Norge. Rødlista for arter 2021. Available online at: http://www.artsdatabanken.no/lister/rodlisteforarter/2021/12133 (Accessed December 2, 2024).
Howard S., Brill R., Hepburn C., Rock J., and Pol M. (2018). Microprocessor-based prototype bycatch reduction device reduces bait consumption by spiny dogfish and sandbar shark. ICES J. Mar. Sci. 75, 2235–2244. doi: 10.1093/icesjms/fsy098
Huse G. and Bakketeig I. E. (2018). “Ressursoversikten 2018,” in Fisken og havet. Available at: https://www.hi.no/hi/nettrapporter/fisken-og-havet/2018/ressursoversikten_2018_ny_til_web_1.
Huveneers C., Rogers P. J., Semmens J. M., Beckmann C., Kock A. A., Page B., et al. (2013). Effects of an electric field on white sharks: in situ testing of an electric deterrent. PLoS One 8 (5), e62730. doi: 10.1371/journal.pone.0062730
ICES (2022). “Spurdog (Squalus acanthias) in subareas 1–10, 12, and 14 (the Northeast Atlantic and adjacent waters),” in Report of the ICES Advisory Committe (ICES Advice 2022). doi: 10.17895/ices.advice.19753588
Kaimmer S. and Stoner A. W. (2008). Field investigation of rare-earth metal as a deterrent to spiny dogfish in the Pacific halibut fishery. Fisheries Res. 94, 43–47. doi: 10.1016/j.fishres.2008.06.015
Kempster R. M., Egeberg C. A., Hart N. S., Ryan L., Chapuis L., Kerr C. C., et al. (2016). How close is too close? The effect of a nonlethal electric shark deterrent on white shark behavior. PLoS One 11 (7), e0157717. doi: 10.1371/journal.pone.0157717
Ketchen K. S. (1972). Size at maturity, fecundity, and embryonic growth of the spiny dogfish (Sqratus acaithia.r) in British Columbia waters. J. Fish. Res. Bd. Canada 29, 1717–1723. Available at: www.nrcresearchpress.com.
Klöcker C. A., Albert O. T., Ferter K., Bjelland O., Lennox R. J., Albretsen J., et al. (2024). Seasonal habitat use and diel vertical migration in female spurdog in Nordic waters. Movement Ecol. 12 (1), 62. doi: 10.1186/s40462-024-00498-2
Marcotte M. M. and Lowe C. G. (2008). Behavioral responses of two species of sharks to pulsed, direct current electrical fields: testing a potential shark deterrent. Mar. Technol. Soc. J. 42, 53–61. doi: 10.4031/002533208786829133
McIntosh P., Barrett L. T., Warren-Myers F., Coates A., Macaulay G., Szetey A., et al. (2022). Supersizing salmon farms in the coastal zone: A global analysis of changes in farm technology and location from 2005 to 2020. Aquaculture 553, 73804. doi: 10.1016/j.aquaculture.2022.738046
McPhee D. P., Blount C., Lincoln Smith M. P., and Peddemors V. M. (2021). A comparison of alternative systems to catch and kill for mitigating unprovoked shark bite on bathers or surfers at ocean beaches. Ocean Coast. Manage. 201, 105492. doi: 10.1016/j.ocecoaman.2020.105492
Moe H., Gaarder R., Sunde L. M., Borthen J., and Olafsen K. (2005). Rømmingssikker not for torsk. Available online at: https://www.sintef.no/globalassets/upload/fiskeri_og_havbruk/publikasjoner/rapporter/rapport-_generell_endelig.pdf (Accessed May 21, 2025).
Myrberg A. A. (2001). The acoustical biology of elasmobranchs. Environ. Biol. Fishes 60, 31–45. doi: 10.1023/A:1007647021634
Nammack M. F., Musick J. A., and Colvocoresses J. A. (1985). Life history of spiny dogfish off the northeastern United States. Trans. Am. Fisheries Soc. 114, 367–376. doi: 10.1577/1548-8659(1985)114<367:lhosdo>2.0.co;2
Nyqvist D., Durif C., Johnsen M. G., De Jong K., Forland T. N., and Sivle L. D. (2020). Electric and magnetic senses in marine animals, and potential behavioral effects of electromagnetic surveys. Mar. Environ. Res. 155, 104888. doi: 10.1016/j.marenvres.2020.104888
O’Connell C. P., Andreotti S., Rutzen M., Meÿer M., Matthee C. A., and He P. (2014a). Effects of the Sharksafe barrier on white shark (Carcharodon carcharias) behavior and its implications for future conservation technologies. J. Exp. Mar. Biol. Ecol. 460, 37–46. doi: 10.1016/j.jembe.2014.06.004
O’Connell C. P., Stroud E. M., and He P. (2014b). The emerging field of electrosensory and semiochemical shark repellents: Mechanisms of detection, overview of past studies, and future directions. Ocean Coast. Manage. 97, 2–11. doi: 10.1016/j.ocecoaman.2012.11.005
Olav Aasen (1962). Norske pigghåmerkinger 1958-61. Available online at: https://imr.brage.unit.no/imr-xmlui/handle/11250/113779 (Accessed May 21, 2025).
Riley M., Bradshaw C. J. A., and Huveneers C. (2022). Long-range electric deterrents not as effective as personal deterrents for reducing risk of shark bite. ICES J. Mar. Sci. 79, 2656–2666. doi: 10.1093/icesjms/fsac199
Ryan L. A., Chapuis L., Hemmi J. M., Collin S. P., McCauley R. D., Yopak K. E., et al. (2018). Effects of auditory and visual stimuli on shark feeding behavior: the disco effect. Mar. Biol. 165 (1), 11. doi: 10.1007/s00227-017-3256-0
Scott K., Piper A. J., Chapman E. C., and Rochas C. M. (2020). Review of the effects of underwater sound, vibration and electromagnetic fields on crustaceans. Seafish Report. Available online at: https://www.seafish.org/document/?id=6ea84e37-c291-4769-8485-b3ac7786b29a (Accessed May 21, 2025).
Sisneros J. A. and Nelson D. R. (2001). Surfactants as chemical shark repellents: Past, present, and future. Environ. Biol. Fishes 60, 117–130. doi: 10.1023/A:1007612002903
Smith E. D. (1974). Electro-physiology of the electrical shark-repel lantt. Transactions of the South African Institute of Electrical Engineers, SAIEE 65 (8), 166–181.
Smith E. D. (1991). Electric shark barrier: initial trials and prospects. PowerEngineering 5, 167–176. doi: 10.1049/pe:19910036
Spaet J. L. Y., Kessel S. T., and Gruber S. H. (2010). Learned hook avoidance of lemon sharks (Negaprion brevirostris) based on electroreception and shock treatment. Mar. Biol. Res. 6, 399–407. doi: 10.1080/17451000903039749
Stehlik L. L. (2007). Essential fish habitat source document: spiny dogfish, Squalus acanthias, life history and habitat characteristics. 2nd edn (Woods Hole, MA: NOAA Technical Memorandum NMFS-NE-203).
Stoner A. W. and Kaimmer S. M. (2008). Reducing elasmobranch bycatch: Laboratory investigation of rare earth metal and magnetic deterrents with spiny dogfish and Pacific halibut. Fisheries Res. 92, 162–168. doi: 10.1016/j.fishres.2008.01.004
Tallack S. M. L. and Mandelman J. W. (2009). Do rare-earth metals deter spiny dogfish? A feasibility study on the use of electropositive “mischmetal” to reduce the bycatch of Squalus acanthias by hook gear in the Gulf of Maine. ICES J. Mar. Sci. 66, 315–322. doi: 10.1093/icesjms/fsn215
Keywords: spiny dogfish, behavior, escapee, conservation, fish welfare, management strategy
Citation: Lal P, Oosterkamp A, Bamber S and Brynildsrud ME (2025) Experience of spiny dogfish Squalus acanthias interactions with aquaculture installations in Norway. Front. Aquac. 4:1539610. doi: 10.3389/faquc.2025.1539610
Received: 04 December 2024; Accepted: 13 May 2025;
Published: 13 June 2025.
Edited by:
Matthew Ajemian, Florida Atlantic University, United StatesReviewed by:
Roger A. Rulifson, East Carolina University, United StatesCharles Bangley, Dalhousie University, Canada
Copyright © 2025 Lal, Oosterkamp, Bamber and Brynildsrud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pradeep Lal, cGxhbEBub3JjZXJlc2VhcmNoLm5v
†These authors have contributed equally to this work
 Pradeep Lal
Pradeep Lal Antonie Oosterkamp2†
Antonie Oosterkamp2†