- Agriculture and Environmental Sciences, School of Biosciences, University of Nottingham, Loughborough, United Kingdom
Neonicotinoids are systemic insecticides used in agriculture to control herbivorous pests by targeting the nervous system. However, the persistence and presence of neonicotinoids in pollen and nectar raises concerns regarding impacts on non-target organisms, particularly pollinators such as bees. Bumblebees (Bombus spp.) are essential for wild plant pollination and crop production but are vulnerable to insecticides due to their foraging behaviors and ecological traits. While commercially available forms of neonicotinoids have been banned in select countries over recent years, they are still utilized extensively in many parts of the world, with limited understanding of impacts on bumblebee physiology and behavior. To investigate neonicotinoid effects on bumblebees, we systematically reviewed studies from Scopus and Web of Science following PRISMA 2020 guidelines. A total of 52 primary studies were identified, revealing a pronounced geographic bias, with 81% of research conducted in the UK and the U.S. (54% and 27%, respectively). Bombus terrestris, B. terrestris audax, and B. impatiens emerged as the most studied species whereas imidacloprid, thiamethoxam, and clothianidin were the most common neonicotinoid compounds tested, represented in 88% of the studies. In comparison, only a single study performed on B. ephippiatus and there are currently no published studies assessing the impact of the compounds nitenpyram or dinotefuran on bumblebee health and behavior. Behavioral alterations, particularly foraging and cognition, were the most prevalent reported effects of neonicotinoids, followed by reproductive health and physiological impacts. This review highlights the need for more geographically and taxonomically diverse research, particularly in regions still using neonicotinoids. The prevalence of sublethal effects raises concerns for colony health and pollination services, yet direct assessments of pollination efficiency remain limited. As such, critical knowledge gaps remain, particularly regarding understudied neonicotinoid compounds and bumblebee species, emphasizing the need for further research to inform sustainable agricultural practices and conservation strategies.
1 Introduction
Bumblebees (Bombus spp.) are essential pollinators that contribute significantly to biodiversity and agricultural productivity (Parrey et al., 2022). They play a crucial role in pollinating various crops, including vegetables, fruits, oilseeds, legumes, fodder crops, and others that cannot be effectively pollinated by honeybees (Abrol et al., 2021; Wahengbam et al., 2019). Bumblebees have become the primary alternative to honeybees for commercial pollination in North America and Europe (Goulson, 2009). Their distinctive biological traits, including large body size, elongated tongue, and ability to perform buzz pollination, enhance their efficiency in pollen release and make them well-adapted to diverse environmental conditions including low temperatures and reduced light levels (Bie et al., 2025). These characteristics position bumblebees as vital pollinators, particularly in temperate regions.
However, bumblebee populations are experiencing alarming declines, with 24% of the 150 Bombus species assessed by the IUCN classified as threatened, and 30% of European species currently classified as of “least concern” are projected to lose at least 30% of ecologically suitable territory within the next 50 years (Cameron and Sadd, 2020; Ghisbain et al., 2024). This decline has been attributed to multiple stressors, including climate change, habitat loss, and pesticide exposure, interacting across spatial and temporal scales to exacerbate population losses (Becher et al., 2018). Among these threats, neonicotinoid insecticides have emerged as a critical concern due to their widespread use and documented adverse effects on pollinators.
The increasing global demand for food security has driven the extensive use of pesticides to enhance crop yields (Popp et al., 2013). Neonicotinoids were developed during the 1980s to overcome environmental concerns associated with older compounds such as organophosphates and combat increasing pesticide resistance among common agricultural pests (Werner and Hitzfeld, 2012; Sánchez-Bayo et al., 2016; Gould et al., 2018). Neonicotinoids rapidly became one of the most widely used insecticides, accounting for a third of the global market within two decades (Ewere et al., 2021). Developed through nicotine-based research, neonicotinoid compounds exhibit enhanced insecticidal properties while exhibiting low toxicity to several non-target organisms such as fish, birds, and mammals (Pang et al., 2020; Bass and Field, 2018).
Neonicotinoids are classified into three primary chemical groups: N-nitroguanidines, comprising the compounds imidacloprid, thiamethoxam, clothianidin, and dinotefuran; nitromethylenes such as nitenpyram; and N-cyanoamidines including acetamiprid and thiacloprid (Goulson, 2013). Their versatile physicochemical properties enable diverse application approaches including foliar sprays, seed treatments, and soil drenches, making them suitable for a range of crops such as maize, sugarbeet, oilseed rape, fruits, and vegetables (Nauen and Jeschke, 2011; Van der Sluijs et al., 2013).
Neonicotinoids are characterized by their small molecular size and high water solubility, allowing them to be efficiently absorbed by plants and transported, primarily via the xylem. This systemic movement enables the compounds and their metabolites to accumulate throughout plant tissues, including within leaves, flowers, and pollen (Bonmatin et al., 2015; Hladik et al., 2018). By targeting nicotinic acetylcholine receptors (nAChRs) in the insect nervous system, neonicotinoids effectively control agricultural pests, including aphids, whiteflies, and leafhoppers (Tomizawa and Casida, 2005; Thany, 2023). While initially thought to have minimal effects on non-target organisms, evidence shows that chronic exposure impairs pollinator foraging, reproduction, and colony health, and they are often associated with high bee toxicities (Hopwood et al., 2012; Stokstad, 2013; Bass and Field, 2018; Jeschke et al., 2011; Basley, 2019; Schulz et al., 2021).
The global decline in bee populations has raised significant concerns due to their vital role in pollination and the potential consequences for ecosystems and agriculture. As part of this, increasing research has highlighted the harmful effects of neonicotinoids, particularly on bee foraging, behavior, reproduction, and pollination services (Ihara and Matsuda, 2018; Pyke, 2022). Consequently, in 2018, the European Union (EU) banned all outdoor uses of clothianidin, imidacloprid, and thiamethoxam (although some exemptions were permitted up until 2024; Dentzman et al., 2025), and in 2020, it withheld renewal of thiacloprid’s approval due to endocrine disruption risks. The U.S. Environmental Protection Agency (EPA) cancelled 12 neonicotinoid products in 2019 with many state-level restrictions in place since 2023 (U.S. EPA, 2019; National Caucus of Environmental Legislators, 2023) and Canada’s Pest Management Regulatory Agency (PMRA) proposed a series of risk mitigation measures and restrictions on use, with reevaluation of measures expected to be published in 2026 (Health Canada, 2020; Health Canada, 2025; Dentzman et al., 2025). In addition, the joint FAO/WHO Meeting on Pesticide Residues introduced intake limits to reduce health risks (Thompson et al., 2020). Despite these regulatory measures, neonicotinoids remain widely used in regions lacking pesticide legislation, with clothianidin, imidacloprid, and thiamethoxam still dominating the global market (Klingelhöfer et al., 2022).
Despite growing evidence for the negative impact of neonicotinoid use, knowledge gaps remain, particularly for less studied taxa such as bumblebees (Dirilgen et al., 2023). However, given the ecological importance of bumblebees and the increasing reliance on pollination services for food production, understanding the extent of the impact of neonicotinoids is essential for informing sustainable agricultural practices and conservation efforts. Therefore, this systematic review aims to critically evaluate existing research on the effects of neonicotinoids on bumble bees, synthesizing both laboratory and field findings. We aimed to 1) identify which compound is the most studied, 2) identify the taxonomic spread of studies using neonicotinoids, and 3) assess the main reported impacts on bumblebee health and physiology. By assessing the broader ecological implications and identifying research gaps, this review will contribute to ongoing discussions on pollinator protection, regulatory measures, and future pesticide use.
2 Methods
A systematic review was conducted following the PRISMA 2020 guidelines. A comprehensive literature search was performed using two academic databases: Scopus and Web of Science, with the most recent search performed in June 2025. To ensure all relevant literature was captured, the following search string and Boolean operators were applied to both databases: (“Neonic*” OR “Neonicotinoid” OR “Neonicotinoids” OR “Imidacloprid” OR “Clothianidin” OR “Thiamethoxam” OR “Acetamiprid” OR “Thiacloprid” OR “Nitenpyram” OR “Dinotefuran”) AND (“Bumblebee*” OR “Bumble bee*” OR “Bombus”). The search was restricted to peer-reviewed, open-access articles within the last 20 years to ensure the review captured recent research. Only English papers were considered.
Following the database search, titles and abstracts were screened independently against the inclusion and exclusion criteria. Briefly, studies were included if they focused on bumblebees, examined neonicotinoid effects, and assessed behavioral, physiological, reproductive, survival, or pollination impacts under field-realistic or controlled conditions. Studies on other pollinators, non-neonicotinoid pesticides, or multiple interacting stressors were excluded.
Duplicates were removed, and full texts of potentially relevant papers were reviewed to confirm eligibility (Figure 1). For each included study, the publication date was noted (Figure 2), and data were extracted on neonicotinoid compound (Figure 3), study location (Figure 4), bumblebee species (Figure 5), and measured impact on bumblebee health or physiology, classed into behavioral, physiological, reproductive, survival, and mortality or pollination efficiency-related effects (Figure 6).
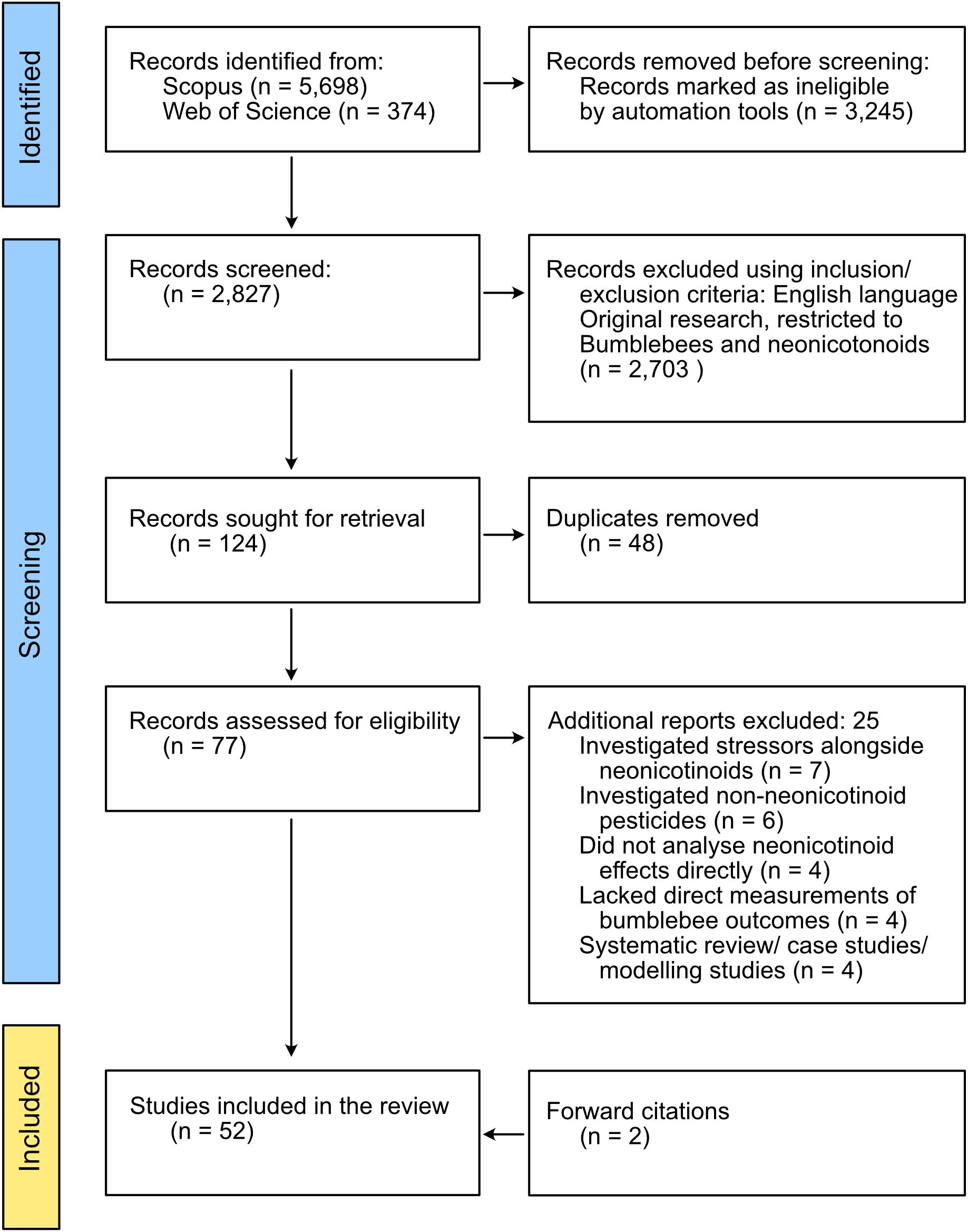
Figure 1. Flowchart of studies selected for the systematic analysis of the impact of neonicotinoids on bumblebees adapted from the PRISMA 2020 guidelines.
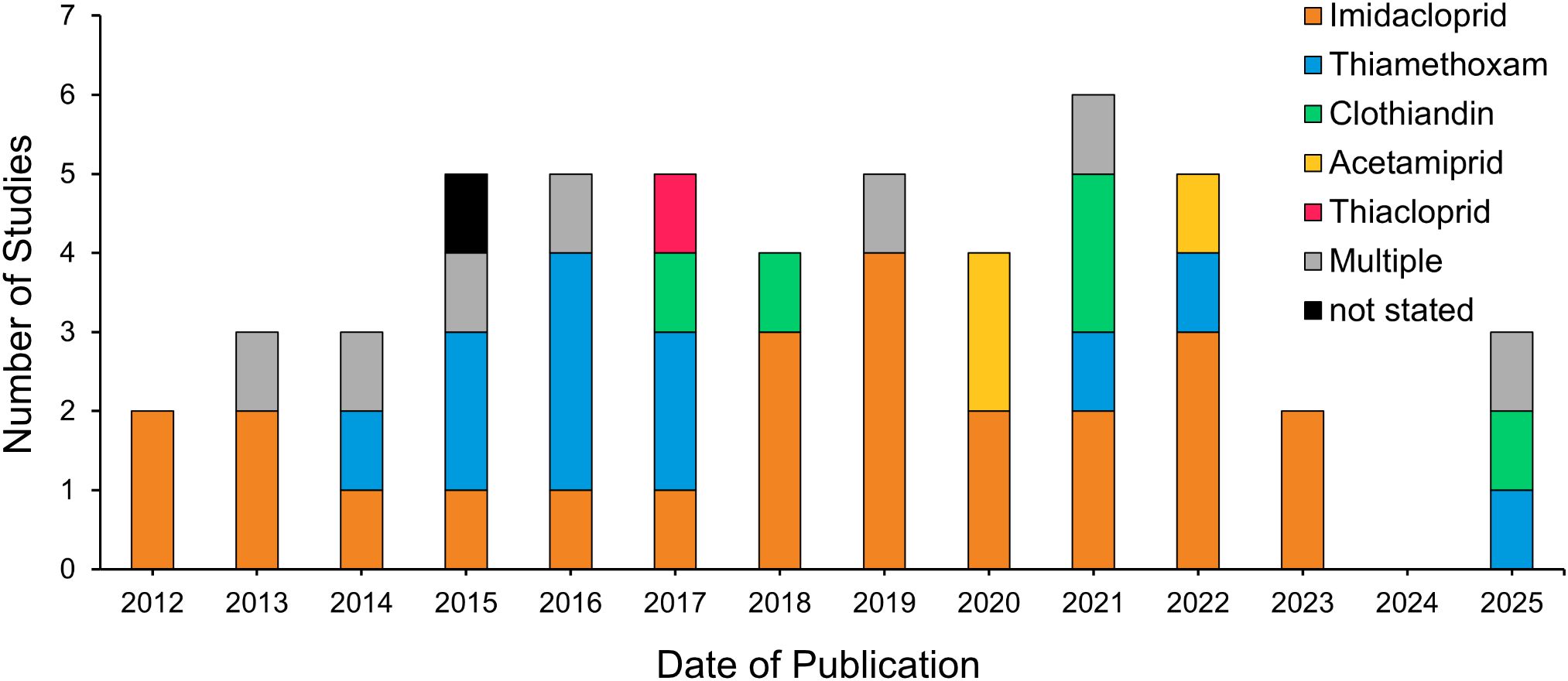
Figure 2. Development of research on the effect of neonicotinoids on bumblebees over time according to compound studied.
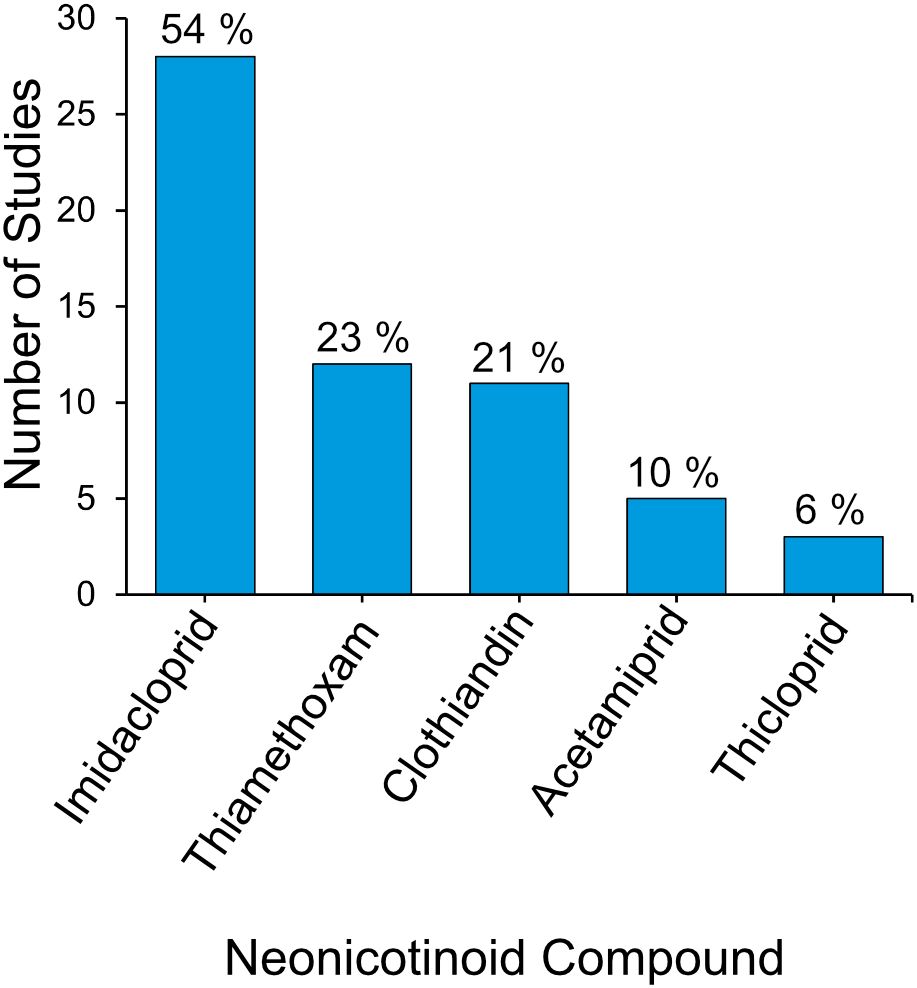
Figure 3. Frequency distribution of studied neonicotinoid compounds across 52 systematically identified studies with percentage of total studies indicated.
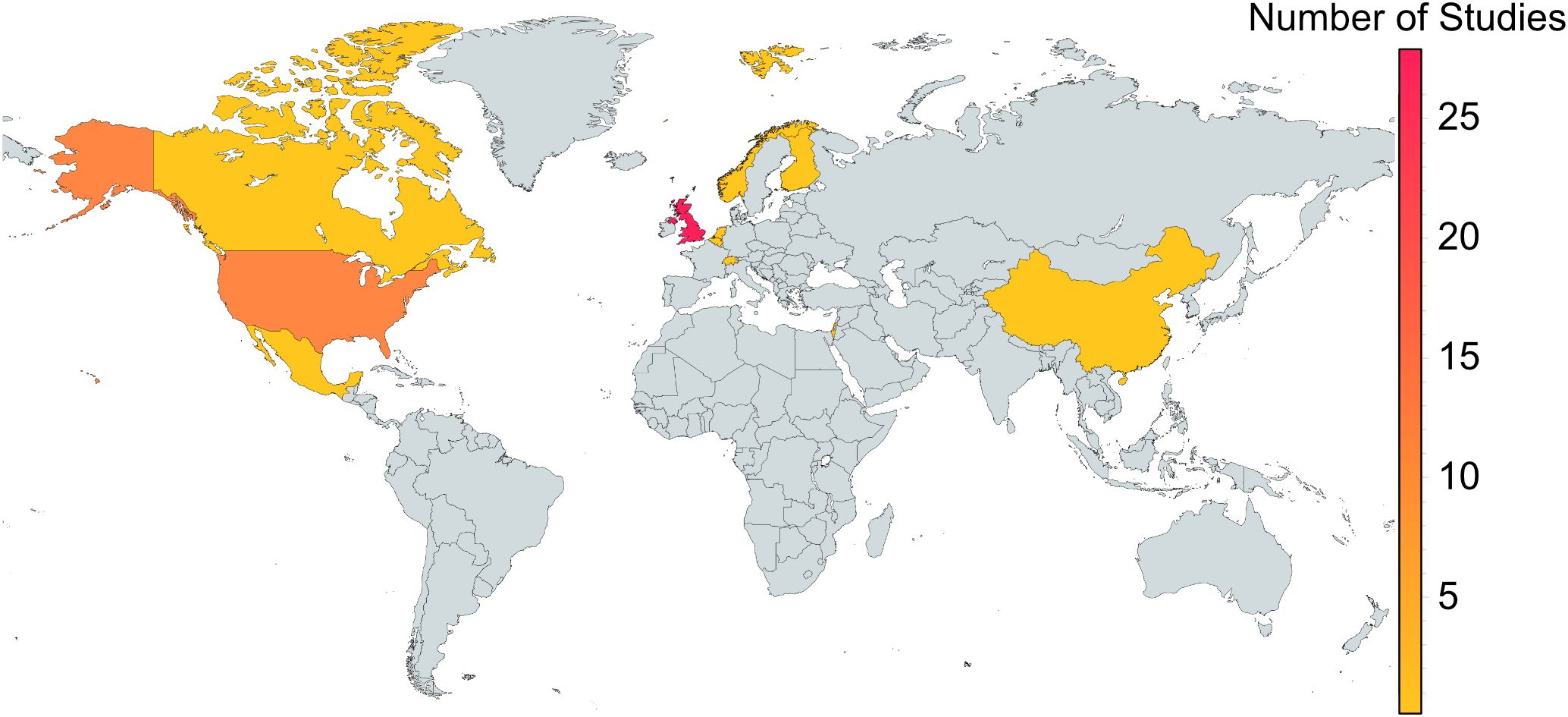
Figure 4. Geographical distribution of research on neonicotinoid impacts on bumblebees. Colors indicate countries ranging from 1 study (yellow) to >28 studies (pink).
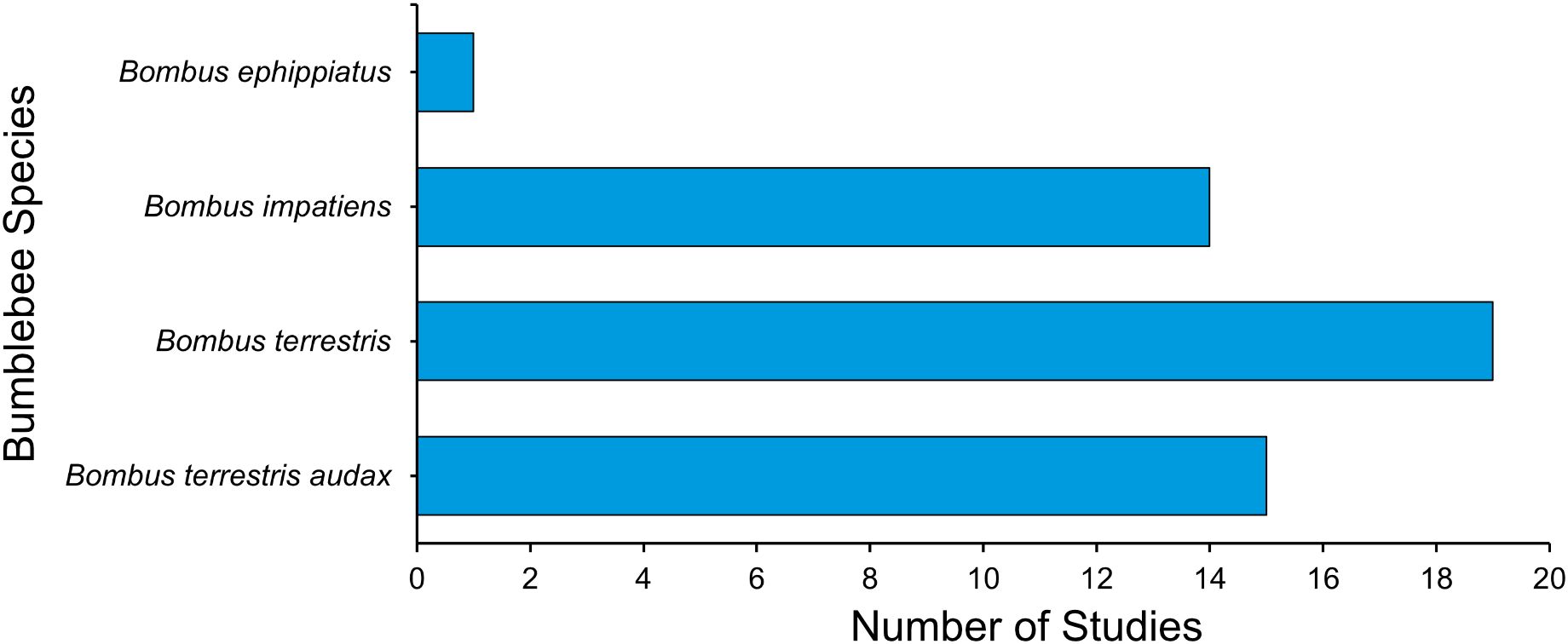
Figure 5. Frequency distribution of bumblebee taxa in the research literature regarding the impact of neonicotinoids on health and physiology across 51 systematically identified studies (one published study does not report on species used).
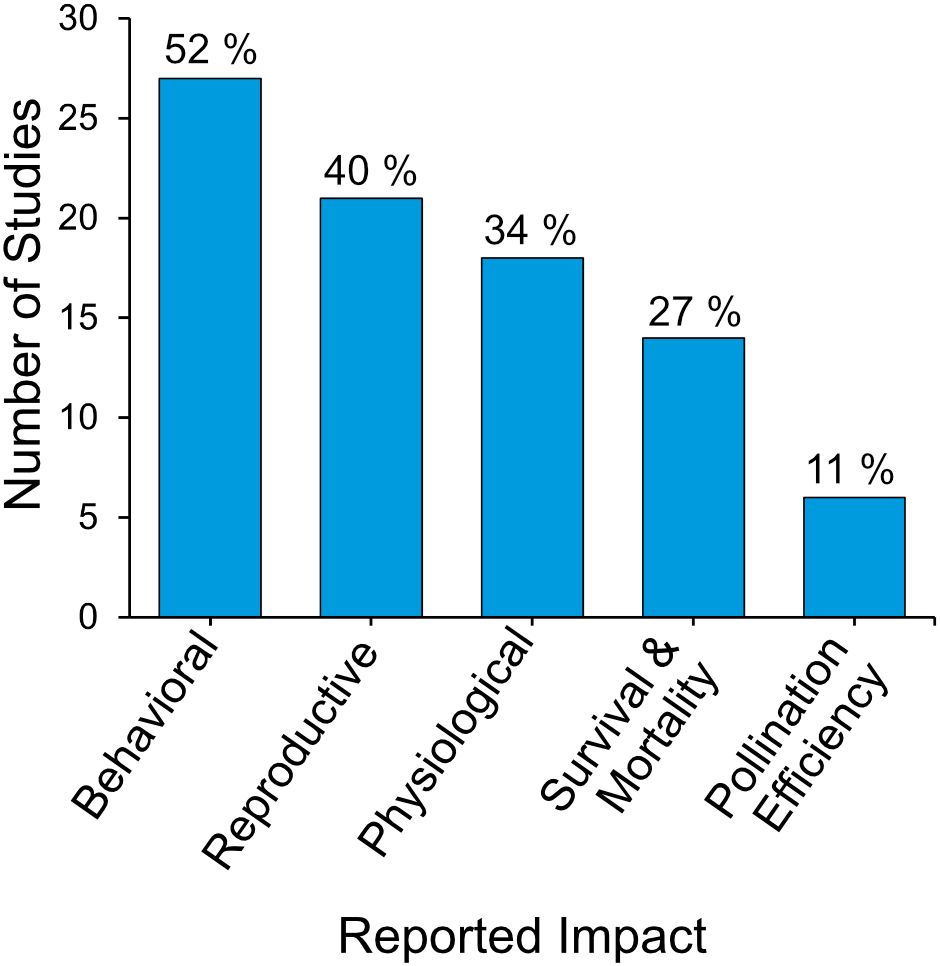
Figure 6. Frequency distribution of the observed neonicotinoid effects on bumblebee health and physiology across 52 systematically identified studies, with the percentage of total studies indicated.
3 Results
A total of 52 original research publications matched our criteria (Figure 1). Full references for all publications and data for each primary research publication are presented in Supplementary Table S1. Relevant literature was published from 2012 onward, with approximately half of the studies published within the last 6 years, demonstrating a rapid expansion of the research field (Figure 2). The highest number of studies was published in 2021 (n = 6).
Imidacloprid was the most commonly studied compound (included in 54% of studies, n = 28), followed by thiamethoxam (23%, n = 12) and clothianidin (19%, n = 11; Figure 3). Acetamiprid and thiacloprid were less frequently studied, accounting for 10% (n = 5) and 6% (n = 3).
Primary research studies were conducted in 10 countries. However, 81% were performed in just two countries: United Kingdom (n = 28) and the United States (n = 14; Figure 4). Two studies were conducted in Switzerland and Norway, whereas single studies were conducted in the Netherlands, Belgium, Finland, Israel, Mexico, China, and Canada.
Studies included in this review represented four bumblebee taxa (Figure 5). Bombus terrestris was the most frequently studied (n = 20), followed by its subspecies Bombus terrestris audax (n = 16). Bombus impatiens was featured in 15 studies, whereas Bombus ephippiatus was notably underrepresented, with only one published study.
Behavioral effects were the most frequently reported impact of neonicotinoids on bumblebees, comprising 52% of studies (n = 27), followed by reproductive (40%, n = 21) and physiological effects (35%, n = 18; Figure 6). Survival and mortality outcomes were documented in 27% of studies (n = 14), whereas pollination efficiency and ecosystem impacts were the least observed, reported in 11% of studies (n = 6).
4 Discussion
With pollinator declines globally recognized, understanding the specific effects of neurotoxic pesticides on bumblebee health and behavior is essential. This systematic review identified 52 studies investigating the impacts of neonicotinoids on bumblebees (Figure 1).
4.1 Neonicotinoid bans and ecological events drive research on bumblebee impacts
The increasing publication trend from 2012 to 2021 (Figure 2) likely reflects growing scientific scrutiny around the use of neonicotinoids. The EU applied a partial ban of neonicotinoids in 2013, followed by the complete ban of three compounds in outdoor field crops in 2018 (Sgolastra et al., 2020; Dentzman et al., 2025). During 2019-2023, emergency pesticide exemptions were made to allow the use of imidacloprid and thiamethoxam in sugar beet, but this decision was reversed in 2023 following appeals (Epstein et al., 2022). Exemptions for neonicotinoid use in the United Kingdom (which left the European Union in 2021) were feasible up to 2024 (Coe et al., 2024; DEFRA, 2025). Currently, the use of cyanoamidine neonicotinoids has not been restricted in the EU, and use of acetamiprid is approved until February 2033, with approval of sulfoxaflor and flupyradifurone, up for renewal in 2025 (Dentzman et al., 2025 and references within).
While slower to implement restrictions on the use of neonicotinoids than the European Union, state-level bans have been implemented (U.S. EPA, 2019; Dentzman et al., 2025). As of 2023, at least 20 states have imposed some form of restriction to protect pollinators (National Caucus of Environmental Regulators, 2023).
The peak in publications in 2021 could have been influenced by the resurgence of virus yellows in sugar beet in 2020 following the EU ban on neonicotinoid seed treatments (Dewar and Qi, 2021), prompting further investigation into the broader consequences of these regulations. Publication delays (Björk and Solomon, 2013) also suggest that research following the bans likely contributed to the later publication surge.
4.2 Neonicotinoid compounds studied reflect commercial use and toxicity profiles: imidacloprid, thiamethoxam, and clothianidin
Imidacloprid, thiamethoxam, and clothianidin were the most frequently studied neonicotinoids (Figures 2, 3). This may reflect the greater risk of nitroguanidine neonicotinoids on pollinators relative to the lower threat cyanoamidine group (Buszewski et al., 2019). An alternative explanation for the prevalence of studies on these compounds has been proposed by Lundin et al. (2015), who identified a positive correlation between the number of studies on each neonicotinoid and its global sales, indicating that compounds with higher commercial usage tend to be more extensively researched. This aligns with findings from Thompson (2020) who identified that in 2012, imidacloprid, thiamethoxam, and clothianidin accounted for 85% of all neonicotinoids sold. Of the three compounds, imidacloprid is the most widely used neonicotinoid, with registered applications on over 140 crops across 120 countries (Sheets, 2010; Jeschke et al., 2011), explaining high research attention. An additional factor which may contribute to the prevalence of imidacloprid prevalence in research may be linked to their strong binding affinity for nAChRs, which influences their toxicological effects and associated risks to bees (Moffat et al., 2016).
In comparison, acetamiprid has been studied less extensively due to its classification as a lower-risk neonicotinoid. Regulatory assessments have found it to pose minimal toxicity to bees at field-realistic concentrations, leading to its continued unrestricted use in the EU since 2021 (House of Commons, 2022). This coincides with the two published studies on acetamiprid in 2020 and 2022 (Figure 2). While high doses can cause sublethal effects, its lower overall risk has contributed to reduced research focus (Varga-Szilay and Tóth, 2022).
Similarly, thiacloprid was among the least studied neonicotinoids, likely due to its comparatively lower acute toxicity to bees and its classification as relatively “bee-safe” (Cabezas and Farinós, 2022; Ellis et al., 2017). Although residue concentrations can be high, the extended time to reach lethal doses reduces immediate risk (Cabezas and Farinós, 2022). Despite this, approval for agricultural use of thiacloprid was discontinued in the EU in 2021, with limited ongoing use in greenhouses (Bai et al., 2025).
Dinotefuran and nitenpyram were absent from the studies identified in this review, likely due to their less prevalent use in agriculture (Camp and Lehmann, 2021). While dinotefuran has been implicated in bee mortality events, research on its sublethal effects remains limited (Hatfield et al., 2021). In contrast, nitenpyram is primarily used as a veterinary flea treatment; however, there have been reported adverse effects on non-target organisms (Rust et al., 2003; Zhu et al., 2020).
Hopwood et al. (2012) show that imidacloprid and clothianidin exhibit high acute toxicity to bumblebees. Although precise LD50 (lethal dose 50%: a determined dose, expected to cause mortality in 50% of treated subjects; Myers, 2017) values have not been established, research indicates that both neonicotinoids are highly harmful upon acute contact exposure. Additionally, imidacloprid is highly toxic when administered orally. Comparatively, clothianidin has slightly higher toxicity than imidacloprid through contact exposure. Hopwood et al. (2012) suggest that the acute toxicity of other neonicotinoids, including acetamiprid, dinotefuran, thiacloprid, and thiamethoxam, remain untested.
The wide range in neonicotinoid toxicities for both target and non-target organisms poses challenges for the regulatory risk assessment conducted during pesticide authorization. In particular, the lesser known impacts of the cyanoamidine group limits our understanding of the wider scale impact of neonicotinoid use (Grout et al., 2020; Dentzman et al., 2025). As such, risk assessments are usually based upon limited standard test species (U.S. EPA, 2021; EFSA, 2013). As such, regulatory threshold levels (RTLs), which determine the acute and chronic thresholds for unacceptable ecological effects, may underestimate actual risk to diverse taxa.
4.3 The geographical distribution of publications shows a bias toward the UK and U.S.
The geographical distribution of research investigating the effects of neonicotinoids on bumblebees exhibits a notable bias, with most studies concentrated in the United Kingdom and the United States (Figure 4). This may reflect capacity and interest in bee-related research, their prominence as major agricultural producers, and also policy requirements for the application of insecticides, which has driven scientific inquiry into the ecological impacts (Donley, 2019). In the U.S. alone, over 1,000 neonicotinoid-containing products are registered with the EPA and remain available on the market (Thompson, 2020). Meanwhile, the EU’s ban may have intensified research efforts to assess the ecological risks posed by these chemicals.
Bumblebees are particularly abundant in temperate regions, where their adaptation to cooler climates makes them key pollinators in these ecosystems (Goulson, 2009). The regional focus is also consistent with bumblebee population trends, as species declines have been widely documented across Europe and North America (Williams and Osborne, 2009). The UK has lost three of its 27 native bumblebee species, with seven more designated as priority species due to significant declines (Reid et al., 2020). These population declines further incentivized research efforts in these regions, as the potential consequences of pollinator losses for ecosystems and food security have become an increasing concern.
Despite extensive research in Europe and North America, studies remain limited in other regions. East Asia, for example, hosts the richest bumblebee diversity globally, with over 50% of known species and approximately 10% of the world’s endemic species concentrated in countries such as China, Mongolia, Japan, and parts of Russia and India (Naeem et al., 2019). Similarly, South America supports diverse bumblebee populations (Krechemer et al., 2020). However, as noted by Dirilgen et al. (2023), stingless bees (Apidae: Apinae: Meliponini) are more commonly used for commercial pollination in South America which could explain the lack of research on bumblebees.
Research in these regions is crucial, particularly as warmer climates typically support higher ectotherm populations, including insect pests and their associated pathogens (Clarke and Pörtner, 2010; Simon and Amarasekare, 2024). Increased temperatures accelerate insect metabolic rates, leading to heightened food consumption and population growth, escalating pest pressure and reliance on insecticides (Deutsch et al., 2018; Schneider et al., 2022). Additionally, the economic challenges faced by warmer, less developed countries (Van de Vliert et al., 2000) may increase their vulnerability to agricultural losses and subsequent reliance on broad-spectrum insecticides.
Globally, the continued widespread use of the three major neonicotinoids is facilitated by the absence of pesticide legislation in approximately 35% of countries (Klingelhöfer et al., 2022). This may, in part, be a result of a lack of national-level reporting of pesticide use in many countries. For example, Tai et al. (2025) suggested that a lack of centralized collection for pesticide data in New Zealand may contribute to masking the true scale and frequency of neonicotinoid use and that current legislation is often restricted to a single taxonomic group. In the case of New Zealand, legislation is restricted to the impact of pesticide use on honeybees only, despite the presence of four bumblebee species and many ground-dwelling native bees (Tai et al., 2025). Lack of regulation or centralized collection has also been reported for many parts of Africa and Asia (WHO, 2018).
4.4 The bumblebee species investigated indicate a taxonomic bias toward commercially significant species: Bombus terrestris and Bombus impatiens
The predominant focus on Bombus terrestris, Bombus terrestris audax, and Bombus impatiens in neonicotinoid research (Figure 5) likely reflects their ecological distribution and commercial significance. Bombus terrestris has a broad natural range across Europe, North Africa, and the Near East (Widmer et al., 1998). Since 1988, B. terrestris has been commercially reared for greenhouse crop pollination in Europe, driving research interest due to their ecological and economic importance (Gosterit and Baskar, 2016). Their ability to establish large colonies and adapt to artificial conditions makes them a practical species for commercial and experimental purposes (Velthuis and van Doorn, 2004). Likewise, B. terrestris audax, a subspecies endemic to Britain and Ireland, is heavily studied in the UK, aligning with the country’s prominence in neonicotinoid research (Goulson, 2010).
In North America, Bombus impatiens is the primary commercially significant species (Winter et al., 2006). Its widespread use in agricultural pollination likely explains its frequent inclusion in neonicotinoid studies. Conversely, Bombus ephippiatus is a Mesoamerican species with limited representation in research, likely due to its restricted distribution (Duennes et al., 2012). However, considering there are over 260 known species worldwide, with native species in all biogeographic regions except for Australia, New Zealand, and sub-Saharan Africa (Cameron and Sadd, 2020); this taxonomic bias indicates critical gaps in our understanding of neonicotinoids on bumblebee health and behavior.
Understanding species-level differences in ecotoxicity is important for both scientific understanding and regulation (Jütte et al., 2023). While B. terrestris and B. impatiens are commercially available, easy to rear and convenient for experimentation, they may not be representative of wild species with smaller populations or different ecologies. For example, B. terrestris has large colonies containing hundreds of workers, which may help buffer against loss. In comparison, B. pseudobaicalensis and B. schrencki have smaller colonies (Cueva Del Castillo et al., 2015) and therefore may be more vulnerable, since losing a few foragers can mean a significant impact on resource intake.
Larger-bodied bumblebees often travel further when foraging (Greenleaf et al., 2007), so any disruptions to navigation or endurance from neonicotinoids may have a greater impact on these species. In addition, it is possible that there will be a difference based on whether a species is a generalist or a specialist when it comes to pollination, with a narrower floral niche which includes impacted plants equating to increased risk of exposure (Jütte et al., 2023). Differences may also relate to the timing of colony activity, with early emerging species more likely to be exposed to high neonicotinoid residues during spring sowing.
It is also possible that species may metabolize insecticides at different rates, making some more resistant than others (Zarevcka, 2013; Manjon et al., 2018). This reflects the findings of Jütte et al. (2023) who found that field-realistic concentrations of a pyrethroid insecticide had differential effects between multiple bee species which they attributed to individual bee weight combined with additional ecological, phylogenetic, or toxicogenomic parameters.
As bumblebees fill different pollination niches, if neonicotinoids selectively impact some species more than others, plant–pollinator networks can become unbalanced, potentially resulting in some plants losing their most effective pollinators. Therefore, risk assessments that rely on a single species underestimate the variability of effects and including multiple species ensures that pesticide approvals account for the most vulnerable pollinators (Jütte et al., 2023).
4.5 Observed effects of neonicotinoids disrupt bumblebee behavior, reproductive success, and ecosystem functioning
Neonicotinoid exposure has been shown to disrupt key bumblebee behaviors (Figure 6). Of our identified studies, foraging behavior was the most frequently reported change with affected bees exhibiting reduced flower visitation rates (Stanley et al., 2015), altered flower preferences (Siviter et al., 2021), and decreased foraging motivation (Lämsä et al., 2018; Muth and Leonard, 2019). Chronic exposure can also impair foraging efficiency by reducing flight endurance, increasing exhaustion rates (Sargent et al., 2021), and affecting homing ability (Stanley et al., 2016; Kenna et al., 2019). Such disruptions can cascade through the colony, reducing resource intake and impairing colony growth.
Learning and memory deficits further exacerbate changes in behavior, with exposed bumblebees demonstrating impaired associative learning, olfactory conditioning (Stanley et al., 2015; Muth et al., 2019), and spatial memory (Samuelson et al., 2016). Since bumblebees rely on learned foraging routes to locate and return to high-quality floral resources, these cognitive impairments likely contribute to colony-wide reductions in food intake. Additionally, exposure reduces responsiveness to food rewards (Smith et al., 2020), decreases sucrose and nectar consumption (Paus-Knudsen et al., 2023; Laycock et al., 2012, Laycock et al., 2014), and diminishes food storage within the colony (Scholer and Krischik, 2014). Disruptions in essential social behaviors, such as nest thermoregulation (Crall et al, 2018) and brood care (Chole et al., 2022), further weaken colony cohesion, reducing reproductive success and survival.
Reproductive effects were also commonly reported, with exposed colonies producing fewer new queens, limiting their ability to establish future generations (Whitehorn et al., 2012; Goulson, 2015). Brood production and overall colony growth were often impaired, with fewer workers and reproductive individuals emerging (Chole et al., 2022; Martínez de Castro Dubernard et al., 2022; Wintermantel et al., 2018). Delayed colony development, including slower nest initiation and egg laying (Wu-Smart and Spivak, 2018), further compromises population stability. Male reproduction was also affected, with reduced drone weight, disrupted production timing, and impaired sperm viability limiting mating success (Straub et al., 2022; Minnameyer et al., 2021). These impairments align with previous research demonstrating neonicotinoid-induced colony decline but extend existing knowledge by emphasizing the vulnerability of reproductive individuals.
Physiologically, neonicotinoids disrupt neurophysiology by interfering with nAChRs, impairing neural development (Martín-Blázquez et al., 2023), and altering brain ion transport-related gene expression (Witwicka et al., 2025). Increased acetylcholinesterase expression (Samson-Robert et al., 2015) suggests impaired neurotransmission, potentially explaining deficits in foraging efficiency and learning ability. Metabolic effects include increased energy demands and reduced detoxification capacity, with exposure elevating oxygen consumption and metabolic activity in flight muscles and the brain (Sargent et al., 2021). Together, these changes may lead to hyperactivity and exhaustion. Reduced detoxification efficiency at higher neonicotinoid concentrations (Aarønes et al., 2021) suggests a threshold where bees struggle to process toxins, increasing overall physiological stress. In addition, exposure also alters nectar-related immune responses (Richman et al., 2022) and upregulates antimicrobial peptide gene expression (Simmons and Angelini, 2017), which could lead to immune overactivation and long-term susceptibility to pathogens.
Survival and mortality effects have been documented, with exposed workers experiencing reduced lifespan and colonies facing premature failure (Laycock et al., 2014; Ellis et al., 2017). Even sublethal doses shorten lifespan by altering nectar chemistry (Richman et al., 2022), which may accelerate colony collapse. Although studied less, widespread bee mortality in certain regions has been associated with large-scale neonicotinoid use (Klingelhöfer et al., 2022).
Direct measures of pollination efficiency and ecosystem impacts were less observed, which is likely a result of few studies being performed in the field relative to laboratory conditions (five out of 52; Supplementary Table S1; Jütte et al., 2023). Other potential reasons for reduced focus in this research area could be the complexity of experimental design required; timeframes needed for study; and potential conflicting factors (beyond neonicotinoid application), which may limit the ability to make a causal link to insecticide use. Additionally, funding and policy requirements have driven focus on establishing whether neonicotinoids directly harm bees, as opposed to longer-term ecosystem-scale studies which are more costly and uncertain.
In contrast, more studies reported behavioral impairments, reduced reproductive success, and increased mortality in bumblebees, traits which are inherently linked to pollination performance and ecosystem functioning. Behavioral effects such as reduced and altered foraging can lead to fewer flower visits and diminished pollen collection (Stanley et al., 2015; Lämsä et al., 2018; Kolano et al., 2021). Compromised buzz pollination efficiency, with shorter buzzing duration, results in lower pollen deposition and reduced crop yield (Whitehorn et al., 2017; Stanley et al., 2015). Furthermore, smaller or poorly structured colonies result in fewer foragers, limiting floral visitation and pollen transfer (McGrady et al., 2021). This reduced reproductive output also compromises the establishment of future colonies, contributing to long-term pollinator population declines. Similarly, increased mortality, particularly among foragers, reduces the duration and scale of pollination activity within the colony, further weakening pollination services. At a broader scale, exposure disrupts plant–pollinator networks, with altered interactions between bumblebees and wild plants, potentially affecting plant reproductive success and overall ecosystem stability (Stanley and Raine, 2016; Arce et al., 2017).
These findings reinforce concerns about the widespread ecological risks of neonicotinoids while highlighting key gaps in current knowledge. The predominance of behavioral effects within the literature emphasizes that the sublethal impacts on foraging, learning, and social behaviors may be more consequential than previously acknowledged. While reproductive and physiological effects were also substantial, survival and pollination impacts were underreported. This suggests that subtle, chronic impairments in behavior and reproduction could undermine colony health before direct mortality is observed. Given that pollination efficiency and ecosystem impacts were the least observed, further research is needed to explore how other disruptions translate into broader ecological consequences.
4.6 Balancing agricultural benefits versus ecological risks: the impact of neonicotinoids on pollinators and the environment
As systemic insecticides, neonicotinoids offer advantages such as high efficacy, targeted action, and ease of application (Simon-Delso et al., 2015). Their use as seed treatments reduces the need for repeated foliar spraying, lowering labor costs, and farmwork exposure. They effectively combat pests resistant to older insecticides like organophosphates and pyrethroids (Venkatesan et al., 2022). This long-term crop protection benefits crops like sugar beet, which is often harvested before flowering, reducing direct pollinator exposure (Odemer et al., 2023).
However, despite their agricultural advantages, neonicotinoids contribute significantly to environmental contamination due to their systemic nature, high water solubility, and persistence in soils and waterways (Stehle et al., 2023). Their persistence in soil, sometimes exceeding 1,000 days, leads to accumulation and prolonged contamination (Bonmatin et al., 2015). They have been detected in non-crop plants like wildflowers at field margins, exposing pollinators even in untreated areas (Stewart et al., 2014).
Some argue that banning neonicotinoids may increase reliance on older, more toxic insecticides, which could pose greater risks to birds, mammals, and fish (Blake, 2018). Others argue that their routine use as seed coatings contradicts integrated pest management principles, which advocate targeted applications based on pest presence (Goulson, 2013). The phenomenon of “delayed toxicity” suggests that lethal effects may only appear after prolonged exposure, complicating environmental risk assessments (Sánchez-Bayo and Tennekes, 2020). While their agricultural benefits are clear, balancing food security with pollinator conservation requires ongoing research into sustainable pest management solutions that minimize ecological harm while ensuring adequate crop protection.
4.7 Limitations and future directions
Despite adhering to PRISMA guidelines, this review acknowledges several limitations. Inconsistent reporting, especially regarding publication bias assessment, complicates the review evaluation process (Moher et al., 2009). It is also important to acknowledge that this review is based exclusively on Scopus and Web of Science studies.
The main limitation of neonicotinoid research on bumblebees result from variation in experimental design (lab vs. field), dose ranges, and exposure durations. Together, this leads to results that are not easily comparable or generalizable. Lab studies provide mechanistic insights but lack realism, whereas field studies provide realism but lack control. Future research should prioritize field-realistic exposures and incorporate more naturalistic or semi-field approaches to reflect real-world conditions (Lundin et al., 2015; Jütte et al., 2023). While lab-based studies permit easy replication and limit confounding factors, doses may not reflect real-world exposure and lack natural variation in diets and population dynamics. This may over- or underestimate the risk depending on experimental setup.
The strong geographical bias toward the UK and the U.S. limits global relevance. This may, in part, be due to restriction to English language only studies. Regions with high bumblebee diversity, such as East Asia and South America, remain underrepresented, constraining the global applicability of existing findings.
Taxonomic bias is another key limitation to understanding the impacts of neonicotinoids on bumblebees (Jütte et al., 2023). The predominance of commercially important species, such as Bombus terrestris and Bombus impatiens, may overlook responses in other species with different sensitivities or ecological roles, which differ in genetics, stress tolerance, foraging ecology, and disease load. Limited species coverage and inconsistent endpoints make it challenging to draw robust conclusions about population-level risks; therefore, broader species inclusion will be necessary for accurate risk assessments.
The focus of most studies on the three main neonicotinoids may neglect the potential effects of lesser-studied compounds. Future research should assess the safety of alternative neonicotinoids, notably those considered lower risk, to support more sustainable pest management strategies.
Finally, although this review categorized observed effects, it did not quantify exposure thresholds. Establishing dose–response relationships is critical for policy development. Future studies should prioritize field-realistic exposures, long-term monitoring, species-specific responses, and broader geographic and climatic coverage. Despite logistical and methodological challenges, these steps are essential for a comprehensive and ecologically relevant understanding of neonicotinoid effects.
5 Conclusion
Neonicotinoids are widely applied systemic insecticides, with increasing evidence suggesting toxic effects on non-target organisms including pollinators. This systematic review identified 52 primary studies published within the last 20 years which investigate the effects of neonicotinoids on bumblebees. Key trends were identified, such as geographical and taxonomic biases and commonly reported neonicotinoid compounds and effects. The findings consistently showed sublethal effects, such as impaired foraging, learning, memory, and reproduction, all of which compromise colony health and long-term population viability. Physiological disruptions, including neurotoxicity and immune stress, were also evident. However, the limited focus on pollination efficiency highlights a critical gap in understanding the broader ecological implications of neonicotinoid exposure. Despite regulatory bans and restrictions, debate continues over the effectiveness of neonicotinoid compounds. This review emphasizes the need for expanded research into alternative pest management strategies that balance agricultural productivity and pollinator conservation. With rising global food demands and increasing pest pressures, addressing current research gaps will be vital to developing ecologically sustainable agricultural systems that protect pollinators like bumblebees.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
DD: Methodology, Conceptualization, Writing – original draft, Writing – review & editing, Investigation, Visualization, Formal analysis, Data curation. AG: Funding acquisition, Supervision, Project administration, Writing – review & editing, Visualization, Conceptualization, Validation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was performed by DD as part of her final research project for BSc (Hons) in Environmental Science at the University of Nottingham. AG was supported by the BBSRC (grant number BB/Y513866/1).
Acknowledgments
We wish to thank Dr Reinhard Stöger for critical feedback on the manuscript and the two reviewers for constructive feedback during the peer review process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frbee.2025.1657493/full#supplementary-material
References
Aarønes M. R., Paus-Knudsen J. S., Nielsen A., Rundberget J. T., and Borgå K. (2021). Within-body distributions and feeding effects of the neonicotinoid insecticide clothianidin in bumblebees (Bombus terrestris). Environ. Toxicol. Chem. 40, 2781–2790. doi: 10.1002/etc.5154
Abrol D. P., Mondal A., and Shankar U. (2021). Importance of bumble bees for crop pollination and food security. J. Palynology 57, 9–37.
Arce A. N., David T. I., Randall E. L., Ramos Rodrigues A., Colgan T. J., Wurm Y., et al. (2017). Impact of controlled neonicotinoid exposure on bumblebees in a realistic field setting. J. Appl. Ecol. 54, 1199–1208. doi: 10.1111/1365-2664.12792
Bai L., Pan S., Sun Y., Shan Y., Song X., Wang D., et al. (2025). Spatiotemporal dissipation, metabolic dynamics and bumblebees’ toxicity risk of the neonicotinoid insecticide thiacloprid in greenhouse conditions. J. Hazardous Materials 491, 137897. doi: 10.1016/j.jhazmat.2025.137897
Basley K. (2019). The effect of neonicotinoid pesticides on non-target organisms (Doctoral dissertation, University of Sussex, Thesis). https://hdl.handle.net/10779/uos.23467235.v1
Bass C. and Field L. M. (2018). Neonicotinoids. Curr. Biol. 28, R772–R773. doi: 10.1016/j.cub.2018.05.061
Becher M. A., Twiston-Davies G., Penny T. D., Goulson D., Rotheray E. L., and Osborne J. L. (2018). Bumble-BEEHAVE: A systems model for exploring multifactorial causes of bumblebee decline at individual, colony, population and community level. J. Appl. Ecol. 55, 2790–2801. doi: 10.1111/1365-2664.13165
Bie M., Song K., Dong H., Zhao W., Lin H., Shi D., et al. (2025). Advancing sustainable agriculture through bumblebee pollination: bibliometric insights and future directions. Sustainability 17, 2177. doi: 10.3390/su17052177
Björk B. C. and Solomon D. (2013). The publishing delay in scholarly peer-reviewed journals. J. informetrics 7, 914–923. doi: 10.1016/j.joi.2013.09.001
Blake R. (2018). EU neonicotinoid ban removes vital tools in global fight against pests. Outlooks Pest Manage. 29, 197–200. doi: 10.1564/v29_oct_02
Bonmatin J. M., Giorio C., Girolami V., Goulson D., Kreutzweiser D. P., Krupke C., et al. (2015). Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci pollut. Res. 22, 35–67. doi: 10.1007/s11356-014-3332-7
Buszewski B., Bukowska M., Ligor M., and Staneczko-Baranowska I. (2019). Aholistic study of neonicotinoids neuroactive insecticides—proper-ties, applications, occurrence, and analysis. Environ. Sci. pollut. Res. 26, 34723–34740. doi: 10.1007/s11356-019-06114-w
Cabezas G. and Farinós G. P. (2022). Sensitivity of buff-tailed bumblebee (Bombus terrestris L.) to insecticides with different mode of action. Insects 13, 184. doi: 10.3390/insects13020184
Cameron S. A. and Sadd B. M. (2020). Global trends in bumble bee health. Annu. Rev. entomology 65, 209–232. doi: 10.1146/annurev-ento-011118-111847
Camp A. A. and Lehmann D. M. (2021). Impacts of neonicotinoids on the bumble bees Bombus terrestris and Bombus impatiens examined through the lens of an adverse outcome pathway framework. Environ. Toxicol. Chem. 40, 309–322. doi: 10.1002/etc.4939
Chole H., de Guinea M., Woodard S. H., and Bloch G. (2022). Field-realistic concentrations of a neonicotinoid insecticide influence socially regulated brood development in a bumblebee. Proc. R. Soc. B 289, 20220253. doi: 10.1098/rspb.2022.0253
Clarke A. and Pörtner H. O. (2010). Temperature, metabolic power and the evolution of endothermy. Biol. Rev. 85, 703–727. doi: 10.1111/j.1469-185X.2010.00122.x
Coe S., Stewart I., and Sutherland N. (2024). Environmental impact of neonicotinoids and other pesticides. Available online at: https://researchbriefings.files.parliament.uk/documents/CDP-2024-0047/CDP-2024-0047.pdf?ref=hir.harvard.edu (Accessed August 28, 2025).
Crall J. D., Switzer C. M., Oppenheimer R. L., Ford Versypt A. N., Dey B., Brown A., et al. (2018). Neonicotinoid exposure disrupts bumblebee nest behavior, social networks, and thermoregulation. Science 362, 683–686. doi: 10.1126/science.aat1598
Cueva Del Castillo R., Sanabria-Urbán S., and Serrano-Meneses M. A. (2015). Trade-offs in the evolution of bumblebee colony and body size: a comparative analysis. Ecol. Evol. 5, 3914–3926. doi: 10.1002/ece3.1659
DEFRA (2025). Statement of reasons for the decision on the application for emergency authorisation of the use of Cruiser SB on sugar beet crops in England in 2025. Available online at: https://www.gov.uk/government/publications/neonicotinoid-product-as-seed-treatment-for-sugar-beet-emergency-authorisation-application/statement-of-reasons-for-the-decision-on-the-application-for-emergency-authorisation-of-the-use-of-cruiser-sb-on-sugar-beet-crops-in-england-in-2025 (Accessed August 28, 2025).
Dentzman K., Franklin D., Avemegah E., and Goldberger J. R. (2025). An overview of agricultural neonicotinoid regulation in the EU, Canada, and the United States. Pest management science. doi: 10.1002/ps.70126
Deutsch C. A., Tewksbury J. J., Tigchelaar M., Battisti D. S., Merrill S. C., Huey R. B., et al. (2018). Increase in crop losses to insect pests in a warming climate. Science 361, 916–919. doi: 10.1126/science.aat3466
Dewar A. M. and Qi A. (2021). The virus yellows epidemic in sugar beet in the UK in 2020 and the adverse effect of the EU ban on neonicotinoids on sugar beet production. Outlooks Pest Manage. 32, 53–59. doi: 10.1564/v32_apr_02
Dirilgen T., Herbertsson L., O’Reilly A. D., Mahon N., and Stanley D. A. (2023). Moving past neonicotinoids and honeybees: A systematic review of existing research on other insecticides and bees. Environ. Res. 235, 116612. doi: 10.1016/j.envres.2023.116612
Donley N. (2019). The USA lags behind other agricultural nations in banning harmful pesticides. Environ. Health 18, 44. doi: 10.1186/s12940-019-0488-0
Duennes M. A., Lozier J. D., Hines H. M., and Cameron S. A. (2012). . Geographical patterns of genetic divergence in the widespread Mesoamerican bumble bee Bombus ephippiatus (Hymenoptera: Apidae). Mol. Phylogenet. Evol. 64, 219–231. doi: 10.1016/j.ympev.2012.03.018
EFSA (2013). Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge-of-field surface waters. EFSA J. 11, 3290. doi: 10.2903/j.efsa.2013.3290
Ellis C., Park K. J., Whitehorn P., David A., and Goulson D. (2017). The neonicotinoid insecticide thiacloprid impacts upon bumblebee colony development under field conditions. Environ. Sci Technol. 51, 1727–1732. doi: 10.1021/acs.est.6b04791
Epstein Y., Chapron G., and Verheggen F. (2022). What is an emergency? Neo-nicotinoids and emergency situations in plant protection in the EU. Ambio 51, 1764–1771. doi: 10.1007/s13280-022-01703-5
Ewere E. E., Reichelt-Brushett A., and Benkendorff K. (2021). Impacts of neonicotinoids on molluscs: what we know and what we need to know. Toxics 9, 21. doi: 10.3390/toxics9020021
Ghisbain G., Thiery W., Massonnet F., Erazo D., Rasmont P., Michez D., and Dellicour S.(2024). Projected decline in European bumblebee populations in the twenty-first century. Nature 628, 337–341. doi: 10.1038/s41586-023-06471-0
Gosterit A. and Baskar V. C. (2016). Impacts of commercialization on the developmental characteristics of native Bombus terrestris (L.) colonies. Insectes Sociaux 63, 609–614 (2016). doi: 10.1007/s00040-016-0507-x
Gould F., Brown Z. S., and Kuzma J. (2018). Wicked evolution: can we address the sociobiological dilemma of pesticide resistance? Science 360, 728–732. doi: 10.1126/science.aar3780
Goulson D. (2009). Bumblebees: behaviour, ecology, and conservation (Oxford University Press). doi: 10.1093/oso/9780199553068.001.0001
Goulson D. (2010). Impacts of non-native bumblebees in Western Europe and North America. Appl. Entomology Zoology 45, 7–12. doi: 10.1303/aez.2010.7
Goulson D. (2013). An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 50, 977–987. doi: 10.1111/1365-2664.12111
Goulson D. (2015). Neonicotinoids impact bumblebee colony fitness in the field; a reanalysis of the UK’s Food & Environment Research Agency 2012 experiment. PeerJ 3, e854. doi: 10.7717/peerj.854
Greenleaf S. S., Williams N. M., Winfree R., and Cremen C.(2007). Bee foraging ranges and their relationship to body size. Oecologia 153, 589–596. doi: 10.1007/s00442-007-0752-9
Grout T., Koenig P., Kapuvari J., and McArt S. (2020). Neonicotinoid insecticides in new york state: economic benefits and risks to pollinators. Available online at: https://cornell.app.box.com/v/2020-neonicotinoid-report (Accessed August 28, 2025).
Hatfield R. G., Strange J. P., Koch J. B., Jepsen S., and Stapleton I. (2021). Neonicotinoid pesticides cause mass fatalities of native bumble bees: a case study from Wilsonville, Oregon, United States. Environ. Entomology 50, 1095–1104. doi: 10.1093/ee/nvab059
Health Canada (2020). Update on the neonicotinoid pesticides. Available online at: https://www.Canada.ca/en/health-Canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/fact-sheets-other-resources/update-neonicotinoid-pesticides-2020.html (Accessed 06/19/2025).
Health Canada (2025). Neonicotinoid insecticides. Available online at: https://www.Canada.ca/en/health-Canada/services/consumer-product-safety/pesticides-pest-management/growers-commercial-users/neonicotinoid-insecticides.html (Accessed August 28, 2025).
Hladik M. L., Main A. R., and Goulson D. (2018). Environmental risks and challenges associated with neonicotinoid insecticides. Environ. Sci. Technol. 52, 3329–3335. doi: 10.1021/acs.est.7b06388
Hopwood J., Vaughan M., Shepherd M., Biddinger D., Mader E., Black S. H., et al. (2012). Are neonicotinoids killing bees. A review of research into the effects of neonicotinoid insecticides on bees, with recommendations for action (USA: Xerces Society for Invertebrate Conservation).
House of Commons (2022). Government approval for the use of neonicotinoids and the impact on bees. Number CDP 2022/0024. Available online at: https://researchbriefings.files.parliament.uk/documents/CDP-2022-0024/CDP-2022-0024.pdf (Accessed 06/19/2025).
Ihara M. and Matsuda K. (2018). Neonicotinoids: molecular mechanisms of action, insights into resistance and impact on pollinators. Curr. Opin. Insect Sci 30, 86–92. doi: 10.1016/j.cois.2018.09.009
Jeschke P., Nauen R., Schindler M., and Elbert A. (2011). Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 59, 2897–2908. doi: 10.1021/jf101303g
Jütte T., Wernecke A., Klaus F., Pistorius J., and Dietzsch A. C.. (2023). Risk assessment requires several bee species to address species-specific sensitivity to insecticides at field-realistic concentrations. Sci. Rep. 13, 22533. doi: 10.1038/s41598-023-48818-7
Kenna D., Cooley H., Pretelli I., Ramos Rodrigues A., Gill S. D., and Gill R. J. (2019). Pesticide exposure affects flight dynamics and reduces flight endurance in bumblebees. Ecol. Evol. 9, 5637–5650. doi: 10.1002/ece3.5143
Klingelhöfer D., Braun M., Brüggmann D., and Groneberg D. A. (2022). Neonicotinoids: A critical assessment of the global research landscape of the most extensively used insecticide. Environ. Res. 213, 113727. doi: 10.1016/j.envres.2022.113727
Kolano P., Borgå K., and Nielsen A. (2021). Temperature sensitive effects of the neonicotinoid clothianidin on bumblebee (Bombus terrestris) foraging behaviour. J. Pollination Ecol. 28, 138–152. doi: 10.26786/1920-7603(2021)633
Krechemer F. D. S., Marchioro C. A., and Butt N. (2020). Past, present and future distributions of bumblebees in South America: identifying priority species and areas for conservation. J. Appl. Ecol. 57, 1829–1839. doi: 10.1111/1365-2664.13650
Lämsä J., Kuusela E., Tuomi J., Juntunen S., and Watts P. C. (2018). Low dose of neonicotinoid insecticide reduces foraging motivation of bumblebees. Proc. R. Soc. B: Biol. Sci. 285, 20180506. doi: 10.1098/rspb.2018.0506
Laycock I., Cotterell K. C., O’Shea-Wheller T. A., and Cresswell J. E. (2014). Effects of the neonicotinoid pesticide thiamethoxam at field-realistic levels on microcolonies of Bombus terrestris worker bumble bees. Ecotoxicology Environ. Saf. 100, 153–158. doi: 10.1016/j.ecoenv.2013.10.027
Laycock I., Lenthall K. M., Barratt A. T., and Cresswell J. E. (2012). Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 21, 1937–1945. doi: 10.1007/s10646-012-0927-y
Lundin O., Rundlöf M., Smith H. G., Fries I., and Bommarco R. (2015). Neonicotinoid insecticides and their impacts on bees: a systematic review of research approaches and identification of knowledge gaps. PloS One 10, e0136928. doi: 10.1371/journal.pone.0136928
Manjon C., Troczka B. J., Zaworra M., Beadle K., Randall E., Hertlein G., et al. (2018). Unravelling the molecular determinants of bee sensitivity to neonicotinoid insecticides. Curr. biology: CB 28, 1137–1143.e5. doi: 10.1016/j.cub.2018.02.045
Martín-Blázquez R., Calhoun A. C., Sadd B. M., and Cameron S. A. (2023). Gene expression in bumble bee larvae differs qualitatively between high and low concentration imidacloprid exposure levels. Sci. Rep. 13, 9415. doi: 10.1038/s41598-023-36232-y
Martínez de Castro Dubernard A., Goulson D., Solís-Montero L., and Vandame R. (2022). Effects of imidacloprid on survival and nest development in the neo-tropical bumblebee Bombus ephippiatus. Apidologie 53, 34. doi: 10.1007/s13592-022-00946-1
McGrady C. M., Strange J. P., López-Uribe M. M., and Fleischer S. J. (2021). Wild bumble bee colony abundance, scaled by field size, predicts pollination services. Ecosphere 12, e03735. doi: 10.1002/ecs2.3735
Minnameyer A., Strobl V., Bruckner S., Camenzind D. W., Van Oystaeyen A., Wäckers F., et al. (2021). Eusocial insect declines: insecticide impairs sperm and feeding glands in bumblebees. Sci Total Environ. 785, 146955. doi: 10.106/j.scitotenv.2021.146955
Moffat C., Buckland S. T., Samson A. J., McArthur R., Chamosa Pino V., Bollan K. A., et al. (2016). Neonicotinoids target distinct nicotinic acetylcholine receptors and neurons, leading to differential risks to bumblebees. Sci. Rep. 6, 24764. doi: 10.1038/srep24764
Moher D., Liberati A., Tetzlaff J., Altman D. G., and Prisma Group (2009). ). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med. 6, e1000097. doi: 10.1371/journal.pmed.1000097
Muth F., Francis J. S., and Leonard A. S. (2019). Modality-specific impairment of learning by a neonicotinoid pesticide. Biol. Lett. 15, 20190359. doi: 10.1098/rsbl.2019.0359
Muth F. and Leonard A. S. (2019). A neonicotinoid pesticide impairs foraging, but not learning, in free-flying bumblebees. Sci. Rep. 9, 4764. doi: 10.1038/s41598-019-39701-5
Myers R. C. (2017). “Acute toxicity testing by the dermal route,” in Health risk assessment dermal and inhalation exposure and absorption of toxicants. Eds. Wang R. G. M., Knaak J. B., and Maibach H. I. (CRC Press, Boca Raton). doi: 10.1201/9780203711989
Naeem M., Liu M., Huang J., Ding G., Potapov G., Jung C., et al. (2019). Vulnerability of East Asian bumblebee species to future climate and land cover changes. Agriculture, Ecosystems & Environment. 277, 11–20. doi: 10.1016/j.agee.2019.03.002
National Caucus of Environmental Legislators (2023). How are U.S. States improving protections for pollinator species in 2023. Available online at: http://www.ncelenviro.org/articles/how-are-u-s-states-improving-protections-for-pollinator-species-in-2023/ (Accessed August 28, 2025).
Nauen R. and Jeschke P. (2011). “Basic and applied aspects of neonicotinoid insecticides,” in Green trends in insect control, The Royal Society of Chemistry. Eds. Lopez O. and Fernandez-Bolanos J., 132–162.
Odemer R., Friedrich E., Illies I., Berg S., Pistorius J., and Bischoff G. (2023). Potential risk of residues from neonicotinoid-treated sugar beet in flowering weeds to honey bees (Apis mellifera L.). Environ. Toxicol. Chem. 42, 1167–1177. doi: 10.1002/etc.5602
Pang S., Lin Z., Zhang W., Mishra S., Bhatt P., and Chen S. (2020). Insights into the microbial degradation and biochemical mechanisms of neonicotinoids. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.00868
Parrey A. H., Raina R. H., Saddam B., Pathak P., Kumar S., Uniyal V. P., et al. (2022). Role of bumblebees (Hymenoptera: Apidae) in pollination of high land ecosystems: A review. Agric. Rev. 43, 368–373. doi: 10.18805/ag.R-2159
Paus-Knudsen J. S., Sveinsson H. A., Grung M., Borgå K., and Nielsen A. (2023). The neonicotinoid imidacloprid impairs learning, locomotor activity levels, and sucrose solution consumption in bumblebees (Bombus terrestris). Environ. Toxicol. Chem. 42, 1337–1345. doi: 10.1002/etc.5611
Popp J., Pető K., and Nagy J. (2013). Pesticide productivity and food security. A review. Agron. Sustain. Dev. 33, 243–255. doi: 10.1007/s13593-012-0105-x
Pyke G. H. (2022). Neonicotinoids and optimal foraging theory. Environ. Adv. 7, 100161. doi: 10.1016/j.envadv.2021.100161
Reid R. J., Troczka B. J., Kor L., Randall E., Williamson M. S., Field L. M., et al. (2020). Assessing the acute toxicity of insecticides to the buff-tailed bumblebee (Bombus terrestris audax). Pesticide Biochem. Physiol. 166, 104562. doi: 10.1016/j.pestbp.2020.104562
Richman S. K., Maalouf I. M., Smilanich A. M., Marquez Sanchez D., Miller S. Z., and Leonard A. S. (2022). A neonicotinoid pesticide alters how nectar chemistry affects bees. Funct. Ecol. 36, 1063–1073. doi: 10.1111/1365-2435.14016
Rust M. K., Waggoner M. M., Hinkle N. C., Stansfield D., and Barnett S. (2003). Efficacy and longevity of nitenpyram against adult cat fleas (Siphonaptera: Pulicidae). J. Med. entomology 40, 678–681. doi: 10.1603/0022-2585-40.5.678
Samson-Robert O., Labrie G., Mercier P. L., Chagnon M., Derome N., and Fournier V. (2015). Increased acetylcholinesterase expression in bumble bees during neonicotinoid-coated corn sowing. Sci. Rep. 5, 12636. doi: 10.1038/srep12636
Samuelson E. E., Chen-Wishart Z. P., Gill R. J., and Leadbeater E. (2016). Effect of acute pesticide exposure on bee spatial working memory using an analogue of the radial-arm maze. Sci. Rep. 6, 38957. doi: 10.1038/srep38957
Sánchez-Bayo F., Goka K., and Hayasaka D. (2016). Contamination of the aquatic environment with neonicotinoids and its implication for ecosystems. Front. Environ. Sci. 4. doi: 10.3389/fenvs.2016.00071
Sánchez-Bayo F. and Tennekes H. A. (2020). Time-cumulative toxicity of neonicotinoids: experimental evidence and implications for environmental risk assessments. Int. J. Environ. Res. Public Health 17, 1629. doi: 10.3390/ijerph17051629
Sargent C., Ebanks B., Hardy I. C., Davies T. E., Chakrabarti L., and Stöger R. (2021). Acute imidacloprid exposure alters mitochondrial function in bumblebee flight muscle and brain. Front. Insect Sci 1. doi: 10.3389/finsc.2021.765179
Schneider L., Rebetez M., and Rasmann S. (2022). The effect of climate change on invasive crop pests across biomes. Curr. Opin. Insect Sci 50, 100895. doi: 10.1016/j.cois.2022.100895
Scholer J. and Krischik V. (2014). Chronic exposure of imidacloprid and clothianidin reduce queen survival, foraging, and nectar storing in colonies of Bombus impatiens. PloS One 9, e91573. doi: 10.1371/journal.pone.0091573
Schulz R., Bub S., Petschick L. L., Stehle S., and Wolfram J. (2021). Applied pesticide toxicity shifts toward plants and invertebrates, even in GM crops. Science 372, 81–84. doi: 10.1126/science.abe1148
Sgolastra F., Medrzycki P., Bortolotti L., Maini S., Porrini C., Simon-Delso N., et al. (2020). Bees and pesticide regulation: lessons from the neonicotinoid experience. Biol. Conserv. 241, 108356. doi: 10.1016/j.biocon.2019.108356
Sheets L. P. (2010). “Imidacloprid: a neonicotinoid insecticide,” in Hayes’ handbook of pesticide toxicology (Academic Press, p), 2055–2064. doi: 10.1016/B978-0-12-374367-1.00095-1
Simmons W. R. and Angelini D. R. (2017). Chronic exposure to a neonicotinoid increases expression of antimicrobial peptide genes in the bumblebee Bombus impatiens. Sci. Rep. 7, 44773. doi: 10.1038/srep44773
Simon M. W. and Amarasekare P. (2024). Predicting the fundamental thermal niche of ectotherms. Ecology 105, e4289. doi: 10.1002/ecy.4289
Simon-Delso N., Amaral-Rogers V., Belzunces L. P., Bonmatin J. M., Chagnon M., Downs C., et al. (2015). Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ. Sci pollut. Res. 22, 5–34. doi: 10.1007/s11356-014-3470-y
Siviter H., Johnson A. K., and Muth F. (2021). Bumblebees exposed to a neonicotinoid pesticide make suboptimal foraging decisions. Environ. Entomology 50, 1299–1303. doi: 10.1093/ee/nvab087
Smith D. B., Arce A. N., Ramos Rodrigues A., Bischoff P. H., Burris D., Ahmed F., et al. (2020). Insecticide exposure during brood or early-adult development reduces brain growth and impairs adult learning in bumblebees. Proc. R. Soc. B 287, 20192442. doi: 10.1098/rspb.2019.2442
Stanley D. A., Garratt M. P., Wickens J. B., Wickens V. J., Potts S. G., and Raine N. E. (2015). Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature 528, 548–550. doi: 10.1038/nature16167
Stanley D. A. and Raine N. E. (2016). Chronic exposure to a neonicotinoid pesticide alters the interactions between bumblebees and wild plants. Funct. Ecol. 30, 1132–1139. doi: 10.1111/1365-2435.12644
Stanley D. A., Russell A. L., Morrison S. J., Rogers C., and Raine N. E. (2016). Investigating the impacts of field-realistic exposure to a neonicotinoid pesticide on bumblebee foraging, homing ability and colony growth. J. Appl. Ecol. 53, 1440–1449. doi: 10.1111/1365-2664.12689
Stehle S., Ovcharova V., Wolfram J., Bub S., Herrmann L. Z., Petschick L. L., et al. (2023). Neonicotinoid insecticides in global agricultural surface waters–exposure, risks and regulatory challenges. Sci Total Environ. 867, 161383. doi: 10.1016/j.scitotenv.2022.161383
Stewart S. D., Lorenz G. M., Catchot A. L., Gore J., Cook D., Skinner J., et al. (2014). Potential exposure of pollinators to neonicotinoid insecticides from the use of insecticide seed treatments in the mid-southern United States. Environ. Sci Technol. 48, 9762–9769. doi: 10.1021/es501657w
Stokstad E. (2013). Pesticides under fire for risks to pollinators. Science 340, 674–676. doi: 10.1126/science.340.6133.674
Straub L., Minnameyer A., Camenzind D., Kalbermatten I., Tosi S., Van Oystaeyen A., et al. (2022). Thiamethoxam as an inadvertent anti-aphrodisiac in male bees. Toxicol. Rep. , 9, 36–45. doi: 10.1016/j.toxrep.2021.12.003
Tai F. K., Northcott G. L., Beggs J. R., Mortensen A. N., and Pattemore D. E. (2025). Scarcity of pesticide data in New Zealand with a focus on neonicotinoids: A review. Sci Total Environ. , 970, 179044. doi: 10.1016/j.scitotenv.2025.179044
Thany S. H. (2023). Molecular mechanism of action of neonicotinoid insecticides. Int. J. Mol. Sci. 24, 5484. doi: 10.3390/ijms24065484
Thompson D. A. (2020). An assessment of neonicotinoid exposure risks through drinking water in Iowa (Doctoral dissertation, The University of Iowa).
Thompson D. A., Lehmler H. J., Kolpin D. W., Hladik M. L., Vargo J. D., Schilling K. E., et al. (2020). A critical review on the potential impacts of neonicotinoid insecticide use: current knowledge of environmental fate, toxicity, and implications for human health. Environ. Science: Processes Impacts 22, 1315–1346. doi: 10.1039/C9EM00586B
Tomizawa M. and Casida J. E. (2005). Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 45, 247–268. doi: 10.1146/annurev.pharmtox.45.120403.095930
U.S. EPA (2019). Product cancellation order for certain pesticide registrations. Available online at: https://www.federalregister.gov/documents/2019/05/20/2019-10447/product-cancellation-order-for-certain-pesticide-registrations (Accessed 12.03.2025).
U.S. EPA (2021). Technical overview of ecological risk assessment—Analysis phase: ecological effect characterization (Washington, DC: US Environmental Protection Agency). Available online at: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/technical-overview-ecological-risk-assessment-0.
Van der Sluijs J. P., Simon-Delso N., Goulson D., Maxim L., Bonmatin J. M., and Belzunces L. P. (2013). Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. sustainability 5, 293–305. doi: 10.1016/j.cosust.2013.05.007
Van de Vliert E., Kluwer E. S., and Lynn R. (2000). Citizens of warmer countries are more competitive and poorer: Culture or chance? J. Economic Psychol. 21, 143–165. doi: 10.1016/S0167-4870(99)00040-9
Varga-Szilay Z. and Tóth Z. (2022). Is acetamiprid really not that harmful to bumblebees (Apidae: Bombus spp.)? Apidologie 53, 2. doi: 10.1007/s13592-022-00909-6
Velthuis H. H. and van Doorn A. (2004). “The breeding, commercialization and economic value of bumblebees,” in Solitary bees: conservation, rearing and management in pollination. Eds. Freitas B. M. and Pereira J. O. P. (Fortaleza, Brazil: Imprensa Universitária).
Venkatesan T., Chethan B. R., and Mani M. (2022). “Insecticide resistance and its management in the insect pests of horticultural crops,” in Trends in horticultural entomology. Ed. Mani M. (Springer, Singapore). doi: 10.1007/978-981-19-0343-4_14
Wahengbam J., Raut A. M., Satinder Pal S. P., and Banu A. N. (2019). Role of bumble bee in pollination. Ann. Biol. 35, 290–295.
Werner I. and Hitzfeld B. (2012). 50 years of ecotoxicology since silent spring – a review. Gaia 21, 217–224. doi: 10.14512/gaia.21.3.13
Whitehorn P. R., O’Connor S., Wackers F. L., and Goulson D. (2012). Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351–352. doi: 10.1126/science.1215025
Whitehorn P. R., Wallace C., and Vallejo-Marín M. (2017). Neonicotinoid pesticide limits improvement in buzz pollination by bumblebees. Sci. Rep. 7, 15562. doi: 10.1038/s41598-017-14660-x
WHO (2018). “Global situation of pesticide management in agriculture and public health,” in Report of a 2018 WHO–FAO survey (Geneva, Switzerland: WHO). Available online at: https://iris.who.int/bitstream/handle/10665/329971/9789241516884-eng.pdf.
Widmer A., Schmid-Hempel P., Estoup A., and Scholl A. (1998). Population genetic structure and colonization history of Bombus terrestris (Hymenoptera: Apidae) from the Canary Islands and Madeira. Heredity 81, 563–572. doi: 10.1046/j.1365-2540.1998.00407.x
Williams P. H. and Osborne J. L. (2009). Bumblebee vulnerability and conservation world-wide. Apidologie 40, 367–387. doi: 10.1051/apido/2009025
Winter K., Adams L., Thorp R., Inouye D., Day L., Ascher J., et al. (2006). “Importation of non-native bumble bees into North America: potential consequences of using Bombus terrestris and other non-native bumble bees for greenhouse crop pollination in Canada, Mexico, and the United States,” in A white paper of the north american pollinator protection campaign (NAPPC).
Wintermantel D., Locke B., Andersson G. K., Semberg E., Forsgren E., Osterman J., et al. (2018). Field-level clothianidin exposure affects bumblebees but generally not their pathogens. Nat. Commun. 9, 5446. doi: 10.1038/s41467-018-07914-3
Witwicka A., López-Osorio F., Chaudhry-Phipps H., and Wurm Y. (2025). A neonicotinoid pesticide causes tissue-specific gene expression changes in bumble bees. Sci Total Environ. 959, 178262. doi: 10.1016/j.scitotenv.2024.178262
Wu-Smart J. and Spivak M. (2018). Effects of neonicotinoid imidacloprid exposure on bumble bee (Hymenoptera: Apidae) queen survival and nest initiation. Environ. Entomology 47, 55–62. doi: 10.1093/ee/nvx175
Keywords: behavior, bumblebees (Bombus spp.), colony, insecticides, neonicotinoids, mortality, physiology, pollinator
Citation: Dennis DJ and Gibbs AJ (2025) The effect of neonicotinoids on bumblebees (Bombus spp.): a systematic review. Front. Bee Sci. 3:1657493. doi: 10.3389/frbee.2025.1657493
Received: 01 July 2025; Accepted: 15 September 2025;
Published: 06 October 2025.
Edited by:
Helen Wallace, Queensland University of Technology, AustraliaReviewed by:
Neelendra K. Joshi, University of Arkansas, United StatesCaroline Strang, The University of Western Ontario, Canada
Copyright © 2025 Dennis and Gibbs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandra J. Gibbs, QWxleGFuZHJhLmdpYmJzQG5vdHRpbmdoYW0uYWMudWs=
 Daisy J. Dennis
Daisy J. Dennis Alexandra J. Gibbs
Alexandra J. Gibbs