- 1Merck & Co., Inc., Rahway, NJ, United States
- 2College of Pharmacy, University of Rhode Island, Kingston, RI, United States
- 3IMO Health, Rosemont, IL, United States
Background: Systematic literature reviews (SLRs) are critical to health research and decision-making but are often time- and labor-intensive. Artificial intelligence (AI) tools like large language models (LLMs) provide a promising way to automate these processes.
Methods: We conducted a systematic literature review on the cost-effectiveness of adult pneumococcal vaccination and prospectively assessed the performance of our AI-assisted review platform, Intelligent Systematic Literature Review (ISLaR) 2.0, compared to expert researchers.
Results: ISLaR demonstrated high accuracy (0.87 full-text screening; 0.86 data extraction), precision (0.88; 0.86), and sensitivity (0.91; 0.98) in article screening and data extraction tasks, but lower specificity (0.79; 0.42), especially when extracting data from tables. The platform reduced abstract and full-text screening time by over 90% compared to human reviewers.
Conclusion: The platform has strong potential to reduce reviewer workload but requires further development.
1 Introduction
Systematic literature reviews (SLRs) are recognized as the most rigorous form of evidence review and synthesis (Aromataris and Pearson, 2014; Clarke and Chalmers, 2018; Grant and Booth, 2009; Munn et al., 2018). In the health economics field, SLRs on the relative cost-effectiveness of different interventions are increasingly used in health care system decision-making (Anderson, 2010; Jacobsen et al., 2020; Luhnen et al., 2018; Mandrik et al., 2021). However, SLRs require substantial investment of time and resources (Allen and Olkin, 1999; Borah et al., 2017; Bullers et al., 2018; Michelson and Reuter, 2019; Shemilt et al., 2016). Best-practice guidelines strongly recommend that SLR tasks be independently executed by at least 2 expert reviewers, which reduces the error rate and improves quality but also increases the overall workload (Higgins et al., 2024; Gartlehner et al., 2020; Page et al., 2021; Shemilt et al., 2016). These time- and labor-intensive processes can also introduce reviewer errors (Clark et al., 2021; Wang et al., 2020), as well as long gaps between SLR initiation and final publication (Beller et al., 2013; Borah et al., 2017). Long production times can in turn affect the longevity of the findings: an estimated 7% of SLRs are already out of date (defined as availability of new findings that would affect the conclusions of the synthesis) at the time of publication (Shojania et al., 2007). “Living SLRs,” a dynamic review format that allows ongoing online updates, have been proposed as a solution to this problem (Elliott et al., 2014; Wijkstra et al., 2021), but still require labor-intensive ongoing screening of newly published articles.
Many of the tasks involved in SLRs are amenable to automation using artificial intelligence (AI) (Beller et al., 2018; Bolaños et al., 2024; Michelson and Reuter, 2019; Shemilt et al., 2016; Tsertsvadze et al., 2015). Until recently, most work in this field used natural language processing (NLP) and similar text mining approaches (Blaizot et al., 2022; Bolaños et al., 2024; Cowie et al., 2022; de la Torre-López et al., 2023; van Dinter et al., 2021). Within the last 2 years, however, there has been extensive interest in the use of generative large language models (LLMs), which are more versatile and accessible than previous generations of AI tools (Lu et al., 2024; O’Connor et al., 2024; Sallam, 2023). Applications of LLMs in SLRs include construction of literature search terms (Alshami et al., 2023; Wang Z. et al., 2025; Wang et al., 2023), article screening (Akinseloyin et al., 2024; Alshami et al., 2023; Guo et al., 2024; Khraisha et al., 2024; Kohandel Gargari et al., 2024; Landschaft et al., 2024; Li et al., 2024; Matsui et al., 2024; Syriani et al., 2023; Tran et al., 2024; Wang Z. et al., 2025; Wang S. et al., 2024), data extraction (Alshami et al., 2023; Dunn et al., 2022; Gartlehner et al., 2024; Ghosh et al., 2024; Khraisha et al., 2024; Landschaft et al., 2024; Wang Z. et al., 2025), and article content synthesis/analysis (Alshami et al., 2023; Wang Z. et al., 2025).
Effective use of LLMs requires careful construction and iteration of the text “prompts” used to instruct the model. We recently developed a user-friendly LLM-based SLR platform, Intelligent Systematic Literature Review (ISLaR) 2.0, for semi-autonomous “human-in-the-loop” abstract and full-text article screening and data extraction (Wang et al., 2025a). Briefly, ISLaR 2.0 is based on ChatGPT4-Turbo (OpenAI, 2024) and incorporates an interface designed to help researchers who are experts in the topic of the SLR develop an effective LLM prompt by entering information such as the purpose of the SLR, article inclusion/exclusion criteria, and examples of relevant text and data elements (Wang et al., 2025a; Wang et al., 2025b). The platform automates the entire SLR process, from retrieving eligible articles in PubMed and Embrace to screening abstracts and full texts and extracting data from included studies. A key feature of ISLaR 2.0 is its human-in-the-loop design, which allows researchers to review screening results and iteratively refine their criteria as needed. Unlike many existing LLM-based tools that focus on a single task (e.g., search or extraction), ISLaR 2.0 integrates the full SLR workflow into one platform. We have performed initial tests of ISLaR’s performance by comparing its article selection and data extraction outputs to those of expert human reviewers in simulated SLR tasks (Wang et al., 2025a; Wang et al., 2025b). In these initial studies, our platform performed comparably to other published automated tools in terms of accuracy (abstract screening, 73.8–86.0%; full-text article screening, 78.3–85.7%; extraction of data from abstracts, 74.8–96.3%), and better than most other tools in terms of consistently high sensitivity (90.1–95.7%, 75.0–91.7%, and 90.3–97.6%, respectively) (Wang et al., 2025b).
This study aimed to prospectively assess the performance of ISLaR 2.0 compared to expert human reviewers in a full SLR, to identify areas where ISLaR can supplement human reviewers as well as those where human reviewers still outperform AI. In contrast to our previous work, which involved screening a subset of curated articles and extracting a limited number of data elements from study abstracts, this evaluation involved screening the full set of articles retrieved by the literature search and comprehensive extraction of relevant data from the full texts of included studies. The SLR’s research question focused on the cost-effectiveness of pneumococcal vaccination to prevent pneumococcal disease (PD) among adult’s ≥18 years of age, and specifically on studies that provide direct comparisons of vaccination costs and benefits. A cost-effectiveness analysis was chosen for this case study as SLRs of this kind involve unique challenges, including heterogeneity in study design, interventions, populations, and settings (Anderson, 2010; Husereau et al., 2022; Jacobsen et al., 2020; Mandrik et al., 2021). Our prospective case-study SLR thus required extraction of complex comparative outcomes in the correct context and permitted comprehensive comparison of AI and human reviewer decisions, providing a rigorous test of our platform’s performance and an important contribution to the literature on the automation of SLRs in the field of health economics.
2 Materials and methods
2.1 Search strategy, study selection criteria, and data extraction fields
The SLR was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 and was pre-registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42024562351) (Cassell et al., 2024).
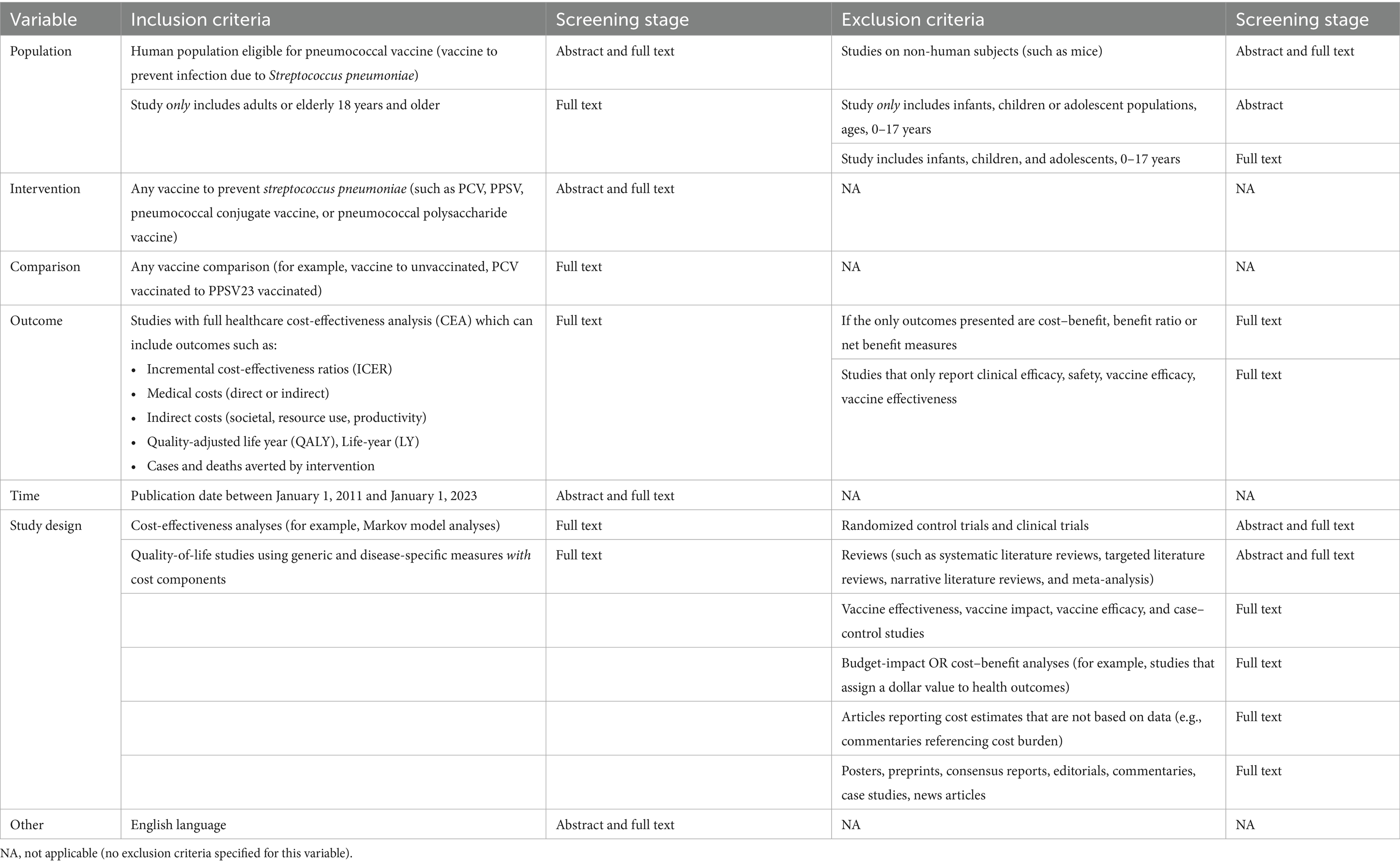
Search strings were manually developed for the PubMed and EMBASE literature databases to identify studies potentially related to the cost-effectiveness of pneumococcal vaccination among adult’s ≥18 years of age (Supplementary Table 1). Study selection was then based on the Population, Intervention, Comparison, Outcome, Time, and Study design (PICOTS) criteria listed in Table 1. We included peer-reviewed studies published in English between January 1, 2011 and January 1, 2023 that conducted cost-effectiveness analyses for any pneumococcal vaccine, compared to no vaccination or any standard-of-care vaccination, among adult’s ≥18 years of age. We excluded studies that reported only clinical outcomes with no cost component, or that reported cost–benefit, benefit ratio, or net benefit measures as the only health economic outcomes. Randomized controlled trials were also excluded, as were reviews, posters, published conference abstracts, preprints, and other non-primary and/or non-peer-reviewed publication types. Different criteria were applied at the abstract and full-text screening stages to emphasize sensitivity at the abstract screening stage and then apply more stringent exclusion criteria to the full texts. This approach is consistent with guidelines created by the Professional Society for Health Economics and Outcomes Research (ISPOR) for the conduct of SLRs that focus on cost-effectiveness outcomes (Mandrik et al., 2021; Wang et al., 2025a; Wang et al., 2025b). For example, the age-based population exclusion criterion applied at the abstract stage was designed to discard studies that included only populations <18 years of age; at the full-text screening stage, we discarded studies that included any population <18 years of age.
The selection of relevant data elements for extraction was guided by the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 statement (Husereau et al., 2022). The data extracted from full-text articles included study design parameters and outcomes, categorized into 23 text-based fields (i.e., data expected to be found in the text of the publication, such as model type and study limitations) and 18 table-based fields [i.e., data expected to be found in the study’s tables, such as quality-adjusted life years (QALYs) and incremental cost-effectiveness ratios (ICERs); Supplementary Table 2]. Extracted data were classified as follows:
• Element: data field of interest, such as “model type”, “number of deaths”, or “conclusion”.
• Value: exact value of each element (e.g., “1 year” for the “model cycle” element).
• Study cohort: study subpopulation specific to a given element and its value (e.g., individuals receiving study vaccine versus individuals receiving comparator vaccine, or adults <65 years of age versus adults ≥65 years of age; left blank for elements and values that applied to the entire study, such as model type).
For text-based values, we also extracted the corresponding text spans from full-text articles; i.e., direct quotations of the section of text within the full-text publication from which each element and/or its value was identified. This quoted text span was used to identify potential explanations for errors made by ISLaR. Text spans were not available for data extracted by ISLaR from study tables; any discrepancies in table-based data elements were therefore resolved manually by the human reviewer team.
No assessment of quality or bias, meta-analysis, or other detailed content analysis of the included studies was performed.
2.2 ISLaR prompt development
ISLaR 2.0 is an LLM-based tool designed by IMO Health, Rosemont, IL, US, in collaboration with the study’s authors, to conduct SLRs (Wang et al., 2025a). The tool is based on ChatGPT4-Turbo (GPT-4-turbo-2024-04-09) (OpenAI, 2024).
The text prompt included the full set of instructions for conducting the SLR, and a zero-shot strategy was employed (Supplementary Table 3). The prompt was constructed based on information on the SLR’s study PICOTS (Population, Intervention, Comparator, Outcome, Time, Study design) inclusion and exclusion criteria and data extraction framework, provided by authors who are experts in PD and pneumococcal vaccination via ISLaR’s semi-structured user interface (Table 1). The prompt also included background information such as definitions of pneumococcal vaccination, relevant study types, PD outcomes, vaccines, and other study variables. In addition, the subject matter expert authors provided examples of study text spans for each data field of interest, which were included to improve ISLaR’s ability to recognize and extract relevant data (Supplementary Table 2). The prompt based on this information was developed iteratively (2 versions) using a human-in-the-loop approach. After initial assessment of the screening decisions made by ISLaR using the first version of the prompt, 2 modifications were made. The first version of the population inclusion/exclusion criterion prompt stated “Exclude studies if they include infants, children, or adolescent populations, ages 0–17 years”; however, ISLaR failed to accurately apply this criterion, and so the prompt was updated in the second version to “Make sure to exclude the article if it involves any infants or children or adolescents or any participants below 18 years of age”. To avoid the conflations of non-Anglophone study locations with non-English-language publications that we observed with the first version of the prompt, the English language criterion was also updated, from “English language only” to “Studies in English language only”; in addition, the country acronyms that were originally included in the background knowledge prompt were removed, as they may have contributed to some of these errors. All study results reported below reflect the use of the second and final iteration of the prompt.
2.3 Study selection
The human reviewer team searched PubMed and EMBASE manually and entered the titles and abstracts of all studies into Microsoft Excel. A single reviewer identified and removed duplicates based on PubMed ID (PMID), or title and first author for studies not indexed in PubMed. Three reviewers then independently screened the abstracts of the remaining unique studies, applying the subset of PICOTS criteria that were assessed during the abstract screening stage and recording all reasons for exclusion. Any discrepancies between reviewers were resolved following independent review by an additional researcher. The full texts of the studies that passed the abstract screening (excluding any supplementary materials, which could not be assessed by ISLaR) were then independently screened by two reviewers using the subset of PICOTS criteria that were assessed at this stage; again, reasons for study exclusion were recorded. Any discrepancies between these reviewers were resolved following secondary review by two independent researchers. All study team members manually recorded the time taken to complete each screening task.
ISLaR searched PubMed using public application programming interfaces (APIs)—specifically, “E-utilities” for the abstract search and PubMed Central APIs for full text retrieval (National Library of Medicine National Center for Biotechnology Information, n.d.)—and EMBASE using the same standard search interface that was used by the human reviewers. The results were reduplicated based on PMID when available, or digital object identifier (DOI) for articles without a PMID. At the abstract screening stage, ISLaR assessed the study’s title and abstract. At the full-text screening and data extraction stages, ISLaR assessed the entire text of the published study, excluding any supplementary material. For studies where the full text was not available on the journal’s website, Amazon Textract was used to convert manually downloaded study PDF files to text (Amazon Web Services, Inc, n.d.). At both screening stages, ISLaR categorized each study as relevant (included) or irrelevant (excluded) and recorded the reason(s) for exclusion of each study. For this study, ISLaR was run fully autonomously, without any human-in-the-loop intervention.
2.4 Data extraction
Four human reviewers independently extracted relevant data from each remaining study into a standard Excel template containing the data fields listed in Supplementary Table 2. Any discrepancies were resolved following secondary review by an additional researcher. For each data element of each included study (e.g., number of deaths due to a given PD), the reviewers extracted the applicable study cohort (e.g., subgroup of the overall study cohort stratified by age or other characteristic, such as “adults 18–64 years of age”) and value (e.g., “1,516 deaths”), and, if the data were extracted from text rather than a table, the relevant text span from the study (e.g., “1,516 deaths due to PD were recorded among adults 18–64 years of age”).
ISLaR also extracted all available data elements and their corresponding value and study cohort, as well as the applicable text span from the study. Missing values were left blank by both the human reviewer team and ISLaR. All extracted data and metadata (e.g., reasons for study exclusion) were exported from ISLaR’s user interface into Excel for analysis. As with screening, data extraction was performed entirely by ISLaR. A third human reviewer then compared the values extracted by ISLaR and by the human reviewers with the values reported in the full texts and made the final decision on whether each value was correct.
2.5 Comparative analysis
Study selection performance was assessed separately for the abstract screening and full-text screening stages of the process, with the human reviewers’ selections considered the gold standard. Human reviewers included subject matter experts in the field of pneumococcal vaccine epidemiology and economic evaluation. For analysis of screening results, each study was categorized as a true positive (TP; study included by human reviewers and ISLaR), true negative (TN; study excluded by human reviewers and ISLaR), false positive (FP; study excluded by human reviewers but included by ISLaR), or false negative (FN; study included by human reviewers but excluded by ISLaR). For the assessment of ISLaR’s data extraction performance, we randomly selected 21 studies (33% of the original TN set of studies, before manual refinement) that were classified as TPs at the full-text screening stage. The data extracted by ISLaR from these 21 studies were compared to the values present in the full texts of the published studies, which were considered the gold standard. Each ISLaR-extracted value was assessed as a TP (ISLaR extracted the correct value from the text), TN (ISLaR output “not applicable” (“NA”) or blank when a value was not present in the text), FP (ISLaR provided an incorrect value, or provided any value when a value was not present in the text), or FN (ISLaR output “NA” or left the field blank for a value that was present in the text).
For each study selection and data extraction performance comparison, the TP/TN/FP/FN categories were used to assess ISLaR’s performance via the following metrics:
• Accuracy:
• Precision/positive predictive value (PPV):
• Recall/sensitivity:
• Specificity:
• F1 value:
• F2 value:
• Matthew’s correlation coefficient (MCC):
• Work saved over sampling at 95% recall (WSS@95%):
The nature of each discrepancy was also noted (for example, which of the PICOTS criteria was/were misclassified for each FN or FP study).
ISLaR-extracted data were also compared to the corresponding human-extracted data to identify examples of both ISLaR-specific and human-specific errors compared to the gold-standard full texts, but no performance metrics were calculated for this comparison. Inter-rater agreement of abstract and full text screening was assessed through Cohen’s Kappa (Supplementary Table 4).
3 Results
3.1 Study selection
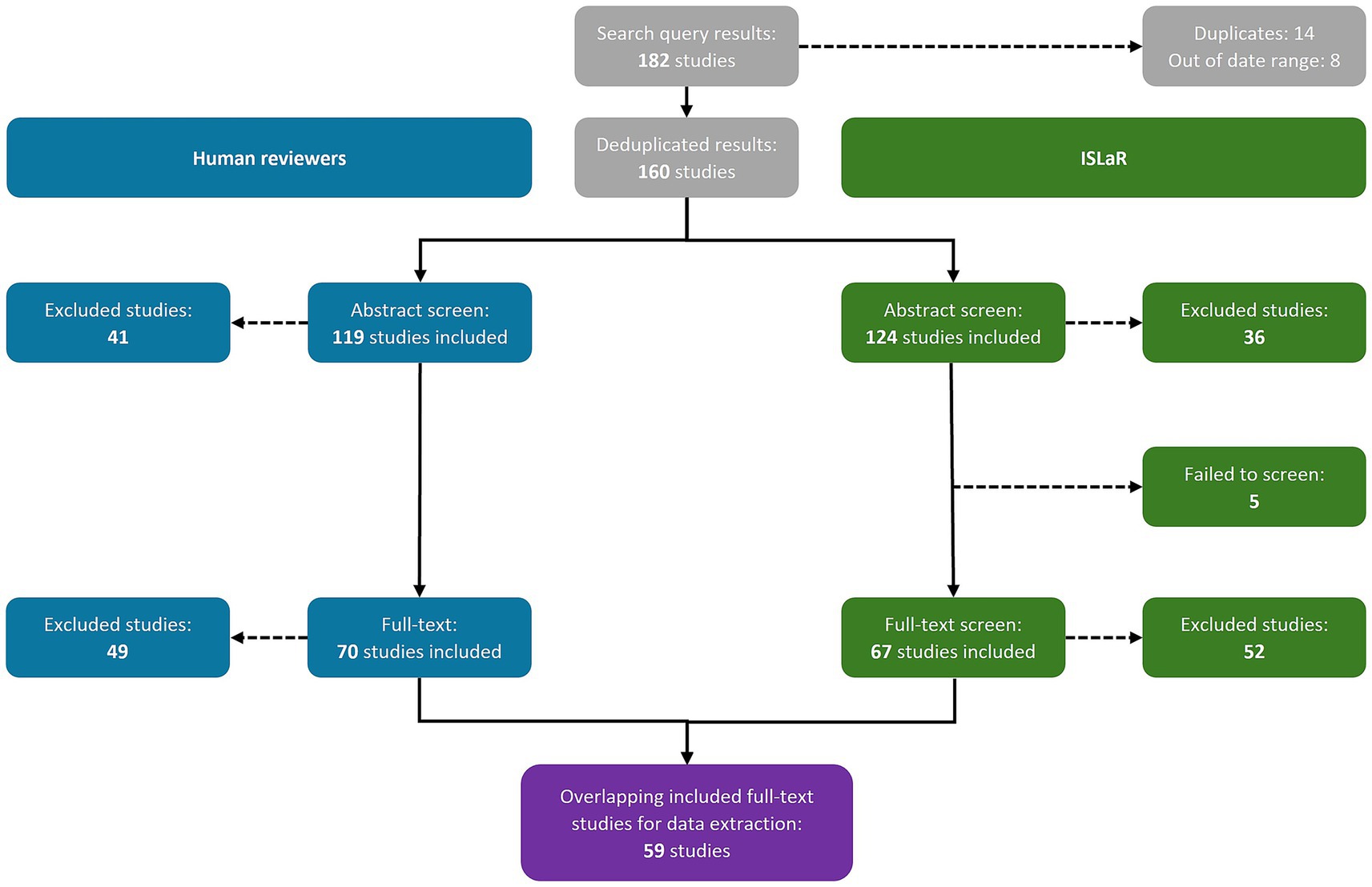
The database search returned 182 studies: 145 from PubMed and 37 from EMBASE (Supplementary Table 1). Both the human reviewers and ISLaR correctly identified and removed 14 duplicates and 8 studies published outside the desired date range, retaining a total of 160 studies for abstract screening (Figure 1). All studies, and their classifications at the abstract and full-text screening stages, are listed in Supplementary Table 5.
3.1.1 Abstract screening
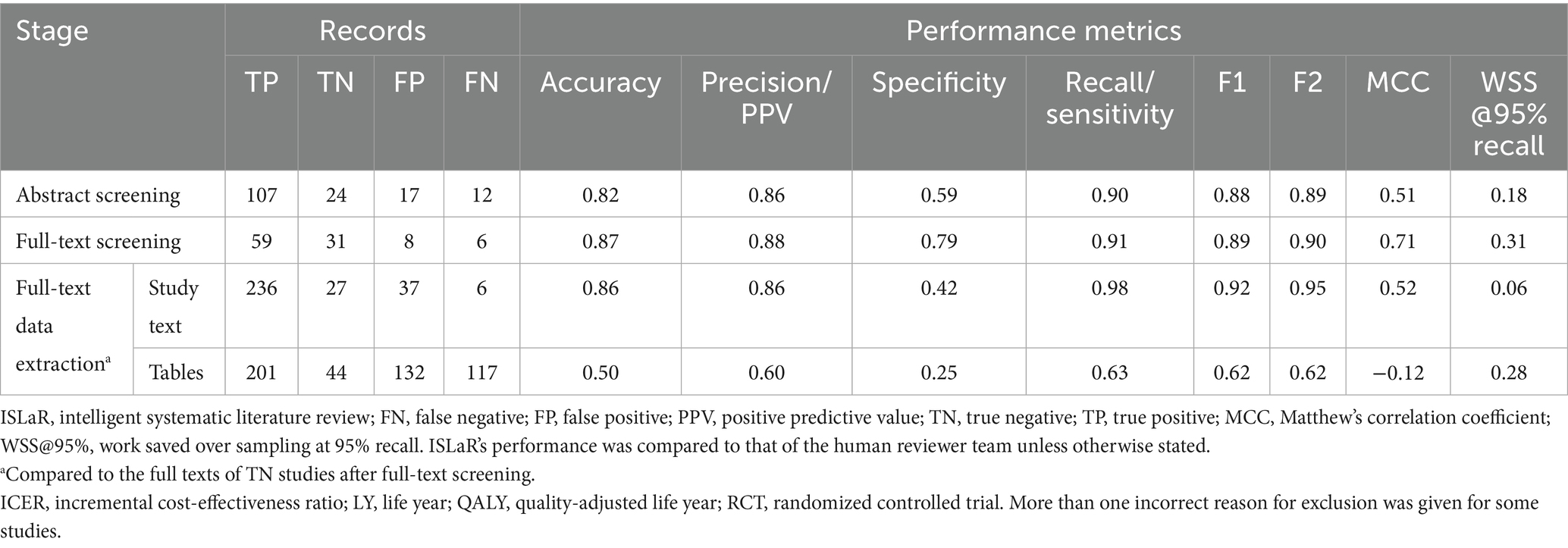
The human reviewers included 119 study abstracts and excluded 41, while ISLaR included 124 study abstracts [107 true positives (TPs) and 17 false positives (FPs), compared to the human reviewers] and excluded 36 [24 true negatives (TNs) and 12 false negatives (FNs); Figure 1; Table 2]. ISLaR thus had high recall/sensitivity (0.90), accuracy (0.82), precision/positive predictive value (PPV; 0.86), and F1 value (0.88) for abstract screening, but lower specificity (0.59; Table 2). There were 22 discrepancies between the decisions of different human reviewers that had to be resolved via independent review by an additional researcher; in these cases, the independent reviewer’s decision was considered correct moving forward. The studies for which inter-reviewer discrepancies occurred included 4/17 FP and 2/12 FN abstracts [i.e., 6/29 (20.7%) of all abstract screening errors made by ISLaR].
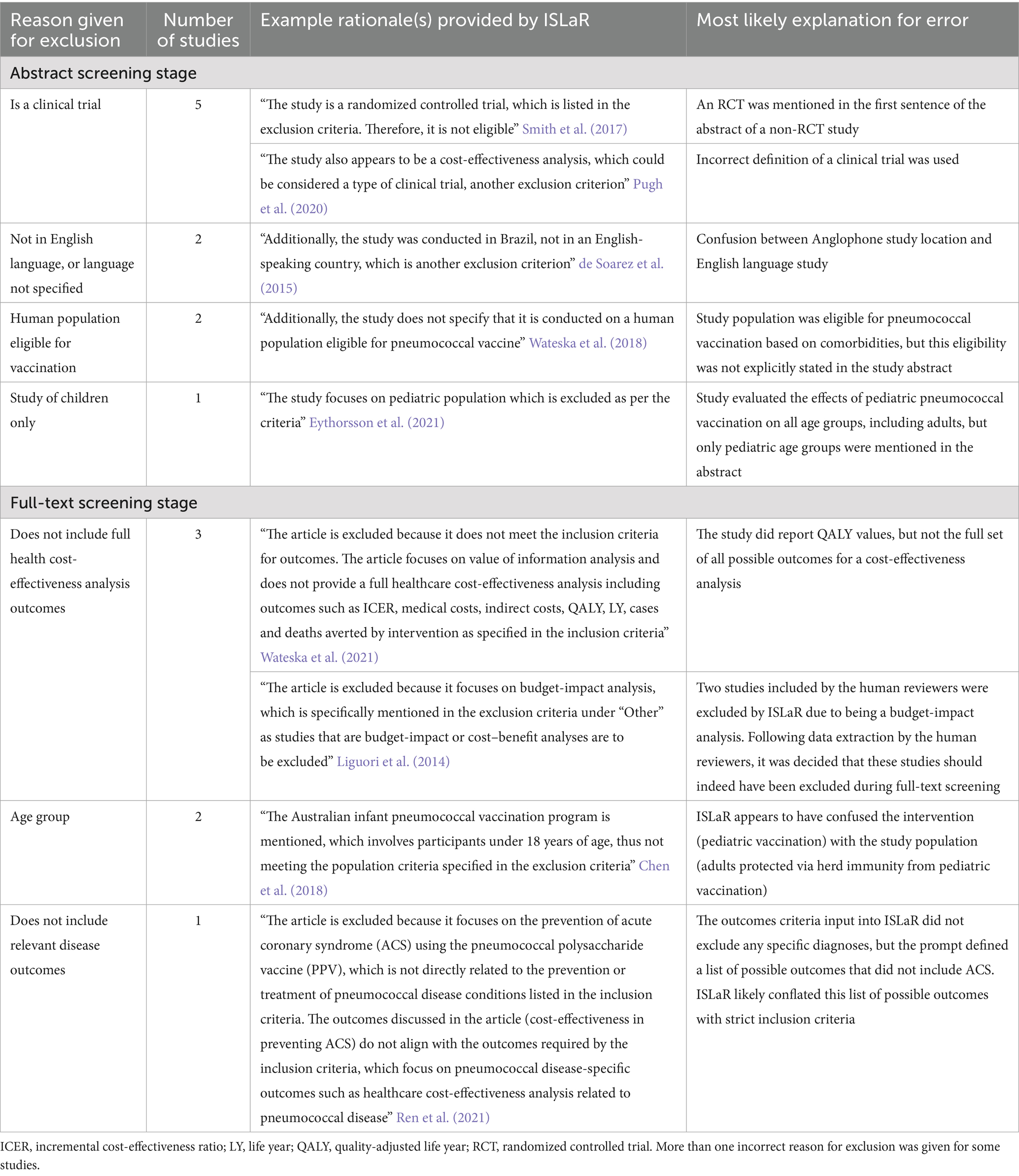
In the review conducted by ISLaR, the primary reasons for the false exclusion of studies are outlined in Table 3. At the abstract screening stage, five studies were erroneously identified as randomized controlled trials (RCTs), either due to ISLaR inferring an incorrect definition of an RCT, or confusion between the design of the study in question and a mention of a separate RCT publication within the background section of the abstract. Additionally, we observed two cases where English-language study abstracts were incorrectly identified as non-English language (due to non-Anglophone study location), despite re-wording the language criterion used in the final version of the prompt to attempt to avoid this issue. Among 12 FN abstracts, eight had a secondary exclusionary reason that was either clearly wrong or a misinterpretation of the Population, Intervention, Comparison, Outcome, Time, and Study design (PICOTS) criteria. Examples of secondary reasons for exclusion provided for FN abstracts included classification of case–control studies and cost-effectiveness analyses as RCTs, classification of primary data analyses as reviews, failure to recognize that adult’s ≥60 years of age were a population eligible for pneumococcal vaccination, and failure to recognize that a study was published in the English language. Incorrect secondary exclusion criteria were also identified for 10/24 TN abstracts.
ISLaR’s FP abstract selections also included errors in recognizing English-language studies (potentially due to some non-English-language studies having an English-language version of the abstract), as well as posters, preprints, and non-primary studies such as editorials, commentaries, and news articles that were correctly excluded by the human reviewers. Human reviewers also excluded nine abstracts that did not assess cost-effectiveness outcomes in an adult population, but that ISLaR erroneously included; in six cases the human reviewers’ exclusion decision was based on information that was also available to ISLaR (for example, use of the words “in children” in the study title), whereas in the other cases the decision was based on expert knowledge that had not been included in the ISLaR prompt (for example, knowledge that the study’s intervention vaccine is only licensed for use in pediatric populations).
3.1.2 Full-text screening
At the full-text review stage, the human reviewers excluded 49 additional studies, retaining 70 for data extraction and detailed review (Figure 1). In contrast, ISLaR was unable to assess the full text of five articles and excluded an additional 52 studies following full-text review. When comparing the subset of studies that were assessed by both the human reviewer team and ISLaR at the full-text stage, ISLaR excluded 37 studies (31 TNs and six FNs compared to the human reviewer gold standard) and retained 67 (59 TPs and 8 FPs) for data extraction (Table 2). ISLaR’s recall (0.91), accuracy (0.87), precision (0.88), and F1 value (0.89) at the full-text screening stage were thus slightly higher than the corresponding values from the abstract screening phase. As expected due to the use of more stringent inclusion and exclusion criteria for the full-text screen, there was a much greater improvement in the specificity score: from 0.59 at the abstract screening phase to 0.79 at the full-text screening phase. There were 16 discrepancies between the decisions of different human reviewers; the studies for which these discrepancies occurred included 3/8 FP and 2/6 FN full-text studies [i.e., 5/14 (35.7%) of the full-text screening errors made by ISLaR].
Two full-text studies were erroneously excluded by ISLaR due to inaccurate classification of the study population’s age group (for example, when an infant vaccination program was mentioned in a study that included an assessment of the impact of pediatric vaccination on adults via herd immunity) and one because of inaccurate classification of the study’s outcomes (Table 3). In addition, three FN studies were excluded due to lack of relevant cost-effectiveness analysis outcomes. After data extraction the human reviewer team concluded that two of these three studies were budget-impact analyses that should indeed have been excluded during full-text screening (i.e., these studies were actually TNs). The ISLaR accuracy and recall metrics for full-text screening listed in Table 2 are thus slight underestimates.
As an informal sensitivity analysis, we prompted ISLaR to assess the full texts of all studies it had excluded at the abstract screening phase. Only 3/36 (8.3%) of these excluded studies were considered relevant to the SLR when reassessed using the full text.
3.1.3 Human–ISLaR comparison
Cohen’s kappa values comparing human–human and human–ISLaR screening results are shown in Supplementary Table 4. At the abstract screening stage, agreement between human reviewers was moderate (κ = 0.65) and higher than the agreement between ISLaR and the composite human decision (κ = 0.50). At the full-text screening stage, the human–human agreement (κ = 0.75) was similar to the human–ISLaR agreement (κ = 0.73). It should be noted that the human–ISLaR comparison was made against the composite human decision, in which discrepancies between human reviewers were resolved by a third reviewer.
3.2 Data extraction
A total of 59 TP full-text studies were included by both the human review team and ISLaR, and thus proceeded to the data extraction phase. The data extracted from 21 randomly selected studies from among this group were used to assess ISLaR’s performance. Compared to the full texts of the respective studies, ISLaR had high accuracy (0.86), precision/PPV (0.86), recall/sensitivity (0.98), and F1 value (0.92) when extracting data elements from study texts, but relatively low specificity (0.42; Table 2). Most FPs came from misattributing cost denomination years, where ISLaR pulled a year mentioned elsewhere in the text and incorrectly classified it as the monetary denomination year. For these text-based data extractions, ISLaR extracted the correct value 87.8% of the time when compared to the gold-standard values present in the studies’ full texts. However, it extracted the correct value 98.4% of the time compared to the values initially extracted by the human reviewers, due to errors and omissions in the human reviewers’ data extractions.
The model’s performance was worse across all metrics when extracting data from study tables rather than study texts (Table 2); overall, ISLaR extracted the correct data from Tables 48.3% of the time compared to the gold-standard values present in the studies’ full texts and 63.9% of the time compared to the human reviewers. Consistent with these results, ISLaR’s F2 and MCC values were high for text-based extractions (F2 = 0.95; MCC = 0.52), but much lower for table-based extractions (F2 = 0.62; MCC = −0.12). For table extractions, FPs generally reflected ISLaR extracting a value from the wrong cell of the relevant table. For example, ISLaR had difficulties accurately extracting study cohort information from large tables, especially when table subheadings were used to differentiate between results from different subpopulations, such as age groups. ISLaR also often recorded “NA” for a value that was present in a table. In addition, there were several instances of ISLaR extracting data from a cost-effectiveness Markov model input table, which in many studies includes similar variables and is formatted similarly to the outcomes data tables; this distinction was not included in ISLaR’s prompt. These extractions were only logged as errors in cases where ISLaR did not also provide an accurate data extraction from the study’s outcomes tables.
We identified several cases where ISLaR successfully extracted data elements that were not found or incorrectly extracted by one (but not both) human reviewer(s): ISLaR’s extracted value was correct for 72.9% of all single-reviewer errors in extracting text-based data, and 27.9% of all single-reviewer errors in extracting table-based data. For 10 data values, ISLaR correctly extracted a data element that was not identified (i.e., was marked as “NA”) by any human reviewer.
3.3 Time to completion
Abstract screening was completed in a mean of 2.99 s per abstract by ISLaR (at low user volume times, i.e., outside standard office hours) and 66.12 s per abstract by the human reviewers. At the full-text screening stage, the average time taken for ISLaR to screen each full text was 7.49 s, compared to 80.86 s for the human reviewers. The average reduction in review time was approximately 63 s for abstracts and 73 s for full-text review.
4 Discussion
In this study, we prospectively compared the performance of an LLM-based platform to that of expert human reviewers in an SLR on the cost-effectiveness of adult pneumococcal vaccination. ISLaR screened articles with high sensitivity, accuracy, and precision, but lower specificity, in 4.5 and 9.3% of the time taken by human reviewers to screen abstracts and full texts, respectively. In data extraction tasks, ISLaR performed markedly better across all metrics when extracting data elements from study texts compared to study tables. For 20.7% of ISLaR’s abstract screening errors, 35.7% of its full-text screening errors, and 61.0% of its data extraction errors, there was a corresponding discrepancy between human reviewers.
ISLaR’s time savings and performance metrics were broadly in line with those reported in other studies of LLM-based SLR automation (Alshami et al., 2023; Dunn et al., 2022; Gartlehner et al., 2024; Guo et al., 2024; Khraisha et al., 2024; Kohandel Gargari et al., 2024; Landschaft et al., 2024; Li et al., 2024; Matsui et al., 2024; Tran et al., 2024; Wang Z. et al., 2025). The average time taken for the human reviewers to screen each study may not be representative of other SLRs, as cost-effectiveness analyses have a standardized outcome reporting format; ISLaR’s relative time savings may thus be greater for other study types. The low specificity score in the abstract screening task was due to the intentional use of less stringent inclusion and exclusion criteria at this stage compared to the full-text screening stage; as expected, this score improved substantially in the full-text screening task. However, even in this second, more stringent screen, ISLaR’s specificity score was lower than its sensitivity score. This finding is consistent with several other studies (Alshami et al., 2023; Guo et al., 2024; Khraisha et al., 2024; Kohandel Gargari et al., 2024; Matsui et al., 2024; Tran et al., 2024), although a smaller number of studies have reported both high sensitivity and high specificity (Landschaft et al., 2024; Li et al., 2024). Since most SLRs ultimately exclude a substantial number of potentially eligible articles (Sampson et al., 2011; Wang et al., 2020; Yaffe et al., 2012), the use of highly sensitive LLM tools to rapidly screen out obviously irrelevant articles would in itself substantially reduce researchers’ workloads while ensuring that almost all relevant articles are retained for detailed manual review. ISLaR and other LLM-based tools could also find similar applications in first-pass screening of newly published articles that may be eligible for inclusion in living SLRs (Elliott et al., 2014; Wijkstra et al., 2021). Future avenues of development could include exploration of article selection formats other than the current binary include/exclude decision, such as numerical relevance scores and dynamic article ranking systems similar to those included in some NLP-based automation tools (Akinseloyin et al., 2024; Bolaños et al., 2024; Oude Wolcherink et al., 2023; van Dijk et al., 2023).
While ISLaR performed well in extracting data from the texts of included studies, its performance in extracting data from tables was substantially weaker and, in our opinion, not yet adequate for use in SLRs. This pattern was reflected across multiple performance metrics. For example, F2 and MCC values were high for text-based extractions but dropped significantly for table-based extractions. Further, ISLaR’s table data extraction errors required additional manual effort to understand and resolve compared to its text data extraction errors, due to frequent occurrences of missing contextual information (such as study cohort), formatting errors in the extracted data, and references to the wrong table number in the data extraction notes. Wang Z. et al. (2025) reported a potentially related phenomenon whereby their LLM-based tool performed best at accurately extracting study design variables (which would be expected to occur in study texts), and worst at extracting outcomes and other numerical data (which would be more likely to appear in study tables). It would thus be advisable to maintain a human-in-the-loop model at least until the performance of LLMs on data extraction tasks improves. Future studies should assess the additional reviewer time needed to correct mistakes introduced by ISLaR or other LLMs in human-in-the-loop models. Other data extraction errors could potentially be mitigated by fine-tuning ISLaR’s underlying LLM to improve its performance on biomedical texts, or using a different LLM that has already been optimized for this purpose (Landschaft et al., 2024; Luo et al., 2024; Robinson et al., 2023; Wu et al., 2024). Prompt development is also a critical aspect of optimizing LLM performance, as the clarity and consistency of user-provided inclusion and exclusion criteria strongly influence system performance and the risk of bias.
Despite the issues with data extraction from tables, we observed several cases where ISLaR was able to correctly extract data that were marked as not present by one or more human reviewers. These human errors were likely due to reviewer fatigue, highlighting a potential benefit of automation in terms of better reproducibility and lower risk of bias (Alshami et al., 2023; Guo et al., 2024; Matsui et al., 2024). In particular, ISLaR’s ability to identify correct values that human reviewers missed highlights its notable potential to complement human effort and reduce the impact of fatigue or oversight. Even modest reductions in fatigue-related mistakes could meaningfully improve the consistency and reliability of SLR outputs, especially for tasks that require sustained attention to repetitive details. However, the reproducibility of LLMs can sometimes be affected by changes in the underlying model that are not transparently documented and available to users, and which can change the outputs generated in response to the same prompt over time (Chen et al., 2023; Gartlehner et al., 2024; O’Connor et al., 2024; Syriani et al., 2023). Reproducibility was not formally assessed in the current study, but we note that when asked to reassess its full-text article exclusion decisions, ISLaR changed its response for 3 of 36 articles. Conducting all tasks for a given stage of an SLR in a single session, and/or replicating tasks to obtain a consensus, may help to mitigate these issues.
Although we have demonstrated the potential utility of ISLaR in rapidly performing SLR tasks to ease researcher workload, there are many challenges and barriers that must be addressed before AI tools can be routinely used in practice (Bolaños et al., 2024; de la Torre-López et al., 2023; Doyal et al., 2023; O’Connor et al., 2019; van Altena et al., 2019). Researchers’ current lack of trust in the accuracy and quality of SLR automation tools has been identified as a major barrier to uptake, and transparency from the developers and users of these tools has been suggested as a means to overcome this challenge (Bolaños et al., 2024; de la Torre-López et al., 2023; Gates et al., 2019; O’Connor et al., 2019; van Altena et al., 2019). The ability of LLMs to document the rationale behind each screening and data extraction decision is a major advantage over earlier AI approaches, improving transparency and aiding in the interpretation of the models’ outputs, as well as informing the future development of models and prompts for better performance (Bolaños et al., 2024; de la Torre-López et al., 2023). For example, the decision rationales provided by ISLaR highlight the platform’s difficulty in distinguishing certain study variables from background information; others have reported similar problems (Landschaft et al., 2024). However, some of ISLaR’s decision rationales—particularly secondary exclusion reasons for studies—were more difficult to interpret. Future studies will explore the impact of limiting ISLaR’s rationale output to a single reason to improve interpretability and, ultimately, transparency. Additionally, further work should explore how the metrics calculated here compare to more classical ML baselines for SLRs.
Our work to make ISLaR’s decisions transparent to users also aligns with global efforts to update SLR best-practice guidelines to include advice on the use of automation tools. For example, the 2020 update of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) checklist now prompts SLR authors to document any automation tools used in article selection, data extraction, and risk of bias assessments, as well as the study inclusion and exclusion decisions made by these tools (Page et al., 2021). Similarly, the Professional Society for Health Economics and Outcomes Research (ISPOR) Criteria for Cost (-Effectiveness) Review Outcomes (CiCERO) checklist and guidance document stress that any automation tools used in an SLR should be reported transparently (Mandrik et al., 2021). In its complementary efforts to advance the development of and uptake of AI tools for evidence synthesis, the International Collaboration for the Automation of Systematic Reviews (ICASR) also emphasizes the importance of transparent reporting of performance metrics (Beller et al., 2018; O’Connor et al., 2024). More recently, ICASR has published guidance on the responsible use of AI in evidence synthesis (Responsible AI in Systematic Evidence synthesis, or RAISE), underscoring the need for transparency in reporting and the careful application of AI to ensure responsible use (Thomas et al., 2025).
4.1 Strengths and limitations
Our choice of case-study SLR was a major strength of the current analysis as it enabled us to perform a rigorous and comprehensive assessment of ISLaR’s performance in a prospective comparison to expert human researchers. This analysis included assessment of the reasons for ISLaR’s errors as well as areas of particular strength for both ISLaR and human reviewers, to improve transparency and inform the future development and use of the platform.
However, the current study has some known limitations. We used stringent search queries that returned relatively few articles for screening; ISLaR might perform differently if working from a broader search query that retrieves more ineligible studies. For example, ChatGPT-4 has been reported to have higher accuracy when screening more imbalanced datasets (i.e., groups of articles that included a higher proportion of irrelevant studies) (Khraisha et al., 2024). In addition, our data extraction process used only the main texts of included articles; future development should focus on allowing ISLaR to assess and extract data from supplementary materials, as well as gray literature, which may contain additional relevant information (Lawrence et al., 2014; Mandrik et al., 2021; Paez, 2017). Finally, the performance of ISLaR in the case-study SLR suggests that the platform is not yet ready for fully autonomous use and requires a “human-in-the-loop” model. However, we have not yet been able to identify a means of highlighting which of ISLaR’s decisions are most likely to be incorrect and to require additional scrutiny by a human reviewer. This is an important area for improvement.
In conclusion, our analysis of ISLaR’s performance in a prospective SLR demonstrates that the platform has potential to reduce the workload involved in an SLR, particularly during the article screening stage and parts of the data extraction process. It could also be used for the ongoing screening of newly published articles for potential inclusion in living SLRs. However, the platform is not yet ready for autonomous use, and the current version requires oversight from expert researchers.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
KC: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. AO: Data curation, Writing – original draft, Writing – review & editing. MR-M: Data curation, Writing – original draft, Writing – review & editing. BC: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. YLH: Writing – review & editing. DW: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. NC: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, United States. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
The authors thank Cath Ennis, in collaboration with ScribCo for medical writing assistance. We would also like to thank Neto Coubily and Katie Feehan for their assistance in abstract screening.
Conflict of interest
KC, AO, BC, YLH, DW, and NC are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA who may own stock/or hold stock options. MR-M is an employee of IMO Health, which received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, to conduct this study.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frai.2025.1662202/full#supplementary-material
References
Akinseloyin, O., Jiang, X., and Palade, V. (2024). A question-answering framework for automated abstract screening using large language models. J. Am. Med. Inform. Assoc. 31, 1939–1952. doi: 10.1093/jamia/ocae166
Allen, I. E., and Olkin, I. (1999). Estimating time to conduct a meta-analysis from number of citations retrieved. JAMA 282, 634–635. doi: 10.1001/jama.282.7.634
Alshami, A., Elsayed, M., Ali, E., Eltoukhy, A. E. E., and Zayed, T. (2023). Harnessing the power of Chat GPT for automating systematic review process: methodology, case study, limitations, and future directions. Systems 11:351. doi: 10.3390/systems11070351
Amazon Web Services, Inc (n.d.). Amazon Textract [Computer software]. Amazon Web Services, Inc. Retrieved Jan 1, 2023, Available from https://aws.amazon.com/textract/
Anderson, R. (2010). Systematic reviews of economic evaluations: utility or futility? Health. Econ. 19, 350–364. doi: 10.1002/hec.1486
Aromataris, E., and Pearson, A. (2014). The systematic review: an overview. Am. J. Nurs. 114, 53–58. doi: 10.1097/01.NAJ.0000444496.24228.2c
Beller, E. M., Chen, J. K., Wang, U. L., and Glasziou, P. P. (2013). Are systematic reviews up-to-date at the time of publication? Syst. Rev. 2:36. doi: 10.1186/2046-4053-2-36
Beller, E., Clark, J., Tsafnat, G., Adams, C., Diehl, H., Lund, H., et al. (2018). Making progress with the automation of systematic reviews: principles of the international collaboration for the automation of systematic reviews (ICASR). Syst. Rev. 7:77. doi: 10.1186/s13643-018-0740-7
Blaizot, A., Veettil, S. K., Saidoung, P., Moreno-Garcia, C. F., Wiratunga, N., Aceves-Martins, M., et al. (2022). Using artificial intelligence methods for systematic review in health sciences: a systematic review. Res. Synth. Methods 13, 353–362. doi: 10.1002/jrsm.1553
Bolaños, F., Salatino, A., Osborne, F., and Motta, E. (2024). Artificial intelligence for literature reviews: opportunities and challenges. Artif. Intell. Rev. 57:259. doi: 10.1007/s10462-024-10902-3
Borah, R., Brown, A. W., Capers, P. L., and Kaiser, K. A. (2017). Analysis of the time and workers needed to conduct systematic reviews of medical interventions using data from the PROSPERO registry. BMJ. Open 7:e012545. doi: 10.1136/bmjopen-2016-012545
Bullers, K., Howard, A. M., Hanson, A., Kearns, W. D., Orriola, J. J., Polo, R. L., et al. (2018). It takes longer than you think: librarian time spent on systematic review tasks. J. Med. Libr. Assoc. 106, 198–207. doi: 10.5195/jmla.2018.323
Cassell, K., Crossrow, N., and Ologunowa, A. (2024). A systematic review of the cost-effectiveness of adult pneumococcal vaccines and comparison to results derived from an AI-driven systematic literature review. Prospero. Available at: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024562351
Chen, C., Beutels, P., Wood, J., Menzies, R., MacIntyre, C. R., McIntyre, P., et al. (2018). Retrospective cost-effectiveness of the 23-valent pneumococcal polysaccharide vaccination program in Australia. Vaccine 36, 6307–6313. doi: 10.1016/j.vaccine.2018.08.084
Chen, L., Zaharia, M., and Zou, J. (2023). How is Chat GPT’s behavior changing over time? arXiv. 2307.09009. doi: 10.48550/arXiv.2307.09009
Clark, J., McFarlane, C., Cleo, G., Ishikawa Ramos, C., and Marshall, S. (2021). The impact of systematic review automation tools on methodological quality and time taken to complete systematic review tasks: case study. JMIR Med. Educ. 7:e24418. doi: 10.2196/24418
Clarke, M., and Chalmers, I. (2018). Reflections on the history of systematic reviews. BMJ. Evid. Based. Med. 23, 121–122. doi: 10.1136/bmjebm-2018-110968
Cowie, K., Rahmatullah, A., Hardy, N., Holub, K., and Kallmes, K. (2022). Web-based software tools for systematic literature review in medicine: systematic search and feature analysis. JMIR Med. Inform. 10:e33219. doi: 10.2196/33219
de la Torre-López, J., Ramírez, A., and Romero, J. R. (2023). Artificial intelligence to automate the systematic review of scientific literature. Computing 105, 2171–2194. doi: 10.1007/s00607-023-01181-x
de Soarez, P. C., Sartori, A. M., Freitas, A. C., Nishikawa, A. M., and Novaes, H. M. (2015). Cost-effectiveness analysis of universal vaccination of adults aged 60 years with 23-valent pneumococcal polysaccharide vaccine versus current practice in Brazil. PLoS One 10:e0130217. doi: 10.1371/journal.pone.0130217
Doyal, A. S., Sender, D., Nanda, M., and Serrano, R. A. (2023). Chat GPT and artificial intelligence in medical writing: concerns and ethical considerations. Cureus 15:e43292. doi: 10.7759/cureus.43292
Dunn, A., Dagdelen, J., Walker, N., Lee, S., Rosen, A. S., Ceder, G., et al. (2022). Structured information extraction from complex scientific text with fine-tuned large language models. arXiv. 2212.05238. doi: 10.48550/arXiv.2212.05238
Elliott, J. H., Turner, T., Clavisi, O., Thomas, J., Higgins, J. P., Mavergames, C., et al. (2014). Living systematic reviews: an emerging opportunity to narrow the evidence-practice gap. PLoS Med. 11:e1001603. doi: 10.1371/journal.pmed.1001603
Eythorsson, E., Asgeirsdottir, T. L., Erlendsdottir, H., Hrafnkelsson, B., Kristinsson, K. G., and Haraldsson, A. (2021). The impact and cost-effectiveness of introducing the 10-valent pneumococcal conjugate vaccine into the pediatric immunization programmer in Iceland-a population-based time series analysis. PLoS One 16:e0249497. doi: 10.1371/journal.pone.0249497
Gartlehner, G., Affengruber, L., Titscher, V., Noel-Storr, A., Dooley, G., Ballarini, N., et al. (2020). Single-reviewer abstract screening missed 13 percent of relevant studies: a crowd-based, randomized controlled trial. J. Clin. Epidemiol. 121, 20–28. doi: 10.1016/j.jclinepi.2020.01.005
Gartlehner, G., Kahwati, L., Hilscher, R., Thomas, I., Kugley, S., Crotty, K., et al. (2024). Data extraction for evidence synthesis using a large language model: a proof-of-concept study. Res. Synth. Methods 15, 576–589. doi: 10.1002/jrsm.1710
Gates, A., Guitard, S., Pillay, J., Elliott, S. A., Dyson, M. P., Newton, A. S., et al. (2019). Performance and usability of machine learning for screening in systematic reviews: a comparative evaluation of three tools. Syst. Rev. 8:278. doi: 10.1186/s13643-019-1222-2
Ghosh, M., Mukherjee, S., Ganguly, A., Basuchowdhuri, P., Naskar, S. K., and Ganguly, D. (2024). AlpaPICO: extraction of PICO frames from clinical trial documents using LLMs. Methods 226, 78–88. doi: 10.1016/j.ymeth.2024.04.005
Grant, M. J., and Booth, A. (2009). A typology of reviews: an analysis of 14 review types and associated methodologies. Health. Inf. Libr. J. 26, 91–108. doi: 10.1111/j.1471-1842.2009.00848.x
Guo, E., Gupta, M., Deng, J., Park, Y. J., Paget, M., and Naugler, C. (2024). Automated paper screening for clinical reviews using large language models: data analysis study. J. Med. Internet Res. 26:e48996. doi: 10.2196/48996
Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (editors). (2024). Cochrane handbook for systematic reviews of interventions version 6.5. Cochrane. Available from: https://aws.amazon.com/textract/
Husereau, D., Drummond, M., Augustovski, F., de Bekker-Grob, E., Briggs, A. H., Carswell, C., et al. (2022). Consolidated health economic evaluation reporting standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II good practices task force. Value Health 25, 10–31. doi: 10.1016/j.jval.2021.10.008
Jacobsen, E., Boyers, D., and Avenell, A. (2020). Challenges of systematic reviews of economic evaluations: a review of recent reviews and an obesity case study. Pharmaco. Econ. 38, 259–267. doi: 10.1007/s40273-019-00878-2
Khraisha, Q., Put, S., Kappenberg, J., Warraitch, A., and Hadfield, K. (2024). Can large language models replace humans in systematic reviews? Evaluating GPT-4’s efficacy in screening and extracting data from peer-reviewed and grey literature in multiple languages. Res. Synth. Methods 15, 616–626. doi: 10.1002/jrsm.1715
Kohandel Gargari, O., Mahmoudi, M. H., Hajisafarali, M., and Samiee, R. (2024). Enhancing title and abstract screening for systematic reviews with GPT-3.5 turbo. BMJ Evid. Bas. Med. 29, 69–70. doi: 10.1136/bmjebm-2023-112678
Landschaft, A., Antweiler, D., Mackay, S., Kugler, S., Ruping, S., Wrobel, S., et al. (2024). Implementation and evaluation of an additional GPT-4-based reviewer in PRISMA-based medical systematic literature reviews. Int. J. Med. Inform. 189:105531. doi: 10.1016/j.ijmedinf.2024.105531
Lawrence, A., Houghton, J. W., Thomas, J., and Weldon, P. (2014). Where is the evidence? Realizing the value of grey literature for public policy and practice. Swin. Instit. Soc. Res. doi: 10.4225/50/5580B1E02DAF9
Li, M., Sun, J., and Tan, X. (2024). Evaluating the effectiveness of large language models in abstract screening: a comparative analysis. Syst. Rev. 13:219. doi: 10.1186/s13643-024-02609-x
Liguori, G., Parlato, A., Zamparelli, A. S., Belfiore, P., Galle, F., Di Onofrio, V., et al. (2014). Adult immunization with 13-valent pneumococcal vaccine in Campania region, South Italy: an economic evaluation. Hum. Vaccin. Immunother. 10, 492–497. doi: 10.4161/hv.26888
Lu, Z., Peng, Y., Cohen, T., Ghassemi, M., Weng, C., and Tian, S. (2024). Large language models in biomedicine and health: current research landscape and future directions. J. Am. Med. Inform. Assoc. 31, 1801–1811. doi: 10.1093/jamia/ocae202
Luhnen, M., Prediger, B., Neugebauer, E. A. M., and Mathes, T. (2018). Systematic reviews of economic evaluations in health technology assessment: a review of characteristics and applied methods. Int. J. Technol. Assess. Health Care 34, 537–546. doi: 10.1017/S0266462318000624
Luo, L., Ning, J., Zhao, Y., Wang, Z., Ding, Z., Chen, P., et al. (2024). Taiyi: a bilingual fine-tuned large language model for diverse biomedical tasks. J. Am. Med. Inform. Assoc. 31, 1865–1874. doi: 10.1093/jamia/ocae037
Mandrik, O. L., Severens, J. L. H., Bardach, A., Ghabri, S., Hamel, C., Mathes, T., et al. (2021). Critical appraisal of systematic reviews with costs and cost-effectiveness outcomes: an ISPOR good practices task force report. Value. Health 24, 463–472. doi: 10.1016/j.jval.2021.01.002
Matsui, K., Utsumi, T., Aoki, Y., Maruki, T., Takeshima, M., and Takaesu, Y. (2024). Human-comparable sensitivity of large language models in identifying eligible studies through title and abstract screening: 3-layer strategy using GPT-3.5 and GPT-4 for systematic reviews. J. Med. Internet Res. 26:e52758. doi: 10.2196/52758
Michelson, M., and Reuter, K. (2019). The significant cost of systematic reviews and meta-analyses: a call for greater involvement of machine learning to assess the promise of clinical trials. Contemp. Clin. Trials Commun. 16:100443. doi: 10.1016/j.conctc.2019.100443
Munn, Z., Stern, C., Aromataris, E., Lockwood, C., and Jordan, Z. (2018). What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med. Res. Methodol. 18:5. doi: 10.1186/s12874-017-0468-4
National Library of Medicine National Center for Biotechnology Information. APIs. Available online at: https://www.ncbi.nlm.nih.gov/home/develop/api/
O’Connor, A. M., Clark, J., Thomas, J., Spijker, R., Kusa, W., Walker, V. R., et al. (2024). Large language models, updates, and evaluation of automation tools for systematic reviews: a summary of significant discussions at the eighth meeting of the international collaboration for the automation of systematic reviews (ICASR). Syst. Rev. 13:290. doi: 10.1186/s13643-024-02666-2
O’Connor, A. M., Tsafnat, G., Thomas, J., Glasziou, P., Gilbert, S. B., and Hutton, B. (2019). A question of trust: can we build an evidence base to gain trust in systematic review automation technologies? Syst. Rev. 8:143. doi: 10.1186/s13643-019-1062-0
Oude Wolcherink, M. J., Pouwels, X., van Dijk, S. H. B., Doggen, C. J. M., and Koffijberg, H. (2023). Can artificial intelligence separate the wheat from the chaff in systematic reviews of health economic articles? Expert Rev. Pharm. Out. Res. 23, 1049–1056. doi: 10.1080/14737167.2023.2234639
Paez, A. (2017). Grey literature: an important resource in systematic reviews. J. Evid. Based Med. doi: 10.1111/jebm.12266
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. doi: 10.1136/bmj.n71
Pugh, S., Wasserman, M., Moffatt, M., Marques, S., Reyes, J. M., Prieto, V. A., et al. (2020). Estimating the impact of switching from a lower to higher valent pneumococcal conjugate vaccine in Colombia, Finland, and the Netherlands: a cost-effectiveness analysis. Infect. Dis. Ther. 9, 305–324. doi: 10.1007/s40121-020-00287-5
Ren, S., Attia, J., Li, S. C., and Newby, D. (2021). Pneumococcal polysaccharide vaccine is a cost saving strategy for prevention of acute coronary syndrome. Vaccine 39, 1721–1726. doi: 10.1016/j.vaccine.2021.02.019
Robinson, A., Thorne, W., Wu, B. P., Pandor, A., Essat, M., Stevenson, M., et al. (2023). Bio-SIEVE: exploring instruction tuning large language models for systematic review automation. arXiv. 2308.06610. doi: 10.48550/arXiv.2308.06610
Sallam, M. (2023). Chat GPT utility in healthcare education, research, and practice: systematic review on the promising perspectives and valid concerns. Healthcare Basel 11:887. doi: 10.3390/healthcare11060887
Sampson, M., Tetzlaff, J., and Urquhart, C. (2011). Precision of healthcare systematic review searches in a cross-sectional sample. Res. Synth. Methods 2, 119–125. doi: 10.1002/jrsm.42
Shemilt, I., Khan, N., Park, S., and Thomas, J. (2016). Use of cost-effectiveness analysis to compare the efficiency of study identification methods in systematic reviews. Syst. Rev. 5:140. doi: 10.1186/s13643-016-0315-4
Shojania, K. G., Sampson, M., Ansari, M. T., Ji, J., Doucette, S., and Moher, D. (2007). How quickly do systematic reviews go out of date? A survival analysis. Ann. Intern. Med. 147, 224–233. doi: 10.7326/0003-4819-147-4-200708210-00179
Smith, K. J., Zimmerman, R. K., Nowalk, M. P., and Lin, C. J. (2017). Cost-effectiveness of the 4 pillars practice transformation program to improve vaccination of adults aged 65 and older. J. Am. Geriatr. Soc. 65, 763–768. doi: 10.1111/jgs.14588
Syriani, E., David, I., and Kumar, G. (2023). Assessing the ability of Chat GPT to screen articles for systematic reviews. arXiv. 2307.06464. Available at: https://www.sciencedirect.com/science/article/pii/S2590118424000303?via%3Dihub
Thomas, J., Flemyng, E., and Noel-Storr, A. (2025). Responsible use of AI in evidence SynthEsis (RAISE): recommendations and guidance (version 2; updated 3 June 2025). Washington DC: Center for Open Science.
Tran, V. T., Gartlehner, G., Yaacoub, S., Boutron, I., Schwingshackl, L., Stadelmaier, J., et al. (2024). Sensitivity and specificity of using GPT-3.5 Turbo models for title and abstract screening in systematic reviews and Meta-analyses. Ann. Intern. Med. 177, 791–799. doi: 10.7326/M23-3389
Tsertsvadze, A., Chen, Y. F., Moher, D., Sutcliffe, P., and McCarthy, N. (2015). How to conduct systematic reviews more expeditiously? Syst. Rev. 4:160. doi: 10.1186/s13643-015-0147-7
van Altena, A. J., Spijker, R., and Olabarriaga, S. D. (2019). Usage of automation tools in systematic reviews. Res. Synth. Methods 10, 72–82. doi: 10.1002/jrsm.1335
van Dijk, S. H. B., Brusse-Keizer, M. G. J., Bucsan, C. C., van der Palen, J., Doggen, C. J. M., and Lenferink, A. (2023). Artificial intelligence in systematic reviews: promising when appropriately used. BMJ Open 13:e072254. doi: 10.1136/bmjopen-2023-072254
van Dinter, R., Tekinerdogan, B., and Catal, C. (2021). Automation of systematic literature reviews: a systematic literature review. Inf. Softw. Technol. 136:106589. doi: 10.1016/j.infsof.2021.106589
Wang, Z., Cao, L., Danek, B., Jin, Q., Lu, Z., and Sun, J. (2025). Accelerating clinical evidence synthesis with large language models. npj Digit. Med. 8:509. doi: 10.1038/s41746-025-01840-7
Wang, D., Datta, S., Glasgow, J., Lee, K., Paek, H., Zhang, J., et al. (2025a). AI-assisted systematic literature review for the analysis of the economic burden of pneumococcal disease [In preparation].
Wang, D., Datta, S., Huang, Y. -L., Zheng, Y., Cassell, K., Paek, H., et al. (2025b). A performance evaluation of artificial intelligence-assisted systematic literature review for studies assessing economic burden, epidemiology, and clinical outcomes [In preparation].
Wang, Z., Nayfeh, T., Tetzlaff, J., O'Blenis, P., and Murad, M. H. (2020). Error rates of human reviewers during abstract screening in systematic reviews. PLoS One 15:e0227742. doi: 10.1371/journal.pone.0227742
Wang, S., Scells, H., Koopman, B., and Zuccon, G. (2023). Can Chat GPT write a good Boolean query for systematic review literature search? 46th international ACM SIGIR conference on Research and Development in information retrieval. Taipei, Taiwan: Association for Computing Machinery.
Wang, S., Scells, H., Zhuang, S., Potthast, M., Koopman, B., and Zuccon, G. (2024). Zero-shot generative large language models for systematic review screening automation. arXiv :2401.06320. doi: 10.48550/arXiv.2401.06320
Wateska, A. R., Nowalk, M. P., Jalal, H., Lin, C. J., Harrison, L. H., Schaffner, W., et al. (2021). Is further research on adult pneumococcal vaccine uptake improvement programs worthwhile? Α value of information analysis. Vaccine 39, 3608–3613. doi: 10.1016/j.vaccine.2021.05.037
Wateska, A. R., Nowalk, M. P., Zimmerman, R. K., Smith, K. J., and Lin, C. J. (2018). Cost-effectiveness of increasing vaccination in high-risk adults aged 18-64 years: a model-based decision analysis. BMC Infect. Dis. 18:52. doi: 10.1186/s12879-018-2967-2
Wijkstra, M., Lek, T., Kuhn, T., Welbers, K., and Steijaert, M. (2021). Living literature reviews. Proceedings of the 11th Knowledge Capture Conference (K-CAP ’21), December 2–3, 2021, Virtual Event, USA, New York, NY, USA: ACM, 8 pages. doi: 10.1145/3460210.3493567
Wu, C., Lin, W., Zhang, X., Zhang, Y., Xie, W., and Wang, Y. (2024). PMC-LLaMA: toward building open-source language models for medicine. J. Am. Med. Inform. Assoc. 31, 1833–1843. doi: 10.1093/jamia/ocae045
Keywords: artificial intelligence, systematic literature review, data extraction, reviewer workload, health technology assessment, large language models
Citation: Cassell K, Ologunowa A, Rastegar-Mojarad M, Chun B, Huang YL, Wang D and Cossrow N (2025) Analysis of article screening and data extraction performance by an AI systematic literature review platform. Front. Artif. Intell. 8:1662202. doi: 10.3389/frai.2025.1662202
Edited by:
Nicola Zeni, University of Bergamo, ItalyReviewed by:
Michelle Angrish, United States Environmental Protection Agency (EPA), United StatesLiuna Geng, Nanjing University, China
Copyright © 2025 Cassell, Ologunowa, Rastegar-Mojarad, Chun, Huang, Wang and Cossrow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kelsie Cassell, a2Vsc2llLmNhc3NlbGxAbWVyY2suY29t
 Kelsie Cassell
Kelsie Cassell Abiodun Ologunowa
Abiodun Ologunowa Majid Rastegar-Mojarad
Majid Rastegar-Mojarad Bianca Chun1
Bianca Chun1 Yi-Ling Huang
Yi-Ling Huang Nicole Cossrow
Nicole Cossrow