- 1Ecology Department, Instituto de Biociências, Universidade de São Paulo - USP, São Paulo, SP, Brazil
- 2Environmental Sciences Laboratory, Universidade Estadual do Norte Fluminense Darcy Ribeiro - UENF, Campos dos Goytacazes, RJ, Brazil
Introduction
Solitary bees represent the vast majority of Apiformes species (Michener, 2007). With the exception of the subfamily Nomadinae (which are cleptoparasitic bees), the solitary lifestyle is present in all other Apiformes subfamilies, totaling approximately 15,500 species (Danforth et al., 2019). This represents approximately 77% of bee diversity. In solitary bees, there is no cooperation or intergenerational contact (Michener, 1974). The female builds the nest alone, provisioning and defending it (Batra, 1984; Alves dos Santos, 2002, Neff, 2008). In each cell built, the female lays an egg. After hatching, the larva passes through four or five stages, consuming the food supplied by the mother before pupating. The adult then emerges and restarts the species cycle. These phases can last a few weeks or many months. In any case, offspring and mothers never meet.
Some solitary bees build nests in pre-existing cavities, such as holes left by beetles in wood-rotting tree trunks or various hollow branches. In this case, nests of these species can be obtained by offering artificial traps, such as bamboo segments or perforated wood (Krombein, 1967; Garófalo et al., 2004). This technique greatly facilitates the collection of data on the biology of the species (Alves dos Santos, 2002) and enables their use on a larger scale for commercial purposes (e.g., Osmia cornuta and Megachile rotundata, both used for crop pollination) (Stephen, 1961; Richards, 1984; Bosch and Kemp, 2002; Muniz et al., 2024).
However, the vast majority of solitary bee species (three-quarters) nest in the ground, excavating their own nests (Danforth et al., 2019; Antoine and Forrest, 2021). In this case, to study them, the first challenge is to find the nest, which is often just a small hole in the ground. Many species form aggregations, which are several nests in proximity, and when the species is active, there is strong movement in the area, signaling the presence of nests (Cane, 2024). In some species, these aggregations are permanent, meaning they become active annually and can increase significantly in size (Batra, 1999; Cane, 2003).
After finding a solitary bee nest on the ground, the second challenge is excavating it to reach the brood cells. Luckily, the nests are shallow (Celary, 2004) or have few branches, making them easy to excavate. However, several species have deep nests in hard or sandy soil, requiring time-consuming and careful excavation (Bohart et al., 1972; Roberts, 1973; Gaglianone, 2000). These two steps make it difficult to obtain data on the biology of most solitary species. Access to the nest provides a unique opportunity to obtain data on the plants on which the species depends entirely, the potential enemies, seasonality of the species, offspring development, nest architecture, and more.
To illustrate the current knowledge gaps regarding solitary bees, we draw on data from two important groups of the Neotropical apifauna: the Eucerini tribe, which is well represented on all continents except Australia, and the subfamily Diphaglossinae, which is restricted to the Americas. These groups are commonly recorded in surveys of Neotropical bee fauna, although usually at low abundances, which further hampers the understanding of their biology. In this review, we examined the literature published over the past 10 decades addressing the biology of these bees as indexed in the Web of Science database.
Knowledge of the biology of Neotropical Eucerini
The tribe Eucerini comprises 780 species (Michener, 2007), with some very speciose genera, such as Eucera, from the Northern Hemisphere. Eucerine bees show particularly high genetic diversity in the Western Hemisphere (Dorchin et al., 2018), with 31 genera, 16 subgenera, and 247 species (Urban et al., 2023), occurring from Quebec, Canada, to Chubut, Argentina. Representatives of this tribe are popularly known as long-horned bees, as the males have extremely long antennae (Figure 1), sometimes twice the size of females. There are reports of male roosting in flowers or branches, in aggregates of several species (Alcock, 1998; Mahlmann et al., 2014; Silva and Andrade, 2022).

Figure 1. Males of the groups discussed in the text: Trichocerapis mirabilis (note the typical long antennae) and Ptiloglossa pretiosa on flower of Lamanonia ternata (Cunoniaceae). Photos credit: Adriana Tiba and Julio Pupim.
All Eucerini species nest on the ground. A compilation of research published over the last 100 years on nesting in Eucerini revealed that only approximately 4% of the species have been studied (32 species) (Table 1). The studies describe the nests, the number and arrangement of brood cells, and in many cases, also provide data on the immatures, associated parasites, and plants used. However, even among the 32 species studied, there are gaps in some of this information. Some of the most complete studies in terms of description about the species are those by Rozen (1964) on Svastra obliqua in Florida; by Parker et al. (1981) on Melissodes agilis in Utah, USA; and by Michelette et al. (2000) on Canephorula apiformis in San Juan, Argentina.
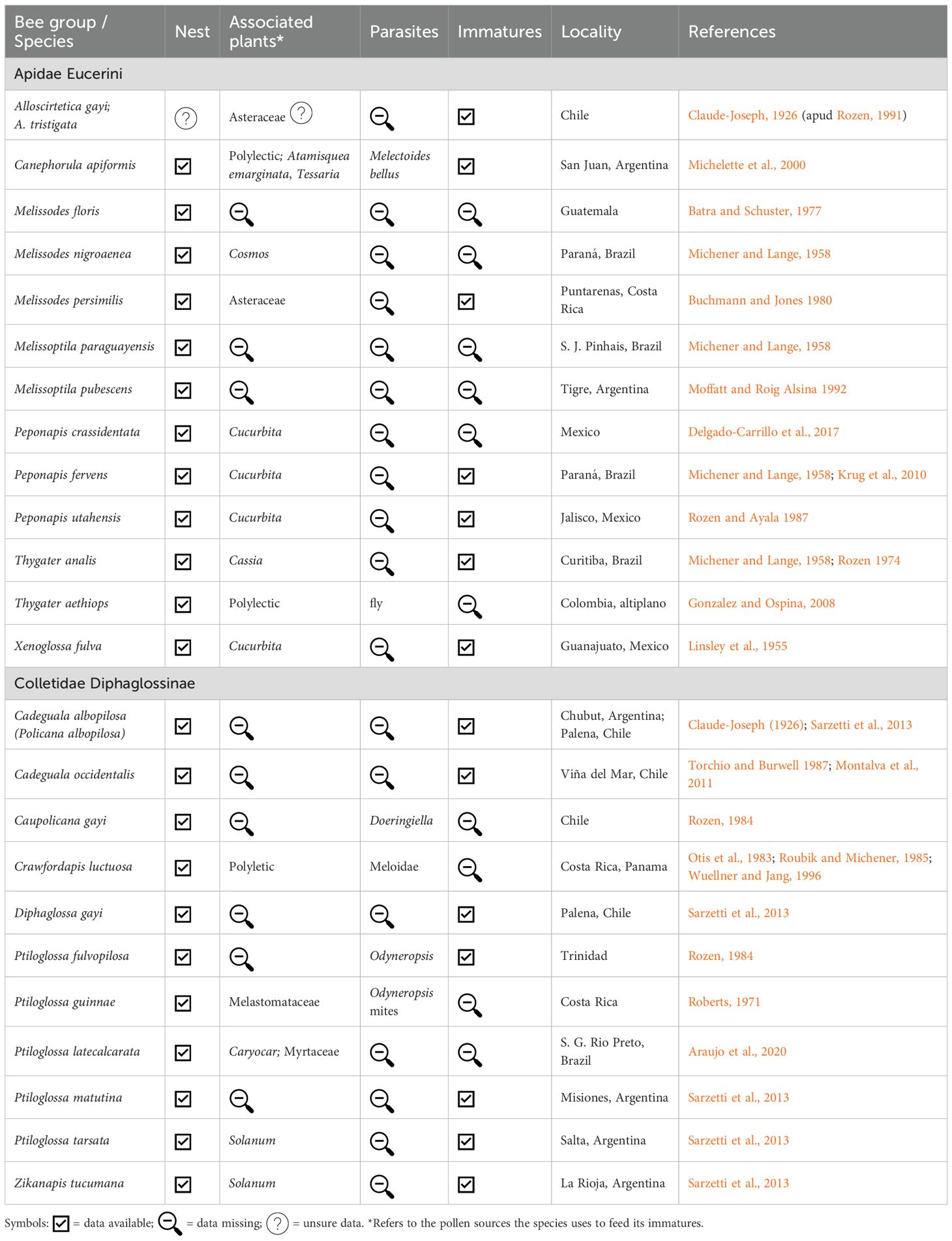
Table 1. Studies on nesting biology of Neotropical solitary bee’s species of the tribe Eucerini and subfamily Diphaglossinae.
Common to most Eucerini are the oval-shaped cells, vertically oriented, with a thin cell lining, eggs placed on top of the provision, pollen packed into the base of cells, liquid layer covering pollen masses. The mature larva places its feces against the cell cap and then spins a thin cocoon, constructed of a number of coarse and fine layers of silk. Rozen (1991) compared the anatomical structures of the mature larvae of the Eucerini. Several species are polylectic, such as Eucera hamata (Miliczky, 1985) and Thygater aethiops (Gonzalez and Ospina, 2008), and others are oligolectic, such as the pumpkin specialist Peponapis and Xenoglossa (Hurd et al., 1971). Nests of three Peponapis species have been described. They form small nesting aggregations (six to eight nests) adjacent to Cucurbita fields, which they pollinate (Hurd et al., 1971). Usually, the brood cells reveal 100% pollen from Cucurbita (Krug et al., 2010).
Of the 32 Eucerini species studied, 14 occur in the Neotropics, but there are numerous hiatuses. For example, there are no nest studies on the genus Florilegus (11 species) or Gaesischia (31 species), only one study on Alloscirtetica (44 species), and two studies on Melissoptila (54 species). Thus, even for the most common and specious genera in the Americas, we have no information on their biology, as their nests have never been located. The Brazilian Eucerini species are well-resolved systematically due to the dedication of Danúncia Urban with this tribe (Urban et al., 2023). Several species are oligolectic, such as Florilegus (specialist on Pontederia) or Gaesischia (specialist on Vernonia) (Schlindwein, 1998). However, their nests remain undetected and may require more intensive and careful field efforts to be found.
Knowledge of the biology of Diphaglossinae
The subfamily Diphaglossinae is composed of large bees of the Colletidae family, restricted to the New World (Michener, 2007). The subfamily is divided into three tribes, 12 genera, and approximately 130 species (Danforth et al., 2019). Like the Eucerini, all Diphaglossinae species are solitary and nest on the ground. The species are notable for their beautiful colors and abundant hairiness (Figure 1). A common characteristic of most species in this subfamily is their crepuscular habit, which makes locating their nests in the wild even more difficult, as the nest is closed all day. Females are active for only a brief window of time at twilight (Danforth et al., 2019).
Investigations on the nesting biology of Diphaglossine bees correspond to studies on 14 species, approximately 10% of their representatives (Table 1). Of these 14 studies, 11 were conducted on Neotropical species and the other 3 in Arizona (Linsley and Cazier, 1970; Rozen, 1984). In South America, the study conducted in Argentina and Chile with five species is noteworthy (Sarzetti et al., 2013). Recurrent parasites belong to the genera Triepeolus and Odyneropsis, both of the tribe Nomadini.
Common to the studied species is the nest with a deep vertical tunnel, with lateral branches radiating from the main burrow in various directions, each ending in a single cell. The branches are filled with soil after oviposition. The brood cells are large to very large (corresponding to the large size of the bees), elongated, with a diameter somewhat larger than the diameter of the burrow, and circular in cross section. The brood cells are vertically oriented and curved at the top, which can reach 90° or more (Ptiloglossa and Crawfordapis) (Rozen, 1984; Sarzetti et al., 2013). The cells are lined with a cellophane-like layer and contain semiliquid provisions. For some species, huge and permanent aggregations have been reported (Roberts, 1971; Otis et al., 1983; Roubik and Michener, 1985).
A recent study of Ptiloglossa latecalcarata conducted in the Brazilian cerrado revealed a curious fact: the presence of monofloral pollen in the brood cells. In this case, the recorded pollen was of Caryocar brasiliense (Caryocaraceae), known as pequi, described as a chiropterophilous plant, and visited by nocturnal bees at twilight (Araujo et al., 2020). The flowers of this species open in the evening and provide resources until dawn, supplying a significant amount of pollen for nocturnal bees. However, it is known that P. latecalcarata is not a specialist on Caryocar but rather and opportunist for the plant with nocturnal anthesis and massing flowering around the nest. A similar behavior was observed on Campomonesia phaea (Myrtaceae) (Cordeiro et al., 2017). The females have a short window of time to forage and collect pollen and nectar for the offspring provision. Thus, the plant that is nearby can be the target pollen source.
Discussion
Using these two groups of Apiformes, Eucerini and Diphaglossinae, we tried to illustrate the gaps of knowledge on solitary bees in the Neotropics. However, we believe that similar limitations exist in other biogeographical regions in the world. We attribute these gaps to many factors, but we highlight one less discussed: descriptive or natural history papers are “out of fashion” and are not seen as high-impact results by journals or modern researchers. We disagree with this trend, as basic data on the biology of any species fuel discussions of “advanced papers” addressing evolutionary questions, phylogenetic relationships, population genetics, and species interaction networks, among others. Classic studies of the biology of species can yield extensive insights into the group (Gaglianone, 2005). The following two examples illustrate how such studies provide valuable information.
With accumulated information on the immatures of many cleptoparasitic species and observations of the strategies of females in nests of all the tribes in the Neotropics, Rozen (2003) contributed substantially to the understanding of the evolution and phylogenetic relationships within the extensive cleptoparasitic subfamily, Nomadinae, the oldest clade of parasitic bees (Sless et al., 2021). Gathering such information was only possible through meticulous nest excavation and description of the larval anatomy of different lineages of cleptoparasitic bees, primarily from Neotropical regions, over many years.
Through access to the nest of the European Andrena marginata, Stenmark (2013) was able to obtain a huge amount of data on foraging behavior, pollen provision, pollen utilization, development, sex ratio, and nest architecture in Sweden. However, the substantial highlight was that with his results, he was able to estimate the critical pollen resources needed for a nest and for an entire bee population, that is, predicting the carrying capacity (K-value) of bee populations in the habitat. Thus, he proposed a model with easily measured variables (the plants available in the area) that can be used as a tool in bee conservation planning. These two cases demonstrate the importance of information on species’ biology obtained through field observation and experimentation.
Bees, like all insects, face numerous threats. The fact that they have been less studied already poses a risk to solitary bees (Alves dos Santos et al., 2025). We cannot protect organisms if we do not know where they live and what they depend on. In Europe, the list of endangered species includes approximately 60% of bee species with insufficient data (Nieto et al., 2014). This deficiency prevents a conclusive assessment of the species’ conservation status. For the Neotropical region, this percentage is certainly much higher.
In conclusion, it would be very important for basic natural history studies to be given greater prestige by journals and the scientific community, taking into account the contribution they can make in several cutting-edge areas. Thus, we encourage young scientists to leave the comfort of air conditioning and venture into fieldwork, where things happen. Soga and Gaston (2025) mention several negative impacts on science and education associated with the reduction in fieldwork experience. Furthermore, observing the activity of females building nests is very pedagogic, as well as very enjoyable. With a good protocol and some instruments, a wealth of data can be achieved.
Author contributions
IA: Conceptualization, Investigation, Validation, Writing – original draft, Writing – review & editing. MG: Conceptualization, Validation, Writing – review & editing.
In memoriam
We dedicate this paper to our dear colleague, Fernando do Amaral Silveira, a great bee systematist, who died prematurely, leaving a gap among bee researchers in Brazil.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. We are grateful to the funding agencies that allow us to continue our studies with native bees: CNPq 314563/2021-0; CNPq 401575/2022-5 to IAS; CNPq 311577/2021-0; and FAPERJ: E26-204.123/2024 to MCG.
In memoriam
We dedicate this paper to our dear colleague, Fernando do Amaral Silveira, a great bee systematist, who died prematurely, leaving a gap among bee researchers in Brazil.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alcock J. (1998). Sleeping aggregations of the bee Idiomelissodes duplocincta (Cockerell) (Hymenoptera: Anthophorini) and their possible function. J. Kansas Entomological Soc. 71, 74–84.
Alves dos Santos I., Martins H. O. J., and Sabino W. (2025). Solitary bees facing climate change. Sociobiology 72, e11380.
Antoine C. M. and Forrest J. R. (2021). Nesting habitat of ground-nesting bees: a review. Ecol. Entomology 46, 143–159. doi: 10.1111/een.12986
Araujo F. F., Araújo P. D. C. S., Siqueira E., Alves-dos-Santos I., Oliveira R., Dötterl S., et al. (2020). Nocturnal bees exploit but do not pollinate flowers of a common bat-pollinated tree. Arthropod-Plant Interact. 14, 785–797. doi: 10.1007/s11829-020-09784-3
Batra S. W. T. (1984). Solitary bees. Sci. Am. 250, 120–127. doi: 10.1038/scientificamerican0284-120
Batra S. W. T. (1999). Biology of Andrena (Scrapteropsis) fenningeri Viereck (Hymenoptera: Andrenidae), harbinger of spring. Proceedings of the Entomological Society of Washington 101, 106–122.
Batra S. W. T. and Schuster J. C. (1977). Nests of Centris, Melissodes and Colletes in Guatemala (Hymenoptera, Apoidea). Biotropica, Lawrence 9, 135–138.
Bohart G. E., Torchio P. F., Maeta Y., and Rust R. W. (1972). Notes on the biology of Emphoropsis pallida Timberlake. J. Kansas Entomol. Soc 45, 381–392.
Bosch J. and Kemp W. P. (2002). Developing and establishing bee species as crop pollinators: the example of Osmia spp. (Hymenoptera: Megachilidae) and fruit trees. Bull. Entomol. Res. 92, 3–16. doi: 10.1079/BER2001139
Buchmann S. L. and Jones C. E. (1980). Observations on the Nesting Biology of Melissodes persimilis Ckll. (Hymenoptera: Anthophoridae). Pan-Pacific Entomologist 56, 200–206.
Cane J. H. (2003). Annual displacement of soil in nest tumuli of alkali bees (Nomia melanderi) (Hymenoptera: Apiformies: Halictidae) across an agricultural landscape. J. Kans. Entomol. Soc 76, 172–176.
Cane J. H. (2024). The extraordinary alkali bee, nomia melanderi (Halictidae), the world’s only intensively managed ground-nesting bee. Annu. Rev. Entomology 69, 99–116. doi: 10.1146/annurev-ento-020623-013716
Celary W. (2004). A comparative study on the biology of Macropis fulvipes (Fabricius,1904) and Macropis europaea Warncke 1973(Hymenoptera: apoidea: melittidae). Foliabiologica (Kraków) 52, 81–85.
Claude-Joseph F. (1926). Recherches biologiques sur les Hymenopteres du Chili. Ann. Sci. Nat. Zool. Ser. 9, 113–268.
Cordeiro G. D., Pinheiro M., Dötterl S., and Alves-dos-Santos I. (2017). Pollination of Campomanesia phaea (Myrtaceae) by night-active bees: a new nocturnal pollination system mediated by floral scent. Plant Biol. 19, 132–139. doi: 10.1111/plb.12520
Danforth B. N., Minckley R. L., and Neff J. L. (2019). The solitary bees: biology, evolution, conservation (Princeton: Princeton University Press).
Delgado-Carrillo O., Lopezaraiza-Mikel M., Ashworth L., Aguilar R., Lobo J.A., and Quesada M. (2017). A scientific note on the first record of nesting sites of Peponapis crassidentata (Hymenoptera: Apidae). Apidologie 48, 644–647.
Dorchin A., Danforth B. N., and Griswold T. (2018). A new genus of eucerine bees endemic to southwestern North America revealed in phylogenetic analyses of the Eucera complex (Hymenoptera: Apidae: Eucerini). Arthropod Systematics Phylogeny 76, 215–234. doi: 10.3897/asp.76.e31927
Gaglianone M. C. (2000). Behavior on flowers, structures associated to pollen transport and nesting biology of Perditomorpha brunerii and Cephalurgus anomalus (Hymenoptera: Colletidae, Andrenidae). Rev. Biol. Trop. 48, 89–99.
Gaglianone M. C. (2005). Nesting biology, seasonality, and flower hosts of Epicharis nigrita (Friese 1900) (Hymenoptera: Apidae: Centridini), with a comparative analysis for the genus. Stud. Neotropical Fauna Envir. 40, 191–200. doi: 10.1080/01650520500250145
Garófalo C. A., Martins C. F., and Alves dos Santos I. (2004). “The Brazilian solitary bee species caught in trap nests,” in Solitary Bees, Conservation, Rearing and Management for Pollination. Eds. Freitas B. M. and Pereira J. O. (Imprensa Universitária, Fortaleza), 77–84.
Gonzalez V. and Ospina M. (2008). Nest structure, seasonality, and host plants of Thygater aethiops (Hymenoptera: Apidae, Eucerini) in the Andes. J. Hymenoptera Res. 17, 110–115.
Hurd P. D., Linsley E. G., and Whitaker T. W. (1971). Squash And Gourd Bees (Peponapis, Xenoglossa) and the origin of the cultivated Cucurbita. Evolution 25, 218–234.
Krombein K. V. (1967). Trap nesting wasps and bees. Life histories, nests and associates (Washington, D.C.: Smithsonian Institution Press), 570.
Krug C., Alves dos Santos I., and Cane J. (2010). Visiting bees of Cucurbita flowers (Cucurbitaceae) with emphasis on the presence of Peponapis fervens Smith (Eucerini, Apidae), S. Catarina, Southern Brazil. Oecologia Australis 14, 128–139. doi: 10.4257/oeco.2010.1401.06
Linsley E. G. and Cazier M. A. (1970). Some competitive relationships among matinal and late afternoon foraging activities of caupolicanine bees in southeastern Arizona (Hymenoptera, Colletidae). J. Kansas Entomol Soc. 43, 251–261.
Linsley E. G., MacSwain J. W., and Smith R. F. (1955). Biological observations on Xenoglossa fulva Smith with some generalizations on biological characters of other eucerine bees. Bull. South. California Acad. Sci. 54, 128–141.
Mahlmann T., Hipólito J., and Oliveira F. F. (2014). Male sleeping aggregation of multiple Eucerini bee genera (Hymenoptera: Apidae) in Chapada Diamantina, Bahia, Brazil. Biodiversity Data J. 2, 15–56. doi: 10.3897/BDJ.2.e1556
Michelette E. R. F., Camargo J. M. F., and Rozen J. G. Jr (2000). Biology of the bee Canephorula apiformis and its cleptoparasite Melectoides bellus: nesting habits, floral preferences, and mature larvae. Am. Museum Novitates 3308, 1–23. doi: 10.1206/0003-0082(2000)308<0001:BOTBCA>2.0.CO;2
Michener C. D. (1974). The Social Behavior of the Bees (Cambridge: Harvard University Press), 404. xii _.
Michener C. D. and Lange R. B. (1958). Observations on the ethology of neotropical anthophorine bees. Univ. Kansas Sci. Bull. 39, 69–96.
Miliczky E. R. (1985). Observations on the nesting biology of Tetralonia hamata Bradley with a description of its mature larva. J. Kansas Entomol. Soc. 58, 686–700.
Moffatt L. and Roig Alsina A. (1992). Communal nesting in the bee Melissoptila pubescens (Smith) (Hymenoptera: Anthophoridae). Revista de la Sociedad Entomológica Argentina 51, 1–4.
Montalva J., Sepúlveda Y., and Baeza R. (2011). Cadeguala occidentalis (Haliday, 1836) (Hymenoptera: Colletidae: Diphaglossinae): biología de nidificación y morfología de los estados inmaduros. Boletín de Biodiversidad de Chile 5, 3–21.
Muniz V. I. M. S., Santos L. F., Oliveira P. A., Silveira D. R., Lima T. E. B., Sousa M. M. B. A., et al. (2024). “Rational rearing and management of the resin-collecting bee Epanthidium tigrinum,” in Rearing, multiplication, and management of native bees for agricultural pollination in Brazil, vol. 1 . Eds. Freitas B. M. and Bezerra A. D. M. (Universidade Federal do Ceará, Fortaleza), 276.
Neff J. L. (2008). Components of nest provisioning behavior in solitary bees (Hymenoptera: Apoidea). Apidologie 39, 30–45. doi: 10.1051/apido:2007055
Nieto A., Roberts S. P. M., Kemp J., Rasmont P., Kuhlmann M., Criado M. G., et al. (2014). European Red List of bees (Luxembourg: Publication Office of the European Union).
Otis G. W., McGinley R. J., Garling L., and Malaret L. (1983). Biology and systematics of the bee genus Crawfordapis (Colletidae, Diphaglossinae). Psyche 89, 279–296.
Parker F. D., Tepedino V. J., and Bohart G. E. (1981). Notes on the biology of a common sunflower bee, Melissodes (Eumelissodes) agilis Cresson. J. New York Entomol. Soc. 89, 43–52.
Richards K. W. (1984). Alfalfa leafcutter bee management in western Canada. Apic. Canada Publ. 1495E.
Roberts R. B. (1971). Biology of the crepuscular bee Ptiloglossa guinnae n. sp. with notes on associated bees, mites, and yeasts. J. Kansas Entomol. Soc 44, .283–.294. doi: 10.2307/25082419
Roberts R. B. (1973). Nest architecture and immature stages of the bee Oxaea flavescens and the status of Oxaeidae. J. Kansas Entomol. Soc 46, 437–446.
Roubik D. W. and Michener C. D. (1985). Nesting biology of crawfordapis in Panama. J. Kansas Entomol. Soc 57, 662–671.
Rozen J. G. (1964). The biology of Svastra obliqua obliqua (Say), with a taxonomic description of its larva. Am. Museum Novitates 2170, 1–13.
Rozen J. G. (1974). Nest biology of the eucerine bee Thygater analis. J. N. Y. Entomol. Soc. 82, 230–234.
Rozen J. G. (1984). Nesting biology of diphaglossine bees (Hymenoptera, Colletidae). Am. Museum Novitates 2786, 1–33.
Rozen J. G. (1991). Nesting biology and mature larva of the bee idiomelissodes duplocincta (Hymenoptera: anthophoridae: eucerini). Am. Museum Novitates 3012, 1–11.
Rozen J. G. (2003). Eggs, ovariole numbers, and modes of parasitism of cleptoparasitic bees, with emphasis on Neotropical species (Hymenoptera: Apoidea). Am. Museum Novitates 3413, 1–36. doi: 10.1206/0003-0082(2003)413<0001:EONAMO>2.0.CO;2
Rozen J. G. and Ayala R. (1987). Nesting biology of the squash bee Peponapis utahensis (Hymenoptera; Anthophoridae; Eucerini). J. N. Y. Entomol. Soc. 95, 28–33.
Sarzetti L., Genise J., Sanchez M. V., Farina J., and Molina A. (2013). Nesting behavior and ecological preferences of five Diphaglossinae species (Hymenoptera, Apoidea, Colletidae) from Argentina and Chile. J. Hymenopt Res. 33, 63–82. doi: 10.3897/jhr.33.5061
Schlindwein C. (1998). Frequent oligolecty characterizing a diverse bee–plant community in a xerophytic bushland of subtropical Brazil. Stud. Neotropical Fauna Environ. 33, 46–59. doi: 10.1076/snfe.33.1.46.2168
Silva W. P. and Andrade R. R. (2022). Male Sleeping Aggregation of Melissodes (Ecplectica) nigroaenea (Smith, 1854) (Hymenoptera, Apidae, Eucerini) in Brazilian Cerrado. Sociobiology 69. doi: 10.13102/sociobiology.v69i2.5459
Sless T.J.L., Branstetter M.G., Gillung J.P., Krichilsky E. A., Tobin K.B., Straka J., et al (2022). Phylogenetic relationships and the evolution of host preferences in the largest clade of brood parasitic bees (Apidae: Nomadinae). Mol Phylogenet Evol. 166. doi: 10.1016/j.ympev.2021.107326
Soga M. and Gaston K. J. (2025). Extinction of experience among ecologists. Trends Ecol. Evol. 40, 212–215. doi: 10.1016/j.tree.2024.12.010
Stenmark M. (2013). Critical floral resource levels and nesting biology of the mining bee Andrena marginata (Hymenoptera: Andrenidae). Entomologisk Tidskrift 134(3), 135–148.
Stephen W. P. (1961). Artificial nesting sites for the propagation of the leaf-cutter bee, MegaChile (Eutricharaea) rotundata, for alfalfa production. J. Econ. Entomol. 54, 989–9993. doi: 10.1093/jee/54.5.989
Torchio P. F. and Burwell B. (1987). Notes on the biology of Cadeguala occidentalis (Hymenoptera: Colletidae) and a review of colletid pupae. Ann. Entomol. Soc. Am. 80, 781–789.
Urban D., Moure J. S., and Melo G. A. R. (2023). Eucerini Latreille, 1802. In: Moure J. S., Urban D., and Melo G. A. R. (Orgs). Catalogue of Bees (Hymenoptera, Apoidea) in the Neotropical Region - online version. Available online at: https://www.moure.cria.org.br/catalogue (Accessed September 8, 2025).
Keywords: apifauna, biodiversity conservation, ground nests, nesting biology, pollinators
Citation: Alves-dos-Santos I and Gaglianone MC (2025) Knowledge gaps on Neotropical solitary bees. Front. Bee Sci. 3:1670631. doi: 10.3389/frbee.2025.1670631
Received: 21 July 2025; Accepted: 27 August 2025;
Published: 15 September 2025.
Edited by:
Helen Wallace, Queensland University of Technology, AustraliaReviewed by:
Taimy Cantillo, State University of Feira de Santana, BrazilCopyright © 2025 Alves-dos-Santos and Gaglianone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabel Alves-dos-Santos, aXNhYmVsaGFAdXNwLmJy
 Isabel Alves-dos-Santos
Isabel Alves-dos-Santos Maria Cristina Gaglianone
Maria Cristina Gaglianone