- 1Department of Radiology, University of Calgary, Calgary, AB, Canada
- 2Biomedical Engineering Graduate Program, University of Calgary, Calgary, AB, Canada
- 3Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada
- 4Alberta Children’s Hospital Research Institute, University of Calgary, Calgary, AB, Canada
- 5Department of Radiology, University of Michigan, Ann Arbor, United States
- 6Department of Clinical Neurosciences, University of Calgary, Calgary, AB, Canada
The retinal age gap, defined as the difference between the predicted retinal age and chronological age, is an emerging biomarker for many eye conditions and even non-ocular diseases. Machine learning (ML) models are commonly used for retinal age prediction. However, biases in ML models may lead to unfair predictions for some demographic groups, potentially exacerbating health disparities. This retrospective cross-sectional study evaluated demographic biases related to sex and ethnicity in retinal age prediction models using retinal imaging data (color fundus photography [CFP], optical coherence tomography [OCT], and combined CFP + OCT) from 9,668 healthy individuals (mean age 56.8 years; 52% female) in the UK Biobank. The RETFound foundation model was fine-tuned to predict retinal age, and bias was assessed by comparing mean absolute error (MAE) and retinal age gaps across demographic groups. The combined CFP + OCT model achieved the lowest MAE (3.01 years), outperforming CFP-only (3.40 years) and OCT-only (4.37 years) models. Significant sex differences were observed only in the CFP model (p < 0.001), while significant ethnicity differences appeared only in the OCT model (p < 0.001). No significant sex/ethnicity differences were observed in the combined model. These results demonstrate that retinal age prediction models can exhibit biases, and that these biases, along with model accuracy, are influenced by the choice of imaging modality (CFP, OCT, or combined). Identifying and addressing sources of bias is essential for safe and reliable clinical implementation. Our results emphasize the importance of comprehensive bias assessments and prospective validation, ensuring that advances in machine learning and artificial intelligence benefit all patient populations.
Introduction
Retinal imaging has become a valuable, non-invasive data source for studying aging and ocular and systemic health (Li and Lin, 2024). Due to its shared embryological origins with the central nervous system, the retina can reflect vascular and neurodegenerative changes that are otherwise difficult to assess. High-resolution retinal imaging enables the direct visualization of microvasculature and neural tissue, offering insights into ocular, cardiovascular, and neurological health. Recent advances in machine learning (ML) have enabled the estimation of biological retinal age from retinal images, leading to the concept of the retinal age gap, which denotes the difference between predicted retinal age and true chronological age (Nielsen et al., 2025a; Nielsen et al., 2025b). Recent studies suggest that increased retinal age gaps may indicate elevated risk for stroke, Parkinson’s disease, and mortality, supporting a shift toward biological age markers as more accurate indicators of health than chronological age alone (Grimbly et al., 2024).
Despite these promising results, concerns are growing regarding the fairness and generalizability of ML tools across diverse populations (Norori et al., 2021). For example, if an ML model underestimates retinal age in certain demographic groups, clinicians may misjudge disease risk, leading to potentially missed diagnoses and exacerbated health disparities. Previous literature has shown how biases from imbalanced demographics, spurious correlations, or mismatches between training and deployment populations can reduce model reliability (Jacoba et al., 2023). As ML becomes more common in ophthalmology, understanding how such biases can influence predictive performance is critical. For example, Burlina et al. (2021) showed that imbalanced training sets can reduce diagnostic consistency in diabetic retinopathy detection, while Lin et al. (2023) found that ML models may misclassify glaucoma in underrepresented groups. However, it remains unclear whether and to what extent retinal age prediction models may be affected by biases leading to unfair biological age predictions for different demographic groups.
In this work, we exemplarily investigate bias differences in the context of ML models trained on two commonly used retinal imaging modalities: color fundus photography (CFP) and optical coherence tomography (OCT). More specifically, we evaluate how RETFound, a widely used retinal image analysis model performs for retinal age prediction across these modalities and examine whether differences in model prediction errors exist within two demographic factors, namely sex and ethnicity.
Materials and methods
Dataset
We utilized data from the UK Biobank, a large population-based repository of participants who underwent detailed health assessments, including retinal imaging (Bycroft et al., 2018). Our final cohort comprised 9,668 participants, selected through a multi-step filtering process designed to ensure data quality and create a healthy cohort for model training and evaluation. First, we performed rigorous quality control on both CFP and OCT images to remove scans with motion artifacts or any other significant acquisition issues. CFP image quality was assessed using a deep learning-based method proposed by Fu et al. (2019), while OCT images were evaluated using the image quality score provided by the Topcon Advanced Boundary Segmentation (TABS) algorithm, as described by Chen et al. (2024). A primary inclusion criterion was the availability of a matched pair of high-quality CFP and OCT images for each participant. Following this, we excluded participants with any self-reported medical conditions, based on the criteria developed by Zhu et al. (2023). Finally, only images from the right eye of each unique participant were included in the final dataset. This approach was chosen to maximize the number of individuals in our cohort, as requiring high-quality images from both eyes would have significantly reduced the sample size. Additionally, using only one eye per participant avoids the need for statistical correction for within-subject inter-eye correlation. In the next step, self-reported ethnicities with less than 200 participants were excluded to ensure robust statistical analyses. The dataset was split into training (50%), validation (10%), and test (40%) sets, stratified by age, sex, and self-reported ethnicity to ensure balanced representation. Demographics for each split are shown in Table 1.
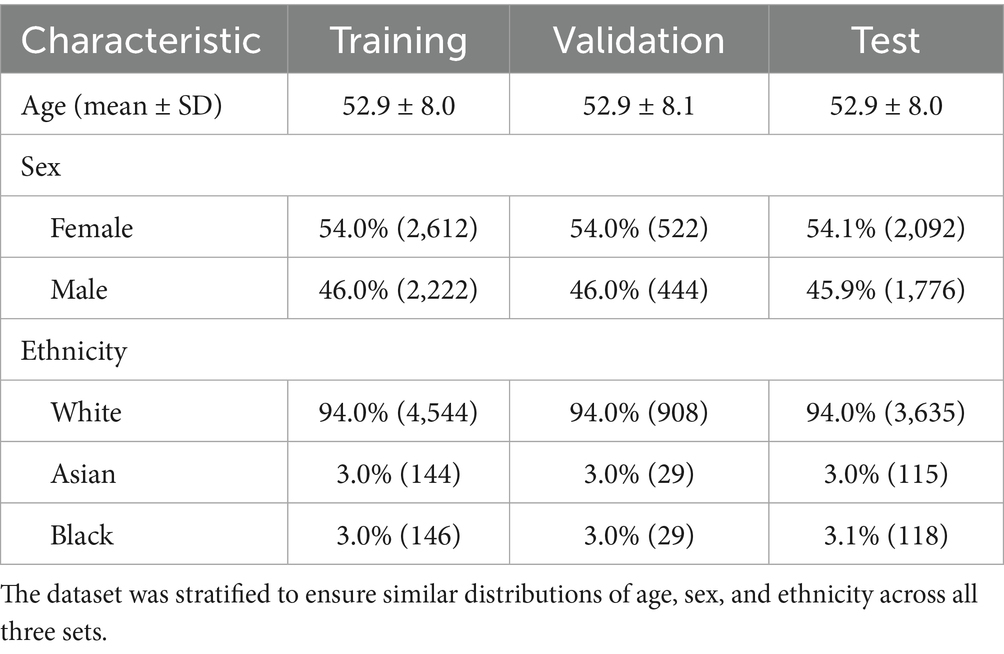
Table 1. Demographic information for participants included in the training, validation, and test sets.
Model architecture and training
We employed the publicly available RETFound foundation model, which was previously successful in many retinal image analysis tasks (Zhou et al., 2023). Model weights were fine-tuned for retinal age prediction based on the RETFound authors’ guidelines across three configurations: (1) CFP only, (2) OCT only, and (3) combined CFP + OCT. The combined approach used late-fusion, concatenating single-modality representations before the final layer. Fine-tuning was used to minimize mean squared error loss between predicted and chronological age, using the Adam optimizer with early stopping based on validation loss. Training was conducted in PyTorch 1.13.1 on an NVIDIA RTX 3090 GPU.
Statistical analysis
Retinal age prediction performance was evaluated using mean absolute error (MAE) between predicted biological age and chronological age. To assess demographic bias, we adapted the approach by Piçarra and Glocker (2023), previously applied to assess sex and ethnicity bias in brain age prediction. Kruskal-Wallis tests were used to compare retinal age gaps across sex and ethnicity groups. To correct for multiple comparisons (three model types, two subgroup categories), we applied a Bonferroni-adjusted significance threshold of .
Results
The combined CFP + OCT model yielded the lowest overall MAE (3.01 years), outperforming the CFP-only (3.40) and OCT-only (4.37) models (Table 2). Sex-based performance varied: the combined model showed minimal difference (females: 2.98; males: 3.04), CFP slightly favored males (3.34 vs. 3.45), and OCT favored females (4.11 vs. 4.67). The combined model also achieved the lowest MAE across ethnic groups: White (3.01), Asian (2.75), and Black (3.16). Kruskal–Wallis tests revealed significant sex bias in the CFP model (p < 0.001), but not in the OCT (p = 0.798) or combined models (p = 0.019; not significant after correction). Ethnicity bias was significant in OCT (p < 0.001), but not in the CFP (p = 0.032) or combined models (p = 0.131).
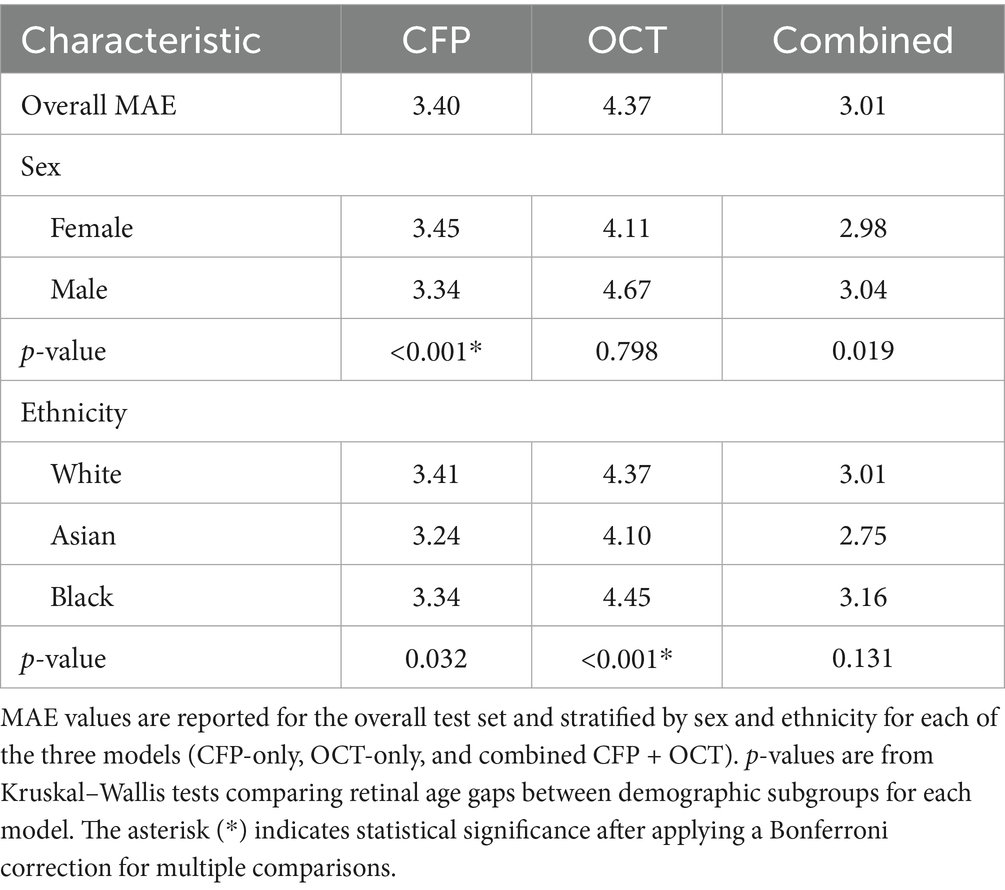
Table 2. Model performance, measured by mean absolute error (MAE) in years, and bias analysis results.
Discussion
The results of this study highlight that finetuning ML models for retinal age prediction can result in significant performance differences between sex and ethnicity groups. This aligns with prior work in ophthalmic imaging artificial intelligence, where models for classifying conditions like age-related macular degeneration, diabetic retinopathy, and glaucoma have shown performance disparities between demographic groups (Luo et al., 2024). These findings highlight the importance of evaluating and understanding biases before clinical deployment of ML models. An unrecognized bias could have downstream effects on disease detection and patient care. Thus, thorough bias analyses and prospective validation across diverse populations are of paramount importance (Krause, 2024).
Our findings further demonstrate that combining multiple imaging modalities may improve predictive performance while helping to reduce bias. The CFP + OCT model achieved the lowest MAE, indicating superior accuracy, and showed no significant differences between sex or ethnicity groups. A possible explanation for these results is that CFP and OCT introduce different sources of bias, where CFP was significantly associated with sex-related bias, while OCT showed significant bias related to ethnicity. We hypothesize that these modality-specific biases may reflect true biological differences in retinal aging across demographic groups, which are captured uniquely by each imaging modality. For example, a recent study by Böttger et al. suggested that sex-specific retinal vascular traits can be detected in CFP images (Böttger et al., 2025). Furthermore, Varma et al. (1994) found that males have 2–3% larger optic discs than females, measurable via CFP. Similarly, Wagner-Schuman et al. (2011) and Poon et al. (2018) reported ethnicity-related differences in central retinal thickness detectable using OCT. Therefore, by leveraging data from both modalities, the combined CFP + OCT model may gain a more comprehensive bias-free understanding of retinal aging, overcoming the biases of the single modality approaches. However, further research is necessary to explore multimodal strategies as a means of enhancing fairness in retinal ML models and to better understand the origins of these biases. For instance, it may be argued that the predictive power of our fine-tuned models may rely heavily on the foundational feature representations learned by the RETFound model during its extensive pretraining. It is plausible that the pretraining dataset enabled RETFound to learn feature representations that are more robust or discriminative for predicting age in certain groups, contributing to the observed performance differences. Future work could incorporate visual interpretability methods, such as saliency maps, to identify the specific retinal features driving these predictions and better understand the anatomical basis of model bias (Stanley et al., 2022).
While this study offers valuable insights into bias in retinal age prediction models, certain limitations warrant further investigation. Notably, the UK Biobank predominantly consists of Caucasian participants, with a limited representation of other ethnic groups. Additionally, our definition of a healthy cohort relies on the absence of self-reported disease. Although this aligns with previous UK Biobank retinal age studies (Zhu et al., 2023; Zhang et al., 2023; Hu et al., 2022), this approach may unintentionally include participants with undiagnosed or subclinical conditions that could affect the performance of the retinal age prediction models. Furthermore, our sample size was also constrained by the availability of matched, high-quality CFP and OCT images from healthy participants within a single visit. Moreover, this study relied on self-reported ethnicity, which is an interpretable but broad categorization. Future research could benefit from correlating prediction errors with genetic principal components to uncover ancestry-related associations that might be overlooked by discrete categories. Additionally, exploring socioeconomic factors as potential biases in retinal age prediction models would be beneficial for future studies. Finally, while this work offers valuable insights based on a large UK-based population cohort, external validation is essential. Broadening the analysis to encompass more diverse datasets and additional machine learning architectures will further strengthen the generalizability of the results.
Conclusion
This work demonstrates that imaging modality selection (CFP vs. OCT vs. combined) affects both performance and bias profiles of retinal age prediction models. As the retinal age gap emerges as a promising biomarker for disease detection, understanding and mitigating bias sources is crucial for safe, reliable implementation. Our findings underscore the need for thorough bias analyses and prospective evaluation to ensure ophthalmic artificial intelligence advancements benefit all patient populations.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: accessing the UK Biobank requires registration. Requests to access these datasets should be directed to https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access.
Ethics statement
The studies involving humans were approved by UK Biobank has approval from the North West Multi-centre Research Ethics Committee (MREC) as a Research Tissue Bank (RTB) approval. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CN: Project administration, Visualization, Validation, Data curation, Formal analysis, Methodology, Writing – review & editing, Software, Conceptualization, Writing – original draft, Investigation. ES: Validation, Writing – review & editing, Methodology. MW: Validation, Methodology, Conceptualization, Writing – review & editing. NF: Methodology, Validation, Funding acquisition, Supervision, Conceptualization, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Lions Sight Centre and the Canada Research Chairs Program.
Acknowledgments
We are very grateful to all the individuals that took part in the UK Biobank study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Böttger, L., Bontempi, D., Trofimova, O., Beyeler, M. J., Bors, S., Iuliani, I., et al. (2025). Sex-specific disease association and genetic architecture of retinal vascular traits. bioRxiv; doi: 10.1101/2025.07.16.665150
Burlina, P., Joshi, N., Paul, W., Pacheco, K. D., and Bressler, N. M. (2021). Addressing artificial intelligence Bias in retinal diagnostics. Transl. Vis. Sci. Technol. 10:13. doi: 10.1167/tvst.10.2.13
Bycroft, C., Freeman, C., Petkova, D., Band, G., Elliott, L. T., Sharp, K., et al. (2018). The UK biobank resource with deep phenotyping and genomic data. Nature 562, 203–209. doi: 10.1038/s41586-018-0579-z
Chen, R., Zhang, S., Peng, G., Meng, W., Borchert, G., Wang, W., et al. (2024). Deep neural network-estimated age using optical coherence tomography predicts mortality. GeroScience 46, 1703–1711. doi: 10.1007/s11357-023-00920-4
Fu, H., Wang, B., Shen, J., Cui, S., Xu, Y., Liu, J., et al.“Evaluation of retinal image quality assessment networks in different color-spaces” in International conference on medical image computing and computer-assisted intervention 2019 Oct 10. eds. D. Shen, T. Peters, L. H. Staib, C. Essert, S. Zhou, and P.-T. Yap, et al. (Cham: Springer International Publishing), 48–56. doi: 10.1007/978-3-030-32239-7_6
Grimbly, M. J., Koopowitz, S. M., Chen, R., Sun, Z., Foster, P. J., He, M., et al. (2024). Estimating biological age from retinal imaging: a scoping review. BMJ Open Ophthalmol. 9:e001794. doi: 10.1136/bmjophth-2024-001794
Hu, W., Wang, W., Wang, Y., Chen, Y., Shang, X., Liao, H., et al. (2022). Retinal age gap as a predictive biomarker of future risk of Parkinson’s disease. Age Ageing 51:afac062. doi: 10.1093/ageing/afac062
Jacoba, C. M. P., Celi, L. A., Lorch, A. C., Fickweiler, W., Sobrin, L., Gichoya, J. W., et al. (2023). Bias and non-diversity of big data in artificial intelligence: focus on retinal diseases: “Massachusetts eye and ear special issue”. Semin. Ophthalmol. 38, 433–441. doi: 10.1080/08820538.2023.2168486
Krause, D. (2024). Addressing the challenges of auditing and testing for AI Bias: a comparative analysis of regulatory frameworks. SSRN Working Paper (posted 28 Jan 2025; date written 10 Dec 2024). doi: 10.2139/ssrn.5050631
Li, R., and Lin, H. (2024). A retinal biomarker of biological age based on composite clinical phenotypic information. Lancet Healthy Longev. 5:100603. doi: 10.1016/S2666-7568(24)00109-0
Lin, M., Xiao, Y., Hou, B., Wanyan, T., Sharma, M. M., Wang, Z., et al. (2023). Evaluate underdiagnosis and overdiagnosis bias of deep learning model on primary open-angle glaucoma diagnosis in under-served populations. AMIA Jt. Summits Transl. Sci. Proc. AMIA Jt. Summits Transl. Sci. 2023, 370–377.
Luo, Y., Khan, M. O., Tian, Y., Shi, M., Dou, Z., Elze, T., et al. (2024). FairVision: equitable deep learning for eye disease screening via fair identity scaling. arXiv (v3, 12 Apr 2024). doi: 10.48550/arXiv.2310.02492
Nielsen, C., Wilms, M., and Forkert, N. D. (2025a). The retinal age gap: an affordable and highly accessible biomarker for population-wide disease screening across the globe. Proc. R. Soc. B Biol. Sci. 292:20242233. doi: 10.1098/rspb.2024.2233
Nielsen, C., Wilms, M., and Forkert, N. D. (2025b). A novel foundation model-based framework for multimodal retinal age prediction. IEEE J. Transl. Eng. Health Med. 13, 299–309. doi: 10.1109/JTEHM.2025.3576596
Norori, N., Hu, Q., Aellen, F. M., Faraci, F. D., and Tzovara, A. (2021). Addressing bias in big data and AI for health care: a call for open science. Patterns 2:100347. doi: 10.1016/j.patter.2021.100347
Piçarra, C., and Glocker, B. (2023). “Analysing race and sex bias in brain age prediction” in Clinical image-based procedures, fairness of AI in medical imaging, and ethical and philosophical issues in medical imaging. eds. S. Wesarg, E. P. Antón, J. S. Baxter, M. Erdt, K. Drechsler, and C. O. Laura, et al. (Cham: Springer Nature Switzerland), 194–204.
Poon, L. Y.-C., Antar, H., Tsikata, E., Guo, R., Papadogeorgou, G., Freeman, M., et al. (2018). Effects of age, race, and ethnicity on the optic nerve and peripapillary region using spectral-domain OCT 3D volume scans. Transl. Vis. Sci. Technol. 7:12. doi: 10.1167/tvst.7.6.12
Stanley, E. A. M., Wilms, M., Mouches, P., and Forkert, N. D. (2022). Fairness-related performance and explainability effects in deep learning models for brain image analysis. J. Med. Imaging 9:061102. doi: 10.1117/1.JMI.9.6.061102
Varma, R., Tielsch, J. M., Quigley, H. A., Hilton, S. C., Katz, J., Spaeth, G. L., et al. (1994). Race-, age-, gender-, and refractive error—related differences in the normal optic disc. Arch. Ophthalmol. 112, 1068–1076. doi: 10.1001/archopht.1994.01090200074026
Wagner-Schuman, M., Dubis, A. M., Nordgren, R. N., Lei, Y., Odell, D., Chiao, H., et al. (2011). Race- and sex-related differences in retinal thickness and foveal pit morphology. Invest. Ophthalmol. Vis. Sci. 52, 625–634. doi: 10.1167/iovs.10-5886
Zhang, S., Chen, R., Wang, Y., Hu, W., Kiburg, K. V., Zhang, J., et al. (2023). Association of retinal age gap and Risk of kidney failure: a UK biobank study. Am. J. Kidney Dis. 81, 537–544.e1. doi: 10.1053/j.ajkd.2022.09.018
Zhou, Y., Chia, M. A., Wagner, S. K., Ayhan, M. S., Williamson, D. J., Struyven, R. R., et al. (2023). A foundation model for generalizable disease detection from retinal images. Nature 622, 156–163. doi: 10.1038/s41586-023-06555-x
Keywords: retinal age prediction, machine learning, bias, multimodal imaging, retinal imaging
Citation: Nielsen C, Stanley EAM, Wilms M and Forkert ND (2025) Assessment of demographic bias in retinal age prediction machine learning models. Front. Artif. Intell. 8:1653153. doi: 10.3389/frai.2025.1653153
Edited by:
Alessandro Bria, University of Cassino, ItalyReviewed by:
Volha V. Malechka, Harvard Medical School, United StatesMichael J. Beyeler, Université de Lausanne, Switzerland
Zhaohua Yu, Uppsala University, Sweden
Copyright © 2025 Nielsen, Stanley, Wilms and Forkert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher Nielsen, Y3NuaWVsc2VAdWNhbGdhcnkuY2E=
 Christopher Nielsen
Christopher Nielsen Emma A. M. Stanley
Emma A. M. Stanley Matthias Wilms
Matthias Wilms Nils D. Forkert
Nils D. Forkert