- Bioinformatics Research Group, SRI International, Menlo Park, CA, United States
We present a tool for multi-omics data analysis that enables simultaneous visualization of up to four types of omics data on organism-scale metabolic network diagrams. The tool’s interactive web-based metabolic charts depict the metabolic reactions, pathways, and metabolites of a single organism as described in a metabolic pathway database for that organism; the charts are constructed using automated graphical layout algorithms. The multi-omics visualization facility paints each individual omics dataset onto a different “visual channel” of the metabolic-network diagram. For example, a transcriptomics dataset might be displayed by coloring the reaction arrows within the metabolic chart, while a companion proteomics dataset is displayed as reaction arrow thicknesses, and a complementary metabolomics dataset is displayed as metabolite node colors. Once the network diagrams are painted with omics data, semantic zooming provides more details within the diagram as the user zooms in. Datasets containing multiple time points can be displayed in an animated fashion. The tool will also graph data values for individual reactions or metabolites designated by the user. The user can interactively adjust the mapping from data value ranges to the displayed colors and thicknesses to provide more informative diagrams.
1 Introduction
Scientists are faced with a coming deluge of single- and multi-omics datasets, such as transcriptomics data plus metabolomics data, reaction flux measurements plus transcriptomics data, and transcriptomics data plus proteomics data. Although these data hold great promise, they present sizable analysis challenges; data analysis is a major bottleneck to discovery from multi-omics technologies. Developing computational methods to extract new understanding from complex multi-omics data presents significant challenges in terms of conveying the aspects of a biological system that are changing, and facilitating comparisons of the different omics measurements for the same biological subsystem.
We present a tool for multi-omics data analysis that enables simultaneous visualization of up to four types of omics data on organism-scale metabolic charts. The tool is an expansion of an earlier tool called the Cellular Overview Paley et al. (2021); Paley and Karp (2006). The Cellular Overview is a web-based interactive metabolic chart that depicts the metabolic reactions, pathways, and metabolites of a single organism as described in a metabolic pathway database for that organism. The tool generates its organism-specific metabolic charts using automated graphical layout algorithms Paley and Karp (2006); Karp and Paley (1994) and provides semantic zooming of its diagrams. The tool was quite popular for analysis of single-omics datasets because it directly conveys to the user the changes in activation levels of different metabolic pathways in the context of the full metabolic network.
This article reports significant multi-omics extensions to the Cellular Overview. The tool can now read a multi-omics dataset from a single file or from a set of files. That multi-omics dataset can contain up to four single-omics datasets. Each of those datasets specifies on which of four “visual channels” within the Cellular Overview the dataset will appear. Those channels are the color and thickness of reaction edges within the diagram, and the color and thickness of metabolite nodes within the diagram. For example, a multi-omics dataset could depict transcriptomics data as the color of the metabolic-reaction edges within the diagram, proteomics data as the thickness of reaction edges, one type of metabolomics data as the color of metabolite nodes, and a second type of metabolomics data (possibly measured using a different technology) as the thickness of metabolite nodes. Each of the visual channels can be animated, with manual stepping of the animation if desired. The user can precisely control the mapping from the color assignments and thicknesses within the diagram to the associated data values. The user can also magnify regions of the display and graph sequences of data values.
The multi-omics Cellular Overview is part of the larger Pathway Tools (PTools) software system Karp et al. (2019, 2020). PTools is an extensive bioinformatics software system whose capabilities include genome informatics, pathway informatics, omics data analysis, comparative analysis, and metabolic modeling. Pathway informatics features include metabolic reconstruction, pathway search, pathway visualization, and metabolic route search. Thus, the metabolic pathway databases on which the Cellular Overview omics-data analysis tools depend can be created through the metabolic reconstruction component of PTools. PTools is used in a wide spectrum of life-science applications, facilitating studies of sequenced bacteria, plants, and animals, and enabling metabolic engineering. No other software system known to us enables a user to install one software tool to access such a large number of seamlessly integrated capabilities. PTools contains another multi-omics analysis tool called the Omics Dashboard Paley et al. (2017); Paley and Karp (2024) that provides a hierarchical model for analyzing multi-omics datasets; the multi-omics Cellular Overview provides an alternative, metabolism-centric approach to analyzing multi-omics data.
The remainder of this article discusses related work on metabolic network-based omics visualization. It describes in more detail what we mean by a multi-omics dataset, and the file formats in which the tools accept multi-omics data. We then describe the visualizations produced by the multi-omics Cellular Overview and the controls provided with the diagram.
2 Related work on metabolic network-based omics visualization
Here we compare the PTools Cellular Overview with related tools for visual pathway-based analysis of omics data. The comparison considers several dimensions of these tools: Do they support analysis of multi-omics data, and how? How are their underlying diagrams produced, e.g., must the metabolic network diagrams be drawn manually (which is quite time consuming), or are they produced automatically? Do the underlying diagrams support important functionality such as semantic zooming and animated displays?
Three approaches have been used to produce drawings of large metabolic networks, independent of their use for analysis of omics data. Approach 1: General graph layout algorithms have been used by Cytoscape Shannon et al. (2003) and VisANT Hu et al. (2007). Diagrams produced by these algorithms bear little resemblance to textbook pathway diagrams, which means these diagrams are less familiar to biologists and therefore more time consuming to learn. Such diagrams are also less useful because the biologists who originally created biological pathway drawings adopted specialized graphical conventions for a reason—they speed the understanding of pathways. Approach 2: Manually drawn pathways have been used by KEGG-related tools and Escher. Although manually drawn diagrams do use biological pathway drawing conventions, the only way to scale this approach to thousands of genomes is to create large “uber pathway diagrams” that combine pathways from many organisms, and thus a single uber diagram can be re-used across many organisms. However, such diagrams necessarily contain many pathways that are not present in any one organism, making the diagrams confusing and larger than necessary. Furthermore, these diagrams must be updated manually when the underlying pathway DB is updated. Approach 3: Pathway-specific layout algorithms that use biological drawing conventions have been used by PTools. This approach produces organism-specific diagrams containing just those pathways present in a given organism. This approach scales to thousands of organisms and can be updated automatically so that the diagram always reflects the latest version of each pathway. These algorithms are difficult to develop.
Table 1 compares omics pathway-painting tools along the following six dimensions. 1) Do their diagrams use general layout algorithms, manual uber drawings, or pathway-specific algorithms? 2) Do they paint omics data on full metabolic network diagrams, or onto single-pathway diagrams only? 3) How many omics datatypes can the tool visualize simultaneously? 4) Can the tool depict omics pop-ups that graph omics data for a given gene or metabolite, which provide more precise quantitative information than the colored grid squares that many diagrams produce? 5) Can the tool produce animated displays to depict multiple time points or conditions? 6) Does the whole-network diagram support semantic zooming that alters the amount of information displayed as the user zooms in and out, such as gene and metabolite names?
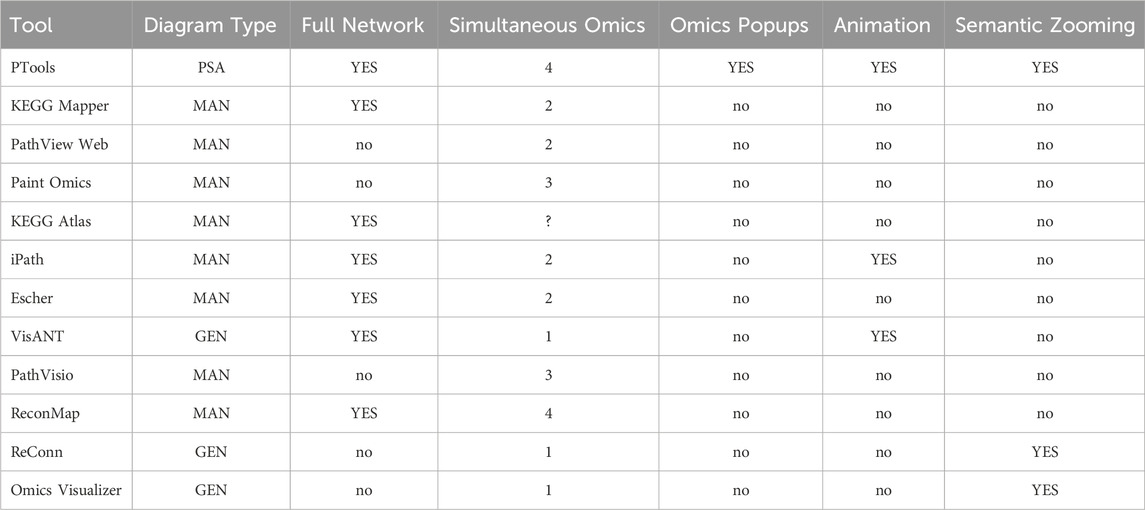
Table 1. Comparison of omics painting tools. Meanings of table cells across the full table: YES = this capability is present in this tool; no = this capability is not present in this tool; “?” = unclear whether this tool has this capability. Diagram Type: MAN = diagram generated manually; PSA = Diagram generated by pathway-specific graph-layout algorithm; GEN = diagram generated by general layout algorithm.
The software tools are described in Table 1 are as follows: PTools Karp et al. (2020) and KEGG Mapper Kanehisa and Sato (2020), which are the only tools that paint data onto both full metabolic network diagrams and individual metabolic pathways. PathView Web Luo et al. (2017) and PaintOmics 3 Hernaández-de Diego et al. (2018) paint multi-omics data onto single-pathway diagrams, not onto full metabolic network diagrams; the same is true of BioMiner Bauer et al. (2015) (not listed in table—defunct, website not active). KEGG Atlas Okuda et al. (2008) (defunct) and iPath 2.0 Yamada et al. (2011) paint data onto full metabolic network diagrams. Escher King et al. (2015) paints data onto any network diagrams manually created by the user, which can be for individual pathways, collections of pathways, or full metabolic networks. VisANT Hu et al. (2007) paints data onto network diagrams generated with general pathway-layout algorithms. PathVisio Kutmon et al. (2015) displays data on manually generated network diagrams. ReconMap Noronha et al. (2017) paints up to 4 types of data onto a single manually drawn diagram of the full human metabolic network. Reconn Ligtenberg et al. (2013) is a Cytoscape plug-in. Omics Visualizer Legeay et al. (2020) supports multiple values per node, showing nodes as circles and splitting the circle into pie sections, each one describing a different omics value and colored differently. It does not color edges. PTools is the most powerful tool, providing organism-specific metabolic-network diagrams that are generated automatically, support semantic zooming, and enable visualization of up to four omics datasets simultaneously with animation and omics popups.
2.1 PTools limitations
Scaling to larger metabolic networks is not likely to be an issue for PTools because metabolic networks for known organisms simply do not become that much larger than the E. coli network. The time to load two datasets containing reaction flux data for 3209 reactions, plus two datasets containing metabolomics data for 1796 compounds, consisting of 20 time points (columns), was 20 s. This is a large number of time points, and most metabolomics datasets are much sparser. Should the size of the dataset double for a larger metabolic network, we believe this time would still be acceptable.
3 Multi-omics file formats
We consider a multi-omics dataset that can be analyzed using the Cellular Overview to be a collection of from one to four single-omics datasets. The single-omics datasets can be measurements of transcript abundance (e.g., transcriptomics), protein abundance (e.g., proteomics), reaction flux, or metabolite abundance (e.g., metabolomics). For that matter, a single-omics dataset can contain any numeric values that the user wants to map to the reaction edges or metabolite nodes of the metabolic network diagram. Each of these single-omics datasets can contain one or more time points or conditions, which are represented as columns in the corresponding file. It is preferable, but not required, that each single-omics dataset contain the same number of columns (time points or conditions).
A multi-omics dataset can be provided to the Cellular Overview in two ways: as a single file and as a set of files, one per single-omics dataset. This approach provides flexibility both to those users who prefer to separate out each single-omics dataset into a separate file, and to those users who prefer to put all their data into one file. Another design goal for the file format is to capture the settings of parameters for the Cellular Overview so that the user can store these parameters into the file once, rather than having to enter them every time they invoke the Cellular Overview.
3.1 Single-file multi-omics format
This format enables up to four single-omics datasets to be combined together into one text file. The file is organized with a header section at the beginning that specifies how many single-omics datasets will follow. The header also defines Cellular Overview parameters for each dataset including which data columns from the single-omics dataset should be used, the type of data in that dataset (e.g., “gene” versus “compound”), an identifying text label for that dataset, and a unique identifier for that dataset (e.g., “Table 1”).
Next, there is a file section for each single-omics dataset. Each single-omics dataset is encoded as a table consisting of a set of rows and columns. Each row describes a single gene, protein, metabolite, etc., depending on the type of data in that single-omics dataset. For example, for transcriptomics data, the first column in each row is the name or identifier of a gene. Subsequent columns, which are tab-delimited, list numeric data values for the transcriptomics measurements at different time points or conditions.
An example file in single-file format is provided as Supplementary File S1. The file format is documented online in Section 9.3.7 of Web Site User’s Guide for Pathway Tools-Based Web Sites (2024).
3.2 Multi-file multi-omics format
The multi-file format separates the sections within the single-file format into multiple files. The header section from the single-file format becomes a separate file called the master file. The master file is where parameter values are assigned for each omics dataset, and the master file lists the file names for each single-omics dataset. Each data table section from the single-file format is stored in a separate file. An example of multi-file format can be found at https://biocyc.org/web-services.shtml#R138.
4 Multi-omics cellular overview visualizations
This section demonstrates the use of the Multi-Omics Cellular Overview on multi-omics datasets for Acinetobacter baylyi and Synechocystis PCC 6803.
4.1 Single time point dataset for Acinetobacter baylyi
Figure 1 depicts a multi-omics dataset using the Cellular Overview. The diagram is organized to show metabolic pathways and reactions inside the cell membrane, with individual reactions on the right side, and pathways on the left. Pathways flow downwards. The diagram is divided so that biosynthetic pathways are drawn on the left portion of the pathway area and catabolic pathways are on the right side. Further subdivisions group pathways into categories such as cofactor biosynthesis and carbohydrate degradation. Edges (lines) in the diagram represent reactions, and nodes represent metabolites. Node shapes communicate the type of metabolite, e.g., triangles are amino acids and squares are carbohydrates; shaded nodes are phosphorylated compounds. As the user zooms in and out of the diagram with a mouse wheel or trackpad, the names of pathways, metabolites, genes, and enzymes appear. The menu to the right (not shown) enables the user to search the diagram by gene name, metabolite name, enzyme name, etc.
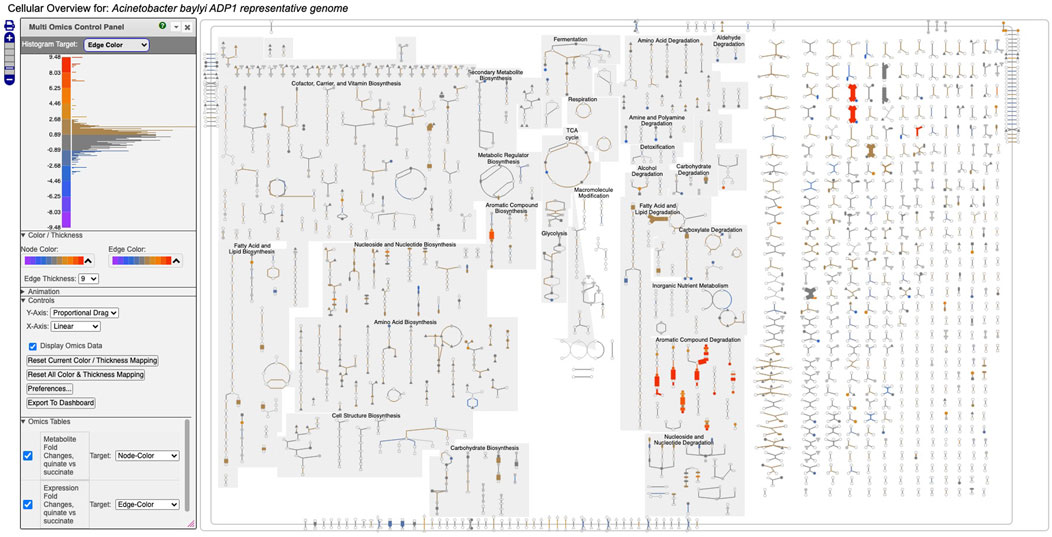
Figure 1. Full Cellular Overview for A. baylyi painted with multi-omics data. On the left is the control panel for the Cellular Overview. The histogram at the top of the control panel shows the assignment of data value ranges to edge colors; red and orange colors represent data values from 4.48 to 9.48.
Figure 1 depicts a multi-omics dataset for Acinetobacter baylyi ADP1 in which growth of A. baylyi was compared under succinate versus quinate as the carbon source Stuani et al. (2014). The component single-omics datasets were 1) a transcriptomics dataset, 2) a gene essentiality dataset, and 3) a metabolomics dataset. All three datasets had a measurement at one time point only. The input data files specified that dataset 1) should be targeted to edge colors, dataset 2) should be targeted to edge thicknesses, and dataset 3) should be targeted to node colors.
Follow these steps to recreate Figure 1.
1. Visit BioCyc.org in a web browser.
2. Click the Change Current Database button and type Acinetobacter baylyi ADP1.
3. Run this command from the top menu: Tools
4. Run this command from the right-sidebar operations menu: Upload Multi-Omics Data from File.
5. Click the Choose File button in the dialog.
6. Provide Supplementary File S1 as the input file.
7. Click the Submit button in the dialog.
Figure 1 shows the resulting Cellular Overview diagram painted with the preceding multi-omics dataset. Although changes are evident in many areas of metabolism, they are particularly strong in the area Aromatic Compound Degradation, which contains many red nodes and edges and lies to the bottom-right of center. Zooming in on that region initially presents the diagram in Figure 2, which shows that metabolite changes (node colors), gene expression changes (edge colors), and gene essentiality (edge thicknesses) are well coordinated (they are all high). As we further zoom Figure 3, metabolite shapes (e.g., circular nodes) are replaced by metabolite names; the names are colored with omics data. The new carbon source, quinate, appears in a pathway near the middle of the figure, quinate degradation I. The end product of that pathway is protocatechuate, which is the input to the pathway at the top center of Figure 3, namely aromatic compounds degradation via beta-ketoadipate. Thus, the Cellular Overview has clearly shown the pathways that the cell activates to catabolize quinate.
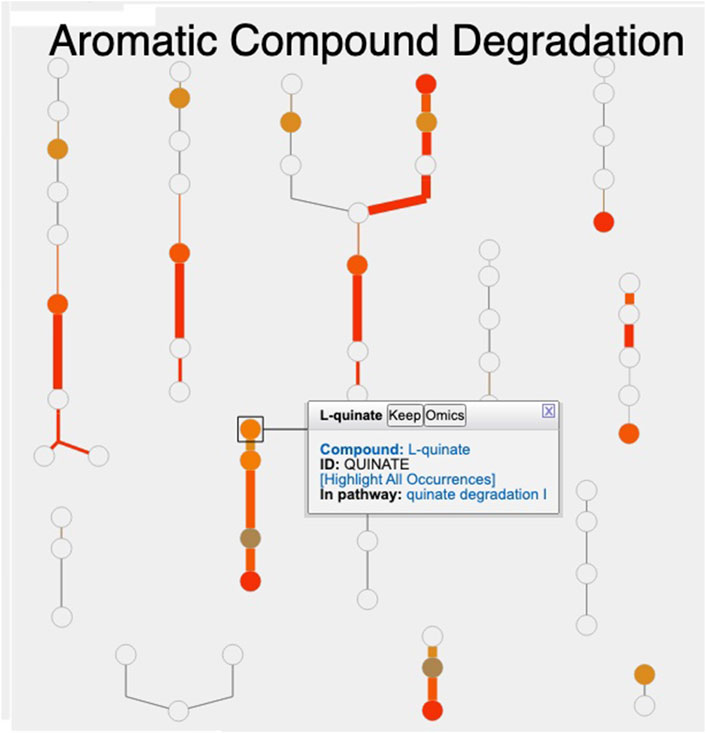
Figure 2. Visualization of A. baylyi multi-omics data on the Aromatic Compound Degradation region of the Cellular Overview; the remaining regions of the Cellular Overview have been cropped away.
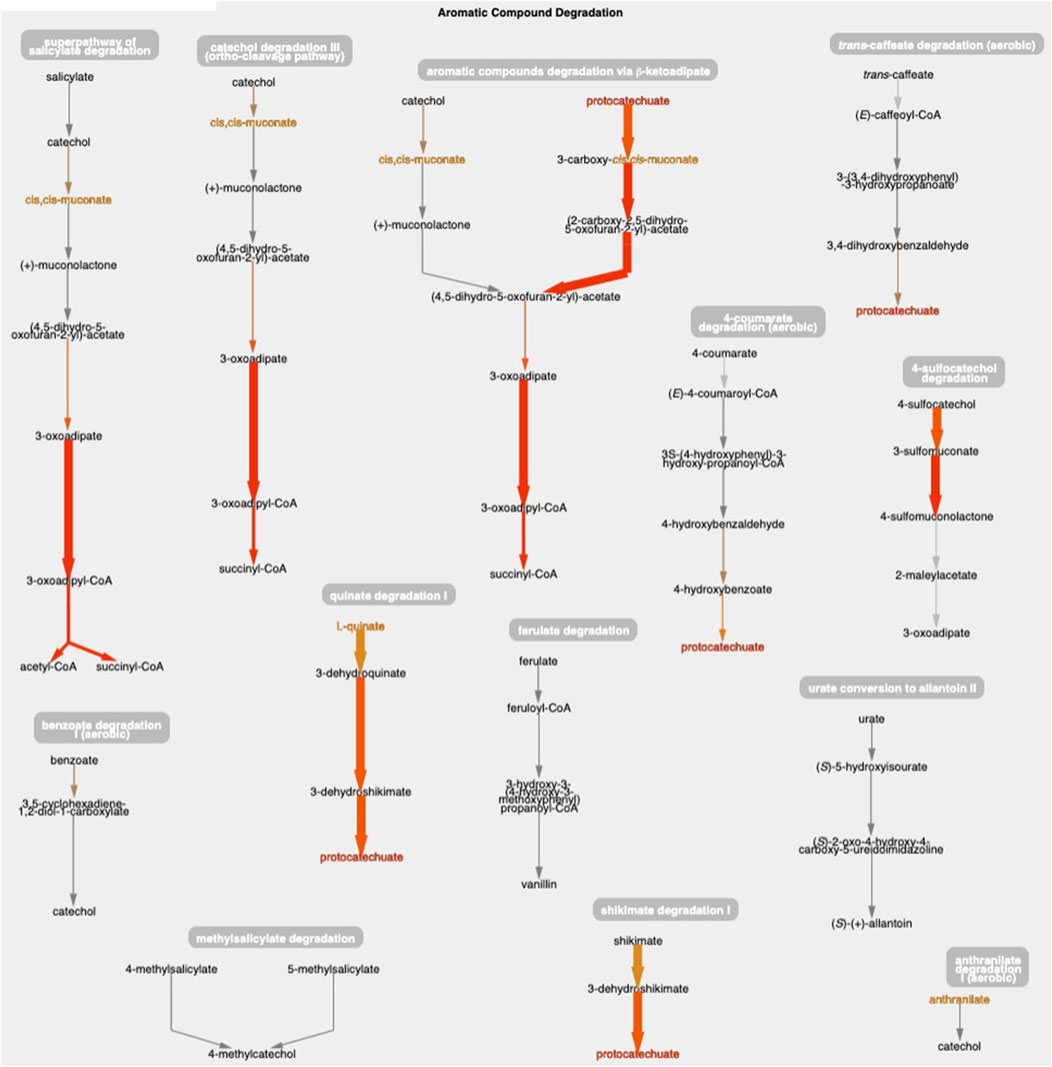
Figure 3. The Aromatic Compound Degradation region of the A. baylyi Cellular Overview zoomed to a higher magnification than Figure 2, causing metabolite nodes to become visible.
The only two amino acids whose abundances are increased during growth on quinate are the aromatic amino acids tyrosine and phenylalanine, which can be found in the Amino Acid Biosynthesis region of the Cellular Overview. Stuani et al. (2014) noted this fact, and postulated that products of quinate degradation in the periplasm may be leaking into the cytoplasm where they become inputs to the biosynthetic pathways for these two amino acids.
4.2 Multiple time point dataset for Synechocystis PCC 6803
Figure 4 depicts a multi-omics dataset for the cyanobacterium Synechocystis sp. PCC 6803 from a study of the effects of the transition from light to dark on this organism Angermayr et al. (2016). Five time points were taken at 1 h, 3 h, 11.75 h (right before the transition), 13 h, and 15 h. The authors reported transcriptomics and proteomics data at all time points. They also provided limited metabolomics data consisting of a list of the most changed metabolites, with a corresponding score for each, but no time points.
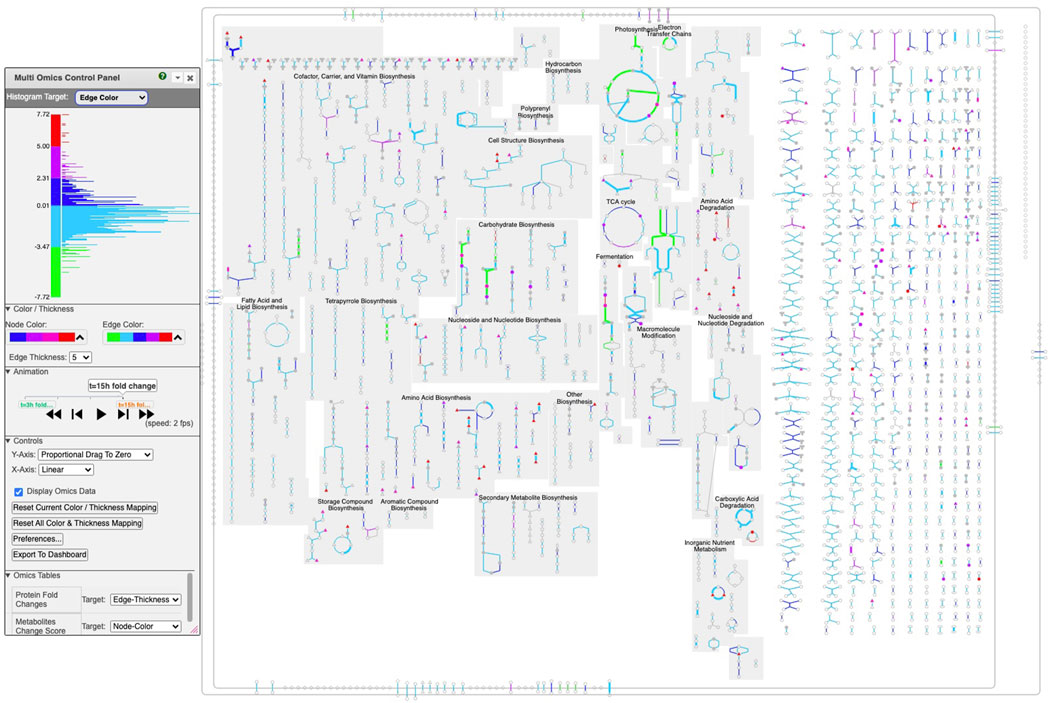
Figure 4. Multi-time point dataset for Synechocystis PCC 6803 shown on the Cellular Overview. The omics viewer control panel is on the left side of the diagram. In this diagram, edge color is assigned to transcriptomics data, edge thickness is assigned to proteomics data, and node color is assigned to metabolomics data. The histogram shown in the image describes the color distribution for depicting transcriptomics data. The red, purple, orange, and black rectangles point to data described further in Figures 5–8, respectively.
We generated the input file (Supplementary File S2) used to create Figures 4–8 by computing fold changes relative to the 1 h timepoint, so that transcriptomics and proteomics datasets each consist of 4 datapoints. Only one time point is available for the metabolomics data, consisting of the reported scores. The input file specifies that the transcriptomics dataset should be targeted to edge colors, the proteomics dataset should be targeted to edge thicknesses, and the metabolomics dataset should be targeted to node colors.
Follow these steps to recreate Figure 4.
1. Visit BioCyc.org in a web browser
2. Click the Change Current Database button and type “Synechocystis sp. PCC 6803 substr. Kazusa”
3. Run this command from the top menu: Tools
4. Run this command from the right-sidebar operations menu: Upload Multi-Omics Data from File
5. Click the Choose File button in the dialog
6. Provide Supplementary File S2 as the input file
7. Click the Submit button in the dialog
Depending on your selection of color palettes for the different datasets and the ranges you specify for the histograms, your image may differ in appearance from Figures 4–8.
Click on the play button to start the animation. The diagram will move through the time points, showing significant changes in many of the pathways and reactions.
We now present several examples of where using this tool can help users to observe interesting phenomena found in these omics data.
Example 1. Using the animation feature, the tool makes it easy to see multiple changes that occur simultaneously. For example, Figure 5 shows the “superpathway of electron transport in the thylakoid membrane” at the latest time point (towards the end of the dark period). One can see how photosystem II (green line) has low transcription (green color) and high protein concentration (thick line) at this time point. At the same time, transcription of the succinate dehydrogenase complex (purple line) and Ndh2 (NADH-quinone oxidoreductase) is significantly higher (no proteomic data is available for these two complexes), indicating that, during the dark, the main sources for reducing power are succinate and NADH.
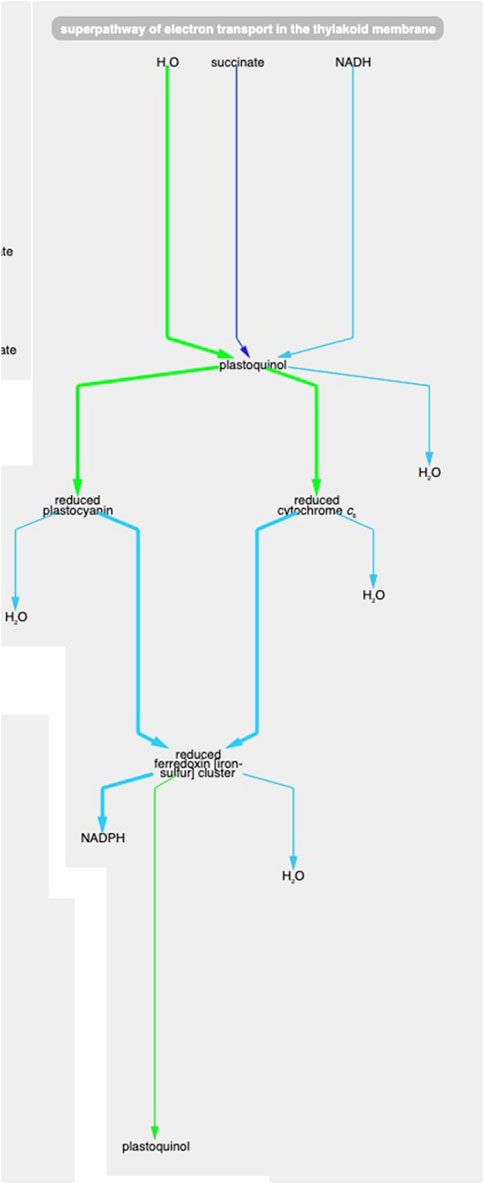
Figure 5. The Synechocystis pathway “superpathway of electron transport in the thylakoid membrane” extracted from Figure 4, at the latest time point. The pathway combines several electron transport pathways that occur in the thylakoid membrane, including components of linear photosynthetic electron transport, respiratory electron transport, cyclic electron transport, and photoprotection by flavodiiron proteins.
Example 2. Glutamate and glutamine are among the most dynamic metabolites in this study Angermayr et al. (2016). They commented that GlnA (glutamine synthetase type I) shows strong downregulation in the dark, while GlnN (glutamine synthetase type III) protein levels showed no significant change. By hovering the mouse on the glutamine synthetase reaction in the “L-glutamine biosynthesis” pathway and selecting “Omics,” a popup diagram appears that clearly illustrates this observation (Figure 6).
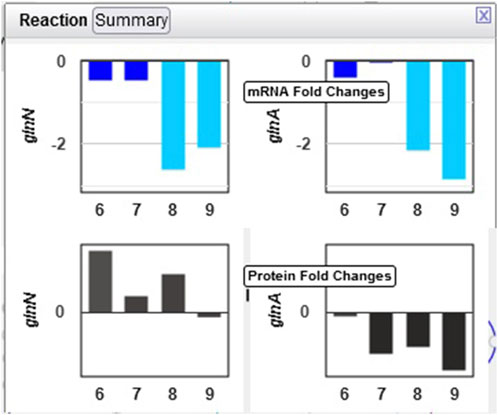
Figure 6. Omics pop-ups graphing Synechocystis transcriptomics and proteomics data for the glnA and glnN genes and their products, which catalyze the ATP-dependent conversion of L-glutamate to L-glutamine. The transcription of both genes is strongly downregulated in the dark, but proteomics data indicate a much stronger decline in the amount of GlnA than of GlnN.
Example 3. The only amino acid whose synthesis was enhanced in the dark was L-alanine. The only L-alanine biosynthetic route demonstrated so far in Synechocystis is desulfuration of L-cysteine, and three enzymes are known to catalyze this reaction. Opening the relevant popup suggests that the responsible enzyme under these conditions is the cysteine desulfurase encoded by the sll0704 gene (shown in Figure 7 as SGL_RS02380, the accession ID assigned by RefSeq).
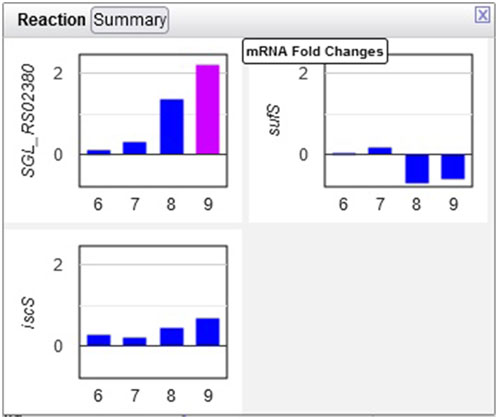
Figure 7. Transcriptomics data for three Synechocystis cysteine desulfurases. Transcription of the sll0704 gene (shown as SGL RS02380) is strongly upregulated in the dark, whereas the transcription of the iscS and sufS genes does not change significantly.
Example 4: The authors noted that chlorophyll content increased in the first three hours of the light period. Looking at the multiomics data for ChlP (Figure 8), the enzyme that converts chlorophyllide a to chlorophyll a and part of the “chlorophyll a biosynthesis II” pathway, one can see a strong delay in the degradation of the enzyme, as the levels of the enzyme continue to grow in the dark despite a very strong decline in transcription.
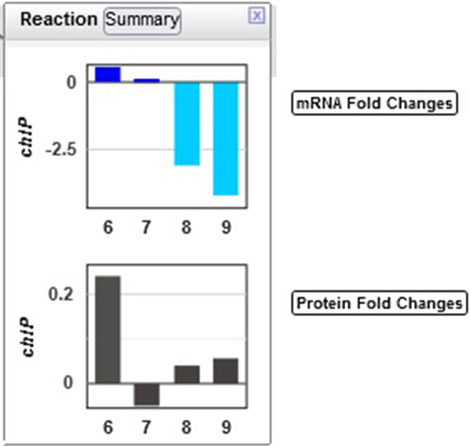
Figure 8. Transcriptomics and proteomics data for the Synechocystis chlP gene and its product, geranylgeranyl diphosphate reductase, which catalyzes a reaction during chlorophyll a biosynthesis.
4.3 Omics viewer controls
Several omics-viewer controls enable the user to tune the diagram to meet their needs more effectively. The controls are shown in the left side of Figure 4.
With the top control, the user can adjust the mapping of colors and thicknesses to data values. Currently the histogram at the top left shows the number of Cellular Overview data points that are mapped to each of the y-axis values. For example, the most data points are in the dark blue and light blue regions that correspond to data values near 0. The histogram is labeled “Histogram Target: Edge Color” to indicate that the diagram is currently depicting edge colors, but the user can also select node colors, node thicknesses, and edge thicknesses.
Currently the control shows that for edge colors there is a linear mapping between data values from −7.72 to 7.72 and the five colors (green to red) shown. If the user wanted the red and green colors at the extremes to encompass a larger set of data values, they could use the mouse to click on the axis label “4.80” and drag that label downwards to expand the length of the red color scale with all of the other regions of the scale decreasing in size. Similarly a user could grab the label “-4.80” and drag it upwards.
With the next control pane, labeled “Color/Thickness,” the user can choose among several provided color scales, each containing a different set of colors. The user can also change the maximum edge thickness the tool uses.
The next pane (“Controls”) enables the user to make other adjustments such as selecting how the y-axis dragging works and resetting the color mapping to the defaults.
The next pane (“Omics Tables,” visible in Figure 1) shows the mapping from each provided single-omics dataset to its target within the Cellular Overview. For example, it shows that expression fold changes are mapped to edge colors. The user can alter the mapping as desired.
If the user mouses over a node or edge within the diagram, a tooltip appears that both identifies that metabolite or reaction and enables the user to display omics pop-ups for that entity and then graph its omics data.
5 Conclusions
We have illustrated the use of the extended Cellular Overview multi-omics visualization capabilities using multi-omics datasets for A. baylyi and Synechocystis. For A. baylyi, the tool identified likely pathways that degrade quinate, and confirmed an observation from the original publication of this dataset that two aromatic amino acids increased in abundance. For Synechocystis, the tool identified a number of changes in the metabolic state of the organism, including which cysteine desulfurase isozyme is likely to be responsible for the increased level of L-arginine.
The multi-omics Cellular Overview supports coordinated analysis of multi-omics datasets on automatically generated organism-specific metabolic network diagrams. Up to four single-omics datasets can be analyzed simultaneously including transcriptomics, proteomics, and metabolomics data. The datasets are provided via either a single input file or through separate files for each single-omics dataset. The user specifies for each dataset whether it should be displayed as node colors, node thicknesses, edge colors, or edge thicknesses. The user can alter the mapping of data values to the color and thickness scales via interactive sliders. Datasets containing multiple time points can be displayed as animations with the ability to single-step the animations. The tool can also graph the data values for individual nodes and edges.
6 Materials and methods
The software that generates Cellular Overview layouts is written in Common Lisp. The software that displays Cellular Overview diagrams and multi-omics data is written in JavaScript, and has been tested within the Chrome, Firefox, and Safari browsers. Although the functionality described herein can be verified through the interactive BioCyc website, all software described in this article is freely available to research institutions for research purposes as part of the Pathway Tools software, including source code, by request to cHRvb2xzLXN1cHBvcnRAYWkuc3JpLmNvbQ==.
For the example datasets used in this paper, we downloaded the publicly available Supplementary Data Files for Stuani et al. (2014) and Angermayr et al. (2016). Where log2 fold change columns were not already present, we computed them (relative to the first timepoint for the Synechocystis data, and based on the provided fold change column for the Acinetobacter data). The gene essentiality data for the Acinetobacter dataset was provided as normalized optical-density growth yields. We subtracted this value from 1 for each gene to generate a measure of essentiality such that higher values represent greater essentiality. The individual component datasets were extracted and combined into a single tab-delimited file for each example, each with an added header section. The resulting files are available as Supplementary File S1 (the Acinetobacter example) and Supplementary File S2 (the Synechocystis example).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
AS: Software, Visualization, Writing–review and editing. RC: Writing–review and editing, Validation. SP: Validation, Writing–review and editing. PK: Validation, Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Visualization, Writing–original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grant NSF2109898 from the National Science Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbinf.2024.1395981/full#supplementary-material
SUPPLEMENTARY FILE S1 | Single-file mult-omics dataset for A. baylyi.
SUPPLEMENTARY FILE S2 | Single-file mult-omics dataset for Synechocystis PCC 6803.
References
Angermayr, S. A., van Alphen, P., Hasdemir, D., Kramer, G., Iqbal, M., van Grondelle, W., et al. (2016). Culturing synechocystis sp. strain pc 6803 with n2 and co2 in a diel regime reveals multiphase glycogen dynamics with low maintenance costs. Appl. Environ. Microbiol. 82, 4180–4189. doi:10.1128/aem.00256-16
Bauer, C., Stec, K., Glintschert, A., Gruden, K., Schichor, C., Or-Guil, M., et al. (2015). BioMiner: paving the way for personalized medicine. Cancer Inf. 14, CIN.S20910–63. doi:10.4137/cin.s20910
Hernaández-de Diego, R., Tarazona, S., Martínez-Mira Balzano-Nogueira, L., Furio-Tarí, T., Pappas, G. J., Conesa, A., et al. (2018). PaintOmics 3: a web resource for the pathway analysis and visualization of multi-omics data. Nuc Acids Res. 46, W503–W509. doi:10.1093/nar/gky466
Hu, Z., Ng, D. M., Yamada, T., Chen, C., Kawashima, S., Mellor, J., et al. (2007). VisANT 3.0: new modules for pathway visualization, editing, prediction and construction. Nuc Acids Res. 35, W625–W632. doi:10.1093/nar/gkm295
Kanehisa, M., and Sato, Y. (2020). KEGG Mapper for inferring cellular functions from protein sequences. Protein Sci. 29, 28–35. doi:10.1002/pro.3711
Karp, P. D., Midford, P., Billington, R., Kothari, A., Krummenacker, M., Ong, W., et al. (2019). Pathway Tools version 23.0 update: software for pathway/genome informatics and systems biology. Brief. Bioinform 22, 109–126. doi:10.1093/bib/bbz104
Karp, P. D., Midford, P., Paley, S., Krummenacker, M., Billington, R., Kothari, A., et al. (2020). Pathway Tools version 24.0: integrated software for pathway/genome informatics and systems biology [v4]. Available at: http://arxiv.org/abs/1510.03964v4.
Karp, P. D., and Paley, S. M. (1994). “Automated drawing of metabolic pathways,” in Proc third international conference on bioinformatics and genome research. Editors H. Lim, C. Cantor, and R. Robbins (World Scientific Publishing Co.), 225–238.
King, Z. A., Drager, A., Ebrahim, A., Sonnenschein, N., Lewis, N. E., and Palsson, B. O. (2015). Escher: a web application for building, sharing, and embedding data-rich visualizations of biological pathways. PLoS Comput. Biol. 11, e1004321. doi:10.1371/journal.pcbi.1004321
Kutmon, M., van Iersel, M. P., Bohler, A., Kelder, T., Nunes, N., Pico, A. R., et al. (2015). PathVisio 3: an extendable pathway analysis toolbox. PLoS Comput. Biol. 11, e1004085. doi:10.1371/journal.pcbi.1004085
Legeay, M., Doncheva, N. T., Jensen, J. H. M. L. J., and Jensen, L. J. (2020). Visualize omics data on networks with omics visualizer, a cytoscape app. F1000Res 9, 157. doi:10.12688/f1000research.22280.2
Ligtenberg, W. P., Bosnacki, D., and Hilbers, P. A. (2013). Reconn: a cytoscape plug-in for exploring and visualizing reactome. J. Bioinform Comput. Biol. 11, 1350004. doi:10.1142/s0219720013500042
Luo, W., Pant, G., Bhavnasi, Y. K., Blanchard, J. R., and Brouwer, C. (2017). Pathview web: user friendly pathway visualization and data integration. Nucleic Acids Res. 45, W501–W508. doi:10.1093/nar/gkx372
Noronha, A., Danielsdottir, A. D., Gawron, P., Johannsson, F., Jonsdottir, S., Jarlsson, S., et al. (2017). ReconMap: an interactive visualization of human metabolism. Bioinformatics 33, 605–607. doi:10.1093/bioinformatics/btw667
Okuda, S., Yamada, T., Hamajima, M., Itoh, M., Katayama, T., Bork, P., et al. (2008). KEGG Atlas mapping for global analysis of metabolic pathways. Nuc Acids Res. 36, W423–W426. doi:10.1093/nar/gkn282
Paley, S., Billington, R., Herson, J., Krummenacker, M., and Karp, P. D. (2021). Pathway tools visualization of organism-scale metabolic networks. Metabolites 11, 64. doi:10.3390/metabo11020064
Paley, S., and Karp, P. (2024). The omics dashboard for interactive exploration of metabolomics and multi-omics data. Metabolites 14, 65. doi:10.3390/metabo14010065
Paley, S. M., and Karp, P. D. (2006). The Pathway Tools cellular overview diagram and omics viewer. Nuc Acids Res. 34, 3771–3778. doi:10.1093/nar/gkl334
Paley, S. M., Parker, K., Spaulding, A., Tomb, J., O’Maille, P., and Karp, P. D. (2017). The Omics Dashboard for interactive exploration of gene-expression data. Oxfordshire: Nuc Acids Res.
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. doi:10.1101/gr.1239303
Stuani, L., Lechaplais, C., Salminen, A. V., Segurens, B., Durot, M., Castelli, V., et al. (2014). Novel metabolic features in acinetobacter baylyi adp1 revealed by a multiomics approach. Metabolomics 10, 1223–1238. doi:10.1007/s11306-014-0662-x
Web Site User’s Guide for Pathway Tools-Based Web Sites (2024). Deletetitle. Available at: https://biocyc.org/PToolsWebsiteHowto.shtml.
Keywords: omics, omics analysis, multi-omics, multi-omics analysis, metabolic networks
Citation: Swart A, Caspi R, Paley S and Karp PD (2024) Visual analysis of multi-omics data. Front. Bioinform. 4:1395981. doi: 10.3389/fbinf.2024.1395981
Received: 04 March 2024; Accepted: 28 August 2024;
Published: 10 September 2024.
Edited by:
Andrew David Yates, European Bioinformatics Institute (EMBL-EBI), United KingdomReviewed by:
Sayed-Amir Marashi, University of Tehran, IranStefan P. Albaum, Bielefeld University, Germany
Copyright © 2024 Swart, Caspi, Paley and Karp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter D. Karp, cGthcnBAYWkuc3JpLmNvbQ==
 Austin Swart
Austin Swart Ron Caspi
Ron Caspi Suzanne Paley
Suzanne Paley Peter D. Karp
Peter D. Karp