Abstract
Introduction:
Commercial production of Atlantic halibut (Hippoglossus hippoglossus) has many obstacles, such as low and highly variable egg quality and survival rates during early developmental stages. We re-evaluated commonly used egg quality markers of Atlantic halibut in 17 egg batches from 11 different females in 2019.
Methods:
At a commercial hatchery in Norway, abnormal phenotypes were observed for several developmental milestones. The parameters fertilization success, percentage of normal early blastomere morphology, percentage of normally developing embryos at blastopore closure, and survival prior to hatching were analyzed to identify egg quality indicators. Small-scale egg incubators were utilized to track eggs with abnormal early blastomere symmetry individually until hatching.
Results:
The observed egg quality parameters varied between the egg groups, with an average of 62 ± 18% fertilization success (31–88%), 55 ± 23% survival until hatching (25–99%), and 49 ± 20% hatching success (22–85%). The percentage of normal early blastomeres, 69 ± 10% (51–85%) was not a significant predictor of hatching success in flask incubations but was a significant predictor in 96-well plate incubations. The development of individual eggs was followed in 96-well plates (n = 509), where eggs with normal early blastomere morphology had 83% higher hatching success than those with abnormal blastomeres (52% vs 22%). The percentage of normally developing embryos at blastopore closure 81 ± 18% (41–99%) was the strongest predictor overall and was significantly associated with both survival until hatching and hatching success.
Discussion:
Survival until hatching and the proportion of embryos with successful blastopore closure can both serve as egg quality indicators, offering potential enhancements to commercial hatchery management practices.
1 Introduction
The Atlantic halibut (Hippoglossus hippoglossus L.) is the largest flatfish in the North Atlantic, with a long cultural history as a valuable game fish (Haug, 1990). Commercial farming of Atlantic halibut began in the late 1990s, following successful juvenile production. Since 2007, Norway has consistently farmed 1,500–2,500 metric tons annually and remains the only country reporting commercial production (FAO, 2023), although aquaculture investments in Chile aim to establish this species (Gallardo et al., 2022). Despite decades of development, Atlantic halibut aquaculture continues to face challenges, including stagnating production. Pathogens such as nodaviruses, IPNV, and Atlantic halibut reovirus (AHR) cause losses during early life stages (Bergh et al., 2001; Blindheim et al., 2015), but variable egg quality is the main obstacle to consistent juvenile production, resulting in low survival during incubation, poor hatching success, and high mortality in the yolk-sac stage (Brown, 2002; Engelsen et al., 2004; Migaud et al., 2013; Pittman et al., 1990b).
In captivity, halibut can produce up to 12 egg batches per season, yielding as many as 1 million eggs per female (Norberg et al., 1991). Gametes are stripped by hand, and timing relative to ovulation is critical to avoid overripening and reduced quality (Bromage et al., 1994). Commonly used parameters to assess egg quality in pelagic fish include fertilization rate, early blastomere morphology, and hatching success (Brooks et al., 1997; Kjørsvik et al., 1990; Migaud et al., 2013). Additional traits, such as egg size, buoyancy, oil droplet distribution, and ovarian fluid characteristics, have also been linked to egg quality in halibut and other species (Bobe and Labbé, 2010; Kjørsvik and Holmefjord, 1995; Shields et al., 1997).
Halibut eggs of good quality achieve high fertilization and hatching rates with low deformity levels, increasing the likelihood of producing viable larvae (Brooks et al., 1997; Holmefjord et al., 1993; Kjørsvik and Holmefjord, 1995; Kjørsvik et al., 1990; Skaalsvik et al., 2015). Broodstock are typically kept in land-based facilities, where water temperature and photoperiod are manipulated to control spawning. Broodstock held at 7–8°C during spawning showed lower fecundity, shorter spawning periods, and reduced egg quality compared with fish maintained below 6.5°C (Brown et al., 2006). Similarly, photoperiod manipulation is used to extend or shift the spawning season, as changes in day length stimulate maturation in cold-water marine finfish (Bromage et al., 2001; Holmefjord et al., 1993; Naess et al., 1996; Smith et al., 1991).
However, egg quality indicators such as fertilization success and early embryo morphology (8/16-cell stage) have proven inconsistent in hatchery operations, and hatching success is difficult to evaluate at commercial scale, where egg batches are often pooled (Bobe and Labbé, 2010; Migaud et al., 2013). Introducing simpler and more reliable egg quality measures before pooling could improve quality control, hatching success, and juvenile output. The aim of this study was to develop a more consistent method for evaluating egg quality markers suitable for commercial hatcheries by following the development of multiple egg batches up to hatching. Fertilization success, normal eight-cell blastomere morphology, embryonic development at blastopore closure, and survival prior to hatching were correlated with hatching success. Embryonic development and anomalies were tracked from fertilization to hatching to identify more reliable markers and optimize hatchery protocols.
2 Materials and methods
2.1 Ethical statement
Fertilized eggs and yolk-sac larvae are developmental stages outside the scope of the Guidelines of the European Union on the protection of animals used for scientific purposes (Directive 2010/63/EU), and approval from the Norwegian Food Safety Authority was not needed. All sampled Atlantic halibut yolk-sac larvae were anesthetized and euthanized with an overdose of tricaine methane-sulfonate (MS-222 Finquel®, Argent Chemical Laboratories Inc., USA) mixed with seawater and briefly rinsed in fresh seawater prior to handling.
2.2 Broodstock husbandry, egg collection and hatchery incubation
Stripping of eggs and milt took place as part of routine husbandry, following normal hatchery protocols at the commercial hatchery at Nordic Halibut AS (Midsund, Norway). Halibut broodstock were held in round flat bottom tanks with a diameter of 10 m at a water depth of 80 cm. Between 70 and 100 broodstock were held in one tank, with a male to female ratio of 1:3. The facility pumped seawater from a depth of 200 m, using an intake located 1000 m from the facility. The seawater had a stable temperature of 8°C and a salinity of 34.4 ppt and was filtered through a sand filter. Three groups of fish were maintained under distinct photoperiod regimes: an ambient photoperiod (natural spring spawning), a photoperiod advanced by four months (autumn spawning), and a photoperiod delayed by three months (summer spawning) (Bjornsson et al., 1998). The temperature was lowered to 6°C two months before and throughout the subsequent spawning season and was kept at 8°C for the rest of the year. Temperature and oxygen saturation were monitored (OxyGuard Commander). When needed, temperature was reduced using a heat pump, and oxygen was increased by oxygen addition. Broodstock were fed three times a week ad libitum with an extruded mix of 22 mm Skretting-Mar Vitalis pellets, freshwater, and gluten according to the manufacturer’s recommendations. Frozen gutted herring was fed once per week. The fish were not fed during the spawning period. Females for stripping were selected based on visual assessment of gonad development, palpation, and the operators’ experience. Once an initial batch was obtained, the same female fish was routinely stripped every 20 to 22 degree days (°d). Two males were stripped, and the milt with the highest sperm count was used to fertilize all eggs from a single stripping session. Sperm concentration was measured in spermatozoa/mL using a photometric sperm counter (Minitube SDM 6), according to hatchery and manufacturer protocols. No predefined threshold was applied and the male with the higher concentration was selected for fertilization. The average concentration across all batches was 8.3 × 109 spermatozoa/mL (range 2.2–13.9 × 109). Eggs were fertilized by gently mixing 1 mL of milt per liter of eggs in ovarian fluid, followed by the addition of 3–5 L of seawater. After 30 minutes, the eggs from individual females were incubated in 250 L cylindroconical upwelling incubators with seawater flow-through at 6°C. The flow in the incubators was adjusted to the egg buoyancy.
2.3 Sampling protocol
A schematic overview of the study is presented in Figure 1. Embryonic development was observed at the following milestones (Figure 1A): eight-cell stage (8C), sixteen-cell stage (16C), blastula stage (BL), 50% epiboly (50%EB), 10-somite stage (10S), blastopore closure/end gastrulation (50°d), and hatching (H). An overview of developmental times in hours and°d is shown in Table 1. All egg groups were initially incubated in large commercial incubators prior to experimental setup (Figure 1B). At each observation point, eggs and larvae were transferred into a transparent Z-counter plate and photographed for later examination using a stereomicroscope with 4× magnification (Wild-Leitz; Zeiss Axiocam ERC5s). Normal development was defined as embryos showing regular cleavage patterns, symmetrical and intact blastomeres, and stage-appropriate morphology. Abnormal development included irregular or incomplete cleavage, fragmentation, abnormal blastodisc formation, loose cell aggregates, disturbed blastopore closure, bent notochords, or other deformities, following criteria described by Kjørsvik et al. (1990) and Cameron et al. (1992).
Figure 1
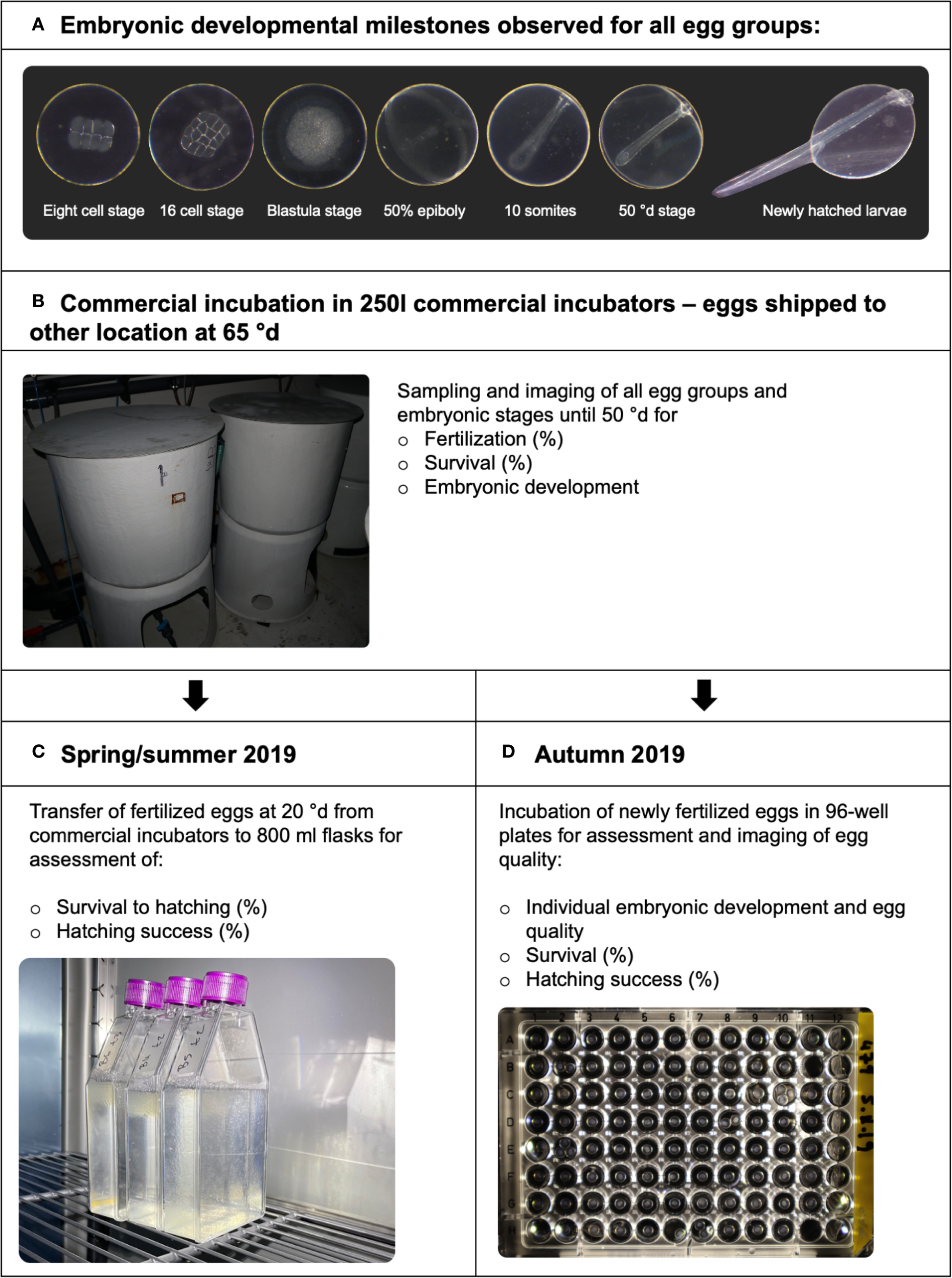
Overview of the experimental design and embryonic developmental monitoring for Atlantic halibut (Hippoglossus hippoglossus). Developmental stages, types of data collected, and the incubation systems used at each stage are indicated. (A) all egg groups were sampled and observed at these developmental milestones. (B) Newly fertilized eggs from all individual females were incubated in the large commercial incubators. All large incubators were emptied when embryos were approx. 65°d after fertilization, for shipment before hatching to other localities. (C) In spring/summer 2019, fertilized eggs at 20°d from the large incubators were transferred to 800 mL cell culture flasks for assessment of further development and viability. (D) In autumn 2019, newly fertilized eggs were incubated individually in 96-well plates. Please see text for more information.
Table 1
| Developmental stage | Hpf | °d | Figure 2 |
|---|---|---|---|
| Eight-cell stage (8C) | 14–16 | 4 | A |
| Sixteen-cell stage (16C) | 18 | 4.5 | D |
| Blastula stage (BL) | 50 | 12.5 | G |
| 50% Epiboly (50%EB) | 109 | 27 | J |
| Ten somite stage (10S) | 135 | 34 | M |
| Blastopore closure (50°d) | 200 | 50 | P |
| Hatching (H) | 335 | 84 | Not shown |
Sampling time points of Atlantic halibut (Hippoglossus hippoglossus) embryos at key developmental stages.
Hours post fertilization, hpf; °d, degree days (6°C × days post fertilization). Figure 2 column indicates the corresponding panel showing normal development at each stage. These sampling points were used in morphological scoring and subsequent analyses of egg quality predictors.
2.3.1 Incubation and sampling (spring/summer and autumn 2019)
Egg groups were selected opportunistically from viable batches identified by hatchery staff, with the number and timing of samples determined by the availability of batches and the capacity for sampling. Seventeen fertilized egg batches from 11 females were collected across three consecutive spawning seasons in 2019 (Table 2): two batches in spring (April), five in summer (July), and ten in autumn (November/December). Seven egg groups were incubated in 800 mL cell culture flasks and ten in 96-well plates.
Table 2
| Female ID | Date | Spawning period | Batch size (mL) | Small-scale incubation type |
|---|---|---|---|---|
| 618 | 01/04/2019 | 7 | 2600 | 800 mL cell culture flask |
| 1140 | 01/04/2019 | 1 | 1000 | 800 mL cell culture flask |
| 548 | 10/07/2019 | 4 | 1850 | 800 mL cell culture flask |
| 704 | 10/07/2019 | 4 | 1600 | 800 mL cell culture flask |
| 906 | 10/07/2019 | 1 | 1100 | 800 mL cell culture flask |
| 527 | 13/07/2019 | 10 | 1050 | 800 mL cell culture flask |
| 1138 | 13/07/2019 | 2 | 1500 | 800 mL cell culture flask |
| 479 | 02/11/2019 | 6 | 2300 | 96-well plate |
| 1132 | 02/11/2019 | 1 | 2200 | 96-well plate |
| 479 | 05/11/2019 | 6 | 1900 | 96-well plate |
| 927 | 05/11/2019 | 1 | 1900 | 96-well plate |
| 1132 | 05/11/2019 | 1 | 2450 | 96-well plate |
| 479 | 08/11/2019 | 6 | 2600 | 96-well plate |
| 927 | 08/11/2019 | 1 | 1900 | 96-well plate |
| 1132 | 08/11/2019 | 1 | 1400 | 96-well plate |
| 1338 | 08/11/2019 | 1 | 1100 | 96-well plate |
| 479 | 12/11/2019 | 6 | 1950 | 96-well plate |
List of incubation type and batch size of Atlantic halibut (Hippoglossus hippoglossus) eggs sampled from broodstock.
Female ID, date, spawning period, which indicates the number of years the female produced eggs, and batch size (mL). Total Females = 11, egg batches = 17.
In spring/summer 2019, approximately 150–300 eggs per group (collected at ~20°d) were transferred into 800 mL cell culture flasks (Figure 1C) containing seawater with 0.25 mg/L oxytetracycline (Terramycin 100 mg/mL, Zoetis, USA), following Skaalsvik et al. (2015). Antibiotics were required to prevent microbial infections in static, small-volume incubations. Preliminary tests without antibiotics resulted in microbial overgrowth and near-complete mortality, making a no-antibiotic control infeasible. Flasks were kept in darkness at 6°C until hatching (82–84°d). Dead eggs were removed and counted every 3–5 days, and newly hatched larvae were photographed under a microscope to estimate survival and hatching success.
In autumn 2019, newly fertilized eggs were incubated individually in 96-well plates (Figure 1D) filled with seawater containing the same antibiotic concentration as above. Evaporation was compensated with demineralized water. Each embryo was photographed at major developmental milestones using a digital camera (Canon EOS M100), which allowed developmental progression to be tracked for individual eggs. At setup, the number of fertilized eggs per plate was recorded. At hatching (~84°d), hatched larvae and live unhatched eggs were counted. The intention was to place one egg per well, but in some cases multiple eggs were inadvertently introduced. Such wells were excluded from further analysis. In total, 509 fertilized eggs could be reliably followed from the eight-cell stage until hatching. Eggs were excluded if fertilization status could not be confirmed, if multiple eggs were inadvertently introduced into the same well, or if their position could not be consistently tracked during incubation. Six developmental milestones were used to describe normal and abnormal embryogenesis.
2.3.2 Embryonic viability
Fertilization success and rates of normal blastomere morphology were determined from pictures of 8-cell stage embryos. Between 100 and 300 individuals were assessed for each egg batch from the commercial incubators. Eggs were classified into three categories: fertilized (visible cell division), unfertilized (transparent), and dead (opaque or whitish eggs with collapsed yolk and loss of buoyancy). Fertilization success was calculated as the proportion of fertilized eggs relative to the total number of fertilized and unfertilized eggs (Equation 1).
Where is the number of eggs with visible cleavage and is the number of transparent undeveloped eggs.
The percentage of eggs with normal early blastomere morphology was calculated from the fertilized fraction of each egg batch, as described by Kjørsvik and Lønning (1983); Kjørsvik et al. (1984) and Shields et al. (1997) (Equation 2).
Where is the number of fertilized eggs with symmetric, evenly sized blastomeres.
The frequency of normal embryos at 50°d stage was calculated by dividing the number of normally developing embryos by the total number of live embryos at that stage (Equation 3).
Where is the number of embryos without visible abnormalities, and is the number of total live embryos at this stage.
The survival rates from the commercial incubators, determined by daily removal and measurement of dead eggs, were used to estimate the hatching success and survival until hatching in the 800 mL incubators (Equation 4). These adjustments ensured that metrics for small-scale incubations reflected the overall batch survival rate up to the point of transfer.
Where is the survival fraction of fertilized eggs up to the point transfer to the small-scale-incubators, and is the number of eggs that were removed from the commercial incubators until the eggs were transferred to the 800 mL incubation flasks.
Hatching success was defined as the percentage of fertilized eggs that hatched, while survival until hatching represented the percentage of fertilized eggs alive at hatching, including both hatched larvae and unhatched but still viable eggs. The survival until 20°d ( was multiplied with the metrics from the incubations to account for any mortality that occurred prior to small-scale incubation.
Hatching success and survival in the 96-well plates were determined as the percentage of fertilized eggs that hatched or survived until hatching, respectively (Equations 5, 6).
2.3.3 Development of individual embryos in 96-well plates
The 96-well plates were further used to follow individual eggs from fertilization until hatching (Figure 1D). At 8C–16C stage, each embryo was categorized as either normal or abnormal early blastomere morphology. The further individual development of these embryos (normal or abnormal, see Figure 2) was then followed until the embryo died or hatched (Figure 3), and the developmental stage of death was recorded. The developmental stages at death were categorized into “before completion of gastrulation” (8C until 50%EB), or “after completion of gastrulation” (10S until hatching). Eggs that did not hatch at the final sampling stage but were seemingly alive were considered as “alive” and their phenotype was similarly assessed as either normal or abnormal after gastrulation. During the initial phase of incubation, some wells contained more than one egg at set-up, and in other cases eggs adhered to the plate lids and were displaced between wells when opened. In both situations, wells where an individual egg could not be reliably tracked were excluded from analysis (see Section 2.3.1).
Figure 2
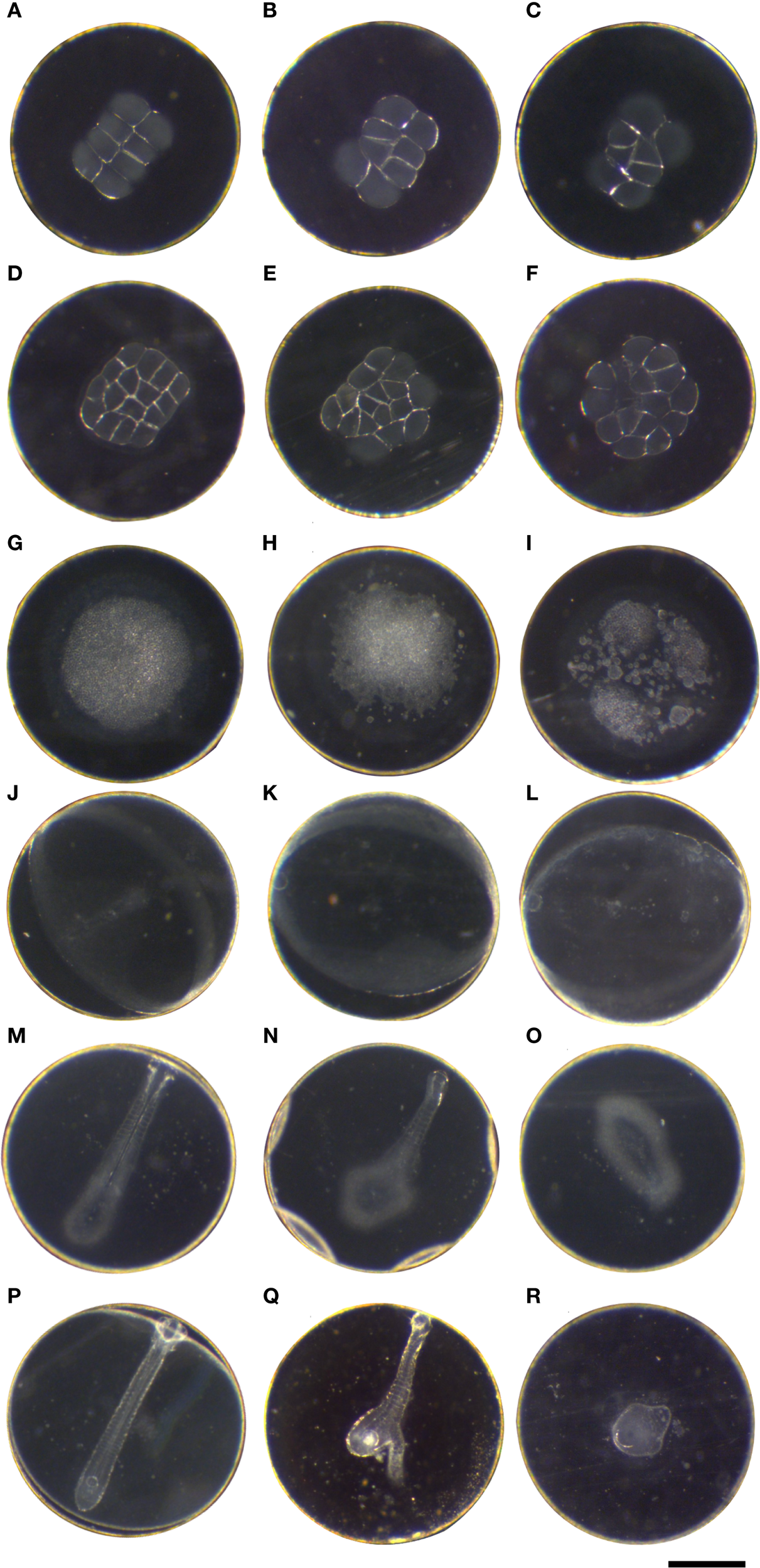
Atlantic halibut (Hippoglossus hippoglossus) embryonic development observed from eight-cell stage until blastopore closure (end of gastrulation at 50°d) at 6°C. Pictured are normal phenotypes (left column) as well as common abnormalities. Each image depicts a different embryo. Each row represents a developmental stage. The eggs within a column have no relationship and do not depict the development of a given phenotype. Embryonic stages in the left column are categorized as normal development. (A–C) eight-cell stage, 14 hpf/3.5°d; (D–F) 16C stage, 18 hpf/4.5°d; (G–I) blastula stage 50hpf/12.5°d; (J–L) 50% epiboly, 109 hpf/27°d; (M–O) approximately 10 somite stage, 135 hpf/34°d; (P–R) blastopore closure 50°d stage, 200 hpf (Scale bar = 1 mm).
Figure 3
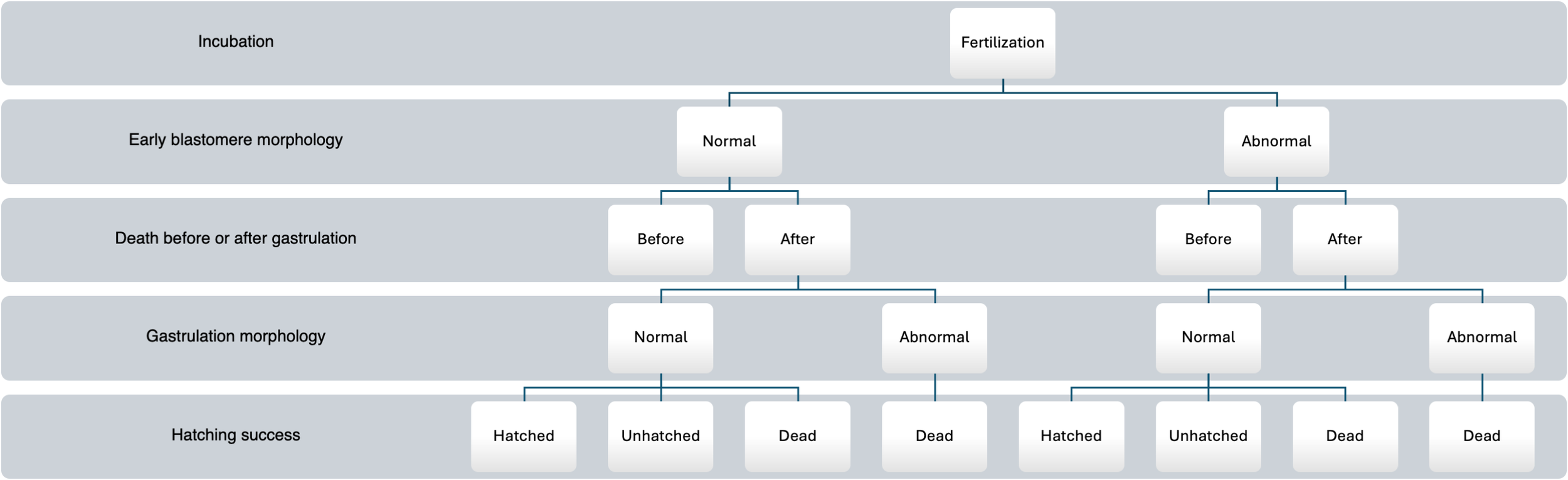
Flow chart of the experiment following the individual development of Atlantic halibut (Hippoglossus hippoglossus) eggs incubated in 96-well plates. At fertilization, eggs were incubated in one well each, and their mode of development was registered at 8C stage (early blastomere morphology), if eggs died during incubation, it was registered whether they died before or after gastrulation. The eggs that survived gastrulation were classified into either normal or abnormal morphology based on the deformities described in Figure 2. None of the eggs with abnormal morphology after gastrulation hatched. At the hatching stage, it was registered whether an egg had hatched, was alive but unhatched, or dead.
2.4 Statistical analyses
Statistical analyses were conducted using RStudio version 2022.7.2.576 (RStudio Team, 2022). The relationships between egg quality parameters and hatching success were analyzed using beta regression models. Hatching success was used as the dependent variable, with fertilization success, the percentage of normal early blastomeres (8C stage), and the percentage of normally developing embryos at blastopore closure (50°d) included as predictors. Analyses were conducted separately for eggs incubated in 800 mL flasks and those in 96-well plates, as incubation method was confounded with egg batch. To avoid boundary issues, proportional data equal to 0 or 1 were adjusted by adding or subtracting a small constant (ϵ = 0.0001). Model fit was evaluated using pseudo-R² values, and significance of predictors was assessed from model coefficients (p ≤ 0.05). Survival until hatching was additionally analyzed in a separate beta regression model as a dependent variable, using the same predictors.
Because each egg batch was incubated in only one incubation system, incubation method and batch effects could not be fully delineated. Comparisons between systems should therefore be interpreted with caution. To evaluate whether initial egg quality differed between systems, we analyzed embryo morphology at 50°d from samples taken in the commercial flow-through incubators (ensuring comparability across batches), using a generalized linear mixed model (GLMM) with a binomial error structure, including incubation method as a fixed effect and batch ID as a random intercept. To assess the robustness of the GLMM results to potential batch effects, we performed a leave-one-batch-out sensitivity analysis. The GLMM was refitted iteratively while excluding each batch in turn, and the fixed effect estimate for incubation method was compared across models. In addition, we performed an exploratory beta regression with the proportion of normal embryos at 50°d as the response, testing fertilization rate, 8-cell morphology, number of spawning periods, and stripped egg volume as predictors, in order to evaluate whether broodstock variables explained variation in embryo quality.
The differences in hatching success between individual 8C stage embryos (on 96-well plates) with normal or abnormal blastomere morphology on the 96-well plates were analyzed using a chi-squared test to assess the effects of phenotype on hatching success. The data were further subset to include only fertilized eggs that died during incubation, and a chi-squared test was used to analyze potential differences in timing of death (before or after gastrulation) and early blastomere morphology (normal versus abnormal). Another subset of the data included eggs that were alive at hatching but did not hatch. Fisher’s exact test was applied to detect possible differences in the morphology after blastopore closure (normal versus abnormal) of these unhatched eggs and their early blastomere morphology (Upton, 1992). Statistical significance was established at p< 0.05.
3 Results
3.1 Embryonic development
The normal embryonic development is illustrated in the left column of Figure 2, and observed developmental anomalies at those stages are shown in the two next columns. In typical normal early cleavage stages (8 and 16 cell stage, 14–18 hpf), the blastomeres were of similar size, symmetry and shape and had consistent cell margins (Figures 2A, D). Typical blastomere anomalies consisted of poorly resolved margins with no clear separation to the intracellular space or the yolk (Figures 2B, C), and little to no bilateral symmetry (Figures 2E, F). At the multi-layered BL stage (Figure 2G, 50 hpf, 12.5°d), the yolk syncytium was visible, and observed anomalies were frayed edges of the blastula with a granular outer perimeter and single cells outside the blastomeres (Figures 2H, I). Severely malformed embryos at this stage were mostly a collection of loose cells (Figure 2I). However, no evidence of these malformations was observed after onset of gastrulation, i.e. loose cells around the developing germ ring. At 50% epiboly (50%EB, 109 hpf, 27°d), the embryonic shield was pronounced (Figure 2J), and in abnormally developing embryos it was either only faintly visible as part of the germ ring (Figure 2K) or not visible at all (Figure 2L), and sometimes droplets were observed surrounding the germ ring (Figures 2K, L). Towards the end of epiboly/gastrulation at the 10 somite stage (10S) (Figure 2M, 135 hpf, 34°d), a common abnormality was partial body axis formation, with segmentation present, no bilateral symmetry, or no visible head present (Figures 2N, O). In some deformed embryos a simple head differentiated without any optic bulbs (not shown). At blastopore closure (50°d stage, 200 hpf, Figure 2P), abnormal embryos were frequently observed without any body axis formation or tissue differentiation, they appeared as an aggregation of undifferentiated cells, round in appearance and with a completed gastrulation (Figures 2Q, R). The severity of these malformations varied from nearly normal appearing embryos to an undifferentiated clump of cells (Figure 2R). The embryos hatched at 335 hpf, 84°d.
3.2 Egg quality parameters and predictors of hatching success
Developmental success varied widely among batches, with fertilization success ranging from 31–88%, normal 8-cell embryos 51–85%, normal 50°d embryos 41–99%, survival until hatching 25–99%, and hatching success 22–85% (Figure 4). Sample sizes for each parameter and batch are provided in Supplementary Table S1. Among females with repeated batches, fertilization success varied considerably within individuals. Female 479 exhibited fertilization rates between 55–79% across four batches, female 1132 between 31–70% across three batches, and female 927 between 34–58% across two batches (Table 3). A GLMM confirmed significantly lower probabilities of normal development at 50°d in 96-well plates compared with flasks (Estimate = –1.20 ± 0.57 SE, z = –2.11, p = 0.035). Eggs incubated in 800 mL flasks generally exhibited higher initial quality than those in 96-well plates. Because 50°d morphology was scored in the commercial flow-through incubators before any small-scale incubation, this GLMM reflects pre-existing differences between the groups later assigned to flasks or plates, rather than effects of the small-scale system itself. A leave-one-batch-out sensitivity analysis produced consistently negative estimates, indicating that this result was not driven by any single batch. Beta regression models (Table 4, Figure 5) identified the proportion of normal embryos at 50°d as the strongest predictor of both hatching success and survival until hatching. In flasks, 50°d morphology was the only significant predictor, whereas fertilization rate and 8C morphology were not significant. In plates, hatching success was also associated with fertilization rate (negative effect) and 8C morphology in addition to 50°d morphology. Survival until hatching was analyzed in the same way, and yielded equivalent results, but is not presented in detail due to its close dependence on hatching success. In an additional exploratory beta regression using 50°d morphology as the response variable across all batches (n = 17), spawning period was not a significant predictor. In contrast, stripped egg volume showed a weak negative association with 50°d morphology (Estimate = –0.0011 ± 0.0004 SE, z = –2.87, p = 0.004), although the effect size was small and the model explained only a modest proportion of the variance (pseudo-R² = 0.34). Leave-one-female-out analyses confirmed that this association was not driven by a single individual.
Figure 4
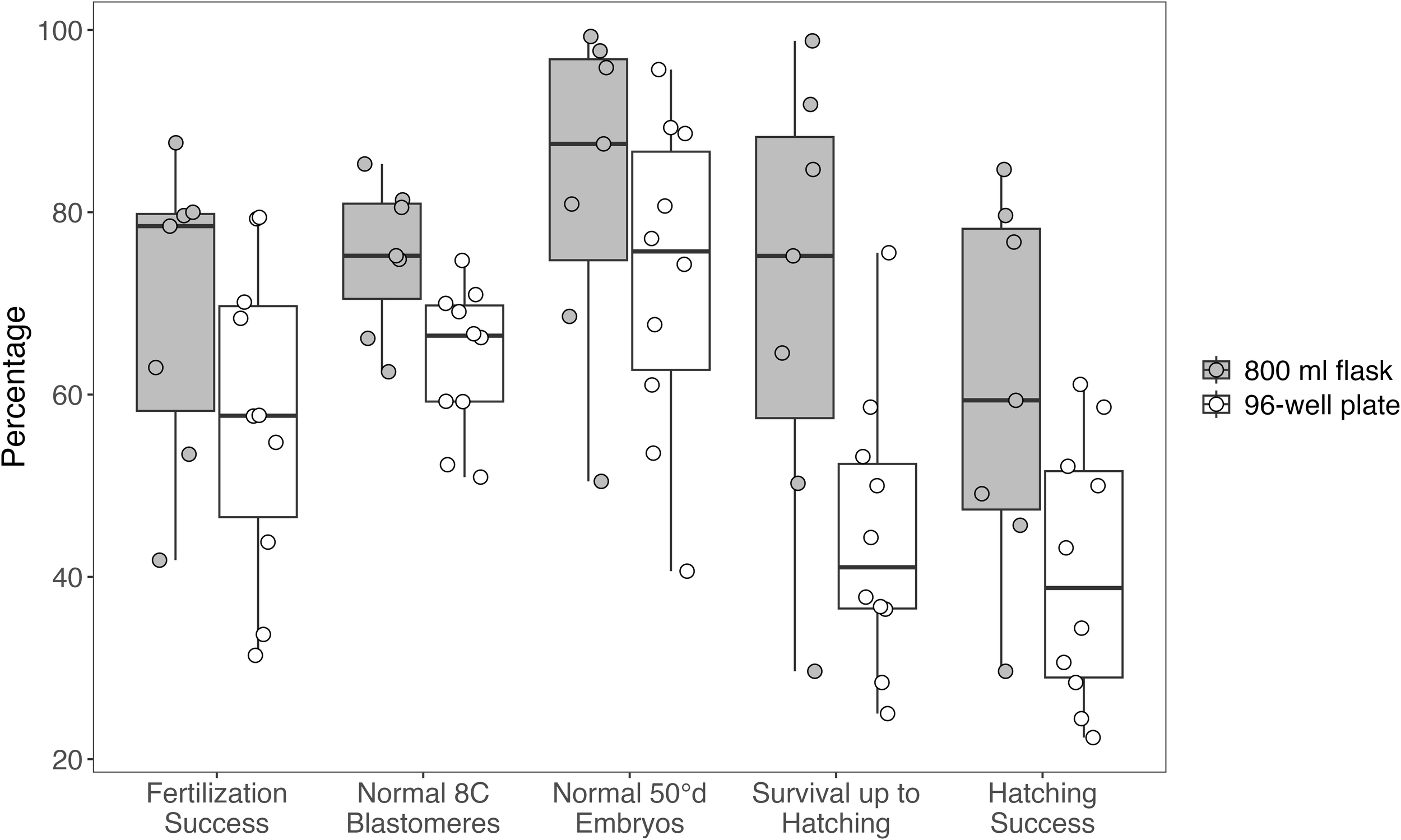
Distribution of Atlantic halibut (Hippoglossus hippoglossus) egg quality parameters for small-scale incubations in 800 mL flasks (n = 7) and 96-well plates (n = 10). Note that different egg groups were used for the two incubation methods. Box plots display the median, interquartile range, and full range of values, with individual data points overlaid. Parameters assessed include fertilization success, proportion of embryos with normal 8-cell blastomeres, proportion of normal embryos at 50°d, survival up to hatching, and hatching success. Data for fertilization success, normal 8C blastomeres, and normal 50°d embryos were collected from commercial incubators and are independent of the incubation method. The small-scale incubations (800 mL flasks and 96-well plates) were used exclusively to assess survival up to hatching and hatching success.
Table 3
| Female ID | Date | Fertilization rate (%) | % normal embryos 8C stage | % normal embryos 50°d | Survival prior to hatching (%) | Hatching rate (%) |
|---|---|---|---|---|---|---|
| 618 | 01/04/2019 | 78 | 66 | 50 | 30 | 30 |
| 1140 | 01/04/2019 | 80 | 63 | 99 | 85 | 85 |
| 548 | 10/07/2019 | 88 | 75 | 88 | 65 | 59 |
| 704 | 10/07/2019 | 63 | 81 | 81 | 75 | 49 |
| 906 | 10/07/2019 | 42 | 81 | 96 | 92 | 77 |
| 527 | 13/07/2019 | 80 | 85 | 98 | 99 | 80 |
| 1138 | 13/07/2019 | 53 | 75 | 69 | 50 | 46 |
| 479 | 02/11/2019 | 55 | 70 | 77 | 53 | 52 |
| 1132 | 02/11/2019 | 44 | 59 | 96 | 59 | 59 |
| 479 | 05/11/2019 | 68 | 75 | 60 | 36 | 34 |
| 1132 | 05/11/2019 | 70 | 52 | 86 | 38 | 24 |
| 927 | 05/11/2019 | 34 | 71 | 93 | 76 | 61 |
| 1132 | 08/11/2019 | 31 | 51 | 91 | 50 | 50 |
| 479 | 08/11/2019 | 79 | 66 | 41 | 28 | 28 |
| 927 | 08/11/2019 | 58 | 59 | 96 | 44 | 43 |
| 1338 | 08/11/2019 | 58 | 69 | 90 | 37 | 31 |
| 479 | 12/11/2019 | 79 | 67 | 68 | 25 | 22 |
| Mean values ± SD | 62 ± 18% | 69 ± 10% | 81 ± 18% | 55 ± 23% | 49 ± 20% |
Overview of egg quality parameters from sampled production Atlantic halibut (Hippoglossus hippoglossus) egg batches during three reproductive seasons (n = 17) in 2019 ± standard deviation.
Hatching rates above the double line are based on 800 mL small-scale incubators, hatching rates below based on 96-well plate incubations.
Table 4
| Predictor | Estimate | Std. Error | 95% CI | z value | p-value |
|---|---|---|---|---|---|
| Flask incubations (n = 7) | |||||
| Intercept | -1.75 | 1.16 | -4.03–0.53 | -1.50 | 0.133 |
| Fertilization rate | -0.35 | 0.60 | -1.52–0.82 | -0.58 | 0.561 |
| Normal 8C embryos (%) | -2.49 | 1.42 | -5.27–0.30 | -1.75 | 0.080 |
| Normal 50°d embryos (%) | 5.24 | 0.60 | 4.07–6.41 | 8.78 | <0.001* |
| Plate incubations (n = 10) | |||||
| Intercept | -2.06 | 1.11 | -4.24–0.13 | -1.85 | 0.065 |
| Fertilization rate | -2.40 | 0.54 | -3.47–1.34 | -4.42 | <0.001* |
| Normal 8C embryos (%) | 2.89 | 0.98 | 0.96–4.82 | 2.94 | 0.003* |
| Normal 50°d embryos (%) | 1.62 | 0.63 | 0.38–2.85 | 2.57 | 0.010* |
Results of beta regression models testing the relationship between egg quality markers and hatching success in Atlantic halibut (Hippoglossus hippoglossus).
Separate models were fitted for eggs incubated in 800 mL flasks (A) and in 96-well plates (B). Estimates, standard errors (SE), z values, p-values, and 95% confidence intervals (CI) are reported for each predictor. Significant predictors (p ≤ 0.05) are shown in bold and marked with an asterisk (*). Pseudo-R² and dispersion (φ) values are reported as indicators of model fit. Models assumed a logit link, maximum likelihood estimation, response proportions constrained to (0,1) with epsilon-adjustment for boundary values.
Pseudo-R²: Flask = 0.93, Plate = 0.87.
Dispersion (φ): Flask = 93.4, Plate = 108.3.
Figure 5
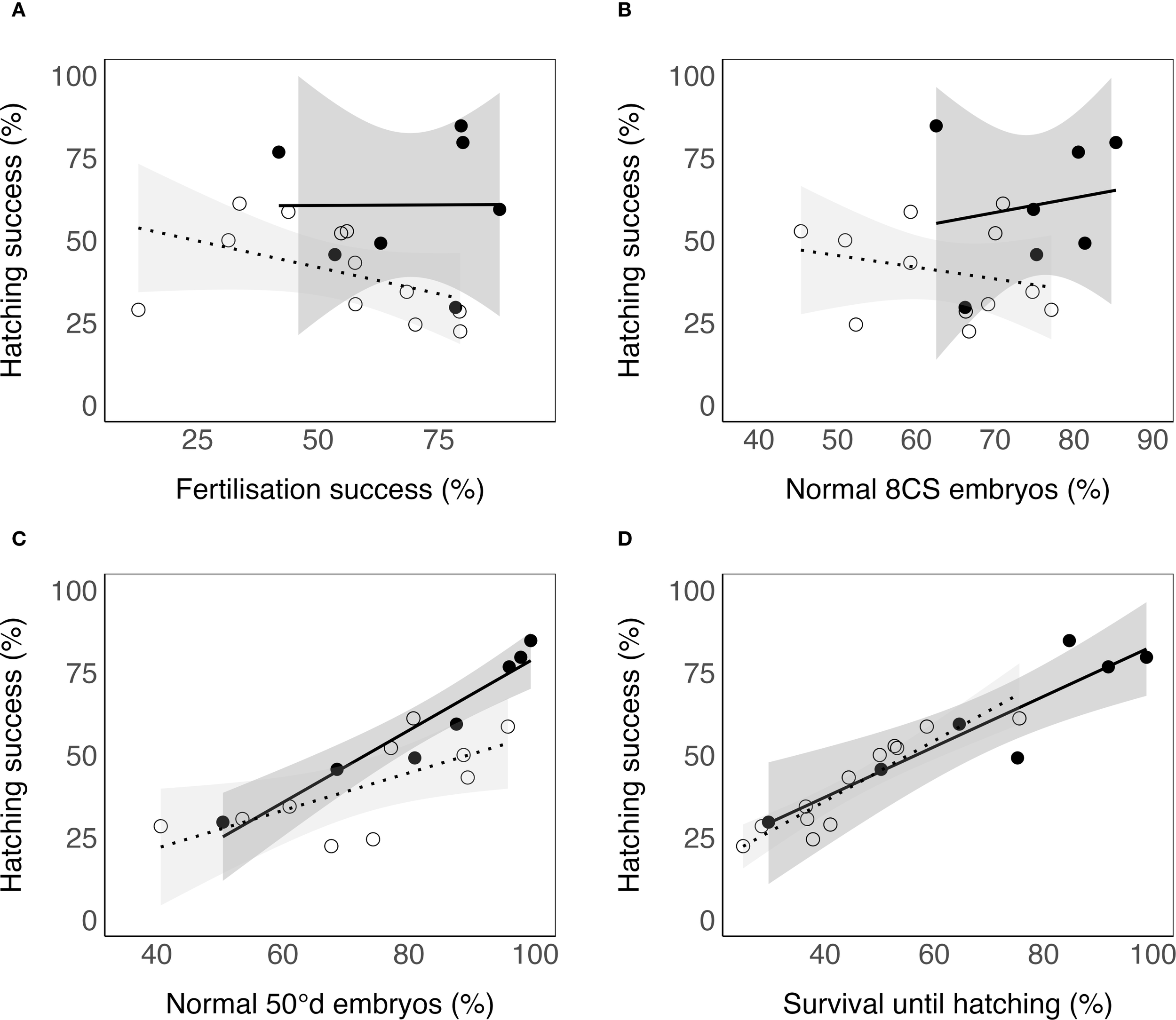
Relationships between selected egg quality markers and hatching success of Atlantic halibut (Hippoglossus hippoglossus). (A) Fertilisation success (%). (B) Normal 8-cell stage (8CS) embryos (%). (C) Normal embryos at 50°d stage (%). (D) Survival until hatching (%). Solid dots represent egg batches incubated in 800 mL flasks, while open dots represent batches incubated in 96-well plates. Note that different egg groups were used for the two incubation methods. Solid and dotted lines indicate linear regression fits for the flask and plate data, respectively, with shaded areas showing 95% confidence intervals. Statistical results from beta regression models are presented in Table 4.
3.3 Fate of individual embryos of different qualities
The individual development of 509 fertilized eggs was followed in the 96-well plates from fertilization until either death or hatching (Figure 6). Early cleavage stages were classified as normal (n = 441) or abnormal (n = 68), based on blastomere morphology (Figures 2A-C). Hatching success was significantly higher in eggs with normal morphology at the 8-cell stage (χ² = 19.2, p< 0.001, Table 5, Figure 6A). Eggs with abnormal morphology at this stage were more likely to die before gastrulation (χ² = 27.4, p< 0.001, Figure 6A). Among the eggs that survived the gastrulation period, those with abnormal early blastomere morphology were more likely to develop abnormal phenotypes post-gastrulation (See Figures 2K–R) compared to eggs with normal 8-cell morphology (χ² = 8.982, p = 0.011, Figure 6B). Because relatively few abnormal 8C embryos survived through gastrulation (n=36), numbers in this group were low at later stages. Hatching outcomes were strongly associated with gastrulation status (χ² = 199.6, p< 0.001): embryos with normal gastrulation had a 72% chance of hatching, whereas none with abnormal gastrulation hatched (Figure 6C). Full individual-level data for all embryos, including well position, 8C morphology, developmental fate, gastrulation phenotype, and hatching outcome, are provided in Supplementary Table S2.
Figure 6
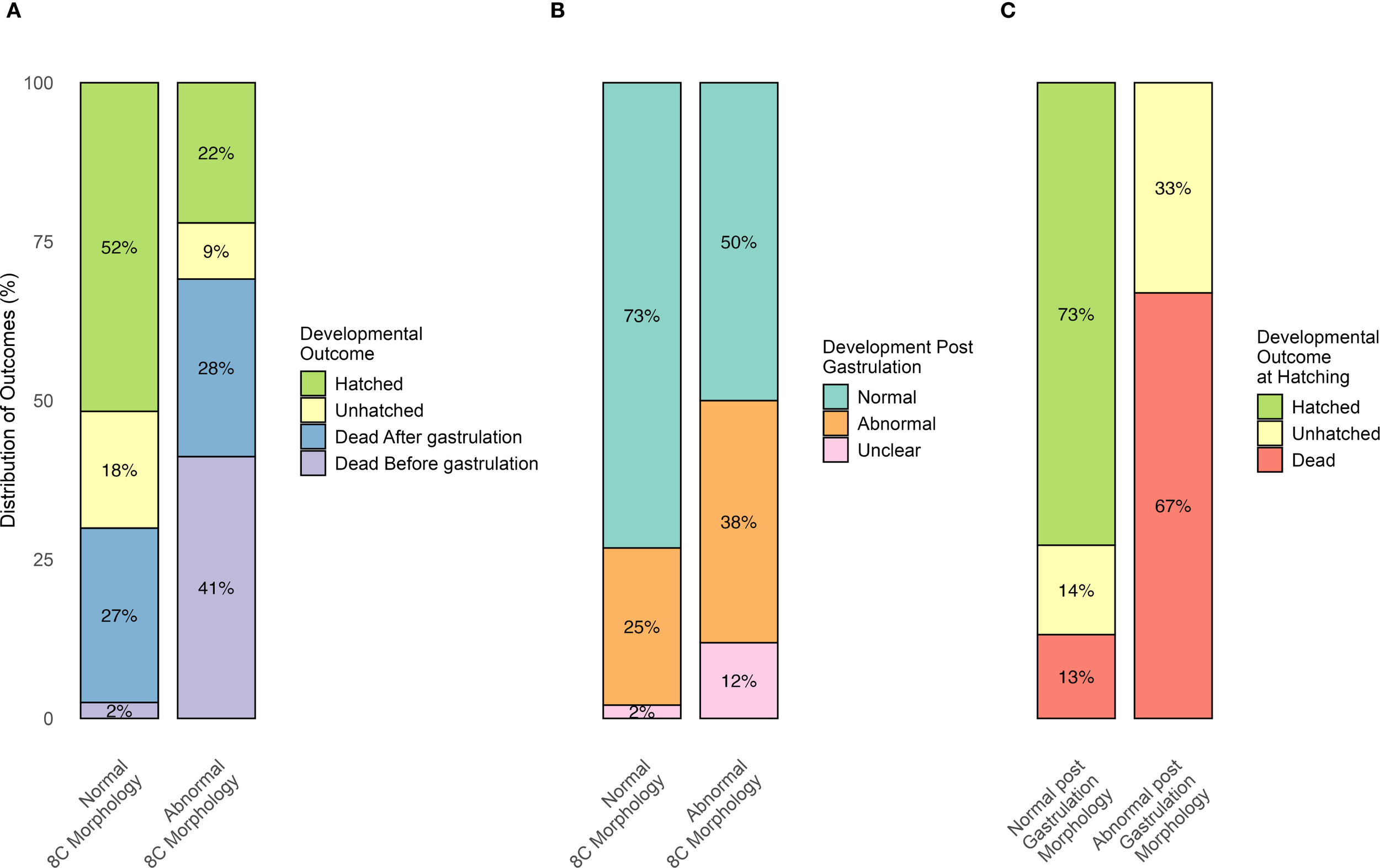
Results from 96-well plate small-scale incubations tracking the developmental fate of individual Atlantic halibut (Hippoglossus hippoglossus) eggs from eight egg batches. (A) Percentages of eggs classified as either “Normal” or “Abnormal” at the 8-cell stage that followed one of four outcomes: hatched, alive but unhatched at the hatching stage, died after gastrulation, or died before gastrulation. (B) Percentages of eggs classified as “Normal” or “Abnormal” at the 8-cell stage, which survived gastrulation and subsequently developed into either “Normal” or “Abnormal” post-gastrulation embryos. (C) Percentages of embryos classified as having either ‘Normal’ or ‘Abnormal’ post-gastrulation morphology, which were subsequently categorized into three developmental outcomes: hatched, unhatched, or dead.”.
Table 5
| Category | Number of eggs: | Percentage of eggs: | ||
|---|---|---|---|---|
| Normal 8C morphology | Abnormal 8C morphology | Normal 8C morphology | Abnormal 8C morphology | |
| Total number | 441 | 68 | 86% | 14% |
| Total dead eggs | 132 | 47 | 30% | 69% |
| Dead before gastrulation | 11* | 28 | 3%* | 41% |
| Dead after gastrulation | 121 | 19 | 27% | 28% |
| Normal PGM | 314* | 21 | 73%* | 50% |
| Abnormal PGM | 106* | 16 | 25%* | 38% |
| Hatched | 228* | 15 | 52%* | 22% |
Summary of outcomes from 96-well plate incubations of fertilized Atlantic halibut (Hippoglossus hippoglossus) eggs with normal and abnormal 8C morphology.
The first row (Total number) includes all fertilized eggs, with percentages representing the proportion of normal and abnormal 8C stage eggs, respectively. Subsequent rows refer specifically to eggs categorized as normal or abnormal 8C morphology. PGM, post gastrulation morphology. Asterisks indicate statistically significant differences between normal and abnormal 8C embryos within the same row (*p ≤ 0.05).
4 Discussion
The embryonic development in Atlantic halibut has been thoroughly described (Blaxter et al., 1983; Lønning et al., 1982; Pittman et al., 1990a; Rollefsen, 1934), and early egg quality markers such as normal early cell cleavages have been used more or less successfully for halibut under various experimental conditions (Brown, 1998; Kjørsvik and Holmefjord, 1995; Mommens et al., 2010; Shields et al., 1997; Skaalsvik et al., 2015). As experienced previously and in the present incubation trials, these early markers have not been reliable for commercial production, and the present study therefore focused on common embryonic deformities observed in a commercial hatchery during the whole developmental period.
In Atlantic halibut, fertilization rate was not indicative of egg viability, in line with results from previous research (Bromage et al., 1994; Kjørsvik, 1990). Large variations in fertilization rates were observed, both between batches of different females as well as in subsequent batches of the same female. Early cleavage blastomere morphology (8–16 cell stage) has been suggested as an indicator of egg viability in Atlantic cod (Gadus morhua) (Kjørsvik and Lønning, 1983; Kjørsvik et al., 1984) and halibut (Shields et al., 1997). In the present study, 8C morphology was not a significant predictor of hatching success in flask incubations, whereas in plate incubations it explained part of the observed variability. Fertilization rate also appeared as a negative predictor in the plate model, but this effect is likely an artifact, possibly linked to individual female effects and the limited sample size, rather than a biologically meaningful relationship. Additionally, while the number of broodfish spawning seasons did not influence egg quality, larger stripped batches were associated with reduced quality. A possible explanation is incomplete stripping of the previous batch, with retention of some ovulated eggs that subsequently lost viability due to post-ovulatory ageing (Bromage et al., 1994; Norberg et al., 1991).
Although flask-incubated groups appeared to perform better than plate-incubated groups overall, this difference is best explained by intrinsic batch quality rather than incubation method. Taken together, these results indicate that while 8C morphology can sometimes reflect variation in embryo performance under certain incubation conditions, it is a weak and inconsistent marker compared to the much stronger predictive power of 50°d morphology. Survival until hatching has also been used as an indicator of egg quality in halibut (e.g. Yilmaz et al., 2022), but this metric is impractical in commercial settings where batches are pooled or shipped before hatching. By contrast, assessing the proportion of normal embryos at 50°d enables hatchery managers to evaluate quality prior to pooling or shipment, prioritize the most promising egg groups, and reduce within-silo variation. Importantly, sorting eggs based on quality at this stage may improve not only immediate hatching outcomes but also long-term production results. For example, in turbot (Scophthalmus maximus), larvae originating from high-quality egg batches were more likely to complete metamorphosis and showed higher long-term survival compared to those from poorer-quality batches (Kjørsvik et al., 2003).
Our results elucidated very different developmental trajectories of individual eggs with either normal or abnormal 8C morphology throughout their development. In contrast to eggs exhibiting normal 8C early cleavage patterns, those with abnormal early cleavage showed significantly higher mortality rates prior to gastrulation (GR stage), higher tendency for abnormal development post-gastrulation, and with markedly reduced hatching success (as shown in Figure 5). The present study supports earlier observations in Atlantic cod that abnormal early cleavage pattern do not necessarily preclude successful embryonic development (Avery et al., 2009). Notably, nearly all embryos with normal morphology after gastrulation successfully hatched, whereas none of the embryos exhibiting abnormal development post-gastrulation reached hatching. These findings underscore the critical role of post-gastrulation developmental normality in predicting hatching success. Such commonly observed later abnormal phenotypes were also described by Brown (1998) as disintegrated blastomeres (similar to Figure 2I) and “developmental arrest” (similar to Figure 2Q), which were associated with poor hatching success. However, these phenotypes were described as relatively rare, and their impact on hatching success was not quantified.
During the autumn spawning season, subsequent egg batches from three fish showed variable and declining hatching rates (females 479, 1132 and 927), similarly to Atlantic cod, in which egg size and egg quality decreased towards the end of the spawning period (Fernández Míguez et al., 2024). However, the broodstock in the commercial hatchery were visually inspected and stripped when experienced operators determined that they were ready. Mature females were stripped for subsequent batches at intervals of 82–84 hours (20–22°d at 6°C). This approach assumed two conditions: a) that the first batch was obtained shortly after ovulation, and b) that subsequent ovulations occurred within 82–84 hours. Atlantic halibut females ovulate at intervals of 72–96 hours (Bromage et al., 1994; Norberg et al., 1991). As little as 4–6 hours between ovulation and spawning can significantly reduce egg quality in marine pelagic finfish eggs, and close monitoring of ovulatory cycles is necessary to avoid overripening (Bromage et al., 1994; Norberg et al., 1991). The commercial hatchery required monitoring of up to 70 females during one reproductive season and individual monitoring of ovulatory cycles as described for example by Norberg et al. (1991) was not feasible. A stripping protocol that does not overlap with the individual ovulatory rhythms could explain the observed variance in egg quality and the declining hatching success we observed in the subsequent egg groups, especially if the factual ovulatory rhythm is shorter than the period in which the fish are stripped. Closer monitoring, possibly fewer broodfish, and more individual female follow-up could also improve hatchery protocols.
The nature of the observed developmental abnormalities in this study implies potential disruptions in the coordination or expression of the various developmental pathways governing body axis formation. Molecular analysis of egg quality thus far focused on the identification of differentially expressed genes between good and poor-quality eggs (Mommens et al., 2010; Reading et al., 2018). Furthermore, Yilmaz et al. (2022) recently found that impaired protein and energy homeostasis in newly fertilized eggs (one cell stage) was a molecular hallmark for poor quality halibut eggs, which indicates a lower developmental competence in these embryos. These approaches could be further explored by focusing on analysis of molecular pathways that are responsible for embryonic body axis formation and tissue differentiation. For example, the pathways Nodal and Wnt/β-Catenin are responsible for localization and specialization of the dorsal region, or the embryonic shield on the zebrafish (Danio rerio) embryo (Lu et al., 2011; Schier and Talbot, 2003, 2005). The embryonic shield is further defined by inhibition of certain pathways such as the Bone Morphogenetic Protein (BMP) pathway (Kondo, 2007). Identifying the differential expression of those pathways and other developmental processes related to organ and tissue development in eggs of good and poor quality at various developmental milestones could pinpoint the molecular determinants of normal embryonic development in Atlantic halibut and the possible disruption of normal embryonic development in domesticated Atlantic halibut eggs of poor quality.
Future studies could explore the implications of the suggested egg quality marker, particularly its effects on long-term outcomes such as larval metamorphosis success, survival, and growth. Possible effects on egg quality from stripping time in relation to ovulation time in this batch spawning fish should also be explored. Specifically, investigations into the developmental trajectories of larvae from egg groups with both poor and good 50°d morphology, whether reared separately or in pooled groups, would provide valuable insights. Such studies could lead to improved strategies to optimize larval performance and standardize production outcomes in commercial hatchery systems.
One limitation of this study was the absence of a no-antibiotic control group in the small-scale incubations. Preliminary tests without antibiotics resulted in widespread microbial overgrowth and mortality in stagnant water, precluding any meaningful developmental assessment. Although this limits direct comparisons to flow-through hatchery systems, which typically do not rely on antibiotic water treatment, all experimental groups received identical treatment, ensuring internal consistency and valid interpretation of relative differences. Another limitation is that the study was conducted at a single hatchery during one production year, using only a fraction of the available broodfish, which constrains the generalizability of the findings. The proposed egg quality marker should therefore be validated across multiple hatcheries, broodfish, and years, ideally within routine commercial operations.
5 Conclusions
Egg batches stripped from commercial broodstock exhibited highly variable developmental success and survival rates up to hatching. The percentage of eggs with normal development at blastopore closure (~50°d) emerged as the strongest predictor of hatching success, establishing it as a promising candidate egg quality marker for Atlantic halibut eggs in commercial hatcheries. Egg groups with more than 90% normal development at blastopore closure displayed consistently higher hatching success with less variability, whereas groups below 70% normal development showed poor performance, with hatching success rates below 50%. These values may serve as initial reference points for hatchery decision-making (e.g., for prioritization or silo allocation), although broader validation would be required before defining strict operational thresholds. Applying such criteria could help reduce within-silo variability and improve production predictability, especially if confirmed across more batches and seasons. Brackets of 10% increments in the proportion of normally developed embryos at 50 °d (e.g., >90%, 80–90%, 70–80%) are proposed as an initial staging system for egg quality, allowing hatchery managers to optimize resource allocation based on available quality levels rather than binary cut-offs.
The developmental trajectories of eggs with good and poor 8C morphology revealed that while poor 8C morphology was associated with lower hatching rates, its frequency was not directly predictive of overall hatching success. Instead, the presence of abnormal phenotypes post-gastrulation had a more pronounced impact, as none of the eggs with post-gastrulation abnormalities successfully hatched. Future research could focus on key factors influencing these outcomes, such as the timing of egg stripping and its effects on hatching success and normal embryonic development. Further investigation into the molecular mechanisms underlying abnormal embryonic development after gastrulation, as well as the long-term larval quality and survival of egg groups sorted using this new quality marker, would provide additional insights. These efforts could enhance the understanding and application of egg quality assessment tools, ultimately improving productivity and consistency in Atlantic halibut aquaculture.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because Fertilized eggs and yolk-sac larvae are developmental stages outside the scope of the Guidelines of the European Union on the protection of animals used for scientific purposes (Directive 2010/63/EU), and approval from the Norwegian Food Safety Authority was not needed.
Author contributions
NN: Conceptualization, Data curation, Formal analysis, Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing. MF: Investigation, Writing – review & editing. LB: Funding acquisition, Project administration, Writing – review & editing. EK: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work and PhD scholarship were fully funded by the Research Council of Norway (project “Juvenile production in Atlantic halibut aquaculture” project number: 281802), Nordic Halibut AS, Norway and NTNU. NTNU funded a five-month salary grant to NN, due to delays during the COVID-19 restrictions. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
We would like to thank Nordic Halibut AS in Midsund, Norway, for accommodating the authors’ research stay, for allowing access to the facilities and for providing technical support whenever needed. Further acknowledgement goes to Tora Bardal (Department of Biology, NTNU) for her support and technical expertise.
Conflict of interest
Author LB was employed by the company Nordic Halibut AS.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The authors used ChatGPT 4o (OpenAI) to assist with language and readability editing during manuscript preparation. All content was reviewed, verified, and edited by the authors, who take full responsibility for the accuracy and integrity of the manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/faquc.2025.1673217/full#supplementary-material
Supplementary Table 1Sample sizes (n) for each Atlantic halibut (Hippoglossus hippoglossus) egg batch and developmental parameter (fertilization success, normal 8-cell embryos, normal 50°d embryos, survival until hatching, and hatching success).
Supplementary Table 2Individual-level dataset from the 96-well plate incubation experiment regarding Atlantic halibut (Hippoglossus hippoglossus) eggs. Each row represents a single Atlantic halibut embryo tracked from fertilization until hatching or death. Columns include batch ID, well position, 8-cell stage (8C) morphology (normal/abnormal), hatching outcome (alive/dead), developmental stage of death, gastrulation status (normal/abnormal/unclear), and whether mortality occurred before or after gastrulation.
Abbreviations
°d, Degree days; hpf, Hours past fertilization; 8C, Eight-cell stage; 16C, Sixteen-cell stage; BL, Blastula stage; GR, Germ ring stage; 50%EB, 50% epiboly stage; 10S, 10 somite stage; 50°d, 50-degree day stage; H, Hatching stage; PGM, Post-gastrulation morphology.
References
1
Avery T. S. Killen S. S. Hollinger T. R. (2009). The relationship of embryonic development, mortality, hatching success, and larval quality to normal or abnormal early embryonic cleavage in Atlantic cod, Gadus morhua. Aquaculture289, 265–273. doi: 10.1016/j.aquaculture.2008.12.011
2
Bergh Ø. Nilsen F. Samuelsen O. B. (2001). Diseases, prophylaxis and treatment of the Atlantic halibut Hippoglossus hippoglossus: a review. Dis. Aquat. Organisms48, 57–74. doi: 10.3354/dao048057 , PMID:
3
Bjornsson B. T. Halldorsson O. Haux C. Norberg B. Brown C. L. (1998). Photoperiod control of sexual maturation of the Atlantic halibut (Hippoglossus hippoglossus): plasma thyroid hormone and calcium levels. Aquaculture166, 117–140. doi: 10.1016/S0044-8486(98)00276-2
4
Blaxter J. H. S. Danielssen D. Moksness E. Oiestad V. (1983). Description of the early development of the halibut Hippoglossus-Hippoglossus and attempts to rear the larvae past 1st feeding. Mar. Biol.73, 99–107. doi: 10.1007/Bf00396290
5
Blindheim S. Nylund A. Watanabe K. Plarre H. Erstad B. Nylund S. (2015). A new aquareovirus causing high mortality in farmed Atlantic halibut fry in Norway. Arch. Virol.160, 91–102. doi: 10.1007/s00705-014-2235-8 , PMID:
6
Bobe J. Labbé C. (2010). Egg and sperm quality in fish. Gen. Comp. Endocrinol.165, 535–548. doi: 10.1016/j.ygcen.2009.02.011 , PMID:
7
Bromage N. Bruce M. Basavaraja N. Rana K. Shields R. Young C. et al . (1994). Egg quality determinants in finfish the role of overripening with special reference to the timing of stripping in the Atlantic halibut Hippoglossus hippoglossus. J. World Aquaculture Soc.25, 13–21. doi: 10.1111/j.1749-7345.1994.tb00799.x
8
Bromage N. Porter M. Randall C. (2001). The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture197, 63–98. doi: 10.1016/S0044-8486(01)00583-X
9
Brooks S. Tyler C. R. Sumpter J. P. (1997). Egg quality in fish: what makes a good egg? Rev. Fish Biol. Fisheries7, 387–416. doi: 10.1023/A:1018400130692
10
Brown N. (1998). Egg quality, triploidy induction and weaning of the Atlantic halibut Hippoglossus hippoglossus. (Stirling: University of Stirling).
11
Brown N. P. (2002). Flatfish farming systems in the Atlantic region. Rev. Fisheries Sci.10, 403–419. doi: 10.1080/20026491051712
12
Brown N. P. Shields R. J. Bromage N. R. (2006). The influence of water temperature on spawning patterns and egg quality in the Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture261, 993–1002. doi: 10.1016/j.aquaculture.2006.08.025
13
Cameron P. Berg J. Dethlefsen V. Von Westernhagen H. (1992). Developmental defects in pelagic embryos of several flatfish species in the southern North Sea. Netherlands. J. Sea Res.29, 239–256. doi: 10.1016/0077-7579(92)90024-9
14
Engelsen R. Asche F. Skjennum F. Adoff G. (2004). “New species in aquaculture: Some basic economic aspects,” in Culture of cold-water marine fish. Eds. MoksnessE.KjørsvikE.OlsenY. (Oxford: Blackwell Publishing), 487–515.
15
FAO (2023). Global aquaculture production 1950-2020 (FishStatJ). In: FAO Fisheries and Aquaculture Division (Rome: Food and Agriculture Organization of the United Nations (FAO)). Available online at: www.fao.org/fishery/statistics/software/fishstatj/en (Accessed June 17, 2024).
16
Fernández Míguez M. Presa P. Puvanendran V. Tveiten H. Hansen Ø.J. Pérez M. (2024). Gene Expression and Phenotypic Assessment of Egg Quality across Developmental Stages of Atlantic Cod throughout the Spawning Season. Int. J. Mol. Sci.25, 7488. doi: 10.3390/ijms25137488 , PMID:
17
Gallardo P. Bueno G. W. Araneda C. Benfey T. (2022). Status of Atlantic halibut (Hippoglossus hippoglossus) aquaculture production technology in Chile. Aquaculture Rep.22, 100958. doi: 10.1016/j.aqrep.2021.100958
18
Haug T. (1990). Biology of the Atlantic halibut, Hippoglossus hippoglossus (L. 1758). Adv. Mar. Biol.26, 1–70. doi: 10.1016/S0065-2881(08)60198-4
19
Holmefjord I. Gulbrandsen J. Lein I. Refstie T. Léger P. Huse I. et al . (1993). An intensive approach to Atlantic halibut fry production. J. World Aquaculture Soc.24, 275–284. doi: 10.1111/j.1749-7345.1993.tb00016.x
20
Kjørsvik E. (1990). The effects of different incubation conditions on the eggs of halibut, hippoglossus-hippoglossus (L). J. Fish Biol.37, 655–657. doi: 10.1111/j.1095-8649.1990.tb05900.x
21
Kjørsvik E. Hoehne-Reitan K. Reitan K. (2003). Egg and larval quality criteria as predictive measures for juvenile production in turbot (Scophthalmus maximus L.). Aquaculture227, 9–20. doi: 10.1016/S0044-8486(03)00492-7
22
Kjørsvik E. Holmefjord I. (1995). “Atlantic halibut (Hippoglossus hippoglossus) and cod (Gadus morhua),” in Broodstock management and egg and larval quality. Eds. BromageN. R.RobertsR. J. (Oxford: Blackwell Science), 169–196.
23
Kjørsvik E. Lønning S. (1983). Effects of egg quality on normal fertilization and early development of the cod, Gadus morhua L. J. Fish Biol.23, 1–12. doi: 10.1111/j.1095-8649.1983.tb02877.x
24
Kjørsvik E. Mangor-Jensen A. Holmefjord I. (1990). Egg quality in fishes. Adv. Mar. Biol.26, 71–113. doi: 10.1016/s0065-2881(08)60199-6
25
Kjørsvik E. Stene A. Lønning S. (1984). “Morphological, physiological and genetical studies of egg quality in cod (Gadus morhua L.),” in The propagation of cod Gadus morhua L.: an international symposium, Arendal, 14–17 June 1983 (Arendal: Havforskningsinstituttet), 67–86.
26
Kondo M. (2007). Bone morphogenetic proteins in the early development of zebrafish. FEBS J.274, 2960–2967. doi: 10.1111/j.1742-4658.2007.05838.x , PMID:
27
Lønning S. Kjørsvik E. Haug T. Gulliksen B. (1982). The early development of the halibut, Hippoglossus hippoglossus (L.), compared with other marine teleosts. Sarsia67, 85–91. doi: 10.1080/00364827.1982.10420534
28
Lu F.-I. Thisse C. Thisse B. (2011). Identification and mechanism of regulation of the zebrafish dorsal determinant. Proc. Natl. Acad. Sci.108, 15876–15880. doi: 10.1073/pnas.1106801108 , PMID:
29
Migaud H. Bell G. Cabrita E. McAndrew B. Davie A. Bobe J. et al . (2013). Gamete quality and broodstock management in temperate fish. Rev. Aquaculture5, S194–S223. doi: 10.1111/raq.12025
30
Mommens M. Fernandes J. M. Bizuayehu T. T. Bolla S. L. Johnston I. A. Babiak I. (2010). Maternal gene expression in Atlantic halibut (Hippoglossus hippoglossus L.) and its relation to egg quality. BMC Res. Notes3, 1–11. doi: 10.1186/1756-0500-3-138 , PMID:
31
Naess T. Harboe T. Mangor-Jensen A. Naas K. E. Norberg B. (1996). Successful first feeding of Atlantic halibut larvae from photoperiod-manipulated broodstock. Progressive Fish-Culturist58, 212–214. doi: 10.1577/1548-8640(1996)058<0212:Tnsffo>2.3.Co;2
32
Norberg B. Valkner V. Huse J. Karlsen I. Grung G. L. (1991). Ovulatory rhythms and egg viability in the atlantic halibut (Hippoglossus-hippoglossus). Aquaculture97, 365–371. doi: 10.1016/0044-8486(91)90328-5
33
Pittman K. Bergh Ø. Opstad I. Skiftesvik A. B. Skjolddal L. Strand H. (1990a). Development of eggs and yolk sac larvae of halibut (Hippoglossus hippoglossus L.). J. Appl. Ichthyol.6, 142–160. doi: 10.1111/j.1439-0426.1990.tb00573.x
34
Pittman K. Skiftesvik A. Berg L. (1990b). Morphological and behavioural development of halibut, Hippoglossus hippoglossus (L.) larvae. J. Fish Biol.37, 455–472. doi: 10.1111/j.1095-8649.1990.tb05876.x
35
Reading J. B. Andersen L. K. Ryu Y.-W. Mushirobira Y. Todo T. Hiramatsu N. (2018). Oogenesis and egg quality in finfish: yolk formation and other factors influencing female fertility. Fishes3, 45. doi: 10.3390/fishes3040045
36
Rollefsen G. (1934). The eggs and larvae of the halibut (Hippoglossus vulgaris). Det Kongelige Norske Videnskabers Selskab Forhandlinger7, 20–23.
37
RStudio Team (2022). RStudio: Integrated development for R (Boston, MA, USA: RStudio, PBC). Available online at: https://www.rstudio.com/ (Accessed June 17, 2023).
38
Schier A. F. Talbot W. S. (2003). Nodal signaling and the zebrafish organizer. Int. J. Dev. Biol.45, 289–297. doi: 10.1387/ijdb.11291859
39
Schier A. F. Talbot W. S. (2005). Molecular genetics of axis formation in zebrafish. Annu. Rev. Genet.39, 561–613. doi: 10.1146/annurev.genet.37.110801.143752 , PMID:
40
Shields R. Brown N. Bromage N. (1997). Blastomere morphology as a predictive measure of fish egg viability. Aquaculture155, 1–12. doi: 10.1016/S0044-8486(97)00105-1
41
Skaalsvik T. H. Bolla S. L. Thornqvist P.-O. Babiak I. (2015). Quantitative characteristics of Atlantic halibut (Hippoglossus hippoglossus L.) egg quality throughout the reproductive season. Theriogenology83, 38–47. doi: 10.1016/j.theriogenology.2014.07.043 , PMID:
42
Smith P. Bromage N. Shields R. Ford L. Gamble J. Gillespie M. et al . (1991). “Photoperiod controls spawning time in the atlantic halibut (Hippoglossus-hippoglossus, L),” in Proceedings of the Fourth International Symposium on the Reproductive Physiology of Fish. (Norwich, UK: University of East Anglia), 172.
43
Upton G. J. (1992). Fisher’s exact test. J. R. Stat. Society: Ser. A (Statistics Society)155, 395–402. doi: 10.2307/2982890
44
Yilmaz O. Jensen A. M. Harboe T. Mogster M. Jensen R. M. Mjaavatten O. et al . (2022). Quantitative proteome profiling reveals molecular hallmarks of egg quality in Atlantic halibut: impairments of transcription and protein folding impede protein and energy homeostasis during early development. BMC Genomics23, 635. doi: 10.1186/s12864-022-08859-0 , PMID:
Summary
Keywords
Atlantic halibut, Hippoglossus hippoglossus , egg quality, gastrulation, aquaculture, fertilization success, embryonic development
Citation
Niepagen N, Ferrer Vidal M, Berg L and Kjørsvik E (2025) Egg quality markers in domesticated broodstock of Atlantic halibut (Hippoglossus hippoglossus) with relevance for optimization of hatchery protocols. Front. Aquac. 4:1673217. doi: 10.3389/faquc.2025.1673217
Received
25 July 2025
Accepted
16 September 2025
Published
25 September 2025
Corrected
29 September 2025
Volume
4 - 2025
Edited by
Charles Weirich, NOAA National Sea Grant Office, United States
Reviewed by
Renzo Pepe-Victoriano, Arturo Prat University, Chile
Pablo Gallardo, University of Magallanes, Chile
Stefano Lancerotto, Hellenic Centre for Marine Research (HCMR), Greece
Updates
Copyright
© 2025 Niepagen, Ferrer Vidal, Berg and Kjørsvik.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nils Niepagen, nils.niepagen@ntnu.no
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.