- 1Department of Aquaculture and Fish Diseases, Faculty of Veterinary Science, University of Thessaly, Karditsa, Greece
- 2Department of Pharmacology & Toxicology, Faculty of Veterinary Science, University of Thessaly, Karditsa, Greece
- 3Laboratory of Cellular Immunology, Department of Microbiology, Hellenic Pasteur Institute, Athens, Greece
- 4Department of Marine Sciences, School of The Environment, University of The Aegean, University Hill, Mytilene, Greece
- 5Institute of Marine Biology, Biotechnology and Aquaculture, Hellenic Centre for Marine Research, Athens, Greece
- 6Institute of Environment and Sustainable Development (IESD), University Research Center of Ioannina (URCI), Ioannina, Greece
- 7Faculty of Agriculture, University of Ioannina, Arta, Greece
A rise of intensive Mediterranean aquaculture has been associated with vulnerability to bacterial infections, necessitating alternative approaches to conventional antibiotics. This study evaluated the antibacterial and bactericidal activity of essential oils derived from fifteen medicinal plants against four key bacterial pathogens affecting Mediterranean marine aquaculture: Aeromonas veronii biovar veronii, Aeromonas veronii biovar sobria, Vibrio harveyi, and Tenacibaculum maritimum. Essential oils were screened using disc diffusion assays, and the most effective oils—thyme, oregano, cinnamon, and absinthe—underwent further evaluation through broth microdilution methods. Results demonstrated that these four oils exhibited notable inhibitory and bactericidal effects, with thyme and oregano showing the strongest overall activity across multiple pathogens. Notably, this is among the first studies to document the in vitro efficacy of essential oils against Tenacibaculum maritimum, a major pathogen with limited treatment options. The findings support the potential use of selected essential oils as sustainable and natural antibacterial agents in fish health management, contributing to reduced reliance on antibiotics in aquaculture.
1 Introduction
Aquaculture, recognized as one of the fastest-growing sectors of food production by the Food and Agriculture Organization of the United Nation (FAO, 2022), can face challenging conditions due to farm intensity and environmental effects (Mavraganis et al., 2020). These conditions can foster the proliferation of bacterial and fungal infections (Abdel-Latif et al., 2023), as well as viral and parasitic diseases (Wright et al., 2023). Notably, the global aquaculture industry suffers substantial losses due to various opportunistic Gram-negative and Gram-positive bacteria. Among these, pathogens such as Aeromonas, Vibrio, Edwardsiella, Flavobacterium, Photobacterium, Pseudomonas, Yersinia, Lactococcus, Renibacterium, and Streptococcus play key roles (Pridgeon and Klesius, 2012; Irshath et al., 2023). Specifically in Mediterranean aquaculture, pathogens like Aeromonas veronii, Photobacterium damselae, Vibrio harveyi, and Tenacibaculum maritimum lead to significant annual fish losses (Arun and Midhun, 2023; Manchanayake et al., 2023; Triga et al., 2023).
The emergence of infections in fish, leading to disease outbreaks, poses a significant challenge for the aquaculture sector, as it can lead to considerable economic losses due to morbidity and mortality. The prevalent practice of maintaining high fish-rearing densities in aquaculture facilitates the transfer and dissemination of pathogenic microorganisms, often being a primary factor in catastrophic outbreaks (Rohani et al., 2022). Intensive farming practices impose substantial stresses on cultured aquatic species, compromising their innate immune defenses against various disease-causing bacterial and viral pathogens. Effective husbandry and overall management, encompassing aspects like biosecurity, nutrition, genetics, system management, and water quality, play a pivotal role in the success of aquaculture production across various intensive culture farming methods, regardless of whether they involve individual or multiple fish species reared in dense populations (Muktar and Tesfaye, 2016).
In regions such as China, India, and Vietnam, fish diseases are estimated to contribute to over 30% of the overall production loss (Mohd-Aris et al., 2019). The susceptibility of aquaculture facilities to disease outbreaks is heightened by the opportunistic nature of several bacterial and viral pathogens, along with parasites that may exist in the environment or as asymptomatic carriers in some fish. This susceptibility hampers the development of an efficient, cost-effective, and stable aquaculture process (Jørgensen, 2020). The onset and progression of fish diseases depend on the interplay between the pathogen, host, and environment. Stressful conditions, including high population density, temperature fluctuations, and hypoxia, can expedite the spread of pathogenic bacteria, leading to significant disease outbreaks (Ben Hamed et al., 2018).
A notable threat worldwide is the bacterium Aeromonas veronii Hickman-Brenner et al., 1987, causing mortality outbreaks in various farmed fish species, including Nile tilapia (Oreochromis niloticus Linnaeus 1758), African catfish (Clarias gariepinus Burchell 1822), rajputi (Puntius gonionotus Bleeker 1850), and several others (Smyrli et al., 2019). Symptoms include exophthalmia, ulcers, and haemorrhagic septicaemia. Aeromonas veronii bv. veronii has particularly impacted European seabass (D. labrax) culture in Greece and in Mediterranean (Smyrli et al., 2017, 2019). Tenacibaculum maritimum is an opportunistic bacterial pathogen causing ‘flexibacteriosis’ in marine fish, primarily affecting cultured European sea bass (Dicentrarchus labrax) (Kolygas et al., 2012) and finally, Vibriosis in Mediterranean marine aquaculture used to be attributed to Vibrio anguillarum, but Vibrio harveyi has emerged as a severe fish pathogen (Triga et al., 2023).
In order to address all the above bacterial pathogens, antibiotics are employed, and in numerous instances, there are clear signs of antibiotic resistance (Caputo et al., 2023), particularly evident during Vibirio harveyi outbreaks in European sea bass (Droubogiannis et al., 2023). V. harveyi is primarily affecting larger sea bass and prompts an upsurge in antibiotic usage. Conversely, several accounts highlight the recurrence of infestations, frequently manifesting shortly after the completion of antibiotic treatments. Moreover, there are reports of simultaneous infections involving other vibrios and/or Photobacterium damselae (Abdel-Aziz et al., 2013; Liu et al., 2016; Mohamad et al., 2019; Zhang et al., 2020). Overall, the widespread use of antibiotics in aquaculture is concerning due to ecological and resistance issues (Hossain et al., 2022; Wang et al., 2022; Feng et al., 2022a, b). Organizations like the World Health Organization (WHO) and FAO have established guidelines to restrict antibiotic use in aquaculture (World Health Organization, 2017; The FAO Action Plan on Antimicrobial Resistance 2021–2025, 2021), underlining the importance of exploring alternative therapies.
In this context, essential oils (EOs) derived from Medicinal Aromatic Plants (MAPs) offer potential alternatives. EOs, composed of lipophilic mixtures responsible for plant aroma, possess antibacterial, antifungal, antiviral, and insecticidal properties (Inamuddin et al., 2023; Mustafa et al., 2023; Rani et al., 2023). The mechanism of action of EOs depends on their chemical composition (Nazzaro et al., 2013), which can vary among plant species (Zuazo et al., 2019). Thus, there is a growing interest in utilizing EOs as antibiotics and fungicides in aquaculture to combat bacterial infections.
In addition to their antibacterial properties, there is a large body of evidence to suggest that essential oils (EOs) also have immunostimulatory effects in animals, including fish (Anastasiou and Buchbauer, 2017; Valdivieso-Ugarte et al., 2019; Alagawany et al., 2020; Firmino et al., 2021).
1.1 Enhanced white blood cell production and phagocytic activity
EOs can stimulate the production of white blood cells, which are crucial for combating infections, and activate phagocytes, the cells responsible for engulfing and eliminating pathogens. These effects indicate a wider potential benefit of EOs for preventing pathological and welfare issues in farmed fish (Mabrok and Wahdan, 2018). For example, rainbow trout fed a diet containing thyme essential oil had significantly higher levels of white blood cells and phagocytic activity compared to controls (Yousefi et al., 2022).
1.2 Improved antioxidative status and immune gene expression
Dietary administration of specific essential oils, such as origanum essential oil, has been shown to enhance antioxidative status, modulate immune-related genes, and increase resistance to bacterial infections. For instance, supplementation with origanum essential oil improved antioxidative status, immune-related genes, and disease resistance in common carp challenged with Aeromonas hydrophila (Abdel-Latif et al., 2020).
These benefits of EOs indicate a potential positive impact on the overall health and disease resistance of farmed fish. The immunostimulatory properties of EOs in fish can enhance their immune responses, potentially leading to improved disease resistance and overall welfare in aquaculture. These findings underscore the potential of EOs as natural and sustainable alternatives for promoting the health and well-being of farmed fish.
The aim of the present work is to investigate the antibacterial properties of 15 EOs which are locally cultivated or easily sourced from commercial producers in the Mediterranean region, against common fish pathogens of Mediterranean aquaculture, aiming to identify EOs with potent inhibitory effects, particularly against Aeromonas, Vibrio, and Tenacibaculum. The antibacterial potential of these essential oils is largely attributed to major constituents such as thymol, carvacrol, eugenol, and cinnamaldehyde, which are known to disrupt bacterial membranes and interfere with metabolic processes (Ultee et al., 2000; Burt and Reinders, 2003; Nazzaro et al., 2013).
2 Materials and methods
2.1 MAPs and EOs
EOs were sourced from these MAPs: Ginger (Gingiber officinale), cutleaf geranium (Geranium dissectum), Basil (Ocimum basilicum), Tea tree (Malaleuca alternifolia), Cinnamon (Cinnamomum verum), Clove (Syzygiuma romaticum, also known as Eugenia caryophyllata), Oregano (Origanum vulgare), Patchouli (Pogostemon cablin), English Lavender (Lavandula angustifolia), Frankincense or olibanum (Boswellia carterii), Blue gum (Eucalyptus globulus), and Sweet Marjoram (Origanum majorana) were procured from a commercial company. Thyme (Thymus vulgaris), Aloe (Aloe vera), and Wormwood or Absinthe (Artemisia absinthium) EOs were extracted manually. For the extraction process, dried samples from the aerial parts (leaves, flowers, and stems) of these plants underwent a 3-hour water distillation using a Clevenger-type apparatus, yielding 2.0–2.6% v/w. The resulting EOs were subsequently dried using anhydrous sodium sulfate and stored at temperature 5°C (± 1°C).
The essential oils (EOs) selected in this study represent plants that are either widely available or already recognized for their use in food, medicine, or aquaculture-related applications. EOs of thyme and oregano are rich in thymol and carvacrol, clove contains eugenol, and cinnamon provides cinnamaldehyde, all of which are widely recognized for strong antibacterial effects through membrane disruption and enzyme inhibition (Ultee et al., 2000; Burt and Reinders, 2003). Alcohol-containing oils, including basil, lavender, and tea tree, contribute compounds such as linalool and terpinen-4-ol with similar mechanisms of action (Lee et al., 2009; Nazzaro et al., 2013). Additional oils, including ginger, eucalyptus, patchouli, frankincense, marjoram, aloe, and wormwood, were included to broaden the chemical classes evaluated and to capture compounds with antioxidant or immunomodulatory potential. With this range of tested essential oils, the study aimed to evaluate representatives of different chemical classes and plant origins, providing a first step toward identifying natural alternatives to antibiotics that can contribute to ensuring the health and sustainability of aquatic ecosystems (Dawood et al., 2021; Park et al., 2016).
2.2 Maintenance and preparation of cultures
Cultures of Aeromonas veronii bv. veronii and Aeromonas veronii bv. veronii were obtained from naturally infected European sea bass (Dicentrarchus labrax) collected from Chios Island and the Saronikos region (Greece), respectively. Similarly, Vibrio harveyi and Tenacibaculum maritimum were isolated from naturally infected sea bass sourced from Euboea Island (Greece). All these isolates were preserved on tryptone soy broth (TSB) slants at a temperature of 4°C. Inocula for the experiments (A. veronii bv. veronii, A. veronii bv. sobria and V. harveyi) were prepared by cultivating the isolates for 16 hours in Mueller–Hinton Broth (MHB; Oxoid) while Tenacibaculum maritimum was cultivated in marine broth (MB; Condalab), all at a temperature of 23°C.
2.3 Disc diffusion assay
Bacterial cultures 18 hours old were appropriately diluted using sterile physiological saline solution (0.85% w/v sodium chloride) based on the McFarland standard (bioMérieux, Marcy l’Etoile, France) to achieve an inoculum of around 106 CFU ml-1. A 5ml volume of each inoculum (A. veronii bv. veronii, A. veronii bv. sobria and V. harveyi) was applied to the surface of Mueller–Hinton agar (MHA; Oxoid) plates that had been surface-dried to remove excess moisture and allowed to make contact for 1 minute. Subsequently, sterile 6mm filter paper discs (Sigma-Aldrich) were positioned on the plates, and immediately 10 µl of each EO was administered separately. Sterile physiological saline solution was applied to separate discs as a negative control to ensure that any inhibition observed was due to the EO and not the solvent or disc material. Plates were then incubated at 23°C for 24 hours. The inhibition zones were measured to the nearest millimeter defined at complete growth inhibition according to EUCAST disk diffusion reading guide (EUCAST, 2024). Each assay was repeated ten times for every EO. These standardized reading practices improve inter-study comparability even though categorical breakpoints are not available for essential oils.
2.4 Colorimetric determination of bacteriostatic and bactericidal concentrations
EOs demonstrating the most potent antibacterial effects in the disc diffusion assay (namely absinthe, thyme, cinnamon, and oregano), underwent further analysis to determine their bacteriostatic and bactericidal concentrations using a modified (Wang et al., 2021; Palaniyappan et al., 2023) colorimetric broth microdilution technique (Salvat et al., 2001). This evaluation involved setting up three sterile 96-well microplates with lids (VWR International). The process involved arranging 200 μl portions of 2% EO in sterile Mueller-Hinton Broth (MHB; A.veronii bv. veronii, A. veronii bv. sobria, V. harveyi) or Marine Broth (MB; Tenacibaculum maritimum) in wells of row A, while wells in rows B to H received 100 μl of sterile MHB (or MB). To enhance the stability and emulsification of the essential oil within the broths, an incorporation of soy lecithin (Oxoid) at a concentration of 0.25% (w/v) was introduced. Serial two-fold dilutions were carried out from row A to row H, with the highest dilution in row H. The inoculums, prepared from 16-hour cultures adjusted according to the McFarland standard, were further diluted with MHB (or MB) to achieve an approximate concentration of 106 CFU ml-1. Each well then received 100 μl of the inoculum and resazurin sodium salt (Sigma-Aldrich).
The microplates were incubated at temperatures of 23°C for 96 hours. A color change from blue to pink or mauve indicated bacterial growth. An extension of Salvat et al.’s method (Burt and Reinders, 2003) involved plating aliquots of 5 μl from the wells that remained blue onto Mueller-Hinton Agar (MHA; A. veronii bv. veronii, A. veronii bv. sobria, V. harveyi) or MA (Tenacibaculum maritimum), followed by a 24-hour incubation at 23°C. The experiment was conducted in six replicates for each microassay.
The bacteriostatic concentration was established as the lowest concentration at which bacteria in at least five out of the six replicates failed to grow in MHB (or MB) but were able to grow when plated on MHA (or MA). The bactericidal concentration was determined as the lowest concentration at which bacteria in at least five of the six replicates did not grow in MHB (or MB) and were not cultured after plating onto MHA (or MA). The method used for MIC and MBC determinations followed CLSI-style broth microdilution methodology commonly applied in EO studies, thereby increasing comparability with standardized antimicrobial testing. A limitation, however, is that CLSI and EUCAST clinical breakpoints are established only for antibiotics and not for essential oils, which prevents direct categorization of EO activity into susceptible, intermediate, or resistant classes (EUCAST, 2024; Xiao et al., 2020; Fokas et al., 2025).
2.5 Statistical analysis
Data were analyzed using SPSS v.1.0.0.1406. Inhibition zone diameters from the disc diffusion assays (10 replicates per each of tested essential oil) were compared using one-way ANOVA, followed by Tukey’s post hoc test to identify significant pairwise differences. MIC and MBC values obtained from broth microdilution assays (six replicates per each tested essential oil) were compared among oils using the non-parametric Kruskal–Wallis test, as distributional assumptions were not met. Correlations between inhibition zone diameters and MIC values were assessed using Pearson’s correlation coefficient to evaluate consistency of results between the two methods. Statistical significance was accepted at p<0.05. The level Significant differences are indicated in Table 1 by bold lettering and explained in the footnotes.
2.6 Bioethics
All MAPs and EOs were acquired from licensed commercial enterprises in adherence to regional law 3937/2011 concerning biodiversity conservation. The handling, storage, and disposal of all biological agents were carried out in accordance with Directives 2000/54/EU and 89/391/EEC. The experimental strategy employed no animals in any aspect of the experimentation taking into account article 4 of Directive 2010/63/EU. The overall experimental protocol’s principles are in accordance with the Hellenic presidential decree 56/2013(106). The bacterial isolates originated from prior isolations from naturally infected fish, and their species-level characterization was accomplished using molecular techniques.
3 Results and discussion
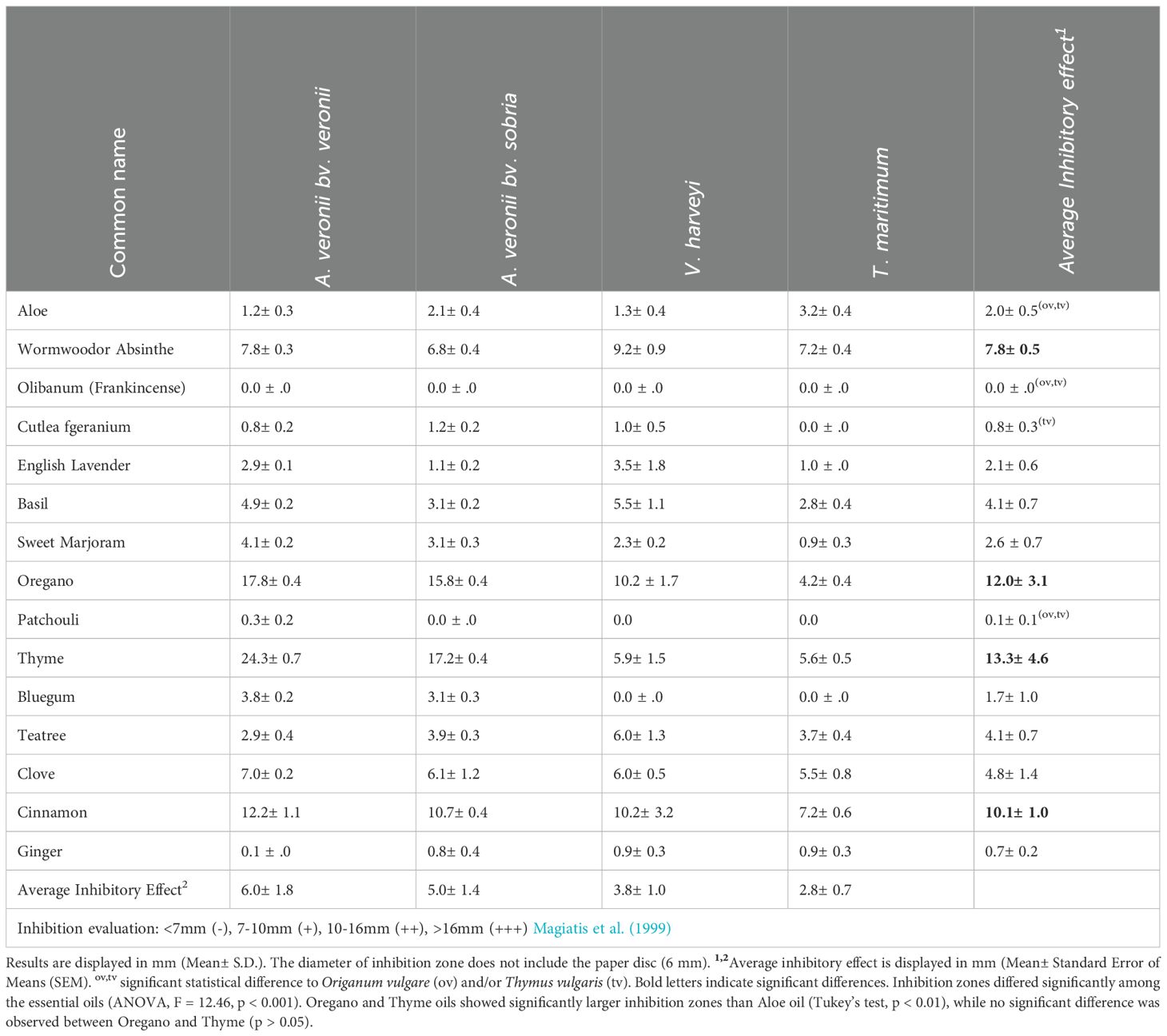
Disc diffusion assay results are provided in Table 1, which shows the bacteriostatic activity of 15 EOs against the four tested fish pathogens. Several essential oils exhibited notable inhibitory effects against the tested pathogens. Oregano and Thyme oils showed significantly larger inhibition zones than Aloe oil (Tukey’s test, p < 0.01), while no significant difference was observed between Oregano and Thyme (p > 0.05). Among the Asphodelaceae family, Aloe vera demonstrated mild inhibitory activity, with zones of inhibition ranging from 1.2 to 3.2mm. The findings exhibited a notable statistical distinction from Oregano and Thyme. Artemisia absinthium from the Asteraceae family exhibited relatively higher inhibition zones, ranging from 6.8 to 9.2mm, suggesting a potential antibacterial efficacy. Remarkably, essential oils such as Thymus vulgaris (Thyme) from the Lamiaceae family displayed substantial inhibition against all pathogens, with zones of inhibition ranging from 5.6 to 24.3mm. This suggests the pronounced antimicrobial potential of Thyme essential oil across the board.
Intriguingly, Oregano (Origanum vulgare) from the same family showed a substantial inhibitory effect against A. veronii bv. veronii and A. veronii bv. sobria, with inhibition zones of 15.8 and 17.8 mm, respectively. This highlights Oregano’s potency in targeting these specific pathogens. Cinnamomum verum (Cinnamon) from the Lauraceae family displayed promising inhibition zones against all tested pathogens, ranging from 7.2 to 12.2mm. Cinnamon’s consistent inhibitory effect could be attributed to its rich composition of active compounds. In contrast, certain essential oils, such as Boswellia carterii (Frankincense or Olibanum) from the Burseraceae family and Pogostemon cablin (Patchouli) from the Lamiaceae family, exhibited negligible inhibitory effects against all tested pathogens.
The calculated average inhibitory effect across all essential oils showed variations in effectiveness against the pathogens. Thyme, with an average inhibitory effect of 13.3mm, and Oregano, with 12.0 mm, emerged as the most potent essential oils, followed by Cinnamon and Absinthe, with an average inhibitory effect of 10.1mm and 7.8mm, respectively.
3.1 Colorimetric determination of bacteriostatic and bactericidal concentrations
The results of colorimetric determination of bacteriostatic and bactericidal concentrations are provided in Table 2. The results indicate that EO of Artemisia absinthium exhibited a promising antibacterial effect across the tested pathogens. The results obtained by the disc diffusion (inhibition zones) correlated well with the MIC values (Pearson’s r = 0.84, p < 0.001), supporting consistency between the two methods.
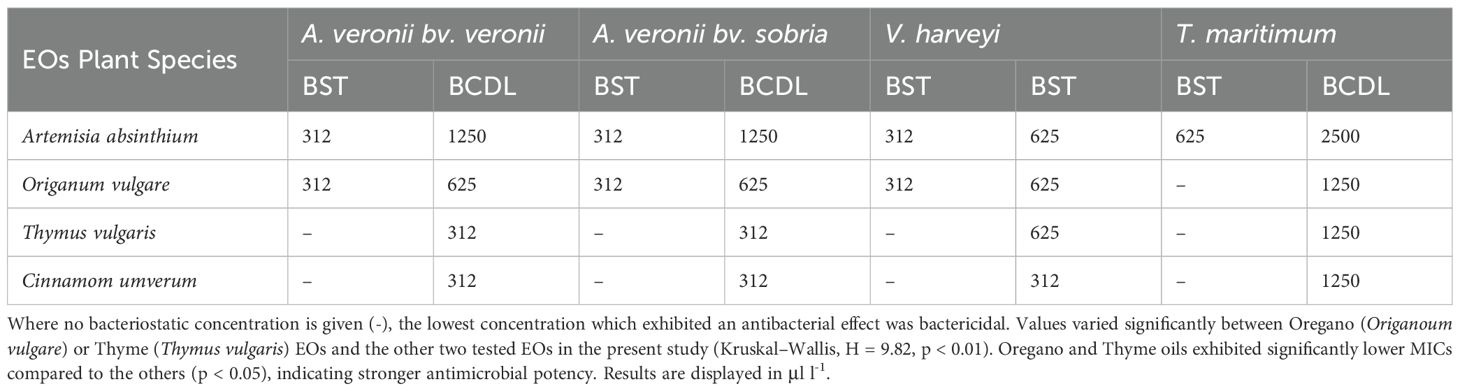
Table 2. EOs bacteriostatic (BST) and bactericidal (BCDL) concentrations against the four fish pathogens at 23°C.
With concentrations ranging from 312 to 625 μl/l for both bacteriostatic and bactericidal actions, A. absinthium showcases a relatively consistent performance. This suggests that it could be a versatile option for controlling various fish infections. Oregano oil exhibited notable antimicrobial effects, with concentrations varying between 312 and 1250 μl/l., notably, the absence of a specific bacteriostatic concentration for V. harveyi suggests that the lowest effective concentration was also bactericidal. Thyme oil demonstrated significant antimicrobial effects across the pathogens, displaying consistent bactericidal concentrations. Cinnamon oil showed relatively consistent performance against A. veronii bv. sobria and V. harveyi with bacteriostatic and bactericidal concentrations of 312 μl/l.
The objective of this study was to examine the antibacterial characteristics of 15 essential oils (EOs) against prevalent fish pathogens found in Mediterranean aquaculture. The primary goal was to identify EOs that exhibit strong inhibitory effects, specifically targeting Aeromonas, Vibrio, and Tenacibaculum. The escalating demand for sustainable aquaculture practices has prompted a search for effective alternatives to conventional antimicrobials in controlling fish pathogens (Nisa et al., 2023). EOs, derived from various MAPs, have gained considerable attention for their potential antimicrobial properties against a wide array of pathogens (Tiwari et al., 2009; Athanassopoulou et al., 2018; Moosavi-Nasab et al., 2019). This includes not only bacteria but also ectoparasites and endoparasites that pose significant threats to fish health and aquaculture productivity (Athanassopoulou et al., 2004; Karagouni et al., 2005; Tsantilas et al., 2005; Athanassopoulou et al., 2009; Dawood et al., 2021). EOs offer a natural and eco-friendly approach to combating these parasitic infections, reducing the reliance on synthetic chemicals and addressing concerns related to antibiotic resistance and environmental impact (Caruso, 2016; Luis et al., 2019; Okeke et al., 2022; Deekshit et al., 2023). By harnessing the inherent antimicrobial properties of essential oils, researchers and aquaculture practitioners are exploring innovative strategies to mitigate the impact of fish pathogens, ensuring the health and sustainability of aquatic ecosystems (Dawood et al., 2021).
The antibacterial activity of EOs studied in this work can be attributed to their main chemical constituents. The results agree with earlier reports that the activity of essential oils is linked to the presence of bioactive phenolic and alcohol components. Compounds such as thymol, carvacrol, eugenol, and cinnamaldehyde are known to damage bacterial membranes and interfere with enzyme activity, leading to cell death (Ultee et al., 2000; Burt and Reinders, 2003). Other constituents, including Linalool and Terpinen-4-ol, have similar disruptive effects on bacterial membranes (Lee et al., 2009; Nazzaro et al., 2013).
While EOs generally require higher concentrations to achieve bacteriostatic or bactericidal effects compared to antibiotics, their multi-target modes of action, effectiveness against biofilms, and reduced risk of resistance development make them valuable complementary agents in aquaculture settings where antibiotic resistance is a growing concern. For instance, oregano, thyme, cinnamon, and absinthe oils have been reported to exert strong inhibitory effects against Aeromonas veronii and Vibrio harveyi when tested by agar dilution and well diffusion methods (Starliper et al., 2015; Tural et al., 2019; Domínguez-Borbor et al., 2020). These findings are consistent with our results and highlight the potential of selected essential oils as alternatives to conventional antibiotics, for example oregano, absinthe, cinnamon, and thyme, which exhibited the highest antimicrobial activity against the pathogens tested in this study. This ranking was substantiated by studies assessing EOs from the same plant species against A. veronni and V. harveyi, utilizing the agar dilution method and well tests (Lee et al., 2009; Starliper et al., 2015; Tural et al., 2019; Domínguez-Borbor et al., 2020; Dawood et al., 2021). Our findings are in line with the work of Bektaş and Özdal (2022) (Athanassopoulou et al., 2018) who investigated the effectiveness of essential oils from Eucalyptus camaldulensis against fish pathogens, including Aeromonas caviae. Furthermore, Kot et al. (2019). explored the antimicrobial activities of specific phytochemicals, including trans-cinnamaldehyde and thymol, against Aeromonas species, indicting the broader potential of plant-derived compounds in combating fish pathogens.
A further crucial consideration revolves around the impact of lecithin as an emulsifier. A number of authors propose that, despite its favorable emulsifying qualities, lecithin could diminish the in vitro antibacterial effectiveness of EOs. This could be due to lecithin’s potential to physically obstruct the interaction between EOs and bacterial cells, possibly by augmenting the availability of phospholipids (Ismaiel and Pierson, 1990; Keweloh et al., 1991; Ultee et al., 2000; Burt and Reinders, 2003). Thus, it’s conceivable that the antibacterial and bactericidal potential of EOs might be even more robust without the presence of lecithin.
As emphasized earlier, variations in EO composition within the same plant species, influenced by factors like harvesting season and geographic location, are acknowledged. Nonetheless, studies performed with specific EO batches should consistently demonstrate reliable reproducibility. The findings of the present work are encouraging and provide support for future research on the potential use of essential oils (EOs) to enhance the antibacterial and bactericidal effects in Mediterranean farmed fish species. This concept is consistent with the findings of Zhang et al (Dawood et al., 2021), who reviewed the use of essential oils in animal production for the purpose of managing food-borne pathogens.
The results of the present work are relevant to the Mediterranean aquaculture industry as they underscore the substantial in vitro antibacterial properties of Absinthe, Thyme, Oregano, and Cinnamon EOs. In fact, these EOs stand out as the most consistently effective across aquaculture studies, with antimicrobial properties which have been widely reported in a range of pathogens (Firmino et al., 2021; Poudel et al., 2021; Dawood et al., 2021). The stronger antimicrobial activity of oregano, thyme, cinnamon, and absinthe oils can be explained by their high content of compounds such as thymol, carvacrol, eugenol, and cinnamaldehyde. This is consistent with earlier work by Mandalakis et al. (2021), who reported strong activity of oregano- and carvacrol-rich oils against Aeromonas veronii bv. sobria.
Particularly intriguing is the prospect of amplifying the antibacterial and bactericidal effects of EOs through simultaneous administration, an area ripe for exploration in future research. Moreover, essential oils can reduce production costs and enhance market appeal for antibiotic-free aquaculture products (Karagouni et al., 2005).
The relatively high MIC values of essential oils compared to antibiotics (Chouhan et al., 2017) may restrict their direct use in aquaculture, since larger doses raise concerns of toxicity and cost-effectiveness. Nevertheless, the growing problem of antibiotic resistance underscores the need to explore such natural alternatives. Although EOs may not always reach the low MIC levels of antibiotics, their activity against resistant strains and eco-friendly profile make them promising candidates for sustainable disease management. Although stability of the tested essential oils was not a limiting factor in the present study due to the in vitro and short incubation periods, long-term application in aquaculture systems may be affected by the degradation of certain compounds. Ganosi et al. (2023) showed that while major constituents such as carvacrol and thymol remain relatively stable for several months, many minor components degrade within 10–70 days, particularly under elevated temperature or sunlight. This indicates that although the main antibacterial activity can be retained, the overall chemical profile of the oils changes over time. This highlights the need for further research aimed at enhancing their efficacy, for example through nanoencapsulation, which improves stability, bioavailability, and sustained release of active compounds, or through synergistic combinations of essential oils with each other as a combination of strategies to enhance stability, bioavailability, and antimicrobial potency of EOs (Chouhan et al., 2017; Valdivieso-Ugarte et al., 2019; Firmino et al., 2021). It should be stated that the effectiveness of EOs is expected to vary according to dosage, species, and exposure time, and acute toxicity tests are necessary to define safe margins before use in live fish. Sublethal effects, such as behavioral stress or immunosuppression, may also occur if concentrations are not carefully controlled (Lanzerstorfer et al., 2021). Therefore, following the results of the present in vitro study, further in vivo testing is required to establish safe and effective application protocols for aquaculture systems.
To our knowledge, the results of the present work constitute a maiden effort in investigating the antibacterial potential of the tested essential oils against Tenacibaculum maritimum, a significant pathogen affecting European sea bass (Dicentrarchus labrax). Infections occur mainly in small fingerlings, creating serious problems in nursery stations (Avendaño-Herrera et al., 2008). At present, no commercial vaccine is available, and trials with autogenous vaccines in Mediterranean farms have provided limited protection, resulting in continued reliance on antibiotic treatments (Kolygas et al., 2012; Mabrok et al., 2022). Moreover, the persistence of T. maritimum remains a concern even after sea bass are transferred to open sea cages.
As a potential solution, the use of oral essential oil-based nutraceutical supplements could help reduce the overall dependence on antibiotics. The prospects for scaling up the use of essential oils in commercial aquaculture are promising, given the available research reports on the benefits in enhancing growth, feed efficiency, immunity, and disease resistance in fish, but successful application will depend on optimizing delivery methods and ensuring cost-effectiveness, for example through microencapsulation or incorporation into aquafeeds (Valdivieso-Ugarte et al., 2019; Liu et al., 2023). An interesting observation of the present study is that Tenacibaculum maritimum is less susceptible to the inhibitory and killing effects of EOs compared to the other fish pathogens tested. It should also be noted that soy lecithin, which was included in the broth assays as an emulsifier to disperse the hydrophobic essential oils, may have interacted with some of their active compounds and reduced their apparent antimicrobial activity. This possible interference represents a limitation of the experimental design and may partly explain the lower susceptibility observed in T. maritimum. In addition, the reduced sensitivity could also reflect resistance mechanisms inherent to this bacterium, including intrinsic resistance, efflux pumps, biofilm formation, or altered cell membrane composition, which are consistent with its documented tolerance to several antibacterial substances (Avendaño-Herrera et al., 2008; Kolygas et al., 2012; Mabrok et al., 2022).
4 Conclusions
The results of the present study highlight the complex interactions between EO constituents and bacterial species, necessitating further investigation to fully harness their potential in aquaculture health management and are consistent with existing literature on the antimicrobial potential of these EOs, attributed to their bioactive constituents. The study underscores the importance of leveraging natural alternatives, such as EOs, to combat bacterial infections in aquaculture and reduce reliance on antibiotics, and conclusively, Absinthe, Thyme, Oregano and Cinnamon can play this significant role toward the development of plant-based therapeutics tailored for Mediterranean fish farming systems and support sustainable disease management strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MK: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization. VK: Investigation, Writing – review & editing. IP: Conceptualization, Investigation, Methodology, Resources, Software, Supervision, Writing – original draft, Writing – review & editing. EK: Writing – original draft, Writing – review & editing, Funding acquisition, Resources. DT: Writing – review & editing, Methodology. VB: Writing – original draft, Writing – review & editing. YK: Writing – original draft, Writing – review & editing, Funding acquisition, Investigation, Methodology. CN: Writing – original draft, Writing – review & editing. FA: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research has been co-financed by Greek and European Union (EuropeanRegional Development Fund) funds in the context of “Research–Create–Innovate” within the Operational Program Competitiveness, Entrepreneurship and Innovation (EPANEK) of the NSRF 2014–2020, project code: Τ6ΥΒΠ-00246O. Acronym: AltMedSea-bream.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Aziz M., Eissa A. E., Hanna M., and Okada M. A. (2013). Identifying some pathogenic vibrio/Photobacterium species during mass mortalities of cultured gilthead seabream (Sparus aurata) and European seabass (Dicentrarchuslabrax) from some Egyptian coastal provinces. Int. J. Vet. Sci. Med. 1, 87–95. doi: 10.1016/j.ijvsm.2013.10.004
Abdel-Latif H. M. R., Abdel-Tawwab M., Khafaga A. F., and Dawood M. A. O. (2020). Dietary origanum essential oil improved antioxidative status, immune-related genes, and resistance of common carp (Cyprinus Carpio L.) to Aeromonas hydrophila infection. Fish Shellfish Immunol. 104, 1–7. doi: 10.1016/j.fsi.2020.05.056
Abdel-Latif H. M. R., Yilmaz S., and Kucharczyk D. (2023). Editorial: Functionality and applications of phytochemicals in aquaculture nutrition. Front. Vet. Sci. 10. doi: 10.3389/fvets.2023.1218542
Alagawany M., Farag M. R., Salah A. S., and Mahmoud M. A. (2020). The role of oregano herb and its derivatives as immunomodulators in fish. Rev. Aquaculture 12, 2481–2492. doi: 10.1111/raq.12453
Anastasiou C. and Buchbauer G. (2017). Essential oils as immunomodulators: Some examples. Open Chem. 15, 352–370. doi: 10.1515/chem-2017-0037
Arun D. and Midhun S. J. (2023). “Chapter 2. Microbiome of fish,” in Recent Advances in Aquaculture Microbial Technology. Eds. Mathew J., Jose M. S., R. E. k., and Kumar A. (United Kingdom: Academic Press), 15–33. doi: 10.1016/B978-0-323-90261-8.00011-0
Athanassopoulou F., Karagouni E., Dotsika E., Ragias V., Tavla J., Christofilloyanis P., et al. (2004). Efficacy and toxicity of orally Administratedanti-coccidial drugs for innovative treatments of Myxobolus sp. Infection in puntazzopuntazzo. Dis. Aquat. Organ. 62, 217–226. doi: 10.3354/dao062217
Athanassopoulou F., Kotou E., and Watsos E. (2018). Study of the bacteriostatic activity of an artemia enrichment compound based on plant extracts from Angelica Sp. J. Hellenic Vet. Med. Soc 51, 293. doi: 10.12681/jhvms.15688
Athanassopoulou F., Pappas I. S., and Bitchava K. (2009). An overview of the treatments for parasitic disease in Mediterranean aquaculture. Options Méditerr Ser. A. 86, 65–83. Available online at: https://om.ciheam.org/om/pdf/a86/00801063.pdf.
Avendaño-Herrera R., Núñez S., Barja J. L., and Toranzo A. E. (2008). Evolution of drug resistance and minimum inhibitory concentration to enrofloxacin in Tenacibaculum maritimum Strains isolated in fish farms. Aquacult. Int. 16, 1–11. doi: 10.1007/s10499-007-9117-y
Bektaş S. and Özdal M. (2022). Antimicrobial activity of Eucalyptus (Eucalyptus camaldulensis) essential oil against fish pathogen bacterium, Aeromonas caviae. Mar. Sc. Technol. Bull. 11, 467–474. doi: 10.33714/masteb.1184165
Ben Hamed S., Tavares Ranzani-Paiva M. J., Tachibana L., De Carla Dias D., Ishikawa C. M., and Esteban M. A. (2018). Fish pathogen bacteria: Adhesion, parameters influencing virulence and interaction with host cells. Fish Shellfish Immunol. 80, 550–562. doi: 10.1016/j.fsi.2018.06.053
Burt S. A. and Reinders R. D. (2003). Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 36, 162–167. doi: 10.1046/j.1472-765X.2003.01285.x
Caputo A., Bondad-Reantaso M. G., Karunasagar I., Hao B., Gaunt P., Verner-Jeffreys D., et al. (2023). Antimicrobial resistance in aquaculture: A global analysis of literature and national action plans. Rev. Aquacult. 15, 568–578. doi: 10.1111/raq.12741
Caruso G. (2016). Antibiotic resistance in fish farming environments: A global concern. J. Fish. Sci. 10, 9–13. Available online at: https://www.itmedicalteam.pl/articles/antibiotic-resistance-in-fish-farming-environments-a-globalconcern.pdf.
Chouhan S., Sharma K., and Guleria S. (2017). Antimicrobial activity of some essential oils—present status and future perspectives. Medicines (Basel) 4, 58. doi: 10.3390/medicines4030058
Dawood M. A. O., El Basuini M. F., Zaineldin A. I., Yilmaz S., Hasan M. T., Ahmadifar E., et al. (2021). Antiparasitic and antibacterial functionality of essential oils: An alternative approach for sustainable aquaculture. Pathogens 10, 185. doi: 10.3390/pathogens10020185
Deekshit V. K., Maiti B., Krishna Kumar B., Kotian A., Pinto G., Bondad-Reantaso M. G., et al. (2023). Antimicrobial resistance in fish pathogens and alternative risk mitigation strategies. Rev. Aquacult. 15, 261–273. doi: 10.1111/raq.12715
Domínguez-Borbor C., Sánchez-Rodríguez A., Sonnenholzner S., and Rodríguez J. (2020). Essential oils mediated antivirulence therapy against vibriosis in Penaeus vannamei. Aquaculture 529, 735639. doi: 10.1016/j.aquaculture.2020.735639
Droubogiannis S., Pavlidi L., Skliros D., Flemetakis E., and Katharios P. (2023). Comprehensive characterization of a novel bacteriophage, vB_VhaS_MAG7 against a fish pathogenic strain of Vibrio harveyi and its in vivo efficacy in phage therapy trials. Int. J. Mol. Sci. 24, 8200. doi: 10.3390/ijms24098200
EUCAST (2024). EUCAST reading guide for broth microdilution and disk diffusion (Växjö, Sweden: European Committee on Antimicrobial Susceptibility Testing). Available online at: https://www.eucast.org (Accessed June 25, 2025).
FAO (2021). The FAO Action Plan on Antimicrobial Resistance 2021–2025 (Rome, Italy: Food and Agriculture Organization).
FAO (2022). “The state of world fisheries and aquaculture,” in Towards Blue Transformation. State World Fish. Aquacult. (SOFIA) vol. 2022. (Food and Agriculture Organization, Rome, Italy).
Feng Y., Hu J., Chen Y., Xu J., Yang B., and Jiang J. (2022a). Ecological effects of antibiotics on aquaculture ecosystems based on microbial community in sediments. Ocean Coast. Manage. 224, 106173. doi: 10.1016/j.ocecoaman.2022.106173
Feng Y., Hu J., Chen Y., Xu J., Yang B., and Jiang J. (2022b). Ecological response to antibiotics re-entering the aquaculture environment with possible long-term antibiotics selection based on enzyme activity in sediment. Environ. Sci. pollut. Res. Int. 29, 19033–19044. doi: 10.1007/s11356-021-17114-0
Firmino J. P., Vallejos-Vidal E., Balebona M. C., Ramayo-Caldas Y., Cerezo I. M., Salomón R., et al. (2021). Diet, immunity, and microbiota interactions: An integrative analysis of the intestine transcriptional response and microbiota modulation in gilthead seabream (Sparus aurata) fed an essential oils-based functional diet. Front. Immunol. 12. doi: 10.3389/fimmu.2021.625297
Fokas R., Anastopoulou Z., and Vantarakis A. (2025). Antimicrobial activity of Greek native essential oils against Escherichia coli O157:H7 and antibiotic-resistant strains harboring pNorm plasmid, mecA, mcr-1 and blaOXA genes. Antibiotics 14, 741. doi: 10.3390/antibiotics14080741
Ganosi E., Barda C., Grafakou M.-E., Rallis M. C., and Skaltsa H. (2023). An In-Depth Stability Study of the Essential Oils from Mentha × piperita, Mentha spicata, Origanum vulgare, and Thymus vulgaris: The Impact of Thermal and Storage Conditions. Separations 10, 488. doi: 10.3390/separations10090488
Hickman-Brenner F. W., MacDonald K. L., Steigerwalt A. G., Fanning G. R., Brenner D. J., and Farmer J. J. 3rd (1987). Aeromonas veronii, a new ornithine decarboxylase-positive species that may cause diarrhea. J. Clin. Microbiol. 25, 900–06.
Hossain A., Habibullah-Al-Mamun M., Nagano I., Masunaga S., Kitazawa D., and Matsuda H. (2022). Antibiotics, antibiotic-resistant bacteria, and resistance genes in aquaculture: Risks, current concern, and future thinking. Environ. Sci. pollut. Res. Int. 29, 11054–11075. doi: 10.1007/s11356-021-17825-4
Inamuddin, A. T., and Cruz J. N. (2023). Essential Oils: Extraction Methods and Applications (New Jersey, USA: John Wiley & Sons).
Irshath A. A., Rajan A. P., Vimal S., Prabhakaran V.-S., and Ganesan R. (2023). Bacterial pathogenesis in various fish diseases: Recent advances and specific challenges in vaccine development. Vaccines 11, 470. doi: 10.3390/vaccines11020470
Ismaiel A. A. and Pierson M. D. (1990). Effect of sodium nitrite and origanum oil on growth and toxin production of Clostridium botulinum in TYG broth and ground pork. J. Food Prot. 53, 958–960. doi: 10.4315/0362-028X-53.11.958
Jørgensen L. V. G. (2020). Zebrafish as a model for fish diseases in aquaculture. Pathogens 9, 609. doi: 10.3390/pathogens9080609
Karagouni E., Athanassopoulou F., Lytra A., Komis C., and Dotsika E. (2005). Antiparasitic and immunomodulatory effect of innovative treatments against Myxobolus sp. Infection in Diplodus puntazzo. Vet. Parasitol. 134, 215–228. doi: 10.1016/j.vetpar.2005.07.020
Keweloh H., Diefenbach R., and Rehm H. J. (1991). Increase of phenol tolerance of Escherichia coli by alterations of the fatty acid composition of the membrane lipids. Arch. Microbiol. 157, 49–53. doi: 10.1007/BF00245334
Kolygas M. N., Gourzioti E., Vatsos I. N., and Athanassopoulou F. (2012). Identification of Tenacibaculum maritimum Strains from marine farmed fish in Greece. Vet. Rec. 170, 623. doi: 10.1136/vr.100778
Kot B., Kwiatek K., Janiuk J., Witeska M., and Pękala-Safińska A. (2019). Antibacterial activity of commercial phytochemicals against Aeromonas species isolated from fish. Pathogens 8, 142. doi: 10.3390/pathogens8030142
Lanzerstorfer P., Sandner G., Pitsch J., Mascher B., Aumiller T., and Weghuber J. (2021). Acute, reproductive, and developmental toxicity of essential oils assessed with alternative in vitro and in vivo systems. Arch. Toxicol. 95, 673–691.
Lee S., Najiah M., Wendy W., and Nadirah M. (2009). Chemical composition and antimicrobial activity of the essential oil of Syzygium aromaticum flower bud (clove) against fish systemic bacteria isolated from aquaculture sites. Front. Agric. China. 3, 332–336. doi: 10.1007/s11703-009-0052-8
Liu H., Zhao Z., Xu W., Cheng M., Chen Y., Xun M., et al. (2023). Preparation, characterization, release and antibacterial properties of cinnamon essential oil microcapsules. Coatings 13, 973.
Liu L., Ge M., Zheng X., Tao Z., Zhou S., and Wang G. (2016). Investigation of Vibrio alginolyticus, V. harveyi and V. parahaemolyticus in large yellow croaker, PseudosciaenaCrocea (Richardson) reared in Xiangshan Bay, China. Aquacult. Rep. 3, 220–224. doi: 10.1016/j.aqrep.2016.04.004
Luis A. I. S., Campos E. V. R., de Oliveira J. L., and Fraceto L. F. (2019). Trends in aquaculture sciences: From now to use of nanotechnology for disease control. Rev. Aquacult. 11, 119–132. doi: 10.1111/raq.12229
Mabrok M., Algammal A. M., Sivaramasamy E., Hetta H. F., Atwah B., Alghamdi S., et al. (2022). Tenacibaculosis caused by Tenacibaculum maritimum: Updated knowledge of this marine bacterial fish pathogen. Front. Cell. Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.1068000
Mabrok M. A. E. and Wahdan A. (2018). The immune modulatory effect of oregano (Origanum vulgare L.) essential oil on Tilapia zillii following intraperitoneal infection with Vibrio Anguillarum. Aquacult. Int. 26, 1147–1160. doi: 10.1007/s10499-018-0274-y
Magiatis P., Melliou E., Skaltsounis A. L., Chinou I. B., and Mitaku S. (1999). Chemical composition and antimicrobial activity of the essential oils of Pistacia lentiscus var. chia. Planta Med. 65, 749–752. doi: 10.1055/s-2006-960856
Manchanayake T., Salleh A., Amal M. N. A., Yasin I. S. M., and Zamri-Saad M. (2023). Pathology and pathogenesis of vibrio infection in fish: A review. Aquacult. Rep. 28, 101459. doi: 10.1016/j.aqrep.2022.101459
Mandalakis M., Anastasiou T. I., Martou N., Keisaris S., Greveniotis V., Katharios P., et al. (2021). Antibacterial Effects of Essential Oils of Seven Medicinal-Aromatic Plants Against the Fish Pathogen Aeromonas veronii bv. sobria: To Blend or Not to Blend? Molecules 26, 2731. doi: 10.3390/molecules26092731
Mavraganis T., Constantina C., Kolygas M., Vidalis K., and Nathanailides C. (2020). Environmental issues of aquaculture development. Egypt. J. Aquat. Biol. Fish. 24, 441–450. doi: 10.21608/ejabf.2020.85857
Mohamad N., Mohd Roseli F. A., Azmai M. N. A., Saad M. Z., Yasin M., Zulkiply N. A., et al. (2019). Natural concurrent infection of Vibrio harveyi and V. alginolyticus in cultured hybrid groupers in Malaysia. J. Aqua Anim. Health 31, 88–96. doi: 10.1002/aah.10055
Mohd-Aris A., Muhamad-Sofie M. H. N., Zamri-Saad M., Daud H. M., and Ina-Salwany M. Y. (2019). Y. Live vaccines against bacterial fish diseases: A review. Vet. World. 12, 1806–1815. doi: 10.14202/vetworld.2019.1806-1815
Moosavi-Nasab M., Mirzapour-Kouhdasht A., and Oliyaei N. (2019). “Application of essential oils for shelf-life extension of seafood products,” in Essential Oils – Oils of Nature. Ed. El-Shemy H. A. (Florida, USA: IntechOpen). doi: 10.5772/intechopen.86574
Muktar Y. and Tesfaye S. (2016). Present status and future prospects of fish vaccination: A review. J. Veterinar. Sci. Technol. 7, 299. doi: 10.4172/2157-7579.1000299
Mustafa A., El-Kashef D. H., Abdelwahab M. F., Gomaa A. A.-R., Mustafa M., Abdel-Wahab N. M., et al. (2023). “Investigation of antiviral effects of essential oils,” in Essential Oils. Ed. Inamuddin (New Jersey, USA: John Wiley & Sons, Limited), 99–123. doi: 10.1002/9781119829614.ch5
Nazzaro F., Fratianni F., De Martino L., Coppola R., and De Feo V. (2013). Effect of essential oils on pathogenic bacteria. Pharm. (Basel). 6, 1451–1474. doi: 10.3390/ph6121451
Nisa M., Dar R. A., Fomda B. A., and Nazir R. (2023). Combating food spoilage and pathogenic microbes via bacteriocins: A natural and eco-friendly substitute to antibiotics. Food Control. 149, 109710. doi: 10.1016/j.foodcont.2023.109710
Okeke E. S., Chukwudozie K. I., Nyaruaba R., Ita R. E., Oladipo A., Ejeromedoghene O., et al. (2022). Antibiotic resistance in aquaculture and aquatic organisms: A review of current nanotechnology applications for sustainable management. Environ. Sci. pollut. Res. Int. 29, 69241–69274. doi: 10.1007/s11356-022-22319-y
Palaniyappan S., Sridhar A., Kari Z. A., Téllez-Isaías G., and Ramasamy T. (2023). Evaluation of phytochemical screening, pigment content, in vitro antioxidant, antibacterial potential and GC-MS metabolite profiling of green seaweed Caulerpa racemosa. Mar. Drugs 21, 278. doi: 10.3390/md21050278
Park J. W., Wendt M., and Heo G. J. (2016). Antimicrobial activity of essential oil of Eucalyptus globulus against fish pathogenic bacteria. Lab. Anim. Res. 32, 87–90. doi: 10.5625/lar.2016.32.2.87
Poudel D. K., Rokaya A., Ojha P. K., Timsina S., Satyal R., Dosoky N. S., et al. (2021). The chemical profiling of essential oils from different tissues of Cinnamomum camphora L. and their antimicrobial activities. Molecules 26, 5132. doi: 10.3390/molecules26175132
Pridgeon J. W. and Klesius P. H. (2012). Major bacterial diseases in aquaculture and their vaccine development. CAB Rev. 7, 1–16. doi: 10.1079/PAVSNNR20127048
Rani M., Jindal S., Anand R., Sharma N., Ranjan K. R., Mukherjee M. D., et al. (2023). “Plant essential oils and their constituents for therapeutic benefits,” in Essential Oils. Ed. Inamuddin (New Jersey, USA: John Wiley & Sons, Limited), 977–1008. doi: 10.1002/9781119829614.ch42
Rohani M. F., Islam S. M., Hossain M. K., Ferdous Z., Siddik M. A. B., Nuruzzaman M., et al. (2022). Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: upgrading growth, reproduction, immunity and disease resistance in fish. Fish. Shellfish. Immunol. 120, 569–589. doi: 10.1016/j.fsi.2021.12.037
Salvat A., Antonnacci L., Fortunato R. H., Suarez E. Y., and Godoy H. M. (2001). Screening of some plants from Northern Argentina for their antimicrobial activity. Lett. Appl. Microbiol. 32, 293–297. doi: 10.1046/j.1472-765x.2001.00923.x
Smyrli M., Prapas A., Rigos G., Kokkari C., Pavlidis M., and Katharios P. (2017). Aeromonas veronii infection associated with high morbidity and mortality in farmed european seabass Dicentrarchuslabrax in the Aegean sea, Greece. Fish Pathol. 52, 68–81. doi: 10.3147/jsfp.52.68
Smyrli M., Triga A., Dourala N., Varvarigos P., Pavlidis M., Quoc V. H., et al. (2019). Comparative study on A novel pathogen of European seabass. Diversity of Aeromonas veronii in the Aegean Sea. Microorganisms 7, 504. doi: 10.3390/microorganisms7110504
Starliper C. E., Ketola H. G., Noyes A. D., Schill W. B., Henson F. G., Chalupnicki M. A., et al. (2015). An investigation of the bactericidal activity of selected essential oils to Aeromonas spp. J. Adv. Res. 6, 89–97. doi: 10.1016/j.jare.2013.12.007
Tiwari B. K., Valdramidis V. P., O’Donnell C. P., Muthukumarappan K., Bourke P., and Cullen P. J. (2009). Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 57, 5987–6000. doi: 10.1021/jf900668n
Triga A., Smyrli M., and Katharios P. (2023). Pathogenic and opportunistic Vibrio spp. Associated with vibriosis incidences in the Greek aquaculture: The role of Vibrio harveyi as the principal cause of vibriosis. Microorganisms 11, 1197. doi: 10.3390/microorganisms11051197
Tsantilas H., Golomazou E., and Athanassopoulou F. (2005). Alternative medicine in aquaculture. J. Hellenic Vet. Med. Soc 56, 249–255. doi: 10.12681/jhvms.15087
Tural S., Durmaz Y., Urçar E., and Turhan S. (2019). Antibacterial activity of thyme (Thymus vulgaris L.), laurel (Lauris Nobilis L.), rosemary (Rosmarinus officinalis L.) and parsley (Petroselinum crispum L.) essential oils against some fish pathogenic bacteria. Acta Aquat Turc. 15, 440–447. doi: 10.22392/actaquatr.549380
Ultee A., Kets E. P., Alberda M., Hoekstra F. A., and Smid E. J. (2000). Adaptation of the food-borne pathogen Bacillus cereus to carvacrol. Arch. Microbiol. 174, 233–238. doi: 10.1007/s002030000199
Valdivieso-Ugarte M., Gomez-Llorente C., Plaza-Díaz J., and Gil Á. (2019). Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 11, 2786. doi: 10.3390/nu11112786
Wang W., Li Q., Lai Q., Lin Y., Zhang Q., and Chen J. (2021). Screening of anti-vibrio activity of marine fungal methanolic fractions to control vibriosis in white shrimp (litopenaeusvannamei). Aquacult Res. 52, 5517–5526. doi: 10.1111/are.15425
Wang X., Lin Y., Zheng Y., and Meng F. (2022). Antibiotics in mariculture systems: A review of occurrence, environmental behavior, and ecological effects. Environ. pollut. 293, 118541. doi: 10.1016/j.envpol.2021.118541
World Health Organization (2017). WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals (Geneva: World Health Organization).
Wright A., Li X., Yang X., Soto E., and Gross J. (2023). Disease prevention and mitigation in US finfish aquaculture: A review of current approaches and new strategies. Rev. Aquacult. 15, 1638–1653. doi: 10.1111/raq.12807
Xiao S., Cui P., Shi W., and Zhang Y. (2020). Identification of essential oils with activity against stationary phase Staphylococcus aureus. BMC Complement Altern. Med. 20, 99. doi: 10.1186/s12906-020-02898-4
Yousefi M., Ghafarifarsani H., Hoseini S. M., Hoseinifar S. H., Abtahi B., Vatnikov Y. A., et al. (2022). Effects of dietary thyme essential oil and prebiotic administration on rainbow trout (Oncorhynchus mykiss) welfare and performance. Fish Shellfish Immunol. 120, 737–744. doi: 10.1016/j.fsi.2021.12.023
Zhang X.-H., He X., Austin B., and Harveyi V. (2020). Vibrio harveyi: A serious pathogen of fish and invertebrates in mariculture. Mar. Life Sci. Technol. 2, 231–245. doi: 10.1007/s42995-020-00037-z
Keywords: medicinal aromatic plants, essential oils, Aeromonas veronii, Tenacibaculum maritimum, Vibrio harveyi
Citation: Kolygas MN, Kostou V, Pappas IS, Karagouni E, Toubanaki DK, Bakopoulos V, Kotzamanis YP, Nathanailides C and Athanassopoulou F (2025) In vitro antibacterial activity of essential oils from medicinal plants against major fish pathogens in Mediterranean aquaculture. Front. Aquac. 4:1665877. doi: 10.3389/faquc.2025.1665877
Received: 15 July 2025; Accepted: 18 September 2025;
Published: 07 October 2025.
Edited by:
Gulnihal Ozbay, Delaware State University, United StatesReviewed by:
Sumanta Kumar Mallik, Directorate of Cold Water Fisheries Research (ICAR), IndiaDavid Hunnicutt, St. Norbert College, United States
Copyright © 2025 Kolygas, Kostou, Pappas, Karagouni, Toubanaki, Bakopoulos, Kotzamanis, Nathanailides and Athanassopoulou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cosmas Nathanailides, Y29zbWFzZmF4QHlhaG9vLmNvbQ==
 Markos N. Kolygas
Markos N. Kolygas Vasiliki Kostou1
Vasiliki Kostou1 Ioannis S. Pappas
Ioannis S. Pappas Evdokia Karagouni
Evdokia Karagouni Dimitra K. Toubanaki
Dimitra K. Toubanaki Vasileios Bakopoulos
Vasileios Bakopoulos Yannis P. Kotzamanis
Yannis P. Kotzamanis Cosmas Nathanailides
Cosmas Nathanailides Fotini Athanassopoulou
Fotini Athanassopoulou