Abstract
Introduction:
This study investigated the use of locally sourced food processing by-products to formulate sustainable aquaculture feed in alignment with circular economy principles.
Methods:
An experimental diet (Food Waste Diet; FW-D) was formulated using 50% fishmeal from tuna viscera and 20% brewer’s spent yeast (BSY) sourced in Okinawa, Japan. The Malabar grouper (Epinephelus malabaricus) were reared in a recirculating aquaculture system for 8 weeks and compared against a commercial diet (CO-D). Growth performance, liver somatic index, insulin-like growth factor 1 (IGF-1) gene expression, and muscle taste-related components were analyzed.
Results:
FW-D-fed fish showed slightly lower growth performance, possibly due to decreased protein content. However, liver histology revealed no signs of metabolic stress, and IGF-1 gene expression levels were higher in the FW-D group, indicating active growth signaling. FW-D-fed fish also exhibited significantly increased muscle levels of unsaturated fatty acids, particularly docosahexaenoic acid (DHA), a nutrient beneficial for human health. Sensory analysis indicated comparable overall taste quality between the two groups. FW-D fillets had greater chewiness and reduced fishy odor, whereas CO-D fillets were richer in fatty taste.
Discussion:
These findings suggest that FW-D, composed of locally available by-products, can offer a nutritionally advantageous and economically viable alternative to conventional feeds. Despite a slight reduction in growth, the enhanced DHA content and acceptable sensory attributes of FW-D-fed fish support its application in sustainable aquaculture systems. This approach contributes to resource recycling and value creation within a local circular economy.
1 Introduction
The global rise in food waste has become a serious environmental, social, and economic challenge (Aleisa and Al-Jarallah, 2024). Thus, Sustainable Development Goals (SDGs) Target 12.3 aims to decrease food waste by half and reduce losses along the entire food supply chain by 2030 (FAO, 2021). Achieving this target necessitates active efforts from all sectors, including government, industry, and academia. Food waste is not merely a source of environmental burden but also holds potential as a valuable resource through recycling and reuse, gaining attention as a means of producing new value. One approach involves the conversion of food waste into animal feed. In Japan, “Food Waste Feed” is called eco-feed, and refers to the feed produced by utilizing food waste. The use of Food Waste Feed plays a crucial role in the efficient use of resources via food recycling and in improving the self-sufficiency feed rate. For instance, the recycling of food waste as feed for pigs and cattle is progressing in Japan, and some companies process food waste collected from supermarkets and food factories into fermented liquid feed and provide it to pig farmers. This initiative has been recognized as contributing to a substantial reduction in feed costs and to the circular economy and SDGs (Nakaishi and Takayabu, 2022).
Large amounts of fish waste are generated in Okinawa Prefecture, Japan, where fishing is thriving. Additionally, Okinawa has a thriving beer industry, and byproducts generated during beer production, such as malt residue and brewer’s spent yeast (BSY), have the potential to be valuable feed resources. BSY is recognized as the second most important byproduct of the beer brewing industry (Ferreira et al., 2010). The main BSY component is Saccharomyces cerevisiae, a high-protein industrial food waste with a protein content ranging from 40% to 50%. Furthermore, its amino acid composition is well-balanced (Marson et al., 2020). BSY is a cost-effective nitrogen source and a nutritionally rich feed containing high vitamin and mineral levels (Ferreira et al., 2010). Thus, BSY, food waste rich in organic matter, is gaining traction as a protein source in aquaculture feed.
Contrastingly, the Malabar grouper (Epinephelus malabaricus), known in Japan as “Yaito-hata” is a carnivorous fish species distributed in the warm waters from the Red Sea to the Indo-West Pacific region. Malabar groupers have high commercial value in Asian countries because of the ease of aquaculture, rapid growth to commercial size, and superior meat texture and flavor (Lin and Shiau, 2003). Especially in the Asia-Pacific region, including Okinawa Prefecture, an increasing interest exists in the development of Malabar grouper aquaculture. However, despite its commercial appeal, one of the greatest challenges that the aquaculture industry faces is the high cost of feed, which significantly affects overall production efficiency. Approximately 70% of the operational costs in fish aquaculture are associated with feed prices. Furthermore, one of the critical challenges affecting aquaculture is the rising feed costs. Thus, active efforts are being made to develop fish feed that decreases the costs for implementing such feeds in aquaculture. However, most of these studies have focused on growth experiments that replaced expensive fishmeal with plant-based proteins. For instance, plant-based proteins such as sunflower meal, soybean meal, and rapeseed meal have been used as substitutes for fishmeal in aquaculture production, with some reports revealing high effectiveness in certain fish species (Mohamed et al., 2018; Li et al., 2013; Bu et al., 2018). Nevertheless, fishmeal still accounts for the majority of the protein sources in aquaculture feed. This is especially true for carnivorous fish species, which use animal protein at much higher rates than plant-based proteins compared to omnivorous or herbivorous species, to promote stable growth (Glencross et al., 2007). Thus, the authors envisioned constructing a regional circular aquaculture system using fish and beer yeast waste from Okinawa Prefecture as a protein source for feed. This approach aims to decrease feed costs and promote the effective utilization of locally generated waste. However, few attempts have been made to investigate Malabar grouper growth using feed containing beer yeast. Additionally, the impact of using animal protein as a substitute for fishmeal in aquaculture feed has been explored in multiple studies on groupers (Wang et al., 2008; Ye et al., 2019). However, little attention has been paid to the effects of aquaculture on the taste quality of fish meat.
This study investigated the effects of feed, primarily composed of fishmeal and BSY prepared from waste generated in Okinawa Prefecture, on the growth and taste quality of Malabar groupers. To assess its effects on growth, we examined the alterations in growth parameters in the fish body and measured the transcription levels of insulin-like growth factor 1 (IGF-1), which is secreted by hepatocytes via interactions with growth hormones released from the pituitary gland during growth regulation (Metzger et al., 2012). Furthermore, we assessed the Hepatosomatic Index (HSI) as an indicator of liver energy metabolism because energy metabolism derived from feed induces dynamic alterations in liver glycogen concentration and cell size (Podolska et al., 2024). Furthermore, we measured the components associated with the taste of fish meat (free amino acids (FAAs), fatty acids (FAs) in crude lipid, and crude fat) and conducted a sensory evaluation using a scoring method to assess the effects of feed on the taste quality of fish meat.
2 Materials and methods
2.1 Diets and bio-economical analysis
Table 1 shows the ingredients and proximate composition analyses (AOAC, 1995) of the commercial diet (CO-D) and food waste diet (FW-D). The control group of Malabar grouper was fed CO-D (Madai EP Major, Marubeni Nisshin Feed, Tokyo, Japan), consisting primarily of fish powder along with soybean pulp, flour, fish oil, vitamin and mineral premix, and rice bran. The ingredients for the FW-D test group included fishmeal made from unused parts of tuna caught in Okinawa, Japan (Kyodokakou, Ltd., Okinawa, Japan) and brewer’s spent yeast (BSY) obtained from a local brewery (Orion Breweries, Ltd., Okinawa, Japan), which was incorporated without additional sterilization or drying treatments. The FW-D formulation was based on the CO-D composition and the fishmeal ratio (50%) was maintained. However, half of the soybean pulp (10%), a portion of the fish oil (2%), and all rice bran (8%) in CO-D were replaced with BSY. The FW-D ingredients were passed via a 250-μm sieve and subsequently thoroughly mixed with fish oil, followed by mechanical mixing with water (30% diet mix). Moist pellets, 4.0 mm in diameter, were pressure-pelleted using a twin-screw extruder (HG-ZLSP150B; Haige, Gunma, Japan), dried at room temperature, and subsequently sealed in ziplock bags. CO-D and FW-D were stored at −20°C until use.
Table 1
| Ingredients (%) | CO-D | FW-D |
|---|---|---|
| Fishmeal | 50.0 | 50.0 |
| Soybean pulp | 20.0 | 10.0 |
| Flour, starch | 14.0 | 14.0 |
| Fish oil | 6.0 | 4.0 |
| Vitamin and mineral premix a | 2.0 | 2.0 |
| Rice bran | 8.0 | − |
| Yeast extract | − | 20.0 |
| Proximate composition (g/100 g) | ||
| Nitrogen-free extract (NFE) | 27.3 | 26.2 |
| Crude protein | 41.7 | 35.2 |
| Crude fat | 10.3 | 10.7 |
| Crude fiber | 1.4 | 1.5 |
| Ash | 9.1 | 8.9 |
| Moisture | 10.2 | 17.6 |
| Energy (kcal/100 g) | 369.0 | 341.5 |
Feed ingredients and nutrition values of CO-D and FW-D.
CO-D: Commercial diet; Food waste diet: FW-D.
a Vitamin and mineral contents (kg−1 mixture): Vitamin A, 1.8×109 IU; Vitamin D3, 2.4×108 IU; Vitamin E, 42.00 g; Vitamin K3, 1.44 g; Vitamin B1, 4.20 g; Vitamin B2, 4.20 g; Vitamin B6, 4.20 g; Vitamin B12, 0.04 g; calcium pantothenate, 26.09 g; nicotinic acid, 24.00 g; biotin, 0.12 g; inositol, 120.00 g; folic acid, 0.48 g; Zinc, 6.00 g; Iodine, 0.12 g; Iron, 6.00 g; Copper, 0.90 g; Manganese, 4.80 g; Aluminum, 0.07 g. (Purchased from Kyodo Kako Co., Ltd.).
Bio-economical analysis of both diets was assessed using the following formula:
-
Feeding cost (US$) = cost of diet × feed consumed
-
Gross income (US$) = final body weight × price of fish
-
Profit (US$) = gross income − feeding cost.
2.2 Experimental fish collection
Juvenile Malabar groupers used in this study were obtained from the Okinawa Prefectural Sea Farming Center, Okinawa, Japan and reared at Sesoko Station, Tropical Biosphere Research Center, University of the Ryukyus, Okinawa, Japan. Fish were acclimated in fiber-reinforced plastic tanks (3 metric ton capacity) with running seawater at ambient temperature for 4 weeks and daily fed with CO-D.
The study was conducted in compliance with the Animal Care and Use Committee guidelines of the University of the Ryukyus and the regulations for the care and use of laboratory animals in Japan.
2.3 Experimental design
Acclimated fish (17.3 ± 1.0 cm in total length and 77.8 ± 12.4 g in body weight) were randomly divided into two groups (300 individuals per group) and cultured in two identical tanks (3 metric ton capacity) with aerated running seawater, maintained at ambient water temperature and photoperiod as shown in Supplementary Figure S1. Additional aeration was provided throughout the experimental period to maintain sufficient dissolved oxygen levels and prevent hypoxic conditions. Under these conditions, one group was daily fed the control diet (CO-D) group and the other was fed daily the test diet (FW-D) group at 5% of the body weight. Thirty fish were collected from the tanks every 2 weeks, and the total length and body weight of each individual were measured under anesthesia with 0.01% 2-phenoxyethanol (Kanto Kagaku, Tokyo, Japan). Six fish were anesthetized and sacrificed by decapitation at the start and end of the 8-week experiment. Prior to sampling, all fish were fasted for 24 hours to ensure standardized physiological conditions and reduce dietary influence on measured parameters. The edible muscles of the sacrificed fish were individually collected by homogenizing the entire dorsal and ventral muscle portion after filleting, and livers were also collected and weighed, and a portion was subsequently immersed in TRIzol (Roche, Mannheim, Germany) and immediately frozen in liquid nitrogen. All collected samples were stored at −80°C until use.
Growth performance was assessed using the following formula:
-
Weight gain rate (WGR, %) = (final weight (g) − initial weight (g))/initial weight (g) × 100
-
Specific growth rate (SGR, %/day) = [ln (final weight (g)) − ln (initial weight (g))]/days of feeding trial × 100
-
Feed conversion rate (FCR) = dry feed intake (g)/weight gain (g)
-
Protein intake (PI) = total feed intake (g) × protein in the diet (%)/days
-
HSI = liver weight (g)/body weight (g) × 100.
2.4 RNA extraction and cDNA synthesis
We extracted RNA from homogenized liver tissue using the TriPure Isolation Reagent (Roche Diagnostics, Indianapolis, IN, USA), following the manufacturer’s protocol. The total RNA concentration was measured using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Following an RNA quality check using 2% agarose gel electrophoresis (Takara Bio, Kusatsu, Japan), cDNA was synthesized using the PrimeScript™ RT Reagent Kit with gDNA Eraser (Takara Bio), following the manufacturer’s instructions. All cDNA samples were stored at −30°C.
2.5 Quantitative reverse transcription-polymerase chain reaction (RT-PCR) assay procedures
The mRNA levels of IGF-I in the liver were quantified using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) and SYBR Green Premix PCR kit (Takara Bio). Primer sets for IGF-I were previously published by Zhu et al. (2022). The final volume of each qPCR reaction mixture was 10 μL, containing SYBR Premix Ex Taq II (5 μL; Tli RNase H Plus) (Takara Bio), forward and reverse primers (0.3 μL; 0.3 M), nuclease-free water (2.4 μL), and cDNA template (2 μL; 1000 ng/μL). The qPCR conditions were as follows: 95°C for 2 min (denaturation), 39 cycles at 95°C for 15 s (denaturation) and 60°C for 1 min (annealing and extension), which demonstrated a melting point from 65°C to 95°C with a 0.5°C increment every 5 s. Two technical replicates were performed for the qPCR. Specific primer assays were conducted using serial dilutions of liver cDNA, which showed amplification efficiencies of approximately 100%. The mRNA level of the target genes in each sample was normalized to the amount of Malabar grouper Ef1α and β-actin as the reference controls.
2.6 Histological assessments
For microscopic preparation, small liver tissue pieces were fixed with 4% paraformaldehyde. The fixed tissues were routinely processed for paraffin embedding. The tissue was fractionated with a 5 μm thickness and subsequently stained with hematoxylin and eosin (HE). Finally, the tissues were assessed and imaged using an optical microscope with a single-lens reflex camera (DS126371; Cannon, Tokyo, Japan).
2.7 Lipid content analysis
The total lipid content was determined as described by Bligh and Dyer (1959) using chloroform/methanol (2:1 in volume, CM solution). Briefly, 2 g of the minced fish muscle was extracted with 30 mL of the CM solution by ultrasonication (UT-105S; Sharp, Osaka, Japan) for 30 min. The solution was filtered under vacuum, and the filtrate was subsequently evaporated in a rotary evaporator (R-124, BÜCHI, Flawil, Switzerland). The concentrate was mixed with 20 mL petroleum ether and 10 g anhydrous sodium sulfate. Furthermore, 10 mL of the organic phase was collected and centrifuged (5702, Eppendorf, Hamburg, Germany) at 600 × g for 5 min. Five milliliters of the organic phase was collected in a weighed flask, and the solvent was evaporated in a rotary evaporator. The lipid residue was weighed (n=6) and the results were expressed as g/100 g fresh weight (FW).
2.8 FA composition analysis
FAs in crude lipid were examined as previously described with slight modifications (Kimura et al., 2013). Briefly, 1 g of minced fish muscle was extracted with 10 mL of hexane/isopropanol (3:2 in volume, HI solution) using ultrasonication for 10 min, the suspension was centrifuged, and the pellet was rinsed with HI solution (2 mL). The entire liquid phase was mixed with water (15 mL) and anhydrous sodium sulfate (1.5 g), and the mixture was placed overnight at 4°C. The organic phase was collected and subsequently evaporated to dryness using a rotary evaporator. The dried extracts were methylated using a Fatty Acid Methylation Kit for glycerides (Nacalai Tesque, Kyoto, Japan) according to the manufacturer’s protocol. FA methyl ester (FAME) samples were examined using a GC-2010-FID system (Shimadzu Corp., Japan) equipped with a DB-WAX column (30 m × 0.32 mm, 0.25 μm; Agilent J&W, Santa Clara, CA, USA). The column oven was increased from 50°C to 200°C at 25°C/min and subsequently increased to 230°C at 3°C/min. The gas flow for N2 was set at 50 cm/s, and the injector and FID detector temperatures were each set at 250°C. FA was quantified by comparing its retention time with those of 16 standard FAME mixtures (Cayman Chemical, Ann Arbor, MI, USA). The levels of each FA were expressed as a percentage (%) of FW.
2.9 FAA analysis
The FAA composition of fish edible muscles was determined using high-performance liquid chromatography (HPLC) with a PRR-2A peristaltic pump (Shimadzu Corp., Kyoto, Japan) for o-phthalaldehyde (OPA) post-column derivatization. Briefly, minced fish muscle was placed in 10 times the volume of purified water. The mixture was homogenized for 5 min and subsequently centrifuged (CR 22 GIII; Hitachi, Tokyo, Japan) at 20,600 × g for 5 min. Equal volumes of the supernatant and chloroform were mixed and the pH was adjusted to 2.5. The mixture was analyzed using HPLC following filtration. The FAAs were separated using an Na-type cation resin chromatography column (100 mm × 6.0 mm i.d., Shimadzu Corp., Kyoto, Japan) using a gradient solvent program consisting of 3 types of commercial mobile phase (amino acid mobile phase kit Na type, Shimadzu Corp., Kyoto, Japan) at a 0.6 mL/min flow rate. The 17 amino acids standard solution (2.5 mM) was purchased from Fuji Film Wako Pure Chemical Industries (Osaka, Japan). Following the column separation, the FAAs were derivatized with OPA to obtain a fluorescent substance. The OPA-derivatized amino acids (λx = 350 nm, λm = 450 nm) were detected with an RF-10AXL fluorescence detector (Shimadzu Corp., Kyoto, Japan). Each FAA’s concentration was expressed in mg/100 g FW.
2.10 Sensory evaluation
Sensory evaluation of the fish muscles (CO-D and FW-D groups) was conducted according to the procedure described by Hu et al. (2013). The panel consisted of 10 males and 11 females aged 20–50 years belonging to the University of the Ryukyus (Okinawa, Japan), and they were individuals with prior experience in sensory evaluation of fish, having participated in multiple similar assessments in previous studies. The evaluation trial was conducted at 25 ± 2°C in the sensory laboratory at the University. The fish fillets (4 cm × 8 cm × 1 cm) were heated with microwave (980 W) for 90 s and used in this trial. Assessments of food taste was divided into the following seven categories: umami, fatty taste, oily feel, fishy smell, whitish color, chewiness, and overall flavor. Each panel gave each selected attribute, with a 7-point score (from −3 = “slightly applicable” to +3 = “very applicable”) for each category according to the taste of the heated fish muscle.
2.11 Statistical analysis
Data are shown as the mean ± standard error of the mean. Prior to conducting t-tests, data normality was assessed using the Shapiro–Wilk test. All variables yielded p-values > 0.05, confirming that the data followed a normal distribution. Comparisons between the two groups were performed using Welch’s t-test in the R project. Other data were analyzed using the independent samples t-test (SPSS 20.0; SPSS Inc., Michigan Avenue, Chicago, IL, USA). Statistical significance was set at P < 0.05.
3 Results
3.1 Proximate analysis of the diets
Table 1 presents the compositional similarities between CO-D and FW-D on a wet matter basis. The crude protein levels in the CO-D and FW-D groups were 41.7% and 35.2%, respectively, with the FW-D group being 6.5% lower than the CO-D group. The crude fat levels were 10.3% and 10.7%, and the nitrogen-free extract levels were similar at 27.3% and 26.2%, respectively. The crude fiber and ash contents in FW-D were primarily derived from fishmeal and were comparable to those in CO-D. The total fatty acid content revealed similar values for CO-D (4551.5 mg/100 g) and FW-D (4569.7 mg/100 g) (Table 2). The total saturated fatty acid content of CO-D was 1.46 times higher than that of FW-D. Stearic acid (C18:0) had the highest content in the CO-D and FW-D. The C18:0 content of CO-D was 1.98 times higher than that of FW-D. Contrastingly, FW-D exhibited a 1.15 times higher unsaturated fatty acid content than CO-D. Among the n-9 unsaturated fatty acids, oleic acid (C18:1) was present at extremely high levels in CO-D and FW-D. For n-6 unsaturated fatty acids, linoleic acid (C18:2) was the characteristic fatty acid in CO-D and FW-D. In terms of n-3 polyunsaturated fatty acids, eicosapentaenoic acid (EPA, C20:5) and docosahexaenoic acid (DHA, C22:6) were present at high concentrations. The EPA levels in CO-D and FW-D were similar; however, FW-D had a 2.53 times higher DHA content than CO-D.
Table 2
| Components | Fishmeal | Yeast extract | Soybean pulp | Flour | Vitamin premix | Fish oil | CO-D | FW-D |
|---|---|---|---|---|---|---|---|---|
| Fatty acid (mg/100 g) | ||||||||
| Saturated (S) | ||||||||
| 14:0 | 210.9 | 0.0 | 0.0 | 0.0 | 0.0 | 2045.0 | 178.9 | 187.3 |
| 16:0 | 39.9 | 112.6 | 30.4 | 99.5 | 11.3 | 12045.4 | 1069.1 | 543.5 |
| 18:0 | 278.4 | 29.1 | 0.0 | 0.0 | 0.0 | 2861.0 | 202.6 | 260.5 |
| ∑S | 529.2 | 141.7 | 30.4 | 99.5 | 11.3 | 16951.4 | 1450.6 | 991.3 |
| Unsaturated (U) | ||||||||
| (n-9) 16:1 | 358.5 | 69.6 | 0.0 | 0.0 | 0.0 | 3269.0 | 220.0 | 325.3 |
| 18:1 | 977.3 | 16.5 | 15.2 | 102.5 | 0.0 | 9252.6 | 1121.9 | 878.2 |
| (n-6) 18:2 | 159.1 | 0.0 | 77.8 | 317.1 | 0.0 | 916.1 | 508.1 | 168.4 |
| 20:2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 148.8 | 12.2 | 6.0 |
| 20:4 | 107.4 | 0.0 | 0.0 | 0.0 | 0.0 | 1220.9 | 49.6 | 102.6 |
| 22:4 | 117.3 | 0.0 | 0.0 | 0.0 | 0.0 | 1027.9 | 82.0 | 99.7 |
| (n-3) 18:3 | 54.5 | 0.0 | 0.0 | 0.0 | 0.0 | 486.7 | 87.5 | 46.7 |
| 18:4 | 33.4 | 0.0 | 0.0 | 0.0 | 0.0 | 631.9 | 45.0 | 42.0 |
| 20:4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 297.8 | 18.9 | 11.9 |
| 20:5 (EPA) | 281.4 | 0.0 | 0.0 | 0.0 | 0.0 | 4477.3 | 319.4 | 319.8 |
| 21:5 | 44.1 | 0.0 | 0.0 | 0.0 | 0.0 | 246.1 | 23.3 | 31.9 |
| 22:5 (DPA) | 31.1 | 0.0 | 0.0 | 0.0 | 0.0 | 172.2 | 10.5 | 22.4 |
| 22:6 (DHA) | 1324.6 | 159.9 | 0.0 | 0.0 | 0.0 | 20649.1 | 602.5 | 1523.5 |
| ∑U | 3488.7 | 246 | 93 | 419.6 | 0 | 42796.4 | 3100.9 | 3578.4 |
| Total (∑S + ∑U) | 4017.9 | 387.7 | 123.4 | 519.1 | 11.3 | 59747.8 | 4551.5 | 4569.7 |
Saturated (S) and unsaturated (U) fatty acid of CO-D and FW-D.
CO-D, Commercial diet; FW-D, Food waste diet.
EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic acid.
3.2 Growth performance and feed utilization efficiency
Table 3 reveals the growth performance and feed utilization efficiency of Malabar groupers cultured for eight weeks. The weekly transition of body weight and total length throughout the experiment is provided in Supplementary Figure S2. The final body weight (FW) of Malabar groupers in the FW-D group tended to be lower than those in the CO-D group. Furthermore, the WGR and SGR tended to be lower in the FW-D group (P = 0.61). Furthermore, the PI calculated from feed intake was lower in the FW-D group than in the CO-D group, indicating a tendency toward a higher feed requirement rate in the FW-D group.
Table 3
| Parameters | CO-D | FW-D | SW W (P) | P |
|---|---|---|---|---|
| Growth performance | ||||
| IW (g) | 75.70 ± 2.61 | 80.00 ± 1.79 | 0.94 (0.42) | 0.18 |
| FW (g) | 101.90 ± 4.09 | 92.60 ± 3.41 | 0.97 (0.20) | 0.83 |
| WGR (%) | 44.66 ± 17.94 | 37.76 ± 11.54 | 0.88 (0.28) | 0.61 |
| SGR (%/day) | 0.65 ± 0.23 | 0.57 ± 0.15 | 0.87 (0.22) | 0.64 |
| FCR | 8.31± 3.73 | 8.83 ± 3.26 | 0.74 (0.25) | 0.87 |
| PI | 1.84 | 1.62 | ||
| Bio-economica analysis of the feeds | ||||
| Cost of feed (US $/kg) | 3.88 | 1.35 | ||
| Feeding cost (US $) | 0.96 | 0.35 | ||
| Gross income (US $) | 1.62 | 1.40 | ||
| Profit (US $) | 0.66 | 1.13 | ||
Growth performance, feed utilization and bio-economica analysis of the cultured Malabar grouper fed with CO-D and FW-D for 8 week.
CO-D, Commercial diet; Food waste diet: FW-D.
IW, Initial weight; FW, Final weight; WGR, Weight gain rate; SGR, Specific growth rate; FCR, Feed conversion ratio; PI, Protein intake. Values are mean of triplicate groups and presented as mean ± SD (n = 30). Values in the same column having different superscript letters are significantly different (P < 0.05; independent-samples t-test) among treatments. The “SW W (P)” column indicates results of the Shapiro–Wilk test used to assess normality of the data distribution before applying the t-test. All variables satisfied normality assumptions (P > 0.05) for parametric testing. The lack of superscript letter indicates no significant differences among treatments.
The bio-economical analysis of the feeds is calculated using an exchange rate of 1 US$ = 156 JPY. Gross income: Price of fish: 16.03 US$/kg; Profit: All other costs are assumed same for all groups and ignored.
3.3 Liver indices including histological evaluation and insulin-like growth factor 1 transcript level
No significant differences in the HSI were noted between the CO-D and FW-D groups, although the FW-D group revealed a slightly decreasing trend (Figure 1). In liver sections stained with HE, the hepatocyte cytoplasm was stained red, indicating acidity, and the fat regions remained unstained and appeared white (Figure 2). No differences were observed in the degree of fat deposition between the CO-D and FW-D groups. When examining igf1 mRNA expression in the liver, no significant differences were observed between the CO-D and FW-D groups, although the FW-D group showed a slightly increasing trend (Figure 3).
Figure 1

Hepatosomatic index of the cultured Malabar grouper fed with CO-D and FW-D. CO-D, Commercial diet; FW-D, Food waste diet. Values are expressed as the mean ± standard deviation. (n = 6). The lack of superscript letters indicates that no significant differences were found among the treatments.
Figure 2
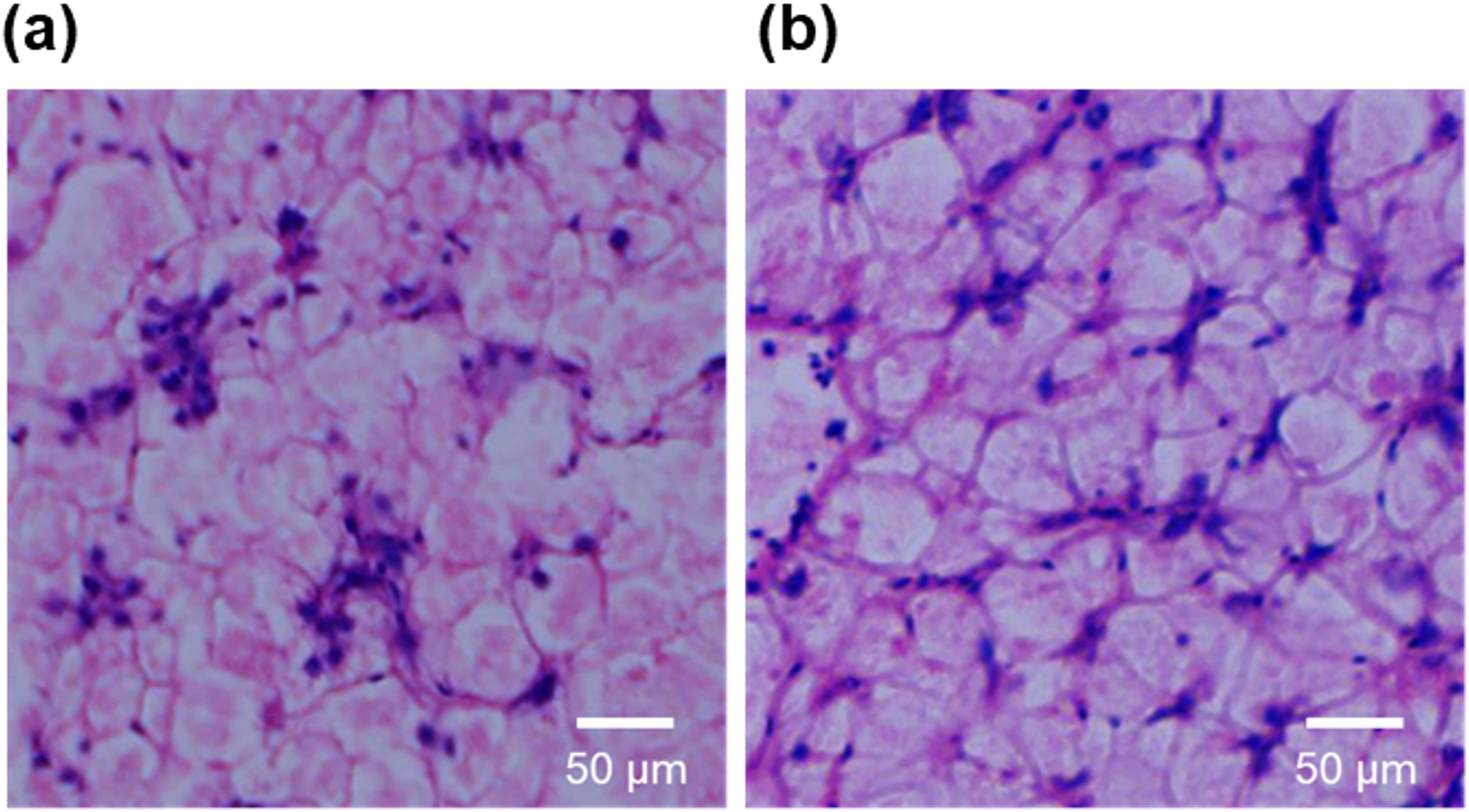
The histology of the cultured Malabar grouper liver tissue (hematoxylin and eosin staining, 100×): fed with CO-D (a) and FW-D (b). CO-D, Commercial diet; FW-D, Food waste diet.
Figure 3
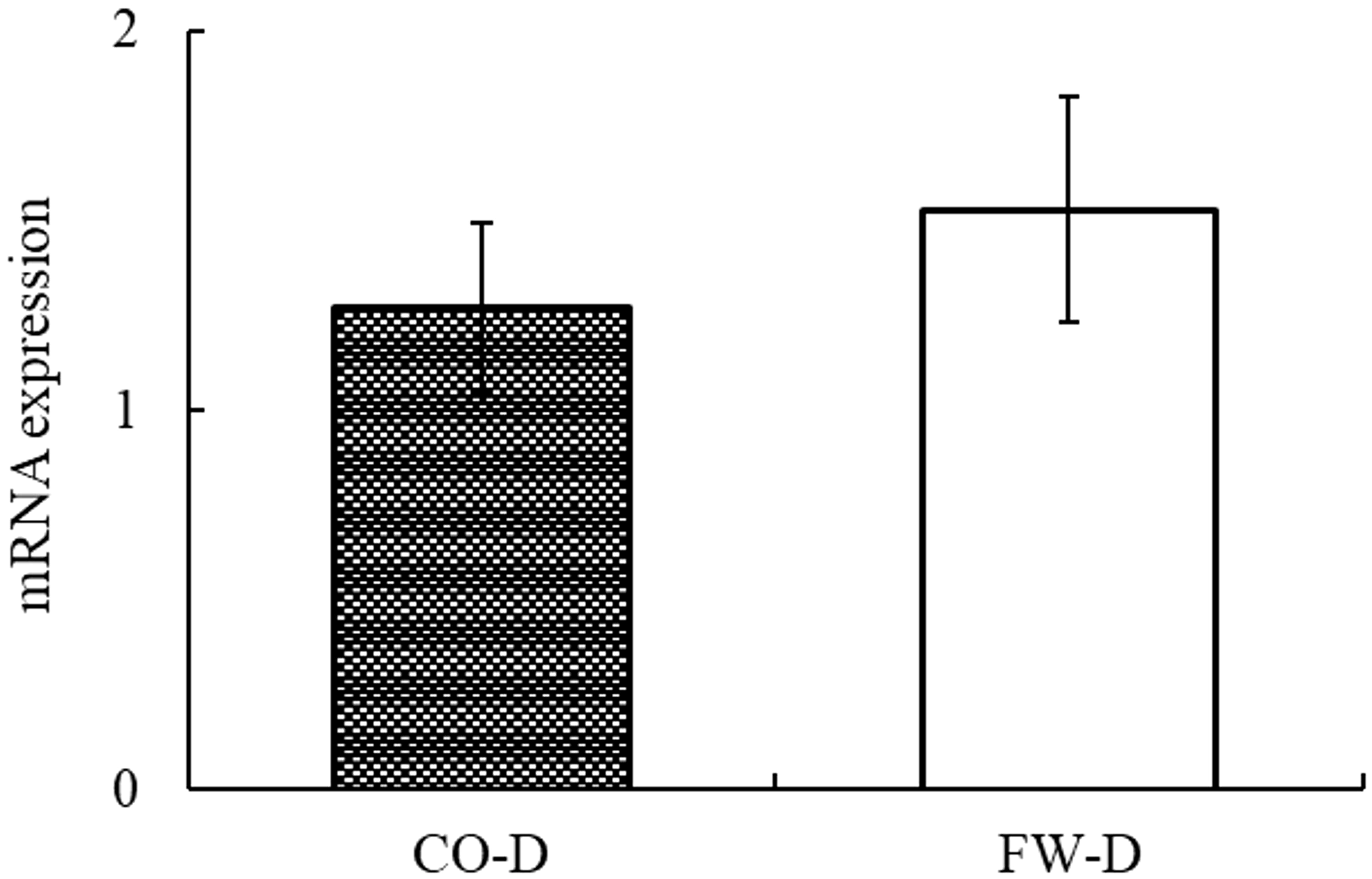
Transcript levels of IGF-1 in the liver of the cultured Malabar grouper fed with CO-D and FW-D. CO-D, Commercial diet; FW-D, Food waste diet. Values are expressed as the mean ± standard deviation. (n = 6). The lack of superscript letters indicates that no significant differences were found among the treatments.
3.4 Crude fat and fatty acid composition in the Malabar grouper muscle
Table 4 reveals the crude fat content and fatty acid composition of the muscle of Malabar groupers fed various diets. The crude fat content in the muscle after eight weeks of farming was significantly lower in the FW-D group than in the CO-D group (P = 0.001). Among the saturated fatty acids (S), palmitic acid (16:0) was the most abundant in both groups, with significantly higher levels in the CO-D group. Oleic acid (18:1n-9) was the most abundant unsaturated fatty acid (U) in both groups, followed by docosahexaenoic acid (DHA, 22:6n-3), linoleic acid (18:2n-6), and eicosapentaenoic acid (EPA, 20:5n-3). C18:1 and C18:2 were substantially higher in the CO-D group. Both EPA and DHA demonstrate pharmacological functions as dietary components. No differences were observed in the EPA composition between the CO-D and FW-D groups; however, DHA was substantially higher in the FW-D group.
Table 4
| Components | CO-D | FW-D | SW W (P) | P |
|---|---|---|---|---|
| Crude lipid (%) | 0.69 ± 0.06 | 0.51 ± 0.06 | 0.95 (0.66) | 0.001 |
| Fatty acid (%) | ||||
| Saturated (S) | ||||
| 14:0 | 3.68 ± 0.42 b | 2.94 ± 0.25 a | 0.93 (0.42) | 0.008 |
| 16:0 | 20.86 ± 0.24 b | 19.37 ± 0.86 a | 0.87 (0.23) | 0.019 |
| 18:0 | 6.01 ± 0.29 a | 6.50 ± 0.37 b | 0.89 (0.11) | 0.030 |
| ΣS | 30.55 ± 0.95 b | 28.81 ± 1.48 a | 0.97 (0.91) | 0.005 |
| Unsaturated (U) | ||||
| (n-9) 18:1 | 25.74 ± 1.44 b | 22.26 ± 1.64 a | 0.95 (0.65) | 0.005 |
| (n-6) 18:2 | 13.41 ± 0.33 b | 10.65 ± 1.02 a | 0.95 (0.64) | 0.001 |
| 20:2 | 0.41 ± 0.01 b | 0.39 ± 0.02 a | 0.92 (0.30) | 0.047 |
| 20:4 | 1.17 ± 0.08 a | 1.69 ± 0.27 b | 0.91 (0.20) | 0.005 |
| 22:4 | 3.10 ± 0.17 | 3.04 ± 0.20 | 0.84 (0.13) | 0.722 |
| (n-3) 18:3 | 2.30 ± 0.05 b | 1.81 ± 0.24 a | 0.87 (0.17) | 0.005 |
| 18:4 | 0.70 ± 0.02 b | 0.62 ± 0.05 a | 0.86 (0.15) | 0.008 |
| 20:4 | 0.79 ± 0.03 b | 0.70 ± 0.03 a | 0.90 (0.15) | 0.002 |
| 20:5 (EPA) | 4.99 ± 0.14 b | 4.50 ± 0.26 a | 0.95 (0.63) | 0.006 |
| 21:5 | 0.42 ± 0.04 | 0.49 ± 0.03 | 0.96 (0.76) | 0.208 |
| 22:5 (DPA) | 0.44 ± 0.03 a | 0.56 ± 0.08 b | 0.91 (0.24) | 0.010 |
| 22:6 (DHA) | 15.99 ± 1.64 a | 24.47 ± 0.20 b | 0.94 (0.44) | 0.002 |
| ΣU | 69.45 ± 3.98 a | 71.19 ± 4.04 b | 0.93 (0.41) | 0.010 |
| Total (ΣS + ΣU) | 100.00 | 100.00 | ||
Crude lipid and fatty acid composition of the cultured Malabar grouper muscle fed with CO-D and FW-D for 8 weeks.
CO-D, Commercial diet; FW-D, Food waste diet.
EPA-eicosapentaenoic acid, DPA-docosapentaenoic acid, DHA-docosahexaenoic acid. Values are mean of triplicate groups and presented as mean ± SD (n = 6). Values in the same column having different superscript letters are significantly different (P < 0.05; independent-samples t-test) among treatments. The “SW W (P)” column indicates results of the Shapiro–Wilk test used to assess normality of the data distribution before applying the t-test. All variables satisfied normality assumptions (P > 0.05) for parametric testing. The lack of superscript letter indicates no significant differences among treatments.
3.5 Free amino acid composition associated with flavor in the Malabar grouper muscle
Table 5 reveals the FAA composition of the Malabar grouper muscle. While the essential amino acids (EAA) in the CO-D and FW-D groups were nearly equivalent (P = 0.868), a significant increase in non-essential amino acids (NEAA) was noted in the CO-D group compared to the FW-D group (P = 0.052). Among all groups, glycine, an amino acid linked to sweetness, demonstrated the highest proportion of all amino acids. The proportion of glycine in the FW-D group was approximately 7% lower than that in the CO-D group (CO-D: 60.1%; FW-D: 52.9%). The levels of other sweetness-associated amino acids (threonine, serine, and alanine) did not differ between the CO-D and FW-D groups. For bitter amino acids, lysine levels were substantially higher in the CO-D group, whereas leucine and histidine levels were considerably lower than those in the FW-D group. No differences were noted in the levels of the other bitter amino acids (methionine, isoleucine, phenylalanine, and arginine) between the two groups.
Table 5
| Components | CO-D | FW-D | SW W (P) | P | taste |
|---|---|---|---|---|---|
| Free amino acids (mg/100g) | |||||
| EAA | |||||
| Val | 3.79 ± 1.06 b | 2.16 ± 0.82 a | 0.96 (0.75) | 0.015 | SwB |
| Met | 1.74 ± 0.79 b | 1.54 ± 0.20 a | 0.93 (0.44) | 0.023 | B |
| Ilu | 1.74 ± 0.79 | 1.54 ± 0.20 | 0.93 (0.41) | 0.349 | B |
| Leu | 1.5 ± 0.26 | 2.48 ± 0.39 | 0.89 (0.11) | 0.064 | B |
| Thr | 35.11 ± 4.32 | 35.59 ± 3.55 | 0.97 (0.90) | 0.122 | Sw |
| Phe | 14.55 ± 2.70 | 14.3 ± 1.92 | 0.95 (0.62) | 0.313 | B |
| His | 6.21 ± 0.74 a | 22.51 ± 3.36 a | 0.83 (0.12) | 0.003 | B |
| Lys | 13.96 ± 2.99 b | 5.16 ± 0.93 a | 0.83 (0.12) | 0.031 | B |
| Arg | 15.83 ± 2.18 | 14.71 ± 2.57 | 0.92 (0.25) | 0.753 | B |
| ΣEAA | 94.27 ± 7.73 | 99.32 ± 5.43 | 0.94 (0.52) | 0.868 | |
| NEAA | |||||
| Ser | 34.76 ± 4.50 | 32.77 ± 3.53 | 0.92 (0.32) | 0.421 | Sw |
| Pro | 71.83 ± 12.09 | 99.27 ± 11.59 | 0.98 (0.99) | 0.277 | SB |
| Gly | 427.66 ± 28.74 b | 338.85 ± 24.57 a | 0.91 (0.21) | 0.032 | Sw |
| Ala | 26.7 ± 4.51 | 25.3 ± 4.25 | 0.83 (0.12) | 0.398 | Sw |
| Asp | 16.87 ± 4.48 | 14.78 ± 1.90 | 0.89 (0.12) | 0.267 | S |
| Glu | 24.92 ± 3.31 | 25.94 ± 3.40 | 0.91 (0.20) | 0.586 | U |
| Cys2 | 2.41 ± 0.27 | 0.00 ± 0.00 | 0.88 (0.16) | 0.725 | N |
| Tyr | 3.91 ± 0.60 | 4.44 ± 0.37 | 0.97 (0.93) | 0.437 | N |
| ΣNEAA | 609.07 ± 29.31 | 541.36 ± 28.12 | 0.95 (0.52) | 0.052 | |
| Total (ΣEAA + ΣNEAA) | 703.35 ± 31.63 | 640.68 ± 26.66 | 0.96 (0.73) | 0.074 | |
Free amino acid composition of the cultured Malabar grouper muscle fed with CO-D and FW-D for 8 weeks.
CO-D, Commercial diet; FW-D, Food waste diet.
EAA, Essential amino acid; NEAA, Non-essential amino acid. The taste attributes are represented as follows: SwB, Sweet-Bitter; B, Bitter; Sw, Sweet; SB, Sour-Bitter; S, Sour; U, Umami; N, Non-taste. Values are mean of triplicate groups and presented as mean ± SD (n = 6). Values in the same column having different superscript letters are significantly different (P < 0.05; independent-samples t-test) among treatments. The “SW W (P)” column indicates results of the Shapiro–Wilk test used to assess normality of the data distribution before applying the t-test. All variables satisfied normality assumptions (P > 0.05) for parametric testing. The lack of superscript letter indicates no significant differences among treatments.
3.6 Sensory evaluation
Figure 4 illustrates the sensory evaluation findings for cooked Malabar grouper muscle after 8 weeks on CO-D and FW-D. The assessment included flavor attributes (umami, fat flavor, and overall taste), color, fishy odor, and texture (oiliness and chewiness). Furthermore, no difference in umami was noted between the CO-D and FW-D groups, with umami scores above the baseline (0). The fat flavor score tended to be lower in the FW-D group than in the CO-D group. No differences in color were observed between the CO-D and FW-D groups. Fishy odor was substantially higher in the CO-D group than in the FW-D group. Chewiness scores tended to be higher in the FW-D group. No difference was noted in the oiliness scores between the groups, with both groups scoring below the baseline.
Figure 4
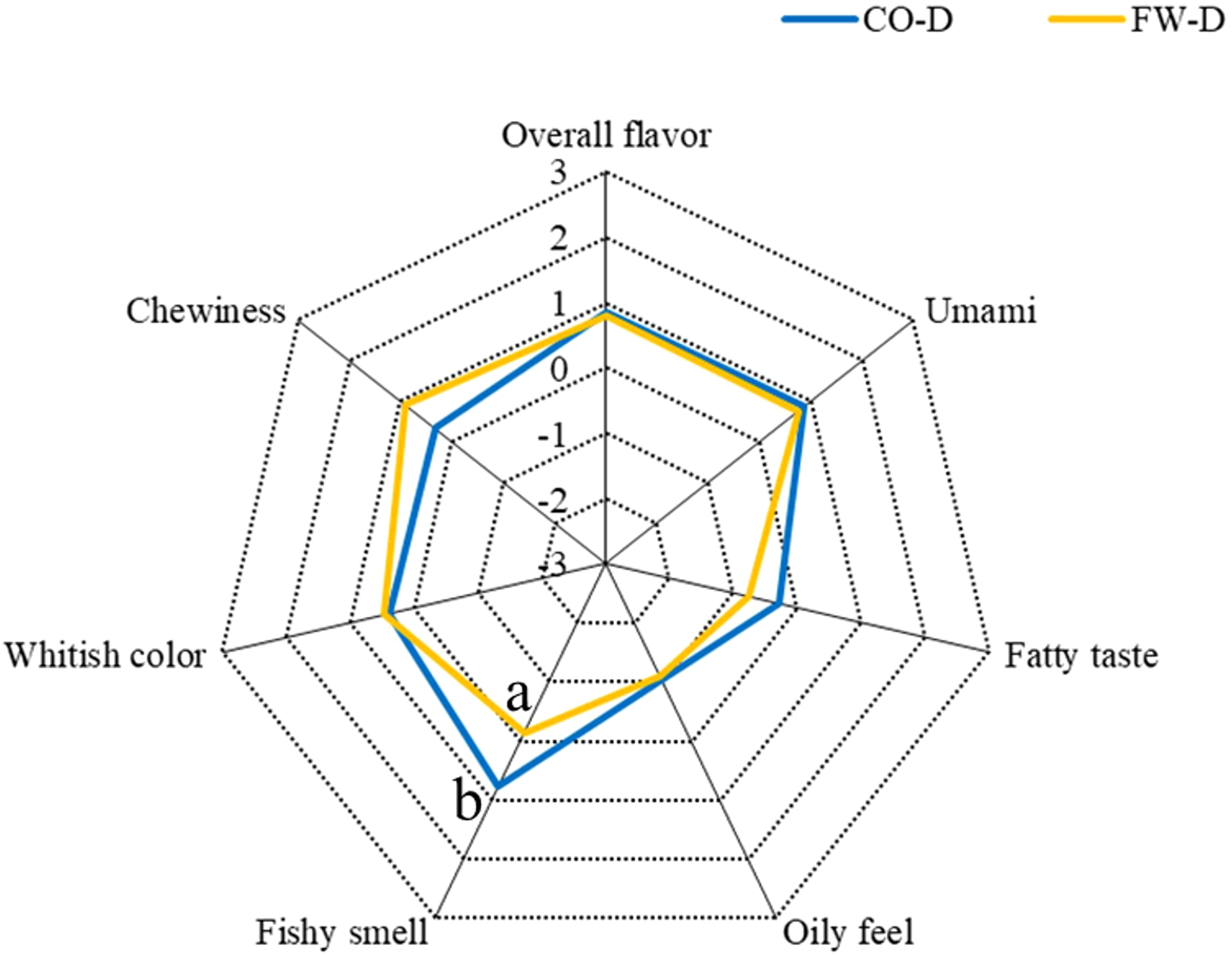
Sensory values of the cultured Malabar grouper fillets fed with CO-D and FW-D. CO-D, Commercial diet; FW-D, Food waste diet. All sensory attributes were assessed using a 7-point scale (from -3 = “slightly applicable” to +3 = “very applicable”). Different superscript letters indicate values that are significantly different according to the Student’s t-test (p < 0.05). The lack of superscript letters indicates that no significant differences were found among the treatments.
4 Discussion
This study aimed to assess the potential use of local food waste obtained via a circular economic approach as a sustainable feed for Malabar grouper aquaculture. The estimated total production cost for eight weeks of farming using FW-D, a low-cost feed formulated with fishmeal and BSY produced from by-products in Okinawa Prefecture (estimated price of FW-D: 1.35 US$/kg), was approximately 65% lower than that of farming with the commonly used CO-D feed in Japan’s aquaculture industry (price: 3.88 US$/kg). Additionally, the gross income of 1 kg of the aquacultured fish in the FW-D group could potentially produce a revenue difference of approximately 5.72 US$ compared to the CO-D group (Table 3). This study compared the growth performance, muscle composition, and sensory evaluation of Malabar groupers fed FW-D and CO-D for 8 weeks. The results showed differences in growth efficiency, muscle composition, and sensory qualities such as taste, aroma, and texture between the two feed types, providing valuable insights into FW-D’s potential as a sustainable feed alternative.
4.1 Growth performance and feed utilization efficiency
The total length and wet weight of the Malabar groupers revealed a trend of higher growth in the FW-D group than in the CO-D group for up to six weeks (See Supplementary Figure S2); however, at eight weeks, the values were lower in the FW-D group. Furthermore, the WGR and SGR were considerably lower in the FW-D group than in the CO-D group at 8 weeks. This suggests that although FW-D supports overall weight gain, it promotes growth less efficiently than CO-D. Given the observed growth decline at 8 weeks, a longer-term feeding trial may be valuable to fully assess the chronic effects of FW-D on growth performance and feed efficiency, particularly in light of its potential cost advantages. The observed decrease in PI in the FW-D group suggests limitations in FW-D’s amino acid profile prepared in this study, which may have contributed to lower growth efficiency. This implied that FW-D’s overall crude protein content was lower than that of CO-D. While this 6.5% difference in crude protein is non-negligible for carnivorous species like the Malabar grouper, it is also important to consider protein quality factors such as amino acid composition and digestibility. BSY has been reported to provide high nutritional value, including nucleic acids and vitamins, yet its amino acid profile—while generally comparable to fishmeal—may present lower levels of certain essential amino acids such as methionine and lysine depending on the yeast strain and processing method (Yamada and Sgarbieri, 2005). However, the actual digestibility and amino acid availability of the BSY used in this study remain unknown, as they may vary depending on the origin and treatment of the yeast. Therefore, future studies should analyze the amino acid profile and assess apparent digestibility coefficients (ADCs) specific to locally sourced BSY in Malabar grouper to optimize feed formulation. Protein is an essential macronutrient in fish feed, acts as an energy source for ATP generation, and is the body’s vital structural component. The required protein levels and utilization efficiencies markedly vary among fish species, reflecting their natural feeding behaviors. Carnivorous fish such as groupers (including Malabar groupers), which predominantly prey on crustaceans and fish, require higher protein levels than herbivorous and omnivorous fish species because protein levels positively correlate with nutritional requirements (Hu et al., 2013). Given the observed decrease in PI in the FW-D group, this indicates that the fish fed FW-D were unable to sustain comparable growth rates over time, likely due to the combined effects of insufficient protein quantity and potential limitations in protein quality. Consequently, as the protein demand increased with growth, the fish fed FW-D appeared unable to sustain the same growth rate, likely reflecting the compounded effects of lower protein quantity and potential limitations in protein quality. Fish grown on protein-deficient diets place a metabolic burden on the liver, where IGF-1 stimulates glycogen and fat metabolism to produce ATP for growth (Wood et al., 2005). Thus, we compared IGF-1 expression levels in the livers of Malabar groupers at eight weeks using RT-PCR and observed a non-significant increase in IGF-1 expression in the FW-D group. Moreover, the HSI tended to decrease in the FW-D group compared to that in the CO-D group, although the difference was not significant. Previous studies have reported that in carnivorous fish, prolonged fasting results in protein catabolism in the liver and the use of liver fat and glycogen for energy production, leading to a reduction in liver size (Wang et al., 2006). Thus, the slight energy deficiency in FW-D may have contributed to the observed tendency toward a decrease in the HSI.
Histological observations of the liver revealed that hepatocytes in CO-D and FW-D groups maintained a continuous cord-like arrangement without inflammation or fibrosis signs, indicating that neither diet caused hepatic toxicity. Additionally, the amount of fat within the hepatocytes was similar between the two groups, suggesting that liver fat catabolism is unlikely to be a factor in the decrease in HSI. Despite lower protein and energy levels, one factor that may have contributed to the growth support of the Malabar groupers in the FW-D group was the presence of BSY unique to FW-D. Estévez et al. (2021) found that diets supplemented with BSY improved growth performance indicators, such as WGR, SGR, and daily growth index in Gilthead seabream. This improvement was attributed to the rich nutrients in the BSY, especially proteins, carbohydrates, nucleic acids, and vitamins. Additionally, BSY supplementation’s positive effect on the growth of hybrid catfish (Pangasianodon hypophthalmus × Pangasius bocourti) has also been shown in growth trials using 45% fishmeal substitution with BSY (Pongpet et al., 2016). The addition of BSY to fish feed also increased protein levels in the muscle after feeding. Oliva-Teles and Gonçalves (2001) investigated the body composition of European sea bass after partially substituting fishmeal with BSY and observed elevated protein levels in the bass muscle. Furthermore, no significant differences were observed in the SGR values across all experimental groups compared to the control, with slight improvements in the FCR observed up to a 30% substitution rate. Thus, FW-D, with a lower protein content and energy than CO-D, did not impose a metabolic burden on the liver possibly because of the growth factors in the BSY. However, energy compensation through glycogen catabolism in the liver may have occurred, which was challenging to histologically confirm in the present study. To address this possibility more directly, future studies should consider quantifying hepatic glycogen levels in FW-D-fed fish to better understand the role of liver energy reserves in compensating for dietary energy differences. Further studies focusing on growth monitoring and hepatic glycogen quantification will help clarify the metabolic adaptations induced by FW-D.
The FW-D group demonstrated lower values of saturated fatty acid composition in the muscle than the CO-D group, albeit not significantly. In most cases, palmitic acid (C16:0) is the primary saturated fatty acid in fish, which is consistent with the saturated fatty acid composition noted in the CO-D and FW-D groups. Although the C16:0 content in the CO-D was approximately 1.97 times higher than that in the FW-D group, the proportion in the muscle was almost unaltered. This result contrasts with studies revealing that dietary fatty acid composition significantly affects the fatty acid profile of fish tissues (Tadesse, 2010). This may be because C16:0 is a primary product of fatty acid synthase and is produced during the initial stage of de novo fatty acid synthesis. This suggests that C16:0 levels in muscle may be maintained by biosynthesis rather than by dietary C16:0 intake, consistent with the findings of Akpinar et al. (2009). In the muscle tissue, monounsaturated fatty acids (MUFA) constituted the largest proportion of unsaturated fatty acids in both feed groups, with oleic acid (C18:1n-9) being the predominant MUFA. This finding is consistent with those of studies on different fish species, including Japanese sea bass and Nile tilapia (Luo et al., 2010). In animals, C18:1 is formed via the oxidative desaturation of stearic acid (C18:0), suggesting a correlation between the C18:0 and C18:1 composition in cells. The C18:1 composition observed in this study may be associated with increased C18:0 levels in the muscle tissue (Tadesse, 2010). The high C18:1 content in all the tissues may also be due to its abundance in the diet.
Contrastingly, the quality and nutritional value of fish flesh were closely associated with polyunsaturated fatty acids (PUFAs), especially C20:5n-3 (EPA) and C22:6n-3 (DHA), which were essential for normal growth and metabolism in groupers and crucial nutrients for humans. In the present study, DHA levels exceeded EPA levels in both feed groups. Wang et al. (2005) reported similar findings, suggesting that one reason may be EPA was more susceptible to oxidation than DHA. Although the muscle EPA levels were higher in the CO-D group than in the FW-D group, the difference was insignificant. However, EPA content was identical in both diets (319 mg/100 g), indicating that muscle EPA levels cannot be completely explained by diet alone. One reason for this may be that groupers convert dietary alpha-linolenic acid (C18:3n-3) into EPA through elongation. The C18:3 content in the CO-D was approximately 1.87 times higher than that in the FW-D, whereas in the muscle, it was approximately 1.27 times higher. Thus, some dietary C18:3 may have been converted into EPA and other PUFAs and stored during metabolic processes. Notably, groupers lack or demonstrate limited ability to convert C18:3 to DHA (Wu and Chen, 2012). In the present study, the DHA content was significantly higher in the FW-D group than in the CO-D group, which was closely associated with the DHA content in both feeds. The high DHA content in FW-D may be attributed to fishmeal and fish oil and BSY as a DHA source. Thus, when designing grouper feed, including high C18:3 and DHA levels to maintain normal growth and key metabolic functions in fish may be necessary. Furthermore, EPA and DHA demonstrate health benefits, including antihyperlipidemic effects (Micallef and Garg, 2009), improvements in non-alcoholic fatty liver disease (Guo et al., 2022), cardiovascular disease prevention (Zhong et al., 2023), and antidiabetic effects (Borja-Magno et al., 2023), suggesting that meat from Malabar groupers farmed with FW-D may have potential health benefits for consumers.
4.2 Quality and sensory evaluation of fish meat
FAAs in fish meat are closely associated with fish growth, nutritional value, and even taste. We hypothesized that the composition of the fish diet influences the taste of fish meat since fish metabolize the protein obtained from the diet and generate and accumulate different amino acids within their bodies. First, the total free amino acid content in fish muscle was lower in the FW-D group than in the CO-D group, which was consistent with the amino acid composition of the proteins in each diet (data not shown). Furthermore, the EAA content in the fish muscle was nearly the same between the groups, whereas the NEAA content was lower in the FW-D group. This finding also corresponds with the EAA and NEAA composition in the proteins of both diets. This suggests that FAA accumulation in the Malabar grouper meat depends on the EAA and NEAA contents in the protein structure of the diet. Differences in the FAA content of fish meat are closely associated with its, especially the umami and sweetness intensity (Hossain et al., 2024). Among the FAAs, glutamic acid and aspartic acid are recognized as the primary components responsible for the umami taste. Fish meat with high concentrations of these amino acids tends to have a strong umami flavor, which is typically preferred by consumers. Other amino acids such as glycine and alanine contribute to the sweetness and flavor profile of fish meat. All these components, including glutamic acid, aspartic acid, glycine, and alanine, demonstrated considerably lower values in the FW-D group than in the CO-D group. However, in the sensory evaluation conducted in this study, consumer panelists perceived the umami taste of fish meat to be similar between the CO-D and FW-D groups, generally rating it as slightly sweet and umami. Although the contents of sweet and umami FAAs were lower in the FW-D group than in the CO-D group, this difference may have been too subtle to be detected by the panelists. Alterations in the sweetness and umami of free amino acids may be explained by their absolute content and synergistic effects from the combination of specific free amino acids (Hwang et al., 2020). Additionally, the temperature of the samples may influence taste perception (Skinner et al., 2018), which warrants further investigation.
The fat sensation in fish fillets considerably influences the flavor, texture, and satisfaction linked to food. The optimal amount of fat that defines the food taste differs depending on the food type; if the fat content deviates from what is typical for the food in question, it can result in greasiness or leanness (Albendea et al., 2023). Grouper fish fillets are typically considered mild within the category of white fish fillets, and in the sensory evaluation, both sample groups were rated as mild. The lack of a difference in fat sensation between the two sample groups was likely correlated with the crude fat content of the fish fillets. However, the fat taste in the CO-D group was rated as “slightly easier to perceive” than in the FW-D group. The fat taste, recently identified as a new type of taste beyond the five traditional basic tastes (sweet, sour, salty, bitter, and umami), is thought to be primarily triggered by fatty acids (Running et al., 2015). When fats are broken down into free fatty acids, they bind to specific receptors on taste buds, allowing fat taste perception, and oleic and linoleic acids are thought to influence preference (Yasumatsu et al., 2018). Although the fat taste in this study was below the standard level for both samples, the CO-D group had a higher value than the FW-D group. This may be attributed to the considerably higher C18:1 and C18:2 content in the CO-D fish fillets than in the FW-D group. Nevertheless, the fat sensation and taste in both samples aligned with what would be expected from the mild fish fillets typical of the Malabar grouper.
Excessive odors can decrease consumer preferences and acceptability. Fish fillets spoil more rapidly than other meat types (such as beef, pork, or chicken), and freshness is correlated with fish odor, which may stem from factors such as microbial trimethylamine production and PUFA oxidation (Miyasaki et al., 2011). Fish spoilage bacteria multiply at near-room temperatures, producing amine odors, such as trimethylamine (Ganeko et al., 2008). In this sensory evaluation, fish odor was substantially affected by diet, with the FW-D group demonstrating a lower fish odor than the CO-D group. This result suggests that the cause is unlikely to be microbial given that both feed groups used the same methods for fish fillet sampling and storage. Instead, it may be associated with the antioxidant components derived from the brewer’s yeast in the FW-D feed. Suzuki et al. (2019) reported that the addition of brewer’s yeast to the diet improved the fish meat flavor, a phenomenon also noted in pork, where brewer’s yeast supplementation elevated the flavor and odor of fillets.
The color of Malabar grouper fillets is important in purchasing decisions, as it is one of the first sensory attributes that consumers notice. A concern exists that feeds rich in plant-based ingredients can lead to darker or duller fillets, potentially leading consumers to link them to less fresh products (Pleić et al., 2022). In this sensory evaluation, no difference in fillet color (whitish) was noted between the two groups, indicating that diet did not influence fillet color. This outcome was expected, as neither diet contained ingredients with colorants such as carotenoids. Furthermore, muscle texture characteristics play a crucial role in the consumer acceptance of a product. Nutrient accumulation within the muscle and the animal’s growth performance may influence these characteristics and are sensitive to dietary manipulation. Chewiness in this sensory evaluation was not substantially different but was affected by diet, with the FW-D group demonstrating greater elasticity than the CO-D group. Alterations in fillet texture can be explained by factors such as muscle cell density, fat content, and collagen content within the muscles (Matos et al., 2012). Histological assessment of the muscle tissue could clarify the reasons for the observed differences in this parameter. Muscle segments consist of bundles of muscle fibers arranged in a regular pattern in the lateral muscles of fish. Each short fiber connects to a muscle septum composed of extremely thin collagen, which coagulates upon heating. The septum transforms into a gelatinous substance, resulting in a softer and more fragile texture (Wang et al., 2022). Therefore, the cooked fish fillets may exhibit a chewier texture if the collagen content in the muscle septum of the FW-D group was lower than that of the CO-D group, which suggests the requirement for continued measurement of collagen content in fish fillets. In addition, although the differences in chewiness and fat flavor were not statistically significant, the consistent trends observed may still reflect biologically or perceptually relevant effects. These aspects warrant further investigation in future studies using larger sample sizes and more sensitive evaluation methods. The panel’s final evaluation of the overall flavor balance for both the diet groups received a positive score (> +1 or “somewhat favorable”) in > 90% of the cases. This indicates that fish fillets from both groups were equally tasty, showing that the Malabar grouper farmed with FW-D is a high-quality product that is well-accepted by consumers.
5 Conclusions
The use of FW-D formulated from locally sourced fishmeal and BSY provides a sustainable alternative to conventional CO-D. Herein, FW-D use in Malabar grouper farming led to a slight decrease in growth rate compared with CO-D; however, the potential benefits for fish quality, such as DHA content increase and fish odor reduction, were substantial. Additionally, decreasing the reliance on imported diet ingredients and using local waste streams provides economic and environmental advantages that may outweigh the minor reduction in growth efficiency. Thus, FW-D offers promising advantages in terms of nutritional quality, cost reduction, and sustainability, although CO-D remains effective in promoting stable growth. Nevertheless, this study was conducted over a relatively short culture period and used fish populations from a single geographic location, which may limit the broader applicability of the findings. Future studies should extend the feeding duration and include multiple sites or fish sources to enhance generalizability and validate the observed effects. Overall, this innovative approach holds potential for the future; however, further research is required to optimize the composition of FW-D to increase protein quality and overall growth performance and to maintain or improve FW-D’s positive effects on fish quality and sensory characteristics.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Medical Research Ethics Committee of the University of the Ryukyus (Okinawa, Japan). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by the Animal Care and Use Committee of the University of the Ryukyus (Okinawa, Japan). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MT: Writing – original draft, Formal Analysis, Writing – review & editing, Data curation, Investigation, Visualization, Conceptualization, Methodology. SU: Methodology, Writing – review & editing, Formal Analysis, Data curation, Supervision, Visualization. CY: Investigation, Resources, Writing – review & editing, Data curation. YI: Writing – review & editing, Investigation, Data curation, Resources. TS: Writing – review & editing, Data curation. AT: Conceptualization, Resources, Writing – original draft, Funding acquisition, Project administration, Methodology, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by COI-NEXT (JPMJPF2012 to AT).
Acknowledgments
We thank the staff of the Okinawa Prefectural Sea Farming Center, Okinawa, Japan, and Sesoko Station, Tropical Biosphere Research Center, University of the Ryukyus, Okinawa, Japan, for providing fish and facilities, respectively. We would like to thank Editage (www.editage.jp) for the English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/faquc.2025.1650008/full#supplementary-material
References
1
Akpinar M. A. Gorgun S. Akpinar A. E. (2009). A comparative analysis of the fatty acid profiles in the liver and muscles of male and female Salmo trutta macrostigma. Food Chem.112, 6–8. doi: 10.1016/j.foodchem.2008.05.025
2
Albendea P. Tres A. Rafecas M. Vichi S. Solà-Oriol D. Verdú M. et al . (2023). Effect of feeding olive pomace acid oil on pork lipid composition, oxidative stability, colour, and sensory acceptance. Animal17, 100879. doi: 10.1016/j.animal.2023.100879
3
Aleisa E. Al-Jarallah R. (2024). Optimizing life cycle sustainability based on municipal solid waste streams and treatment potentials. Environ. Syst. Decis.44, 887–905. doi: 10.1007/s10669-024-09978-7
4
Association of Official Analytical Chemists (A.O.A.C.) (1995). Official Methods of Analysis. 16th ed (Arlington, VA: AOAC).
5
Bligh E. G. Dyer W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol.37, 911–917. doi: 10.1139/o59-099
6
Borja-Magno A. I. Furuzawa-Carballeda J. Guevara-Cruz M. Arias C. Granados J. Bourges H. et al . (2023). Supplementation with EPA and DHA omega-3 fatty acids improves peripheral immune cell mitochondrial dysfunction and inflammation in subjects with obesity. J. Nutr. Biochem.120, 109415. doi: 10.1016/j.jnutbio.2023.109415
7
Bu X. Y. Wang Y. Y. Chen F. Y. Tang B. B. Luo C. Z. Wang Y. et al . (2018). An evaluation of replacing fishmeal with rapeseed meal in the diet of Pseudobagrus ussuriensis: growth, feed utilization, nonspecific immunity, and growth-related gene expression. J. World Aquac. Soc49, 1068–1080. doi: 10.1111/jwas.12470
8
Estévez A. Padrell L. Iñarra B. Orive M. San Martin D. (2021). Brewery by-products (yeast and spent grain) as protein sources in gilthead seabream (Sparus aurata) feeds. Aquaculture543, 736921. doi: 10.1016/j.aquaculture.2021.736921
9
FAO (2021).Food loss and food waste. Available online at: https://www.fao.org/food-loss-and-food-waste/flw-data (Accessed February 5, 2025).
10
Ferreira I.M.P.L.V.O. Pinho O. Vieira E. Tavarela J. G. (2010). Brewer’s Saccharomyces yeast biomass: characteristics and potential applications. Trends Food Sci. Technol.21, 77–84. doi: 10.1016/j.tifs.2009.10.008
11
Ganeko N. Shoda M. Hirohara I. Bhadra A. Ishida T. Matsuda H. et al . (2008). Analysis of volatile flavor compounds of sardine (Sardinops melanostica) by solid phase microextraction. J. Food Sci.73, S83–S88. doi: 10.1111/j.1750-3841.2007.00608.x
12
Glencross B. D. Booth M. Allan G. L. (2007). A feed is only as good as its ingredients – a review of ingredient evaluation strategies for aquaculture feeds. Aquac. Nutr.13, 17–34. doi: 10.1111/j.1365-2095.2007.00450.x
13
Guo X. F. Wang C. Yang T. Ma W. J. Zhai J. Zhao T. et al . (2022). The effects of fish oil plus vitamin D3 intervention on nonalcoholic fatty liver disease: a randomized controlled trial. Eur. J. Nutr.61, 1931–1942. doi: 10.1007/s00394-021-02772-0
14
Hossain M. J. Alam A. N. Lee E. Y. Hwang Y. H. Joo S. T. (2024). Umami characteristics and taste improvement mechanism of meat. Food Sci. Anim. Resour.44, 515–532. doi: 10.5851/kosfa.2024.e29
15
Hu L. Yun B. Xue M. Wang J. Wu X. Zheng Y. et al . (2013). Effects of fish meal quality and fish meal substitution by animal protein blend on growth performance, flesh quality and liver histology of Japanese seabass (Lateolabrax japonicus). Aquaculture20, 52–61. doi: 10.1016/j.aquaculture.2012.10.025
16
Hwang Y. H. Ismail I. Joo S. T. (2020). Identification of umami taste in sous-vide beef by chemical analyses, equivalent umami concentration, and electronic tongue system. Foods9, 251. doi: 10.3390/foods9030251
17
Kimura R. Takahashi N. Lin S. Goto T. Murota K. Nakata R. et al . (2013). DHA attenuates postprandial hyperlipidemia via activating PPARα in intestinal epithelial cells. J. Lipid Res.54, 3258–3268. doi: 10.1194/jlr.M034942
18
Li S. Zhu D. Li K. Yang Y. Lei Z. Zhang Z. (2013). Soybean curd residue: composition, utilization, and related limiting factors. ISRN Ind. Eng.2013, 1–8. doi: 10.1155/2013/423590
19
Lin Y. H. Shiau S. Y. (2003). Dietary lipid requirement of grouper, Epinephelus malabaricus, and effects on immune responses. Aquaculture225, 243–250. doi: 10.1016/S0044-8486(03)00293-X
20
Luo G. Xu J. Teng Y. Ding C. Yan B. (2010). Effects of dietary lipids level on growth, digestive enzyme, feed utilization and fatty acid composition of Japanese sea bass (Lateolabrax japonicas L.) reared in freshwater. Aquacult. Res.4, 210–219. doi: 10.1111/j.1365-2109.2009.02319.x
21
Marson G. V. de Castro R. J. S. Belleville M. P. Hubinger M. D. (2020). Spent brewer’s yeast as a source of high added value molecules: a systematic review on its characteristics, processing and potential applications. World J. Microbiol. Biotechnol.36, 95. doi: 10.1007/s11274-020-02866-7
22
Matos E. Gonçalves A. Bandarra N. Colen R. Nunes M. L. Valente L. M. P. et al . (2012). Plant proteins and vegetable oil do not have detrimental effects on post-mortem muscle instrumental texture, sensory properties and nutritional value of gilthead seabream. Aquaculture358–359, 205–212. doi: 10.1016/j.aquaculture.2012.07.009
23
Metzger D. C. Luckenbach J. A. Shimizu M. Beckman B. R. (2012). Normalizing for biology: Accounting for technical and biological variation in levels of reference gene and insulin-like growth factor 1 (igf1) transcripts in fish livers. Comp. Biochem. Physiol. A163, 7–14. doi: 10.1016/j.cbpa.2012.04.014
24
Micallef M. A. Garg M. L. (2009). Anti-inflammatory and cardioprotective effects of n-3 polyunsaturated fatty acids and plant sterols in hyperlipidemic individuals. Atherosclerosis204, 476–482. doi: 10.1016/j.atherosclerosis.2008.09.020
25
Miyasaki T. Hamaguchi M. Yokoyama S. (2011). Change of volatile compounds in fresh fish meat during ice storage. J. Food Sci.76, C1319–C1325. doi: 10.1111/j.1750-3841.2011.02388.x
26
Mohamed S. H. Magdy A. S. Eman Y. M. Mohamed A. E. Ehab R.-H. Simon J. D. (2018). Growth and physiological responses of Nile tilapia, Oreochromis niloticus fed dietary fermented sunflower meal inoculated with Saccharomyces cerevisiae and Bacillus subtilis. Aquaculture495, 592–601. doi: 10.1016/j.aquaculture.2018.06.018
27
Nakaishi T. Takayabu H. (2022). Production efficiency of animal feed obtained from food waste in Japan. Environ. Sci. pollut. Res.29, 61187–61203. doi: 10.1007/s11356-022-20221-1
28
Oliva-Teles A. Gonçalves P. (2001). Partial replacement of fishmeal by brewers yeast (Saccaromyces cerevisae) in diets for sea bass (Dicentrarchus labrax) juveniles. Aquaculture202, 269–278. doi: 10.1016/S0044-8486(01)00777-3
29
Pleić I. L. Bušelić I. Messina M. Hrabar J. Žuvić L. Talijančić I. et al . (2022). A plant-based diet supplemented with Hermetia illucens alone or in combination with poultry by-product meal: one step closer to sustainable aquafeeds for European seabass. J. Anim. Sci. Biotechnol.13, 77. doi: 10.1186/s40104-022-00725-z
30
Podolska M. Nadolna-Ałtyn K. Pawlak J. Horbowy J. (2024). The presence of nematodes in the liver of Baltic cod, Gadus morhua, is associated with a decline in condition factors and hepatosomatic index of the host. Fish. Res.273, 106958. doi: 10.1016/j.fishres.2024.106958
31
Pongpet J. Ponchunchoovong S. Payooha K. (2016). Partial replacement of fishmeal by brewer’s yeast (Saccharomyces cerevisiae) in the diets of Thai Panga (Pangasianodon hypophthalmus × Pangasius bocourti). Aquac. Nutr.22, 575–585. doi: 10.1111/anu.12280
32
Running C. A. Craig B. A. Mattes R. D. (2015). Oleogustus: the unique taste of fat. Chem. Senses40, 507–516. doi: 10.1093/chemse/bjv036
33
Skinner M. Eldeghaidy S. Ford R. Giesbrecht T. Thomas A. Francis S. et al . (2018). Variation in thermally induced taste response across thermal tasters. Physiol. Behav.188, 67–78. doi: 10.1016/j.physbeh.2018.01.017
34
Suzuki M. Masuda T. Kawamoto T. Tajima S. Uchikura K. Kurita T. (2019). Effects of feeding liquid Brewer’s yeast on growth performance, carcass characteristics, and meat quality of finishing pigs. Jpn. J. Swine Sci.56, 23–32. doi: 10.5938/youton.56.2_23
35
Tadesse Z. (2010). Diet composition impacts the fatty acids contents of Nile tilapia, (Oreochromis niloticus L.) in Ethiopian high land lakes. Verh. Internet. Assoc. Limnol.30, 1363–1368. doi: 10.1080/03680770.2009.11902333
36
Wang J. T. Liu Y. J. Tian L. X. Mai K. S. Du Z. Y. (2005). Effects of dietary lipids level on growth performance, lipid deposition and hepatic lipogenesis in juvenile cobia (Rachycentron canadum). Aquaculture249, 439–447. doi: 10.1016/j.aquaculture.2005.04.038
37
Wang T. Hung C. C. Y. Randall D. J. (2006). The comparative physiology of food deprivation: from feast to famine. Annu. Rev. Physiol.68, 223–251. doi: 10.1146/annurev.physiol.68.040104.105739
38
Wang Y. Li K. Han H. Zheng Z. X. Bureau D. P. (2008). Potential of using a blend of rendered animal protein ingredients to replace fish meal in practical diets for malabar grouper (Epinephelus malabricus). Aquaculture281, 113–117. doi: 10.1016/j.aquaculture.2008.03.033
39
Wang Y. Wang X. Lin C. Yu M. Chen S. Guo J. et al . (2022). Heat-induced structural changes in fish muscle collagen related to texture development in fish balls: Using eel ball as a study model. Food Sci. Nutr.10, 329–341. doi: 10.1002/fsn3.2462
40
Wood A. W. Duan C. Bern H. A. (2005). Insulin-like growth factor signaling in fish. Int. Rev. Cytol.243, 215–285. doi: 10.1016/S0074-7696(05)43004-1
41
Wu F. C. Chen H. Y. (2012). Effects of dietary linolenic acid to linoleic acid ratio on growth, tissue fatty acid profile and immune response of the juvenile grouper Epinephelus malabaricus. Aquaculture324–325, 111–117. doi: 10.1016/j.aquaculture.2011.10.030
42
Yamada E. A. Sgarbieri V. C. (2005). Yeast (Saccharomyces cerevisiae) protein concentrate: preparation, chemical composition, and nutritional and functional properties. J. Agric. Food Chem.53, 3931–3936. doi: 10.1021/jf0400821
43
Yasumatsu K. Iwata S. Inoue M. Ninomiya Y. (2018). Fatty acid taste quality information via GPR120 in the anterior tongue of mice. Acta Physiol.226, e13215. doi: 10.1111/apha.13215
44
Ye H. Zhou Y. Su S. Wang A. Tan X. Sun Z. et al . (2019). Effects of replacing fish meal with rendered animal protein blend on growth performance, hepatic steatosis and immune status in hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Aquaculture511, 734203. doi: 10.1016/j.aquaculture.2019.734203
45
Zhong N. Han P. Wang Y. Zheng C. (2023). Associations of polyunsaturated fatty acids with cardiovascular disease and mortality:a study of NHANES database in 2003-2018. BMC Endocr. Disord.23, 185. doi: 10.1186/s12902023-01412-4
46
Zhu Y. Fukunaga K. Udagawa S. Shimabukuro A. Takemura A. (2022). Effects of selected light wavelengths on the transcript levels of photoreceptors and growth-related hormones and peptides in the Malabar grouper Epinephelus malabaricus. Aquac. Rep.27, 101393. doi: 10.1016/j.aqrep.2022.101393
Summary
Keywords
aquaculture, food waste, grouper, growth, brewer’s spent yeast, sensory evaluation
Citation
Takahashi M, Udagawa S, Yamauchi C, Imura Y, Seong T and Takemura A (2025) Effect of cultivation feed diet using local food processing by-products on the growth and flavor factor in the Malabar grouper Epinephelus malabaricus. Front. Aquac. 4:1650008. doi: 10.3389/faquc.2025.1650008
Received
19 June 2025
Accepted
24 July 2025
Published
11 August 2025
Volume
4 - 2025
Edited by
Lars Christian Gansel, Norwegian University of Science and Technology, Norway
Reviewed by
Gulab Khedkar, Dr. Babasaheb Ambedkar Marathwada University, India
Manjun Yang, Sun Yat-sen University, China
Updates
Copyright
© 2025 Takahashi, Udagawa, Yamauchi, Imura, Seong and Takemura.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Makoto Takahashi, macott@cs.u-ryukyu.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.