Abstract
Black soldier fly (Hermetia illucens) larvae are insects capable of valorizing various waste streams into protein that has the potential to replace fish meal (FM) in fish diets. To evaluate the feasibility of replacing FM with black soldier fly meal (BSFM) in the diet of red drum (Sciaenops ocellatus) juveniles, a two-part study was conducted that included a palatability trial followed by an 8-week feeding trial with isonitrogenous and isoenergetic formulations. Red drum is a marine and brackish-water fish species found in the Western Atlantic, ranging from Massachusetts, southern Florida, and the Gulf to northern Mexico. The palatability trial was conducted with BSFM replacing FM in diets at 0% (Control), 25% (BSFM25), 50% (BSFM50), and 100% (BSFM100) levels. The palatability assessment showed that the red drum feeding response was statistically similar between the Control and BSFM25 diets. The feeding trial included the diets above plus BSFM replacing FM at 75% (BSFM75), and the effects on growth, feeding efficiency, whole-body biochemical composition, intestinal/liver histomorphology, and intestinal microbiome were assessed. Better growth and feeding efficiency (P<0.05) were observed in fish fed the BSFM25 diet compared to those fed the BSFM50, BSFM75, and BSFM100 diets. Whole-body saturated fatty acids increased with higher levels of BSFM in the diet, while the levels of arachidonic acid (ARA), eicosapentanoic acid (EPA), and docosahexaenoic acid (DHA) remained statistically similar in BSFM25 and BSFM50 compared to the Control. Liver histomorphology revealed increased hepatic vacuolization with increasing levels of BSFM. Intestinal microbiota presented high alpha diversity abundance among the treatments, but the fish fed the BSFM diets tended to have genera associated with chitinase and lipase activity. Overall, dietary FM replacement with BSFM is an adequate alternative protein source with replacement up to 50%, but levels over 75% caused a reduction in growth and hepatic lipid accumulation.
Highlights
-
Formulations with 10.7 g/100g (DM) BSFM showed no negative impact on red drum palatability responses.
-
Adequate BSFM inclusion ranges between 10.7 and 21.4 g/100g (DM) in diets for red drum growth and feeding efficiency.
-
Whole-body ARA, EPA, and DHA levels remained stable up to the BSFM50 treatment, ensuring nutritional benefits for human consumption.
1 Introduction
Seafood consumption has steadily increased over the years and its global demand will further expand due to population growth and the rising awareness of its health benefits (FAO et al., 2024). The contribution of aquaculture to total finfish and shellfish production has resiliently sufficed in meeting this demand. However, it has been estimated that millions of additional tons of seafood will be necessary in the coming years (FAO et al., 2024). Traditionally, the aquaculture industry utilizes fish meal (FM) obtained from wild pelagic fish species, such as herrings, sardines, and menhaden, as the main source of animal dietary protein (Tacon and Metian, 2008, 2015). This is because FM is considered the optimal protein source for aquafeed production due to its exceptional nutritional quality, including a well-balanced amino acid profile, high protein digestibility, and palatability (NRC, 2011). However, the growing disparity between the supply and demand of FM, along with its rising prices, exerts economic pressure on the aquaculture production sector (Siddaiah et al., 2023; Tacon and Metian, 2008). Consequently, overreliance on FM for feed formulation is increasingly recognized as an untenable strategy. Currently, the protein content in commercial aquafeeds is derived from a blend of FM, animal by-products, and plant-based ingredients, such as poultry by-products, blood meal, soybean meal, canola meal, and/or corn protein concentrate (Fisher et al., 2020). These formulations are designed to meet species-specific nutritional requirements while amending challenges related to antinutritional factors (ANFs), palatability, and imbalanced amino acid profile, and to changes in commodity prices and availability (Gatlin et al., 2007). Thus, there is urgent pressure to explore nutritional and alternative strategies for FM substitution and to develop cost-effective long-term aquafeed production systems that can enhance the growth and profitability of the aquaculture industry.
Black soldier fly (Hermetia illucens) larvae (BSFL) are the most extensively researched insect species as an alternative protein source for aquaculture diets (Hua, 2021; Priyadarshana et al., 2022). This is largely due to their ability to convert low-quality, low-cost waste substrate (food waste, animal manure, and various agricultural by-products) into nutrient-rich ingredients (Ellawidana et al., 2023; Makkar et al., 2014). The crude protein content in BSFL meal (BSFM) ranges from 42% to 60% and crude fat from 10% to 30%, depending on the processing methods and substrate growth media (NRC, 2011). This malleable nutritional content provides an opportunity for tailoring nutritional profiles for various aquaculture species (Wang et al., 2019). Additionally, the significance of BSFM rises when considering its FM-like amino acid composition and retention of various essential minerals for fish, such as iron, zinc, potassium, phosphorus, manganese, and magnesium (Henry et al., 2015; NRC, 2011).
The growth performance benefits of BSFM have been extensively reported across several finfish species, including Atlantic salmon (Salmo salar), gilthead seabream (Sparus aurata), rainbow trout (Oncorhynchus mykiss), largemouth bass (Micropterus salmoides) (Fischer et al., 2022), and red drum (Sciaenops ocellatus) (Fisher et al., 2020; Moutinho et al., 2024; Randazzo et al., 2021; Yamamoto et al., 2022). However, the optimal inclusion level in formulated diets that support proper growth while avoiding adverse physiological effects such as disruptions in metabolism and/or liver/intestine histomorphology abnormalities varies depending on the species (Fischer et al., 2022; Randazzo et al., 2021; Romano et al., 2023; Siddaiah et al., 2023).
The incorporation of BSFM as an alternative protein source for carnivorous fish has garnered interest in aquaculture largely due to their typically high protein requirements and sensitivity to ANF’s within various plant-based proteins (Henry et al., 2015). Research indicates that BSFM can maintain growth performance in Atlantic salmon (Salmo salar) (Belghit et al., 2019) and European sea bass (Dicentrarchus labrax) without negatively affecting feed efficiency (Magalhães et al., 2017). Yamamoto et al. (2022) reported that replacing 65% of FM with BSFM resulted in different growth outcomes depending on the larvae’s substrate. Specifically, fish fed BSFM derived from larvae reared on spent brewer’s grain showed significantly lower weight gain, while those fed BSFM from larvae raised on the Gainesville diet exhibited growth comparable to the control group (Yamamoto et al., 2022). While a reduction in omega-3 fatty acid levels is often observed in fish fed diets with high BSFM inclusions (Lock et al., 2016; Yamamoto et al., 2022), enrichment strategies have been proposed to counterbalance such effects (Erbland et al., 2020). Histology assessments in rainbow trout revealed that levels of BSFM at 22.7–45 g/100g dry matter (DM) to replace or complement vegetable protein-rich ingredients in diets deprived of FM showed no adverse liver or intestinal changes (Randazzo et al., 2021). However, inclusion levels of 9 g/kg DM induced inflammation and liver damage in largemouth bass (Peng et al., 2021; Fischer et al., 2022), indicating this species is particularly sensitive. In contrast, prawn (Palaemon adspersus) fed BSFM showed reduced oxidative stress markers, indicating enhanced resilience under stressful conditions (Mastoraki et al., 2020), while soybean meal-induced intestinal enteritis was mitigated by BSFL inclusion in the diets of rainbow trout (Kumar et al., 2021). These positive findings may be due to bioactive compounds in BSFM, such as chitin, lauric acid, and/or antimicrobial peptides, that could provide a valuable advantage as in-feed prebiotics and antibiotics (Surendra et al., 2020).
Red drum is a marine teleost, native to the western Atlantic coast, ranging from Massachusetts to northern Mexico, including the Gulf and southern Florida, where it inhabits both coastal and estuarine waters (Matlock, 1990). It is highly valued for both commercial and recreational purposes due to its rapid growth, high reproductive capacity, and resilience to environmental changes (Castillo and Gatlin, 2018; Liao et al., 2010). This species is recognized as a high-level predator, feeding on fish, mollusks, and arthropods with easy adaptability to consume pelleted diets under farming conditions (Davis, 1990; Matlock, 1990). Thus, considering that red drum shows potential for aquafarming, the objective of this study was to evaluate BSFM replacing FM as a protein ingredient in the diet of juvenile red drum on an equal protein basis. To this end, this study assessed red drum palatability response and growth performance, whole-body proximate composition, amino/fatty acid profiles, liver/intestines histology, and gut microbiota.
2 Materials and methods
The use of red drum was performed under scientific research protocols of Florida Atlantic University and Institutional Animal Care and Use Committee (IACUC Protocol numbers: A20–29 and A-23-24), complying with all local and/or international animal welfare laws and guidelines.
2.1 Source of experimental fish and quarantine
Red drum from Duke Energy – Crystal River Mariculture Center (Citrus County, Florida, United States) were used in both a palatability study and a feeding trial, which was conducted at Harbor Branch Oceanographic Institute – Florida Atlantic University. Upon arrival for each study, the fish were quarantined (250L RAS) and fed a commercial diet until the desired size was reached to start either the palatability or the growth study. Water quality parameters were kept within acceptable ranges for red drum: dissolved oxygen (130.5%–156.3%), temperature (28.4 ± 0.3°C), pH (average 7.8 ± 0.12), salinity (average 29.9 ± 0.67 ppt), alkalinity (average 227.3 ± 46.4 mg/L), total ammonia nitrogen (TAN, average 0.03 ± 0.03 mg/L), and NO2-N (average 0.02 ± 0.01 mg/L).
2.2 Palatability experiment
2.2.1 Diet preparation
The BSFM protein was procured from Stratium LLC (NOCO, Buffalo, USA) and the proximate composition (48.58% crude protein and 12.97% crude fat), along with the amino acid profile, was analyzed (Supplementary Table 1). The BSFL were collected during the early to mid-larval stage following Stratium LLC protocols, dried, and cold-pressed to produce the BSFM protein. Based on the measured nutritional profile, the experimental diets were designed to meet the nutritional requirements of red drum (Serrano et al., 1992) (Table 1) and were formulated to be isonitrogenous (45.5% crude protein) and isocaloric (508 Kcal/100g, gross energy) with BSFM replacing 0% (Control), 25% (BSFM25), 50% (BSFM50), and 100% (BSFM100) of FM. The control diet was formulated with a crude protein content provided by 54.55% fish meal, 13.65% poultry meal, and 31.80% soybean protein concentrate.
Table 1
| Ingredient | Experimental diets (g/100g) | ||||
|---|---|---|---|---|---|
| Control | BSFM25 | BSFM50 | BSFM75 | BSFM100 | |
| Fish meal | 40.0 | 30.0 | 20.0 | 10.0 | 0.0 |
| Poultry meal | 10.7 | 10.7 | 10.7 | 10.7 | 10.7 |
| Soybean protein concentrate | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 |
| BSFM | 0.0 | 10.7 | 21.4 | 32.2 | 42.9 |
| Corn starch | 9.5 | 9.7 | 9.9 | 10.7 | 12.0 |
| Menhaden oil | 12.8 | 11.3 | 9.8 | 8.0 | 6.0 |
| CMC | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Vitamin premix | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Mineral premix | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Stay C | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Monocalcium phosphate | 0.5 | 1.2 | 1.8 | 2.0 | 2.0 |
| Lecithin | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Total (g) | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Diet composition | |||||
| Crude protein* | 47.3 | 46.6 | 45.7 | 44.7 | 43.6 |
| Moisture | 3.3 | 3.4 | 3.3 | 2.9 | 4.2 |
| Crude fat | 18.9 | 17.5 | 16.3 | 14.8 | 13.3 |
| Crude fiber | 2.7 | 3.5 | 4.9 | 5.3 | 6.2 |
| Ash | 12.8 | 12.2 | 12.0 | 11.1 | 9.9 |
| Gross energy (Kcal/100g) | 516.51 | 510.29 | 507.91 | 505.88 | 499.64 |
Experimental diet formula and composition.
The diets have BSFM replacing 0% (Control), 25% (BSFM25), 50% (BSFM50), 75% (BSFM75), and 100% (BSFM100) of FM. Vitamin premix was ARS/702 and mineral premix was ARS/1440 in aquafeed products (Fish Technology Center, Bozeman, MT). Data are expressed on an “as is” basis. *Crude protein = %N x 6.25.
Dry ingredients were mixed for 15 min using a V-Blender, followed by the addition of wet ingredients and thoroughly mixed until an adequate consistency was reached for pelleting. The resulting moist dough was screw-pressed using a lab-pelletizer with 3- and 4-mm diameter die plates. Diet strands were placed in a forced air oven at 60°C for 24h until the moisture content was less than 10%. Then, all the diets were broken into appropriate pellet sizes, sieved for fines, and stored at -20°C until use. A sample of each diet was collected for proximate, amino acid and fatty acid composition analyses (Tables 2, 3).
Table 2
| Amino acids | Control | BSFM25 | BSFM50 | BSFM75 | BSFM100 |
|---|---|---|---|---|---|
| Essential | |||||
| Arginine | 3.09 | 2.91 | 2.75 | 2.68 | 2.44 |
| Histidine | 1.09 | 1.09 | 1.09 | 1.11 | 1.09 |
| Isoleucine | 2.09 | 2.02 | 1.99 | 1.98 | 1.90 |
| Leucine | 3.38 | 3.24 | 3.16 | 3.13 | 3.00 |
| Lysine | 3.40 | 3.15 | 2.96 | 2.81 | 2.61 |
| Methionine | 0.98 | 0.92 | 0.82 | 0.74 | 0.64 |
| Phenylalanine | 1.97 | 1.92 | 1.88 | 1.89 | 1.83 |
| Threonine | 1.84 | 1.74 | 1.69 | 1.65 | 1.59 |
| Tryptophan | 0.50 | 0.52 | 0.53 | 0.54 | 0.53 |
| Valine | 2.37 | 2.31 | 2.33 | 2.38 | 2.34 |
| Non-essential | |||||
| Alanine | 2.77 | 2.64 | 2.59 | 2.56 | 2.46 |
| Aspartic Acid | 4.52 | 4.30 | 4.20 | 4.15 | 4.02 |
| Cysteine | 0.46 | 0.45 | 0.46 | 0.46 | 0.45 |
| Glycine | 3.35 | 3.02 | 2.83 | 2.69 | 2.40 |
| Glutamic Acid | 6.88 | 6.49 | 6.28 | 6.20 | 5.92 |
| Hydroxylysine | 0.09 | 0.05 | 0.03 | 0.03 | 0.02 |
| Hydroxyproline | 0.72 | 0.56 | 0.47 | 0.39 | 0.29 |
| Proline | 2.35 | 2.29 | 2.28 | 2.34 | 2.29 |
| Serine | 1.69 | 1.61 | 1.57 | 1.58 | 1.59 |
| Taurine † | 0.36 | 0.31 | 0.27 | 0.23 | 0.19 |
| Tyrosine | 1.37 | 1.49 | 1.59 | 1.72 | 1.77 |
| Total amino acids | 45.42 | 43.21 | 41.93 | 41.41 | 39.53 |
Profile of amino acid composition of diets fed to juvenile red drum (Sciaenops ocellatus).
The diets have BSFM replacing 0% (Control), 25% (BSFM25), 50% (BSFM50), 75% (BSFM75), and 100% (BSFM100) of FM.
Results are expressed on an “as is” basis. *Crude protein = %N x 6.25. †Conditionally essential amino acids.
Table 3
| Fatty acids | Control | BSFM25 | BSFM50 | BSFM75 | BSFM100 |
|---|---|---|---|---|---|
| C12:0 | 0.07 | 2.56 | 3.66 | 4.91 | 7.51 |
| C13:0 | 0.04 | 0.05 | 0.02 | 0.02 | 0.03 |
| C14:0 | 2.07 | 2.61 | 2.61 | 2.25 | 2.35 |
| C15:0 | 0.38 | 0.48 | 0.25 | 0.21 | 0.22 |
| C16:0 | 4.49 | 4.57 | 5.32 | 4.72 | 5.01 |
| C17:0 | 0.26 | 0.34 | 0.17 | 0.14 | 0.15 |
| C18:0 | 2.54 | 3.32 | 2.04 | 1.78 | 2.28 |
| C20:0 | 0.12 | 0.15 | 0.11 | 0.10 | 0.11 |
| C21:0 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 |
| C22:0 | 0.12 | 0.15 | 0.11 | 0.11 | 0.11 |
| C23:0 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 |
| C24:0 | 0.10 | 0.11 | 0.06 | 0.09 | 0.09 |
| ΣSFA A | 10.21 | 14.35 | 14.38 | 14.36 | 17.89 |
| C14:1 | 0.04 | 0.06 | 0.05 | 0.06 | 0.07 |
| C15:1 | 0.08 | 0.11 | 0.07 | 0.06 | 0.06 |
| C16:1 | 13.67 | 17.20 | 9.37 | 7.81 | 7.98 |
| C17:1 | 0.08 | 0.07 | 0.05 | 0.01 | 0.03 |
| C18:1n-9(Z) | 2.60 | 4.26 | 2.42 | 2.40 | 3.73 |
| C18:1n-9(E) | 0.87 | 1.35 | 0.35 | 0.37 | 0.30 |
| C20:1 | 0.34 | 0.44 | 0.19 | 0.18 | 0.20 |
| C22:1 | 0.09 | 0.09 | 0.00 | 0.08 | 0.00 |
| C24:1 | 0.19 | 0.12 | 0.06 | 0.11 | 0.09 |
| Σ MUFA B | 17.97 | 23.70 | 12.55 | 11.08 | 12.46 |
| C18:3n-3 (LNA) | 2.55 | 3.46 | 1.38 | 0.92 | 0.94 |
| C20:3n-3 | 0.17 | 0.22 | 0.13 | 0.08 | 0.10 |
| C20:5n3 (EPA) | 6.48 | 8.79 | 3.36 | 2.70 | 2.25 |
| C22:6n3 (DHA) | 3.68 | 4.98 | 1.90 | 1.41 | 1.20 |
| Σ n-3 PUFA C | 12.89 | 17.46 | 6.77 | 5.12 | 4.49 |
| C20:2n6 | 0.12 | 0.15 | 0.10 | 0.12 | 0.12 |
| C20:4n-6 (ARA) | 0.38 | 0.47 | 0.26 | 0.19 | 0.18 |
| C20:3n-6 | 0.13 | 0.15 | 0.10 | 0.06 | 0.07 |
| C18:2n-6 (LA) | 0.98 | 2.58 | 1.87 | 2.33 | 3.75 |
| C18:3n-6 | 0.86 | 1.10 | 0.41 | 0.40 | 0.44 |
| Σ n-6 PUFA D | 2.47 | 4.45 | 2.74 | 3.10 | 4.56 |
| Σ PUFA E | 28.24 | 39.36 | 16.28 | 13.33 | 13.53 |
| Σ SFA:Σ PUFA F | 0.36 | 0.36 | 0.88 | 1.08 | 1.32 |
| Σ n-3: Σ n-6 G | 5.22 | 3.92 | 2.47 | 1.65 | 0.99 |
Fatty acid profile of diets fed to juvenile red drum (Sciaenops ocellatus) expressed in µg/mg.
The diets have BSFM replacing 0% (Control), 25% (BSFM25), 50% (BSFM50), 75% (BSFM75), and 100% (BSFM100) of FM. SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids. ASum of all fatty acids without double bonds; BSum of all fatty acids with a single double bond; CSum of all n-3 fatty acids; DSum of all n-6 fatty acids; ESum of all fatty acids with ≥ 2 double bonds; FRatio between the sum of all SFA and the sum of all PUFA; GRatio between the sum of all n-3 and the sum of all n-6 fatty acids.
2.2.2 Acclimatization
After quarantine, the fish (5 g average) were fed a commercial diet and grown out in four 250L tanks of a 24-tank recirculating aquaculture system (RAS) until the target experimental size of 90–100 g/fish was reached to commence the palatability study. The RAS was maintained at a photoperiod set at 12 hours of light and 12 hours of darkness (lights on at 07:00). During this time, the fish underwent a habituation period so they became accustomed to human presence during the feeding assessment in order to observe apparent satiation behaviors (Al-Souti et al., 2019: Suresh et al., 2011). When the target weight was reached, 360 fish were evenly redistributed into 12 experimental tanks within the same RAS and the mean body weight (MBW) was recorded for each tank. The fish then underwent a 14-day acclimatization and habituation period to adjust to the new stocking density and tank conditions before commencing the palatability assessment.
2.2.3 Assessment of palatability by feed consumption
Palatability was determined as feed consumption (mg feed/gram of fish biomass) for the Control and experimental diets according to Al-Souti et al. (2019); Glencross et al. (2007), and Suresh et al. (2011). Thus, the palatability assessment involved feeding the fish beyond apparent satiation and observing their feed rejection behaviors (Glencross et al., 2007). After the initial rejection behavior was noted, the fish were given an additional 10 min to ensure all the individuals within the tank exhibited rejection. Then, the uneaten feed was removed from the tank using a siphon connected to a mesh net at one end, which collected the uneaten feed. The collected feed was then dried in a forced-air oven at 60°C for 12 h to reach a constant weight. Feed consumption was calculated as mg feed intake/gram of fish biomass (Suresh et al., 2011).
The palatability assessment consisted of four trial periods over 5 consecutive days of observation. During the 5-day observation period, the palatability assessment was only performed during the morning feeding (09:00), while the evening feeding (15:00) consisted of a fixed 1.5% MBW ration of the experimental diet being tested for that replicate tank treatment. Each trial included a 14-day washout period between the observation period of the trial and the next trial. The washout period consisted of feeding all fish from each experimental tank at a 1.5% MBW ration per day with a commercial feed. For the observation period of each trial, one of the five test diets was randomly assigned to each tank, with three replicates per treatment. Treatment diets were systematically reassigned to tanks for each trial so that each tank was presented with a different diet every trial, ultimately receiving four of the five diets during the four palatability assessment trials. Total tank biomass was measured the day after each trial’s observation period and feed rations were adjusted accordingly.
Data were verified for normality using the Shapiro–Wilk test and for homogeneity of the variance using Levene’s test. The normality assumption was checked for each set of data collected from each trial. Then, all the data were submitted to a linear mixed-effects model to account for repeated measures data collection for each trial. Data were statistically analyzed using SPSS version 27 (SPSS, Chicago, IL, USA).
2.3 Feeding trial
2.3.1 Diet preparation
Five experimental diets were designed for the feeding trial. Diets were formulated to be isonitrogenous (45.5% crude protein) and isocaloric (508 Kcal/100g, gross energy) with BSFM replacing 0% (Control), 25% (BSFM25), 50% (BSFM50), 75% (BSFM75), and 100% (BSFM100) of FM. To this end, the same formulation and manufacturing procedure as in the palatability experiment were used in the feeding trial as described in section 2.2.1, using a 1.5 mm diameter die plate as the fish were smaller than in the palatability trial.
2.3.2 Experimental conditions
A total of 400 red drum (approximately 39 g initial weight per fish) were evenly distributed into a 20 tank RAS (250L per tank) with a photoperiod of 12 hours of light and 12 hours of darkness (lights on at 07:00). The treatments were randomly assigned to each tank with four replicates per treatment and the fish were fed their respective experimental diets. The fish were fed twice at 5% initial MBW (11:00 and 15:00) every day for 8 weeks. At the beginning of the trial, the MBW of all 20 fish was recorded for each tank. The weighing procedure was repeated every 2 weeks during the 8-week trial for each tank and the feeding rations were adjusted accordingly.
2.3.3 Sample collection
At the end of the trial, fish from each tank were counted, the MBW was recorded, and the fish were sampled 24-h postprandial. Five fish from each tank were euthanized with an overdose of buffered MS-222 at 300 mg/L to assess viscerosomatic index parameters. The fish were individually weighed, measured, and dissected to calculate hepatosomatic, intestinosomatic indices, and Fulton condition factor (HPI%, ISI%, and K factor, respectively). From these five fish, two replicates of liver and mid-intestine samples were preserved in 10% buffered formalin for histomorphology analysis and three replicates of whole intestines were aseptically collected with heat sterilized dissecting equipment and placed into a 15 mL Falcon tube and stored at -80°C for gut microbiome analysis. Another five fish from each tank were euthanized with an overdose of buffered MS-222 at 300 mg/L and stored at -80°C for whole-body proximate composition, amino acids, and fatty acids analyses.
2.3.4 Calculations for growth parameters
Growth performance analysis was calculated between the fish groups fed different diets formulated with BSFM replacing FM. The parameters were calculated as follows:
-
survival (%) = [final population/initial population] × 100;
-
percentage weight gain (PWG) = [(final body weight – initial body weight)/(initial body weight)] × 100;
specific growth rate (SGR) (Ricker, 1975) = [(lnWf – lnWi)/Δt] × 100, where Wi is the initial average weight, Wf is the final average weight, and Δt is the time between measurements;
-
feed efficiency (FE) = wet weight gain (g)/dry feed consumed (g);
-
protein efficiency ratio (PER, %) = [weight gain (g, wet weight)/protein intake (g, dry weight)] × 100;
-
Fulton condition factor (Ricker, 1975), K = [fish weight (g)/fish length 3, (cm)] × 104;
-
viscerosomatic index (VSI, %) = [viscera weight (g)/body weight (g)] × 100;
-
hepatosomatic index (HSI, %) = [liver weight (g)/body weight (g)] × 100;
-
intestinosomatic indices (ISI, %) = [intestine weight (g)/body weight (g)] × 100.
2.3.5 Proximate composition, amino acid, and fatty acid analysis
Experimental diets and whole-body fish samples were analyzed for proximate composition and amino acid profile at the Experiment Station Chemical Laboratories (ESCL), University of Missouri, Columbia, Missouri (Tables 1, 2) according to standard procedures (AOAC, 2003). The diet and whole-body fish samples were freeze-dried, ground, and homogenized prior to lipid extraction. Lipids were extracted according to modified methods described by Folch et al. (1957) and Parrish (1999). The resulting fatty acid (FA) extracts were methylated to produce fatty acid methyl esters (FAMEs) (Lepage and Roy, 1984) for analysis with gas chromatography-mass spectrometry (GC-MS). Then, the samples were analyzed on a Clarus 680/600 T GC-MS (Perkin-Elmer, Waltham, Mass., USA) using a 30 m Thermo Fisher TR-5 general purpose column with a 250-µm diameter. Hydrogen was used as the carrier gas at a flow rate of 0.8 mL/min. The GC inlets were held at a constant temperature of 250°C. Samples were individually injected into the column (using an 82-vial autosampler) at a volume of 1.0 µl with a 61.5/1 split ratio. The oven temperature was programmed from 40°C (1 min) to 200°C (2 min) at a rate of 15°C/min and then to 250°C at a rate of 3.0°C/min. QP GC-MS was carried out using 70eV EI and these data were evaluated using full scan acquisition over the m/z range of 45–600 with quantification based on the Total Ion Chromatogram (TIC). Fatty acids were identified by comparison to a 37-component FAME standard (Supelco 37 FAME Mix, Millipore Sigma, Burlington, Mass., USA). The ratio of the internal standard (C19:0) peak area was used for quantification (Table 3).
2.3.6 Histology of the intestines and liver
Mid-intestine and liver samples were collected and immediately fixed in Davidson’s solution for 48 hours, washed in 70% ethanol to remove residual fixative, and stored in fresh 70% ethanol until further processing. Samples were dehydrated in a series of ethanol solutions using a Shandon Excelsior ES Tissue Processor, cleared in xylenes, and embedded in paraffin wax with a Histostar Embedding Station (Thermo Scientific, Waldorf, Germany). Sections were cut to a 5 µm thickness using an HM 355 S-2 Rotary Microtome (Thermo Scientific, Waldorf, Germany) and mounted on glass slides. Slides were dried overnight on a slide warmer to ensure proper adhesion before staining. Sections were stained with hematoxylin and eosin (H&E) using a Leica AutoStainer XL (Leica Biosystems, Wetzlar, Germany) to evaluate general liver and intestinal histomorphology. Eight fish tissue samples (n=8/treatment) with two sections per fish were examined under a Keyence BZ-X All-in-One Fluorescence Microscope (Keyence Corporation, Osaka, Japan). First, a 2× magnification scan captured the entire tissue, and the Keyence navigation system randomly selected five fields (area of 1.6 mm2) per section to be analyzed under 10× magnification.
Histological scoring (i.e., 0: normal, 1: minimal to mild, 2: mild, 3: moderate, 4: moderate to severe) indicating arbitrary increasing inflammatory severity was done visually per section, similar to that reported by Palma et al. (2021) and Romano et al. (2023). Intestinal assessment included arbitrary inflammation severity along with the measurements of the length of the serosa, muscularis, submucosa, lamina propria, and mucosa, and goblet cell count in 20 folds per section. Liver samples were evaluated for inflammation severity and presence of intracytoplasmic vacuoles using a Keyence hybrid cell application to quantify the number of vacuoles and the total area of vacuoles per area (µm2). Histological scoring and analysis of the intestines and livers of fish fed the Control diet (Figures 1, 2) served as a baseline for assessing the effects of the increased BSFM protein levels replacing fish meal in the other treatments.
Figure 1

5 µm histological sections of intestines from juvenile Red Drum (Sciaenops ocellatus) fed diet with BSFM replacing FM at 0% (Control, a), 25% (BSFM25, b), 50% (BSFM50, c), 75% (BSFM75, d) and 100% (BSFM100, e) stained with hematoxylin and eosin (H&E) at 10× magnification. Red arrows indicate goblet cells, blue arrows denote edema, and structural layers are labeled as serosa (S), muscularis (M), lamina propria (LP), and mucosa (Mu). Black circles highlight lymphocyte aggregation.
Figure 2
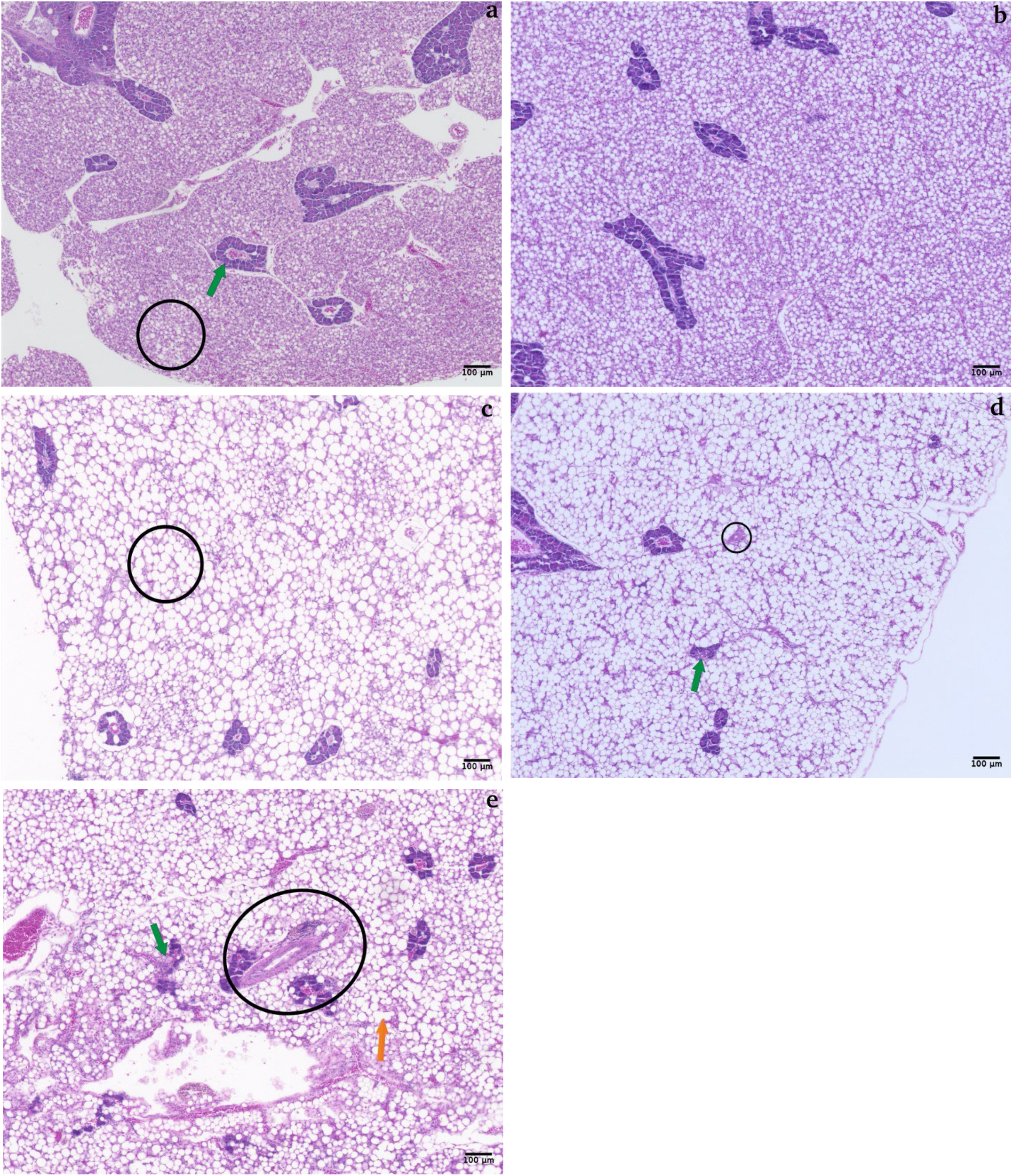
5µm histological sections of livers from juvenile Red drum (Sciaenops ocellatus) fed diets with BSFM replacing fishmeal at 0% (Control, a), 25% (BSFM25, b), 50% (BSFM50, c), 75% (BSFM75, d), and 100% (BSFM100, e), stained with hematoxylin and eosin (H&E) at 10× magnification. Green arrows indicate pancreatic exocrine tissue health, orange arrows denote vacuole degeneration, and black circles highlight regions of overall tissue health in lower BSFM treatments (a–c), and possible fibrotic tissue formation in higher BSFM diets (d, e). Lymphocyte infiltration is shown in (d, e). possible fibrotic tissue formation in higher BSFM diets (d, e). Lymphocyte infiltration is shown in (d, e).
2.3.7 Microbiome analysis
Whole intestine samples were sent to Azenta Life Sciences for 16S DNASeq. Sequences underwent quality control and trimming (TrimGalore v. 0.6.10) (Krueger, 2020). A QIIME2 (Quantitative Insights into Microbial Ecology 2) (v. amplicon-2024.2) snakemake (v. 7.32.4) pipeline was used to run sequences through DADA2 (v. 2024.2.0) for denoising and designating amplicon sequence variants (ASVs), with defaults other than forward and reverse truncation set to 225 bp (Bolyen et al., 2019; Bradshaw et al., 2020; Callahan et al., 2016; Köster and Rahmann, 2012). RESCRIPt (v. 2024.2.0) was used to create a classifier with the publicly available 341f and 805r V3/V4 16S primers against the SILVA 138.1 database, which was then used in VSEARCH (v. 2.22.1) to annotate the ASVs (Pruesse et al., 2007; Robeson et al., 2021; Rognes et al., 2016; Yilmaz et al., 2014). This annotation was used to remove mitochondria, chloroplasts, eukaryotes, and unassigned sequences. ASVs less than 350bp were removed (Bolyen et al., 2019).
Growth performance parameters, condition indices, histology data, and body composition (proximate and amino acid profiles) were statistically analyzed using SPSS version 27 (SPSS, Chicago, IL, USA), after being validated for normality using the Shapiro–Wilk test and homogeneity using Levene’s test. Statistical differences were identified by means of one-way ANOVA followed by a pair-wise comparison of means using Tukey’s post hoc HSD test. Results are expressed as means ± standard error of the mean (SEM). Significance levels were established at ɑ=0.05. Second-degree polynomial regression analysis (Snedecor and Cochran, 1978) was used to determine the correlation between the growth/amino acid/fatty acid parameters and dietary BSFM level in juvenile red drum using SPSS version 27 (SPSS, Chicago, IL, USA).
The fatty acid profiles in samples of whole-body Florida pompano juveniles underwent permutational analysis of variance (PERMANOVA with 9999 permutations), including a posteriori pairwise comparison. The PERMANOVA was tested with one factor: diet treatment (Control, BSFM25, BSFM50, BSFM75, and BSFM100). Assumptions of multivariate homoscedasticity were verified with a PERMDISP test, and data were transformed (arcsine square root) when necessary. Analyses were run using a Bray–Curtis similarity matrix with PRIMER 7 (v. 7.1.12) and PERMANOVA+ (v.1.0.2).
Data obtained from the histological analyses were analyzed using SPSS version 27 (SPSS, Chicago, IL, USA). Welch’s ANOVA was used with the Games–Howell test for equal variances not assumed for the serosa, lamina propria, mucosa, and goblet cell count. For inflammation severity, muscularis, submucosa, vacuole number, and total vacuole area, normality and homoscedasticity (Levene’s test) was confirmed, and statistical differences were identified by means of one-way ANOVA followed by post hoc pair-wise comparisons of the means using Tukey’s HSD test. Results are expressed as means ± SEM.
For the microbiome, R (v. 4.2.0) and RStudio (2023.06.1) were used to analyze the final ASV table with the following libraries loaded: phyloseq (v. 1.42.0), vegan (v. 2.6-4), tidyverse (v. 2.0.0), FSA (v.0.9.4), reshape (v. 0.8.9), DESeq2 (v. 1.38.3), pairwiseAdonis (v. 0.4), metagenomeSeq (v. 1.40.0), venn (v. 1.11), rcompanion (v. 2.4.21), ANCOMBC2 (v. 2.0.2), and car (v. 3.1-1) (Dusa, 2022; Fox and Weisberg, 2019; Lin et al., 2022; Lin and Das Peddada, 2020; Love et al., 2021; Mangiafico, 2022; McMurdie and Holmes, 2013; Ogle et al., 2022; Oksanen et al., 2022; Paulson et al., 2024; Wickham, 2007; Wickham et al., 2019). To remove rare ASVs and reduce noise at the community level, ASVs with less than 11 occurrences across all samples were removed. (Sun et al., 2013). Alpha diversity (Shannon, Simpson, and Fisher indexes) was calculated using untransformed data. Correlations between these indices and observed ASVs were tested with Spearman rank tests. Kruskal–Wallis and Dunn pairwise tests were used to test for differences between diets based upon Shannon diversity. Differences between diets based upon the Bray–Curtis dissimilarity of variance stabilizing transformation (DESeq2) data were visualized using a principal coordinate of analysis (PCoA) and tested using overall and pairwise permutational analysis of variance (PERMANOVA) tests. The Benjamini–Hochberg correction was used to adjust for multiple testing in pairwise tests (Dunn and PERMANOVA). Tests were considered statistically significant at ɑ=0.5. Differentially abundant genera between the Control and each of the BSFM diets were tested for via ANOCMBC2 with default parameters. Scripts associated with analysis can be found on GitHub (https://github.com/djbradshaw2/red_ drum_BSFM_2024).
3 Results
3.1 Assessment of palatability by feed consumption
The palatability assessment of fish fed BSFM25 was statistically similar to the Control diet (Figure 3). Lower palatability (P = 0.0001) was observed in fish fed the BSFM50 diet compared to those fed the Control, BSFM25, and BSFM100 diets. Fish fed the BSFM100 diet presented similar feed consumption to those fed the Control and BSFM25 diets (P<0.05).
Figure 3
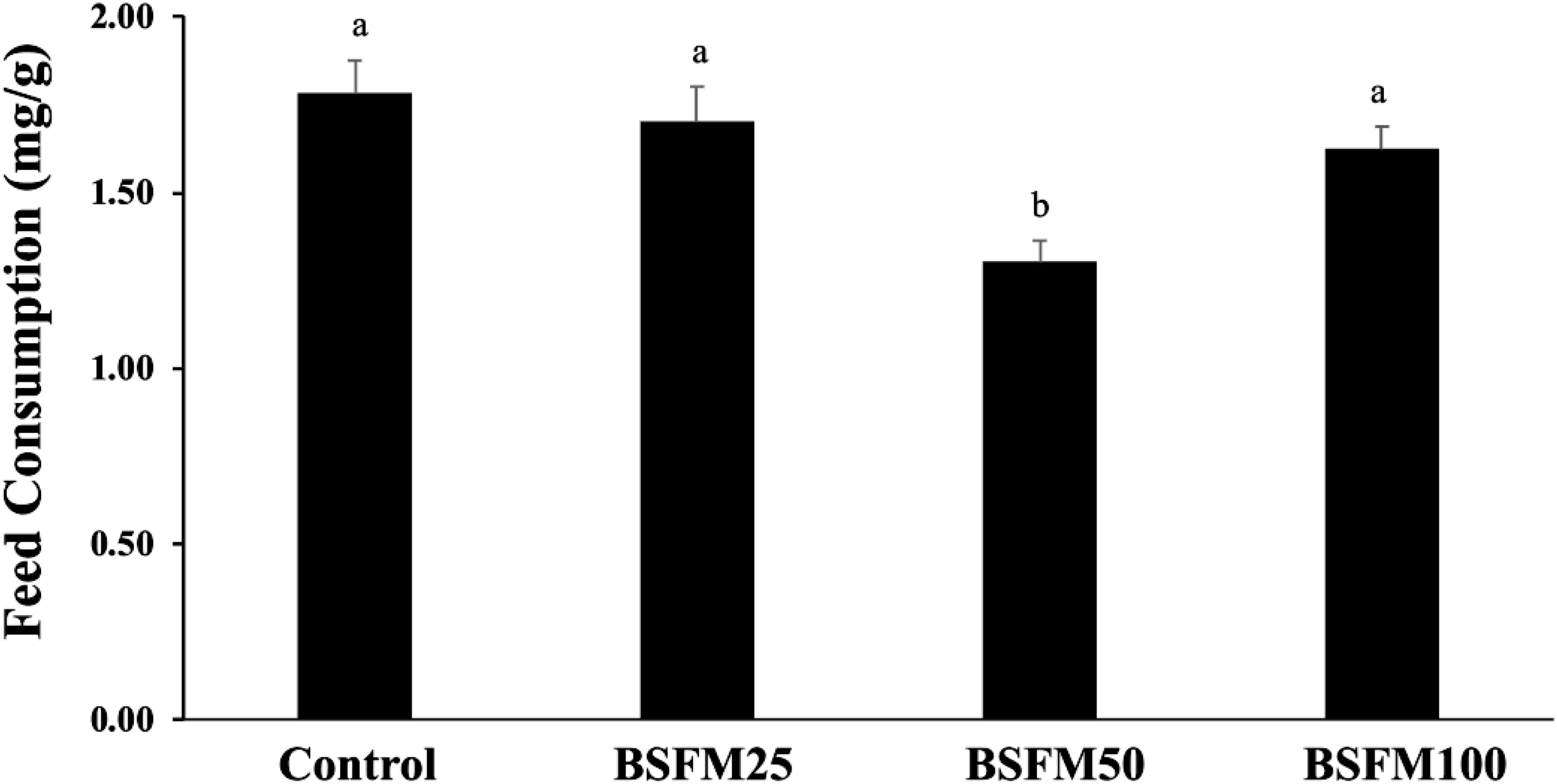
Palatability of black soldier fly meal (BSFM) based on feed consumption (mg feed/gram of fish biomass). The diets have BSFM replacing 0% (Control), 25% (BSFM25), 50% (BSFM50), 75% (BSFM75), and 100% (BSFM100) of FM. Data are shown as the mean with ± S.E.M. (n=60). Superscript letters indicate statistical differences among groups identified by Tukey’s post hoc test (one-way ANOVA, p<0.05).
3.2 Growth performance
Mean MBW was recorded per treatment biweekly in the 2, 4, 6, and 8 weeks (Figure 4). At 2 weeks, greater weight was observed in fish fed the BSFM50 diet in comparison to those fed the BSFM100 diet (ANOVA; P = 0.002). At 4 weeks, a greater weight was recorded in fish fed BSFM50 compared to the Control, BSFM75, and BSFM100 diets (ANOVA; P < 0.0001). At 6 weeks, greater weight was observed in fish fed the Control, BSFM25, and BSFM50 diets compared with the BSFM75 and BSFM100 diets (ANOVA; P < 0.0001). At the end of the 8-week experiment, greater weight was recorded in fish fed the Control, BSFM25, and BSFM50 diets compared to the BSFM75 and BSFM100 diets (ANOVA; P < 0.0001).
Figure 4
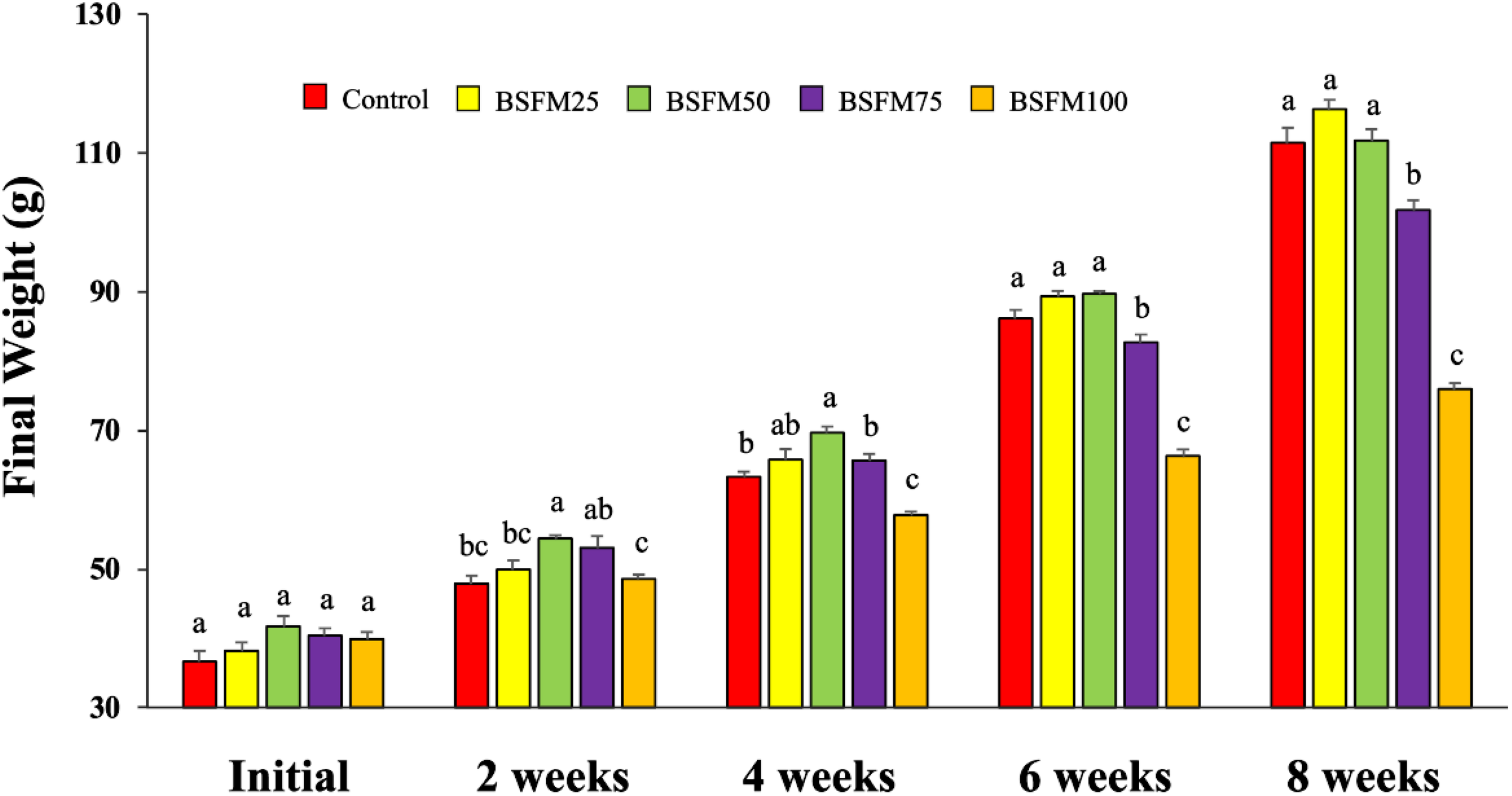
The diets have black soldier fly meal (BSFM) replacing 0% (Control), 25% (BSFM25), 50% (BSFM50), 75% (BSFM75), and 100% (BSFM100) of FM. The graph shows the effects of BSFM inclusion on the final weight of red drum. Data are shown as the mean MBW with ± S.E.M. (n=4). Superscript letters indicate statistically significant differences among groups.
Fish readily accepted the experimental diets and at the end of the 8-week experimental period, the survival rate was high (above 97.5%) with no differences among the dietary treatments (Table 4). Higher PWG, SGR, and FE (P<0.05) were calculated in fish fed the BSFM25 diet compared to those fed the BSFM50, BSFM75, and BSFM100 diets. Higher PER (P<0.05) was recorded in fish fed the BSFM25 diet compared to those fed the BSFM50, BSFM75, and BSFM100 diets, but it was not different from those fed the Control diet. The Fulton factor (K factor) of fish fed the BSFM25 diet was higher than those fed the BSFM50, BSFM75, and BSFM100 diets. The VSI presented no differences (P>0.05) among dietary treatments. The HSI of fish fed the BSFM25 diet was higher than the Control fish, but not different from fish fed the BSFM50, BSFM75, or BSFM100 diets. A higher ISI was measured in fish fed the Control diet compared to those in the BSFM100 treatment.
Table 4
| Parameter | Control | BSFM25 | BSFM50 | BSFM75 | BSFM100 |
|---|---|---|---|---|---|
| Survival (%) | 100.0 ± 0.00 | 100.0 ± 0.00 | 97.50 ± 1.44 | 100.0 ± 0.00 | 100.0 ± 0.00 |
| PWG | 204.00 ± 6.71a | 204.74 ± 5.08a | 175.11 ± 3.46b | 152.29 ± 7.13c | 90.40 ± 1.52d |
| SGR | 1.98 ± 0.04a | 1.99 ± 0.03a | 1.81 ± 0.02b | 1.65 ± 0.05c | 1.15 ± 0.01d |
| FE | 0.49 ± 0.01a | 0.49 ± 0.02a | 0.44 ± 0.01b | 0.39 ± 0.01c | 0.26 ± 0.02d |
| PER | 1.02 ± 0.03ab | 1.06 ± 0.02a | 0.96 ± 0.01bc | 0.88 ± 0.03c | 0.61 ± 0.01d |
| K factor | 1.02 ± 0.01ab | 1.06 ± 0.01a | 1.01 ± 0.01b | 1.01 ± 0.01b | 1.00 ± 0.01b |
| VSI | 4.62 ± 0.12 | 4.96 ± 0.22 | 4.85 ± 0.12 | 4.82 ± 0.22 | 4.30 ± 0.18 |
| HSI | 0.89 ± 0.06b | 1.50 ± 0.29a | 1.28 ± 0.08ab | 1.28 ± 0.08ab | 1.10 ± 0.08ab |
| ISI | 1.24 ± 0.08a | 1.20 ± 0.13ab | 0.96 ± 0.05ab | 0.98 ± 0.05ab | 0.89 ± 0.07b |
Growth performance, feeding efficiency, and indices of juvenile red drum (Sciaenops ocellatus) fed experimental diets in an 8-week feeding trial.
The diets have BSFM replacing 0% (Control), 25% (BSFM25), 50% (BSFM50), 75% (BSFM75), and 100% (BSFM100) of FM. Results are means ± S.E.M. Statistical differences between experimental diets were identified by means of one-way ANOVA (p < 0.05) followed by further comparing group means using Tukey’s post hoc test.Superscript letters indicate significant defferences among experimental groups.
3.3 Whole-body proximate, amino acid, and fatty acid composition
There were no significant differences in the whole-body proximate composition of crude protein, crude fat, crude fiber, and ash among the dietary treatments (P>0.05) (Table 5). Essential and non-essential amino acids (EAA and NEAA, respectively) showed no statistical differences between treatments except for taurine. Lower taurine content was found in fish fed the BSFM100 diet compared to those fed the Control and BSFM25 diets (P<0.05).
Table 5
| Control | BSFM25 | BSFM50 | BSFM75 | BSFM100 | |
|---|---|---|---|---|---|
| Crude protein* | 63.27 ± 3.62 | 60.83 ± 3.25 | 64.41 ± 2.44 | 65.00 ± 0.15 | 62.53 ± 4.53 |
| Crude fat | 13.92 ± 1.23 | 13.92 ± 0.91 | 15.46 ± 1.08 | 14.22 ± 0.66 | 11.25 ± 1.02 |
| Crude fiber | 0.59 ± 0.14 | 0.47 ± 0.04 | 0.56 ± 0.11 | 0.58 ± 0.04 | 0.52 ± 0.03 |
| Ash | 16.49 ± 0.77 | 17.14 ± 2.25 | 18.53 ± 0.56 | 17.92 ± 0.78 | 21.41 ± 2.02 |
| Essential amino acids | |||||
| Arginine | 3.62 ± 0.21 | 3.82 ± 0.27 | 3.88 ± 0.14 | 3.93 ± 0.12 | 3.82 ± 0.21 |
| Histidine | 1.22 ± 0.07 | 1.29 ± 0.11 | 1.31 ± 0.06 | 1.31 ± 0.06 | 1.23 ± 0.07 |
| Isoleucine | 2.40 ± 0.17 | 2.50 ± 0.20 | 2.55 ± 0.11 | 2.60 ± 0.13 | 2.46 ± 0.15 |
| Leucine | 3.90 ± 0.25 | 4.09 ± 0.34 | 4.16 ± 0.17 | 4.23 ± 0.19 | 4.05 ± 0.23 |
| Lysine | 4.54 ± 0.30 | 4.77 ± 0.39 | 4.86 ± 0.19 | 4.93 ± 0.22 | 4.70 ± 0.29 |
| Methionine | 1.55 ± 0.10 | 1.62 ± 0.13 | 1.65 ± 0.06 | 1.68 ± 0.07 | 1.61 ± 0.09 |
| Phenylalanine | 2.17 ± 0.14 | 2.27 ± 0.18 | 2.31 ± 0.09 | 2.35 ± 0.10 | 2.25 ± 0.13 |
| Threonine | 2.34 ± 0.14 | 2.43 ± 0.18 | 2.46 ± 0.09 | 2.52 ± 0.09 | 2.43 ± 0.13 |
| Tryptophan | 0.62 ± 0.05 | 0.60 ± 0.05 | 0.62 ± 0.02 | 0.64 ± 0.02 | 0.64 ± 0.03 |
| Valine | 2.70 ± 0.17 | 2.82 ± 0.22 | 2.89 ± 0.12 | 2.91 ± 0.13 | 2.77 ± 0.16 |
| Non-essential amino acids | |||||
| Alanine | 3.89 ± 0.21 | 4.05 ± 0.28 | 4.12 ± 0.16 | 4.19 ± 0.11 | 4.10 ± 0.24 |
| Aspartic acid | 5.21 ± 0.32 | 5.43 ± 0.42 | 5.52 ± 0.21 | 5.61 ± 0.22 | 5.43 ± 0.30 |
| Cysteine† | 0.53 ± 0.03 | 0.55 ± 0.05 | 0.57 ± 0.02 | 0.55 ± 0.02 | 0.55 ± 0.04 |
| Glycine | 5.29 ± 0.29 | 5.48 ± 0.33 | 5.57 ± 0.27 | 5.62 ± 0.16 | 5.54 ± 0.42 |
| Glutamic Acid | 7.84 ± 0.48 | 8.24 ± 0.65 | 8.40 ± 0.30 | 8.54 ± 0.29 | 8.26 ± 0.45 |
| Hydroxylysine | 0.14 ± 0.02 | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.15 ± 0.01 | 0.16 ± 0.02 |
| Hydroxyproline | 1.32 ± 0.09 | 1.31 ± 0.09 | 1.34 ± 0.10 | 1.33 ± 0.09 | 1.33 ± 0.13 |
| Proline | 2.78 ± 0.15 | 2.88 ± 0.18 | 2.95 ± 0.14 | 3.00 ± 0.08 | 2.97 ± 0.21 |
| Serine | 1.97 ± 0.10 | 2.07 ± 0.16 | 2.08 ± 0.07 | 2.11 ± 0.04 | 2.08 ± 0.10 |
| Taurine† | 0.36 ± 0.03a | 0.31 ± 0.03a | 0.27 ± 0.01ab | 0.21 ± 0.01bc | 0.20 ± 0.01c |
| Tyrosine† | 1.43 ± 0.11 | 1.59 ± 0.15 | 1.65 ± 0.06 | 1.54 ± 0.08 | 1.49 ± 0.08 |
| Total amino acids | 55.86 ± 3.23 | 58.30 ± 4.32 | 59.34 ± 2.18 | 59.99 ± 1.92 | 58.09 ± 3.23 |
Whole-body proximate and amino acid composition of juvenile red drum (Sciaenops ocellatus) fed experimental diets in an 8-week feeding trial.
The diets have BSFM replacing 0% (Control), 25% (BSFM25), 50% (BSFM50), 75% (BSFM75), and 100% (BSFM100) of FM. W/W%= grams per 100 grams of sample. * Crude protein = %N x 6.25. † Conditionally essential amino acids. Results are means ± SEM (n = 4) expressed on an “as is” basis unless otherwise indicated. Superscript letters indicate one-way ANOVA (p<0.05) confirmed by Tukey post hoc test.
The whole-body fatty acid profiles presented differences in lauric acid (C12:0), myristic acid (C14:0), stearic acid (C18:0), palmitoleic acid (C16:1), oleic acid [C18:1n-9 (Z)], elaidic acid [C18:1n-9 (E)], linoleic acid (C18:2n-6, LA), arachidonic acid (C20:4n-6, ARA), eicosatrienoic acid (C20:3n-6), eicosapentanoic acid (C20:5n-3, EPA), and docosahexenoic acid (C22:6n-3, DHA) (Table 6). Lauric acid (C12:0) content in the whole body increased with increasing levels of dietary BSFM in the treatments, with the highest values in fish fed BSFM75 and BSFM100. Fish fed the BSFM50 diet had higher myristic acid (C14:0) content compared to those fed the Control diet, likewise stearic acid (C18:0) compared to Control and BSFM100 diets. A higher sum of all saturated fatty acids (SFA, ΣSFA) was measured in fish fed BSFM50 compared to those fed the Control diet. Palmitoleic (C16:0) and oleic (C18:1n-6) acids presented higher content in fish fed BSFM50 than in those fed the BSFM100 and the Control diet, respectively. Monounsaturated fatty acids (Σ MUFA) showed no difference among dietary treatments [Pseudo-F (4, 15) = 0.86, P (perm) = 0.609; P (MC) = 0.581]. LA content was statistically higher in fish fed BSFM75 and BSFM100 compared to those fed the Control diet. ARA, EPA, and DHA showed higher content in fish fed the Control, BSFM25, and BSFM50 diets than those fed the BSFM100 diet. Higher Σ n-3 PUFA was observed in fish fed the Control, BSFM25, and BSFM50 diets compared to those fed the BSFM100 diet. Higher Σ n-6 PUFA was observed in fish fed BSFM50, BSFM75, and BSFM100 compared to those fed the Control diet. The Σ n-3: Σ n-6 ratio in fish fed the Control and BSFM25 diets was higher than those fed the BSFM50, BSFM75, and BSFM100 diets.
Table 6
| Fatty acids | Control | BSFM25 | BSFM50 | BSFM75 | BSFM100 |
|---|---|---|---|---|---|
| C12:0 | 0.02 ± 0.03a | 0.36 ± 0.11a | 1.19 ± 0.23b | 1.74 ± 0.27c | 1.77 ± 0.33c |
| C13:0 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| C14:0 | 1.83 ± 0.22b | 1.93 ± 0.16ab | 2.48 ± 0.39a | 1.99 ± 0.28ab | 2.20 ± 0.16ab |
| C15:0 | 0.32 ± 0.11 | 0.37 ± 0.09 | 0.39 ± 0.05 | 0.33 ± 0.05 | 0.22 ± 0.07 |
| C16:0 | 4.54 ± 0.74 | 4.28 ± 0.66 | 5.80 ± 0.70 | 4.83 ± 0.71 | 5.02 ± 0.54 |
| C17:0 | 0.24 ± 0.07 | 0.27 ± 0.07 | 0.30 ± 0.04 | 0.25 ± 0.04 | 0.18 ± 0.05 |
| C18:0 | 3.04 ± 0.42b | 3.36 ± 0.49ab | 3.97 ± 0.3a | 3.30 ± 0.16ab | 3.04 ± 0.54b |
| C20:0 | 0.14 ± 0.02 | 0.13 ± 0.02 | 0.16 ± 0.01 | 0.14 ± 0.01 | 0.12 ± 0.01 |
| C21:0 | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| C22:0 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.13 ± 0.01 | 0.12 ± 0.00 | 0.10 ± 0.01 |
| C23:0 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.01± 0.00 |
| C24:0 | 0.11 ± 0.02 | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 |
| ΣSFA A | 10.43 ± 0.80b | 10.98 ± 1.43ab | 14.61 ± 0.88c | 12.86 ± 0.59ac | 12.80 ± 1.53abc |
| C14:1 | 0.04 ± 0.02 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| C15:1 | 0.06 ± 0.01 | 0.07 ± 0.02 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.05 ± 0.01 |
| C16:1 | 12.30 ± 2.03 ab | 13.36 ± 2.47 ab | 15.32 ± 2.34 a | 12.02 ± 1.15 ab | 9.57 ± 2.67 b |
| C17:1 | 0.12 ± 0.08 | 0.16 ± 0.03 | 0.16 ± 0.06 | 0.18 ± 0.03 | 0.12 ± 0.02 |
| C18:1n-9 (Z) | 3.90 ± 1.05 b | 5.09 ± 0.95 ab | 6.21 ± 0.94 a | 6.02 ± 0.88 ab | 5.64 ± 1.21 ab |
| C18:1n-9 (E) | 1.17 ± 0.48 a | 1.07 ± 0.15 ab | 1.20 ± 0.30 a | 0.93 ± 0.26 ab | 0.54 ± 0.07 b |
| C20:1 | 0.54 ± 0.22 | 0.59 ± 0.13 | 0.65 ± 0.10 | 0.63 ± 0.15 | 0.49 ± 0.12 |
| C22:1 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.02 | 0.08 ± 0.01 | 0.05 ± 0.05 |
| C24:1 | 0.19 ± 0.05 | 0.23 ± 0.07 | 0.22 ± 0.03 | 0.18 ± 0.03 | 0.16 ± 0.06 |
| Σ MUFA B | 18.42 ± 3.71 | 20.72 ± 3.56 | 23.99 ± 3.63 | 20.15 ± 2.33 | 16.67 ± 4.11 |
| C18:2n-6 (LA) | 2.61 ± 1.45 b | 3.44 ± 0.51 ab | 6.19 ± 0.58 ac | 7.11 ± 1.78 c | 6.75 ± 2.00 c |
| C18:3n-6 | 0.46 ± 0.12 | 0.47 ± 0.13 | 0.52 ± 0.05 | 0.43 ± 0.08 | 0.31 ± 0.06 |
| C18:3n-3 (LNA) | 1.38 ± 0.51 | 1.45 ± 0.52 | 1.52 ± 0.31 | 1.26 ± 0.39 | 0.75 ± 0.25 |
| C20:2n-6 | 0.21 ± 0.08 | 0.26 ± 0.04 | 0.31 ± 0.05 | 0.29 ± 0.04 | 0.27 ± 0.04 |
| C20:4n-6 (ARA) | 0.80 ± 0.14 a | 0.77 ± 0.12 a | 0.77 ± 0.08 a | 0.57 ± 0.10 ab | 0.46 ± 0.08 b |
| C20:3n-6 | 0.17 ± 0.02 ab | 0.16 ± 0.02 ab | 0.20 ± 0.03 a | 0.15 ± 0.03 ab | 0.13 ± 0.03 b |
| C20:3n-3 | 0.25 ± 0.08 | 0.34 ± 0.08 | 0.31 ± 0.06 | 0.29 ± 0.06 | 0.27 ± 0.08 |
| C20:5n-3 (EPA) | 5.68 ± 1.72 a | 5.75 ± 1.75 a | 6.42 ± 0.45 a | 4.94 ± 1.10 ab | 2.78 ± 0.85 b |
| C22:2n-6 | 0.13 ± 0.03 | 0.14 ± 0.03 | 0.14 ± 0.04 | 0.14 ± 0.01 | 0.14 ± 0.05 |
| C22:6n-3 (DHA) | 6.00 ± 1.47 a | 6.02 ± 1.28 a | 6.72 ± 1.26 a | 5.26 ± 1.09 ab | 3.28 ± 0.85 b |
| Σ PUFA C | 17.70 ± 5.45 | 18.80 ± 4.12 | 23.10 ± 2.69 | 20.44 ± 4.47 | 15.14 ± 4.16 |
| Σ n-3 PUFA D | 13.31 ± 3.67 a | 13.56 ± 3.51 a | 14.97 ± 1.96 a | 11.76 ± 2.51 ab | 7.08 ± 1.98 b |
| Σ n-6 PUFA E | 4.39 ± 1.80 b | 5.24 ± 0.78 ab | 8.13 ± 0.73 a | 8.69 ± 1.97 a | 8.06 ± 2.21 a |
| Σ SFA:Σ PUFA F | 0.64 ± 0.20 | 0.59 ± 0.06 | 0.64 ± 0.11 | 0.66 ± 0.16 | 0.88 ± 0.17 |
| Σ n-3: Σ n-6 G | 3.19 ± 0.57 a | 2.58 ± 0.46 a | 1.84 ± 0.08 b | 1.36 ± 0.04 bc | 0.88 ± 0.06 c |
Whole-body fatty acid profile expressed in µg/mg of juvenile red drum (Sciaenops ocellatus) fed experimental diets in an 8-week feeding trial.
The diets have BSFM replacing 0% (Control), 25% (BSFM25), 50% (BSFM50), 75% (BSFM75), and 100% (BSFM100) of FM. Values are the means (µg/mg) of four fish from each of five experimental groups with standard error of the mean (SEM). Different superscript letters designate significant (P < 0.05) differences. SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids. ASum of all fatty acids without double bonds; B Sum of all fatty acids with a single double bond; C Sum of all fatty acids with ≥ 2 double bonds; D Sum of all n-6 fatty acids; F Ratio between the sum of all SFA and the sum of all PUFA. G Ratio between the sum of all n-3 and the sum of all n-6 fatty acids.
3.4 Regression analysis
The second-degree polynomial equation best described the relationship between growth performance parameters and increasing dietary levels of BSFM (Table 7). The regression slope indicated a positive relationship between SGR, PER, and final weight and increasing dietary BSFM levels, while FCR had a negative relationship. The quadratic term indicated a concave relationship between SGR, PER, and final weight and graded levels of dietary BSFM, while the FCR relationship was convex. The R-squared (R2) values explained the proportion of the variance in the parameters that was predictable in the regression model.
Table 7
| Growth parameter | Regression | Equation | P | R2 |
|---|---|---|---|---|
| SGR | Quadratic | y = – 0.000113x2 + 0.0032x +1.98 | 0.000 | 0.952 |
| FCR | Quadratic | y = 0.0002x2 – 0.01x + 2.09 | 0.000 | 0.949 |
| PER | Quadratic | y = – 0.000070x2 + 0.0029x +1.02 | 0.000 | 0.936 |
| Final weight | Quadratic | y = – 0.15x2 + 8.42x +2119.8 | 0.000 | 0.829 |
| Essential amino acids | ||||
| Methionine | Quadratic | y = – 12253.9x2 + 21948.2x – 7507.2 | 0.000 | 0.833 |
| Lysine | Quadratic | y = – 2580.4x2 + 16380.1x – 23643.5 | 0.000 | 0.831 |
| Threonine | Quadratic | y = – 27525.7x2 + 97167.7x – 83376.9 | 0.000 | 0.830 |
| Arginine | Quadratic | y = – 3207.5x2 + 18863.2x – 25325.5 | 0.000 | 0.823 |
| Leucine | Quadratic | y = – 10485.7x2 + 68803.8x – 1105030.5 | 0.000 | 0.821 |
| Isoleucine | Quadratic | y = – 36364.1x2 + 148968.7x – 150260.4 | 0.000 | 0.803 |
| Phenylalanine | Quadratic | y = – 72537.2x2 + 280636.9x – 269117.0 | 0.000 | 0.760 |
| Histidine | – | n/a | ns | – |
| Tryptophan | – | n/a | ns | – |
| Valine | – | n/a | ns | – |
| Fatty acids | ||||
| C12:0 | Quadratic | y = – 24.58x2 – 95.95x + 2218.09 | 0.000 | 0.829 |
| EPA | Cubic | y = + 26.15x3 – 105.01x2 – 45.34x + 2485.99 | 0.000 | 0.805 |
| Σn3/n6 ratio | Quadratic | y = – 103.37x2 + 195.53x + 2312.5 | 0.000 | 0.787 |
| DHA | Cubic | y = + 121.54x3 – 281.11x2 – 63.12x + 2453.68 | 0.000 | 0.767 |
| Σ n-3 PUFA | Cubic | y = + 3.12x3 – 24.89x2 – 20.50x + 2466.28 | 0.000 | 0.753 |
| Σ SFA | Quadratic | y = – 24.16x2 – 101.59x + 2211.51 | 0.000 | 0.747 |
| LA | Quadratic | y = – 164.41x2 – 219.08x + 2203.54 | 0.000 | 0.703 |
| Σ n-6 PUFA | Cubic | y = – 1683.58x3 – 481.55x2 + 1607.63x + 2741.49 | 0.003 | 0.568 |
| ARA | Cubic | y = + 174837.2x3 – 29084.2x2 – 226.57x + 2329.6 | 0.001 | 0.445 |
| LNA | Linear | y = + 196.11x + 2068.43 | 0.004 | 0.379 |
Best-fit regression models for growth parameters and amino and fatty acids with P and R2 values for diets with BSFM replacing 0% (Control), 25% (BSFM25), 50% (BSFM50), 75% (BSFM75), and 100% (BSFM100) of FM.
The essential amino acid content in the diets presented a positive regression slope and concave quadratic term in relation to the final weight of fish fed increasing dietary levels of BSFM. Neither linear (P>0.05), quadratic, nor cubic models could be fitted for histidine, tryptophan, and valine (Table 7).
Fatty acid content in diets presented cubic, quadratic, and linear relationships with final weight. Lauric acid (C12:0), ∑n3/n6 ratio, ∑ SFA, and LA best fitted the quadratic model. EPA, DHA, ∑n-3 PUFA, ∑n-6 PUFA, and ARA best fitted the cubic model. The best-fit model for LNA was a linear model.
3.5 Histology of intestines and liver
The intestines of fish fed the Control diet showed goblet cells evenly dispersed throughout the mucosal epithelium with regular fold thickness in the serosa, muscularis, submucosa, and lamina propria (Table 8). The Control diet served as a baseline to determine histological variations with fish fed BSFM75 and BSFM100, showing aggregating lymphocytes, thickening of the lamina propria, and reduced goblet cell count (Figures 1d, e). Histological scoring of inflammation severity in the intestine was lowest in fish fed BSFM50 compared to those fed the Control, BSFM75, and BSFM100 diets (P<0.05) (Table 8; Figure 1). Histological measurements of the intestine showed higher serosa length in fish fed BSFM75 and BSFM100 compared to those fed the Control, BSFM25, and BSFM50 diets (P<0.05). There was no difference in the lamina propria width in the intestines of fish fed the BSFM25, BSFM50, and BSFM75 diets with respect to other treatments. Wider mucosal epithelium in BSFM50 and lower goblet cell counts were measured in fish fed the BSFM75 and BSFM100 diets compared to those fed the other diets.
Table 8
| Control | BSFM25 | BSFM50 | BSFM75 | BSFM100 | |
|---|---|---|---|---|---|
| Intestine | |||||
| Inflammation severity | 1.28 ± 0.11a | 0.89 ± 0.08ab | 0.73 ± 0.01b | 1.34 ± 0.10a | 1.53 ± 0.11a |
| Serosa length (µm) | 6.37 ± 0.20b | 6.58 ± 0.14b | 6.51 ± 0.10b | 7.71 ± 0.15a | 7.78 ± 0.20a |
| Muscularis length (µm) | 65.42 ± 2.65 | 63.04 ± 2.21 | 72.67 ± 2.60 | 70.76 ± 2.11 | 66.40 ± 3.54 |
| Submucosa length (µm) | 83.84 ± 2.94 | 80.56 ± 2.71 | 86.44 ± 3.15 | 89.24 ± 2.21 | 83.90 ± 3.42 |
| Lamina propria width (µm) | 75.68 ± 3.72a | 52.29 ± 2.11c | 48.05 ± 2.72c | 47.11 ± 1.80c | 65.05 ± 3.55a |
| Mucosa width (µm) | 30.98 ± 1.60b | 26.91 ± 1.14b | 32.23 ± 0.59a | 25.66 ± 1.08b | 28.38 ± 1.20b |
| Goblet cells/area | 155.88 ± 10.83a | 112.54 ± 5.42b | 98.32 ± 3.91b | 68.89 ± 6.06c | 59.02 ± 2.79c |
| Liver | |||||
| Inflammation severity | 0.40 ± 0.07d | 0.36 ± 0.06d | 0.74 ± 0.08c | 1.23 ± 0.09b | 1.68 ± 0.10a |
| Vacuole number | 3372 ± 212b | 3014 ± 98b | 4276 ± 158ab | 4158 ± 135ab | 4373 ± 206a |
Histological scoring of the intestines and livers of juvenile red drum (Sciaenops ocellatus) fed experimental diets in an 8-week feeding trial.
The diets have BSFM replacing 0% (Control), 25% (BSFM25), 50% (BSFM50), 75% (BSFM75), and 100% (BSFM100) of FM.Superscript letters indicate significant defferences among experimental groups.
The livers of fish fed the Control diet showed regular vacuoles and sinusoid organization with discrete margins and intact pancreatic exocrine tissue (Figure 2a), while fish fed BSFM100 presented swollen, coalescing vacuoles, signs of pancreatic exocrine tissue breakdown, and fibrotic tissue formation (Figure 2d). Liver inflammation severity scores were the highest in BSFM100 compared to fish fed the other diets (P<0.05). A higher vacuole number was observed in the BSFM100 treatment with respect to the Control and BSFM25 treatment (Table 8; Figure 2).
3.6 Gut microbiome
After taxonomy-based filtering, rare ASV removal, and eliminating samples with <1,000 sequences, there were 2,912 remaining ASVs, which represented 27 phyla, including 26 named phyla, 151 orders (125 named), 64 classes (59 named), 263 families (186 named), and 444 genera (299 named). The mean Shannon diversity was 3.26 +/- 0.69 across all samples with the highest mean diversity across all BSFM25 samples (3.52 +/- 0.58) and the lowest mean across all BSFM75 samples (3.04 +/- 0.83) (Supplementary Figure 1a). Kruskal–Wallis testing revealed that there were no differences between treatments across all samples (chi-squared = 3.7005, p-value = 0.448) and pairwise upon Dunn testing. PCoA analysis did not reveal a distinct visual separation of isolates by BSFM percentage, with the best view of the PCoA only representing 9.7% of the total variation (Supplementary Figure 1b). PERMANOVA analysis of Bray–Curtis dissimilarities confirms that there were no differences in the overall microbiome between treatments either overall (R2 = 0.070, Pseudo-F value = 1.04, and p-value= 0.22) or pairwise.
The most common genera across all samples were Psudomonas (12.3+/-17.6), Muribaculaceae (7.6+/-11.0%), Escherichia-Shigella (4.9+/-5.7%), Vibrio (4.2+/-8.4%), Serratia (4.0+/-5.8%), and Enterovibrio (2.8+/-10.5%) (Figure 5). Of the genera that had a relative percentage greater than 1% across all samples Photobacterium (4.2x in BSFM25, 1.7x in BSFM50, 142.5x in BSFM75, and 120.5x in BSFM100), Stenotrophomonas (3.0x, 1.7x, 7.4x, and 2.2x), Vibrio (2.4x, 14.8x, 4.9x, and 22.5x), Sphingomonas (2.8x, 4.5x, 1.2x, and 3.5x), Bacteroides (1.7x, 2.4x, 1.1x, and 1.5x), and Bacillus (1.6x, 1.7x, 1.5x, and 1.6x) were higher in all BSFM diets compared to the Control diet (Supplementary Table 1, Supplementary Figures 1a, b). In contrast, Staphylococcus (4.4x, 5.7x, 1.8x, and 15.4x) and Marinobacter (2.2x, 5.6x, 2.6x, and 17.3x) were higher in the Control diet than in all the BSFM diets. ANCOMBC2 did not identify any differentially abundant genera in any BSFM diets in comparison with the Control diet.
Figure 5
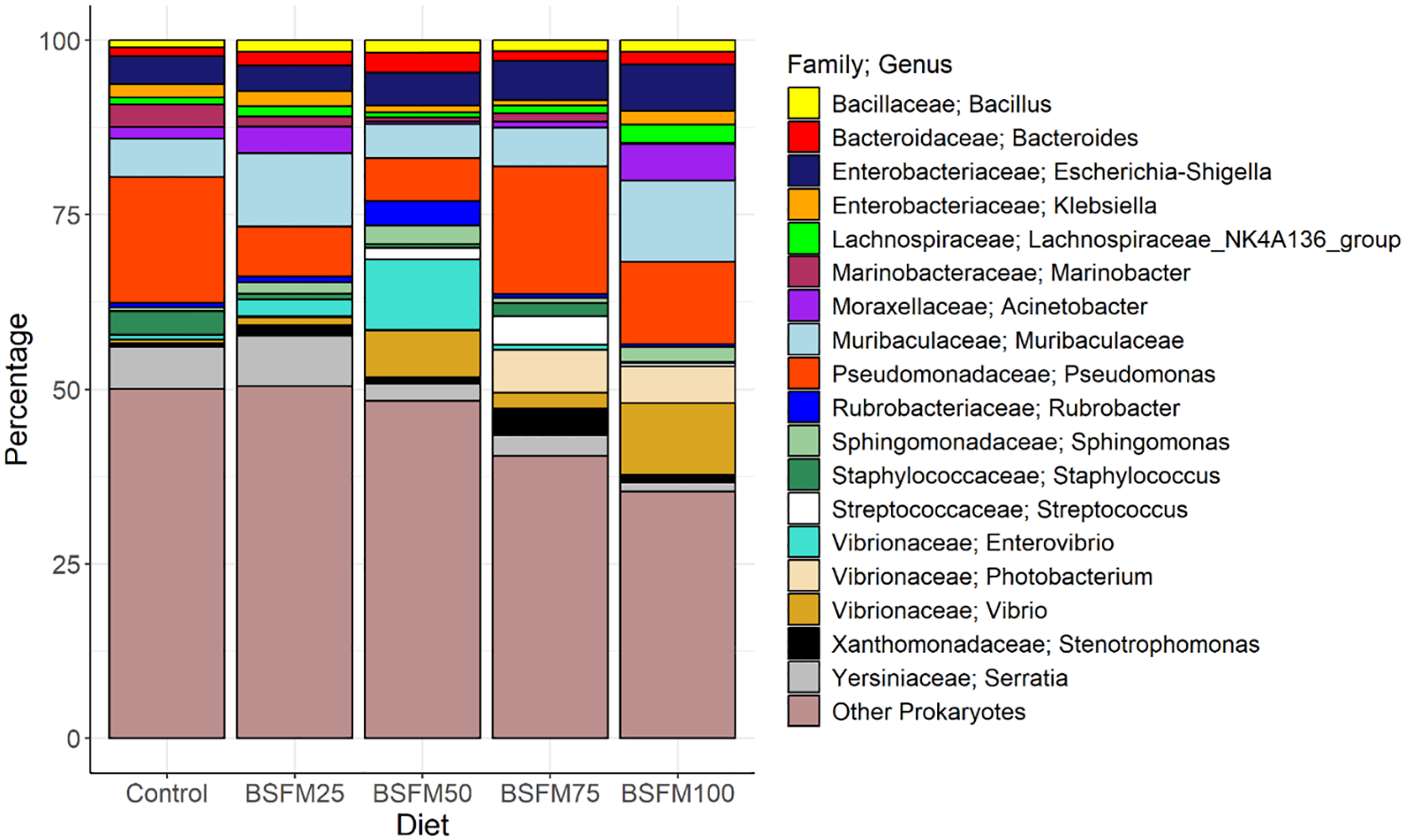
Stacked bar charts of the genera that represented greater than 1% of the population across all samples separated by diet for diets with BSFM replacing FM at 0% (Control), 25% (BSFM25), 50% (BSFM50), 75% (BSFM75) and 100% (BSFM100). Control is 100% fish meal while BSFM stands for black soldier fly meal with the numbers next to it in each diet name representing the BSFM replacement percentage. Family/Genus is represented by color with Other Prokaryotes containing the genera that represented less than 1% of the total population.
4 Discussion
The palatability of feed is a crucial factor when replacing FM with alternative protein sources in diets. The chemosensory properties of a new ingredient in formulated feeds will influence consumption as less or unpalatable diets lead to increased waste from uneaten feeds with clear negative impacts on operating costs (Al-Souti et al., 2019). In this study, the palatability assessment of the BSFM25 diet resulted in similar behavioral responses compared to fish fed the Control diet. This indicates that the fish feeding response towards diets formulated with BSFM (10.7 g/100g in diets) replacing FM as a protein source showed an adequate chemosensory response associated with feeding consumption. To our knowledge, these results constitute the first report on the palatability of feed with BSFM replacing FM in diets for red drum. The palatability response to the BSFM50 diet suggests that pellets containing BSFM at 21.4 g/100g in diets were less palatable for red drum. This could be due to higher levels of saturated fatty acids and off-flavor and odor-active compounds added with increased inclusion of dietary BSFM, as reported in Huseynli et al. (2023) (off-flavor/odor-active compounds were not analyzed here). Exploring methods to enhance diet palatability could be worthwhile. It was curious, however, that this trend did not continue, showing no difference in feed consumption recorded in fish fed BSFM100 compared to the Control. This could be related to the fish compensating for an imbalanced nutrient content, or less digestible energy, in diets formulated with no FM. Observations of the excessive hepatic vacuolization seem to support the suggestion that there was decreased digestible energy for red drum that was stored as lipids in the liver. Higher feed intake due to dietary imbalances has been reported in several species, including gilthead sea bream (Sparus aurata), Nile tilapia (Oreochromis niloticus), and pirarucu (Arapaima gigas) (Mattos et al., 2016; Montoya et al., 2012; Norambuena et al., 2012). Overall, as fish rely on their senses of smell, taste, and sight to discriminate between feeds that provide pleasant or unpleasant chemosensory feedback associated with eating, it is paramount to evaluate the palatability of a new ingredient when formulating aquafeeds.
In the feeding trial, BSFM replacing FM showed high final weight in fish fed BSFM50 from 2 weeks onwards, indicating that BSFM up to 21.4 g/100g is feasible in diets for red drum as an alternative protein source (Figure 4). By the end of the 8-week feeding trial, the growth parameters showed that the BSFM25 treatment (10.7 g/100g in diet) led to similar growth and feeding efficiency compared to the Control. It should be highlighted that most of the total amino acids in the BSFM25, BSFM50, BSFM75, and BSFM100 diets were numerically lower than in the Control diet (Table 2), which may explain the lower growth in fish fed increasing dietary inclusions of BSFM. Accordingly, Yamamoto et al. (2022) demonstrated that red drum had similar growth when fed a FM-based control diet compared to a diet replacing 65% of FM with BSFLM (10.0 g/100g in diet) (when the larvae were fed a Gainesville diet), but the diets were supplemented with essential amino acids and taurine. Moreover, another study showed that it was possible to completely replace FM when using a mixture of animal and plant-based proteins, but essential amino acids were supplemented, including taurine (Suehs and Gatlin, 2022). Velasquez et al. (2015) found that adding 1% of taurine to red drum diets significantly improved growth when fed a plant-based diet when either extruded or cold pelleted. In this study, taurine was not supplemented, which was reflected in both decreasing dietary and whole-body taurine levels in the red drum fed diets with increasing BSFM inclusions. Thus, it may be possible that higher inclusions of BSFM are possible if amino acids are supplemented in the red drum diets. However, there are indications that the reduced growth in the red drum may also have been due to some adverse histopathological changes to their internal organs.
Some studies have shown that BSFM can cause hepatic vacuolization as observed in zebrafish (Danio rerio) (Cardinaletti et al., 2019; Zarantoniello et al., 2020) and rainbow trout (Randazzo et al., 2021) and, in some cases, inflammation and localized necrosis/hemorrhaging, as observed in Jian carp (Cyprinus carpio) (Li et al., 2017) and largemouth bass (Fischer et al., 2022). In this study, the most obvious pathology was hepatic vacuolization, especially in fish fed the BSFM100 diet, which was likely due to excessive lipid accumulation. In particular, coalescing lipid vacuoles and early fibrotic tissue appeared in the livers of fish fed BSFM75, followed by severe vacuolization and hepatocyte degeneration in those fed BSFM100. The main fatty acid within BSFM is lauric acid (C12), which substantially increased with increasing dietary BSFM inclusions that eventually became seven-fold higher in the whole bodies of red drum fed the BSFM100 diet compared to the Control. This finding is consistent with those in rainbow trout (Cardinaletti et al., 2019; Bruni et al., 2020; Randazzo et al., 2021) and in red drum (Yamamoto et al., 2022). Thus, it appears that red drum are unable to adequately process such high levels of dietary lauric acid and thus, it is stored in the liver. Excessive dietary chitin within BSFLM may have acted as an ANF (Kroeckel et al., 2012), and some of the suggested actions include decreasing bile production and thus reducing the efficiency of lipases (Weththasinghe et al., 2021). Even low chitin inclusion levels of 1% reduced feed intake and growth in Atlantic salmon (Olsen et al., 2006). In this study, the BSFL were harvested during early to mid-developmental stages, when chitin content is relatively low (data not analyzed), to produce the BSFM protein. However, more research is required to evaluate the responses of red drum to BSFL-derived chitin.
Generally, the lipid composition of the fish reflects that of the diets (Turchini et al., 2009), which was similarly the trend in this study. The results in this study showed that EPA, DHA, and Σ n-3 PUFA levels (Table 6) were similar in fish fed BSFM25 and BSFM50 compared to those in fish fed the Control diet. This indicates that including BSFM as a protein alternative up to 21.4 g/100g in diets for red drum maintains high levels of EPA, DHA, and Σ n-3 PUFA, beneficial for human consumption (Young, 2009). The results showed that most whole-body fatty acid profiles reflected the fatty acid profile of the experimental diets, as previously reported in red drum (Yamamoto et al., 2022). An accumulation of saturated fatty acids (Σ SFA) and reduced concentration of LC-PUFA was observed in fish fed increasing levels of BSFM (Table 6), as also reported in Nile tilapia, rainbow trout, and meagre (Argyrosomus regius) when fed increasing levels of BSFM in diets (Agbohessou et al., 2021; Guerreiro et al., 2020; Renna et al., 2017).
Lauric acid is characteristically high in BSFM, and in animals, lauric acid has antimicrobial and anti-obesity properties that can potentially enhance post-harvest quality and provide health benefits to the consumer (Alfhili and Aljuraiban, 2021). The results in this study showed that lauric (C12) acid content in the whole-body analyses increased with increasing BSFM levels in the diets, with the highest values in fish fed BSFM75 and BSFM100. Previous investigations reported species-specific responses, with Atlantic salmon rapidly utilizing lauric acid for energy (Belghit et al., 2019) while rainbow trout and largemouth bass stored it in muscle and the liver (Bruni et al., 2020; Fischer et al., 2022). Overall, the intestines of fish fed BSFM50 were observed, and scoring indicated a better condition compared to the other treatments, possibly due to the health benefits of lauric acid, as previously reported by Kumar et al. (2021) and Randazzo et al. (2021). There were, however, trends that consistently changed with increasing BSFM inclusions, such as the goblet cell count. The goblet cell count decreased with increasing dietary levels of BSFM, with the lowest values in fish fed BSFM75 and BSFM100. A reduction in goblet cells could be indicative of insufficient or imbalanced amino acid or fatty acid nutrients required for mucin synthesis and epithelial maintenance (Seo et al., 2022). This mucus secretion enhances intestinal lubrication, motility, and digestion, thus making the goblet cell count an indicator of gut health (Seo et al., 2022). Overall, it appeared that red drum fed the Control, BSFM25, and BSFM50 diets showed similar intestinal structures, with intact mucosal folds and goblet cell distribution. However, inflammation and tissue alterations were observed in BSFM75, with compressed folds and lymphocyte aggregation, while BSFM100 showed edema and a low number of goblet cells, demonstrating an increased inflammatory response.
The intestinal health of fish is greatly influenced by the microbiome, which can either aid in digestion and produce beneficial byproducts or others that are pathogenic and can produce toxins (Xiong et al., 2019). In this study, the microbiome did not have any overall differences across BSFM diets in either alpha diversity (Shannon’s) or beta diversity (Bray–Curtis), which is in contrast to results of Yamamoto et al. (2022) when red drum were fed diets where 65% of FM was replaced by BSFM. Alpha diversity differences between experimental diets with BSFM are not consistent in the literature, with some studies reporting only one alpha metric (Yamamoto et al., 2022) while others reported higher alpha diversity (Oktay et al., 2024). Although ANCOMBC2 did not identify any differentially abundant genera, there were patterns that could be explored based on the Control and across all BSFM diets. Thus, Yamamoto et al. (2022) identified distinct shifts in intestinal microbiota composition depending on the BSFM substrate. Streptococcus and Flavobacterium were more abundant in the control group, while a higher percentage of Lactobacillus and Corynebacterium appeared in fish fed BSFM from larvae reared on spent brewer’s grain. Actinomyces, Enterococcus, Gracilibacillus, and Oceanobacillus were higher in fish fed BSFM from larvae raised on the Gainesville substrate. None of these genera had similar patterns in this study, which is consequential considering the salinity differences between the brackish water used in the study by Yamamoto et al. (2022) compared with the higher salinity of 29.9 ppt in this study. This is consistent with Bradshaw et al. (2023), who described microbial changes between increasing raring salinity conditions in Florida pompano. Photobacterium, Vibrio, and Bacillus, which are consistently found in fish gut microbiomes (Ghanbari et al., 2015), were also significantly higher in the gut microbiome of red seabream (Pagrus major) in BSFM samples in comparison to their control samples (Oktay et al., 2024). This study also found that there were higher Paenibacillus and Oceanobacillus levels in the BSFM samples. Bacillus is a well-known probiotic that has been associated with immune stimulation and improved disease resistance in various fish species (Ma et al., 2022; Oktay et al., 2024). Both Vibrio and Photobacterium are associated with chitinase and some of their species are considered fish pathogens, while Photobacterium is further associated with lipase (Gomaa, 2021; Labella et al., 2018; Oktay et al., 2024). Stenotrophomonas has a limited nutritional spectrum and is a nitrate reducer whose type species (S. maltophilia) is a human pathogen and is typically found in the soil environment, but can also produce chitinase and lipase (Palleroni and Bradbury, 1993; Ryan et al., 2009). While many of the genera that were found to be exclusively higher in fish fed BSFM diets may contribute to digestion by producing digestive enzymes, some are also linked to pathogenesis in fish.
It can be difficult to determine the cause(s) for lower growth, and thus statistical analyses were run to potentially detect any relationships. Polynomial regression described the relationship between growth, amino, and fatty acids and the R2 value accounting for the degree of change due to BSFM replacing FM in experimental diets (Table 7). The R2 values for SGR, FCR, and PER appeared compellingly high, indicating that the variables analyzed are well-suited with relatively little variation attributable to factors other than increasing dietary levels of BSFM. Similar occurrences were found for the amino acids and fatty acids in quadratic terms with convex (>0) and concave (<0) shapes, indicating synergy or independence among the factors, respectively. It should be acknowledged that a high correlation between variables does not mean causation. Overall, these polynomial models can help feed formulators establish multiple correlations, integrating amino acids and fatty acids to optimize growth while achieving higher R² values by balancing protein and fat from diverse sources in varying proportions.
5 Conclusions
This study provided new data on the optimal inclusion levels of BSFM, which found that BSFM can replace 25% of FM based on the growth performance, feeding efficiency, and acceptable palatability in red drum. However, these results were negatively impacted when BSFM replaced 50% or above of FM, which was likely due to a combination of deficiencies in dietary amino acids and deleterious changes to the liver and intestinal histopathology that indicate reduced nutrient utilization. Overall, BSFM proved to be a viable alternative protein source, although ways to increase the nutrient load of BSFL to meet the needs of red drum and the influence of chitin on the intestinal microbiome and histopathology should be explored.
Statements
Data availability statement
The data presented in the study are deposited in the NCBI Sequence Read Archive repository, accession number BioProject PRJNA1194735.
Ethics statement
The use of red drum was performed under scientific research protocols of Florida Atlantic University and Institutional Animal Care and Use Committee (IACUC Protocol numbers: A20-29 and A-23-24) complying with all local and/or international animal welfare laws and guidelines. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JP: Data curation, Supervision, Methodology, Investigation, Conceptualization, Writing – review & editing, Writing – original draft, Software, Project administration, Visualization, Funding acquisition, Resources, Validation, Formal analysis. MR: Conceptualization, Writing – review & editing. DB: Supervision, Conceptualization, Visualization, Writing – review & editing, Investigation, Software, Methodology, Data curation, Resources, Funding acquisition, Validation, Project administration, Writing – original draft, Formal analysis. SM: Data curation, Methodology, Conceptualization, Investigation, Formal analysis, Writing – review & editing. LC: Formal analysis, Writing – review & editing. JP: Writing – review & editing, Formal analysis, Methodology, Investigation. RP: Validation, Visualization, Writing – review & editing, Conceptualization. NR: Writing – review & editing, Conceptualization, Writing – original draft, Investigation, Resources, Validation, Visualization, Methodology. PW: Funding acquisition, Writing – review & editing, Formal analysis, Resources, Writing – original draft, Visualization, Project administration, Methodology, Investigation, Validation, Conceptualization, Data curation, Supervision, Software.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the U.S. Department of Agriculture, Agricultural Research Service by Cooperative Agreement Numbers 59-6034-9-007 and 59-6034-4-003 with Florida Atlantic University’s Harbor Branch Oceanographic Institute.
Acknowledgments
The authors are grateful to the staff of Aquaculture and Stock Enhancement, Harbor Branch, FAU, for their help during fish stocking, feeding, and sampling. The authors also extend a special thanks to Zachary Nilles, Christopher Robinson, Alex Dodge, Erica Slaten, Maddy Wheeler, Richard Mulroy, Stephanie Rutkowski, Tyler Bianchine, Michael Aina, and Jenna Baggett for helping with daily RAS maintenance. Thanks to Susan Laramore and Caitlyn Courtemanche for histology assistance. The authors would also like to thank Eric Latimer, Duke Energy Crystal River Mariculture Center, and Brian Gorski, Coastal Conservation Association Florida (CCA Florida), for donating the red drum for this project.
Conflict of interest
Author SM was employed by the company Nofima AS. Author RP was employed by the company River Stratium LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
Any mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply a recommendation or endorsement by the U.S. Department of Agriculture (USDA). USDA is an equal opportunity provider and employer.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/faquc.2025.1619878/full#supplementary-material
References
1
Agbohessou P. S. Mandiki S. N. M. Gougbédji A. Megido R. C. Hossain M. S. De Jaeger P. et al . (2021). Total replacement of fish meal by enriched-fatty acid Hermetia illucens meal did not substantially affect growth parameters or innate immune status and improved whole body biochemical quality of Nile tilapia juveniles. Aquaculture Nutr.27, 880–896. doi: 10.1111/anu.13232
2
Alfhili M. A. Aljuraiban G. S. (2021). Lauric acid, a dietary saturated medium-chain fatty acid, elicits calcium-dependent eryptosis. Cells10. doi: 10.3390/cells10123388
3
Al-Souti A. Gallardo W. Claereboudt M. Mahgoub O. (2019). Attractability and palatability of formulated diets incorporated with chicken feather and algal meals for juvenile gilthead seabream, Sparus aurata. Aquaculture Rep.14. doi: 10.1016/j.aqrep.2019.100199
4
AOAC (2003). Official Methods of Analysis of the Association of Official Analytical Chemists. 15th edn (Arlington, Virginia: Association of Official Analytical Chemists).
5
Belghit I. Waagbø R. Lock E. J. Liland N. S. (2019). Insect-based diets high in lauric acid reduce liver lipids in freshwater Atlantic salmon. Aquaculture Nutr.25, 343–357. doi: 10.1111/anu.12860
6
Bolyen E. Rideout J. R. Dillon M. R. Bokulich N. A. Abnet C. C. Al-Ghalith G. A. et al . (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol.37, 8. doi: 10.1038/s41587-019-0209-9
7
Bradshaw D. J. II Dickens N. J. Trefry J. H. McCarthy P. J. (2020). Defining the sediment prokaryotic communities of the Indian River Lagoon, FL, USA, an Estuary of National Significance. PLoS One15, e0236305. doi: 10.1371/JOURNAL.PONE.0236305
8
Bradshaw D. J. Perricone C. S. King L. E. Allmon E. B. Sepúlveda M. Riche M. et al . (2023). Commercial production of Florida pompano (Trachinotus carolinus) larvae at low salinity induces variable changes in whole-larvae microbial diversity, gene expression, and gill histopathology. Front. Mar. Sci.10. doi: 10.3389/fmars.2023.1158446
9
Bruni L. Randazzo B. Cardinaletti G. Zarantoniello M. Mina F. Secci G. et al . (2020). Dietary inclusion of full-fat Hermetia illucens prepupae meal in practical diets for rainbow trout (Oncorhynchus mykiss): Lipid metabolism and fillet quality investigations. Aquaculture529, 735678. doi: 10.1016/J.AQUACULTURE.2020.735678
10
Callahan B. J. McMurdie P. J. Rosen M. J. Han A. W. Johnson A. J. A. Holmes S. P. (2016). DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods13, 7. doi: 10.1038/nmeth.3869
11
Cardinaletti G. Randazzo B. Messina M. Mugetti D. Radaelli G. Giorgini E. et al . (2019). Effects of dietary inclusion of full-fat Hermetia illucens prepupae in zebrafish (Danio rerio). Aquaculture498, 1–6. doi: 10.1016/j.aquaculture.2018.08.052
12
Castillo S. Gatlin D. M. (2018). Dietary requirements for leucine, isoleucine and valine (branched-chain amino acids) by juvenile red drum Sciaenops ocellatus. Aquaculture Nutr.24, 1056–1065. doi: 10.1111/anu.12644
13
Davis J. T. (1990). Southern Regional Aquaculture Center. NINETEENTH ANNUAL PROGRESS REPORT. Stoneville, Mississippi 38776.
14
Dusa A. (2022). venn: Draw Venn Diagrams. Available online at: https://CRAN.R-project.org/package=venn (Accessed October 10, 2024).
15
Ellawidana D. Mutucumarana R. K. H.A. D. R. Magamage M. S. (2023). Nutritional composition and apparent metabolizable energy (AME) value of black soldier fly larvae (Hermetia illucens L.) full-fat meal for broiler chickens. Turkish J. Agric. - Food Sci. Technol.11, 1825–1833. doi: 10.24925/turjaf.v11i10.1825-1833.5992
16
Erbland P. Alyokhin A. Perkins L. B. Peterson M. (2020). Dose-dependent retention of omega-3 fatty acids by black soldier fly larvae (Diptera: Stratiomyidae). J. Economic Entomology113, 1221–1226. doi: 10.1093/jee/toaa045
17
FAO IFAD UNICEF WFP WHO . (2024). The State of Food Security and Nutrition in the World 2024 – Financing to end hunger, food insecurity and malnutrition in all its forms. Rome. doi: 10.4060/cd1254en
18
Fischer H. Romano N. Renukdas N. Kumar V. Sinha A. K. (2022). Comparing black soldier fly (Hermetia illucens) larvae versus prepupae in the diets of largemouth bass, Micropterus salmoides: Effects on their growth, biochemical composition, histopathology, and gene expression. Aquaculture546. doi: 10.1016/j.aquaculture.2021.737323
19
Fisher H. J. Collins S. A. Hanson C. Mason B. Colombo S. M. Anderson D. M. (2020). Black soldier fly larvae meal as a protein source in low fish meal diets for Atlantic salmon (Salmo salar). Aquaculture521. doi: 10.1016/j.aquaculture.2020.734978
20
Folch J. Lees M. Stanley G. H. S. (1957). A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry. 226 (1), 497–509. doi: 10.1016/S0021-9258(18)64849-5
21
Fox J. Weisberg S. (2019). An R Companion to Applied Regression (Third) (Sage). Available online at: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (Accessed October 10, 2024).
22
Gatlin D. M. Barrows F. T. Brown P. Dabrowski K. Gaylord T. G. Hardy R. W. et al . (2007). “Expanding the utilization of sustainable plant products in aquafeeds: A review,” in Aquaculture Research, vol. 38, 551–579. doi: 10.1111/j.1365-2109.2007.01704.x
23
Ghanbari M. Kneifel W. Domig K. J. (2015). A new view of the fish gut microbiome: Advances from next-generation sequencing. Aquaculture448, 464–475. doi: 10.1016/j.aquaculture.2015.06.033
24
Glencross B. D. Booth M. Allan G. L. (2007). A feed is only as good as its ingredients - A review of ingredient evaluation strategies for aquaculture feeds. Aquaculture Nutrition13, 17–34. doi: 10.1111/j.1365-2095.2007.00450.x
25
Gomaa E. Z. (2021). Microbial chitinases: properties, enhancement and potential applications. Protoplasma258, 4. doi: 10.1007/S00709-021-01612-6
26
Guerreiro I. Castro C. Antunes B. Coutinho F. Rangel F. Couto A. et al . (2020). Catching black soldier fly for meagre: Growth, whole-body fatty acid profile and metabolic responses. Aquaculture516, 734613. doi: 10.1016/J.AQUACULTURE.2019.734613
27
Henry M. Gasco L. Piccolo G. Fountoulaki E. (2015). “Review on the use of insects in the diet of farmed fish: Past and future,” in Animal Feed Science and Technology . (Elsevier B.V) 23 (1), 1–22. doi: 10.1016/j.anifeedsci.2015.03.001
28
Hua K. (2021). A meta-analysis of the effects of replacing fish meals with insect meals on growth performance of fish. Aquaculture530. doi: 10.1016/j.aquaculture.2020.735732
29
Huseynli L. Parviainen T. Kyllönen T. Aisala H. Vene K. (2023). Exploring the protein content and odor-active compounds of black soldier fly larvae for future food applications. Future Foods7, 100224. doi: 10.1016/J.FUFO.2023.100224
30
Köster J. Rahmann S. (2012). Snakemake-a scalable bioinformatics workflow engine. Bioinformatics28, 2520–2522. doi: 10.1093/bioinformatics/bts480
31
Kroeckel S. Harjes A. G. E. Roth I. Katz H. Wuertz S. Susenbeth A. et al . (2012). When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute — Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture364–365, 345–352. doi: 10.1016/J.AQUACULTURE.2012.08.041
32
Krueger F. (2020). Trim Galore (Babraham Bioinformatics). Available online at: https://github.com/FelixKrueger/TrimGalore (Accessed October 10, 2024).
33
Kumar V. Fawole F. J. Romano N. Hossain M. S. Labh S. N. Overturf K. et al . (2021). Insect (black soldier fly, Hermetia illucens) meal supplementation prevents the soybean meal-induced intestinal enteritis in rainbow trout and health benefits of using insect oil. Fish Shellfish Immunol.109, 116–124. doi: 10.1016/J.FSI.2020.12.008
34
Labella A. M. Castro M. D. ManChado M. Borrego J. J. (2018). “Description of new and amended clades of the genus photobacterium,” in Microorganisms 2018, vol. 6 (1), 24. doi: 10.3390/MICROORGANISMS6010024
35
Lepage G. Roy C. C. (1984). Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J. Lipid Res.25, 1391. doi: 10.1016/S0022-2275(20)34457-6
36
Li S. Ji H. Zhang B. Zhou J. Yu H. (2017). Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture477, 62–70. doi: 10.1016/J.AQUACULTURE.2017.04.015
37
Liao Y. C. Chen L. S. Shao K. T. (2010). The predatory Atlantic red drum, Sciaenops ocellatus, has invaded the western Taiwanese coast in the Indo-West Pacific. Biol. Invasions12, 1961–1965. doi: 10.1007/s10530-009-9642-x
38
Lin H. Das Peddada S. (2020). Analysis of compositions of microbiomes with bias correction. Nat. Commun.11, 1. doi: 10.1038/s41467-020-17041-7
39
Lin H. Eggesbø M. Das Peddada S. (2022). Linear and nonlinear correlation estimators unveil undescribed taxa interactions in microbiome data. Nat. Commun.13, 1–16. doi: 10.1038/s41467-022-32243-x
40
Lock E. R. Arsiwalla T. Waagbø R. (2016). Insect larvae meal as an alternative source of nutrients in the diet of Atlantic salmon (Salmo salar) postsmolt. Aquaculture Nutr.22, 1202–1213. doi: 10.1111/anu.12343
41
Love M. Anders S. Huber W. (2021). DESeq2: Differential gene expression analysis based on the negative binomial distribution. Available online at: https://github.com/mikelove/DESeq2 (Accessed October 10, 2024).
42
Ma S. Yu D. Liu Q. Zhao M. Xu C. Yu J. (2022). Relationship between immune performance and the dominant intestinal microflora of turbot fed with different Bacillus species. Aquaculture549, 737625. doi: 10.1016/J.AQUACULTURE.2021.737625
43
Magalhães R. Sánchez-López A. Leal R. S. Martínez-Llorens S. Oliva-Teles A. Peres H. (2017). Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture476, 79–85. doi: 10.1016/J.AQUACULTURE.2017.04.021
44
Makkar H. P. S. Tran G. Heuzé V. Ankers P. (2014). “State-of-the-art on use of insects as animal feed,” in Animal Feed Science and Technology, vol. 197. (Elsevier B.V), 1–33. doi: 10.1016/j.anifeedsci.2014.07.008
45
Mangiafico S. (2022). rcompanion: Functions to Support Extension Education Program Evaluation. Available online at: https://CRAN.R-project.org/package=rcompanion (Accessed October 10, 2024).
46
Mastoraki M. Vlahos N. Patsea E. Chatzifotis S. Mente E. Antonopoulou E. (2020). The effect of insect meal as a feed ingredient on survival, growth, and metabolic and antioxidant response of juvenile prawn Palaemon adspersus (Rathke 1837). Aquaculture Res.51, 3551–3562. doi: 10.1111/are.14692
47
Matlock G. C. (1990). The life history of red drum. Available online at: https://www.researchgate.net/publication/252321167 (Accessed October 10, 2024).
48
Mattos B. O. de Nascimento-Filho E. C. T. dos Anjos-Santos A. Sánchez-Vázquez F. J. Fortes-Silva R. (2016). Daily self-feeding activity rhythms and dietary self-selection of pirarucu (Arapaimagigas). Aquaculture465, 152–157. doi: 10.1016/J.AQUACULTURE.2016.09.005
49
McMurdie P. J. Holmes S. (2013). phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS One8. doi: 10.1371/journal.pone.0061217
50
Montoya A. Zamora S. Sánchez-Vázquez F. J. (2012). Dietary selection by gilthead sea bream (Sparus aurata) provided with unbalanced mixed-macronutrient feeds dispensed from self-feeders. Aquaculture358–359, 35–40. doi: 10.1016/J.AQUACULTURE.2012.06.008
51
Moutinho S. Peres H. Martins N. Serra C. Santos R. A. Monroig Ó. et al . (2024). Use of black soldier fly (Hermetia illucens) larvae meal in diets for gilthead seabream juveniles: Effects on growth-related gene expression, intermediary metabolism, digestive enzymes, and gut microbiota modulation. Aquaculture580, 740357. doi: 10.1016/J.AQUACULTURE.2023.740357
52
Norambuena F. Estévez A. Sánchez-Vázquez F. J. Carazo I. Duncan N. (2012). Self-selection of diets with different contents of arachidonic acid by Senegalese sole (Solea Senegalensis) broodstock. Aquaculture, 364–365, 198–205. doi: 10.1016/j.aquaculture.2012.08.016
53
NRC (2011). NRC (National Research Council). Nutrient requirements of fish and shrimp (Washington D.C: National Academy Press).
54
Ogle D. H. Doll J. C. Wheeler P. Dinno A. (2022). FSA: Stock Fisheries Analysis. Available online at: https://github.com/fishR-Core-Team/FSA (Accessed October 10, 2024).
55
Oksanen J. Simpson G. L. Blanchet F. G. Kindt R. Legendre P. Minchin P. R. et al . (2022). vegan: Community Ecology Package. Available online at: https://CRAN.R-project.org/package=vegan (Accessed October 10, 2024).
56
Oktay O. Seong T. Kabeya N. Morioka S. Liu C. M. Kobayashi T. et al . (2024). Can black soldier fly meal in diets improve gut microbiota diversity, nutrient digestibility, and growth response of marine fish? A study on red sea bream Pagrus major. Fisheries Sci.90, 773–786. doi: 10.1007/S12562-024-01807-9/FIGURES/3
57
Olsen R. E. Suontama J. Langmyhr E. Mundheim H. Ringø E. Melle W. et al . (2006). The replacement of fish meal with Antarctic krill, Euphausia superba in diets for Atlantic salmon, Salmo salar. Aquaculture Nutr.12, 280–290. doi: 10.1111/j.1365-2095.2006.00400.x
58
Palleroni N. J. Bradbury J. F. (1993). Stenotrophomonas, a new bacterial genus for Xanthomonas maltophilia (Hugh 1980) Swings et al. 1983. Int. J. Systematic Bacteriology43, 606–609. doi: 10.1099/00207713-43-3-606/CITE/REFWORKS
59
Palma M. Bledsoe J. W. Tavares L. C. Romano N. Small B. C. Viegas I. et al . (2021). Digesta and plasma metabolomics of rainbow trout strains with varied tolerance of plant-based diets highlights potential for non-lethal assessments of enteritis development. Metabolites11. doi: 10.3390/metabo11090590
60
Parrish C. C. (1999) Determination of total lipid, lipid classes, and fatty acids in aquatic samples. Lipids in Freshwater Ecosystems. ArtsM. T.WainmanB. C.. (New York: Springer). 4–20. doi: 10.1007/978-1-4612-0547-0_2
61
Paulson J. Olson N. Braccia D. Wagner J. Talukder H. Pop M. et al . (2024).metagenomeSeq: Statistical analysis for sparse high-throughput sequncing. Bioconductor package. Available online at: http://www.cbcb.umd.edu/software/metagenomeSeq (Accessed October 10, 2024).
62
Peng K. Mo W. Xiao H. Wang G. Huang Y. (2021). Effects of black soldier fly pulp on growth performance, histomorphology and lipid metabolism gene expression of Micropterus salmoides. Aquaculture Rep.20. doi: 10.1016/j.aqrep.2021.100737
63
Priyadarshana M. K. C. Walpita C. N. Ruwandeepika H. A. D. Magamage M. P. S. (2022). Effects of black soldier fly, Hermetia illucens (Linnaeus 1758), larvae incorporated feed on histomorphology, gut microbiota and blood chemistry of cultured fishes: A review. Asian Fisheries Sci.35, 269–281. doi: 10.33997/j.afs.2022.35.3.005
64
Pruesse E. Quast C. Knittel K. Fuchs B. M. Ludwig W. Peplies J. et al . (2007). SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res.35, 7188–7196. doi: 10.1093/NAR/GKM864
65
Randazzo B. Zarantoniello M. Gioacchini G. Cardinaletti G. Belloni A. Giorgini E. et al . (2021). Physiological response of rainbow trout (Oncorhynchus mykiss) to graded levels of Hermetia illucens or poultry by-product meals as single or combined substitute ingredients to dietary plant proteins. Aquaculture538. doi: 10.1016/j.aquaculture.2021.736550
66
Renna M. Schiavone A. Gai F. Dabbou S. Lussiana C. Malfatto V. et al . (2017). Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss, Walbaum) diets. J. Anim. Sci. Biotechnol.8. doi: 10.1186/s40104-017-0191-3
67
Ricker W. E. (1975). Computation and interpretation of biological statistics of fish populations. Bull. Fisheries Res. Board Canada191, 1–382.
68
Robeson M. S. O’Rourke D. R. Kaehler B. D. Ziemski M. Dillon M. R. Foster J. T. et al . (2021). RESCRIPt: Reproducible sequence taxonomy reference database management. PloS Comput. Biol.17, e1009581. doi: 10.1371/JOURNAL.PCBI.1009581
69
Rognes T. Flouri T. Nichols B. Quince C. Mahé F. (2016). VSEARCH: A versatile open source tool for metagenomics. PeerJ2016. doi: 10.7717/PEERJ.2584
70
Romano N. Datta S. N. Pande G. S. J. Sinha A. K. Yamamoto F. Y. Beck B. H. et al . (2023). Dietary inclusions of black soldier fly (Hermetia illucens) larvae frass enhanced production of channel catfish (Ictalurus punctatus) juveniles, stevia (Stevia rebaudiana), and lavender (Lavaridula angustifolia) in an aquaponic system. Aquaculture575. doi: 10.1016/j.aquaculture.2023.739742
71
Ryan R. P. Monchy S. Cardinale M. Taghavi S. Crossman L. Avison M. B. et al . (2009). The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat. Rev. Microbiol.7, 7. doi: 10.1038/nrmicro2163
72
Seo B. S. Park S. J. Hwang S. Y. Lee Y. I. Lee S. H. Hur S. W. et al . (2022). Effects of decreasing fishmeal as main source of protein on growth, digestive physiology, and gut microbiota of olive flounder (Paralichthys olivaceus). Animals12. doi: 10.3390/ani12162043
73
Serrano J. A. Nematipour G. R. Gatlin D. M. (1992). Dietary protein requirement of the red drum (Sciaenops ocellatus) and relative use of dietary carbohydrate and lipid. Aquaculture101, 283–291. doi: 10.1016/0044-8486(92)90031-F
74
Siddaiah G. M. Kumar R. Kumari R. Chandan N. K. Debbarma J. Damle D. K. et al . (2023). Dietary fishmeal replacement with Hermetia illucens (Black soldier fly, BSF) larvae meal affected production performance, whole body composition, antioxidant status, and health of snakehead (Channa striata) juveniles. Anim. Feed Sci. Technol.297. doi: 10.1016/j.anifeedsci.2023.115597
75
Snedecor G. W. Cochran W. G. (1978). Statistical methods. 6th ed (Ames, IA: Iowa State University Press).
76
Suehs C. M. Gatlin D. M. III. (2022). Corn-fermented protein as a replacement for fish meal in diets for Nile tilapia (Oreochromis niloticus). J. World Aquaculture Soc.53, 1107–1120.
77
Sun M. Y. Dafforn K. A. Johnston E. L. Brown M. V. (2013). Core sediment bacteria drive community response to anthropogenic contamination over multiple environmental gradients. Environ. Microbiol.15, 2517–2531. doi: 10.1111/emi.2013.15.issue-9
78
Surendra K. C. Tomberlin J. K. van Huis A. Cammack J. A. Heckmann L. H. L. Khanal S. K. (2020). “Rethinking organic wastes bioconversion: Evaluating the potential of the black soldier fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae) (BSF),” in Waste Management, (Elsevier Ltd) 117, 58–80. doi: 10.1016/j.wasman.2020.07.050
79
Suresh A. V. Kumaraguru vasagam K. P. Nates S. (2011). Attractability and palatability of protein ingredients of aquatic and terrestrial animal origin, and their practical value for blue shrimp, Litopenaeus stylirostris fed diets formulated with high levels of poultry byproduct meal. Aquaculture319, 132–140. doi: 10.1016/j.aquaculture.2011.06.039
80
Tacon A. G. J. Metian M. (2008). Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture285, 146–158. doi: 10.1016/J.AQUACULTURE.2008.08.015
81
Tacon A. G. J. Metian M. (2015). Feed matters: Satisfying the feed demand of aquaculture. Rev. Fisheries Sci. Aquaculture23, 1–10. doi: 10.1080/23308249.2014.987209
82
Turchini G. M. Torstensen B. E. Ng W. K. (2009). Fish oil replacement in finfish nutrition. Rev. Aquaculture1 (1), 10–57. Wiley-Blackwell. doi: 10.1111/j.1753-5131.2008.01001.x
83
Velasquez A. Pohlenz C. Barrows F. T. Gaylord T. G. Gatlin D. M. (2015). Assessment of taurine bioavailability in pelleted and extruded diets with red drum Sciaenops ocellatus. Aquaculture449, 2–7. doi: 10.1016/J.AQUACULTURE.2015.03.034
84
Wang G. Peng K. Hu J. Yi C. Chen X. Wu H. et al . (2019). Evaluation of defatted black soldier fly (Hermetia illucens L.) larvae meal as an alternative protein ingredient for juvenile Japanese seabass (Lateolabrax japonicus) diets. Aquaculture507, 144–154. doi: 10.1016/j.aquaculture.2019.04.023
85
Weththasinghe P. Hansen J. O. Nokland D. Lagos L. Rawski M. Overland M. (2021). Full-fat black soldier fly larvae (Hermetia illucens) meal and paste in extruded diets for Atlantic salmon (Salmo salar): Effect on physical pellet quality, nutrient digestibility, nutrient utilization and growth performance. Aquaculture530, 735785. doi: 10.1016/j.aquaculture.2020.735785
86
Wickham H. (2007). Reshaping data with the reshape package. J. Stat. Software21, 1–20. doi: 10.18637/JSS.V021.I12
87
Wickham H. Averick M. Bryan J. Chang W. McGowan L. D. François R. et al . (2019). Welcome to the tidyverse. J. Open Source Software4, 1686. doi: 10.21105/joss.01686
88
Xiong J. B. Nie L. Chen J. (2019). “Current understanding on the roles of gut microbiota in fish disease and immunity,” in Zoological research, (NLM (Medline) 40 (2), 70–76. doi: 10.24272/j.issn.2095-8137.2018.069
89
Yamamoto F. Y. Suehs B. A. Ellis M. Bowles P. R. Older C. E. Hume M. E. et al . (2022). Dietary fishmeal replacement by black soldier fly larvae meals affected red drum (Sciaenops ocellatus) production performance and intestinal microbiota depending on what feed substrate the insect larvae were offered. Anim. Feed Sci. Technol.283. doi: 10.1016/j.anifeedsci.2021.115179
90
Yilmaz P. Parfrey L. W. Yarza P. Gerken J. Pruesse E. Quast C. et al . (2014). The SILVA and “all-species living tree project (LTP)” taxonomic frameworks. Nucleic Acids Res.42, D643–D648. doi: 10.1093/nar/gkt1209
91
Young K. (2009). Omega-6 (n-6) and omega-3 (n-3) fatty acids in tilapia and human health: A review. International Journal of Food Sciences and Nutrition60 (Suppl 5), 203–211. doi: 10.1080/09637480903140503
92
Zarantoniello M. Randazzo B. Truzzi C. Giorgini E. Marcellucci C. Vargas-Abúndez J. A. et al . (2020). A six-months study on Black Soldier Fly (Hermetia illucens) based diets in zebrafish. Sci. Rep.10, 859. doi: 10.1038/s41598-020-57738-3
Summary
Keywords
red drum, black soldier fly meal, growth performance, ARA, DHA, EPA
Citation
Paredes JF, Riche M, Bradshaw D, Mejri S, Chin LS, Perez J, Popa R, Romano N and Wills PS (2025) Evaluation of the effect of black soldier fly (Hermetia illucens L.) larvae meal in the diet of red drum (Sciaenops ocellatus) juveniles on production performance and feed palatability. Front. Aquac. 4:1619878. doi: 10.3389/faquc.2025.1619878
Received
28 April 2025
Accepted
29 May 2025
Published
02 July 2025
Volume
4 - 2025
Edited by
D. K. Meena, Central Inland Fisheries Research Institute (ICAR), India
Reviewed by
Manjula Magamage, Sabaragamuwa University, Sri Lanka
Roberta Schiavone, University of Salento, Italy
Updates
Copyright
© 2025 Paredes, Riche, Bradshaw, Mejri, Chin, Perez, Popa, Romano and Wills.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan F. Paredes, paredesj@fau.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.